1. Introduction
The presence of plastics in the natural environment was detected for the first time at the beginning of the 20th century [
1]. Since then, plastics production has considerably grown by going from 2 million tons in 1950 to 400.3 million tons in 2022 [
2]. Plastics were originally synthetic organic polymers that derived from the polymerisation of monomers extracted from fossil fuels (oil or gas) [
3]. Nowadays, plastics can be classified into different groups according to the raw materials employed to produce them and their biodegradability. The groups include conventional plastics, which are produced from non-renewable energies and are not biodegradable, as well as plastics whose raw material comes from fossil fuels (petrobased plastics) and can be biodegraded. Other group comprises biobased plastics, which are synthesized from the biomass or from natural resources, and are divided into two groups depending on whether they can be biodegraded or not. Except for conventional plastics, the other groups enter the bioplastics category [
4,
5].
One of the most widely used plastics in the farming sector is polyethylene (PE) [
6,
7,
8,
9]. Low-density polyethylene (LDPE) is a raw material that is generally employed to produce the plastic films utilized in most greenhouses and for the sacks used for storing special fertilizers [
10]. In Europe, it has been estimated that 40,000 km
2 of farmland are covered by these films [
11]. LDPE is a synthetic hydrophobic high-molecular-weight polymer characterized by its hardness, resistance to chemicals, flexibility and clearness [
12]. These characteristics enable LDPE to be present in the industrial, farming and domestic sectors [
13]. As LDPE is a petrobased plastic that cannot be biodegraded in the short term, it remains in the natural environment for long periods of time [
7,
14]. PHAs are a family of biobased plastics produced by different species of bacteria under nutrient-limiting (nitrogen and phosphorus) conditions with excess carbon [
15,
16]. Poly(hydroxybutyrate) (PHB) is the most studied polymer and is the best known member of the PHAs family [
17]. Poly(lactic) acid (PLA) has been proposed for producing horticultural products to reduce the environmental problems that derive from the large quantities of the plastics employed in the sector [
8,
18,
19]. It was the first polymer to be generated from renewable raw material that can be commercialized on a large scale [
15], and its properties are similar to those of polystyrene and polyethylene [
20].
Today conventional plastics are so widely employed in the agricultural sector because they possess the necessary mechanical properties to do so. However, in most applications, the retrieval and recycling of the employed plastics are impossible. So plastic waste accumulates on farmland which, in Europe, corresponds to 5-6% of all the plastic waste on this continent [
21]. This accumulation can affect the activity of different organisms, such as earthworms or microorganisms, and also physical soil properties (e.g., porosity and the structure of aggregates [
8,
22]. They also act as a surface for different pollutants to be adsorbed, which means that these compounds might accumulate in soil [
7,
23]. Different studies have considered using bioplastics as a solution to these sustainability problems [
7,
10,
15,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33] because they are produced from renewable energies, in a more energy-efficient way, and can also be composted and recycled [
25]. The main disadvantage of biobased plastics as opposed to conventional plastics is their low mechanical resistance [
34]. It is for this reason that developing biodegradable plastics using an optimum combination between suitable mechanical properties and their own biodegradation represents a multidisciplinary challenge, as well as an opportunity to improve the sustainability of agricultural practices [
24,
27]. Carbon is one of the most important elements as regards soil productivity because different organisms (bacteria, fungi and protozoa) use it as substrate to increase both their number and biomass [
35]. Directly adding microorganisms (bioaugmentation) or organic amendments (biostimulation) is expected to facilitate biodegradation processes in soil [
36,
37]. Different studies have investigated the role of amendments on the biodegradation of distinct polymers in soil [
7,
38], but have given inconsistent results. Therefore, more studies that clarify the role of not only biological activity, but also of the microbial communities that live in soil during plastics biodegradation processes, are necessary.
For all these reasons, the need to conduct studies to acquire information about plastics biodegradability and their final destination, and to develop strategies that help to remove plastics from the natural environment by reliable reproducible testing methods, is revealed. To do so, the specific objectives of the present work were to: (i) evaluate the biodegradability, in the short term, of three plastics widely used for various purposes: PHB, PLA and LDPE by following the procedure described in the ISO 17556 Standard [
39], which includes the necessary procedures for measuring the biodegradation of plastics in soil; (ii) study the effect of adding organic amendment to soil on the biodegradation of the selected plastics.
2. Materials and Methods
2.1. Materials Characterisation
2.1.1. Soil
The soil sample employed to conduct this work corresponds to a Bt horizon of a calcic xeralf (Alfisol-type soil) situated on the mountainous Mondúver massif (La Safor, Valencia, Spain). Its location is: 39°02'24.3"N 0°21'14.5"W. Soil was described in the field and was sampled following the procedure described by the FAO [
40]. The field sample was duly registered in the laboratory. Then it was spread over a plastic tray and plant materials and coarse fragments were separated. It was dried at 40-50 °C in a drying chamber and ground with a wooden rolling pin to be sieved at 2 mm. This allowed an air-dried fine earth fraction to be obtained to perform the analyses (FEF). Moisture content was determined by gravimetric procedure. The pH was measured in 1:5 soil:water suspension (v/v) using a GLP pHmeter (Crison™, Barcelona, Spain) [
41]. Electric conductivity (EC) was measured in an aqueous extract at 1:5 soil:water (w/v) by a PC 2700 conductimeter (EUTECH Instruments, Singapore) [
42]. Particle size was determined by the Bouyoucos densimeter method [
43]. Organic matter (OM) was determined by oxidation with potassium dichromate in the presence of sulphuric acid and by titration with ferrous ammonium sulphate [
44]. Total nitrogen (N) was measured by an elemental EA 1110 CNHS Auto-Analyser (CE Instruments, Milan, Italy) with heat treatment at a temperature of at least 900 °C in the presence of oxygen following an ISO procedure [
45].
2.1.2. Organic Amendment
The organic amendment employed in this experiment corresponds to a widespread organic amendment in agriculture: cow manure (CM). It was supplied by Desco S.L. company (Valencia, Spain). Moisture was determined by gravimetric procedure. pH and EC were determined following norms UNE-EN 13037 [
46] and UNE-EN 13038 [
47], respectively. OM was determined according to the procedure described in norm UNE-EN 13039 [
48], while the Walkley and Black method [
44] was followed for oxidizable organic carbon. Finally, to determine N content, the procedure described in ISO 13878 [
45] was followed. The dose applied in the experiment was the equivalent to 20 tons of manure per hectare within the range of manure application recommended in agriculture (between 10,000 and 40,000 kg ha
-1) [
49] . The contributed N dose corresponded to a quantity of 200 kg ha
-1, which is slightly higher than that recommended (170 kg ha
-1).
