Submitted:
06 March 2025
Posted:
06 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
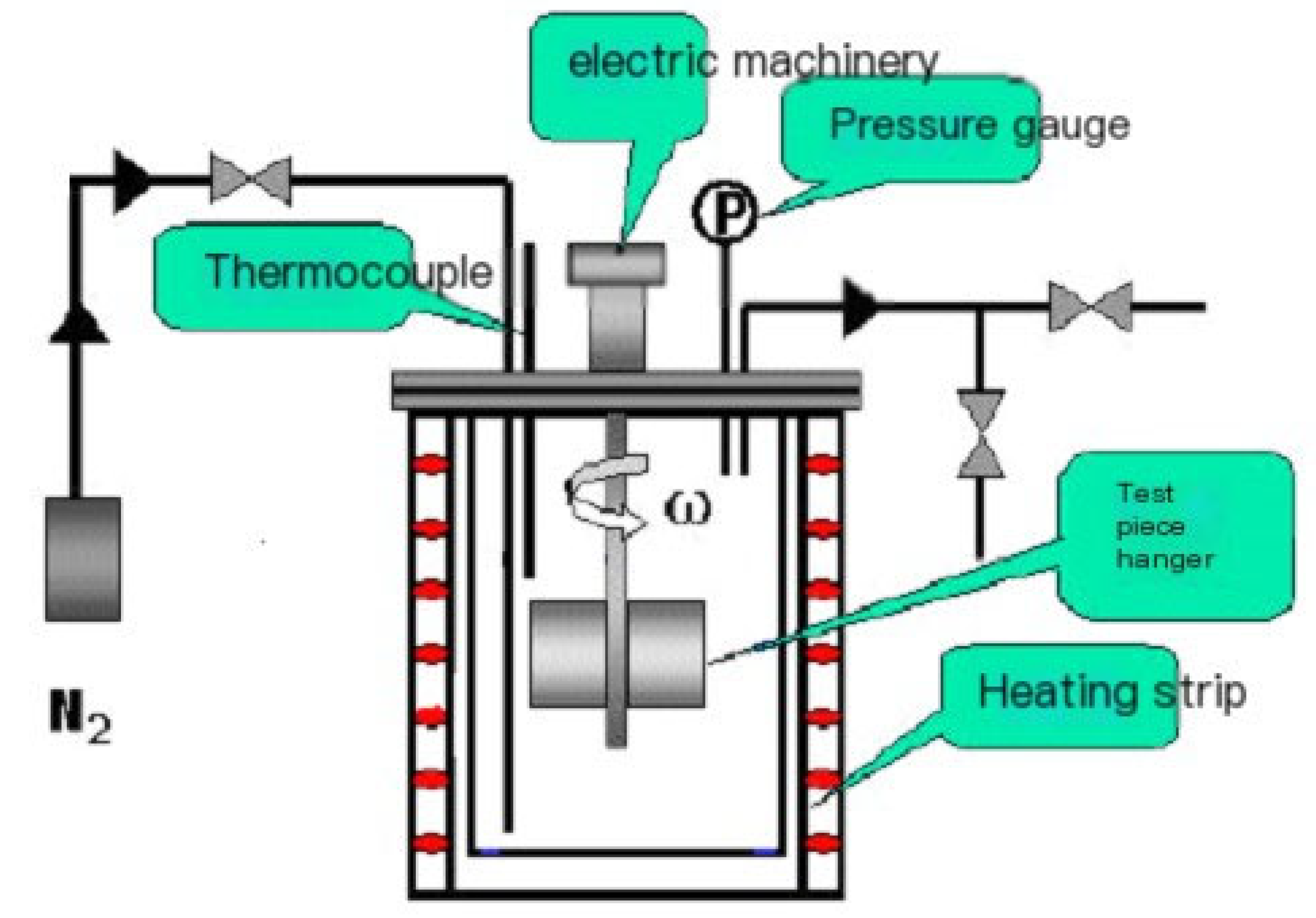
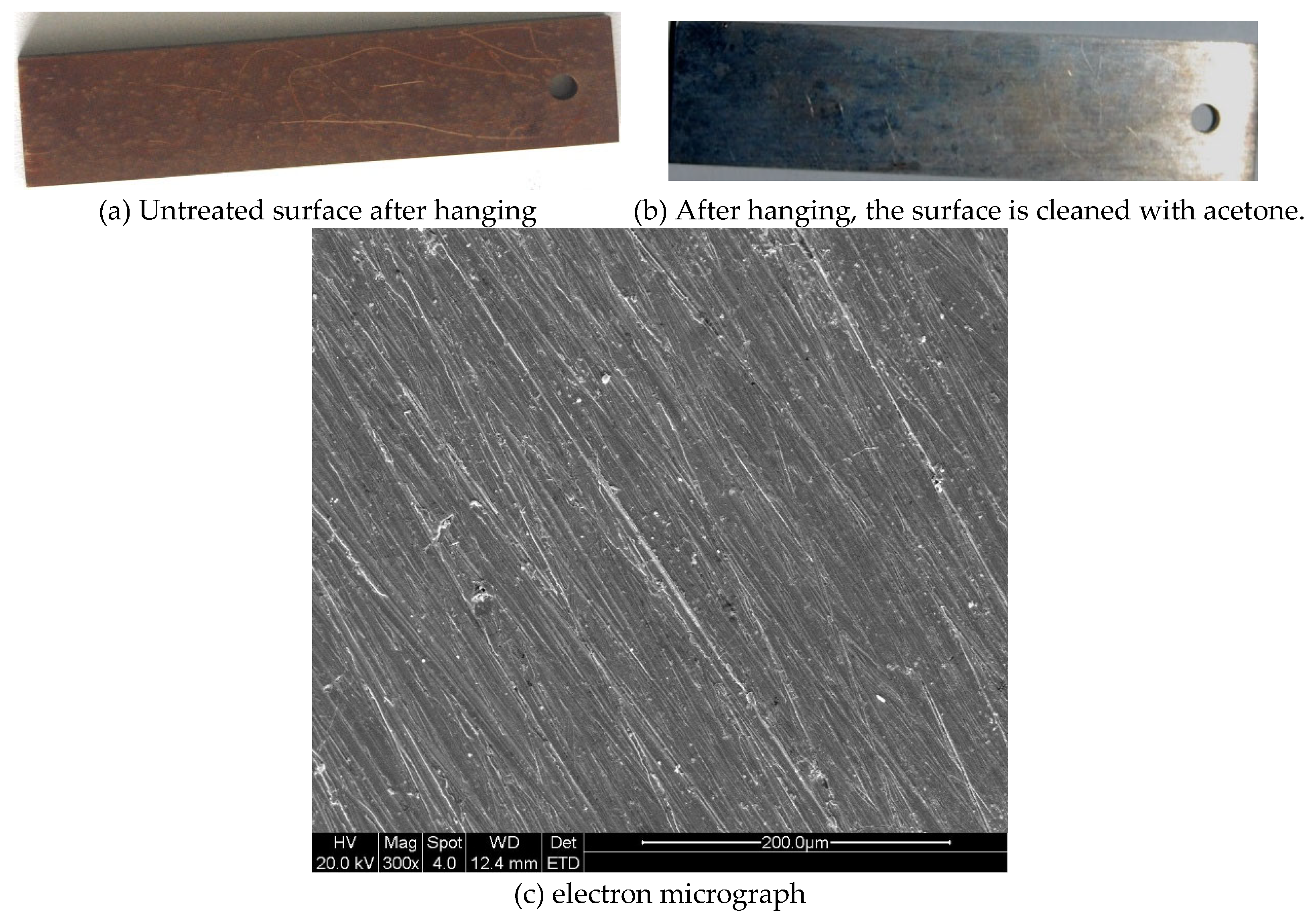
2. Materials and Methods
2.1. Alkylpyridine quaternary ammonium salt was synthesized by alkylation reaction with alkylpyridine and benzyl chloride as raw materials. The main reaction is as follows:
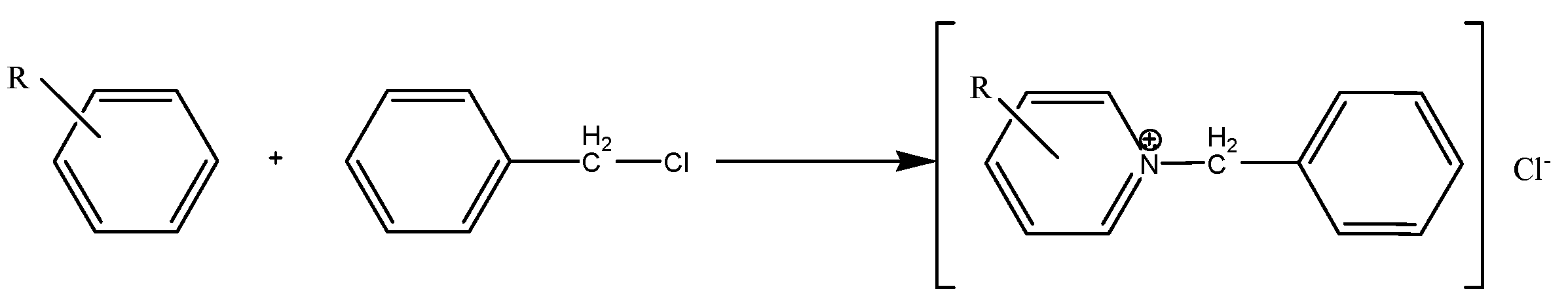
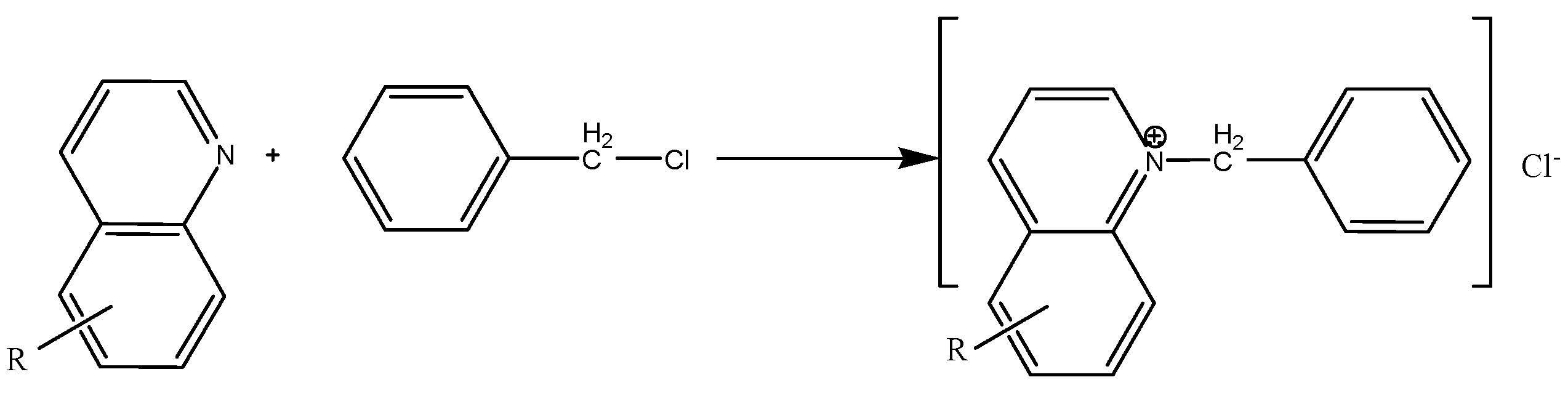
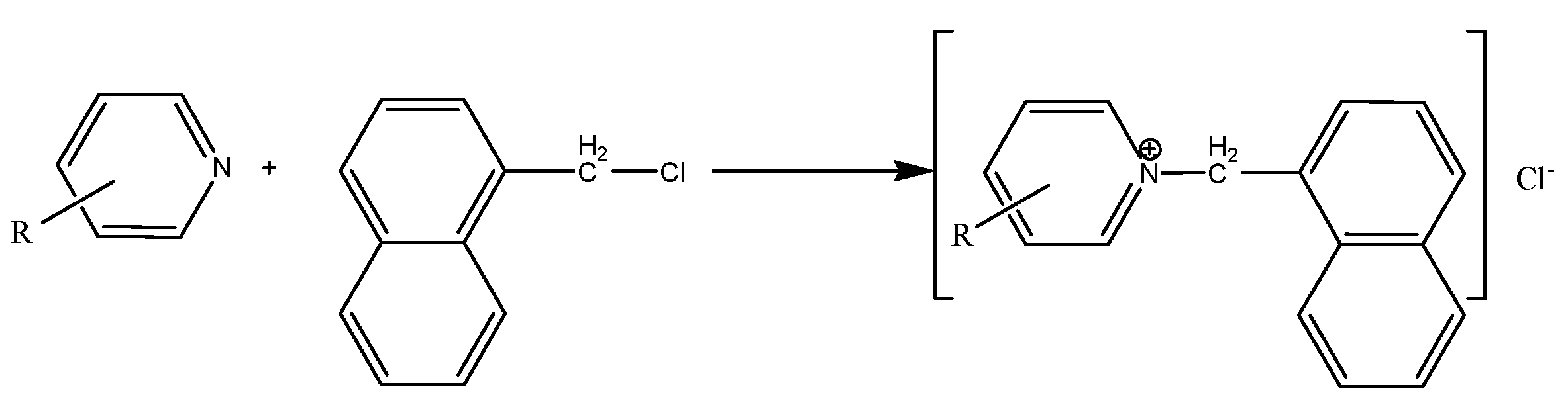
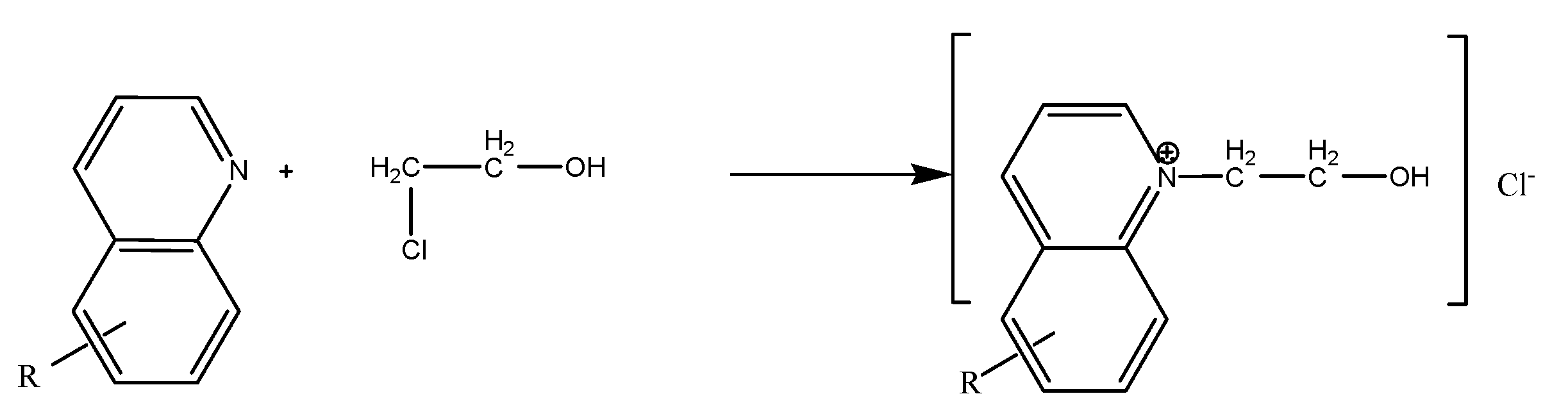
3. Results and Discussion
3.1. Study on Corrosion Inhibition Mechanism of Alkyl Quinoline Quaternary Ammonium Salt
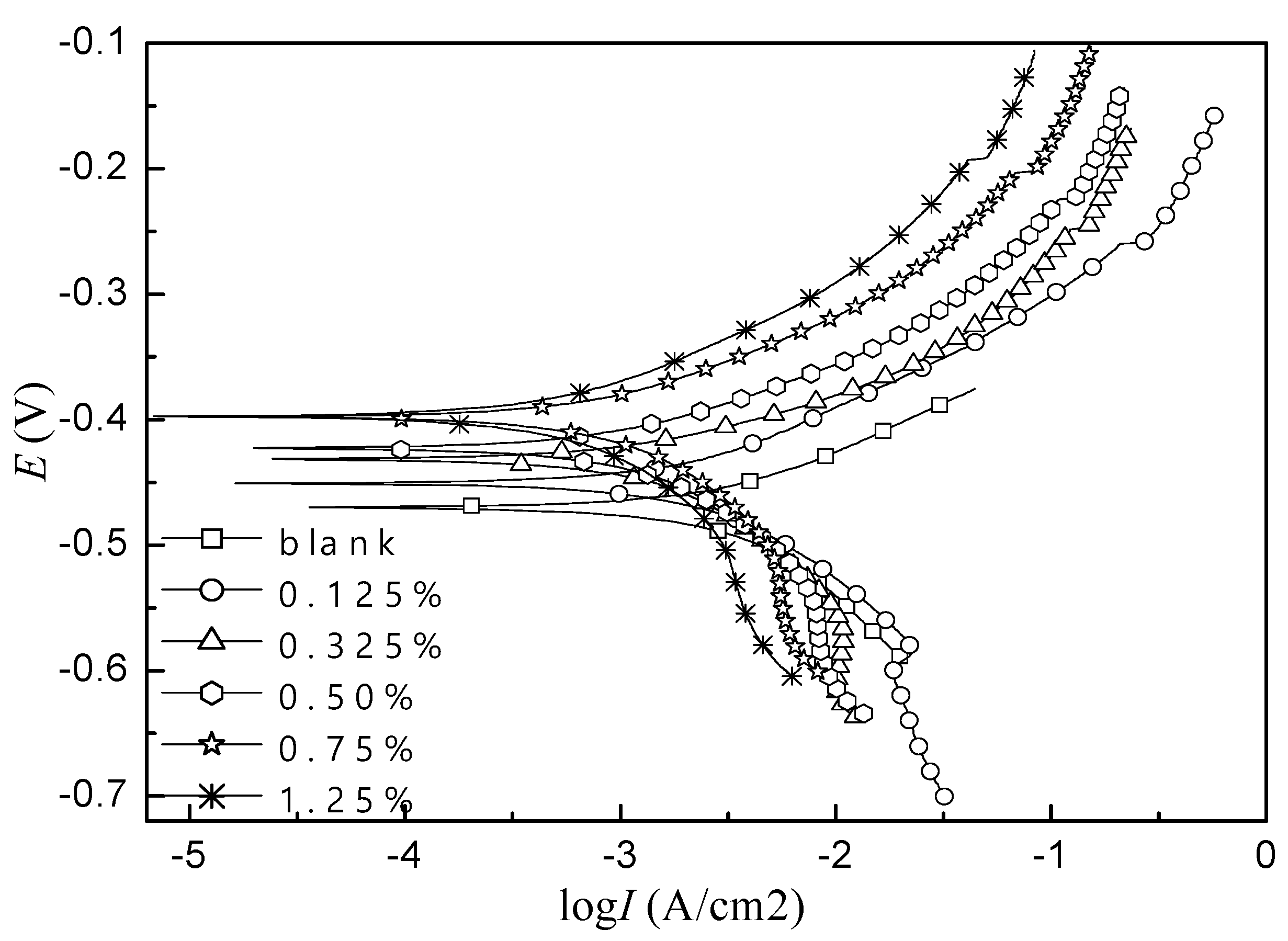
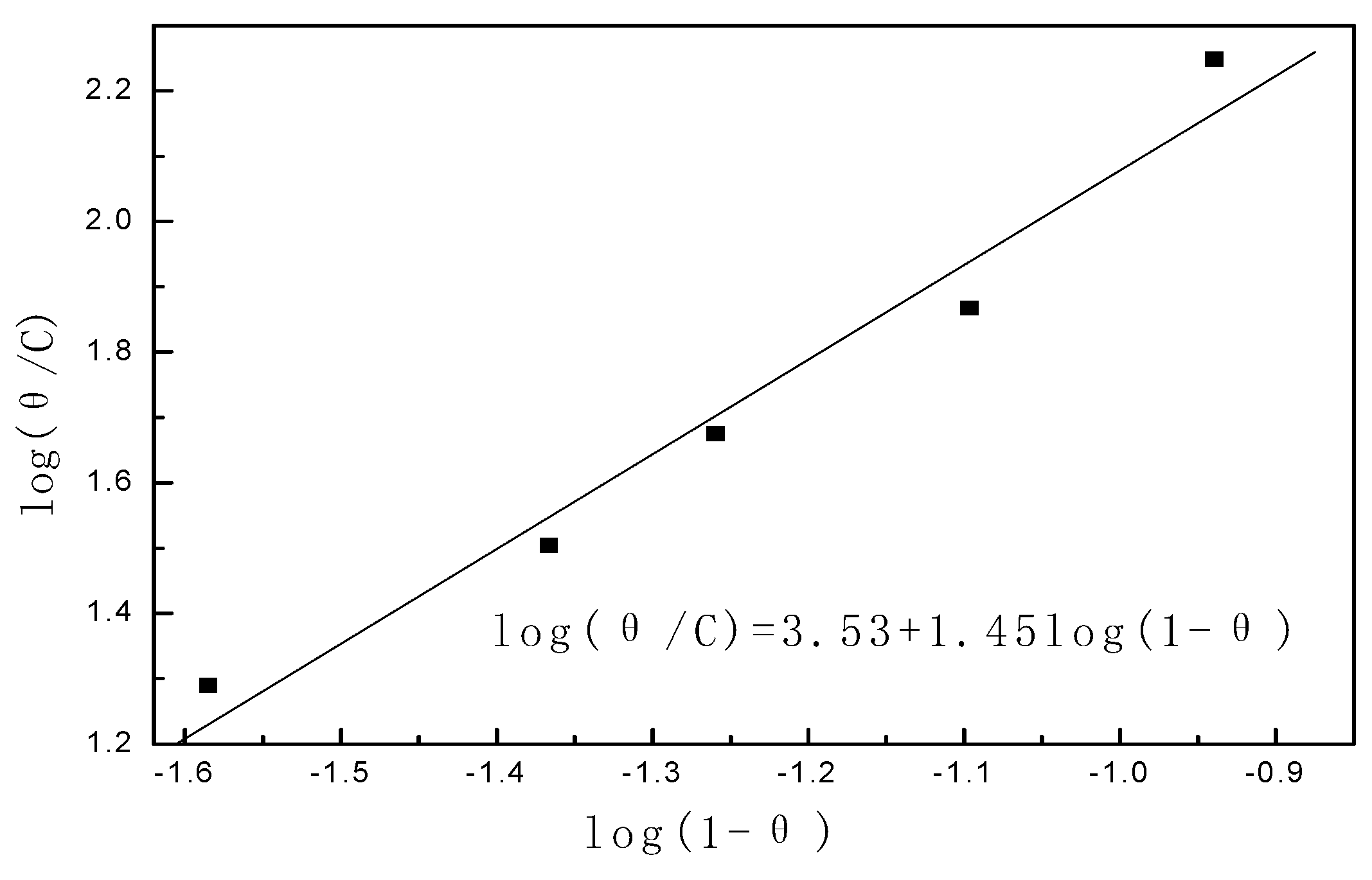
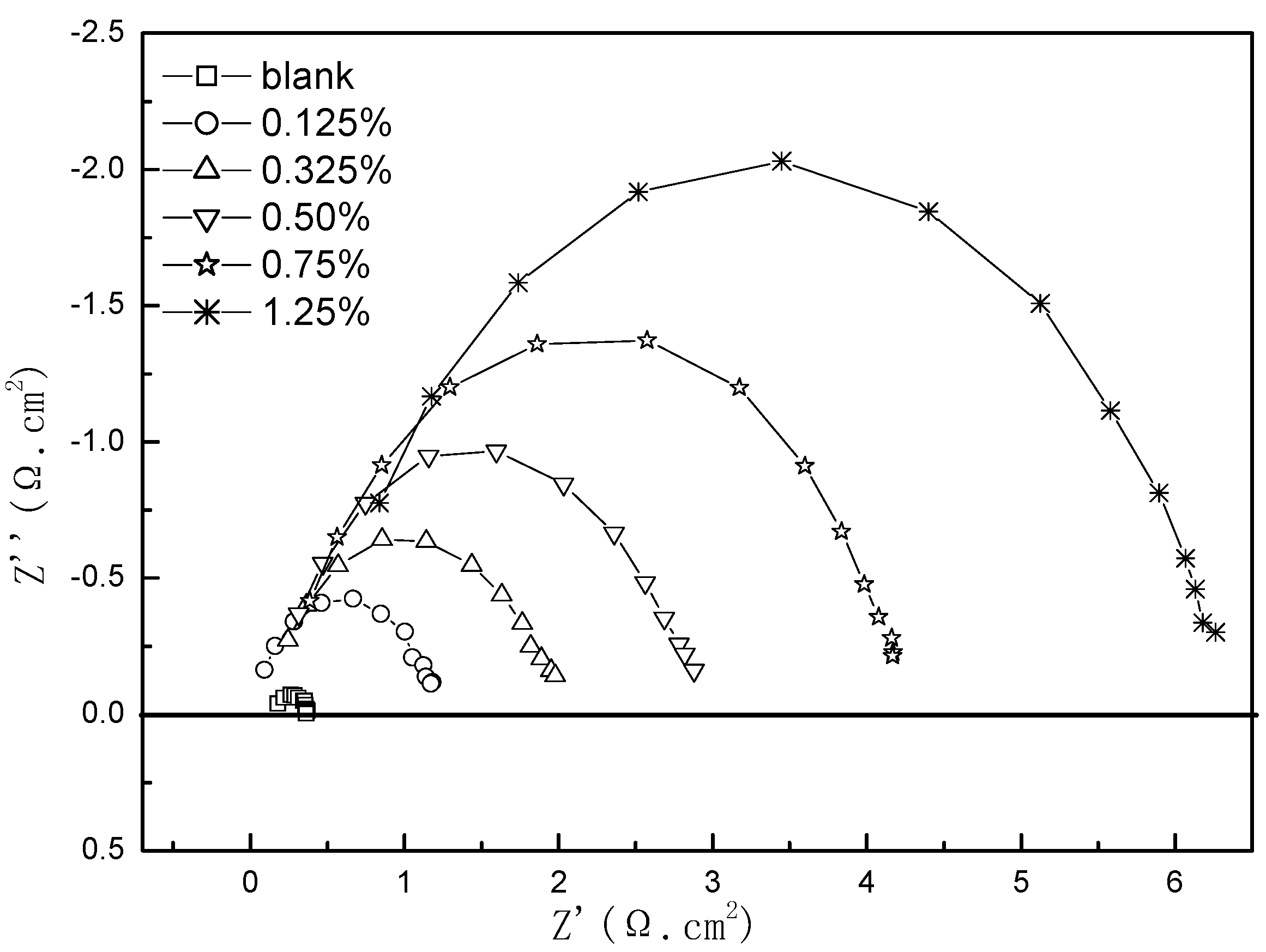
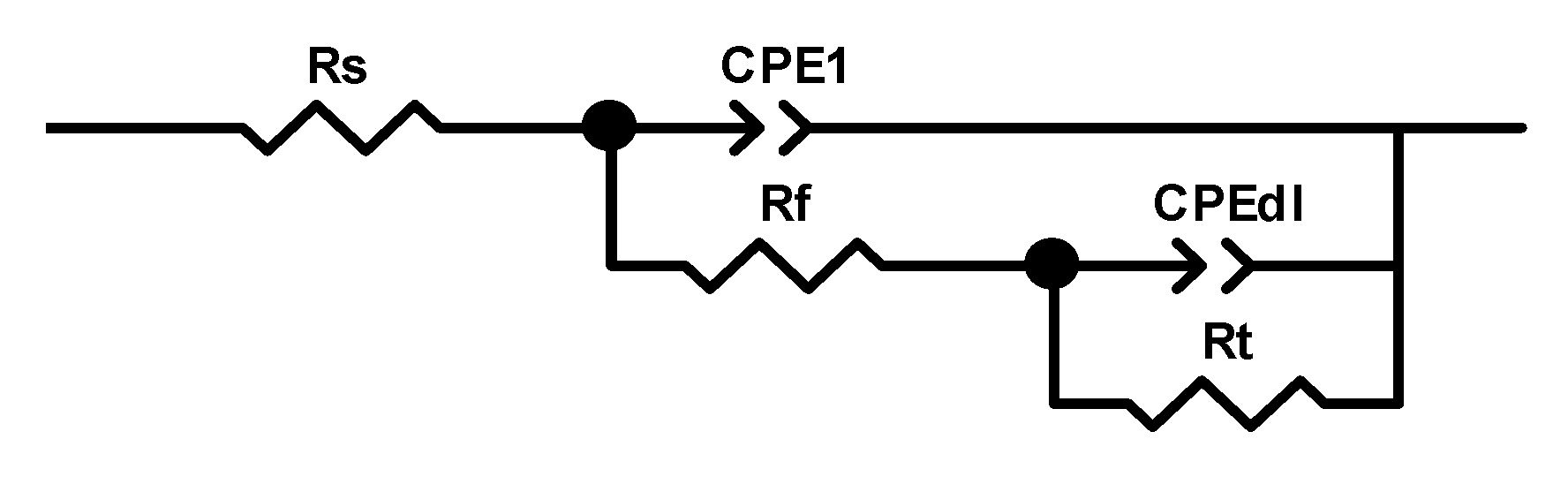
3.2. Primary Screen of Quaternary Ammonium Salts Containing N Heterocyclic Rings
