Submitted:
19 February 2025
Posted:
20 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
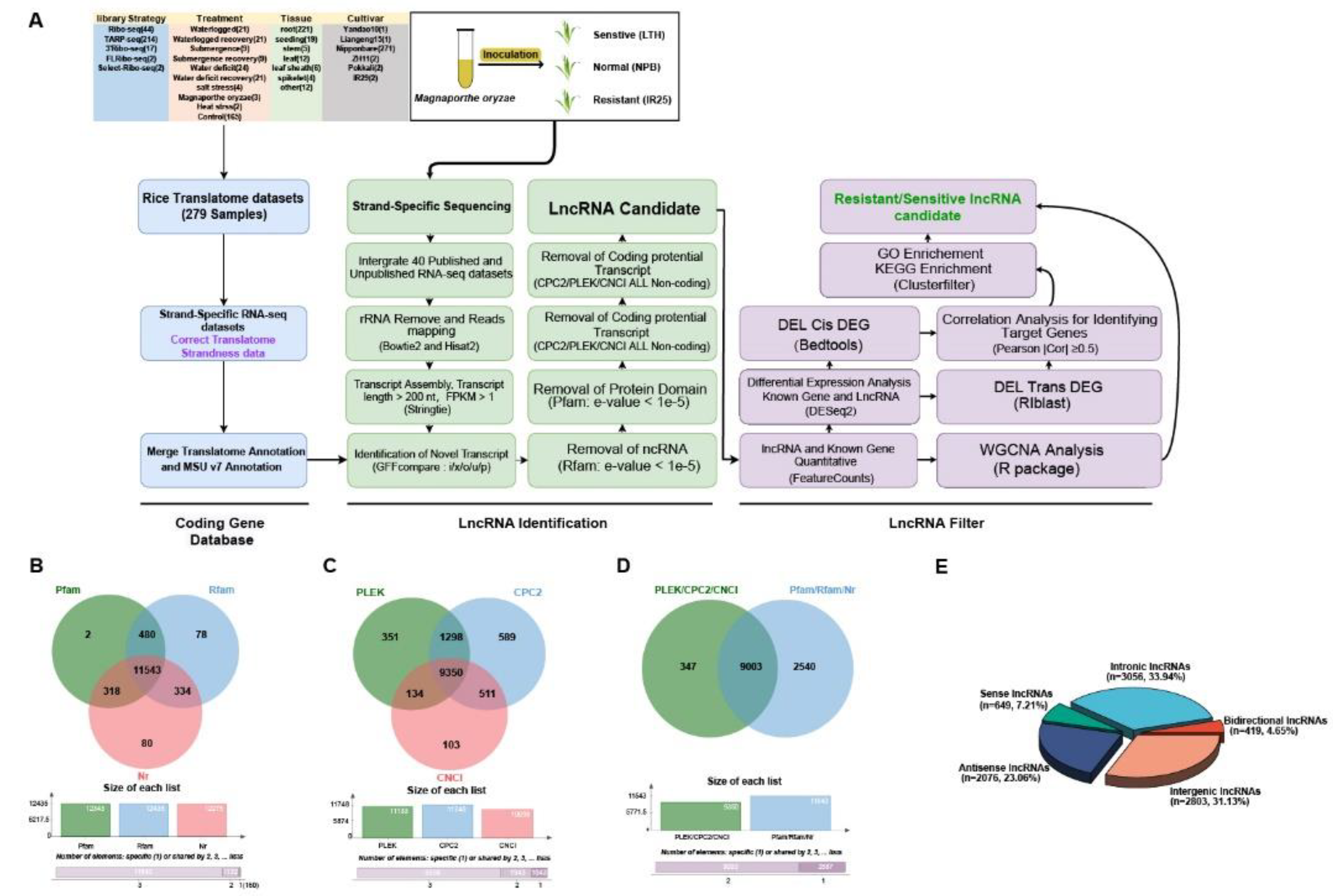
2.1. Analysis of the CodingRNA Dataset Assembly
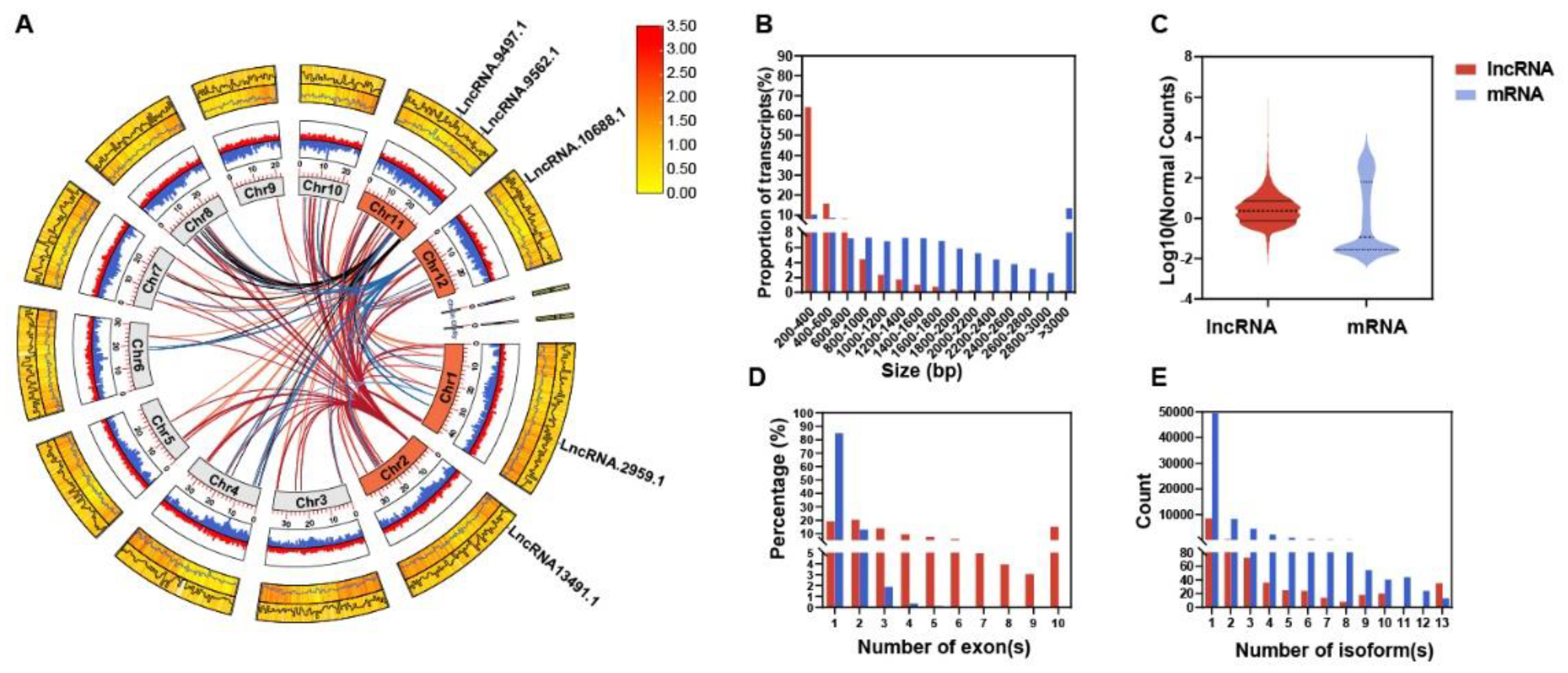
2.2. Integrated Translatome and Transcriptome-Based Lncrna Identification Pipeline and Analysis
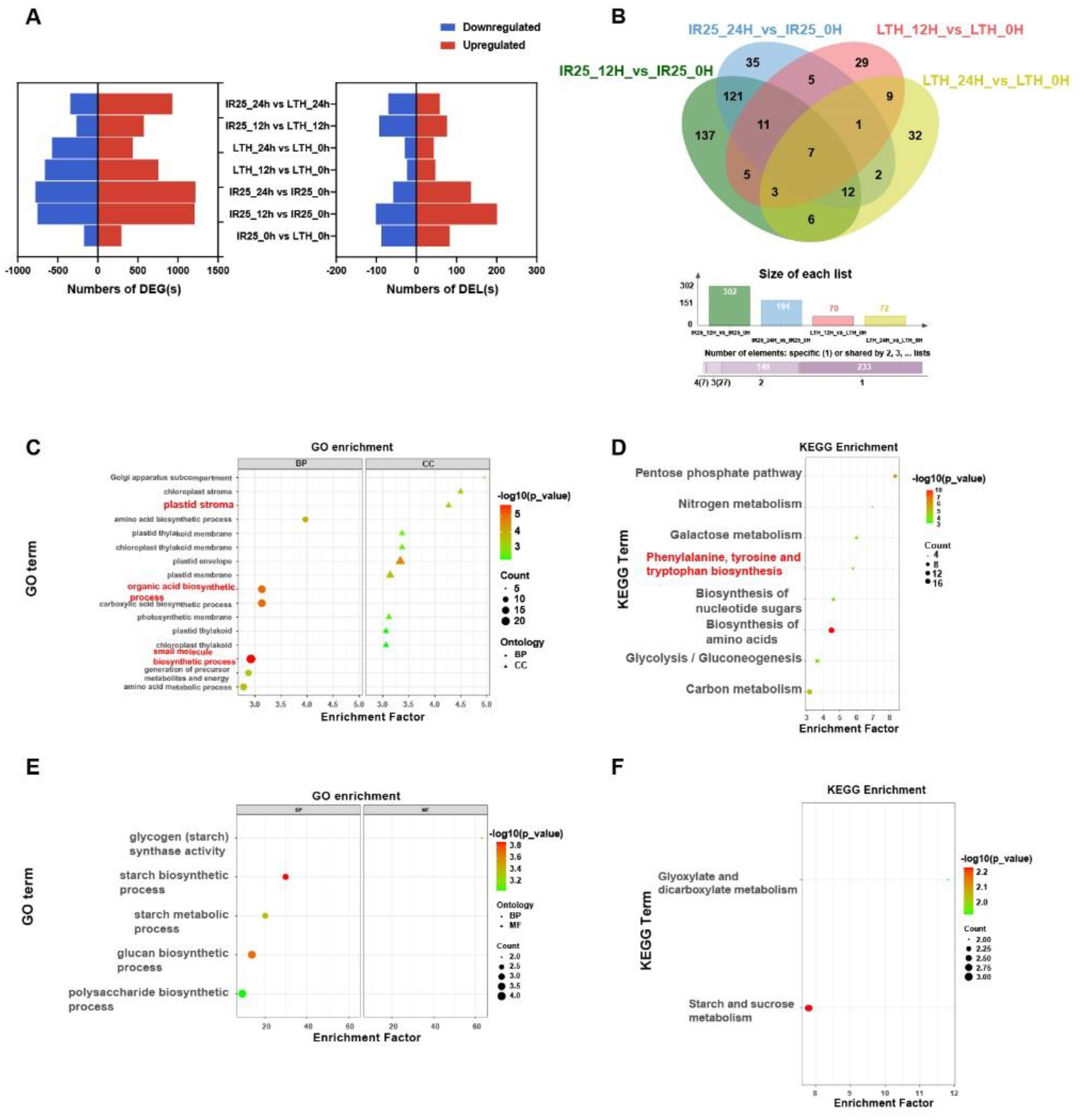
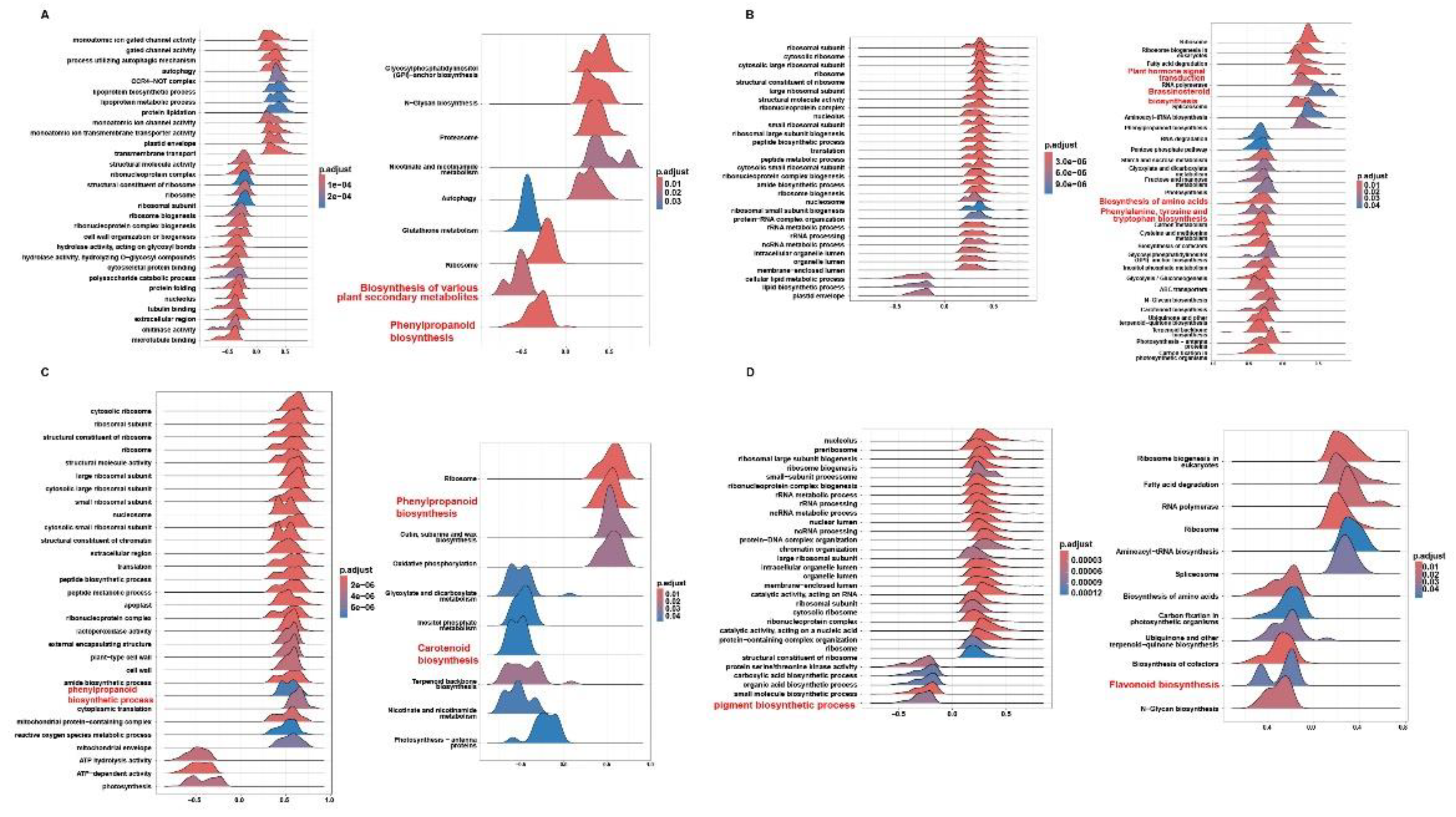
2.3. DELs and Their Differential Expression (DE) Target Genes Regulatory Networks Reveal the Mechanisms Underlying Rice Response to M. oryzae Infection
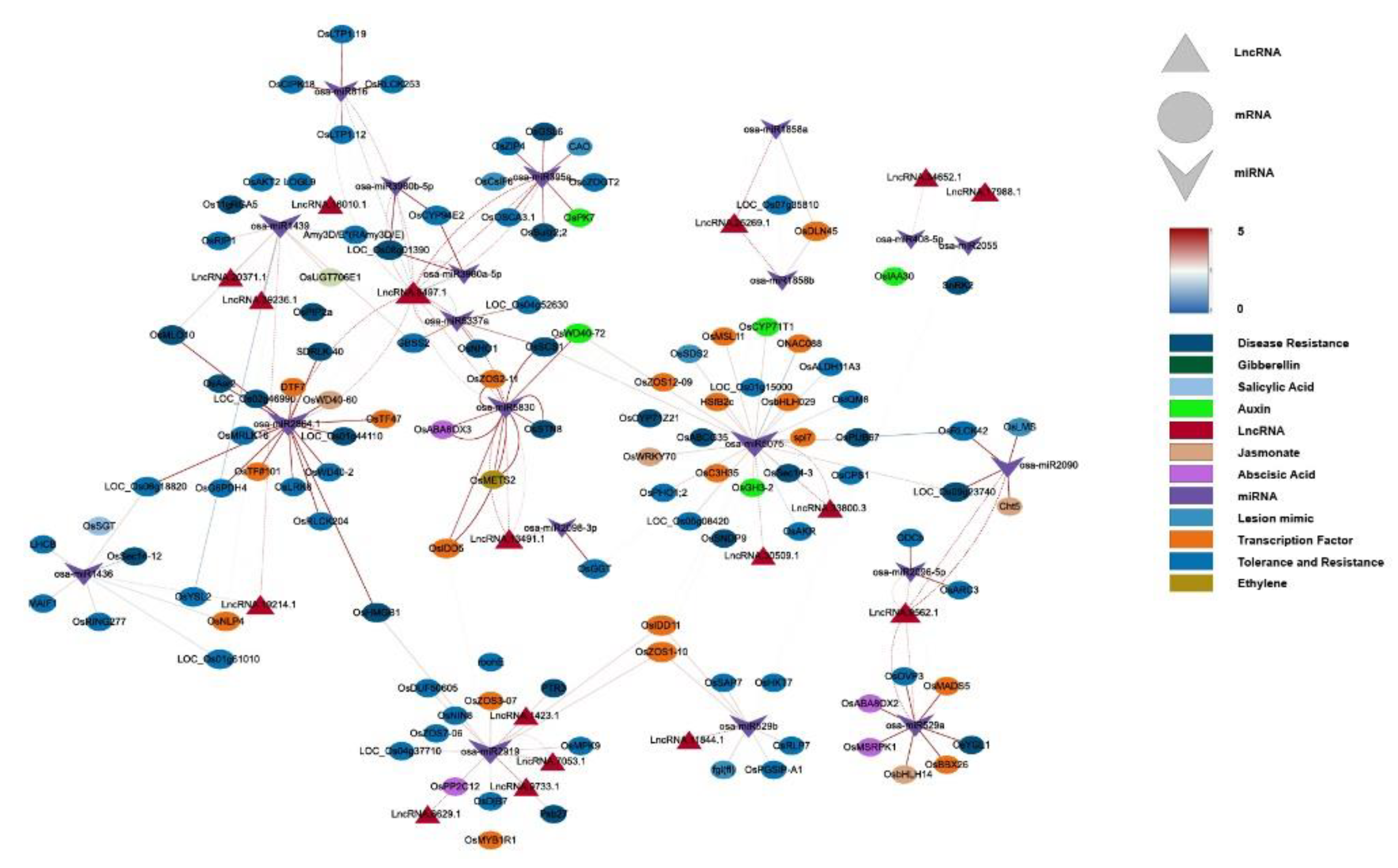
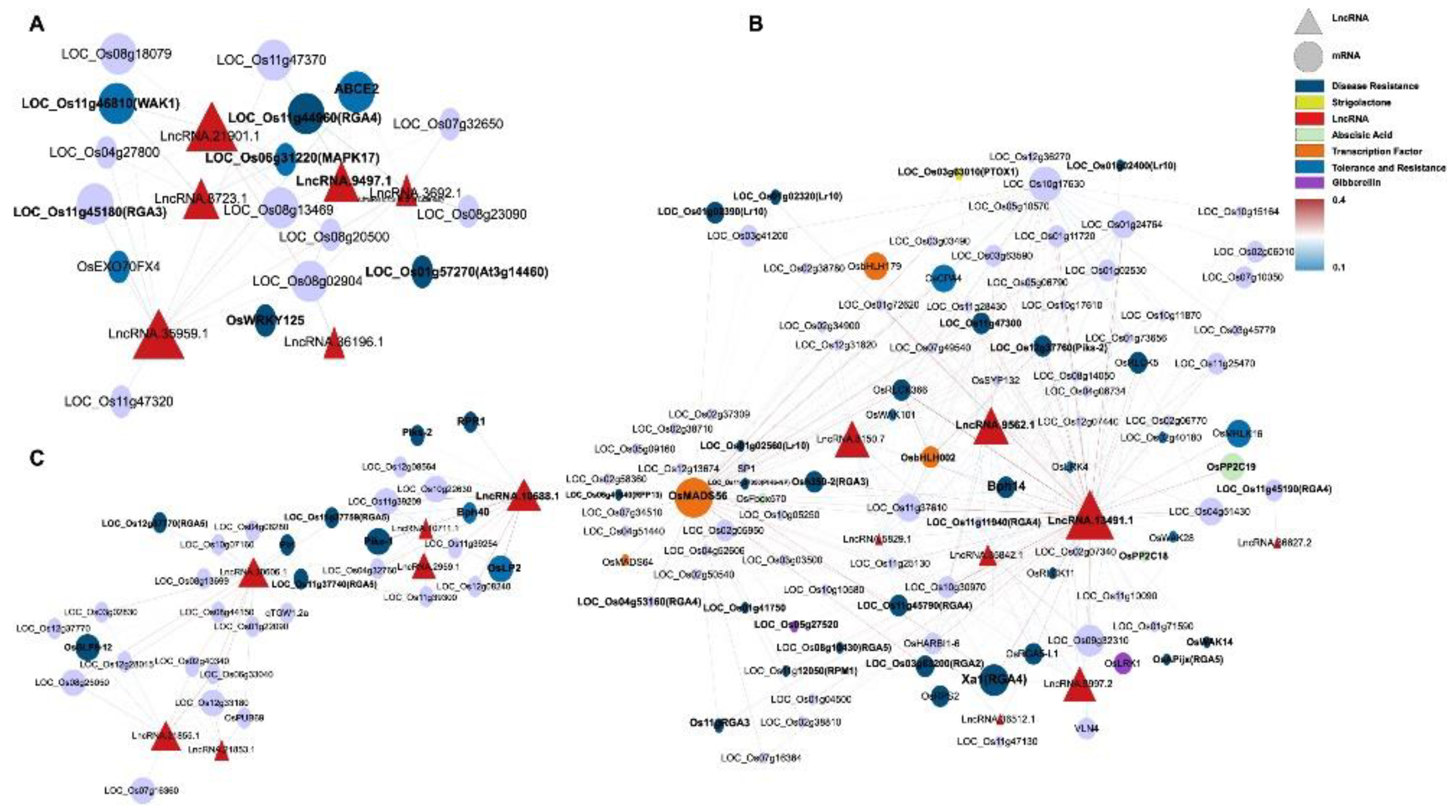
2.4. Construction of a ceRNA Network Reveals the Role of lncRNAs in Rice Blast Resistance
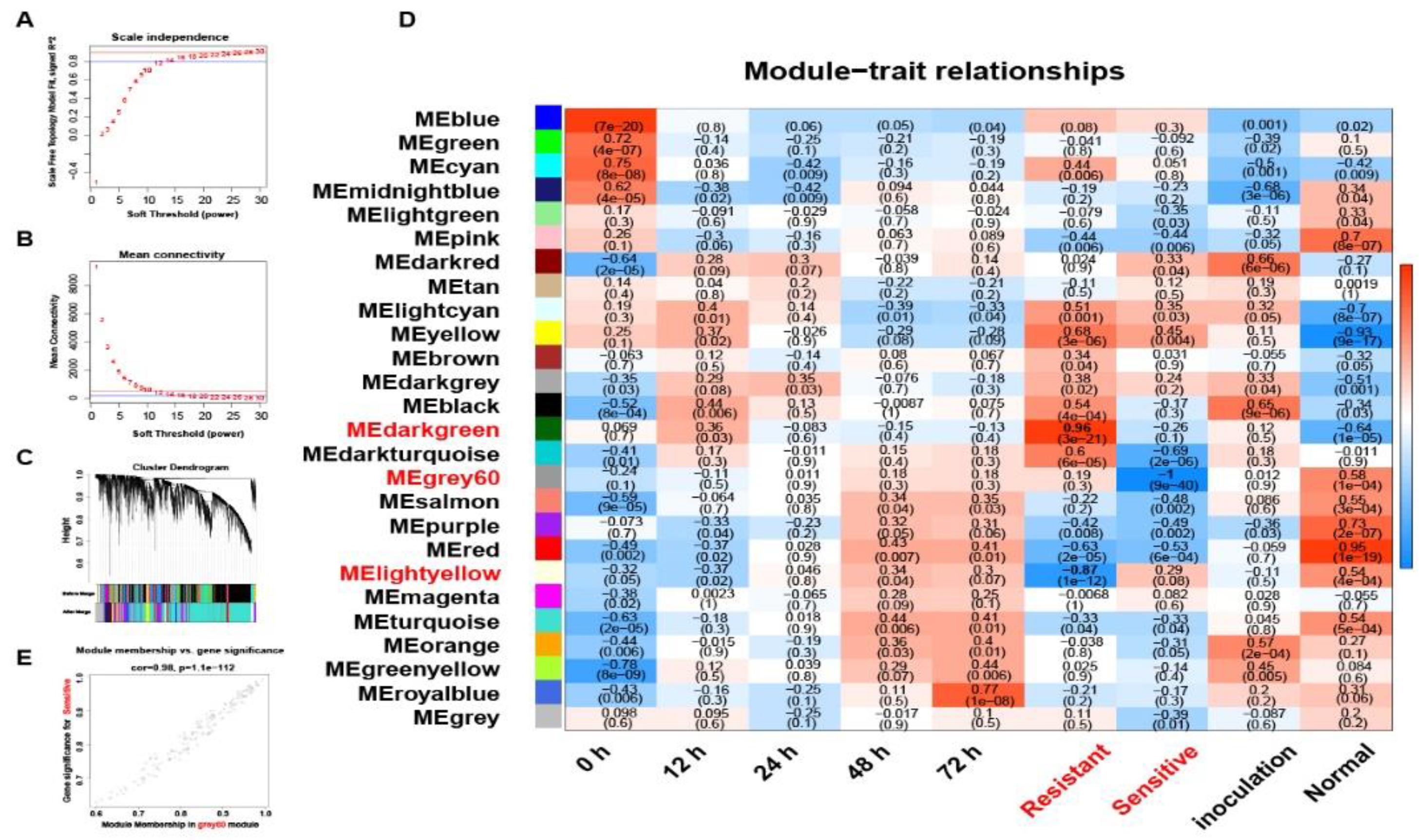
2.5. WGCNA Uncovers Key Hormone, R genes / Proteins, and Receptor Kinase Networks Under M. oryzae Stress
2.6. Mechanistic Insights into the Functional Roles of Key lncRNAs in Rice Blast Resistance
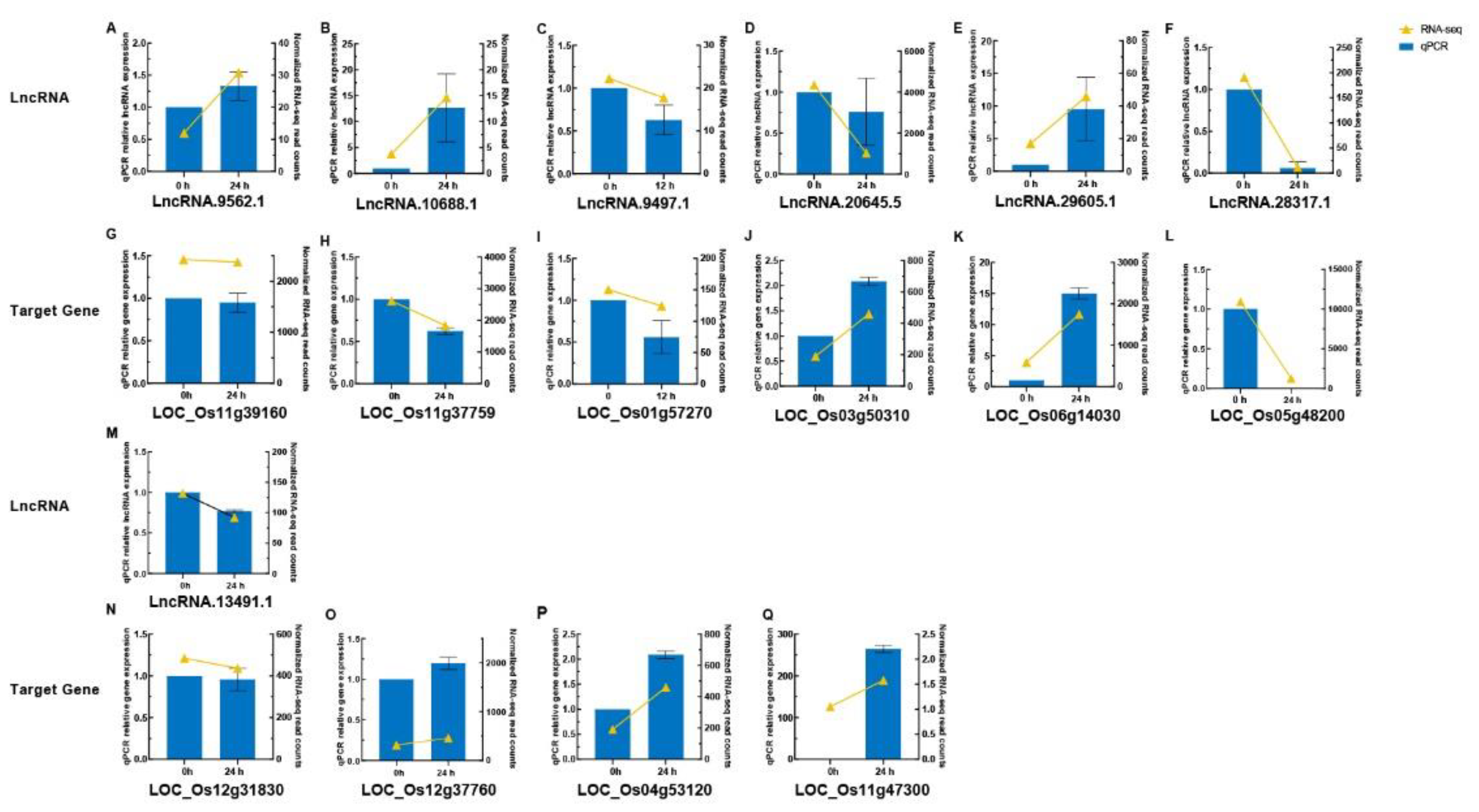
2.7. qRT–PCR Analysis and LncRNA Cloning Validation
3. Discussion
3.1. Optimized lncRNA Identification Pipeline and Its Contribution to Rice Genome Annotation
3.2. LncRNAs Participate in Rice Blast Resistance by Regulating JA, ET, and IAA Signaling Pathways in a Coordinated Manner
3.3. lncRNAs Mediate Immune Responses by Regulating RLKs and R Genes/Proteins and by Participating in ceRNA Networks
4. Materials and Methods
4.1. LncRNA Strand-Specific Library Data Sources
4.2. LncRNA Identification and Classification
