Submitted:
27 February 2025
Posted:
28 February 2025
You are already at the latest version
Abstract
P3HT is one of the most well-known conducting polymers, and it is still considered a promising candidate for new applications in its conductive p-doped state. According to the literature, polymer doping can be assessed through IR spectroscopy: the raise of strong IR absorption bands - the so-called IRAVs – is indeed the signature of polarons, which form on the polymer chain after the hole (or electron) injection by the dopant. While the mechanism of the IRAVs activation has been widely explored in the past and the IRAV bands can be reasonably predicted by means of state-of-the-art DFT calculations on model charged oligomers in vacuo, several peculiar features remain still unclear, and cannot be fully understood based on such idealized models. In addition, changes in the Raman spectrum of doped polymers (RaAV bands) are widely used for monitoring the doping process, but the interpretation is often limited to purely empirical correlations. This paper focuses on the comparative analysis of IRAVs and RaAVs of doped P3HT, aiming at a unified description of the structure of doped P3HT. By means of an experimental campaign on doped P3HT samples in chloroform solution, and on the solid samples cast from the same solutions, we will explore how modifications in the spectra can reveal different features of the molecular structure that can be related to the doping mechanism. In particular, we will discuss the effect of the doping level on the vibrational features of the polymer and the dopant, to unfold the spectroscopic markers of ICT complexes, and the role of the crystalline vs disordered phases.
Keywords:
1. Introduction
- which phases are preferentially probed (e.g. doped/charged domains are preferentially probed by IR);
- the relationship between given spectroscopic markers and specific structural features.
2. Results and Discussion
2.1. Ideal Models of Doped P3HT Chains and General Features of Their Vibrational Spectra
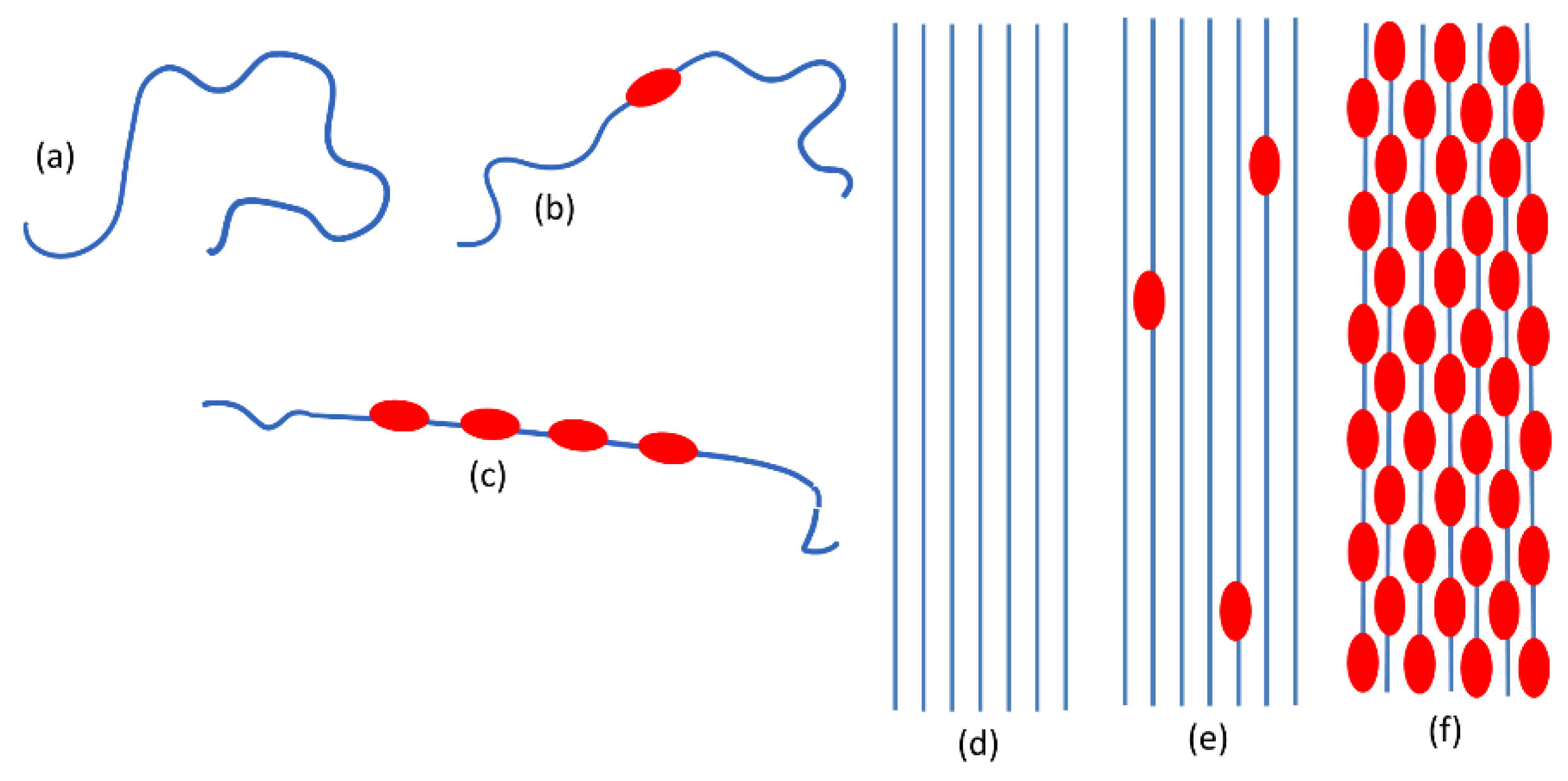
- Neutral chains which have a prevalently disordered conformation (both for the backbone and the alkyl chains). Neutral coils in Scheme 1.a.
- Slightly doped chains where only one or a few backbone segments are planar due to the presence of the polaron defect (even if more than one polaron defect is present they are far apart) while the remaining part of the polymer chain is conformationally distorted. Isolated polarons in Scheme 1.b.
- Heavily doped chains where long planar segments of the backbone are present (Scheme 1.c). In an ideal limiting case, the entire polymer backbone would be planar, and we might even think of an ideal 1D crystal with equally spaced polaron defects.
- Neutral, crystalline P3HT: the structure is the same as that of the pristine polymer with planar backbone chains regularly packed in a 3D crystalline structure (Scheme 1.d).
- P3HT crystals with isolated polarons. It is reasonable to think that the structure be similar to that of the pristine polymer perturbed just in proximity of the isolated polaron defects (Scheme 1.e).
- Chains with distorted conformation in the amorphous phase. Doping in the disordered amorphous phase seems unlikely since ICT would imply planarization of the backbone. Probably only with an excess of F4TCNQ some doping might occur. On the other hand, if ICT does occur, the planarization of the chain would favor clustering of the chains and hence the obtainment of an ordered 3D domain.
