Submitted:
03 March 2025
Posted:
04 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
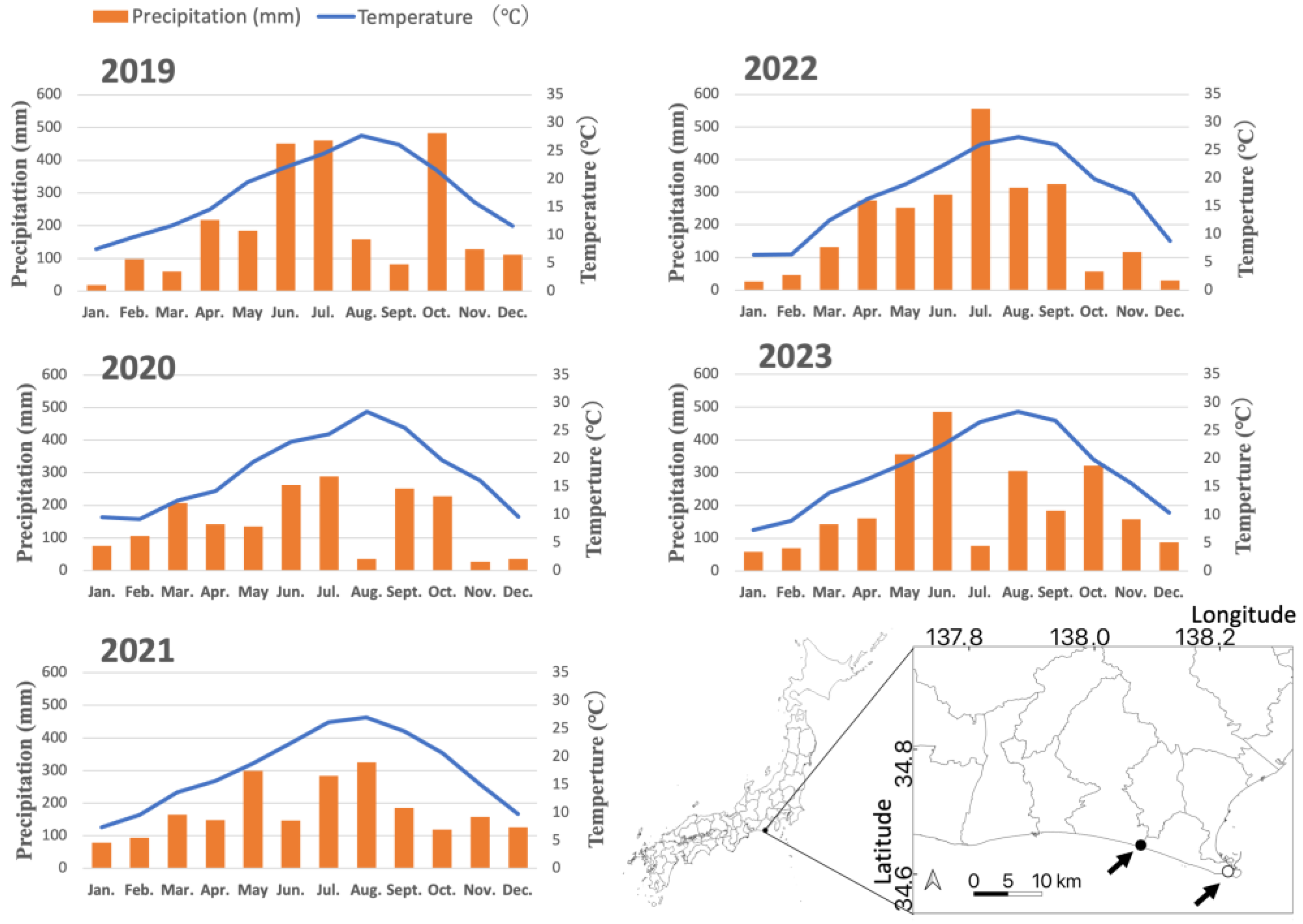
2.1. Study Site
2.2. Tree Health Monitoring
2.3. Statistical Analysis
3. Results
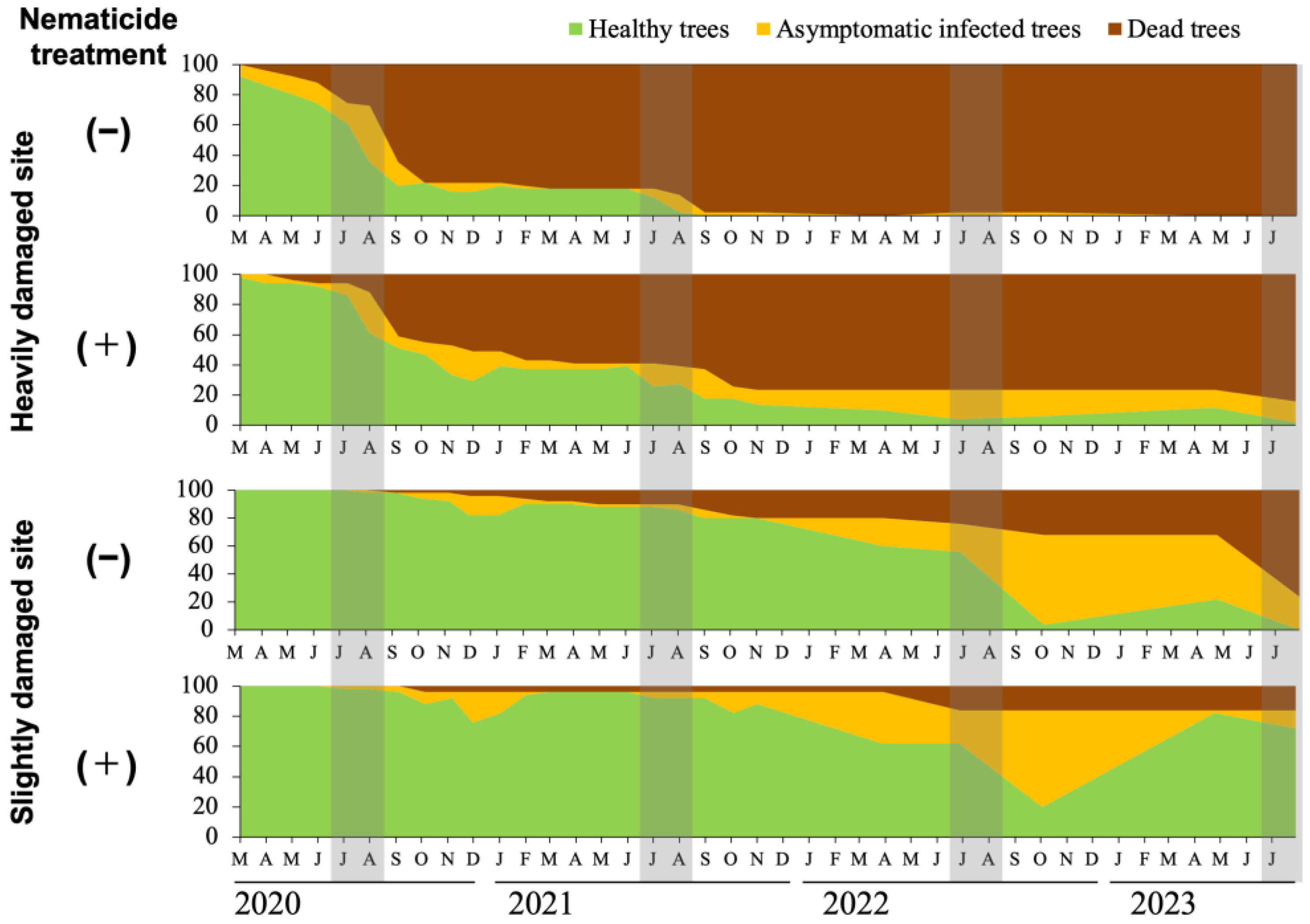
3.1. Relationship Between Initial PWD Damage, Nematicide Treatment, and Damage Progression
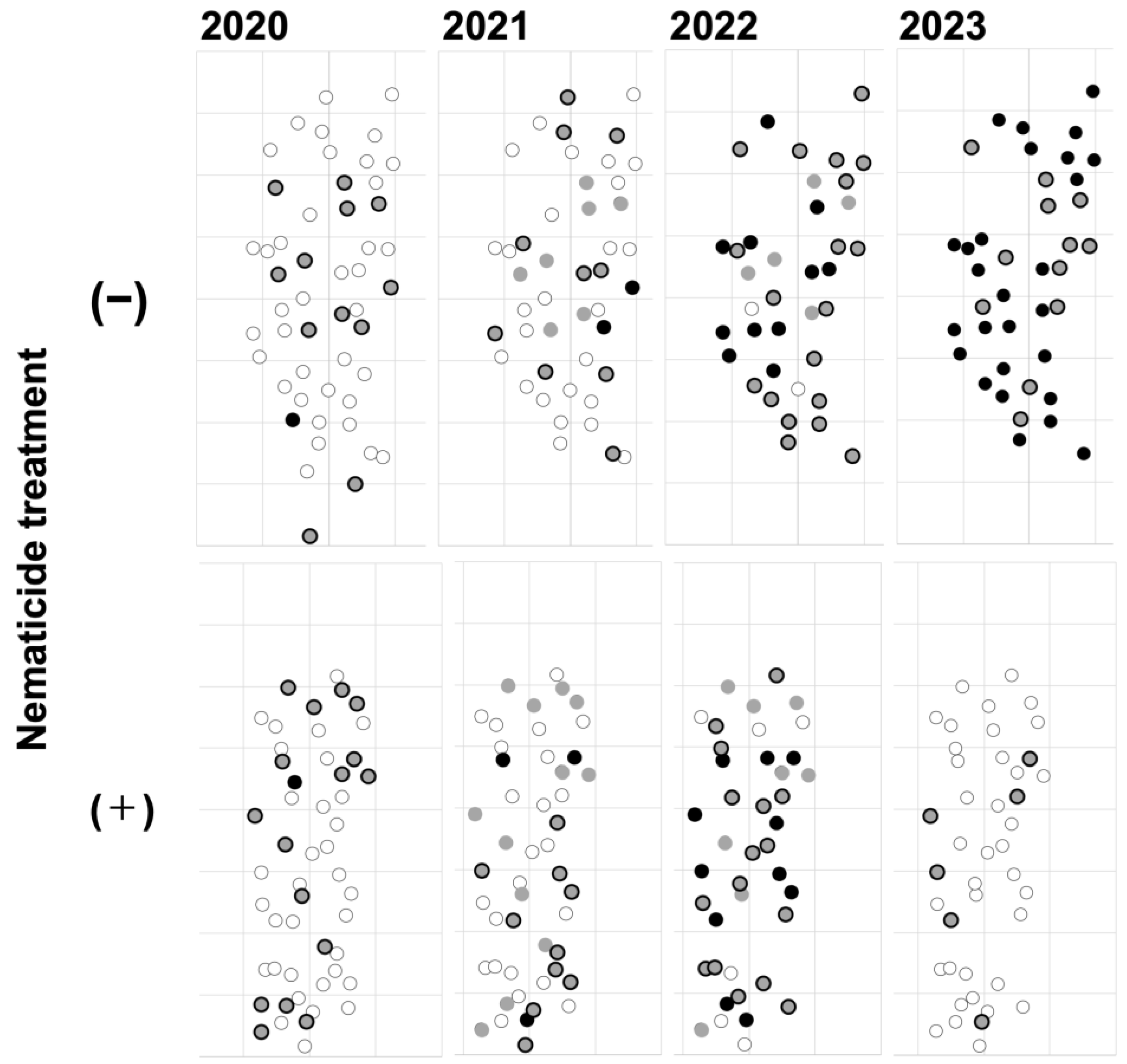
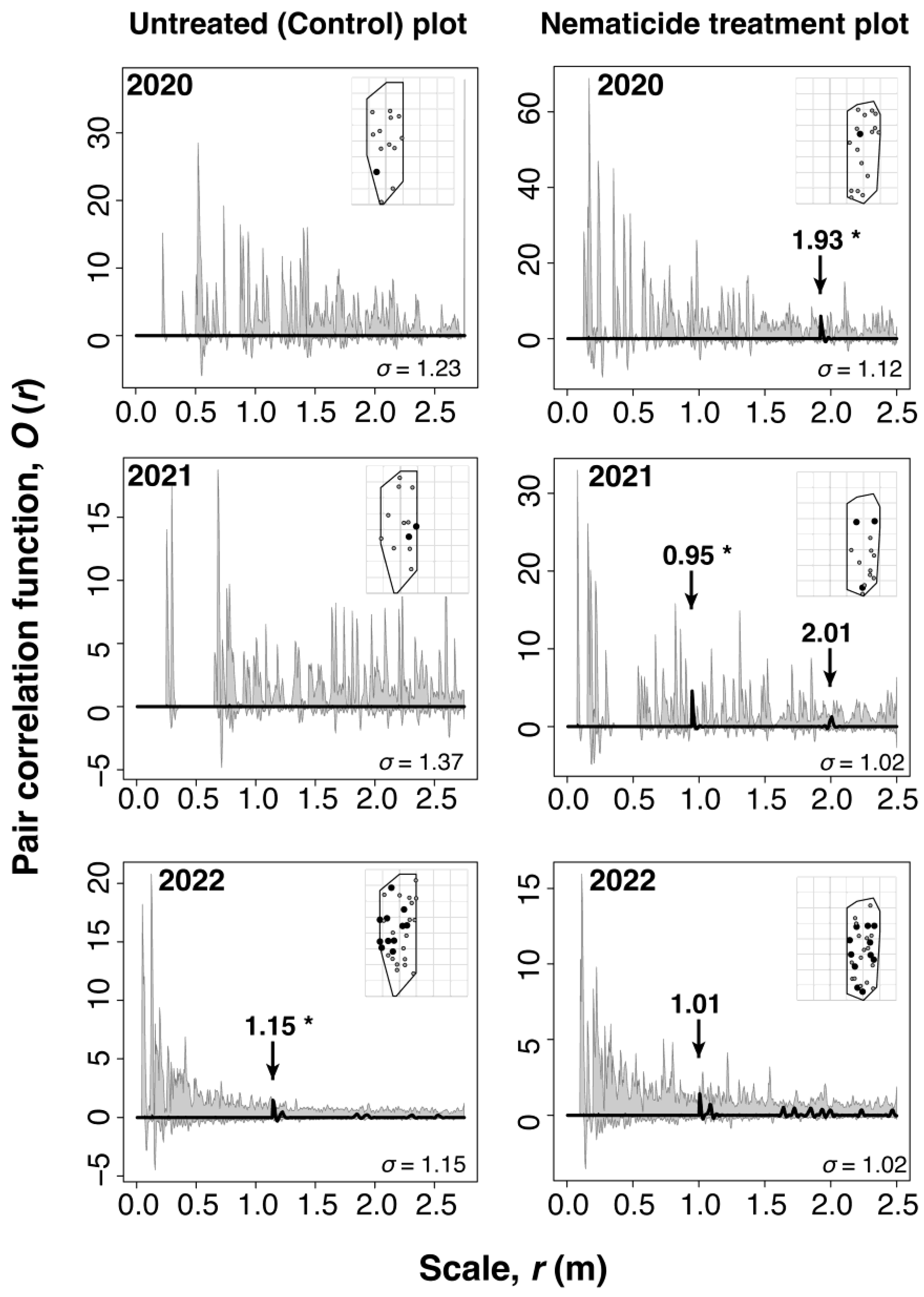
3.2. Spatial Overlap Between Oviposition Target Trees and Asymptomatic Infected Trees in the Slightly Damaged Site
4. Discussion
4.1. Suggestions for PWD Control
4.2. Suggestions for PWD Control Considering Asymptomatic Infected Trees
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamiya, Y. History of Pine Wilt Disease in Japan. J Nematol 1988, 20, 219–226. [Google Scholar] [PubMed]
- Zhao, B.G. Pine Wilt Disease in China. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer Japan: Tokyo, Japan, 2008; ISBN 978-4-431-75655-2. [Google Scholar]
- Hao, Z.; Huang, J.; Li, X.; Sun, H.; Fang, G. A Multi-Point Aggregation Trend of the Outbreak of Pine Wilt Disease in China over the Past 20 Years. Forest Ecology and Management 2022, 505, 119890. [Google Scholar] [CrossRef]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First Report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annual Review of Phytopathology 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine Wilt Disease: A Global Threat to Forestry. Plant Pathology 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Akbulut, S.; Stamps, W.T. Insect Vectors of the Pinewood Nematode: A Review of the Biology and Ecology