1. Introduction
In recent years, interest in functional foods has increased significantly, leading to rapid growth in their global market [
1]. These products were first defined in 1984 by the Japanese government as “Foods for Specified Health Use” (FOSHU), described as “foods containing an ingredient with health functions and officially approved to claim their physiological effects on the human body”. Functional foods offer significant benefits for both physical and mental well-being, which are key factors driving consumer acceptance. Consequently, the growing demand for these products has encouraged research on the incorporation of bioactive compounds into everyday foods [
2]. Among these compounds, polyphenols and dietary fiber have gained particular attention for their well-documented health benefits, including antioxidant, anti-inflammatory, and gut health-promoting properties [
3,
4].
Artichoke (
Cynara cardunculus var. scolymus), a vegetable typical of the Mediterranean Diet, is a rich source of polyphenols and fiber, making it highly valuable nutritionally [
5]. It is cultivated mainly in the Mediterranean region, Canary Islands, Egypt, Asia, and South America, with Italy, Spain, and France being the leading producers. The edible portion of the artichoke, its immature flower, represents about 30% of its fresh weight. Consequently, industrial processing generates 60–85% of bio-waste and by-products, including leaves, outer bracts, and stems, raising concerns about food waste and environmental impact [
6]. Artichoke heads, leaves, and stems are rich in bioactive compounds, including caffeic acid derivatives, flavonoids (e.g., luteolin and apigenin), and dietary fiber such as inulin and pectins. These compounds confer to artichoke strong antioxidant activity, which is associated with protective effects against cardiovascular, hepatic, and neurological disorders [
7]. As a result, artichoke by-products can be considered as valuable ingredients for functional foods, supplements, and animal feed, while their reuse exemplifies a promising circular economy approach aimed at reducing the risk of metabolic and age-related disorders [
8]. Several studies have demonstrated the successful incorporation of artichoke by-products into both non-food and food matrices, such as bread and pasta, to enhance their health-promoting properties [
6,
9,
10].
The incorporation of agricultural and food industry residues into staple foods such as pasta offers a dual benefit: reducing food waste and improving the nutritional profile of widely consumed products. Carpentieri et al. [
11] conducted an extensive analysis of the use of bioactive compounds derived from agri-food by-products, such as dietary fibers, proteins, antioxidants, and omega-3 fatty acids, for pasta functionalization. Their review explored how these compounds influence the pasta-making process, as well as its technological, structural, sensory, and nutritional properties. Moreover, they discussed technological strategies, including sustainable emerging technologies, to mitigate the negative impacts of unconventional ingredients and enhance the health-promoting properties of pasta.
Among agri-food by-products, Colombo et al. [
8] reported technological applications involving artichoke by-products, particularly outer bracts and stems. Most studies have focused on bakery products, such as bread and breadsticks, where artichoke powder is used as a partial substitute for conventional flour in varying proportions [
10,
12,
13,
14,
15]. These studies assessed the effects of enrichment from technological, nutritional, and sensory perspectives. Few studies, however, have addressed pasta enrichment, and these typically involve the use of polyphenol extracts as a water substitute, enriching the pasta only with specific bioactive compounds, such as polyphenols, to enhance some properties, like antioxidant activity [
16,
17]. The literature contains very few studies utilizing whole artichoke by-products. Among these, the work of la Gatta et al. [
18] stands out, as they investigated the use of a powder derived from artichoke outer bracts in fresh pasta formulation. This study evaluated the effects from a technological perspective, including protein interactions, as well as nutritional aspects such as glycemic index modulation and sensory characteristics influenced by the presence of volatile compounds typical of the vegetable matrix. Nonetheless, significant knowledge gaps remain regarding the effects of cooking and digestion on bioactive compounds, particularly polyphenols and fiber, their interactions with metabolism, and their potential health benefits for humans.
The here presented study aims to investigate the feasibility of using artichoke by-products, such as outer bracts, to produce pasta enriched with polyphenols and fiber. By incorporating these bioactive compounds into pasta, a commonly consumed product, the functional properties of the pasta can be enhanced, potentially providing health benefits to consumers while promoting sustainability. The research examined the impact of artichoke bracts incorporation on pasta’s technological properties, nutritional quality, antioxidant activity, and sensory attributes. Furthermore, the enriched pasta underwent in vitro digestion to evaluate polyphenol bioaccessibility, focusing on free and bound polyphenols, and the colon availability index as predictive index for the polyphenols absorption and metabolism.
2. Materials and Methods
2.1. Raw Materials
Fresh artichokes
(Cynara scolymus (L.) var. Romanesco) were purchased from a local market (Bari, Italy) and used to obtain artichoke by-products. Specifically, in this study, the external bracts were collected and processed to produce artichoke powder (AP), as previously described by Bavaro et al. [
10]. Commercial re-milled durum wheat semolina (Casillo, Corato, Italy), was also purchased from a local market. According to the label, semolina contained 2% total fat, 70% of total available carbohydrates, 2.8% total dietary fiber, and 12% crude proteins.
2.2. Pasta Making
The pasta was prepared by mixing semolina and water using a mixer (Pasta Mixer, Marcato, Italy) equipped with accessories for
Fettuccine production. This process yielded a dough with a moisture content of 44% (U%). The experimental dough was prepared by replacing 10% of the semolina with AP. A control sample (P-CTR) was produced using 100% re-milled durum wheat semolina. Fresh
Fettuccine, approximately 20 cm long and 1.2 mm thick were produced. The samples were then dried at 40°C for 16 hours using the Biosec dehydrator (Tauro Essiccatori, Italy) to achieve a moisture content <12.5%, in compliance with Italian legal requirements for dry pasta [
19]. The samples were subsequently stored at room temperature under vacuum. Two different batches were produced. The experimental pasta (P-AP) and the control pasta (P-CTR) were subjected to technological, chemical, biological, and sensory analyses.
2.3. Physico-Chemical Characterization
The water-holding capacity of the re-milled durum wheat semolina and AP was determined as previously described [
10] and expressed as the quantity of water held by 1 g of semolina or AP. The pH of pasta doughs was measured with a portable pH-meter (type110, Eutech Instruments, Singapore City, Singapore) supplied with Double Pore D electrode (Hamilton, Bonaduz, Switzerland). Total titratable acidity (TTA) values of pasta doughs were determined according to American Association for Clinical Chemistry method (AACC) 02-31.01 [
20]. Water activity (a
w) was measured with AcquaLab (Decagon Devices, Inc., Pullman, WA, USA).
2.4. Technological Characterization of Pasta
The technological properties of both pasta samples were assessed following the methods described by Schettino et al. [
21].
2.4.1. Optimal Cooking Time
The optimal cooking time (OCT) of the pasta samples was determined according to the American Association of Cereal Chemists (AACC) approved method 66-50 [
22]. Pasta samples were boiled in a 1:10 (w/v) dry pasta: water ratio without adding salt. Every 30 seconds, some
Fettuccine strands were removed, cut, and examined for the disappearance of the white core.
2.4.2. Water Absorption
Water absorption at the OCT was measured by weighing the pasta before and after cooking. It was calculated as ((W1 − W0)/W0) × 100, where W1 is the weight of the cooked pasta and W0 is the weight of the uncooked sample.
2.4.3. Cooking Loss
Cooking loss was determined by measuring the amount of solid matter lost into the cooking water. Portions of 30 g of pasta were cooked in 300 mL of boiling tap water without salt. The pasta samples were cooked for the OCT, and the water volume was brought back to its initial level after cooking. Dry matter in the cooking water was determined from 25 mL of freeze-dried water residue. The residue was weighed and expressed as a percentage of dry material, reported as grams of matter lost per 100 g of pasta.
