Submitted:
06 February 2025
Posted:
10 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
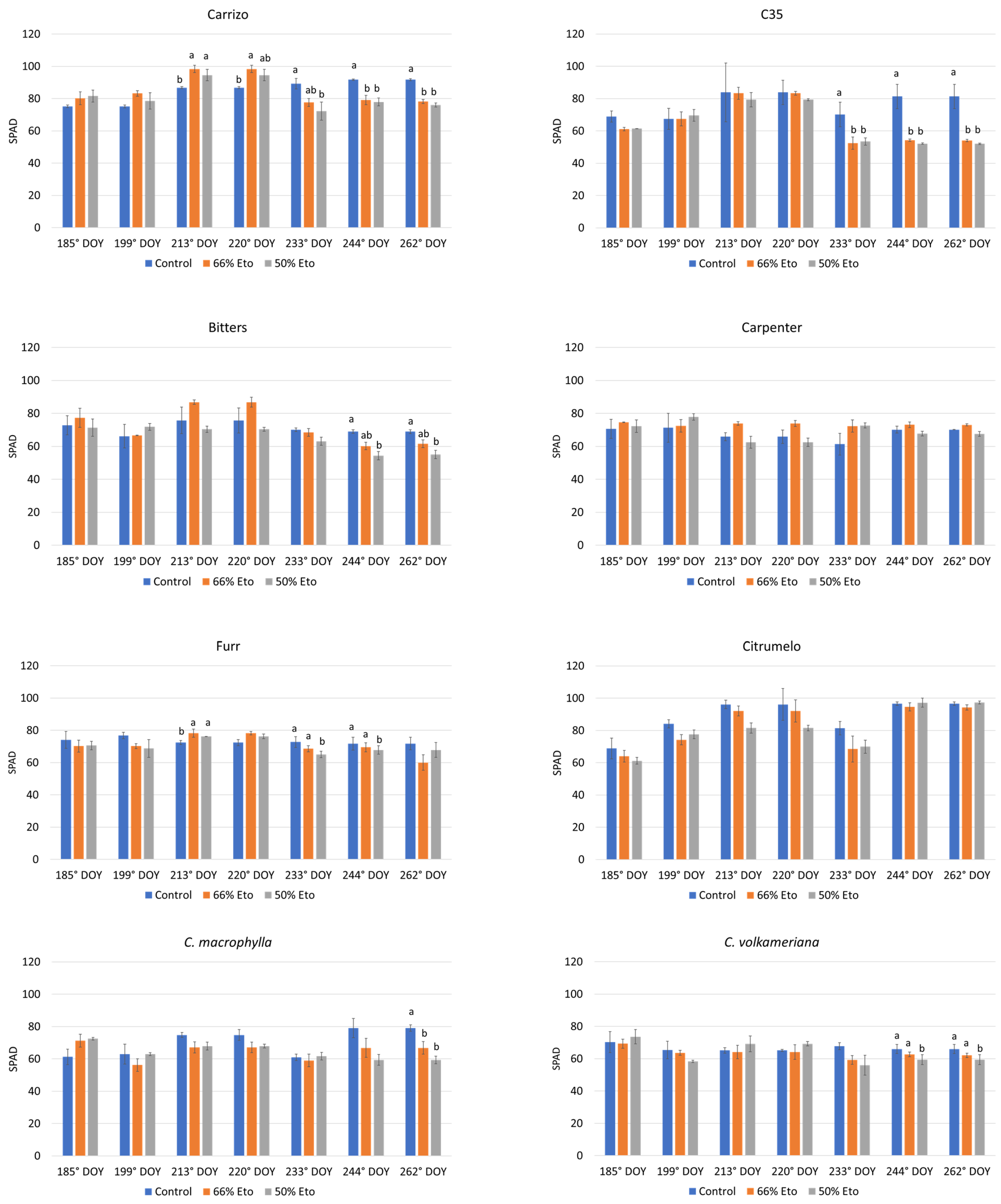
2.1. Morphological Measurements and Leaf Chlorophyll Content Determination (SPAD)
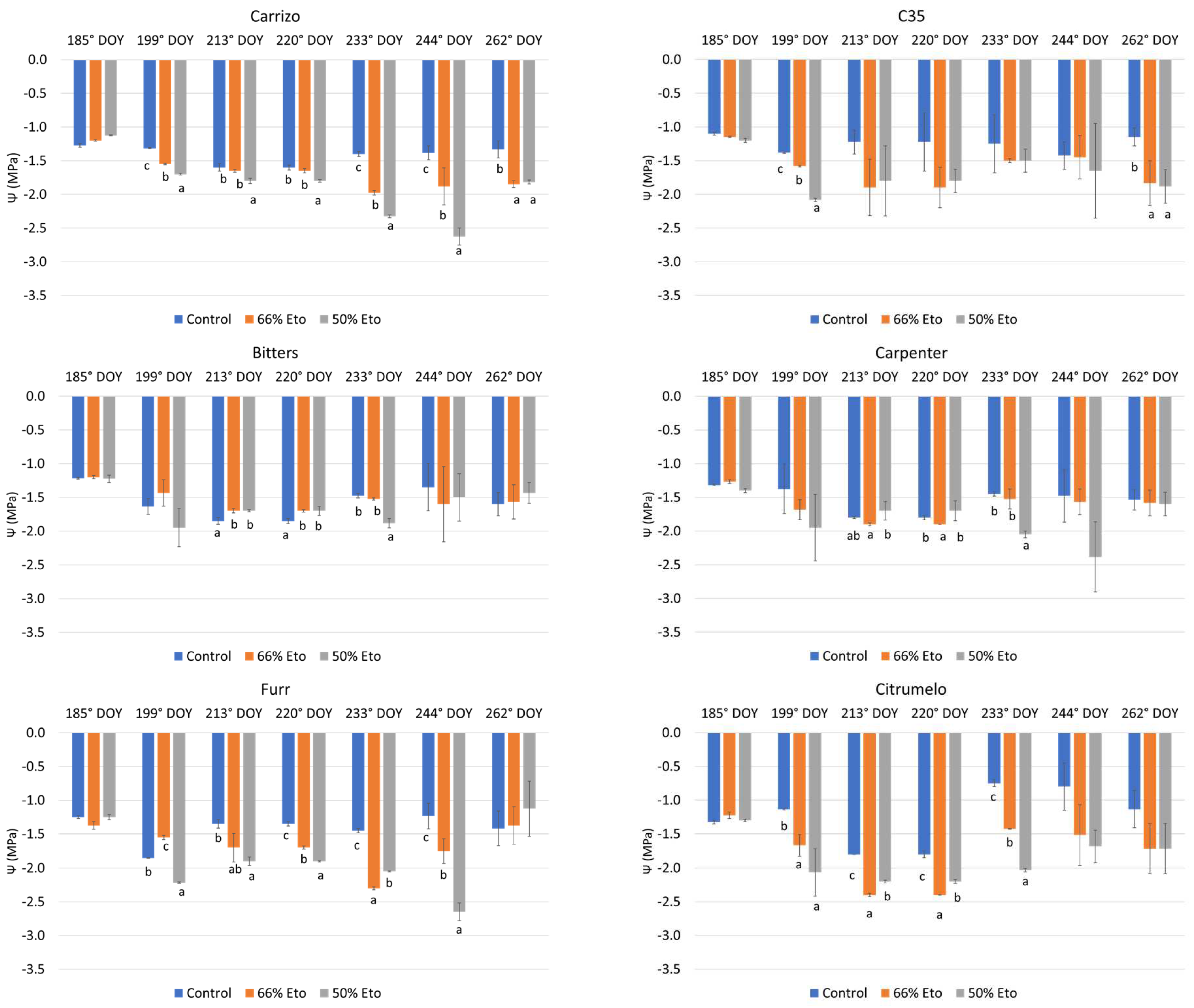
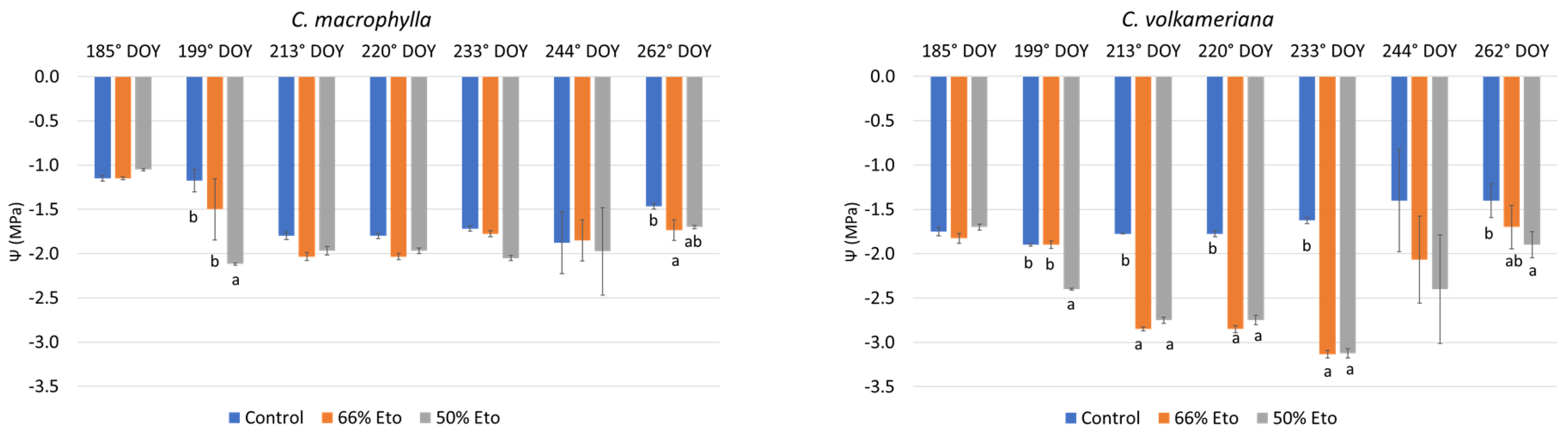
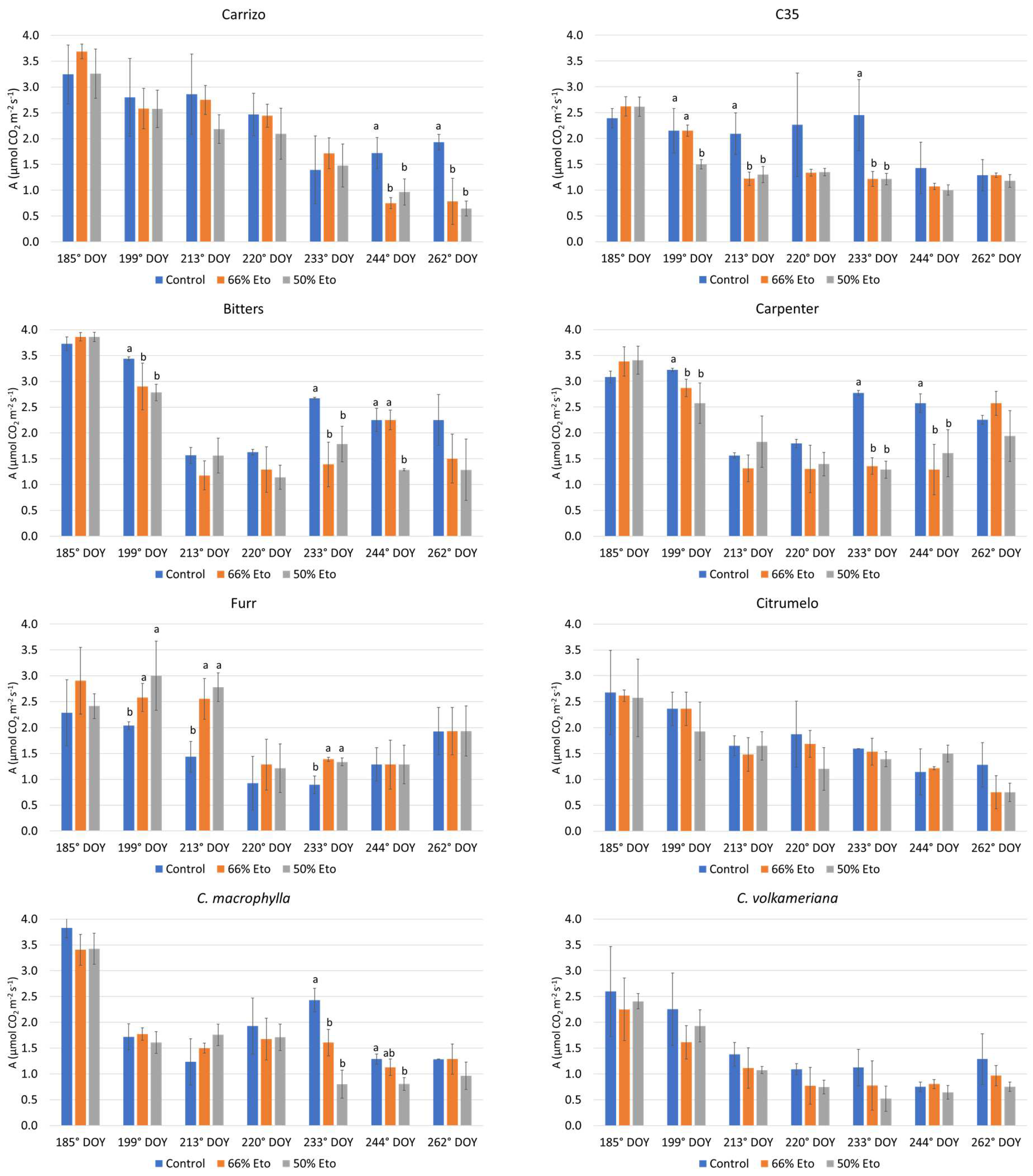
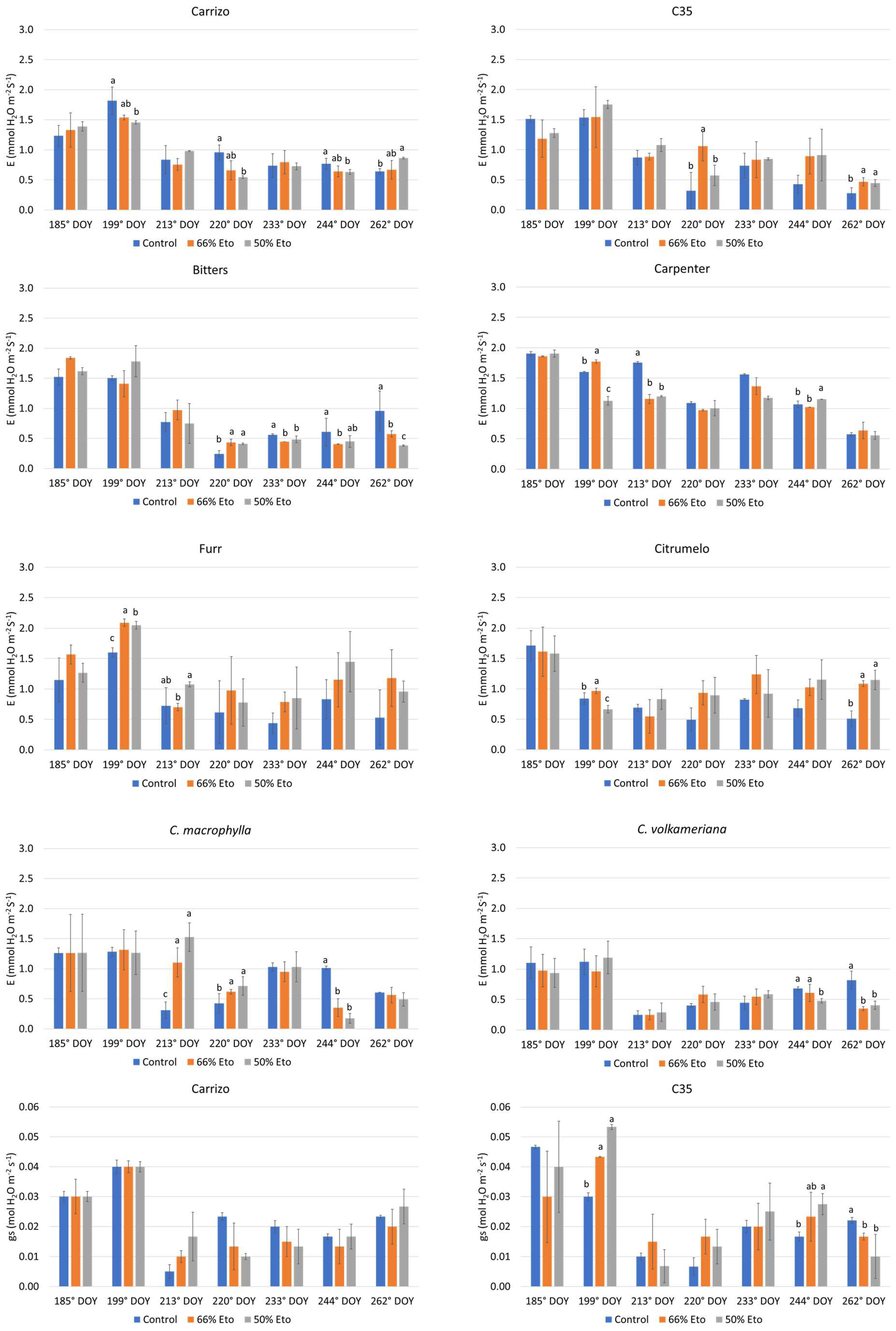
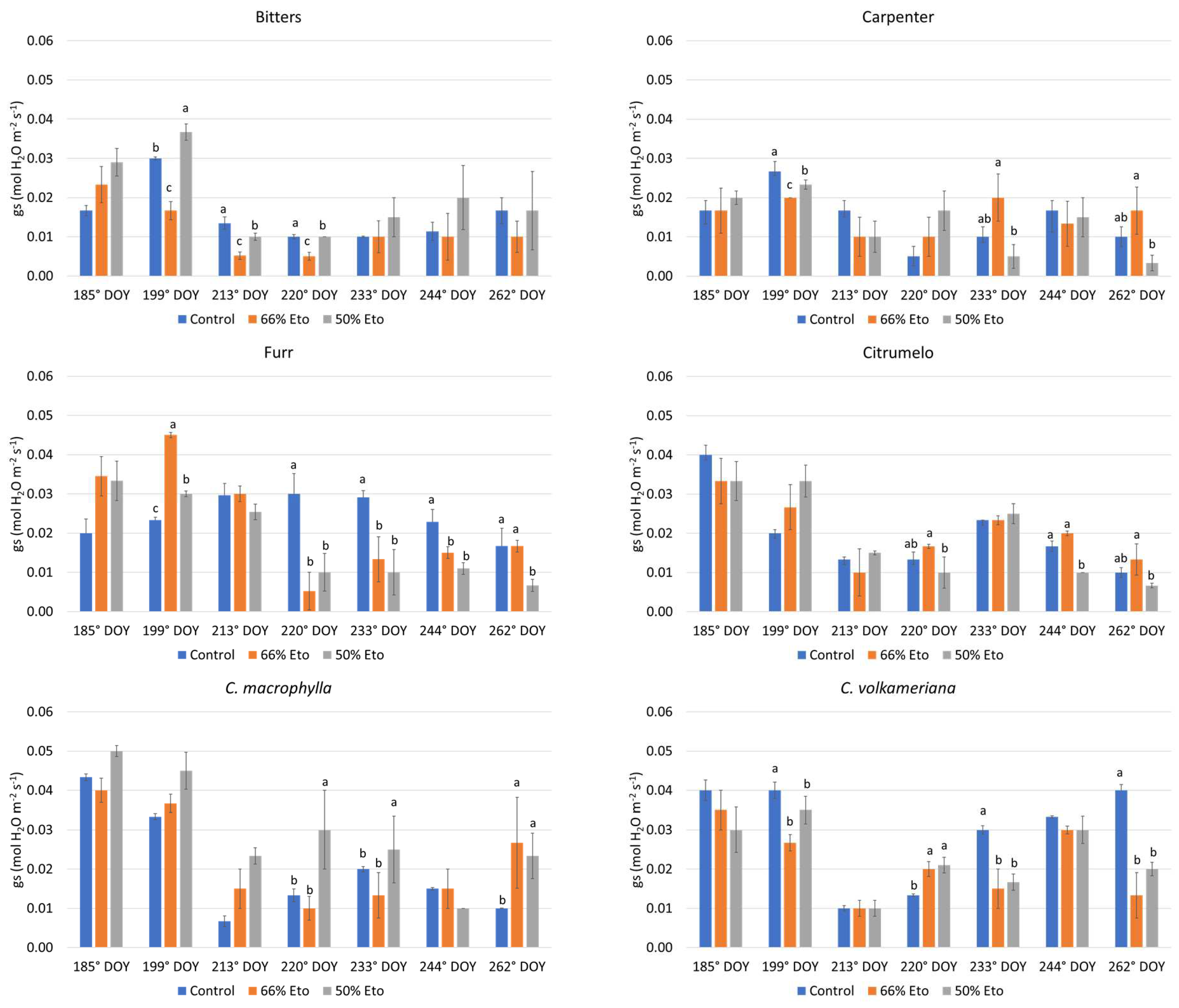
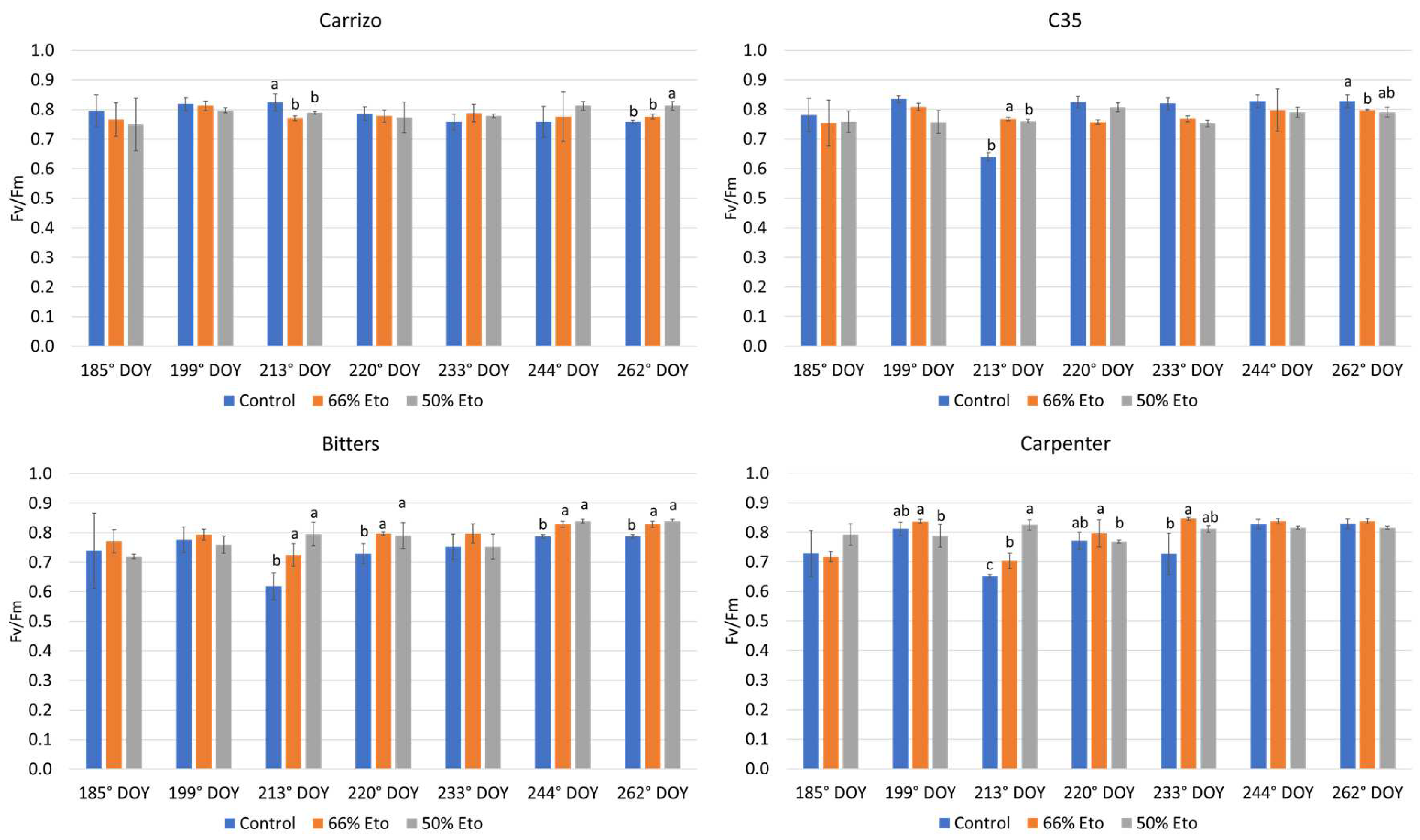
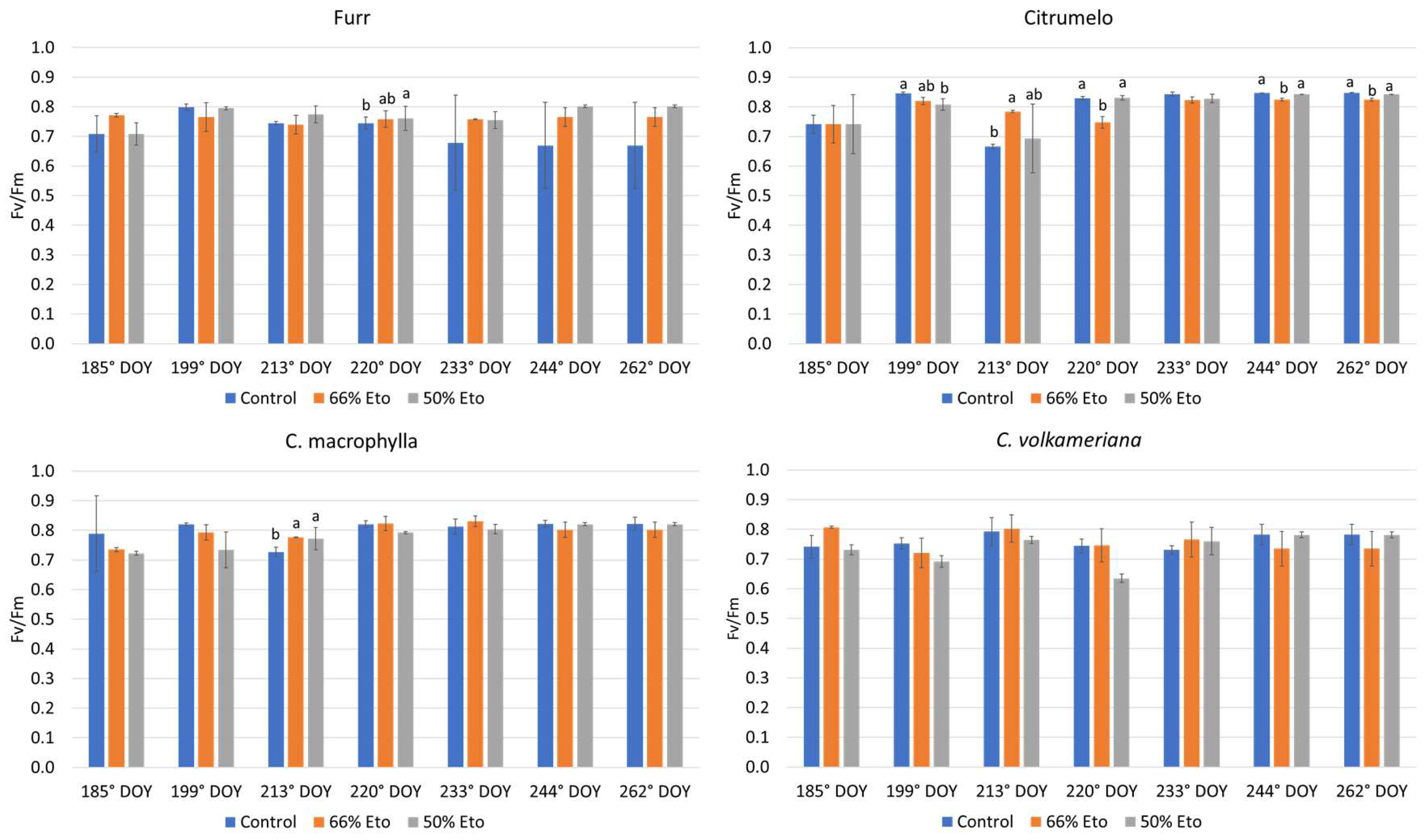
2.2. Plant Water Status and Physiological Monitoring of the Plants
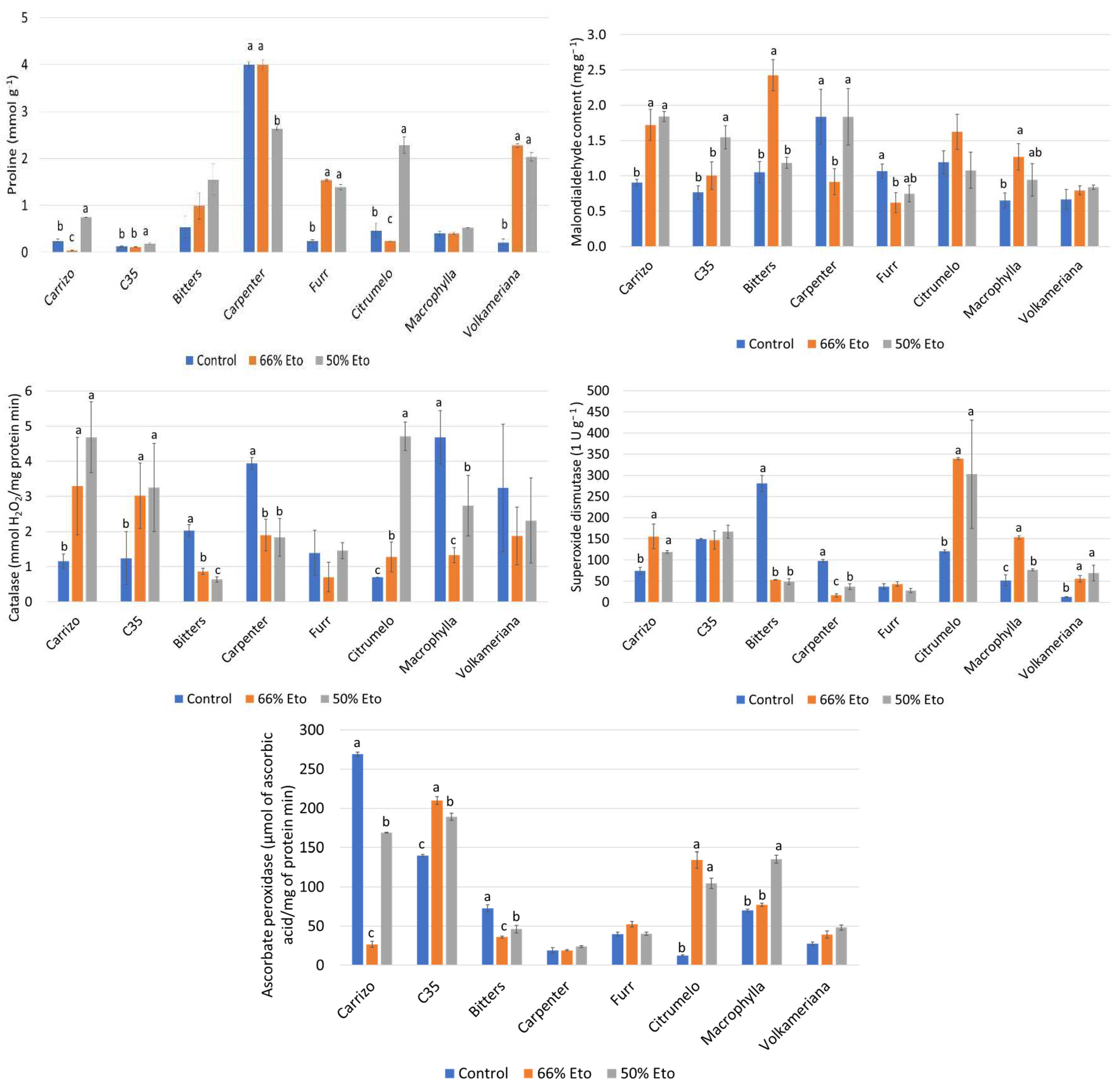
2.3. Proline Accumulation, Malondialdehyde Determination and Antioxidant Enzyme Activities
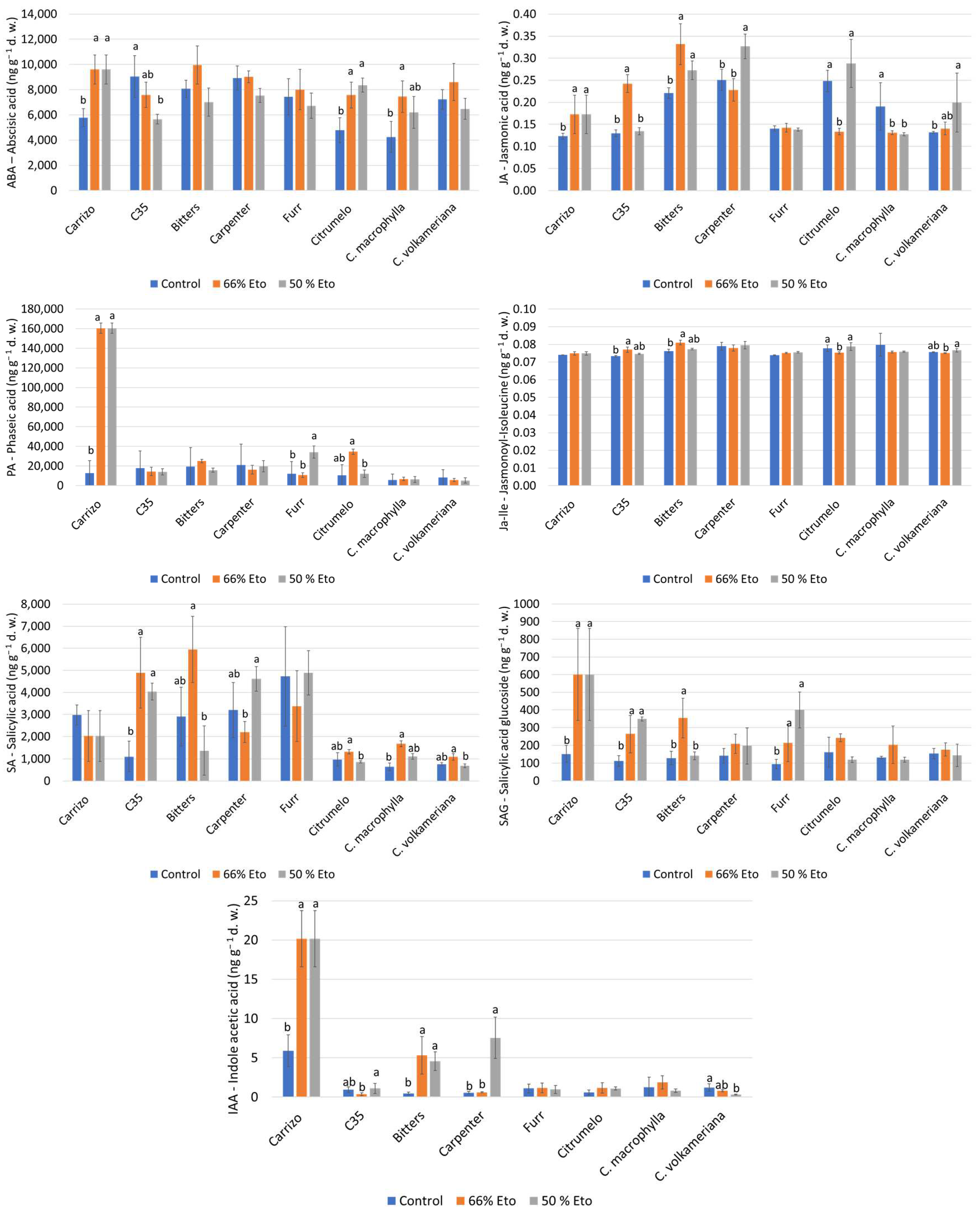
2.4. Hormone Analyses
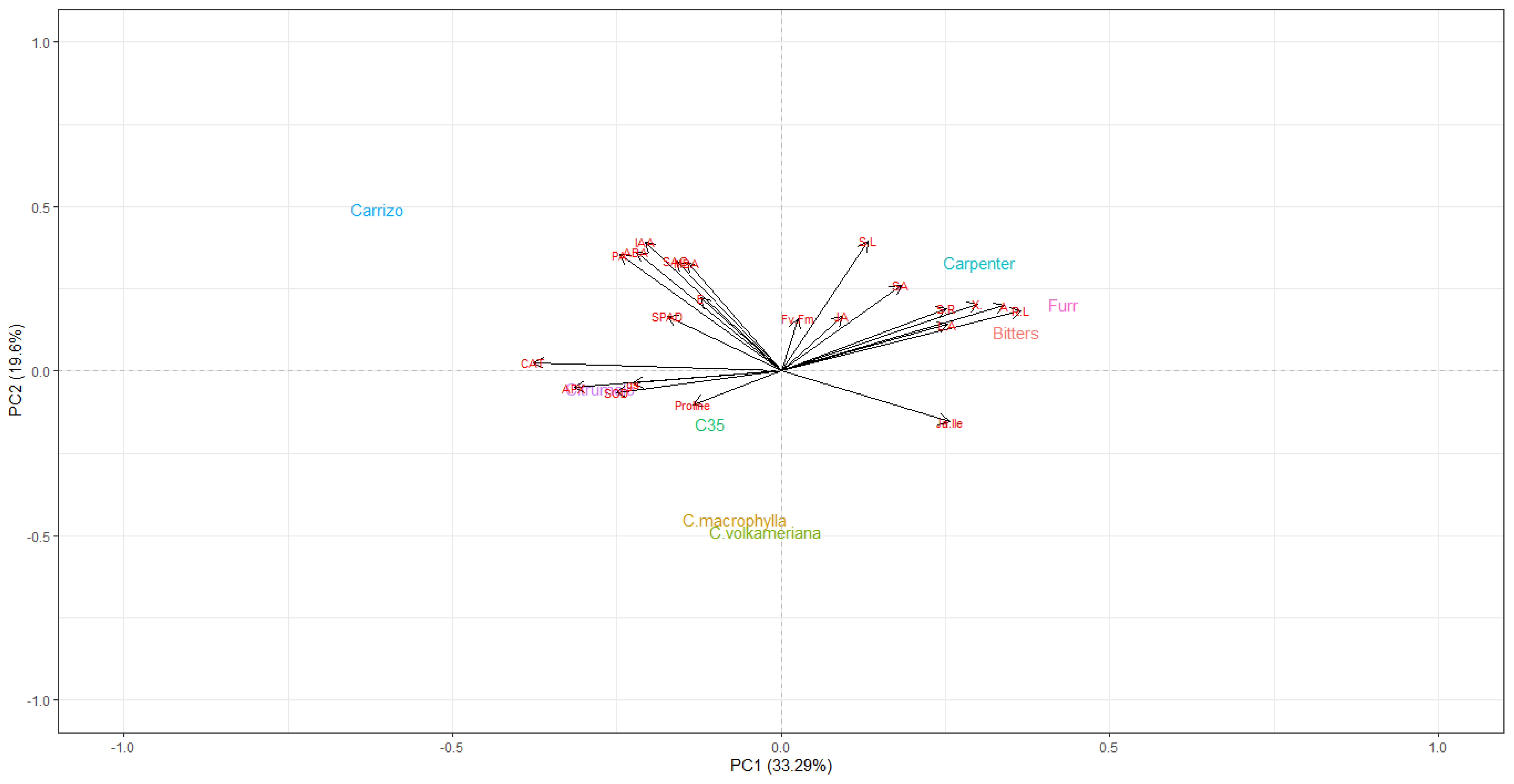
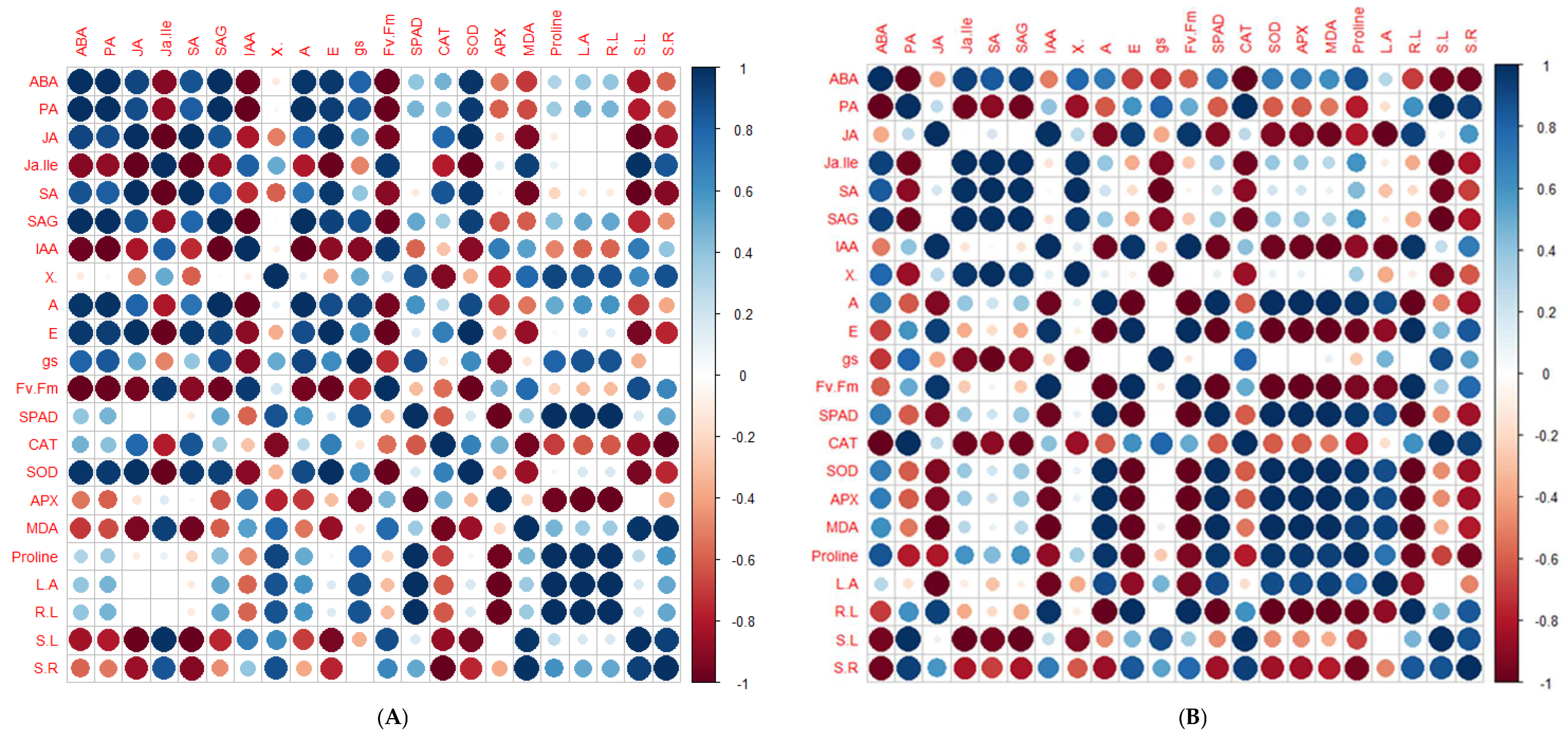
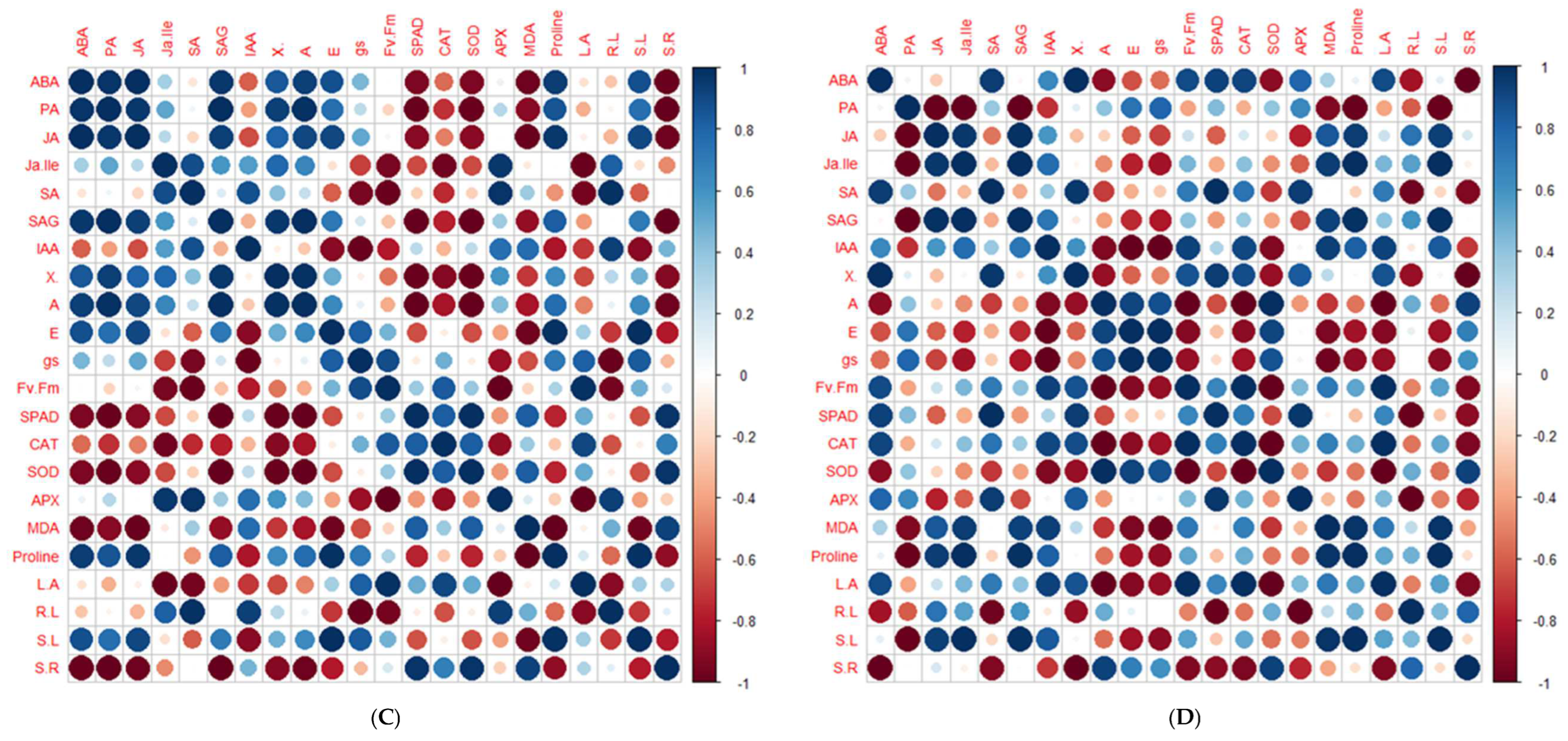
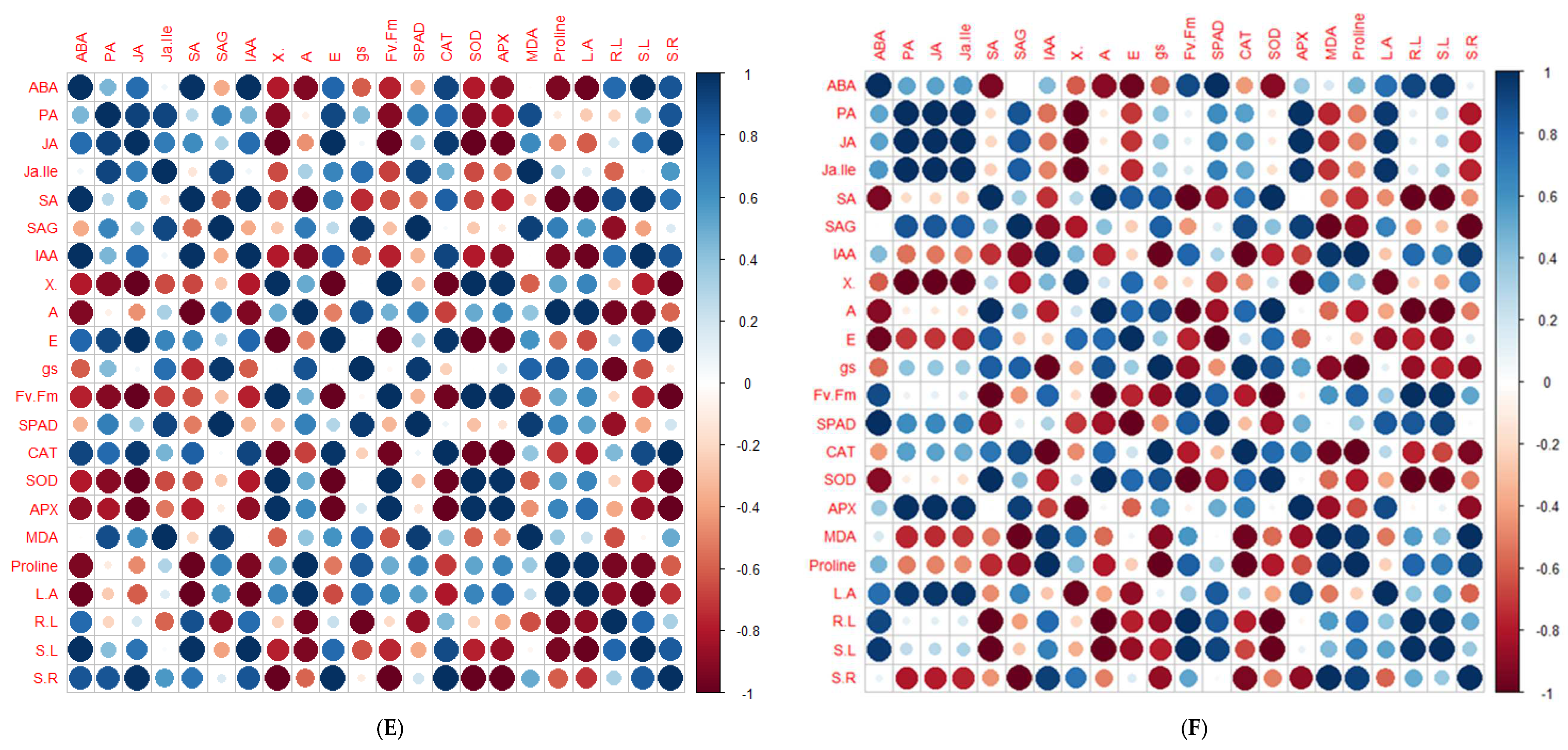
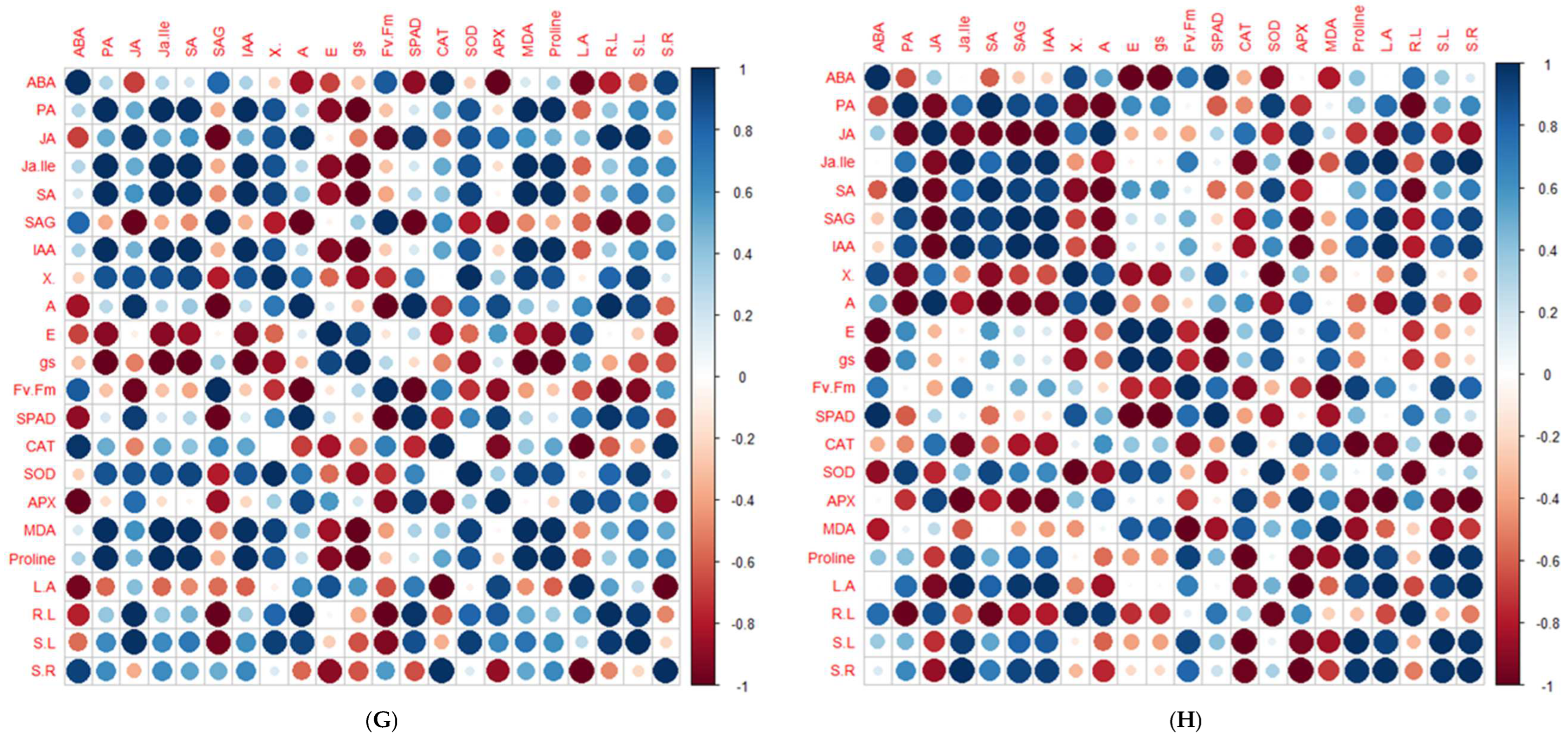
2.5. Principal Component Analysis and Pearson’s Correlation Test
3. Materials and Methods
3.1. Plant Material and Salt Treatments
3.2. Morphological Analyses and Leaf Chlorophyll Content Determination (SPAD)
3.3. Plant Water Status and Physiological Monitoring of the Plants
3.4. Proline and Malondialdehyde Accumulation, and Antioxidant Enzyme Activities
3.5. Hormone Analyses
3.6. Statistical analysis
4. Discussion
5. Conclusions
Acknowledgments
References
- Ziogas, V.; Tanou, G.; Belghazi, M.; Filippou, P.; Fotopoulos, V.; Grigorios, D.; Molassiotis, A. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol. Biol. 2015, 89, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Durán, L.; Gmitter, Jr., F. G.; Arjona-López, J.M.; Calero-Velázquez, R.; Hervalejo, Á., Arenas-Arenas, F.J. Water-stress influences on three new promising HLB-tolerant Citrus rootstocks. Horticulturae 2021, 7, 336. [Google Scholar] [CrossRef]
- Sampaio, A.H.R.; Silva, R.O.; Brito, R.B.F.; dos Santos Soares Filho, W.; da Silva Gesteira, A.; Souza, L.D.; Coelho Filho, M.A. Sweet orange acclimatisation to water stress: a rootstock dependency. Sci. Hortic. 2021, 276, 109727. [Google Scholar] [CrossRef]