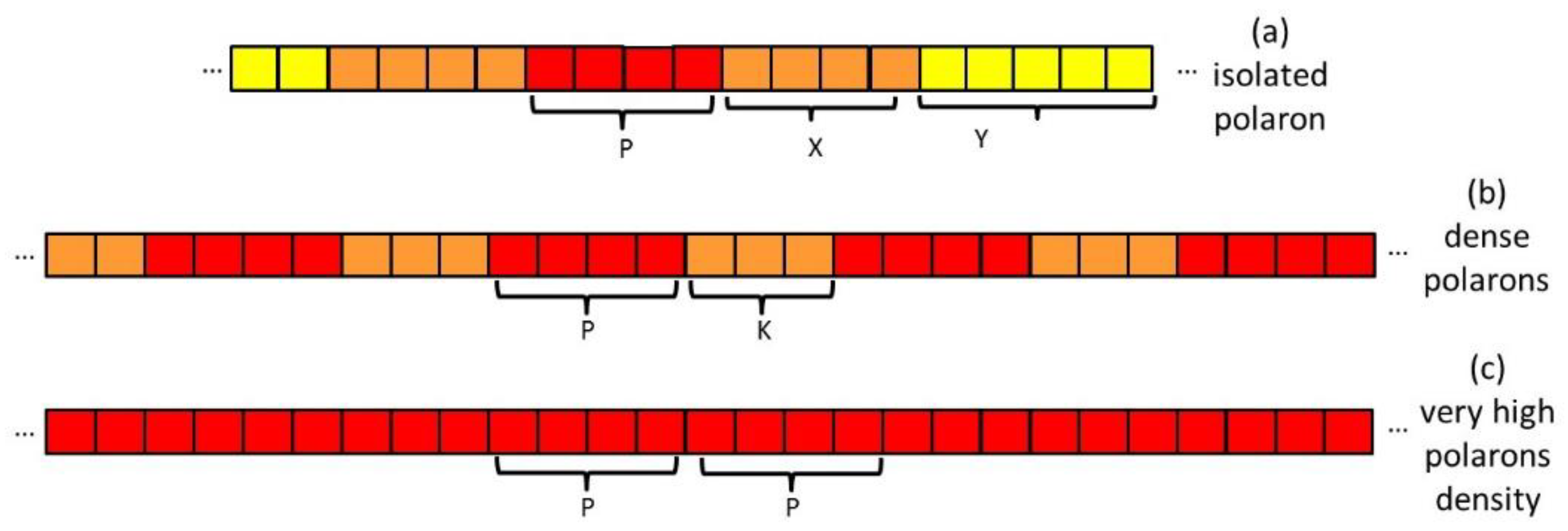
- Isolated polaron (Scheme 2.a). We can distinguish three regions of the polymer chain: 1) a charge defect of about 4-5 thiophene rings characterized by large spin density and polarization of the CC bonds (red region); 2) a perturbed region which extends to a certain number X of rings with perturbed geometry and force constants (orange region); 3) a planar and neutral region which extends for Y rings (yellow region). In the case of crystalline samples Y can be very large because of the planarization induced by the crystalline field.
- Region with high polaron concentration (Scheme 2.b). The charge defects are similar to the previous case and are separated by a perturbed region (K rings). We can assume that in this case the third region characterized by planar, neutral rings is lacking.
- Region with very high polaron concentration (Scheme 2.c) can be foreseen e.g. in the case of excess doping. In this case, the perturbed regions, which separate the individual charge defects, are vanishing small. The discussion of the Raman spectra (Section 3.4) will show that postulating the existence of such limiting structure is needed to explain experiments on samples prepared with a large excess of dopants.
