1. Introduction
In the Iberian Peninsula, three species of rodents with very similar characteristics (Wood mouse, Algerian mouse and Common vole) currently coexist in its central plateau. Two of them have been using the resources and adapting to the conditions of the area for a long time (Wood mouse, Algerian mouse) but the other one has just arrived (Common vole) [
1,
2,
3]. By comparing their behavior with the use of resources and in the occupation of habitats, we intend to understand how they have adapted to the conditions of the occupied environment. We believe that to coexist three rodent species with similar characteristics should segregate their niches and habitats to prevent intense competition for resources from eliminating any of the competing species, as established by the principle of competitive exclusion [
4]. The adaptations acquired over time by each species in this respect should show up in the experiment we have set up. In this experiment, we compared the behaviour of the three rodent species living here in the occupation of three types of habitat and their behaviour during the processing of one of the most abundant resources in the area, the seeds of the oak species, the acorns. In recent years, the relationship between rodent species and oak has attracted the attention of several research groups [
5,
6,
7,
8,
9,
10,
11], but in many of them the interpretation of the outcome of these relationships has been changing depending on the pairs of species studied because in some cases rodents participate and collaborate in seed dispersal, but in others they only act as predators [
12].
From an ecological point of view, relationships between species are studied in terms of the benefits or detriments that each species obtains during the relationship [
13]. The outcome of the relationship for each species can be beneficial if the target species gains individuals or resources, detrimental if it loses individuals or resources, and neutral if there is no change in the number of individuals or resources available to it. On the basis of these premises, several types of relationships have been defined: Competition, where both species involved suffer detriment; Amensalism, where one suffers detriment and the other is undisturbed; Commensalism, one species benefits, the other is not altered; Predation, the predator species benefits, the prey species is harmed; Neutralism, neither species suffers variation; and Mutualism, both species benefit. However, by observing these relationships in various types of species, it has been shown that the relationship can change over time. For example, an asymmetrical competitive relationship in which one species suffers greater harm than the other may end up as amensalism. Predation, if the prey species does not suffer a significant depletion of its resources, can become commensalism, an example being the galls of hymenopterans produced on oak species.
Our research is oriented in this direction. We want to test whether a predation of acorns by rodents can be transformed into a relationship more akin to mutualism. Some authors have found that the relationship between scatter-hoarding rodents and oak species varies in their outcomes [
6,
7,
8,
9,
10,
11]. At one end of the range is antagonism, a relationship called predation which rodents species takes resources from oaks species that they suffer costs [
12]. At the other end is mutualism, where both species derive benefits. However, at the extreme of mutualism, the benefits must be symmetrical for both species. Therefore, relationships between rodent and oak species will never reach mutualism because the rodent always obtains more benefits than the plant. However, the contribution of some rodent species with the transport and storage of acorns to the oak dispersal process brings the relationship closer to mutualism and separates it from predation [
14,
15,
16].
Refs. [
10,
17,
18] proposed a mathematical model to assess whether the outcome of scatter-hoarding relationship is antagonism or mutualism [
19,
20]. When the benefits of transportation and burial are greater than the costs caused by predation during this process of scatter-hoarding, we can call the relationship mutualistic.
This range of results in rodent-oak relationships led us to believe that the observed variations may be due to the duration of the relationships. Of the three species investigated here, the two that have been consuming acorns for the longest time may have acquired adaptations that the third recently arrived species has not been able to acquire due to lack of time.
