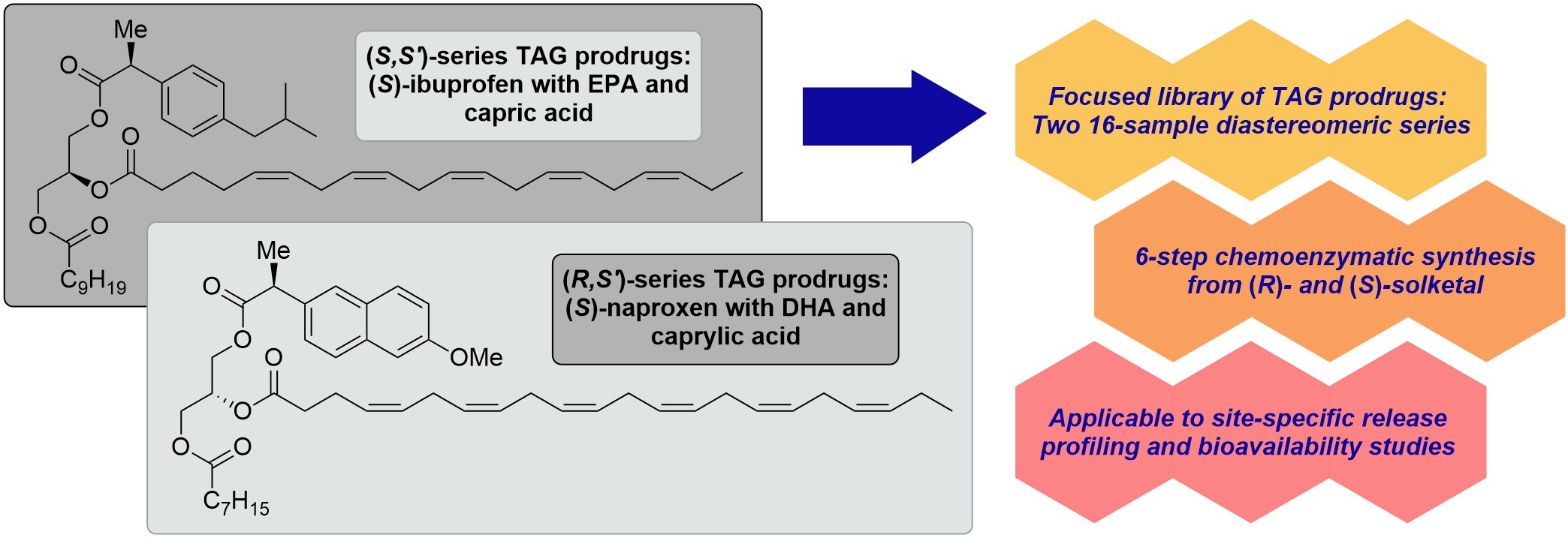
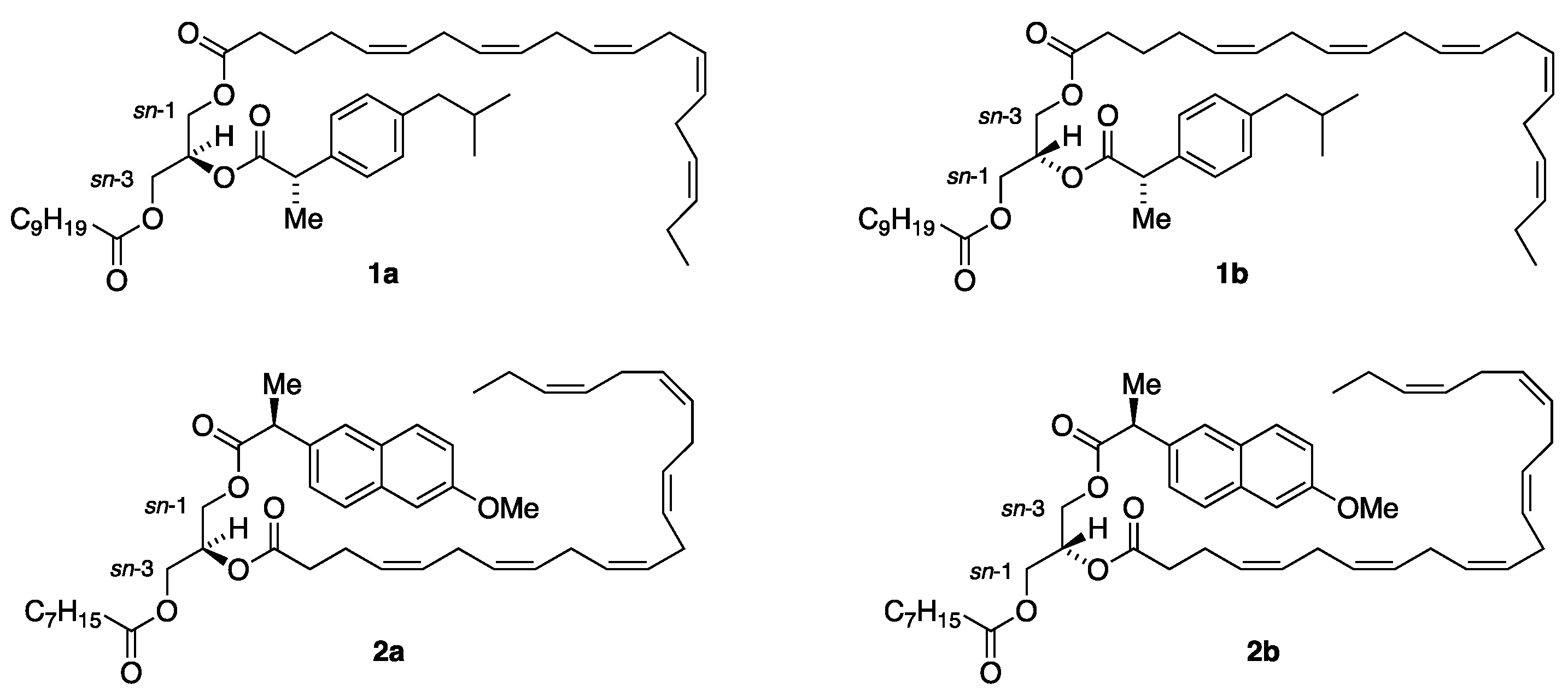
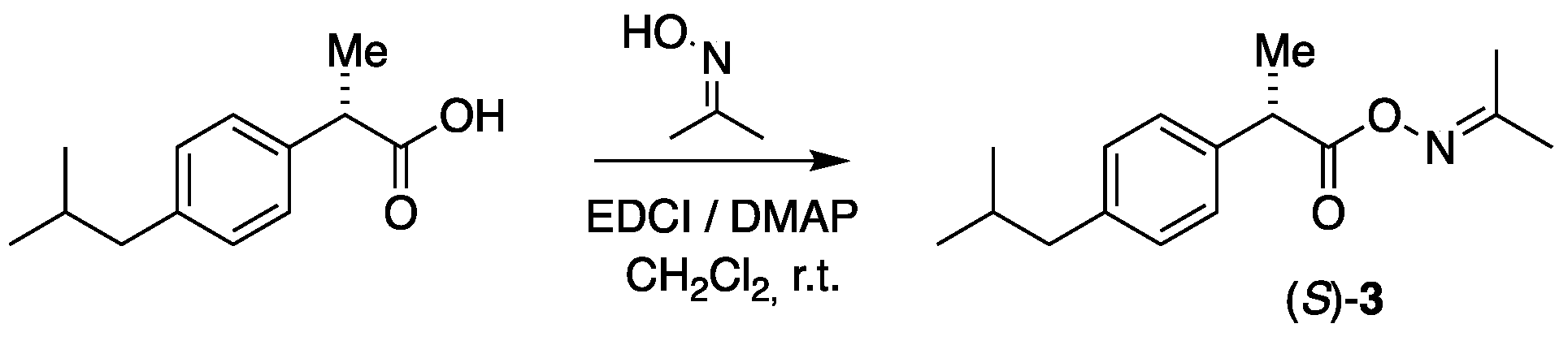
3.2. Activation of Drugs as Oximes
3.2.1. Synthesis of (S)-propan-2-one-O-(2-(4-isobutylphenyl)propanoyl Oxime, (S)-3
To a solution of (S)-ibuprofen (94 mg, 0.465 mmol), DMAP (16 mg, 0.131 mmol) and EDCI (105 mg, 0.553 mmol) in CH2Cl2 (2 mL) were added acetoxime (34 mg, 0.465 mmol) and the solution stirred on a magnetic stirrer at room temperature for 3-4 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of ethyl acetate/petroleum ether (3:2) as eluent. The solvent was removed in vacuo on a rotary evaporator and the crude product applied to a silica gel flash chromatography using ethyl acetate/petroleum ether (1:1) as eluent, which afforded the product (S)-3 as a slightly yellow liquid in a quantitative yield (115 mg, 0.465 mmol). [α]20D = -7.18 (c. 7.3, CH2Cl2). IR (NaCl, νmax / cm-1): 3058 (vs), 2958 (vs), 2931 (vs), 2853 (vs), 1758 (vs), 1653 (s). 1H NMR (400 MHz, CDCl3) δH: 7.27-7.17 (m, 2H, Ibu-2,6), 7.14-7.01 (m, 2H, Ibu-3,5), 3.79 (q, J=7.2 Hz, 1H, CHCH3), 2.44 (d, J=7.2 Hz, 2H, CH2CH), 1.99 (s, 3H, NC(CH3)2), 1.83 (nonet, J=6.7 Hz, 1H, CH(CH3)2), 1.83 (s, 3H, NC(CH3)2), 1.56 (d, J=7.2 Hz, 3H, CHCH3), 0.88 (d, J=6.7 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 171.9, 164.4 (N=C), 140.7, 137.5 (2), 129.4 (2), 127.3, 45.1, 44.3, 30.3, 22.5 (2), 22.1, 18.5, 16.9 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C16H23NO2Na 284.1621; found, 284.1618.
