1. Introduction
Pine wilt disease (hereafter abbreviated as PWD), responsible for significant damage to pine forests throughout Japan except for Hokkaido, peaked around 1980, resulting in an annual loss of over 2 million cubic meters of pine trees nationwide. Following this peak, the disease appeared to gradually subside. However, this apparent decline can be attributed to the significant reduction in pine forests susceptible to the disease. Presently, pine wilt disease is spreading to the last remaining forests of Japanese red pine,
Pinus densiflora Sieb. et Zucc. in cold high-altitude areas and high-latitude regions in Japan [
1]. Additionally, due to the increased global movement of materials, this disease, which has devastated Japanese forests, spread to other East Asian countries [
2,
3] and reached Portugal in Europe by the end of the 20th century [
4], later spreading to Spain [
5] and Armenia [
6].
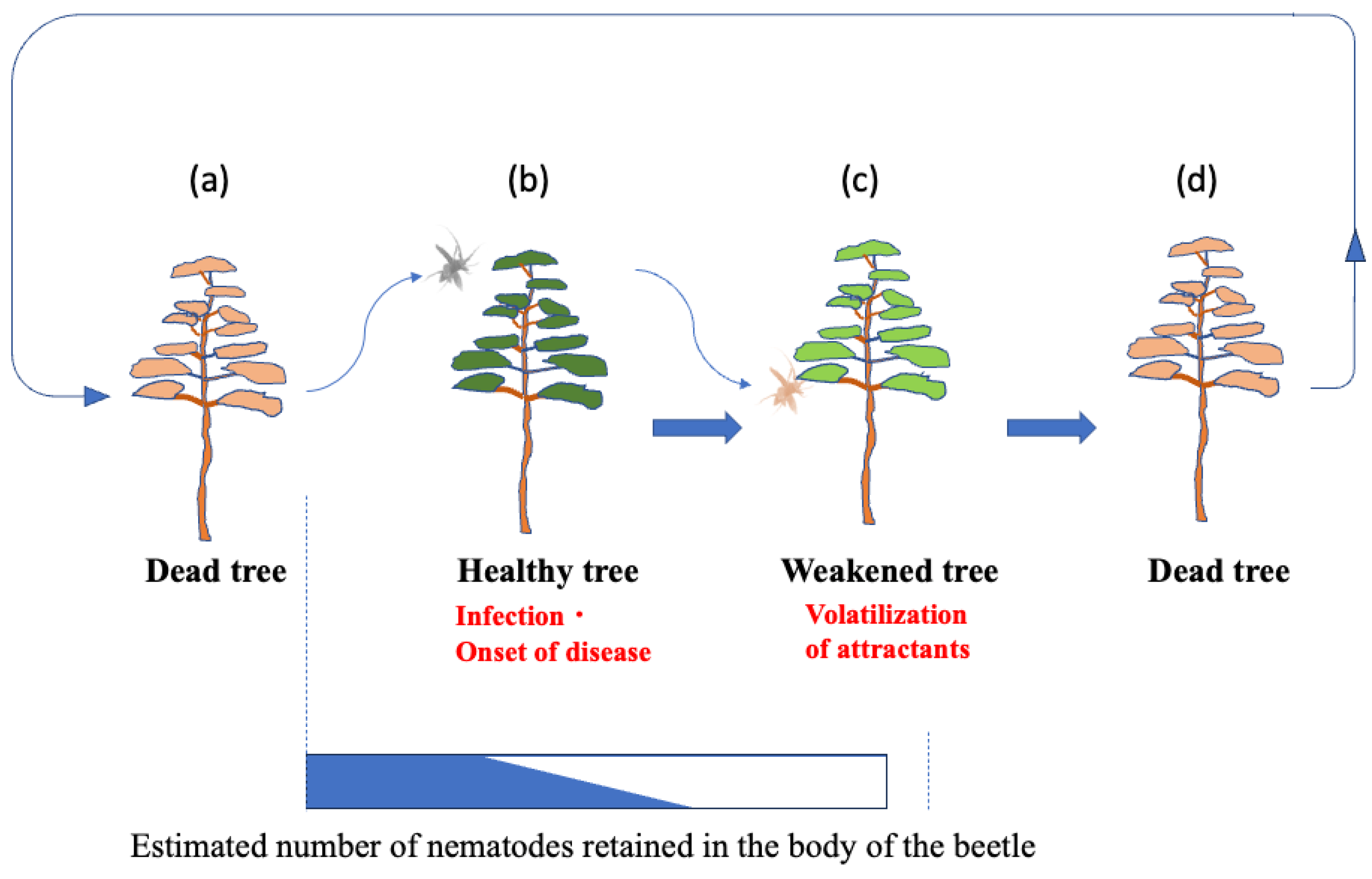
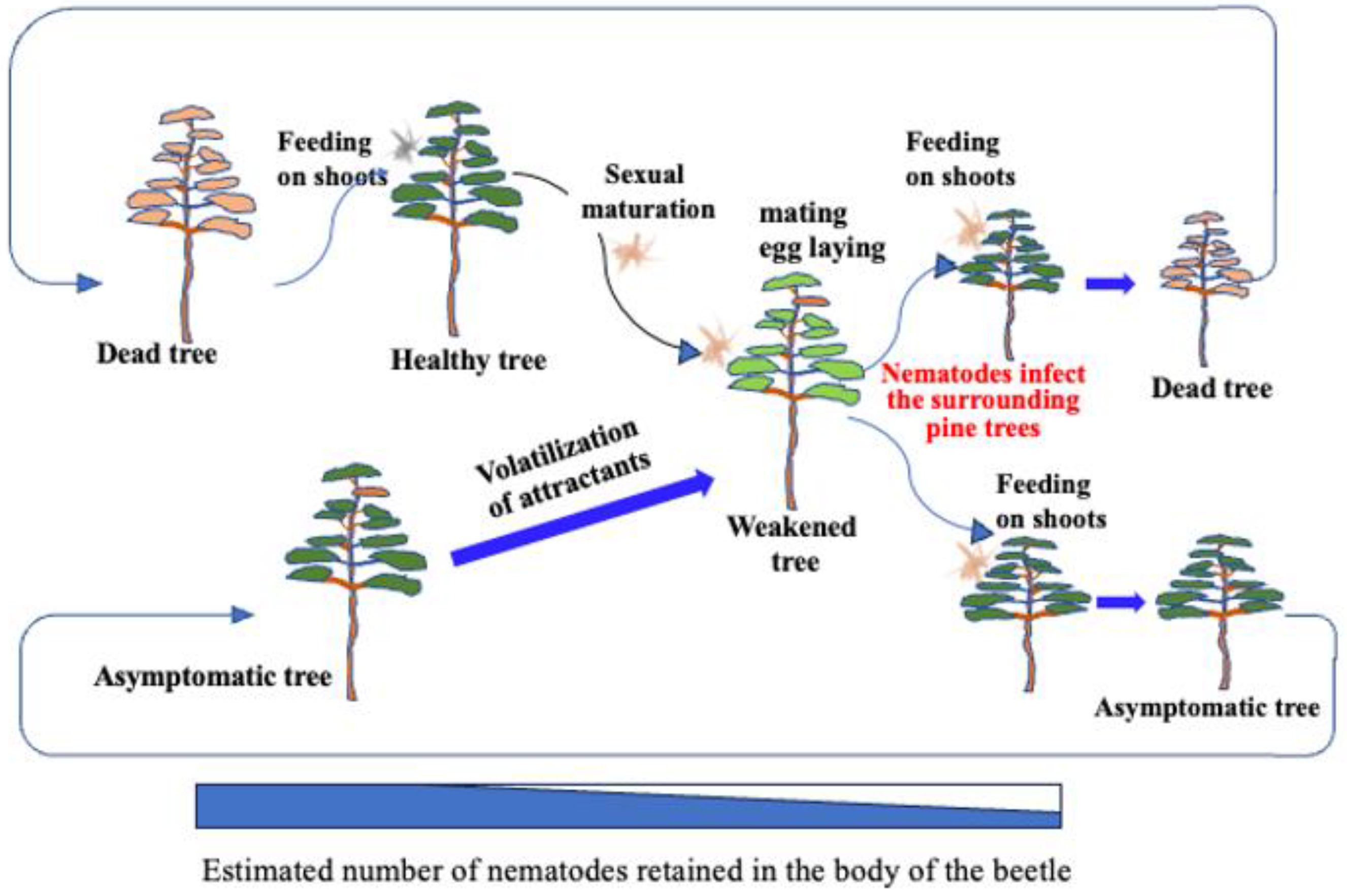
The established infection cycle of pine wilt disease (PWD) has been understood as follows: Pine sawyer beetles emerge from pine trees that died due to PWD the previous year with immature reproductive organs [
7]. To mature their reproductive organs, beetles fly to healthy pines and vigorously feed on the bark of young branches creating feeding scars. This period of sexual immaturity lasts about 5-15 days [
8] for males and 16-30 days [
9] for females, during which the feeding activity creates scars on the bark. Nematodes retained within the beetle's body enter the pine tissues through these feeding scars, initiating infection (primary infection). As the host pine becomes diseased, it emits volatile compounds such as ethanol and terpenes, attracting sexually mature beetles that fly to weakened pines for mating and oviposition. Beetles overwinter as matured larvae within the dead pine wood, pupate the following spring, and emerge as adults carrying numerous nematodes from the dead wood. These adults then fly to healthy pines, perpetuating the infection cycle (
Figure 1).
Based on this understanding of the infection cycle, two primary methods have been utilized to control this forest epidemic in Japan. One strategy entails the preventive application of insecticides on host pine trees to deter the vector, Japanese pine sawyer beetle,
Monochamus alternatus Hope (Japanese pine sawyer beetle, hereafter referred to as
Monochamus beetle, or beetle), from feeding on young branches. This prevents the pathogen, the pine wood nematode,
Bursaphelenchus xylophilus (Steiner & Bu ̈hrer) Nickle (hereafter abbreviated as PWN) harbored within the beetle's body from invading the tree tissues via the beetle’s feeding scars. The other method involves the eradication of dead trees, where both the nematodes and beetle larvae breed, through felling and removing by burning or fumigation. Despite the understanding that thorough implementation of these methods should prevent damage, in reality, even after felling and removing all dead trees in the forest in early spring, a few wilting trees due to PWD occur surrounding the stumps of removed dead trees in early summer [
