Submitted:
21 January 2025
Posted:
22 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Bacterial Strains
2.2. Extraction of Surfactin
2.3. Cell Culture
2.4. Experimental Design
2.5. Sampling and Histology Detection
2.6. Cytokines Detection
2.7. RNA Isolation and Gene Expression
2.8. Western Blotting Assay
2.9. Immunofluorescence Assay
2.10. ROS Detection
2.11. Antioxidant Enzyme Detection
2.12. Statistical Analysis
3. Results
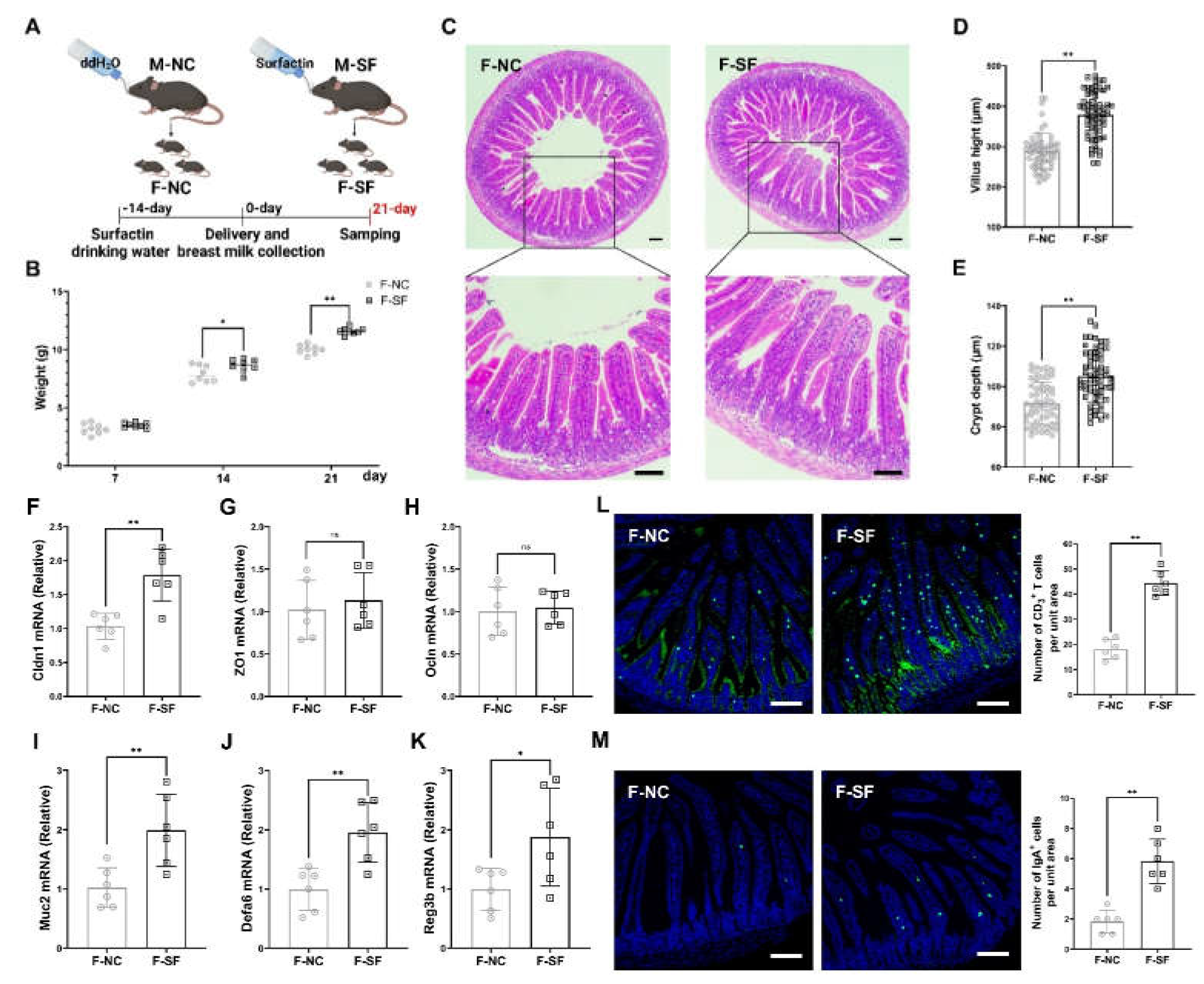
3.1. Maternal Surfactin Administration Enhances Offspring Intestinal Development and Intestinal Innate Mucosal Immunity
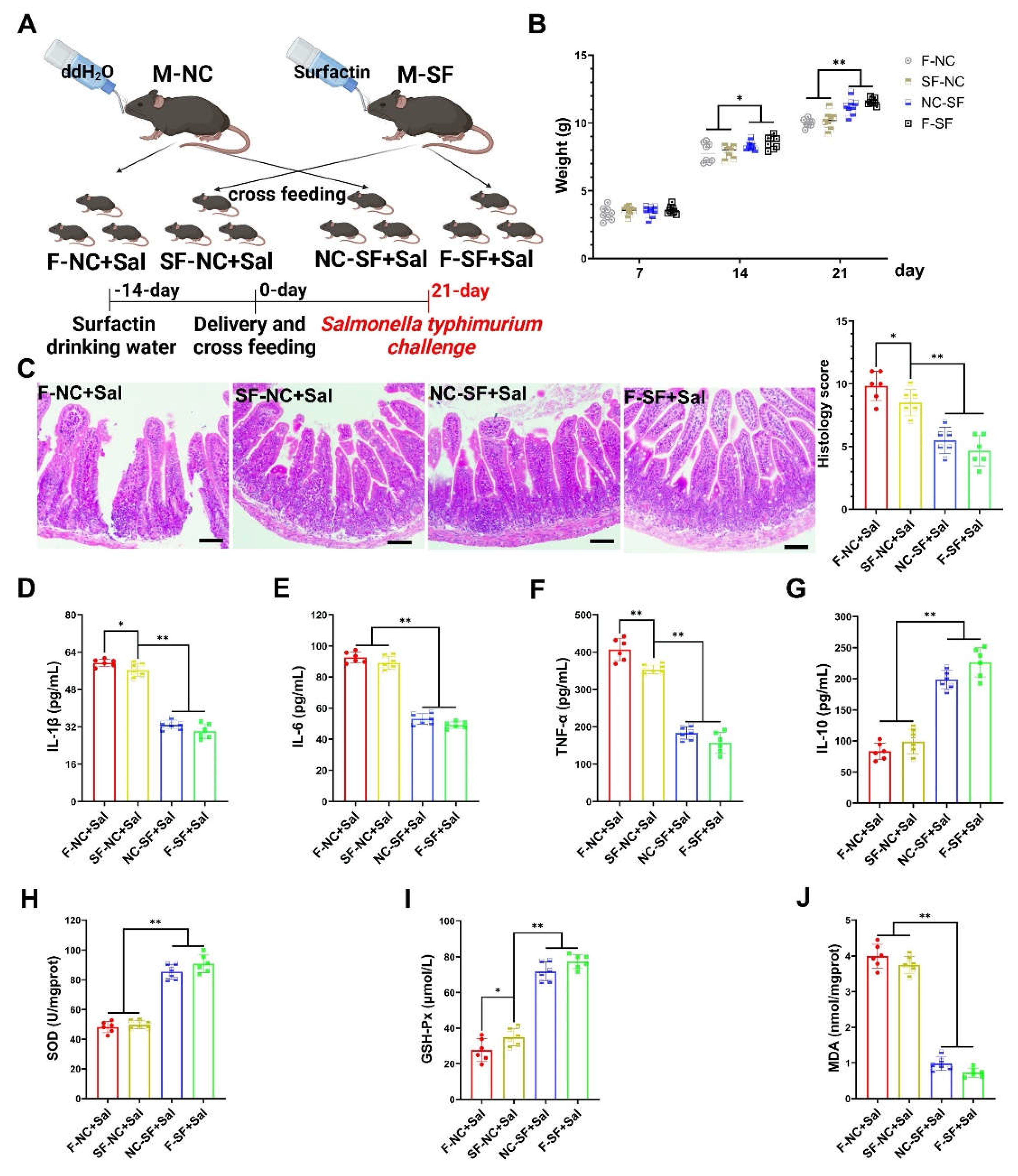
3.2. Maternal Surfactin Administration Mitigates Intestinal Inflammatory Injury in Offspring
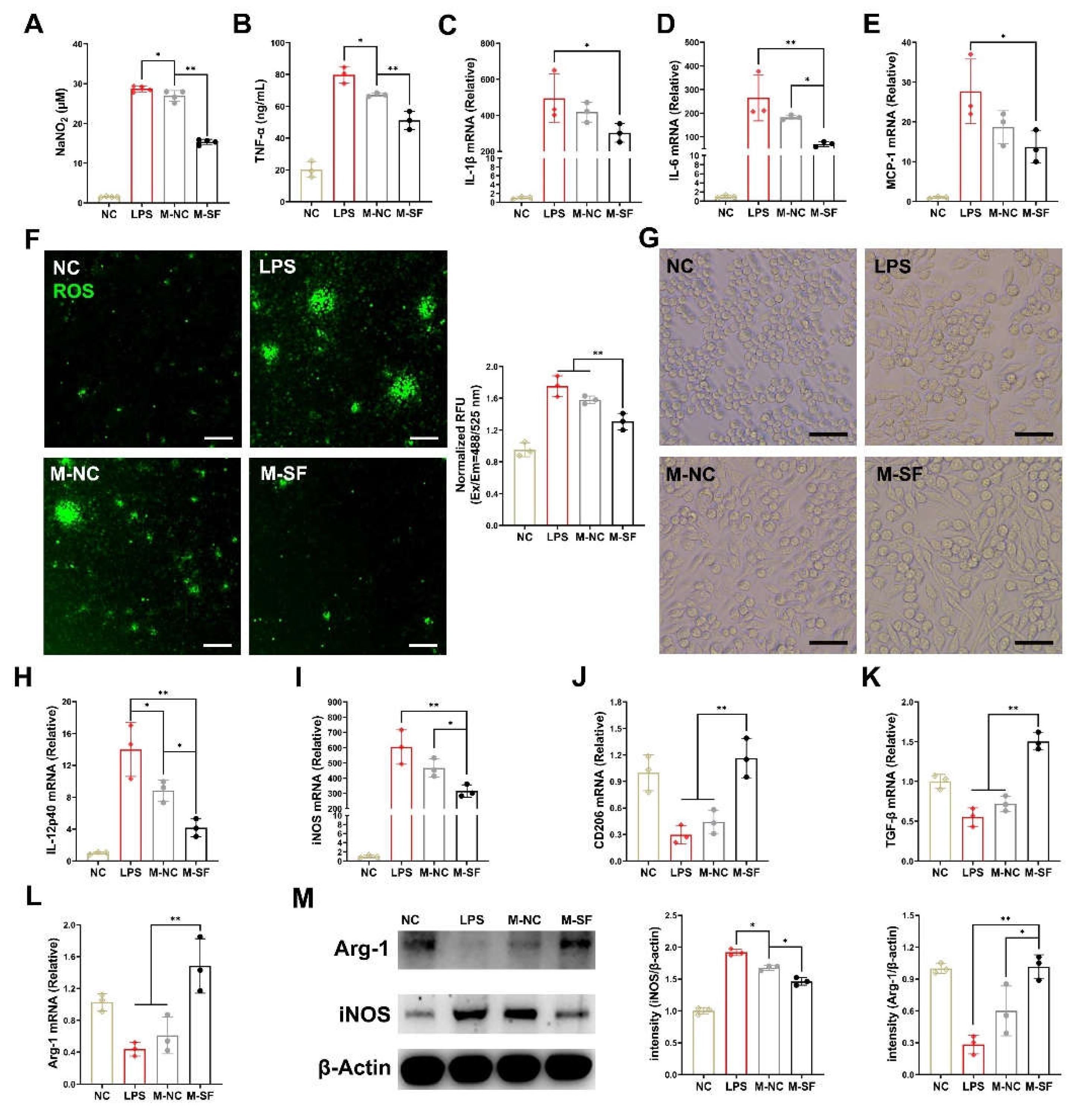
3.3. Breast Milk from Surfactin-Fed Dams Ameliorates Offspring's Inflammatory and Oxidative Stress via Macrophage Polarization Regulation
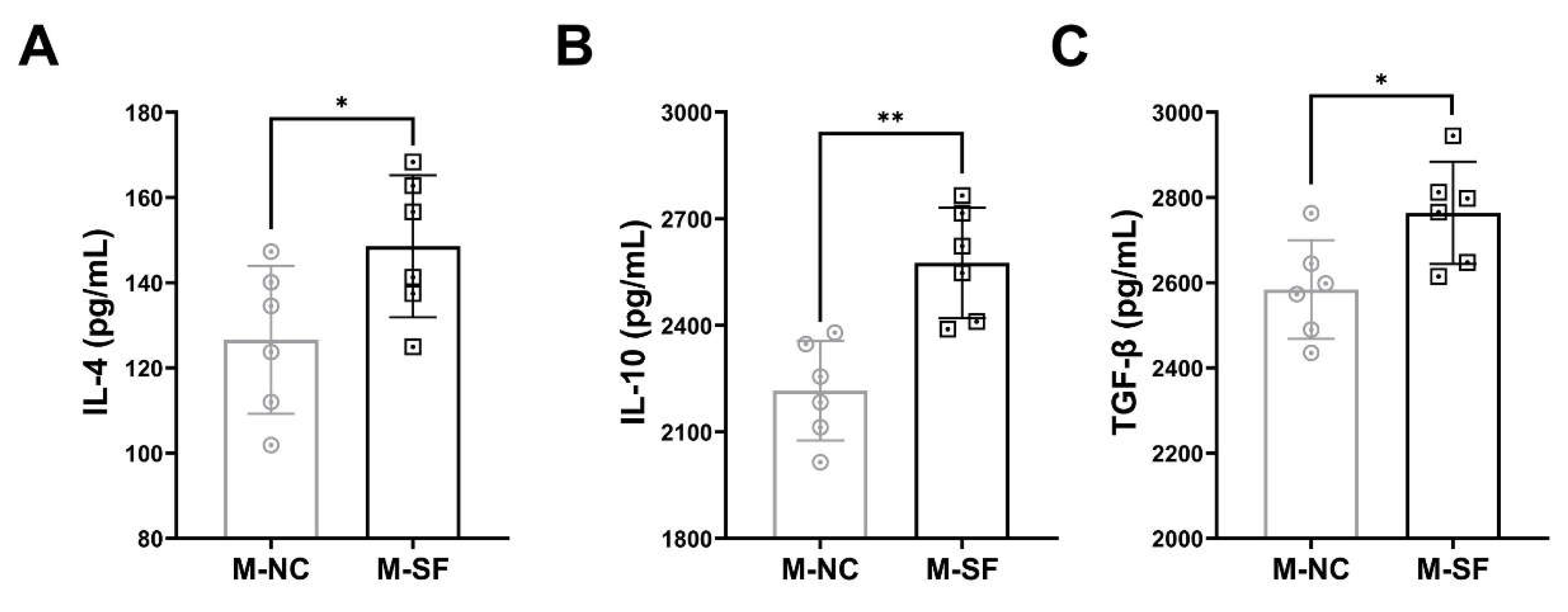
3.4. Impact of Maternal Surfactin Feeding on the Content of Anti-Inflammatory Factors in Breast Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E.; Child Health Epidemiology Reference Group of the World Health, O.; Unicef. Global causes of diarrheal disease mortality in children . PLoS ONE 2013, 8, e72788. [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Hashimoto-Hill, S.; Alenghat, T. Neonatal microbiota-epithelial interactions that impact infection. Front Microbiol 2022, 13, 955051. [Google Scholar] [CrossRef] [PubMed]