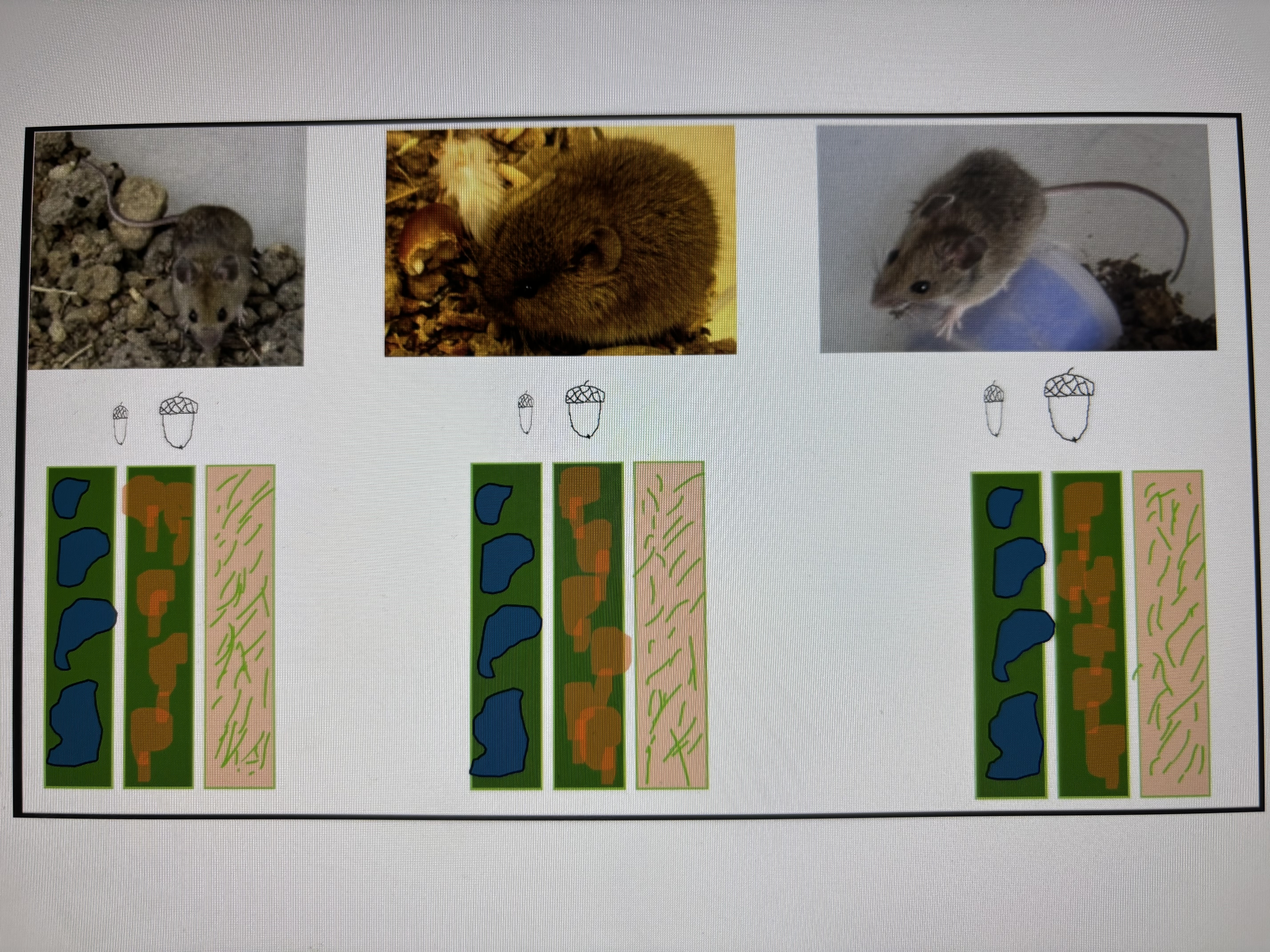
To test this, we placed the three rodent species in semi-wild conditions with three different habitat types to study their occupancy. We fed them acorns of the two most abundant oak species in the area to observe how they treat these seeds.
The objective we want to achieve is to test whether the duration of the rodent-oak relationship modifies the relationship through the adaptations that each species acquires. Does the time elapsed since the start of acorn consumption make any difference to the treatment of acorns during consumption? To test this, we compared the behaviour of three rodent species with different time spent handling acorns.
Another objective is to test whether there is habitat segregation between rodent species. This would allow coexistence without unduly increasing competition. Are there habitat preferences between rodent species? To verify this, we studied the occupation of different habitats by the three rodent species.
Finally, we want to know the degree of involvement of each rodent species in the process of dissemination of oak acorns through the transport and storage of acorns. Does each rodent species transport and store acorns? Where do they store them? To find out, we tracked the fate of the acorns after processing by rodents.
3. Results
The results of the experiment were estimated as the number of acorns consumed daily by each rodent species in each habitat of each of the two oak species. In
Table 1 we present the results of the GLMM model in which we have observed the interactions between rodent and oak species, habitat type and time course estimated as number of acorns consumed.