3.2.2. Synthesis of (S)-propan-2-one-O-(2-(6-methoxynaphthalen-2-yl)propanoyl Oxime, (S)-4
The same procedure was followed as described for (S)-3 using (S)-naproxen (105 mg, 0.456 mmol), DMAP (14 mg, 0.115 mmol) and EDCI (109 mg, 0.569 mmol) in CH2Cl2 (2 mL) were added acetoxime (33 mg, 0.451 mmol). Purification on silica gel flash chromatography using ethyl acetate/petroleum ether (1:1) as eluent, followed by recrystallization from n-hexane, afforded the product (S)-4 as a white solid in a quantitative yield (130 mg, 0.456 mmol). M.p. 42.3-43.4°C. [α]20D = -12.3 (c. 16.5, CH2Cl2). IR (NaCl, νmax / cm-1): 3052 (vs), 2956 (vs), 2932 (vs), 2863 (vs), 2848 (vs), 1753 (vs), 1652 (s). 1H NMR (400 MHz, CDCl3) δH: : 7.72-7.67 (m, 3H, Nap-1,4,8), 7.44 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.11 (d, J=2.5 Hz, 1H, Nap-5), 3.96 (q, J=7.2 Hz, 1H, CHCH3), 3.91 (s, 3H, OCH3), 1.99 (s, 3H, NC(CH3)2), 1.83 (s, 3H, NC(CH3)2), 1.65 (d, J=7.2 Hz, 3H, CHCH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 171.9, 164.4, 157.8, 135.4, 133.8, 129.4, 129.0, 127.3, 126.4, 126.1, 119.1, 105.7, 55.4, 44.6, 22.1, 18.7, 17.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C17H19NO3Na 308.1257; found, 308.1251.
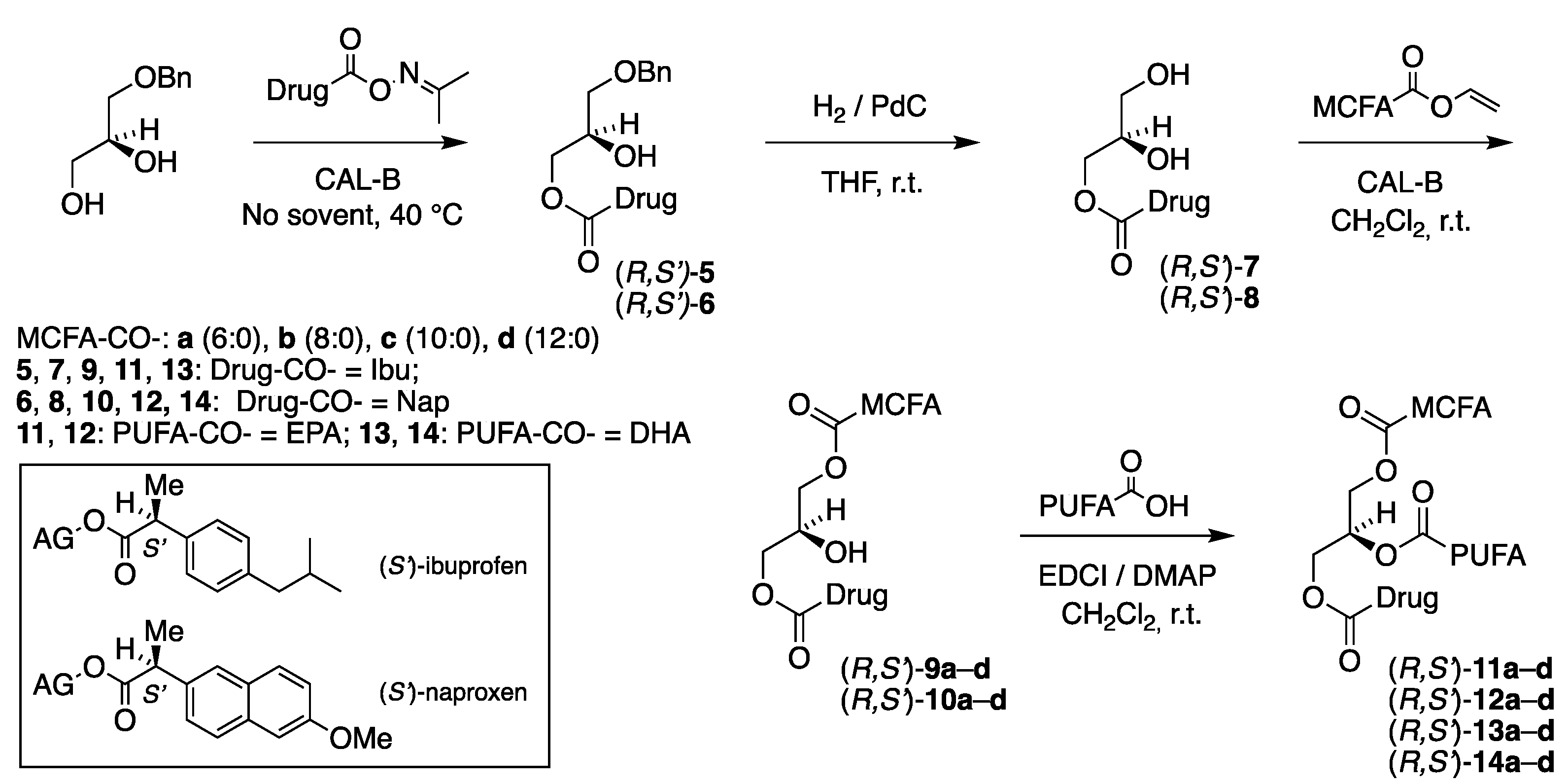
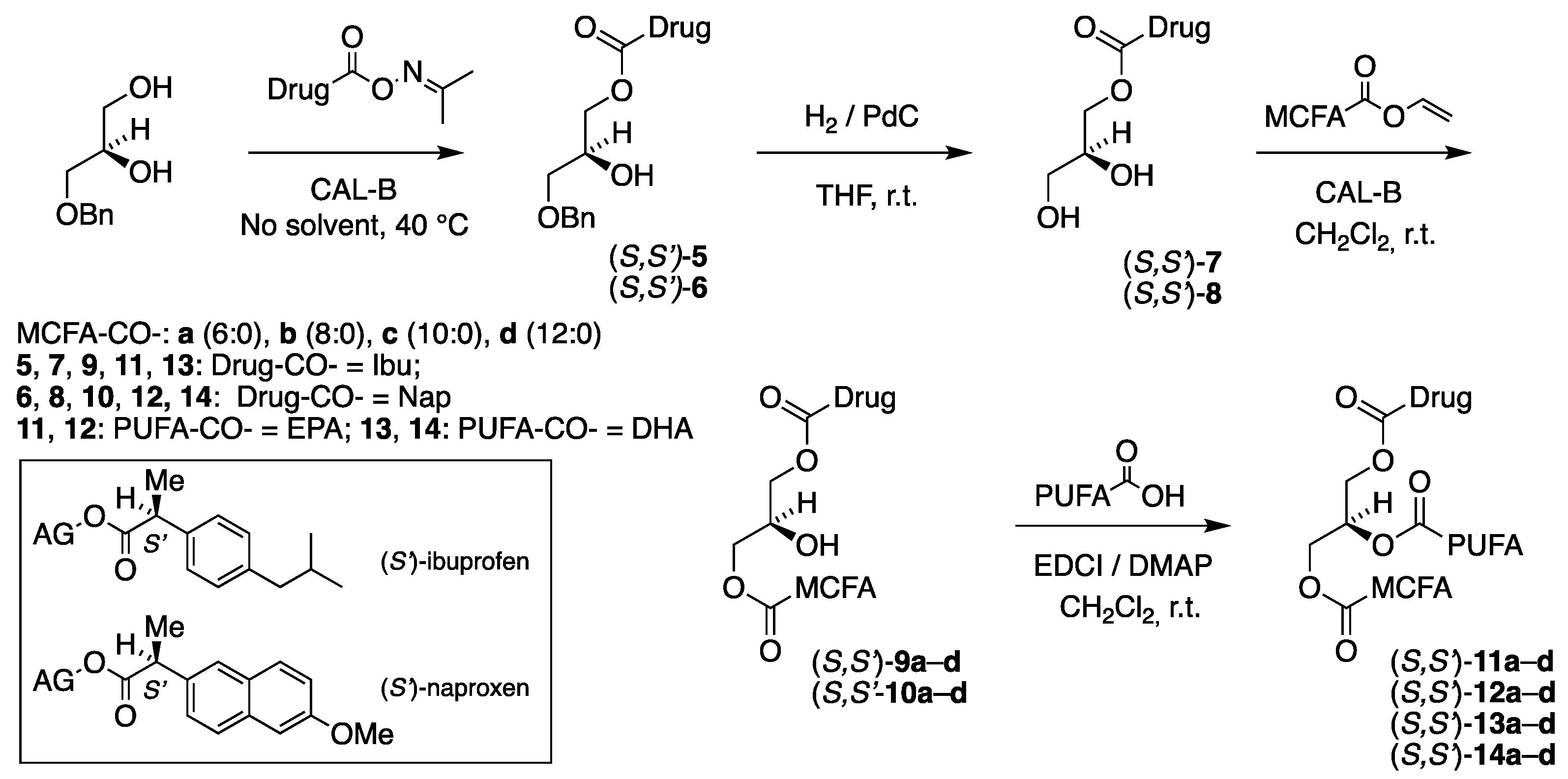
3.3. Enzymatic Coupling of the Drugs: Synthesis of (R,S')-5, (S,S')-5, (R,S')-6 and (S,S')-6
3.3.1. Synthesis of 1-O-benzyl-3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (R,S')-5
To a mixture of 1-O-benzyl-sn-glycerol (100 mg, 0.549 mmol) and ibuprofen acetoxime ester (S)-3 (163 mg, 0.659 mmol), immobilized CAL-B (40 mg) was added and the resulting mixture stirred at 40 °C for 31 h under nitrogen atmosphere. The lipase preparation was separated by filtration and the solvent removed in vacuo on a rotary evaporator. The concentrate was applied to a 4% boric acid impregnated flash silica gel chromatography using petroleum ether/ethyl acetate (3:2) as an eluent. The first fraction from the column was contaminated with some oxime starting material and required a repeated chromatography. The combined fractions afforded the product (R,S')-5 as a colorless liquid in 90% yield (175 mg, 0.472 mmol). [α]20D = +25.0 (c. 14.0, CH2Cl2). IR (NaCl, νmax / cm-1): 3458 (br s), 3089 (s), 3462 (br s), 3089 (s), 3062 (s), 3028 (s), 2954 (vs), 2925 (vs), 2868 (vs), 1736 (vs), 1607. 1H NMR (400 MHz, CDCl3) δH: 7.38-7.27 (m, 5H, Ph-H), 7.21-7.16 (m, 2H, Ibu-2,6), 7.10-7.06 (m, 2H, Ibu-3,5), 4.47 (s, 2H, CH2Ph), 4.18 (dd, J=11.4, 4.7 Hz, 1H, CH2 sn-3), 4.13 (dd, J=11.4, 6.1 Hz, 1H, CH2 sn-3), 3.99-3.95 (m, 1H, CH sn-2), 3.72 (q, J=7.2 Hz, 1H, CHCH3), 3.44 (dd, J=9.6, 4.5 Hz, 1H, CH2 sn-1), 3.37 (dd, J=9.6, 5.9 Hz, 1H, CH2 sn-1), 2.44 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 1.84 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.49 (d, J=7.2 Hz, 3H, CHCH3), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.9, 140.8, 137.8, 137.7, 129.5 (2), 128.6 (2), 128.0 (2), 127.8 (2), 127.3, 73.6, 70.8, 69.0, 65.7, 45.2, 30.3, 22.5, 18.53 (2), 18.47 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C23H30O4Na 393.2036; found, 393.2030.