4.3. Identification of cis- and trans-Targets and Known lncRNAs
4.4. Differential Expression Analysis and Functional Enrichment
4.5. Transcription Factor Identification and lncrna Localization
4.6. Weighted Gene Coexpression Network Analysis (WGCNA)
4.7. Single Key lncRNA Analysis
4.8. Competing Endogenous RNA (ceRNA) Network Construction
4.9. qRT-PCR Method and lncRNA Cloning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattick, J.S.; Rinn, J.L. Discovery and Annotation of Long Noncoding RNAs. Nat Struct Mol Biol 2015, 22, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Koch, L. Screening for lncRNA Function. Nat Rev Genet 2017, 18, 70–70. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I. Evolution to the Rescue: Using Comparative Genomics to Understand Long Non-Coding RNAs. Nat Rev Genet 2016, 17, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ivanov, M.; Dittrich, A.N.; Nelson, A.D.; Marquardt, S. LncRNA FLAIL Affects Alternative Splicing and Represses Flowering in Arabidopsis. The EMBO Journal 2023, 42, e110921. [Google Scholar] [CrossRef]
- X, Z.; J, L.; B, L.; H, G.; Y, L.; Y, Q. Global Identification of Arabidopsis lncRNAs Reveals the Regulation of MAF4 by a Natural Antisense RNA. Nature communications 2018, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Sun, F.; Hu, J.; Zha, X.; Su, W.; Yang, J. Overexpressing lncRNA LAIR Increases Grain Yield and Regulates Neighbouring Gene Cluster Expression in Rice. Nat Commun 2018, 9, 3516. