Submitted:
20 February 2025
Posted:
24 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Testing Procedure
2.3. Instrumentation
2.4. Measurement Outcomes
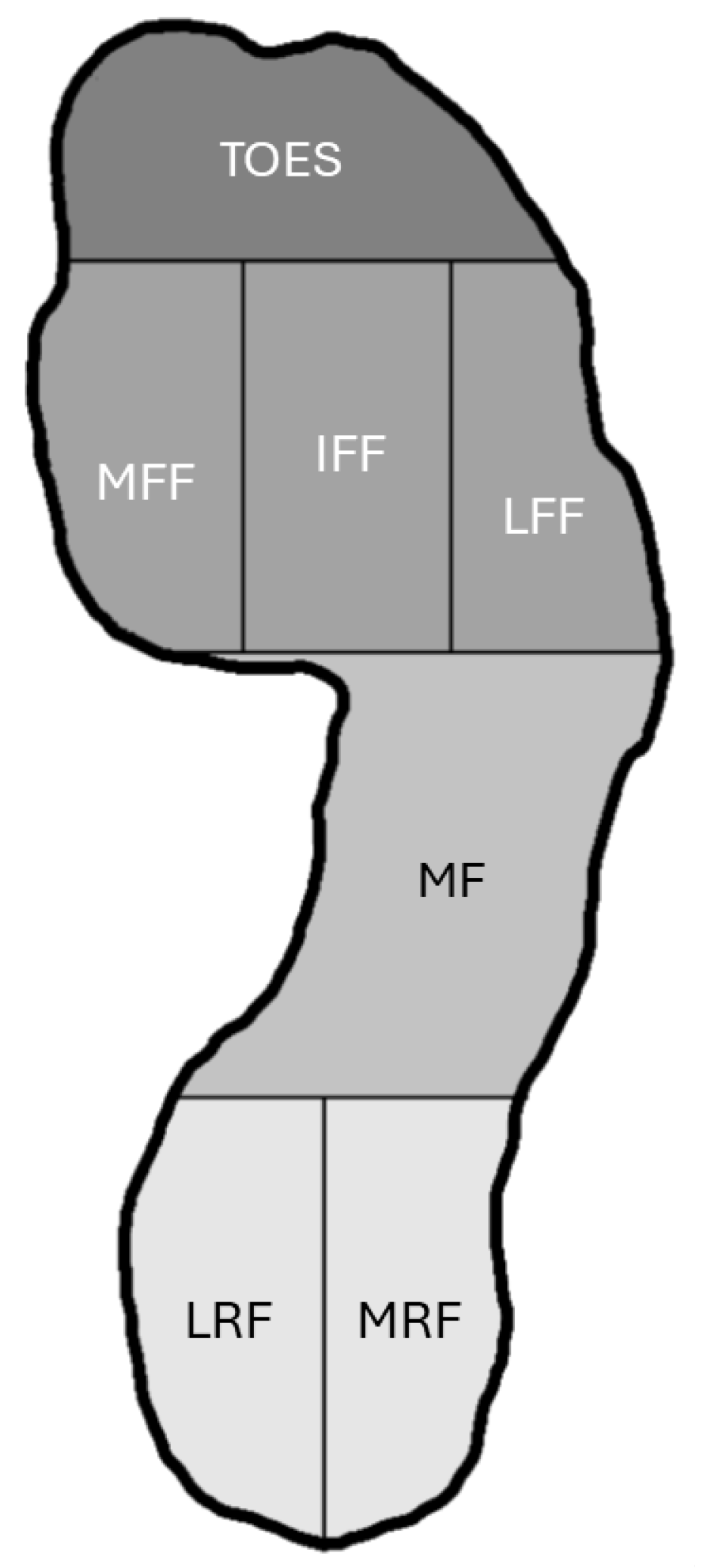
2.5. Reliability Study
2.6. Statistical Analysis
3. Results
3.1. Spatial Parameters