2.1.3. Plastics and the Reference Material
The plastics used in this experiment were: PHB (C
4H
6O
2); PLA (C
3H
4O
2) and LDPE (C
2H
4). The reference material (RM) was microcrystalline cellulose (C
6H
10O
5). Oxidizable organic carbon was determined for each plastic, as well as the theoretical oxygen demand (ThOD), which is understood as the stoichiometric quantification of the oxygen required to completely oxidize a compound [
50] that gives way to CO
2 and H
2O. In our case, ThOD is given by the stoichiometry of the following chemical reactions:
| PHB: |
C4H6O2 + 9/2O2
|
4CO2 + 3H2O |
 |
| PLA: |
C3H4O2 + 3O2
|
3CO2 + 2H2O |
 |
| LDPE: |
C2H4 + 3O2
|
2CO2 + 2H2O |
 |
| Microcrystalline cellulose (MR): |
C6H10O5 + 6O2
|
6CO2 + 5H2O |
 |
2.2. Experimental Design
The procedure applied to carry out the following test is described in norm ISO 17556 [
39], which includes the necessary procedures to measure the ultimate aerobic biodegradability of plastic materials in soil by measuring biochemical oxygen demand (BOD) in a respirometer or the amount of generated CO
2. The norm can be applied to natural or synthetic polymers, copolymers or mixtures of polymers. In our case, the applied method was to determine BOD with a respirometer. This study evaluated the biodegradation in soil of PHB, PLA and LDPE, with different treatments. The used reactors were respirometers (WTW®, OxiTop™-C and OxiTop™-IDS, Weilheim, Germany), which indirectly measure the quantity of consumed oxygen. The employed soil corresponded to FEF. Two blanks were made, one only with soil (Bl-) and the other with both soil and CM (Bl+), and a test was run with soil by adding CM as the RM and another in which soil, plastic and CM had been biologically inactivated in an autoclave (Inact). For all the plastics, two treatments were carried out (
Table 1): one with soil + plastic (-) and another with soil, plastic and CM (+). For PHB: PHB– (soil with PHB) and PHB+ (soil with PHB and CM). For PLA: PLA– (soil with PLA) and PLA+ (soil with PLA and CM). For LDPE: LDPE– (soil with LDPE) and LDPE+ (soil with LDPE and CM). To each container, 21 mL of water were added, which corresponded to 60% of this soil’s water holding capacity (WHC), which was 35%. The exact quantities of soil, plastic, CM and water added to each container shown in
Table 1.
The plastic material was introduced into pieces that were no bigger than 5 mm x 5 mm in size. For biological inactivation purposes, the corresponding containers were sterilized in an R2LPS autoclave (J.P. SELECTA®, Barcelona, Spain) at 121 °C for 20 minutes along with the parts required to place the respirometer, except for the head. Once autoclaved, the parts and the head were placed inside a laminar flow chamber superficially sterilized with UV light to start the experiment. Containers were left in the dark at 21 °C ± 1 °C in an incubator (WTW®, TS606/3, Weilheim, Germany) for 20 days.
2.3. Calculations
Biodegradability was determined according to the expression in Eq. (1) [
39] :
where: Dt is the percentage of the test material’s (plastic) biodegradation in time t; DBO
T is the BOD that corresponds to the container containing the plastic material that is expressed as milligrams of consumed oxygen per kilogram of dry soil (mg O
2 kg
-1); BOD
B is the BOD that corresponds to the control blank (mg O
2 kg
-1), which is the test soil; ThOD is the theoretical oxygen demand of the tested material and is expressed as milligrams of oxygen per gram of plastic material (mg O
2 g
-1); ρT is the concentration of the test material (plastic) in the reaction mixture inside the container, expressed as g kg
-1.
For the soil treatments with only soil (PHB–; PLA–; LDPE– and the RM), Bl- was deducted, while Bl+ was deducted from the treatments with CM (PHB+; PLA+ and LDPE+). The difference was divided by the ThOD of the corresponding plastic by multiplying it by the quantity of this added plastic in the two replicas (Eq. 1).
The amount of consumed oxygen was obtained indirectly by measuring the negative pressure that took place inside the respirometer, which included a CO
2 trap by adding NaOH lentils. Both consumed oxygen and the soda trap caused depression, which was captured by the head for short time intervals throughout the incubation time. After 20 experiment days, the results were obtained, which showed decreased pressure in hectopascal units (hPa). The pressure values expressed as hPa were converted into atmospheres (atm) and temperature into Kelvin (°K) grades. Using the ideal gases equation (Eq. 2) and Eq. 3, the consumed O
2 were calculated for each time in all the treatments, and were expressed per kg of dry soil.
where: P is pressure (atm); V is the container’s gas volume (L); n is the number of moles; R is the constant of ideal gases (L atm mol
-1 °K
-1); T is temperature (°K).
Additionally, the change in the weight of the plastics between the beginning and the end of the experiment was determined. The different strips were washed and weighed before and after the process to determine the mass loss.
2.4. Statistical Analysis
All the calculations were done using Microsoft Office-Excel™ (version 2017). To determine differences in the results between treatments, a one-factor ANOVA was used with Tukey’s post hoc test. The employed statistical package was SPSS® (IBM®, version 28).
3. Results and Discussion
3.1. Materials’ Characterisation
3.1.1. Soil
According to the sampling, the profile presented the following genetic horizons: Ah, AB, Bt and R. Soil was slightly basic (pH = 7.49). There were no salinity problems because the EC value was 0.127 dS m
-1 (< 0.2 dS m
-1). Soil organic matter content was 3.05%, and the soil’s texture class is clayey with 56% clay, 30% silt and 14% sand; in this sense, Briassoulis and Mistriotis (2018) [
28] indicate that fine texture natural soils are ideal for biodegradation tests of bio-based materials.
3.1.2. Organic Amendment
The moisture content in the organic amendment employed in the tests was 60%. The percentage of OM and oxidizable organic carbon of CM was 83% and 28.17%, respectively.
3.1.3. Plastics and the Reference Material
The plastic with the highest percentage of oxidizable organic carbon was PHB with 39.11%, followed by PLA with 13.25% and LDPE with 0.72%. As for the ThOD, the compound that required the most oxygen per gram was LDPE (3,420 mg O2 g-1), followed by PHB (1,670 mg O2 g-1), PLA (1,330 mg O2 g-1) and, by microcrystalline cellulose (RM) (1,185 mg O2 g-1).
3.2. O2 (BOD) Consumption
The amount of consumed O
2 over the 20 days that the experiment lasted in each treatment shown in
Table 2.