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, F.; Zhang, G.; Li, X.; Wen, C.; Li, H. A Long Noncoding RNA Functions in Pumpkin Fruit Development through S-Adenosyl-L-Methionine Synthetase. Plant Physiology 2024, 195, 940–957. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Lin, F.; He, G.; Terzaghi, W.; Zhu, D.; Deng, X.W. Arabidopsis Noncoding RNA Mediates Control of Photomorphogenesis by Red Light. Proceedings of the National Academy of Sciences 2014, 111, 10359–10364. [Google Scholar] [CrossRef]
- Marquardt, S.; Raitskin, O.; Wu, Z.; Liu, F.; Sun, Q.; Dean, C. Functional Consequences of Splicing of the Antisense Transcript COOLAIR on FLC Transcription. Mol Cell 2014, 54, 156–165. [Google Scholar] [CrossRef]
- Seo, J.S.; Sun, H.-X.; Park, B.S.; Huang, C.-H.; Yeh, S.-D.; Jung, C.; Chua, N.-H. ELF18-INDUCED LONG-NONCODING RNA Associates with Mediator to Enhance Expression of Innate Immune Response Genes in Arabidopsis. The Plant Cell 2017, 29, 1024–1038. [Google Scholar] [CrossRef]
- Cui, J.; Luan, Y.; Jiang, N.; Bao, H.; Meng, J. Comparative Transcriptome Analysis between Resistant and Susceptible Tomato Allows the Identification of lncRNA16397 Conferring Resistance to Phytophthora Infestans by Co-Expressing Glutaredoxin. The Plant Journal 2017, 89, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Long Non-Coding RNAs and Their Biological Roles in Plants. Genomics, Proteomics & Bioinformatics 2015, 13, 137–147. [CrossRef]