- ECC modes are collective CC stretching modes which involve a whole planar backbone segment (yellow, red and orange regions of Scheme 2). The presence of structural defects can localize these modes, and the coupling with the alkyl chains through the linking C-C bond can activate IR and Raman modes which could not be seen in the spectrum of the pristine highly crystalline polymer.
- Frequency of ECC-like modes is sensitive to conjugation. It decreases for increasing conjugation length. Indeed, the comparison between solution and solid-state spectra of pristine P3HT shows a remarkable decrease in the ECC band frequency due to the planarization of the backbone. Moreover, upon doping, also the charge injection delocalized along the chain contributes to the lowering of the frequency through the change of the CC force constants associated to changes of BLA, towards a more quinoid structure.
- Raman intensity of ECC modes. In general, the polarizability and hence the Raman intensity associated to the stretching of a polar bond is lower than that of an apolar bond. Therefore, it is reasonable to assume that the modes with the highest Raman intensities are those associated to co-planar and neutral ring sequences. It is also well known that the intensity of collective modes is stronger than that of localized modes. From these observations one can conclude that the Raman spectrum is dominated by the vibrational modes of the planar, neutral thiophene segments. Thus, for example, in the Raman spectrum of pristine polythiophene the dominant contribution is that of the crystalline phase, while the amorphous fraction gives an almost negligible contribution. On the other hand, the Raman spectrum of heavily doped P3HT (presence of highly polar bonds) is weak and clear evidences of this will be given in Section 3.4.
- Intensity of the IRAV modes. Differently from the Raman spectrum, polar bonds give rise to a large infrared intensity. This explains the huge intensity of the ECC modes responsible for the IRAV bands, if we realize that they represent the vibrations of the polar CC bonds in the polaron defect and in the highly perturbed adjacent units.
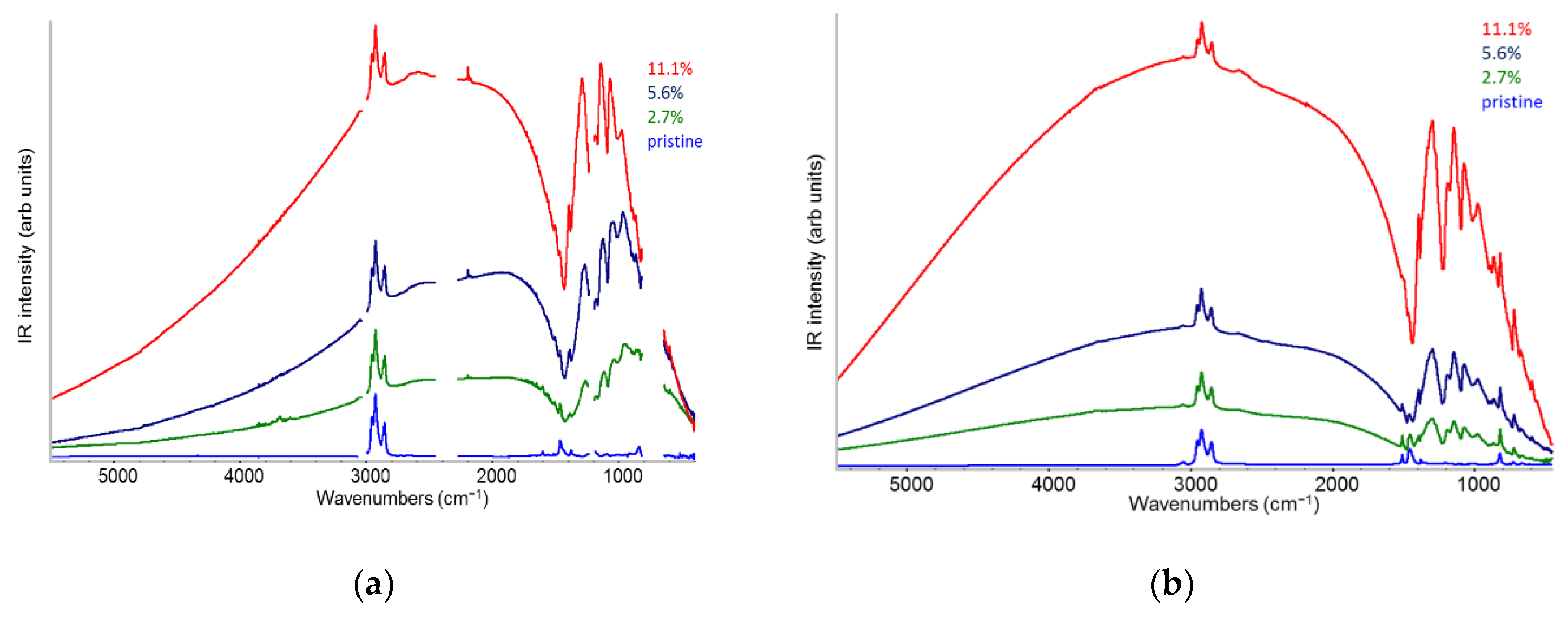
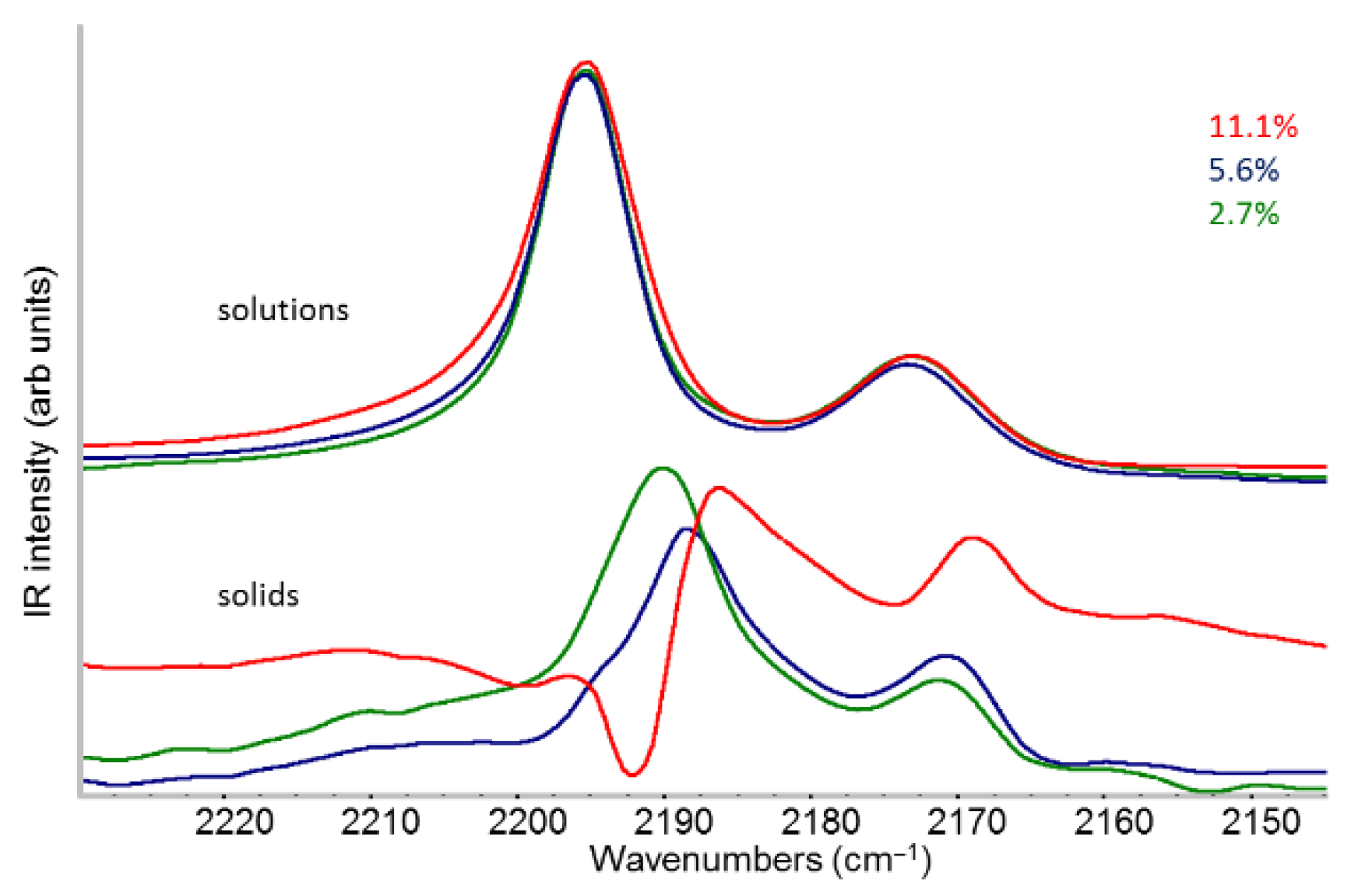
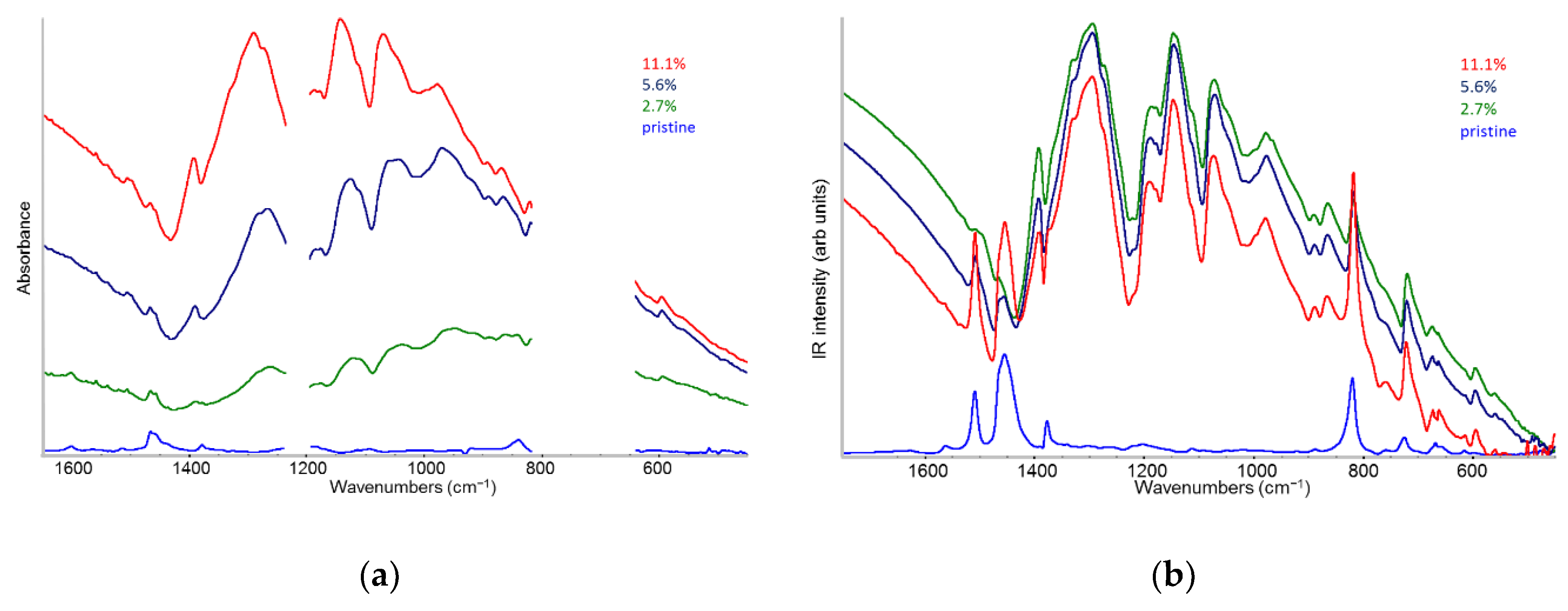
2.2. IR Features of F4TCNQ: Evidence of ICT Doping in Solution and in Solid-State
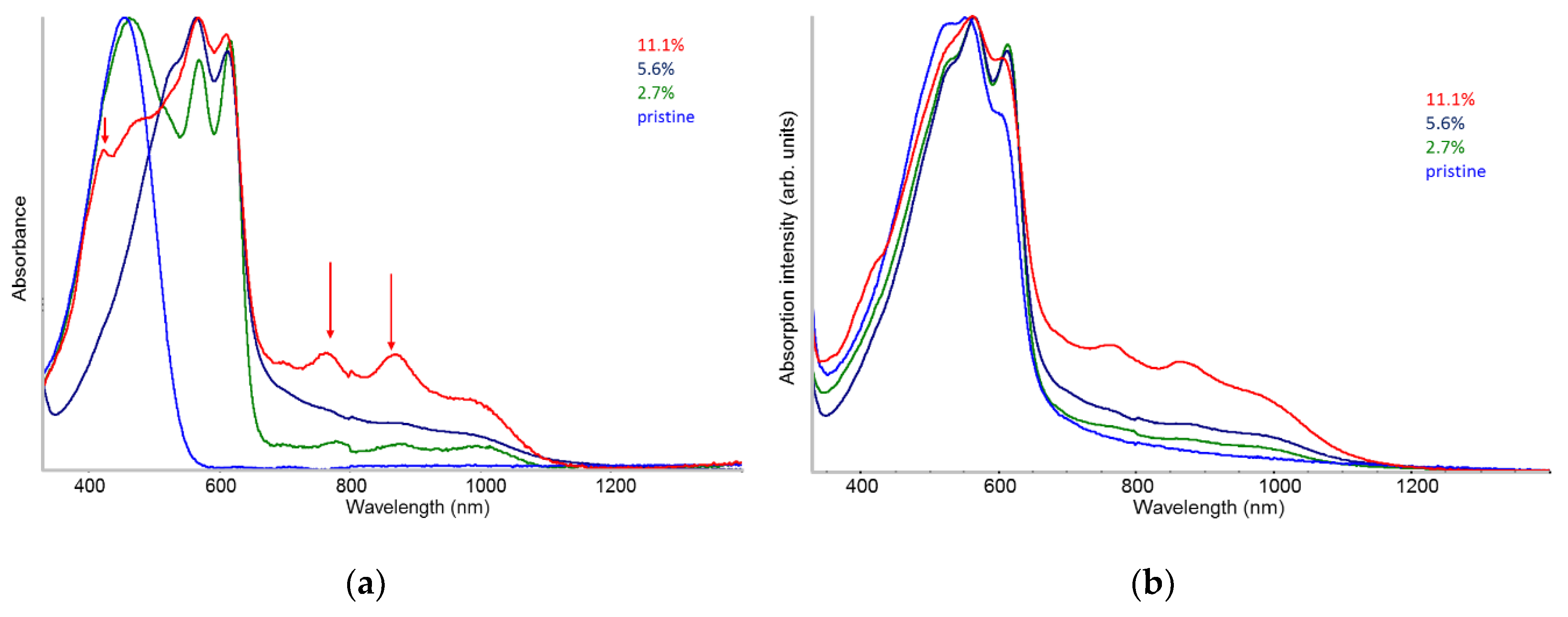
2.3. UV-vis-NIR Absorption in Solution and in Solid-State
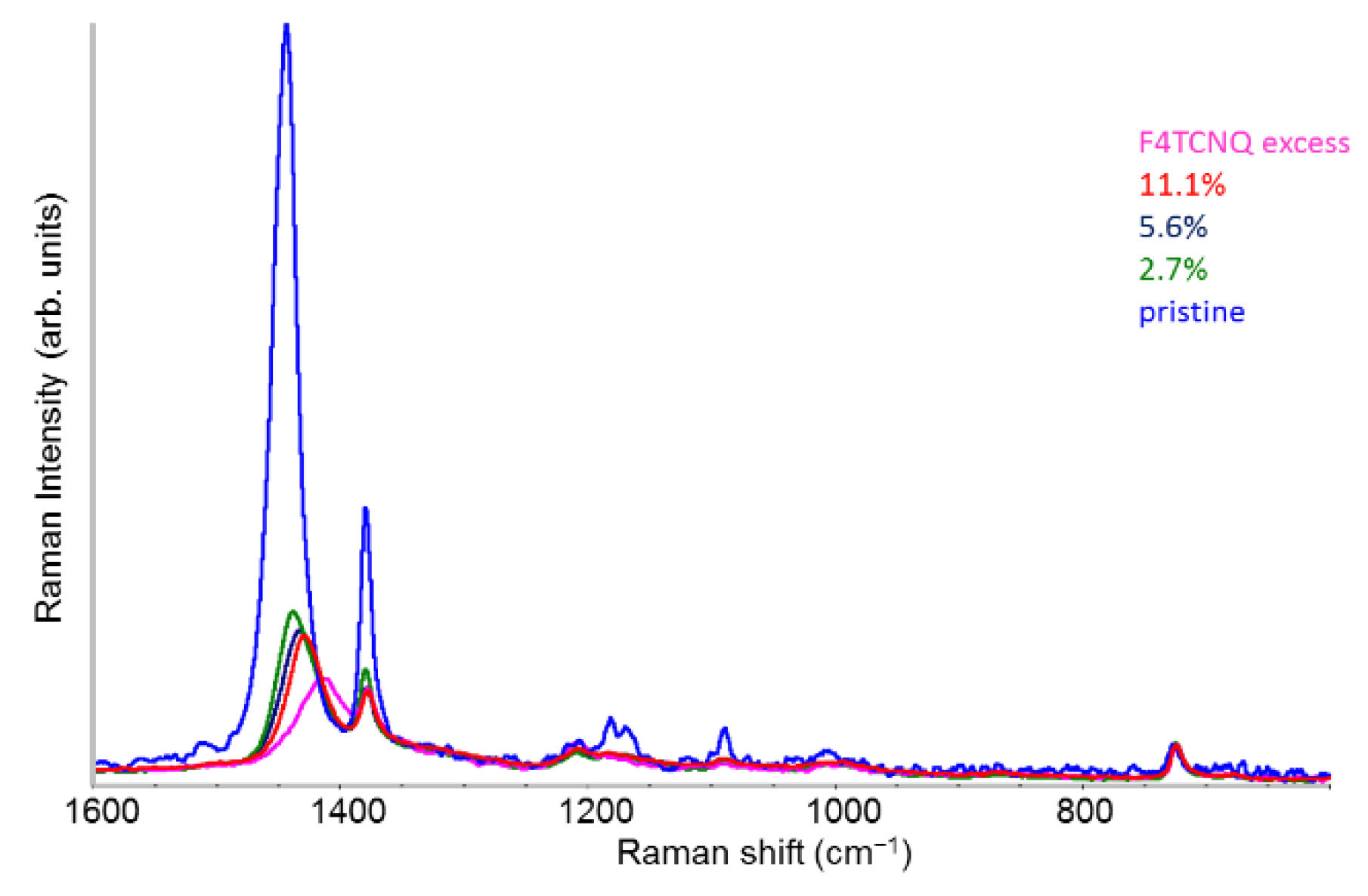
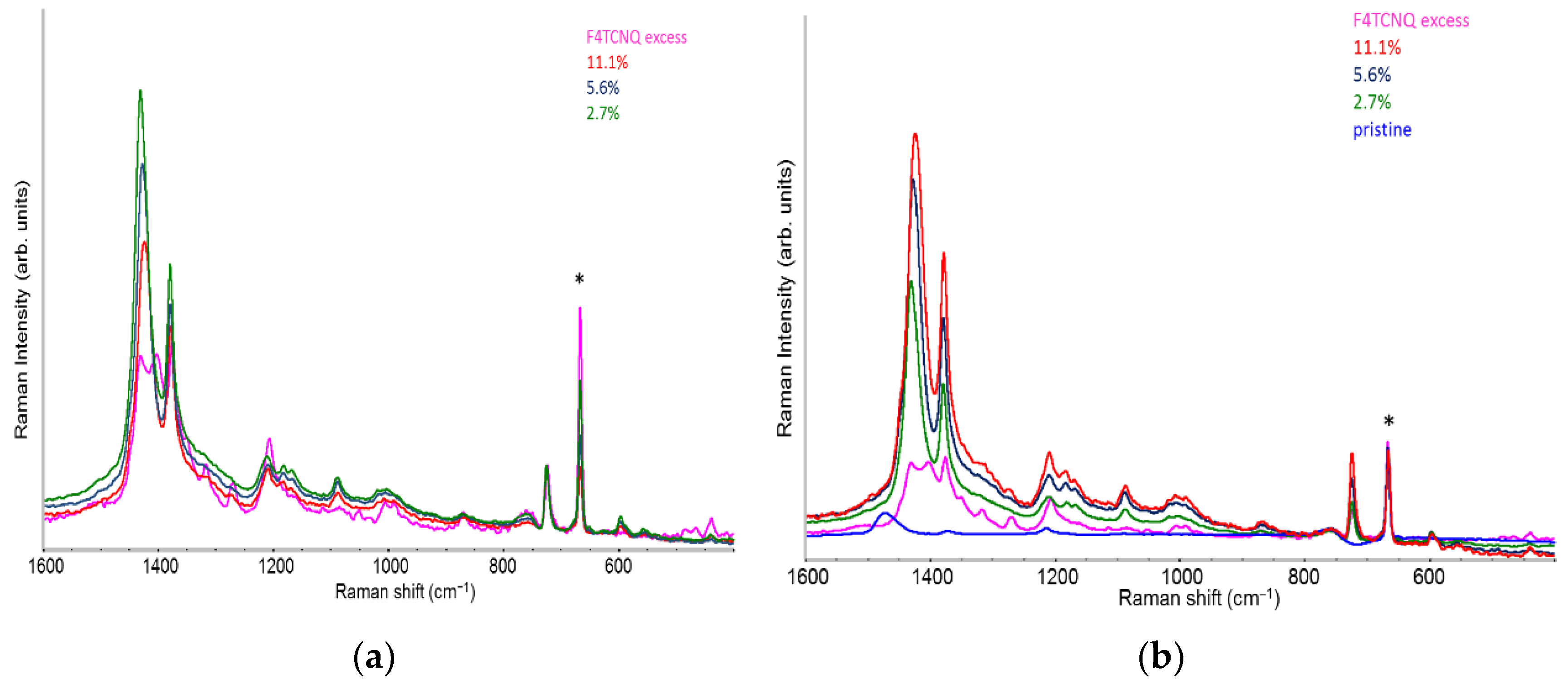
2.4. Raman Spectra of Doped P3HT: Evidence of Chain Planarization in Solution and Interplay of Different Phases
- a variety of different phases present in the material: for example, un-doped polymer chains and chains/crystal domains doped to a different extent, namely chains belonging to crystals which host diluted polaron defects (Scheme 1.e) or chains belonging to co-crystals with a dense polarons population (Scheme 1.f).
