Submitted:
02 April 2025
Posted:
03 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. FT-IR
2.2.2. FESEM
2.2.3. TUNA
2.2.4. Self-Repairing Efficiency Evaluation Tests
3. Results and Discussion
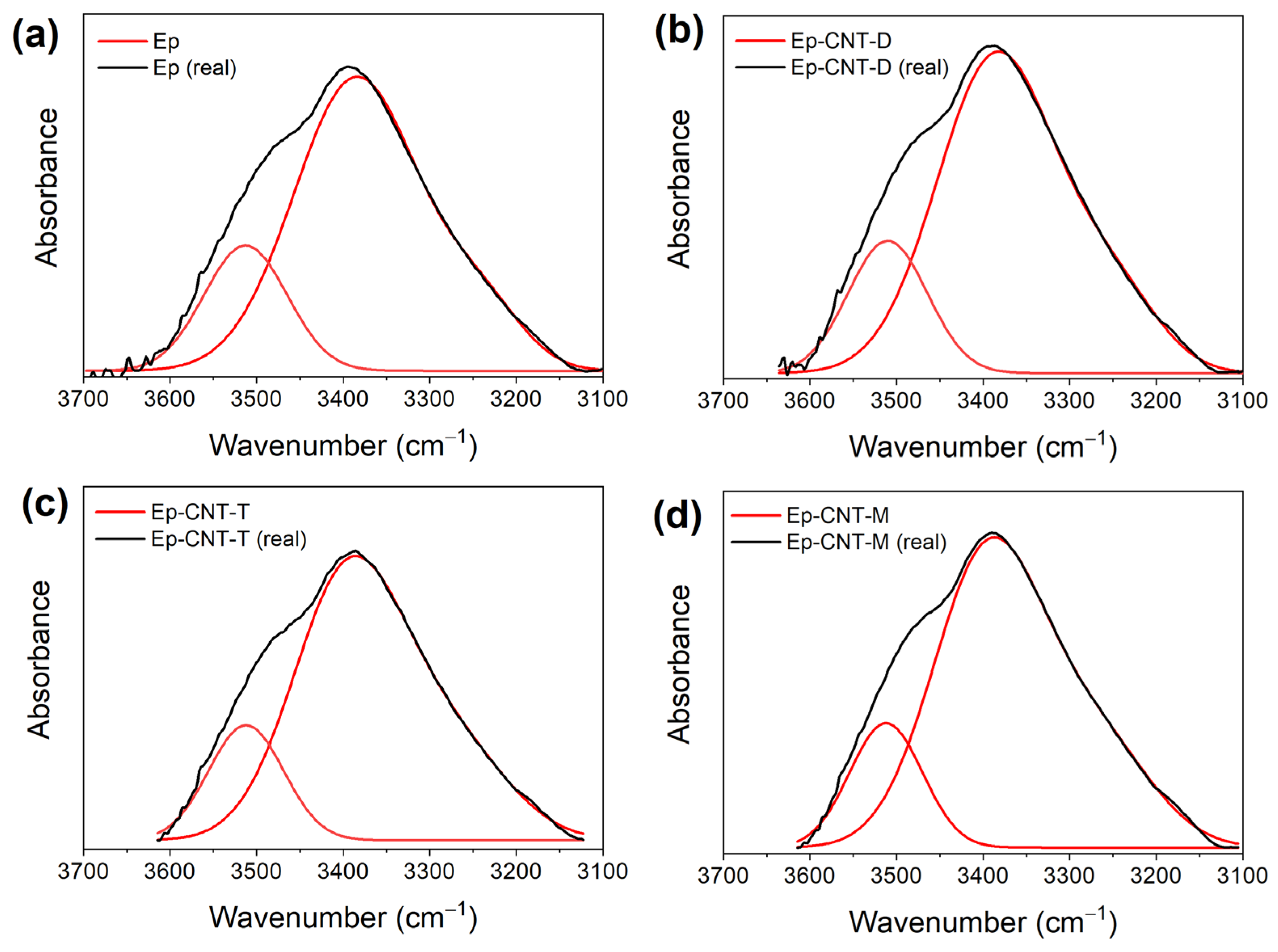
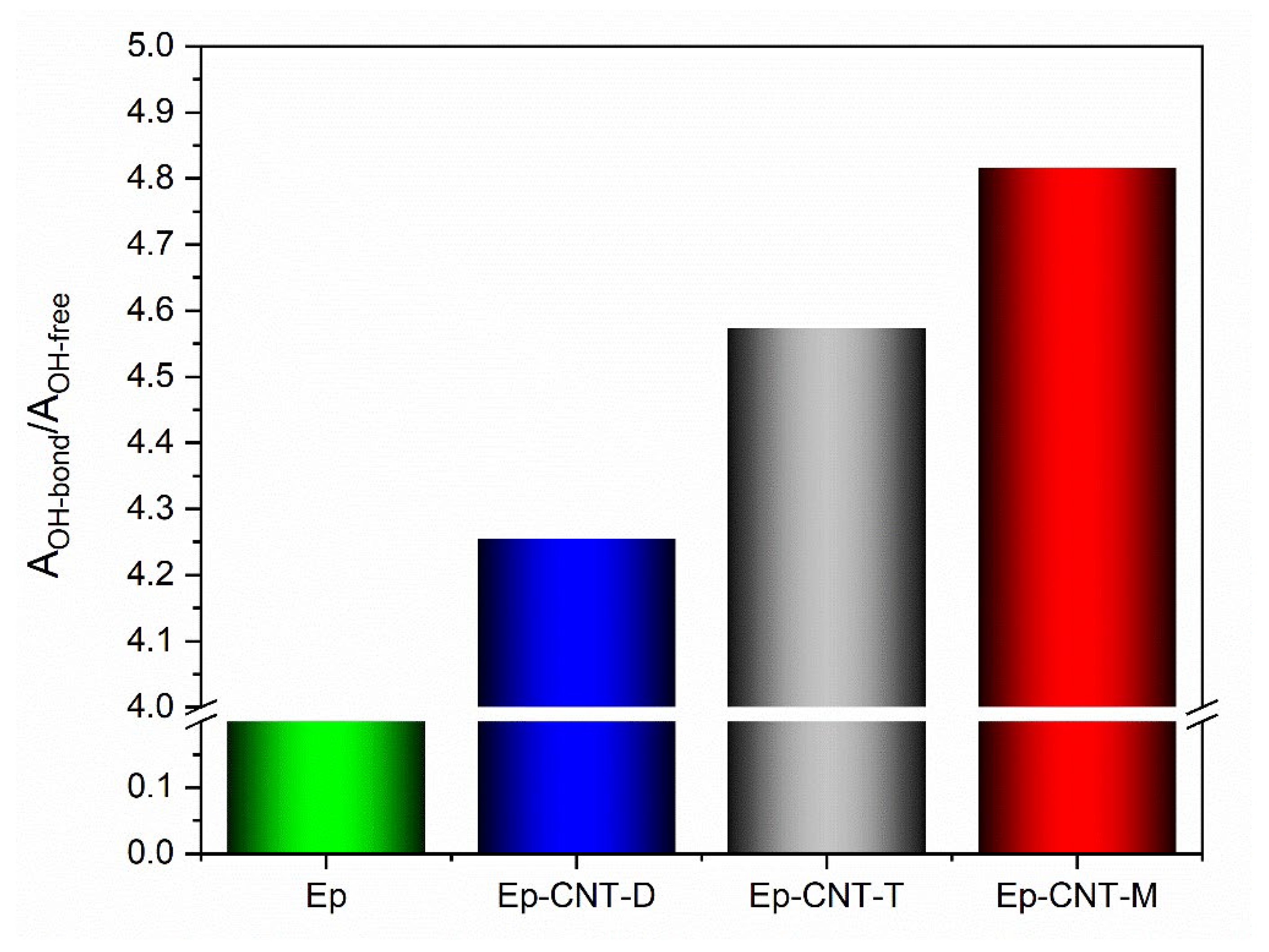
3.1. FT-IR Analysis
3.1.1. FT-IR Investigation of the System Based on Filler D
3.1.2. FT-IR Investigation of the System Based on Filler T
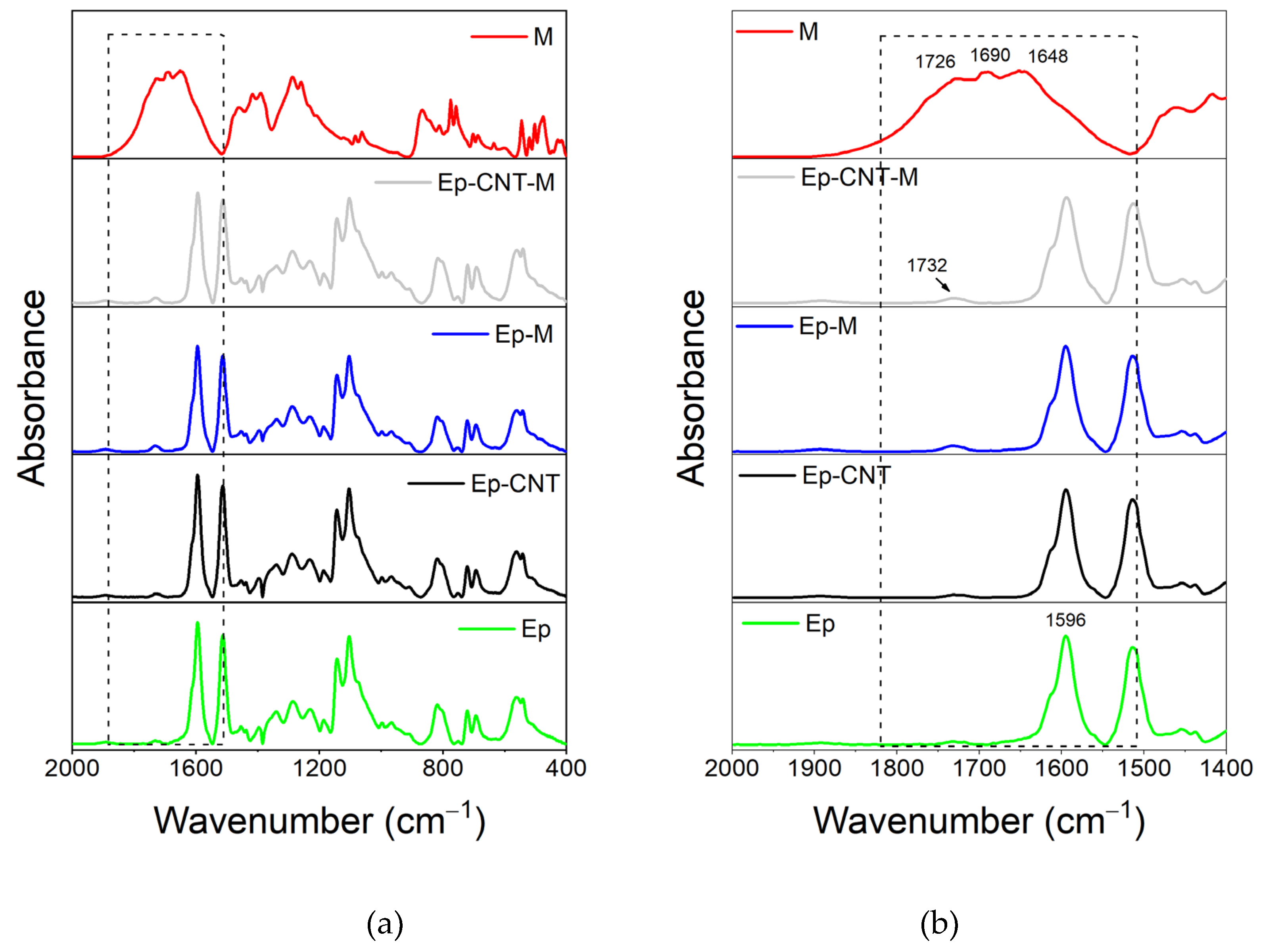
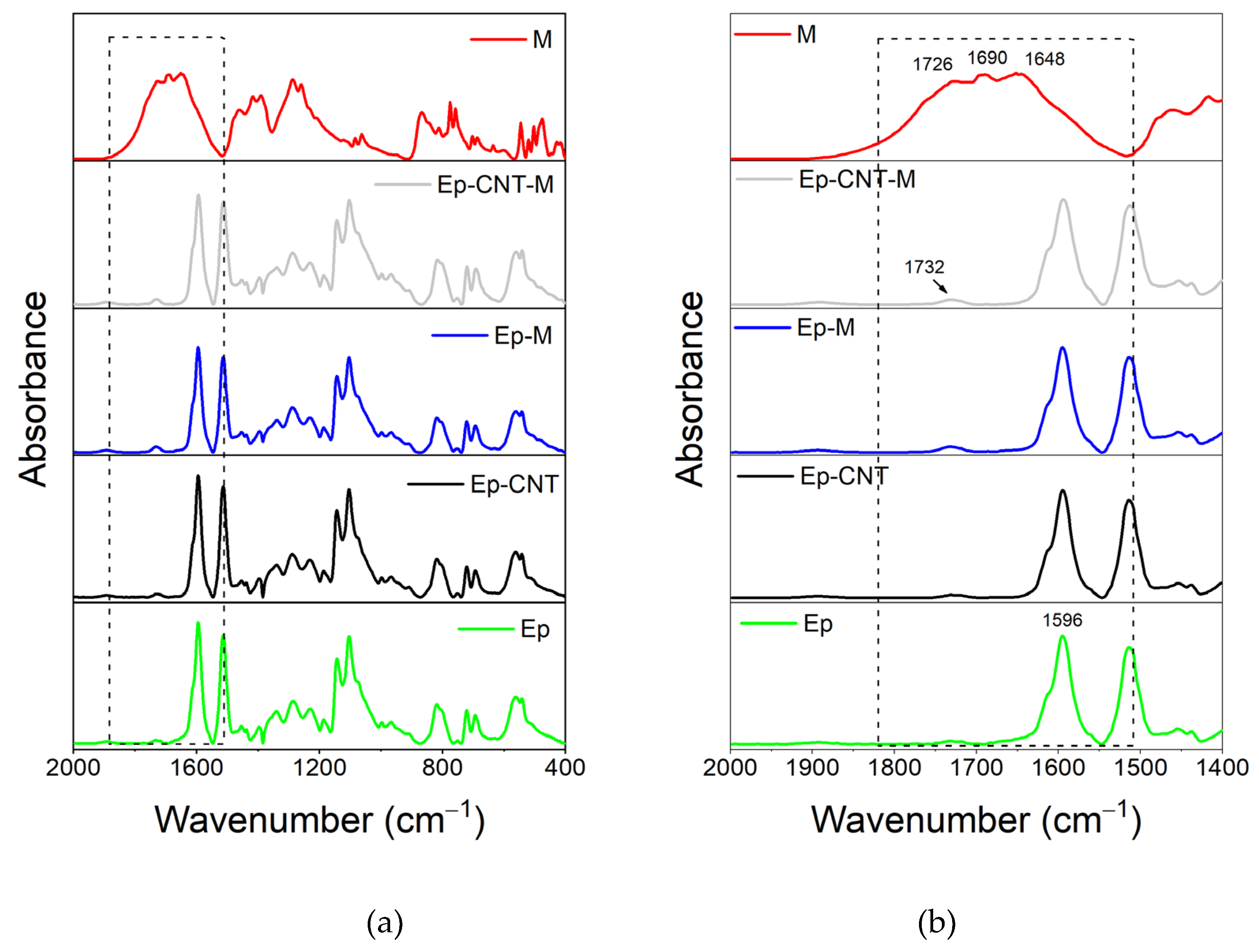
3.1.3. FT-IR Investigation of the System Based on Filler M
3.2. Additional FT-IR Analysis
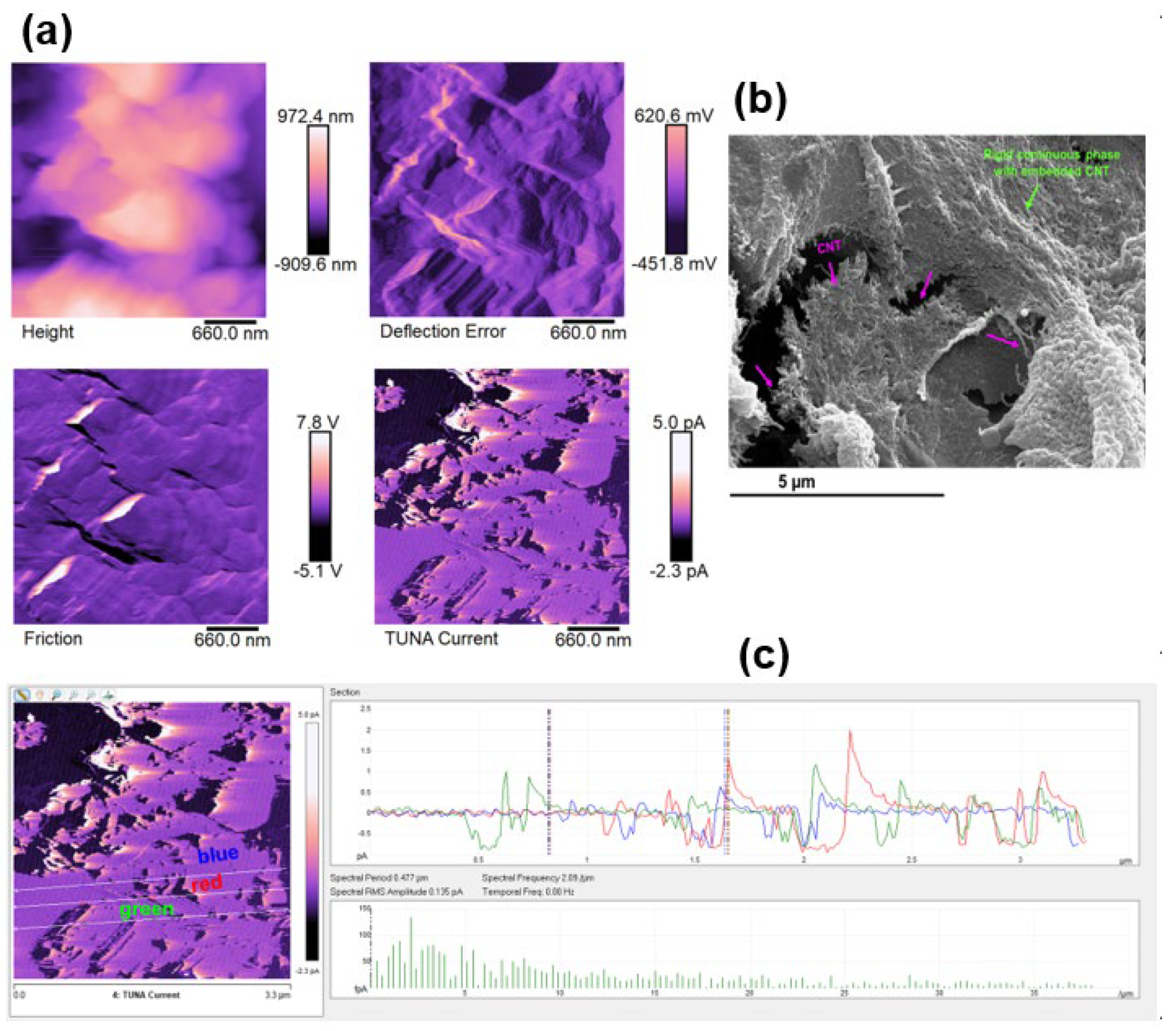
3.3. Morphological Investigation by FESEM and TUNA
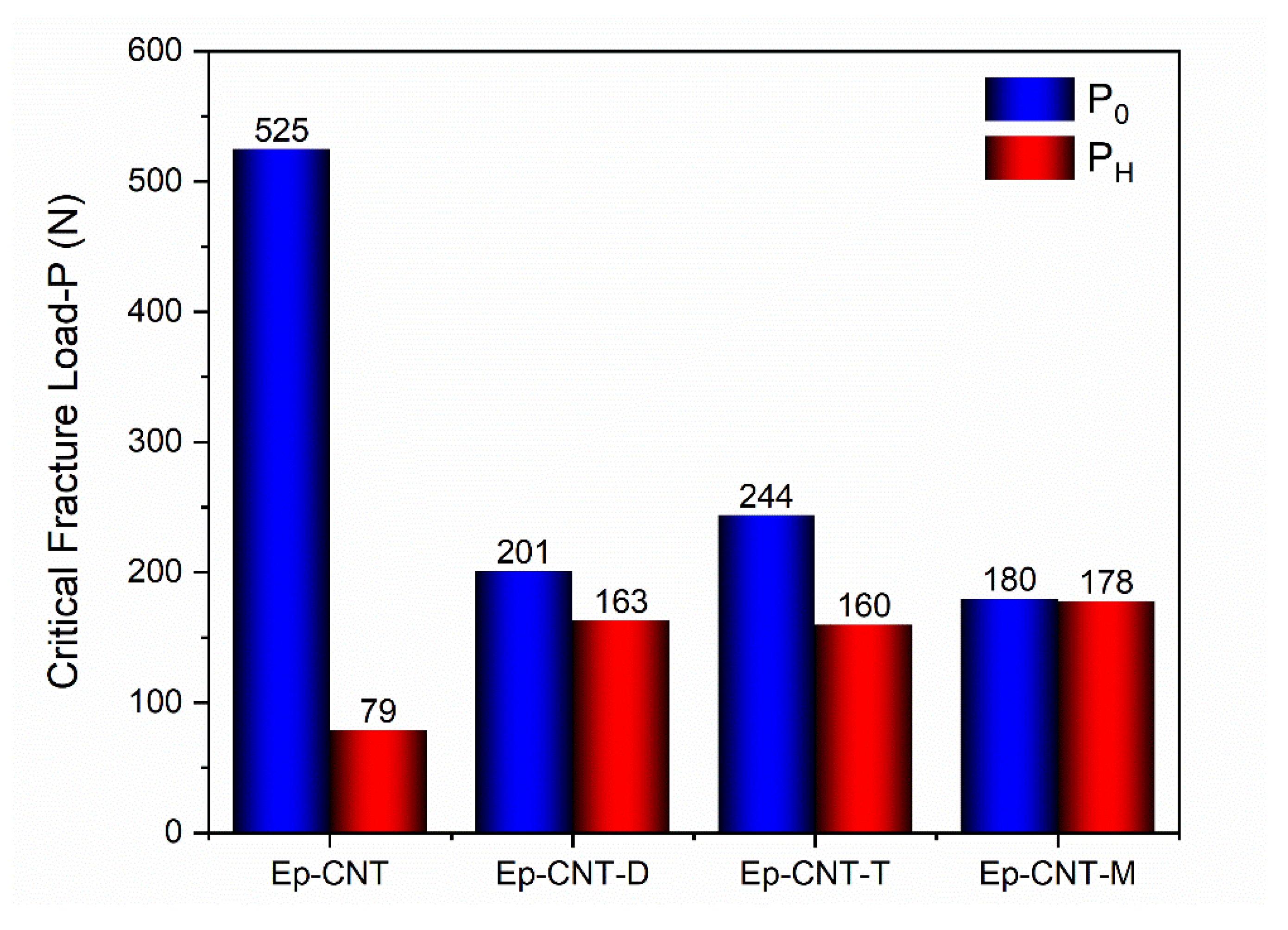
3.4. Self-Repairing Efficiency Evaluation Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, K.; Zhao, G.; Hu, D.; Li, R.; Wei, Q.; Fu, Q.; Deng, H. Magnetic and electrically conductive polyurethane composites with high content of two functional fillers base on “Root” inspired microstructure. Compos. B Eng. 2023, 252, 110512. [CrossRef]
- Yun, G.; Tang, S.Y.; Lu, H.; Zhang, S.; Dickey, M.D.; Li, W. Hybrid-Filler Stretchable Conductive Composites: From Fabrication to Application. Small Sci. 2021, 1, 2000080. [CrossRef]
- Osman, A.; Elhakeem, A.; Kaytbay, S.; Ahmed, A. A comprehensive review on the thermal, electrical, and mechanical properties of graphene-based multi-functional epoxy composites. Adv Compos Hybrid Mater 2022, 5, 547–605. [CrossRef]
- Kopsidas, S.; Olowojoba, G.B. Multifunctional epoxy composites modified with a graphene nanoplatelet/carbon nanotube hybrid. J. Appl. Polym. Sci. 2021, 138, e50890. [CrossRef]
- Longo, R.; Guadagno, L.; Lamberti, P. Electromagnetic Characterization of Polycaprolactone electrospun nanofibers filled with Fe3 O4 Nanoparticles,” 2020 4th International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT), Istanbul, Turkey, 2020, pp. 1–5. [CrossRef]
- Fauche, R.; Windey, R.; Pfeiffer, H.; Seveno, D.; Wevers, M. Effects of Water Uptake on the Electrical Properties of Carbon Black-Epoxy Nanocomposites. ACS Appl. Nano Mater. 2024, 7, 3610–3619. [CrossRef]
- Shen, P.; Jiang, Z.; Viktorova, J.; Pollard, B.; Kumar, A.; Stachurski, Z.; Connal, L.A. Conductive and Self-Healing Carbon Nanotube–Polymer Composites for Mechanically Strong Smart Materials. ACS Appl. Nano Mater. 2023, 6, 986–994. [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Reversible Self-Healing Carbon-Based Nanocomposites for Structural Applications. Polymers 2019, 11, 903. [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder,W.H.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. B Eng. 2019, 157, 1–13. [CrossRef]
- Nik Md Noordin Kahar, N.N.F.; Osman, A.F.; Alosime, E.; Arsat, N.; Mohammad Azman, N.A.; Syamsir, A.; Itam, Z.; Abdul Hamid, Z.A. The Versatility of Polymeric Materials as Self-Healing Agents for Various Types of Applications: A Review. Polymers 2021, 13, 1194. [CrossRef]