2.4.4. Hydration Test
Aliquots of 5 grams of pasta were placed in beakers containing 100 mL of tap water and incubated in a thermostatic bath at 25°C. After 5, 10, 15, 30, 60, 90, and 180 minutes of incubation, each aliquot was removed from the water, drained for approximately 1 minute, blotted with tissue paper, and weighed. The results were expressed as ((W1 − W0)/W0) × 100, where W1 represents the weight of the hydrated sample and W0 represents the weight of the dry sample.
2.4.5. Instrumental Colour
Colour values were measured on grinded pasta samples by a colorimeter (CR-400, Konica Minolta, Osaka, Japan) with a D65 illuminant, using attachment for granular materials CR-A50. The values were obtained using CIE colour system coordinates L (lightness), a* (red-green) and b* (yellow-blue) on five measurements for each sample. Colour difference, ∆E*ab, was calculated using the formula:
where ∆a, ∆b and ∆L are the differences for L, a, and b values between sample and standard reference [
23].
2.5. Nutritional Properties
The nutritional characterization and proximate composition of the enriched pasta (P-AP) and the control pasta (P-CTR) were carried out by BonassisaLab S.p.a. (Foggia, Italy), an accredited food analysis laboratory. Fat content was determined using the acid hydrolysis method. Protein content was calculated as total nitrogen × 6.25. Total dietary fiber was measured using the AOAC (2000) method 985.29[
24], and carbohydrate content was calculated by subtracting the fiber fraction from the total carbohydrates.
2.5.1. Starch Hydrolysis and Predicted Glycaemic Index (pGI)
The predicted glycaemic index (pGI) of P-CTR and P-AP pasta samples was determined using an in vitro model slightly modified from the method described by Canale et al. [
25]. This method is based on starch hydrolysis and sugar release during digestion, as described by Goñi et al. [
26]. Samples were pre-dried in a thermo-ventilated oven at 40 ± 1 °C for approximately 24 hours until a constant weight was achieved and then ground into a fine flour using a coffee grinder (Moulinex, Lourdes, France). Briefly, 100 mg of dry pasta was digested sequentially. Firstly, samples were digested with pepsin (Sigma-Aldrich P7125, 0.1 g/mL) in an HCl–KCl buffer (pH 1.5) at 40 °C for 1 hour. After pepsin digestion, samples were incubated with α-amylase (Sigma-Aldrich A3176; 48 U mg/g of pasta) in Tris-Maleate buffer (pH 6.9) at 37 °C using an orbital shaker. During incubation, aliquots of 1 mL were removed at 0, 30, 60, 90, 120, 150, and 180 minutes. Each aliquot was heated at 100 °C for 5 minutes to inactivate the enzyme, cooled, and centrifuged at 10,000 ×g at 4 °C. For each supernatant, 500 µL was incubated with amyloglucosidase (330 U/mL) in 1.5 mL of sodium acetate buffer (pH 4.75) at 60 °C for 45 minutes. The glucose released during digestion was quantified using a Varioskan Flash Spectral Scanning Multimode Reader (Thermo Fisher Scientific) at 510 nm, employing a commercial enzymatic kit (K-GLUC, Megazyme) based on the glucose oxidase/peroxidase (GOPOD) enzyme system. The glucose release over time (0–180 min) was plotted to calculate the area under the curve (AUC). The hydrolysis index (HI) was determined as:
White bread was used as the reference sample. The predicted glycaemic index (pGI) was calculated using the formula:
2.5.2. Protein Solubility and Electrophoresis
The solubility of proteins in control and enriched pasta samples was determined in triplicate, as described by Bonomi et al. [
27]. Briefly, finely ground samples (0.5 g) were dissolved in either 10 mL of 50 mM sodium phosphate buffer (pH 7.0) containing 0.1 M NaCl, or 10 mL of 50 mM sodium phosphate buffer (pH 7.0) containing 0.1 M NaCl, 8M urea and 10 mM DTT. After stirring at room temperature for 60 min, samples were centrifuged at 10,000×g for 20 min at 15°C to remove insoluble materials. The protein content in the supernatant was assessed using a colorimetric method [
28].
Ten µg of proteins extracted in the presence of urea and DTT were diluted with 15 µL of loading buffer (0.125 M Tris-HCl, pH 6.8, 20% glycerol, 2% SDS, 0.02% bromophenol blue, 5% 2-mercaptoethanol) and heated in boiling water for 10 min. SDS-PAGE analysis was performed on a 12% polyacrylamide gel using a Mini-PROTEAN apparatus (Bio-Rad, USA). Following staining with Coomassie Blue and destaining steps, proteins were visualized using a ChemiDocTM XRS+ (Bio-Rad) and images were analyzed with Image LabTM Software (Bio-Rad).
2.6. Free and Bound Polyphenols Characterization
The free phenolic compounds were extracted from both cooked and uncooked P-CTR and P-AP pasta samples following the method reported by Bavaro et al. [
10]. Briefly, polyphenols were extracted using ultrasounds with aqueous methanol (80% v/v), at matrix/solvent ratios of 1:5. The mixtures were sonicated (37 kHz, 50% potency, 30 °C, 10 min) with the Fisherbrand FB11203 ultrasonic bath, shaken (150 rpm, 20 min in the dark), centrifuged (4500 × g, 10 min), and filtered at 0.45 µm. Exhaustive extraction was ensured by repeating the solvent addition, shaking, and combining the supernatants, which were stored at −20 °C. The remaining pellets of uncooked pasta samples underwent alkaline hydrolysis for bound polyphenols extraction, involving treatment with 2M NaOH (2 hours), acidification (pH 2 with 6M HCl), and successive extraction with ethyl acetate for three times. The extracts were evaporated to dryness with a Rotavapor (BUCHI Italia s.r.l), and then reconstituted in methanol (15% v/v) and filtered (0.45 µm).
Polyphenol profiles were analyzed by HPLC-DAD using the Agilent 1260 Infinity Series Chromatograph system, supplied with Agilent Open Lab CDS Chem Station Software (Palo Alto, CA, USA). The system was equipped with 1260 HIP Degasser, G1312B binary pump, G1316A Thermostat, and G4212B DAD detector. Separation was performed on a 5 μm Phenomenex Luna C18 (4.6 × 250 mm) column (Phenomenex Torrance, CA, USA). The mobile phase consisted of MeOH (solvent A) and acetic acid/water (5:95 v/v) (solvent B) with the following gradient: 0–25 min, 15–40 % A, 25–30 min, 40 % A (isocratic), 30–45 min, 40–63 % A, 45–47 min, 63 % A (isocratic), 47–52 min, 63–100 % A, 52–56 min, 100 % A (isocratic). The flow rate was constant at 1 mL/min. Main phenolic compounds were identified by comparing spectra and retention times with those of available standards. Results were expressed as mg of compound per 100 g of dry weight sample (DW).
2.7. Antioxidant Activity
Methanolic extracts were assayed to determine antioxidant activity using 2,2-diphenyl-1-pycrilhydracyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and Ferric Ion Reducing Antioxidant Power (FRAP) assays. The radical scavenging activities were calculated using microplate methods with a Varioskan Flash Spectral Scanning Multimode Reader (Thermo Fisher Scientific).
The DPPH assay was performed as described by Brand-Williams et al. [
29] by preparing a 1mM solution of DPPH in methanol. For the calibration curve, Trolox was used at concentrations ranging from 0.005 mM to 1 mM. To determine antioxidant capacity, 20 µL of each methanolic extract or Trolox standard was added to 180 µL of the DPPH stock solution. After 30 min of incubation at room temperature in darkness, the absorbance was measured at 517 nm.