- different ECC-like normal modes, which mainly involve different domains – chain segments - of each doped chain. Scheme 2 helps to illustrate this feature, which can be corroborated considering that the chain segments affected by the doping (the red and orange boxes in Scheme 2.b) show a more quinoid geometry of the thiophene rings as well as CC stretching force constants different from those of the pristine case. This kind of phenomena often cause localization of the vibrational modes in the regions featuring different structural characteristic and a different vibrational dynamic.
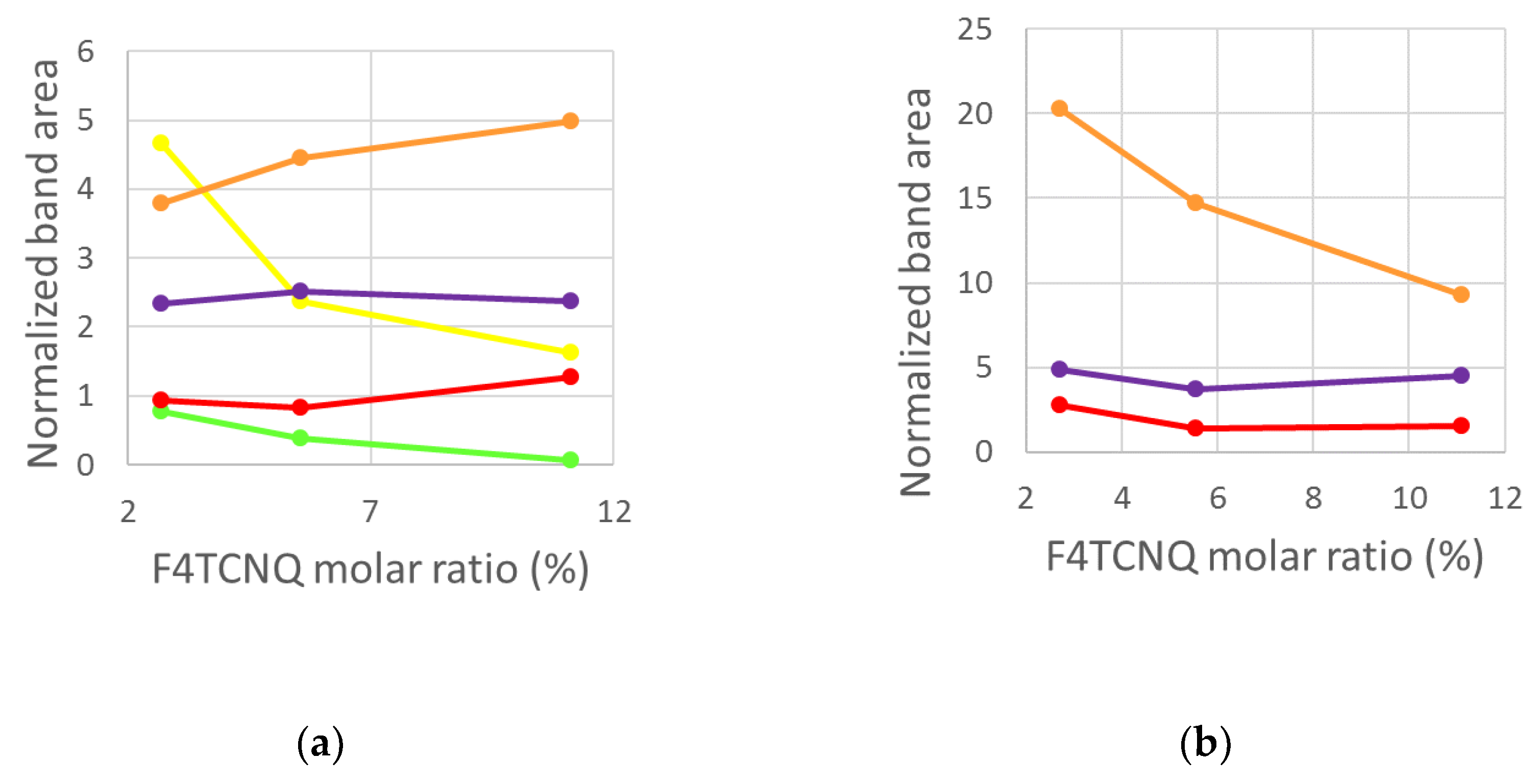
- Two Lorentzian components for a good fit of pristine, neutral domains. They correspond to the hairy A and hairy B crystalline phases of P3HT illustrated in ref. [28], with ECC modes at slightly different frequencies (1452, 1443 cm-1) respectively (modes of “yellow segments”).
- A Lorentzian component at about 1430 cm-1 which describes the RaAV component identified by means of spectra subtraction illustrated in Figure 7 (modes of “orange” segments).
- Two Lorentzian components for the highly perturbed phase, peaking at 1420 and 1410 cm-1 respectively (modes of “red segments”).