3.3.2. Synthesis of 3-O-benzyl-1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (S,S')-5
The same procedure was followed as described for (R,S')-5 using 3-O-benzyl-sn-glycerol (100 mg, 0.549 mmol), ibuprofen acetoxime ester (S)-3 (163 mg, 0.659 mmol), and immobilized CAL-B (45 mg). Purification on a 4% boric acid impregnated flash silica gel column using pet. ether/ethyl acetate (3:2) as eluent afforded the product (S,S')-5 as a colorless liquid in 92% yield (187 mg, 0.505 mmol). As before, the first fraction from the column was contaminated with some oxime starting material and required a repeated chromatography. [α]20D = +20.7 (c. 11.0, CH2Cl2). IR (NaCl, νmax / cm-1): 3458 (br s), 3089 (s), 3062 (s), 3028 (s), 2954 (vs), 2925 (vs), 2865 (vs), 1740 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.37-7.27 (m, 5H, Ph-H), 7.18 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.08 (d, J=8.1 Hz, 2H, Ibu-3,5), 4.47 (s, 2H, CH2Ph), 4.16 (d, J=5.2 Hz, 2H, CH2 sn-1), 3.98-3.94 (m, 1H, CH sn-2), 3.72 (q, J=7.2 Hz, 1H, CHCH3), 3.42 (dd, J=9.6, 4.5 Hz, 1H, CH2 sn-3), 3.36 (dd, J=9.6, 5.9 Hz, 1H, CH2 sn-3), 2.44 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 1.84 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.49 (d, J=7.2 Hz, 3H, CHCH3), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.9, 140.8, 137.9, 137.7, 129.5 (2), 128.6 (2), 128.0 (2), 127.9 (2), 127.3, 73.6, 70.8, 69.0, 65.6, 45.2, 30.3, 22.5, 18.52 (2), 18.46 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C23H30O4Na 393.2036; found, 393.2031.
3.3.3. Synthesis of 1-O-benzyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol, (R,S')-6
The same procedure was followed as described for (R,S')-5 using 1-O-benzyl-sn-glycerol (100 mg, 0.549 mmol), naproxen acetoxime ester (S)-4 (172 mg, 0.604 mmol), and immobilized CAL-B (38 mg). Purification on a 4% boric acid impregnated flash silica gel column using pet. ether/ethyl acetate (3:2) as eluent resulted in a first fraction contaminated with the starting material that as before required repeated chromatography. Recrystallization of the combined fractions from n-hexane afforded the product (R,S')-6 as a white solid in 69% yield (149 mg, 0.378 mmol). M.p. 51.7-52.1°C. [α]20D = +53.8 (c. 1.9, CH2Cl2). IR (NaCl, νmax / cm-1): 3538 (br s), 3057 (s), 2973 (vs), 2936 (vs), 2909 (vs), 2864 (vs), 1719 (vs), 1632 (s), 1605 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.71-7.65 (m, 3H, Nap-1,4,8), 7.38 (dd, J=8.4, 1.9 Hz, 1H, Nap-3), 7.34-7.22 (m, 5H, Ph-H), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 4.40 (s, 2H, CH2Ph), 4.19 (dd, J=11.5, 4.8 Hz, 1H, CH2 sn-3), 4.14 (dd, J=11.5, 6.0 Hz, 1H, CH2 sn-3), 4.00-3.87 (m, 1H, CH sn-2), 3.91 (s, 3H, OCH3), 3.87 (q, J=7.2 Hz, 1H, CHCH3), 3.40 (dd, J=9.6, 4.4 Hz, 1H, CH2 sn-1), 3.32 (dd, J=9.6, 6.0 Hz, 1H, CH2 sn-1), 2.31 (d, J=5.2 Hz, 1H, OH), 1.58 (d, J=7.2 Hz, 3H, CHCH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.8, 157.9, 137.8, 135.6, 133.9, 129.4, 129.1, 128.6, 128.0 (2), 127.8 (2), 127.4, 126.3, 129.1, 119.2, 105.8, 73.6, 70.9, 69.0, 65.8, 55.5, 45.5, 18.5 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C24H26O5Na 417.1672; found, 417.1663.