- Arjona-López, J.M.; Aparicio-Durán, L.; Gmitter Jr, F.G.; Romero-Rodríguez, E.; Grosser, J.W.; Hervalejo, A.; Arenas-Arenas, F.J. ; Physiological influence of water stress conditions on novel hlb-tolerant citrus rootstocks. Agronomy 2022, 13, 63. [Google Scholar] [CrossRef]
- Consoli, S.; Caggia, C.; Russo, N.; Randazzo, C.L.; Continella, A.; Modica, G.; Cacciola, S.O.; Faio, L.; Reverberi, M.; Baglieri, A.; et al. Sustainable use of citrus waste as organic amendment in orange orchards. Sustainability 2023, 15, 2482. [Google Scholar] [CrossRef]
- de Oliveira Sousa, A.R.; Ribas, R.F.; Coelho Filho, M.A.; Freschi, L.; Ferreira, C.F.; dos Santos Soares Filho, W.; Freschi, L.; Fortes Ferreira, C.; dos Santos Soares Filho, W.; Pérez-Molina, J.P.; et al. Drought tolerance memory transmission by citrus buds. Plant Sci. 2022, 320, 111292. [Google Scholar] [CrossRef]
- Miranda, M.T.; Silva, S.F.; Silveira, N.M.; Pereira, L.; Machado, E.C.; Ribeiro, R.V. ; Root osmotic adjustment and stomatal control of leaf gas exchange are dependent on citrus rootstocks under water deficit. J. Plant Growth Regul. 2021, 40, 11–19. [Google Scholar] [CrossRef]
- Intrigliolo, F.; Giuffrida, A.; Rapisarda, P.; Calabretta, M.; Roccuzzo, G. SPAD as an indicator of nitrogen status in Citrus. In Proceeding of the IXth international citrus congress, Orlando, FL; 2000; pp. 665–667. [Google Scholar] [CrossRef]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi. J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Tanaka, A.; Ito, H. Chlorophyll degradation and its physiological function. Plant And Cell Physiology 2024, 093. [Google Scholar] [CrossRef]
- Yang, L.T.; Pan, J.F.; Hu, N.J.; Chen, H.H.; Jiang, H.X.; Lu, Y.B.; Chen, L.S. Citrus physiological and molecular response to boron stresses. Plants 2021, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Forner-Giner, M.Á.; Rodríguez-Gamir, J.; Primo-Millo, E.; Iglesias, D.J. Hydraulic and chemical responses of citrus seedlings to drought and osmotic stress. J. Plant Growth Regul. 2011, 30, 353–366. [Google Scholar] [CrossRef]