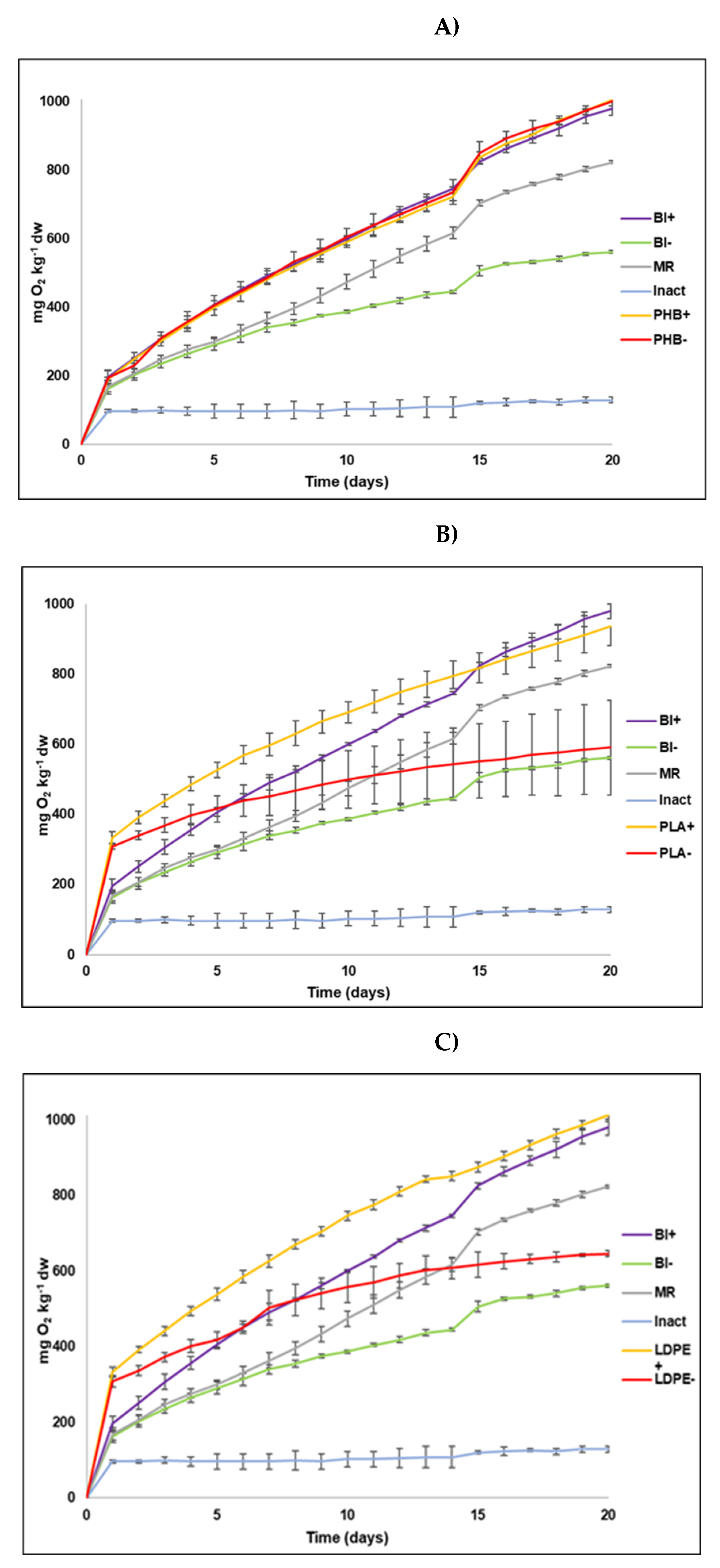
All the data are expressed as mg of consumed O2 per kg of dry soil (d.w.). As expected, in all cases in the sterilized material (Inact), consumed oxygen remained very low, and almost constant throughout the experiment (between 96 mg kg-1 at the beginning and 129 mg kg-1 at the end of the experiment). It is also important to stress that no sign of any alteration was noted in plastics. For the other treatments, the plastic-free control soil without amendment (Bl-) displayed the least biological activity, and consumed accumulated oxygen was 561 mg kg-1. The consumed oxygen in the soil with the CM amendment (Bl+) was 980 mg kg-1, which was almost double that of the control soil and was slightly higher than that in the RM soil, whose value was 823 mg kg-1. These results indicate that by adding both CM and the RM stimulates soil’s biological activity.
The soil treatments without amendments with PLA (PLA-) and LDPE (LDPE-) showed intermediate and slightly greater activity than Bl-. There were no significant differences for the oxygen consumed between both treatments (
Table 3), with 590 mg kg
-1 and 646 mg kg
-1 respectively for PLA- and LDPE-. For the other treatments, PHB+, PHB-, PLA+ and LDPE+ led to a greater edaphic microbiota response, with consumed oxygen values of 1,006 mg kg
-1, 1,000 mg kg
-1, 936 mg kg
-1 and 1,012 mg kg
-1, respectively. There were no significant differences among any of the treatments (
Table 3). Although these values were not much higher than those of the soil with amendment (Bl+), they indicated that adding manure and/or plastics PHB and PLA also stimulated soil’s biological activity. It is also important to stress soil’s positive microbial response to the addition of PHB, as reflected in the oxygen consumption of treatment PHB- (1,000 mg kg
-1).
The comparison made of oxygen consumption evolution in soil to the different treatments and for the three plastic types shown in
Figure 1. For PHB (
Figure 1A), no change whatsoever was noted for the sterile soil (Inact). The untreated soil (Bl-) presented the least activity, followed by the soil with the RM. The response of the other treatments (Bl+, PHB+ and PHB-) was greater and similar for each treatment. As previously indicated, soil biological activity responds in the same way to the addition adding of both CM (PHB+) and PHB (PHB-), and both jointly and separately. This finding revealed that this polymer derived from poly(hydroxybutyrate) acid very positively influenced soil biological activity stimulation. This was logical because the carbon content that was easily oxidizable in this plastic was higher than that of manure (see
Section 3.1.3). With PLA (
Figure 1B), for the first 15 days, the soil with both plastic and CM (PLA+) was the treatment with the most consumed oxygen, although in the end it was similar to the soil with amendment (Bl+) and the soil with microcrystalline cellulose (RM). The soil with plastic alone (PLA-) and the control soil (Bl-) consumed approximately half the oxygen that the previous treatments did, with the first treatment consuming more than the control soil. This may also be due to the oxidizable C content (13.25%) of this plastic material. Regarding LDPE (
Figure 1C), as with PLA, the soil with this plastic and CM (LDPE+), and the soil with amendment (Bl+) followed by the RM, were the treatments that led to a greater microorganisms’ response. For the first 15 days, although the consumption in LDPE+ was slightly higher, these three treatments obtained similar values. Similarly, to what occurred with PHB and PLA, the soil with the RM obtained a slightly lower value than that for LDPE+ and Bl+, which confirms the effect of organic amendment on soil’s biological activity. Except for the sterile soil (Inact), and as in previous cases, the poorest response was obtained for the soil with only plastic (LDPE-) and the control soil (Bl-).