3.3.4. Synthesis of 3-O-benzyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol, (S,S')-6
The same procedure was followed as described for (R,S')-5 using 3-O-benzyl-sn-glycerol (76 mg, 0.417 mmol), naproxen acetoxime ester (S)-4 (172 mg, 0.439 mmol), and immobilized CAL-B (42 mg). Purification on a 4% boric acid impregnated flash silica gel column using pet. ether/ethyl acetate (3:2) as eluent resulted in a first fraction contaminated with the starting material that as before required repeated chromatography. Recrystallization of the combined fractions from n-hexane afforded the product (S,S')-6 as a white solid in 65% yield (107 mg, 0.272 mmol). M.p. 63.2-63.5°C. [α]20D = +47.7 (c. 1.7, CH2Cl2). IR (NaCl, νmax / cm-1): 3540 (br s), 3053 (s), 2972 (vs), 2940 (vs), 2904 (vs), 2862 (vs), 1718 (vs), 1630 (s), 1607 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.71-7.64 (m, 3H, Nap-1,4,8 ), 7.38 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.34-7.21 (m, 5H, Ph-H), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 4.37 (s, 2H, CH2Ph), 4.17 (d, J=5.5 Hz, 2H, CH2 sn-1), 4.00-3.90 (m, 1H, CH sn-2), 3.91 (s, 3H, OCH3), 3.88 (q, J=7.2 Hz, 1H, CHCH3), 3.37 (dd, J=9.6, 4.4 Hz, 1H, CH2 sn-3), 3.31 (dd, J=9.6, 6.2 Hz, 1H, CH2 sn-3), 2.32 (d, J=4.8 Hz, 1H, OH), 1.58 (d, J=7.2 Hz, 3H, CHCH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.8, 157.8, 137.8, 135.6, 133.9, 129.4, 129.1, 128.6, 128.0 (2), 127.8 (2), 127.4, 126.3, 126.1, 119.2, 105.8, 73.5, 70.8, 68.9, 65.7, 55.5, 45.5, 18.5 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C24H26O5Na 417.1672; found, 417.1671.
3.4. Removal of the benzyl protective group: Synthesis of (R,S')-7, (S,S')-7, (R,S')-8 and (S,S')-8
3.4.1. Synthesis of 3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (R,S')-7
Pd/C catalyst (8 mg) was placed into a 25 mL flame-dried two-necked round-bottom flask equipped with a magnetic stirrer under nitrogen atmosphere at room temperature and the flask sealed with a septum. A solution of 1-O-benzyl-3-[(S)-2-(4-isobutylphenyl)-propanoyl]-sn-glycerol (R,S')-5 (40 mg, 0.108 mmol) dissolved in dry THF (3.2 mL) was added with a syringe, followed by n-hexane (5.2 mL). A balloon filled with hydrogen gas was then mounted on a syringe and stuck through the septum. The mixture was stirred while the hydrogen gas was blown through the flask to replace the nitrogen atmosphere with hydrogen. Then a tiny drop of perchloric acid was added and the solution stirred vigorously at room temperature while being monitored with TLC. When the reaction came to completion according to the TLC (approximately 12 minutes) the flask was promptly opened and the acid neutralized by adding NaHCO3 (s). Then the solution was filtered, and the solvent removed in vacuo on a rotary evaporator. The crude product was applied to a 4% boric acid impregnated flash silica gel chromatography using petroleum ether/ethyl acetate (2:3) as eluent, wich afforded the product (R,S')-7 as a pale-yellow oil, in 98% yield (30 mg, 0.107 mmol). [α]20D = +42.9 (c. 3.5, CH2Cl2). IR (NaCl, νmax / cm-1): 3423 (br s), 3063 (s), 3025 (s), 2954 (vs), 2923 (vs), 2867 (vs), 1740 (vs) 1H NMR (400 MHz, CDCl3) δH: 7.19 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.10 (d, J=8.1 Hz, 2H, H-4,6 Ibu), 4.22 (dd, J=11.6, 4.6 Hz, 1H, CH2 sn-3), 4.10 (dd, J=11.4, 6.1 Hz, 1H, CH2 sn-3), 3.87-3.80 (m, 1H, CH sn-2), 3.74 (q, J=7.2 Hz, 1H, CHCH3), 3.57 (dd, J=11.5, 4.0 Hz, 1H, CH2 sn-1), 3.45 (dd, J=11.5, 5.6 Hz, 1H, CH2 sn-1), 3.10-2.75 (bm, 2H, OH), 2.45 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 1.85 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.51 (d, J=7.2 Hz, 3H, CHCH3), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 175.2, 140.8, 137.4, 129.5 (2), 127.1 (2), 70.2, 65.4, 63.2, 45.1, 45.1, 30.2, 22.4 (2), 18.4 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C16H24O4Na 303.1567; found, 303.1563.
3.4.2. Synthesis of 1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (S,S')-7
The same procedure was followed as described for (R,S')-7 using Pd/C (15 mg), 3-O-benzyl-1-[(S)-2-(4-isobutylphenyl)-propanoyl]-sn-glycerol (S,S')-5 (50 mg, 0.135 mmol), THF (4.0 mL) and n-hexane (6.5 mL). Purification on a 4% boric acid impregnated flash silica gel column using pet. ether/ethyl acetate (2:3) as eluent afforded the product (S,S')-7 as a colorless liquid in 93% yield (35 mg, 0.125 mmol). [α]20D = +33.9 (c. 2.0, CH2Cl2). IR (NaCl, νmax / cm-1): 3455 (br s), 3060 (s), 3028 (s), 2954 (vs), 2925 (vs), 2865 (vs), 1742 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.19 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.10 (d, J=8.1 Hz, 2H, H-4,6 Ibu), 4.22 (dd, J=11.6, 4.6 Hz, 1H, CH2 sn-1), 4.10 (dd, J=11.4, 6.2 Hz, 1H, CH2 sn-1), 3.87-3.80 (m, 1H, CH sn-2), 3.74 (q, J=7.2 Hz, 1H, CHCH3), 3.57 (dd, J=11.5, 4.0 Hz, 1H, CH2 sn-3), 3.45 (dd, J=11.5, 5.6 Hz, 1H, CH2 sn-3), 2.45 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 2.35-2.19 (bs, 1H, OH), 1.82-1.92 (bs, 1H, OH), 1.85 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.51 (d, J=7.2 Hz, 3H, CHCH3), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 175.2, 140.8, 137.5, 129.5 (2), 127.1 (2), 70.2, 65.4, 63.2, 45.1, 45.1, 30.2, 22.4 (2), 18.4 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C16H24O4Na 303.1567; found, 303.1569.