The ABTS free radical scavenging activity was performed as described by Re et al. [
30]. A 7 mM ABTS stock solution was mixed with 2.45 mM potassium persulfate to obtain the ABTS cation radical solution, which was stored in darkness for 16 h prior to use. The ABTS solution was subsequently diluted to achieve an absorbance of approximately 0.700 at 734 nm. Then, 180 µL of the diluted solution was mixed with 20 µL of the extract or Trolox standard solution, and the absorbance was measured after 2 minutes.
The antioxidant capacity of extracts was also determined using the FRAP assay, as proposed by Benzie and Strain [
31]. Known concentrations of ferrous sulfate solution (FeSO
4), ranging from 0.1 to 1 mM, were used to prepare the calibration curve. The FRAP working reagent consisted of 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mM HCl, and 20 mM FeCl
3, in a ratio of 10:1:1 (v/v). The absorbance of the standards and each extract was measured at 593 nm after 4 minutes of incubation at 37°C.
In all assays, the extracts were analyzed in triplicate. The results of the ABTS and DPPH tests were expressed as μmol of Trolox equivalents (TE) per gram of dry weight (DW), while those for the FRAP assay were expressed as μmol of ferrous sulfate equivalents (FSE).
2.8. In Vitro Digestion and Polyphenol Bioaccessibility Evaluation
The bioaccessibility of polyphenols of pasta samples was assessed using a three-stage in vitro digestion model simulating the oral, gastric, and small intestinal phases, as described by Bavaro et al. [
32], with minor modifications. Briefly, both control (P-CTR) and enriched (P-AP) pasta samples were cooked at their optimal cooking time (OCT) and subjected to simulated in vitro digestion. Cooked pasta (6 g) was mixed with oral phase solution (6mL), saline solution (3mL), and α-amylase (10.6 mg/g pasta), vortexed, and incubated at 37 °C with shaking (85 rpm) for 10 minutes. The gastric phase was initiated adding a porcine pepsin solution (19 mg/mL in 0.1 M HCl) and adjusting the pH to 3.0 ± 0.1 using 1.0 M HCl. The solutions were then incubated at 37 °C, 85 rpm for 1 hour. For the intestinal phase, a mixture containing pancreatin (30 mg/mL), lipase (15 mg/mL), and porcine bile salts (30 mg/mL) in 0.1 M NaHCO
3 was added, and the solutions were incubated at 37 °C, 85 rpm for 2 hours. After the small intestinal phase, the samples were centrifuged (10,000 rpm, 4 °C, 1 h). After the small intestinal phase, the samples were centrifuged (10,000 rpm, 4 °C, 1 h). The supernatants were filtered (0.45 μm) and analyzed using HPLC-DAD to calculate the bioaccessibility of polyphenols. This was expressed as the percentage ratio of each phenolic compound released from cooked pasta in digested versus undigested samples.
The digested pasta pellets underwent bound polyphenol extraction to evaluate the colon-available index (CAI, %) as described by Lucas-González et al. [
33].
2.9. Sensory Analysis
2.9.1. Panel Test Design and Execution
Twelve expert evaluators with over 70 hours of training in sensory analysis of a wide variety of foods, including pasta, performed the descriptive analysis (DA).Sensory tests were carried out at IBE-CNR Sensory Lab (Bologna, Italy), in individual booths equipped with tablets running specific software for sensory data acquisition (FIZZ, Biosystemès, Couternon, France), according to the standard protocol UNI 8589:19901. Before executing the tests, participants were informed of the main research outcomes and gave consent for their data to be used. Participation in the research was voluntary, and the right to privacy and data protection was respected in accordance with current legislation (GDPR 2016/679).
2.9.2. Descriptive Analysis (DA)
Judges received 30 g of both samples, control (P-CTR) and enriched (P-AP), cooked at the optimal cooking time (OCT), in plastic plates labelled with three-digit random numbers. The samples were served on a tray at room temperature (20 ± 2 °C) and presented to assessors monadically, in a balanced order. Tests were carried out in duplicate and under the conditions described in the standard ISO 13299:2016 [
34] for descriptive analysis using intensity scales (ISO 8586:2023) [
35]. The sample evaluation order was randomized using a balanced Latin square design [
36]. Twenty-five sensory attributes were selected from the literature [
37] or added as new terms to create an appropriate lexicon list for the descriptive analysis. Five olfactory and six aromatic attributes were chosen: wheat pasta, whole grain, vegetal, artichoke, legumes odor and flavor, and spicy tea flavor. Moreover, five gustatory and nine texture attributes were also evaluated: sweet, acid, bitter, salty, umami; firmness, roughness, nerve, graininess, gummy, adhesiveness, fibrousness, flouriness, and astringency. The descriptors were rated on a 9-point scale from “no perception” to the “highest intensity perceivable”. The panelists were also asked to rate the products’ overall liking on a 9-point hedonic scale from ”1: extremely disliked; “5: neither liked nor disliked”; to “9: extremely liked”, as proposed for novel food by research [
38]. Panelists used water to rinse their mouths between samples.
2.10. Statistical Analysis
The results were presented as mean values ± standard deviations. Data were subject to statistical analysis using Statistica 12.0 software (StatSoft, Inc., Tulsa, OK, USA). Data on physico-chemical properties, polyphenols content, and antioxidant activity were compared using one-way ANOVA followed by Tukey’s test to determine significant different (p < 0.05). Pearson’s correlation coefficient (r, p < 0.05) was calculated to assess correlations between antioxidant activities and polyphenols content. Sensory data were analyzed using IBM SPSS V. 27 and R programming language ver.4.3.1 (R Core Team 2023. _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria) and SensoMineR: Sensory Data Analysis for R package version 1.2. One-way ANOVA analysis was performed on DA sensory scores, and the Tukey post hoc test was carried out to test the differences between samples. The significance level was fixed at p < 0.05. Mean DA intensity values were used to generate a spider plot and represent the pasta sensory profiles.
3. Results and Discussion
The incorporation of artichoke powder (AP) into pasta formulation demonstrated notable impacts on its technological, nutritional, and functional characteristics.
3.1. Technological Properties
Table 1 summarizes the significant the physico-chemical and technological differences between control pasta (P-CTR) and enriched pasta (P-AP) produced.
After drying, the
Fettuccine obtained (
Figure 1) showed water activity (a
w) values of approximately 0.4. P-AP and P-CTR samples exhibited a similar optimal cooking time (OCT), indicating that the addition of AP did not significantly alter cooking performance (
Table 1).
As previously observed [
10], the replacement of 10% of semolina with AP, whose pH values (ca 5.1) are lower than those of re-milled durum wheat semolina (ca 6.2), determined a slight reduction in the pH of P-AP compared to the control samples (P-CTR). The titratable acidity of P-AP pasta, expressed as the volume (mL) of 0.1 M NaOH required to reach a pH value of 8.3, was also significantly higher than that of the control P-CTR, being 4.50 and 0.75, respectively. The raw materials used for pasta production presented different water-holding capacity, specifically it was 1.1 and 6.6 g water/g for re-milled durum wheat semolina and artichoke powder (AP), respectively. These results are consistent with findings reported in the literature [
39]. The different absorption capacities of the ingredients used resulted significantly (
p < 0.05) higher water absorption at OCT in P-AP (+35% compared to P-CTR).