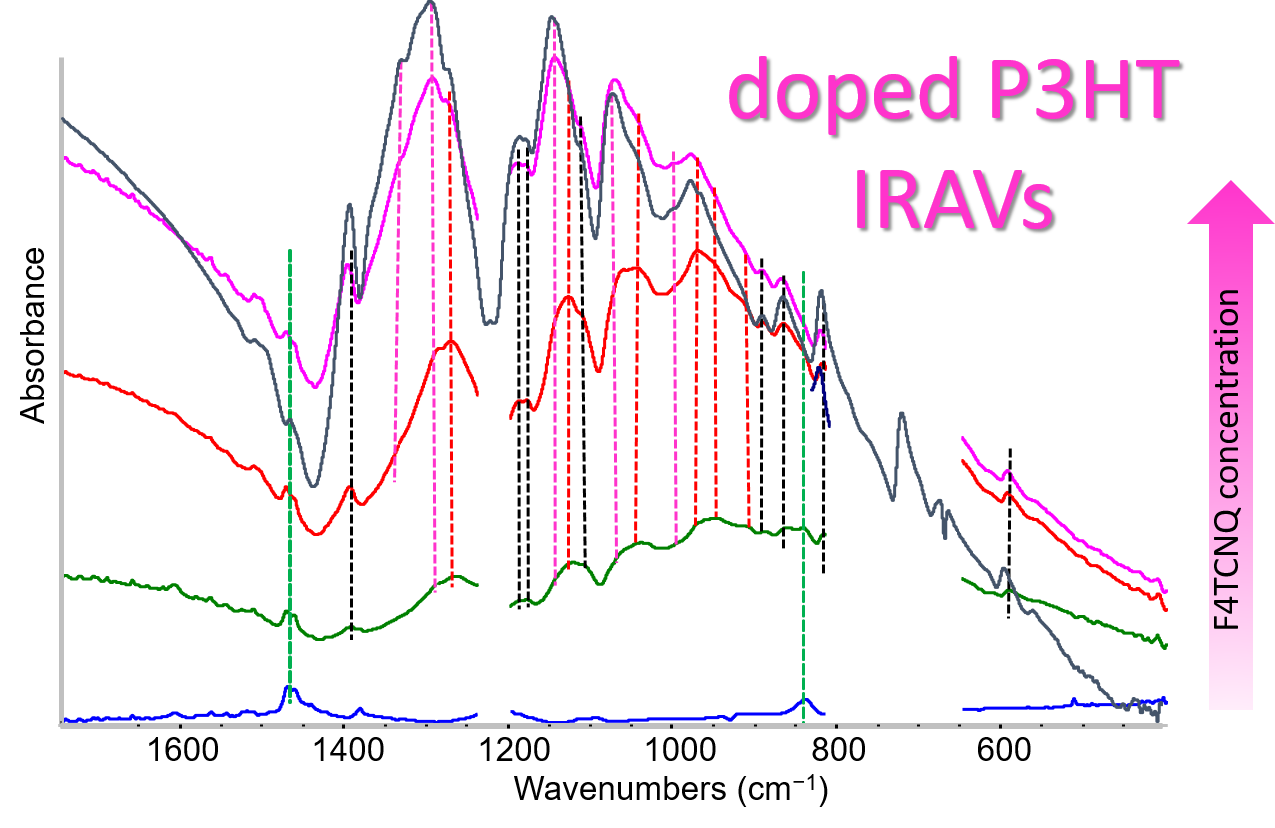
2.5. IR Spectra: Analysis of the IRAV Bands: Polarons on Isolated Chains and Polarons in 3-D Domains
3. Materials and Methods
4. Conclusions
- The evidence of the presence of different structures in solutions is revealed by the evolution of the IR pattern depending on the level of doping. We observe: (i) incipient IRAVs in the low doping case (dilute polarons on single P3HT chains; (ii) IRAVs of ordered doped domains (chains with a dense polaron population forming small aggregates in the solution) starting from 11.1% dopant concentration.
- In solid films obtained by casting from solutions, for all doping levels the IR spectra converge to the same IRAVs pattern that is attributed to the normal modes of the polarons belonging to solid crystalline domains, irrespective of the polaron density along the chains. IRAVs do not distinguish co-crystals of kind Π or IL, nor domains with diluted polaron defects.
- The spectra of solutions suggests that there is an optimal dopant concentration (~10%) that facilitates the formation of pre-aggregates with ordered local structure already in solution.
- The existence of planar (doped) chains in solution is proven by the appearance of the vibronic structure in the UV-vis absorption spectra, which shows an evolution highlighting clustering phenomena.
- Differently from IRAV bands, which probe charged domains and are not sensitive to sample morphology, the Raman spectra are rich of information about the polaron environment. The analysis of the different components which give rise to the main RaAV band provides a description of the doped P3HT chains as made by domains of different kinds, characterized by thiophene rings featuring a different geometry and more or less polar bonds. While the evolution of the Raman spectra with increasing doping gives meaningful insights into the evolution of the sample morphology, a quantitative estimate of the relative abundance of the different domains is not affordable so far. Indeed, the intrinsic Raman activity of the ECC normal modes confined in different domains is different, and a reliable estimate of its value is not yet available. Experiments strongly corroborate the hypothesis that it drastically decreases with increasing CC bond polarity.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, T.; Shirakawa, H.; Ikeda, S. Simultaneous polymerization and formation of polyacetylene film on the surface of concentrated soluble Ziegler-type catalyst solution. J. Polym. Sci., Polym. Chem. Ed. 1974, 12, 11. [Google Scholar]
- Bright, A. A.; Cohen, M. J.; Garito, A. F.; Heeger, A. J.; Mikulski, C. M.; MacDiarmid, A. G. Epitaxial crystalline films of the metallic polymer (SN)x. Appl. Phys. Lett. 1975, 26, 612. [Google Scholar]
- Shirakawa, H.; Louis, E. J.; MacDiarmid, A. G.; Chiang, C.K.; Heeger, A. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Commun. 1977, 578. [Google Scholar] [CrossRef]
- Chiang, C. K.; Fincher, Jr., C. R.; Park, Y. W.; Heeger, A. J.; Shirakawa, H.; Louis, E. Electrical Conductivity in Doped Polyacetylene. J. Phys. Rev. Lett. 1977, 39, 1098. [Google Scholar]
- Skotheim, T.A. Handbook of Conducting Polymers, Dekker: New York, USA 1986.
- Brédas, J.L.; Silbey, R. Conjugated Polymers: The Novel Science and Technology of Highly Conducting and Nonlinear Optically Active Materials. Springer: Dordrecht, Netherlands 1991.