of Monochamus Species. Forest Pathology 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential Distribution of Pine Wilt Disease Under Future Climate Change Scenarios. PLOS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine Wilt Disease: A Threat to European Forestry. Eur J Plant Pathol 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Annual Report on Forest and Forestry in Japan: Fiscal Year 2023. Available online: https://www.rinya.maff.go.jp/j/kikaku/hakusyo/r5hakusyo/index.html (accessed on 14 February 2025).
- Kiyohara, T.; Tokushige, Y. Inoculation Experiments of a Nematode, Bursaphelenchus sp., onto Pine Trees. Journal of the Japanese Forestry Society 1971, 53, 210–218, (in Japanese with English summary). [Google Scholar] [CrossRef]
- Mamiya, Y.; Enda, N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) By Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Kishi, Y. Invasion of Pine Trees by Bursaphelenchus lignicolus M. & K. (Nematoda: Aphelenchoidae) from Monochamus alternatus HOPE (Coleoptera: Cerambycidae). Journal of the Japanese Forestry Society 1978, 60, 179–182. (in Japanese). [Google Scholar] [CrossRef]
- Togashi, K. Transmission Curves of Bursaphelenchus xylophilus (Nematoda : Aphelenchoididae) from Its Vector, Monochamus alternatus (Coleoptera : Cerambycidae), to Pine Trees with Reference to Population Performance. Applied Entomology and Zoology 1985, 20, 246–251. [Google Scholar] [CrossRef]
- Linit, M.J. Transmission of Pinewood Nematode Through Feeding Wounds of Monochamus carolinensis (Coleoptera: Cerambycidae). J Nematol 1990, 22, 231–236. [Google Scholar]
