Submitted:
17 January 2025
Posted:
20 January 2025
You are already at the latest version
Abstract
Sirtuin-1 (SIRT1), a histone deacetylase enzyme expressed in ocular tissues with intracellular localization, plays a protective role against multiple ocular diseases such as retinal degeneration, cataracts, and optic neuritis. The correlation between diabetic retinopathy (DR) and decreased levels of SIRT1 has stimulated investigation into natural therapeutic compounds that function as agonists. Curcumin (CUR), which upregulates SIRT1 protein expression, has been identified as a molecule with agonist properties. However, the challenge lies in delivering CUR effectively to the deeper layers of the eye, as retinal, due to its poor solubility and inadequate ocular penetration after topical administration. Within this context, the development of self-nanoemulsifying drug delivery systems (SNEDDS) for CUR topical ocular delivery can represent a novel approach. In accordance with our prior research, optimized SNEDDS loaded with CUR were characterized post-reconstitution with simulated tear fluid (STF) at a 1:10 ratio.Optimal parameters were achieved, ensuring mean globule size<50 nm, PDI <0.2, emulsification time <40 sec, clear appearance (transmittance >95%), viscosity, pH, and osmolarity close to physiological values. An entrapment efficiency (EE%) of approximately 99% and absence of drug precipitation were noted upon resuspension with STF. CUR loaded in these systems exhibited better stability than free CUR up to 1 week exposed to different conditions and demonstrated burst release following sustained release under simulated