Submitted:
25 March 2025
Posted:
26 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Research Method
2.1. Connection to Real-Life Application
2.2. Computer Modeling Tool
2.3. Controllable Variables
2.4. Some Assumptions
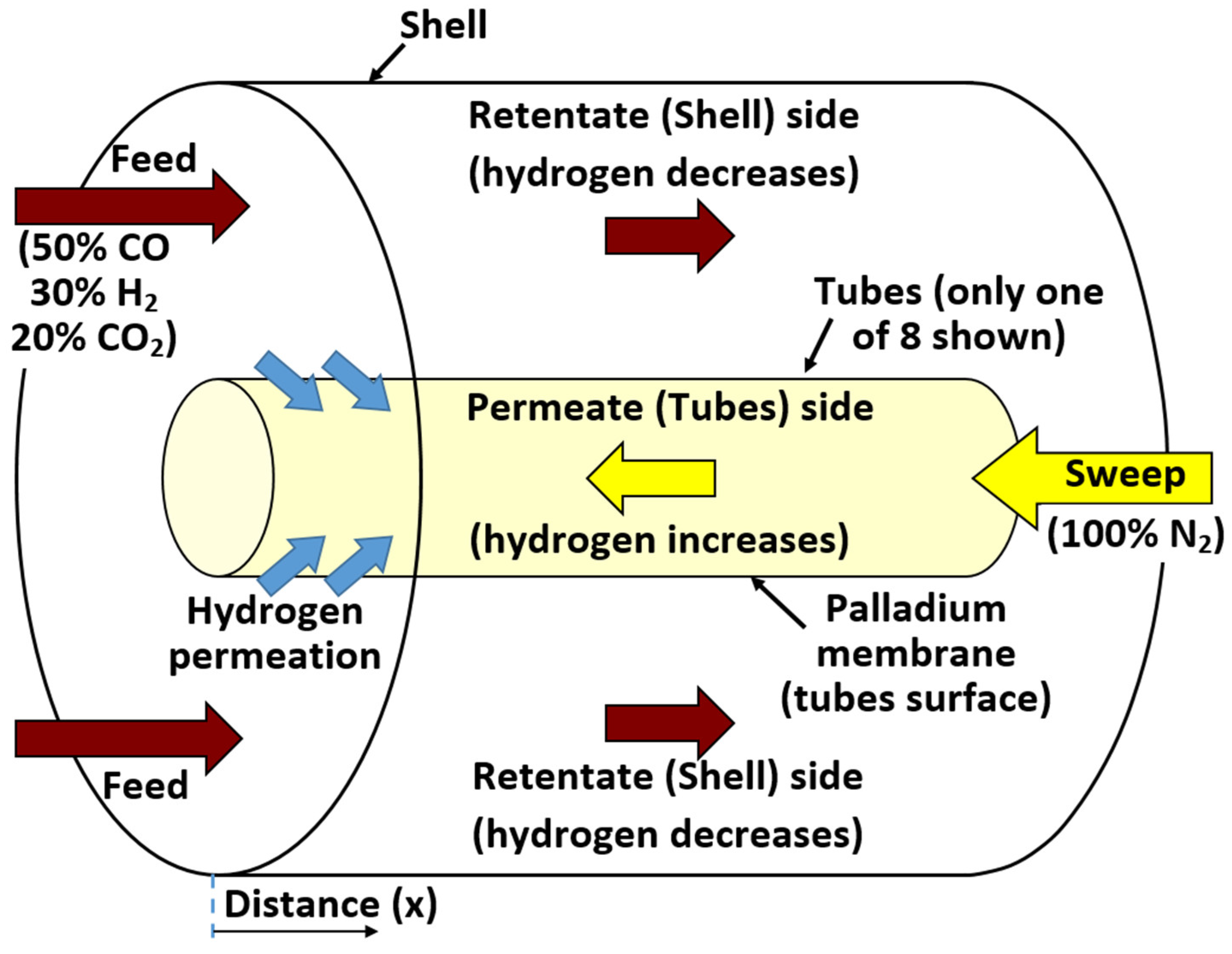
2.5. Flow Setup
3. General Model Settings
3.1. Fixing Common Parameters
3.2. Underlying Geometry
3.3. Fixed Conditions
4. Hydrogen Permeation Metrics
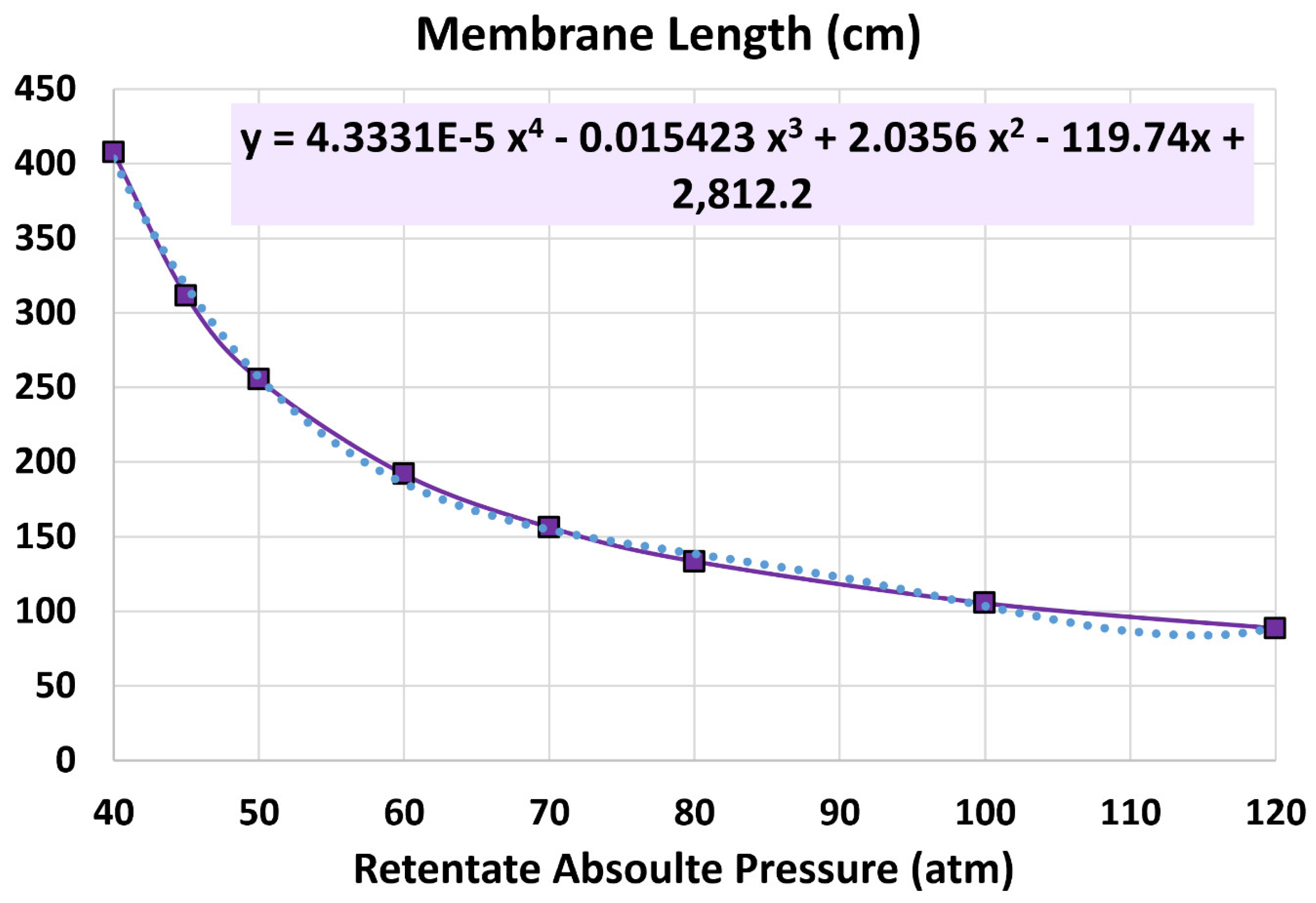
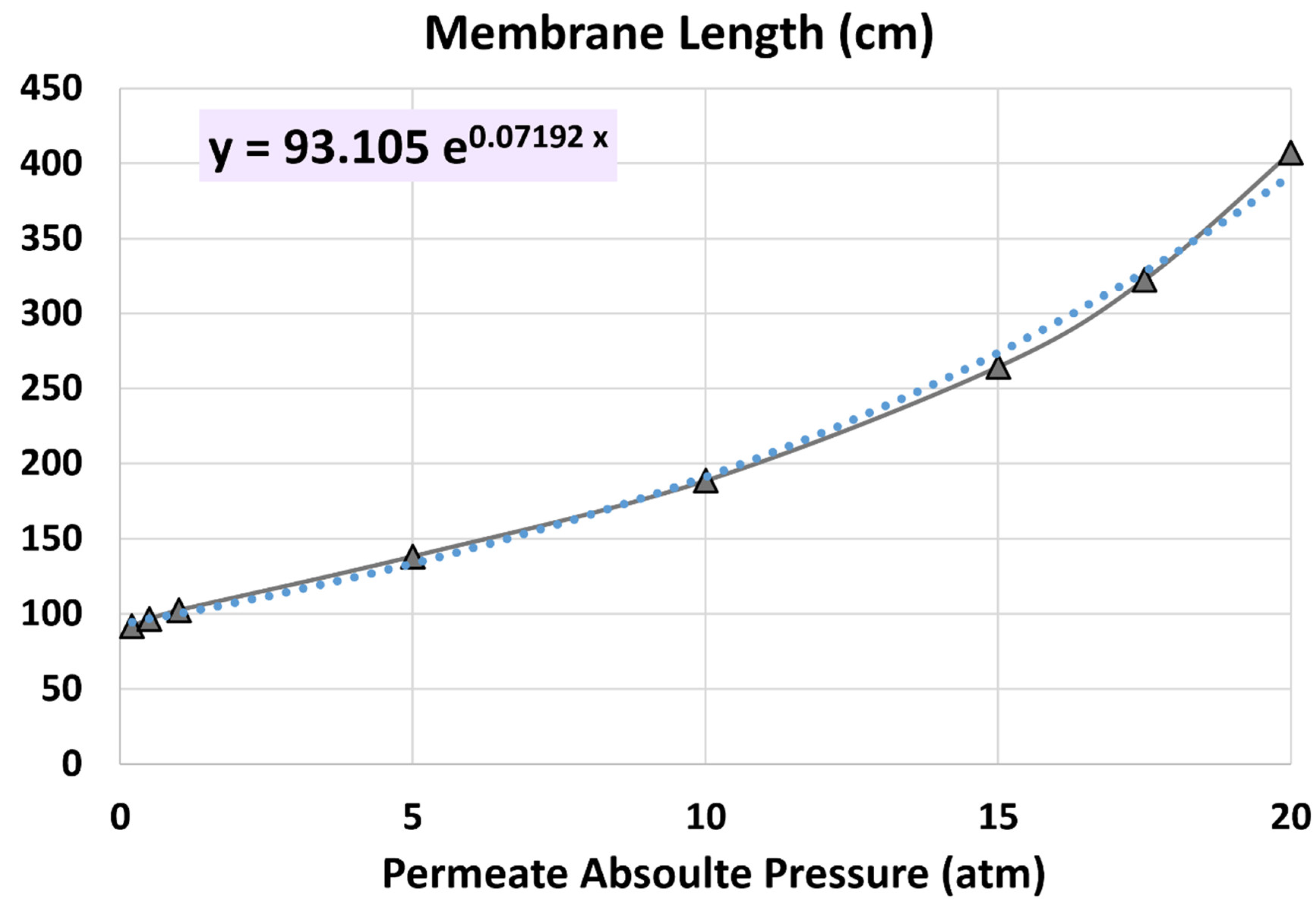
4.1. Membrane Length
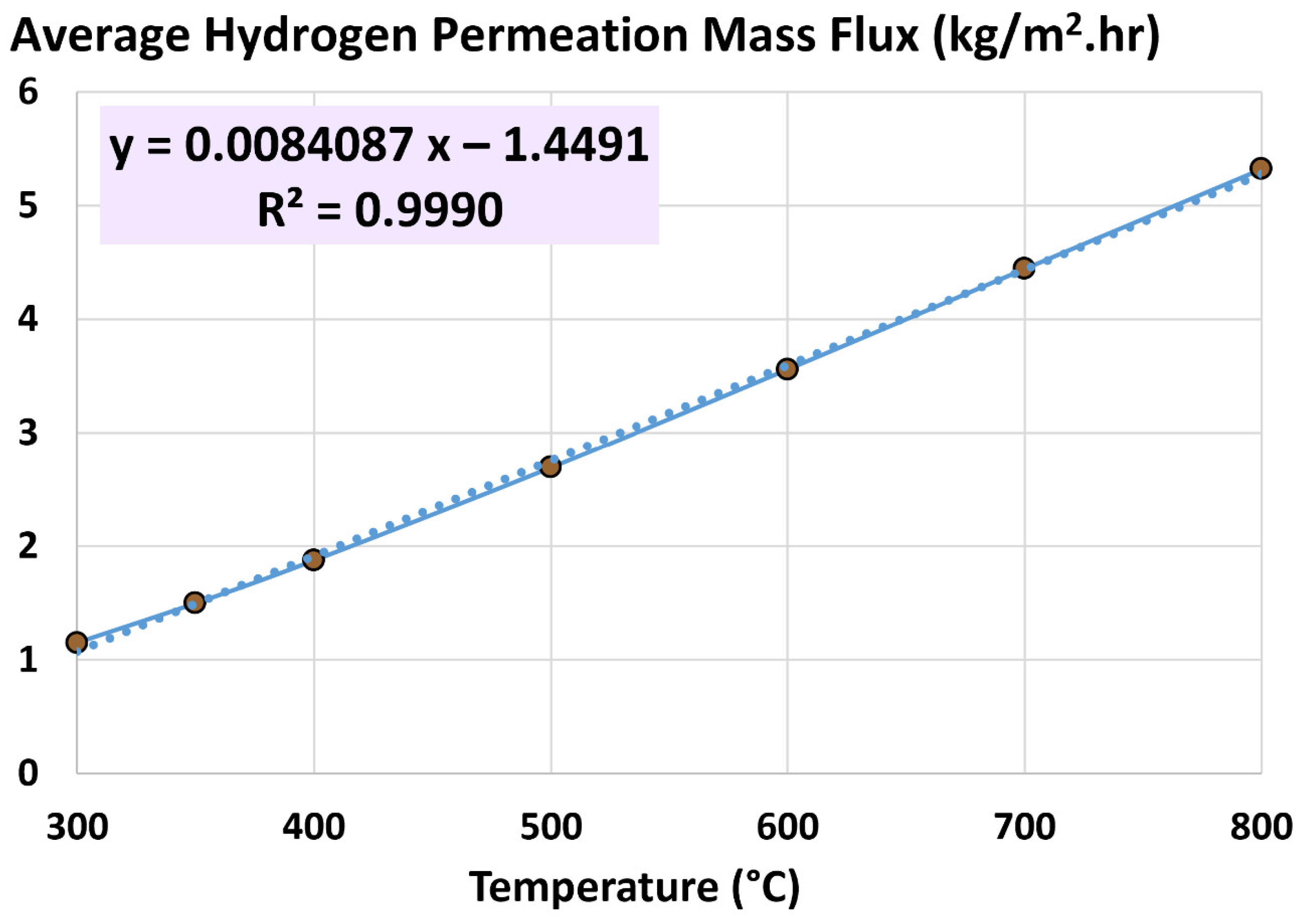
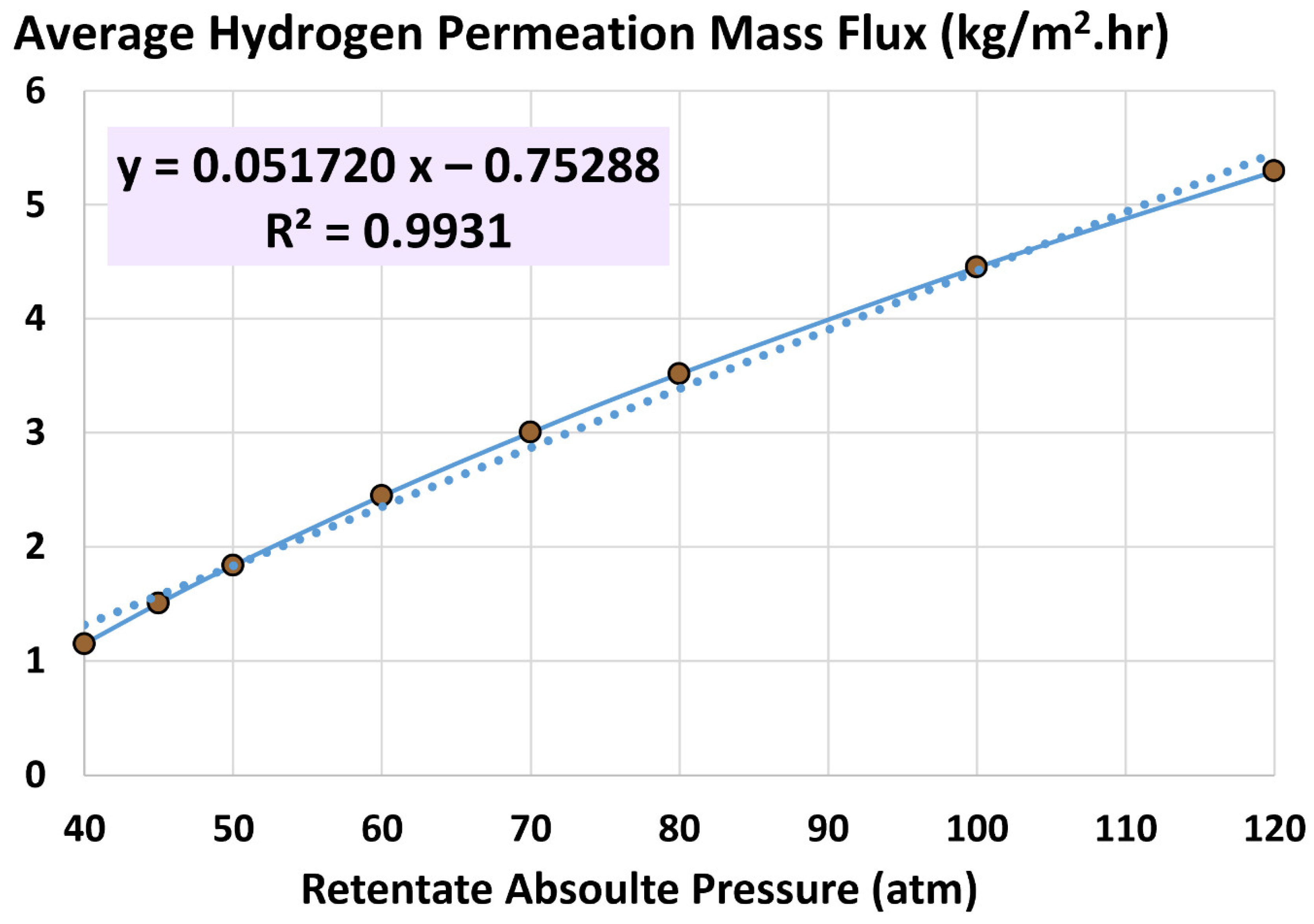
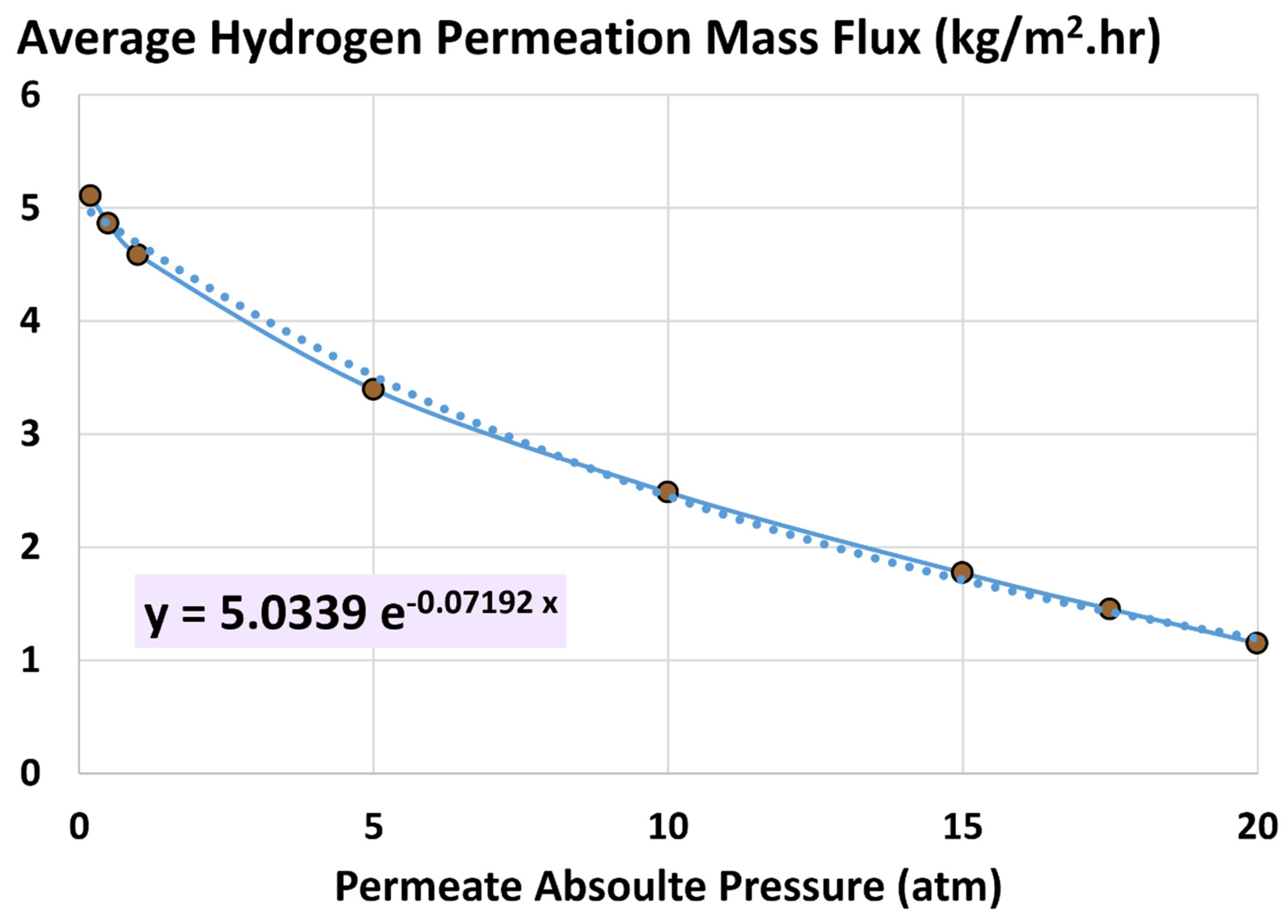
4.2. Average Hydrogen Permeation Mass Flux
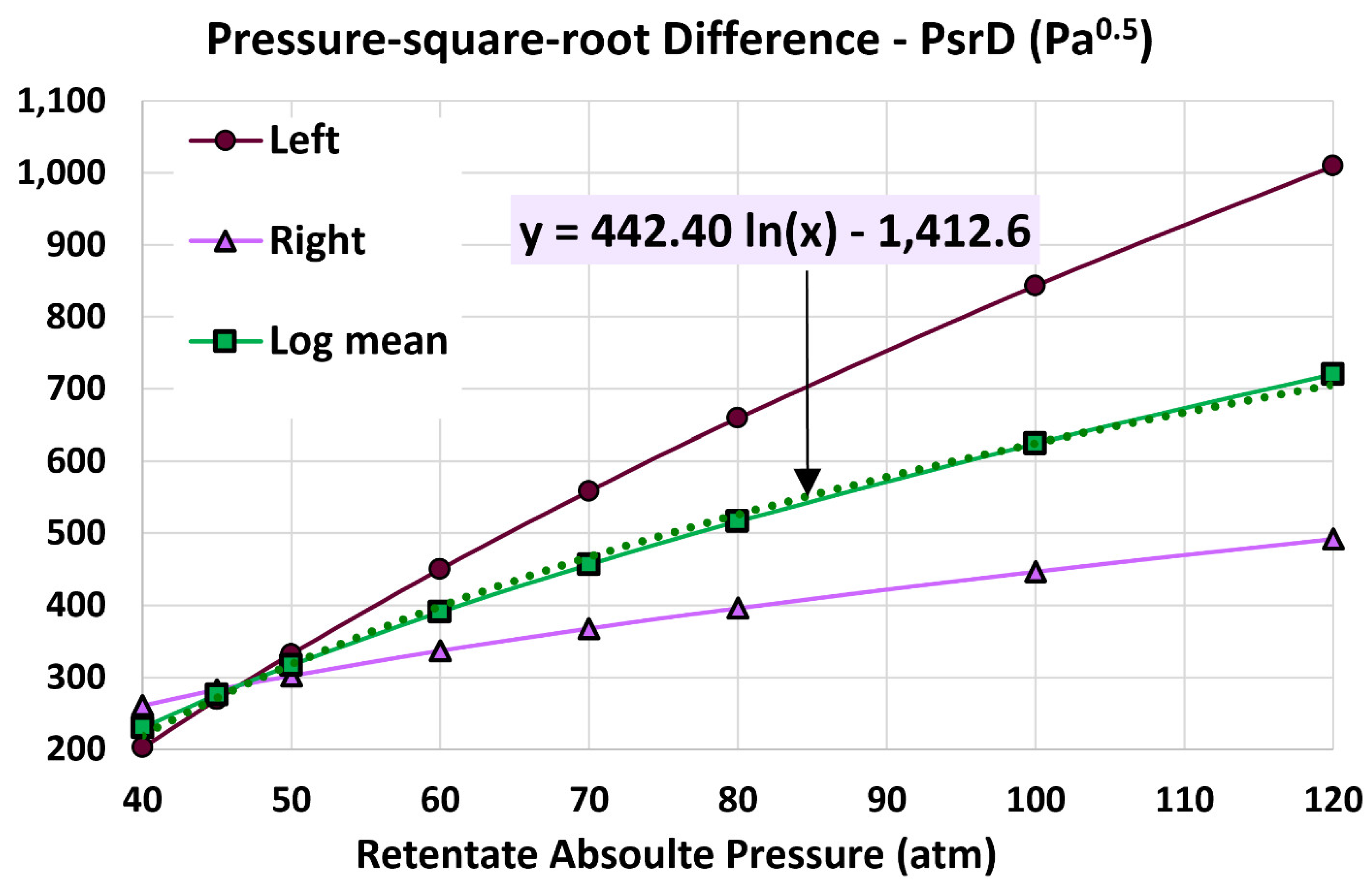
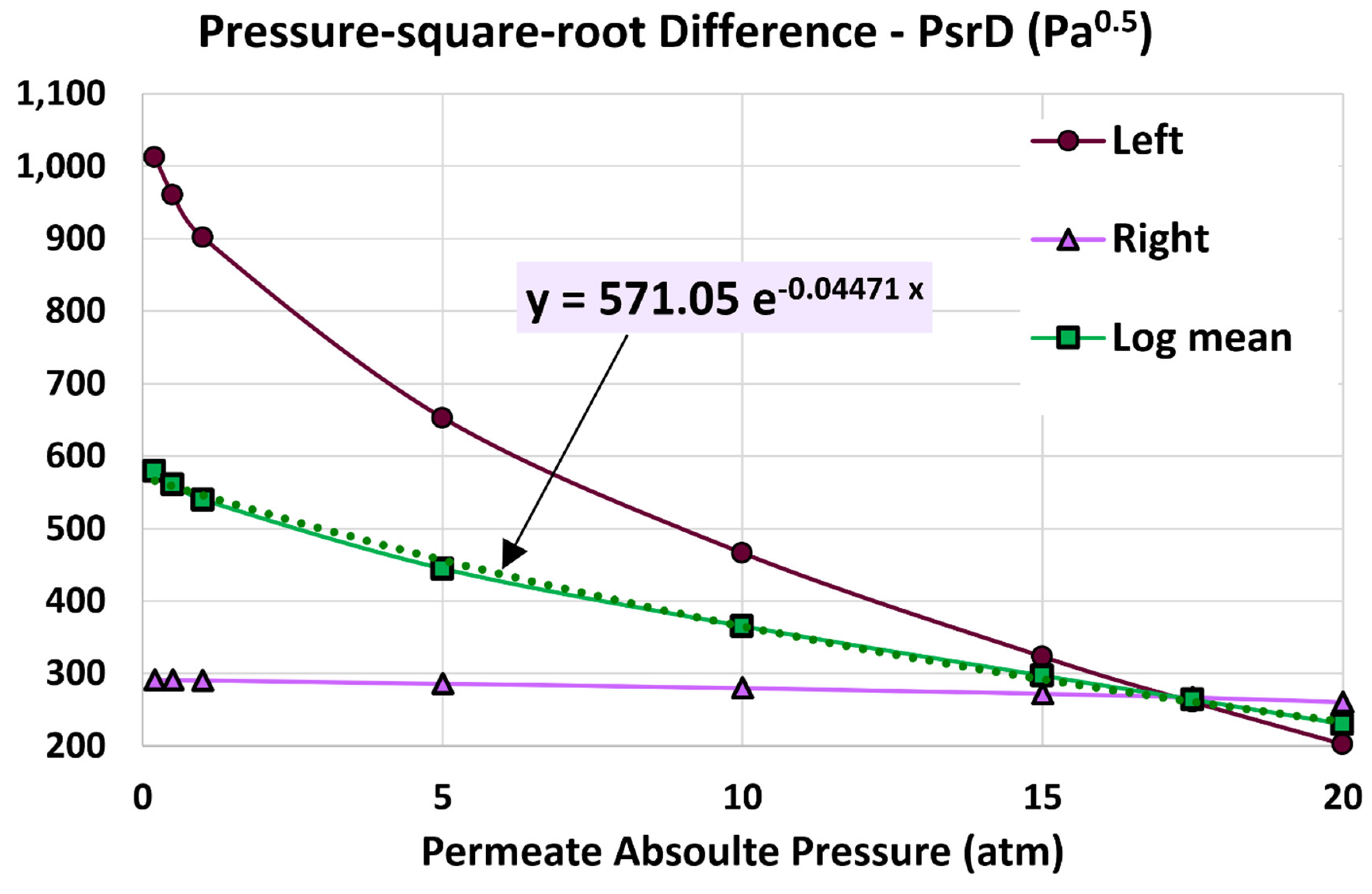
4.3. Log Mean Pressure-Square-Root Difference
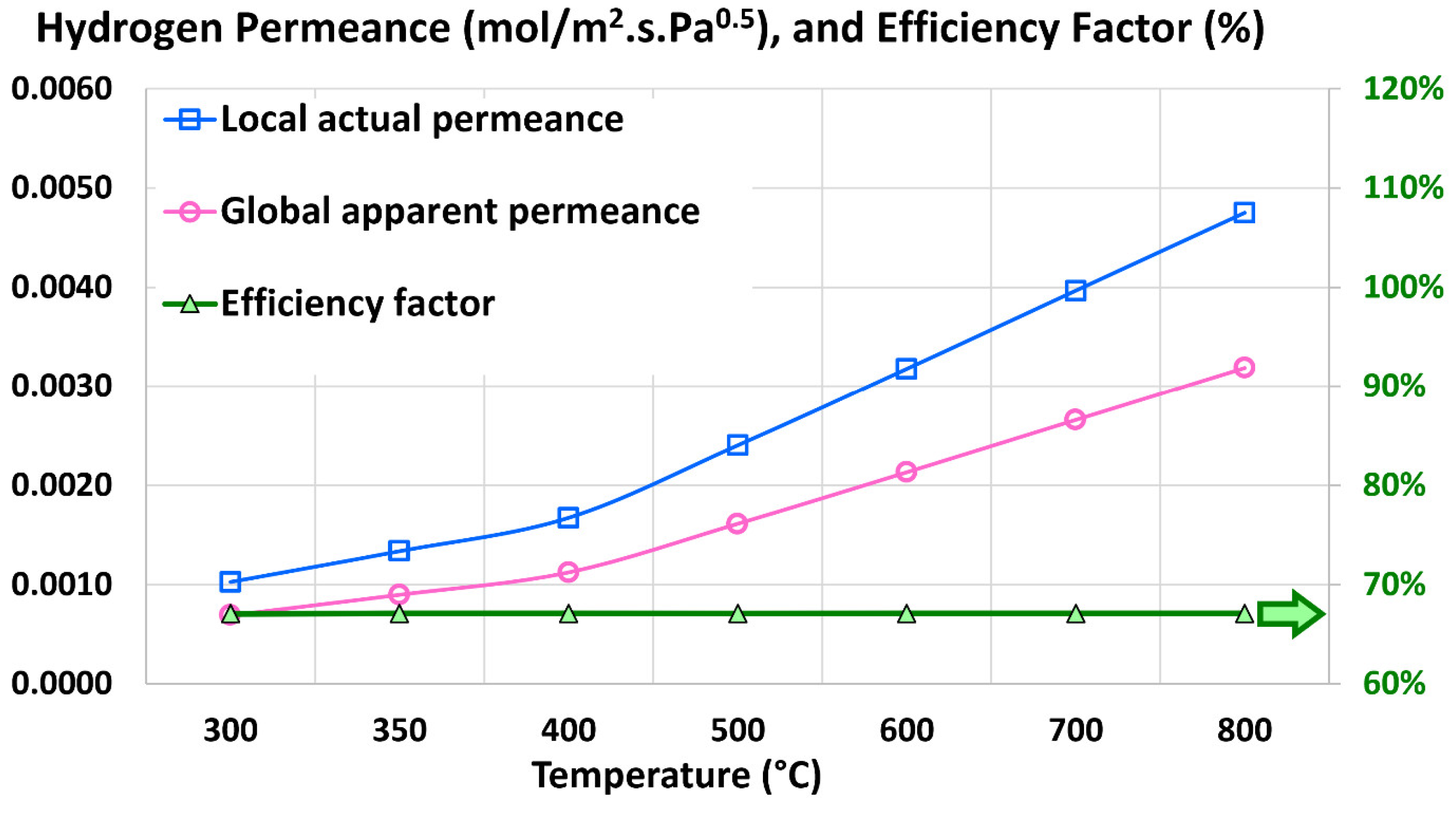
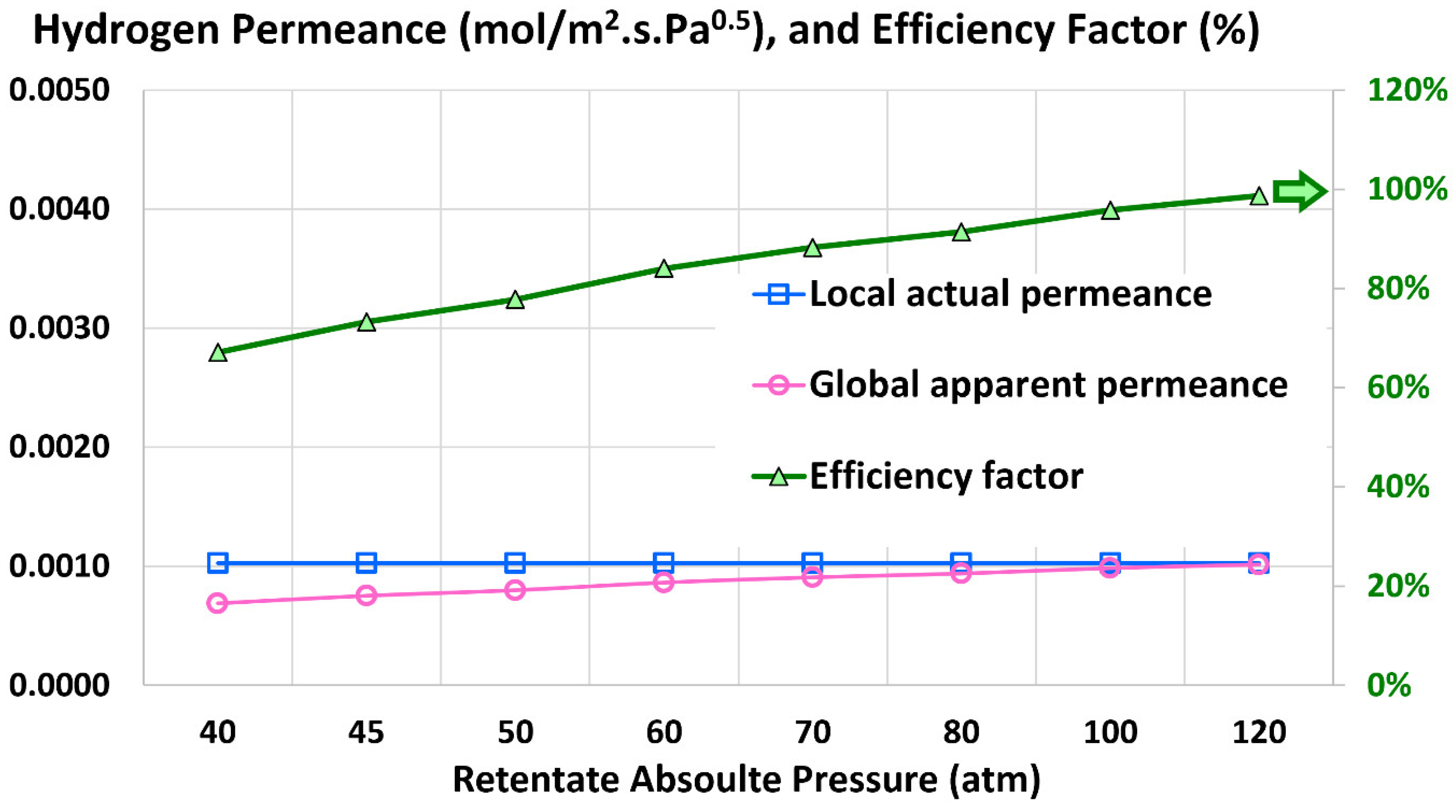
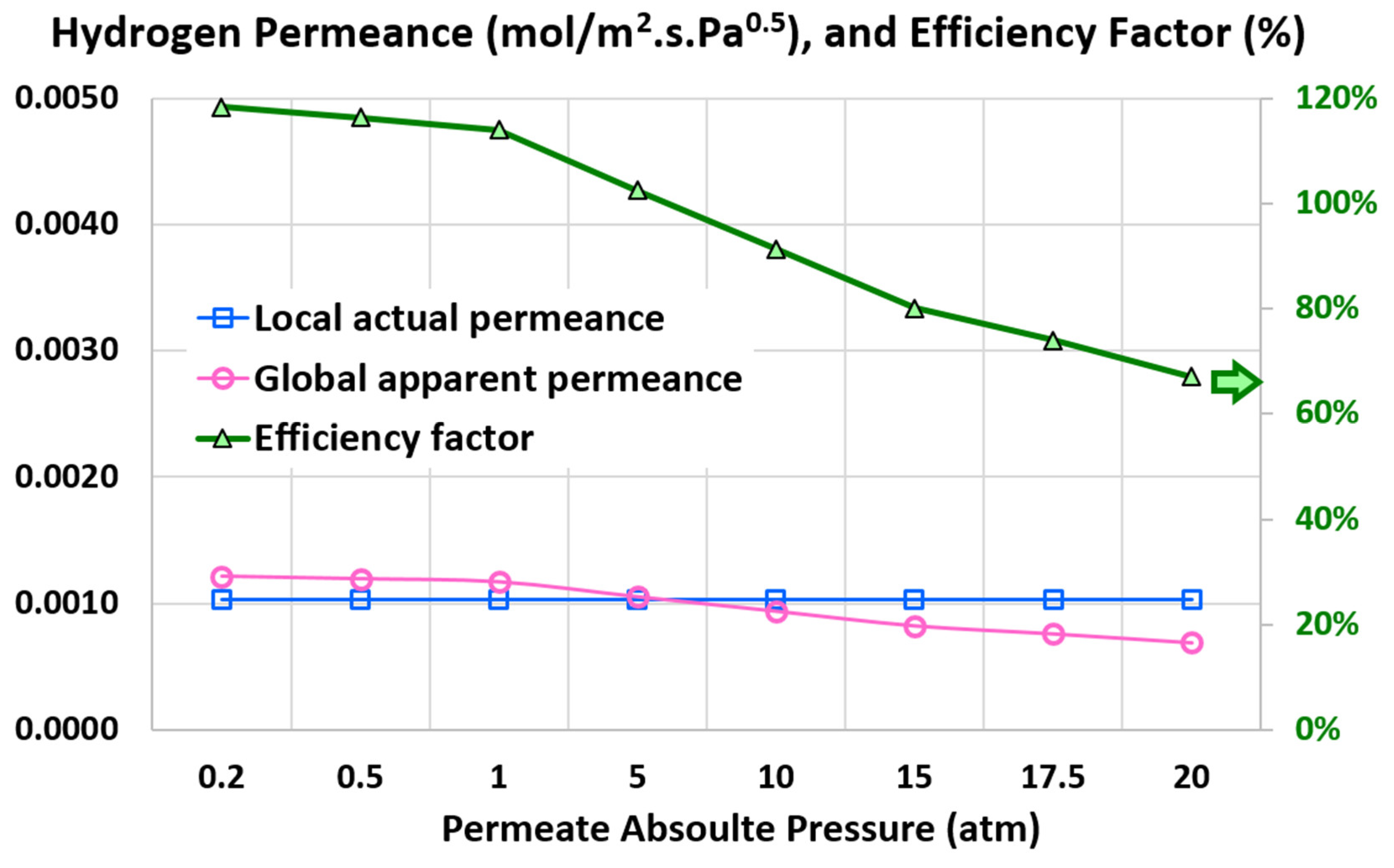
4.4. Global Apparent Permeance
4.5. Efficiency Factor
5. Results
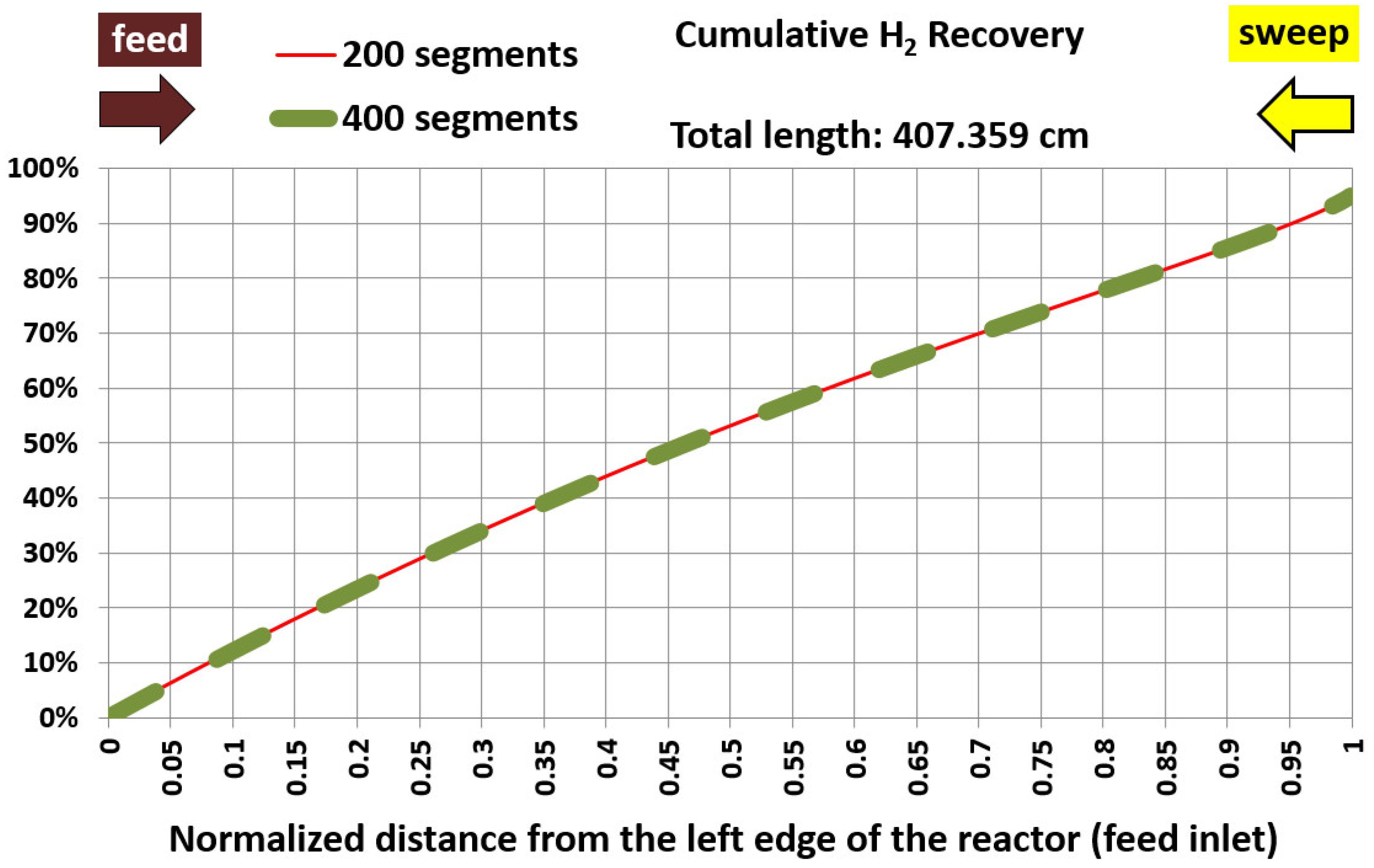
5.1. Base Case and Spatial Resolution Test
5.2. Influence of Temperature
- 300 °C (base)
- 350 °C
- 400 °C
- 500 °C
- 600 °C
- 700 °C
- 800 °C
5.3. Influence of Retentate Pressure
- 40 atm (base)
- 45 atm
- 50 atm
- 60 atm
- 70 atm
- 80 atm
- 100 atm
- 120 atm
5.4. Influence of Permeate Pressure
- 20 atm (base)
- 17.5 atm
- 15 atm
- 10 atm
- 5 atm
- 1 atm
- 0.5 atm
- 0.2 atm
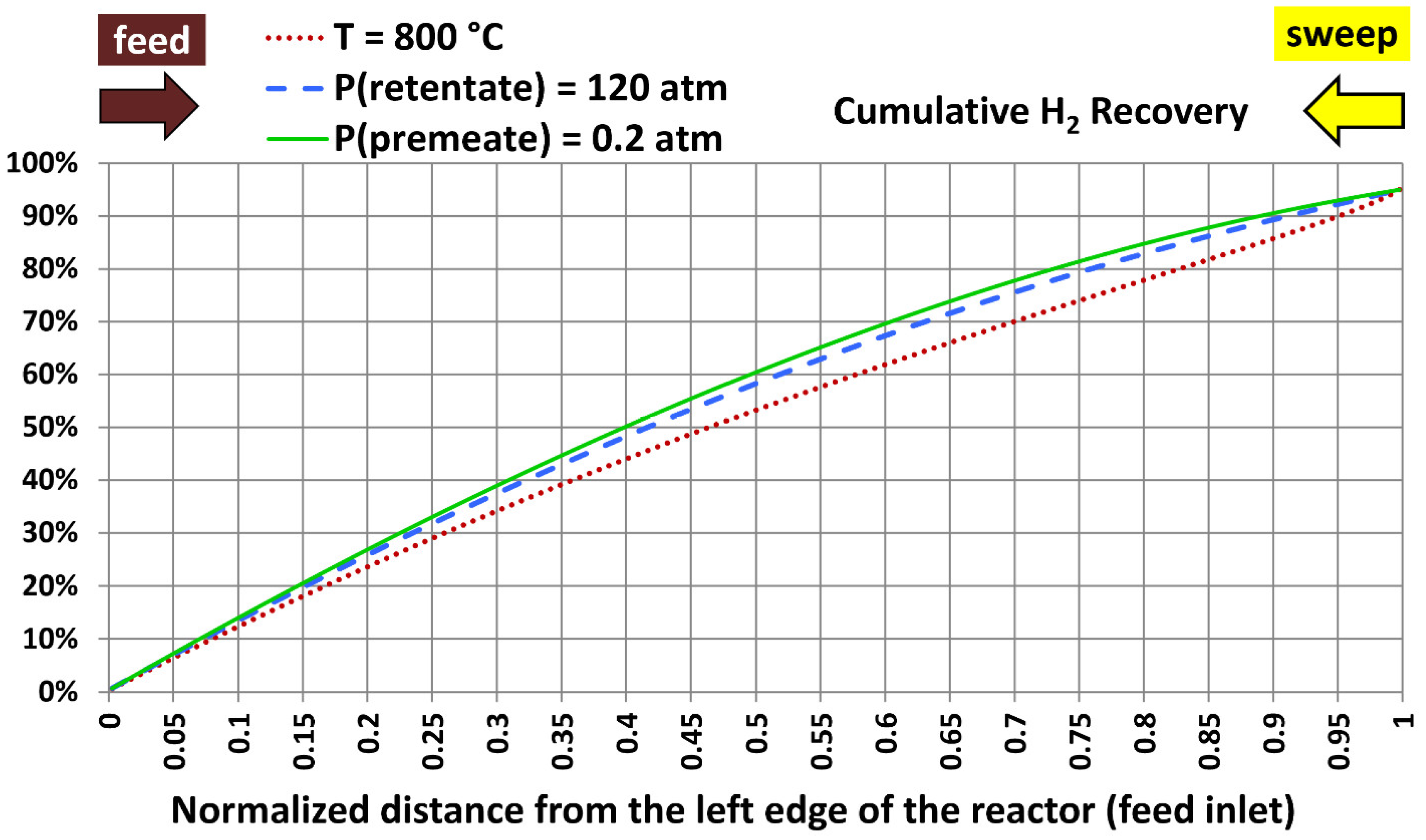
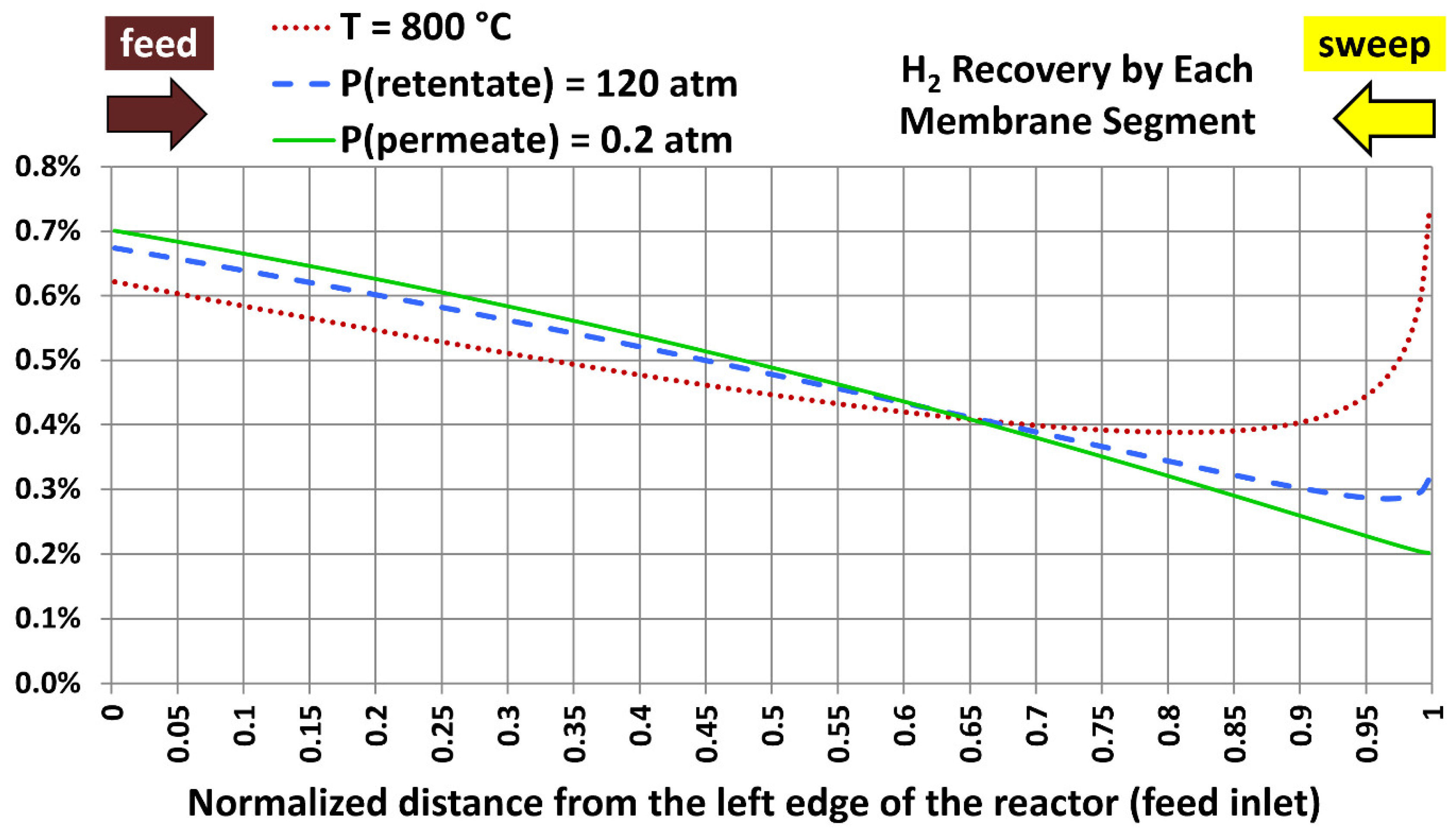
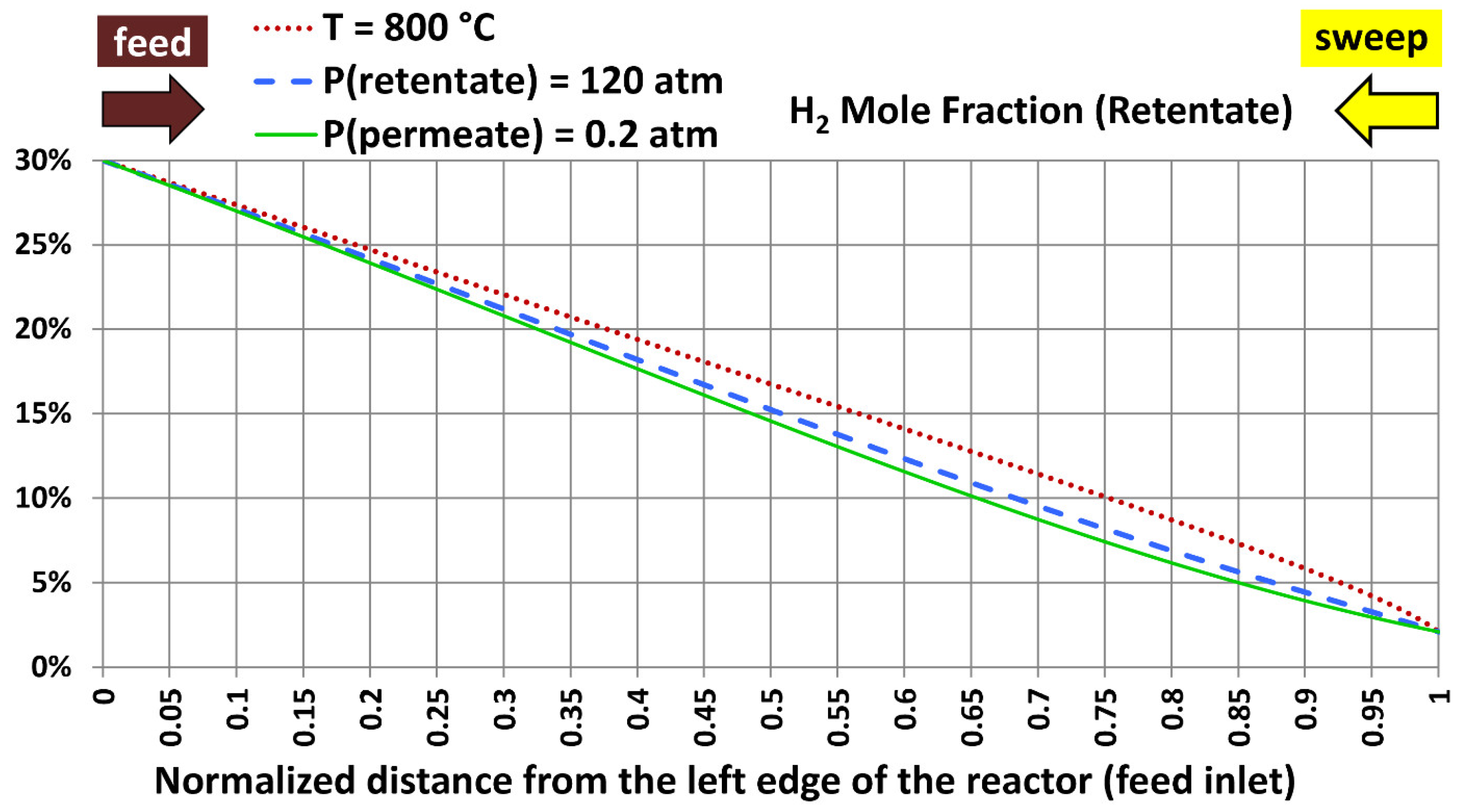
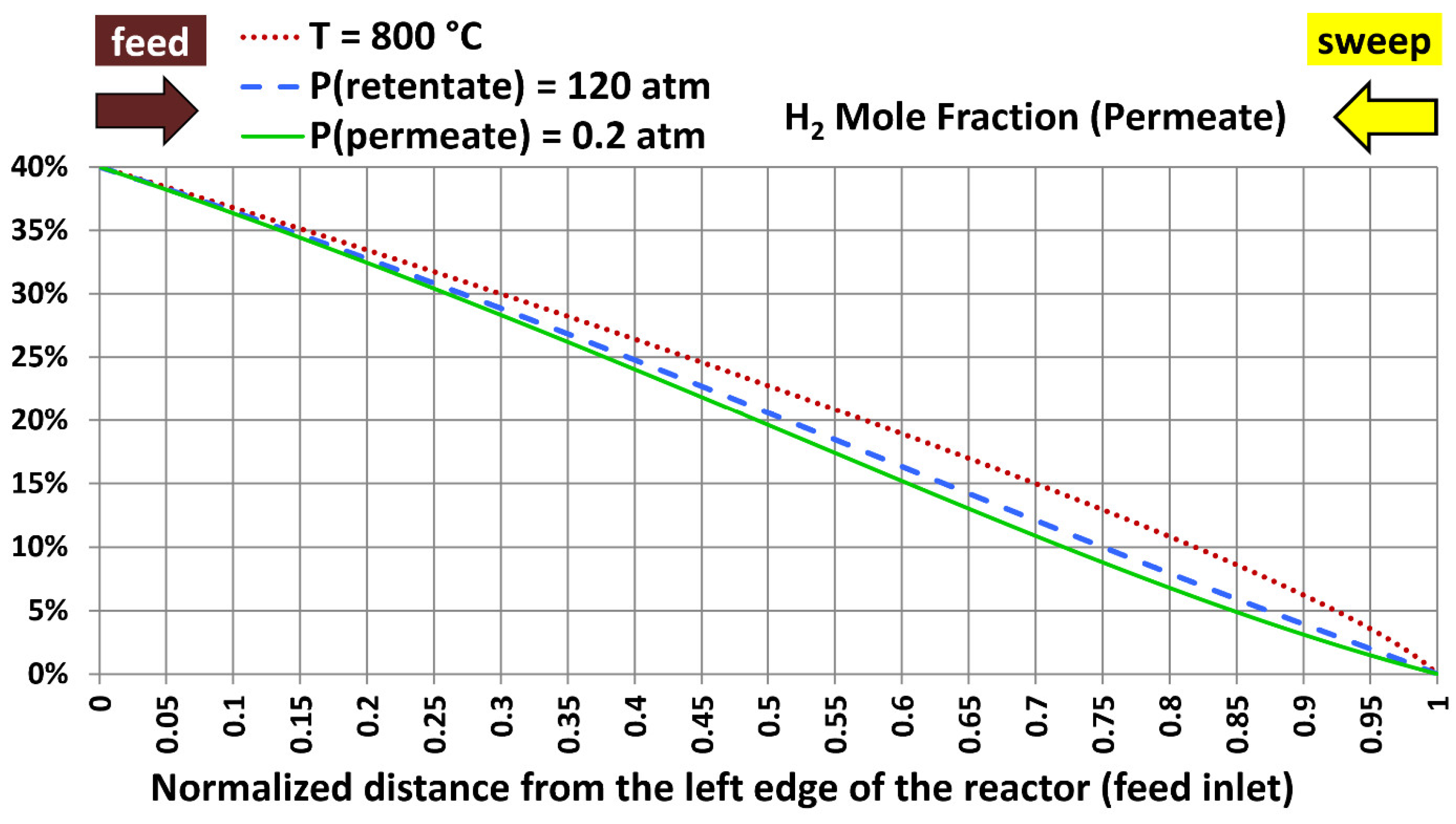
5.5. Profiles with Extreme Design Variables
6. Discussion
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Continuous Plug-Flow Reactor Modeling
Appendix B. Modelling Hydrogen Permeation
Appendix B.1. Segmental Plug-Flow Reactor
Appendix B.2. Modeling Algorithm
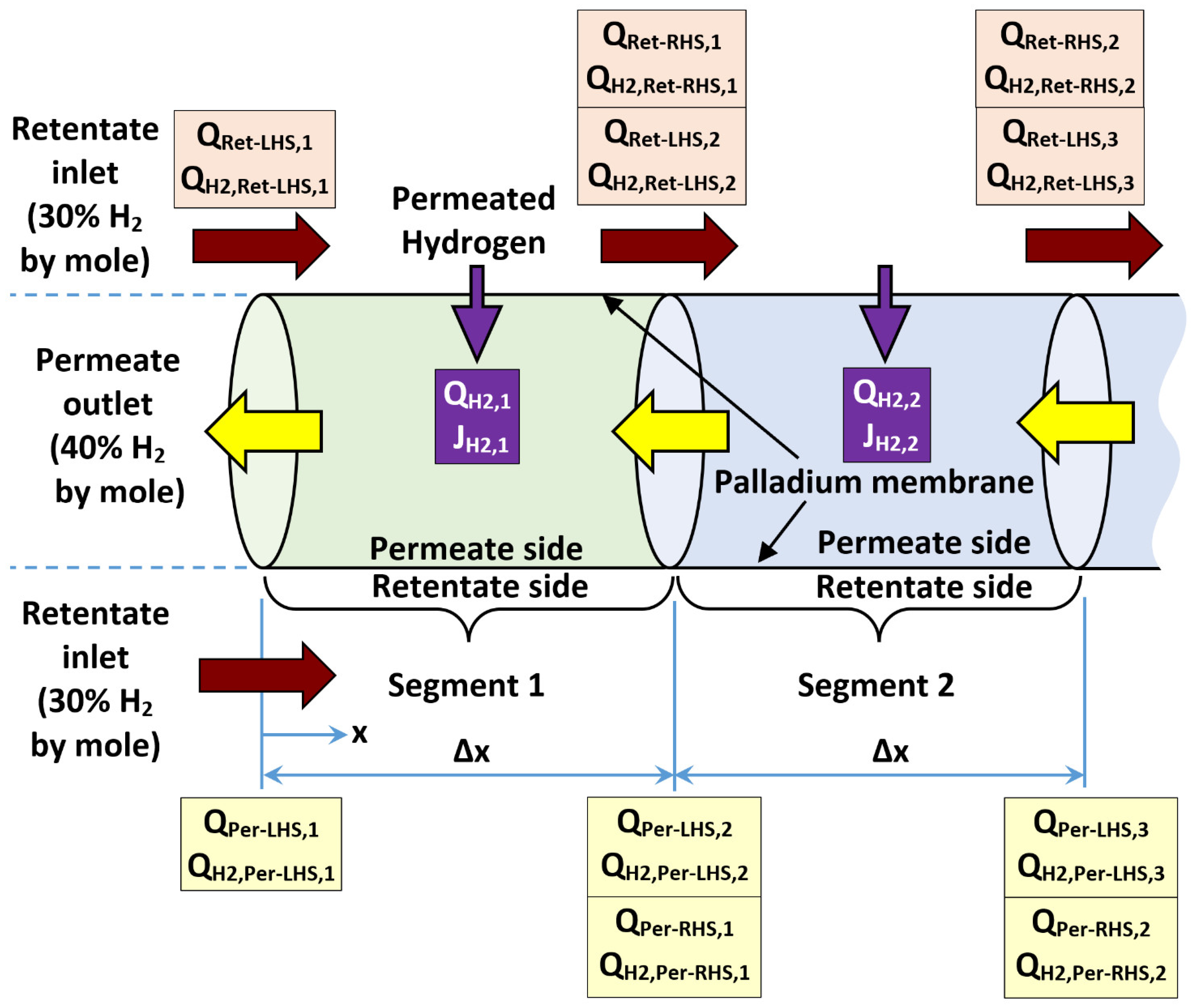
- a)
- Start with a known hydrogen mole fraction in the retentate at the LHS (XH2,Ret-LHS,i), standard volume flow rate of retentate at the LHS (QRet-LHS,i), hydrogen mole fraction of permeate at the LHS (XH2,Per-LHS,i), and standard volume flow rate of permeate at the LHS (QPer-LHS,i) of the segment (say segment number i).