- Zhang, F.; Zhang, L.; Yaseen, M.; Huang, K. A review on the self-healing ability of epoxy polymers. J Appl Polym Sci. 2021, 138, e50260. [CrossRef]
- Guadagno, L.; Vertuccio, L.; Barra, G.; Naddeo, C.; Sorrentino, A.; Lavorgna, M.; Raimondo, M.; Calabrese, M. Eco-friendly polymer nanocomposites designed for self-healing applications. Polymer 2021, 223, 123718. [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Bocchetta, P. Self-Healing Nanocomposites—Advancements and Aerospace Applications. J. Compos. Sci. 2023, 7, 148. [CrossRef]
- Banerjee, P.; Kumar, S.; Bose, S. Thermoreversible Bonds and Graphene Oxide Additives Enhance the Flexural and Interlaminar Shear Strength of Self-Healing Epoxy/Carbon Fiber Laminates. ACS Appl. Nano Mater. 2021, 4, 6821–6831. [CrossRef]
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications. Polymers 2021, 13, 201. [CrossRef]
- Gong, Z.; Huang, J.; Cao, L.; Xu, C.; Chen, Y. Self-healing epoxidized natural rubber with ionic/coordination crosslinks. Mater. Chem. Phys. 2022, 285, 126063. [CrossRef]
- Roy, N.; Bruchmann, B.; Lehn, J.M. DYNAMERS: dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [CrossRef]
- Yu, K.; Xin, A.; Wang, Q. Mechanics of self-healing polymer networks crosslinked by dynamic bonds. J. Mech. Phys. Solids 2018, 121, 409–431. [CrossRef]
- Shang, W.; Hou, G.; Ren, R.; Li, X.; Weng, Y.; Liu, J. Molecular dynamics simulation insight into topological structure dependence of self-healing polymer nanocomposites. Phys. Chem. Chem. Phys. 2023, 25, 19046–19057. [CrossRef]
- Islam, S.; Bhat, G.S. Progress and challenges in self-healing composite materials. Mater. Adv. 2021, 2, 1896–1926. [CrossRef]
- Smoleń, P.; Czujko, T.; Komorek, Z.; Grochala, D.; Rutkowska, A.; Osiewicz-Powęzka, M. Mechanical and Electrical Properties of Epoxy Composites Modified by Functionalized Multiwalled Carbon Nanotubes. Materials 2021, 14, 3325. [CrossRef]
- Jouni, M.; Fedorko, P.; Celle, C.; Djurado, D.; Chenevier, P.; Faure-Vincent, J. Conductivity vs functionalization in single-walled carbon nanotube films. SN Appl. Sci. 2022, 4, 132. [CrossRef]
- Vertuccio, L.; Calabrese, E.; Raimondo, M.; Catauro, M.; Sorrentino, A.; Naddeo, C.; Longo, R.; Guadagno, L. Effect of Temperature on the Functionalization Process of Structural Self-Healing Epoxy Resin. Aerospace 2023, 10, 476. [CrossRef]
- Liu, T.; Zhao, H.; Zhang, D.; Lou, Y.; Huang, L.; Ma, L.; Hao, X.; Dong, L.; Rosei, F.; Lau, W.M. Ultrafast and high-efficient self-healing epoxy coatings with active multiple hydrogen bonds for corrosion protection. Corros. Sci. 2021, 187, 109485. [CrossRef]
- Wang, N.; Feng, X.; Pei, J.; Cui, Q.; Li, Y.; Liu, H.; Zhang, X. Biobased Reversible Cross-Linking Enables Self-Healing and Reprocessing of Epoxy Resins. ACS Sustainable Chem. Eng. 2022, 10, 3604–3613. [CrossRef]
