Submitted:
10 April 2025
Posted:
10 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Flowering Phenology
2.2. Floral Biology and Rewards
3.3. Pollination Components
3.4. Reproductive Success
3. Discussion
4. Materials and Methods
4.1. Study System
4.2. Flowering Phenology
4.3. Floral Biology and Rewards
4.4. Pollinator Components
2.5. Reproductive Success
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemoine-Rodríguez, R.; Inostroza, L.; Zepp, H. The global homogenization of urban form. An assessment of 194 cities across time. Landsc. Urban Plan. 2020, 2004, 103949. [CrossRef]
- Richards, D. R.; Belcher, R. N. Global changes in urban vegetation cover. Remote Sens. 2019, 12, 23. [CrossRef]
- McDonald, R. I.; Kareiva, P.; Forman, R. T. T. The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol. Conserv. 2008, 141, 1695–1703. [CrossRef]
- Teixido, A.L.; Kaminski, L.A.; Fuzessy, L.F.; Oliveira, P.C.; Souza, C.S.; Gomes, I.N.; Maruyama, P.K. Anthropogenic impacts on plant-animal mutualisms: A global synthesis for pollination and seed dispersal. Biol. Conserv. 2022, 266, 109461. [CrossRef]
- United Nations. World Urbanization Prospects. United Nations, N.Y., 2018, 125 p.
- Myers, N.; Mittermeier, R. A.; Mittermeier, C. G.; da Fonseca, G. A. B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature. 2000, 403, 853–858. [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra L,R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA. 2012, 109, 16083–16088. [CrossRef]
- Van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science. 2020, 368, 417–420. [CrossRef]
- Luck, G.W. A review of the relationships between human population density and biodiversity. Biol. Rev. 2007, 82, 607–645. [CrossRef]
- Tan, P.Y.; Zhang, J.; Masoudi, M.; Alemu, J.B.; Edwards, P.J.; Grêt-Regamey, A.; Richards, D.R.; Saunders, J.; Song, X.P.; Wong, L.W. A conceptual framework to untangle the concept of urban ecosystem services. Landsc. Urban Plan. 2020, 200, 103837. [CrossRef]
- Bernard, E.; de Lucena Damasceno, L.T.; de Frias, A.V.C.; Hintze, F. Assessing the Effects of Urbanization on Bats in Recife Area, Atlantic Forest of Brazil. In: Urban Bats: Biology, Ecology, and Human Dimensions; Ortega, J., Dixon, M.D., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 123–136.
- Harrison, T.; Winfree, R. Urban drivers of plant-pollinator interactions. Func. Ecol. 2015, 29, 879-888. [CrossRef]
- Russo, D.; Ancilloto, L. 2015. Sensitivity of bats to urbanization: a review. Mamm. Biol. 2015, 80, 205‒2012. [CrossRef]
- Silva, J.L.S.; Pontes de Oliveira, M.T.; Cruz-Neto, O.; Tabarelli-Lopes, A.V. Plant–pollinator interactions in urban ecosystems worldwide: A comprehensive review including research funding and policy actions. Ambio. 2020, 50, 884‒990. [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination– A systematic review. Biol. Conserv. 2020, 241, 108321. [CrossRef]
- Youngsteadt, E.; Keighron, M. C. Urban pollination ecology. Annu. Rev. Ecol. Evol. Syst., 2023, 54, 21–42. [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals?. Oikos. 2011, 120, 321–326. [CrossRef]
- Delaplane, K.S. Crop Pollination by Bees, Volume 1: Evolution, Ecology, Conservation, and Management; CABI: Wallingford, UK, 2021; 141 pp.