- b)
- Compute (QH2,Per-LHS,i), which is the standard volume flow rate of the hydrogen content in the permeate stream at the LHS of the current segment being analyzed (say segment i), as follows:
- c)
- Compute (ΔPH20.5)LHS,i, which is the difference in the partial pressures of hydrogen raised to the power of 0.5 (this difference is the driving force for hydrogen permeation through the palladium membrane) at the LHS of the current segment being analyzed (say segment i), as follows:
- d)
- Compute (JH2,i), which is a predicted (first-iteration) segment-level molar flux of permeating hydrogen through the palladium membrane based on the conditions at LHS of the current segment being analyzed (say segment i), as follows:
- e)
- Convert the LHS-based first-iteration molar flux (JH2,i) to a predicted (first-iteration) segment-level standard volume flow rate of permeating hydrogen (QH2,i) for the current segment being analyzed (say segment i).
- f)
- Compute (XH2,Ret-RHS,i) and (XH2,Per-RHS,i), which are predicted (first-iteration) mole fractions of hydrogen in the retentate stream and the permeate stream, respectively at the RHS of the current segment being analyzed (say segment i), as follows:
- g)
- Compute (ΔPH20.5)RHS,I, which is the difference in the partial pressures of hydrogen raised to the power of 0.5 (as the driving force for hydrogen permeation) at the RHS of the current segment being analyzed (say segment i), as follows:
- h)
- Compute (ΔPH20.5)i, which is the difference in the partial pressures of hydrogen raised to the power of 0.5 assigned to the current segment being analyzed (say segment i). It is taken as the arithmetic average of the LHS value and the RHS value, as follows:
- i)
- Compute (JH2,i), which is a corrected (second-iteration) segment-level molar flux of permeating hydrogen through the palladium membrane, which includes driving forces for permeation at both sides of the current segment being analyzed (say segment i), as follows:
- j)