- Li, J.; Du, X.; Zhang, A.; Wen, J.; Shuai, L.; Li, S.; Zhu, M.; Nie, Y. Hydrogen-bonded polymeric materials with high mechanical properties and high self-healing capacity. Mater. Chem. Front. 2024, 8, 3828–3858. [CrossRef]
- Guadagno, L.; Foglia, F.; Pantani, R.; Romero-Sanchez, M.D.; Calderón, B.; Vertuccio, L. Low-Voltage Icing Protection Film for Automotive and Aeronautical Industries. Nanomaterials 2020, 10, 1343. [CrossRef]
- Krajewski, D.; Oleksy, M.; Oliwa, R.; Bulanda, K.; Czech, K.; Mazur, D.; Masłowski, G. Methods for Enhancing the Electrical Properties of Epoxy Matrix Composites. Energies 2022, 15, 4562. [CrossRef]
- Suherman, H.; Dweiri, R.; Sulong, A.B.; Zakaria, M.Y.; Mahyoedin, Y. Improvement of the Electrical-Mechanical Performance of Epoxy/Graphite Composites Based on the Effects of Particle Size and Curing Conditions. Polymers 2022, 14, 502. [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M. A review on recent advances on the mechanical and conductivity properties of epoxy nanocomposites for industrial applications. Polym. Bull. 2023, 80, 3449–3487. [CrossRef]
- Raimondo, M.; Donati, G.; Milano, G.; Guadagno, L. Hybrid composites based on carbon nanotubes and graphene nanosheets outperforming their single-nanofiller counterparts. FlatChem 2022, 36, 100431. [CrossRef]
- Ali, A.; Rahimian Koloor, S.S.; Alshehri, A.H.; Arockiarajan, A. Carbon Nanotube Characteristics and Enhancement Effects on the Mechanical Features of Polymer-based Materials and Structures – A Review. J. Mater. Res. Technol. 2023, 24, 6495–6521. [CrossRef]
- Lavagna, L.; Nisticò, R.; Musso, S.; Pavese, M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: a review. Mater. Today Chem. 2021, 20, 100477. [CrossRef]
- Deshpande, P.P.; Radue, M.S.; Gaikwad, P.; Bamane, S.; Patil, S.U.; Pisani, W.A.; Odegard, G.M. Prediction of the Interfacial Properties of High-Performance Polymers and Flattened CNT-Reinforced Composites Using Molecular Dynamics. Langmuir 2021, 37,11526–11534. [CrossRef]
- Castro, V.G.; Costa, I.B.; Medeiros, F.S.; Siqueira, É.J.; Kasama, A.H.; Figueiredo, K.C.S.; Lavall, R.L.; Silva, G.G. Improved Functionalization of Multiwalled Carbon Nanotubes in Ultra-Low Acid Volume: Effect of Solid/Liquid. J. Braz. Chem. Soc. 2019, 30, 2477–2487. [CrossRef]
- Vennerberg, D.C.; Quirino, R.L.; Jang, Y.; Kessler, M.R. Oxidation Behavior of Multiwalled Carbon Nanotubes Fluidized with Ozone. ACS Appl. Mater. Interfaces 2014, 6, 1835–1842. [CrossRef]
- Samorì, C.; Sainz, R.; Ménard-Moyon, C.; Toma, F.M.; Venturelli, E.; Singh, P.; Ballestri, M.; Prato, M.; Bianco, A. Potentiometric titration as a straightforward method to assess the number of functional groups on shortened carbon nanotubes. Carbon 2010, 48, 2447–2454. [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A Review on Carbon Nanotubes and Graphene as Fillers in Reinforced Polymer Nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [CrossRef]
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 2011, 49, 1919–1930. [CrossRef]