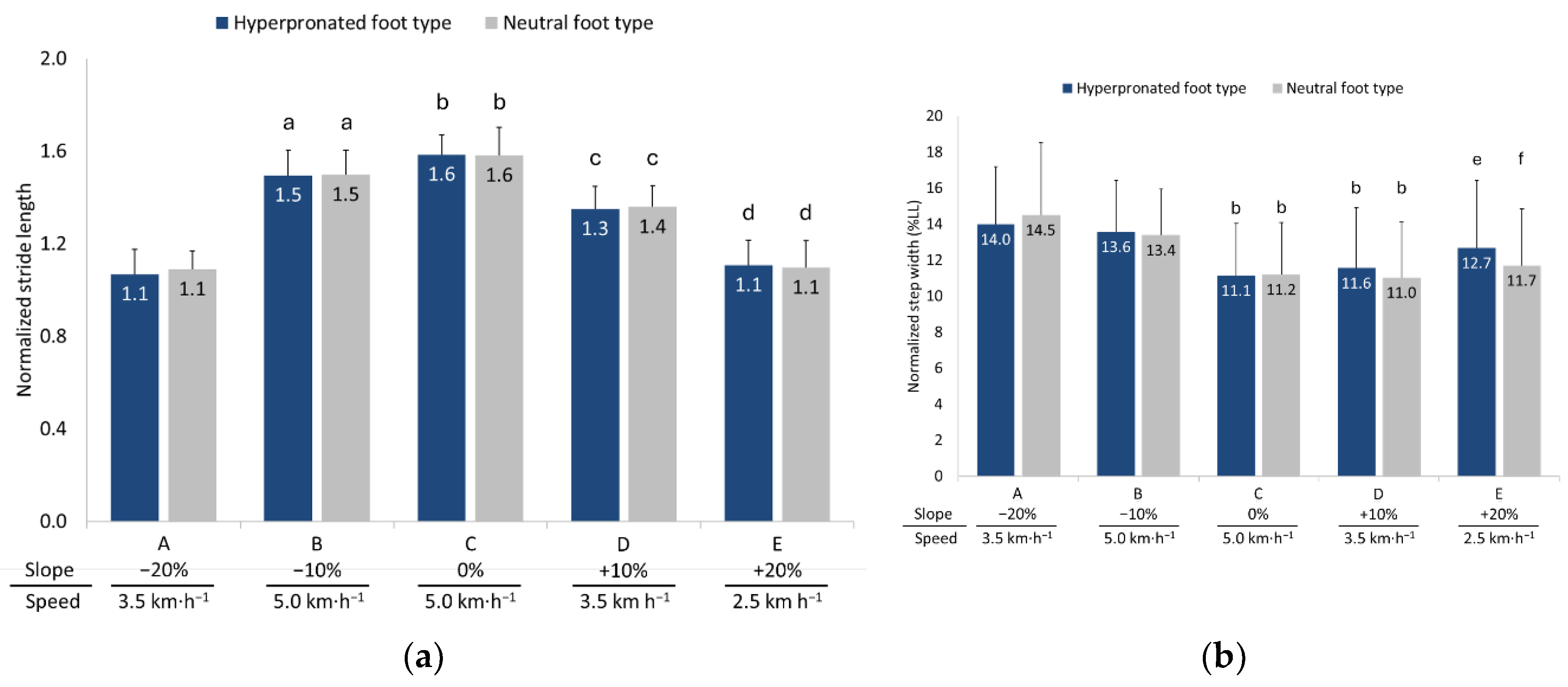
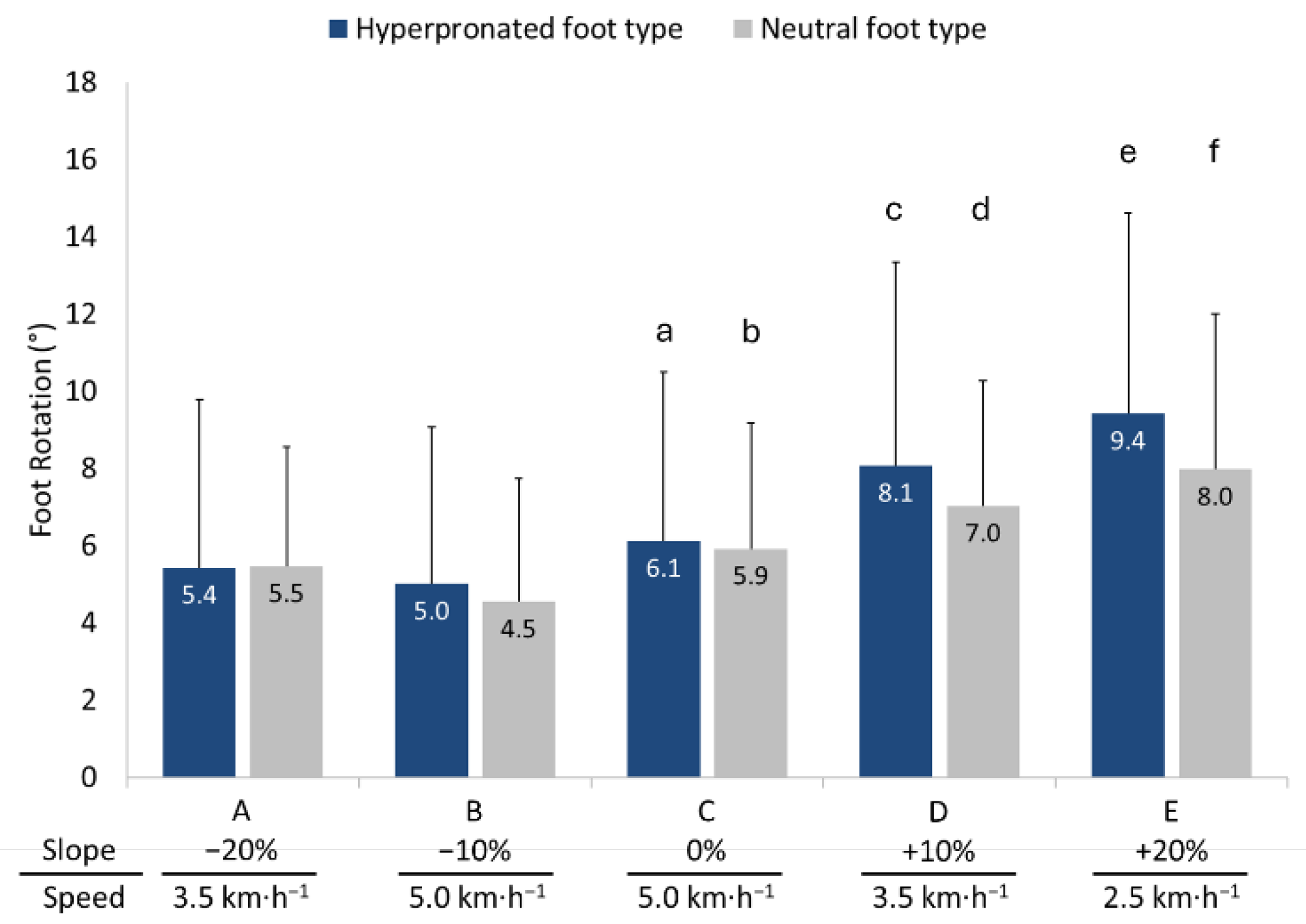
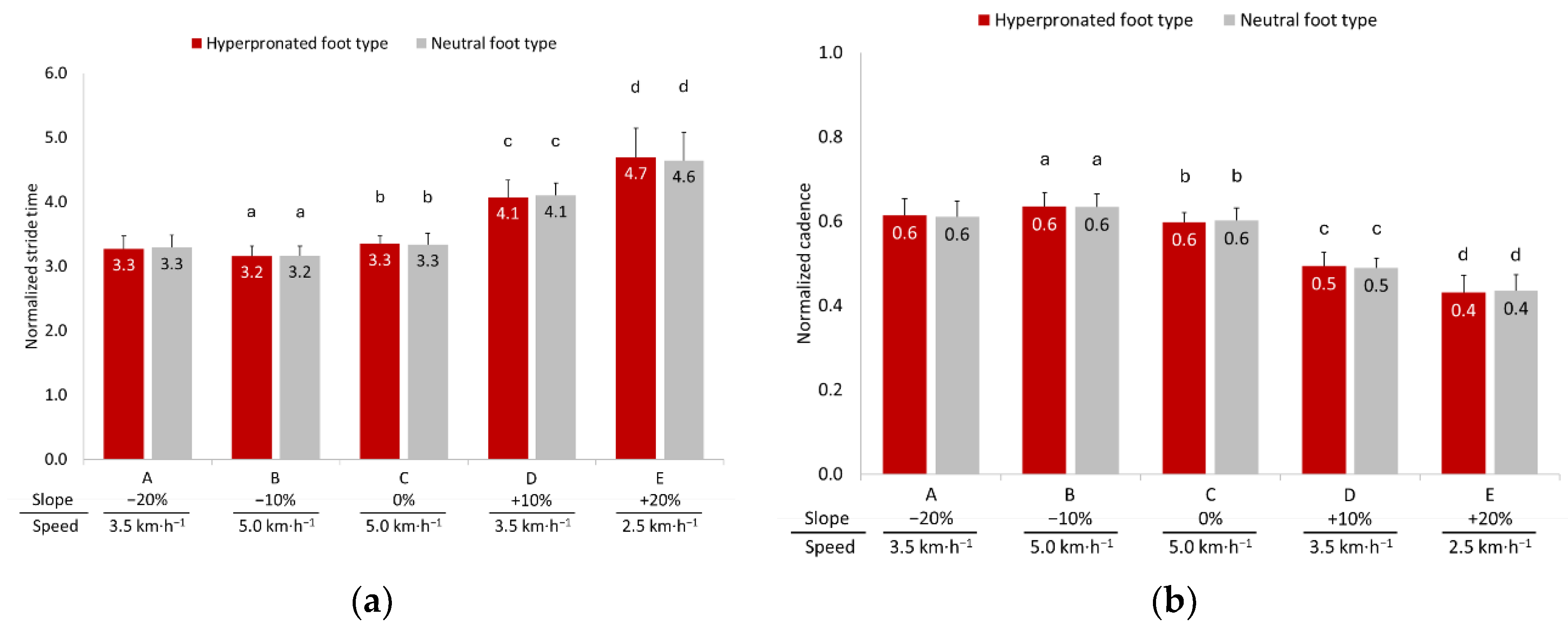
3.2. Temporal Parameters
3.3. Gait Phase Analysis
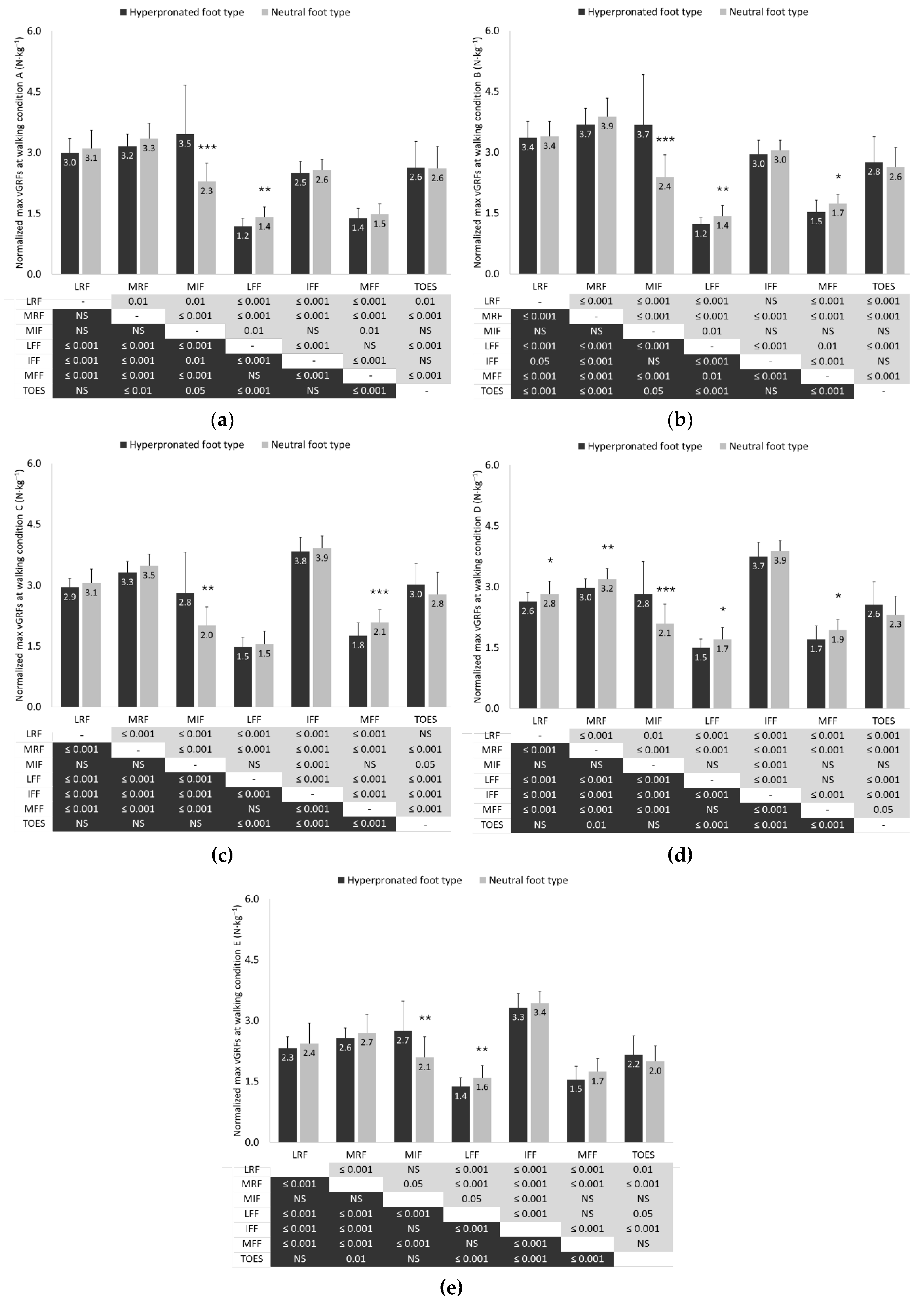
3.4. Vertical Ground Reaction Forces
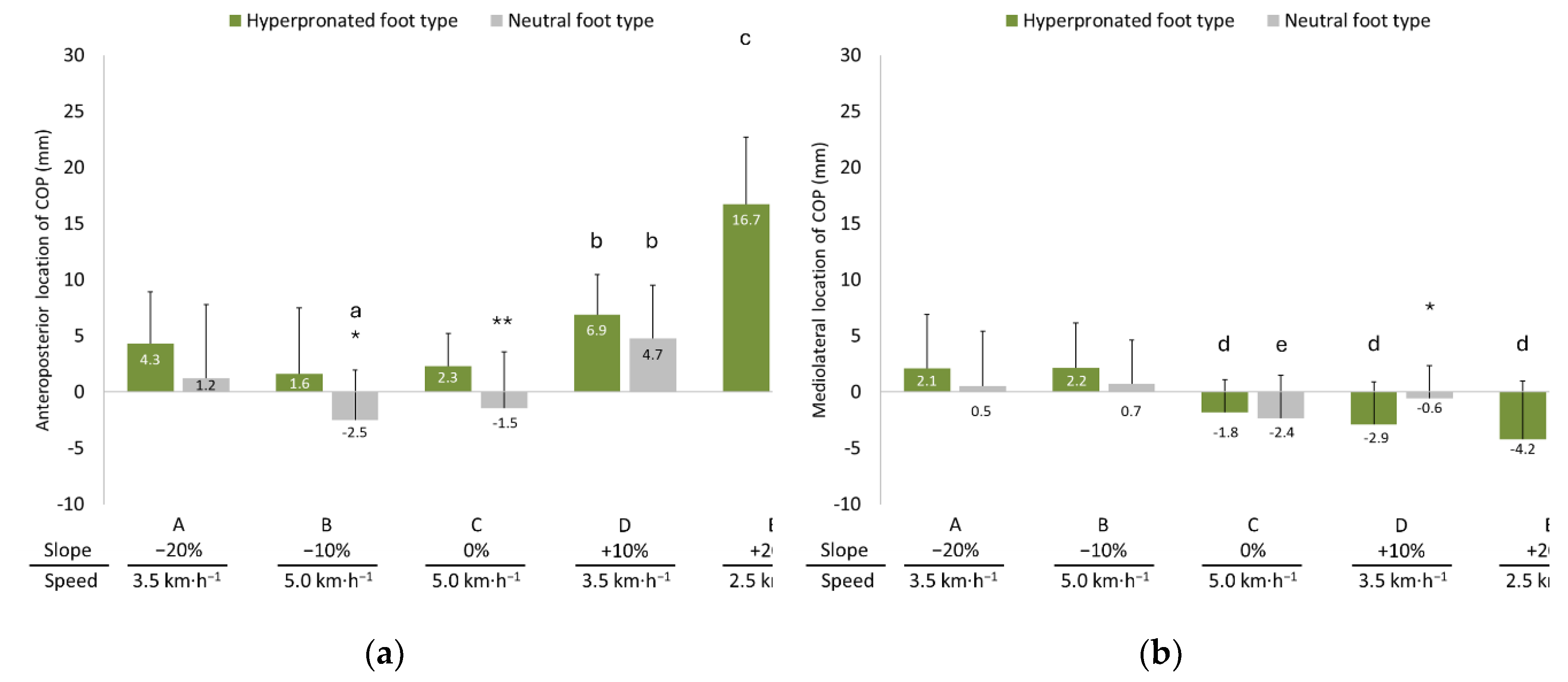
3.5. Anteroposterior and Mediolateral Locations of COP
4. Discussion
4.1. Spatiotemporal Parameters and Gait Phase Analysis
4.2. Vertical Ground Reaction Forces
4.3. Anteroposterior and Mediolateral Locations of COP
4.4. Limitations
4.5. Clinical Significance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Horwood, A.M.; Chockalingam, N. Defining Excessive, over, or Hyper-Pronation: A Quandary. Foot 2017, 31, 49–55.
- Brosky, J.A.; Balazsy, J.E. Functional Anatomy of the Foot and Ankle. In Orthopaedic Physical Therapy Secrets: Third Edition; Elsevier Inc., 2017; pp. 581–586 ISBN 9780323286855.