3.4.3. Synthesis of 3-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol, (R,S')-8
The same procedure was followed as described for (R,S')-7 using 1-O-benzyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol (R,S')-6 (130 mg, 0.330 mmol), THF (9.5 mL), n-hexane (16.5 mL) and Pd/C catalyst (25 mg). Purification on a 4% boric acid impregnated flash silica gel column using pet. ether/ethyl acetate (2:3) as eluent, followed by recrystallization from n-hexane, afforded the product (R,S')-8 as white, thin, needle-like crystals in 93% yield (93 mg, 0.306 mmol). M.p. 42.7-43.4°C. [α]20D = +43.5 (c. 2.2, CH2Cl2). IR (NaCl, νmax / cm-1): 3459 (br), 3058 (vs), 2980 (vs), 2940 (vs), 2878 (vs), 1732 (vs), 1634 (s), 1606 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.71-7.61 (m, 3H, Nap-1,4,8), 7.38 (dd, J=8.6, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 4.23-4.07 (m, 2H, CH2 sn-3), 3.90 (s, 3H, OCH3), 3.96-3.84 (m, 1H, CH sn-2), 3.81 (q, J=7.2 Hz, 1H, CHCH3), 3.57 (m, 1H, CH2 sn-1), 3.45 (m, 1H, CH2 sn-1), 1.59 (d, J=7.2 Hz, 3H, CHCH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 175.2, 157.9, 135.4, 133.9, 129.4, 129.0, 127.4, 126.1 (2), 119.3, 105.8, 70.2, 65.7, 63.3, 55.4, 45.5, 18.5 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C17H20O5Na 327.1203; found, 327.1201.
3.4.4. Synthesis of 1-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol, (S,S')-8
The same procedure was followed as described for (R,S')-7 using 3-O-benzyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol (S,S')-6 (126 mg, 0.319 mmol), THF (9.5 mL), n-hexane (15 mL) and Pd/C catalyst (17 mg). Purification on a 4% boric acid impregnated flash silica gel column using pet. ether/ethyl acetate (2:3) as eluent, followed by recrystallization from n-hexane, afforded the product (S,S')-8 as white solid in 99% yield (96 mg, 0.315 mmol). M.p. 58.9-59.7°C. [α]20D = +34.3 (c. 1.0, CH2Cl2). IR (NaCl, νmax / cm-1): 3455 (br s), 3056 (vs), 2982 (vs), 2945 (vs), 2874 (vs), 1734 (vs), 1633 (s), 1605 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.71-7.61 (m, 3H, Nap-1,4,8), 7.39 (dd, J=8.6, 1.5 Hz, 1H, Nap-3), 7.15 (dd, J=8.9, 2.4 Hz, 1H, Nap-7), 7.12 (d, J=2.4 Hz, 1H, Nap-5), 4.18 (d, J=4.9 Hz, 2H, CH2 sn-1), 3.92 (s, 3H, OCH3), 3.92-3.88 (m, 1H, CHCH3), 3.88-3.82 (m, 1H, CH sn-2), 3.56 (dd, J=11.1, 4.9 Hz, 1H, CH2 sn-3), 3.45 (dd, J=11.1, 5.6 Hz, 1H, CH2 sn-3), 2.30-184 (m, 2H, OH), 1.59 (d, J=7.2 Hz, 3H, CHCH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 175.2, 157.9, 135.4, 133.9, 129.4, 129.1, 127.5, 126.1 (2), 119.3, 105.8, 70.2, 65.7, 63.3, 55.5, 45.5, 18.5 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C17H20O5Na 327.1203; found, 327.1212.
3.5. The enzymatic coupling of the MCFAs: Synthesis of (R,S')-9a, (S,S')-9a, (R,S')-10a and (S,S')-10a
3.5.1. Synthesis of 1-hexanoyl-3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (R,S')-9a
Immobilized CAL-B (18 mg) was added to a solution of 3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol (R,S')-7 (37 mg, 0.132 mmol), and vinyl hexanoate (21 mg, 0.145 mmol) in CH2Cl2 (3.5 mL). The resulting mixture was stirred at room temperature for 7 h. The lipase preparation was separated by filtration and the solvent removed in vacuo on rotary evaporator. The concentrate was applied to a 4% boric acid impregnated flash silica gel chromatography using petroleum ether/ethyl acetate (7:3) as eluent. This afforded the product (R,S')-9a as a colorless liquid in 80% yield (40 mg, 0.106 mmol). [α]20D = +22.7 (c. 3.0, CH2Cl2). IR (NaCl, νmax / cm-1): 3321 (br s), 2956 (vs), 2931 (vs), 2870 (vs), 1740 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.19 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.10 (d, J=8.1 Hz, 2H, H-4,6 Ibu), 4.21-3.94 (m, 5H, CH2 sn-1/3, CH sn-2), 3.74 (q, J=7.2 Hz, 1H, CHCH3), 2.44 (d, J=6.8 Hz, 2H, CH2CH(CH3)2), 2.34-2.27 (m, 2H, CH2COO SFA), 1.84 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.67-1.57 (m, 2H, CH2CH2COO), 1.50 (d, J=7.2 Hz, 3H, CHCH3), 1.37-1.22 (m, 4H, CH2), 0.90 (t, J=6.9 Hz, 3H, CH2CH3), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.9 (C=O Ibu), 174.0 (C=O SFA), 140.9, 137.6, 129.6 (2), 127.2 (2), 68.5, 65.5, 65.0, 45.2 (2), 34.2, 31.4, 30.3, 24.7, 22.5 (2), 22.4, 18.5, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C22H34O5Na 401.2298; found, 401.2300.
3.5.2. Synthesis of 3-hexanoyl-1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (S,S')-9a
Immobilized CAL-B (17 mg) was added to a solution of 1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol (S,S')-7 (25 mg, 0.089 mmol), and vinyl hexanoate (14 mg, 0.098 mmol) in CH2Cl2 (2 mL). The resulting mixture was stirred at room temperature for 7 h. The lipase preparation was separated by filtration and the solvent removed in vacuo on rotary evaporator. The concentrate was applied to a 4% boric acid impregnated flash silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent. This afforded the product (S,S')-9a as a colorless liquid in 94% yield (32 mg, 0.085 mmol). [α]20D = +21.0 (c. 0.4, CH2Cl2). IR (NaCl, νmax / cm-1): 3465 (br s), 2975 (vs), 2941 (vs), 2864 (vs), 2834 (vs), 1738 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.20 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.10 (d, J=8.1 Hz, 2H, H-4,6 Ibu), 4.21-3.97 (m, 5H, CH2 sn-1/3, CH sn-2), 3.74 (q, J=7.2 Hz, 1H, CHCH3), 2.44 (d, J=6.8 Hz, 2H, CH2CH(CH3)2), 2.39-2.28 (m, 1H, OH), 2.34-2.27 (m, 2H, CH2COO SFA), 1.84 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.68-1.55 (m, 2H, CH2CH2COO), 1.51 (d, J=7.2 Hz, 3H, CHCH3), 1.38-1.24 (m, 4H, CH2), 0.90 (t, J=6.9 Hz, 3H, CH2CH3), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.9 (C=O Ibu), 174.0 (C=O SFA), 140.9, 137.6, 129.6 (2), 127.2 (2), 68.5, 65.4, 65.0, 45.2, 45.2, 34.2, 31.4, 30.3, 24.7, 22.5 (2), 22.4, 18.5, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C22H34O5Na 401.2298; found, 401.2294.
3.5.3. Synthesis of 1-hexanoyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol, (R,S')-10a
Immobilized CAL-B (15 mg) was added to a solution of 3-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol (R,S')-8 (27 mg, 0.089 mmol), and vinyl hexanoate (14 mg, 0.098 mmol) in CH2Cl2 (2.4 mL). The resulting mixture was stirred at room temperature for 2 h after which more CAL-B (5 mg) was added to speed up the reaction. After further 3.5 h reaction TLC monitoring indicated a complete reaction. The lipase preparation was separated by filtration and the solvent removed in vacuo on rotary evaporator. The concentrate was applied to a 4% boric acid impregnated flash silica gel chromatography using petroleum ether/ethyl acetate (7:3) as eluent. This afforded the product (R,S')-10a as a colorless liquid in 92% yield (33 mg, 0.082 mmol). [α]20D = +22.6 (c. 2.5, CH2Cl2). IR (NaCl, νmax / cm-1): 3459 (br s), 2946 (vs), 2930 (vs), 2870 (vs), 1740 (vs), 1635 (s), 1605 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.86-7.58 (m, 3H, Nap-1,4,8), 7.47-7.30 (m, 1H, Nap-3), 7.19-7.04 (m, 2H, Nap-5,7), 4.33-3.62 (m, 9H, CH2 sn-1/3, CH sn-2, OCH3, CHCH3), 2.24-2.19 (m, 3H, OH, CH2COO), 1.61-1.52 (m, 5H, CH2CH2COO, CHCH3), 1.32-1.23 (m, 4H, CH2), 0.87 (t, J=6.7 Hz, 3H, CH2CH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Nap), 173.3 (C=O SFA), 157.9, 135.4, 133.9, 129.4, 129.1, 127.4, 126.10, 126.07, 119.2, 105.8, 68.4, 65.6, 65.0, 55.4, 45.4, 34.1, 31.4, 24.7, 22.4, 18.5, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C23H30O6Na 425.1935; found, 425.1933.