ocular conditions. Mucoadhesion studies were performed in STF for both CUR-SNEDDS and CUR-SNEDDS positively charged with dimethyldidodecylammonium bromide (DDAB). SNEDDS exhibited no adverse effects on the viability of human corneal epithelial (HCE) cells up to 3 μM and demonstrated superior antioxidant activity compared to neat CUR on retinal cells (ARPE-19) exposed to hydroquinone. Cell uptake studies confirmed enhanced accumulation of CUR within retinal cells (around 20% of CUR internalized) following exposure to CUR-SNEDDS compared to neat CUR (0-0.5% of CUR internalized). CUR-SNEDDS, at lower concentrations, were found to effectively induce SIRT1 expression. The cytocompatibility, antioxidant properties, and enhanced cellular uptake suggest that these developed systems hold promise as formulations for the delivery of CUR to the retina.
Keywords:
1. Introduction
2. Results
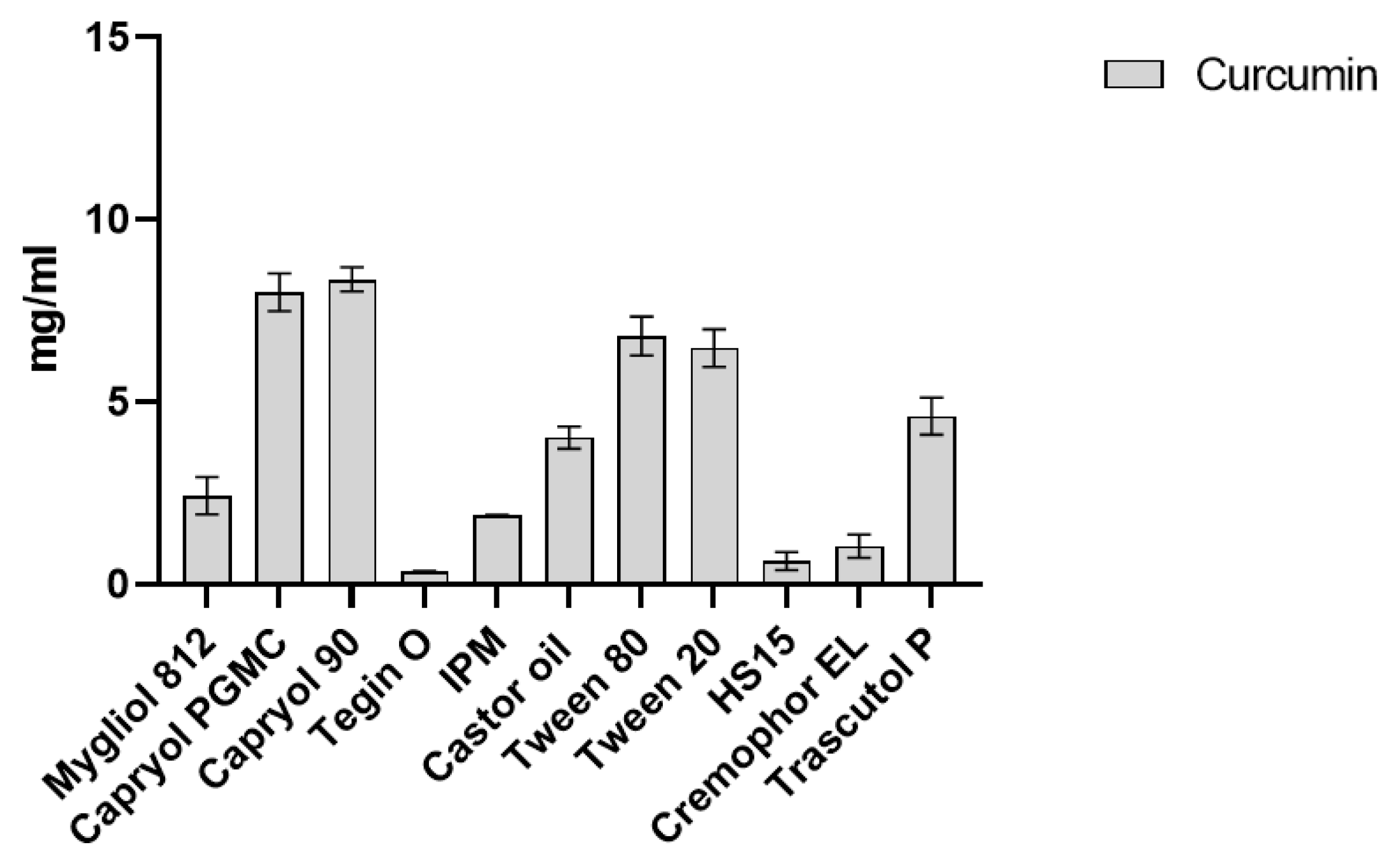
2.1. CUR Solubility in Different Vehicles
2.2. Physicochemical Characterization
| Sample | Size (nm) ± SD | PDI ± SD | ZP ± SD | Trasmittance (%) | Time of emulsific (sec.) |
|---|---|---|---|---|---|
| A | 13.26 ± 0.07 | 0.105 ± 0.017 | -4.02 ± 1.02 | 100 | ~12.04 |
| AC | 13.44 ± 0.19 | 0.095 ± 0.021 | -4.12 ± 0.88 | 99 | ~31.22 |
| AC+ | 14.02 ± 0.22 | 0.112 ± 0.032 | +2.92 ± 0.09 | 99 | ~32.44 |
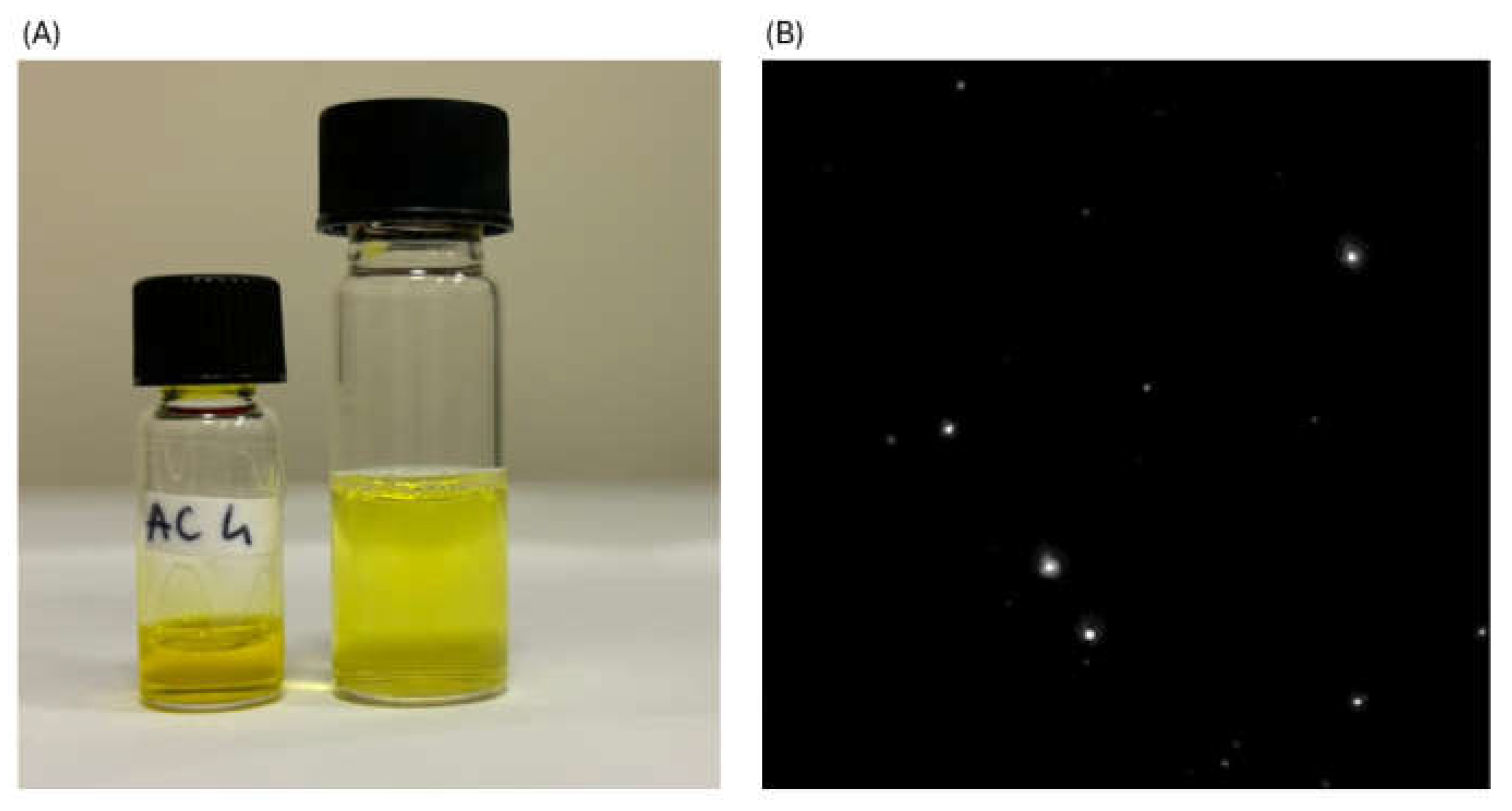
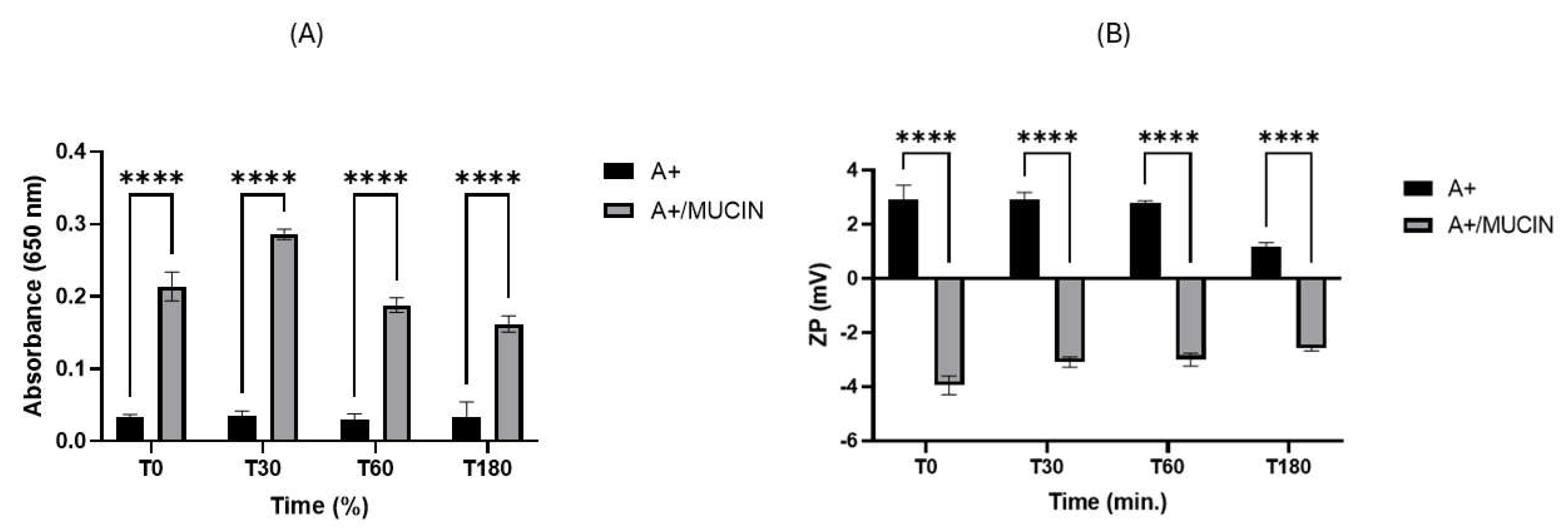
2.3. Mucoadhesive Properties of Cationic CUR-SNEDDS
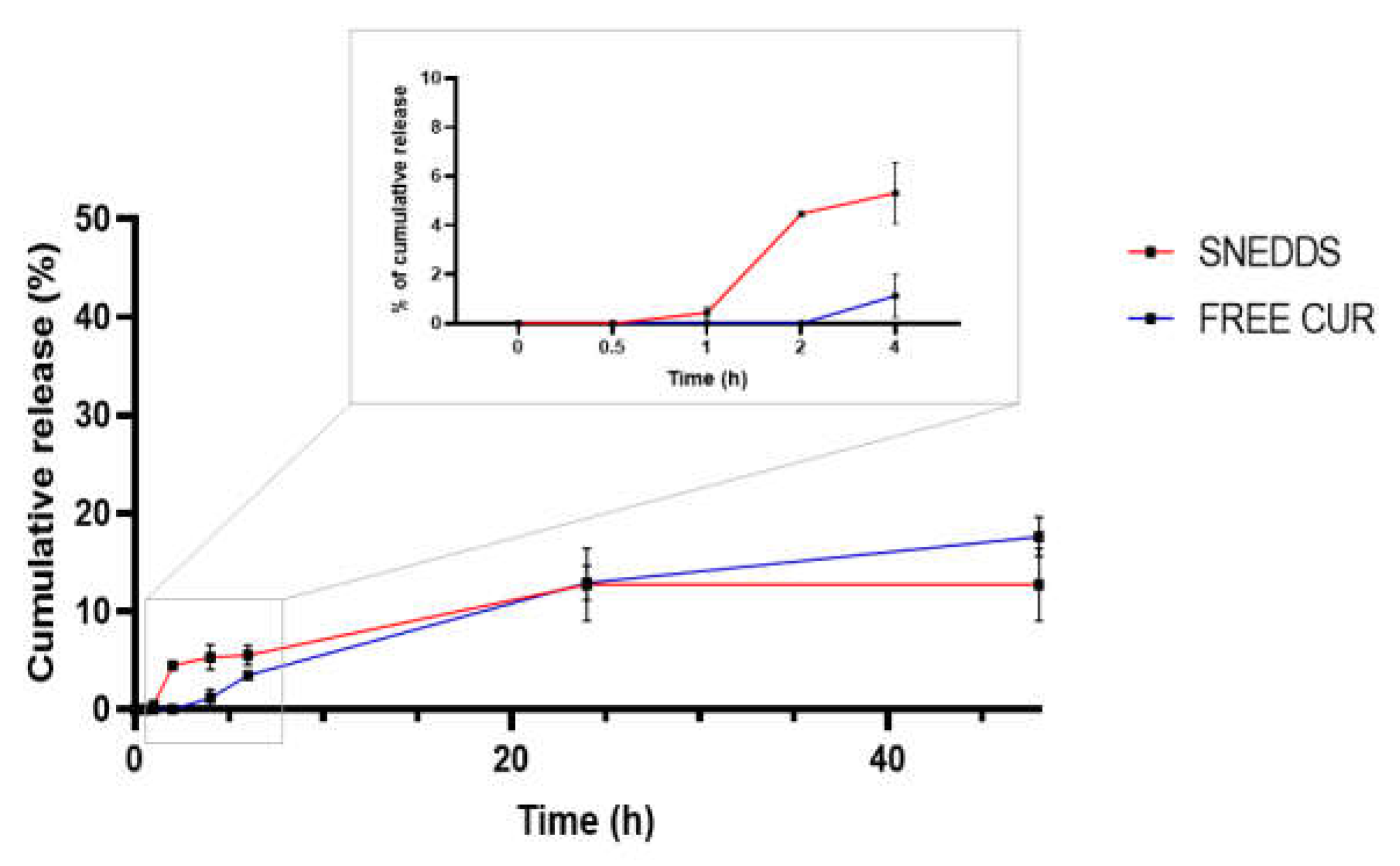
2.4. EE% and In Vitro Release
2.5. Stability Studies
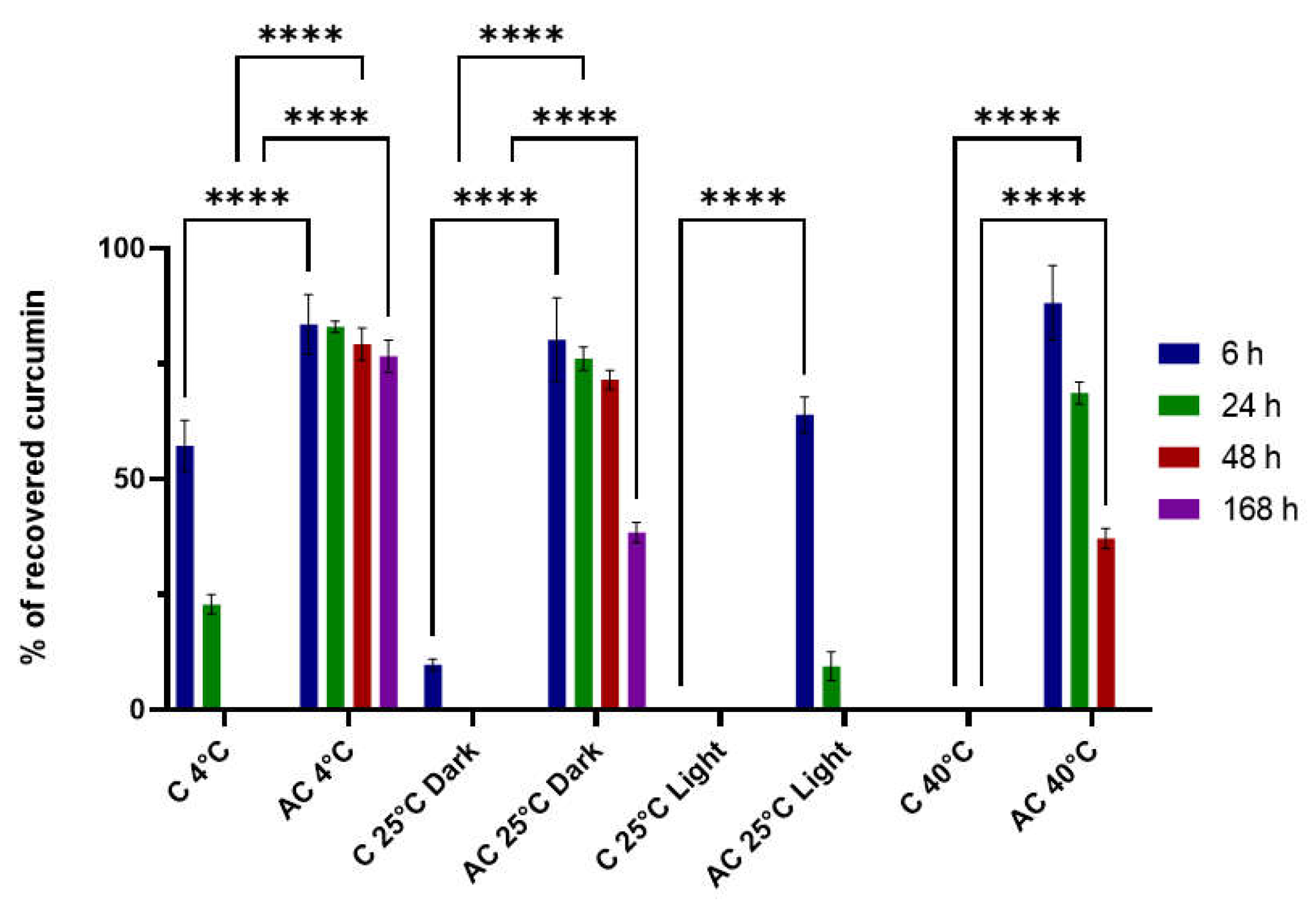
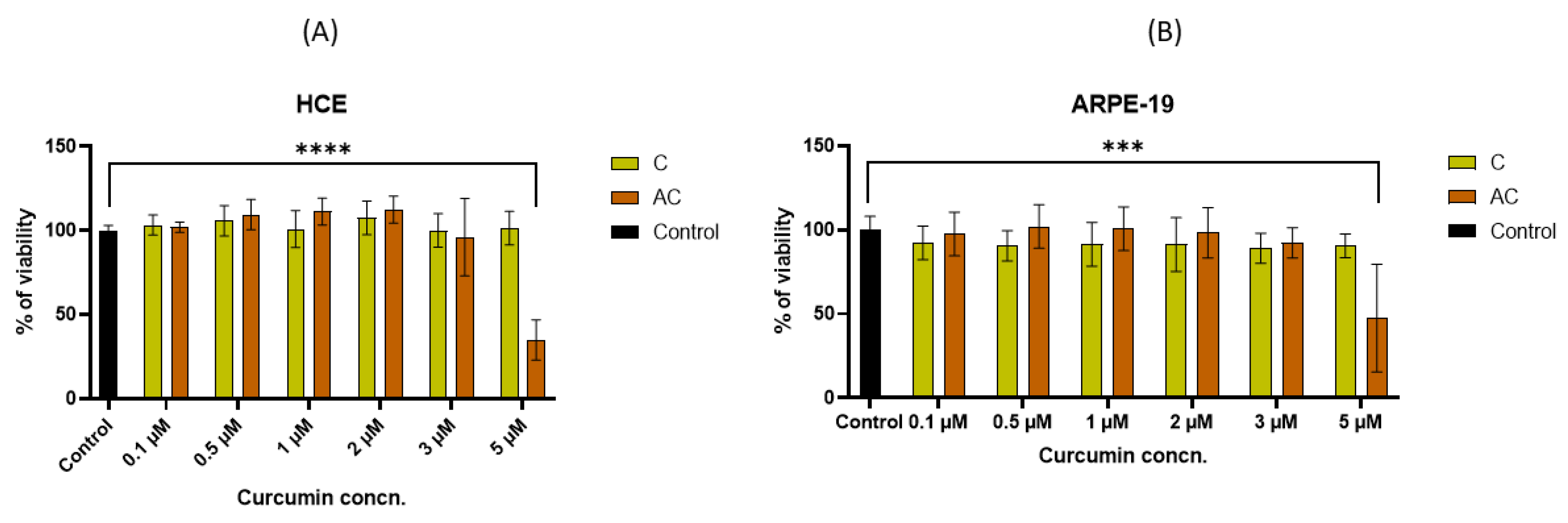