- dos Santos, I.C.; de Almeida, A.A.F.; Pirovani, C.P.; Costa, M.G.C.; da Conceição, A.S.; dos Santos Soares Filho, W.; Filho, M.A.C.; Gesteira, A.S. Physiological, biochemical and molecular responses to drought conditions in field-grown grafted and ungrafted citrus plants. Environ. Exper. Bot. 2019, 162, 406–420. [Google Scholar] [CrossRef]
- Miranda, M.T.; da Silva, S.F.; Moura, B.B.; Hayashi, A.H.; Machado, E.C.; Ribeiro, R.V. Hydraulic redistribution in Citrus rootstocks under drought. Theor. Exp. Plant Physiol. 2018, 30, 165–172. [Google Scholar] [CrossRef]
- Miranda, M.T.; Pires, G.S.; Pereira, L.; de Lima, R.F.; da Silva, S.F.; Mayer, J.L.; Azevedo, F., A.; Machado, C.E.; Jansen, S.; et al. Rootstocks affect the vulnerability to embolism and pit membrane thickness in Citrus scions. Plant, Cell & Environment 2024, 37, 1. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A. The role of abscisic acid in drought stress: How aba helps plants to cope with drought stress. In: Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, LS. (eds) Drought Stress Tolerance in Plants, Vol 2. Springer, Cham. 2016; pp. 123–151. [CrossRef]
- Arbona, V.; Zandalinas, S.I.; Manzi, M.; González-Guzmán, M.; Rodriguez, P.L.; Gómez-Cadenas, A. Depletion of abscisic acid levels in roots of flooded Carrizo citrange (Poncirus trifoliata L. Raf. × Citrus sinensis L. Osb.) plants is a stress-specific response associated to the differential expression of PYR/PYL/RCAR receptors. Plant Mol. Biol., 2017, 93, 623–640. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci., 2022, 23, 1084. [Google Scholar] [CrossRef]
- Dhakarey, R.; Raorane, M.L.; Treumann, A.; Peethambaran, P.K.; Schendel, R.R.; Sahi, V.P.; Hause, B.; Bunzel, M.; Henry, A.; Kohli, A.; et al. Physiological and Proteomic Analysis of the Rice Mutant cpm2 Suggests a Negative Regulatory Role of Jasmonic Acid in Drought Tolerance. Front Plant Sci 2017, 8, 190. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: the molecular point of view. Plant Cell Rep. 2021; 40, 1471–1494. [Google Scholar]
