Submitted:
06 February 2025
Posted:
07 February 2025
You are already at the latest version
Abstract
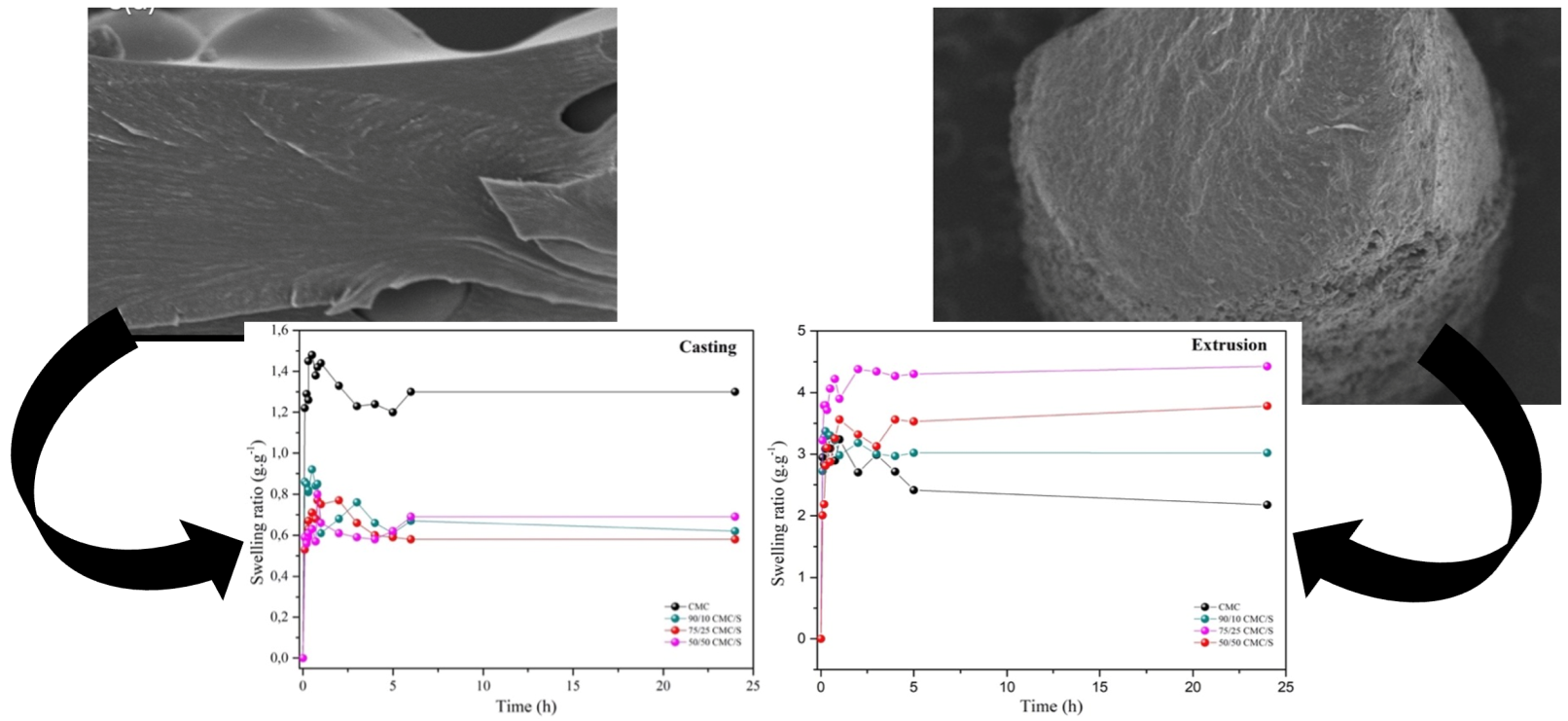
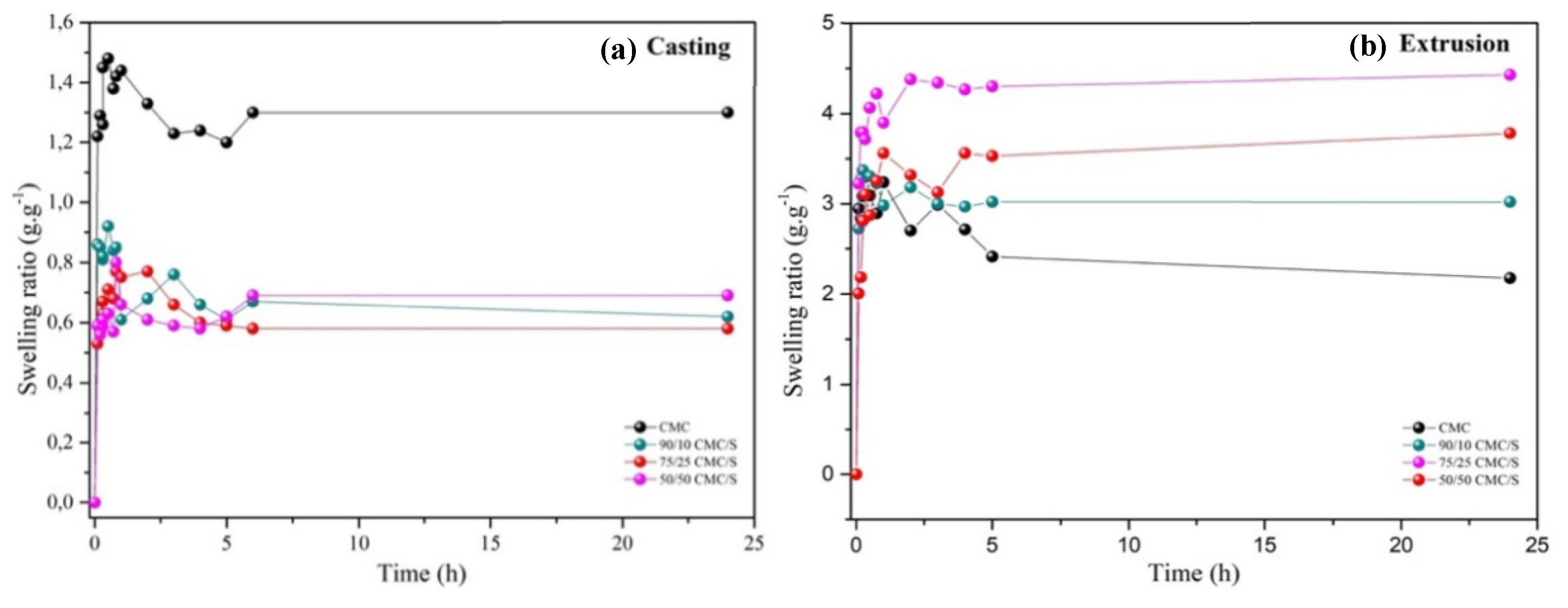
This study discusses the preparation of biopolymeric hydrogels (Biomaterial) via different techniques, such as casting and extrusion, to compare the effects of the process and the use of citric acid as a crosslinker on hydrogel morphology, physicochemical properties, and swelling degree. Casting is widely used for its low cost and space-saving nature, but up-scaling is problematic. Extrusion offers a way to produce materials in large quantities; these materials can undergo mechanical and thermal energy, which can significantly alter their properties. The samples obtained by extrusion had porous surfaces, which are critical for water penetration and swelling of superabsorbent hydrogels. In contrast, the hydrogels produced by casting did not form pores, resulting in a lower degree of swelling. Extrusion increased the swelling degree threefold due to the formation of pores, influencing water absorption and diffusion dynamics, especially in samples with higher starch content, where crosslinking occurred more effectively.