2.6. Cytocompatibility Studies
2.7. Curcumin Uptake in ARPE-19 Cells and SIRT1 Expression
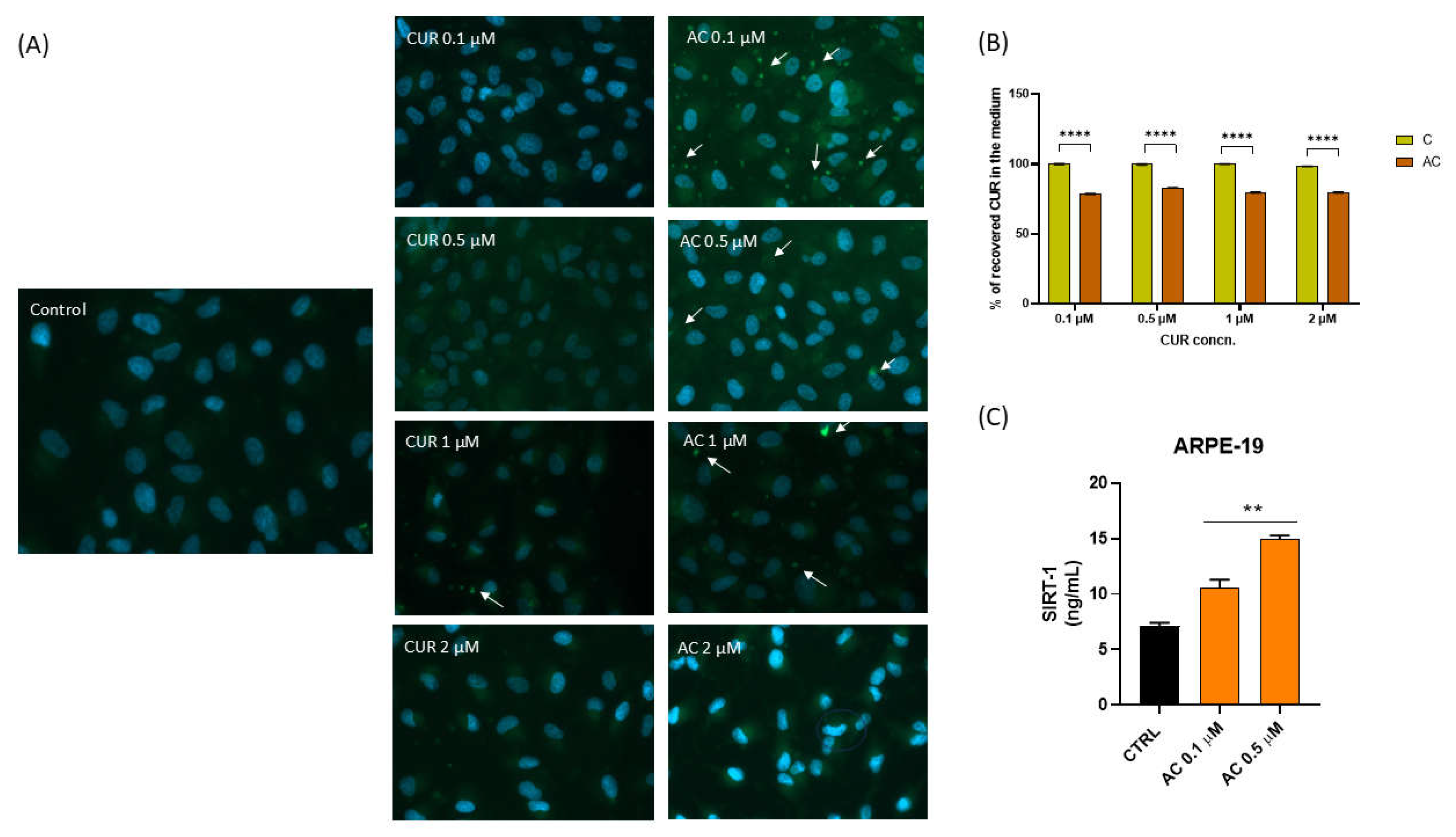
2.8. Antioxidant Activity
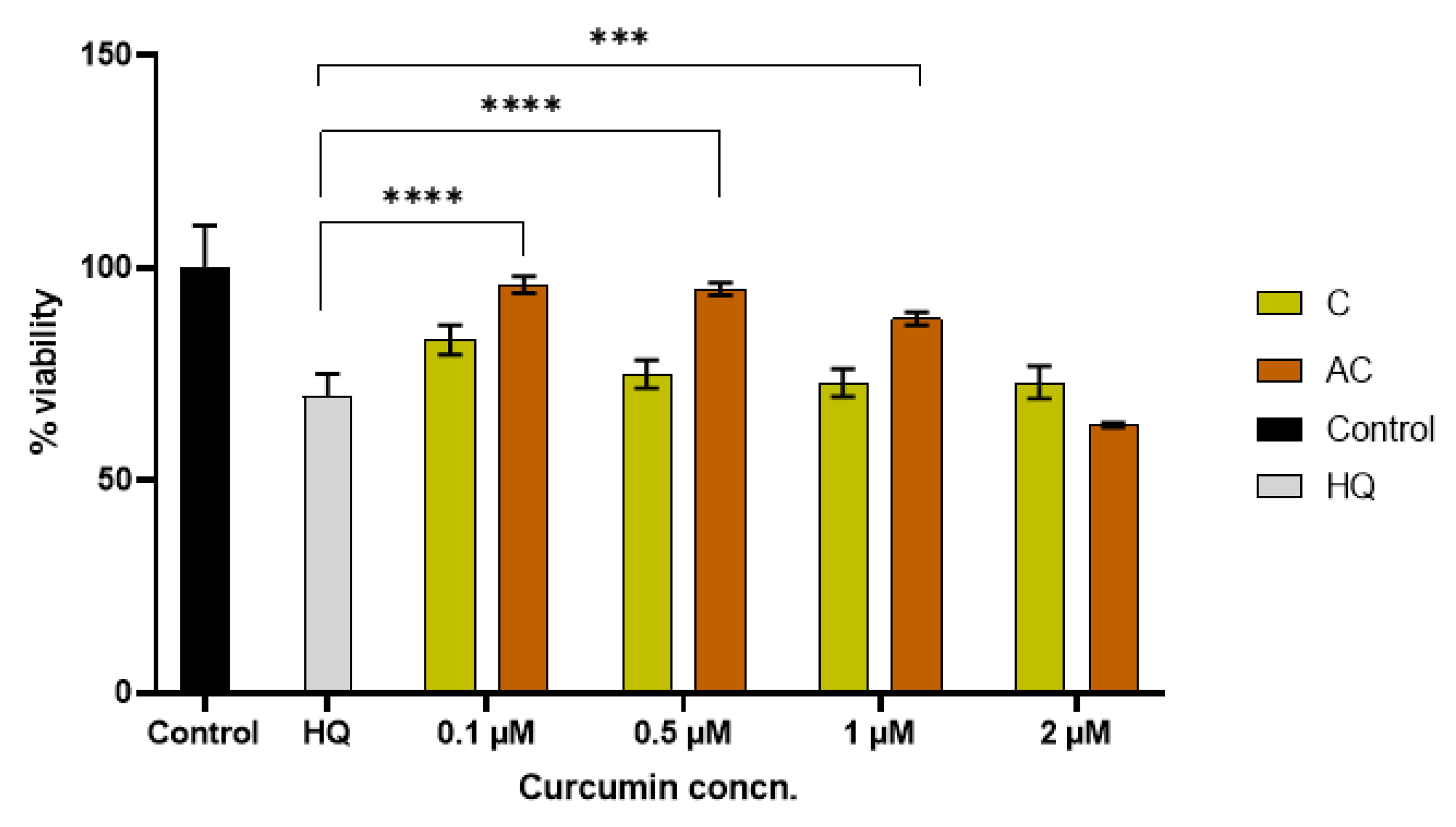
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Excipients Screening
4.3. Preparation of CUR-SNEDDS
| Sample | Capryol® PGMC (%w/w) | Tween ® 80 (%w/w) | Transcutol ® P (%w/w) | CUR (mg/ml) |
|---|---|---|---|---|
| A | 15.04 | 55.18 | 28.21 | |
| AC | 15.04 | 55.18 | 28.21 | 1 |
4.4. Physicochemical Characterization and Morphology
4.4.1. Globule Size, Polydispersity Index (PDI) and Zeta Potential (ZP)
4.4.2. Morphology
4.4.3. Clarity, pH, Viscosity and Emulsification Time
4.5. Entrapment Efficiency (EE) % and In Vitro Release
4.6. HPLC Method
4.7. Stability Studies
4.8. Cationic CUR-SNEDDS
4.9. Cytocompatibility Study of CUR and CUR-SNEDDS
4.9.1. Human Corneal Epithelium (HCE) Cells
4.9.2. Human Retinal Pigment Epithelia (ARPE-19) Cells
4.10. SIRT1 Expression Levels in ARPE-19 Cells
4.11. Cell Viability in Simulated Oxidative Stress Conditions
4.12. Cell-Uptake Investigation in Retinal Cell
4.13. Statistical Analysis
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safi, S. Z., Qvist, R., Kumar, S., Batumalaie, K.,; Ismail, I. S. B. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. BioMed Res Int. 2014, 2014, 801269. [Google Scholar]
- Ahmad, A. , & Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J Immunoassay Immunochem. 2020, 41(3), 257–271. [Google Scholar] [PubMed]
- Hammer, S.S. , et al. Fasting and fasting-mimicking treatment activate SIRT1/LXRα and alleviate diabetes-induced systemic and microvascular dysfunction. Diabetologia. 2021. [Google Scholar] [CrossRef]