3.5.4. Synthesis of 3-hexanoyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol, (S,S')-10a
Immobilized CAL-B (15 mg) was added to a solution of 1-[(S)-2-(6-methoxynaphthalen-2-yl)]-sn-glycerol (S,S')-8 (33 mg, 0.108 mmol), and vinyl hexanoate (28 mg, 0.198 mmol) in CH2Cl2 (3 mL). The resulting mixture was stirred at room temperature for 3 h after which more CAL-B (5 mg) was added to speed up the reaction. After further 5.5 h reaction TLC monitoring indicated a complete reaction. The lipase preparation was separated by filtration and the solvent removed in vacuo on rotary evaporator. The concentrate was applied to a 4% boric acid impregnated flash silica gel chromatography using petroleum ether/ethyl acetate (7:3) as eluent. This afforded the product (S,S')-10a as a colorless liquid in 70% yield (30 mg, 0.075 mmol). [α]20D = +21.5 (c. 0.6, CH2Cl2). IR (NaCl, νmax / cm-1): 3466 (br s), 2969 (vs), 2972 (vs), 1735 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.73-7.68 (m, 2H, Nap-4,8), 7.66 (d, J=1.9 Hz, 1H, Nap-1), 7.39 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.11 (d, J=2.5 Hz, 1H, Nap-5), 4.21-3.98 (m, 5H, CH2 sn-1/3, CH sn-2), 3.91 (s, 3H, OCH3), 3.90 (q, J=7.2 Hz, 1H, CHCH3), 2.28 (t, J=7.6 Hz, 2H, CH2COO), 1.61-1.57 (m, 2H, CH2CH2COO), 1.59 (d, J=7.2 Hz, 3H, CHCH3), 1.32-1.23 (m, 4H, CH2), 0.88 (t, J=6.9 Hz, 3H, CH2CH3) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Nap), 174.8 (C=O Nap), 174.0 (C=O SFA), 157.9, 135.4, 133.9, 129.4, 129.1, 127.5, 126.2, 126.1, 119.3, 105.8, 68.5, 65.6, 65.0, 55.5, 45.5, 34.2, 31.4, 24.7, 22.4, 18.6, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C23H30O6Na 425.1935; found, 425.1939.
3.6. Coupling of EPA: Synthesis of (R,S')-11a, (S,S')-11a, (R,S')-12a and (S,S')-12a
3.6.1. Synthesis of 2-[5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoyl]-1-hexanoyl-3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (R,S')-11a
To a solution of 1-hexanoyl-3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol (R,S')-9a (15 mg, 0.040 mmol) and EPA as a free acid (13 mg, 0.044 mmol) in CH2Cl2 (2 mL) were added DMAP (6 mg, 0.043 mmol) and EDCI (12 mg, 0.058 mmol). The solution was stirred on a magnetic stirrer at room temperature for 23 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (9:1) as eluent, which afforded the product (R,S')-11a as a yellow oil, in 96% yield (26 mg, 0.039 mmol). [α]20D = +8.29 (c. 2.8, CH2Cl2). IR (NaCl, νmax / cm-1): 3012 (vs), 2958 (vs), 2927 (vs), 2871 (vs), 1744 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.18 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.08 (d, J=8.1 Hz, 2H, Ibu-3,5), 5.40-5.28 (m, 10H, =CH), 5.23-5.17 (m, 1H, CH sn-2), 4.29 (dd, J=11.9, 4.4 Hz, 1H, CH2 sn-1/3), 4.21 (dd, J=11.9, 5.4 Hz, 1H, CH2 sn-1/3), 4.14 (dd, J=11.9, 5.8 Hz, 1H, CH2 sn-1/3), 4.00 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 3.70 (q, J=7.1 Hz, 1H, CHCH3), 2.89-2.77 (m, 8H, =CHCH2CH=), 2.44 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 2.31-2.20 (m, 4H, CH2COO EPA, CH2COO SFA), 2.13-2.03 (m, 4H, CH2CH2CH= and =CHCH2CH3), 1.84 (nonet, J=6.9 Hz, 1H, CH(CH3)2), 1.69-1.62 (m, 2H, CH2CH2COO EPA), 1.62-1.55 (m, 2H, CH2CH2COO SFA), 1.49 (d, J=7.1 Hz, 3H, CHCH3), 1.33-1.21 (m, 4H, CH2), 0.97 (t, J=7.5 Hz, 3H, CH3 EPA), 0.89 (d, J=6.7 Hz, 6H, CH(CH3)2), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Ibu), 173.3 (C=O SFA), 172.6 (C=O EPA), 140.8, 137.4, 132.2, 129.5 (2), 129.1, 129.0, 128.7, 128.5, 128.4, 128.3, 128.2, 128.0, 127.3 (2), 127.2, 69.1, 62.4, 62.1, 45.2, 45.1, 34.7, 34.1, 33.7, 31.4, 26.7, 26.4 (3), 25.8, 24.8, 24.7, 22.5 (2), 22.4, 20.7, 18.4, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C42H62O6Na 685.4439; found, 685.4412.
3.6.2. Synthesis of 2-[5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoyl]-3-hexanoyl-1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (S,S')-11a
To a solution of 3-hexanoyl-1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol (S,S')-9a (11 mg, 0.029 mmol) and EPA as a free acid (10 mg, 0.032 mmol) in CH2Cl2 (1.5 mL) were added DMAP (4 mg, 0.031 mmol) and EDCI (8 mg, 0.042 mmol). The solution was stirred on a magnetic stirrer at room temperature for 25 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent, which afforded the product (S,S')-11a as a yellow oil, in 89% yield (17 mg, 0.026 mmol). [α]20D = +8.27 (c. 2.2, CH2Cl2). IR (NaCl, νmax / cm-1): 3013 (vs), 2970 (vs), 2873 (vs), 2829 (vs), 1744 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.18 (d, J=8.1 Hz, 2H, Ibu-2,6), 7.08 (d, J=8.1 Hz, 2H, Ibu-3,5), 5.40-5.26 (m, 10H, =CH), 5.23-5.17 (m, 1H, CH sn-2), 4.29 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.19 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.12 (dd, J=11.9, 6.1 Hz, 1H, CH2 sn-1/3), 4.05 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 3.70 (q, J=7.1 Hz, 1H, CHCH3), 2.87-2.77 (m, 8H, =CHCH2CH=), 2.44 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 2.37-2.20 (m, 4H, CH2COO EPA, CH2COO SFA), 2.13-2.02 (m, 4H, CH2CH2CH= and =CHCH2CH3), 1.85 (nonet, J=6.9 Hz, 1H, CH(CH3)2), 1.70-1.54 (m, 4H, CH2CH2COO EPA, CH2CH2COO SFA), 1.49 (d, J=7.1 Hz, 3H, CHCH3), 1.33-1.21 (m, 4H, CH2), 0.97 (t, J=7.5 Hz, 3H, CH3 EPA), 0.89 (d, J=6.8 Hz, 6H, CH(CH3)2), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Ibu), 173.3 (C=O SFA), 172.7 (C=O EPA), 140.8, 137.5, 132.2, 129.5 (2), 129.1, 129.0, 128.7, 128.5, 128.4, 128.3, 128.2, 128.0, 127.3 (2), 127.2, 69.0, 62.6, 62.1, 45.2, 45.1, 34.7, 34.1, 33.7, 31.4, 26.7, 25.8 (3), 25.7, 24.9, 24.7, 22.5 (2), 22.4, 20.7, 18.4, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C42H62O6Na 685.4439; found, 685.4436.