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban agriculture in the developing world: A review. Agron. Sustain. Dev. 2013, 33, 695–720. [CrossRef]
- Zezza, A.; Tasciotti, L. Urban agriculture, poverty, and food security: Empirical evidence from a sample of developing countries. Food Policy. 2010, 35, 265‒273. [CrossRef]
- Duchamp, J.E.; Swihart, R.K. Shifts in bat community structure related to evolved traits and features of human-altered landscapes. Landsc. Ecol. 2008, 23, 849‒860. [CrossRef]
- Lüttge, U.; Buckeridge, M. Trees: structure and function and the challenges of urbanization. Tree. 2023, 37, 9‒16. [CrossRef]
- Dzul-Cauich, H. F.; Munguía-Rosas, M. A. Negative effects of light pollution on pollinator visits are outweighed by positive effects on the reproductive success of a bat-pollinated tree. Sci. Nat. 2022, 109, 12. [CrossRef]
- Macgregor, C.J.; Evans, D.M.; Fox; Pocock, M.O. The dark side of street lighting: impacts on moths and evidence for the disruption of nocturnal pollen transport. Glob. Chang. Biol. 2017, 23, 697‒707. [CrossRef]
- Neil, K.; Wu, J. Effects of urbanization on plant flowering phenology: A review. Urban Ecosyst. 2006, 9, 243–257. [CrossRef]
- Silva, V. H. D.; Gomes, I. N.; Cardoso, J. C. F.; Bosenbecker, C.; Silva, J. L. S.; Cruz-Neto, O.; Oliveira, W.; Stewart, A. B.; Lopes, A. V.; Maruyama, P. K. Diverse urban pollinators and where to find them. Biol. Conserv. 2023, 281, 110036. [CrossRef]
- Baldock, K.R.C. Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect. Sci. 2020, 38, 63‒71. [CrossRef]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: a meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412– 1429. [CrossRef]
- Liang, H.; He, Y-D.; Theodorou, P.; Yang, C-F. The effects of urbanization on pollinators and pollination: A meta-analysis. Ecol. Lett. 2023, 26, 1629–1642. [CrossRef]
- Buchholz, S.; Egerer, M.H. Functional ecology of wild bees in cities: towards a better understanding of trait- urbanization relationships. Biodiv. Conserv. 2020, 29, 2779– 2801. [CrossRef]
- Maruyama, P. K.; Silva, J. L. S.; Gomes, I. N.; Bosenbecker, C.; Cruz-Neto, O.; Oliveira, W.; Cardoso, J. C. F.; Stewart, A. B.; Lopes, A. V. A global review of urban pollinators and implications for maintaining pollination services in tropical cities. Ecology of Tropical Cities: Natural and Social Sciences Applied to the Conservation of Urban Biodiversity. 2021. [CrossRef]
- Ramírez-Fráncel, L. A.; García-Herrera, L. V.; Losada-Prado, S.; Reinoso-Flores, G.; Sánchez-Hernández, A.; Estrada-Villegas, S.; Lim, B. K.; Guevara, G. Bats and their vital ecosystem services: A global review. Integr. Zool. 2022, 17, 2‒23. [CrossRef]
- Ratto, F.; Simmons, B. I.; Spake, R.; Zamora-Gutierrez, V.; MacDonald, M. A.; Merriman, J. C.; Tremlett, J. C.; Poppy, G. M.; Peh, K. S-H.; Dicks, L. V. Global importance of vertebrate pollinators for plant reproductive success: A meta-analysis. Front. Ecol. Environ. 2018, 16, 82–90. [CrossRef]
- Jung, K.; Threlfall, C.G. Urbanization and Its Effects on Bats—A Global Meta-Analysis. In: Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: New York, NY. 2016; pp. 13–33. [CrossRef]
- Russo, D.; Coleman, J.L.; Ancillotto, L.; Korine, C. Ecosystem Services by Bats in Urban Areas. In: Urban Bats: Biology, Ecology, and Human Dimensions; Moretto, L., Coleman, J.L., Davy, C.M., Fenton, M.B., Korine, C., Patriquin, K.J., Eds.; Springer: New York, NY. 2022; pp. 167–180. [CrossRef]
- Lu, P.; Yu, Q.; Liu, J.; Lee, X. Advance of tree-flowering dates in response to urban climate change. Agric. For. Meteorol. 2006, 138, 120–131. [CrossRef]
- Baumann, P.R. Urban heat island lesson. Geocarto International. 2009, 24, 473–483. [CrossRef]
- Neil, K.; Wu, J.; Bang, C.; Faeth, S. Urbanization affects plant flowering phenology and pollinator community: effects of water availability and land cover. Ecol. Process. 2014, 3, 17. [CrossRef]
- Mendes, P.; Vieira, T.B.; Oprea, M.; Ditchfield, A.D. Long-distance movement of Artibeus lituratus (chiroptera: phyllostomidae) in the state of espírito santo, Brazil. Ecotropica. 2009, 15, 43–46.