- Mimura, T. , Kaji, Y., Noma, H., Funatsu, H., & Okamoto, S. The role of SIRT1 in ocular aging. Exp Eye Res 2013, 116, 17–26. [Google Scholar]
- Zhou, M. , Luo, J., & Zhang, H. Role of Sirtuin 1 in the pathogenesis of ocular disease. Int J of Mol Med. 2018, 42(1), 13–20. [Google Scholar]
- Li, P. , Zhang, L., Zhou, C., Lin, N., & Liu, A. Sirt 1 activator inhibits the AGE-induced apoptosis and p53 acetylation in human vascular endothelial cells. J Toxicol Sci. 2015, 40, 615–624. [Google Scholar]
- He, M. , Long, P., Yan, W., Chen, T., Guo, L., Zhang, Z., & Wang, S. ALDH2 attenuates early-stage STZ-induced aged diabetic rats retinas damage via SIRT1/Nrf2 pathway. Life Sci. 2018, 215, 227–235. [Google Scholar]
- Escribano-Lopez, I. , Diaz-Morales, N., Iannantuoni, F., Lopez-Domenech, S., de Marañon, A. M., Abad-Jimenez, Z.,... & Victor, V. M. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Rep. 2018. [Google Scholar]
- Giovannini, L. , & Bianchi, S. Role of nutraceutical SIRT1 modulators in AMPK and mTOR pathway: Evidence of a synergistic effect. Nutrition 2017, 34, 82–96. [Google Scholar]
- Zendedel, E. , Butler, A. E., Atkin, S. L., & Sahebkar, A. Impact of CURon sirtuins: A review. J Cell Biochem. 2018, 119, 10291–10300. [Google Scholar]
- Ren, B. C. , Zhang, Y. F., Liu, S. S., Cheng, X. J., Yang, X., Cui, X. G.,... & Li, X. Y. CURalleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via SIRT1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. 2020, 24, 12355–12367. [Google Scholar]