3.2.1. Evaluation of Corrosion Inhibition Performance of Quaternary Ammonium Salts Obtained by Reaction of Alkyl Pyridine and Benzyl Chloride at Low Temperature
|
Acid temperature (°C) |
Acid concentration (100%) |
Inhibitor concentration (%) |
Corrosion rate (g/m2 • h) |
| 60 | 15%HCl | 0.5 | 4.5 |
| 60 | 12%HCl+3%HF | 0.5 | 3.8 |
| 60 | multi-hydrogen acid | 0.5 | 2.9 |
| 90 | 15%HCl | 1 | 3.5 |
| 90 | 12%HCl+3%HF | 1 | 3.1 |
| 90 | multi-hydrogen acid | 1 | 2.7 |
3.2.2. Evaluation of Corrosion Inhibition Performance of Quaternary Ammonium Salts Obtained by Reaction of Alkyl Quinoline and Benzyl Chloride at Low Temperature
|
Acid temperature (°C) |
Acid concentration (100%) |
Inhibitor concentration (%) |
Corrosion rate (g/m2•h) |
| 60 | 15%HCl | 0.5 | 2.8 |
| 60 | 12%HCl+3%HF | 0.5 | 2.1 |
| 60 | multi-hydrogen acid | 0.5 | 1.5 |
| 90 | 15%HCl | 1 | 2.3 |
| 90 | 12%HCl+3%HF | 1 | 1.9 |
| 90 | multi-hydrogen acid | 1 | 1.2 |
3.2.3. Evaluation of Corrosion Inhibition Performance of Quaternary Ammonium Salts Obtained by Reaction of Alkyl Quinoline and Chloroethanol at Low Temperature