- Sueki, D.G.; Cleland, J.A.; Wainner, R.S. A Regional Interdependence Model of Musculoskeletal Dysfunction: Research, Mechanisms, and Clinical Implications. Journal of Manual & Manipulative Therapy 2013, 21, 90–102. [CrossRef]
- Riegger-Krugh, C.; Keysor, J.J. Skeletal Malalignments of the Lower Quarter: Correlated and Compensatory Motions and Postures. Journal of Orthopaedic & Sports Physical Therapy 1996, 23, 164–170. [CrossRef]
- Yazdani, F.; Razeghi, M.; Karimi, M.T.; Raeisi Shahraki, H.; Salimi Bani, M. The Influence of Foot Hyperpronation on Pelvic Biomechanics during Stance Phase of the Gait: A Biomechanical Simulation Study. Proc Inst Mech Eng H 2018, 232, 708–717. [CrossRef]
- Powers, C.M. The Influence of Altered Lower-Extremity Kinematics on Patellofemoral Joint Dysfunction: A Theoretical Perspective. Journal of Orthopaedic & Sports Physical Therapy 2003, 33, 639–646. [CrossRef]
- Neal, B.S.; Griffiths, I.B.; Dowling, G.J.; Murley, G.S.; Munteanu, S.E.; Franettovich Smith, M.M.; Collins, N.J.; Barton, C.J. Foot Posture as a Risk Factor for Lower Limb Overuse Injury: A Systematic Review and Meta-analysis. J Foot Ankle Res 2014, 7. [CrossRef]
- Hintermann, B.; Nigg, B.M. Pronation in Runners. Sports Medicine 1998, 26, 169–176. [CrossRef]
- Pérez-Morcillo, A.; Gómez-Bernal, A.; Gil-Guillen, V.F.; Alfaro-Santafé, J.; Alfaro-Santafé, J.V.; Quesada, J.A.; Lopez-Pineda, A.; Orozco-Beltran, D.; Carratalá-Munuera, C. Association between the Foot Posture Index and Running Related Injuries: A Case-Control Study. Clinical Biomechanics 2019, 61, 217–221. [CrossRef]
- Kaufman, K.R.; Brodine, S.K.; Shaffer, R.A.; Johnson, C.W.; Cullison, T.R. The Effect of Foot Structure and Range of Motion on Musculoskeletal Overuse Injuries. Am J Sports Med 1999, 27, 585–593. [CrossRef]
- Almeheyawi, R.N.; Bricca, A.; Riskowski, J.L.; Barn, R.; Steultjens, M. Foot Characteristics and Mechanics in Individuals with Knee Osteoarthritis: Systematic Review and Meta-analysis. J Foot Ankle Res 2021, 14. [CrossRef]
- Razeghi, M.; Batt, M.E. Foot Type Classification: A Critical Review of Current Methods. Gait Posture 2002, 15, 282–291. [CrossRef]
- Shin, H.S.; Lee, J.H.; Kim, E.J.; Kyung, M.G.; Yoo, H.J.; Lee, D.Y. Flatfoot Deformity Affected the Kinematics of the Foot and Ankle in Proportion to the Severity of Deformity. Gait Posture 2019, 72, 123–128. [CrossRef]
- Buldt, A.K.; Levinger, P.; Murley, G.S.; Menz, H.B.; Nester, C.J.; Landorf, K.B. Foot Posture Is Associated with Kinematics of the Foot during Gait: A Comparison of Normal, Planus and Cavus Feet. Gait Posture 2015, 42, 42–48. [CrossRef]
- Marouvo, J.; Sousa, F.; Fernandes, O.; Castro, M.A.; Paszkiel, S. Gait Kinematics Analysis of Flatfoot Adults. Applied Sciences (Switzerland) 2021, 11. [CrossRef]
- Okamura, K.; Egawa, K.; Ikeda, T.; Fukuda, K.; Kanai, S. Relationship between Foot Muscle Morphology and Severity of Pronated Foot Deformity and Foot Kinematics during Gait: A Preliminary Study. Gait Posture 2021, 86, 273–277. [CrossRef]
- Zhang, X.; Aeles, J.; Vanwanseele, B. Comparison of Foot Muscle Morphology and Foot Kinematics between Recreational Runners with Normal Feet and with Asymptomatic Over-Pronated Feet. Gait Posture 2017, 54, 290–294. [CrossRef]
- Abdul Razak, A.H.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot Plantar Pressure Measurement System: A Review. Sensors (Switzerland) 2012, 12, 9884–9912.
- Burnie, L.; Chockalingam, N.; Holder, A.; Claypole, T.; Kilduff, L.; Bezodis, N. Commercially Available Pressure Sensors for Sport and Health Applications: A Comparative Review. Foot 2023, 56.
- Arzehgar, A.; Nia, R.G.N.N.; Hoseinkhani, M.; Masoumi, F.; Sayyed-Hosseinian, S.-H.; Eslami, S. An Overview of Plantar Pressure Distribution Measurements and Its Applications in Health and Medicine. Gait Posture 2025, 117, 235–244. [CrossRef]
- Fallahtafti, F.; Wurdeman, S.R.; Yentes, J.M. Sampling Rate Influences the Regularity Analysis of Temporal Domain Measures of Walking More than Spatial Domain Measures. Gait Posture 2021, 88, 216–220. [CrossRef]
- Veilleux, L.N.; Raison, M.; Rauch, F.; Robert, M.; Ballaz, L. Agreement of Spatio-Temporal Gait Parameters between a Vertical Ground Reaction Force Decomposition Algorithm and a Motion Capture System. Gait Posture 2016, 43, 257–264. [CrossRef]
- Fujishita, H.; Ikuta, Y.; Maeda, N.; Komiya, M.; Morikawa, M.; Arima, S.; Sakamitsu, T.; Obayashi, H.; Fukuhara, K.; Ushio, K.; et al. Effects of Rearfoot Eversion on Foot Plantar Pressure and Spatiotemporal Gait Parameters in Adolescent Athletes. Healthcare (Switzerland) 2023, 11. [CrossRef]
- Chuckpaiwong, B.; Nunley, J.A.; Mall, N.A.; Queen, R.M. The Effect of Foot Type on In-Shoe Plantar Pressure during Walking and Running. Gait Posture 2008, 28, 405–411. [CrossRef]
- Mei, Q.; Gu, Y.; Xiang, L.; Yu, P.; Gao, Z.; Shim, V.; Fernandez, J. Foot Shape and Plantar Pressure Relationships in Shod and Barefoot Populations. Biomech Model Mechanobiol 2020, 19, 1211–1224. [CrossRef]
- Buldt, A.K.; Forghany, S.; Landorf, K.B.; Levinger, P.; Murley, G.S.; Menz, H.B. Foot Posture Is Associated with Plantar Pressure during Gait: A Comparison of Normal, Planus and Cavus Feet. Gait Posture 2018, 62, 235–240. [CrossRef]
- Han, J.T.; Koo, H.M.; Jung, J.M.; Kim, Y.J.; Lee, J.H. Differences in Plantar Foot Pressure and COP between Flat and Normal Feet During Walking. J Phys Ther Sci 2011, 23, 683–685. [CrossRef]
- Ledoux, W.R.; Hillstrom, H.J. The Distributed Plantar Vertical Force of Neutrally Aligned and Pes Planus Feet. Gait Posture 2002, 15, 1–9. [CrossRef]
- Wong, Y.S. Influence of the Abductor Hallucis Muscle on the Medial Arch of the Foot: A Kinematic and Anatomical Cadaver Study. Foot Ankle Int 2007, 28, 617–620. [CrossRef]
- Semaan, M.B.; Wallard, L.; Ruiz, V.; Gillet, C.; Leteneur, S.; Simoneau-Buessinger, E. Is Treadmill Walking Biomechanically Comparable to Overground Walking? A Systematic Review. Gait Posture 2022, 92, 249–257.