- Torow, N.; Marsland, B.J.; Hornef, M.W.; Gollwitzer, E.S. Neonatal mucosal immunology. Mucosal Immunol 2017, 10, 5–17. [Google Scholar] [CrossRef]
- Westrom, B.; Arevalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Perez-Cano, F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front Immunol 2020, 11, 1153. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front Immunol 2017, 8, 584. [Google Scholar] [CrossRef]
- Cederlund, A.; Kai-Larsen, Y.; Printz, G.; Yoshio, H.; Alvelius, G.; Lagercrantz, H.; Stromberg, R.; Jornvall, H.; Gudmundsson, G.H.; Agerberth, B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS ONE 2013, 8, e53876. [Google Scholar] [CrossRef]
- Garofalo, R. Cytokines in human milk. J Pediatr 2010, 156, S36–S40. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Grossi, E.; Drago, L. Role of the Human Breast Milk-Associated Microbiota on the Newborns' Immune System: A Mini Review. Front Microbiol 2017, 8, 2100. [Google Scholar] [CrossRef]
- Karcz, K.; Krolak-Olejnik, B. Vegan or vegetarian diet and breast milk composition - a systematic review. Crit Rev Food Sci Nutr 2021, 61, 1081–1098. [Google Scholar] [CrossRef]
- Petersohn, I.; Hellinga, A.H.; van Lee, L.; Keukens, N.; Bont, L.; Hettinga, K.A.; Feskens, E.J.M.; Brouwer-Brolsma, E.M. Maternal diet and human milk composition: An updated systematic review. Front Nutr 2023, 10, 1320560. