Submitted:
05 February 2025
Posted:
05 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
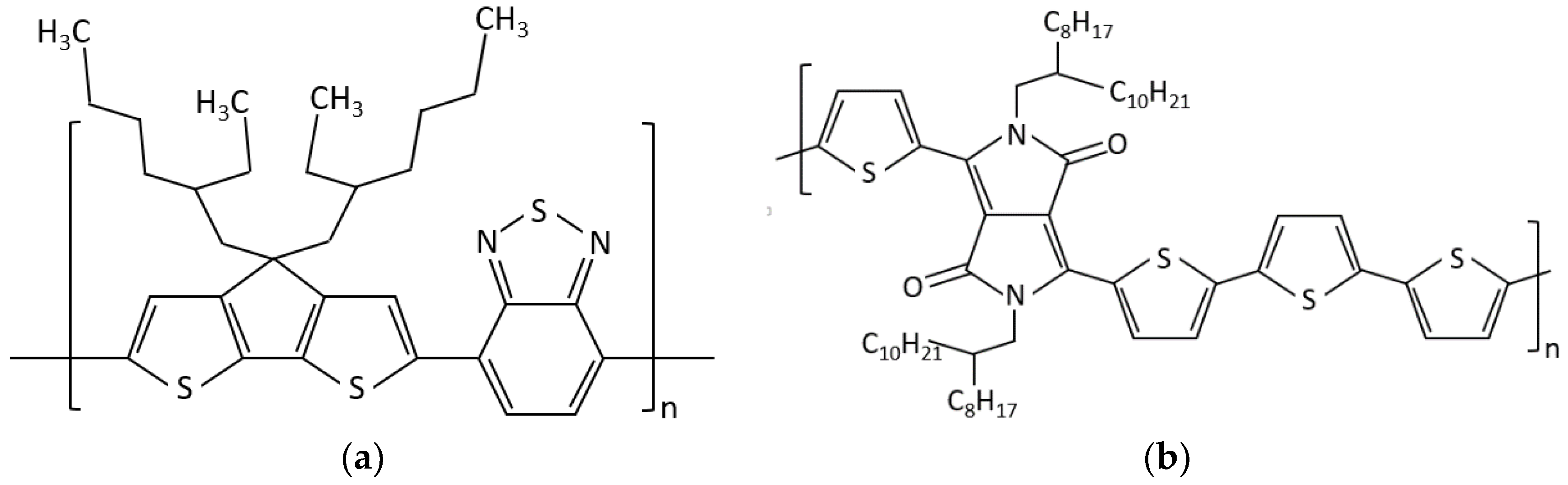
2.1. Materials
2.2. Samples Preparation
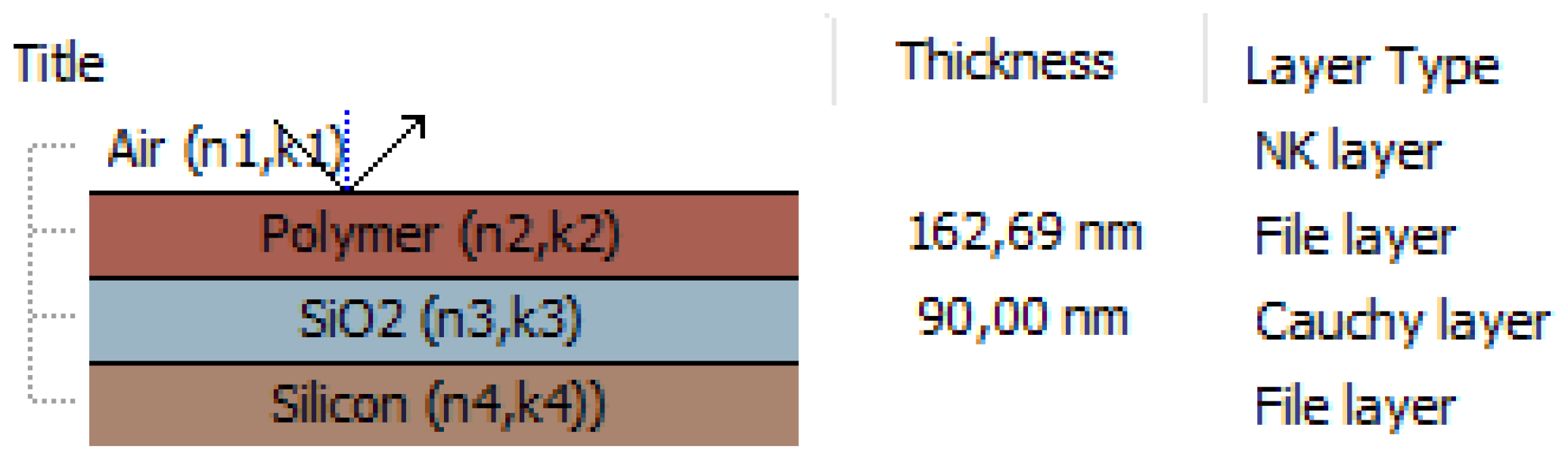
2.3. Methods
3. Results and Discussion