- Zhao, X.; Zhan, X. Electron transporting semiconducting polymers in organic electronics. Chem. Soc. Rev. 2011, 40, 3728–3743. [Google Scholar]
- Fratini, S.; Nikolka, M.; Salleo, A.; Schweicher, G.; Sirringhaus, H. Charge transport in high-mobility conjugated polymers and molecular semiconductors. Nature Mater., 2020, 19, 491–502. [Google Scholar]
- Kim, T.; Yang, S.J.; Sung, S.J.; Kim, Y.S.; Chang, M.S.; Jung, H.; Park, C.R. Highly reproducible thermocontrolled electrospun fiber based organic photovoltaic devices. ACS Appl. Mater. Interfaces 2015, 7, 4481–4487. [Google Scholar]
- Zhao, W.; Ding, J.; Zou, Y.; Di, C.A.; Zhu, D. Chemical doping of organic semiconductors for thermoelectric applications. Chem. Soc. Rev. 2020, 49, 7210–7228. [Google Scholar]
- Lim, E.; Peterson, K.A.; Su, G.M.; Chabinyc, M.L. Thermoelectric Properties of Poly(3-hexylthiophene) (P3HT) Doped with 2,3,5,6-Tetrafluoro-7,7,8,8-tetracyanoquinodimethane (F4TCNQ) by Vapor-Phase Infiltration”. Chem. Mater. 2018, 30, 998–1010. [Google Scholar] [CrossRef]
- Lüssem, B.; Keum, C.M.; Kasemann, D.; Naab, B.; Bao, Z.; Leo, K. Doped Organic Transistors. Chem. Rev. 2016, 116, 13714–13751. [Google Scholar]
- Untilova, V.; Biskup, T.; Biniek, L.; Vijayakumar, V.; Brinkmann, M. Control of Chain Alignment and Crystallization Helps Enhance Charge Conductivities and Thermoelectric Power Factors in Sequentially Doped P3HT:F4TCNQ Films. Macromolecules. 2020, 53, 2441–2453. [Google Scholar] [CrossRef]
- Su, W. P.; Schrieffer, J. R.; Heeger, A. Solitons in Polyacetylene. J. Phys. Rev. Lett. 1979, 42, 1698. [Google Scholar] [CrossRef]
- Ehrenfreund, E.; Vardeny, Z.; Brafman, O.; Horovitz, B. Amplitude and phase modes in trans-polyacetylene: Resonant Raman scattering and induced infrared activity. Phys. Rev. B 1987, 36, 1535–1553. [Google Scholar] [CrossRef]
- Painelli, A.; Girlando, A.; Del Freo, L.; Soos, Z. G. Infrared intensity and local vibrations of charged solitons. Phys. Rev. B 1997, 56, 15100−15108. [Google Scholar] [CrossRef]
- Gussoni, M.; Castiglioni, C.; Zerbi, G. Vibrational spectroscopy of polyconjugated materials: polyacetylene and polyenes. In Spectroscopy of advanced materials. Clark, R.J.H.; Hester, R.E., Ed.; Wiley: New York, USA, 1991; pp. 251–353. [Google Scholar]
- Castiglioni, C.; Lopez Navarrete, J.T.; Zerbi, G.; Gussoni, M. A simple interpretation of the vibrational spectra of undoped, doped and photoexcited polyacetylene: Amplitude mode theory in the GF formalism. Solid State Commun. 1988, 65, 625–630. [Google Scholar] [CrossRef]
- Brinkmann, M. Structure and morphology control in thin films of regioregular poly(3-hexylthiophene). J. Polym. Sci. B 2011, 49, 1218–1233. [Google Scholar] [CrossRef]
- Hamidi-Sakr, A.; Biniek, L.; Bantignies, J.-L.; Maurin, D.; Herrmann, L.; Leclerc, N.; Lévêque, P.; Vijayakumar, V.; Zimmermann, N.; Brinkmann, M. A Versatile Method to Fabricate Highly In-Plane Aligned Conducting Polymer Films with Anisotropic Charge Transport and Thermoelectric Properties: The Key Role of Alkyl Side Chain Layers on the Doping Mechanism. Adv. Funct. Mater. 2017, 27, 1700173. [Google Scholar] [CrossRef]
- Lee, S.; Moon, G.D.; Jeong, U. Continuous production of uniform poly(3-hexylthiophene) (P3HT) nanofibers by electrospinning and their electrical properties. J. Mater. Chem. 2009, 19, 743–748. [Google Scholar] [CrossRef]
- Chen, J.Y.; Kuo, C.C.; Lai, C.S.; Chen, W.C.; Chen, H.L. Manipulation on the morphology and electrical properties of aligned electrospun nanofibers of poly(3-hexylthiophene) for field-effect transistor applications. Macromolecules. 2011, 44, 2883–2892. [Google Scholar] [CrossRef]
- Watts, K.E.; Neelamraju, B.; Ratcliff, E.L.; Pemberton, J.E. Stability of Charge Transfer States in F4TCNQ-Doped P3HT. Chem. Mater. 2019, 31, 6986–699. [Google Scholar]
- Hase, H.; Berteau-Rainville, M.; Charoughchi, S.; Bodlos, W.; Orgiu, E.; Salzmann, I. Critical dopant concentrations govern integer and fractional charge-transfer phases in doped P3HT. J. Phys. Mat. 2022, 6, 014004. [Google Scholar] [CrossRef]
- Jacobs, I.E.; Cendra, C.; Harrelson, T.F.; Bedolla Valdez, Z.I.; Faller, R.; Salleo, A.; Moulé, A. J. Polymorphism controls the degree of charge transfer in a molecularly doped semiconducting polymer, Mater. Horizons 2018, 5, 655–660. [Google Scholar]
- Scholes, D.T.; Yee, P.Y.; Lindemuth, J.R.; Kang, H.; Onorato, J.; Ghosh, R.; Luscombe, C. K.; Spano, F. C.; Tolbert, S. H.; Schwartz B., J. The Effects of Crystallinity on Charge Transport and the Structure of Sequentially Processed F4TCNQ-Doped Conjugated Polymer Films. Adv. Funct. Mater. 2017, 27, 1702654. [Google Scholar] [CrossRef]
- Nightingale, J.; Wade, J.; Moia, D.; Nelson, J.; Kim, J.S. Impact of Molecular Order on Polaron Formation in Conjugated Polymers. J. Phys. Chem. C. 2018, 122, 29129–29140. [Google Scholar] [CrossRef]
- Brambilla, L.; Kim, J.-S.; Kim, B.J.; Hernandez, V.; Lopez Navarrete, J.T.; Zerbi, G. Poly(3-hexylthiophene-2.5-diyl): Evidence of different polymer chain conformations in the solid state from a combined study of regioregularity control and Raman spectroscopy. Journal of Molecular Structure 2020, 1221, 128882. [Google Scholar] [CrossRef]
- Casalegno, M.; Famulari, A.; Meille, S.V. Modeling of Poly(3-hexylthiophene) and Its Oligomer’s Structure and Thermal Behavior with Different Force Fields: Insights into the Phase Transitions of Semiconducting Polymers. Macromolecules 2022, 55(7), 2398–2412. [Google Scholar] [CrossRef]
- Stanfield, D.A.; Wu, Y.; Tolbert, S.H.; Schwartz, B.J. Controlling the Formation of Charge Transfer Complexes in Chemically Doped Semiconducting Polymers. Chem. Mater. 2021, 33, 2343–2356. [Google Scholar] [CrossRef]
- Pingel, P.; Zhu, L.; Park, K.S.; Vogel, J.O.; Eung-Gun Kim, S.J.; Rabe, J.P.; Brédas, J.-L.; Koch, N. Charge-Transfer Localization in Molecularly Doped Thiophene-Based Donor Polymers. J. Phys. Chem. Lett. 2010, 1, 2037–2041. [Google Scholar] [CrossRef]
- Zhu, L.; Kim, E.-G.; Yi, Y.; Brédas, J.-L. Charge Transfer in Molecular Complexes with 2,3,5,6-Tetrafluoro-7,7,8,8- tetracyanoquinodimethane (F4-TCNQ): A Density Functional Theory Study. Chem. Mater. 2011, 23, 5149–5159. [Google Scholar] [CrossRef]
- Duong, D.T.; Wang, C.; Antono, E.; Toney, M.F.; Salleo, A. The chemical and structural origin of efficient p-type doping in P3HT. Org Electron., 2013, 14, 1330–1336. [Google Scholar]
- Müller, L.; Nanova, D.; Glaser, T.; Beck, S.; Pucci, A.; Kast, A.K.; Schröder, R.R; Mankel, E.; Pingel, P.; Neher, D.; Kowalsky, W.; Lovrincic, R. Charge-Transfer-Solvent Interaction Predefines Doping Efficiency in p-Doped P3HT Films. Chem. Mater. 2016, 28, 4432–4439. [Google Scholar]
