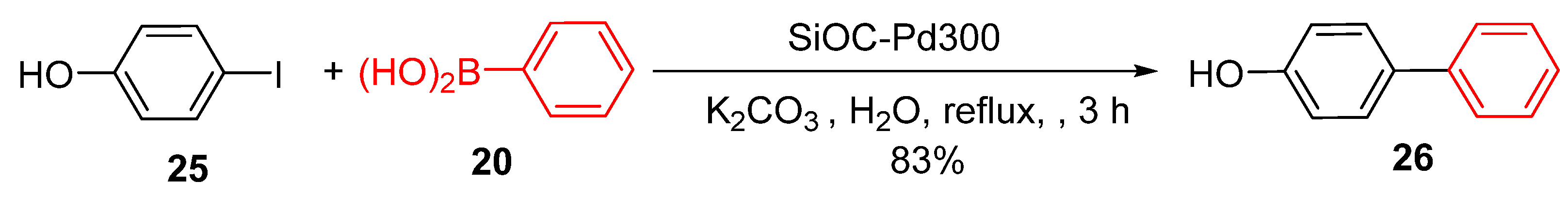1. Introduction
The increasing demand for environmentally friendly and efficient chemical processes has positioned sustainable catalysis at the forefront of modern chemical syntheses. As industries worldwide strive to reduce their environmental impact, there is growing emphasis on developing catalytic systems that are highly effective while minimizing waste, energy consumption, and hazardous materials. Sustainable catalytic processes play a major role in achieving these goals by enabling the production of chemical compounds with high efficiency and low ecological footprints [
1,
2,
3,
4,
5,
6].
Palladium nanoparticles (PdNPs), with their exceptional surface-to-volume ratios, have emerged as transformative tools in this context, offering unparalleled catalytic activity, selectivity and reusability. Various forms of nanocatalysts have been developed, including supported, Schiff-based, graphene-based, thin-film, mixed metal oxide, magnetic and core-shell nanocatalysts [
7]. Among these, palladium-based nanocatalysts have garnered extensive attention in both academia and industry due to their versatility in carbon-carbon and carbon-heteroatom cross-coupling reactions as well as other catalytic processes like hydrogenation, oxidation, reduction and esterification [
8,
9,
10,
11,
12]. Their exceptional catalytic activity, selectivity and reusability render them invaluable for applications ranging from fine chemical synthesis to large-scale industrial processes where they are used in the production of various valuable compounds such as natural products, biologically active molecules and pharmaceuticals [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. For instance, several drugs and intermediates such as naproxen (an anti-inflammatory agent) [
28,
29], prosulfuron (herbicide) [
30], Singulair [
31,
32], Eletriptan [
31,
33,
34,
35] BVDU (antiviral ), [
36] Axitinib (antitumor), [
37] 2-ethylhexyl-
p-methoxycinnamate (UV sunscreen B agent) [
38] and co-monomers of styrene polymers [
39] have been made by Heck reactions.
Palladium nanoparticles (PdNPs) have attracted significant attention in organic synthesis because of their remarkable advantages over traditional catalysts. Unlike bulk metal catalysts, PdNPs offer a much higher surface-area-to-volume ratio which enhances catalytic activity and selectivity. This enables the use of smaller quantities of catalyst, leading to cost-effective reactions and reduced environmental impacts [
40,
41,
42,
43,
44,
45]. Additionally, PdNPs demonstrate exceptional recyclability and reusability which are critical factors in sustainable chemistry. Their ability to maintain catalytic performance over multiple cycles makes them ideal for large-scale industrial applications by reducing the need for frequent replacements and minimizing waste [
45].
Recent studies have highlighted the efficiency of PdNPs in cross-coupling reactions by using sustainable approaches. Pd nanocatalysts are often immobilized on supports such as inorganic carbonaceous materials, silica, metal oxide-based hybrids, biopolymers, organic polymeric hybrids and magnetic nanostructures [
46,
47,
48,
49]. These supports enhance the catalytic environment and create synergy between the support and the nanocatalyst [
41,
46,
50,
51]. For instance, PdNPs supported on fibrous nanosilica (KCC-1) or stabilized by heteroatom-donor-modified polystyrene-based polymer ionic liquids have demonstrated high catalytic efficiency and recyclability in Suzuki–Miyaura cross-coupling reactions under mild conditions, achieving significantly higher turnover numbers (TONs) than conventional Pd catalysts [
52,
53]. Another innovative approach utilizes iron to reduce the quantity of palladium required, enhancing sustainability and cost-effectiveness [
54].
The selectivity of PdNPs for catalytic reactions is another distinguishing feature. This selectivity allows for precise control over reaction pathways resulting in higher yields of desired products with fewer by-products [
55]. Advanced designs, such as water-soluble dendrimer-encapsulated PdNPs, offer remarkable stability and efficiency in various cross-coupling reactions under mild conditions, supporting green chemistry principles [
56,
57]. These dendrimer-encapsulated nanoparticles, with their small size distributions, enable better characterization in kinetic studies of carbon-carbon coupling reactions [
56,
57].
Recent innovations include the development of Pd-loaded Cucurbit [
7] uril-modified iron oxide nanoparticles for green solvents as well as nitrogen-doped carbon nanosheets, which have achieved ninefold increases in turnover frequencies for Suzuki reactions compared to conventional Pd/C catalysts [
58,
59].
Biomass-derived boron carbon nitride has achieved high yields and recyclability in Suzuki-Miyaura and CN arylation reactions [
60]. These systems demonstrate the integration of biodegradable and renewable materials and the potential of eco-friendly supports. Bio-based supports, such as kenaf-cellulose modified with poly(amidoxime) ligands and bacterial supports, such as
Paracoccus yeei, highlight the potential of biodegradable materials to reduce environmental impact while maintaining excellent catalytic efficiency [
61,
62].
These advances, coupled with the rational design of nanostructured supports that enhance substrate selectivity and reduce catalyst deactivation [
46], underscore the potential of sustainable methods for optimizing catalytic performance while addressing environmental concerns. This review provides a comprehensive overview of recent innovations in PdNP synthesis and catalytic applications, focusing on strategies to enhance efficiency, selectivity and sustainability. By critically analyzing these developments and identifying gaps in current methodologies, this review highlights key areas for future research to overcome limitations and guide the design of next-generation PdNP catalysts for widespread industrial adoption.
2. Palladium Nanoparticles Supported on Biopolymers for Cross-Coupling Reactions
Biopolymers and biomass-derived polymers, such as cellulose, starch, pectin, agarose, chitosan, proteins and enzymes, have recently gained attention as supports for metal nanoparticles in catalytic applications [
47]. Their advantages, including low toxicity, cost efficiency, high biocompatibility, availability and abundance, make them superior to traditional supports. Among these, palladium (Pd) nanoparticles supported on biopolymers have shown remarkable potential in cross-coupling reactions [
63].
2.1. Palladium Nanoparticles Supported on on Chitosan
Chitosan (CS), a biopolymer known for its biocompatibility, biodegradability and abundant chelating functional groups, has gained significant attention as a support material for catalytic systems [
64,
65,
66]. Its surface amine (-NH₂) and hydroxyl (-OH) groups enable strong coordination with transition metal species, making it ideal for stabilizing nanoparticles [
67,
68,
69,
70]. Additionally, the ability of chitosan to form composites, beads and blends enhances its physical and mechanical properties, allowing it to serve as a robust support for heterogeneous catalysts [
71,
72,
73,
74].
Building on these properties, Dohenduo et al. developed a novel supramolecular Pd(II) catalyst supported on chitosan grafted with L-asparagine and an EDTA linker, named Pd@ASP–EDTA–CS (
Figure 1) [
75].
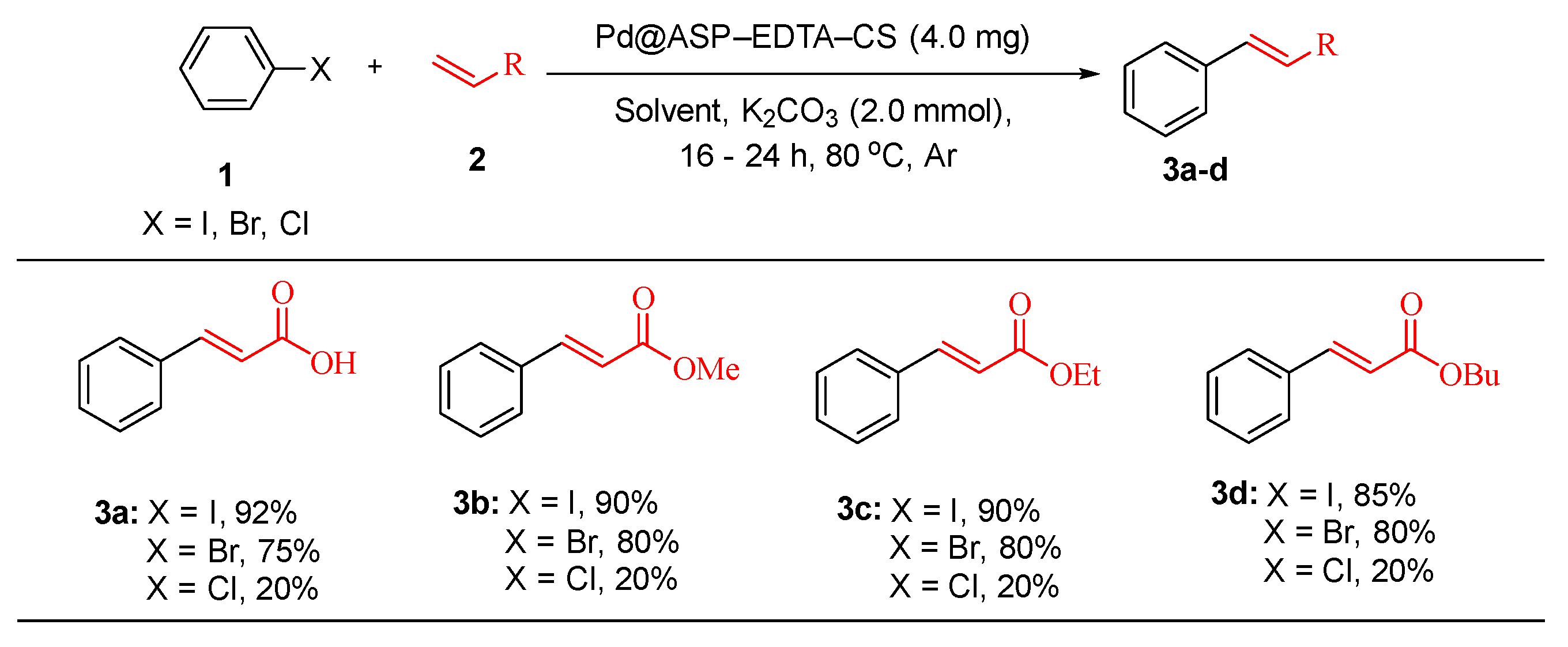
This catalyst was successfully employed in the Heck cross-coupling reaction of aryl halides (
1) with alkenes (
2) to produce biologically active cinnamic acid derivatives (
3) in good to excellent yields (
Scheme 1). The key advantages of this catalyst include its high catalytic activity, excellent thermal stability and easy recovery via simple filtration. Notably, the catalyst demonstrated more than five cycles of reusability with no significant loss in efficiency and no detectable leaching of Pd into the reaction medium or final products [
75].
The reaction conditions were optimized using iodobenzene and methyl acrylate, achieving the best yields under 4.0 mg catalyst loading, DMF or acetonitrile solvents and a reaction temperature of 80 °C. Under these conditions, methyl acrylate gave the highest yield (90%), whereas ethyl and butyl acrylates furnished slightly lower yields (85% each). However, substrates with electron-withdrawing groups (EWGs) on aryl halides afforded trace yields, highlighting the limitations of the system [
75]. This study highlights the potential of Pd@ASP–EDTA–CS as a sustainable and effective catalytic system for cross-coupling reactions offering significant advancements in green chemistry.