- Dichio, B.; Xiloyannis, C.; Sofo, A.; Montanaro, G. Osmotic regulation in leaves and roots of olive trees during a water deficit and rewatering. Tree Physiol. 2006, 26, 179–185. [Google Scholar] [CrossRef]
- Modica, G.; Di Guardo, M.; Puglisi, I.; Baglieri, A.; Fortuna, S.; Arcidiacono, F.; Costantino, D.; La Malfa, S.; Gentile, A.; Arbona, V.; et al. Novel and widely spread citrus rootstocks behavior in response to salt stress. Environ Exp Bot 2024, 225, 105835. [Google Scholar] [CrossRef]
- Vanella, D.; Consoli, S.; Continella, A.; Chinnici, G.; Milani, M.; Cirelli, G.L.; D’Amico, M.; Maesano, G.; Gentile, A.; La Spada, P.; et al. Environmental and Agro-Economic Sustainability of Olive Orchards Irrigated with Reclaimed Water under Deficit Irrigation. Sustainability, 2023, 15, 15101. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of antioxidants to Paraquat in Pea Leaves (Relationships to Resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef]
- Ushimaru, T.; Maki, Y.; Sano, S.; Koshiba, K.; Asada, K. ; Tsuji, HInduction of enzymes involved in the ascorbate-dependent antioxidative system, namely, ascorbate peroxidase, monodehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oryza sativa) seedlings germinated under water. Plant Cell Physiol., 1997, 38, 541–549. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Masia, A. Superoxide dismutase and catalase activities in apple fruit during ripening and post-harvest and with special reference to ethylene. Physiol. Plant. 1998, 104, 668–672. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil, 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Khedr, A.H.; Abbas, M.A.; Wahid, A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version, 2016, 2, 1–189. [Google Scholar]
- Rodríguez-Gamir, J.; Primo-Millo, E.; Forner, J.B.; Forner-Giner, M.A. Citrus rootstock responses to water stress. Sci. Hortic. 2010, 126, 95–102. [Google Scholar] [CrossRef]
- Stagno, F.; Intrigliolo, F.; Consoli, S.; Continella, A.; Roccuzzo, G. Response of orange trees to deficit irrigation strategies: Effects on plant nutrition, yield, and fruit quality. J. Irrig. Drain. Eng. 2015, 141, 04015014. [Google Scholar] [CrossRef]