- Convert the corrected, segment-level molar flux (JH2,i) to a corresponding updated (refined) segment-level standard volume flow rate of permeating hydrogen (QH2,i) for the current segment being analyzed (say segment i), as follows:
- k)
- Compute (RH2,i), which is the hydrogen recovery due to the current segment being analyzed (say segment i), as follows:
- l)
- Optional: Compute (H2,i), which is the cumulative hydrogen recovery, due to all previous segments of the membrane reactor in addition to the current segment being analyzed (say segment i), as follows:
- m)
- Compute (QRet-RHS,i) and (QH2,Ret-RHS,i), which are the standard volume flow rate of the retentate stream and the hydrogen content in that retentate stream, respectively at the RHS of the current segment being analyzed (say segment i), as follows:
- n)
- Compute (XH2,Ret-RHS,i), which is the corrected (second-iteration) mole fraction of hydrogen in the retentate stream at the RHS of the current segment being analyzed (say segment i), as follows:
- o)
- Compute (QPer-RHS,i) and (QH2,Per-RHS,i), which are the standard volume flow rate of the permeate stream and the hydrogen content in that permeate stream, respectively at the RHS of the current segment being analyzed (say segment i), as follows:
- p)
- Compute (XH2,Per-RHS,i), which is the corrected (second-iteration) mole fraction of hydrogen in the permeate stream at the RHS of the current segment being analyzed (say segment i), as follows:
- q)
- Set the obtained RHS conditions of the current segment being analyzed (say segment i) as LHS conditions at the next adjacent segment to be analyzed (segment i+1), and repeat the computation procedure sequentially for all remaining segments until the last membrane segment (segment n).
- sub>∙ (ΔPH20.5)LHS,i
- JH2,i
- QH2,i
- XH2,Ret-RHS,i and XH2,Per-RHS,i
- (ΔPH20.5)RHS,i
- (ΔPH20.5)i
- JH2,i
- QH2,i
- RH2,i
- Optional: H2,i
- QRet-RHS,i and QH2,Ret-RHS,i
- XH2,Ret-RHS,i
- QPer-RHS,i and QH2,Per-RHS,i
- XH2,Per-RHS,i
- r)