- Kim, M.-K. Foot Pressure Analysis of Adults with Flat and Normal Feet at Different Gait Speeds on an Ascending Slope. J Phys Ther Sci 2015, 27, 3767–3769. [CrossRef]
- Kim, M.-K.; Lee, Y.-S. A Three-Dimensional Gait Analysis of People with Flat Arched Feet on an Ascending Slope. J Phys Ther Sci 2014, 26, 1437–1440. [CrossRef]
- Resende, R.A.; Pinheiro, L.S.P.; Ocarino, J.M. Effects of Foot Pronation on the Lower Limb Sagittal Plane Biomechanics during Gait. Gait Posture 2019, 68, 130–135. [CrossRef]
- Requelo-Rodríguez, I.; Castro-Méndez, A.; Jiménez-Cebrián, A.M.; González-Elena, M.L.; Palomo-Toucedo, I.C.; Pabón-Carrasco, M. Assessment of Selected Spatio-Temporal Gait Parameters on Subjects with Pronated Foot Posture on the Basis of Measurements Using Optogait. A Case-Control Study. Sensors 2021, 21. [CrossRef]
- Redmond, A.C.; Crosbie, J.; Ouvrier, R.A. Development and Validation of a Novel Rating System for Scoring Standing Foot Posture: The Foot Posture Index. Clinical Biomechanics 2006, 21, 89–98. [CrossRef]
- Knutson, G.A. Anatomic and Functional Leg-Length Inequality: A Review and Recommendation for Clinical Decision-Making. Part I, Anatomic Leg-Length Inequality: Prevalence, Magnitude, Effects and Clinical Significance. Chiropr Osteopat 2005, 13, 11. [CrossRef]
- Bunnell, W.P. An Objective Criterion for Scoliosis Screening. The Journal of Bone & Joint Surgery, Am vol 1984, 66, 1381–1387.
- Farahmand, B.; Ebrahimi Takamjani, E.; Yazdi, H.R.; Saeedi, H.; Kamali, M.; Bagherzadeh Cham, M. A Systematic Review on the Validity and Reliability of Tape Measurement Method in Leg Length Discrepancy. Med J Islam Repub Iran 2019, 33, 46. [CrossRef]
- Powden, C.J.; Hoch, J.M.; Hoch, M.C. Reliability and Minimal Detectable Change of the Weight-Bearing Lunge Test: A Systematic Review. Man Ther 2015, 20, 524–532. [CrossRef]
- Tobler, W. Three Presentations on Geographical Analysis and Modeling: Non- Isotropic Geographic Modeling; Speculations on the Geometry of Geography and Global Spatial Analysis. Technical Report 1993, 93.
- Pingel, T.J. Modeling Slope as a Contributor to Route Selection in Mountainous Areas. Cartogr Geogr Inf Sci 2010, 37, 137–148. [CrossRef]
- Meyer, C.; Killeen, T.; Easthope, C.S.; Curt, A.; Bolliger, M.; Linnebank, M.; Zörner, B.; Filli, L. Familiarization with Treadmill Walking: How Much Is Enough? Sci Rep 2019, 9, 1–10. [CrossRef]
- Paillard, T. Effects of General and Local Fatigue on Postural Control: A Review. Neurosci Biobehav Rev 2012, 36, 162–176. [CrossRef]
- Hof, A.L. Scaling Gait Data to Body Size. Gait Posture 1996, 4, 222–223. [CrossRef]
- Zebris medical GmbH Software Manual Zebris FDM 2.0.x; Am Galgenbühl 14, D-88316 Isny im Allgäu, Germany, 2022;.
- Cao, Y.; Zhuang, H.; Zhang, X.; Guo, R.; Pang, H.; Zheng, P.; Xu, H. Impact of Foot Progression Angle on Spatiotemporal and Plantar Loading Pattern in Intoeing Children during Gait. Sci Rep 2024, 14. [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016, 15, 155–163. [CrossRef]
- Hillstrom, H.J.; Song, J.; Kraszewski, A.P.; Hafer, J.F.; Mootanah, R.; Dufour, A.B.; Chow, B.S.; Deland, J.T. Foot Type Biomechanics Part 1: Structure and Function of the Asymptomatic Foot. Gait Posture 2013, 37, 445–451. [CrossRef]
- Farahpour, N.; Jafarnezhad, A.A.; Damavandi, M.; Bakhtiari, A.; Allard, P. Gait Ground Reaction Force Characteristics of Low Back Pain Patients with Pronated Foot and Able-Bodied Individuals with and without Foot Pronation. J Biomech 2016, 49, 1705–1710. [CrossRef]
- Barton, C.J.; Levinger, P.; Webster, K.E.; Menz, H.B. Kinematics Associated with Foot Pronation in Individuals with Patellofemoral Pain Syndrome: A Case-control Study. J Foot Ankle Res 2011, 4. [CrossRef]
- Dodelin, D.; Tourny, C.; L’Hermette, M. The Biomechanical Effects of Pronated Foot Function on Gait. An Experimental Study. Scand J Med Sci Sports 2020, 30, 2167–2177. [CrossRef]
- Wang, Y.; Qi, Y.; Ma, B.; Wu, H.; Wang, Y.; Wei, B.; Wei, X.; Xu, Y. Three-Dimensional Gait Analysis of Orthopaedic Common Foot and Ankle Joint Diseases. Front Bioeng Biotechnol 2024, 12. [CrossRef]