- Morrison, D.W. Foraging ecology and energetics of the frugivorous bat Artibeus jamaicensis. Ecology. 1978, 59, 716‒723. [CrossRef]
- Biella, P.; Tommasi1, N.; Guzzetti, L.; Pioltelli, E.; Labra, M.; Galimberti, A. City climate and landscape structure shape pollinators, nectar and transported pollen along a gradient of urbanization. J. Appl. Ecol. 2022, 59, 1586–1595. [CrossRef]
- Villareal, A.G.; Freeman, C.E. Effects of temperature and water stress on some floral nectar characteristics in Ipomopsis longiflora (Polemoniaceae) under controlled conditions. Int. J. Plant Sci. 1990, 151, 5–9. [CrossRef]
- Ayuntamiento de Mérida. Estudio-diagnóstico del arbolado urbano en parques públicos de Mérida. Mérida, México. 2018. Available at: http://www.merida.gob.mx/sustentable/contenidos/doc/ArboladoUrbano_Parques.pdf. Last accessed: 16/03/2025.
- Ortega, J.; Castro-Arellano, I. Artibeus jamaicensis. Mammalian Species. 2001, 662, 1–9. [CrossRef]
- Ávila-Gómez A.S.; Moreno, C.; García-Morales, R.; Zuria, I., Sánchez-Rojas, G.; Briones-Salas, M. 2015. Deforestation thresholds for phyllostomid bat populations in tropical landscapes in the Huasteca region, Mexico. Trop. Conserv. Sci. 2015, 8, 646‒661. [CrossRef]
- Rodríguez-Durán, A.; Otero, W. Species richness and diversity of a West Indian bat assemblage in a fragmented ecosystem. Acta Chiropterol. 2011, 13, 439‒445. [CrossRef]
- Dzul-Cauich, H.F.; Stoner, K.; Ibarra-Cerdeña, C.N.; Munguía-Rosas, M.A. Living away from specialized pollinators: The pollination system of Ceiba pentandra in the Yucatan Peninsula. Ecol. Evol. 2025, 15, e70974. [CrossRef]
- Guimarães-Alves, S.; Gaglianone, M. C. Bee guilds’ responses to urbanization in neotropics: A case study. Diversity. 2021, 13, 365. [CrossRef]
- Gribel, R.; Gibbs, P. E.; Queiróz, A. L. Flowering phenology and pollination biology of Ceiba pentandra (Bombacaceae) in Central Amazonia. Journal of Tropical Ecology. 1999, 15, 247‒263. [CrossRef]