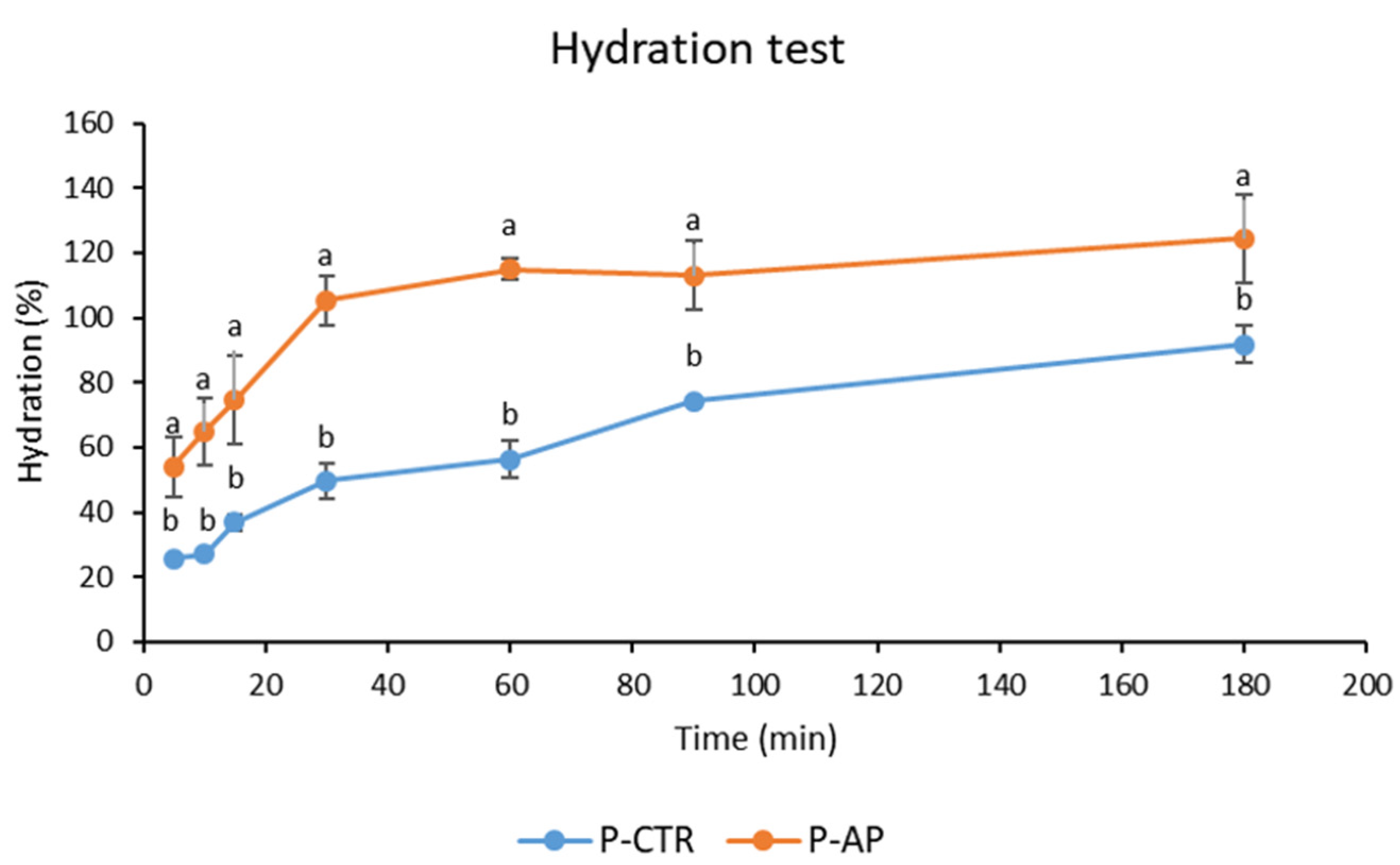
In
Figure 2 are reported the kinetics of water absorption at 25 °C of pasta samples. The substitution of semolina with 10% artichoke powder increased the water absorption of the pasta. Consequently, P-AP pasta showed faster and greater water absorption compared to P-CTR. Thus, the differing water absorption capacities of pasta samples were influenced by their distinct compositions, particularly the presence of hydrophilic macromolecules such as fibers, notably inulin, which is abundant in AP [
10]. Finally, consistent with previous studies [
40], cooking loss values for P-AP were higher (9.4 g/100g pasta) than P-CTR (4.6 g/100g), which could be attributed to the reduction of the gluten network in the P-AP dough.
The results related to colorimetric differences between control pasta (P-CTR) and pasta enriched with artichoke powder (P-AP), analyzed using the CIE color system, showed that the incorporation of artichoke powder had a significant effect (
p < 0.05) on pasta color. These changes are likely due to the natural pigments and polyphenols in artichokes, which influence color through oxidation and heat interactions. In particular, the enriched pasta showed a significant decrease in lightness (L*) compared to P-CTR. This darkening effect was expected, as artichoke powder contains natural pigments such as polyphenols, chlorophylls, and flavonoids, which contribute to a darker appearance. Additionally, the enriched pasta showed a higher a* coordinate, indicating a significant shift toward a more reddish coloration and a decrease in yellowness compared to the control sample [
11]. Similar findings have been reported in previous studies on the incorporation of artichoke powder in baked products [
10,
15].
3.2. Polyphenol Content and Antioxidant Activity
To maximize the benefits of a diet, it is essential to understand the presence of bioactive compounds, their distribution in food, and how food formulation, processing, and cooking can impact the availability of these beneficial components.
Table 2 reports the characterization by HPLC-DAD of free phenolic compounds extracted from control (P-CTR) and enriched (P-AP) pasta samples before and after the cooking process. The results revealed a significant increase (
p < 0.05) in polyphenol content in P-AP, where the most abundant phenolic compounds were chlorogenic acid, 3,5-dicaffeoylquinic acid, and 1,5-dicaffeoylquinic acid, accounting for 70% of the total identified polyphenols in P-AP. Flavonoids, including apigenin-7-O-glucoside, luteolin, and apigenin aglycone, were present at 17%. These results are consistent with our previous study on bread enriched with artichoke powder [
10]. The main effect of processing was a reduction of about 42% in the free identified polyphenols due to cooking losses. After cooking, chlorogenic acid, 3,5-dicaffeoylquinic acid, and 1,5-dicaffeoylquinic acid remained the main identified compounds, followed by flavonoids. The reduction in polyphenol content after cooking has also been reported by other authors in studies on pasta enriched with different vegetable matrices [
18,
41,
42].
Table 2 also reports polyphenol recovery after simulated digestion. Notably, there was an increase of approximately 47% in the total identified polyphenols compared to cooked P-AP pasta. This increase can be attributed to the release of bound polyphenols during digestion. To confirm this finding, the quantification and identification of bound polyphenols in P-AP pasta, compared to its control, were performed and are shown in
Table 3.
The polyphenol content was found to be 19.41 mg/100 g DW, a value comparable to the increase observed after digestion (15 mg/100g DW). The main identified compounds bound to the artichoke powder were caffeic acid, ferulic acid, apigenin, and coumaric acid. In particular, caffeic acid accounted for 66.5% of the total identified compounds, followed by nearly 4 mg/100 g DW of ferulic acid—a typical wheat flour compound also presents in the control (P-CTR)—1.19 mg/100 g DW of apigenin-7-O-glucoside, and 1.1 mg/100 g DW of coumaric acid. In P-AP pasta, the flavonoid apigenin accounted for approximately 6.1%, and the obtained results are comparable to those reported for bread enriched with the same artichoke powder [
10]. After digestion, only 3% of bound polyphenols were recovered in the fraction used to calculate the colon availability index (CAI). This parameter represents the polyphenols not released in the upper GI tract but available in the colon for further microbial metabolism [
33]. The highest CAI was observed for apigenin, including both apigenin-7-O-glucoside and apigenin (44%), followed by coumaric acid (0.42%). It is also interesting to note the presence of luteolin aglycone, albeit in small amounts, which is directly available for colonic metabolism. Several authors have reported that polyphenols reaching the colon undergo biochemical transformations such as hydrolysis, cleavage, reduction, and deglycosylation, leading to the production of low-molecular-weight derivatives. These compounds, through their interaction with gut microbiota, may enhance bioavailability and provide additional health benefits [
5,
43,
44]. Recently, Cheng et al. [
45] explained how bioactive compounds such as polyphenols, despite their low bioavailability, can exert significant health benefits. The authors suggest that polyphenols influence and regulate gut microbiota composition by promoting microbial metabolism, leading to the production of trimethylamine N-oxide (TMAO) and short-chain fatty acids (SCFAs). Additionally, they are metabolized into more bioavailable compounds with enhanced bioactivity [
45].