- Heo, J.B.; Sung, S. Vernalization-Mediated Epigenetic Silencing by a Long Intronic Noncoding RNA. Science 2011, 331, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Sung, S. Vernalization-Triggered Intragenic Chromatin Loop Formation by Long Noncoding RNAs. Developmental Cell 2017, 40, 302–312.e4. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.-F.; Feng, Y.-Z.; He, H.; Lian, J.-P.; Yang, Y.-W.; Lei, M.-Q.; Zhang, Y.-C.; Chen, Y.-Q. Transcriptional Landscape of Pathogen-Responsive lncRNAs in Rice Unveils the Role of ALEX1 in Jasmonate Pathway and Disease Resistance. Plant Biotechnology Journal 2020, 18, 679–690. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Y.; Li, Q.; Cao, Y.; Yang, D.; Liu, S.; Wang, X.; Mi, Y.; Liu, Y.; Ding, C.; et al. A lncRNA Fine-Tunes Salicylic Acid Biosynthesis to Balance Plant Immunity and Growth. Cell Host & Microbe 2022, 30, 1124–1138.e8. [Google Scholar] [CrossRef]
- Jiang, N.; Cui, J.; Shi, Y.; Yang, G.; Zhou, X.; Hou, X.; Meng, J.; Luan, Y. Tomato lncRNA23468 Functions as a Competing Endogenous RNA to Modulate NBS-LRR Genes by Decoying miR482b in the Tomato-Phytophthora Infestans Interaction. Hortic Res 2019, 6. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Under Pressure: Investigating the Biology of Plant Infection by Magnaporthe Oryzae. Nat Rev Microbiol 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Mitchell, T.; Hu, Y.; Liu, X.; Dai, L.; Wang, G.-L. Recent Progress and Understanding of the Molecular Mechanisms of the Rice–Magnaporthe Oryzae Interaction. Molecular Plant Pathology 2010, 11, 419–427. [Google Scholar] [CrossRef]