The fact that the Inact treatment presented slightly higher consumed oxygen on the first days, which then remained practically invariable for the rest of the time (
Table 2 and
Figure 1), firstly suggests that the autoclaving process was correctly performed and, secondly, that the small amount of consumed oxygen in these containers was not caused by biological activity, but by some short-lasting physicochemical mechanism. When comparing the amount of consumed oxygen between this treatment (129 mg kg
-1) and the control soil (Bl-, 561 mg kg
-1), physicochemical degradation played an imperceptible role, unlike biological degradation that was the main process to eliminate these polymers in soils, as previously demonstrated in different studies [
4,
13,
16,
21,
22,
51,
52].
Another point to highlight is the fact that except for PLA+, the consumed oxygen in all the treatments was above that of its corresponding blank (
Table 2). For the soil with amendment (Bl+), the quantity of consumed oxygen almost doubled that of the soil with no amendment (Bl-) because organic amendment was added. Indeed, adding CM very possibly led to bioaugmentation and/or biostimulation processes that, on the one hand, had acted as an input of microorganisms and, on the other hand, as a source of nutrients that stimulated microbial activity. Such an effect has been described by different studies: for instance, Hamarashid et al. (2010) [
35] indicate that organic C is fundamental for bacteria, fungi and protozoa in soil, which use it as substrate to increment both their number and biomass. Chaturvedi et al. (2013) and Li et al. (2013) [
36,
37] observed that directly adding microorganisms or organic amendments facilitates biodegradation processes in soil.
Our results also indicated that adding cellulose (RM) to soil led to the slightly lesser stimulation of edaphic microbiota than that achieved by adding PHB (
Figure 1), which confirmed the results of other previous studies [
16,
21], and suggests that PHB is a highly biodegradable polymer. Indeed it has been used as a positive control in many tests and plays the same role as microcrystalline cellulose did in the present study [
21]. The similar oxygen consumption in both treatments (1,006 mg kg
-1 for PHB+ and 1,000 mg kg
-1 for PHB-) also denotes high compound biodegradability because the soil with this plastic and without amendment (PHB-) consumed practically the same quantity of oxygen as the treatment with CM (PHB+). This situation did not occur in PLA and LDPE. In these cases, the treatments with CM (PLA+ and LDPE+) consumed almost double the oxygen than the soils with no amendment (PLA- and LDPE-). As previously mentioned, the fact that oxygen consumption increased when CM was added is related to bioaugmentation and biostimulation processes because adding manure should increase both the microbial population and nutrients content, especially C and N, which considerably stimulates microbial activity.
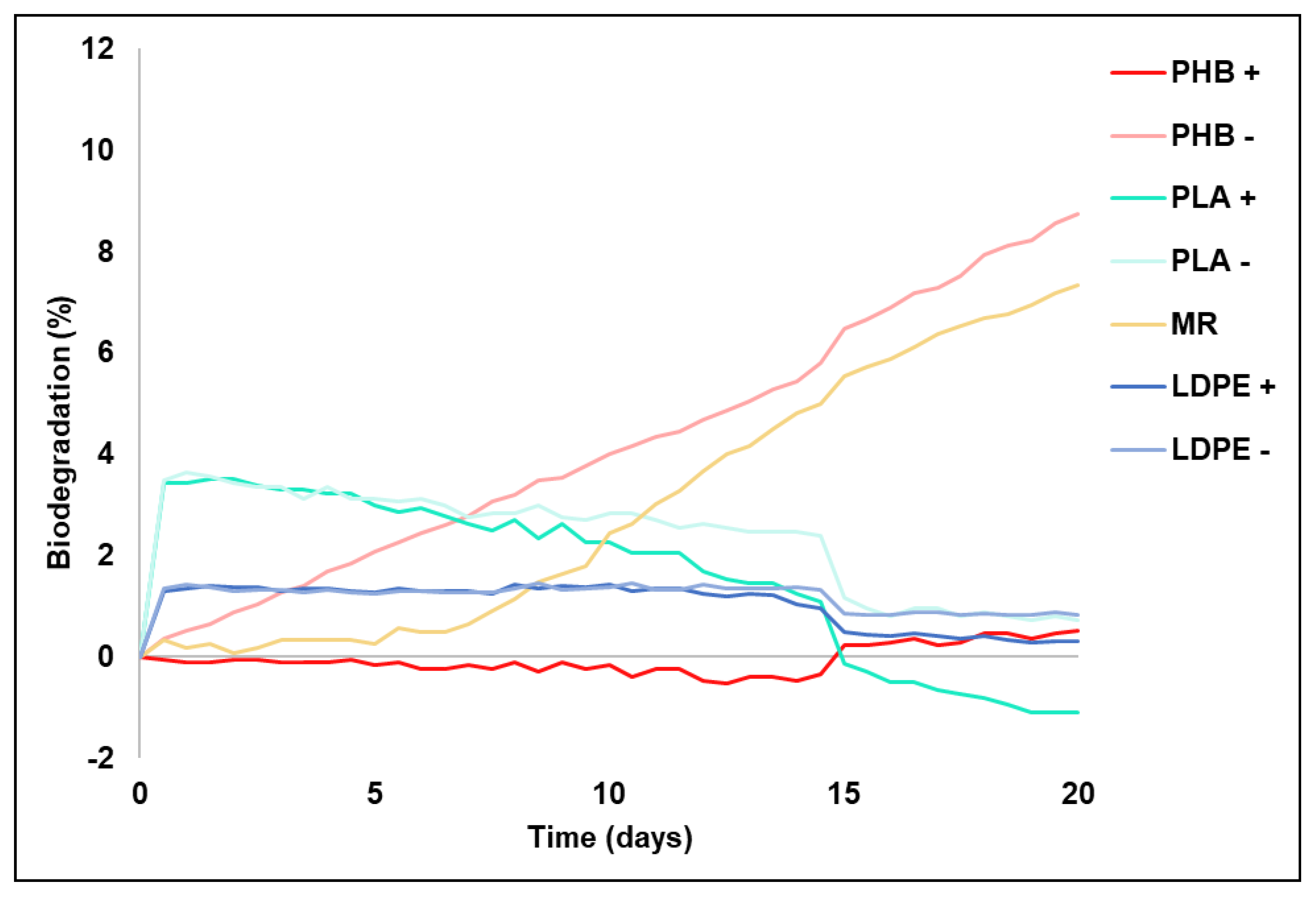
3.3. Plastic Materials Biodegradability
Table 3 shows the plastics biodegradation percentages obtained at the end of the experiment. For the RM, which acted as a positive control, biodegradation was 7.37%. Significant differences were found for PHB between the treatment with CM (PHB+) and that without CM (PHB-), whose biodegradation percentages were respectively 0.52% and 8.76%. The PHB+ biodegradation percentages were slightly higher than those of cellulose (RM), respectively with 8.76% and 7.37%, which confirmed high PHB biodegradability. These results are similar to those reported by other authors [
16,
21,
22,
43,
54,
55,
56,
57]. With PLA, the biodegradability in the soil with (PLA+) and that without (PLA-) amendment was -1.10% and 0.73%, respectively. The fact that negative values were obtained is explained by the bigger quantity of oxygen consumed in the control soil; that is, the control value is subtracted from the problem sample value to eliminate the part of the oxygen consumed by soil; this result has also been indicated by Briassoulis and Dejean (2010) [
58] in their experiment to measure biodegradation in soil with different plastic films. Those authors also noted greater CO
2 production in blanks than in soils with plastics. This biodegradability evolution shown in
Figure 2 for PLA, along with such low biodegradability percentages, are interesting for being a polymer that is classified as biodegradable. In line with this, both Rudnik and Briassoulis (2011) and Tokiwa and Calabia (2006) [
15,
20] indicate that the biodegradation of this polymer needs higher temperatures, such as those required to accomplish composting processes (60 °C).