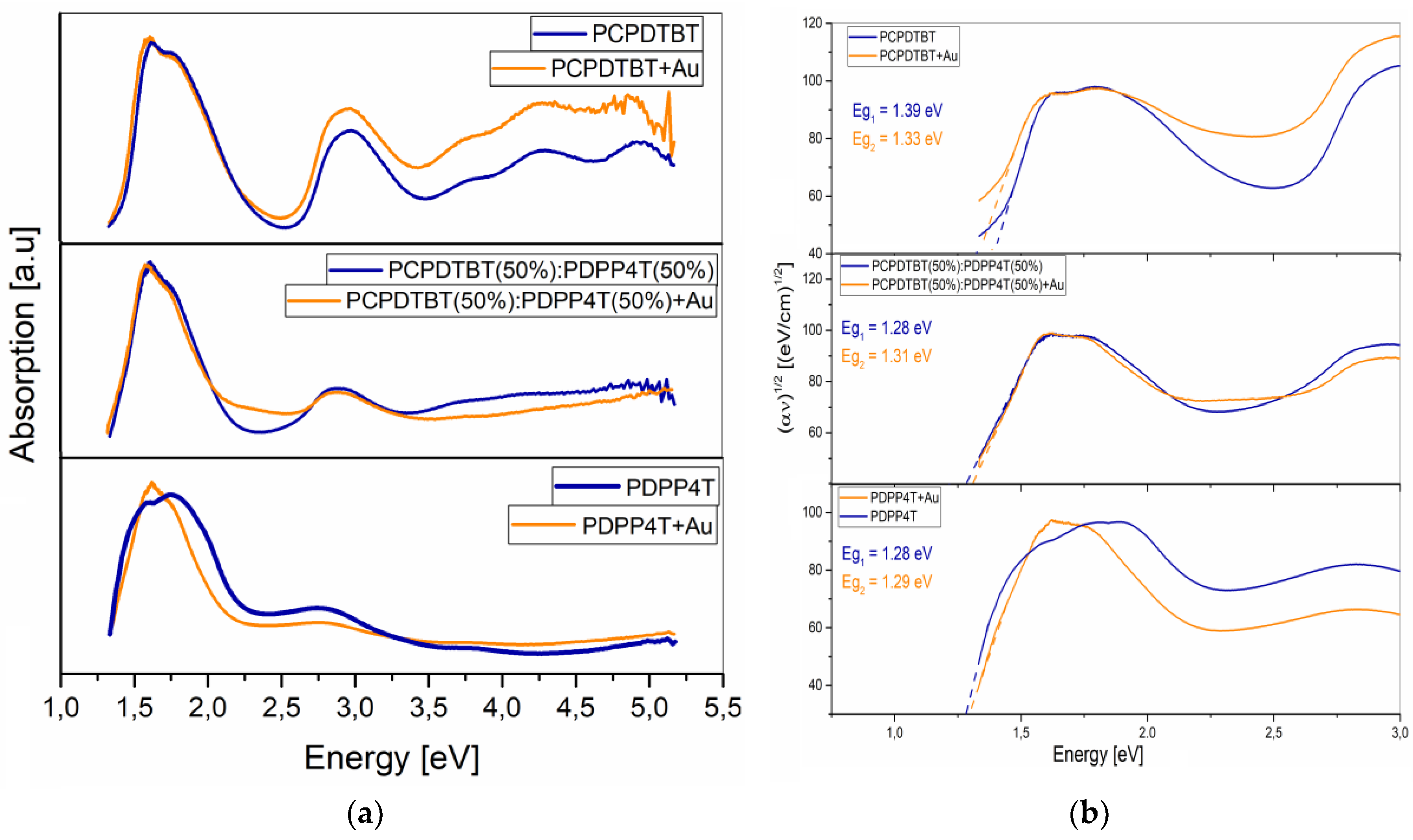
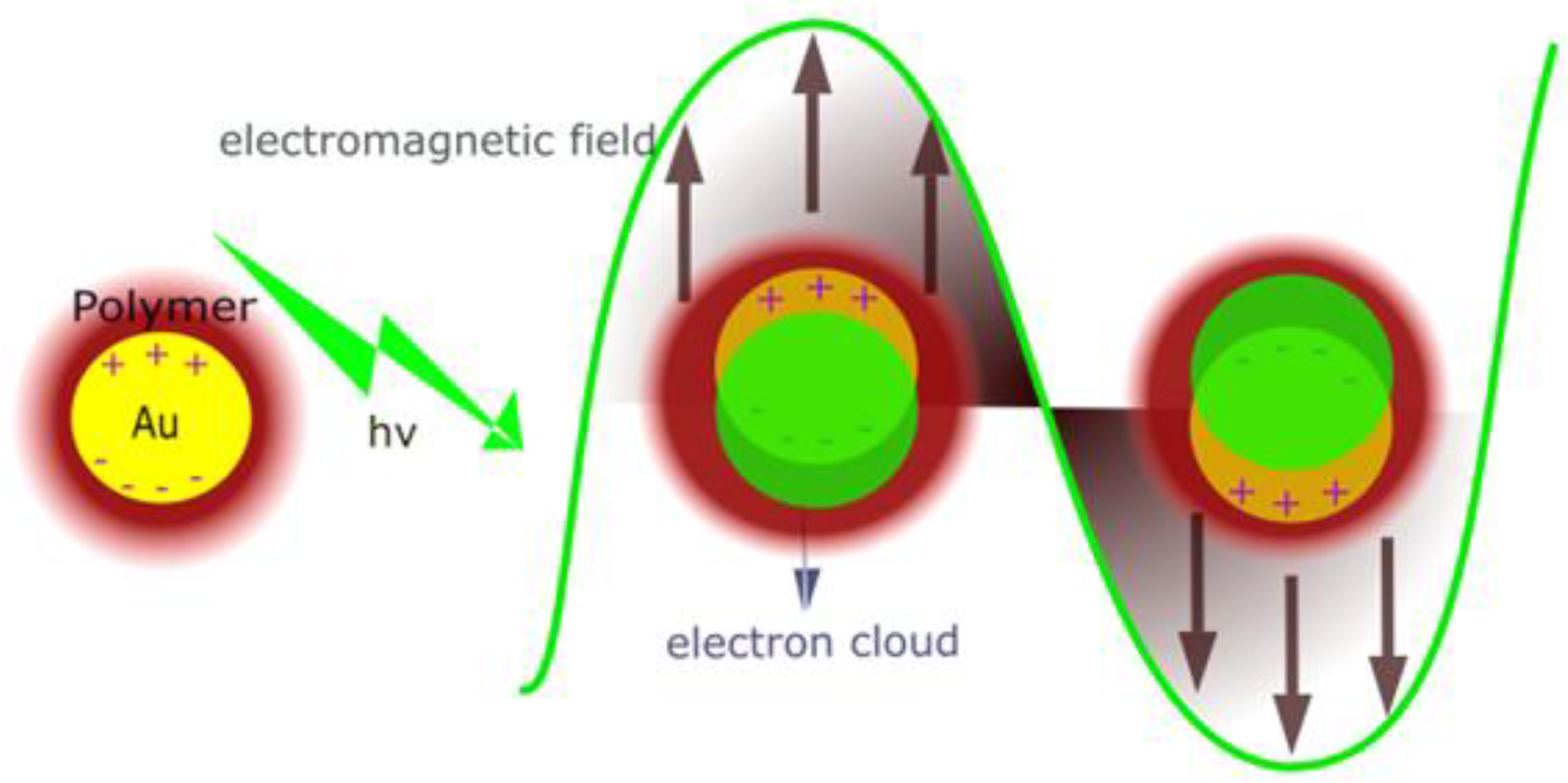
3.1. Optical Analysis
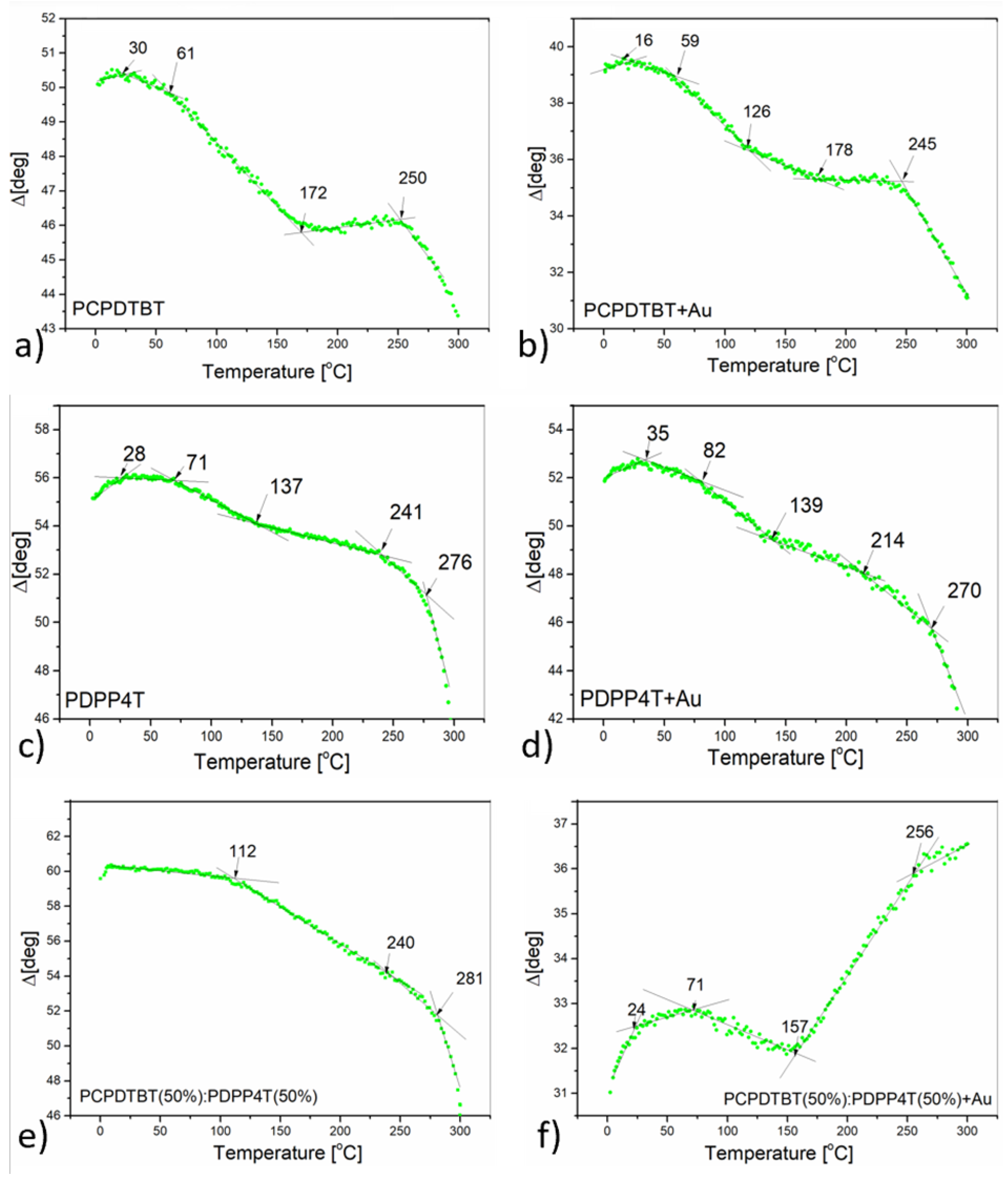
3.2. Thermal Analysis
3.3. XRD Analysis
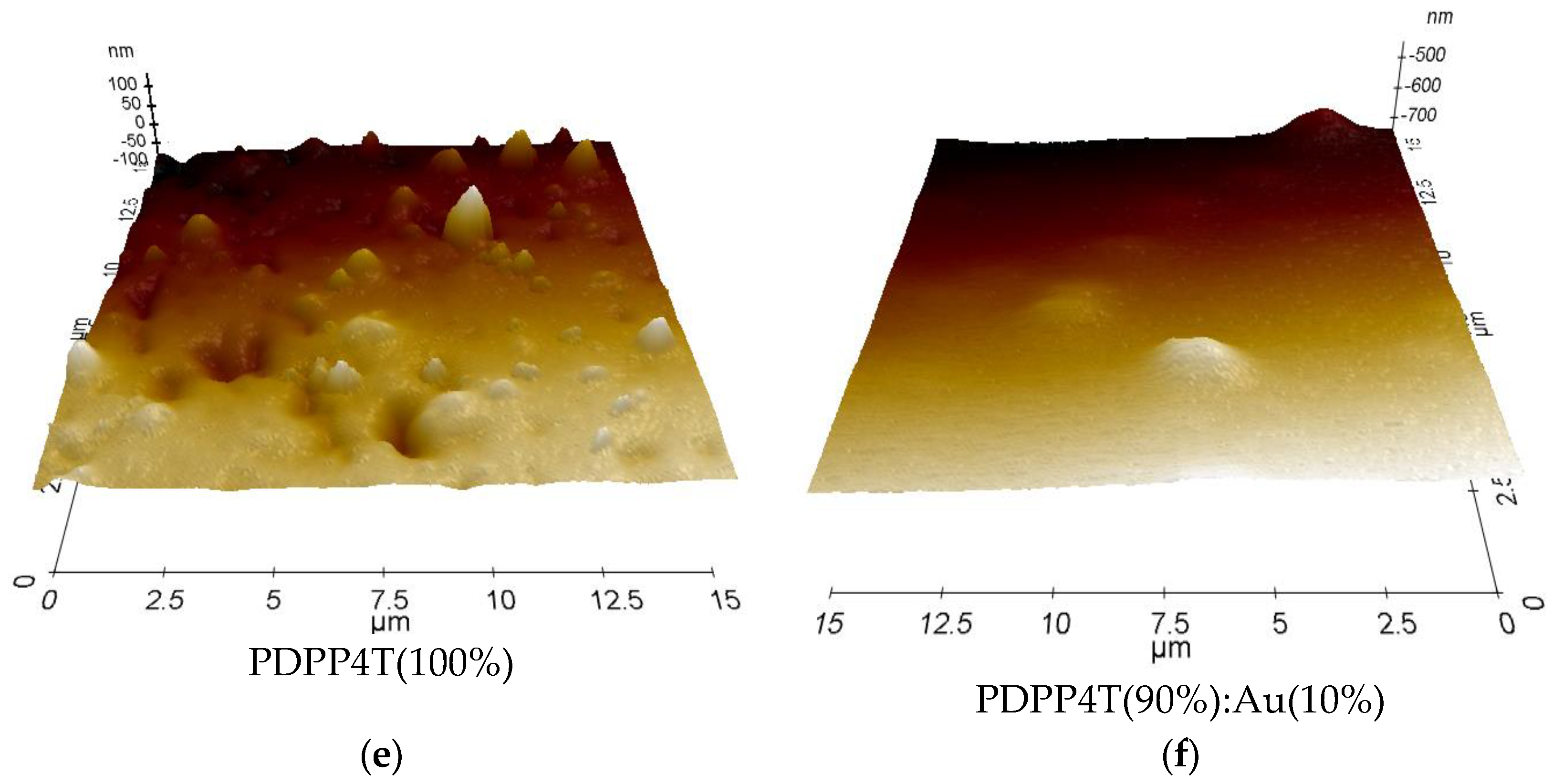
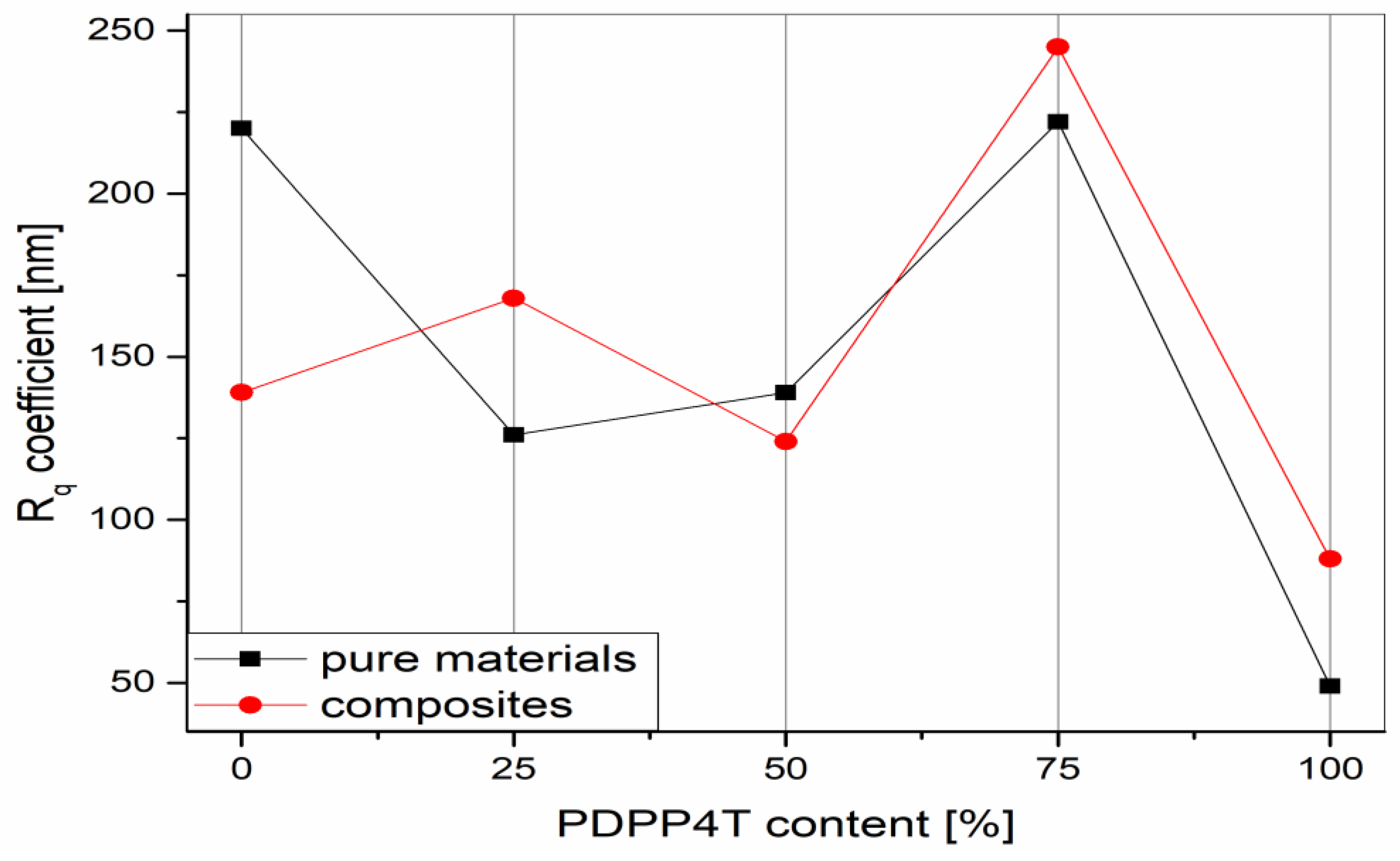
3.4. Microscopic Analysis:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
References
- A.K. Sulaiman, C.C. Yap, F. Touati, Binary blend based dye sensitized photo sensor using PCPDTBT and MEH-PPV composite as a light sensitizer, Synth. Met. 210 (2015) 392-397; 10.1016/j.synthmet.2015.11.005.
- Y. Li, Y. Wei, K. Feng, Y. Hao, J. Pei, Y. Zhang, B. Sun, Introduction of PCPDTBT in P3HT:Spiro-OMeTAD blending system for solid-state hybrid solar cells with dendritic TiO2/Sb2S3 nanorods composite film, J. Sol. St. Chem., 276 (2019) 278-284; 10.1016/j.jssc.2019.05.020.