- Sakulkoo, W.; Osés-Ruiz, M.; Oliveira Garcia, E.; Soanes, D.M.; Littlejohn, G.R.; Hacker, C.; Correia, A.; Valent, B.; Talbot, N.J. A Single Fungal MAP Kinase Controls Plant Cell-to-Cell Invasion by the Rice Blast Fungus. Science 2018, 359, 1399–1403. [Google Scholar] [CrossRef]
- Li, W.; Chern, M.; Yin, J.; Wang, J.; Chen, X. Recent Advances in Broad-Spectrum Resistance to the Rice Blast Disease. Current Opinion in Plant Biology 2019, 50, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Ran, L.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A Natural Allele of a Transcription Factor in Rice Confers Broad-Spectrum Blast Resistance. Cell 2017, 170, 114–126.e15. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; He, W.; Wang, T.; Yang, Y.; Xu, Q.; Zhao, X.; Yang, L.; Zhang, H.; Li, X.; Lv, Y.; et al. A Complete Assembly of the Rice Nipponbare Reference Genome. Molecular Plant 2023, 16, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza Sativa Nipponbare Reference Genome Using next Generation Sequence and Optical Map Data. Rice 2013, 6, 4. [Google Scholar] [CrossRef]
- Xiong, Q.; Ma, B.; Lu, X.; Huang, Y.-H.; He, S.-J.; Yang, C.; Yin, C.; Zhao, H.; Zhou, Y.; Zhang, W.-K.; et al. Ethylene-Inhibited Jasmonic Acid Biosynthesis Promotes Mesocotyl/Coleoptile Elongation of Etiolated Rice Seedlings[OPEN]. Plant Cell 2017, 29, 1053–1072. [Google Scholar] [CrossRef]
- White, M.; Carbonare, L.D.; Puerta, M.L.; Iacopino, S. ; Martin; Edwards; Dunne, K.; Pires, E.; Levy, C.; McDonough, M.; et al. Structures of the Arabidopsis Thaliana Oxygen-Sensing Plant Cysteine Oxidases PCO4 and PCO5 Enable Targeted Manipulation of Their Activity. 2020.