- Lobo, J. A.; Quesada, M.; Stoner, K. E. Effects of pollination by bats on the mating system of Ceiba pentandra (Bombacaceae) populations in two tropical life zones in Costa Rica. Am. J. Bot. 2005, 92, 370‒376. [CrossRef]
- Finer, M.S.; Morgan, M.T. Effects of natural rates of geitonogamy on fruit set in Asclepias speciosa (Apocynaceae): evidence favoring the plant's dilemma. Am. J. Bot. 2003, 90, 1746‒1750. [CrossRef]
- Herrerías-Diego, Y.; Quesada, M.; Stoner, K. E.; Lobo, J. A.; Hernández-Flores, Y.; Montoya, G. S. Effect of forest fragmentation on fruit and seed predation of the tropical dry forest tree Ceiba aesculifolia. Biol. Conserv, 2008, 141, 241−248. [CrossRef]
- Dick, C. W.; Bermingham, E.; Lemes, M. R.; Gribel, R. Extreme long-distance dispersal of the lowland tropical rainforest tree Ceiba pentandra L. (Malvaceae) in Africa and the Neotropics. Mol. Ecol. 2007, 16, 3039-3049. [CrossRef]
- Lobo, J. A.; Quesada, M.; Stoner, K. E.; Fuchs, E. J.; Herrerías-Diego, Y.; Rojas, J.; Saborio, G. Factors affecting phenological patterns of bombacaceous trees in seasonal forests in Costa Rica and Mexico. Am. J. Bot. 2003, 90, 1054-1063. [CrossRef]
- Elmqvist, T.; Cox, P. A.; Rainey, W. E.; Pierson, E. D. Restricted pollination on oceanic islands: pollination of Ceiba pentandra by flying foxes in Samoa. Biotropica. 1992, 24, 15−23. [CrossRef]
- Singaravelan, N.; Marimuthu, G. Nectar feeding and pollen carrying from Ceiba pentandra by pteropodid bats. J. Mamm. 2004, 85, 1‒7. [CrossRef]
- Pennington, T. D., Sarukhán, J. . Árboles tropicales de México: manual para la identificación de las principales especies. Mexico City; UNAM. 2005, 523 p.