10,
11]. To understand the reasons behind these puzzling patterns of damage occurrence, one of the authors conducted a thorough investigation over four years focusing on all 72, 40-year-old Korean pine,
Pinus koraiensis Sieb. et Zucc trees planted in an arboretum [
12]. This investigation involved tracking the locations of dead trees while continuously monitoring resin secretion [
13], a physiological indicator of pine health, in all trees starting from the year when two dead trees first appeared due to pine wilt. It is important to note that the damage in this forest continued to increase, even though all dead wood had been completely removed by early summer, prior to the appearance of the beetles. This removal was intended to prevent damage from occurring and spreading further throughout the forest. As a key to solving this mystery, we incorporated the concept of “
asympto-matic infected trees.” These are trees that become infected with nematodes in a given year and experience a decline in resin exudation but survive without visible symptoms until the following year or later. As a result, they are overlooked during the eradication of dead trees by early summer, and later develop visible symptoms, contributing to disease progression [
12].
To reassess existing theories on the PWD infection cycle and clarify the role of asympto- matic carrier trees in disease spread, it is essential to quantify the number of nematodes carried by beetles arriving at these trees for reproduction. Additionally, a detailed investigation into the relationship between their feeding behavior during reproduction and nematode transmission to healthy trees is required. Therefore, in this study, we initially observed the behavior of beetles towards both asymptomatic carrier trees and healthy trees nearby, and assessed nematode infection in the healthy trees through cage release experiments.
Furthermore, to elucidate the conditions under which asymptomatic carrier trees arise as breeding targets for beetles, we inoculated 3- or 5-year-old Japanese black pines, Pinus thunbergii Parl, in nurseries, as well as potted 3-year-old Japanese black pines, with a small number of nematodes in early and late summer. We then observed the progression of disease until the following summer. Additionally, we regularly harvested inoculated seedlings and isolated nematodes from various parts of the seedlings to determine their distribution within the host plant and its association with their physiological and external symptoms.
2. Materials and Methods
The following in-cage experiments and the field inoculation experiments were implemented at Suzuka-city, Mie Prefecture (34°91’N, 136°52’ E.)
1. In-cage insect release experiments: Investigation of beetle reproductive behavior and transition of retained nematodes to surrounding healthy trees
As some of the results of the in-cage experiments have already been published in other Japanese journal [
14], a summary of the methods and results is given here to aid understanding of the overall purpose of this experiment.