- S. Banerjee, P. Singh, P. Purkayastha, S. Kumar Ghosh, Evolution of Organic Light Emitting Diode (OLED) Materials and their Impact on Display Technology, Chem. Asian J. 2024, e202401291. [CrossRef]
- Debashish Nayak, Ram Bilash Choudhary, A survey of the structure, fabrication, and characterization of advanced organic light emitting diodes, Microelectronics Reliability,144, 2023, 114959. [CrossRef]
- L. Luo, Z. Liu. Recent progress in organic field-effect transistor-based chem/bio-sensors , VIEW. 2022, 3, 20200115. [CrossRef]
- Yujie Zhao, Wei Wang, Zihan He, Boyu Peng, Chong-An Di, Hanying Li, High-performance and multifunctional organic field-effect transistors, Chinese Chemical Letters,34/9, 2023,108094. [CrossRef]
- Kim, O. et al. Efect of PVP-capped ZnO nanoparticles with enhanced charge transport on the performance of P3HT/PCBM polymer solar cells. Polymers 11, 1818, (2019). [CrossRef]
- Liu, C. et al. Decreased charge transport barrier and recombination of organic solar cells by constructing interfacial nanojunction with annealing-free ZnO and Al flms. ACS Appl. Mater. Interfaces 9, 22068–22075. (2017). [CrossRef]
- Mohtaram, F. et al. Electrospun ZnO nanofber interlayers for enhanced performance of organic photovoltaic devices. Sol. Energy 197, 311–316. (2020). [CrossRef]
- Swart, H. C. et al. P3HT: PCBM based solar cells: A short review focusing on ZnO nanoparticles bufer layer, post-fabrication annealing and an inverted geometry. J. Mater. Sci. Eng. B 5, 12–35. (2015). [CrossRef]
- Wanninayake, A. P., Church, B. C. & Abu-Zahra, N. Efect of ZnO nanoparticles on the power conversion efciency of organic photovoltaic devices synthesized with CuO nanoparticles. AIMS Mater. Sci. 3(3), 927–937. (2016). [CrossRef]
- Jiaqi Dong, Chuxuan Yan, Yingzhi Chen, Wenjie Zhou, Yu Peng, Yue Zhang, Lu-Ning Wang, Zheng-Hong Huang, Organic semiconductor nanostructures: optoelectronic properties, modification strategies, and photocatalytic applications, Journal of Materials Science & Technology, 113, 2022, 175-198. [CrossRef]
- Zhongkai Cheng, Nasir Javed, and Deirdre M. O’Carroll, Optical and Electrical Properties of Organic Semiconductor Thin Films on Aperiodic Plasmonic Metasurfaces, ACS Applied Materials & Interfaces 2020 12 (31), 35579-35587. [CrossRef]
- Balakrishnan T. S., Sultan M. T. H., Shahar F. S., Basri A. A., Shah A. U. M., Sebaey T. A., Łukaszewicz A., Józwik J., Grzejda R.: Fatigue and impact properties of kenaf/glass-reinforced hybrid pultruded composites for structural applications, Materials, 17, 2 (2024) 302. [CrossRef]
- Hajduk, B., Bednarski, H., Jarka, P. et al. Thermal and optical properties of PMMA films reinforced with Nb2O5 nanoparticles. Sci Rep 11, 22531 (2021). [CrossRef]
- Huang Y., Sultan M. T. H., Shahar F. S., Grzejda R., Łukaszewicz A.: Hybrid fiber-reinforced biocomposites for marine applications: A review, Journal of Composites Science, 8, 10 (2024) 430. [CrossRef]
- Shahar F. S., Sultan M. T. H., Grzejda R., Łukaszewicz A., Oksiuta Z., Krishnamoorthy R. R.: Harnessing the potential of natural composites in biomedical 3D printing, Materials, 17, 24 (2024) 6045. [CrossRef]
- Ibrahim N. I., Sultan M. T. H., Łukaszewicz A., Shah A. U. M., Shahar F. S., Józwik J., Najeeb M. I., Grzejda R.: Characterization and isolation method of Gigantochloa scortechinii (Buluh Semantan) cellulose nanocrystals, International Journal of Biological Macromolecules, 272, Part 1 (2024) 132847. [CrossRef]
- T-C. Wang, Y-H. Su, Y-K.Hung, C-S. Yeh, L-W. Huang, W. Gomulya, L-H. Lai, M.A. Loi, J-S. Yang, JJ. Wu, Charge collection enhancement by incorporation of gold–silica core–shell nanoparticles into P3HT:PCBM/ZnO nanorod array hybrid solar cells, Phys. Chem. Chem. Phys. 17 (2015) 19854-19861; 10.1039/c5cp03081a.
- H. Gao, J. Meng, J. Sun, J. Deng, Enhanced performance of polymer solar cells based on P3HT:PCBM via incorporating Au nanoparticles prepared by the micellar method, Journal of Materials Science: Mat. in Electr. 31(2020) 10760–10767; 10.1007/s10854-020-03626-x.
- S. Pillai, K. R. Catchpole, T. Trupke, M. A. Green, Surface plasmon enhanced silicon solar cells, Journal of Applied Physics 101, 093105, 2007. [CrossRef]
- Majid Sharifi, Farnoosh Attar, Ali Akbar Saboury, Keivan Akhtari, Nasrin Hooshmand, Anwarul Hasan, Mostafa A. El-Sayed, Mojtaba Falahati, Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy, Journal of Controlled Release, 311–312, 2019, 170-189. [CrossRef]
- Hajduk, B.; Jarka, P.; Bednarski, H.; Janeczek, H.; Kumari, P.; Farcas, A. Thermal Transitions and Structural Characteristics of Poly(3,4-ethylenedioxythiophene/cucurbit[7]uril) Polypseudorotaxane and Polyrotaxane Thin Films. Materials 2024, 17, 1318. [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Nitschke, P.; Janeczek, H. Phase diagram of P3HT:PC70BM thin films based on variable-temperature spectroscopic ellipsometry. Polym. Test. 2020, 84, 106383. [CrossRef]
- Wang, T.; Pearson, A.J.; Dunbar, A.D.F.; Staniec, P.A.; Watters, D.C.; Coles, D.; Yi, H.; Iraqi, A.; Lidzey, D.G.; Jones, R.A.L. Competition between substrate-mediated π-π stacking and surface-mediated Tg depression in ultrathin conjugated polymer films. Eur. Phys. J. E 2012, 35, 12. [CrossRef]
- Kim, J.H.; Jang, J.; Zin, W.-C. Estimation of the thickness dependence of the glass transition temperature in various thin polymer films. Langmuir 2000, 16, 4064–4067. [CrossRef]
- Keddie, J.L.; Jones, R.A.; Cory, R.A. Size-dependent depression of the glass transition temperature in polymer films, Europhys. Eur. Lett. 1994, 27, 59–64, 10.1209/0295-5075/27/1/011.
- T. Pratyusha, G. Sivakumar, A. Yella, D. Gupta, Novel Ternary Blend of PCDTBT, PCPDTBT and PC70BM for the Fabrication of Bulk Heterojunction Organic Solar Cells, Mater. Today: Proceedings 4 (2017) 5067–5073; 10.1016/j.matpr.2017.04.115.
- F.S.U. Fischer, D. Trefz, J. Back, N. Kayunkid, B. Tornow, S. Albrecht, K.G Yager, G. Singh, A. Karim, D. Neher, M. Brinkmann, S. Ludwigs, Highly Crystalline Films of PCPDTBT with Branched Side Chains by Solvent Vapor Crystallization: Influence on Opto-Electronic Properties, Adv. Mater., 27 (2015) 1223–1228; 10.1002/adma.201403475.
- Z. Ahmad, F. Touati, F.F. Muhammad, M. Ani Najeeb, R. A. Shakoor Effect of ambient temperature on the efficiency of the PCPDTBT:PC71BM BHJ solar cells, Z. Ahmad et al., Appl. Phys. A 123 (2017) 486; 10.1007/s00339-017-1098-8.
- Y. Liu, F. Liu, H.W. Wang, D. Nordlund, Z. Sun, S. Ferdous Thomas P. Russell, Sequential Deposition: Optimization of Solvent Swelling for High-Performance Polymer Solar Cells, ACS Appl. Mater. Interfaces, 7 (2015) 653-661; 10.1021/am506868g.