|
Acid temperature (°C) |
Acid concentration (100%) |
Inhibitor concentration (%) |
Corrosion rate (g/m2•h) |
| 60 | 15%HCl | 0.5 | 5.2 |
| 60 | 12%HCl+3%HF | 0.5 | 4.3 |
| 60 | multi-hydrogen acid | 0.5 | 4.1 |
| 90 | 15%HCl | 1 | 4.1 |
| 90 | 12%HCl+3%HF | 1 | 3.9 |
| 90 | multi-hydrogen acid | 1 | 3.3 |
3.2.4. Evaluation of the Performance of Alkyl Quinoline Quaternary Ammonium Salts of Compound Propargyl Alcohol at High Temperature
3.2.5. Performance of Alkyl Quinoline Quaternary Ammonium Salt after Compounding Propargyl Alcohol and Cuprous Iodide at 180°C
3.3. Improvement of Acid Solubility of Alkyl Quinoline Quaternary Ammonium Salts
3.3. Improved Temperature Resistance of Alkyl Quinoline Quaternary Ammonium Salt Corrosion Inhibitor
3.3.1. Corrosion Inhibition Performance at 160-190°C
3.3.2. See Table 12 for Corrosion Inhibition Performance at 200°C
3.3.3. Corrosion Inhibition Performance at High Temperature in the Presence of Partial Hydrogen Sulfide
3.3.4. Inhibitory Performance of Ultra-High Temperature Acidizing Corrosion Inhibitor Under Dynamic Condition
| Acid type | Inhibitor concentration | Dispersant concentration | Temperature (°c) |
Pressure (atmospheric pressure) |
Corrosion rateG/ (m2.h) | Remark |
| multi-hydrogen acid | 5.5% | 7% | 195 | 160 | 82. 4 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 87. 8 | Slight blackening |
|
| 15%HCl | 5.5% | 7% | 195 | 160 | 98. 1 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 99. 3 | Slight blackening |
|
| 12%HCl+ 3%HF |
5.5% | 7% | 195 | 160 | 89. 2 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 93. 4 | Slight blackening |
4. Conclusions
References
- XIE R, WEI Y, LUO K, et al. Effect of environmental factors on the corrosion behavior of CoCrNi and CoCrFeMnNi alloys in 3.5 wt% NaCl solution [J]. Journal of Materials Research and Technology, 2025, 35: 2994-3007.