- Compute (H2,i), which is the cumulative hydrogen recovery at the last segment. It is the overall hydrogen recovery by the entire membrane reactor, and it is obtained by simply adding the segment-level hydrogen recovery (RH2,i) for all the (n) segments of the membrane reactor. The total cumulative value is itself the target hydrogen recovery (β). Therefore
References
- Bell, D.A.; Towler, B.F.; Fan, M. , Coal gasification and its applications, William Andrew/Elsevier, Oxford, U.K., 2011.
- Marzouk, O.A.; Huckaby, E.D. , Assessment of syngas kinetic models for the prediction of a turbulent nonpremixed flame, in: Fall Meet. East. States Sect. Combust. Inst. 2009, College Park, Maryland, USA, 2009: pp. 726–751. [CrossRef]
- Couto, N.; Rouboa, A.; Silva, V.; Monteiro, E.; Bouziane, K. , Influence of the Biomass Gasification Processes on the Final Composition of Syngas, Energy Procedia 2013, 36, 596–606. [CrossRef]
- Jayanarasimhan, A.; Pathak, R.M.; Shivapuji, A.M.; Rao, L. , Tar Formation in Gasification Systems: A Holistic Review of Remediation Approaches and Removal Methods, ACS Omega 2024, 9, 2060–2079. [CrossRef]
- [National Energy Technology Laboratory of the United States Department of Energy] NETL, Reactions & Transformations, (2022). https://netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/reaction-transformations (accessed , 2022). 5 May.
- Frolov, S.M.; Panin, K.S.; Smetanyuk, V.A. , Gasification of Liquid Hydrocarbon Waste by the Ultra-Superheated Mixture of Steam and Carbon Dioxide: A Thermodynamic Study, Energies 2024, 17, 2126. [CrossRef]
- Talmadge, M.; Biddy, M.; Dutta, A.; Jones, S.; Meyer, A. , Syngas Upgrading to Hydrocarbon Fuels Technology Pathway, National Renewable Energy Laboratory (NREL), and Pacific Northwest National Laboratory (PNNL) of the United States Department of Energy (DoE)., 2013. https://www.nrel.gov/docs/fy13osti/58052.pdf (accessed , 2024). 31 May.
- Lin, S.; Harada, M.; Suzuki, Y.; Hatano, H. , Hydrogen production from coal by separating carbon dioxide during gasification, Fuel 2002, 81, 2079–2085. [CrossRef]
- Stork, Syngas Fired Steam Boiler, (2022). https://www.stork.com/en/client-cases/syngas-fired-steam-boiler (accessed , 2022). 6 May.