Keywords:
1. Introduction
2. Results and Discussion
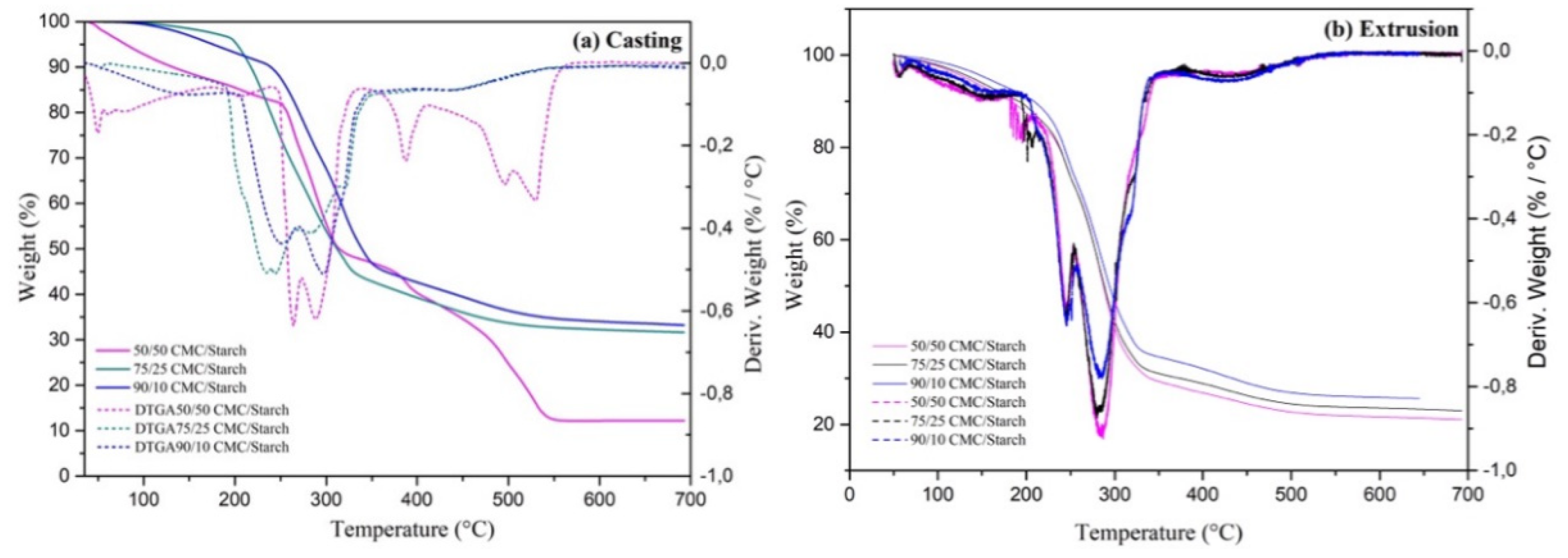
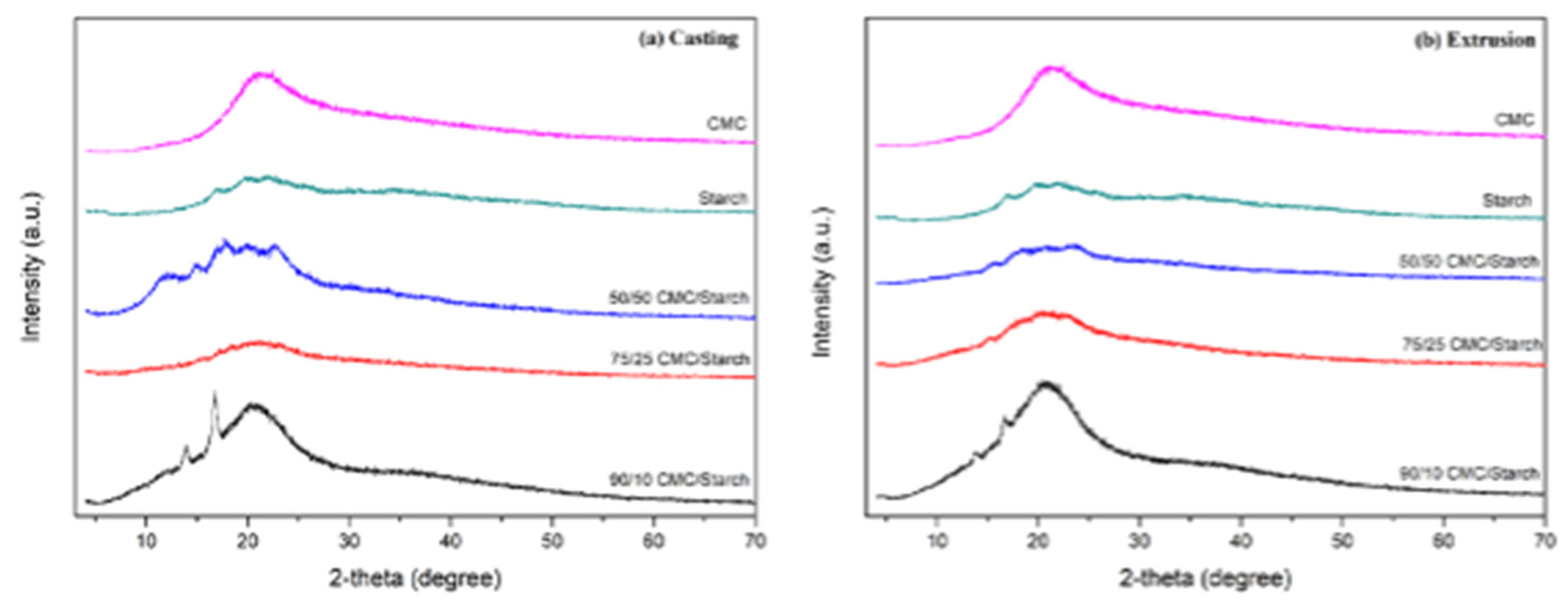
2.1. Characterization
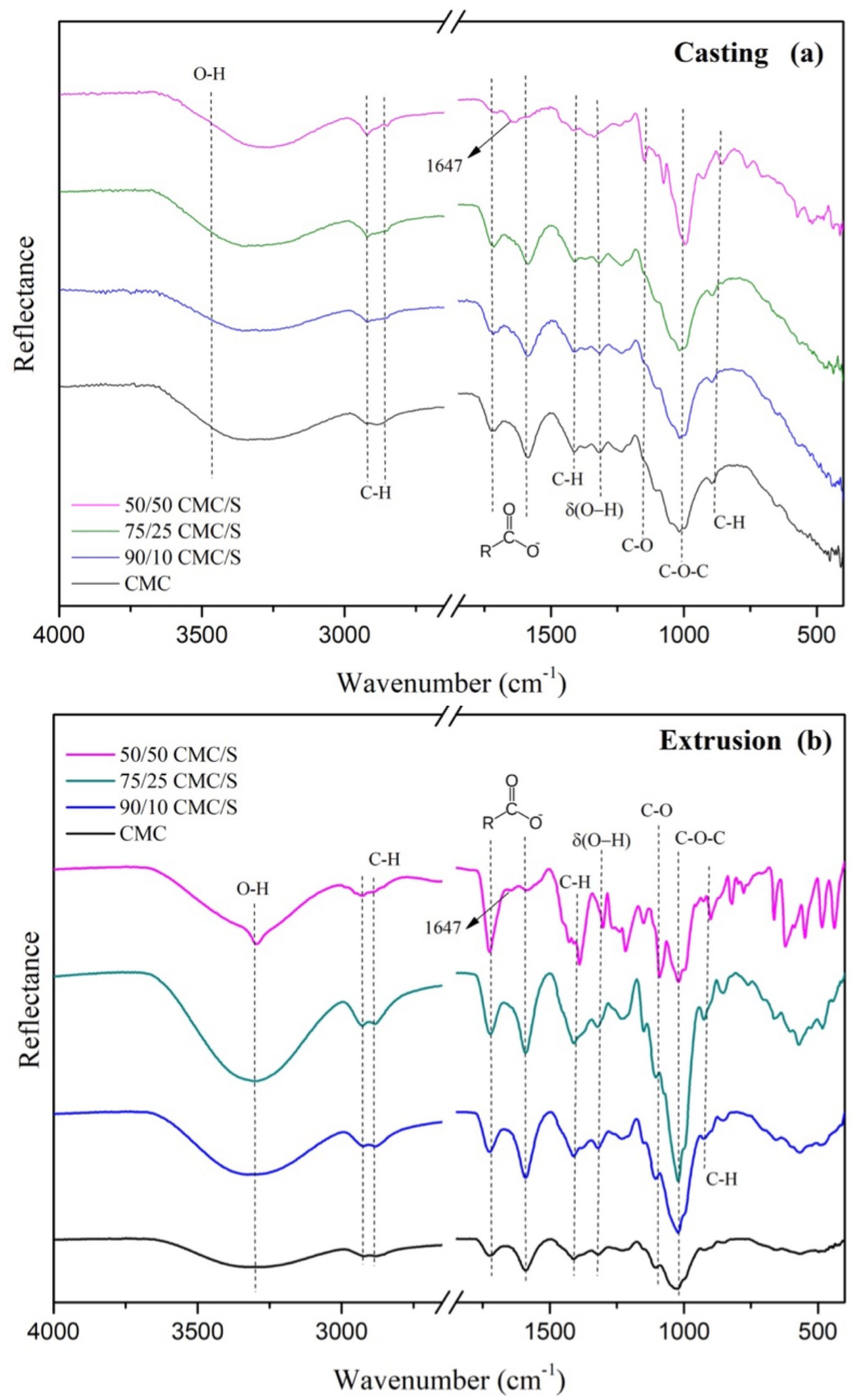
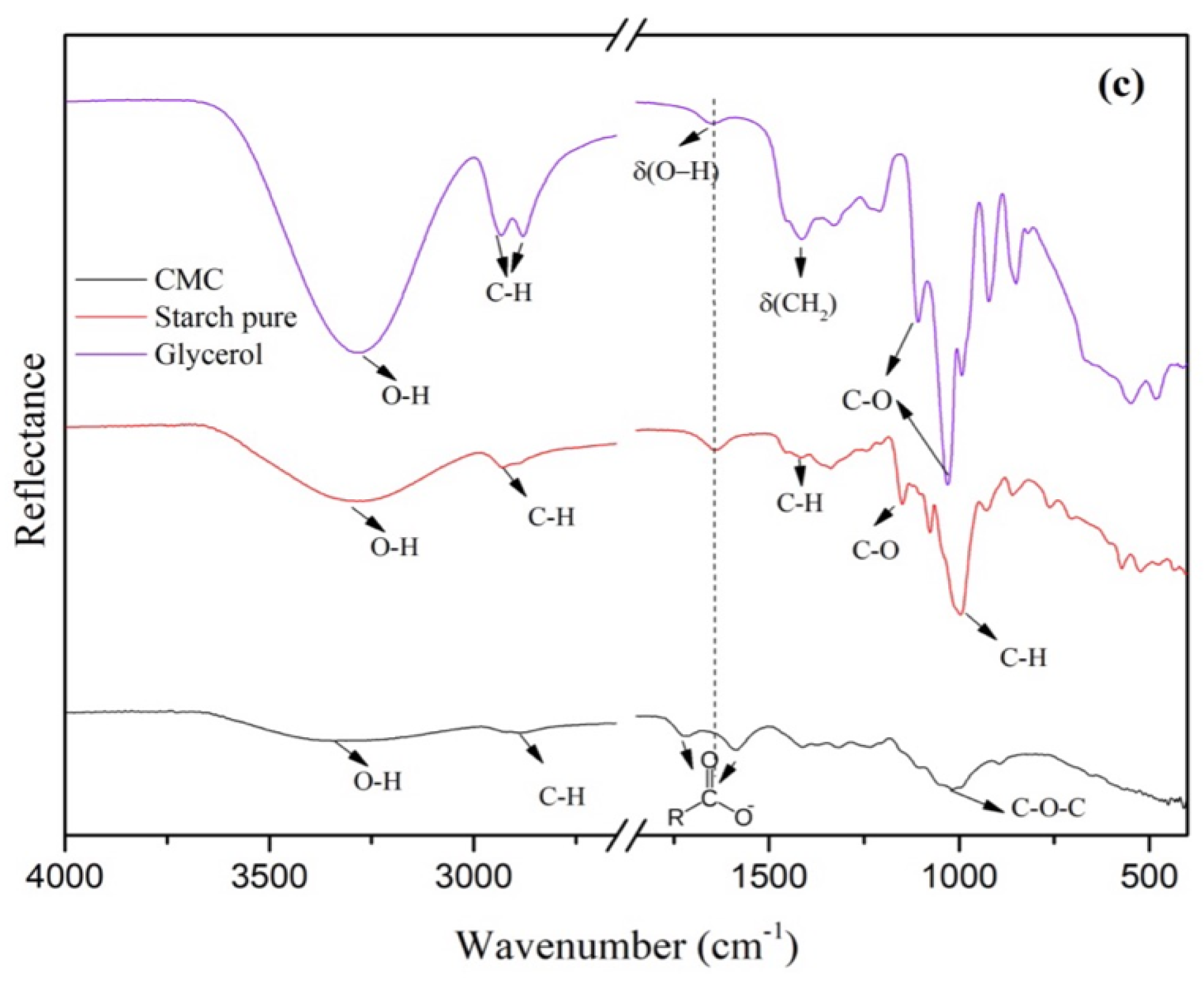
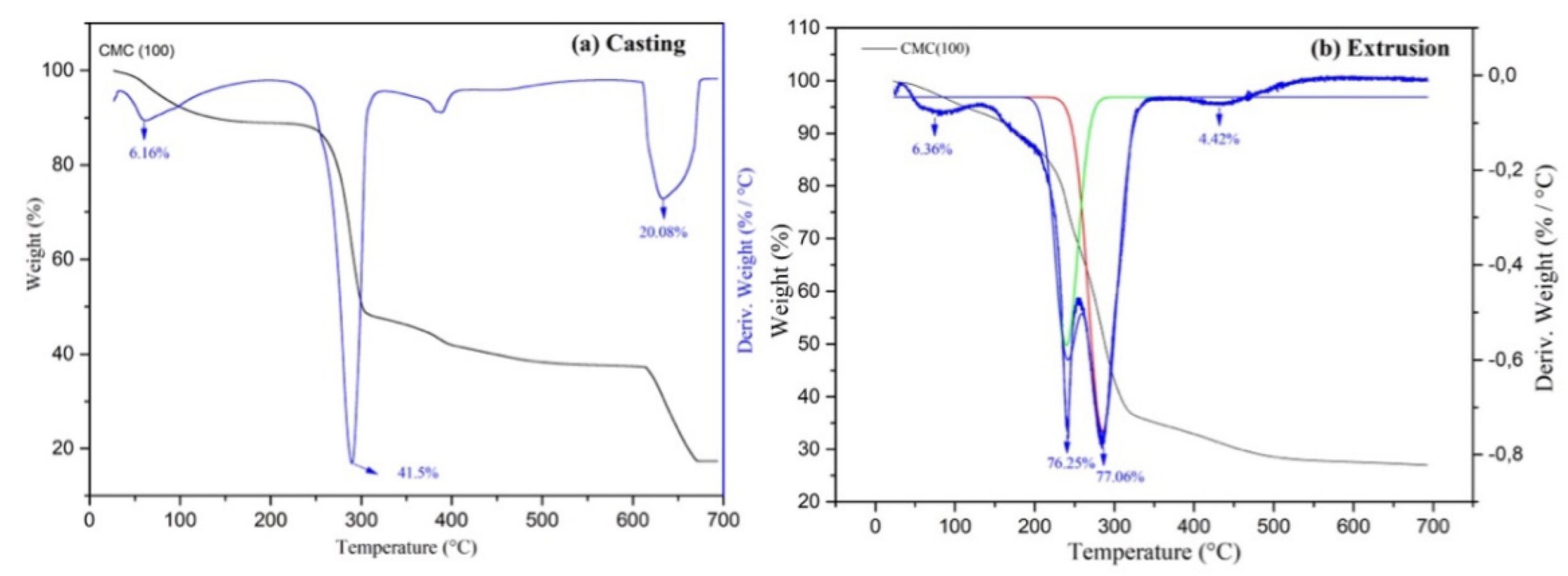
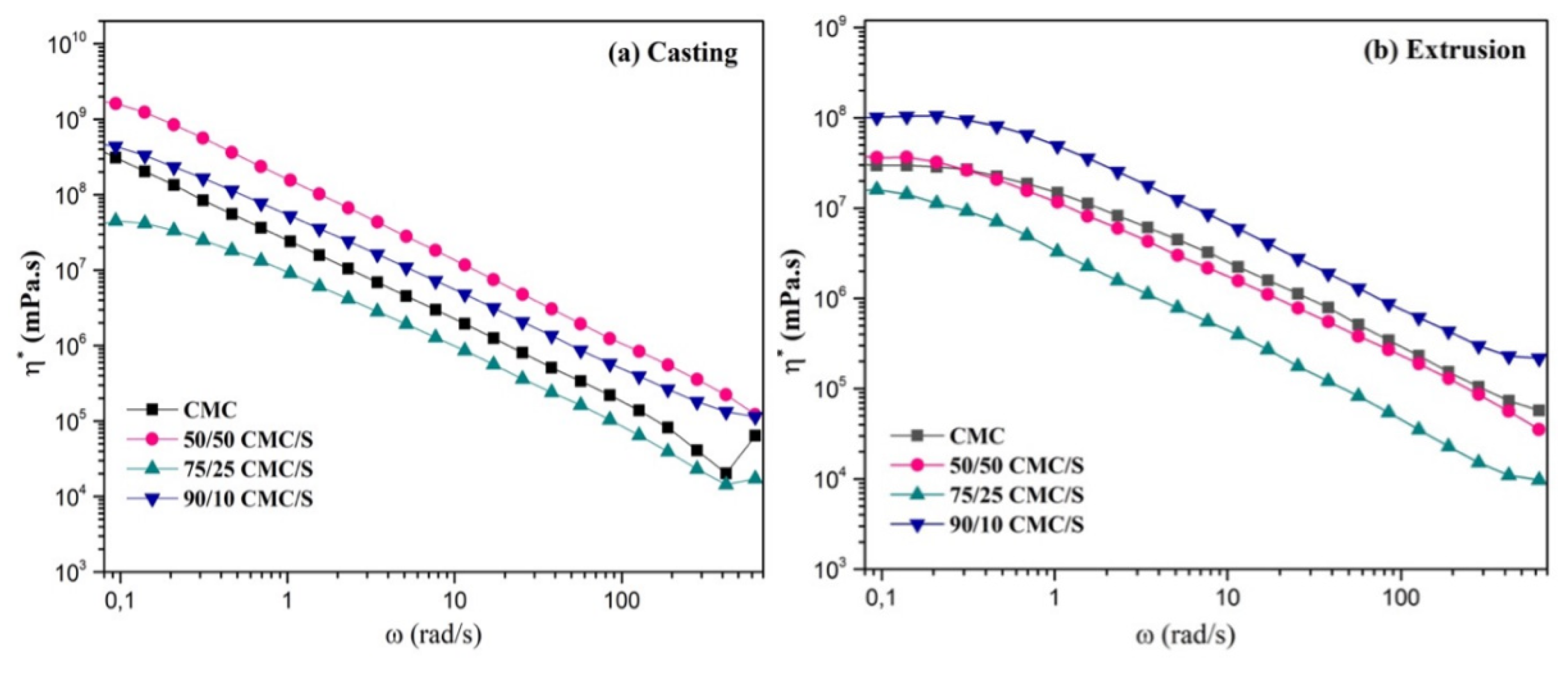
2.2. Mechanical Properties
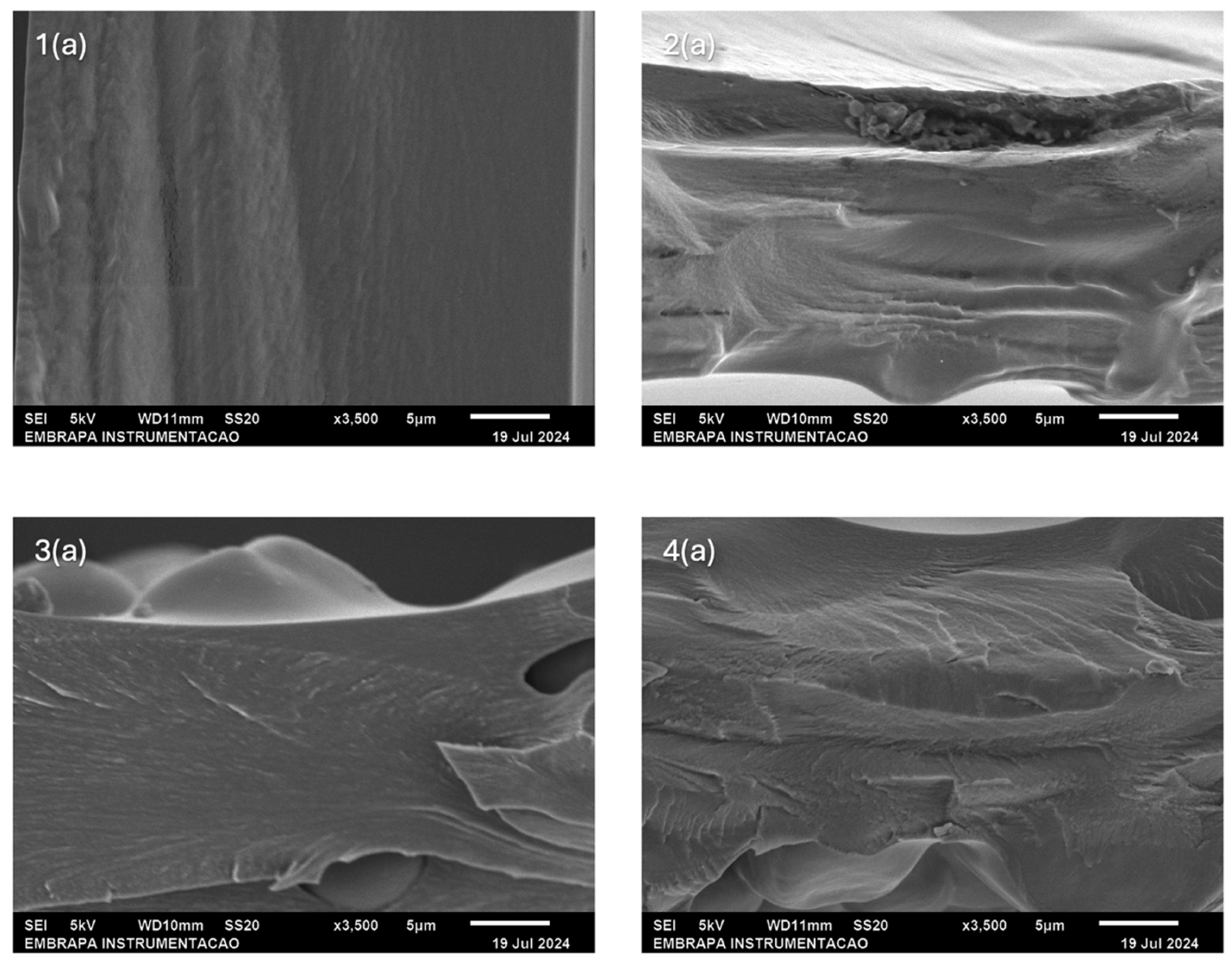
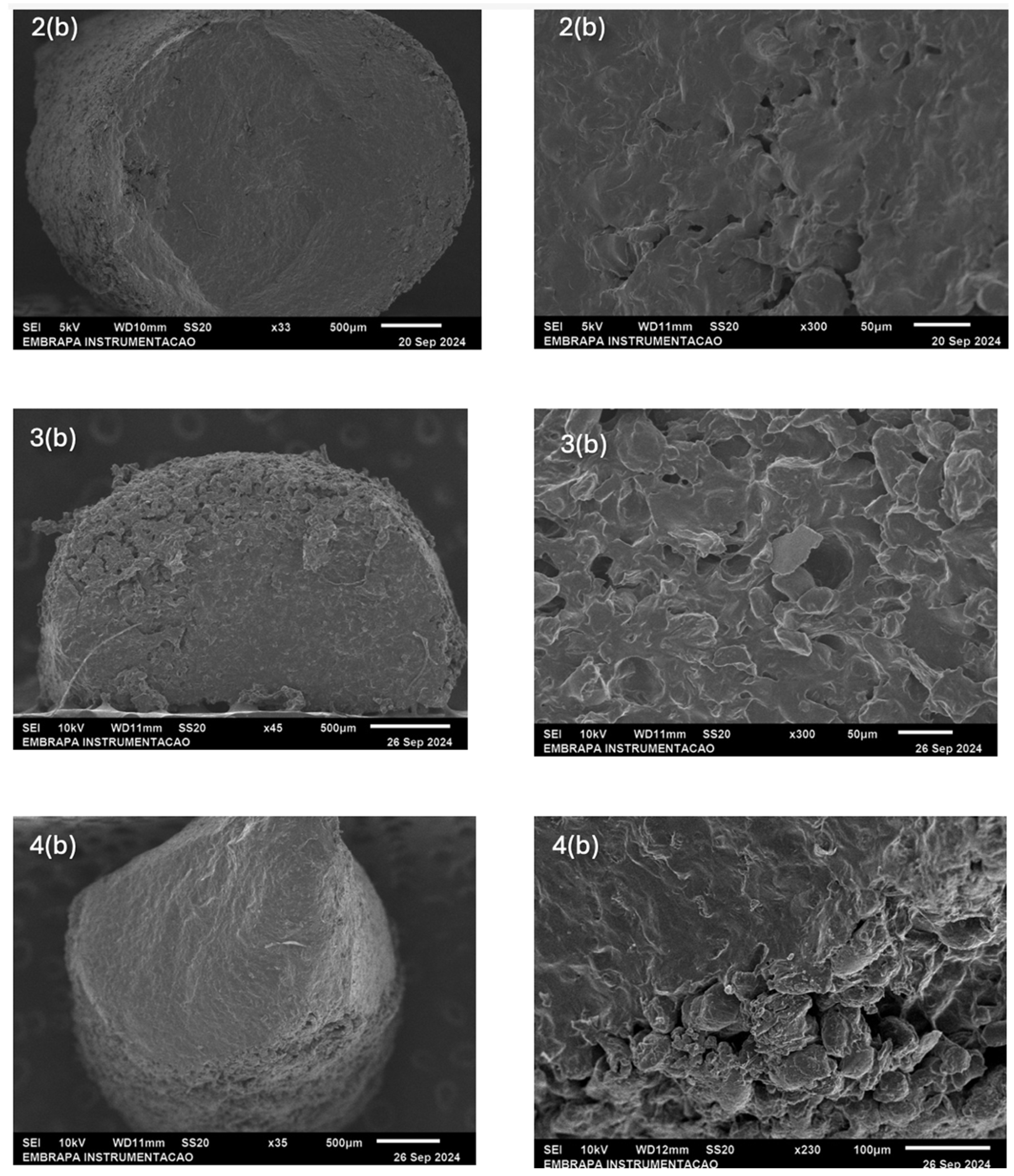
2.3. Morphology
2.4. Swelling Degree
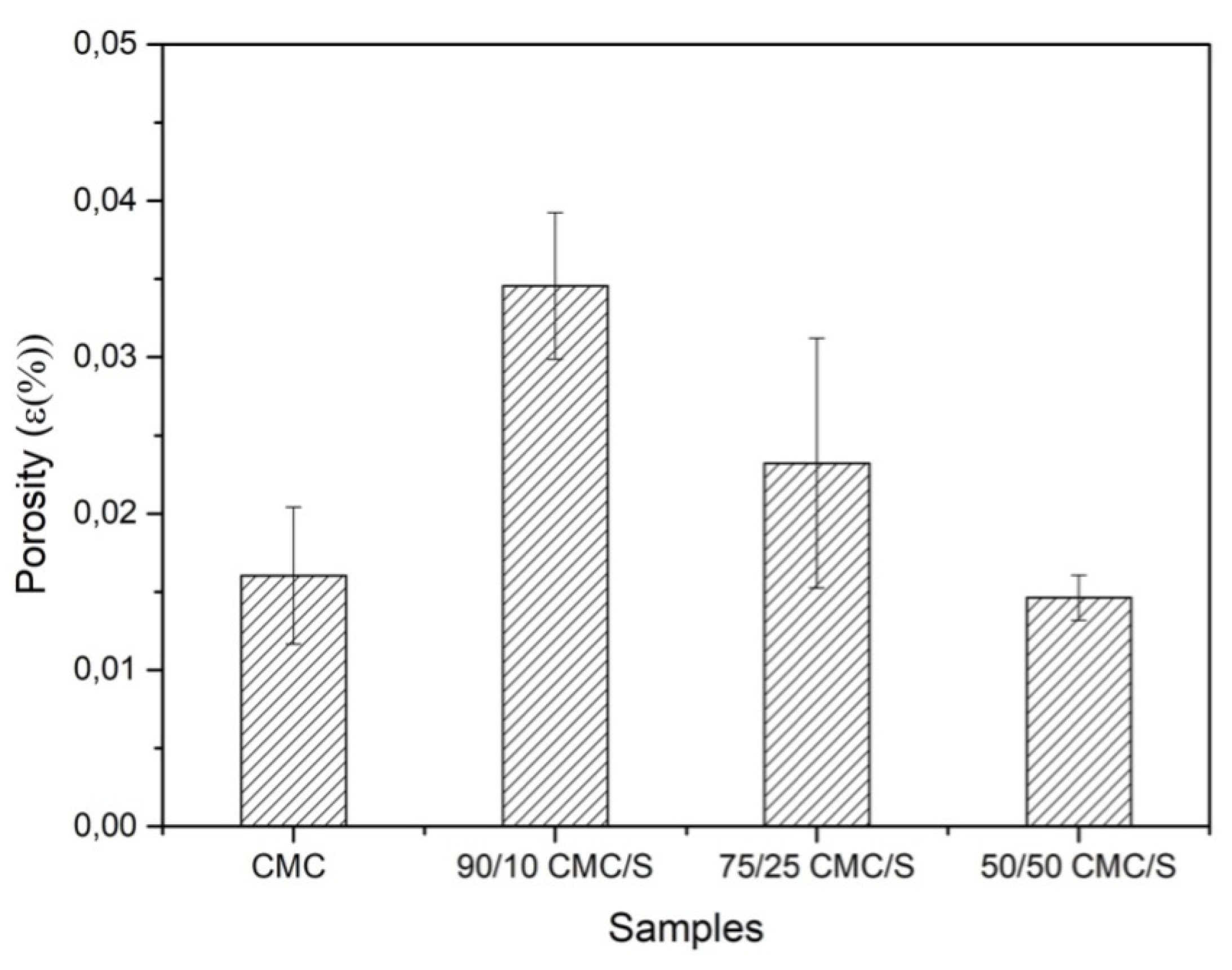
2.5. Porosity Measurement
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Hydrogels with Carboxymethylcellulose (CMC) and Starch (S) by Casting
| Sample | CMC | Starch | Citric acid |
|---|---|---|---|
| CMC | 100 | 0 | 20 |
| CMC/S | 90 | 10 | 20 |
| CMC/S | 75 | 25 | 20 |
| CMC/S | 50 | 50 | 20 |
4.3. Preparation of Hydrogels with Carboxymethylcellulose (CMC) and Starch (S) by Extrusion
| Sample | CMC | Starch | Citric acid |
|---|---|---|---|
| CMC | 100 | 0 | 20 |
| CMC/S | 90 | 10 | 20 |
| CMC/S | 75 | 25 | 20 |
| CMC/S | 50 | 50 | 20 |
4.4. Fourier Transform Infrared Spectrometry (FTIR)
4.5. Thermal Properties
4.6. Mechanical Properties
4.7. Morphology
4.8. X-Ray Diffraction
4.9. Degree of Swelling
4.10. Porosity Measurements
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, C.; Carmo, M. Why Nonconventional Materials Are Answers for Sustainable Agriculture. MRS Energy and Sustainability 2019, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Torres, J.A.; Shviro, M.; Carmo, M.; He, T.; Ribeiro, C. Photocatalytic Materials Applications for Sustainable Agriculture. Prog Mater Sci 2022, 130, 100965. [Google Scholar] [CrossRef]