Table 4 presents the antioxidant capacity of polyphenol extracts from uncooked, cooked, digested, and bound P-AP pasta, compared to the control (P-CTR). Three different methods were used: the ABTS and DPPH assays, which are most suitable for evaluating the radical-scavenging power of artichoke-enriched pasta, and the FRAP assay, which assesses the ability of artichoke polyphenols to counteract heavy metals involved in free radical production. These tests are primarily based on electron transfer to evaluate the capacity of polyphenols to scavenge free radicals through electron donation. In the DPPH assay, hydrogen atom transfer is also involved. For the ABTS and DPPH assays, results are expressed as µmol of Trolox Equivalents/g DW, while for the FRAP assay, results are expressed as µmol of Ferrous Sulfate Equivalent/g DW.
The results showed that enriched pasta (P-AP) had significantly higher (
p < 0.05) antioxidant capacity values across all assays. Specifically, the free polyphenol extracts from uncooked P-AP exhibited the highest antioxidant capacity, both in terms of radical scavenging and the ability to counteract heavy metal reduction (Fe³⁺ to Fe²⁺), compared to the control. Specifically, the highest value was recorded for ABTS (22.26 µmol TE/g DW), followed by DPPH (4.67 µmol TE/g DW) and FRAP (4.28 µmol FSE/g DW). Although the FRAP method has been reported to have a low biological correlation with polyphenol antioxidant activity [
46], combining multiple antioxidant assays helps provide a more comprehensive evaluation. Processing reduced the antioxidant capacity of free polyphenol extracts, but the trend remained similar to that observed in uncooked pasta (
Table 4), with P-AP maintaining a higher antioxidant level than the control. Similar results were reported by la Gatta et al. [
18] for pasta enriched with a low percentage (3%) of lyophilized artichoke waste. Polyphenols in food matrices such as pasta are also present in bound forms. In this study, the antioxidant power of bound polyphenol extracts from P-AP was assessed, considering both cooking and digestive conditions. As observed for free compounds, the highest antioxidant capacity for bound polyphenol extracts was recorded using the ABTS assay (6.99 µmol TE/g DW), followed by FRAP (1.91 µmol FSE/g DW) and DPPH (1.65 µmol TE/g DW). After cooking and digestion, the highest values in P-AP samples were recorded for FRAP (0.393 µmol FSE/g DW), followed by ABTS (0.328 µmol TE/g DW) and DPPH (0.086 µmol TE/g DW).
Moreover, the values obtained from the ABTS assay were higher than those recorded using the DPPH assay, a trend also observed in other studies evaluating the oxidative capacity of plant-based matrices [
15,
47]. As explained by Sadowska-Bartosz & Bartosz [
48], it is recommended to use multiple assays, as each measures different pools of antioxidants in complex food matrices. Additionally, Pearson’s correlation coefficients were used to evaluate the relationship between polyphenol content and antioxidant capacity. A statistically significant (
p < 0.05) positive correlation was found across all assays, with r values of 0.99, 0.95, and 0.90 for DPPH, ABTS, and FRAP, respectively. These results confirm the role of artichoke by-products as rich sources of antioxidants and demonstrate that the polyphenols recovered from each extract contribute to the antioxidant activity of pasta samples.
3.3. Nutritional Properties
Pasta generally contains a high amount of starch and low levels of health-promoting compounds such as dietary fiber, minerals, vitamins, and polyphenols. Several studies have proposed improving the nutritional value of pasta by incorporating functional ingredients, often derived from agri-food by-products, to enhance physiological benefits and reduce disease risks [
11,
49,
50]. The functional ingredients added to pasta mainly include dietary fiber, proteins, omega-3 fatty acids, and polyphenols, whose consumption has been associated with various health benefits, including antidiabetic, antioxidant, anti-inflammatory, and antibiotic effects. As reported in
Table 5, few significant differences (
p > 0.05) were observed in the analyzed nutritional parameters among pasta samples. In fact, both samples showed similar energy values (
p > 0.05), with P-CTR at 1500 KJ (354 Kcal) and P-AP at 1487 KJ (351 Kcal). Notably, P-AP exhibited a significantly higher dietary fiber content (9.8%) compared to P-CTR (2.8%), contributing to its improved health profile.
Moreover, the fiber content in the enriched pasta allows the product to be classified as HIGH FIBER, as it exceeds the 6% dietary fiber threshold required by the nutrition claims listed in the Annex of Regulation (EC) No 1924/2006 [
51], as last amended by Regulation (EU) No 1047/2012 [
52]. It is also noteworthy that both P-AP and its control (P-CTR) are rich in mono- and polyunsaturated fatty acids, likely deriving from semolina, confirming the importance of consuming this cereal-based food. Furthermore, the presence of dietary fiber and phenolic compounds in the enriched pasta contributes to reducing the glycemic index compared to the control, as shown in
Table 5. Specifically, P-AP had a lower predicted glycemic index (pGI) of 56.67 compared to 58.41 for P-CTR, representing a significant benefit in modulating blood glucose levels. The reported pGI is also lower than that reported by la Gatta et al. [
18] for artichoke-enriched pasta, although the authors used a lower enrichment percentage (3%) compared to the present study.
The findings presented here align with previous studies highlighting the role of dietary fiber, particularly artichoke by-products, in moderating glycemic response, as seen in bread enriched with artichoke powder or pasta with chicory inulin [
10,
32].