- IRANPOUR M, BABAEI A, BAGHERZADEH M. Microwave and heat-assisted extracted Melilotus officinalis as a potential eco-friendly corrosion inhibitor for carbon steel in 0.5 M HCl solution [J]. Cleaner Chemical Engineering, 2025, 11.
- THIRUMALAIKUMARASAMY D, SHANMUGAM K, BALASUBRAMANIAN V. Comparison of the corrosion behaviour of AZ31B magnesium alloy under immersion test and potentiodynamic polarization test in NaCl solution [J]. Journal of Magnesium and Alloys, 2014, 2 (1) : 36-49.
- DE GROOT M T, VERMEULEN P. Advanced characterization of alkaline water electrolysis through electrochemical impedance spectroscopy and polarization curves [J]. Journal of Electroanalytical Chemistry, 2024, 974.
- WANG C-J, KUSDIYARTO P, LI Y-H. Potentiodynamic polarization analysis with various corrosion inhibitors on A508/IN-182/IN-52M/308L/316L welds [J]. Kuwait Journal of Science, 2024, 51 (2).
- ABUBAKR H M C, GHAITH M E, EL-SHERIF A A, EL-DEAB M S. Influence of metal ions on cysteine as a corrosion inhibitor for low carbon steel in sulfuric acid: Polarization, EIS, XPS, and DFT analysis [J]. International Journal of Electrochemical Science, 2024, 19 (7).
- AYODELE O O, BABALOLA B J, BORODE A O, et al. Effect of titanium diboride addition on the corrosion behaviour of sintered titanium composites in acidic and sodium chloride environments [J]. Materials Today Communications, 2025, 43.
- SHU X, WANG H, ZHAO J. Microstructures and corrosion behaviors under sodium chloride aqueous conditions of Co-free non-equiatomic Al0.32CrFeTi0.73 (Ni1.50-xMox) (x=0, 0.23) high entropy alloys [J]. Journal of Materials Research and Technology, 2024, 33: 834-44.
- CHIDIEBERE M A, ANADEBE V C, BARIK R C. Assessment of the inhibition performance of ZIF-8 on corrosion Mitigation of API 5L X65 steel in 3.5wt% NaCl: Experimental and theoretical insight [J]. Journal of Materials Research and Technology, 2024, 33: 2879-98.
- ALAMRI A H, RASHEEDA K, KAMAL S J, et al. Pyrimidine derivatives as efficient anticorrosive agents for acid corrosion of mild steel: Electrochemical and computational validation [J]. Arabian Journal of Chemistry, 2024, 17 (6).
- CHAUDHARY M Y, GUPTA M, BANSAL P, et al. Allyl triphenyl phosphonium bromide, an ionic liquid as an eco-friendly and green inhibitor for corrosion of aluminium in hydrochloric acid: Mechanistic insights and experimental validation [J]. Sustainable Chemistry for the Environment, 2025, 9.
- KOKALJ, A. On the use of the Langmuir and other adsorption isotherms in corrosion inhibition [J]. Corrosion Science, 2023, 217.
- ONUKWULI O D, NNANWUBE I A, OCHILI F O, OBIBUENYI J I. Assessing the efficiency of danacid as corrosion inhibitor for aluminium in HCl medium: Experimental, theoretical and optimization studies [J]. Heliyon, 2024, 10 (24).
- DOHARE P, QURAISHI M A, VERMA C, et al. Ultrasound induced green synthesis of pyrazolo-pyridines as novel corrosion inhibitors useful for industrial pickling process: Experimental and theoretical approach [J]. Results in Physics, 2019, 13.
- MU'AZU N D, HALADU S A, ALGHAMDI J M, et al. Inhibition of low carbon steel corrosion by a cationic gemini surfactant in 10wt.% H2SO4 and 15wt.% HCl under static condition and hydrodynamic flow [J]. South African Journal of Chemical Engineering, 2023, 43: 232-44.
- MUSTAFA A M, SAYYID F F, BETTI N, et al. Inhibition of mild steel corrosion in hydrochloric acid environment by 1-amino-2-mercapto-5- (4- (pyrrol-1-yl) phenyl) -1, 3, 4-triazole [J]. South African Journal of Chemical Engineering, 2022, 39: 42-51.
- P P K, G A, MISHMA J N C, et al. New benzisoxazole derivative: A potential corrosion inhibitor for mild steel in 0.5 M hydrochloric acid medium -insights from electrochemical and density functional theory studies [J]. Heliyon, 2023, 9 (10).
- BELKHEIRI A, DAHMANI K, MZIOUD K, et al. Assessment of 14- (4-nitrophenyl) -14H-dibenzo[a, j]xanthene as an effective organic corrosion inhibitor for mild steel in 1 M HCl: Electrochemical, theoretical, and surface analysis [J]. International Journal of Electrochemical Science, 2025, 20 (1).
- ABDULLAH K A, ALLOUSH F A, SALAME C. Investigation of the Monocrystalline Silicon Solar Cell Physical Behavior after Thermal Stress by AC Impedance Spectra [J]. Energy Procedia, 2014, 50: 30-40.
- CHEN X, WANG Z, YU S, LI G. Corrosion inhibition of carbon steel in NaCl solution Using a mixture of alkanol amine and calcium nitrite: Electrochemical and microscopic evaluation [J]. International Journal of Electrochemical Science, 2024, 19 (11).
- LAN W, CHEN Z, ZHANG X, et al. Corrosion resistance analysis of Al2O3-TiO2 composite ceramic coatings on carbon steel pipe surfaces [J]. Alexandria Engineering Journal, 2025, 110: 377-85.
- BELGHITI M E, BOUAZAMA S, ECHIHI S, et al. Understanding the adsorption of newly Benzylidene-aniline derivatives as a corrosion inhibitor for carbon steel in hydrochloric acid solution: Experimental, DFT and molecular dynamic simulation studies [J]. Arabian Journal of Chemistry, 2020, 13 (1) : 1499-519.
- KANNAN P, VARGHESE A, PALANISAMY K, ABOUSALEM A S. Probing the effect of newly synthesized phenyltrimethylammonium tetrachloroaluminate ionic liquid as an inhibitor for carbon steel corrosion [J]. Applied Surface Science Advances, 2021, 6.