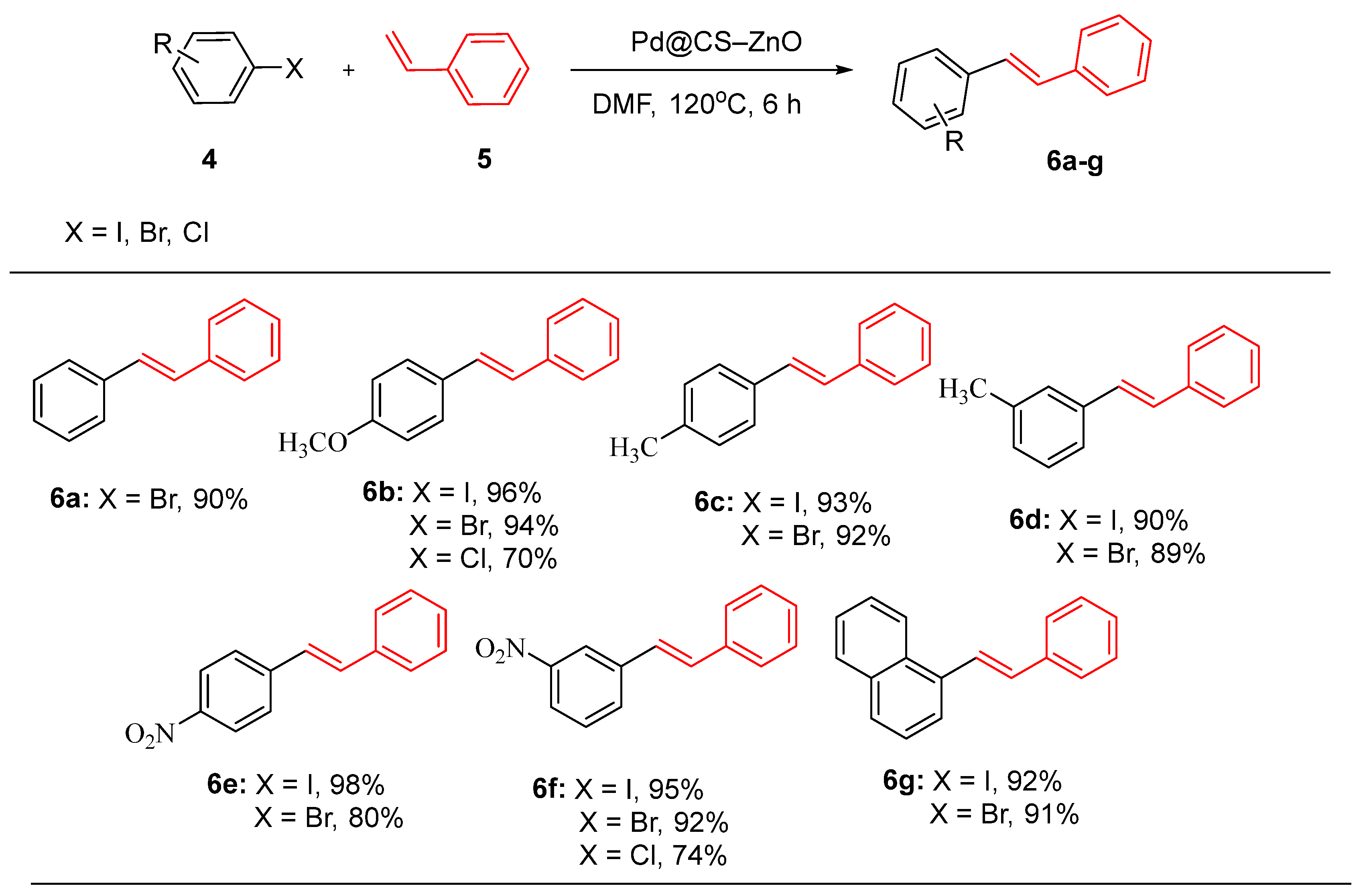
In a related study, Calistan et al. [
76]. developed a novel palladium nanocatalyst (Pd@CS–ZnO) supported on chitosan and ZnO nanoparticles to enhance the efficiency and reusability of Heck coupling reactions [
76]. The study used 1-bromo-4-nitrobenzene as the substrate to determined optimal reaction conditions, reacting with styrene to optimize reaction parameters such as solvent choice, base system, reaction time and catalyst amount, The optimal conditions identified was DMF as the solvent, K₂CO₃ as the base, a catalyst dosage of 30 mg of Pd@CS-ZnO and the reaction time of 6 h. The catalyst was applied to the coupling reactions of various aryl halides (
4) with styrene (
5), achieving yields of 70-98% with high chemical stability, cost efficiency, and eco-friendliness (
Scheme 2). Notably, the catalyst maintained a 91% yield after six cycles, highlighting its reusability. These findings suggest that Pd@CS-ZnO is a competitive candidate for enhanced cross-coupling catalysis, addressing the limitations of traditional homogeneous Pd catalysts, such as cost, toxicity, and separation difficulties, by being phosphine-free and supported on a solid matrix, making it more environmentally friendly and economically viable [
76].
2.2. Palladium Nanoparticles Supported on Kenaf-Cellulose Modified with Poly(amidoxime) Ligands
Islam et al. developed a functionalized biopolymer palladium nanocatalyst (PdNc@PA) by embedding palladium nanoparticles in biodegradable kenaf-cellulose modified with poly(amidoxime) ligands [
61]. The catalyst demonstrated exceptional stability and efficiency in a variety of cross-coupling reactions, including Mizoroki-Heck, Suzuki-Miyaura, and Hiyama reactions, consistently achieving high yields. Notably, its robustness enabled multiple reuse cycles with minimal activity loss, offering a sustainable solution for Pd nanoparticle catalysis. By leveraging biodegradable materials, this work highlights a significant advancement in reducing the environmental impact while maintaining a high catalytic performance.
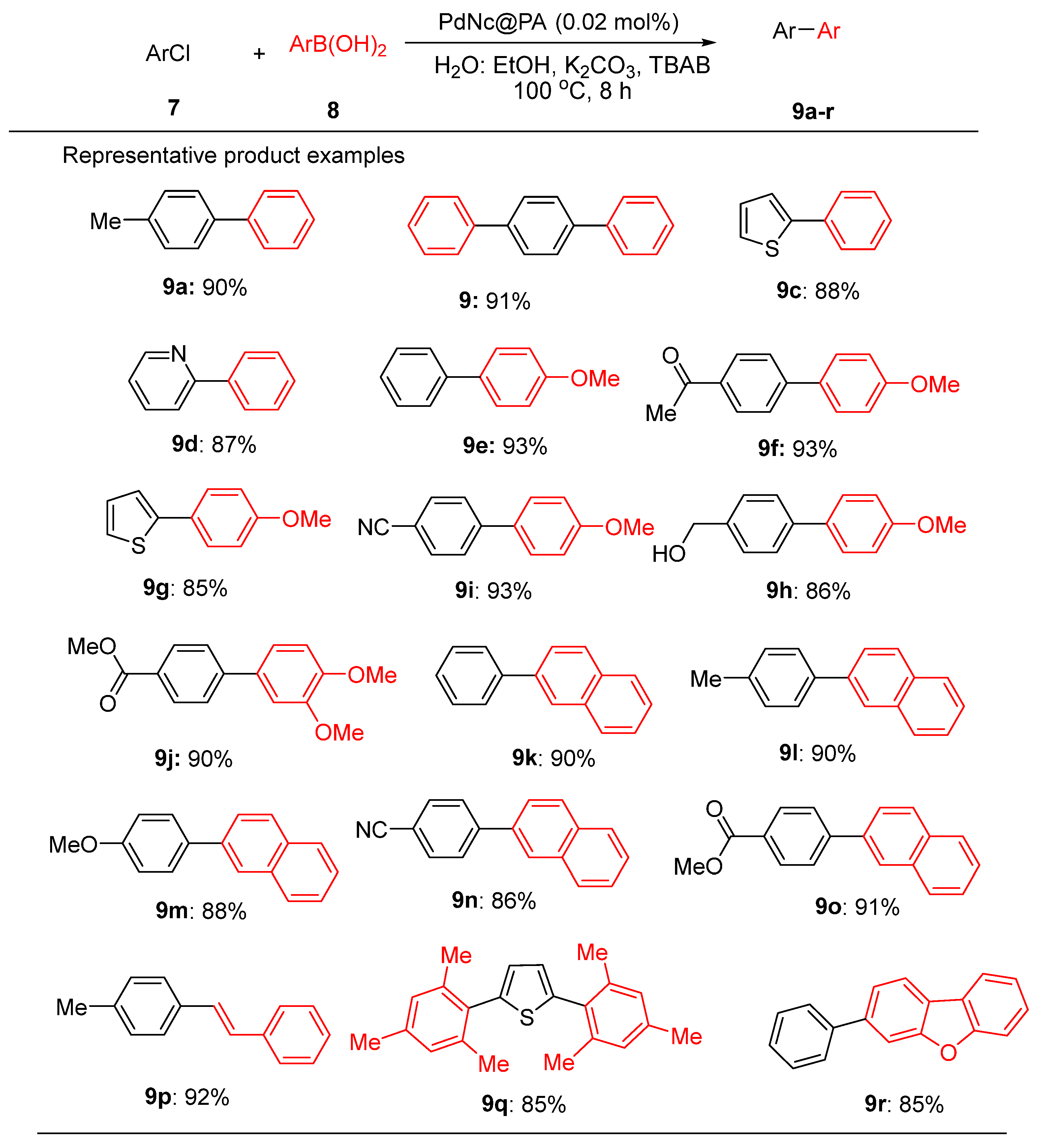
A deeper exploration of the Suzuki-Miyaura reaction involving various arylboronic acids and aryl/heteroaryl chlorides revealed the efficacy of this catalyst in the synthesis of biaryl products
9a-r (
Scheme 3). Aryl and heteroaryl chlorides with phenylboronic acid yielded products
9a-d in excellent yields. 4-Methoxyphenylboronic acid coupled with both activated and deactivated aryl chlorides yielding products
9e-j (85–93%). Sterically hindered naphthylboronic acid yielded products
9k-o in up to 92% yield. Notably, (
E)-styrylboronic acid coupled with 4-chlorotoluene to furnish (
E)-1-methyl-4-styrylbenzene
9p in 84% yield. Mesitylboronic acid produced 2,5-dimesitylthiophene
9q in 85% and dibenzo[b,d]furan-3-ylboronic acid with chlorobenzene yielded
9r in 84% [
61].
PdNc@PA was compared with other catalysts for the Suzuki reactions. While catalysts like Pd/MIL-101 (0.9 mol%, 97% yield) and Pd/WA30 (10 mol%, 100% yield) were effective, PdNc@PA achieved up to 93% yield with significantly lower loading (0.6–0.5 mol%), demonstrating superior catalytic efficiency [
61]. PdNc@PA was a highly efficient catalyst for bond formation at minimal loadings. Diagnostics, including hot filtration tests and ICP-AES analysis, confirmed that the reaction proceeded via a heterogeneous mechanism with no detectable leaching of Pd species, ensuring the stability and reusability of the catalyst.