3.3.1. Protein Solubility and Electrophoretic Migration
The rheological and textural properties of pasta are affected by the constituents of wheat flour. Particularly, proteins may form insoluble aggregates via hydrophobic interactions and disulfide bonds influencing elasticity and adhesiveness [
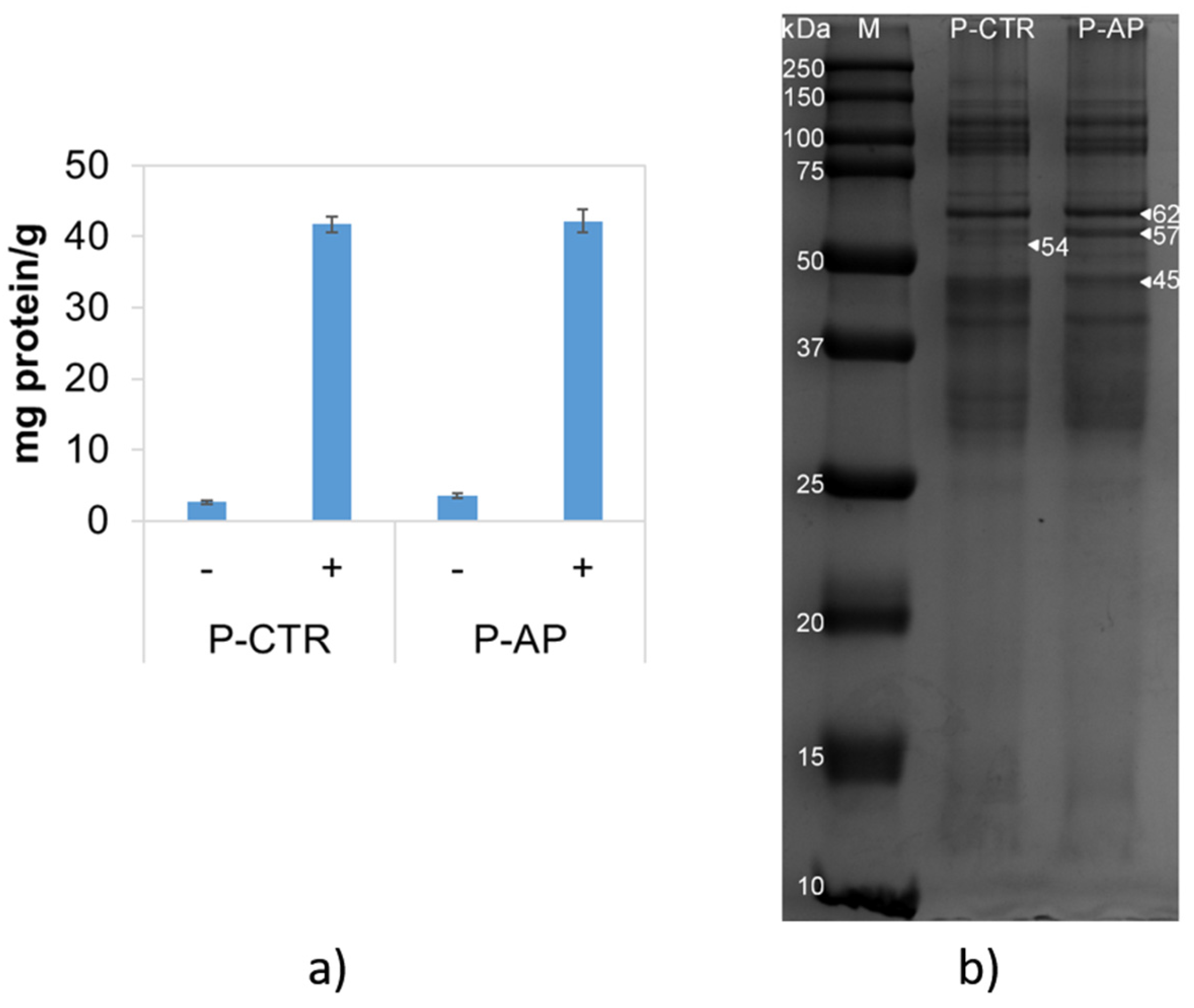
53]. The study of protein solubility provides insights into the amount of proteins able to aggregate. Protein solubility was assessed in both P-CTR and P-AP samples using two extraction buffers (
Figure 3a). No differences were observed between the two samples. The addition of denaturing and reducing agents, such as urea and DTT, resulted in a clear increase in protein solubility -approximately 10-fold- compared to the standard buffer. This suggests the crucial role of hydrophobic interactions and of disulfide bonds in stabilizing insoluble protein aggregates [
53]. SDS-PAGE analysis of proteins extracted in the presence of urea and DTT revealed slight differences in protein composition between the two samples, particularly in the range 40-60 kDa (
Figure 3b). P-AP exhibited a higher abundance of the 62 and 57 kDa bands, while it lacked the 54 kDa band and displayed a lower level of the 45 kDa band. These bands could correspond to glutenin(s), which are closely associated with adhesiveness [
53]. Indeed, the observed differences suggest a possible modification in the protein structure of P-AP pasta that may result from interaction between wheat proteins and bioactive compounds from the artichoke powder.
The analysis of bound polyphenols in enriched pasta was conducted to evaluate the influence of gluten on polyphenol release from the dough, given the strong interactions between phenolics and proteins [
54]. Dietary polyphenols, as chlorogenic acid present in artichoke powder, can bind to gluten, reducing its digestibility and immunogenicity due to the presence of quinic acid in its structure. This interaction may suggest a potential role of polyphenols in modulating gluten digestion and adsorption, which could be relevant for individuals with celiac disease [
55].
3.4. Descriptive Analysis Profile Results
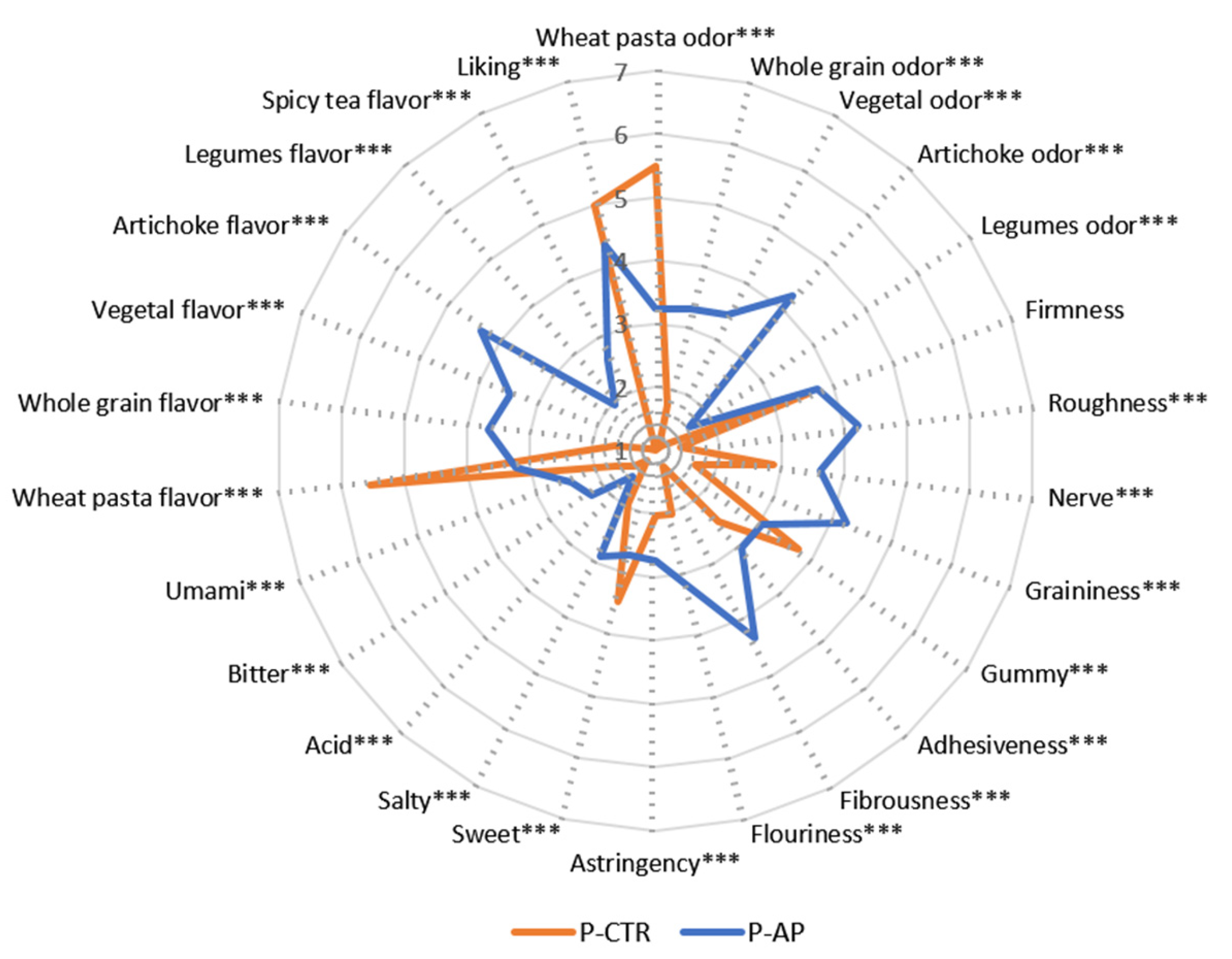
Trained panel sensory data highlighted clear sensory differences between the samples (
Figure 4), with PA enrichment significantly impacting the pasta’s aromatic profile. The control sample (P-CTR) exhibited a higher intensity only for wheat pasta odor and flavor, whereas the enriched sample (P-AP) showed a higher intensity for most of the perceived odors and flavors, including whole grain, vegetal, artichoke, legume, and spicy tea flavors. This is in accordance with la Gatta et al. [
18], who demonstrated the influence of a powder derived from artichoke outer bracts in fresh pasta formulation on its volatile profile. The P-CTR sample was sweeter while exhibiting lower intensity in umami, salty, acidic, bitter, and astringent sensations. The slightly higher astringency and bitterness of P-AP can be attributed to the presence of cynaropicrin in the artichoke [
15,
56].