Slezak et al. (2023) and Wang et al. (2023) [
9,
19] also indicated that this plastic presents low biodegradability at ambient temperature. Many studies have evaluated PLA biodegradability in soils under similar conditions to those herein described with similar results. Kamiya et al. (2007) [
59] did not observe PLA degradation in soil for 120 days, and neither did Hoshino et al. (2001) [
60], who did not detect PLA degradation in soil for 90 days at any of the 19 Japanese locations where tests were performed. Adhikari et al. (2016) [
61] measured PLA biodegradability under similar conditions to ours (in soil for 28 days at 25 °C) and reported a biodegradation percentage of only 13.8% at the end of their experiment. Harmean et al. (2014) [
62] reported a biodegradation percentage of 37.4% after 56 days at 30 °C with 80% humidity. Nevertheless, those studies that have evaluated PLA biodegradability in compost at higher temperatures obtained a much higher biodegradation percentage. Kale et al. (2007) [
63] also evaluated biodegradability in compost for 58 days at 58 °C and obtained a biodegradation percentage of 84%. Likewise, Mihai et al. (2014) [
64] reported 60% PLA biodegradation in compost after exposing it to 58 °C for 30 days. As soil temperatures do not exceed 30 °C under normal conditions and increasing such soil temperatures is unfeasible, PLA biodegradation in soils would very much depend only on time [
9,
15] and, to a certain extent, on soil characteristics. To determine if this polymer is a good candidate to safely and sustainably replace the conventional plastics employed in agriculture, more research is needed to accurately find out the time needed to complete PLA biodegradation under different conditions.
For the treatments with LDPE (LDPE+ and LDPE-), and as
Figure 2 depicts, the biodegradation percentages remained constant at very low levels for 20 days. At the end of the experiment, the biodegradation percentages (
Table 3) for both these treatments were 0.31% for LDPE+ and 0.82% for LDPE-, which confirms the low biodegradability of this plastic, a characteristic that has been demonstrated before by different authors [
7,
8,
13,
21,
65,
66].
Its biodegradability is still very low even when adding microbial inoculum [
66]. Those authors concluded that oxidation prior to LDPE biodegradation is the only option for its alteration, which leads to not only the formation of more attackable monomers, but also to the application of the microbiome from rubbish dumps. According with these results, Beltrán-Sanahuja et al. (2021) [
6] conclude that after one year of exposure to soil with different environmental conditions, conventional materials showed no degradation through several lines of evidence: weight loss, spectroscopy and thermal derived metrics.
3.4. Effect of Organic Amendment on Plastics Biodegradation
Regarding the effect of adding organic amendments on the biodegradation of plastic materials in soils, it has been shown that soils rich in OM present greater microbial activity [
60,
67], and the amount of OM in soil has been positively correlated with materials biodegradation [
7,
35,
38]. So a soil with CM amendment is expected to present a higher biodegradation percentage. Furthermore, the previous section indicates its positive effect on increasing oxygen consumption because biological activity increases. However, it was surprising to note that the soils with plastics and CM amendment herein employed (PHB+, PLA+ and LDPE+) obtained much lower biodegradation percentages than their counterparts with no amendment (PHB-, PLA- and LDPE-) (
Table 3). Thus the oxygen consumption data are not reliable enough for us to discuss the possible benefit of organic amendments during plastics biodegradation processes. The low plastics biodegradability in the reactors with CM might be due to the soil microorganisms that come from a natural environment not having acclimatized in the short term and preferring to consume the source of C from CM that is more easily oxidizable and, thus, plastics would not be attacked. In fact, the main difference found to the other works consulted in this study was that the inocula of the used microorganisms came from isolates from rubbish dumps [
66,
68,
69], which have undergone a long acclimatization process, whereas microorganisms came from a natural environment in our case. Roy et al. (2008) [
70] also mentioned in most studies into the plastics biodegradation that specific and very well identified bacterial strains have been employed. Yet under natural conditions, neither the composition nor the efficiency of microbial communities can be anticipated, nor can their catalytic abilities [
7,
51] . Sen and Raut (2015) [
13] indicated that one of the strategies to facilitate the dissolution and consequent degradation by microorganisms is to use the polymer as the only source of C. Indeed, Park and Kim (2019) [
71] observed 14.7% weight loss in PE particles when they introduced it as the only source of C during a 60-day experiment.
In the present work, the PHB biodegradation percentage in the treatment with CM was 0.52%, while it was 8.76% in the soil with no amendment (
Table 3). There are also other factors that may have an influence, and could also be due to the microbial activity that derives from adding OM not implying greater polymers biodegradation. Microorganisms activity may increase when OM is added to the medium because microorganisms employ this same OM as a source of C, and not the biodegradable polymers present. Hoshino et al. (2001) [
60] demonstrated that the biodegradation of polymers in soil is affected mainly by temperature and the total available N content in soil, whereas adding organic supplements does not offer any benefit. Given these differences in the results and the few studies found about the effect of organic amendments on plastics biodegradation processes, further research is necessary to explain the complex relations that occur among microbial activity, OM and the biodegradation of polymers in soil.