- Heshmati, J. , Golab, F., Morvaridzadeh, M., Potter, E., Akbari-Fakhrabadi, M., Farsi, F.,... & Shidfar, F. The effects of CURsupplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020, 14, 77–82. [Google Scholar]
- Sun, Q. , Jia, N., Wang, W., Jin, H., Xu, J., & Hu, H. Activation of SIRT1 by CURblocks the neurotoxicity of amyloid-β25–35 in rat cortical neurons. Biochem Biophys Res Commun 2014, 448, 89–94. [Google Scholar] [PubMed]
- Bahrami, A. , Montecucco, F., Carbone, F., & Sahebkar, A. Effects of CURon aging: Molecular mechanisms and experimental evidence. BioMed Res Int 2021, 2021, 8972074. [Google Scholar] [PubMed]
- Gbr, A. A. , Abdel Baky, N. A., Mohamed, E. A., & Zaky, H. S. Cardioprotective effect of pioglitazone and CURagainst diabetic cardiomyopathy in type 1 diabetes mellitus: impact on CaMKII/NF-κB/TGF-β1 and PPAR-γ signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 2021, 394, 349–360. [Google Scholar] [PubMed]
- Jiang, D. , Xu, T., Zhong, L., Liang, Q., Hu, Y., Xiao, W., & Shi, J. Research progress of VEGFR small molecule inhibitors in ocular neovascular diseases. Eur J Med Chem. 2023, 115535. [Google Scholar]
- Yang, J. , Miao, X., Yang, F. J., Cao, J. F., Liu, X., Fu, J. L., & Su, G. F. Therapeutic potential of CURin diabetic retinopathy. Int J Mol Med. 2021, 47, 1–12. [Google Scholar]
- Simion, V. , Stan, D., Constantinescu, C. A., Deleanu, M., Dragan, E., Tucureanu, M. M.,... & Calin, M. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J Pharm Pharmacol 2016, 68, 195–207. [Google Scholar]
- Zingale, E. , Bonaccorso, A., D’Amico, A. G., Lombardo, R., D’Agata, V., Rautio, J., & Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar]