- Raimondo, M.; Calabrese, E.; Binder, W.H.; Michael, P.; Rana, S.; Guadagno, L. Tunneling Atomic Force Microscopy Analysis of Supramolecular Self-Responsive Nanocomposites. Polymers 2021, 13, 1401. [CrossRef]
- Liu, Z.; Guan, X.; Li, B.; Yin, H.; Jin, C. Investigation on Junction Contacts of Semiconducting Carbon Nanotube Networks Using Conductive Atomic Force Microscopy. ACS Appl. Mater. Interfaces 2024, 16, 38, 51309–51317. [CrossRef]
- Sa’aya, N.S.N.; Demon, S.Z.N.; Abdullah, N.; Abdul Halim, N. Morphology Studies of SWCNT Dispersed in Conducting Polymer as Potential Sensing Materials. SSP 2021, 317, 189–94. [CrossRef]
- Longo, P.; Mariconda, A.; Calabrese, E.; Raimondo, M.; Naddeo, C.; Vertuccio, L.; Russo, S.; Iannuzzo, G.; Guadagno, L. Development of a new stable ruthenium initiator suitably designed for self-repairing applications in high reactive environments. J. Ind. Eng. Chem. 2017, 54, 234–251. [CrossRef]
- Guadagno, L.; Raimondo, M.; Naddeo, C.; Vertuccio, L.; Russo, S.; Iannuzzo, G.; Calabrese, E. Rheological, Thermal and Mechanical Characterization of Toughened Self-Healing Supramolecular Resins, Based on Hydrogen Bonding. Nanomaterials 2022, 12, 4322. [CrossRef]
- Buaksuntear, K.; Limarun, P.; Suethao, S.; Smitthipong, W. Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers. Int. J. Mol. Sci. 2022, 23, 6902. [CrossRef]
- Chen, L.; Xu, J.; Zhu, M.; Zeng, Z.; Song, Y.; Zhang, Y.; Zhang, X.; Deng, Y.; Xiong, R.; Huang, C. Self-healing polymers through hydrogen-bond cross-linking: synthesis and electronic applications. Mater. Horiz. 2023, 10, 4000–4032. [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Raimondo, M.; Barra, G.; De Nicola, F.; Volponi, R.; Lamberti, P.; Spinelli, G.; Tucci, V. Electrical Current Map and Bulk Conductivity of Carbon Fiber-Reinforced Nanocomposites. Polymers 2019, 11, 1865. [CrossRef]
- Zhang, W.; Yin, L.; Zhao, M.; Tan, Z.; Li, G. Rapid and non-destructive quality verification of epoxy resin product using ATR-FTIR spectroscopy coupled with chemometric methods. Microchem. J. 2021, 168, 106397. [CrossRef]
- Yang, W.; Chai, X.; Tian, Y.; Chen, S.; Cao, Y.; Lu, R.; Jiang, Y.; Li, T. Temperature-dependent FTIR study of a supramolecular mesophase from the self-assembly of melamine and barbituric acid derivatives. Liq. Cryst. 1997, 22, 579–583. [CrossRef]
- Xu, Y.; Zhang, Q. Two-Dimensional Fourier Transform Infrared (FT-IR) Correlation Spectroscopy Study of the Imidization Reaction from Polyamic Acid to Polyimide. Appl. Spectrosc. 2014, 68, 657–662. [CrossRef]
- Nyquist, R.A.; Fiedler, S.L. Infrared study of five-and six-membered type cyclic imides. Vib. Spectrosc. 1995, 8, 365–386. [CrossRef]
- Chandra, S.; Saleem, H.; Sundaraganesan, N.; Sebastian, S. Experimental and theoretical vibrational spectroscopic and HOMO, LUMO studies of 1, 3-dimethylbarbituric acid. Indian J. Chem. 2009, 48A, 1219–1227.