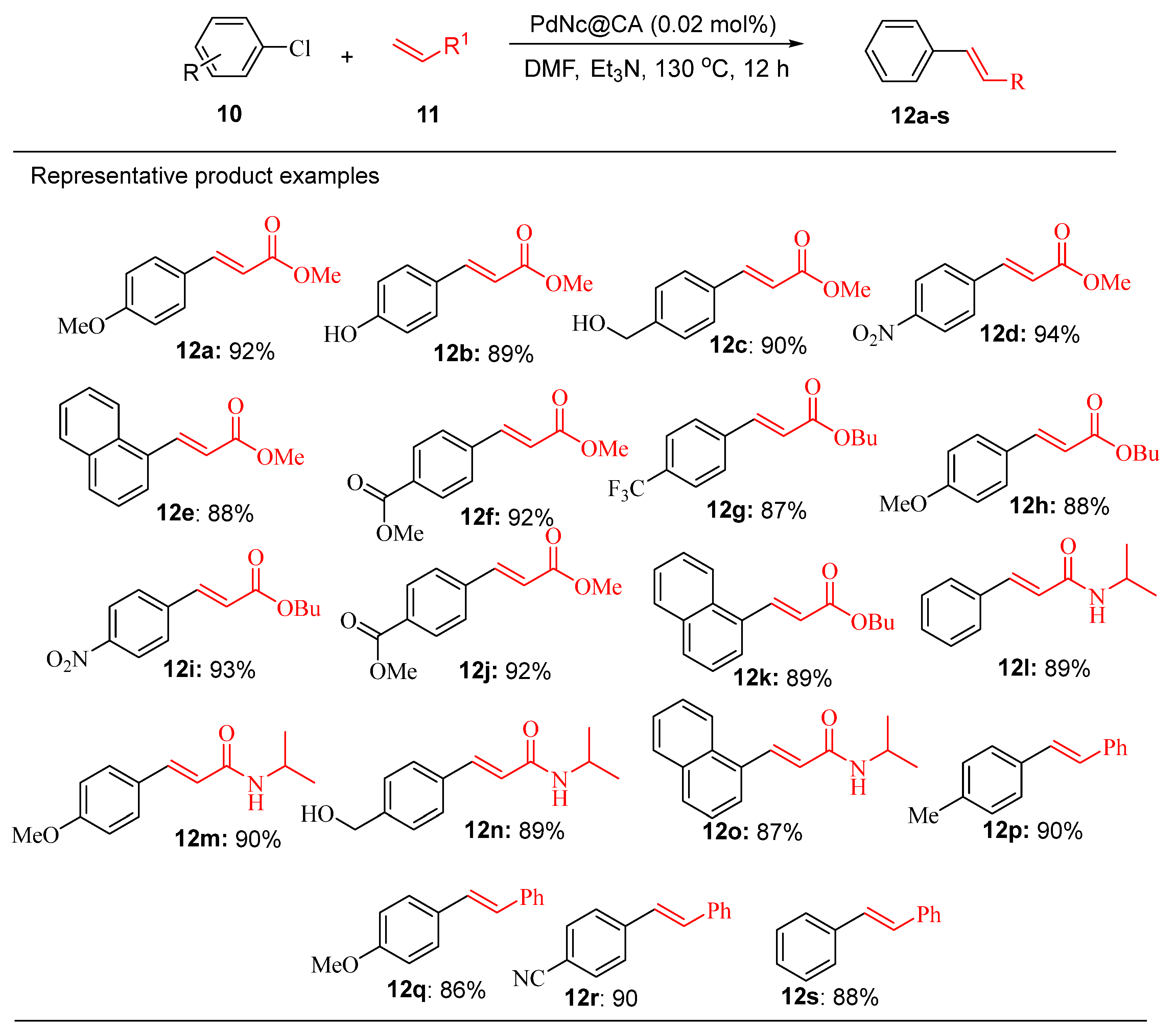
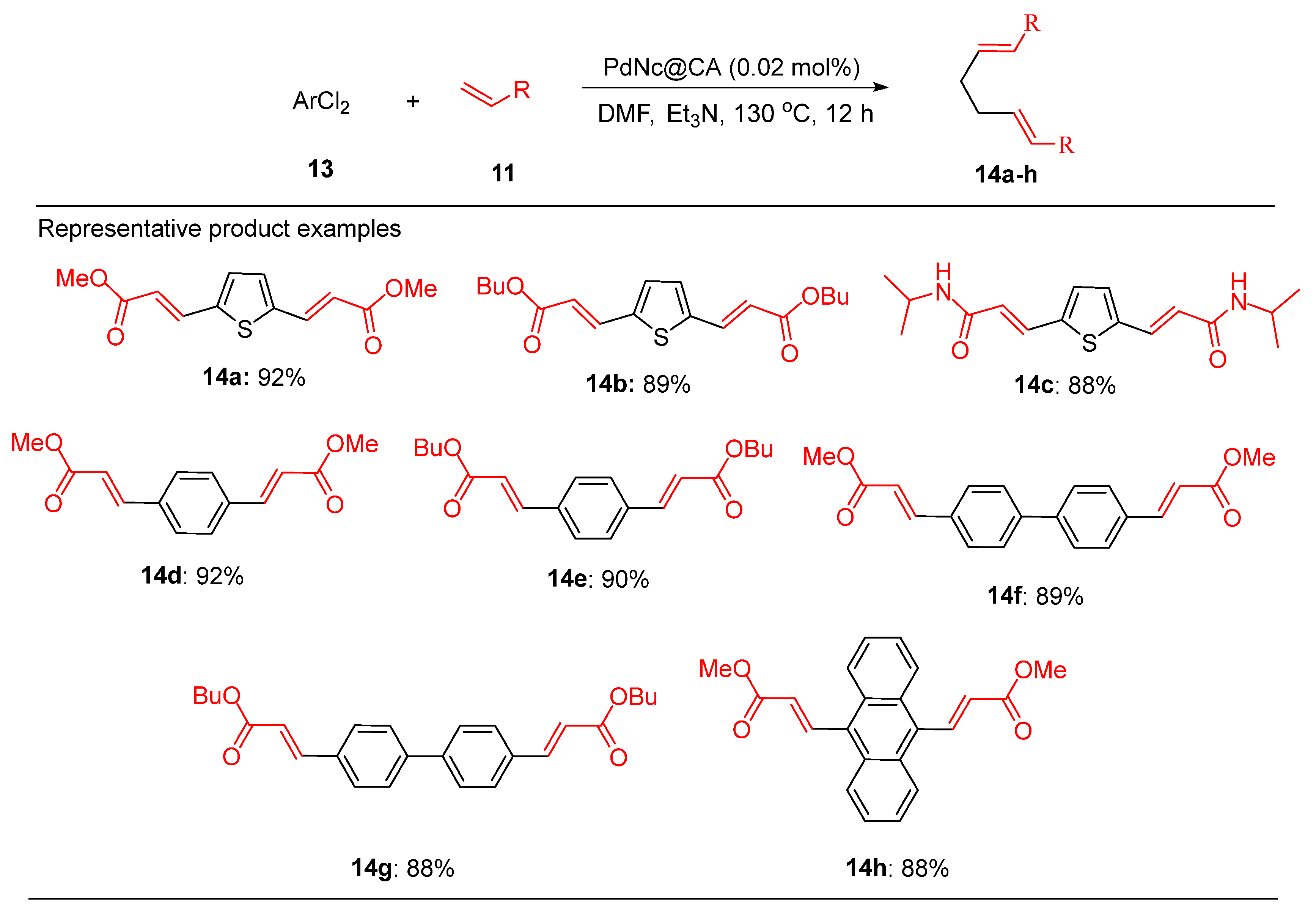
Similarly, the PdNc@PA catalyst demonstrated outstanding efficiency in the Heck-Matsuda reaction of chlorobenzene (
10) with styrene and methyl acrylate (
11) (
Scheme 4 and
Scheme 5). This system outperformed conventional catalysts giving higher yields even at low catalytic loadings, further demonstrating its versatility.
Recyclability studies of PdNc@PA in Mizoroki-Heck and Suzuki-Miyaura reactions showed remarkable durability, with consistent catalytic performance over multiple cycles. Only a slight decline in efficiency was observed, primarily due to minor losses during the decantation process [
61].
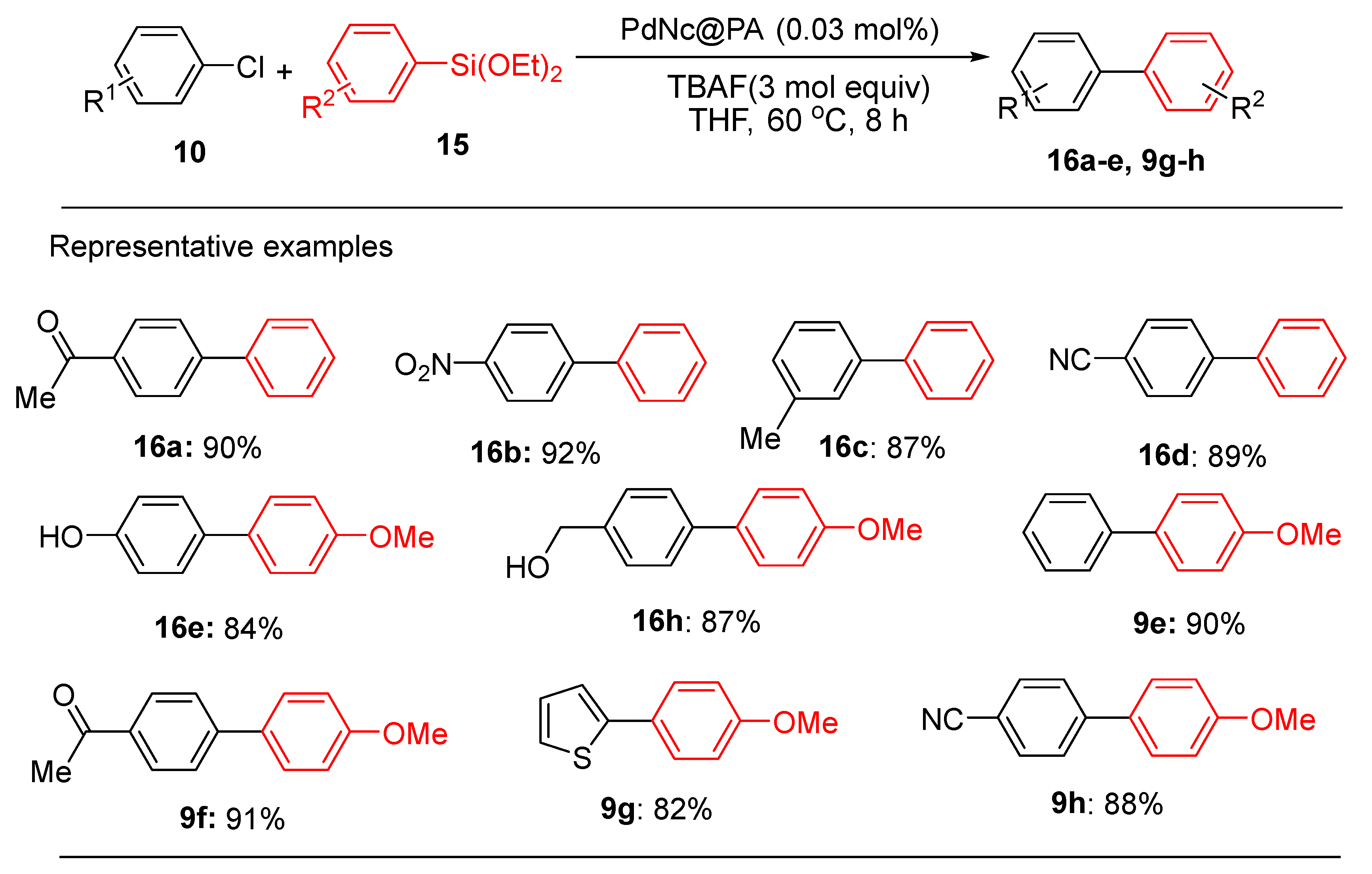
The PdNc@PA catalyst also exhibited the Hiyama coupling of various aryl chlorides with organosilane reagents (
Scheme 6). The organosilicon reagent triethoxy(4-methoxyphenyl)silane (
15) was reacted with a variety of aryl chlorides (
10) to give their respective biaryl derivatives (
16) in yields of up to 91 %. Interestingly, a heteroaryl chloride, 2-chlorothiophene, provided cross-coupling product
9g in 82% yield and readily coupled with triethoxy(phenyl)silane to afford the corresponding biaryl products
16a-h in excellent yields. This operational simplicity and functional group tolerance broaden the scope of its application and further support its environmental and practical advantages.
These results indicated that PdNc@PA is a powerful and sustainable catalyst for cross-coupling reactions that combines efficiency, reusability and environmental benefits. Its versatility across a diverse range of substrates, along with its minimal environmental impact, make it a promising tool for advancing green chemistry. For other previous catalysis using Pd supported on biopolymers, see the reports in the literature [
15,
44,
77].
3. Palladium Nanoparticles Supported on Polymers
Polymers have been widely utilized as supports/stabilizers for nanoparticles [
78,
79]. Their extensive application is attributed to their availability, enhanced stabilization properties of metal nanoparticles and resistance to particle sintering/agglomeration. Recently, the use of novel engineered polymers such as polyorganophosphazenes with an inorganic backbone [
80], polyvinyl pyridines [
79,
81], fibres [
79] and dendrimers [
63,
79,
82] as supports has become increasingly popular. Several of these have been implicated in various cross-coupling reactions for chemical synthesis and industrial applications and many have been previously reviewed [
44]. We herein report the more recent ones.
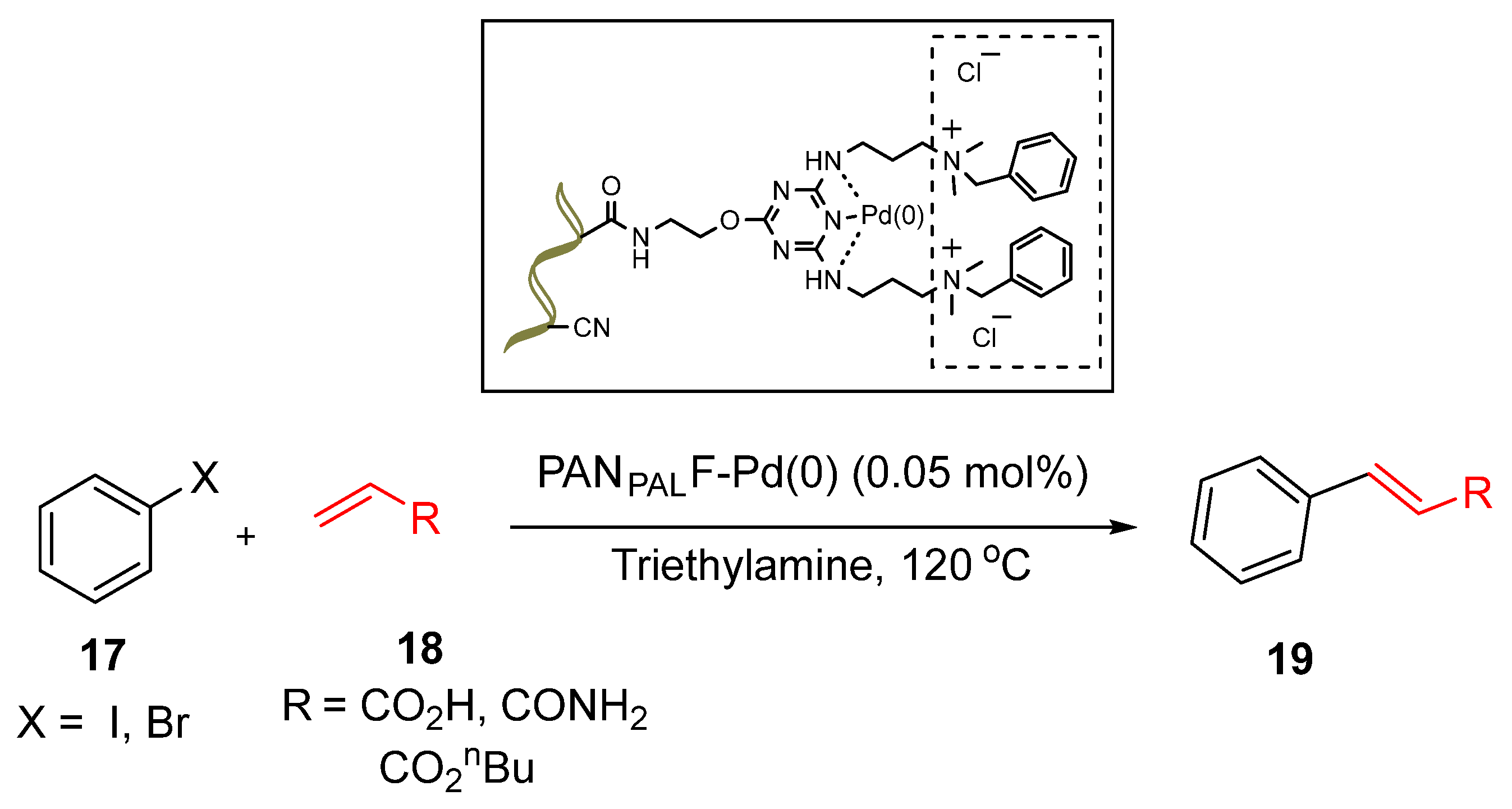
Palladium Nanoparticles Supported on Functionalized Polyacrylonitrile Fiber
Wu et al. [
83]. developed a cutting-edge aza-ligand-functionalized polyacrylonitrile fibre (PANPALF) as a support for Pd(0) nanoparticles. Functionalization of the fibre introduced amino groups and quaternary ammonium salt moieties, which facilitated strong chelation and stabilization of the Pd(0) nanoparticles through an
in-situ reduction process. This innovative PANPALF-Pd(0) catalyst demonstrated exceptional performance in solvent-free Heck coupling reactions of terminal olefins, achieving yields of 43–99% (
Scheme 7). Acrylic acid showed slightly higher reactivity than acrylamide and n-butanol acrylate, indicating that the polarity of the functional groups in olefin had no obvious effect on the reactivity of the Heck reaction [
83]. Similarly, the catalyst displayed a significantly higher turnover number (TON) of 89,000 for iodinated aromatics over brominated ones, attributed to the ease of C-I bond cleavage. Both the electron-donating and electron-withdrawing groups on the halogenated aromatics facilitated smooth reactions, highlighting the broad applicability of the catalyst.