- Kearney, J. Food Consumption Trends and Drivers. Philosophical Transactions of the Royal Society B: Biological Sciences 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int J Environ Res Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent Polymers Used for Agricultural Water Retention. Polym Test 2021, 94, 107021. [Google Scholar] [CrossRef]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of Biopolymer-Based Hydrogels and Their Applications in Agriculture: A Review in Perspective of Synthesis and Their Degree of Swelling for Water Holding. RSC Adv 2023, 13, 24731–24754. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional Cross Linked Hydrophilic Polymeric Network “Hydrogels”: An Agriculture Boom. Agric Water Manag 2021, 253, 106939. [Google Scholar] [CrossRef]
- Felippe, D.; Navroski, M.C.; Pereira, M. de O.; Baptista, K.R.S. de P. Hydrogel in the Seedling Growth of Eucalyptus Dunnii Maiden under Different Irrigation Management. Revista Ambiente & Água 2021, 16, e2582. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable Superabsorbent Hydrogel IncreasesWater Retention Properties of Growing Media and Plant Growth. Agriculture and Agricultural Science Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef]
- El-Asmar, J.; Jaafar, H.; Bashour, I.; Farran, M.T.; Saoud, I.P. Hydrogel Banding Improves Plant Growth, Survival, and Water Use Efficiency in Two Calcareous Soils. Clean (Weinh) 2017, 45, 1700251. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Luan, Q.; Tang, H.; Huang, F.; Xiang, X.; Yang, C.; Bao, Y. Cellulose Anionic Hydrogels Based on Cellulose Nanofibers As Natural Stimulants for Seed Germination and Seedling Growth. J Agric Food Chem 2017, 65, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- González Gómez, H.; Ramírez Godina, F.; Ortega Ortiz, H.; Benavides Mendoza, A.; Robledo Torres, V.; Cabrera De la Fuente, M. Use of Chitosan-PVA Hydrogels with Copper Nanoparticles to Improve the Growth of Grafted Watermelon. Molecules 2017, 22, 1031. [Google Scholar] [CrossRef] [PubMed]
- Babaluei, M.; Mottaghitalab, F.; Seifalian, A.; Farokhi, M. Injectable Multifunctional Hydrogel Based on Carboxymethylcellulose/Polyacrylamide/Polydopamine Containing Vitamin C and Curcumin Promoted Full-Thickness Burn Regeneration. Int J Biol Macromol 2023, 236, 124005. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, A.; Saha, N.; Kitano, T.; Saha, P. Hydrothermal Effect and Mechanical Stress Properties of Carboxymethylcellulose Based Hydrogel Food Packaging. Carbohydr Polym 2015, 117, 559–568. [Google Scholar] [CrossRef]
- Pereira, J.F.; Marim, B.M.; Simões, B.M.; Yamashita, F.; Mali, S. Hydrogels Based on Gelatin, Xanthan Gum, and Cellulose Obtained by Reactive Extrusion and Thermopressing Processes. Prep Biochem Biotechnol 2023, 53, 942–953. [Google Scholar] [CrossRef]