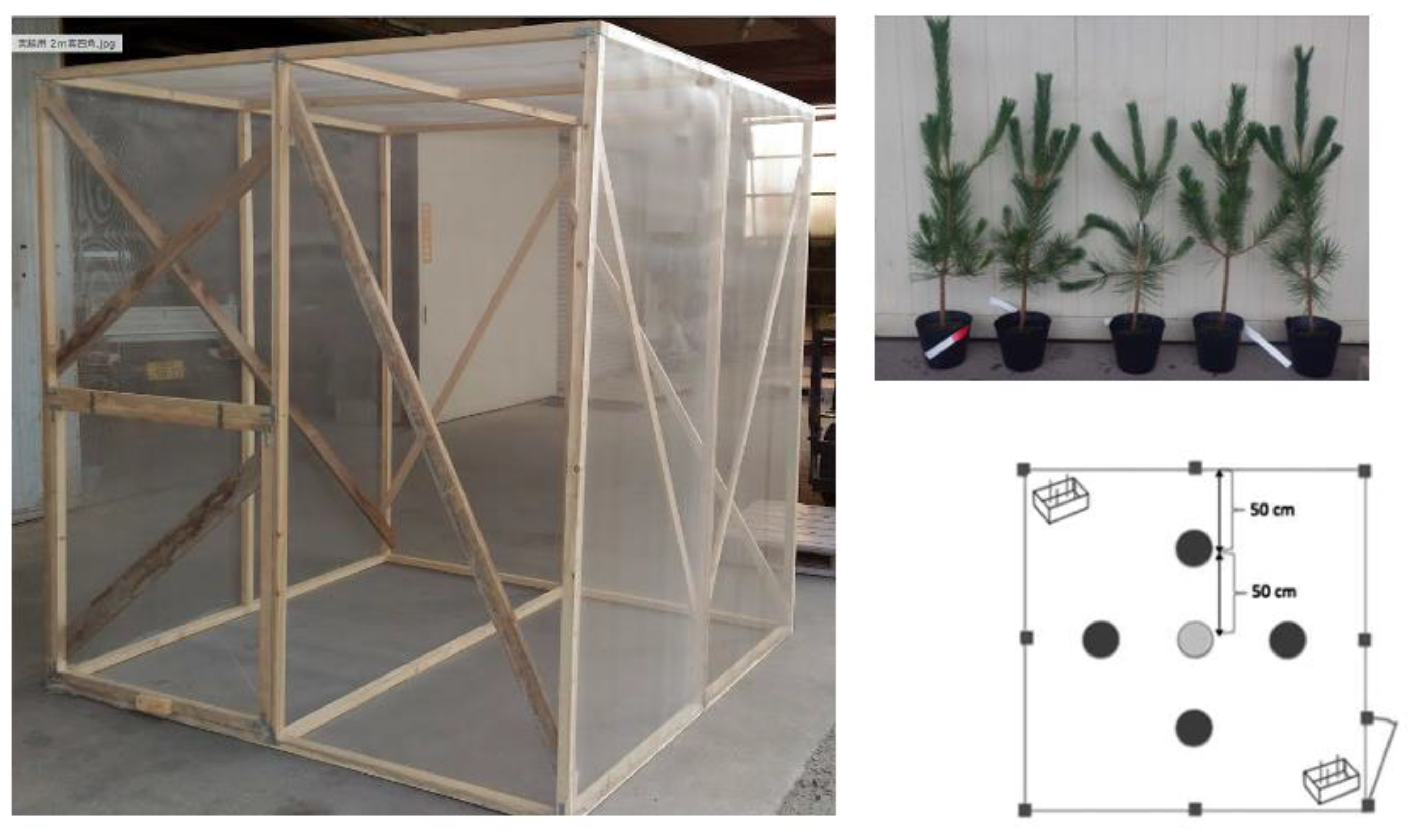
The experiments were conducted in a mesh cage measuring 2 meters in height, width, and depth. About a month before the cage release experiments, one externally healthy three-year-old black pine seedling was designated as the oviposition target tree —essentially an artificially created asymptomatic carrier tree. To induce disease onset, indicated by the cessation of resin exudation, the seedling was pre-inoculated with 5,000 pathogenic nematodes. This seedling was placed at the center of the cage, surrounded by one healthy black pine seedling in each of the four directions. Positioned in two corners of the cage were polystyrene foam boxes (dimensions: height x width x depth = 15 x 18 x 20 centimeters). Within each box, four wooden sticks, each 20 cm in length, were erected to facilitate natural takeoff for the beetles (
Figure 2).
Pine logs that had been killed due to pine wilt in Ueno Forest Park, 43 km away, were transported and stocked in a 1.5 x 2 x 2 m (H x W x D) netted cage. Beetles emerging from the logs were collected daily, and their size, sex and the date of collection were recorded and each beetle was kept in a plastic container (1- 4 L) with a small hole for ventilation until each in-cage release experiment. A short Japanese black pine twig was used as food and replaced with fresh one every two to three days.
Three unmated females were released into one box, and three unmated males were released into the other. After 72 hours, the number of feeding scars on each of the five black pine seedlings and the number of nematodes isolated from each feeding scar were investigated. Following previous reports [
8,
9], mature and immature beetles were discriminated based on the number of days after emergence. The experiments were repeated nine times for sexually immature beetles (males aged 5-10 days, females aged 5-10 days) and, sexually mature beetles (males aged 14-31 days, females aged 19-30 days), respectively. Furthermore, following each release test, each tested beetle was individually dissected, and nematodes remaining in the beetle body were isolated using the Baermann funnel method and counted the number.
2. Examination of the conditions under which asymptomatic carrier trees occur: Experimental inoculation of pine seedlings planted in the field with a small number of nematodes
Some trials of the cage experiments showed that the number of nematodes invading through a single Monochamus beetle feeding scar was generally low. Therefore, in the second series of inoculation experiments, we set the inoculation dose to a lower level of 50 nematodes per site (50 ± 8.3). Two experiments were conducted:
The first experiment aimed to determine how host pines develop disease when infected with a small number of nematodes in different seasons and to examine the distribution and quantity of nematodes in trees that survive as asymptomatic carrier trees.
The second experiment investigated the relationship between the number of beetle feeding scars and the mortality rate of pine saplings.
Due to operational constraints, designated control plots were not established. However, throughout the experiment, we continuously monitored dozens of untreated healthy seedlings growing in the same nursery to compare their symptoms with those of the nematode-inoculated seedlings.