- Okamura, K.; Egawa, K.; Ikeda, T.; Fukuda, K.; Kanai, S. Relationship between Foot Muscle Morphology and Severity of Pronated Foot Deformity and Foot Kinematics during Gait: A Preliminary Study. Gait Posture 2021, 86, 273–277. [CrossRef]
- Sakamoto, K.; Kudo, S. Morphological Characteristics of Intrinsic Foot Muscles among Flat Foot and Normal Foot Using Ultrasonography. Acta Bioeng Biomech 2020, 22, 161–166. [CrossRef]
- Gray, E.G.; Basmajian, J. V. Electromyography and Cinematography of Leg and Foot (“Normal” and Flat) during Walking. Anat Rec 1968, 161, 1–15. [CrossRef]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. Foot Posture Influences the Electromyographic Activity of Selected Lower Limb Muscles during Gait. J Foot Ankle Res 2009, 2. [CrossRef]
- Hunt, A.E.; Smith, R.M. Mechanics and Control of the Flat versus Normal Foot during the Stance Phase of Walking. Clinical Biomechanics 2004, 19, 391–397. [CrossRef]
- Kwon, Y.; Shin, G. Foot Kinematics and Leg Muscle Activation Patterns Are Altered in Those with Limited Ankle Dorsiflexion Range of Motion during Incline Walking. Gait Posture 2022, 92, 315–320. [CrossRef]
- Mohammadi, R.; Phadke, C.P. Effects of Treadmill Incline and Speed on Peroneus Longus Muscle Activity in Persons with Chronic Stroke and Healthy Subjects. Gait Posture 2017, 54, 221–228. [CrossRef]
- Duchateau, J.; Enoka, R.M. Neural Control of Lengthening Contractions. Journal of Experimental Biology 2016, 219, 197–204. [CrossRef]
- Jalaleddini, K.; Nagamori, A.; Laine, C.M.; Golkar, M.A.; Kearney, R.E.; Valero-Cuevas, F.J. Physiological Tremor Increases When Skeletal Muscle Is Shortened: Implications for Fusimotor Control. Journal of Physiology 2017, 595, 7331–7346. [CrossRef]
- Hiemstra, L.A.; Lo, I.K.Y.; Fowler, P.) Effect of Fatigue on Knee Proprioception: Implications for Dynamic Stabilization. Journal of Orthopaedic & Sports Physical Therapy 2001, 31, 59–605.
- Buldt, A.K.; Allan, J.J.; Landorf, K.B.; Menz, H.B. The Relationship between Foot Posture and Plantar Pressure during Walking in Adults: A Systematic Review. Gait Posture 2018, 62, 56–67. [CrossRef]
- Jonely, H.; Brismée, J.-M.; Sizer, P.S.; James, C.R. Relationships between Clinical Measures of Static Foot Posture and Plantar Pressure during Static Standing and Walking. Clinical Biomechanics 2011, 26, 873–879. [CrossRef]
- Rao, S.; Song, J.; Kraszewski, A.; Backus, S.; Ellis, S.J.; Md, J.T.D.; Hillstrom, H.J. The Effect of Foot Structure on 1st Metatarsophalangeal Joint Flexibility and Hallucal Loading. Gait Posture 2011, 34, 131–137. [CrossRef]
- Bednarczyk, E.; Sikora, S.; Kossobudzka-Górska, A.; Jankowski, K.; Hernandez-Rodriguez, Y. Understanding Flat Feet: An in-Depth Analysis of Orthotic Solutions. Journal of Orthopaedic Reports 2024, 3, 100250. [CrossRef]
- Cornwall, M.W.; McPoil, T.G. Relative Movement of the Navicular Bone During Normal Walking. Foot Ankle Int 1999, 20, 507–512. [CrossRef]
- Bolgla, L.A.; Malone, T.R. Plantar Fasciitis and the Windlass Mechanism: A Biomechanical Link to Clinical Practice. J Athl Train 2004, 39, 77–82.
- Strutzenberger, G.; Leutgeb, L.; Claußen, L.; Schwameder, H. Gait on Slopes: Differences in Temporo-Spatial, Kinematic and Kinetic Gait Parameters between Walking on a Ramp and on a Treadmill. Gait Posture 2022, 91, 73–78. [CrossRef]
- Zwirner, J.; Zhang, M.; Ondruschka, B.; Akita, K.; Hammer, N. An Ossifying Bridge – on the Structural Continuity between the Achilles Tendon and the Plantar Fascia. Sci Rep 2020, 10. [CrossRef]
- Cheung, J.T.M.; Zhang, M.; An, K.N. Effect of Achilles Tendon Loading on Plantar Fascia Tension in the Standing Foot. Clinical Biomechanics 2006, 21, 194–203. [CrossRef]
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R. Plantar Fasciitis: A Retrospective Analysis of 267 Cases. Physical Therapy in Sport 2002, 3, 57–65. [CrossRef]
- Kafetzakis, I.; Konstantinou, I.; Mandalidis, D. Effects of Hiking-Dependent Walking Speeds and Slopes on Spatiotemporal Gait Parameters and Ground Reaction Forces: A Treadmill-Based Analysis in Healthy Young Adults. Applied Sciences 2024, 14, 4383. [CrossRef]
- Leroux, A.; Fung, J.; Barbeau, H. Postural Adaptation to Walking on Inclined Surfaces: I. Normal Strategies. Gait Posture 2002, 15, 64–74.