- J.C. Bijleveld, R. A. Melanie Verstrijden, M.M. Wienka, R.A.J. Janssen, Copolymers of diketopyrrolopyrrole and thienothiophene for photovoltaic cells, J. Mater. Chem., 21 (2011) 9224-9231; 10.1039/C1JM10961H.
- Z. Yi, X. Sun, Y. Zhao, Z. Li, Diketopyrrolopyrrole-Based π-Conjugated Copolymer Containing β-Unsubstituted Quintetthiophene Unit: A Promising Material Exhibiting High Hole-Mobility for Organic Thin-Film Transistors, Z. Yi et al., Chem. Mater., 24 (2012) 4350-4356; 10.1021/cm302341m.
- Hajduk, B.; Jarka, P.; Tański, T.; Bednarski, H.; Janeczek, H.; Gnida, P.; Fijalkowski, M. An Investigation of the Thermal Transitions and Physical Properties of Semiconducting PDPP4T:PDBPyBT Blend Films. Materials 2022, 15, 8392. [CrossRef]
- Borgesi, A.; Tallarida, G.; Amore, G.; Cazzaniga, F.; Queirolo, F.; Alessandri, M.; Sassela, A. Influence ofroughness and grain dimension on the optical functions of polycrystalline silicon films. Thin Solid Film. 1998, 313–314, 243–247. [CrossRef]
- Lora L. Spangler, John M. Torkelson, J. Scot Royal, Influence of solvent and molecular weight on thickness and surface topography of spin-coated polymer films, Polymer Engineering and Science 30/11 (1990) 644-653.