Further, in agreement with the technological properties, fiber enrichment in the pasta sample containing artichoke powder (P-AP) proved to influence the structural integrity of the pasta during cooking, enhancing texture attributes such as roughness, nerve, graininess, adhesiveness, fibrousness, and flouriness. The control sample (P-CTR) recorded the highest intensity for gumminess. These findings align with the differences observed between the two pasta samples in protein composition.
Judges expressed their highest appreciation for the control sample (5.0), likely due to familiarity, as consumers tend to appreciate products they are more familiar with. The familiarity and cultural significance of traditional foods, such as regular pasta, make it challenging for consumers to accept new or enriched variants [
57]. Despite that, the enriched pasta received an encouraging evaluation (4.3) and may increase in acceptance by providing information about naturality and health benefits.” Recent studies demonstrate that consumers are not willing to swap potential health benefits for hedonic attributes [
58,
59], and those who are more taste-oriented are skeptical about new product formulas [
60]. However, their opinion now is changing and the adequate information on the health benefits, has the potential to increase consumers’ inclination toward and acceptance of novel functional foods [
2].
5. Conclusions
This study highlights the feasibility of enriching pasta with artichoke powder to improve its nutritional profile and functional properties while addressing food waste concerns. The inclusion of 10% AP led to significant improvements in polyphenol content, antioxidant capacity, and dietary fiber levels. The in vitro digestion study confirmed the bioaccessibility of these bioactive compounds, suggesting potential health benefits, particularly in gut health and metabolic regulation.
From a technological perspective, the increased water absorption and cooking loss indicate that fiber enrichment alters pasta structure, which may require optimization to maintain optimal texture. Sensory analysis revealed that P-AP had distinct vegetal and bitter notes, which may influence consumer acceptance. However, the growing consumer demand for functional foods could enhance the market potential of enriched pasta. Descriptive analysis has allowed us to gain an in-depth understanding of the sensory profile of both enriched and non-enriched pasta, helping to predict consumer appreciation.
Overall, the results demonstrate that artichoke bracts can be effectively upcycled into a value-added food ingredient, supporting sustainable food production. Future research should explore formulation adjustments and consumer acceptance strategies to maximize the appeal of enriched pasta while retaining its health benefits.
Author Contributions
Conceptualization, A.R.B., P.D.B and A.C.; methodology, V.L. and P.D.B; software, A.R.B. and P.D.B.; validation, A.R.B., P.D.B, S.R. and V.L.; formal analysis, A.R.B., P.D.B, V.L. and S.R.; investigation, A.R.B., P.D.B., S.R., M.C., R.T., and V.L.; resources, P.D.B. and A.C.; data curation, A.R.B., P.D.B, V.L., S.R. and A.C.; writing—original draft preparation, A.R.B., P.D.B, S.P., M.C. and A.C.; writing—review and editing, A.R.B., P.D.B, S.P. and A.C..; visualization, A.R.B.; supervision, A.R.B., P.D.B and A.C.; project administration, A.C.; funding acquisition, P.D.B. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project PON ARS01_00783 ALIFUN—“Development of functional foods for the traditional Italian food products innovation”; Project NutrAge: “Nutrition & Active aging” CNR project (FOE-2021, DAB.AD005.225), and “IDENTITA’rete Integrated meDiterranean network for the observation and development of personalized Nutrition paths against malnutrition”; Trajectory 5 project “Nutraceuticals, nutrigemonics and functional foods” of the Health Operational Plan—action line 5.1; ON FOODS “Research and Innovation network on food and nutrition Sustainability, Safety and Security—Working ON Food.” Piano Nazionale di Ripresa e Resilienza (PNNR)—Missione 4 Componente 2 Investimento 1.3—Avviso n. 341 del 15/03/2022 del Ministero dell’Università e del-la Ricerca—“Partenariati estesi alle università, ai centri di ricerca, alle aziende per il finanziamento di progetti di ricerca di base”– NextGenerationEU.- Codice progetto PE00000003.
Institutional Review Board Statement
All procedures for sensory evaluation were carried out in accordance with relevant laws, institutional guidelines, European Code of Conduct for Research Integrity and were approved by Italian National Research Council Ethical Commission, November, 18, 2024, as NUTR-AGE Project activity.
Informed Consent Statement
The study involved only professional sensory judges, all providing their informed consent.
Acknowledgments
The authors want to thank Maria Clementina Spagnuolo for her precious administrative and technical support in the management of the Projects.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AP |
Artichoke powder |
| AUC |
Area under the curve |
| aw
|
Water activity |
| DW |
Dry weight |
| FSE |
Ferrous sulfate equivalent |
| HI |
Hydrolysis index |
| OCT |
Optimal Cooking Time |
| P-AP |
Enriched pasta with artichoke powder |
| P-CTR |
Control Pasta |
| pGI |
predicted Glycaemic Index |
| TE |
Trolox equivalent |
| TTA |
Total titratable acidity |
References
- Alongi, M. , & Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V. , & Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D. ,... & Zhang, C. Effects of dietary fiber on human health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Tarantini, A.; Bruno, A.; Logrieco, A.F.; Gallo, A.; Mita, G.; Valerio, F.; Bleve, G.; Cardinali, A. Functional foods in Mediterranean diet: exploring the functional features of vegetable case-studies obtained also by biotechnological approaches. Aging Clin. Exp. Res. 2024, 36, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, P.; Quizhpe, J.; Rosell, M.d.l.Á.; Peñalver, R.; Nieto, G. Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review. Appl. Sci. 2024, 14, 4940. [Google Scholar] [CrossRef]
- Olas, B. An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements. Nutrients 2024, 16, 599. [Google Scholar] [CrossRef]
- Colombo, R.; Moretto, G.; Pellicorio, V.; Papetti, A. Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties. Foods 2024, 13, 1427. [Google Scholar] [CrossRef]
- Zayed, A. & Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT 2020, 132, 109883. [Google Scholar] [CrossRef]
- Bavaro, A.R.; De Bellis, P.; Montemurro, M.; D’Antuono, I.; Linsalata, V. & Cardinali, A. Characterization and functional application of artichoke bracts: enrichment of bread with health promoting compounds. LWT 2025, 215, 117256. [Google Scholar] [CrossRef]
- Carpentieri, S.; Augimeri, G.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, M.; Damergi, C.; Marzouk, C.B.; Blecker, C. & Bouzouita, N Effect of artichoke (Cynara scolymus L.) by-product on the quality and total phenol content of bread. Mediterr. J. Chem. 2016, 5, 548–553. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R. & Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from Artichoke Processing Industry: Reuse in Bread-Making and Evaluation of the Physico-Chemical Characteristics of the Final Product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef] [PubMed]
- Cannas, M.; Conte, P.; Urgeghe, P.P.; Piga, A.; Alañón, M.E. & Del Caro, A. Artichoke by-products: promising ingredients for breadstick fortification. LWT 2024, 202, 116307. [Google Scholar] [CrossRef]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F. & Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. 2017, 52, 2078–2087. [Google Scholar] [CrossRef]
- Amoriello, T.; Mellara, F.; Ruggeri, S.; Ciorba, R.; Ceccarelli, D.; Ciccoritti, R. Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project. Sustainability 2022, 14, 14778. [Google Scholar] [CrossRef]
- la Gatta, B.; Rutigliano, M.; Liberatore, M.T.; Dilucia, F.; Spadaccino, G.; Quinto, M. & Di Luccia, A. Preservation of bioactive compounds occurring in fresh pasta fortified with artichoke bracts and tomato powders obtained with a novel pre-treatment. LWT 2023, 187, 115298. [Google Scholar] [CrossRef]
- Italian Republic. Decreto del Presidente della Repubblica (DPR) 9 febbraio 2001, n. 187. Regolamento per la revisione della normativa sulla produzione e commercializzazione di sfarinati e paste alimentari, a norma dell’articolo 50 della legge 22 febbraio 1994, n. 146; Ministry of Agricultural, Food and Forestry Policies: Rome, Italy, 2001. [Google Scholar]
- AACC International. Approved Methods of Analysis. In Method 02-31.01. Titratable Acidity—Basic Method; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Schettino, R.; Verni, M.; Acin-Albiac, M.; Vincentini, O.; Krona, A.; Knaapila, A.; Cagno, R.D.; Gobbetti, M.; Rizzello, C.G.; Coda, R. Bioprocessed Brewers’ Spent Grain Improves Nutritional and Antioxidant Properties of Pasta. Antioxidants 2021, 10, 742. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis. 2010. Available online: http://methods.aaccnet.org/ (accessed on 18 March 2025).