- Simões, B.M.; Cagnin, C.; Yamashita, F.; Olivato, J.B.; Garcia, P.S.; de Oliveira, S.M.; Eiras Grossmann, M.V. Citric Acid as Crosslinking Agent in Starch/Xanthan Gum Hydrogels Produced by Extrusion and Thermopressing. LWT 2020, 125. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Kumar Thakur, V. Recent Advances in Starch-Clay Nanocomposites. International Journal of Polymer Analysis and Characterization 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Reid, D.S.; McHugh, T.H.; Berrios, J.D.J.; Krochta, J.M. Thermal Transitions and Extrusion of Glycerol-Plasticized Whey Protein Mixtures. J Food Sci 2008, 73, E169–E175. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Di Giogio, L.; Goyanes, S.; Mauri, A.; Famá, L. Influence of Process (Extrusion/Thermo-Compression, Casting) and Lentil Protein Content on Physicochemical Properties of Starch Films. Carbohydr Polym 2019, 208, 221–231. [Google Scholar] [CrossRef]
- Alam, M.S.; Pathania, S.; Sharma, A. Optimization of the Extrusion Process for Development of High Fibre Soybean-Rice Ready-to-Eat Snacks Using Carrot Pomace and Cauliflower Trimmings. LWT 2016, 74, 135–144. [Google Scholar] [CrossRef]
- Custódio, A.C.; Patrik, R.; Ribeiro, S.; Brunelle, T.; De Lima, S.; Silvano De Araújo, E.; Lopes Barros De Araújo, P. Purificação Simplificada Do Rejeito de Glicerina Bruta Da Produção de Biodiesel Da Biorrefinaria Berso-UFPE: Uma Prática Sustentável Simple Purification Process of Waste Glycerol from Berso-Ufpe Biorefinary Biodiesel Production: A Sustainable Practice Revista Brasileira de Geografia Física; 2022; Vol. 15;