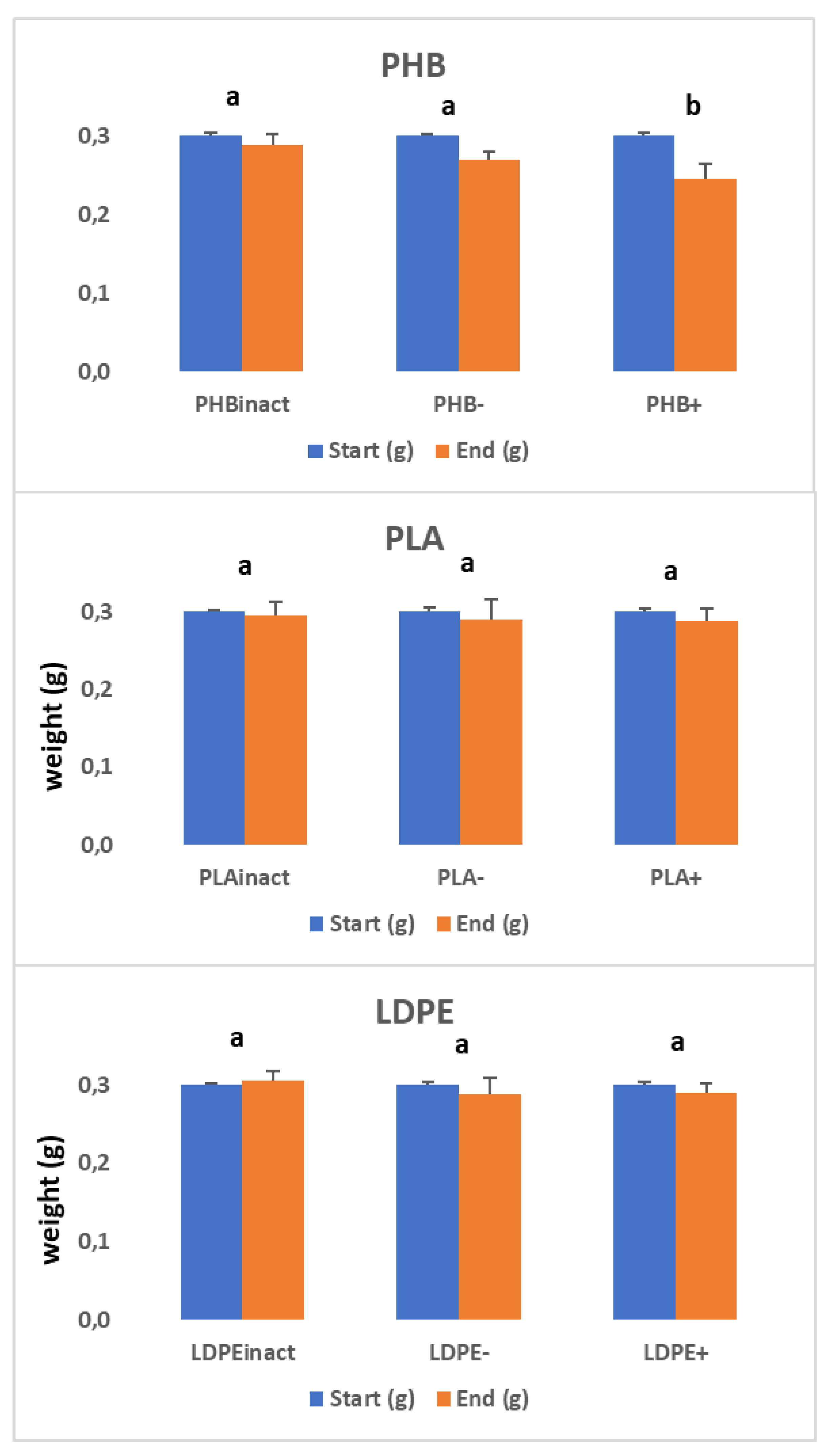
To complete this study,
Figure 3 shows the weight values of the plastics in each treatment at the beginning and end of the experiment. As observed, an important decrease in plastic weight variation was only obtained in the case of PHB: 10.0% for PHB- and particularly significant in the treatment with manure addition (18.4%). No significant differences were observed for PLA and LDPE. This finding confirms the previous results and highlights the different biodegradability of these plastic materials.
4. Conclusions
After evaluating the biodegradability of the three conventional plastic materials (PHB, PLA and LDPE) in a Mediterranean Alfisol-type soil and the effect of adding organic amendment (CM) by the method based on norm ISO 17556 [
39], which is the international norm for measuring the biodegradation of plastics in soil; our results confirm that measuring only O
2 consumption from soil biological activity is not sufficient to evaluate biodegradation effects. PHB behaves similarly to the positive RM (microcrystalline cellulose) compared to PLA and LDPE that barely degrade. Our findings confirm too that the biodegradability order of these three plastic materials is PHB>>>PLA>LDPE.
PHB could be a good alternative for replacing conventional farming plastics owing to its high biodegradability in soil in short term. Conversely, PLA is not a good candidate for replacing conventional plastic materials in the agricultural sector because its biodegradation takes place at temperatures that cannot be naturally reached on farmland. LDPE is a polymer whose biodegradability is very low and, therefore, must be ruled out for any use. Adding CM to our soil did not influence the biodegradability of these three plastic materials. This finding suggests that natural soil biodiversity seems only high enough to permit materials’ biodegradation without having to add amendments of this type. More studies are needed to elucidate how and in what way organic amendments should be applied to facilitate biodegradation processes and to understand the complex relations among microbial communities, soil and polymers biodegradation mechanisms.
Finally, this study indicates that, in order to solve today’s plastics problems, it is necessary to accurately know the biodegradation conditions of the plastics chosen as an alternative so that use does not harm the natural environment, which is currently the case of conventional plastics. There is also the need to more widely investigate the short- and long-term destinations of bioplastics within soil environment because both the nature and properties of soils can significantly impact their biodegradation. Therefore, more studies are necessary to understand these variables in detail. Bioplastics cannot act as a panacea to solve the plastic contamination problem and, although they can replace conventional plastics, we must contemplate other management options to minimize the contamination problem, such as prevention, reduction and reuse in situ.
Author Contributions
Conceptualization, R.B. and L.R-P.; methodology, R.B., L.R-P and O.A-S; validation, R.B. and L.R-P.; formal analysis, N.R., E.F-G., O.A-S.; investigation, R.B., L.R-P and O.A-S.; resources and data curation, R.B. and L.R-P.; writing—original draft preparation, R.B.,N.R.,L.R-P.; writing—review and editing, R.B., L.R-P. and O.A-S.; visualization, supervision and project administration, R.B. and L.R-P.; funding acquisition, R.B. and L.R-P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Unit of Research in Soils, Waste, and Environment (SOREiMA) of the University of València, and the Conselleria of Agriculture, Environment, Climate Change, and Rural Development of the Valencian Government. Grant number: UV-ICA/CSIC2018 and OTR2016-16953INVES respectively.