- Huang, X.M.; Huang, H.B.; Gao, F.F. The growth potential generated in citrus fruit under water stress and its relevant mechanisms. Sci. Hort. 2000, 83, 227–240. [Google Scholar] [CrossRef]
- Treeby, M.T.; Henriod, R.E.; Bevington, K.B.; Milne, D.J.; Storey, R. Irrigation management and rootstock effects on navel orange [Citrus sinensis (L.) Osbeck] fruit quality. Agric. Water Manag. 2007, 91, 24–32. [Google Scholar] [CrossRef]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees, 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Pedroso, F.K.; Prudente, D.A.; Bueno, A.C.R.; Machado, E.C.; Ribeiro, R.V. Drought tolerance in citrus trees is enhanced by rootstock-dependent changes in root growth and carbohydrate availability. Environ. Exp Bot. 2014, 101, 26–35. [Google Scholar] [CrossRef]
- Santana-Vieira, D.; Freschi, L.; Almeida, L.; de Moraes, D.H.S.; Neves, D.M.; dos Santos, L.M.; Bertolde, F.Z.; dos Santos Soares Filho, W.; Filho, M.A.C.; da Silva Gesteira, A. Survival strategies of citrus rootstocks subjected to drought. Sci Rep. 2016, 38775. [Google Scholar] [CrossRef]
- Tobar, S.; Gil, P.M.; Schaffer, B.; Schwember, A.R.; Cautín, R.; Mártiz, J. Physiological and Growth Responses of W. Murcott Tangor Grafted on Four Rootstocks under Water Restriction. Horticulturae 2024, 10, 352. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.; Jifon, J.; Carvajal, M.; Syvertsen, J.P. Gas exchange, chlorophyll and nutrient contents in relation to Na+ and Cl− accumulation in ‘Sunburst’ mandarin grafted on different rootstock. Plant Sci. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S. III.; Pons, T.L. Plant Physiological Ecology. Springer Science, New-York, 2000. [CrossRef]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009; 66, 135–142. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben, El; Hadj, S. ; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hort. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Filippou, P.; Diamantidis, G.; Vasilakakis, M.; Fotopoulos, V.; Molassiotis, A. Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiol. Biochem. 2013, 68, 118–126. [Google Scholar] [CrossRef]