The distribution of the residuals allowed us to observe the good fit of the chosen model. We compared the fit with other models, but the AIC values indicated that the chosen model had the best fit.
There are significant differences between rodent species in the number of acorns consumed. The same differences are observed between the two oak species. More acorns are consumed from one than from the other. There are also differences in the number of acorns consumed between habitats.
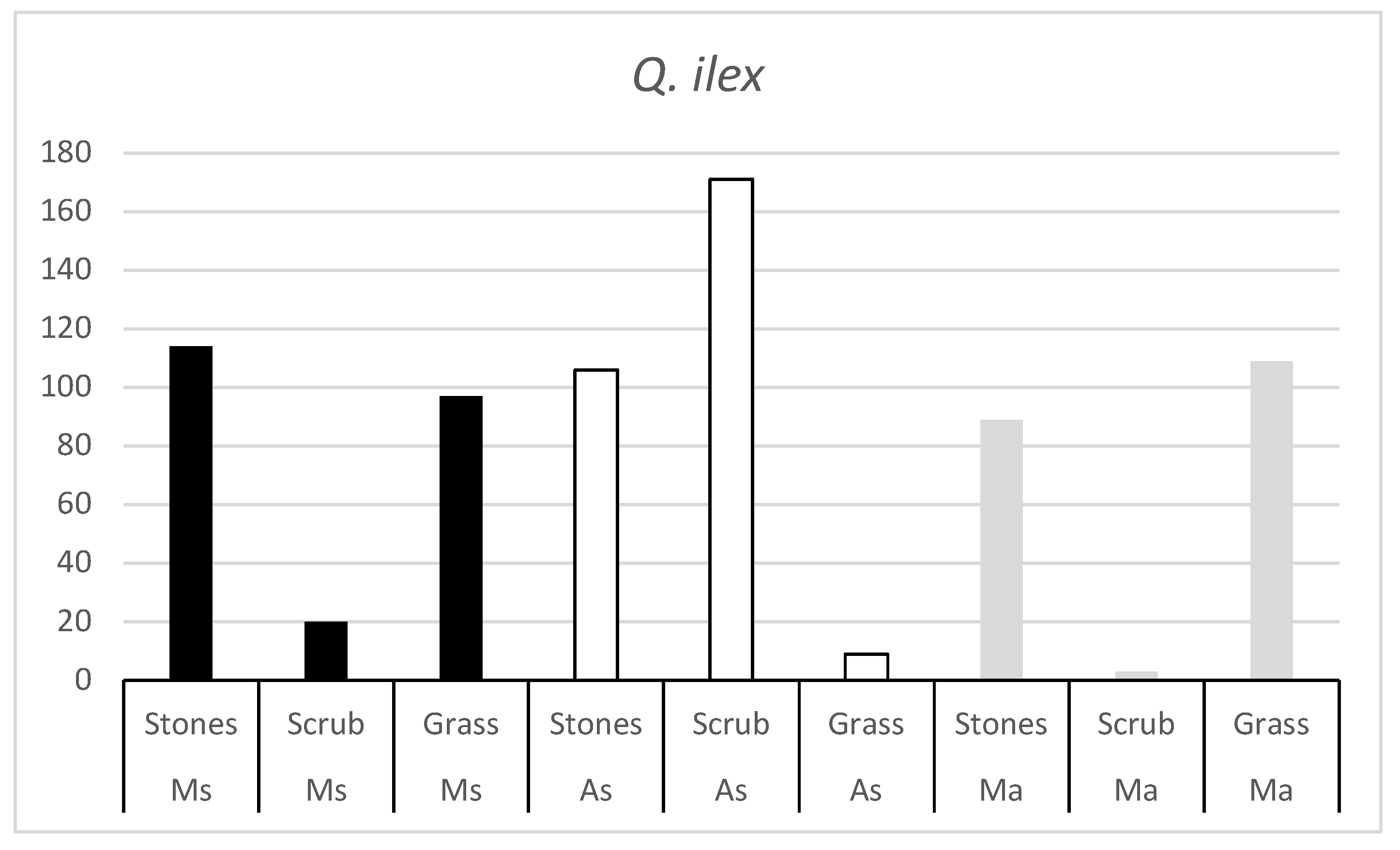
The number of Q. ilex acorns consumed by each rodent species in the three habitats (
Fig 1) shows that in all three rodent species there is a habitat that is little appreciated. The number of acorns consumed in these habitats is very low, indicating that the rodent is not active in these habitats.