- Suxing, S.; Yu, X.; Li, J.; Liu, X.; Sui, L.; Zhang, J.; Fu, Z.; Shao, Y. , Effects of Hydrogen Addition on the Thermal Performance and Emissions of Biomass Syngas Combustion in a Horizontal Boiler, Energies 2024, 17, 2632. [CrossRef]
- Marzouk, O.A. , Portrait of the Decarbonization and Renewables Penetration in Oman’s Energy Mix, Motivated by Oman’s National Green Hydrogen Plan, Energies 2024, 17, 4769. [CrossRef]
- Timilsina, M.S.; Chaudhary, Y.; Shah, A.K.; Lohani, S.P.; Bhandari, R.; Uprety, B. , Syngas composition analysis for waste to methanol production: Techno-economic assessment using machine learning and Aspen plus, Renew. Energy 2024, 228, 120574. [Google Scholar] [CrossRef]
- Cavo, M.; Rivarolo, M.; Gini, L.; Magistri, L. , An advanced control method for fuel cells - Metal hydrides thermal management on the first Italian hydrogen propulsion ship, Int. J. Hydrog. Energy 2023, 48, 20923–20934. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Expectations for the Role of Hydrogen and Its Derivatives in Different Sectors through Analysis of the Four Energy Scenarios: IEA-STEPS, IEA-NZE, IRENA-PES, and IRENA-1. 5°C, Energies 2024, 17, 646. [Google Scholar] [CrossRef]
- Halder, P.; Babaie, M.; Salek, F.; Haque, N.; Savage, R.; Stevanovic, S.; Bodisco, T.A.; Zare, A. , Advancements in hydrogen production, storage, distribution and refuelling for a sustainable transport sector: Hydrogen fuel cell vehicles, Int. J. Hydrog. Energy 2024, 52, 973–1004. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Zero Carbon Ready Metrics for a Single-Family Home in the Sultanate of Oman Based on EDGE Certification System for Green Buildings, Sustainability 2023, 15, 13856. [CrossRef]
- Liu, H.; Lu, H.; Hu, H. , CO2 capture and mineral storage: State of the art and future challenges, Renew. Sustain. Energy Rev. 2024, 189, 113908. [Google Scholar] [CrossRef]
- Bakirci, M. , Smart city air quality management through leveraging drones for precision monitoring, Sustain. Cities Soc. 2024, 106, 105390. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Compilation of Smart Cities Attributes and Quantitative Identification of Mismatch in Rankings, J. Eng. 2022, 2022, 5981551. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Adiabatic Flame Temperatures for Oxy-Methane, Oxy-Hydrogen, Air-Methane, and Air-Hydrogen Stoichiometric Combustion using the NASA CEARUN Tool, GRI-Mech 3. 0 Reaction Mechanism, and Cantera Python Package, Eng. Technol. Appl. Sci. Res. 2023, 13, 11437–11444. [Google Scholar] [CrossRef]
- Jamrozik, A.; Grab-Rogaliński, K.; Tutak, W. , Hydrogen effects on combustion stability, performance and emission of diesel engine, Int. J. Hydrog. Energy 2020, 45, 19936–19947. [Google Scholar] [CrossRef]
- Xie, T.; Wang, P. , Analysis of NO formation in counter-flow premixed hydrogen-air flame, Trans. Can. Soc. Mech. Eng. 2013, 37, 851–859. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Hydrogen Utilization as a Plasma Source for Magnetohydrodynamic Direct Power Extraction (MHD-DPE), IEEE Access 2024, 12, 167088–167107. [CrossRef]
- Goldmeer, J.; Catillaz, J.; Donohue, J. , Hydrogen as a fuel for gas turbines - A pathway to lower CO2, General Electric (GE), 2022. https://www.ge.com/content/dam/gepower-new/global/en_US/downloads/gas-new-site/future-of-energy/hydrogen-fuel-for-gas-turbines-gea34979.pdf (accessed , 2024). 31 May.
- Javoy, S.; Mevel, R.; Paillard, C.E. , A study of N2O decomposition rate constant at high temperature: Application to the reduction of nitrous oxide by hydrogen, Int. J. Chem. Kinet. 2009, 41, 357–375. [Google Scholar] [CrossRef]
- Weiland, N.T.; Strakey, P.A. , Global NOx Measurements in Turbulent Nitrogen-Diluted Hydrogen Jet Flames, in: San Diego, California, United States, 2007. https://www.osti.gov/biblio/913259 (accessed , 2024). 31 May.
- Marzouk, O.A. , Dataset of total emissivity for CO2, H2O, and H2O-CO2 mixtures; over a temperature range of 300-2900 K and a pressure-pathlength range of 0.01-50 atm.m, Data Brief (2025) 111428. [CrossRef]
- Ghiat, I.; Al-Ansari, T. , A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus, J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. , Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies, Energies 2022, 15, 887. 15. [CrossRef]
- Ruan, R.; Wang, G.; Li, S.; Wang, M.; Lin, H.; Tan, H.; Wang, X.; Liu, F. , The effect of alkali and alkaline earth metals (AAEMs) on combustion and PM formation during oxy-fuel combustion of coal rich in AAEMs, Energy 2024, 293, 130695. [CrossRef]
- Marzouk, O.A. , Radiant Heat Transfer in Nitrogen-Free Combustion Environments, Int. J. Nonlinear Sci. Numer. Simul. 2018, 19, 175–188. [Google Scholar] [CrossRef]
- Rajabloo, T.; Valee, J.; Marenne, Y.; Coppens, L.; De Ceuninck, W. , Carbon capture and utilization for industrial applications, Energy Rep. 2023, 9, 111–116. [CrossRef]
- Marzouk, O.A. , Temperature-Dependent Functions of the Electron–Neutral Momentum Transfer Collision Cross Sections of Selected Combustion Plasma Species, Appl. Sci. 2023, 13, 11282. [Google Scholar] [CrossRef]
- Donskoy, I. , Techno-Economic Efficiency Estimation of Promising Integrated Oxyfuel Gasification Combined-Cycle Power Plants with Carbon Capture, Clean Technol. 2023, 5, 215–232. [CrossRef]
- Marzouk, O.A. , Detailed and simplified plasma models in combined-cycle magnetohydrodynamic power systems, Int. J. Adv. Appl. Sci. 2023, 10, 96–108. [Google Scholar] [CrossRef]
- Dinca, C.; Slavu, N.; Cormoş, C.-C.; Badea, A. , CO2 capture from syngas generated by a biomass gasification power plant with chemical absorption process, Energy 2018, 149, 925–936. 149. [CrossRef]
- Marzouk, O.A. , Technical review of radiative-property modeling approaches for gray and nongray radiation, and a recommended optimized WSGGM for CO2/H2O-enriched gases, Results Eng. 2025, 25, 103923. [CrossRef]
- Bhavsar, A.; Hingar, D.; Ostwal, S.; Thakkar, I.; Jadeja, S.; Shah, M. , The current scope and stand of carbon capture storage and utilization ∼ A comprehensive review, Case Stud. Chem. Environ. Eng. 2023, 8, 100368. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. , Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment, Fuel 2023, 342, 127776. [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. , A Review of CO2 Applications in the Processing of Polymers, Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Singh, A.; Saini, V.; Jain, S.; Gour, A. ; Techno-Economic; Environmental, and Policy Perspectives of Carbon Capture to Fuel Technologies, Int. Res. J. Adv. Eng. Hub IRJAEH 2024, 2, 1387–1403. [Google Scholar] [CrossRef]
- Bukar, A.M.; Asif, M. , Technology readiness level assessment of carbon capture and storage technologies, Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- Shabbani, H.J.K.; Othman, M.R.; Janabi, S.K.A.; Barron, A.R.; Helwani, Z. , H2 purification employing pressure swing adsorption process: Parametric and bibliometric review, Int. J. Hydrog. Energy 2024, 50, 674–699. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, S.; Ganguly, S.; Patwardhan, A.V. , Steam reforming of methane and methanol in simulated macro & micro-scale membrane reactors: Selective separation of hydrogen for optimum conversion, J. Nat. Gas Sci. Eng. 2014, 18, 286–295. [Google Scholar] [CrossRef]
- Kassi, A.H.; Al-Hattab, T.A. , A Review: Membrane Reactor for Hydrogen Production: Modeling and Simulation, Eng. Chem. 2023, 4, 17–31. [Google Scholar] [CrossRef]
- Omidifar, M.; Babaluo, A.A.; Jamshidi, S. , H2 permeance and surface characterization of a thin (2 μm) Pd-Ni composite membrane prepared by electroless plating, Chem. Eng. Sci. 2024, 283, 119370. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Barbieri, G. , Hydrogen concentration and purification by membrane process: A multistage analysis, Renew. Energy 2023, 218, 119243. [Google Scholar] [CrossRef]
- Piemonte, V.; Di Paola, L.; De Falco, M.; Iulianelli, A.; Basile, A. , Hydrogen production using inorganic membrane reactors, in: Adv. Hydrog. Prod. Storage Distrib., Elsevier, 2014: pp. 283–316. [CrossRef]
- Marzouk, O.A. , 2030 Ambitions for Hydrogen, Clean Hydrogen, and Green Hydrogen, Eng. Proc. 2023, 56, 14. [Google Scholar] [CrossRef]
- Peters, T.; Caravella, A.; Membranes, P.-B. ; Perspectives; Membranes2019) 25. [CrossRef]
- Marcantonio, V.; De Falco, M.; Capocelli, M.; Bocci, E.; Colantoni, A.; Villarini, M. , Process analysis of hydrogen production from biomass gasification in fluidized bed reactor with different separation systems, Int. J. Hydrog. Energy 2019, 44, 10350–10360. [Google Scholar] [CrossRef]
- Liemberger, W.; Groß, M.; Miltner, M.; Harasek, M. , Experimental analysis of membrane and pressure swing adsorption (PSA) for the hydrogen separation from natural gas, J. Clean. Prod. 2017, 167, 896–907. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. , Hydrogen Separation and Purification from Various Gas Mixtures by Means of Electrochemical Membrane Technology in the Temperature Range 100–160 °C, Membranes 2021, 11, 282. [CrossRef]
- Chang, K.; Li, Q.; Li, Q. , Refrigeration cycle for cryogenic separation of hydrogen from coke oven gas, Front. Energy Power Eng. China 2008, 2, 484–488. [Google Scholar] [CrossRef]
- Kangwanpongpan, T.; Makarov, D.; Cirrone, D.; Molkov, V. , LES model of flash-boiling and pressure recovery phenomena during release from large-scale pressurised liquid hydrogen storage tank, Int. J. Hydrog. Energy 2024, 50, 390–405. [Google Scholar] [CrossRef]
- Ohira, K. , Study of nucleate boiling heat transfer to slush hydrogen and slush nitrogen, Heat Transfer—Asian Res. 2003, 32, 13–28. [CrossRef]
- Harris, P.D.; Barnes, R. , The uses of helium and xenon in current clinical practice, Anaesthesia 2008, 63, 284–293. [CrossRef]
- Xie, Y.; Zhong, K. , Investigation on the Performances of Vuilleumier Cycle Heat Pump Adopting Mixture Refrigerants, Entropy 2017, 19, 446. [CrossRef]
- Usas, S.A.; Ricardez-Sandoval, L. , An optimal sustainable planning strategy for national carbon capture deployment: A review on the state of CO 2 capture in Canada, Can. J. Chem. Eng. (2024) cjce.25249. [CrossRef]
- Marzouk, O.A. , Subcritical and supercritical Rankine steam cycles, under elevated temperatures up to 900°C and absolute pressures up to 400 bara, Adv. Mech. Eng. 2024, 16, 1–18. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Wind Speed Weibull Model Identification in Oman, and Computed Normalized Annual Energy Production (NAEP) From Wind Turbines Based on Data From Weather Stations, Eng. Rep. 7 (2025) e70089. [CrossRef]
- Marzouk, O.A. , Tilt sensitivity for a scalable one-hectare photovoltaic power plant composed of parallel racks in Muscat, Cogent Eng. 2022, 9, 2029243. [CrossRef]
- Shang, Y.; Sang, S.; Tiwari, A.K.; Khan, S.; Zhao, X. , Impacts of renewable energy on climate risk: A global perspective for energy transition in a climate adaptation framework, Appl. Energy 2024, 362, 122994. [Google Scholar] [CrossRef]
- Marzouk, O.A.; Intensity, E.G.; Simulator, P.V.P.P.U.T.N.E. “Aladdin,” Energies 2024, 17, 405. [CrossRef]
- Chipangamate, N.S.; Nwaila, G.T. , Assessment of challenges and strategies for driving energy transitions in emerging markets: A socio-technological systems perspective, Energy Geosci. 2024, 5, 100257. [CrossRef]
- Murmura, M.A.; Cerbelli, S.; Annesini, M.C. , Transport-reaction-permeation regimes in catalytic membrane reactors for hydrogen production. The steam reforming of methane as a case study, Chem. Eng. Sci. 2017, 162, 88–103. [Google Scholar] [CrossRef]
- Oh, D.-K.; Lee, K.-Y.; Park, J.-S. , Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology, Membranes 2020, 10, 338. [CrossRef]
- Berstad, D.; Nekså, P.; Gjøvåg, G.A. , Low-temperature syngas separation and CO2 capture for enhanced efficiency of IGCC power plants, Energy Procedia 2011, 4, 1260–1267. 4. [CrossRef]
- Brdar, R.D.; Jones, R.M. , GE IGCC Technology and Experience with Advanced Gas Turbines, General Electric (GE) Power Systems, 2021. https://www.ge.com/content/dam/gepower-new/global/en_US/downloads/gas-new-site/resources/reference/ger-4207-ge-igcc-technology-experience-advanced-gas-turbines.pdf (accessed , 2022). 6 May.
- Krishnan, G.; Steele, D.; O’Brien, K.; Callahan, R.; Berchtold, K.; Figueroa, J. , Simulation of a Process to Capture CO2 From IGCC Syngas Using a High Temperature PBI Membrane, Energy Procedia 2009, 1, 4079–4088. 1. [CrossRef]
- Wang, T. , An overview of IGCC systems, in: Integr. Gasif. Comb. Cycle IGCC Technol., Elsevier, 2017: pp. 1–80. [CrossRef]
- Pruschek, R.; Oeljeklaus, G.; Kloster, R. , Enhancement of the efficiency of coal-fired power generation systems: final report, Institute for Process Engineering and Power Plant Technology, University of Stuttgart, Stuttgart, Germany, 1998. https://www.osti.gov/etdeweb/biblio/688545 (accessed , 2025). 27 February.
- Marzouk, O.A. , Condenser Pressure Influence on Ideal Steam Rankine Power Vapor Cycle using the Python Extension Package Cantera for Thermodynamics, Eng. Technol. Appl. Sci. Res. 2024, 14, 14069–14078. [Google Scholar] [CrossRef]
- Power, M. , IGCC Integrated coal Gasification Combined Cycle Power Plants, Mitsubishi Power, a brand of Mitsubishi Heavy Industries, Ltd., 2021. https://power.mhi.com/catalogue/pdf/igcc.pdf (accessed , 2022). 6 May.
- Marzouk, O.A. , Chronologically-Ordered Quantitative Global Targets for the Energy-Emissions-Climate Nexus, from 2021 to 2050, in: 2022 Int. Conf. Environ. Sci. Green Energy ICESGE, IEEE [Institute of Electrical and Electronics Engineers], Virtual, 2022: pp. 1–6. [CrossRef]
- Zhao, Y.; Ying, D.; Liu, Y.; Wentao, R. , Relative Stability Analysis Method of Systems Based on Goal Seek, in: 2021 3rd Int. Conf. Ind. Artif. Intell. IAI, 2021: pp. 1–5. [CrossRef]
- Guerrero, H. ; Solver; Scenarios, and Goal Seek Tools, in: H. Guerrero (Ed.), Excel Data Anal. Model. Simul., Springer International Publishing, Cham, 2019: pp. 311–346. [CrossRef]
- Tian, Y.; Qin, C.; Yang, Z.; Hao, D. , Numerical simulation study on the leakage and diffusion characteristics of high-pressure hydrogen gas in different spatial scenes, Int. J. Hydrog. Energy 2024, 50, 1335–1349. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Contrasting the Cartesian and polar forms of the shedding-induced force vector in response to 12 subharmonic and superharmonic mechanical excitations, Fluid Dyn. Res. 2010, 42, 035507. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Torabi, T.; Amiri, T.Y.; Fortunelli, A.; Iulianelli, A. , Parametric and sensitive analysis of Pd-Ag membrane reactor performance in biogas reforming to generate decarbonized hydrogen by Computational Fluid Dynamic-Response Surface Methodology, Fuel 2024, 365, 131205. [CrossRef]
- Marzouk, O.A. , Characteristics of the Flow-Induced Vibration and Forces With 1- and 2-DOF Vibrations and Limiting Solid-to-Fluid Density Ratios, J. Vib. Acoust. 2010, 132, 041013. [Google Scholar] [CrossRef]
- Kassi, A.H.; Al-Hattab, T.A. , A CFD model of natural gas steam reforming in a catalytic membrane reactor: Effect of various operating parameters on the performance of CMR, Int. J. Hydrog. Energy 2024, 56, 780–796. [Google Scholar] [CrossRef]
- Marzouk, O.A.; Nayfeh, A.H. , Characterization of the flow over a cylinder moving harmonically in the cross-flow direction, Int. J. Non-Linear Mech. 2010, 45, 821–833. [Google Scholar] [CrossRef]
- Zakeri, R.; Fazeli, A. , CFD modeling of hydrogen production from glycerol steam reforming in Tesla microchannel reactor, Fuel 2024, 357, 129646. [CrossRef]
- Li, X.; Zhao, Y.; Liu, Z.; Chen, H. , A new methodology for preliminary design of centrifugal impellers with prewhirl, Proc. Inst. Mech. Eng. Part J. Power Energy 2020, 234, 251–262. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Evolutionary Computing Applied to Design Optimization, in: Vol. 2 27th Comput. Inf. Eng. Conf. Parts B, ASMEDC, Las Vegas, Nevada, USA, 2007: pp. 995–1003. [CrossRef]
- Zhang, Z. , Understanding and exploiting the nonlinear behavior of tuned liquid dampers (TLDs) for structural vibration control by means of a nonlinear reduced-order model (ROM), Eng. Struct. 2022, 251, 113524. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Flow control using bifrequency motion, Theor. Comput. Fluid Dyn. 2011, 25, 381–405. [Google Scholar] [CrossRef]
- Marzouk, O.A. , A two-step computational aeroacoustics method applied to high-speed flows, Noise Control Eng. J. 2008, 56, 396. [Google Scholar] [CrossRef]
- San, J.Y.; Worek, W.M.; Lavan, Z. , Entropy Generation in Convective Heat Transfer and Isothermal Convective Mass Transfer, J. Heat Transf. 1987, 109, 647–652. [Google Scholar] [CrossRef]
- [United States Department of Energy] DOE, Department of Energy Fundamentals Training Handbook, Thermodynamics, Heat Transfer, and Fluid Flow, Volume 2 of 3, United States Department of Energy (DOE), 1992. https://www.standards.doe.gov/standards-documents/1000/1012-bhdbk-1992-v2/@@images/file (accessed , 2024). 1 June.
- Enerquip, What’s the difference between parallel flow, counter flow and crossflow heat exchangers?, (2022). https://www.enerquip.com/whats-the-difference-between-parallel-flow-counter-flow-and-crossflow-heat-exchangers (accessed , 2022). 7 May.
- Thermex, Why counter flow heat exchangers are more efficient, (2022). http://www.thermex.co.uk/news/blog/605-why-counter-flow-heat-exchangers-are-more-efficient (accessed , 2022). 7 May.