The PANPALFPd(0) system offers substantial advantages, such as excellent catalytic activity, solvent-free operation and extensive substrate compatibility. Remarkably, the catalyst retained a high yield of 93% even after 11 cycles of reuse, with minimal Pd leaching (0.075%) highlighting its stability and sustainable potential for industrial applications [
83]. These findings underscores the significant role of biopolymer-supported palladium nanoparticles in advancing green chemistry by enhancing catalytic efficiency and recyclability for Heck coupling reactions.
4. Palladium Nanoparticles Supported on Nitrogen-Doped Materials
The development of novel and efficient catalysts remains a cornerstone of research in heterogeneous catalysis, addressing challenges in both fundamental science and industrial applications. Historically, carbon materials have been widely used as supports for catalysts owing to their high surface areas, tunable porosities and functional versatility [
84]. Recently, nitrogen-doped carbon materials have emerged as revolutionary supports for palladium nanoparticles (PdNPs) by offering enhanced catalytic performance through tailored electronic properties and robust metal-support interactions.
Nitrogen doping, in its essence, involves the incorporation of nitrogen atoms into the carbon backbone, whereas nitrogen functionalization typically refers to nitrogen atoms attached to the material’s surface. In situ methods, such as pyrolysis of nitrogen- and carbon-rich precursors, yield materials with nitrogen distributed both in the bulk and on the surface [
85,
86]. In contrast, post-treatment methods, like ammonia pyrolysis, primarily result in surface nitrogen functionalities [
87]. These variations in nitrogen placement influence the electronic properties and catalytic behaviour of the material, tailoring its performance for specific reactions.
Nitrogen functionalities, including pyridinic, graphitic and amine groups, significantly enhance PdNP stabilization, electron transfer and metal-support interactions. This synergy not only improves the stability and efficiency of PdNPs but also broadens their applicability in catalytic processes [
7,
46,
88]. Furthermore, nitrogen-doped supports address traditional challenges such as nanoparticle agglomeration, leaching and low turnover frequencies, which are pivotal in the development of high-performance and sustainable catalytic systems [
46,
59].
These advancements underscore the transformative potential of N-doped materials in catalysis, bridging the gap between innovation and sustainability. In the following sections, we explore their role in addressing the longstanding limitations and enabling new frontiers in PdNP-based catalysis.
4.1. Palladium Nanoparticles Supported on Polydopamine-Functionalized Zn-Al Mixed Metal Oxides (Zn-Al MMO)
Joshani et al. synthesized a PdNP catalyst supported on polydopamine-functionalized Zn-Al MMO showing the use of polydopamine as a green stabilizing biotemplate. This system afforded exceptional yields in the Suzuki-Miyaura coupling of a variety of substrates (
Scheme 8). The reaction was optimized by screening different solvents such as H
2O, EtOH, DMF, toluene and solvent mixtures; an additive base such as K
2CO
3, Et
3N and Na
2CO
3 and the catalyst at a loading of 0.1–0.03 mol% Pd at a temperature of 50
oC. Interestingly, the probe reaction afforded the best conversion with an EtOH/H
2O (1:1) mixture as the solvent with 98 % yield in just 1 h of reaction time at 0.02 mol% Pd catalyst loading, and K
2CO
3 as the base (
Scheme 8).
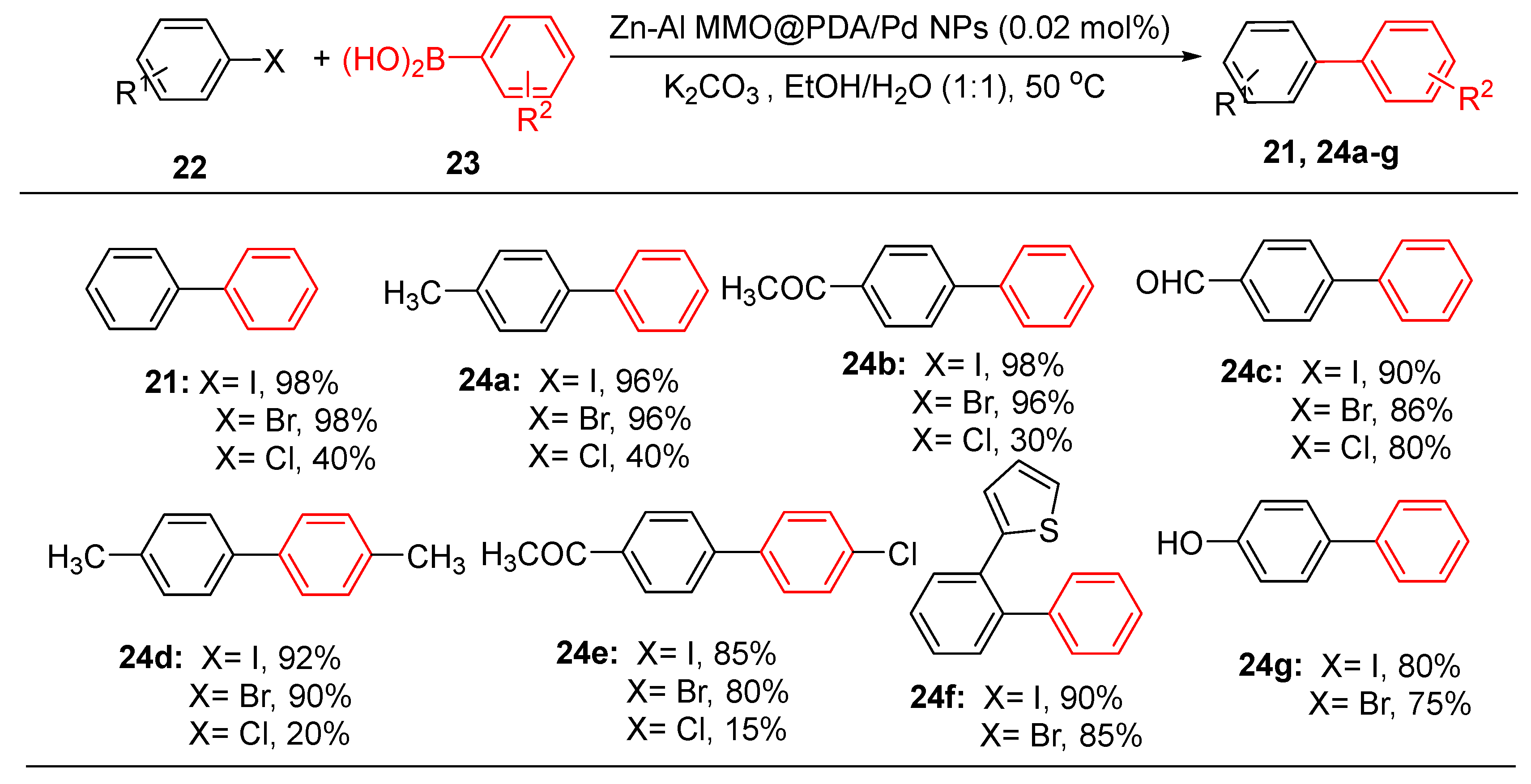
Optimized reaction conditions were used to demonstrate the applicability and versatility of the catalysts across a wide variety of substrates. As seen with other systems, the chloroarenes were more sluggish in the reactions as compared to bromo or iodo analogues, obviously due to their poor leaving capacity. Otherwise, all the reactions took only 1–3 h to produce excellent yields, irrespective of the effect of other organic substitutions, such as electron-donating (CH
3, OCH
3, OH) or withdrawing groups (COCH
3) (
Scheme 9) [
89].
This catalyst displayed remarkable durability and maintained its activity over eight cycles without significant loss. Compared to Pd/N-CNS, the Zn-Al MMO system offered similar recyclability while excelling in accommodating sterically hindered substrates [
89]. The developed Zn-Al MMO@PDA/Pd NPs nanocatalyst was also investigated and found to outperform the previously published technique in terms of yield and reaction time when the optimized reaction conditions were used for the Suzuki-Miyaura coupling of 4-bromoanisole and phenyl boronic acid. Therefore, they concluded that the catalyst has remarkable reusability, maintaining activity over eight consecutive cycles, highlighting emerging trends in sustainable Pd nanoparticle applications for cross-coupling catalysis [
89].
4.2. Palladium Nanoparticles Supported on 3D-Printed Silicon Oxycarbide (SiOC) Monoliths
Building on these advancements, Dory et al. developed a novel 3D-printed silicon oxycarbide monolith functionalized with PdNPs to further enhance catalytic performance using atomic layer deposition (ALD) [
90]. This innovative methodology represents a significant advancement in heterogeneous catalysis, particularly for Suzuki-Miyaura reactions conducted in aqueous media. ALD enables precise control over Pd deposition, ensuring a uniform and well-distributed layer of PdNPs across the monolith surface, which is important for achieving consistent and high catalytic activity.
The 3D-printed architecture of the SiOC monoliths provided a highly optimized catalytic environment characterized by enhanced mass transfer and improved substrate accessibility. These structural features facilitate efficient interactions between the reactants and active sites on the PdNPs, significantly improving their catalytic performance. This was demonstrated by achieving yields of up to 83% in the Suzuki-Miyaura coupling of iodobenzene with phenylboronic acid under aqueous conditions (
Scheme 10).
The monoliths also exhibited remarkable robustness and reusability, thereby addressing the key challenges in sustainable catalysis. The precise and uniform deposition of PdNPs via ALD minimizes deactivation and leaching, ensuring consistent catalytic efficiency over multiple reaction cycles. The Suzuki-Miyaura cross-coupling reaction illustrated in
Scheme 10 further highlights the efficacy of this system in promoting efficient organic transformations [
90].
4.3. Palladium Nanoparticles Supported on Nitrogen-Doped Carbon Nanosheets (N-CNS)
Palladium nanoparticles supported on nitrogen-rich carbon nanosheets (Pd/N-CNS) represent a significant advancement in heterogeneous catalysis, particularly Suzuki cross-coupling reactions. Using an intercalation templating strategy, Chui et al. synthesised two-dimensional nitrogen-rich carbon nanosheets (N-CNS) derived from petroleum asphalt [
59]. These carbon nanosheets were enriched with pyridinic nitrogen species and served as a robust support for palladium nanoparticles by enhancing metal-support interactions and stabilising the nanoparticles [
59]. This nitrogen-rich environment also improved the dispersibility of palladium, which is necessary for achieving high catalytic performance.
The Pd/N-CNS catalyst achieved an impressive turnover frequency (TOF) of 2390 h
-¹ under mild conditions for Suzuki cross-coupling reactions, representing a nearly nine-fold increase in activity compared to commercial Pd/C catalysts. It also maintained its high activity over five consecutive reaction cycles, with a TOF of 2294 h⁻¹, demonstrating exceptional stability and reusability [
59]. Theoretical insights attributed the enhanced performance to efficient electron transfer facilitated by the high density of the pyridinic nitrogen species.
The catalytic efficacy of Pd/N-CNS was further validated in the Suzuki cross-coupling reaction between bromobenzene and phenylboronic acid (
Scheme 11), where it achieved significantly higher conversion rates (85%) compared to other support systems.
These findings highlight the practical advantages of Pd/N-CNS catalysts in facilitating efficient and selective organic transformations. By exploiting the unique properties of nitrogen-doped carbon materials, this work provides a promising framework for the design of efficient, stable and sustainable Pd-based systems with broad applicability in industrial catalysis.