- Chini, A.; Boter, M.; Solano, R. Plant Oxylipins: COI1/JAZs/MYC2 as the Core Jasmonic Acid-signalling Module. The FEBS Journal 2009, 276. [Google Scholar] [CrossRef]
- Bao, D.; Chang, S.; Li, X.; Qi, Y. Advances in the Study of Auxin Early Response Genes: Aux/IAA, GH3, and SAUR. The Crop Journal 2024, 12, 964–978. [Google Scholar] [CrossRef]
- Yang, Z.; Hui, S.; Lv, Y.; Zhang, M.; Chen, D.; Tian, J.; Zhang, H.; Liu, H.; Cao, J.; Xie, W.; et al. miR395-Regulated Sulfate Metabolism Exploits Pathogen Sensitivity to Sulfate to Boost Immunity in Rice. Molecular Plant 2022, 15, 671–688. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, N.; Zhang, J. Glucose-Induced Delay of Seed Germination in Rice Is Mediated by the Suppression of ABA Catabolism Rather Than an Enhancement of ABA Biosynthesis. Plant and Cell Physiology 2009, 50, 644–651. [Google Scholar] [CrossRef]
- Zhai, K.; Liang, D.; Li, H.; Jiao, F.; Yan, B.; Liu, J.; Lei, Z.; Huang, L.; Gong, X.; Wang, X.; et al. NLRs Guard Metabolism to Coordinate Pattern- and Effector-Triggered Immunity. Nature 2022, 601, 245–251. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Li, J.; Zhou, G.; Wang, Q.; Bian, W.; Erb, M.; Lou, Y. Prioritizing Plant Defence over Growth through WRKY Regulation Facilitates Infestation by Non-Target Herbivores. eLife 2015, 4, e04805. [Google Scholar] [CrossRef]
- Otomo, K.; Kenmoku, H.; Oikawa, H.; König, W.A.; Toshima, H.; Mitsuhashi, W.; Yamane, H.; Sassa, T.; Toyomasu, T. Biological Functions of Ent - and Syn -copalyl Diphosphate Synthases in Rice: Key Enzymes for the Branch Point of Gibberellin and Phytoalexin Biosynthesis. The Plant Journal 2004, 39, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, H.; Li, X.; Xiao, J.; Wang, S. Rice GH3 Gene Family: Regulators of Growth and Development. Plant Signaling & Behavior 2011, 6, 570–574. [Google Scholar] [CrossRef]
- Miyamoto, K.; Shimizu, T.; Lin, F.; Sainsbury, F.; Thuenemann, E.; Lomonossoff, G.; Nojiri, H.; Yamane, H.; Okada, K. Identification of an E-Box Motif Responsible for the Expression of Jasmonic Acid-Induced Chitinase Gene OsChia4a in Rice. J Plant Physiol 2012, 169, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Luo, Y.; Zhang, L.; Yao, Y.; Han, L.; Chen, Z.; Wang, L.; Li, Y. OsABA8ox2, an ABA Catabolic Gene, Suppresses Root Elongation of Rice Seedlings and Contributes to Drought Response. The Crop Journal 2020, 8, 480–491. [Google Scholar] [CrossRef]
- Seo, J.; Joo, J.; Kim, M.; Kim, Y.; Nahm, B.H.; Song, S.I.; Cheong, J.; Lee, J.S.; Kim, J.; Choi, Y.D. OsbHLH148, a Basic Helix-loop-helix Protein, Interacts with OsJAZ Proteins in a Jasmonate Signaling Pathway Leading to Drought Tolerance in Rice. The Plant Journal 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63 – OsWRKY76 – OsDREB1B Module Regulates Chilling Tolerance in Rice. The Plant Journal 2022, 112, 383–398. [Google Scholar] [CrossRef]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M.; et al. Ethylene Promotes Submergence-Induced Expression of OsABA8ox1, a Gene That Encodes ABA 8’-Hydroxylase in Rice. Plant Cell Physiol 2007, 48, 287–298. [Google Scholar] [CrossRef]
- Li, H.; Zhou, S.-Y.; Zhao, W.-S.; Su, S.-C.; Peng, Y.-L. A Novel Wall-Associated Receptor-like Protein Kinase Gene, OsWAK1, Plays Important Roles in Rice Blast Disease Resistance. Plant Mol Biol 2009, 69, 337–346. [Google Scholar] [CrossRef]
- Okuyama, Y.; Kanzaki, H.; Abe, A.; Yoshida, K.; Tamiru, M.; Saitoh, H.; Fujibe, T.; Matsumura, H.; Shenton, M.; Galam, D.C.; et al. A Multifaceted Genomics Approach Allows the Isolation of the Rice Pia -blast Resistance Gene Consisting of Two Adjacent NBS-LRR Protein Genes. The Plant Journal 2011, 66, 467–479. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, J.; Zhang, S.; Chen, L.; Yang, T.; Dong, J.; Fu, H.; Ma, Y.; Zhou, L.; Wang, J.; et al. Genome-Wide Association Mapping and Gene Expression Analysis Reveal the Negative Role of OsMYB21 in Regulating Bacterial Blight Resistance in Rice. Rice 2021, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Rohila, J.S.; Yang, Y. Rice Mitogen-Activated Protein Kinase Gene Family and Its Role in Biotic and Abiotic Stress Response. Journal of Integrative Plant Biology 2007, 49, 751–759. [Google Scholar] [CrossRef]
- Cesari, S.; Thilliez, G.; Ribot, C.; Chalvon, V.; Michel, C.; Jauneau, A.; Rivas, S.; Alaux, L.; Kanzaki, H.; Okuyama, Y.; et al. The Rice Resistance Protein Pair RGA4/RGA5 Recognizes the Magnaporthe Oryzae Effectors AVR-Pia and AVR1-CO39 by Direct Binding[W][OA]. Plant Cell 2013, 25, 1463–1481. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 Confers Resistance to Brown Planthopper by Fortifying Sclerenchyma in Rice Leaf Sheaths. Molecular Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef]
- Wu, F.; Sheng, P.; Tan, J.; Chen, X.; Lu, G.; Ma, W.; Heng, Y.; Lin, Q.; Zhu, S.; Wang, J.; et al. Plasma Membrane Receptor-like Kinase Leaf Panicle 2 Acts Downstream of the DROUGHT AND SALT TOLERANCE Transcription Factor to Regulate Drought Sensitivity in Rice. Journal of Experimental Botany 2015, 66, 271–281. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, W.; Liu, M.; Li, Y.; Liu, J.; Franceschetti, M.; Yi, Z.; Zhu, X.; Zhang, Z.; Lu, G.; et al. The Piks Allele of the NLR Immune Receptor Pik Breaks the Recognition of AvrPik Effectors of the Rice Blast Fungus. Journal of integrative plant biology 2022. [Google Scholar] [CrossRef]
- Hayashi, N.; Inoue, H.; Kato, T.; Funao, T.; Shirota, M.; Shimizu, T.; Kanamori, H.; Yamane, H.; Hayano-Saito, Y.; Matsumoto, T.; et al. Durable Panicle Blast-Resistance Gene Pb1 Encodes an Atypical CC-NBS-LRR Protein and Was Generated by Acquiring a Promoter through Local Genome Duplication. The Plant Journal 2010, 64, 498–510. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, K.-K.; Cho, Y.-G. Molecular and Functional Analysis of U-Box E3 Ubiquitin Ligase Gene Family in Rice (Oryza Sativa). International Journal of Molecular Sciences 2021, 22, 12088. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Chen, C.; Shen, Z. Genome-Wide Characterization and Expression Analysis of the Germin-Like Protein Family in Rice and Arabidopsis. International Journal of Molecular Sciences 2016, 17. [Google Scholar] [CrossRef]