- Islebe, G.A.; Sánchez-Sánchez, O.; Valdéz-Hernández, M.; Weissenberger, H. Distribution of Vegetation Types. In; Biodiversity and Conservation of the Yucatán Peninsula; Islebe, G.; Calmé, S.; León-Cortés, J.; Schmook, B., Eds.; Springer: Cham, Switzerland, 2015; pp. 39–53.
- Luna-Nieves, A.; González, E. J.; Cortés-Flores, J.; Ibarra-Manríquez, G.; Maldonado-Romo, A.; Meave, J. Interplay of environmental cues and wood density in the vegetative and reproductive phenology of seasonally dry tropical forest trees. Biotropica, 2022, 54, 500–514. [CrossRef]
- Dafni, A. Pollination Ecology: A Practical Approach; Oxford University Press: Oxford, UK, 1992; 250 pp.
- R Core Team. 2020. R: A language and environment for statistical computing. Vienna, Austria; R Foundation for Statistical Computing.
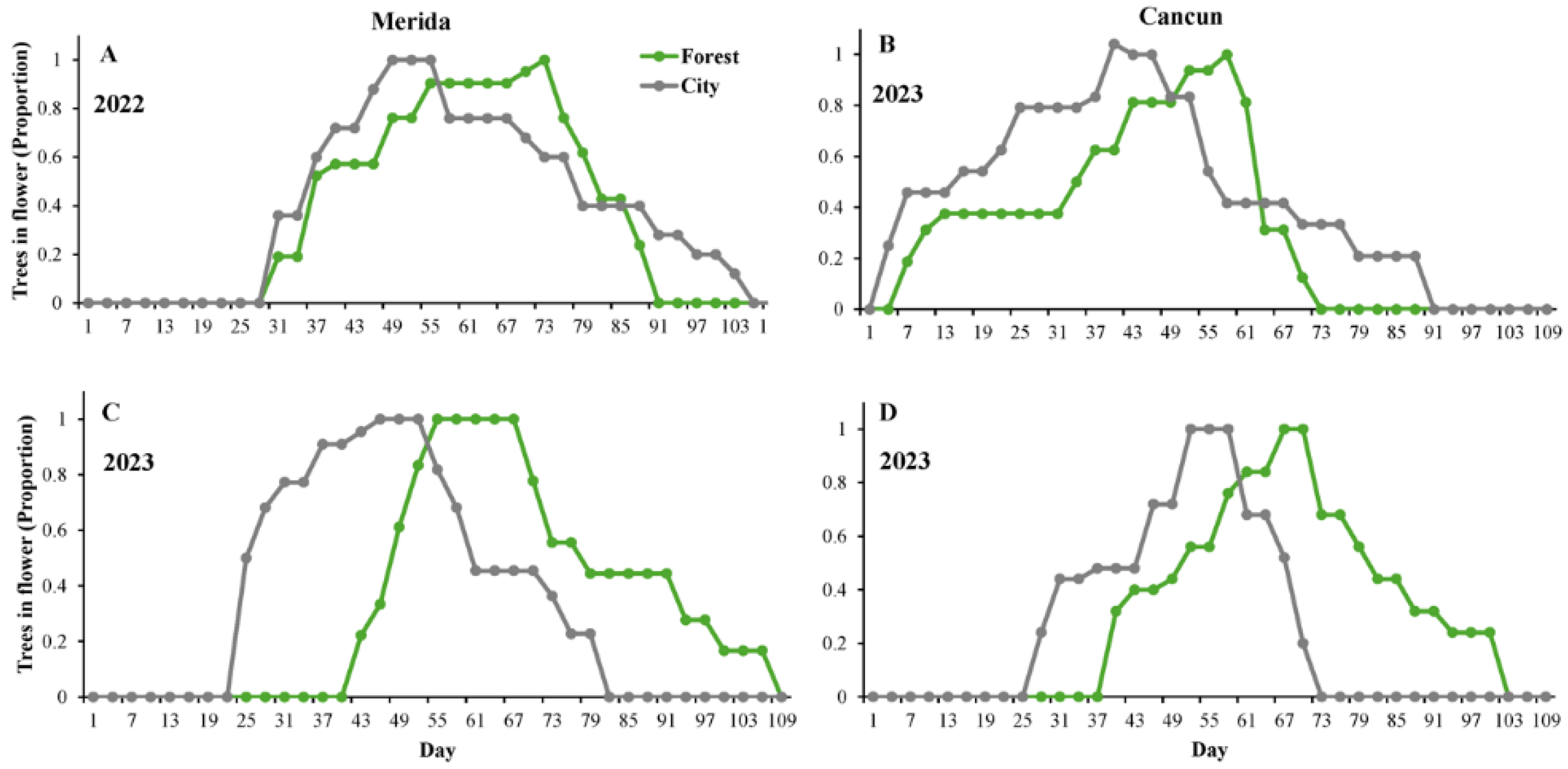
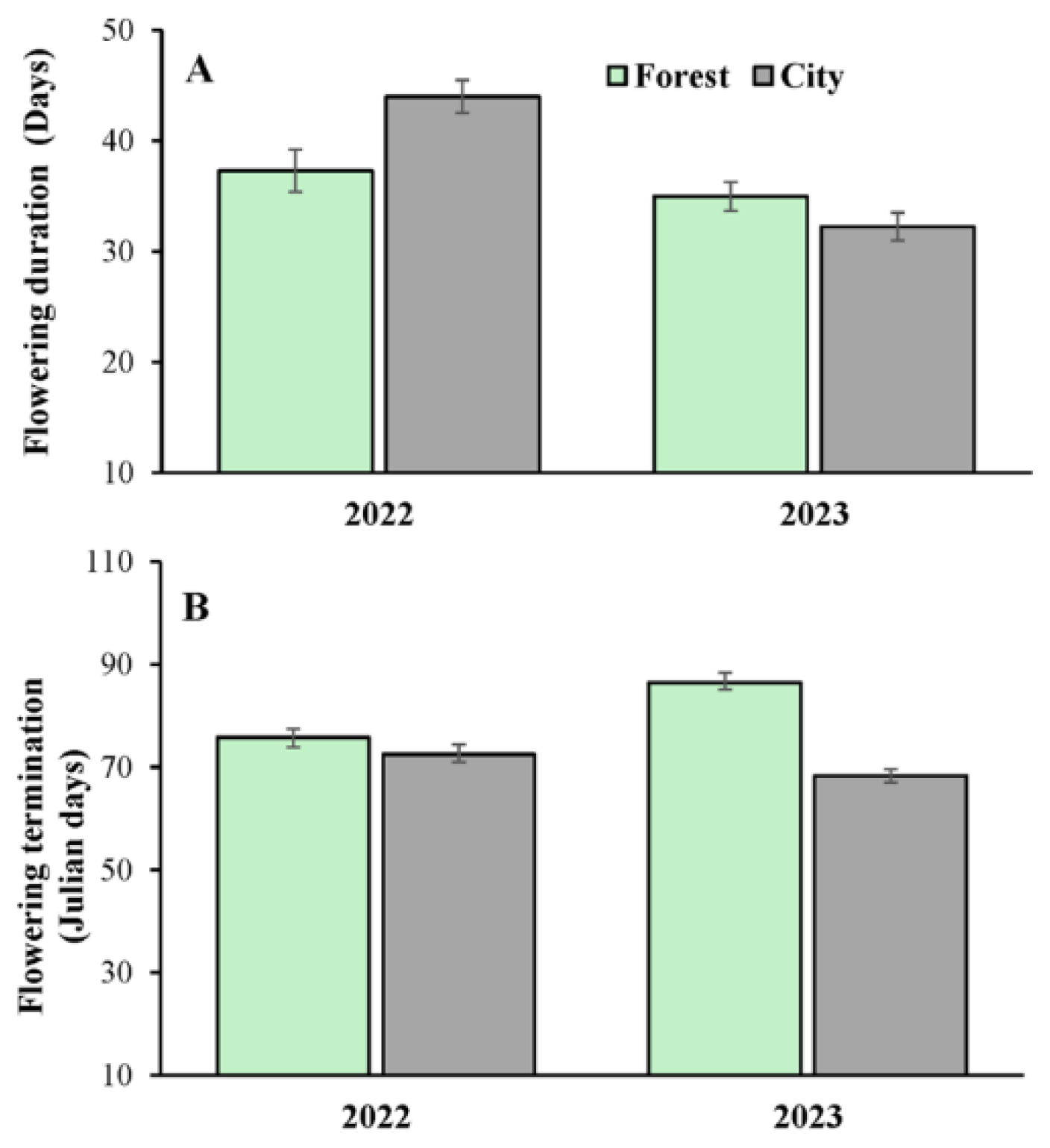
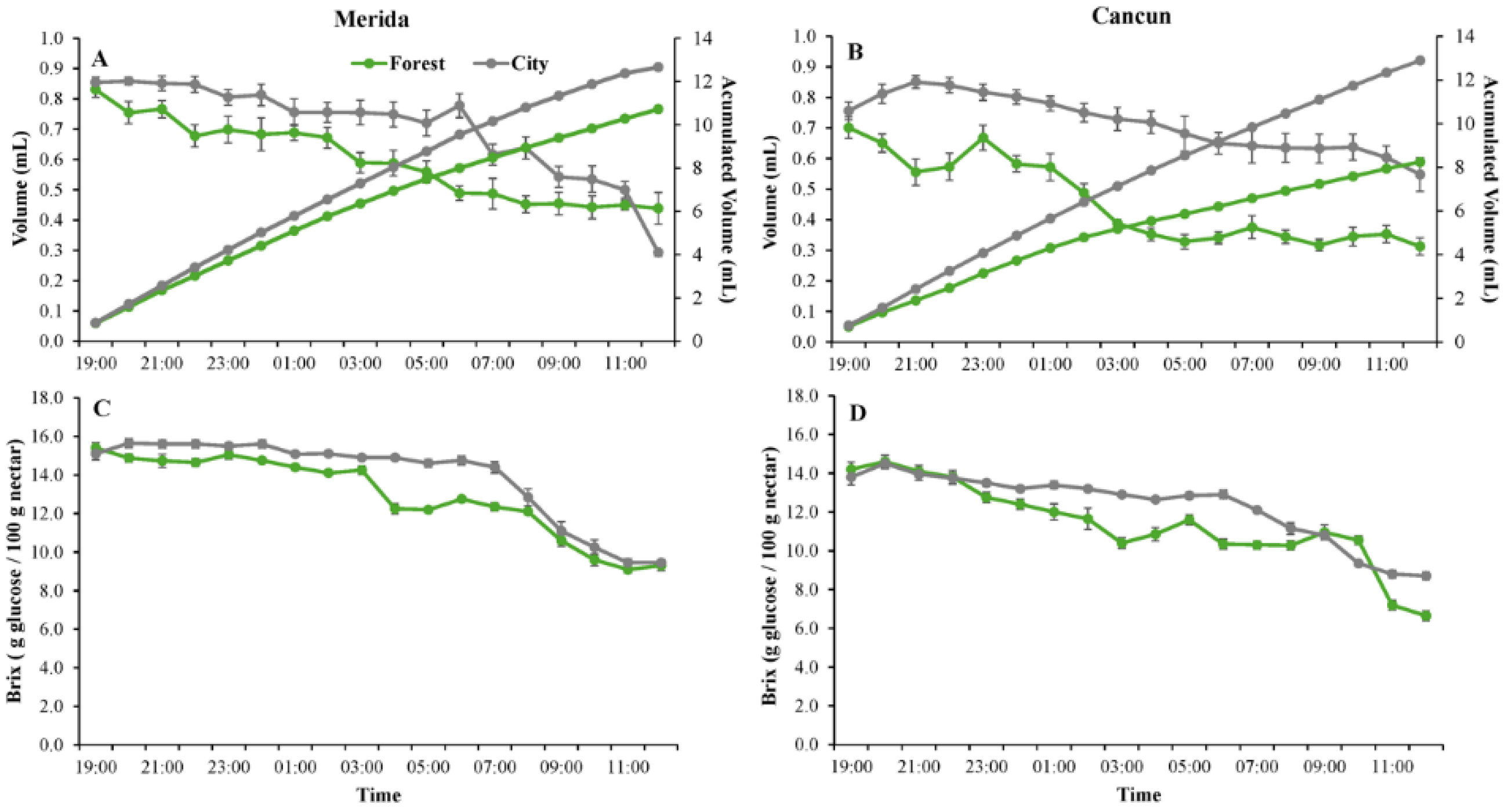
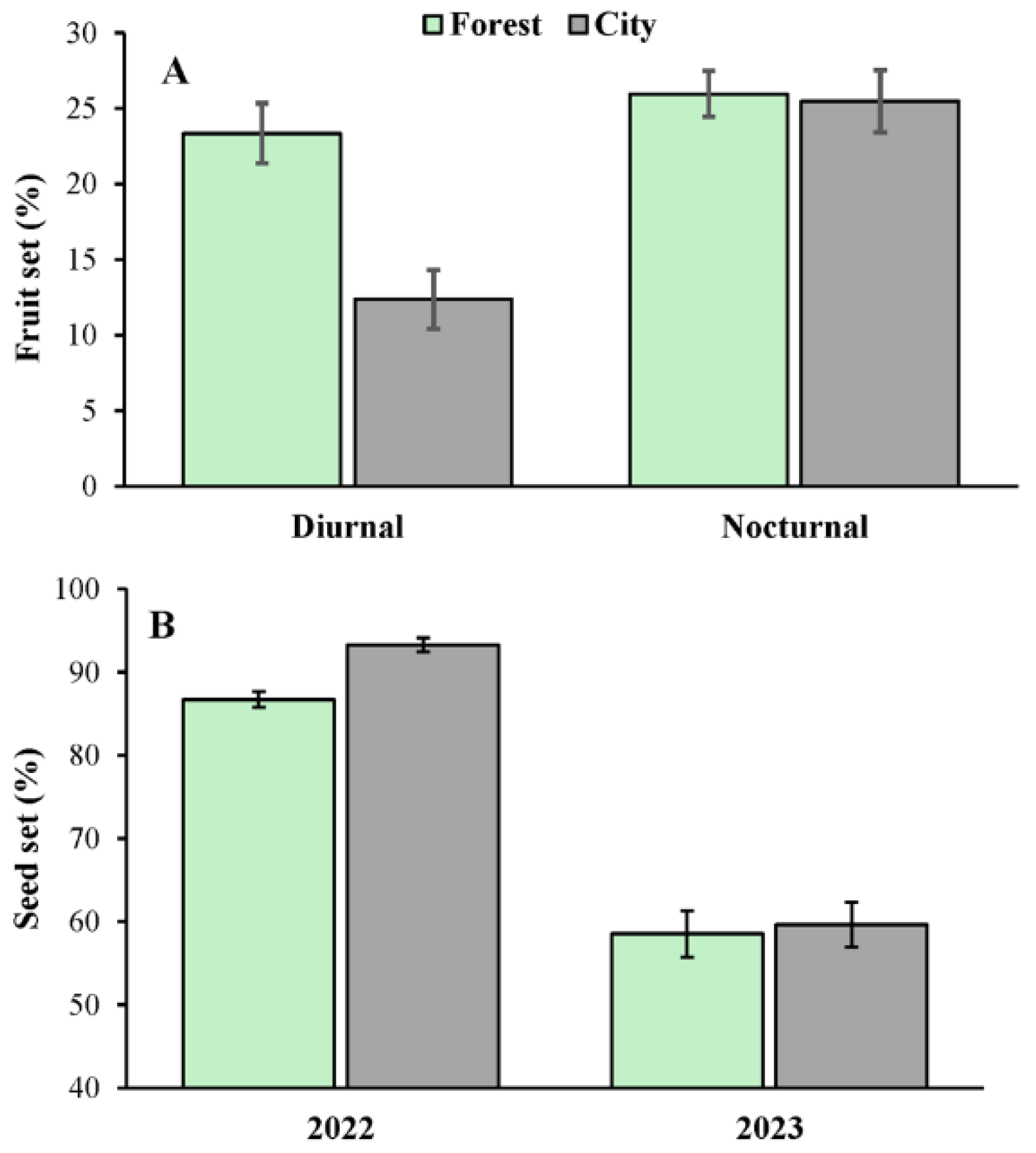
| Response | |||
| Source of variation | Onset | Duration | Termination |
| Urbanization | χ21=157.19** | χ21=1.59 | χ21=122.56** |