2.1. Differences in Disease Development and Nematode Distribution in the Host Tree. According to Infection Time
Previous inoculation experiments have experienced that late inoculation often result in delayed onset of symptoms until the following year [
15]. Additionally, it is known that physiological changes in pines seem to occur in late stages of the pine wilt season (late August to September), affecting the infection of pathogenic nematodes [
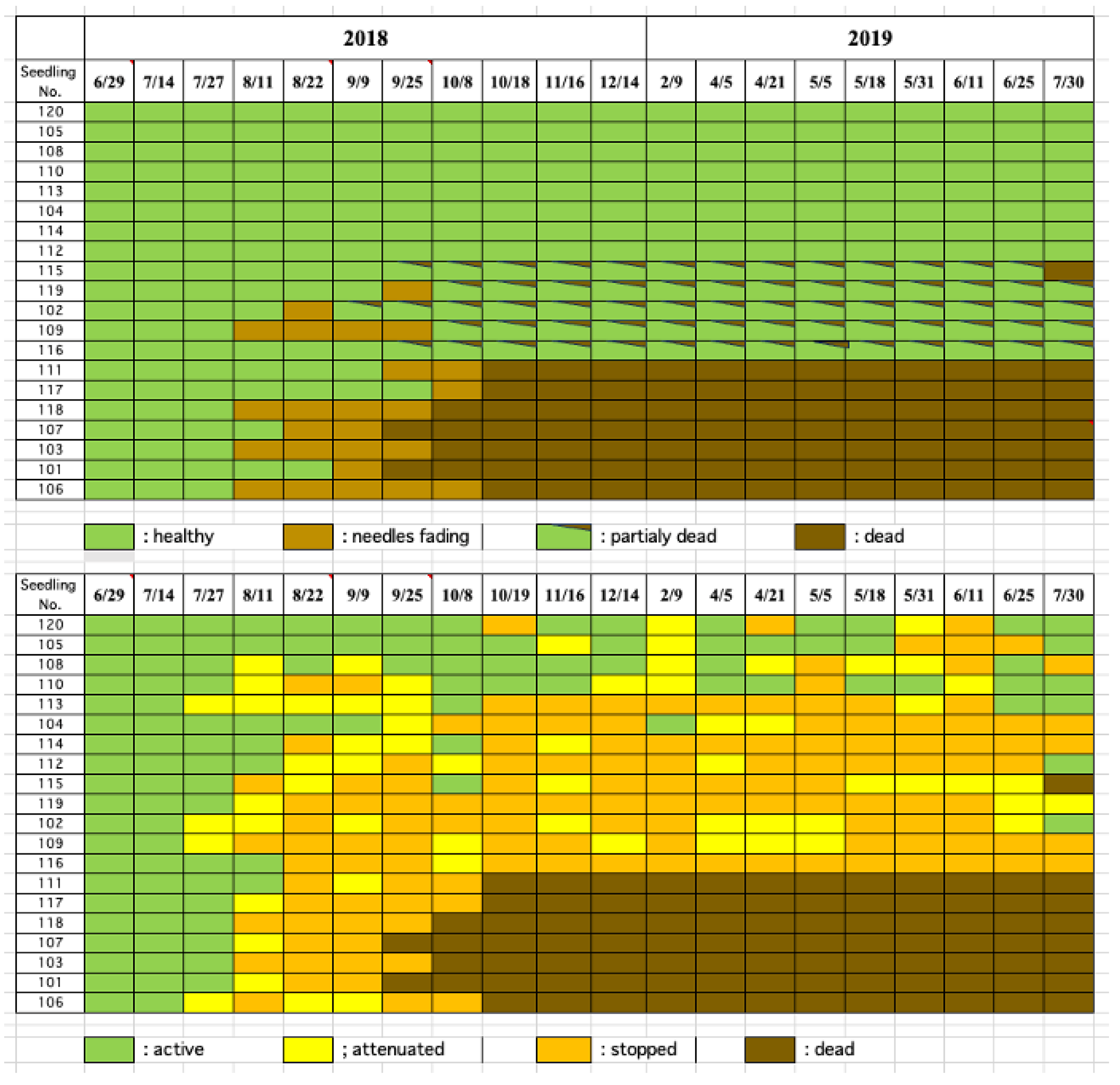
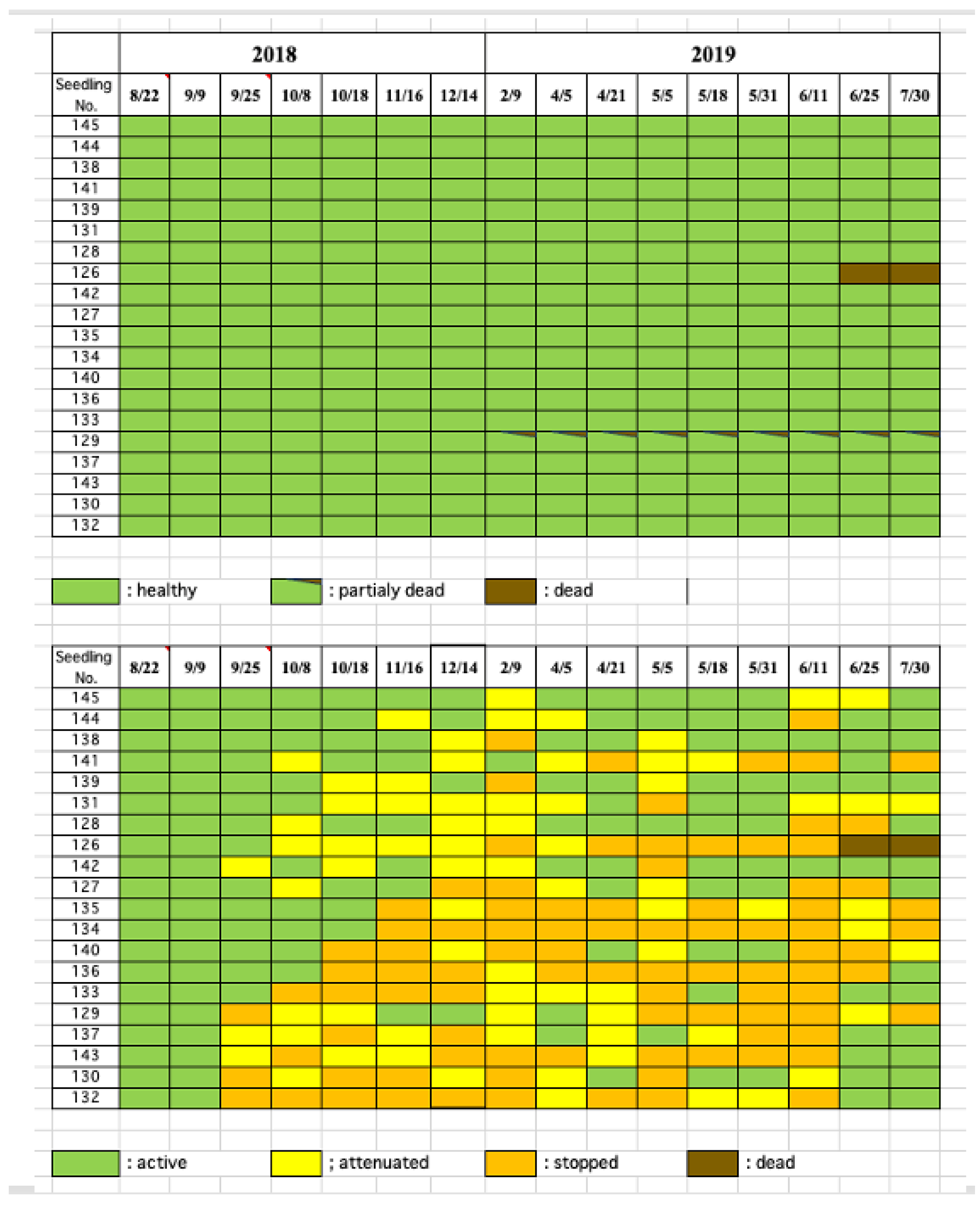
16]. To confirm this, 50 nematodes were inoculated on the one-year-old branches of 80 three-year-old black pine seedlings in the early pine wilt season (1 July 2018) and on 65 seedlings in the late pine wilt season (25 August 2018). Subsequently, 20 seedlings designated from each inoculation timing group were separated from others for periodical observation for disease progression, recording resin exudation status as physiological symptoms and changes in external symptoms through photography from 29 June just prior to nematode inoculation to 30 July of the following year for the early inoculation group, and similarly from 22 August to 30 July of the following year for the late inoculation group.