- Togashi, K. Vector-Nematode Relationships and Epidemiology in Pine Wilt Disease. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer Japan: Tokyo, Japan, 2008; ISBN 978-4-431-75654-5. [Google Scholar]
- Togashi, K. Variation in External Symptom Development of Pine Wilt Disease in Field Grown Pinus thunbergii. Journal of the Japanese Forestry Society 1989, 71, 442–448. [Google Scholar] [CrossRef]
- Kuroda, K. Mechanism of Cavitation Development in the Pine Wilt Disease. European Journal of Forest Pathology 1991, 21, 82–89. [Google Scholar] [CrossRef]
- Fukuda, K. Physiological Process of the Symptom Development and Resistance Mechanism in Pine Wilt Disease. Journal of Forest Research 1997, 2, 171–181. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two Terpene Synthases in Resistant Contribute to Defence Against Bursaphelenchus xylophilus. Plant, Cell & Environment 2021, 44, 257–274. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Li, D.; Liu, Z.; Wang, X.; Zhang, W.; Wen, X.; Zhang, X. Bursaphelenchus xylophilus Venom Allergen Protein BxVAP2 Responds to Terpene Stress, Triggers Plant Defense in Nicotiana benthamiana. Forests 2024, 15, 1929. [Google Scholar] [CrossRef]
- Linit, M.J. Nemtaode-Vector Relationships in the Pine Wilt Disease System. J Nematol 1988, 20, 227–235. [Google Scholar]
- Zhao, L.; Zhang, S.; Wei, W.; Hao, H.; Zhang, B.; Butcher, R.A.; Sun, J. Chemical Signals Synchronize the Life Cycles of a Plant-Parasitic Nematode and Its Vector Beetle. Current Biology 2013, 23, 2038–2043. [Google Scholar] [CrossRef]