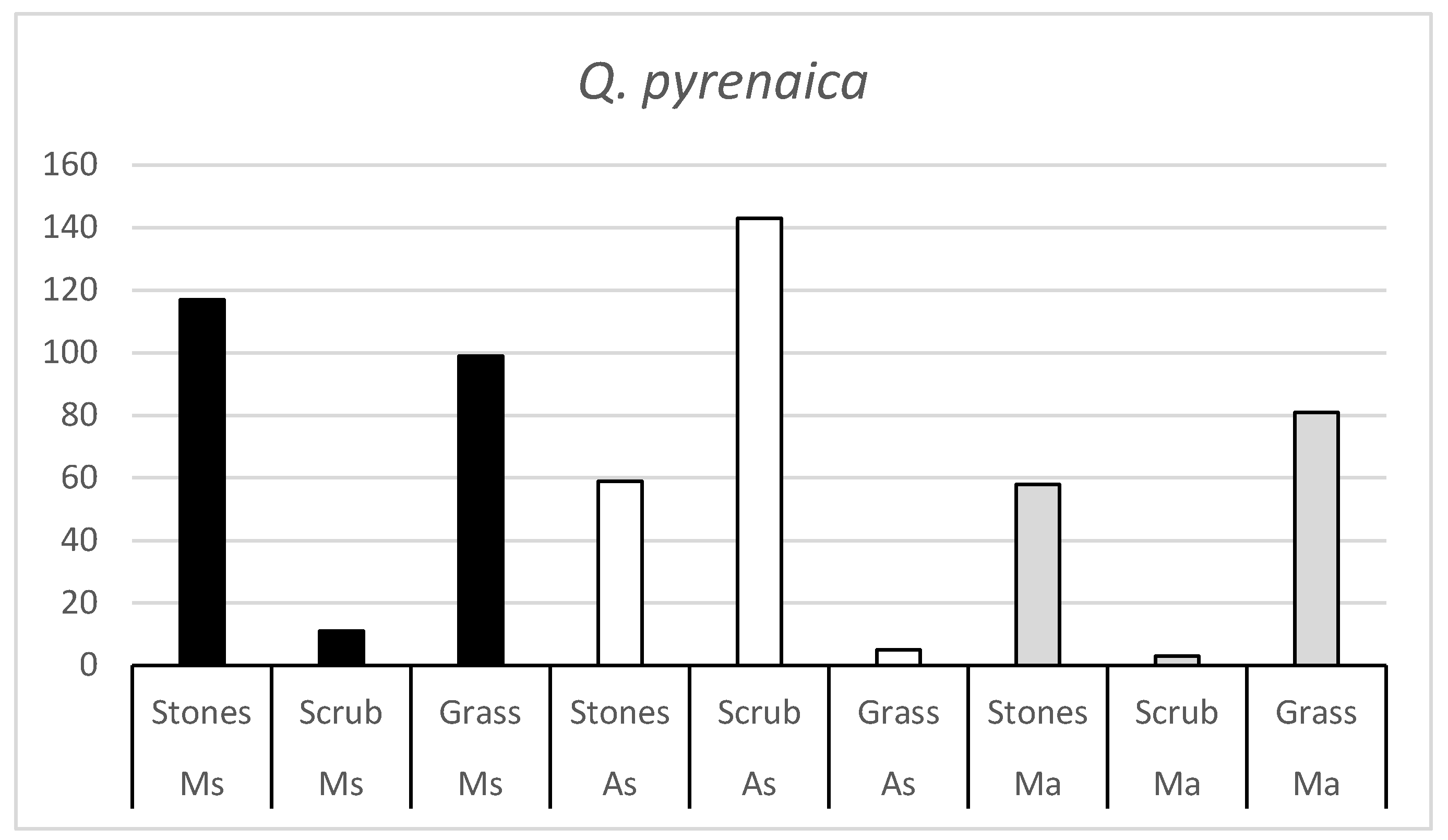
Figure 2 shows the number of
Q. pyrenaica acorns consumed in each habitat by the three rodent species. There are also clearly significant differences in the number of acorns consumed in some habitats within each rodent species.