- NAHLé A, SALIM R, EL HAJJAJI F, et al. Experimental and theoretical approach for novel imidazolium ionic liquids as Smart Corrosion inhibitors for mild steel in 1.0 M hydrochloric acid [J]. Arabian Journal of Chemistry, 2022, 15 (8).
- SHTEFAN V, KANUNNIKOVA N, ZUYOK V. Comparative evaluation of microstructure and electrochemical, high-temperature corrosion rates of titanium- and aluminum-modified black chromium coatings on AISI 304 stainless steel [J]. Surface and Coatings Technology, 2025, 497.
- JIANG H, WANG B, LIU J, et al. Corrosion inhibition of Q235 and X65 steels in CO2-saturated solution by 2-phenyl imidazoline [J]. Arabian Journal of Chemistry, 2023, 16 (6).
- WANG Y, YANG Z, HU H, et al. Indolizine quaternary ammonium salt inhibitors: The inhibition and anti-corrosion mechanism of new dimer derivatives from ethyl acetate quinolinium bromide and n-butyl quinolinium bromide [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 651.
- BELKHEIRI A, DAHMANI K, KHATTABI M, et al. Evaluation of 14- (p-tolyl) -14H-dibenzo[a, j]xanthene as a highly efficient organic corrosion inhibitor for mild steel in 1 M HCl: Electrochemical, theoretical, and surface characterization [J]. International Journal of Electrochemical Science, 2024, 19 (12).
- WU G, LI L, CHEN X, et al. The growth mechanism and corrosion resistance of laser-assisted plasma electrolytic oxidation (PEO) composite coating on AZ31B magnesium alloy [J]. Journal of Magnesium and Alloys, 2024.
- WAHBA R H, EL-SONBATI A Z, DIAB M A, et al. Electrochemical Corrosion Performance of N80 steel in Acidized 10% HCl medium using 4-methyl-1-Phenyl-3- (p-tolyldiazenyl) −2, 3-dihydro-1H-pyrrol-2-ol [J]. Heliyon, 2025, 11 (3).
- GONI L K M O, ALI S A, AL-MUALLEM H A, JAFAR MAZUMDER M A. Synthesis of a new quaternary ammonium salt for efficient inhibition of mild steel corrosion in 15 % HCl: Experimental and theoretical studies [J]. Heliyon, 2024, 10 (19).
- VASTAG G, FELHŐSI I, VRANEŠ M, SHABAN A. Impact of N-decyl-nicotineamide bromide on copper corrosion inhibition in acidic sulfate containing environment: Electrochemical and piezoelectrochemical insights [J]. Heliyon, 2024, 10 (22).
- MERINO E, CHANDRASEKAR A R, PAKSERESHT A, et al. Improved corrosion resistance of AZ31B Mg alloy by eco-friendly flash-PEO coatings [J]. Applied Surface Science Advances, 2024, 20.
- MUTLU I H, EMRE M C, KAYA A O. A comparison of the corrosion resistance of galvanized low steel with solgel method coated ZrO2, ZrO2+Polymer coating [J]. Kuwait Journal of Science, 2023, 50 (4) : 524-38.
- ARIBOU Z, OUAKKI M, EL HAJRI F, et al. Comprehensive assessment of the corrosion inhibition properties of quinazoline derivatives on mild steel in 1.0 M HCl solution: An electrochemical, surface analysis, and computational study [J]. International Journal of Electrochemical Science, 2024, 19 (11).
- CHEN S, FAN J, LEI T, et al. Effect of oxalic acid on the corrosion of 6063 aluminum alloy in ethylene glycol-water solution in presence of ammonium alcohol polyvinyl phosphate [J]. International Journal of Electrochemical Science, 2024, 19 (4).
- LIAO J, CHENG Z, ZHANG W, et al. Temperature sensitivity and transition kinetics of uniform corrosion of zirconium alloys in superheated steam [J]. Heliyon, 2024, 10 (12).
- SHAHI N, SHAH S K, SINGH S, et al. Comparison of dodecyl trimethyl ammonium bromide (DTAB) and cetylpyridinium chloride (CPC) as corrosion inhibitors for mild steel in sulphuric acid solution [J]. International Journal of Electrochemical Science, 2024, 19 (5).
- FAWZY A, ALDUAIJ O K, AL-BAHIR A, et al. A comparative study of pyridine and pyrimidine derivatives based formamidine for copper corrosion inhibition in nitric acid: Experimental and computational exploration [J]. International Journal of Electrochemical Science, 2024, 19 (1).
- ALTALHI A, A. Novel N-heterocyclic Schiff base based on Isatin derivative as a sustainable, eco-friendly, and highly efficiency corrosion inhibitor for carbon steel in sulfuric acid medium: Electrochemical and Computational investigation [J]. International Journal of Electrochemical Science, 2024, 19 (1).
- EL-HAITOUT B, SARDJONO R E, ES-SOUNNI B, et al. Electrochemical and quantum chemical investigation on the adsorption behavior of a schiff base and its metal complex for corrosion protection of mild steel in 15 wt% HCl solution [J]. Heliyon, 2024, 10 (23).
- SHAO H, YIN X, ZHANG K, et al. N-[2- (3-indolyl) ethyl]-cinnamamide synthesized from cinnamomum cassia presl and alkaloid tryptamine as green corrosion inhibitor for Q235 steel in acidic medium [J]. Journal of Materials Research and Technology, 2022, 20: 916-33.
- EMRAYED H F, AMRAGA E A, IBRAHIM D M, FOUDA A E-A S. Corrosion inhibition effect of Schiff base and its metal complexes with [Mn (II) and Cu (II) ] on carbon steel in hydrochloric acid solution: Experimental and surface studies [J]. International Journal of Electrochemical Science, 2025, 20 (1).
- EL-MORSY F E, ALAHMAR N M, ALHEBSHE N S, MADKHALI M M M. One-pot synthesis of Schiff base as an excellent corrosion inhibition for Inconel 800 alloy in sulfuric acid medium: Experimental, DFT calculation and spectroscopic inspections [J]. International Journal of Electrochemical Science, 2025, 20 (3).
- AL-GORAIR A S, AL-HABAL T, EL-SAYED R, et al. Investigations of non-ionic surfactants derived from triazoles and pyrroles as potent corrosion inhibitors for carbon steel in hydrochloric acid [J]. International Journal of Electrochemical Science, 2023, 18 (9).
- MATINE A, LIZOUL B, EL ALAOUI EL ABDALLAOUI H, et al. Investigation of 2-ethoxy-4- (oxiran-2-ylmethyl) phenol as a potentially effective anti-corrosion agent for C38 steel [J]. International Journal of Electrochemical Science, 2024, 19 (12).
- MOHSEN O A, FARAJ M W, DARWESH T M, et al. Indole derivatives efficacy and kinetics for inhibiting carbon steel corrosion in sulfuric acid media [J]. Results in Engineering, 2024, 23.
- BELKHEIRI A, DAHMANI K, MZIOUD K, et al. Advanced evaluation of novel quinoline derivatives for corrosion inhibition of mild steel in acidic environments: A comprehensive electrochemical, computational, and surface study [J]. International Journal of Electrochemical Science, 2024, 19 (10).
- PAN J, HE X, CAO K. Electrochemical and theoretical studies of two amino acid ionic liquids as corrosion inhibitors for mild steel in 3.5 wt% NaCl solution [J]. International Journal of Electrochemical Science, 2025, 20 (2).
- UDUNWA D I, ONUKWULI O D, MENKITI M C, et al. 1-Butyl-3-methylimidazolium methane sulfonate ionic liquid corrosion inhibitor for mild steel alloy: Experimental, optimization and theoretical studies [J]. Heliyon, 2023, 9 (7).
- XHANARI K, FARRUKU M, BERISHA A, et al. 2-Amino-6-methylbenzothiazole as corrosion inhibitor for low carbon steel in acidic solution: Experimental and theoretical studies [J]. Results in Chemistry, 2025, 13.
- BELKHEIRI A, DAHMANI K, ARIBOU Z, et al. In-depth study of a newly synthesized imidazole derivative as an eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Theoretical, electrochemical, and surface analysis perspectives [J]. International Journal of Electrochemical Science, 2024, 19 (10).