Informed Consent Statement
Not applicable.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge AIMPLAS technological center for their assistance in obtaining the plastic materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, C.; Liu, Y.; Chen, W.Q.; Zhu, B.; Qu, S.; Xu, M. Critical review of global plastics stock and flow data. J. Ind. Ecol. 2021, 25(5), 1300–1317. [Google Scholar] [CrossRef]
- Nayanathara, P.G.C.; Sandaruwan, A. The world of plastic waste: A review. Cleaner Materials, 2024, 11, 100220. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull, 2011, 62(12), 2588–2597. [CrossRef]
- Gómez, E.F.; Michel Jr, F.C. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 2013, 98(12), 2583–2591. [CrossRef]
- European Bioplastics. Available online: https://www.european-bioplastics.org/bioplastics/materials/ (accessed on 7 May 2022).
- Beltrán-Sanahuja, A.; Benito-Kaesbach, A.; Sánchez-García, N.; Sanz-Lázaro, C. Degradation of conventional and biobased plastics in soil under contrasting environmental conditions. Sci. Total Environ. 2021, 787, 147678. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; Zhou, Y.; Fei, J. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhao, Y.; Zhou, R.; Lin, J.; Su, T.; Tong, H.; Wang, Z. Biodegradation of polybutylene adipate-co-terephthalate by Priestia megaterium, Pseudomonas mendocina, and Pseudomonas pseudoalcaligenes following incubation in the soil. Chemosphere 2022, 307, 135700. [Google Scholar] [CrossRef]
- Slezak, R.; Krzystek, L.; Puchalski, M.; Krucińska, I.; Sitarski, A. Degradation of bio-based film plastics in soil under natural conditions. Sci. Total Environ. 2023, 866, 161401. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Scarascia, G.; Picuno, P.; Guarde, D.; Dejean, C. Review, mapping and analysis of the agricultural plastic waste generation and consolidation in Europe. Waste Manage. Res. 2013, 31(12), 1262–1278. [Google Scholar] [CrossRef]
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of plastic film, biodegradable paper and bio-based film mulching for summer tomato production: soil properties, plant growth, fruit yield and fruit quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- Negi, H.; Gupta, S.; Zaidi, M.G.H.; Goel, R. Studies on biodegradation of LDPE film in the presence of potential bacterial consortia enriched soil. Biologija 2011, 57(4), 141–147. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3(1), 462–473. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 567, 333–349. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Degradation behaviour of poly(lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Crops Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.; Alves, M.M.; Vicente, A.A. Factors affecting polyhydroxyalkanoates biodegradation in soil. Polym. Degrad. Stab. 2020, 182, 109408. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial degradation of polyhydroxyalkanoates with different chemical compositions and their biodegradability. Environ. Microbiol. 2017, 73(2), 353–367. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Mueller, R.R.; Iannotti, E.L. Use of low MW polylactic acid to stimulate growth and yield of soybeans. Plant Growth Regul. 1996, 19, 223–232. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.; Zhang, W.; Lin, J.; Wang, Z.; Lyu, S.; Tong, H. Biodegradation of polylactic acid by a mesophilic bateria Bacillus safensis. Chemosphere, 2023, 318, 137991. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). App. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Briassoulis, D.; Mistriotis, A.; Mortier, N.; Tosin, M. A horizontal test method for biodegradation in soil of bio-based and conventional plastics and lubricants. J. Clean. Prod. 2020, 242, 118392. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere. 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Briassoulis, D. An overview on the mechanical behaviour of biodegradable agricultural films. J. Polym. Environ. 2004, 12, 65–81. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23(1), 47–56. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51(3), 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based plastic mulching films. Polym. Test. 2018, 67, 99–109. [Google Scholar] [CrossRef]
- Briassoulis, D.; Mistriotis, A. Key parameters in testing biodegradation of bio-based materials in soil. Chemosphere. 2018, 207, 18–26. [Google Scholar] [CrossRef]
- Sander, M. Biodegradation of polymeric mulch films in agricultural soils: concepts, knowledge gaps, and future research directions. Environ. Sci. Technol. 2019, 53, 2304–2315. [Google Scholar] [CrossRef]
- Maraveas, C. Environmental sustainability of plastic in agriculture. Agriculture. 2020, 10(8), 1–15. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef]
- Maraveas, C. The sustainability of plastic nets in agriculture. Sustainibility. 2020, 12(9), 3625. [Google Scholar] [CrossRef]
- Hamarashid, N.; Othman, M.; Hussain, M. Effects of Soil Texture on Chemical Compositions, Microbial Populations and Carbon Mineralization in Soil. Egypt. J. Exp. Biol. 2010, 6(1), 59–64. [Google Scholar]
- Chaturvedi, S.; Kumar, A.; Singh, B.; Nain, L.; Joshi, M.; Satya, S. Bioaugmented composting of Jatropha de-oiled cake and vegetable waste under aerobic and partial anaerobic conditions. J. Basic Microbiol. 2013, 53(4), 327–335. [Google Scholar] [CrossRef]
- Li, Z.; Lu, H.; Ren, L.; He, L. Experimental and modeling approaches for food waste composting: A review. Chemosphere. 2013, 93(7), 1247–1257. [Google Scholar] [CrossRef]
- Agamuthu, P.; Tan, Y.S.; Fauziah, S.H. Bioremediation of Hydrocarbon Contaminated Soil Using Selected Organic Wastes. Procedia Environ. Sci. 2013, 18, 694–702. [Google Scholar] [CrossRef]
- AENOR (Asociación Española de Normalización y Certificación), 2019. Plásticos. Determinación de la biodegradabilidad aeróbica última de materiales plásticos en el suelo mediante la medición de la demanda de oxígeno en un respirómetro o la cantidad de dióxido generada (UNE-EN ISO 17556:2019).
- FAO., 2009. Guía para la descripción de suelos - Cuarta edición. Roma. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fao.org/3/a0541s/a0541s.pdf.
- ISO, 1994a. ISO 10390. Soil Quality. Determination of pH. International Organization for Standardization, Geneva.
- ISO, 1994b. ISO 11265. Soil Quality. Determination of the Specific Electrical Conductivity. International Organization for Standardization, Geneva.
- Day, P.R. Particle fractionation and particle-size analysis. In: Methods of Soil Analysis. Part I. Black, C.A., Ed.; American Society of Agronomy, Madison, USA, 1965; pp.545-567.