- Durrani, I.S.; Jan, A.; Shah, S.; Iqbal, A.; Ahmad, D.; Khan, H.; Naqvi, S.M.S. Bioinformatics Studies of OSGLP8-12 Gene from Oryza Sativa (Japonica) Reveal Its Role in Conferring Resistance against Disease and Stresses. Pakistan Journal of Botany 2020. [Google Scholar] [CrossRef]
- Ji, Z.; Ji, C.; Liu, B.; Zou, L.; Chen, G.; Yang, B. Interfering TAL Effectors of Xanthomonas Oryzae Neutralize R-Gene-Mediated Plant Disease Resistance. Nat Commun 2016, 7, 13435. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.; Nam, J.; Dangl, J. The Arabidopsis Thaliana RPM1 Disease Resistance Gene Product Is a Peripheral Plasma Membrane Protein That Is Degraded Coincident with the Hypersensitive Response. Proceedings of the National Academy of Sciences of the United States of America 1998, 95 26, 15849–15854. [Google Scholar] [CrossRef]
- Zhan, P.; Ma, S.; Xiao, Z.; Li, F.; Wei, X.; Lin, S.; Wang, X.; Ji, Z.; Fu, Y.; Pan, J.; et al. Natural Variations in Grain Length 10 (GL10) Regulate Rice Grain Size. Journal of Genetics and Genomics 2022, 49, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, M.; Abe, A.; Utsushi, H.; Yoshida, K.; Takagi, H.; Fujisaki, K.; Undan, J.; Rakshit, S.; Takaichi, S.; Jikumaru, Y.; et al. The Tillering Phenotype of the Rice Plastid Terminal Oxidase (PTOX) Loss-of-Function Mutant Is Associated with Strigolactone Deficiency. The New phytologist 2014, 202 1, 116–131. [Google Scholar] [CrossRef]
- Vij, S.; Giri, J.; Dansana, P.K.; Kapoor, S.; Tyagi, A. The Receptor-like Cytoplasmic Kinase (OsRLCK) Gene Family in Rice: Organization, Phylogenetic Relationship, and Expression during Development and Stress. Molecular plant 2008, 1 5, 732–750. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.-I.; Kwon, H.; Cho, M.; Kim, B.-G.; Chung, J.; Nam, M.; Song, J.S.; Kim, K.-H.; Yoon, I. The Rice Abscisic Acid-Responsive RING Finger E3 Ligase OsRF1 Targets OsPP2C09 for Degradation and Confers Drought and Salinity Tolerance in Rice. Frontiers in Plant Science 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Gong, Y.; Chong, K.; Xu, Y. Phosphatase OsPP2C27 Directly Dephosphorylates OsMAPK3 and OsbHLH002 to Negatively Regulate Cold Tolerance in Rice. Plant, cell & environment, 2020. [Google Scholar] [CrossRef]
- Loutre, C.; Wicker, T.; Travella, S.; Galli, P.; Scofield, S.; Fahima, T.; Feuillet, C.; Keller, B. Two Different CC-NBS-LRR Genes Are Required for Lr10-Mediated Leaf Rust Resistance in Tetraploid and Hexaploid Wheat. The Plant journal : for cell and molecular biology, 2009; 60 6, 1043–1054. [Google Scholar] [CrossRef]
- Luo, H.; Song, F.; Goodman, R.M.; Zheng, Z. Up-Regulation of OsBIHD1, a Rice Gene Encoding BELL Homeodomain Transcriptional Factor, in Disease Resistance Responses. Plant biology 2005, 7 5, 459–468. [Google Scholar] [CrossRef]
- Wang, A.; Shu, X.; Jing, X.; Jiao, C.; Chen, L.; Zhang, J.; Ma, L.; Jiang, Y.; Yamamoto, N.; Li, S.; et al. Identification of Rice ( Oryza Sativa L.) Genes Involved in Sheath Blight Resistance via a Genome-wide Association Study. Plant Biotechnology Journal 2021, 19, 1553–1566. [Google Scholar] [CrossRef]
- Wang, L.-L.; Jin, J.-J.; Li, L.-H.; Qu, S.-H. Long Non-Coding RNAs Responsive to Blast Fungus Infection in Rice. Rice 2020, 13, 77. [Google Scholar] [CrossRef]
- Jain, P.; Sharma, V.; Dubey, H.; Singh, P.K.; Kapoor, R.; Kumari, M.; Singh, J.; Pawar, D.V.; Bisht, D.; Solanke, A.U.; et al. Identification of Long Non-Coding RNA in Rice Lines Resistant to Rice Blast Pathogen Maganaporthe Oryzae. Bioinformation 2017, 13, 249–255. [Google Scholar] [CrossRef]
- Metraux, J. Recent Breakthroughs in the Study of Salicylic Acid Biosynthesis. Trends in plant science 2002, 7 8, 332–334. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis: A Simple Two-Step Pathway Converts Tryptophan to Indole-3-Acetic Acid in Plants. Molecular plant 2012, 5 2, 334–338. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, C.; Zhao, T.; Yang, L.; Shang, Q.; Wang, G.; Liu, Z.; Gai, Y.; Ji, X. Integrated Analysis of lncRNAs and mRNAs Reveals Complex Gene Network Mediated by lncRNAs and Regulatory Function of MuLRR-RLK-AS in Response to Phytoplasma Infection in Mulberry. Biomolecules 2024, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.; Valli, A.; Todesco, M.; Mateos, I.; Puga, M.I.; Rubio-Somoza, I.; Leyva, A.; Weigel, D.; García, J.; Paz-Ares, J. Target Mimicry Provides a New Mechanism for Regulation of microRNA Activity. Nature Genetics 2007, 39, 1033–1037. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Hou, X.; Wu, S.; Zhang, Q.; Meng, J.; Luan, Y. Genome-Wide Identification of lncRNAs and Analysis of ceRNA Networks During Tomato Resistance to Phytophthora Infestans. Phytopathology 2020, 110, 456–464. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, H.; Li, J.; Sui, N. Comparative Transcriptome Analysis of Sweet Sorghum Provides Insights into New lncRNAs Acting as ceRNAs during Salt Responses. 2019. [Google Scholar] [CrossRef]
- Kajala, K.; Gouran, M.; Shaar-Moshe, L.; Mason, G.A.; Rodriguez-Medina, J.; Kawa, D.; Pauluzzi, G.; Reynoso, M.; Canto-Pastor, A.; Manzano, C.; et al. Innovation, Conservation, and Repurposing of Gene Function in Root Cell Type Development. Cell 2021, 184, 3333–3348.e19. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, J.; Chen, S.; Chen, J.; Liang, Y.; Zhang, C.; Li, Q.; Lai, J.; Li, L. Large-Scale Translatome Profiling Annotates the Functional Genome and Reveals the Key Role of Genic 3’ Untranslated Regions in Translatomic Variation in Plants. Plant Commun 2021, 2, 100181. [Google Scholar] [CrossRef]
- Reynoso, M.A.; Borowsky, A.T.; Pauluzzi, G.C.; Yeung, E.; Zhang, J.; Formentin, E.; Velasco, J.; Cabanlit, S.; Duvenjian, C.; Prior, M.J.; et al. Gene Regulatory Networks Shape Developmental Plasticity of Root Cell Types under Water Extremes in Rice. Developmental Cell 2022, 57, 1177–1192.e6. [Google Scholar] [CrossRef]