In the same way, we have studied the interactions between habitats and species using the mass of acorns consumed per acorn per day by rodent species. In
Table 2 we present the result of the GLMM model in which we tested the interactions between rodent and oak species acting in different habitats. The distribution of residuals allowed us to observe the good fit of the chosen model. We compared the fit with other models, but the AIC values indicated that the chosen one presents the best fit. There are also significant differences in the average mass that each rodent species consumes of each acorn per day. Between the two oak species there are also differences in the average amount consumed per acorn per day. However, between habitats there are no differences in the consumption of the mass of acorns per day.
Algerian mouse ingests the smallest daily mass of each acorn. It is the smallest species [
35]. On the contrary, the largest species, Common vole, consumes the highest mass per acorn per day (
table 3). Rodents generally consume more
Q. ilex than
Q pyrenaica.
Q. ilex has higher concentrations of sugars, proteins and fats [
35], making it the more nutritious species. Possibly for this reason these acorns are preferred to those of the other species. Of the three rodent species Algerian mouse and Wood mouse consume more of the Q. ilex acorns, but Common vole does not show a preference for any of the two oak species.
To check the acorn consumption rate of the three rodent species in the three habitats, we subtracted the number of acorns consumed or mobilized per day during the seven days of the experiment from the initial number of acorns of each species. We fitted a linear model to the values obtained and calculated the slope of the line to compare the speed of acorn mobilization by habitat and rodent species (
Table 4).
Depending on the slope, the species mobilizing acorns the fastest is Wood mouse in the scrub habitat for the two oak species. The acorns of the two oak species are moved less quickly by Algerian mouse in the rock and grass habitats. Common vole also moved acorns of both oak species with speed in grassy and rocky habitats. Wood mouse shows little activity in the grass habitat and Algerian mouse and Common vole do not visit the scrub habitat much.
To check the activity of the three rodent species in the three habitats, we estimated the mean mass of acorns consumed per acorn and days in the three species and the three habitats (
Table 5). The mean value of the mass of acorns consumed in each habitat does not show significant differences. However, significant differences do appear when observing the mean consumption per acorn by each rodent species in each habitat. With this variable, the same trends are observed when using the slope of the straight line adjusted for the number of acorns disappeared per habitat. The same trends are also observed in the mass of acorns consumed by each rodent species. Algerian mouse ingests the least mass but does not differ among the three habitats. The mass consumed is slightly lower in the habitat that is least visited, the shrubland. Wood mouse and Common vole do show significant differences in the habitat least frequented by each.
To check the treatment of acorns by each rodent species, we have classified the remains of acorns located after being mobilized in four different classes: those started to eat at the Basal end (B), those that open at the Apical end (A), where the embryo is located, those eaten Totally (T) and those not used that remain Intact (I). Those initiated by the apical part may or may not keep the embryo.
Table 6 shows the results of the treatment of
Q. ilex acorns by the three rodent species. In the three columns on the left we show the number of acorns of each class and the percentage that this number represents with respect to the total number of acorns provided (450 in the three habitats). The Apical class is divided into two parts because some of the acorns beginning at the apical end may retain the embryo after attack. In the right column, the number of Basal plus Intact and Apical acorns that retain the embryo is summed to compile all acorns of these three classes that retain the embryo after consumption. We also added the percentage that this number represents with respect to the total number of acorns provided (450 in the three habitats). The two rodent species that process the most
Q. ilex acorns are Algerian mouse and Wood mouse. A high percentage of the acorns they process begin to be eaten at the basal end (33 %). Together with the intact acorns that are not used, these acorns reach a percentage of about 89% of acorns that retain the embryo after being handled by these two rodent species. The behavior of Common vole is different. This species leaves many acorns intact, but the acorns it uses start at the apical end, destroying the embryo.
The treatment of
Q. pyrenaica acorns shows similar trends to those used with
Q. ilex (
table 7), although in this case the number of acorns attacked is lower because a higher number remains intact. The proportion of acorns that Algerian mouse and Wood mouse open at the basal end remains around 30%. However, Common vole opens at the apical end, destroying the embryo with almost all the acorns it consumes.
Table 8 shows the destinations of
Q. ilex acorns located after their mobilization by rodents in the different habitats.
Algerian mouse in the two habitats in which it is active shows a preference for depositing the acorns of this oak species in the larder [
22]. It consumes few acorns in situ, leaves a small number of acorns on the surface and stores a significant number of acorns in scattered caches, but this number is half of those deposited in the larder.