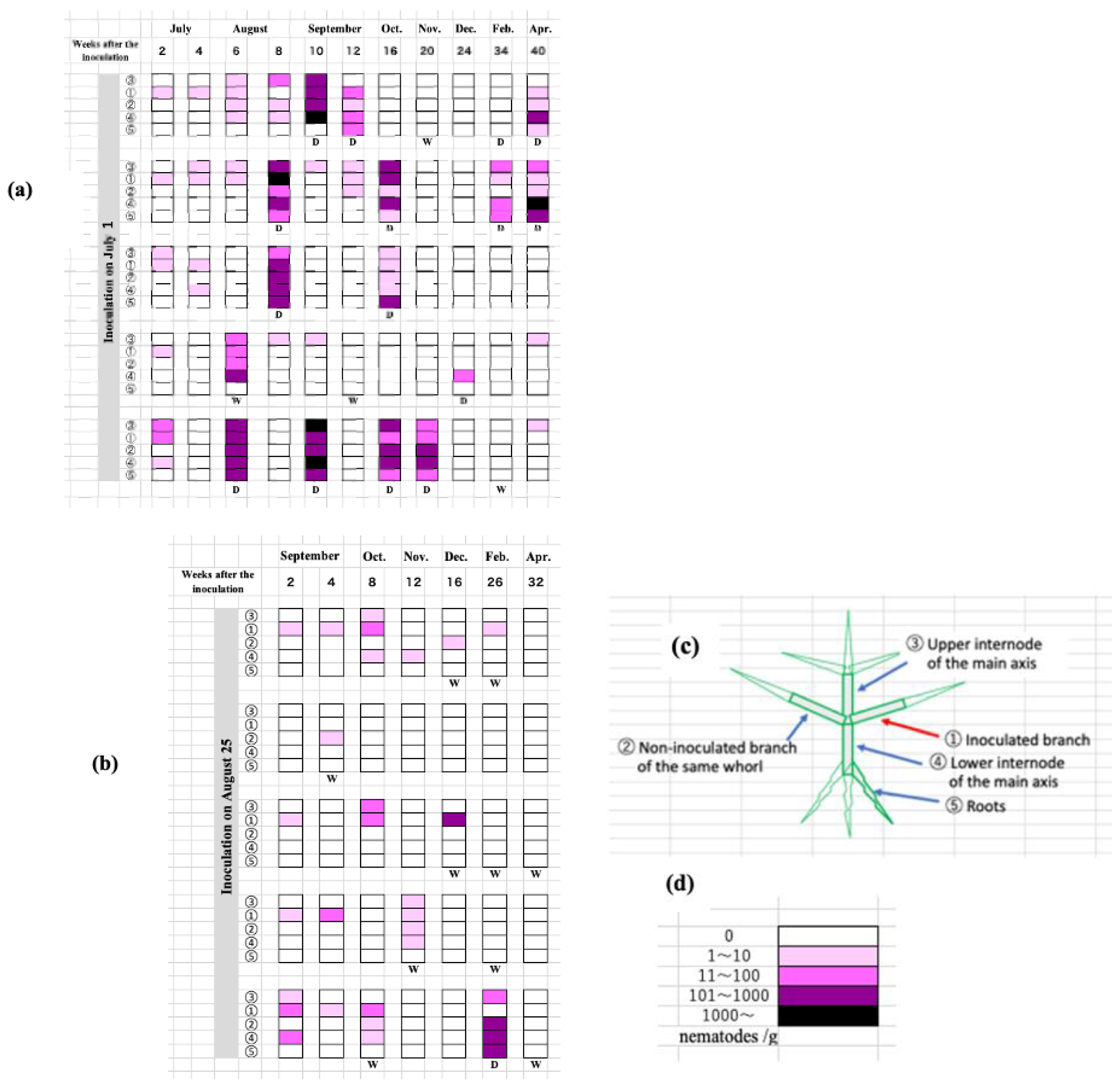
Furthermore, to determine in which part, for how long, and how many nematodes survived, a total of 11 times after inoculation on 1 July, 2018 until April of the following year for the early inoculation group, and 7 times after inoculation on 25 August, 2018 until April of the following year for the late inoculation group, five randomly selected inoculated seedlings from each inoculation timing group were collected periodically, using random number table. From each of the collected seedlings, five tissue samples were taken: (1) inoculation site, (2) another branch of the same whorl of the inoculated branch, (3) the upper part and (4) lower part of the main stem, and (5) the roots. Each sample was shredded and soaked in a small Baermann apparatus for 24 hours, and the isolated nematodes were counted under a stereomicroscope. After nematode isolation, each pine tissue was air-dried, weighed, and the number of nematodes was evaluated per gram of dry weight.