- Yang, X.; Song, B.; Cui, J.; Wang, L.; Wang, S.; Luo, L.; Gao, L.; Mo, B.; Yu, Y.; Liu, L. Comparative Ribosome Profiling Reveals Distinct Translational Landscapes of Salt-Sensitive and -Tolerant Rice. BMC Genomics 2021, 22, 612. [Google Scholar] [CrossRef]
- Reynoso, M.A.; Kajala, K.; Bajic, M.; West, D.A.; Pauluzzi, G.; Yao, A.I.; Hatch, K.; Zumstein, K.; Woodhouse, M.; Rodriguez-Medina, J.; et al. Evolutionary Flexibility in Flooding Response Circuitry in Angiosperms. Science 2019, 365, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Q.; Chen, Z.; Yue, Y.; Liu, Y.; Zhao, Y.; Zhou, D.-X. Histone Deacetylases Control Lysine Acetylation of Ribosomal Proteins in Rice. Nucleic Acids Res 2021, 49, 4613–4628. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Jin, J.-J.; Li, L.-H.; Qu, S.-H. Long Non-Coding RNAs Responsive to Blast Fungus Infection in Rice. Rice (N Y) 2020, 13, 77. [Google Scholar] [CrossRef]
- Fan, J.; Quan, W.; Li, G.-B.; Hu, X.-H.; Wang, Q.; Wang, H.; Li, X.-P.; Luo, X.; Feng, Q.; Hu, Z.-J.; et al. circRNAs Are Involved in the Rice-Magnaporthe Oryzae Interaction1[OPEN]. Plant Physiol 2020, 182, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Tsunematsu, H.; Yanoria, M.J.T.; Ebron, L.A.; Hayashi, N.; Ando, I.; Kato, H.; Imbe, T.; Khush, G.S. Development of Monogenic Lines of Rice for Blast Resistance. Breeding Science 2000, 50, 229–234. [Google Scholar] [CrossRef]
- Wingett, S.; Andrews, S. FastQ Screen: A Tool for Multi-Genome Mapping and Quality Control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality Control of RNA-Seq Experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet.journal 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat Biotechnol 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat Biotechnol 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res 2020, 9, ISCB. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Kalvari, I.; Nawrocki, E.P.; Ontiveros-Palacios, N.; Argasinska, J.; Lamkiewicz, K.; Marz, M.; Griffiths-Jones, S.; Toffano-Nioche, C.; Gautheret, D.; Weinberg, Z.; et al. Rfam 14: Expanded Coverage of Metagenomic, Viral and microRNA Families. Nucleic Acids Res 2021, 49, D192–D200. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A Fast and Accurate Coding Potential Calculator Based on Sequence Intrinsic Features. Nucleic Acids Res 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhou, Z. PLEK: A Tool for Predicting Long Non-Coding RNAs and Messenger RNAs Based on an Improved k-Mer Scheme. BMC Bioinformatics 2014, 15, 311. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing Sequence Intrinsic Composition to Classify Protein-Coding and Long Non-Coding Transcripts. Nucleic Acids Research 2013, 41, e166. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Fukunaga, T.; Hamada, M. RIblast: An Ultrafast RNA-RNA Interaction Prediction System Based on a Seed-and-Extension Approach. Bioinformatics 2017, 33, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Golicz, A.A.; Bhalla, P.L.; Edwards, D.; Singh, M.B. Rice 3D Chromatin Structure Correlates with Sequence Variation and Meiotic Recombination Rate. Commun Biol 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yuan, C.; Zhang, J.; Zhang, Z.; Bai, L.; Meng, Y.; Chen, L.-L.; Chen, M. PlantNATsDB: A Comprehensive Database of Plant Natural Antisense Transcripts. Nucleic Acids Res 2012, 40, D1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhang, Z.; Ling, Y.; Xu, W.; Su, Z. PNRD: A Plant Non-Coding RNA Database. Nucleic Acids Res 2015, 43, D982–989. [Google Scholar] [CrossRef]
- RNAcentral Consortium RNAcentral 2021: Secondary Structure Integration, Improved Sequence Search and New Member Databases. Nucleic Acids Res 2021, 49, D212–D220. [CrossRef]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: An Updated Database Dedicated to Long Non-Coding RNA Annotation in Both Animals and Plants. Nucleic Acids Res 2021, 49, D165–D171. [Google Scholar] [CrossRef]
- Szcześniak, M.W.; Bryzghalov, O.; Ciomborowska-Basheer, J.; Makałowska, I. CANTATAdb 2.0: Expanding the Collection of Plant Long Noncoding RNAs. Methods Mol Biol 2019, 1933, 415–429. [Google Scholar] [CrossRef]
- Di Marsico, M.; Paytuvi Gallart, A.; Sanseverino, W.; Aiese Cigliano, R. GreeNC 2.0: A Comprehensive Database of Plant Long Non-Coding RNAs. Nucleic Acids Res 2022, 50, D1442–D1447. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the next-Generation Sequencing Data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Research 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2. WIREs Computational Statistics 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation (Camb) 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- xuzhougeng Xuzhougeng/Org. Osativa.Eg.Db v0.01 2019.
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: Charting Functional Regulatory Maps in Plants. Nucleic Acids Research 2020, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Hu, S.; Tang, G.; Chen, J.; Yi, Y.; Xie, H.; Lin, J.; Wang, M.; Wang, D.; et al. RNALocate v3.0: Advancing the Repository of RNA Subcellular Localization with Dynamic Analysis and Prediction. Nucleic Acids Research 2024, gkae872. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinformatics 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proceedings of the National Academy of Sciences 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A Plant Small RNA Target Analysis Server (2017 Release). Nucleic Acids Res 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Xu, Q.; Dong, J.; Song, L.; Wang, W.; Shen, F. Long Noncoding RNA lncRNA354 Functions as a Competing Endogenous RNA of miR160b to Regulate Genes in Response to Salt Stress in Upland Cotton. Plant, Cell & Environment 2021, 44, 3302–3321. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinformatics 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