- Ben-Mansour, R.; Haque, M.A.; Habib, M.A.; Paglieri, S.; Harale, A.; Mokheimer, E.M.A. , Effect of temperature and heat flux boundary conditions on hydrogen production in membrane-integrated steam-methane reformer, Appl. Energy 2023, 346, 121407. [Google Scholar] [CrossRef]
- Barbieri, G. , Sweep Gas in a Membrane Reactor, in: E. Drioli, L. Giorno (Eds.), Encycl. Membr., Springer Berlin Heidelberg, Berlin, Heidelberg, 2015: pp. 1–2. [CrossRef]
- Li, Z.; Polfus, J.M.; Xing, W.; Denonville, C.; Fontaine, M.-L.; Bredesen, R. , Factors Limiting the Apparent Hydrogen Flux in Asymmetric Tubular Cercer Membranes Based on LaCO27WCO3. 5MoCO1.5O55.5−δ and La0.87Sr0.13CrOsub>3−δ</sub>, Membranes 2019, 9, 126. [Google Scholar] [CrossRef]
- Xie, D.; Yu, J.; Wang, F.; Zhang, N.; Wang, W.; Yu, H.; Peng, F.; Park, A.-H.A. , Hydrogen permeability of Pd–Ag membrane modules with porous stainless steel substrates, Int. J. Hydrog. Energy 2011, 36, 1014–1026. [Google Scholar] [CrossRef]
- Chein, R.Y.; Chen, Y.C.; Chung, J.N. , Sweep gas flow effect on membrane reactor performance for hydrogen production from high-temperature water-gas shift reaction, J. Membr. Sci. 2015, 475, 193–203. [Google Scholar] [CrossRef]
- Balachandran, U.; Dorris, S.E.; Emerson, J.E.; Lee, T.H.; Lu, Y.; Park, C.Y.; Picciolo, J.J.; Membranes, H.S. , Argonne National Laboratory (ANL) of the United States Department of Energy, 2011. https://publications.anl.gov/anlpubs/2011/03/69523.pdf (accessed , 2024). 31 May.
- Bidica, N.; Ghimis, N.; Monea, B. , Experimental results of deuterium/hydrogen co-current permeation through Nickel membrane, in non-steady-state surface limited regime, Fusion Eng. Des. 2023, 194, 113718. [Google Scholar] [CrossRef]
- Escolástico, S.; Ivanova, M.; Solís, C.; Roitsch, S.; Meulenberg, W.A.; Serra, J.M. , Improvement of transport properties and hydrogen permeation of chemically-stable proton-conducting oxides based on the system BaZr1-x-yYxMyO3-δ, RSC Adv. 2012, 2, 4932. 2. [CrossRef]
- Longhurst, G.R.; Kratville, A. , A Simple System for Measuring Permeation of Hydrogen Through Stainless Steel, Fusion Sci. Technol. 2014, 66, 385–393. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, C.-H.; Lin, Y.-L.; Tsai, C.-W.; Chein, R.-Y.; Yu, C.-T. , Interfacial permeation phenomena of hydrogen purification and carbon dioxide separation in a non-isothermal palladium membrane tube, Chem. Eng. J. 2016, 305, 156–168. [Google Scholar] [CrossRef]
- Chiesa, P.; Romano, M.C.; Kreutz, T.G. , Use of membranes in systems for electric energy and hydrogen production from fossil fuels, in: Handb. Membr. React., Elsevier, 2013: pp. 416–455. [CrossRef]
- Marín, P.; Patiño, Y.; Díez, F.V.; Ordóñez, S. , Modelling of hydrogen perm-selective membrane reactors for catalytic methane steam reforming, Int. J. Hydrog. Energy 2012, 37, 18433–18445. [Google Scholar] [CrossRef]
- Park, Y.; Cha, J.; Oh, H.-T.; Lee, T.; Lee, S.H.; Park, M.G.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Han, J.; Yoon, C.W.; Jo, Y.S. , A catalytic composite membrane reactor system for hydrogen production from ammonia using steam as a sweep gas, J. Membr. Sci. 2020, 614, 118483. [Google Scholar] [CrossRef]
- Brunetti, A.; Caravella, A.; Drioli, E.; Barbieri, G. , CHAPTER 1. Membrane Reactors for Hydrogen Production, in: 2017: pp. 1–29. [CrossRef]
- Li, X.; Li, A.; Lim, C.J.; Grace, J.R. , Hydrogen permeation through Pd-based composite membranes: Effects of porous substrate, diffusion barrier and sweep gas, J. Membr. Sci. 2016, 499, 143–155. [Google Scholar] [CrossRef]
- Meng, X.; Shang, Y.; Meng, B.; Yang, N.; Tan, X.; Sunarso, J.; Liu, S. , Bi-functional performances of BaCe0. 95Tb0.05O3−δ-based hollow fiber membranes for power generation and hydrogen permeation, J. Eur. Ceram. Soc. 2016, 36, 4123–4129. [Google Scholar] [CrossRef]
- Roberts, R.M.; Elleman, T.S. ; H. P. Iii; Verghese, K., Hydrogen Permeability of Sintered Aluminum Oxide, J. Am. Ceram. Soc. 1979, 62, 495–499. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Kjølseth, C.; Serra, J.M. , Outstanding hydrogen permeation through CO 2 -stable dual-phase ceramic membranes, Energy Env. Sci 2014, 7, 3736–3746. [Google Scholar] [CrossRef]
- Qi, X. , Electrical conduction and hydrogen permeation through mixed proton–electron conducting strontium cerate membranes, Solid State Ion. 2000, 130, 149–156. [CrossRef]
- Sakbodin, M.; Schulman, E.; Oh, S.C.; Pan, Y.; Wachsman, E.D.; Liu, D. , Dual utilization of greenhouse gases to produce C2+ hydrocarbons and syngas in a hydrogen-permeable membrane reactor, J. Membr. Sci. 2020, 595, 117557. [Google Scholar] [CrossRef]
- Smith, J.B.; Aasen, K.I.; Wilhelmsen, K.; Käka, D.; Risdal, T.; Berglund, A.; Østby, A.S.; Budd, M.; Bruun, T.; Werswick, B. , Recent development in the HMR pre-combustion gas power cycle, Energy Procedia 2009, 1, 343–351. [CrossRef]
- Tan, X.; Tan, X.; Yang, N.; Meng, B.; Zhang, K.; Liu, S. , High performance BaCe0. 8Y0.2O<sub>>3−a</sub (BCY) hollow fibre membranes for hydrogen permeation, Ceram. Int. 2014, 40, 3131–3138. [Google Scholar] [CrossRef]
- Unruh, D.; Pabst, K.; Schaub, G. , Fischer−Tropsch Synfuels from Biomass: Maximizing Carbon Efficiency and Hydrocarbon Yield, Energy Fuels 2010, 24, 2634–2641. [CrossRef]
- Institute, G.T. , Direct Hydrogen Production from Biomass Gasifier Using Hydrogen-Selective Membrane, Gas Technology Institute (GTI), In collaboration with Natural Resources Research Institute University of Minnesota at Duluth, 2007. https://www.xcelenergy.com/staticfiles/xe/Corporate/RDF-DirectHydrogenProduction-Report%5B1%5D.pdf (accessed , 2024). 31 May.
- Kinouchi, K.; Katoh, M.; Horikawa, T.; Yoshikawa, T.; Wada, M. , HYDROGEN PERMEABILITY OF PALLADIUM MEMBRANE FOR STEAM-REFORMING OF BIO-ETHANOL USING THE MEMBRANE REACTOR, Int. J. Mod. Phys. Conf. Ser. 2012, 06, 7–12. [Google Scholar] [CrossRef]
- Barnoon, P. , Modeling of a high temperature heat exchanger to supply hydrogen required by fuel cells through reforming process, Energy Rep. 2021, 7, 5685–5699. [CrossRef]
- Barnoon, P.; Toghraie, D.; Mehmandoust, B.; Fazilati, M.A.; Eftekhari, S.A. , Comprehensive study on hydrogen production via propane steam reforming inside a reactor, Energy Rep. 2021, 7, 929–941. [CrossRef]
- Fornarelli, F.; Dambrosio, L.; Terlizzi, L.; Camporeale, S.M. , Performance and cost multi objective optimisation of a shell-and-tube LHTES device for mid-temperature applications, J. Energy Storage 2024, 99, 113134. [Google Scholar] [CrossRef]
- Kirincic, M.; Trp, A.; Lenic, K.; Torbarina, F. , Numerical analysis of the influence of geometry parameters on charging and discharging performance of shell-and-tube latent thermal energy storage with longitudinal fins, Appl. Therm. Eng. 2024, 236, 121385. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Ge, R. , Comparison of performance enhancement in a shell and tube based latent heat thermal energy storage device containing different structured fins, Renew. Energy 2023, 206, 994–1006. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Direct Numerical Simulations of the Flow Past a Cylinder Moving With Sinusoidal and Nonsinusoidal Profiles, J. Fluids Eng. 2009, 131, 121201. [Google Scholar] [CrossRef]
- Daneshparvar, M.R.; Beigzadeh, R. , Multi-objective optimization of helical baffles in the shell-and-tube heat exchanger by computational fluid dynamics and genetic algorithm, Energy Rep. 2022, 8, 11064–11077. [CrossRef]
- Marzouk, O.A. , One-way and two-way couplings of CFD and structural models and application to the wake-body interaction, Appl. Math. Model. 2011, 35, 1036–1053. [Google Scholar] [CrossRef]
- Saldanha, W.H.; Arrieta, F.R.P.; Soares, G.L. , State-of-the-Art of Research on Optimization of Shell and Tube Heat Exchangers by Methods of Evolutionary Computation, Arch. Comput. Methods Eng. 2021, 28, 2761–2783. [Google Scholar] [CrossRef]
- Marzouk, O.A.; Huckaby, E.D. , Simulation of a Swirling Gas-Particle Flow Using Different k-epsilon Models and Particle-Parcel Relationships, Eng. Lett. 2010, 18, 7. [Google Scholar] [CrossRef]
- Li, P.; Chen, L.; Xia, S.; Zhang, L. , Entropy Generation Rate Minimization for Methanol Synthesis via a CO2 Hydrogenation Reactor, Entropy 2019, 21, 174. 21. [CrossRef]
- Li, P.; Chen, L.; Xia, S.; Kong, R.; Ge, Y. , Multi-objective optimal configurations of a membrane reactor for steam methane reforming, Energy Rep. 2022, 8, 527–538. [CrossRef]
- Bichkar, P.; Dandgaval, O.; Dalvi, P.; Godase, R.; Dey, T. , Study of Shell and Tube Heat Exchanger with the Effect of Types of Baffles, Procedia Manuf. 2018, 20, 195–200. [CrossRef]
- Pal, E.; Kumar, I.; Joshi, J.B.; Maheshwari, N.K. , CFD simulations of shell-side flow in a shell-and-tube type heat exchanger with and without baffles, Chem. Eng. Sci. 2016, 143, 314–340. [Google Scholar] [CrossRef]
- Salahuddin, U.; Bilal, M.; Ejaz, H. , A review of the advancements made in helical baffles used in shell and tube heat exchangers, Int. Commun. Heat Mass Transf. 2015, 67, 104–108. [Google Scholar] [CrossRef]
- Jamsran, N.; Park, H.; Lee, J.; Oh, S.; Kim, C.; Lee, Y.; Kang, K. , Influence of syngas composition on combustion and emissions in a homogeneous charge compression ignition engine, Fuel 2021, 306, 121774. [CrossRef]
- Kousheshi, N.; Yari, M.; Paykani, A.; Mehr, A.S.; de la Fuente, G.F. , Effect of Syngas Composition on the Combustion and Emissions Characteristics of a Syngas/Diesel RCCI Engine, Energies 2020, 13, 212. [CrossRef]
- Ribeiro, A.M.; Santos, J.C.; Rodrigues, A.E. , PSA design for stoichiometric adjustment of bio-syngas for methanol production and co-capture of carbon dioxide, Chem. Eng. J. 2010, 163, 355–363. [Google Scholar] [CrossRef]
- Giuliano, A.; Freda, C.; Catizzone, E. , Techno-Economic Assessment of Bio-Syngas Production for Methanol Synthesis: A Focus on the Water–Gas Shift and Carbon Capture Sections, Bioengineering 2020, 7, 70. 7. [CrossRef]
- [United States National Institute of Standards and Technology] NIST, NIST Chemistry WebBook - Hydrogen, (2021). https://webbook.nist.gov/cgi/cbook.cgi?Name=h2&Units=SI (accessed , 2022). 7 May.
- [United States National Institute of Standards and Technology] NIST, NIST Chemistry WebBook - Carbon monoxide, (2021). https://webbook.nist.gov/cgi/cbook.cgi?Name=CO&Units=SI (accessed , 2022). 7 May.
- [United States National Institute of Standards and Technology] NIST, NIST Chemistry WebBook - Carbon dioxide, (2021). https://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Units=SI (accessed , 2022). 7 May.
- Kuo, K.K.; Combustion, P.O. ; nd ed; Wiley, J.; Hoboken, 2005.
- Marzouk, O.A. , Estimated electric conductivities of thermal plasma for air-fuel combustion and oxy-fuel combustion with potassium or cesium seeding, Heliyon 10 (2024) e31697. [CrossRef]
- Poinsot, T.; Veynante, D. , Theoretical and Numerical Combustion, 2nd ed, Edwards, Philadelphia, 2005.
- [International Union of Pure and Applied Chemistry] IUPAC, IUPAC Compendium of Chemical Terminology, 3rd ed. Online version 3.0.1, (2019). [CrossRef]
- [United States National Institute of Standards and Technology] NIST, CODATA [Committee on Data for Science and Technology] Value: molar gas constant, (2018). https://physics.nist.gov/cgi-bin/cuu/Value?r (accessed , 2022). 7 May.
- [United States National Institute of Standards and Technology] NIST, NIST Chemistry WebBook - Nitrogen, (2021). https://webbook.nist.gov/cgi/cbook.cgi?Name=n2 (accessed , 2022). 7 May.
- Utamura, M.; Nikitin, K.; Kato, Y. , A generalised mean temperature difference method for thermal design of heat exchangers, Int. J. Nucl. Energy Sci. Technol. 2008, 4, 11. [Google Scholar] [CrossRef]
- IV, J.H.L.; Lienhard V, J.H. , A Heat Transfer Textbook (fifth edition), 5th ed., Phlogiston Press, 2019.
- Marzouk, O.A. , Energy Generation Intensity (EGI) for Parabolic Dish/Engine Concentrated Solar Power in Muscat, Sultanate of Oman, IOP Conf. Ser. Earth Environ. Sci. 2022, 1008, 012013. [Google Scholar] [CrossRef]
- Bandyopadhyay, S. , All forms of energy are equal, but some forms of energy are more equal than others, Clean Technol. Environ. Policy 2021, 23, 2775–2776. [Google Scholar] [CrossRef]
- Demirbas, A. ; Balubaid; M. A.; Basahel; A. M.; Ahmad; M.H.; Sheikh, Octane Rating of Gasoline and Octane Booster Additives, Pet. Sci. Technol. 2015, 33, 1190–1197. [Google Scholar] [CrossRef]
- Kubesh, J.; King, S.R.; Liss, W.E. , Effect of Gas Composition on Octane Number of Natural Gas Fuels, in: San Francisco, California, USA, 1992: p. 92 2359. [CrossRef]
- Leonzio, G. , Methanol Synthesis: Optimal Solution for a Better Efficiency of the Process, Processes 2018, 6, 20. 6. [CrossRef]
- Tan, E.C.D.; Talmadge, M.; Dutta, A.; Hensley, J.; Schaidle, J.; Biddy, M.; Humbird, D.; Snowden-Swan, L.J.; Ross, J.; Sexton, D.; Yap, R.; Lukas, J. , Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons via Indirect Liquefaction Thermochemical Research Pathway to High-Octane Gasoline Blendstock Through Methanol/Dimethyl Ether Intermediates, National Renewable Energy Laboratory (NREL) and Pacific Northwest National Laboratory (PNNL) of the United States Department of Energy, 2015. https://www.nrel.gov/docs/fy15osti/62402.pdf (accessed , 2024). 1 June.
- Vita, A.; Italiano, C. , Fuel and hydrogen related problems for conventional steam reforming of natural gas, in: Curr. Trends Future Dev. Bio- Membr., Elsevier, 2020: pp. 71–89. [CrossRef]
- Marzouk, O.A. , Lookup Tables for Power Generation Performance of Photovoltaic Systems Covering 40 Geographic Locations (Wilayats) in the Sultanate of Oman, with and without Solar Tracking, and General Perspectives about Solar Irradiation, Sustainability 2021, 13, 13209. [CrossRef]
- Heumann, C.; Schomaker, M. ; Shalabh, Introduction to Statistics and Data Analysis: with exercises, solutions and applications in r, 2nd ed., Springer Cham, 2023. https://link.springer.com/book/10.1007/978-3-031-11833-3.
- Marzouk, O.A.; Huckaby, E.D. , A Comparative Study of Eight Finite-Rate Chemistry Kinetics for CO/H2 Combustion, Eng. Appl. Comput. Fluid Mech. 2010, 4, 331–356. [Google Scholar] [CrossRef]
- Wang, S.; Lei, X.; Xu, B.; Jiang, W.; Kong, L.; Yang, B.; Tian, Y.; Liu, Y. , Vacuum evaporation and condensation thermodynamics and evaporation kinetics of pure silver, Mater. 2024, 4, 100189. [CrossRef]
- Marzouk, O.A.; Jul, W.A.M.H.R.; Al Jabri, A.M.K.; Al-ghaithi, H.A.M.A. , Construction of a Small-Scale Vacuum Generation System and Using It as an Educational Device to Demonstrate Features of the Vacuum, Int. J. Contemp. Educ. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Scientific, A. , Alicat flow controller - MC · MCW · MCR · MCV, Alicat Scientific, Inc. (a Halma company), Tucson, Arizona, USA, 2020. https://documents.alicat.com/manuals/Gas_Flow_Controller_Manual.pdf (accessed , 2024). 1 June.