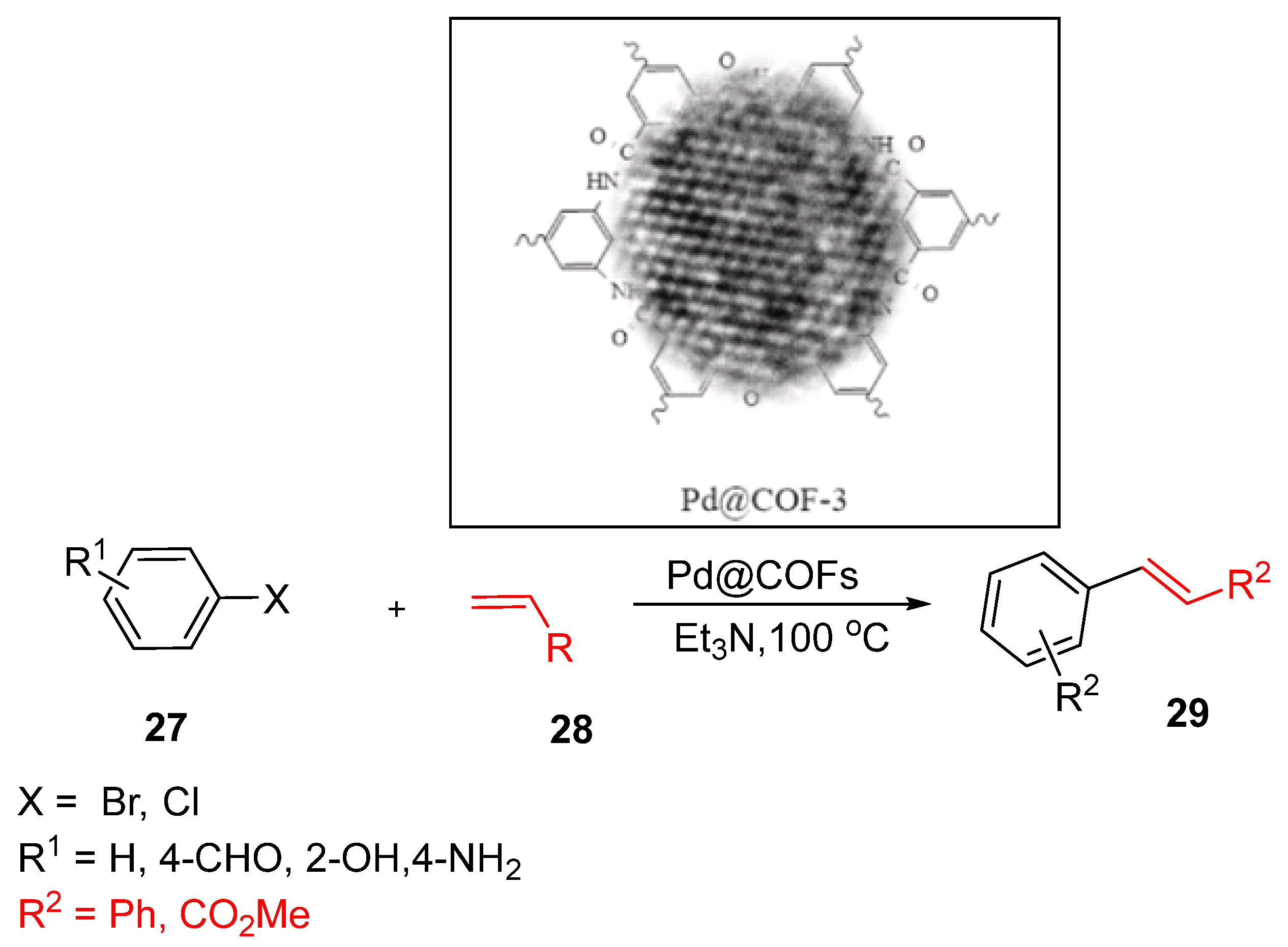
4.4. Palladium Nanocrystals-Embedded Covalent Organic Framework (Pd@COF)
Abdulah
et al., demonstrated the potential of palladium nanocrystals (Pd NCs) embedded in covalent organic frameworks (COFs) as highly efficient heterogeneous catalysts for carbon-carbon (C–C) cross-coupling reactions, particularly the Heck reaction [
91]. The versatility of Pd NCs@COFs in sustainable catalysis has been highlighted in recent studies [
92,
93,
94,
95]. Pd NCs were synthesized in situ within COFs via a solvothermal method using tricarboxylic acids such as H₃L2, H₃L1, H₃BTC and 1,3,5-triazine-2,4,6-triamine moieties. This approach yielded Pd NCs@COFs with particle sizes ranging from 1 to 5 nm, ensuring excellent catalytic performance [
91].
The catalytic efficacy of Pd NCs@COFs was demonstrated by the Heck reaction (
Scheme 12), where vinyl derivatives reacted with aryl halides in the presence of trimethylamine (Et₃N) as a base. Using only 5 mol.% of Pd NCs@COFs, complete conversion (100%) was achieved under mild reaction conditions. Various substrates, including chloro- and bromobenzene derivatives, were successfully employed both with and without functional groups such as formyl (CHO), hydroxyl (O–H), and amine (NH₂). Vinyl derivatives such as styrene and methyl acrylate also exhibited excellent conversions, highlighting the versatility of the catalyst.
A notable advantage of Pd NCs@COFs is their exceptional recyclability, which was investigated under identical reaction conditions over multiple catalytic cycles. Even after the fourth cycle, the catalyst retained over 95% conversion, with no observable palladium leaching, as confirmed by ¹H NMR spectroscopy [
91]. The solid catalyst could be easily separated via filtration or centrifugation, washed with ethanol and reused without significant loss of performance. This stability is attributed to the nitrogen-rich framework of the COFs, which enhances the stabilization of the Pd nanocrystals and prevents agglomeration [
93].
The straightforward synthesis of Pd NCs@COFs further underscores their practical applicability. The addition of Pd salts to COFs facilitates the coordination of Pd²⁺ ions, followed by in situ reduction to Pd⁰, resulting in a robust and efficient catalyst. The high stability and consistent performance of Pd NCs@COFs make them excellent candidates for sustainable catalysis, in alignment with green chemistry principles [
96].
The innovative design and synthesis of Pd NCs@COFs represent a significant advancement in cross-coupling catalysis. By combining exceptional catalytic performance, recyclability and environmental compatibility, Pd NCs@COFs provide a powerful platform for eco-friendly chemical processes, underscoring their potential as next-generation catalysts for organic synthesis [
91].
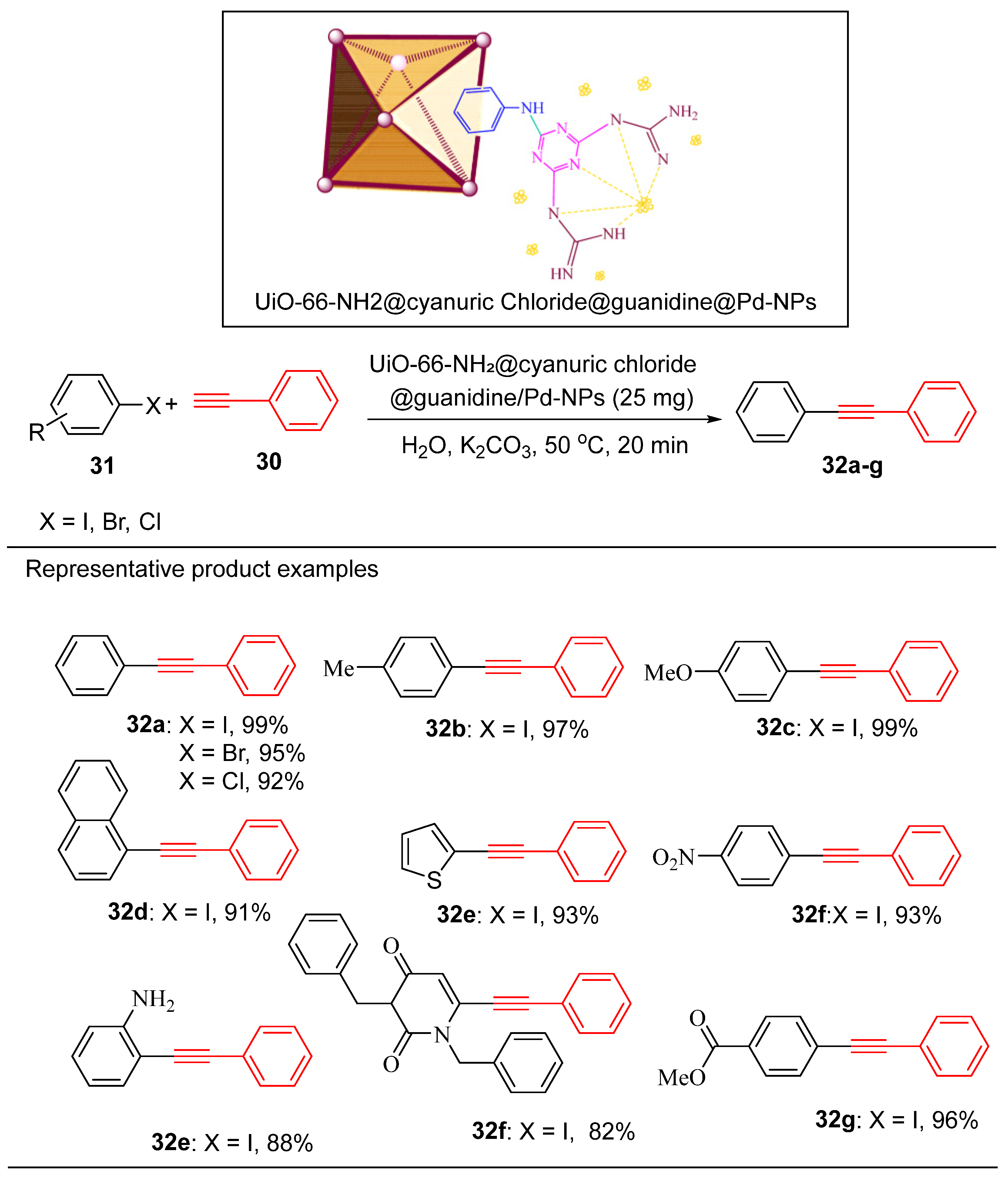
4.5. Palladium Nanoparticle-Decorated Porous Metal–Organic-Framework (Zr)@Guanidine
Metal–organic frameworks (MOFs) have emerged as exceptional supports for immobilizing palladium nanoparticles (Pd-NPs) in recent decades, significantly enhancing their catalytic activity and stability [
97,
98]. MOFs have garnered considerable attention for catalytic C–C coupling reactions owing to their unique properties, including extensive surface area, highly ordered structures, adjustable pore sizes, uniformly distributed metal nodes and excellent chemical modifiability [
10,
84]. Among these, UiO-66-NH₂, a zirconium-terephthalate MOF, is particularly noteworthy because of its remarkable chemical, thermal and mechanical stability, intrinsic open metal sites, large surface area and amino group functionalities, making them excellent candidates for post-synthetic functionalization [
99,
100].
Mohammadi and Vaezi developed UiO-66-NH₂ functionalized with cyanuric chloride and guanidine, a nitrogen-rich organic ligand, via a post-synthetic modification (PSM) approach [
101]. The guanidine moiety, with its amino functionalities, acted as a robust binding centre, modifying the electronic structure of UiO-66-NH₂ and effectively immobilizing Pd²⁺ ions. These ions were subsequently reduced to Pd⁰, resulting in uniformly dispersed Pd NPs across the MOF. This innovative design enhanced the catalyst’s stability and efficiency, particularly in aqueous environments, due to the strong coordination between the Zr₆ cluster secondary building units (SBUs) and the nitrogen-rich linker [
102,
103,
104,
105,
106,
107,
108].