- Jiao, Z.; Zhang, B.; Li, C.; Kuang, W.; Zhang, J.; Xiong, Y.; Tan, S.; Cai, X.; Huang, L. Carboxymethyl Cellulose-Grafted Graphene Oxide for Efficient Antitumor Drug Delivery. Nanotechnol Rev 2018, 7, 291–301. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch—Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose-Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. 2019. [CrossRef]
- Parvaneh, S.; Pourmadadi, M.; Abdouss, M.; Pourmousavi, S.A.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Carboxymethyl Cellulose/Starch/Reduced Graphene Oxide Composite as a PH-Sensitive Nanocarrier for Curcumin Drug Delivery. Int J Biol Macromol 2023, 241. [Google Scholar] [CrossRef] [PubMed]
- Simi, C.K.; Emilia Abraham, T. Hydrophobic Grafted and Cross-Linked Starch Nanoparticles for Drug Delivery. Bioprocess Biosyst Eng 2007, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, C.; Simões, B.M.; Yamashita, F.; Andrello, A.C.; de Carvalho, G.M.; Grossmann, M.V.E. Hydrogels of Starch/Carboxymethyl Cellulose Crosslinked with Sodium Trimetaphosphate via Reactive Extrusion. J Appl Polym Sci 2021, 138. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch—Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Rudhziah, S.; Ahmad, A.; Mohamed, N.S. Biopolymer Electrolyte Based on Derivatives of Cellulose from Kenaf Bast Fiber. Polymers (Basel) 2014, 6, 2371–2385. [Google Scholar] [CrossRef]
- Biswal, D.R.; Singh, R.P. Characterisation of Carboxymethyl Cellulose and Polyacrylamide Graft Copolymer. Carbohydr Polym 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Sauraj; Bano, S.; Kumar, A.; Negi, Y.S. Development of Novel Cross-Linked Carboxymethyl Cellulose/Poly(Potassium 1-Hydroxy Acrylate): Synthesis, Characterization and Properties. Polymer Bulletin 2020, 77, 4555–4570. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Tohamy, H.-A.S.; Salama, A.; Kamel, S. THERMAL PROPERTIES OF CARBOXYMETHYL CELLULOSE ACETATE BUTYRATE; 2019; Vol. 53;
- Ma, X.; Chang, P.R.; Yu, J. Properties of Biodegradable Thermoplastic Pea Starch/Carboxymethyl Cellulose and Pea Starch/Microcrystalline Cellulose Composites. Carbohydr Polym 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Rohr, T.G. UFRRJ INSTITUTO DE TECNOLOGIA CURSO DE PÓS-GRADUAÇÃO EM ENGENHARIA QUÍMICA DISSERTAÇÃO ESTUDO REOLÓGICO DA MISTURA CARBOXIMETILCELULOSE/AMIDO E SUA UTILIZAÇÃO COMO VEÍCULO DE INOCULAÇÃO BACTERIANO; 2007;
- Ochoa-Yepes, O.; Di Giogio, L.; Goyanes, S.; Mauri, A.; Famá, L. Influence of Process (Extrusion/Thermo-Compression, Casting) and Lentil Protein Content on Physicochemical Properties of Starch Films. Carbohydr Polym 2019, 208, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Duquette, D.; Nzediegwu, C.; Portillo-Perez, G.; Dumont, M.J.; Prasher, S. Eco-Friendly Synthesis of Hydrogels from Starch, Citric Acid, and Itaconic Acid: Swelling Capacity and Metal Chelation Properties. Starch/Staerke, 2020; 72. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, P.; Zhang, J.; Wang, A. Study on Superabsorbent Composite XVI. Synthesis, Characterization and Swelling Behaviors of Poly(Sodium Acrylate)/Vermiculite Superabsorbent Composites. Eur Polym J 2007, 43, 1691–1698. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in Healthcare: From Static to Dynamic Material Microenvironments. Acta Mater 2013, 61, 931–944. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; Liu, W.; Chen, J.; Zeng, Z.; Liu, C. Properties of Starch after Extrusion: A Review. Starch—Stärke 2018, 70, 1700110. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Saravacos, G.D. $ Porosity and Pore Size Distribution of Starch Materials; 1993; Vol. 18;
- De Albuquerque, G.; Neto, A.; Monteiro, M.; Dantas, I.; Da Silva, V.; Menegaz, C.; Cristiano, Z. ESTUDOS DE DEGRADAÇÃO TÉRMICA E NO SOLO DE FILMES FORMADOS POR GOMA XANTANA E CARBOXIMETILCELULOSE RETICULADOS COM ÁCIDO CÍTRICO; 2019; Vol. 20;
- Marim, B.M.; Mantovan, J.; Gil-Giraldo, G.A.; Pereira, J.F.; Simões, B.M.; Yamashita, F.; Mali, S. Reactive Extrusion-Assisted Process to Obtain Starch Hydrogels through Reaction with Organic Acids. Polysaccharides 2022, 3, 792–803. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel Biodegradable Superabsorbent Hydrogels Derived from Cotton Cellulose and Succinic Anhydride: Synthesis and Characterization. J Appl Polym Sci 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Vedovello, P.; Fernandes, C.; Tiritan, M.E.; Paranhos, C.M. Chiral Mixed Matrix Membranes with the (S,S)-Whelk-O®1 Selector Supported on Polyethersulfone/MCM-41. New Journal of Chemistry 2024. [CrossRef]
- Chavda, H.; Patel, C. Effect of Crosslinker Concentration on Characteristics of Superporous Hydrogel. Int J Pharm Investig 2011, 1, 17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