- Delozanne, J.; Desgardin, N.; Cuvillier, N.; Richaud, E. Thermal oxidation of aromatic epoxy-diamine networks. Polym. Degrad. Stab. 2019, 166, 174–187. [CrossRef]
- Sharma, A.; Gupta, V.; Mishra, R.; Tandon, P.; Maeda, S.; Kunimoto, K.K. Study of vibrational spectra and molecular structure of intermolecular hydrogen bonded 2-thiohydantoin using Density Functional Theory. J. Mol. Struct. 2011, 1004, 237–247. [CrossRef]
- Sharma, A.; Gupta, V.; Tandon, P.; Rawat, P.; Maeda, S.; Kunimoto, K.K. Experimental (FT-IR, FT-Raman, NMR) and theoretical spectroscopic properties of intermolecular hydrogen bonded 1-acetyl-2-thiohydantoin polymorphs. Spectrochim Acta A Mol Biomol Spectrosc. 2012, 90, 141–151. [CrossRef]
- Deval, V.; Kumar, A.; Gupta, V.; Sharma, A.; Gupta, A.; Tandon, P.; Kunimoto, K.K. Molecular structure (monomeric and dimeric) and hydrogen bonds in 5-benzyl 2-thiohydantoin studied by FT-IR and FT-Raman spectroscopy and DFT calculations. Spectrochim Acta A Mol Biomol Spectrosc. 2014, 132, 15–26. [CrossRef]
- Calabrese, E.; Raimondo, M.; Sorrentino, A.; Russo, S.; Longo, P.; Mariconda, A.; Longo, R.; Guadagno, L. Verification of the Self-Healing Ability of PP-co-HUPy Copolymers in Epoxy Systems. Polymers 2024, 16, 1509. [CrossRef]
- Yoshida, S. Quantitative evaluation of an epoxy resin dispersion by infrared spectroscopy. Polymer journal 2014, 46, 430–434. [CrossRef]
- Liu, T.; Zhang, M.; Guo, X.; Liu, C.; Liu, T.; Xin, J.; Zhang, J. Mild chemical recycling of aerospace fiber/epoxy composite wastes and utilization of the decomposed resin. Polym. Degrad. Stab. 2017, 139, 20–27. [CrossRef]