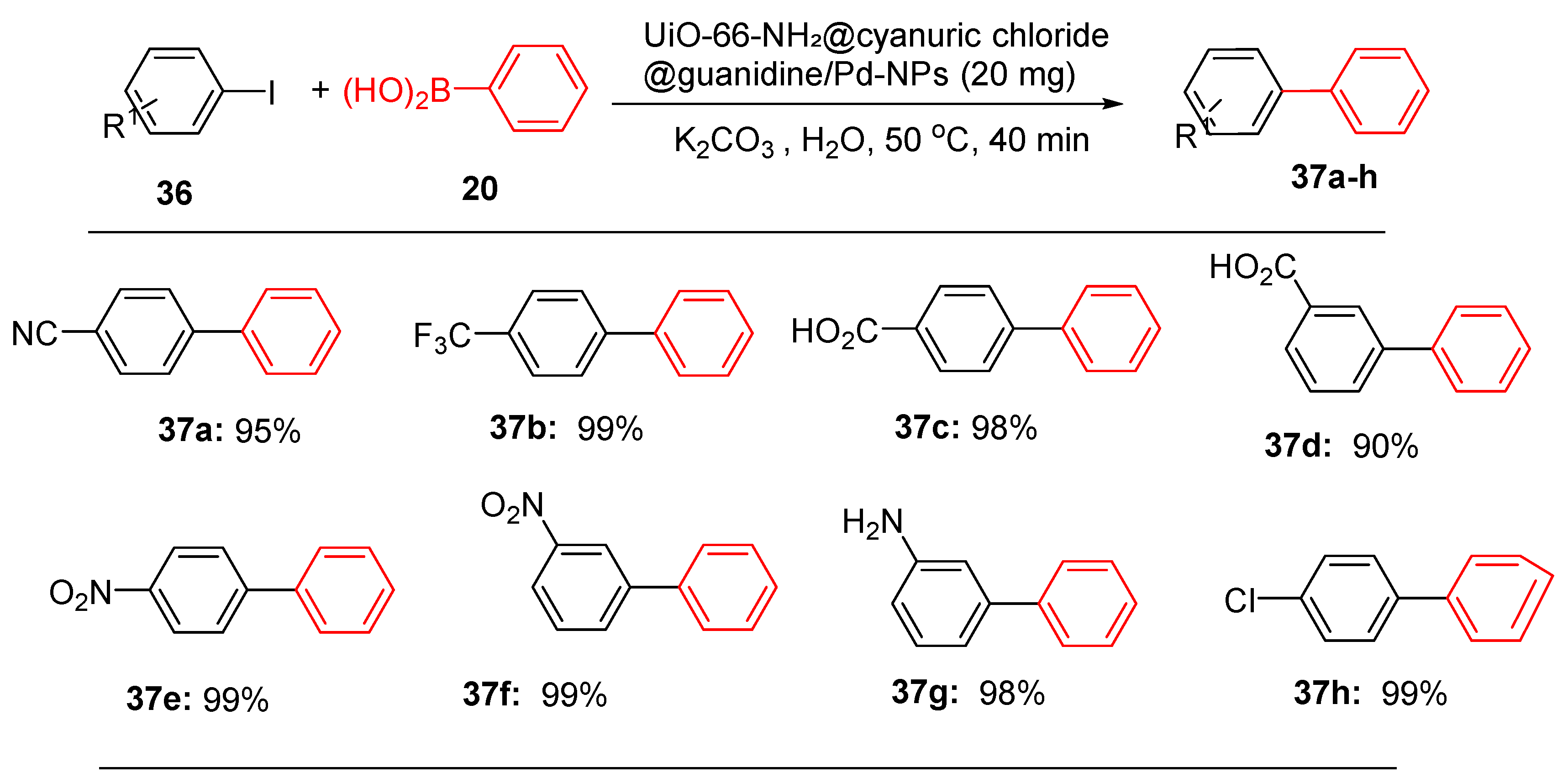
The catalytic efficacy of UiO-66-NH₂@cyanuric chloride@guanidine/Pd-NPs was thoroughly investigated in Suzuki, Heck and Sonogashira cross-coupling reactions [
101]. The reaction conditions were optimized by the reaction of iodobenzene and phenylacetylene with UiO-66-NH2@cyanuric chloride/guanidine/Pd-NPs (
Scheme 13). The optimal reaction conditions were then applied to the Sonogashira reactions of diverse aryl halides and terminal alkynes to afford diverse organic compounds in high yields (
Scheme 13).
The synthesized catalyst was highly efficient and widely applicable, as demonstrated by its ability to catalyze the Sonogashira coupling process in the presence of iodine, bromine, and chlorine derivatives of aromatic compounds. Moreover, the catalyst was efficient in the Sonogashira carbonylative reaction (
Scheme 14).
For the Suzuki reaction, iodobenzene reacted with boronic acids efficiently in water and the optimum conditions (20 mg of catalyst in the presence of K
2CO
3 at 50 °C and 40 min of reaction time) were applied to afford some of these representative compounds (
Scheme 15).
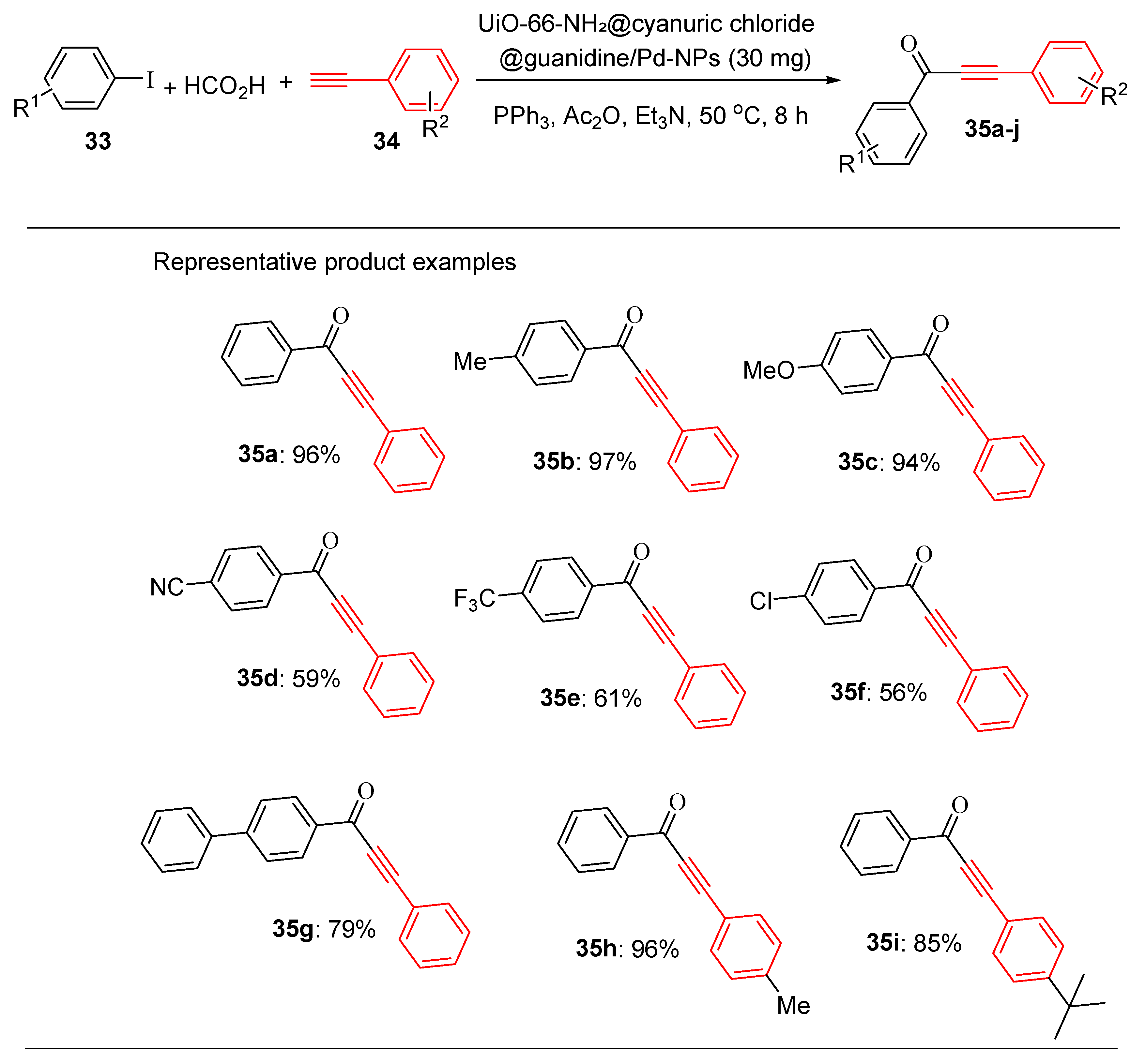
Similarly, the catalyst was also efficient in the Heck reaction involving halobenzene and various alkenes under the same conditions, affording high product yields within 2 h using 30 mg of the catalyst [
101]. These results demonstrate the broad substrate compatibility, high selectivity and exceptional efficiency of the catalyst for diverse C–C coupling reactions. In addition, the catalyst exhibited outstanding performance in carbonylative Sonogashira reactions under mild conditions, further demonstrating its versatility.
A significant feature of UiO-66-NH₂@cyanuric chloride@guanidine/Pd-NPs was their exceptional recyclability and stability. The catalyst retained over 85% of its initial activity after nine consecutive cycles, with no significant loss of efficiency or Pd leaching observed during the reactions. This durability was attributed to the robust nitrogen-rich framework provided by guanidine and the structural integrity of UiO-66-NH₂. The straightforward synthesis and functionalization of the catalyst further enhance its scalability and practicality for industrial applications.
The UiO-66-NH₂@cyanuric chloride@guanidine/Pd-NP catalyst represents a significant advancement in heterogeneous catalysis by combining excellent catalytic performance, sustainability and recyclability. Its ability to operate in water as a green solvent and its compatibility with various substrates underscores its potential for eco-friendly organic synthesis. With continued advancements in the synthesis, stability and catalytic applications of MOF-supported Pd NPs, this study highlights a promising platform for green and sustainable chemistry.
5. Palladium Nanoparticles Supported on Functionalized Synthetic Material
The immobilization of palladium nanoparticles (PdNPs) on non-polymeric synthetic materials offers an innovative approach to catalysis, combining enhanced stability, reusability and efficiency with green chemistry principles. Synthetic supports such as boehmite nanoparticles, polydimethylsiloxane films, amphiphilic frameworks and surfactant-based systems provide robust platforms for stabilizing PdNPs, preventing leaching and optimizing catalytic performance in demanding applications [
109,
110,
111]. These materials create precisely tuned environments for PdNPs, facilitating superior activity and selectivity in key transformations, such as Suzuki-Miyaura, Heck and Sonogashira couplings, while ensuring recyclability and minimal environmental impact [
10,
46].
Each type of synthetic support has unique advantages in catalytic systems. For example, functionalized boehmite nanoparticles enhance catalytic stability in environmentally friendly solvents, such as PEG-400, while minimizing palladium leaching [
109]. Polydimethylsiloxane films improve the dispersion and durability of PdNPs, enabling efficient C–C coupling and pollutant reduction under mild conditions [
110]. Pd nanoparticles (Pd NPs) supported on micellar structures provide a versatile platform for advanced cross-coupling reactions [
112], whereas chiral surfactants offer novel opportunities for asymmetric catalysis with reduced reliance on toxic ligands and solvents [
111]. Together, these systems illustrate the transformative potential of functionalized synthetic materials for advanced PdNP-based catalysis.
This section explores the latest advancements in these synthetic supports, highlighting their contributions to enhancing the stability, sustainability, and efficiency of PdNPs across diverse applications.
5.1. Palladium Nanoparticles Supported on Boehmite Nanoparticles (Pd(0)-SMTU-Boehmite)
Boehmite, a hydrated aluminum oxide, has gained prominence as a support material for palladium nanoparticles (Pd NPs) because of its remarkable thermal stability and its ability to enhance nanoparticle dispersion [
113,
114]. These attributes make it an excellent candidate for various catalytic applications, particularly C–C coupling reactions and other industrial processes. The high surface area and mesoporous structure of boehmite provide an optimal environment for catalytic activity as demonstrated in numerous studies [
115,
116].
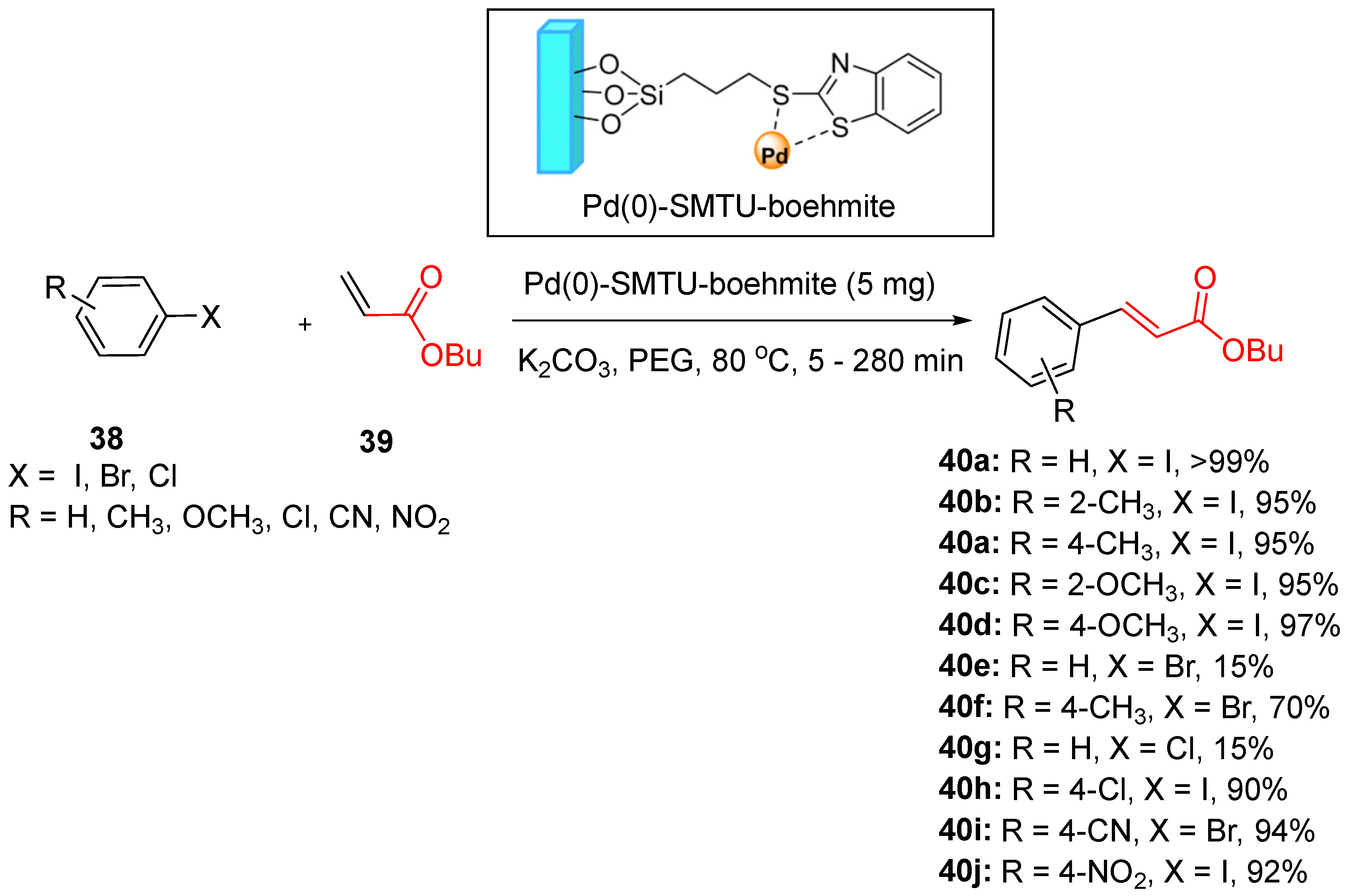
In a notable study, Hajighasemi et al. synthesized palladium complex-supported boehmite nanoparticles (Pd(0)-SMTU-boehmite) and successfully applied them to Mizoroki-Heck and Suzuki-Miyaura C–C coupling reactions. The coupling of iodobenzene with butyl acrylate under optimized conditions of temperature, catalyst loading, base and solvent demonstrated the versatility and efficiency of Pd(0)-SMTU-boehmite in carbon–carbon bond formation [
109]. Iodobenzene was first coupled with butyl acrylate under different operating conditions, such as temperature, amount of catalyst, base and solvent, and the optimum conditions were applied to the coupling of different aryl halides
38 (
Scheme 16). This catalyst exhibited broad applicability with various aryl halides and maintained excellent activity under mild reaction conditions.