- Maji, I. , Mahajan, S., Sriram, A., Medtiya, P., Vasave, R., Khatri, D. K.,... & Singh, P. K. Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J Control Release 2021, 337, 646–660. [Google Scholar]
- Tran, P. , & Park, J. S. Recent trends of self-emulsifying drug delivery system for enhancing the oral bioavailability of poorly water-soluble drugs. J Pharm Inv 2021, 51, 439–463. [Google Scholar]
- ElKasabgy, N. A. Ocular supersaturated self-nanoemulsifying drug delivery systems (S-SNEDDS) to enhance econazole nitrate bioavailability. Int J Pharm 2014, 460, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Rasoanirina, B. N. V. , Lassoued, M. A., Kamoun, A., Bahloul, B., Miladi, K., & Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm Dev Techno 2020, 25, 694–703. [Google Scholar]
- Vikash, B. , Pandey, N. K., Kumar, B., Wadhwa, S., Goutam, U., Alam, A.,... & Singh, S. K. Formulation and evaluation of ocular self-nanoemulsifying drug delivery system of brimonidine tartrate. J Drug Deliv Sci Technol. 2023, 81, 104226. [Google Scholar]
- Beg, S. , Swain, S., Singh, H. P., Patra, C. N., & Rao, M. B. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech 2012, 13, 1416–1427. [Google Scholar]
- Ardito, F. , Perrone, D., Giuliani, M., Testa, N. F., & Muzio, L. L. Effects of CURon squamous cell carcinoma of tongue: an in vitro study. Curr Top Med Chem 2018, 18, 233–243. [Google Scholar]
- Liu, X. , Shen W., Xia W. & Lu P. Early effects of intravitreal anti-VEGF agents on cornea and visual acuity in patients with diabetic retinopathy, Cutan Ocul Toxicol. 2023, 42, 213–218. [Google Scholar] [CrossRef]