- Dagdag, O.; Safi, Z.; Hamed, O.; Jodeh, S.; Wazzan, N.; Haldhar, R.; Safi, S.K.; Berisha, A.; Gouri, M.E. Comparative study of some epoxy polymers based on bisphenolic and aromatic diamines: synthesis, viscosity, thermal properties computational and statistical approaches. J. Polym. Res. 2021, 28, 165. [CrossRef]
- Azzaoui, J.E.; Berradi, M.; El-Aouni, N.; Eddaoukhi, A.; Berradi, O.; Dagdag, O.; Yacoubi, A.E.; Hsissou, R.; Bouchti, M.E.; Bachiri, A.E.; Rafik, M. Synthesis of trifunctional epoxy resin crosslinked with MDA, PDA, and BDA: microscopic, rheological, and thermal studies. Interactions 2025, 246, 35. [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. B Eng. 2017, 128, 30–38. [CrossRef]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Russo, S.; Iannuzzo, G. Effect of carbon nanotube and functionalized liquid rubber on mechanical and electrical properties of epoxy adhesives for aircraft structures. Compos. B Eng. 2017, 129, 1–10. [CrossRef]
- Maddams, W. The scope and limitations of curve fitting. Applied Spectroscopy. 1980, 34, 245-67. https://opg.optica.org/as/abstract.cfm?URI=as-34-3-245.
- Kaupp, G.; Avouris, P.; Bhushan, B.; Von Klitzing, K.; Sakaki, H.; Wiesendanger, R. Atomic force microscopy, scanning nearfield optical microscopy and nanoscratching: Application to rough and natural surfaces. 2006; pp. 1–292. [CrossRef]
- Gautier, B.; Fares, B.; Prudon, G.; Dupuy, J.C. Imaging by atomic force microscopy of the electrical properties difference of the facets of oxygen-ion-induced ripple topography in silicon. Appl. Surf. Sci. 2004, 231–232, 136-140. [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Atomic Force and Scanning Tunneling Microscopy Imaging of Graphene Nanosheets Derived from Graphite Oxide. Langmuir 2009, 25, 10, 5957–5968. [CrossRef]