- Solomonow, M. Ligaments: A Source of Musculoskeletal Disorders. J Bodyw Mov Ther 2009, 13, 136–154. [CrossRef]
- Pereira, B.S.; Andrade, R.; Espregueira-Mendes, J.; Marano, R.P.C.; Oliva, X.M.; Karlsson, J. Current Concepts on Subtalar Instability. Orthop J Sports Med 2021, 9. [CrossRef]
- Mickle, K.J.; Angin, S.; Crofts, G.; Nester, C.J. Effects of Age on Strength and Morphology of Toe Flexor Muscles. Journal of Orthopaedic and Sports Physical Therapy 2016, 46, 1065–1070. [CrossRef]
- Tanaka, T.; Suga, T.; Imai, Y.; Ueno, H.; Misaki, J.; Miyake, Y.; Otsuka, M.; Nagano, A.; Isaka, T. Characteristics of Lower Leg and Foot Muscle Thicknesses in Sprinters: Does Greater Foot Muscles Contribute to Sprint Performance? Eur J Sport Sci 2019, 19, 442–450. [CrossRef]
- Kaneda, K.; Maeda, N.; Ikuta, Y.; Tashiro, T.; Tsutsumi, S.; Arima, S.; Sasadai, J.; Suzuki, Y.; Morikawa, M.; Komiya, M.; et al. The Features of Foot Morphology and Intrinsic Foot Muscle Property in Adolescent Swimmers: An Ultrasound-Based Study. J Hum Kinet 2023, 88, 95–103. [CrossRef]
- Langley, B.; Cramp, M.; Morrison, S.C. Clinical Measures of Static Foot Posture Do Not Agree. J Foot Ankle Res 2016, 9. [CrossRef]
- Chevalier, T.L.; Hodgins, H.; Chockalingam, N. Plantar Pressure Measurements Using an In-Shoe System and a Pressure Platform: A Comparison. Gait Posture 2010, 31, 397–399. [CrossRef]
- Mousavi, S.H.; Khorramroo, F.; Jafarnezhadgero, A. Gait Retraining Targeting Foot Pronation: A Systematic Review and Meta-Analysis. PLoS ONE 2024, 19. [CrossRef]
- Tartaruga, M.P.; Brisswalter, J.; Peyré-Tartaruga, L.A.; Alberton, C.L.; Cadore, E.L.; Tiggemann, C.L.; Silva, E.M.; Kruel, L.F.M.; Ávila, A.O.V.; Coertjens, M. The Relationship between Running Economy and Biomechanical Variables in Distance Runners. Res Q Exerc Sport 2012, 83, 367–375. [CrossRef]
- Esmaeili, A.; Hosseininejad, S.E.; Jafarnezhadgero, A.A.; Dionisio, V.C. The Interaction Effect of Different Footwear Types and Static Navicular Drop or Dynamic Ankle Pronation on the Joint Stiffness of the Lower Limb during Running. Gait Posture 2024, 108, 28–34. [CrossRef]
- Genova, J.M.; Gross, M.T. Effect of Foot Orthotics on Calcaneal Eversion During Standing and Treadmill Walking for Subjects With Abnormal Pronation. Journal of Orthopaedic & Sports Physical Therapy 2000, 30, 664–675. [CrossRef]
| Side | Groups | |||
|---|---|---|---|---|
| HFT (n=21) | NFT (n=21) | Total (n=42) | ||
| Sex (M/F) | 11/10 | 11/10 | 22/20 | |
| Age (yrs) | 24.4 ± 2.7 | 23.8 ± 3.1 | 24.1 ± 2.9 | |
| Body Mass (kg) | 71.0 ± 14.3 | 68.5 ± 9.7 | 69.8 ± 12.1 | |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | |
| Body Mass Index (kg·m-1) | 20.5 ± 3.3 | 19.9 ± 2.3 | 20.2 ± 2.9 | |
| FPI-6 score | R | 10.6 ± 1.1 | 2.4 ± 1.5 ** | 6.5 ± 4.3 |
| L | 10.5 ± 0.9 | 2.6 ± 1.3 ** | 6.5 ± 4.2 | |
| Leg length | R | 88.7± 6.1 | 87.0 ± 4.0 | 87.8 ± 5.1 |
| L | 88.7± 6.1 | 87.0 ± 4.1 | 87.8 ± 5.2 | |
| Ankle dorsi flexion (°) | R | 53.8 ± 5.1 | 50.0 ± 6.0 * | 51.9 ± 5.8 |
| L | 53.6 ± 5.5 | 50.0 ± 5.8 * | 51.8 ± 5.9 | |
| Gait Phases | Group | Walking conditions (slope/speed) | ||||
| A | B | C | D | E | ||
| −20% | −10% | 0% | +10% | +20% | ||
| 3.5 km·h-1 | 5.0 km·h-1 | 5.0 km·h-1 | 3.5 km·h-1 | 2.5 km·h-1 | ||
| Stance phase (%LL) | HFT | 62.4 ± 0.9 | 60.8 ± 0.6 a | 61.8 ± 0.9 b | 64.6 ± 0.9 d | 66.6 ± 1.0 e |
| NFT | 62.9 ± 1.1 | 60.9 ± 1.1 a | 61.7 ± 1.1 c | 64.7 ± 1.3 d | 67.5 ± 1.6 e | |
| Load response (%LL) | HFT | 12.4 ± 0.9 | 10.8 ± 0.6 a | 11.8 ± 0.9 b | 14.6 ± 0.9 d | 16.6 ± 1.0 e |
| NFT | 12.9 ± 1.1 | 10.9 ± 1.1 a | 11.7 ± 1.1 c | 14.7 ± 1.3 d | 17.5 ± 1.6 e | |
| Mid-stance (%LL) | HFT | 37.6 ± 0.9 | 39.2 ± 0.6 a | 38.2 ± 0.9 b | 35.4 ± 0.9 d | 33.4 ± 1.0 e |
| NFT | 37.1 ± 1.1 | 39.1 ± 1.1 a | 38.3 ± 1.1 c | 35.3 ± 1.3 d | 32.5 ± 1.6 e | |
| Pre-swing (%LL) | HFT | 12.4 ± 0.9 | 10.8 ± 0.6 a | 11.8 ± 0.9 b | 14.6 ± 0.9 d | 16.6 ± 1.0 e |
| NFT | 12.9 ± 1.1 | 10.9 ± 1.1 a | 11.7 ± 1.1 c | 14.7 ± 1.3 d | 17.5 ± 1.6 e | |
| Swing phase (%LL) | HFT | 37.6 ± 0.9 | 39.2 ± 0.6 a | 38.2 ± 0.9 b | 35.4 ± 0.9 d | 33.4 ± 1.0 e |
| NFT | 37.1 ± 1.1 | 39.1 ± 1.1 a | 38.3 ± 1.1 c | 35.3 ± 1.3 d | 32.5 ± 1.6 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