- Kirino, H.; Maehara, N.; Shinya, R. How Did Bursaphelenchus Nematodes Acquire a Specific Relationship with Their Beetle Vectors, Monochamus? Front. Physiol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Inhibitory Activity of Fenitrothion Against Maturation Feeding of Japanese Pinesawyer, Monochamus alternatus. Japanese Journal of Applied Entomology and Zoology 1988, 32, 245–251. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Song, M.-Y.; Shin, S.-C.; Park, Y.-S. Effects of Aerial Insecticide Sprays on Ant Communities to Control Pine Wilt Disease in Korean Pine Forests. Applied Entomology and Zoology 2005, 40, 563–574. [Google Scholar] [CrossRef]
- Suh, D.Y.; Jung, J.-K.; Lee, S.K.; Seo, S.-T. Effect of Aerial Spraying of Thiacloprid on Pine Sawyer Beetles (Monochamus alternatus) and Honey Bees (Apis mellifera) in Pine Forests. Entomological Research 2021, 51, 83–89. [Google Scholar] [CrossRef]
- Fujishita, A. Trunk Injection of Chemicals for the Control of the Pine Wilt Disease Caused by Bursaphelenchus xylophilus. Bulletin of the Shizuoka Prefecture Forestry Experiment Station 1985, 13, 23–34, (in Japanese with English summary). [Google Scholar]
- Sousa, E.; Naves, P.; Vieira, M. Prevention of Pine Wilt Disease Induced by Bursaphelenchus xylophilus and Monochamus galloprovincialis by Trunk Injection of Emamectin Benzoate. Phytoparasitica 2013, 41, 143–148. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Yang, T.; Xu, M.; Li, Y.; Pei, Y.; Tang, J.; Zheng, Z.; Sun, Z.; Cheng, G.; Li, X.; Li, H.; Wang, L; Chen, F. Optimized Emamectin Benzoate Trunk Injection: Addressing Temperature Limitations for Pine Wilt Disease Control. Pest Management Science 2025, 81, 892–902. [Google Scholar] [CrossRef]