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- ISO, 1998. ISO 13878. Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis). Organización Internacional de Normalización, Geneva.
- AENOR (Asociación Española de Normalización y Certificación), 2012a. Mejoradores de suelo y sustratos de cultivo. Determinación del pH. (UNE-EN 13037).
- AENOR (Asociación Española de Normalización y Certificación), 2001. Mejoradores de suelo y sustratos de cultivo. Determinación de la conductividad eléctrica. (UNE-EN 13038).
- AENOR (Asociación Española de Normalización y Certificación), 2012b. Mejoradores de suelo y sustratos de cultivo. Determinación del contenido en materia orgánica y de las cenizas (UNE-EN 13039).
- VitiViniCultura.net, 2022. Estiércol: ventajas, tipos, dosis. Aplicación. https://www.vitivinicultura.net/estiercol-viticultura.html#:~:text=Las%20dosis%20que%20se%20recomiendan,de%20esti%C3%A9rcol%20fresco%20por%20Ha. (Accessed: 17/08/2022).
- Raffo, E.; Ruiz, E. Caracterización de las aguas residuales y la demanda bioquímica de oxígeno. Industrial Data 2014, 17(1), 11. [Google Scholar]
- Lucas, N., Bienaime, C., Belloy, C., Queneudec, M., Silvestre, F., Nava-Saucedo, J.E.,. Polymer biodegradation: Mechanisms and estimation techniques. A review. Chemosphere. 2008, 73(4), 429–442. [CrossRef]
- Muenmee, S. , Chiemchaisri, W., Chiemchaisri, C. International Biodeterioration and Biodegradation Enhancement of biodegradation of plastic wastes via methane oxidation in semi-aerobic land fill. Int. Biodeterior. Biodegrad. 2016, 113, 244–255. [Google Scholar] [CrossRef]
- Song, C., Wang, S., Ono, S., Zhang, B., Shimasaki, C., Inoue, M. The biodegradation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/V) and PHB/V-degrading microorganisms in soil. Polym. Adv. Technol. 2003, 14(3–5), 184–188. [CrossRef]
- Sridewi, N. , Bhubalan, K., Sudesh, K. Degradation of commercially important polyhydroxyalkanoates in tropical mangrove ecosystem. Polym. Degrad. Stab. 2006, 91(12), 2931–2940. [Google Scholar] [CrossRef]
- Lim, S.P., Gan, S.N., Tan, I.K.P. Degradation of Medium-Chain-Length Polyhydroxyalkanoates in Tropical Forest and Mangrove Soils. App. Biochem. Biotechnol. 2005, 126(1), 23-33. [CrossRef]
- Shrivastav, A., Mishra, S.K., Pancha, I., Jain, D., Bhattacharya, S., Patel, S., Mishra, S. Biodegradability studies of polyhydroxyalkanoate (PHA) film produced by a marine bacteria using Jatropha biodiesel byproduct as a substrate. World J. Microbiol. Biotechnol. 2011, 27(7), 1531–1541. [CrossRef]
- Thomas, S. , Shumilova, A.A., Kiselev, E.G., Baranovsky, S.V., Vasiliev, A.D., Nemtsev, I.V., Kuzmin, A.P., Sukovatyi, A.G., Avinash, R.P., Volova, T.G. Thermal, mechanical and biodegradation studies of biofiller based poly-3-hydroxybutyrate biocomposites. Int. J. Biol. Macromol. 2020, 155, 1373–1384. [Google Scholar] [CrossRef]
- Briassoulis, D. , Dejean, C. Critical review of norms and standards for biodegradable agricultural plastics part I. Biodegradation in soil. J. Poly. Environ. 2010, 18, 384–400. [Google Scholar] [CrossRef]
- Kamiya, M. , Asakawa, S., Kimura, M. Molecular analysis of fungal communities of biodegradable plastics in two Japanese soils. Soil Sci. Plant Nutr. 2007, 53(5), 568–574. [Google Scholar] [CrossRef]
- Hoshino, A. , Sawada, H., Yokota, M., Tsuji, M., Fukuda, K., Kimura, M. Influence of weather conditions and soil properties on degradation of biodegradable plastics in soil. Soil Sci. Plant Nutr. 2001, 47(1), 35–43. [Google Scholar] [CrossRef]
- Adhikari, D. , Mukai, M., Kubota, K., Kai, T., Kaneko, N., Araki, K.S., Kubo, M. Degradation of bioplastics in soil and their degradation effects on environmental microorganisms. J. Agric. Chem. Environ. 2016, 5, 23–34. [Google Scholar] [CrossRef]
- Harmaen, A.S., Khalina, A., Azowa, I., Hassan, M.A., Tarmian, A., Jawaid, M. Thermal and biodegradation properties of poly(lactic acid)/fertilizer/oil palm fibers blends biocomposites. Polym. Compos. 2015, 36(1), 576-583. [CrossRef]
- Kale, G., Auras, R., Singh, S.P., Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26(8), 1049–1061. [CrossRef]
- Mihai, M. , Legros, N., Alemdar, A. Formulation-Properties Versatility of Wood Fiber Biocomposites Based on Polylactide and Polylactide/Thermoplastic Starch Blends. Polym. Eng. Sci. 2014, 54(6), 1325-1340. [CrossRef]
- Otake, Y., Kobayashi, T., Asabe, H., Murakami, N., Ono, K. Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J. App. Polym. Sc. 1995, 56(13), 1789–1796.
- Sridharan, R. , Krishnaswamy, V.G., Kumar, P.S. Analysis and microbial degradation of Low-Density Polyethylene (LDPE) in Winogradsky column. Environ. Res. 2021, 201, 111646. [Google Scholar] [CrossRef]
- Boluda, R. , Roca-Pérez, L., Iranzo, M., Gil, C., Mormeneo, S. Determination of enzymatic activities using a miniaturized system as a rapid method to assess soil quality. Eur. J. Soil Sci. 2014, 65(2), 286–294. [Google Scholar] [CrossRef]
- Emadian, S.M. , Onay, T.T., Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manage. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Khan, S. , Ali, S.A., Ali, A.S. Biodegradation of low density polyethylene (LDPE) by mesophilic fungus ‘Penicillium citrinum’ isolated from soils of plastic waste dump yard, Bhopal, India. Environ. Technol. 2300. [Google Scholar] [CrossRef]
- Roy, P.K. , Titus, S., Surekha, P., Tulsi, E., Deshmukh, C., Rajagopal, C. Degradation of abiotically aged LDPE film containing pro-oxidant by bacterial consortium. Polym. Degrad. Stab. 2008, 93, 1917–1922. [Google Scholar] [CrossRef]
- Park, S.Y. , Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere. 2019, 222, 527–533. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).