3.6.3. Synthesis of 2-[5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoyl]-1-hexanoyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol, (R,S')-12a
To a solution of 1-hexanoyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol (R,S')-10a (10 mg, 0.025 mmol) and EPA as a free acid (8 mg, 0.027 mmol) in CH2Cl2 (1.3 mL) were added DMAP (3 mg, 0.027 mmol) and EDCI (8 mg, 0.037 mmol). The solution was stirred on a magnetic stirrer at room temperature for 24 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent, which afforded the product (R,S')-12a as a yellow oil, in 80% yield (14 mg, 0.020 mmol). [α]20D = +9.29 (c. 1.4, CH2Cl2). IR (NaCl, νmax / cm-1): 3013 (vs), 2970 (vs), 2940 (vs), 2853 (vs), 1743 (vs), 1635 (s), 1607 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.72-7.66 (m, 2H, Nap-4,8), 7.64 (d, J=1.9 Hz, 1H, Nap-1), 7.37 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 5.48-5.27 (m, 10H, =CH), 5.20 (m, 1H, CH sn-2), 4.30 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.22 (dd, J=11.9, 4.4 Hz, 1H CH2 sn-1/3), 4.16 (dd, J=11.9, 6.0 Hz, 1H, CH2 sn-1/3), 4.03 (dd, J=11.9, 5.8 Hz, 1H, CH2 sn-1/3), 3.90 (s, 3H, OCH3), 3.86 (q, J=7.2 Hz, 1H, CHCH3), 2.90-2.75 (m, 8H, =CHCH2CH=), 2.24 (t, J=7.5 Hz, 2H, CH2COO EPA), 2.12-2.04 (m, 2H, CH2COO SFA), 2.08 (td, J=7.4, 1.6 Hz, 2H, CH2CH2CH=), 2.05-1.96 (m, 2H, =CHCH2CH3), 1.83-1.75 (m, 2H, CH2CH2COO EPA), 1.60-1.51 (m, 5H, CH2CH2COO SFA and CHCH3), 1.34-1.21 (m, 4H, CH2), 0.98 (t, J=7.5 Hz, 3H, CH3 EPA), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.2 (C=O Nap), 173.3 (C=O SFA), 172.6 (C=O EPA), 157.9, 135.3, 133.9, 132.2, 129.4, 129.1, 129.0, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.3, 127.2, 126.3, 126.1, 119.2, 105.7, 69.0, 62.5, 62.1, 55.4, 45.5, 34.1, 33.6, 31.4, 26.4, 25.8 (3), 25.7, 24.6, 24.2, 22.4, 20.7, 18.4, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C43H58O7Na 709.4075; found, 709.4059.
3.6.4. Synthesis of 2-[5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoyl]-3-hexanoyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol, (S,S')-12a
To a solution of 3-hexanoyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol (S,S')-10a (11 mg, 0.027 mmol) and EPA as a free acid (9 mg, 0.030 mmol) in CH2Cl2 (1.3 mL) were added DMAP (4 mg, 0.029 mmol) and EDCI (8 mg, 0.040 mmol). The solution was stirred on a magnetic stirrer at room temperature for 30 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent, which afforded the product (S,S')-12a as a yellow oil, in 95% yield (18 mg, 0.026 mmol). [α]20D = +12.4 (c. 1.5, CH2Cl2). IR (NaCl, νmax / cm-1): 3012 (vs), 2962 (vs), 2934 (vs), 2873 (vs), 1743 (vs), 1635 (s), 1607 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.72-7.66 (m, 2H, Nap-4,8), 7.65 (d, J=1.9 Hz, 1H, Nap-1), 7.37 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 5.44-5.28 (m, 10H, =CH), 5.24 (m, 1H, CH sn-2), 4.30 (dd, J=11.9, 4.1 Hz, 1H, CH2 sn-1/3), 4.20 (dd, J=11.9, 4.4 Hz, 1H CH2 sn-1/3), 4.13 (dd, J=11.9, 6.3 Hz, 1H, CH2 sn-1/3), 4.06 (dd, J=11.9, 5.8 Hz, 1H, CH2 sn-1/3), 3.91 (s, 3H, OCH3), 3.86 (q, J=7.2 Hz, 1H, CHCH3), 2.89-2.75 (m, 8H, =CHCH2CH=), 2.24 (t, J=7.5 Hz, 2H, CH2COO EPA), 2.19-2.11 (m, 2H, CH2COO SFA), 2.07 (td, J=7.4, 1.4 Hz, 2H, CH2CH2CH=), 2.05-1.97 (m, 2H, =CHCH2CH3), 1.85-1.71 (m, 2H, CH2CH2COO EPA), 1.60-1.53 (m, 2H, CH2CH2COO SFA), 1.58 (d, J=7.2 Hz, 3H, CHCH3), 1.34-1.21 (m, 4H, CH2), 0.98 (t, J=7.5 Hz, 3H, CH3 EPA), 0.88 (t, J=6.9 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.2 (C=O Nap), 173.3 (C=O SFA), 172.6 (C=O EPA), 157.9, 135.4, 133.9, 132.2, 129.4, 129.1, 129.0, 128.44, 128.36, 128.3, 128.2, 128.0, 127.3, 127.2, 126.3, 126.1, 119.2, 105.7, 69.0, 62.7, 62.1, 55.4, 45.4, 34.1, 33.6, 31.4, 29.9, 26.6, 25.78, 25.75 (3), 25.7, 24.8, 24.6, 22.4, 20.7, 18.5, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C43H58O7Na 709.4059; found, 709.4059.
3.7. Coupling of DHA: Synthesis of (R,S')-13a, (S,S')-13a, (R,S')-14a and (S,S')-14a
3.7.1. Synthesis of 2-[4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyl]-1-hexanoyl-3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (R,S')-13a
To a solution of 1-hexanoyl-3-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol (R,S')-9a (15 mg, 0.040 mmol) and DHA as a free acid (15 mg, 0.044 mmol) in CH2Cl2 (2 mL) were added DMAP (6 mg, 0.043 mmol) and EDCI (12 mg, 0.058 mmol). The solution was stirred on a magnetic stirrer at room temperature for 23 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (9:1) as eluent, which afforded the product (R,S')-13a as a yellow oil, in 79% yield (22 mg, 0.032 mmol). [α]20D = +6.90 (c. 1.0, CH2Cl2). IR (NaCl, νmax / cm-1): 3013 (vs), 2954 (vs), 2925 (vs), 2854 (vs), 1743 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.18 (d, J=7.8 Hz, 2H, Ibu-2,6), 7.08 (d, J=7.8 Hz, 2H, Ibu-3,5), 5.50-5.24 (m, 12H, =CH), 5.23-5.17 (m, 1H, CH sn-2), 4.29 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.21 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.14 (dd, J=11.9, 5.7 Hz, 1H, CH2 sn-1/3), 4.01 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 3.70 (q, J=7.2 Hz, 1H, CHCH3), 2.89-2.79 (m, 10H, =CHCH2CH=), 2.44 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 2.37-2.19 (m, 6H, CH2CH2COO DHA, CH2COO SFA), 2.08 (quint., J=7.6 Hz, 2H, =CHCH2CH3), 1.83 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.62-1.56 (m, 2H, CH2CH2COO SFA), 1.49 (d, J=7.2 Hz, 3H, CHCH3), 1.36-1.13 (m, 4H, CH2), 0.97 (t, J=7.5 Hz, 3H, CH3 DHA), 0.89 (d, J=6.4 Hz, 6H, CH(CH3)2), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Ibu), 173.3 (C=O SFA), 172.9 (C=O DHA), 140.8, 137.4, 132.2, 129.5 (2), 128.7, 128.5, 128.4, 128.3, 128.2, 128.1, 128.0, 127.9, 127.8, 127.6 (2), 127.3, 127.2, 69.00, 62.5, 62.1, 45.2, 45.1, 34.2, 34.1, 31.4, 30.3, 25.8 (3), 25.7, 25.5, 24.7, 22.7, 22.5 (2), 22.4, 20.7, 18.4, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C44H64O6Na 711.4595; found, 711.4577.