- Yoshimura, A.; Kawasaki, K.; Takasu, F.; Togashi, K.; Futai, K.; Shigesada, N. Modeling the Spread of Pine Wilt Disease Caused by Nematodes with Pine Sawyers as Vector. Ecology 1999, 80, 1691–1702. [Google Scholar] [CrossRef]
- Togashi, K.; Shigesada, N. Spread of the Pinewood Nematode Vectored by the Japanese Pine Sawyer: Modeling and Analytical Approaches. Popul Ecol 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Xia, C.; Chon, T.-S.; Takasu, F.; Choi, W.I.; Park, Y.-S. Simulating Pine Wilt Disease Dispersal With an Individual-Based Model Incorporating Individual Movement Patterns of Vector Beetles. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Yu, R.; Luo, Y.; Zhou, Q.; Zhang, X.; Wu, D.; Ren, L. Early Detection of Pine Wilt Disease Using Deep Learning Algorithms and UAV-based Multispectral Imagery. Forest Ecology and Management 2021, 497, 119493. [Google Scholar] [CrossRef]
- Tahir, S.; Hassan, S.S.; Yang, L.; Ma, M.; Li, C. Detection Methods for Pine Wilt Disease: A Comprehensive Review. Plants 2024, 13, 2876. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, J.; Wang, T.; Zhao, J.; Gadekallu, T.R.; Fang, K. Detection of Pine Wilt Disease Using AAV Remote Sensing With an Improved YOLO Model. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing 2024, 17, 19230–19242. [Google Scholar] [CrossRef]
- Futai, K. Role of Asymptomatic Carrier Trees in Epidemic Spread of Pine Wilt Disease. J For Res 2003, 8, 253–260. [Google Scholar] [CrossRef]
- Futai, K. Two Infectious Diseases: “COVID-19” and “Pine Wilt Disease. ” Forests 2024, 15, 1724. [Google Scholar] [CrossRef]