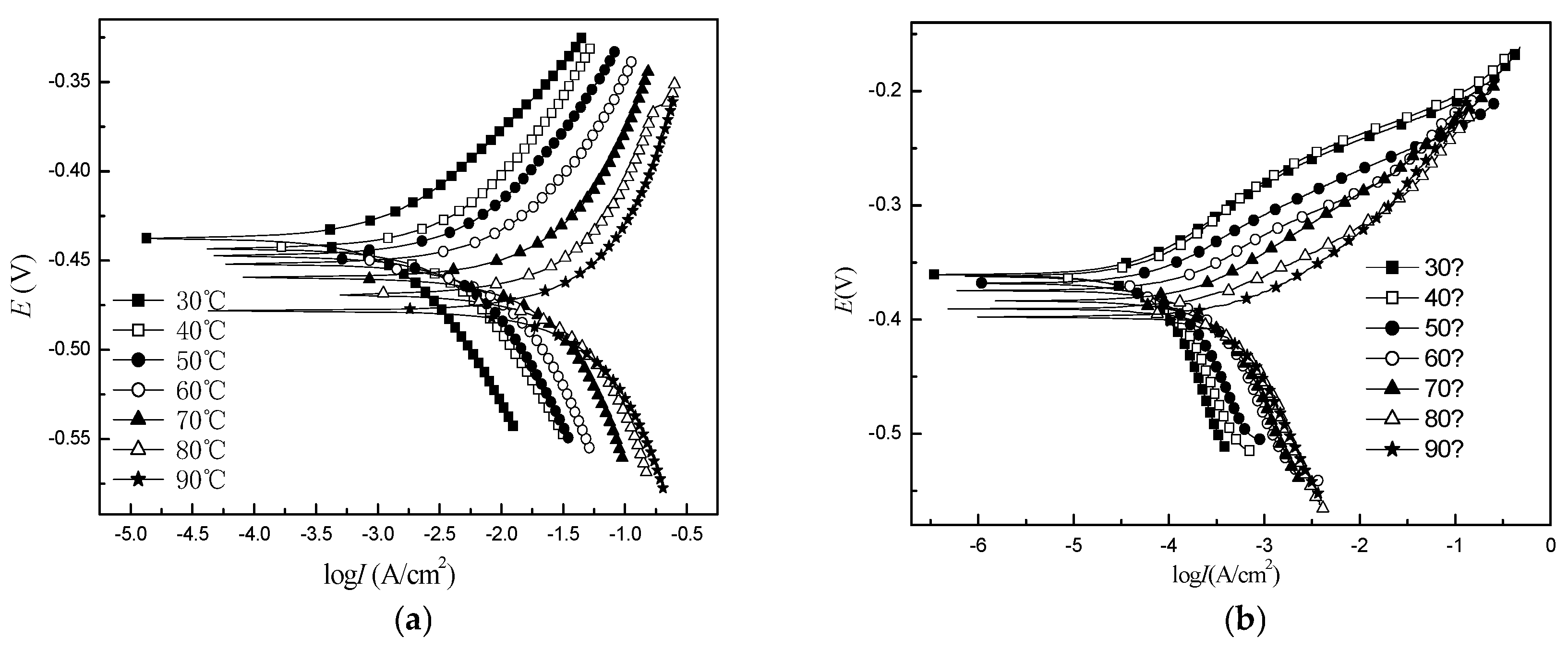
|
Concentration (mol/L) |
Corrosion potential (V) |
Anode Tafel Slope (mV) |
Cathode Tafel Slope (mV) |
corrosion current Density (A/cm2) |
inhibition efficiency (100%) |
| 0 | -0.47 | 79.0 | 84.0 | 2.0×10-2 | -- |
| 0.0050 | -0.45 | 80.4 | 119.1 | 2.3×10-3 | 88.5 |
| 0.0125 | -0.43 | 60.3 | 125.4 | 1.6×10-3 | 92.0 |
| 0.0200 | -0.42 | 64.5 | 110.3 | 1.1×10-3 | 94.5 |
| 0.0300 | -0.40 | 65.9 | 106.4 | 8.6×10-4 | 95.7 |
| 0.0500 | -0.39 | 67.0 | 103.8 | 5.2×10-4 | 97.4 |
|
Concentration (mol/L) |
Rs (Ω.cm2) |
CPE1-T (F/cm2) |
CPE1-P |
Rf (Ω.cm2) |
CPE1-T (F/cm2) |
CPE1-P |
Rt (Ω.cm2) |
| 0 | 0.25 | 9.15e-4 | 0.98 | 0.16 | 7.25e-4 | 0.71 | 0.13 |
| 0.0050 | 0.18 | 1.52e-4 | 0.63 | 1.11 | 5.71e-5 | 0.83 | 1.12 |
| 0.0125 | 0.41 | 3.83e-4 | 0.62 | 1.54 | 1.45e-4 | 0.81 | 1.74 |
| 0.0200 | 0.29 | 2.9e-4 | 0.69 | 2.79 | 1.32e-4 | 0.83 | 2.58 |
| 0.0300 | 0.27 | 3.4e-4 | 0.73 | 4.25 | 2.2e-4 | 0.79 | 3.76 |
| 0.0500 | 0.30 | 4.12e-4 | 0.70 | 6.71 | 2.51e-4 | 0.79 | 5.65 |
|
Corrosion inhibitor concentration (mol/L) |
Temperature (K) |
Corrosion potential (mV) |
Anode Tafel Slope (mV) |
Cathode Tafel Slope (mV) |
Corrosion current Density (A/cm2) |
| 303.2 | -438.5 | 68.4 | 100.7 | 3.5×10-3 | |
| 313.2 | -443.4 | 75.0 | 89.2 | 4.8×10-3 | |
| 323.2 | -447.5 | 71.4 | 94.8 | 7.2×10-3 | |
| 0 | 333.2 | -452.2 | 73.3 | 91.6 | 8.7×10-3 |
| 343.2 | -459.1 | 75.8 | 88.0 | 1.1×10-2 | |
| 353.2 | -469.1 | 80.0 | 83.0 | 1.7×10-2 | |
| 363.2 | -477.9 | 80.3 | 82.6 | 1.9×10-2 | |
| 303.2 | -360.7 | 62.5 | 116.9 | 5.98×10-5 | |
| 313.2 | -362.0 | 67.2 | 103.4 | 7.28×10-5 | |
| 323.2 | -368.1 | 62.1 | 118.3 | 1.01×10-4 | |
| 0.050 | 333.2 | -374.6 | 63.9 | 112.4 | 1.92×10-4 |
| 343.2 | -383.6 | 61.5 | 120.5 | 2.61×10-4 | |
| 353.2 | -390.5 | 51.8 | 189.9 | 3.72×10-4 | |
| 363.2 | 397.7 | 54.9 | 157.5 | 5.20×10-4 |
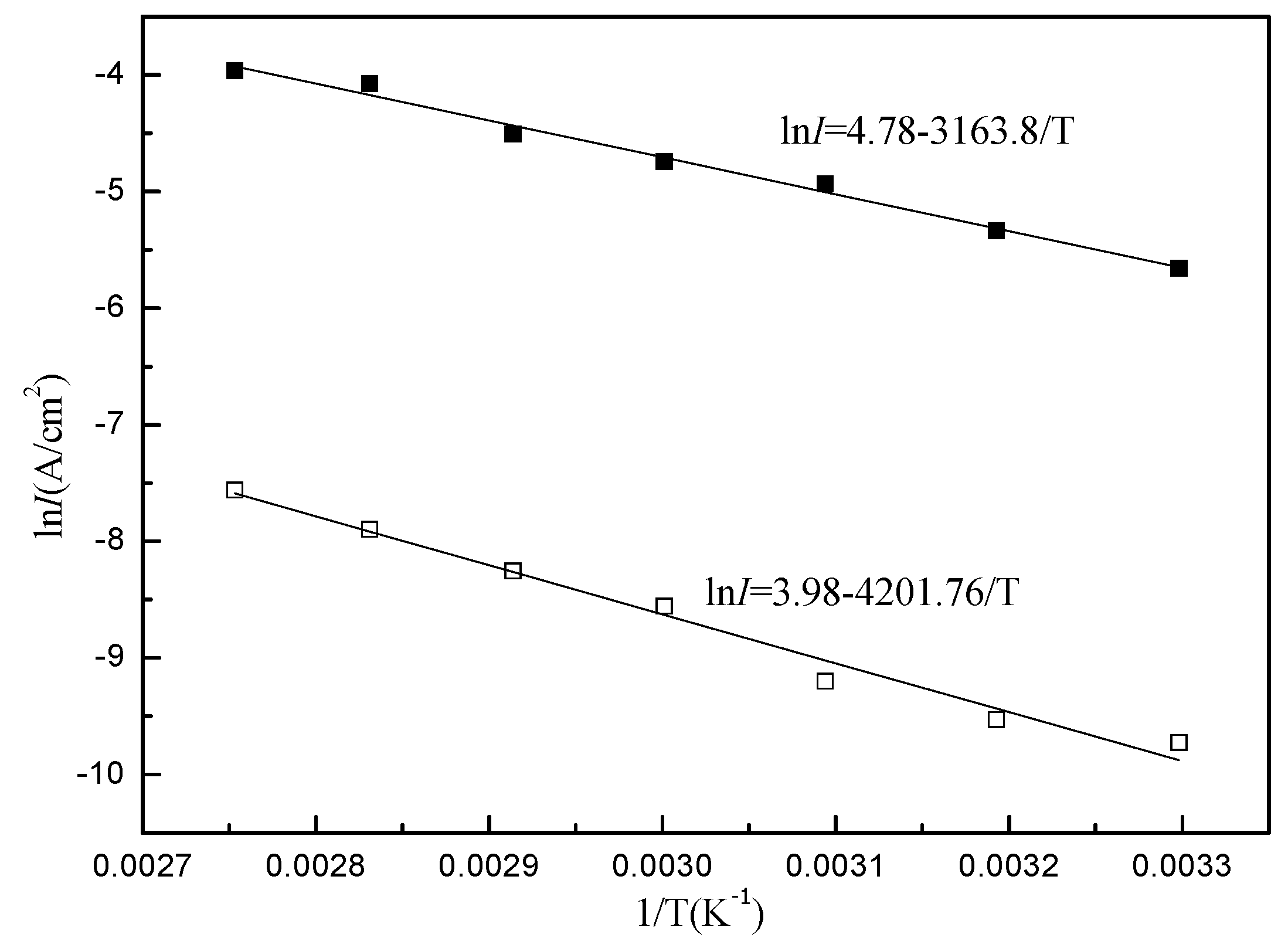
|
Concentration (mol/L) |
Pre-exponential factor A (A/cm2) |
Activation energy Ea (J/mol) |
| 0 | 4.78 | 2.6×104 |
| 0.0056 | 3.98 | 3.5×104 |
| Acid type | inhibitor concentration | temperature (°C) |
pressure (Atmospheric pressure) | corrosion rate g/ (m2 • h) |
Remark |
| multi-hydrogen acid | 2% | 120 | 160 | 7.5 | specimen brightness |
| 3% | 140 | 160 | 12.2 | specimen brightness | |
| 4% | 160 | 160 | 18.7 | specimen brightness | |
| 15%HCl | 2% | 120 | 160 | 12.8 | specimen brightness |
| 3% | 140 | 160 | 18.6 | specimen brightness | |
| 4% | 160 | 160 | 21.2 | specimen brightness | |
| 12%HCl+3%HF | 2% | 120 | 160 | 9.4 | specimen brightness |
| 3% | 140 | 160 | 14.3 | specimen brightness | |
| 4% | 160 | 160 | 17.2 | specimen brightness |
| Acid type | Inhibitor concentration | Temperature (°c) |
Pressure (atmospheric pressure) |
Corrosion rate G/ (m2 • h) |
Remark |
| multi-hydrogen acid | 3% | 140 | 160 | 10.6 | After alcohol and acetone wipe, the test piece is bright |
| 4% | 160 | 160 | 16.1 | ||
| 4.5% | 180 | 160 | 23.2 | ||
| 15%HCl | 3% | 140 | 160 | 15.4 | |
| 4% | 160 | 160 | 19.2 | ||
| 4.5% | 180 | 160 | 26.1 | ||