- Stephanie L. Fronk, Ming Wang, Michael Ford, Jessica Coughlin, Cheng-Kang Mai, Effect of chiral 2-ethylhexyl side chains on chiroptical properties of the narrow bandgap conjugated polymers PCPDTBT and PCDTPT, Chemical Science, 7/ 8 (2016) 5313-5321.
- Yongqiang Wang, Tao An, Jiawei Xue, Realizing high detectivity organic photodetectors in visible wavelength by doping highly ordered polymer PCPDTBT, Organic Electronics, 82 (2020) 105700.
- Lei Wang, Ming Hu, Youdi Zhang, Zhongyi Yuan, Yu Hu, Xiaohong Zhao, Yiwang Chen, High molecular weight polymeric acceptors based on semi-perfluoroalkylated perylene diimides for pseudo-planar heterojunction all-polymer organic solar cells, Polymer 255 (2022) 125114.
- Joseph Nathanael, D. Mangalaraj, N. Ponpandian, Controlled growth and investigations on the morphology and mechanical properties of hydroxyapatite/titania nanocomposite thin films, Composites Science and Technology 70 (2010) 1645–1651.
- Daniela Predoi, Stefan Talu , Stelut Carmen Ciobanu, Simona Liliana Iconaru, Robert Saraiva Matos, Henrique Duarte da Fonseca Filho, Exploring the physicochemical traits, antifungal capabilities, and 3D spatial complexity of hydroxyapatite with Ag+–Mg2+ substitution in the biocomposite thin films, Micron 184 (2024) 103661.