2.2. Relationship Between the Number of Beetle Feeding Scars and Mortality Rate of Pine Saplings: Effect of Multipoint Infection)
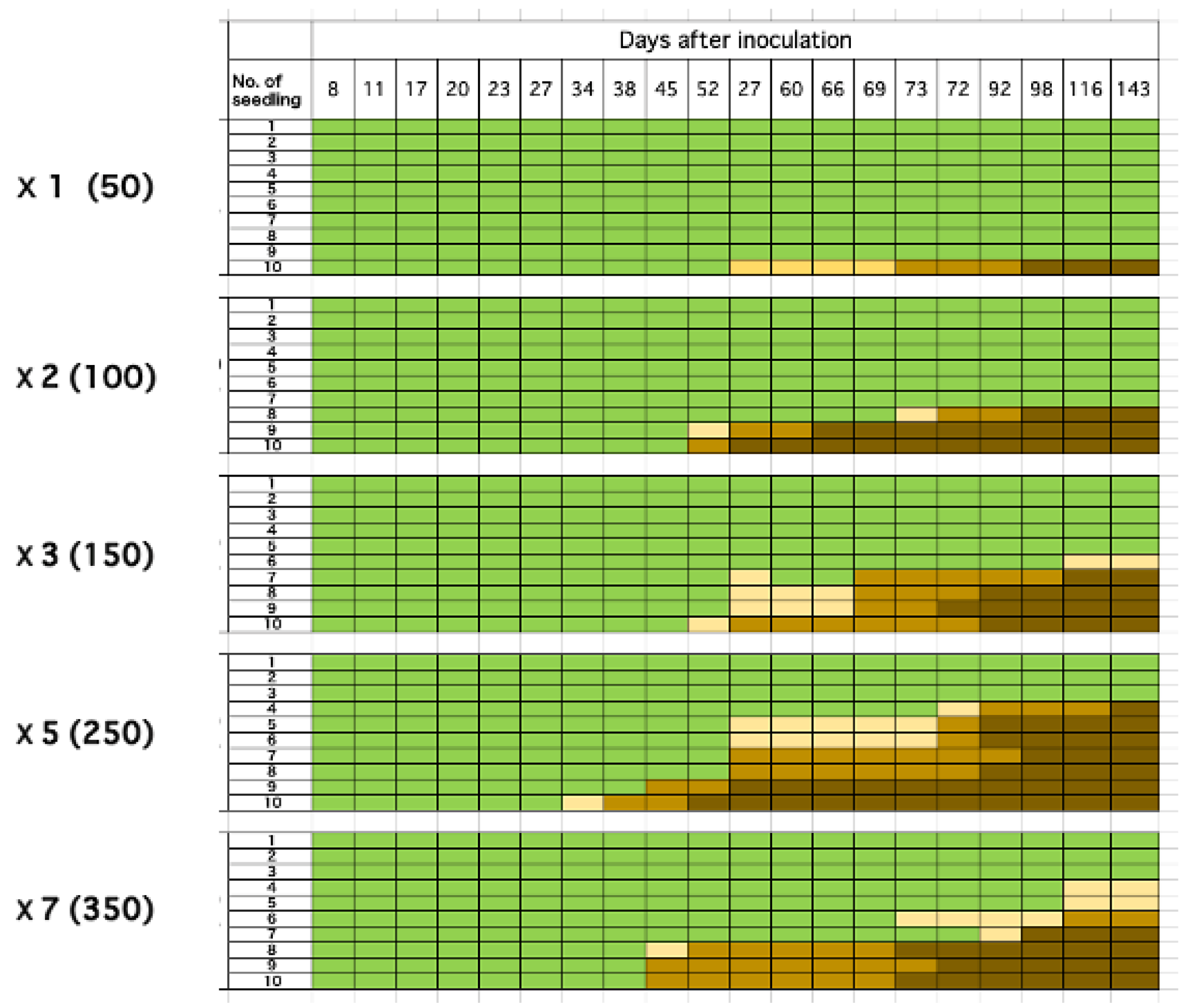
When beetles feed on healthy pines, they create feeding scars in multiple places while. walking on branches. In the cage release experiments, it was observed that, on average, each beetle left about four feeding marks on the seedlings during a three-day observation period. Even with a low number of nematodes per site, an increase in the number of feeding sites and total nematodes infecting a single seedling may increase damage to the host pine and raise the probability of death. To investigate this, 50 five-year old black pine saplings planted in the field were inoculated with 50 nematodes per site, varying the number of inoculated sites (number of nematodes per sapling) from 1 site (50), 2 sites (100), 3 sites (150), 5 sites (250) to 7 sites (350), on 21 July, 2017. Ten saplings were used for each number of inoculation sites (number of inoculated nematodes). After inoculation, resin exudation (symptom) of each sapling was observed regularly.
In experiments 2.1, and 2.2, the inoculum used for inoculation was the highly pathogenic B. xylophilus, Ka-4 strain cultured on Botrytis cinerea (grey mold) colonies. The pathogenicity of the nematodes used for inoculation was confirmed before each experiment by inoculating them into five healthy black pine trees (at a rate of 1,000 nematodes per seedling and 4,500 nematodes per sapling), resulting in nearly complete tree mortality.
In all experiments, the progression of symptoms was assessed based on visual changes observed and judged from photo recordings, while physiological symptoms were assessed based on the amount of resin exuding from pinholes made in the main stem of seedlings two hours after pinning, which is a modification of Oda’s method [
13] for small seedlings.
4. Discussion
Our study challenges conventional assumptions about the infection cycle of pine wilt disease (PWD). In the cage-release experiment, sexually mature beetles exhibited distinct feeding behavior compared to sexually immature beetles. They created significantly more feeding scars on asymptomatic carrier trees placed at the center of the cage than on healthy trees positioned around them (
Tukey’s multiple comparison test, p < 0.05). However, they also left feeding scars evenly across all healthy trees. This suggests that, contrary to previous understanding, sexually mature beetles are not exclusively attracted to weakened or dead trees for reproduction. Instead, they continue vigorous feeding during their reproductive period, moving between oviposition sites and surrounding healthy seedlings [
14].