| 12%HCl+3%HF | 3% | 140 | 160 | 11.9 | |
| 4% | 160 | 160 | 17.8 | ||
| 4.5% | 180 | 160 | 24.7 |
| Acid liquor | Inhibitor concentration | Formula | Acid- solubility |
| multi-hydrogen acid | 5% | 30% quaternary ammonium salt +50% alcohol +20% OP-10 | precipitation |
| 30% quaternary ammonium salt +60% alcohol +10% peregal | precipitation | ||
| 30% quaternary ammonium salt +50% isopropyl alcohol +20% OP-10 | precipitation | ||
| 30% quaternary ammonium salt +60% isopropyl alcohol +10% peregal | homogeneous solution | ||
| 15%HCl | 5% | 30% quaternary ammonium salt +50% alcohol +20% OP-10 | precipitation |
| 30% quaternary ammonium salt +60% alcohol +10% peregal | precipitation | ||
| 30% quaternary ammonium salt +50% isopropyl alcohol +20% OP-10 | precipitation | ||
| 30% quaternary ammonium salt +60% isopropyl alcohol +10% peregal | homogeneous solution | ||
| mud acid | 5% | 30% quaternary ammonium salt +50% alcohol +20% OP-10 | precipitation |
| 30% quaternary ammonium salt +60% alcohol +10% peregal | precipitation | ||
| 30% quaternary ammonium salt +50% isopropyl alcohol +20% OP-10 | precipitation | ||
| 30% quaternary ammonium salt +60% isopropyl alcohol +10% peregal | homogeneous solution |
| Acid type | Inhibitor concentration | Dispersant concentration | Temperature (°c) |
Pressure (atmospheric pressure) |
Corrosion rate G/ (m2 • h) |
Remark |
| multi-hydrogen acid | 4% | 5% | 160 | 160 | 41.6 | specimen brightness |
| 4.5% | 5% | 180 | 160 | 62.8 | specimen brightness | |
| 5% | 5% | 190 | 160 | 76.2 | relatively bright | |
| 15%HCl | 4% | 5% | 160 | 160 | 59.1 | specimen brightness |
| 4.5% | 5% | 180 | 160 | 76.9 | relatively bright | |
| 5% | 5% | 190 | 160 | 83.7 | Slight blackening | |
| 12%HCl+ 3%HF |
4% | 5% | 160 | 160 | 48.6 | specimen brightness |
| 4.5% | 5% | 180 | 160 | 71.4 | relatively bright | |
| 5% | 5% | 190 | 160 | 79.4 | Slight blackening |
| Acid type | Inhibitor concentration | Dispersant concentration | Temperature (°c) |
Pressure (atmospheric pressure) | Corrosion rate G/ (m2• h) |
Remark |
| multi-hydrogen acid | 5.5% | 7% | 195 | 160 | 78.8 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 82.1 | Slight blackening | |
| 15%HCl | 5.5% | 7% | 195 | 160 | 90.2 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 94.5 | Slight blackening | |
| 12%HCl+ 3%HF |
5.5% | 7% | 195 | 160 | 84.2 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 88.5 | Slight blackening |
| Acid type | Inhibitor concentration | Dispersant concentration | Temperature (°c) |
Pressure (atmospheric pressure) |
Corrosion rate G/ (m2 •h) |
Remark |
| multi-hydrogen acid | 5.5% | 7% | 195 | 160 | 79. 1 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 82. 6 | Slight blackening | |
| 15%HCl | 5.5% | 7% | 195 | 160 | 93. 7 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 96. 1 | Slight blackening | |
| 12%HCl+ 3%HF |
5.5% | 7% | 195 | 160 | 86. 7 | Slight blackening |
| 5.5% | 7% | 200 | 160 | 89. 1 | Slight blackening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