On the contrary, it happens with Wood mouse. This rodent species prefers to deposit Q. ilex acorns in scattered caches in the habitat it occupies. Even more so in the stone habitat that it also visits, it removes acorns that it deposits in caches made in the scrub habitat, its preferred habitat, although it also transports acorns from the scrub habitat to the stone habitat where it buries them in caches.
Common vole consumes most of the acorns it processes in situ. The number of acorns abandoned on the surface or introduced into larders is negligible [
22]. It does not bury acorns in caches. It does not engage in this behavior.
The same result was obtained for
Q. pyrenaica acorns (
Table 9). Algerian mouse deposits more acorns of this oak species in the larder than in the other destinations. It makes burials in caches, but the acorns deposited there are half as many as in the larder. Common vole also consumes acorns of this oak species in situ. Wood mouse maintains a preference for burying acorns of this species in scattered caches, moving acorns between preferred habitats.
4. Discussion
The three rodent species chosen for this study share space in the center of the Iberian Peninsula, in the central plateau [
3,
27]. However, as we have been able to verify, they segregate their habitats, perhaps in order not to establish an intense competition for the same resources that would be detrimental to all the competing species [
4]. Wood mouse was the first species to arrive in this region. This species prefers to occupy more intensively the habitat constituted by thickets and small trees. It probably prefers this structure because the thickets protect them from the action of their own predators [
36]. As it has been occupying this region for a long time, it is likely to have had negative experiences with predators such as birds of prey [
2]. These birds are not active in the thickets, which hinder their movements to locate rodents. It is therefore possible that this rodent species seeks the protection of thickets for its feeding activities. According to [
37] this species is not present in the interior of large forests or monospecific formations. In these situations, the dominant woody species does not allow the proliferation of other species, not even the appearance of scrub. They are usually homogeneous and unprotected surfaces where rodents would be stalked from tree branches without any protection. Another habitat frequented by this rodent is rocks. Occupying the galleries between rocks or even moving around the base of rocks provides them with protection from predators. Where they are almost not active is in grassy habitats. These are open areas where the rodent would be visible to birds of prey flying overhead. In this way Wood mouse separates its breeding habits from those of Algerian mouse. This second species does not enter areas of dense scrub. It has occupied the Iberian Peninsula for less time than Wood mouse, but is attacked by the same species of birds of prey. It escapes from them by taking advantage of the galleries in the rocky habitat, which it occupies most intensively. It also moves through open crop and grassy habitats, where it could be attacked by predatory birds. However, it escapes from them by occupying tall grassy pastures. It uses this grass as a protective element, making galleries between it without digging into the ground. The tall grass covers them from above and hides them from the sight of predators [
36]. As we can see, each species uses different strategies to deal with the same problem. Common vole also inhabits crop and pasture areas where it is currently confined in the study region. Here it visits grassy habitats more intensively. It also suffers predation by birds of prey, but its escape strategy is to build galleries, tunnels, which it digs in the ground, unlike the previous species. This species also uses the habitat of stones, where it finds galleries already built by the proximity of the stones, thus escaping the action of predators. This segregation of the habitats that each rodent species occupies allows them to coexist without competition between them for any particular resource displacing or excluding any of the species [
4].
The average mass of acorns that each rodent species ingests depends on its size. The smaller species consumes less acorns and the larger species consumes more average mass of acorns, which is logical as it has to maintain a larger body mass [
38].
Q. ilex is the oak species with the highest mass consumed by the three rodent species, although in Common vole the difference does not reach significant levels. This difference in consumption could be due to the fact that
Q. ilex has higher concentrations of fats, sugars and proteins [
35], which gives it more nutritional properties. To cope with winter hardships,
Q. ilex acorns could provide greater reserves [
39]. There are no significant differences in the amount of acorn mass consumed between habitats. These differences only appear in the habitats least used by each rodent species.