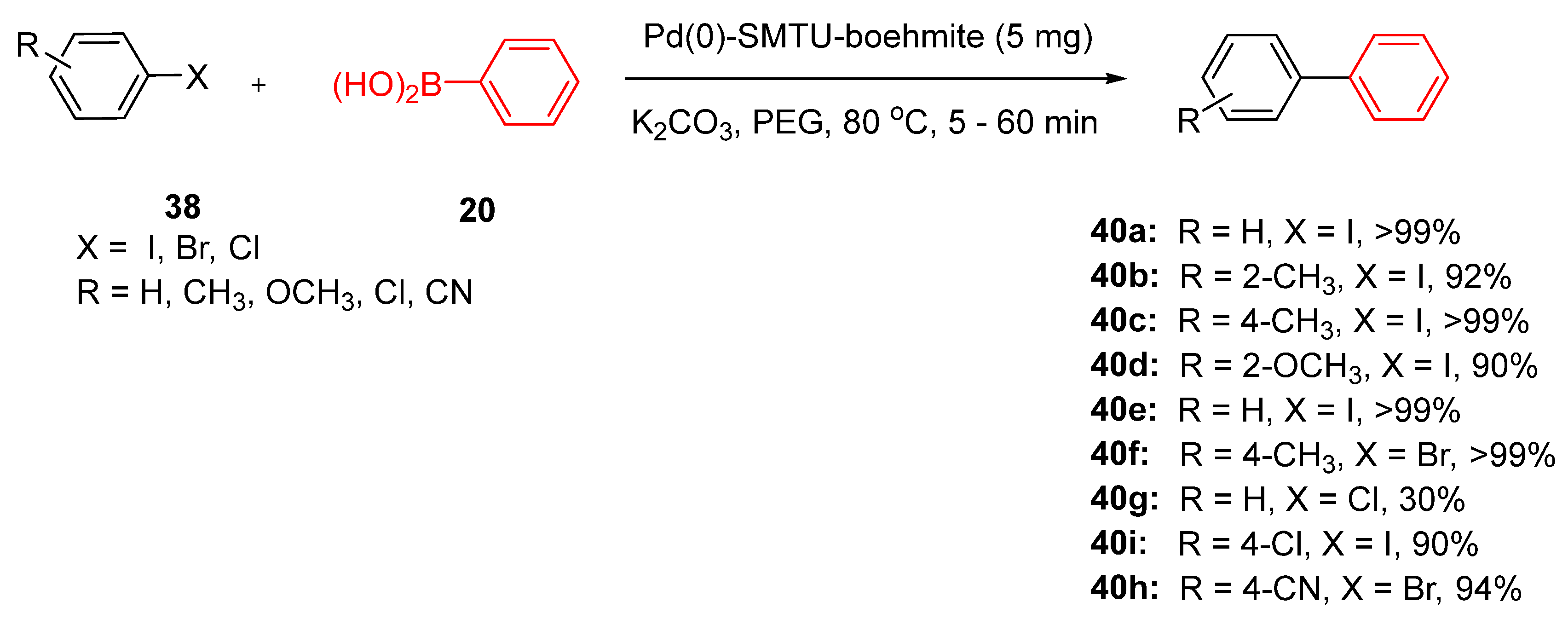
The authors also explored the reactivity of the catalyst in the Suzuki-Miyaura cross-coupling reaction to expand the application of the catalyst in biaryl synthesis through the reaction of various types of aryl halides
38 under optimum reaction conditions (
Scheme 17).
These reactions are conducted under environmentally benign conditions, highlighting the role of this catalyst in advancing green chemistry. Importantly, the catalyst demonstrated excellent recyclability, retaining its activity over multiple cycles with negligible palladium leaching, as verified through ICP-OES and hot filtration tests [
109,
117].
The robustness of boehmite-supported palladium catalysts also extends to high-temperature applications, such as selective oxidation and hydrogenation reactions, where they provide stable and efficient platforms for catalytic transformations [
113,
116] Despite these advantages, challenges in preparing and modifying boehmite surfaces remain, necessitating further research to maximize their catalytic potential. Nevertheless, the outstanding performance of Pd(0)-SMTU-boehmite in cross-coupling reactions underscores its critical role in the development of sustainable catalytic technology.
5.2. Palladium Nanoparticles Supported on Micellular Structures
Palladium nanoparticles (Pd NPs) supported on micellar structures represent a significant advancement in catalytic technologies, particularly for cross-coupling reactions, such as the Suzuki-Miyaura reaction. The amphiphilic architecture of micelles provides a unique aqueous environment that enhances the catalytic efficiency, stability and recyclability of Pd NPs. This approach aligns with green chemistry principles, leveraging water as a solvent while reducing the reliance on hazardous organic reagents. Among these systems, poly(2-(dimethylamino)ethyl methacrylate)-b-poly(lauryl methacrylate) (PDMAEMA-b-PLMA) micelles have demonstrated exceptional performance, achieving yields of 73–98% at room temperature with as little as 0.05 mol% Pd loading, without requiring additional ligands [
112,
118]. This stability is further enhanced by the micellar framework, which prevents agglomeration of Pd NPs and enables multiple catalyst reuse cycles with minimal activity loss [
112,
118].
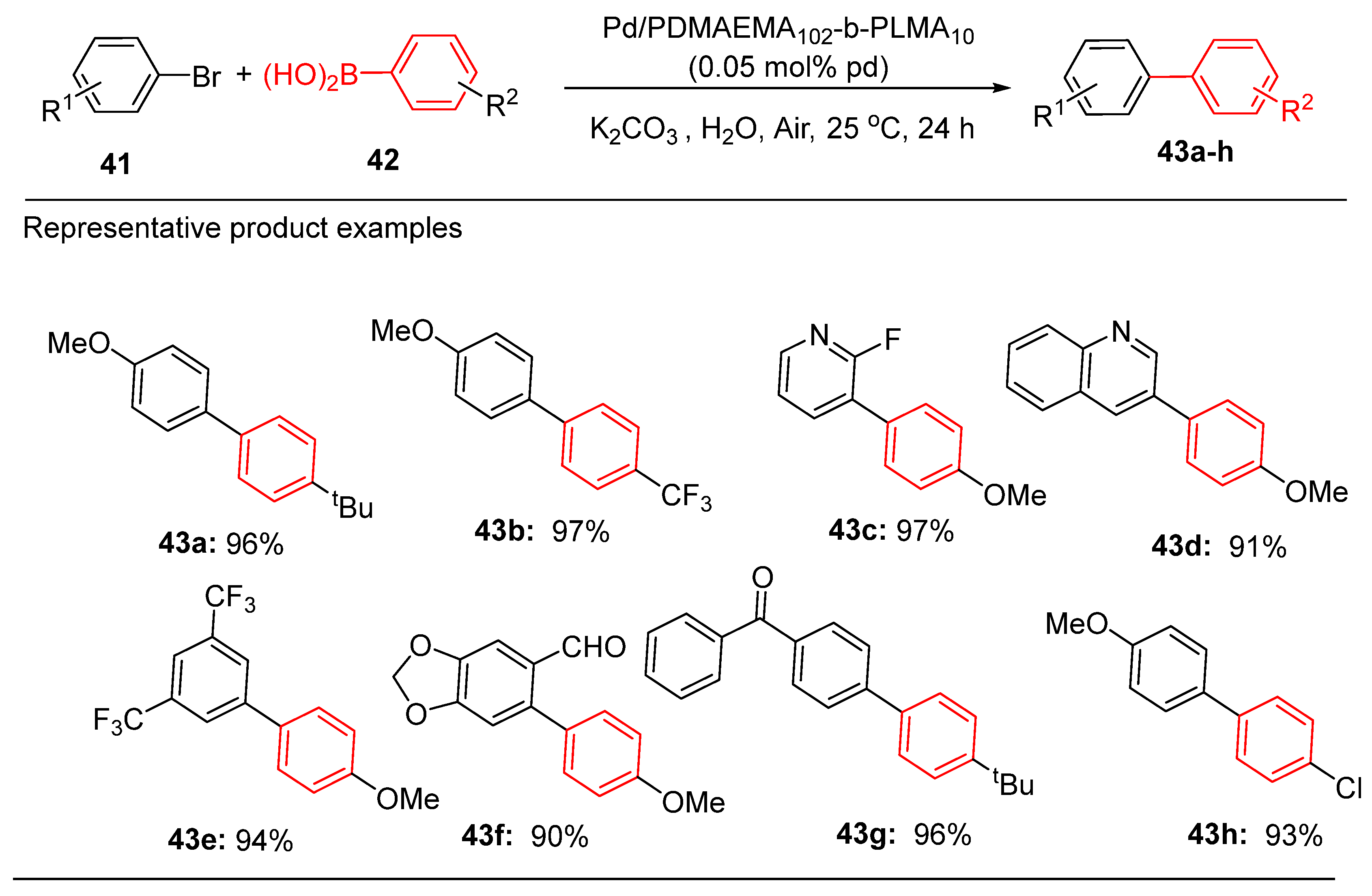
The structural properties of the micelles are critical for determining their catalytic performance. Increasing the hydrophilic PDMAEMA segment significantly improved the efficiency, while increasing the hydrophobic PLMA content diminished the activity. Pd/PDMAEMA102-b-PLMA10, with an optimized Pd:N ratio of 1:10, consistently demonstrated >99% yields in Suzuki-Miyaura cross-coupling reactions [
83]. Conversely, lower Pd:N ratios, such as 1:30, led to reduced catalytic activity, attributed to insufficient stabilization of Pd NPs, resulting in larger, less reactive particles. These micellar systems also exhibit broad substrate compatibility, efficiently coupling electron-rich and electron-poor aryl bromides, including challenging ortho-substituted derivatives. Functionalized arylboronic acids, bulky substituents, and pharmaceutically relevant scaffolds, such as pyridyl and quinolinyl derivatives, were successfully transformed, highlighting the versatility of these catalysts (
Scheme 18) [
83].
Mechanistically, micelles act as nanoreactors providing a confined space that enhances solubilization and electron transfer between Pd NPs and reactants, thereby optimizing reaction rates and selectivity. The synergistic interactions between Pd NPs and the micellar framework create a favorable microenvironment for efficient catalytic transformations [
118,
119]. Despite these promising attributes, challenges remain in scaling these systems for industrial applications. Micelle degradation over extended use and substrate compatibility with aqueous systems require further research. Nonetheless, the continued development of robust micellar designs and innovative surfactants offers the potential to overcome these limitations and expand the application of micellar-supported Pd NPs in sustainable catalysis [
118,
119].
5.3. Palladium Nanoparticles Supported on Polydimethylsiloxane Film
Polydimethylsiloxane (PDMS) films have emerged as innovative supports for palladium nanoparticles (Pd NPs) offering enhanced stability and catalytic performance in diverse applications. Majhi et al. [
110]. developed a novel method for immobilizing Pd NPs on PDMS films demonstrating their efficacy in C–C coupling reactions, including Suzuki-Miyaura and Sonogashira reactions as well as in the reduction of organic pollutants. The PDMS matrix provides a durable and flexible platform for Pd NPs ensuring stability under the reaction conditions while maintaining excellent catalytic efficiency. This approach is particularly significant in the context of sustainable catalysis because PDMS-based systems eliminate the need for high-energy reaction conditions and hazardous solvents. Moreover, the hydrophobic nature of the PDMS films facilitated the dispersion of Pd NPs and enhanced substrate interactions, contributing to improved turnover frequency and selectivity. The use of PDMS as a functionalized support aligns with the emerging trends in green chemistry, providing a robust, reusable and environmentally friendly platform for Pd-based catalysis.