- Qi, D.U.; Zhao, X.H.; Le, X.I.A.; Jiang, C.J.; Wang, X.G.; Yi, H.A.N.; Jinf, W.; Hai-qui, U. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Oustric, J.; Herbette, S.; Morillon, R.; Giannettini, J.; Berti, L.; Santini, J. Influence of rootstock genotype and ploidy level on common clementine (Citrus clementina Hort. ex Tan) tolerance to nutrient deficiency. Front. Plant Sci. 2021, 12, 634237. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Tan, F.; Liu, H.; Zhu, M.; Yang, H.; Bi, G.; Wan, H.; Wang, J.; Xu, R.; et al. Fatty acid metabolic flux and lipid peroxidation homeostasis maintain the biomembrane stability to improve citrus fruit storage performance. Food chem. 2019, 292, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Arbona, V.; Morillon, R.; Gómez-Cadenas, A. Salinity and water deficit, in: Talon, M., Caruso, M., Gmitter, F.G. The genus citrus. Woodhead Publishing, 2020. 291–309. [CrossRef]
- Scialò, E.; Sicilia, A.; Continella, A.; Gentile, A.; Lo Piero, A.R. Transcriptome Profiling and Weighted Gene Correlation Network Analysis Reveal Hub Genes and Pathways Involved in the Response to Polyethylene-Glycol-Induced Drought Stress of Two Citrus Rootstocks. Biology 2024, 13, 595. [Google Scholar] [CrossRef]
- de Campos, M.K.F.; de Carvalho, K.; de Souza, F.S.; Marur, C.J.; Pereira, L.F.P.; Bespalhok Filho, J.C.; Vieira, L.G.E. Drought tolerance and antioxidant enzymatic activity in transgenic ‘Swingle’citrumelo plants over-accumulating proline. Environ. Exp. Bot. 2011, 72, 242–250. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Biotechnology of plant osmotic stress tolerance physiological and molecular considerations. Acta Hortic. 2000, 560, 285–292. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Syvertsen, J.P.; Gimeno, V.; Botía, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 2007, 134, 532–542. [Google Scholar] [CrossRef]