- Arshi, N.; Lu, J.; Joo, Y.K.; Lee, C.G.; Yoon, J.H.; Ahmed, F. ; Study on structural, morphological and electrical properties of sputtered titanium nitride films under different argon gas flow, Mater. Chem. Phys. 2012, 134, 839–844. [Google Scholar] [CrossRef]
- Ralchenko, V.; Sychov, I.; Vlasov, I.; Vlasov, A.; Konov, V.; Khomich, A.; Voronina, S. , Quality of diamond wafers grown by microwave plasma CVD: effects of gas flow rate, Diam. Relat. Mater. 1999, 8, 189–193. [Google Scholar] [CrossRef]
- Rombouts, C.; Bair, M.; Barbe, J.; Wright, J.D.; Kramer, R.; Krajicek, Z. , A Comparison of Primary Gas Flow Standards Spanning the Range from 10 sccm N 2 to 10 slm N 2, NCSLI Meas. 2014, 9, 46–54. 9. [CrossRef]
- Ahmadi, A.; Hadipour, N.L.; Kamfiroozi, M.; Bagheri, Z. , Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde, Sens. Actuators B Chem. 2012, 161, 1025–1029. [Google Scholar] [CrossRef]
- Berg, R.F.; Gooding, T.; Vest, R.E. , Constant pressure primary flow standard for gas flows from 0. 01cm3/min to 100cm3/min (0.007–74μmol/s), Flow Meas. Instrum. 2014, 35, 84–91. [Google Scholar] [CrossRef]
- Fillet, R.; Nicolas, V.; Celzard, A.; Fierro, V. , Solar evaporation performance of 3D-printed concave structures filled with activated carbon under low convective flow, Chem. Eng. J. 2023, 457, 141168. [Google Scholar] [CrossRef]
- Jing, J.; Yang, L.; Tang, X.; He, P.; Tang, K. , Numerical Simulation of Multiphase Flow Erosion in the Gas Well Relief Line Elbow under Supercritical Conditions, J. Pipeline Syst. Eng. Pract. 2023, 14, 04023031. [Google Scholar] [CrossRef]
- Shahid, M.; Chambers, B.; Sankarasubramanian, S. , Methane and oxygen from energy-efficient, low temperature in situ resource utilization enables missions to Mars, AIChE J. 69 (2023) e18010. [CrossRef]
- Cao, Y.; Liang, W.; Law, C.K. , Real Gas Effects in High-Pressure Ignition of n -Dodecane/Air Mixtures, J. Phys. Chem. A 2024, 128, 3604–3612. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, Y.; Dong, Y.; Wen, C.; Yang, Y. , High-pressure supersonic carbon dioxide (CO2) separation benefiting carbon capture, utilisation and storage (CCUS) technology, Appl. Energy 2023, 339, 120975. [Google Scholar] [CrossRef]
- Gaganis, V.; Homouz, D.; Maalouf, M.; Khoury, N.; Polychronopoulou, K. , An Efficient Method to Predict Compressibility Factor of Natural Gas Streams, Energies 2019, 12, 2577. [CrossRef]
- Kartal, M.A.; Atakök, G.; Ersoy, S. , Cooling and Multiphase Analysis of Heated Environmentally Friendly R152A (C2H4F2) Fluid Coming from the Production Process According to Nist Indicators, Appl. Sci. 2024, 14, 4143. [Google Scholar] [CrossRef]
- Manfredi, M.; Persico, G.; Spinelli, A.; Gaetani, P.; Dossena, V. , Design and commissioning of experiments for supersonic ORC nozzles in linear cascade configuration, Appl. Therm. Eng. 2023, 224, 119996. [Google Scholar] [CrossRef]
- Maraggi, L.M.R.; Moscardelli, L.G. , Modeling hydrogen storage capacities, injection and withdrawal cycles in salt caverns: Introducing the GeoH2 salt storage and cycling app, Int. J. Hydrog. Energy 2023, 48, 26921–26936. [Google Scholar] [CrossRef]
- Srinivasan, N.; Zhang, H.; Yang, S. , VLE-Based Phase Field Method to Simulate High-Pressure Diffuse Interface with Phase Change, in: AIAA SCITECH 2024 Forum, American Institute of Aeronautics and Astronautics, Orlando, FL, 2024. [CrossRef]
- Mahmoud, M. , Development of a New Correlation of Gas Compressibility Factor (Z-Factor) for High Pressure Gas Reservoirs, J. Energy Resour. Technol. 136 ( 2013. [CrossRef]
- Marzouk, O.A. , The Sod gasdynamics problem as a tool for benchmarking face flux construction in the finite volume method, Sci. Afr. 10 (2020) e00573. [CrossRef]
- Bischoff, J.L.; Rosenbauer, R.J.; Pitzer, K.S. , The system NaCl-H2O: Relations of vapor-liquid near the critical temperature of water and of vapor-liquid-halite from 300° to 500°C, Geochim. Cosmochim. Acta 1986, 50, 1437–1444. [Google Scholar] [CrossRef]
- Dauchot, J.-P.; Dath, J.-P. , Oxidation of carbon monoxide on thin film catalysts: Characterization by a critical temperature measurement, J. Catal. 1984, 86, 373–383. [Google Scholar] [CrossRef]
- Guo, G.; Wang, F.; Liu, G.-Q.; Luo, S.-J.; Guo, R.-B. , Calculation on the phase equilibrium and critical temperature of CH4/CO2, Process Saf. Environ. Prot. 2018, 113, 369–377. [Google Scholar] [CrossRef]
- Ingebritsen, S.E.; Hayba, D.O. , Fluid flow and heat transport near the critical point of H 2 O, Geophys. Res. Lett. 1994, 21, 2199–2202. [Google Scholar] [CrossRef]
- Karnaukhov, V.A.; Oeschler, H.; Avdeyev, S.P.; Duginova, E.V.; Rodionov, V.K.; Budzanowski, A.; Karcz, W.; Bochkarev, O.V.; Kuzmin, E.A.; Chulkov, L.V.; Norbeck, E.; Botvina, A.S. , Critical temperature for the nuclear liquid-gas phase transition, Phys. Rev. C 2003, 67, 011601. [Google Scholar] [CrossRef]
- Kestin, J.; Korfali, Ö.; Sengers, J.V. , Density expansion of the viscosity of carbon dioxide near the critical temperature, Phys. Stat. Mech. Its Appl. 1980, 100, 335–348. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. , 1.5 Thermodynamic Aspects of Energy, in: Compr. Energy Syst., Elsevier, 2018: pp. 153–211. [CrossRef]
- Tiab, D.; Donaldson, E.C. , Introduction to Petroleum Geology, in: Petrophysics, Elsevier, 2016: pp. 23–66. [CrossRef]
- Behera, U.S.; Prasad, S.K.; Byun, H.-S. , Experimental validation on the phase separation for the 2-(Diisopropylamino)ethyl methacrylate and Poly [2-(diisopropylamino)ethyl methacrylate] in supercritical CO2, J. Mol. Liq. 2024, 393, 123553. [Google Scholar] [CrossRef]
- Shang, Z.; Yang, Y.; Zhang, L.; Sun, H.; Zhong, J.; Zhang, K.; Yao, J. , Hydrogen adsorption and diffusion behavior in kaolinite slit for underground hydrogen storage: A hybrid GCMC-MD simulation study, Chem. Eng. J. 2024, 487, 150517. [Google Scholar] [CrossRef]
- Shen, Y.; Qiu, C.; Liu, D.; Tao, X.; Wan, A.; Zhang, Z.; Gan, Z. , Experimental study on a closed-cycle Joule-Thomson cryocooler working at liquid hydrogen temperature, Appl. Therm. Eng. 2023, 234, 121291. [Google Scholar] [CrossRef]
- Wei-yu, C.; Sun, L.; Zhou, J.; Li, X.; Huang, L.; Xia, G.; Meng, X.; Wang, K. , Toward Predicting Interfacial Tension of Impure and Pure CO 2 -Brine Systems Using Robust Correlative Approaches, ACS Omega (2024) acsomega. 3c0 7956. [CrossRef]
- Xie, Y.; Dong, H.; Zhang, S.; Lu, X.; Ji, X.; CO, S.O. ; CH4; N2 in choline chloride/urea; Environ, G.E.2016) 195–200. [CrossRef]
- Mihara, S.; Sagara, H.; Arai, Y.; Saito, S. , The compressibility factors of hydrogen-methane, hydrogen-ethane and hydrogen-propane gaseous mixtures. , J. Chem. Eng. Jpn. 1977, 10, 395–399. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Thermo Physical Chemical Properties of Fluids Using the Free NIST Chemistry WebBook Database, Fluid Mech. Res. Int. J. 1 ( 2017. [CrossRef]
- [Pacific Northwest National Laboratory of the United States Department of Energy] PNNL, H2 Tools - Hydrogen Compressibility at different temperatures and pressures, (2022). https://h2tools.org/hyarc/hydrogen-data/hydrogen-compressibility-different-temperatures-and-pressures (accessed , 2022). 20 September.
- [Center for Chemical Process Safety of the American Institute of Chemical Engineers] CCPS, Plug Flow Reactor (PFR), (2022). https://www.aiche.org/ccps/resources/glossary/process-safety-glossary/plug-flow-reactor-pfr (accessed , 2022). 20 September.
- Ming, D.; Glasser, D.; Hildebrandt, D.; Glasser, B.; Metzer, M. , Attainable region theory: an introduction to choosing an optimal reactor, Wiley, Hoboken, New Jersey, 2016.
- Rosa, D.; Goes, P.; Manzi, J. , Steady-State Plug Flow Reactor Analysis by means of Minimum Entropy, in: Comput. Aided Chem. Eng., Elsevier, 2017: pp. 1165–1170. [CrossRef]
- Curtis, R.; Nguyen, C.; Lower, S.; Reactions, F.-O. 2021). https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/02%3A_Reaction_Rates/2.03%3A_First-Order_Reactions (accessed , 2022). 20 September.
- Phuakpunk, K.; Chalermsinsuwan, B.; Assabumrungrat, S. , Comparison of chemical reaction kinetic models for corn cob pyrolysis, Energy Rep. 2020, 6, 168–178. [CrossRef]
- Marzouk, O.A.; Nayfeh, A.H. , New Wake Models With Capability of Capturing Nonlinear Physics, in: ASME 2008 27th Int. Conf. Offshore Mech. Arct. Eng. OMAE 2008, ASME [American Society of Mechanical Engineers], Estoril, Portugal, 2009: pp. 901–912. [CrossRef]
- Marzouk, O.A.; Nayfeh, A.H. , A Study of the Forces on an Oscillating Cylinder, in: ASME 2007 26th Int. Conf. Offshore Mech. Arct. Eng. OMAE 2007, ASME [American Society of Mechanical Engineers], San Diego, California, USA, 2009: pp. 741–752. [CrossRef]
- [American Institute of Chemical Engineers] AIChE, Plug Flow Reactor (PFR), (2022). https://www.aiche.org/ccps/resources/glossary/process-safety-glossary/plug-flow-reactor-pfr (accessed , 2022). 9 May.
- Tuckerman, M.E. , Lecture 25: Plug flow reactors and comparison to continuously stirred tank reactors, (2020). https://chem.libretexts.org/Courses/New_York_University/CHEM-UA_652%3A_Thermodynamics_and_Kinetics/Lecture_25%3A_Plug_flow_reactors_and_comparison_to_continuously_stirred_tank_reactors (accessed , 2022). 9 May.
- Marzouk, O.A. , Coupled differential-algebraic equations framework for modeling six-degree-of-freedom flight dynamics of asymmetric fixed-wing aircraft, Int. J. Appl. Adv. Sci. 2025, 12, 30–51. [Google Scholar] [CrossRef]
- Brencio, C.; Fontein, F.W.A.; Medrano, J.A.; Di Felice, L.; Arratibel, A.; Gallucci, F. , Pd-based membranes performance under hydrocarbon exposure for propane dehydrogenation processes: Experimental and modeling, Int. J. Hydrog. Energy 2022, 47, 11369–11384. [Google Scholar] [CrossRef]
- Campo, M.; Tanaka, A.; Mendes, A.; Sousa, J.M. , Characterization of membranes for energy and environmental applications, in: Adv. Membr. Sci. Technol. Sustain. Energy Environ. Appl., Elsevier, 2011: pp. 56–89. [CrossRef]
- Caravella, A.; Hara, S.; Drioli, E.; Barbieri, G. , Sieverts law pressure exponent for hydrogen permeation through Pd-based membranes: Coupled influence of non-ideal diffusion and multicomponent external mass transfer, Int. J. Hydrog. Energy 2013, 38, 16229–16244. [Google Scholar] [CrossRef]
- Olander, D.; Konashi, K.; Yamawaki, M.; Fuel, Z.H.; Mater, C.N. ; Elsevier, 2012: pp. 313–357. [CrossRef]
- Suzuki, A.; Yukawa, H. , A Review for Consistent Analysis of Hydrogen Permeability through Dense Metallic Membranes, Membranes 2020, 10, 120. [CrossRef]
- Koffler, S.A.; Hudson, J.B.; Ansell, G.S. , Hydrogen permeation through alpha-palladium, Trans. Metall. Soc. Am. Inst. Min. Metall. Pet. Eng. 1969, 245, 1735–1740. [Google Scholar]
- Morreale, B.D.; Ciocco, M.V.; Enick, R.M.; Morsi, B.I.; Howard, B.H.; Cugini, A.V.; Rothenberger, K.S. , The permeability of hydrogen in bulk palladium at elevated temperatures and pressures, J. Membr. Sci. 2003, 212, 87–97. [Google Scholar] [CrossRef]
- Yuan, M.; Lee, K.; Van Campen, D.G.; Liguori, S.; Toney, M.F.; Wilcox, J. , Hydrogen Purification in Palladium-Based Membranes: An Operando X-ray Diffraction Study, Ind. Eng. Chem. Res. 2019, 58, 926–934. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Performance analysis of shell-and-tube dehydrogenation module, Int. J. Energy Res. 2017, 41, 604–610. [Google Scholar] [CrossRef]
- Nordio, M.; Wassie, S.A.; Van Sint Annaland, M.; Tanaka, D.A.P.; Sole, J.L.V.; Gallucci, F. , Techno-economic evaluation on a hybrid technology for low hydrogen concentration separation and purification from natural gas grid, Int. J. Hydrog. Energy 2021, 46, 23417–23435. [Google Scholar] [CrossRef]
- Marzouk, O.A. , Levelized cost of green hydrogen (LCOH) in the Sultanate of Oman using H2A-Lite with polymer electrolyte membrane (PEM) electrolyzers powered by solar photovoltaic (PV) electricity, E3S Web Conf. 2023, 469, 00101. [CrossRef]
- Corporation, M. , Use Goal Seek to find the result you want by adjusting an input value, (2022). https://support.microsoft.com/en-us/office/use-goal-seek-to-find-the-result-you-want-by-adjusting-an-input-value-320cb99e-f4a4-417f-b1c3-4f369d6e66c7 (accessed , 2022). 11 May.