- Gao, J.; Roehling, J.D.; Li, Y.; Guo, H.; Moulé, A.J.; Grey, J.K. The effect of 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane charge transfer dopants on the conformation and aggregation of poly(3-hexylthiophene). J. Mater. Chem. C 2013, 1, 5638–5646. [Google Scholar]
- Gao, J.; Niles, E.T.; Grey, J.K. Aggregates promote efficient charge transfer doping of Poly(3- hexylthiophene), J. Phys. Chem. Lett. 2013, 4, 2953–2957. [Google Scholar]
- Tashiro, K.; Kobayashi, M.; Kawai, T.; Yoshino, K. Crystal structural change in poly(3-alkyl thiophene)s induced by iodine doping as studied by an organized combination of X-ray diffraction, infrared/Raman spectroscopy and computer simulation techniques. Polymer (Guildf) 1997, 38, 2867–2879. [Google Scholar]
- Castiglioni, C.; Tommasini, M. Infrared active vibrations in doped π-conjugated materials: the mechanism of activation of Raman modes. Asian J. Phys. 2022, 31, 14221–1776. [Google Scholar]
- Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman spectroscopy of polyconjugated molecules and materials: confinement effect in one and two dimensions. Phil. Trans. Roy. Soc. London. A. 2004, 362, 2425–2459. [Google Scholar]
- Lopez Navarrete, J.T.; Zerbi, G. Lattice dynamics and vibrational spectra of polythiophene. II. Effective coordinate theory, doping induced, and photoexcited spectra. J. Chem. Phys. 1998, 94, 965. [Google Scholar]
- Saporiti, C.; Brambilla, L.; Fazzi, D.; Castiglioni, C. Insights into the Structural and Vibrational Properties of Polaron in Doped Poly(3-alkyl-thiophene), P3HT. J. Phys. Chem. C.; 2024, 128, 5189–5205. [Google Scholar]
- Jacobs, I.E.; Aasen, E.W.; Oliveira, J.L.; Fonseca, T.N.; Roehling, J.D.; Li, J.; Zhang, G.; Augustine, M. P.; Mascal, M.; Moulé, A.J. Comparison of solution-mixed and sequentially processed P3HT:F4TCNQ films: effect of doping-induced aggregation on film morphology. J. Mater. Chem. C 2016, 4, 3454–3466. [Google Scholar]
- Stanfield, D.A.; Mehmedović, Z.; Schwartz, B.J. Vibrational Stark Effect Mapping of Polaron Delocalization in Chemically Doped Conjugated Polymers. Chem. Mater. 2021, 33, 8489–8500. [Google Scholar]
- Ghosh, R.; Chew, A.R.; Onorato, J.; Pakhnyuk, V.; Luscombe, C.K.; Salleo, A.; Spano, F.C. Spectral Signatures and Spatial Coherence of Bound and Unbound Polarons in P3HT Films: Theory Versus Experiment. J. Phys. Chem. C. 2018, 122, 18048–18060. [Google Scholar] [CrossRef]
- Wu, E.C.; Salamat, C.Z.; Ruiz, O.L.; Qu, T.; Kim, A.; Tolbert, S.H.; Schwartz, B. J. Counterion Control and the Spectral Signatures of Polarons, Coupled Polarons, and Bipolarons in Doped P3HT Films. Adv. Funct. Mater. 2023, 33, 2213652. [Google Scholar] [CrossRef]
- Enengl, C.; Enengl, S.; Pluczyk, S.; Havlicek, M.; Lapkowski, M.; Neugebauer, H.; Ehrenfreund, E. Doping-Induced Absorption Bands in P3HT: Polarons and Bipolarons. ChemPhysChem 2016, 17, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, I. H.; Batista, E. R.; Tretiak, S.; Saxena, A.; Smith, D. L.; Martin, R. L. Role of Geometric Distortion and Polarization in Localizing Electronic Excitations in Conjugated Polymers. J. Chem. Theory Comput. 2013, 9, 1144–1154. [Google Scholar] [CrossRef]
- Brambilla, L.; Tommasini, M.; Botiz, I.; Rahimi, K.; Agumba, J. O.; Stingelin, N.; Zerbi, G. Regio-Regular Oligo and Poly(3-HexylThiophene): Precise Structural Markers from the Vibrational Spectra of Oligomer Single Crystals. Macromolecules 2014, 47, 6730−6739. [Google Scholar]
- Brambilla, L.; Capel Ferrón, C.; Tommasini, M.; Hong, K.; López Navarrete, J. T.; Hernández, V.; Zerbi, G. Infrared and Multi- Wavelength Raman Spectroscopy of Regio-Regular P3HT and Its Deutero Derivatives. J. Raman Spectrosc. 2018, 49, 569−580. [Google Scholar] [CrossRef]
- Mosca, S.; Milani, A.; Castiglioni, C.; Hernández Jolín, V.; Meseguer, C.; López Navarrete, J. T.; Zhao, C.; Sugiyasu, K.; Ruiz Delgado, M. C. Raman Fingerprints of Pi-Electron Delocalization in Polythiophene-Based Insulated Molecular Wires. Macromolecules 2022, 55, 3458−3468. [Google Scholar] [CrossRef]
- Saporiti, C.; Brambilla, L.; Tommasini, M.; Del Zoppo, M.; Castiglioni, C.; Zerbi, G. Probing the Charge State and the Intermolecular Environment by Vibrational Spectroscopy: The Peculiar Modulation of Frequencies and Band Intensities of F4TCNQ and Its Anion. Spectrosc. J. 2024, 2, 264–286. [Google Scholar] [CrossRef]
- Spano, F. C.; Silva, C. H- and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014. 65, 477–500. [CrossRef]
- Mansour, A.E.; Valencia, A.M.; Lungwitz, D.; Wegner, B.; Tanaka, N.; Shoji, Y.; Fukushima, T.; Opitz, A.; Cocchi, C.; Koch, N. Understanding the evolution of the Raman spectra of molecularly p-doped poly(3-hexylthiophene-2,5-diyl): signatures of polarons and bipolarons. Phys. Chem. Chem. Phys. 2022, 24, 3109–3118. [Google Scholar] [CrossRef]
- Arrigoni, A.; Brambilla, L.; Castiglioni, C.; Bertarelli, C. Conducting Electrospun Nanofibres: Monitoring of Iodine Doping of P3HT through Infrared (IRAV) and Raman (RaAV) Polaron Spectroscopic Features. Nanomaterials. 2022, 12, 4308. [Google Scholar] [PubMed]
- Arrigoni, A.; Brambilla, L.; Bertarelli, C.; Saporiti, C.; Castiglioni, C. Conducting Electrospun Poly(3-hexylthiophene-2,5-diyl) Nanofibers: New Strategies for Effective Chemical Doping and its Assessment Using Infrared Spectroscopy. Appl. Spectrosc. 2024, 78, 1279–1294. [Google Scholar] [PubMed]
- Yin, J.; Wang, Z.; Fazzi, D.; Shen, Z.; Soci, C. First-Principles Study of the Nuclear Dynamics of Doped Conjugated Polymers. J. Phys. Chem. C. 2016, 120, 1994–2001. [Google Scholar]
- Wang, S.; Fazzi, D.; Puttisong, Y.; Jafari, M.J.; Chen, Z.; Ederth, T.; Andreasen, J. W.; Chen, W. M.; Facchetti, A.; Fabiano, S. Effect of Backbone Regiochemistry on Conductivity, Charge Density, and Polaron Structure of n-Doped Donor-Acceptor Polymers. Chem. Mater. 2019, 31, 3395–3406. [Google Scholar]
- Fityk – Curve fitting and data analysis. Available online: https://fityk.nieto.pl/ (accessed on 10 February 2025).
- Wojdyr, M. Fityk: a general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar]


| P3HT Samples in CHCl3 Solution | Solid P3HT Samples | |||
|---|---|---|---|---|
| Dopant Percentage | Peak Wavenumber (cm-1) | FWHM (cm-1) |
Peak Wavenumber (cm-1) | FWHM (cm-1) |
| 0% (pristine) | 1474 | 42 | 1444 | 25 |
| 2.7% | 1432 | 29 | 1439 | 42 |
| 5.6% | 1428 | 29 | 1433 | 41 |
| 11.1% | 1424 | 33 | 1430 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