- Alemour, B.; Badran, O.; Hassan, M.R. A review of using conductive composite materials in solving lightening strike and ice accumulation problems in aviation. J. Aerosp. Technol. Manag. 2019, 11, 1–23. [CrossRef]
- Guadagno, L.; Vertuccio, L.; Aliberti, F.; Pantani, R.; Raimondo, M.; Catauro, M.; Longo, R. Development of de-icing/self-sensing structural composites via controlled Joule heating curing. Compos. B Eng. 2025, 292, 112079. [CrossRef]
- Chung, D.D.L. A review of multifunctional polymer-matrix structural composites. Compos. B Eng. 2019, 160, 644–660. [CrossRef]
- Long, C.; Xu, J.; Luo, X.; Liu, Z.; Bing, W.; Song, Q.; Yukui, C.; Yi, W.; Gao, X.; Li, C. Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview. Nanotechnol. Rev. 2023, 12, 20230105. [CrossRef]
- De Vivo, B.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L.; Vittoria, V.; Sorrentino, A. Comparison of the physical properties of epoxy-based composites filled with different types of carbon nanotubes for aeronautic applications. Adv. Polym. Tech. 2012, 31, 205–218. [CrossRef]
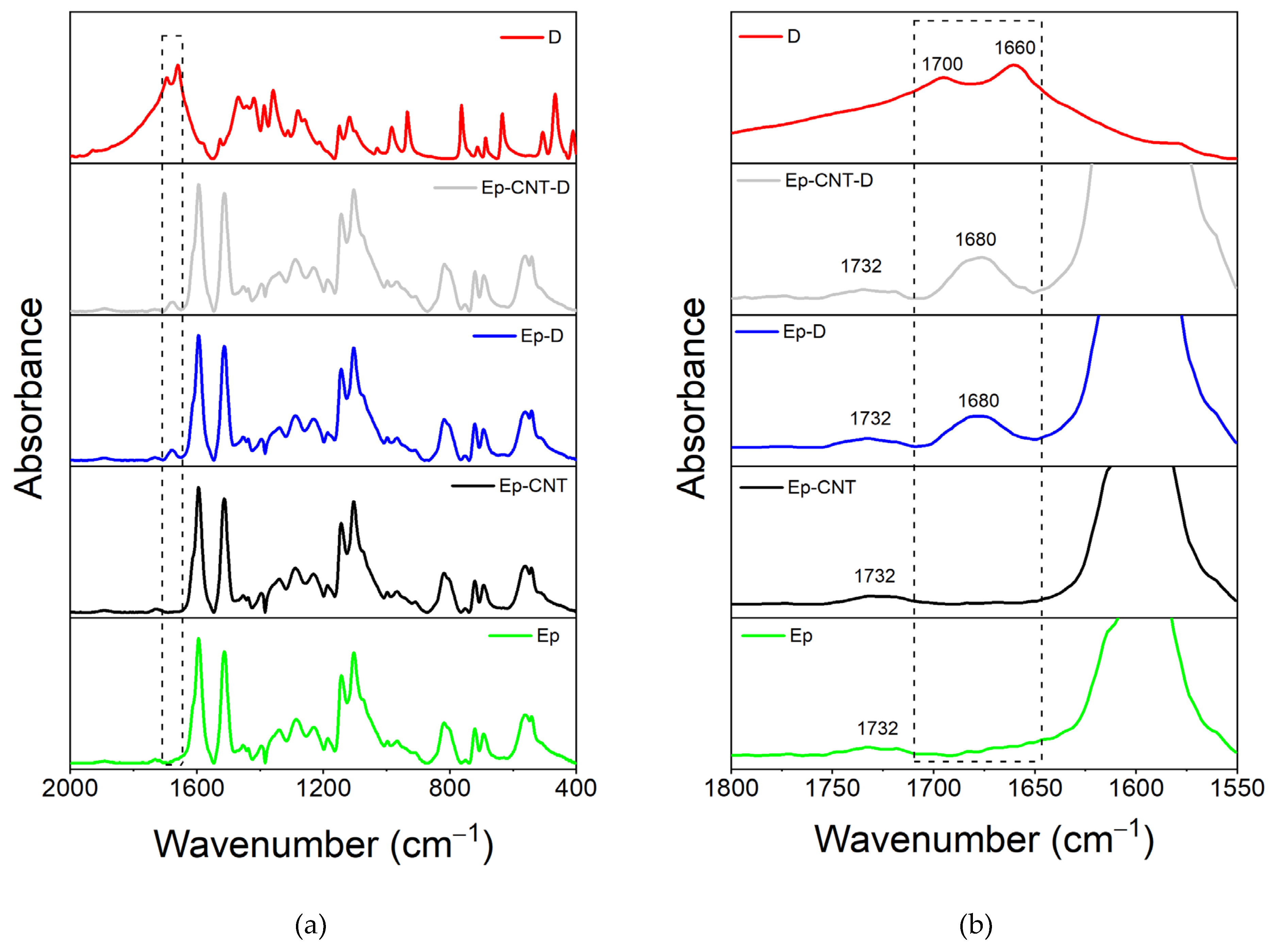
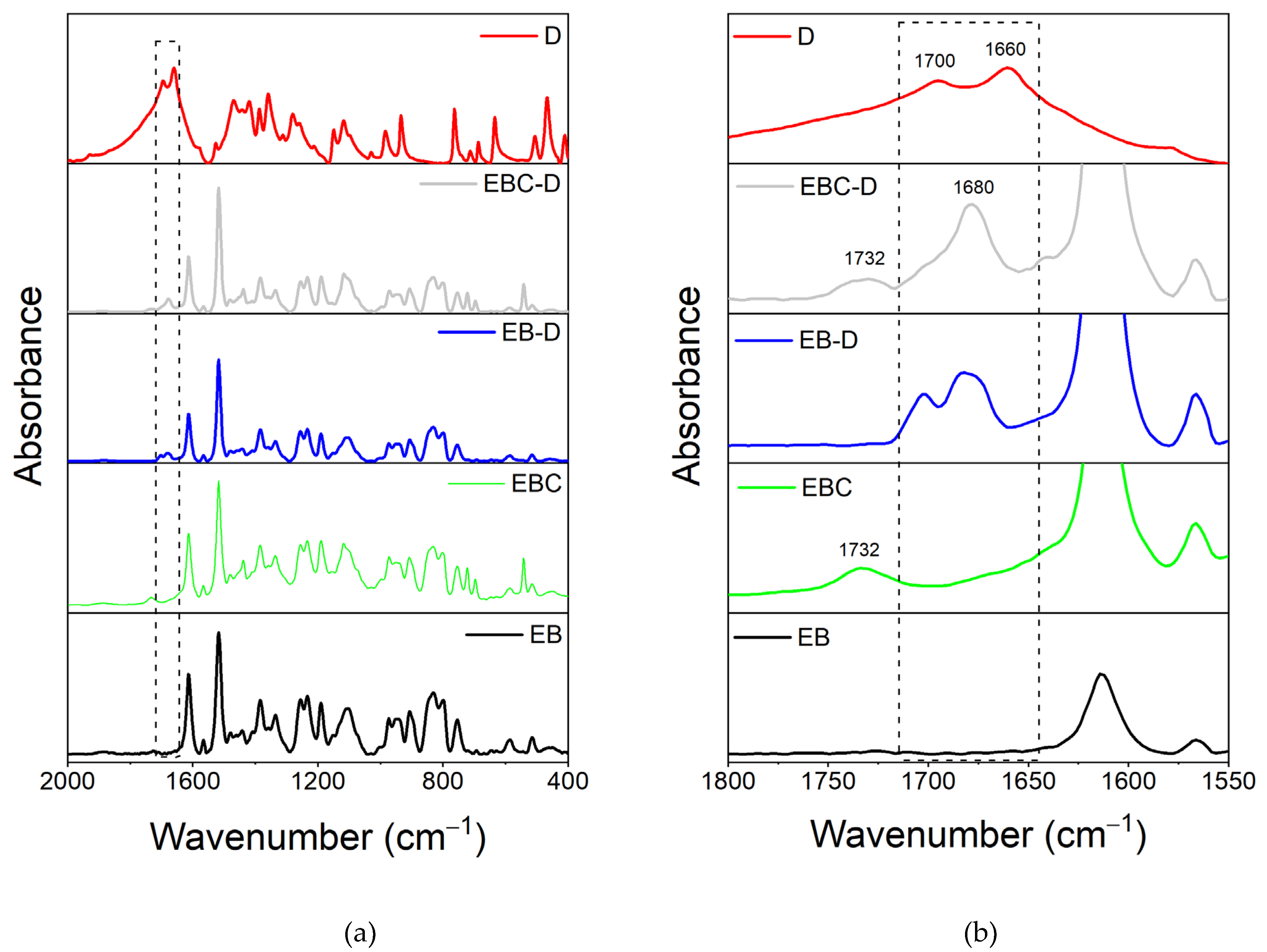
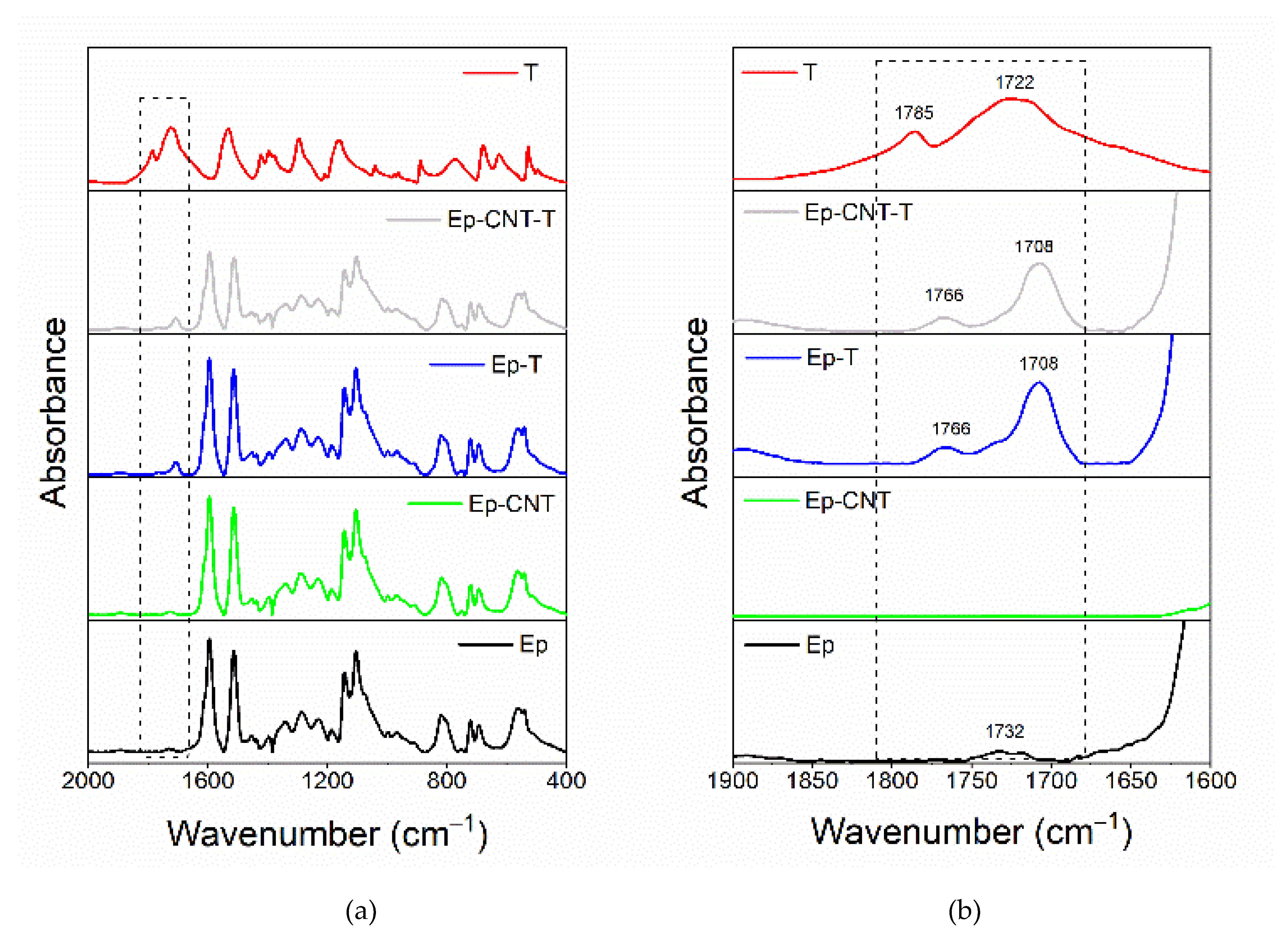
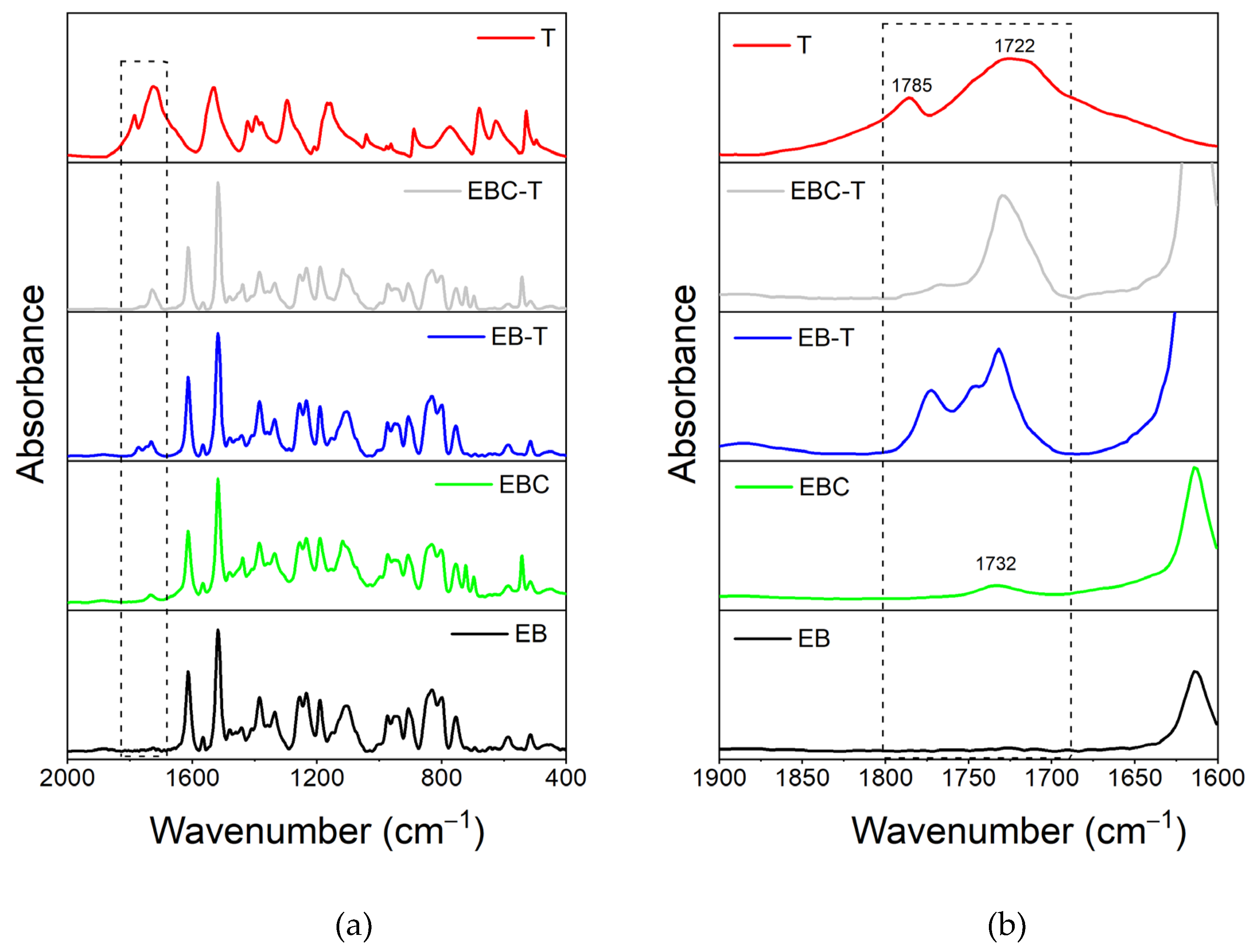
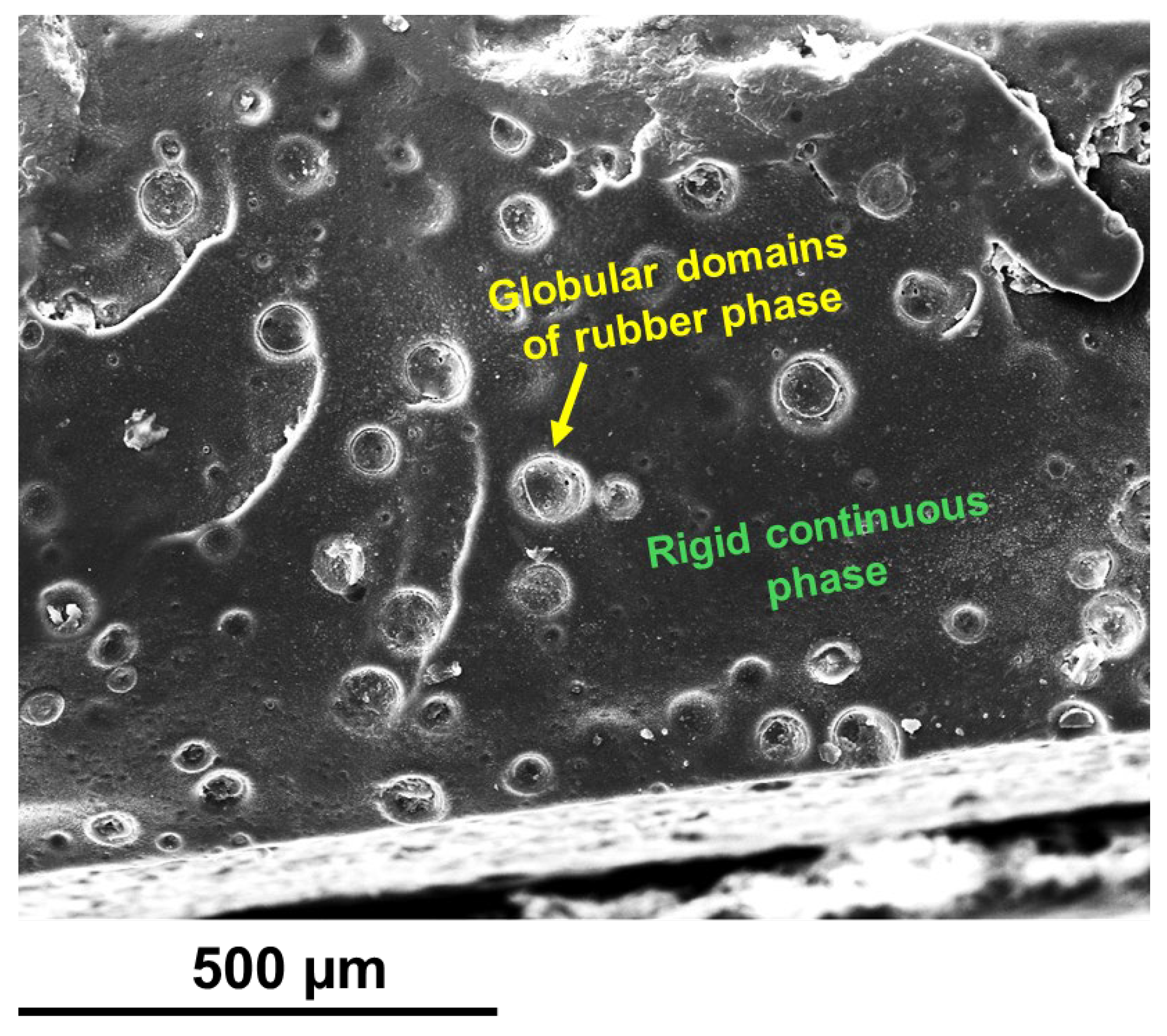
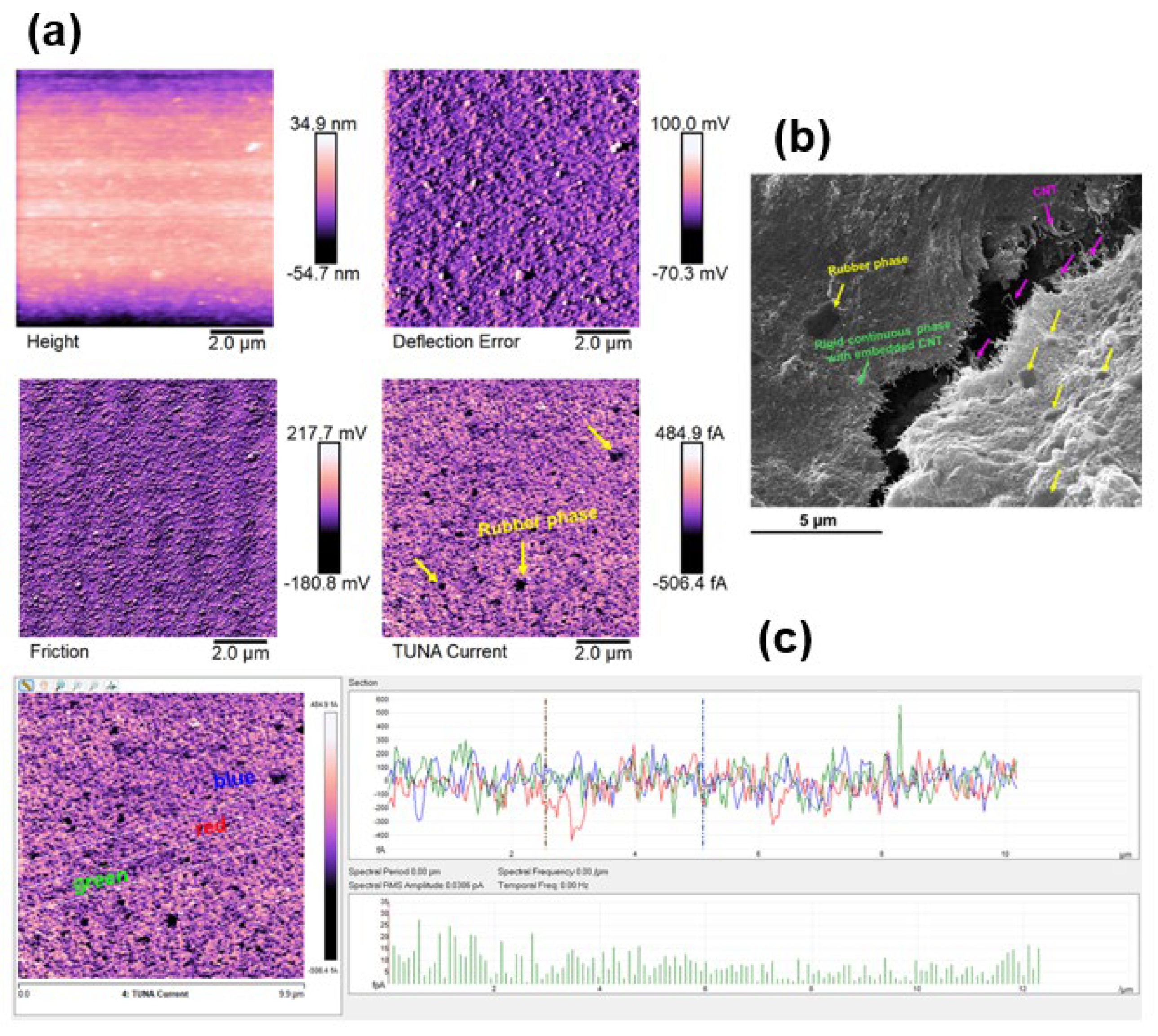
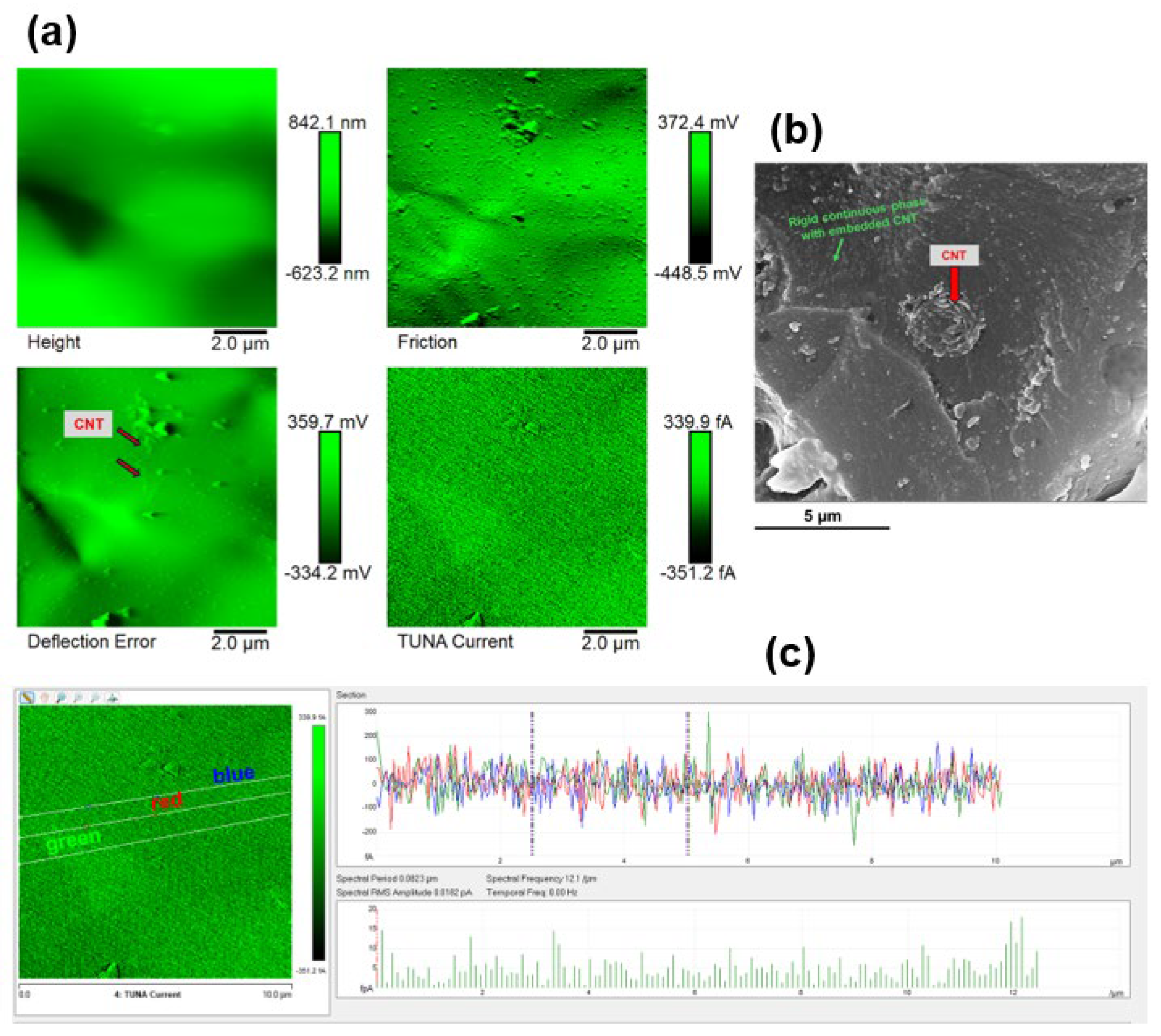
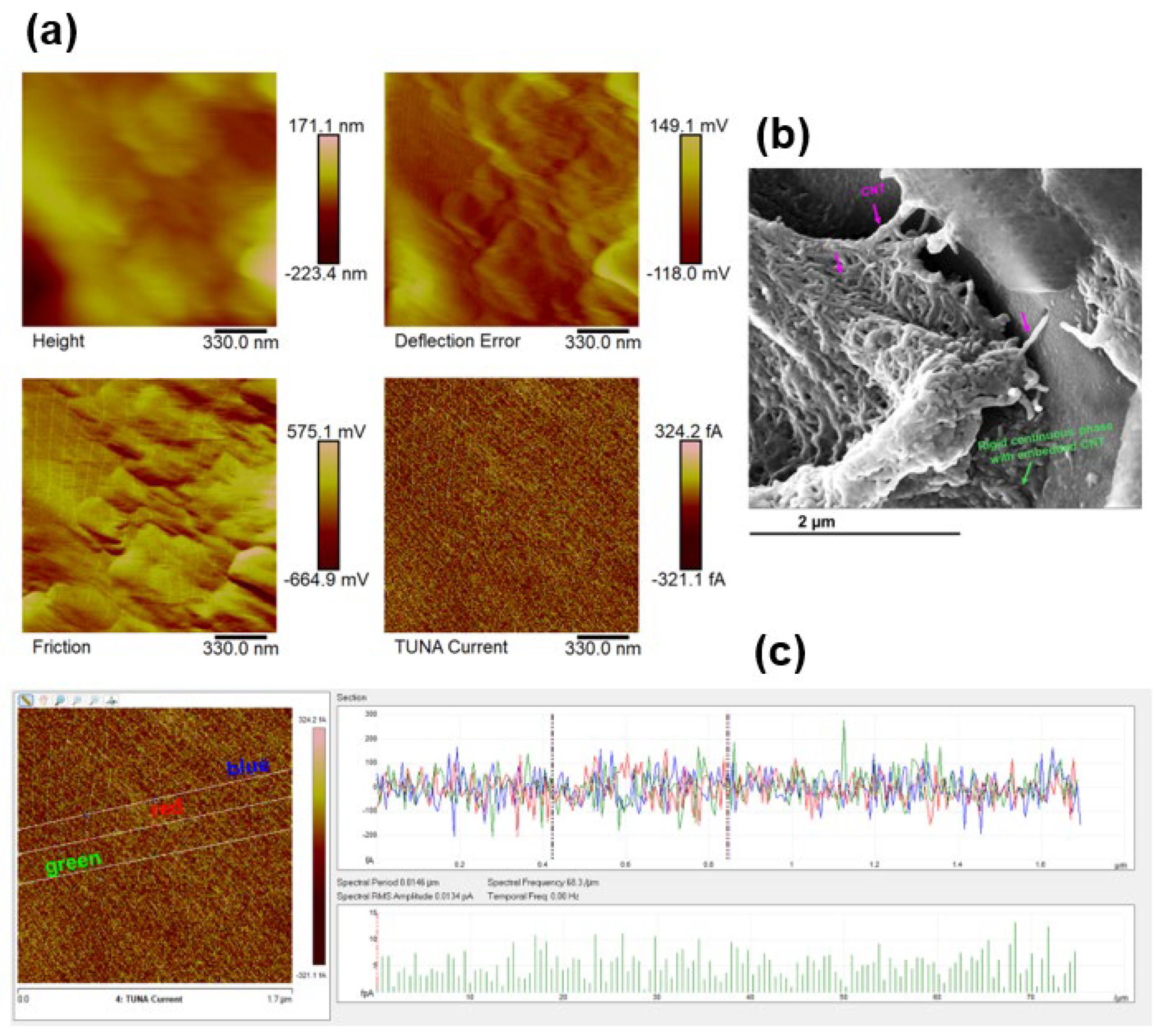