- Naageshwaran, V. , Ranta, V. P., Toropainen, E., Tuomainen, M., Gum, G., Xie, E.,... & Del Amo, E. M. Topical pharmacokinetics of dexamethasone suspensions in the rabbit eye: Bioavailability comparison. Int J Pharm 2022, 615, 121515. [Google Scholar]
- Altamimi, M. , Haq, N., Alshehri, S., Qamar, W., & Shakeel, F. Enhanced skin permeation of hydrocortisone using nanoemulsion as potential vehicle. ChemistrySelect. 2019, 4(34), 10084–10091. [Google Scholar]
- Butt, U. , ElShaer, A., Snyder, L. A., Al-Kinani, A. A., Le Gresley, A., & Alany, R. G. Fatty acid based microemulsions to combat ophthalmia neonatorum caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials. 2018, 8(1), 51. [Google Scholar]
- Jörgensen, A. M. , Friedl, J. D., Wibel, R., Chamieh, J., Cottet, H., & Bernkop-Schnürch, A. Cosolvents in self-emulsifying drug delivery systems (SEDDS): do they really solve our solubility problems? Mol Pharm. 2020, 17(9), 3236–3245. [Google Scholar] [PubMed]
- Vasconcelos, T. , Marques, S., & Sarmento, B. Measuring the emulsification dynamics and stability of self-emulsifying drug delivery systems. Eur J Pharm Biopharm 2018, 123, 1–8. [Google Scholar] [PubMed]
- Puglia, C. , Santonocito, D., Romeo, G., Intagliata, S., Romano, G. L., Strettoi, E.,... & Bucolo, C. Lipid nanoparticles traverse non-corneal path to reach the posterior eye segment: In vivo evidence. Molecules 2021, 26, 4673. [Google Scholar]
- Bonaccorso, A. , Pepe, V., Zappulla, C., Cimino, C., Pricoco, A., Puglisi, G.,... & Carbone, C. Sorafenib repurposing for ophthalmic delivery by lipid nanoparticles: A preliminary study. Pharmaceutics 2021, 13, 1956. [Google Scholar] [PubMed]