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Park, S.C. Immunomodulatory Role of Microbial Surfactants, with Special Emphasis on Fish. Int J Mol Sci 2020, 21, 7004. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Gao, Z.Q.; Zhao, X.Y.; Qi, G.F. Surfactin inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and inflammasomes in macrophages for adjuvant activity. Sci Rep-Uk 2016, 6, 39303. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Liu, H.F.; Wang, X.Q.; Yang, Q. Surfactin induces maturation of dendritic cells. Bioscience Rep 2016, 36, e00387. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Sakamoto, K.; Zeng, M.Y.; Wang, Y.; Hakim, J.; Matus-Acuna, V.; Inohara, N.; Nunez, G. Maternal Immunization Confers Protection to the Offspring against an Attaching and Effacing Pathogen through Delivery of IgG in Breast Milk. Cell Host Microbe 2019, 25, 313–323 e314. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. International journal of clinical and experimental pathology 2014, 7, 4557–4576. [Google Scholar]
- Mirpuri, J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: Implications for public health initiatives. Pediatr Res 2021, 89, 301–306. [Google Scholar] [CrossRef]
- Noel, G.; In, J.G.; Lemme-Dumit, J.M.; DeVine, L.R.; Cole, R.N.; Guerrerio, A.L.; Campbell, J.D.; Kovbasnjuk, O.; Pasetti, M.F. Human Breast Milk Enhances Intestinal Mucosal Barrier Function and Innate Immunity in a Healthy Pediatric Human Enteroid Model. Front Cell Dev Biol 2021, 9, 685171. [Google Scholar] [CrossRef]