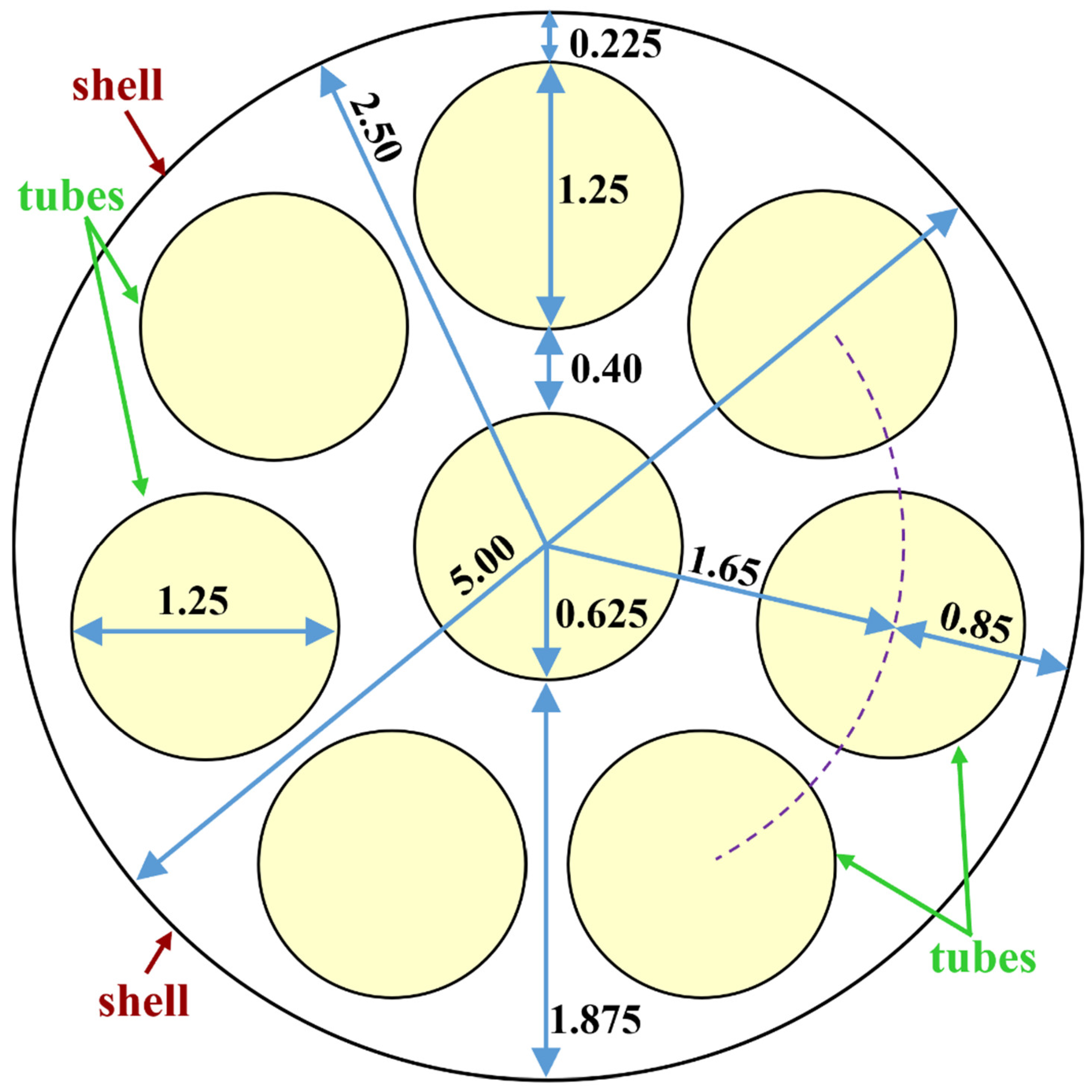
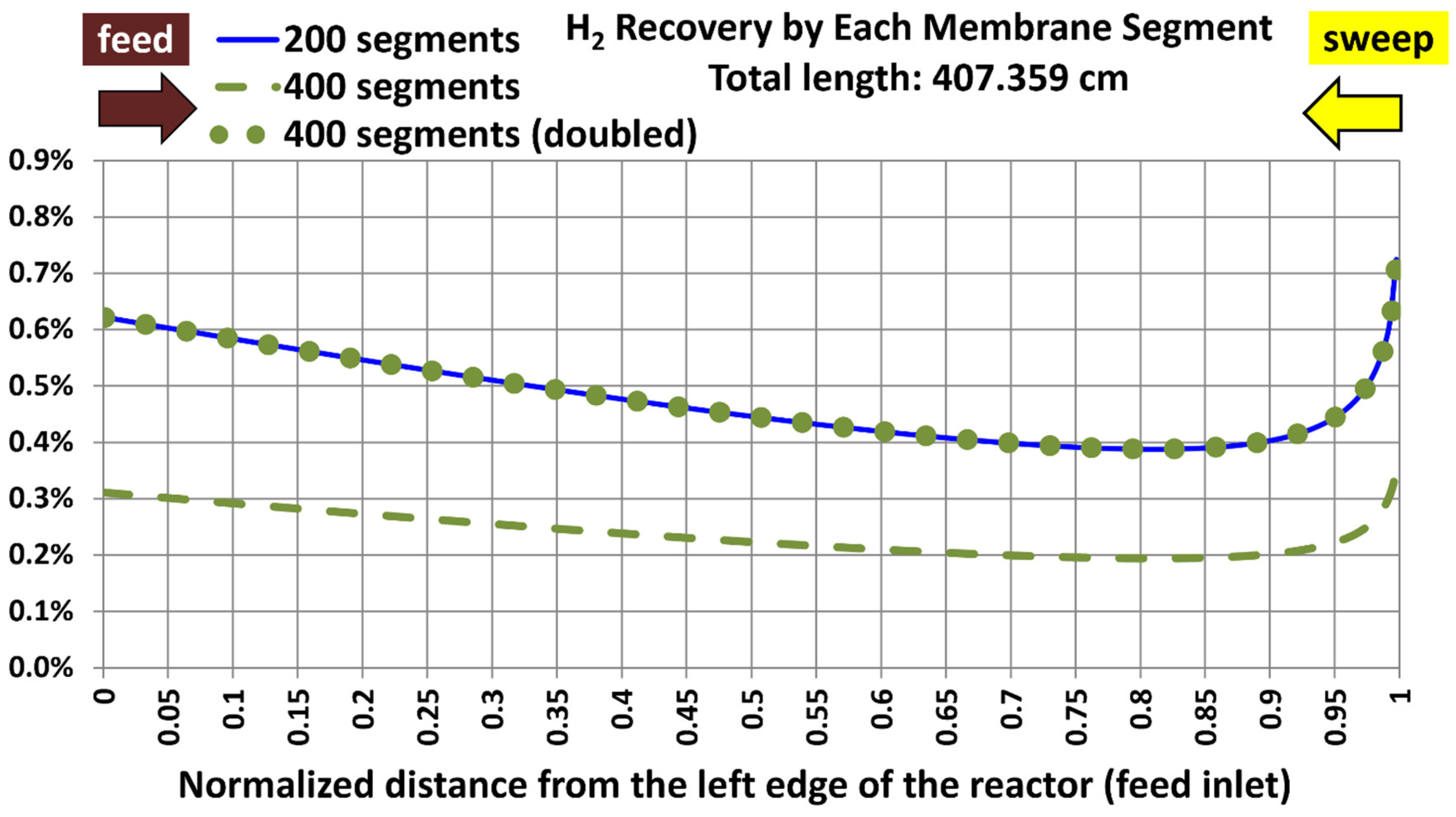
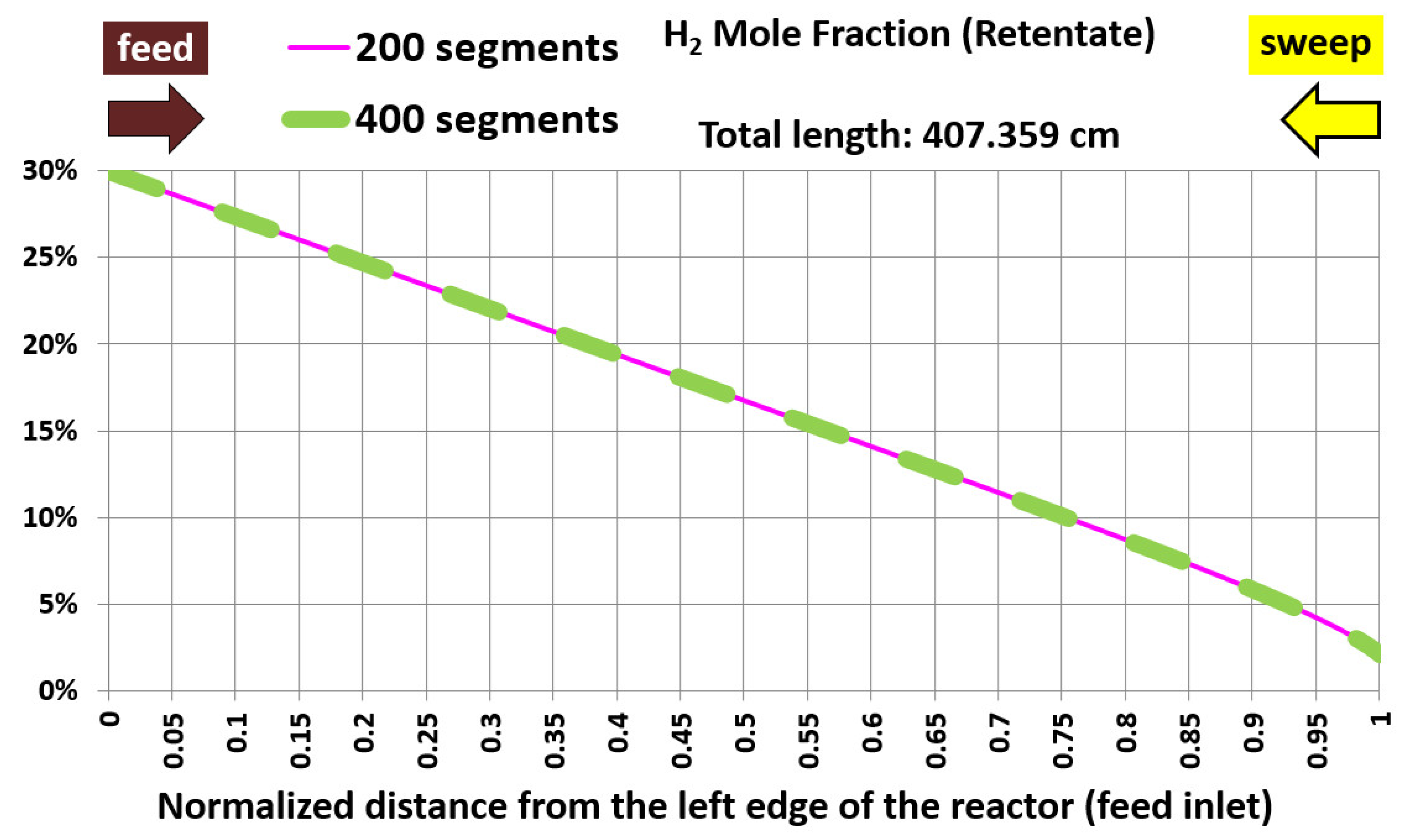
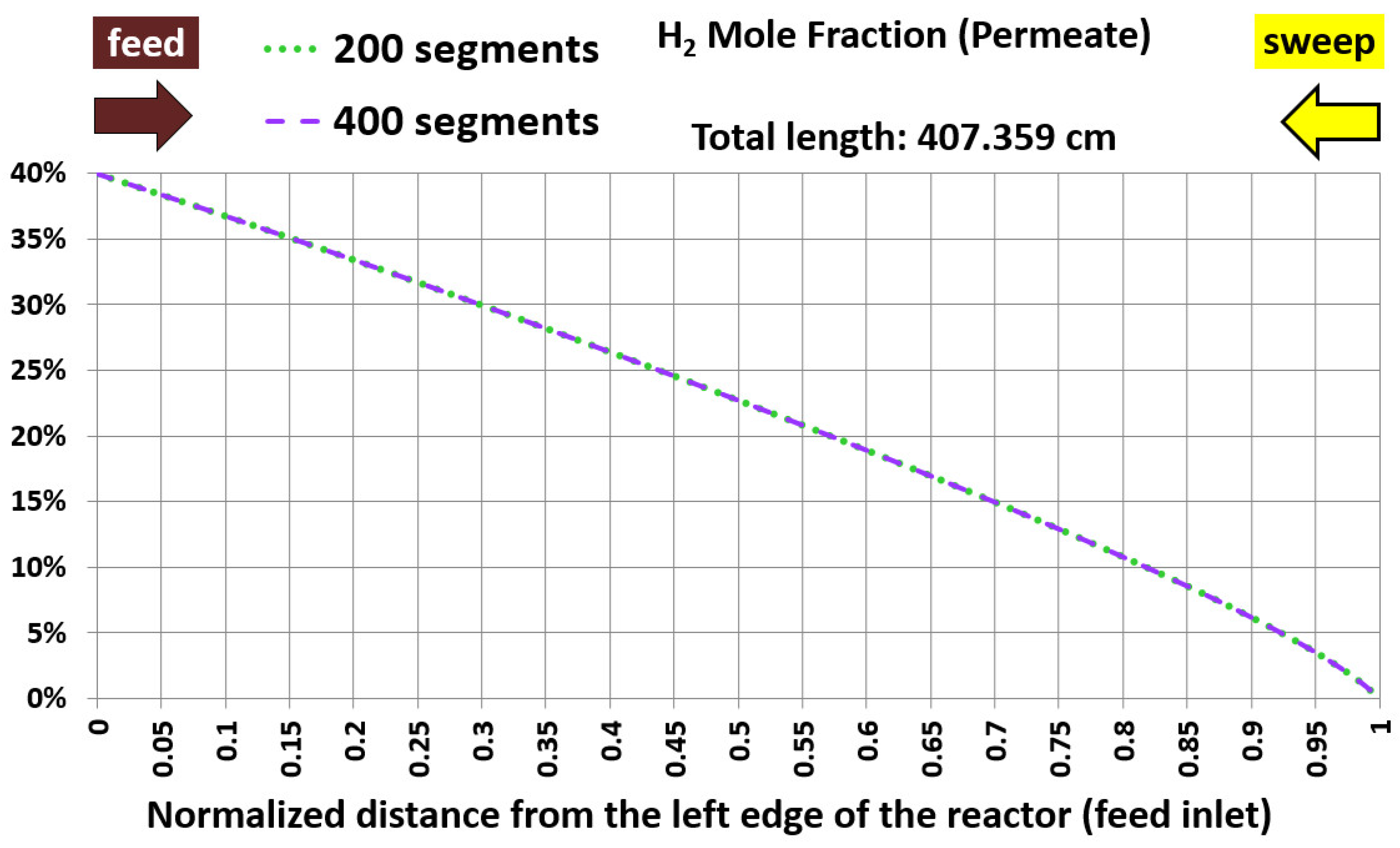
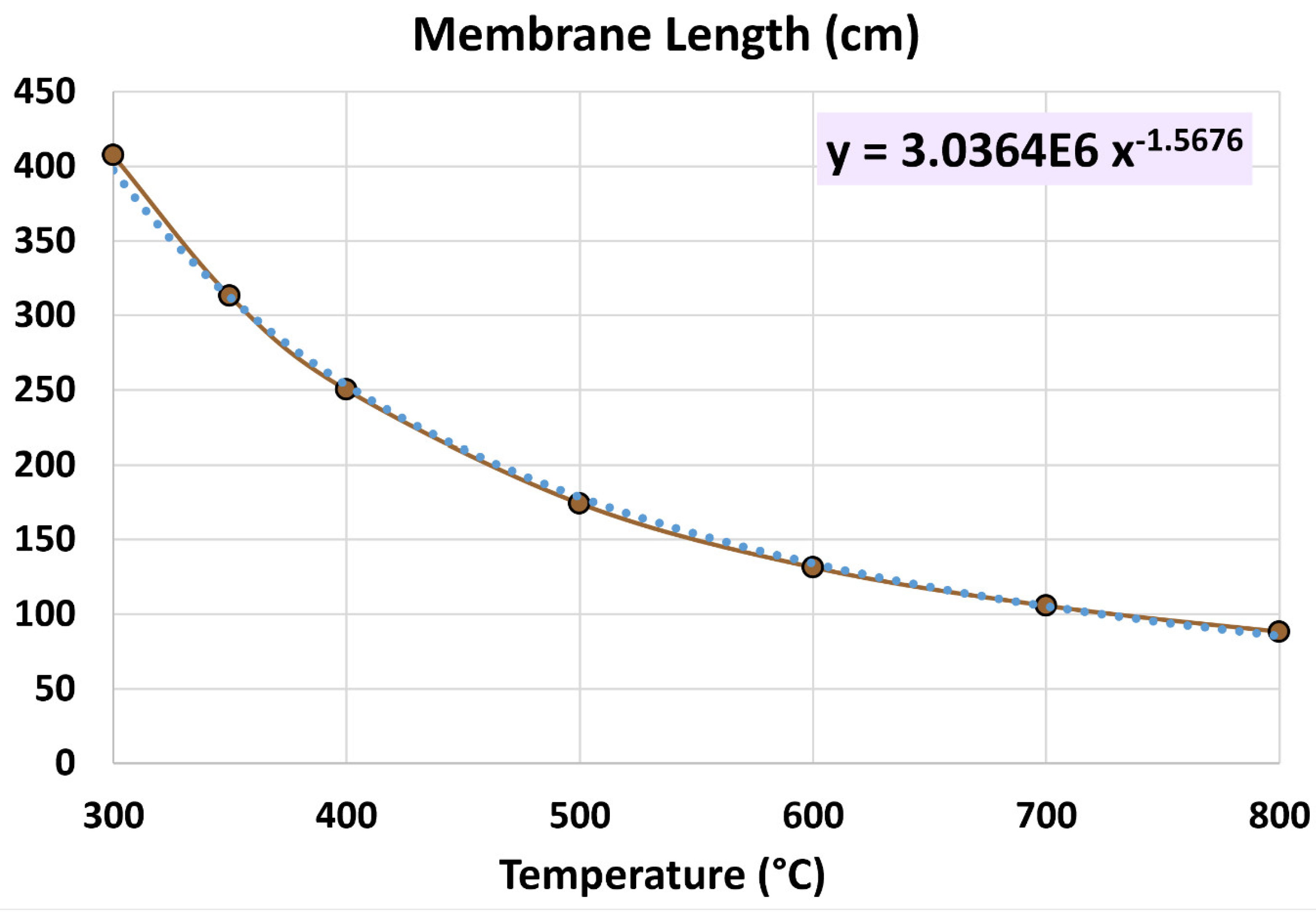
| Geometric feature | Value |
|---|---|
| Shell diameter | 5.000 cm (1.969 in) |
| Tube diameter | 1.250 cm (0.4921 in) |
| Number of tubes | 8 |
| Shell cross-section area (excluding tubes) | 9.817 cm2 (1.522 in2) |
| Tubes cross-section area (all 8 tubes) | 9.817 cm2 (1.522 in2) |
| Shell-to-tubes area ratio | 1: 1 |
| Tube cross-section area (single tube) | 1.227 cm2 (0.1902 in2) |
| Condition | Value |
|---|---|
| Inlet mole fraction, H2 | 30% [134] |
| Inlet mole fraction, CO | 50% [135] |
| Inlet mole fraction, CO2 | 20% [136,137] |
| Molecular weight, H2 | 2.01588 kg/kmol [138] |
| Molecular weight, CO | 28.0101 kg/kmol [139] |
| Molecular weight, CO2 | 44.0095 kg/kmol [140] |
| Molecular weight, mixture | 23.412 kg/kmol |
| Inlet mass fraction, H2 | 0.025832 |
| Inlet mass fraction, CO | 0.598207 |
| Inlet mass fraction, CO2 | 0.375961 |
| Inlet mass flow rate | 60 kg/hr (132.28 lbm/hr) |
| Inlet standard volume flow rate | 970,068 sccm (standard cubic centimeters per minute) |
| Target hydrogen recovery | 95% (by mass, by mole, or by standard volume - identical) |
| Condition | Value |
|---|---|
| Inlet gas | 100% N2 |
| Molecular weight, N2 | 28.0134 kg/kmol [146] |
| Inlet mass flow rate | 30.692 kg/hr (67.664 lbm/hr) |
| Inlet standard volume flow rate | 414,704 sccm (standard cubic centimeters per minute) |
| Target outlet mole fraction of H2 | 40% |
| Fluid property | Value |
|---|---|
| Temperature | 300 °C (572.00 °F) |
| Absolute retentate pressure | 40.0 atm (587.84 psia) |
| Absolute permeate pressure | 20.0 atm (293.92 psia) |
| Result | Value | Absolute percentage change | |
| n = 200 segments | n = 400 segments | ||
| Membrane length (cm) | 407.359 | 407.359 | 0% (identical) |
| Average hydrogen permeation mass flux (kg/m2.hr) | 1.151 | 1.150 | 0.01% |
| Pressure-square-root difference at the left end (Pa0.5) | 202.345 | 202.345 | 0% (identical) |
| Pressure-square-root difference at the right end (Pa0.5) | 260.655 | 268.896 | 3.16% |
| Log mean pressure-square-root difference (Pa0.5) | 230.271 | 234.05 | 1.64% |
| Global apparent hydrogen permeance (mol/m2.s.Pa0.5) | 6.8849 × 10–4 | 6.7732 × 10–4 | 1.62% |
| Efficiency factor (%) | 67.09% | 66.00% | 1.62% |
| Hydrogen recovery (%) | 95.000% | 94.991% | 0.01% |
| Extreme case number | Temperature | Absolute retentate pressure | Absolute permeate pressure |
|---|---|---|---|
| 1 | 800 °C (1,472.00 °F) | 40.0 atm (587.84 psia) | 20.0 atm (293.92 psia) |
| 2 | 300 °C (572.00 °F) | 120.0 atm (1,763.5 psia) | 20.0 atm (293.92 psia) |
| 3 | 300 °C (572.00 °F) | 40.0 atm (587.84 psia) | 0.20 atm (2.9392 psia) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