- Halik, S.; Bergdahl, D.R. Long-Term Survival of Bursaphelenchus xylophilus in Living Pinus sylvestris in an Established Plantation. European Journal of Forest Pathology 1994, 24, 357–363. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Futai, K. Asymptomatic Carrier Trees in Pine Stands Naturally Infected with Bursaphelenchus xylophilus. Nematology 2007, 9, 243–250. [Google Scholar] [CrossRef]
- Bergdahl, D.R.; Halik, S. Persistence of the Pine Wood Nematode in Asymptomatic Scots Pines. In The Pinewood Nematode, Bursaphelenchus xylophilus; Mota, M, Vieira, P., Eds.; Brill: Berlin, Germany, 2004; ISBN 978-90-474-1309-7. [Google Scholar]
- Takeuchi, Y.; Kanzaki, N.; Futai, K. Volatile Compounds in Pine Stands Suffering from Pine Wilt Disease: Qualitative and Quantitative Evaluation. Nematology 2006, 8, 869–879. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Yi, S.; Wei, Y.; Wang, M. Odorant-Binding Protein 19 in Monochamus alternatus Involved in the Recognition of a Volatile Strongly Emitted from Ovipositing Host Pines. Insect Science 2024, 31, 134–146. [Google Scholar] [CrossRef]
- Kato, T.; Kenmochi, A.; Yamada, Y.; Futai, K. Confirmation of the Presence of Asymptomatic Carrier Trees of Pine Wilt Disease After Thorough Control Procedure, and the Effectiveness of Trunk Injection to Suppress Disease Development. Journal of the Japanese Forest Society 2019, 101, 46–51. [Google Scholar] [CrossRef]
- Koiwa, T. Survey of Asymptomatic Carrier Trees of Pine Wilt Disease in Iwate Prefecture, Northeastern Japan. Journal of the Japanese Forest Society 2019, 101, 35–45. [Google Scholar] [CrossRef]