- Hu, G.; Su, Y.; Kang, B.H.; Fan, Z.; Dong, T.; Brown, D.R.; Cheah, J.; Wittrup, K.D.; Chen, J. High-throughput phenotypic screen and transcriptional analysis identify new compounds and targets for macrophage reprogramming. Nat Commun 2021, 12, 773. [Google Scholar] [CrossRef]
- Denizli, M.; Capitano, M.L.; Kua, K.L. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front Cell Infect Microbiol 2022, 12, 940937. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.H.; Seo, W.T.; Yun, H.D.; Hong, S.Y.; Kim, M.K.; Cho, K.M. The production of surfactin during the fermentation of by potential probiotic CSY191 and the resultant growth suppression of MCF-7 human breast cancer cells. Food Chem 2012, 131, 1347–1354. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhao, H.Y.; Meng, F.Q.; Zhou, L.B.; Pang, X.Y.; Lu, Z.X.; Lu, Y.J. Ameliorated effects of a lipopeptide surfactin on insulin resistance in vitro and in vivo. Food Sci Nutr 2022, 10, 2455–2469. [Google Scholar] [CrossRef] [PubMed]
- Pastva, A.M.; Wright, J.R.; Williams, K.L. Immunomodulatory roles of surfactant proteins A and D: Implications in lung disease. Proc Am Thorac Soc 2007, 4, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lunn, P.G. The impact of infection and nutrition on gut function and growth in childhood. P Nutr Soc 2000, 59, 147–154. [Google Scholar] [CrossRef]