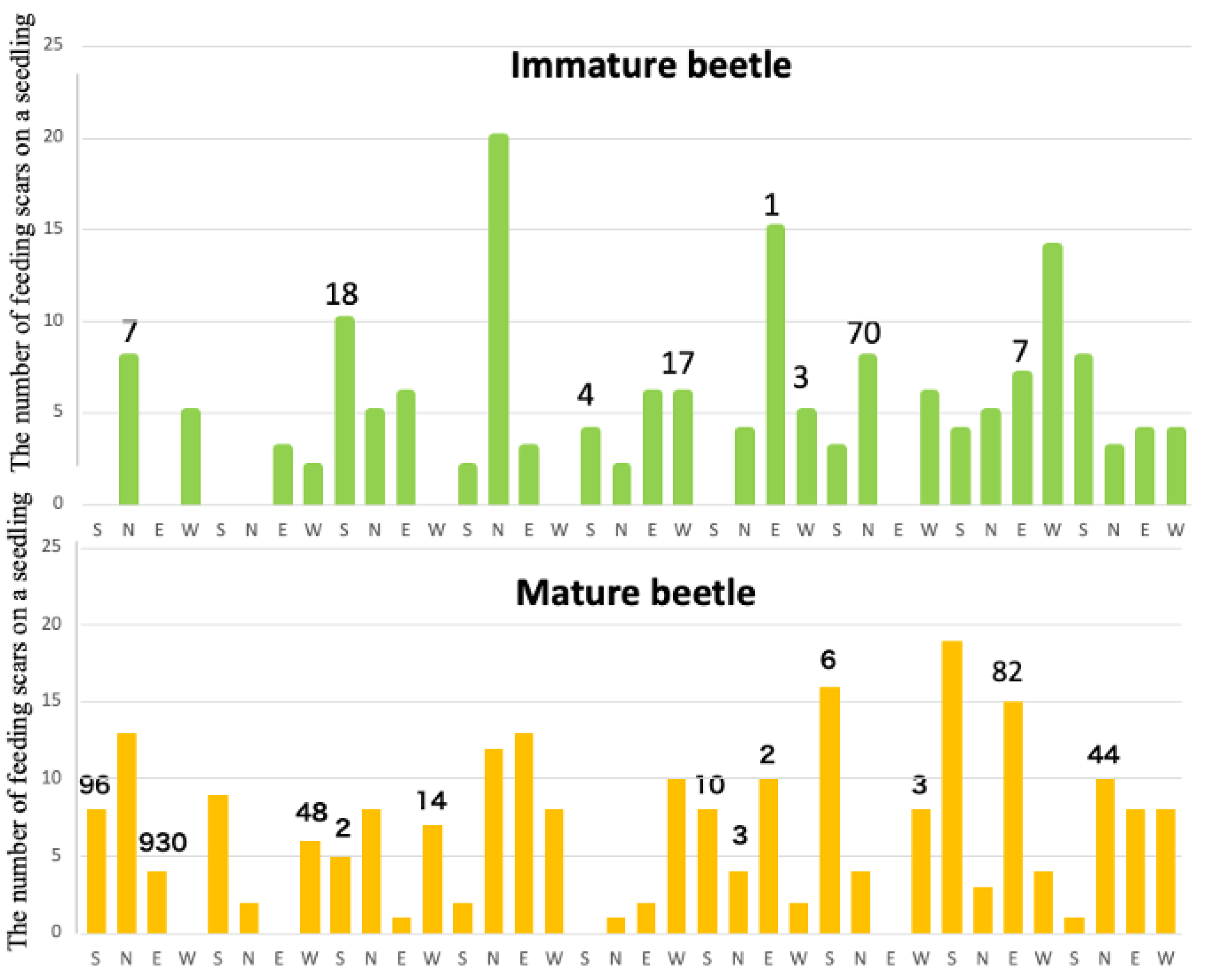
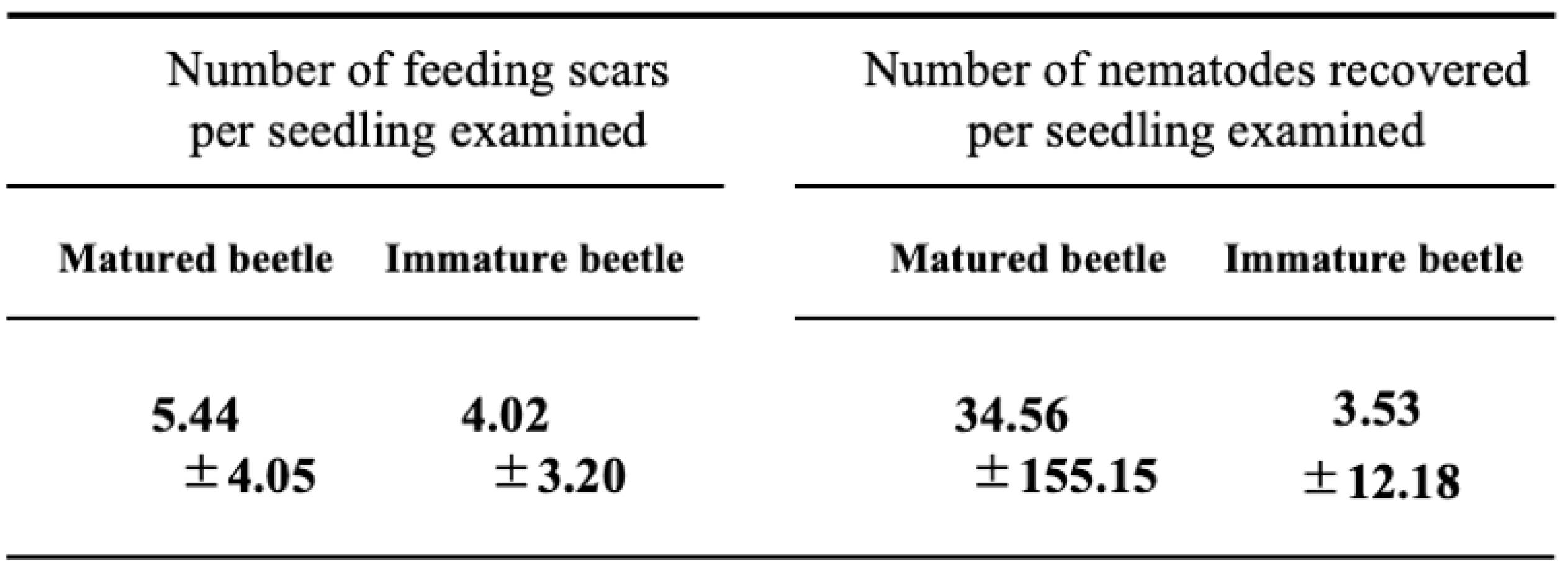
Previously, it was believed that sexually immature
* Monochamus beetles transmitted most. of the nematodes they carried through feeding scars on healthy pines, causing primary infections (
Figure 1). However, our cage-release experiments revealed that nematode transmission by immature beetles was minimal; very few nematodes entered feeding scars, and only a limited number successfully invaded pine tissues (
Table 1). This aligns with earlier reports showing that pine trees fed on by immature beetles within the first 10 days post-emergence exhibited no mortality, whereas trees fed on by sexually mature beetles 2 to 7 weeks post-emergence showed high mortality [
17]. Additionally, previous studies indicate that nematodes use intestinal storage lipid reserves as an internal clock, exiting the beetle only after a certain amount of lipid is consumed [
18].