- Phan, C. M. , Ross, M., Fahmy, K., McEwen, B., Hofmann, I., Chan, V. W.,... & Jones, L. Evaluating viscosity and tear breakup time of contemporary commercial ocular lubricants on an in vitro eye model. Transl Vis Sci Technol. 2023, 12(6), 29–29. [Google Scholar] [PubMed]
- Rathore, C. , Hemrajani, C., Sharma, A. K., Gupta, P. K., Jha, N. K., Aljabali, A. A.,... & Tambuwala, M. M. Self-nanoemulsifying drug delivery system (SNEDDS) mediated improved oral bioavailability of thymoquinone: optimization, characterization, pharmacokinetic, and hepatotoxicity studies. Drug Deliv Transl Res 2023, 13, 292–307. [Google Scholar]
- Chen, X. L. , Liang, X. L., Zhao, G. W., Zeng, Q. Y., Dong, W., Ou, L. Q.,... & Liao, Z. G. Improvement of the bioavailability of CURby a supersaturatable self nanoemulsifying drug delivery system with incorporation of a hydrophilic polymer: in vitro and in vivo characterisation. J Pharm Pharmacol 2021, 73, 641–652. [Google Scholar]
- Yang, Y. , Duan, W., Lin, Y., Yi, W., Liang, Z., Yan, J.,... & Jin, Z. SIRT1 activation by CUR pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013, 65, 667–679. [Google Scholar]
- Bonaccorso, A. , Gigliobianco, M. R., Pellitteri, R., Santonocito, D., Carbone, C., Di Martino, P.,... & Musumeci, T. Optimization of CURnanocrystals as promising strategy for nose-to-brain delivery application. Pharmaceutics 2020, 12, 476. [Google Scholar]
- Koskela, A. , Reinisalo, M., Hyttinen, J. M., Kaarniranta, K., & Karjalainen, R. O. Pinosylvin-mediated protection against oxidative stress in human retinal pigment epithelial cells. Mol Vis 2014, 20, 760. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