- Muhardi, L.; Delsing, D.J.M.; Zakharova, I.; Huysentruyt, K.; Chong, S.Y.; Ng, R.T.; Darma, A.; Hegar, B.; Hasosah, M.; Toro-Monjaraz, E.; et al. Early-Life Gut Health Indicators and Reported Prevalence of Infant Functional Constipation by Healthcare Professionals. Nutrients 2023, 15, 298. [Google Scholar] [CrossRef]
- Prentice, S. They Are what You eat: Can Nutritional Factors during Gestation and early infancy Modulate the Neonatal immune Response? Frontiers in Immunology 2017, 8, 1641. [Google Scholar] [CrossRef]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci Rep-Uk 2017, 7, 45274. [Google Scholar] [CrossRef]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants' Gut and Immune Health. Front Immunol 2021, 12, 604080. [Google Scholar] [CrossRef]
- Ramanan, D.; Sefik, E.; Galvan-Pena, S.; Wu, M.; Yang, L.; Yang, Z.; Kostic, A.; Golovkina, T.V.; Kasper, D.L.; Mathis, D.; et al. An Immunologic Mode of Multigenerational Transmission Governs a Gut Treg Setpoint. Cell 2020, 181, 1276–1290 e1213. [Google Scholar] [CrossRef]
- Chen, S.Z.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.Z.; Xiao, G.G.; Rao, L.; Duo, Y.H. Macrophages in immunoregulation and therapeutics. Signal Transduct Tar 2023, 8, 207. [Google Scholar] [CrossRef]
- Kühl, A.A.; Erben, U.; Kredel, L.I.; Siegmund, B. Diversity of intestinal Macrophages in inflammatory Bowel Diseases. Frontiers in Immunology 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. P Natl Acad Sci USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, S.M.; Wu, Z.H.; Li, T.T.; Li, N.; Zhang, B.; Han, D.D.; Wang, S.L.; Zhao, J.C.; Wang, J.J. Cohousing-mediated