- Alia, M.P.; Matysik, J. Effect of proline on the production of singlet oxygen. Amino acids 2001, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Jung, S. Variation in antioxidant metabolism of young and mature leaves of Arabidopsis thaliana subjected to drought. Plant Sci. 2004, 166, 459–466. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Scialò, E.; Sicilia, A.; Continella, A.; Modica, G.; Lo Piero, A.R. ; On the “Priming” Steps: Initial Results on the Effect of PEG-Induced Drought Stress upon the Oxidative Status and Related Gene Expression in Citrus Rootstocks. Acta Hortic. 2024, 1399, 131–138. [Google Scholar]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef]
- Mahouachi, J.; Arbona, V.; Gómez-Cadenas, A. Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regul. 2007, 53, 43–51. [Google Scholar] [CrossRef]
- Arbona, V.; Gómez-Cadenas, A. Hormonal modulation of citrus responses to flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Vincent, C.I.; Grosser, J.W.; Dutt, M. The response of salt-stressed Valencia sweet orange (Citrus sinensis) to salicylic acid and methyl jasmonate treatments. Plant Physiol. Rep. 2021, 26, 137–51. [Google Scholar] [CrossRef]
- Mahouachi, J.; Socorro, A.R.; Talon, M. Responses of papaya seedlings (Carica papaya L.) to water stress and rehydration: growth, photosynthesis and mineral nutrient imbalance. Plant Soil 2006, 281, 137–146. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Zhang, C. Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Aust. J. Plant Physiol. 2001, 28, 1055–1061. [Google Scholar] [CrossRef]
- Lei, Y.B.; Yin, C.Y.; Li, C.Y. Differences in some morphological, physiological and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol. Plant. 2006, 127, 182–191. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Continella, A.; Pannitteri, C.; La Malfa, S.; Legua, P.; Distefano, G.; Nicolosi, E.; Gentile, A. Influence of different rootstocks on yield precocity and fruit quality of ‘Tarocco Scirè’ pigmented sweet orange. Sci. Hortic. 2018, 230, 62–67. [Google Scholar] [CrossRef]
- Siebert, T.; Krueger, R.; Kahn, T.; Bash, J.; Vidalakis, G. Descriptions of new varieties recently distributed from the Citrus Clonal Protection Program. Citrograph, 2010; pp.20–26.