- Cho, Y.; Jung, K. Countermeasures Against Asymptomatic Carriers in Pine Wilt Disease in Korea. Journal of the Japanese Forest Society 2019, 101, 26–29. [Google Scholar] [CrossRef]
- Ishiguro, H.; Futai, K. When Coming to a Wilting Pine Tree, Monochamus alternatus Causes Pinewood Nematode Infection on the Surrounding Healthy Trees. Tree and Forest Health 2022, 26, 59–64, (in Japanese with English summary). [Google Scholar] [CrossRef]
- Oda, K. Target trees of pine wilt disease and its diagnostic method. Forest Pests 1967, 16, 263–266. (in Japanese). [Google Scholar]
- Wiegand, T.; A. Moloney, K. Rings, Circles, and Null-Models for Point Pattern Analysis in Ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R. Spatstat: An R Package for Analyzing Spatial Point Patterns. Journal of Statistical Software 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Berman, M.; Diggle, P. Estimating Weighted Integrals of the Second-Order Intensity of a Spatial Point Process. Journal of the Royal Statistical Society: Series B (Methodological) 1989, 51, 81–92. [Google Scholar] [CrossRef]
- Togashi, K. Spatial Pattern of Pine Wilt Disease Caused by Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) Within a Pinus thunbergii Stand. Res Popul Ecol 1991, 33, 245–256. [Google Scholar] [CrossRef]
- Naves, P.M.; Camacho, S.; De Sousa, E.M.; Quartau, J.A. Transmission of the Pine Wood Nematode Bursaphelenchus xylophilus Through Feeding Activity of Monochamus galloprovincialis (Col., Cerambycidae). Journal of Applied Entomology 2007, 131, 21–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