microbiota transfer from milk bioactive components-dosed mice ameliorate colitis by remodeling colonic mucus barrier and lamina propria macrophages. Gut Microbes 2021, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, S.Y.; Liu, B.J.; Wang, F.Q.; Lu, Z.Q.; Jin, M.L.; Wang, Y.Z. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef]
| Gene | Sequence (5′-3′) |
| Cldn1 | F- GGGGACAACATCGTGACCG |
| R- AGGAGTCGAAGACTTTGCACT | |
| ZO1 | F- ACCACCAACCCGAGAAGAC |
| R- CAGGAGTCATGGACGCACA | |
| Ocln | F- TTGAAAGTCCACCTCCTTACAGA |
| R- CCGGATAAAAAGAGTACGCTGG | |
| Muc2 | F- TGACGTCTGGTGGAATGGTG |
| R- CAGCGTAGTTGGCACTCTCA | |
| Defa6 | F- CCTTCCAGGTCCAGGCTGAT |
| R- TGAGAAGTGGTCATCAGGCAC | |
| Reg3b | F- ACTCCCTGAAGAATATACCCTCC |
| R- CGCTATTGAGCACAGATACGAG | |
| IL-1β | F- AGTTGACGGACCCCAAAAG |
| R- TTTGAAGCTGGATGCTCTCAT | |
| IL-6 | F- CCAAGAGGTGAGTGCTTCCC |
| R- CTGTTGTTCAGACTCTCTCCCT | |
| MCP-1 | F- AGCCAACTCTCACTGAAGCC |
| R- GGACCCATTCCTTCTTGGGG | |
| iNOS | F- GGAGTGACGGCAAACATGACT |
| R- TCGATGCACAACTGGGTGAAC | |
| IL-12p40 | F- CGCCACACAAATGGATGCAA |
| R- TGTGTCCTGAGGTAGCCGTA | |
| CD206 | F- CTCTGTTCAGCTATTGGACGC |
| R- CGGAATTTCTGGGATTCAGCTTC | |
| TGF-β | F- TTGGATTGCCAGTGCTAACCC |
| R- AACAAGCCACAGTAACATGACA | |
| Arg-1 | F- CGTTGTATGATGCACAGCCG |
| R- CCCCACCCAGTGATCTTGAC | |
| β-Actin | F- GGCTGTATTCCCCTCCATCG |
| R- CCAGTTGGTAACAATGCCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