On the other hand, it has long been assumed that sexually mature beetles arriving at weak ened trees for reproduction carry few nematodes, as most nematodes were thought to have already left the beetle’s body during its earlier feeding activity on healthy pines. However, our experiments challenge this assumption. In the cage-release study, sexually mature beetles were individually maintained on young pine branches before reaching sexual maturity (~10 days for males, ~20 days for females). Even after feeding on pine seedlings for three days during the experiment, dissection revealed that males still retained an average of 850 nematodes and females an average of 1,900. This suggests that sexually mature beetles actually harbor far more nematodes than previously believed when they arrive at weakened trees for reproduction.
Our findings are supported by studies on the timing of nematode transmission.
Togashi [
19]and
Naves et al. [
20] found that peak nematode transmission occurred
20–35 days and
15–42 days after beetle emergence, respectively, coinciding with sexual maturation and attraction to declining trees. Once these beetles begin reproduction, they continue feeding on both the weakened trees they inhabit and nearby healthy trees, thereby establishing new infection routes. However, the role of sexually mature beetles in transmitting nematodes to healthy trees has been largely overlooked.
In our experiments, sexually mature beetles frequently fed on healthy trees surrounding weakened ones (
32 out of 36 healthy trees tested), creating 231 feeding scars—similar in number to those made by immature beetles. When we examined 196 of these scars, 20 contained nematodes, with an average of 35 nematodes per seedling. Importantly, even a small number of invading nematodes can have significant consequences. As demonstrated in
Experiment 2b, an increase in feeding scars, rather than the size of individual scars, leads to greater nematode entry, accelerating disease progression. Interestingly, previous research found no correlation between feeding scar size and the number of transmitted nematodes [
21], reinforcing the idea that total feeding site number is the primary determinant of transmission efficiency.
The results of our nematode inoculation experiment further support the role of latent infec tions. In the early-season inoculation experiment (July 1, 2018), where a small number (
50 nematodes) were introduced, disease progression was slow. Among 20 monitored black pine seedlings,
40% were completely dead, and 20% were partially dead by July of the following year (
Figure 3). Partial wilting is often overlooked in control efforts, yet studies on
Pinus koraiensis forests revealed that even after wilted branches were removed, seven out of 13 trees eventually died [
12]. These findings highlight the importance of monitoring not only symptomatic trees but also asymptomatic carrier trees and those with partial wilting.
Notably, late-season inoculations resulted in fewer cases of severe wilting, with most trees remaining asymptomatic until the following year or later. This suggests that certain infection conditions contribute to the formation of latent carrier trees. One key factor is the low number of infecting nematodes. Some infected trees survive without visible symptoms, yet harbor small nematode populations internally. Increased temperatures and drought conditions [
22] can weaken host resistance, triggering nematode proliferation, disease progression, and the eventual creation of breeding sites for sawyer beetles. Another factor is lower temperatures during late-summer or early-autumn infection, which may enhance host resistance. In essence, conditions that slow disease development contribute to asymptomatic infections. Even when trees at the same site are infected simultaneously, individual variations in these factors can determine whether a tree dies or becomes a latent carrier [
23].
The ease of nematode invasion from beetle feeding scars is a critical factor in host suscepti bility. This property varies by pine species and seasonal conditions [
16]. In susceptible black pines, only about
10% of nematodes at feeding scars successfully invade host tissues [
24]. Since
B. xylophilus reproduces sexually, very low infection densities could reduce mating opportunities, leading to population collapse. However, nematode sex pheromones facilitate encounters between males and females [
25], enhancing population persistence. If reproduction fails, the nematodes disappear, and the tree survives. If successful, the nematode population remains at low density until host resistance declines, triggering rapid proliferation and wilting. This was evident in our experiments, where nematodes were recovered from symptom-free seedlings the following April.
Long-term latent infections have also been observed in other pine species. Bergdahl and Halik [
26] found that in Vermont, USA, despite inoculating 30,000 nematodes, many
Pinus sylvestris (Scots pine) trees survived for 7–11 years as asymptomatic carriers, with nematodes persisting for
2–11 years. The cooler climate and higher resistance of
P. sylvestris compared to
P. thunbergii may have contributed to this prolonged latent phase [
27], but the study nonetheless illustrates how nematodes can persist long-term in asymptomatic carriers.
After dead trees are removed from a forest, sawyer beetles may migrate and feed on remain ing healthy trees. However, these trees take time to develop disease symptoms and reduced resin exudation, which makes them suitable for egg-laying. Research suggests that a
one-month gap exists between beetle emergence and the onset of resin-abnormal trees [
28]. Because
Monochamus beetles lack suitable oviposition sites for
~20 days (males) and ~10 days (females) after emergence, latent infections from previous years provide critical breeding sites, ensuring population continuity. This means that mature beetles contribute significantly to outdoor nematode transmission by introducing small numbers of nematodes into multiple feeding sites on healthy trees surrounding weakened hosts (
Figure 8). This overlooked process underscores the importance of asymptomatic carriers in sustaining PWD, nullifying control efforts, and facilitating disease spread.
Recent advancements in PWD detection methods have improved monitoring [
29]. Aerial detection using remote sensing and UAVs (Unmanned Aerial Vehicle) identifies symptomatic trees at a large scale but remains ineffective for detecting asymptomatic carriers [
30,
31,
32,
33]. Direct detection methods, such as volatile compound analysis [
34,
35], DNA-based techniques [
36,
37,
38], and pH measurement of wood samples [
39], offer high accuracy but are costly and impractical for large-scale surveys.
Traditional resin exudation measurement remains the most practical approach for detecting infected trees in the field [
12,
40].