- D’Imperio, M.; Durante, M.; Gonnella, M.; Renna, M.; Montesano, F.F.; Parente, A.; Mita, G.; Serio, F. Enhancing the Nutritional Value of Portulaca Oleracea L. by Using Soilless Agronomic Biofortification with Zinc. Food Res. Int. 2022, 155, 111057. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Rockville, MA, USA, 2000. [Google Scholar]
- Canale, M.; Sanfilippo, R.; Strano, M.C.; Bavaro, A.R.; Amenta, M.; Bizzini, M.; Allegra, M.; Blangiforti, S.; Spina, A. Technological Properties of Inulin-Enriched Doughs and Breads, Influence on Short-Term Storage and Glycemic Response. Foods 2024, 13, 2711. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Bonomi, F.; D’Egidio, M.G.; Iametti, S.; Marengo, M.; Marti, A.; Pagani, M.A.; Ragg, E.M. Structure–Quality Relationship in Commercial Pasta: A Molecular Glimpse. Food Chem. 2012, 135, 348–355. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Di Biase, M.; Linsalata, V.; D’Antuono, I.; Di Stefano, V.; Lonigro, S.L.; Garbetta, A.; Valerio, F.; Melilli, M.G.; Cardinali, A. Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation. Foods 2024, 13, 1815. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Díez-Riquelme, V.; Viuda-Martos, M.; Ángel Pérez-Álvarez, J.; Sánchez-Zapata, E.; Fernández-López, J. Effect of the Food Matrix on the (Poly)Phenol Stability of Different Plant-Based Meat Products and Their Main Ingredients after in Vitro Gastrointestinal Digestion. Food Funct. 2023, 14, 10796–10813. [Google Scholar] [CrossRef]
-
ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Geneva, Switzerland, 2023.
- Périnel, E.; Pagès, J. Optimal Nested Cross-over Designs in Sensory Analysis. Food Qual. Prefer. 2004, 15, 439–446. [Google Scholar] [CrossRef]
- Societa Italiana di Scienze Sensoriali. Atlante Sensoriale dei Prodotti Alimentari; Sinesio, F.M.E., Spinelli, S., Eds.; Tecniche Nuove: Milan, Italy, 2012. [Google Scholar]
- Galetti, J.A.; Calder,Beth L. ; and Skonberg, D.I. Mechanical Separation of Green Crab (Carcinus Maenas) Meat and Consumer Acceptability of a Value-Added Food Product. J. Aquat. Food Prod. Technol. 2017, 26, 172–180. [Google Scholar] [CrossRef]
- Dadalı, C. Artichoke Bracts as Fat and Wheat Flour Replacer in Cake: Optimization of Reduced Fat and Reduced Wheat Flour Cake Formulation. J. Food Meas. Charact. 2023, 17, 98–107. [Google Scholar] [CrossRef]
- Calasso, M.; Lisi, A.; Ressa, A.; Caponio, G.R.; Difonzo, G.; Minervini, F.; Gargano, M.L.; Vacca, M.; De Angelis, M. Incorporating Fresh Durum Wheat Semolina Pasta Fortified with Cardoncello (Pleurotus Eryngii) Mushroom Powder as a Mediterranean Diet Staple. Antioxidants 2025, 14, 284. [Google Scholar] [CrossRef]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Liberatore, M.T.; Dilucia, F.; Rutigliano, M.; Viscecchia, R.; Spano, G.; Capozzi, V.; Bimbo, F.; Di Luccia, A.; la Gatta, B. Polyphenolic Characterization, Nutritional and Microbiological Assessment of Newly Formulated Semolina Fresh Pasta Fortified with Grape Pomace. Food Chem. 2025, 463, 141531. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022, 48, 100941. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; et al. Interactions between gut microbiota and polyphenols: a mechanistic and metabolomic review. Phytomedicine 2023, 154979. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S. . & Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Padalino, L.; D’Antuono, I.; Durante, M.; Conte, A.; Cardinali, A.; Linsalata, V.; Mita, G.; Logrieco, A.; Del Nobile, M. Use of Olive Oil Industrial By-Product for Pasta Enrichment. Antioxidants 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal Foods Fortified with By-Products from the Olive Oil Industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- European Commission. Commission Regulation (EU) No 1047/2012 of 8 November 2012 amending Regulation (EC) No 1924/2006 with regard to the list of nutrition claims Text with EEA relevance. Off. J. Eur. Union 2012, 310, 36–37. [Google Scholar]
- Zang, P.; Gao, Y.; Chen, P.; Lv, C.; Zhao, G. Recent Advances in the Study of Wheat Protein and Other Food Components Affecting the Gluten Network and the Properties of Noodles. Foods 2022, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Krekora, M.; Nawrocka, A. The Influence of Selected Polyphenols on the Gluten Structure—A Study on Gluten Dough with Application of FT-IR and FT-Raman Spectroscopy. J. Cereal Sci. 2022, 108, 103570. [Google Scholar] [CrossRef]
- Ribeiro, M.; Sousa, T.d.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Review of Structural Features and Binding Capacity of Polyphenols to Gluten Proteins and Peptides In Vitro: Relevance to Celiac Disease. Antioxidants 2020, 9, 463. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A Comprehensive Research Review and Therapeutic Potential as an Anti- Hepatitis C Virus Agent. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Bolha, A.; Blaznik, U.; Korošec, M. Influence of Intrinsic and Extrinsic Food Attributes on Consumers’ Acceptance of Reformulated Food Products: A Systematic Review. Slov. J. Public Health 2020, 60, 72. [Google Scholar] [CrossRef]
- Ballco, P.; Gracia, A. Tackling Nutritional and Health Claims to Disentangle Their Effects on Consumer Food Choices and Behaviour: A Systematic Review. Food Qual. Prefer. 2022, 101, 104634. [Google Scholar] [CrossRef]
- Crucean, D.; Debucquet, G.; Rannou, C.; le-Bail, A. Vitamin B4 as a salt substitute in bread: A challenging and successful new strategy. Sensory perception and acceptability by French consumers. Appetite 2019, 134, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Laureati, M.; De Boni, A.; Saba, A.; Lamy, E.; Minervini, F.; Delgado, A.M.; Sinesio, F. Determinants of Consumers’ Acceptance and Adoption of Novel Food in View of More Resilient and Sustainable Food Systems in the EU: A Systematic Literature Review. Foods 2024, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).