The two oak species used here have been selected because they are the most widespread in the study region [
31]. Possibly, because of their density, they are also the most abundant. The rodent species that first arrived in the Iberian Peninsula is Wood mouse (Pleistocene), during the Neolithic period Algerian mouse arrived from Africa and Common vole has just arrived. The two rodent species that have been occupying the region the longest are likely to have encountered the acorns of the two oak species more frequently during their breeding season, given their abundance [
40]. It is likely that this is why they have incorporated them into their diet, setting aside the nutritional characteristics that their cotyledons possess [
41]. Common vole has only recently arrived and has not previously encountered this food because it does not occupy woodland or wooded areas. Its food consists of fresh green herbaceous plant material, which are the plants it finds in the agricultural areas and pastures where it lives [
3]. In this experiment, its usual food was available in all three habitats, but more densely in the grassy habitat. This is probably the first time it has had acorns of these species to feed on.
All these differences are reflected in the different ways of handling the acorns of each rodent species. The behaviour shown during the processing of acorns is different in the three rodent species. Wood mice and Algerian mice have long consumed acorns. Both rodent species open most of the acorns they process at the basal end away from the position of the embryo at the apical end (between 24 and 33 % of the available acorns and between 71 and 78 % of the acorns attacked). This means that in a first attack 71 % of the acorns processed by these two rodent species retain the embryo and therefore the ability to germinate. Why does this behaviour occur? [
42,
43] seem to suggest a certain awareness of the rodent in this basal opening, but this is not possible, because they spend more effort on this type of opening than on the apical opening. The apical end is narrower and the mouth opening required is smaller. We think that the rodents are rather forced by the characteristics of the acorns to use this basal opening. According to [
44,
45,
46,
47] oak species accumulate high concentrations of tannins in the embryo environment to prevent predators from eating the embryo. Based on the results of this experiment, we believe that the highest amount of tannins is concentrated in the apical shell surrounding the embryo. All acorns consumed in their entirety by Wood mouse and Algerian mouse retained 1/3 of the shell of the apical dome surrounding the embryo intact (
Fig 3). This structure is probably not attacked by the rodents because of the bad taste that the tannins accumulated there give them and they are forced to access the inside of the acorn through the basal end, which is more difficult to access. It is likely that these two rodent species, which have been consuming acorns longer, have had the negative experience of the bad taste of the tannins [
45] at this apical end of the shell, which has forced them to adapt to opening the acorns from the basal part, even though it is more difficult for them.
Common vole shows a different behaviour, opening acorns at the apical end as we have verified by acorn remains and recordings [
35]. Common vole has not previously used this type of feed. The lack of previous experience leads it to ignore the bad taste of tannins at this apical end because it does not know how the rest of the shell tastes.
It is likely that Wood mouse and Algerian mouse, which have been consuming acorns for a long time, have adapted to open the acorns at the basal end because of the bad taste of the tannins in the apical part. But this behaviour favours the plants as it preserves the embryo at least during the first attack [
23]. For this reason, we believe that the relationship of these two rodent species with oak species, which began as acorn predation by adaptation, has shifted towards the extreme of mutualism [
17,
48,
49]. This relationship cannot be called mutualism because it is not total mutualism, but it is close in that direction as the rodents benefit the plant. In a conscious way? We think not. They do this behaviour out of obligation, because of the bad taste of the tannins that the plant places at the apical end [
44,
45]. However, in any case, they favour the oak species with this behaviour [
42]. They provide them with a benefit, that their acorns can germinate after being partly consumed [
43]. The rodents of these two species also benefit, but they already did so when the relationship was one of simple predation.
Wood mice have been in the Iberian Peninsula longer than Algerian mice [
24,
25]. It has probably also been practising this behaviour for longer, which is why it opens a smaller number of acorns at the ‘wrong’ end, the apical end. Algerian mouse opens a greater number of acorns at the apical end than Wood mouse, as if it had not yet fully acquired this behaviour, but the number of acorns it opens at the basal end is still much greater, even though it has been practising this behaviour for less time.