| Site | χ21=24.38** | χ21=3.84 | χ21=53.38** |
| Year | χ21=69.29** | χ21=24.27** | χ21=5.02* |
| Urbanization x Site | χ21=0.04 | χ21=0.01 | χ21=0.12 |
| Urbanization x Year | χ21=1.56 | χ21=10.75** | χ21=25.92** |
| Response | |||||
| Source of variation | Visits | Pollen load | Fruit set | Seed set | Germination |
| Urbanization | χ21=0.06 | χ21=1.19 | χ21=3.83† | χ21=2.69 | χ21=4.76* |
| P. guild | χ21=0.46 | χ21=37.19** | χ21=16.22** | χ21=0.46 | χ21=4.23* |
| Site | χ21=0.01 | χ21=0.79 | χ21=8.95** | χ21=2.14 | χ21=3.12 |
| Year | χ21=0.06 | χ21=11.14** | χ21=34.79** | χ21=66.76** | χ21=5.46* |
| Urbanization x P. guild | χ21=0.95 | χ21= 2.36 | χ21=16.30** | χ21=0.04 | χ21=0.01 |
| Urbanization x Site | χ21=0.11 | χ21= 0.61 | χ21=0.19 | χ21=0.01 | χ21=0.05 |
| Urbanization x Year | χ21=1.06 | χ21= 2.02 | χ21=0.14 | χ21=15.59** | χ21=0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