- Hayat, F.; Li, J.; Liu, W.; Li, C.; Song, W.; Iqbal, S.; Khan, U.; Javed, H.U.; Altaf, M.A.; Tu, P.; et al. Influence of citrus rootstocks on scion growth, hormone levels, and metabolites profile of ‘Shatangju’mandarin (Citrus reticulata Blanco). Horticulturae 2022, 8, 608. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef]
- Balfagón, D.; Rambla, J.L.; Granell, A.; Arbona, V.; Gómez-Cadenas, A. Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ. Exp. Bot. 2022, 195, 104793. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asgharei, M.R. Influence of foliar-applied salicylic acid on growth, gas-exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica 2015, 53, 410–418. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A. Khan Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
| Leaf area (m2) |
Shoot length (cm) | Root length (cm) |
Shoot to Root Ratio (g g−1) |
|||||||||
| Control | 66% Et0 | 50% Et0 | Control | 66% Et0 | 50% Et0 | Control | 66% Et0 | 50% Et0 | Control | 66% Et0 | 50% Et0 | |
| Carrizo | 3.18 a | 2.40 b | 1.62 c | 410.67 | 311.00 | 279.33 | 40.00 a | 38.67 a | 28.00 b | 1.80 a | 1.44 b | 1.43 b |
| C35 | 4.44 a | 2.01 b | 3.20 b | 343.17 a | 235.67 b | 239.63 b | 40.33 a | 31.33 b | 31.50 b | 1.70 a | 1.47 b | 0.93 c |
| Bitters | 1.07 b | 3.47 a | 4.01 a | 397.00 | 396.50 | 384.13 | 45.67 a | 36.33 b | 36.75 b | 2.20 a | 1.80 b | 1.76 b |
| Carpenter | 1.82 | 2.02 | 2.09 | 464.67 | 372.90 | 353.75 | 45.33 | 41.33 | 36.75 | 2.10 | 2.43 | 1.44 |
| Furr | 2.47 b | 2.97 b | 3.70 a | 400.81 a | 287.67 b | 256.34 b | 39.00 | 40.00 | 39.33 | 2.00 | 2.25 | 2.42 |
| Citrumelo | 3.29 a | 2.28 ab | 1.84 b | 263.00 | 260.50 | 283.00 | 40.33 a | 34.50 b | 32.00 b | 1.50 a | 1.44 b | 0.88 c |
| Macrophylla | 1.7 a | 1.74 a | 0.83 b | 252.17 | 191.67 | 150.83 | 33.00 | 31.00 | 30.67 | 1.60 | 1.41 | 1.46 |
| Volkameriana | 3.9 a | 2.19 b | 2.07 b | 225.33 a | 186.83 b | 170.33 b | 34.33 | 30.67 | 29.67 | 3.00 a | 1.24 b | 1.23 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