| Compound | Description | References for chemical formula | Acronym |
|---|---|---|---|
| Tetra glycidyl methylene dianiline* | Tetrafunctional epoxy precursor, viscous liquid |
[44] | E |
| 1,4-butanedioldiglycidylether* | Reactive diluent, liquid |
[44] | B |
| Carboxyl terminated butadiene acrylonitrile** | Toughening Elastomer, viscous liquid |
[9,23] | C |
| 4,4′-diaminodiphenyl sulfone* | Hardening agent, powder |
[44] | H |
| Multiwall carbon nanotubes*** | Conductive filler (3100 Grade), powder |
[9] | CNT |
| 1,3-Dimethylbarbituric acid* | Self-healing filler, powder |
[23,45] | D |
| 2-Thiohydantoin* | Self-healing filler, powder |
[23,45] | T |
| Murexide* | Self-healing filler, powder |
[23,45] | M |
| Sample composition | Description | Acronym |
|---|---|---|
| E+B | Uncured viscous liquid sample | EB |
| E+B+C | Uncured viscous liquid sample | EBC |
| E+B+self-healing filler | Uncured viscous liquid sample | EB-D |
| EB-T | ||
| EB-M | ||
| E+B+C+self-healing filler | Uncured viscous liquid sample | EBC-D |
| EBC-T | ||
| EBC-M | ||
| E+B+C+H | Oven-cured epoxy sample | Ep |
| E+B+C+H+CNT | Oven-cured epoxy sample | Ep-CNT |
| E+B+C+H+self-healing filler | Oven-cured epoxy sample | Ep-D |
| Ep-T | ||
| Ep-M | ||
| E+diluent+C+H+CNT+self-healing filler | Oven-cured epoxy sample | Ep-CNT-D |
| Ep-CNT-T | ||
| Ep-CNT-M |
| Self-healing filler | Structural formula | |
|---|---|---|
| D | 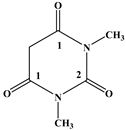 |
|
| T | 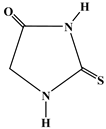 |
|
| M | 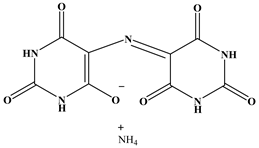 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