- Jae Seung Ha, Kyung Hwan Kim, Dong Hoon Choi. ,5-Bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione-Based DonorAcceptor Alternating Copolymer Bearing 5,5’-Di (thiophen-2-yl)-2,20 -biselenophene Exhibiting 1.5 cm2V-1s-1Hole Mobility in Thin-Film Transistors, J. S. Ha et al., J. Am. Chem. Soc. 133 (2011) 10364–10367.
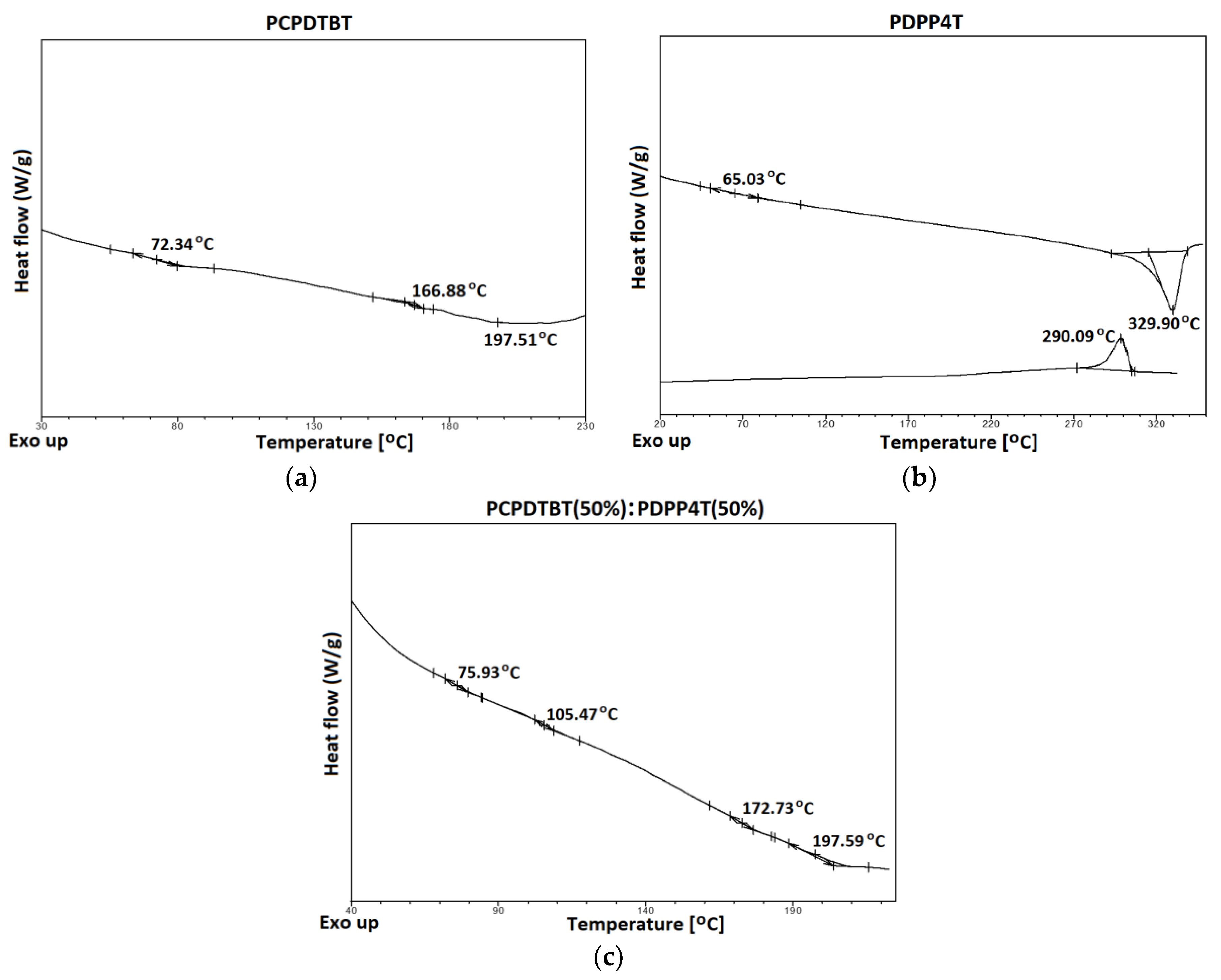
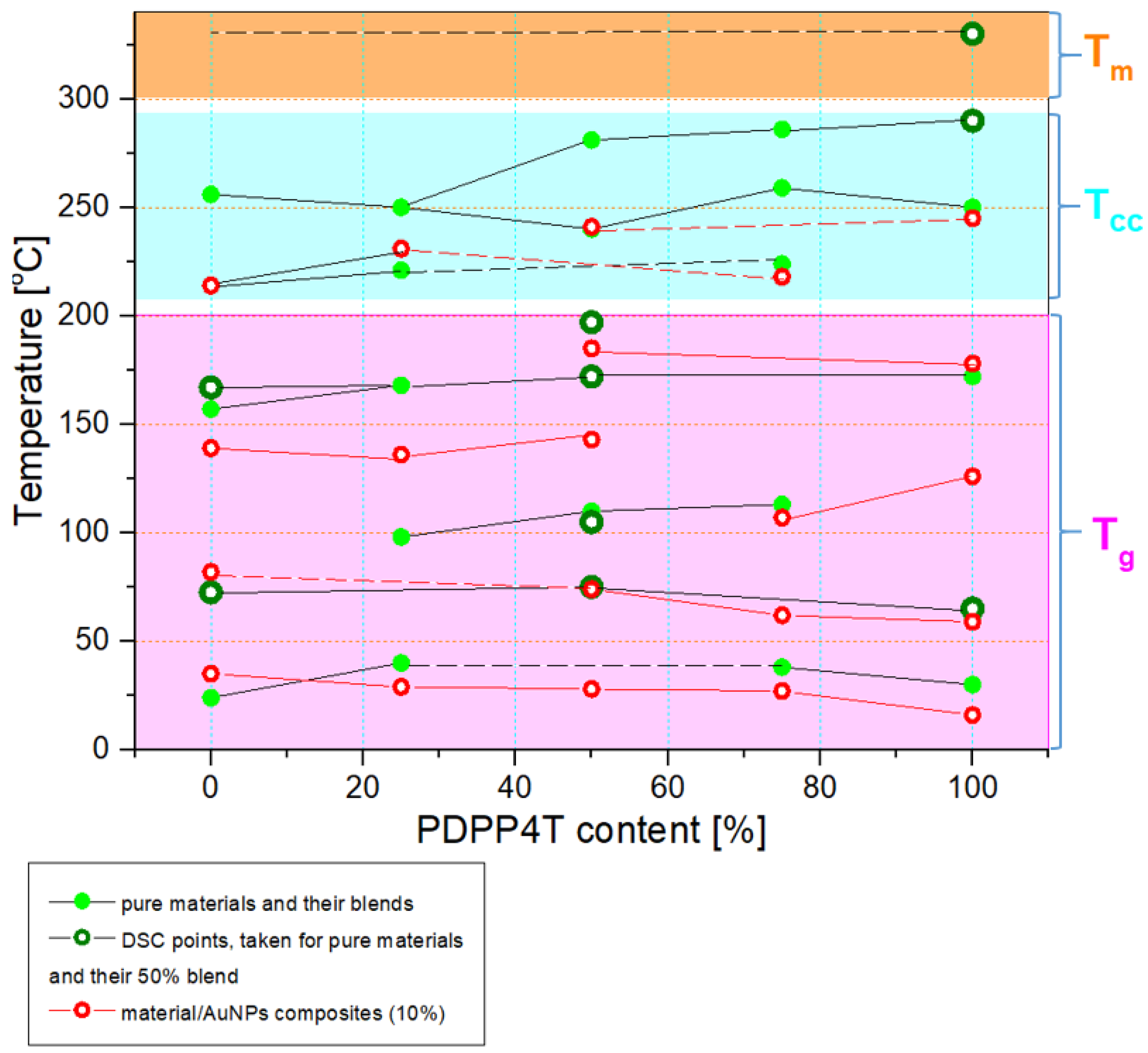
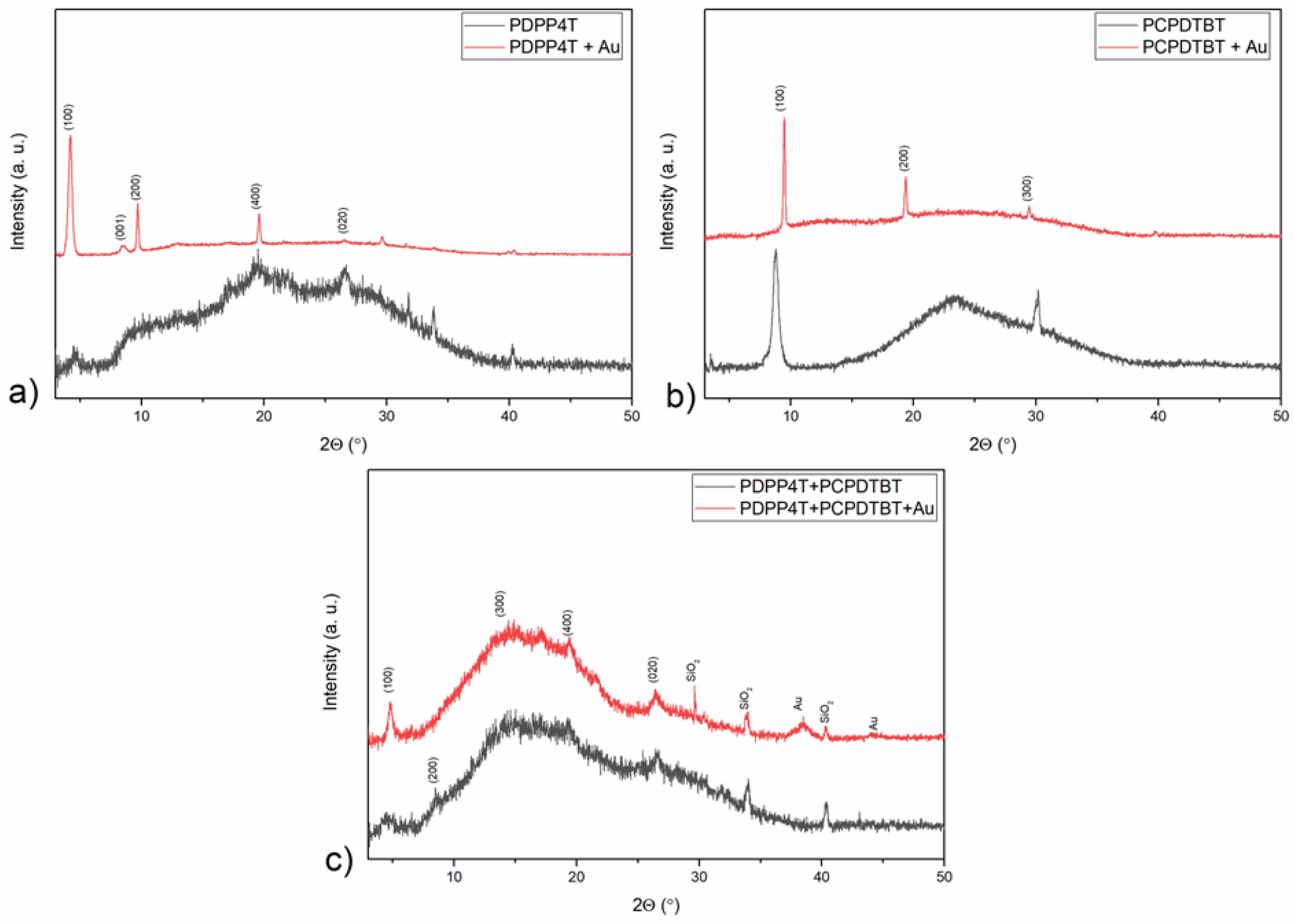
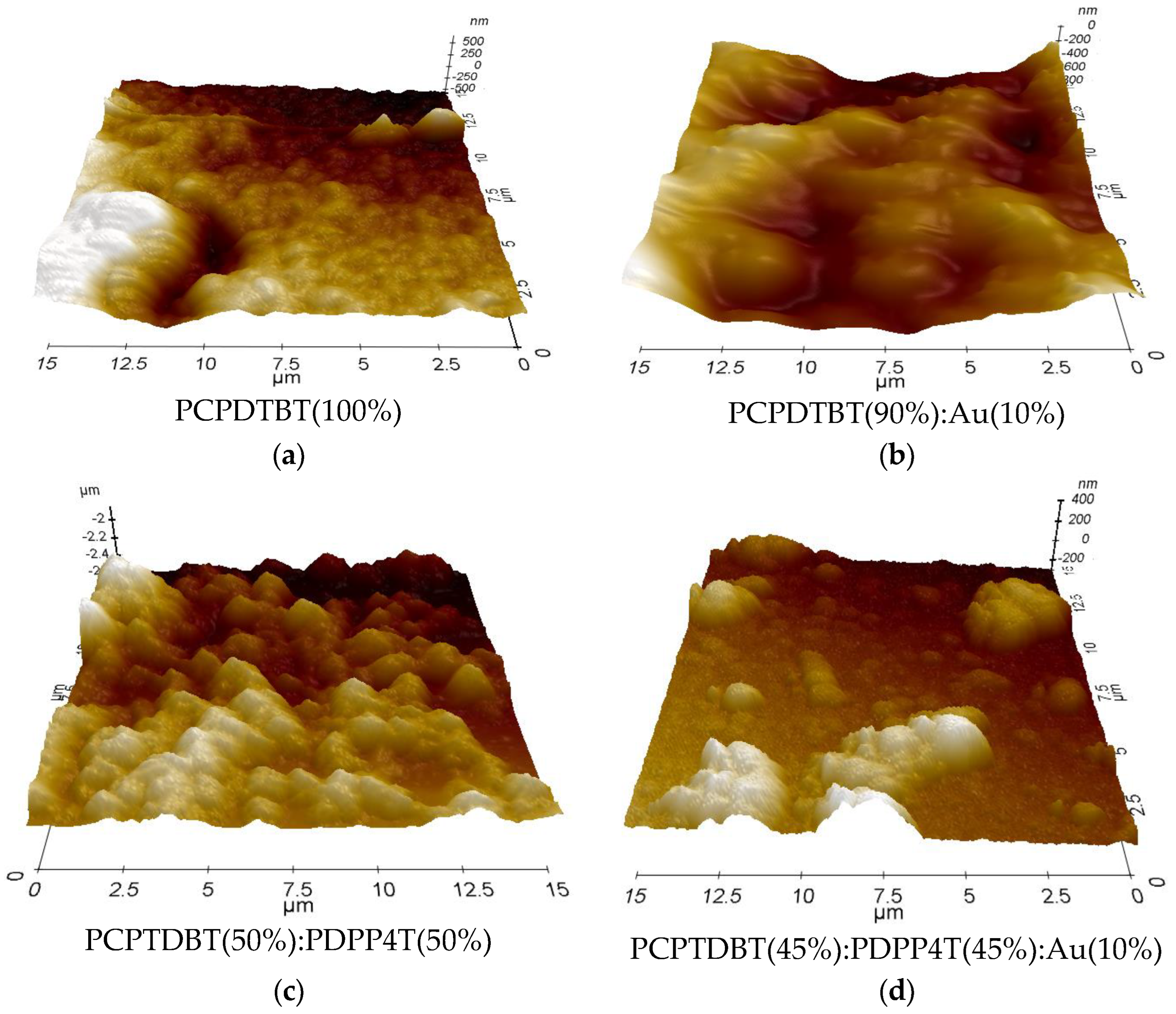
| Sample No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| PCPDTBT [%] | 100 | 75 | 50 | 25 | 0 |
| PDPP4T [%] | 0 | 25 | 50 | 75 | 100 |
| Sample No | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|
| PCPDTBT [%] | 100 | 70 | 45 | 20 | 0 |
| PDPP4T [%] | 0 | 45 | 50 | 70 | 100 |
| Au [%] | 10 | 10 | 10 | 10 | 10 |
| PCPDTBT [%] |
PDPP4T [%] |
Eg [eV] | Eg [eV] (+Au) |
|---|---|---|---|
| 100 |
0 | 1,39 | 1,33 |
| 75 |
25 | 1,32 | 1,38 |
| 50 |
50 | 1,28 | 1,31 |
| 25 |
75 | 1,22 | 1,29 |
| 0 |
100 | 1,28 | 1,29 |
| Sample | Crystal size (nm) |
|---|---|
| PCPDTBT | 16.1 |
| +Au | 47.3 |
| PDPP4T | 18.0 |
| +Au | 31.6 |
| BLENDA | 21.5 |
| +Au | 60.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