3.7.2. Synthesis of 2-[4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyl]-3-hexanoyl-1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol, (S,S')-13a
To a solution of 3-hexanoyl-1-[(S)-2-(4-isobutylphenyl)propanoyl]-sn-glycerol (S,S')-9a (11 mg, 0.029 mmol) and DHA as a free acid (11 mg, 0.032 mmol) in CH2Cl2 (1.5 mL) were added DMAP (4 mg, 0.031 mmol) and EDCI (8 mg, 0.042 mmol). The solution was stirred on a magnetic stirrer at room temperature for 25 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent, which afforded the product (S,S')-13a as a yellow oil, in 65% yield (13 mg, 0.019 mmol). [α]20D = +8.22 (c. 0.9, CH2Cl2). IR (NaCl, νmax / cm-1): 3013 (vs), 2972 (vs), 2874 (vs), 1748 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.18 (d, J=7.8 Hz, 2H, Ibu-2,6), 7.08 (d, J=7.8 Hz, 2H, Ibu-3,5), 5.46-5.27 (m, 12H, =CH), 5.23 (tt, J=6.0, 4.3 Hz, 1H, CH sn-2), 4.29 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.19 (dd, J=11.9, 4.3 Hz, 1H, CH2 sn-1/3), 4.12 (dd, J=11.9, 6.1 Hz, 1H, CH2 sn-1/3), 4.05 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 3.70 (q, J=7.2 Hz, 1H, CHCH3), 2.90-2.80 (m, 10H, =CHCH2CH=), 2.44 (d, J=7.2 Hz, 2H, CH2CH(CH3)2), 2.39-2.18 (m, 6H, CH2CH2COO DHA, CH2COO SFA), 2.13-2.03 (m, 2H, =CHCH2CH3), 1.84 (nonet, J=6.8 Hz, 1H, CH(CH3)2), 1.64-1.57 (m, 2H, CH2CH2COO SFA), 1.49 (d, J=7.2 Hz, 3H, CHCH3), 1.36-1.23 (m, 4H, CH2), 0.97 (t, J=7.5 Hz, 3H, CH3 DHA), 0.89 (d, J=6.4 Hz, 6H, CH(CH3)2), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Ibu), 173.3 (C=O SFA), 172.2 (C=O DHA), 140.9, 137.5, 132.2, 129.5 (2), 128.7, 128.5, 128.4, 128.3, 128.2, 128.1, 128.0, 127.9, 127.8, 127.6 (2), 127.3, 127.2, 69.1, 62.5, 62.1, 45.2, 45.1, 34.2, 34.1, 31.4, 30.3, 25.8 (3), 25.8, 25.7, 24.7, 22.8, 22.5 (2), 22.4, 20.7, 18.4, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C44H64O6Na 711.4595; found, 711.4581.
3.7.3. Synthesis of 2-[4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyl]-1-hexanoyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol, (R,S')-14a
To a solution of 1-hexanoyl-3-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol (R,S')-10a (10 mg, 0.025 mmol) and DHA as a free acid (9 mg, 0.027 mmol) in CH2Cl2 (1.3 mL) were added DMAP (3 mg, 0.027 mmol) and EDCI (8 mg, 0.037 mmol). The solution was stirred on a magnetic stirrer at room temperature for 24 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent, which afforded the product (R,S')-14a as a yellow oil, in 84% yield (15 mg, 0.021 mmol). [α]20D = +8.00 (c. 1.5, CH2Cl2). IR (NaCl, νmax / cm-1): 3009 (vs), 2979 (vs), 2941 (vs), 2837 (vs), 1740 (vs), 1634 (s), 1609 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.72-7.66 (m, 2H, Nap-4,8), 7.65 (d, J=1.9 Hz, 1H, Nap-1), 7.37 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 5.44-5.24 (m, 12H, =CH), 5.21 (tt, J=5.9, 4.5 Hz, 1H, CH sn-2), 4.30 (dd, J=11.9, 4.4 Hz, 1H, CH2 sn-1/3), 4.21 (dd, J=11.9, 4.4 Hz, 1H, CH2 sn-1/3), 4.16 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 4.03 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 3.91 (s, 3H, OCH3), 3.87 (q, J=7.2 Hz, 1H, CHCH3), 2.89-2.76 (m, 10H, =CHCH2CH=), 2.31-2.16 (m, 6H, CH2CH2COO DHA, =CHCH2CH3), 2.13-2.03 (m, 2H, CH2COO SFA), 1.63-1.50 (m, 2H, CH2CH2COO SFA), 1.58 (d, J=7.1 Hz, 3H, CHCH3), 1.33-1.20 (m, 4H, CH2), 0.97 (t, J=7.5 Hz, 3H, CH3 DHA), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.2 (C=O Nap), 173.3 (C=O SFA), 172.1 (C=O DHA), 157.9, 135.3, 133.9, 132.2, 129.5, 129.4, 129.1, 128.7, 128.47 (2), 128.45, 128.4, 128.3, 128.23, 128.16, 128.0, 127.8, 127.3, 127.2, 126.3, 126.1, 119.2, 105.8, 69.2, 62.5, 62.1, 55.5, 45.5, 34.1, 34.0, 31.4, 25.8 (3), 25.73, 25.70, 24.6, 22.7, 22.4, 20.7, 18.4, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C45H60O7Na 735.4231; found, 735.4210.
3.7.4. Synthesis of 2-[4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyl]-3-hexanoyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol, (S,S')-14a
To a solution of 3-hexanoyl-1-[(S)-2-(6-methoxynaphthalen-2-yl)propanoyl]-sn-glycerol (S,S')-10a (11 mg, 0.027 mmol) and DHA as a free acid (10 mg, 0.030 mmol) in CH2Cl2 (1.3 mL) were added DMAP (4 mg, 0.029 mmol) and EDCI (8 mg, 0.037 mmol). The solution was stirred on a magnetic stirrer at room temperature for 30 h. The reaction was disconnected by passing the reaction mixture through a short column packed with silica gel by use of Et2O/CH2Cl2 (1:9). The solvent was removed in vacuo on a rotary evaporator. The residue was applied to a silica gel chromatography using petroleum ether/ethyl acetate (4:1) as eluent, which afforded the product (S,S')-14a as a yellow oil, in 79% yield (15 mg, 0.021 mmol). [α]20D = +12.6 (c. 0.5, CH2Cl2). IR (NaCl, νmax / cm-1): 3012 (vs), 2977 (vs), 2941 (vs), 2878 (vs), 2834 (vs), 1741 (vs), 1635 (s), 1607 (vs). 1H NMR (400 MHz, CDCl3) δH: 7.72-7.66 (m, 2H, Nap-4,8), 7.65 (d, J=1.9 Hz, 1H, Nap-1), 7.37 (dd, J=8.5, 1.9 Hz, 1H, Nap-3), 7.14 (dd, J=8.9, 2.5 Hz, 1H, Nap-7), 7.10 (d, J=2.5 Hz, 1H, Nap-5), 5.44-5.24 (m, 12H, =CH), 5.21 (tt, J=6.0, 4.4 Hz, 1H, CH sn-2), 4.30 (dd, J=11.9, 4.2 Hz, 1H, CH2 sn-1/3), 4.19 (dd, J=11.9, 4.5 Hz, 1H, CH2 sn-1/3), 4.13 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 4.03 (dd, J=11.9, 5.9 Hz, 1H, CH2 sn-1/3), 3.91 (s, 3H, OCH3), 3.85 (q, J=7.1 Hz, 1H, CHCH3), 2.89-2.77 (m, 10H, =CHCH2CH=), 2.30-2.16 (m, 6H, CH2CH2COO DHA, =CHCH2CH3), 2.10-2.03 (m, 2H, CH2COO SFA), 1.60-1.52 (m, 2H, CH2CH2COO SFA), 1.58 (d, J=7.1 Hz, 3H, CHCH3), 1.33-1.20 (m, 4H, CH2), 0.97 (t, J=7.5 Hz, 3H, CH3 DHA), 0.88 (t, J=7.0 Hz, 3H, CH3 SFA) ppm. 13C{H} NMR (101 MHz, CDCl3) δC: 174.3 (C=O Nap), 173.3 (C=O SFA), 172.2 (C=O DHA), 157.9, 135.3, 133.9, 132.2, 129.5, 129.4, 129.1, 128.7, 128.5 (2), 128.5, 128.4, 128.3, 128.2, 128.0, 127.8, 127.4, 127.2, 126.3, 126.1, 119.2, 105.8, 69.1, 62.7, 62.1, 55.5, 45.4, 34.1, 34.0, 31.4, 25.8 (3), 25.74, 25.71, 24.6, 22.7, 22.4, 20.7, 18.5, 14.4, 14.0 ppm. HRMS (ESI) m/z: [M + Na]+ calcd for C45H60O7Na 735.4231; found, 735.4217.