The behaviour of the three rodent species during the handling, transport and storage of acorns is also different. Common vole does not transport acorns. It consumes most of the acorns on the spot where it finds them. A small number are scattered and left on the surface. Nor does it bury acorns. This behaviour can be explained by the fact that, as it is a newcomer species, it does not know or has not had previous experience with the new predators that may be lurking in this region [
36]. Because of this lack of experience, it consumes acorns directly where it finds them without worrying that the predators may be its own specimens. Wood mice and Algerian mice, as they have been in the region longer, have already had negative experiences with predators, especially birds of prey that stalk them from the sky [
36]. For this reason, they seek the protection of bushes, stones or grass so as not to be located. The primary dispersal of the acorns of oak species is by barocory, falling by gravity from the tree to be deposited on the ground under the tree. The points where the rodents locate the acorns are under the producing trees, which do not allow the proliferation of many plants due to competition. Therefore, there is little protection under the treetops where acorns are deposited. If experience leads rodents to look for food under the canopy of these trees, birds of prey also have the experience that the mice they feed on are concentrated under the trees [
36]. For this reason, as they find no protection when locating acorns, the rodents of these two species (Wood mouse and Algerian mouse) must tend to take the acorns as quickly as possible to protected places to process them. It is possible that this is one of the reasons why rodents of these two species initiate the transport of acorns and thus their dissemination. We must understand that transport is an energy expenditure that would not be necessary if there were no important reason for this expenditure.
The differences observed between Common vole and the other two rodent species in the in situ consumption and transport of acorns could be explained by the adaptations and experiences that Wood mouse and Algerian mouse have acquired due to the longer time they have been handling acorns in this region. Some authors provide other causes that also prevent on-site consumption. Any characteristic of acorns that lengthens the handling time prevents their consumption in situ, including the presence of tannins [
5,
50,
51]. To reduce the concentration of tannins it is necessary to store them for a long time [
45].
There are also differences between the three rodent species in the behaviour that leads acorns to their final destination [
52,
53]. Wood mice and Algerian mice consume few acorns in situ because of the aforementioned risk of predation. However, they also leave few acorns on the surface. The fundamental difference between the two species is that Wood mouse places most of the acorns it processes in caches [
54,
55]. This behaviour may be due to the fact that reciprocal theft between conspecifics is widespread in rodent societies of this species [
55]. In order to avoid losing the harvest, each specimen makes shallow accumulations of a small number of acorns in the cache. They are shallow to provide immediate food supplies in the near future without expending a lot of energy burying and digging up acorns [
55]. The caches consist of a small number of acorns because if they are stolen, only a small part of the hard-earned acorns are lost [
54]. This kind of storage favours the dispersal process because if the stored acorns germinate, they have little difficulty in emerging [
53]. This rodent species participates intensively in the process of acorn dissemination. Algerian mice, on the contrary, accumulate a greater number of acorns in the artificial containers constructed to mimic their larders [
22,
41,
53]. This behaviour seems to be more typical of rodents that expect to survive the adverse conditions of winter [
9]. They accumulate a large number of resources so that they do not have to forage during unfavourable winter days [
53,
56]. This kind of storage is deeper than the caches, and if the stored acorns germinate, it is more difficult for them to emerge. The number of acorns stored is also greater than in caches, but the probability of forgetting their location is lower than in the case of caches due to their smaller number and because they are usually resting places, sharing the function of storage and refuge. This species also participates in the process of acorn dispersal. The rodent species that does not participate in the dissemination of acorns is Common vole, firstly because it does not transport acorns and secondly because its behaviour is that of a predator, destroying the embryo of the acorns. It is a hostile species for the acorn dispersal process.
5. Conclusions
The three rodent species, with similar characteristics, occupy the same territory, but separate their niches and habitats to avoid strong competition for resources that would lead to the exclusion of one of them.
The presence of rodent predators in the region is one of the factors determining the choice of habitat for protection.
The presence of these rodent predators may be one of the factors involved in the process of acorn dispersal as it may be one of the causes for the transport of acorns by rodents at the initial stage of the dissemination process. Rodents must seek refuge from predators and therefore take the acorns to protected places for processing.
The length of time a relationship is maintained can lead to adaptations that vary the outcome of the relationship. The two species that have consumed acorns for the longest time have adapted to consume acorns by opening them at the basal end, possibly because of previous unpleasant experiences with tannins. This behaviour preserves the embryo and serves the interests of the plants by allowing their seeds to germinate. This rodent behaviour shifts the relationship from initial predation to mutualism.
Common vole does not store acorns, does not transport them and destroys its embryo behaving like a predatory species. It is hostile to the acorn dispersal process. The other two species do store acorns. Wood mouse in caches which could favour the germination and emergence of acorns. Algerian mouse stores them in larders, which could hinder the emergence of acorns. These two species collaborate in the process of acorn dispersal, bringing the relationship closer to mutualism.