5.4. Palladium Nanoparticles Supported Chiral Surfactants
Chiral surfactants offer a unique platform for stabilizing Pd nanoparticles, enabling asymmetric catalysis with enhanced selectivity and efficiency. Ranganath et al. [
111]. demonstrated the use of chiral surfactants to stabilize Pd NPs in asymmetric Suzuki cross-coupling reactions. The chiral environment provided by these surfactants promotes the formation of enantioselective products while reducing the need for toxic ligands and solvents. This approach aligns with sustainable chemistry principles by minimizing waste and incorporating greener methodologies into catalytic systems. The stabilization of Pd NPs by chiral surfactants ensures their activity and selectivity even under mild reaction conditions, whereas the asymmetric nature of the catalytic environment enables the efficient synthesis of enantioenriched compounds. These advancements reflect the growing importance of combining catalyst efficiency with sustainability, making chiral-surfactant-supported Pd NPs a promising avenue for optimizing cross-coupling reactions and broadening the scope of asymmetric catalysis.
6. Palladium Nanoparticles Supported on Aerobic bacterial Cells (Paracoccus yeei)
The innovative use of aerobic bacterial cells, specifically
Paracoccus yeei, as a sustainable support for palladium nanoparticles (Pd NPs) represents a breakthrough in green catalytic technologies. Rybochkin et al. [
62]. explored this biohybrid system using
Paracoccus yeei VKM B-3302 as the supporting matrix, addressing the high energy consumption and waste associated with traditional Pd catalysts. The resulting biohybrid catalyst exhibited exceptional efficiency in Mizoroki-Heck and Suzuki-Miyaura cross-coupling reactions, achieving catalytic performances comparable to those of the commercial Pd/C catalysts.
A notable advantage of this system is its recyclability, which maintains a consistent activity over five reuse cycles without significant performance degradation. This recyclability not only underscores its economic viability but also its alignment with sustainability goals. The integration of biological supports into catalytic systems highlights the potential of biohybrid catalysts to reduce the environmental impact while maintaining high efficiency. By leveraging the metabolic and structural properties of aerobic bacteria, this approach opens new avenues for designing eco-friendly and cost-effective catalytic systems for industrial applications.
7. Palladium Nanoparticles Supported on Porous Silica Materials
Porous silica materials have emerged as versatile supports for palladium nanoparticles (Pd NPs), offering exceptional stability, recyclability and efficiency in heterogeneous catalysis [
46,
120]. Recent advancements in the synthesis and application of Pd NPs immobilized on zeolites and mesoporous silica-based materials have underscored their significant potential for sustainable cross-coupling reactions, including the Suzuki-Miyaura, Heck, Stille and Sonogashira reactions. [
49,
120,
121,
122] By immobilizing Pd NPs on porous silica structures, these systems mitigate critical issues, such as nanoparticle aggregation and leaching, ensuring consistent catalytic activity across multiple cycles [
44,
120].
Studies have demonstrated that Pd NPs supported on mesoporous silica exhibit remarkable catalytic efficiency and can be reused several times without a significant loss of activity, making them particularly attractive for industrial-scale applications. [
120,
122] The porous structure of silica not only enhances substrate accessibility but also ensures uniform dispersion of active catalytic sites, significantly improving reaction rates and selectivity. Moreover, functionalized porous silica materials allow the precise tuning of catalytic properties paving the way for advanced and sustainable catalytic systems [
120].
As highlighted in the recent literature, the versatility of porous silica supports continues to drive innovation in Pd NP-based catalysis [
49,
120,
121,
122]. These systems address the dual challenges of efficiency and sustainability, reinforcing their role as essential tools for greener chemical syntheses in cross-coupling reactions.
The unique attributes of porous silica materials, as detailed in this section, contribute to a broader discussion of the solid matrices presented in
Section 9.3. This interconnected perspective emphasizes the major role of silica-based supports in advancing Pd NP catalysis across diverse applications.
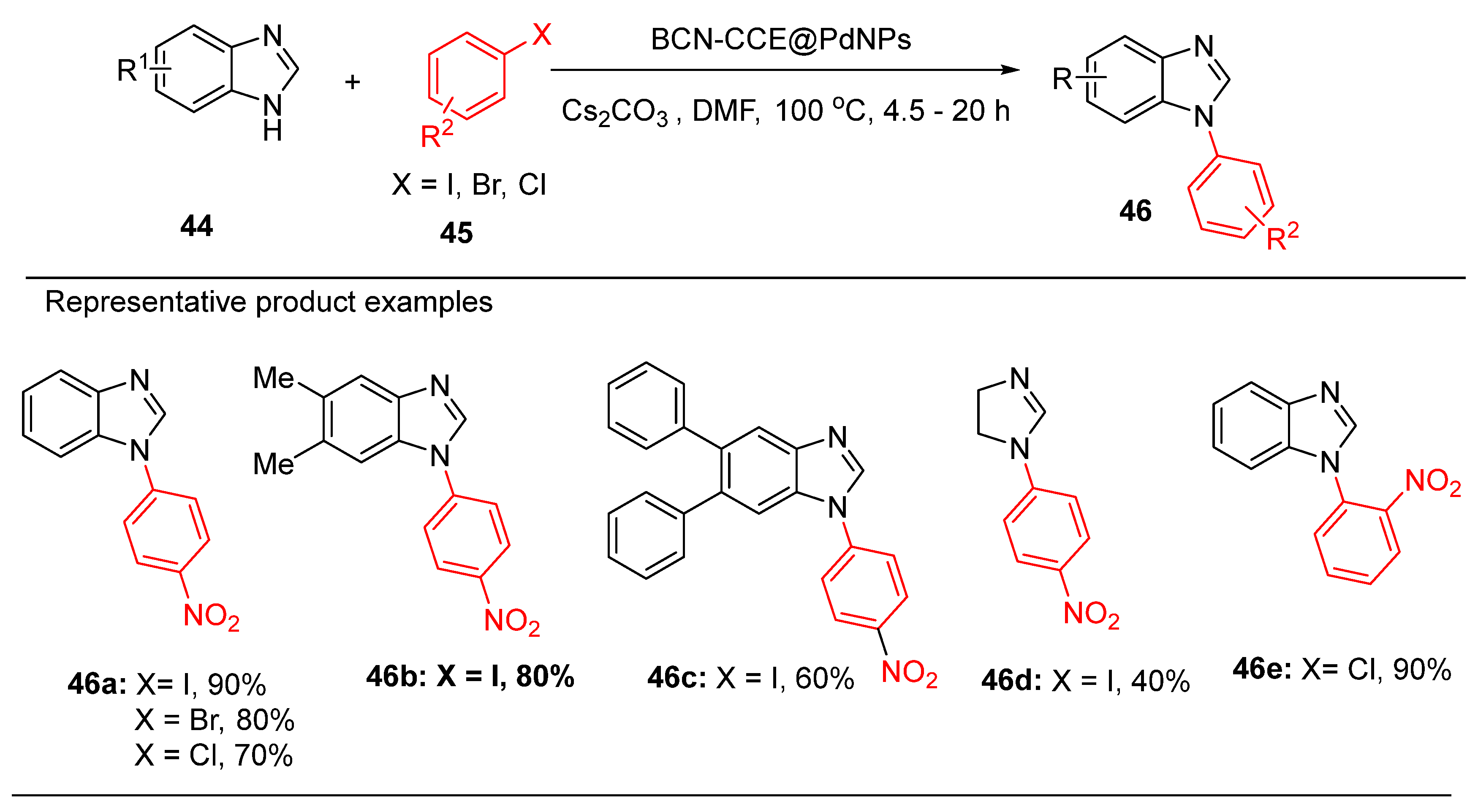
8. Palladium Nanoparticle Immobilized on Coconut Coir Extract Coated Boron Carbon Nitride
Chandrashekharan et al. presented a novel approach to sustainable catalysis through the development of a Pd nanocatalyst immobilized on boron carbon nitride coated with a coconut coir extract (BCN-CCE@Pd) [
60]. This eco-friendly system demonstrated remarkable catalytic efficiency in Suzuki-Miyaura cross-coupling and C–N arylation reactions under mild conditions, achieving excellent product yields (
Scheme 19) [
60].
The synergy between the boron carbon nitride support and the coconut coir extract significantly enhanced the stability and reactivity of the Pd NPs, providing a highly active and reusable catalytic platform.
The catalytic efficiency of the BCN-CCE@Pd nanocatalyst over multiple reaction cycles highlights its potential for industrial applications where sustainability and cost-effectiveness are critical. This study underscores emerging trends in the utilization of natural and renewable materials to support palladium catalysts, paving the way for greener methodologies for cross-coupling catalysis.
9. Palladium Nanoparticles Supported on Metal Oxides and Metal Oxide Hybrid Materials as Catalyst for Cross Coupling Reaction
The strategic integration of palladium nanoparticles (PdNPs) with advanced support materials has led to transformative innovations in catalysis, particularly in cross-coupling and oxidation reactions [
89]. Among these, metal oxides provide an ideal platform for stabilising and dispersing palladium species, enhancing their accessibility, activity and durability [
89,
123]. Atomically dispersed palladium on metal oxides exemplifies this advancement with superior catalytic efficiency and selectivity attributed to uniform active sites and enhanced turnover frequencies [
120]. These catalysts not only improve energy efficiency but also align with sustainable chemistry principles, making them indispensable for precision-driven applications.
This section discusses the synergistic role of PdNPs and metal oxide supports in catalytic applications, focusing on cutting-edge systems. [
89,
120,
123,
124,
125,
126] These innovations illustrate how tailored support materials can amplify the catalytic potential of palladium while addressing key challenges in scalability, environmental impact, and mechanistic understanding. By exploring these advancements, this section highlights the critical contributions of metal-oxide-supported PdNPs to modern industrial and environmental catalysis.
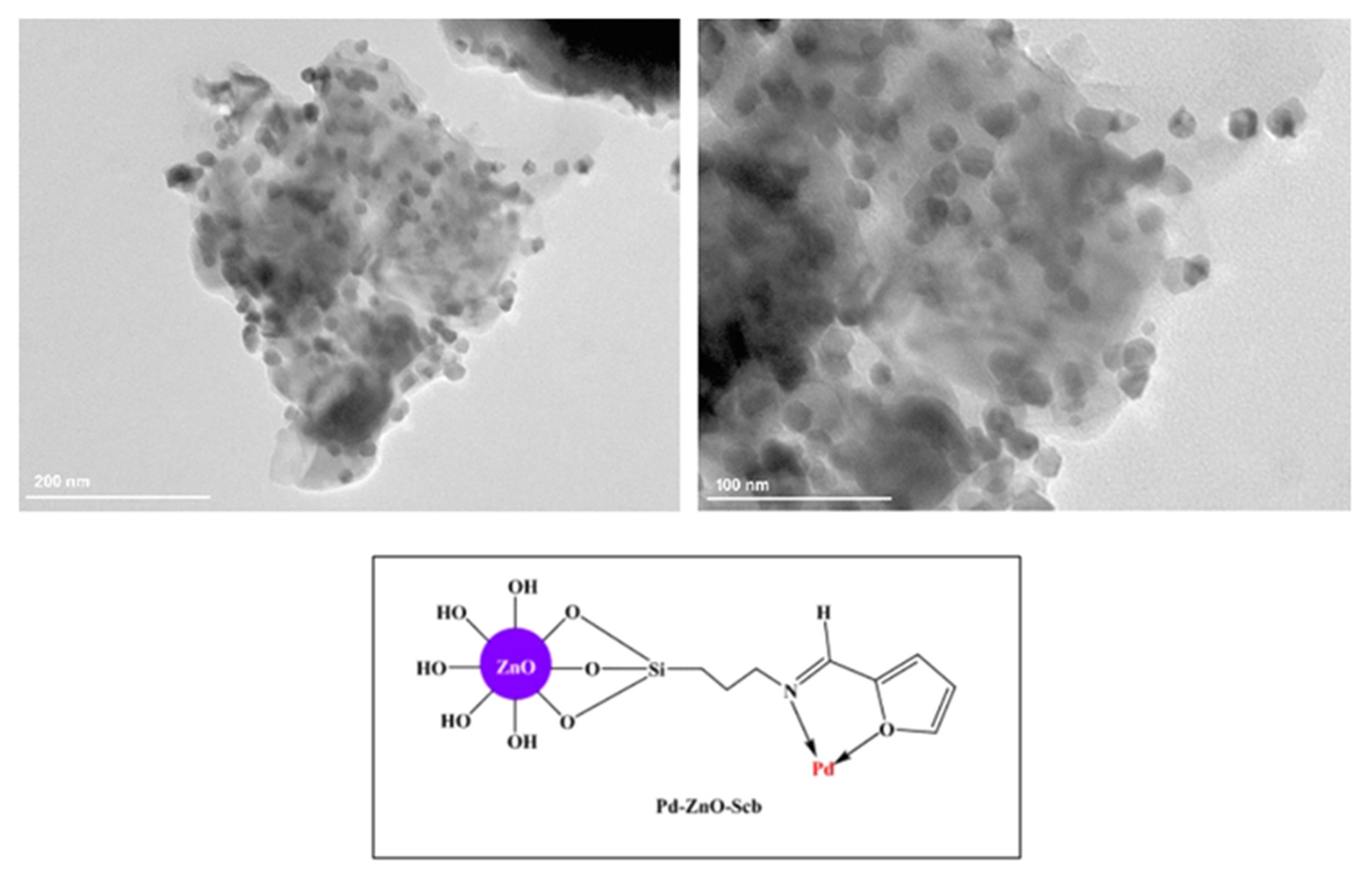
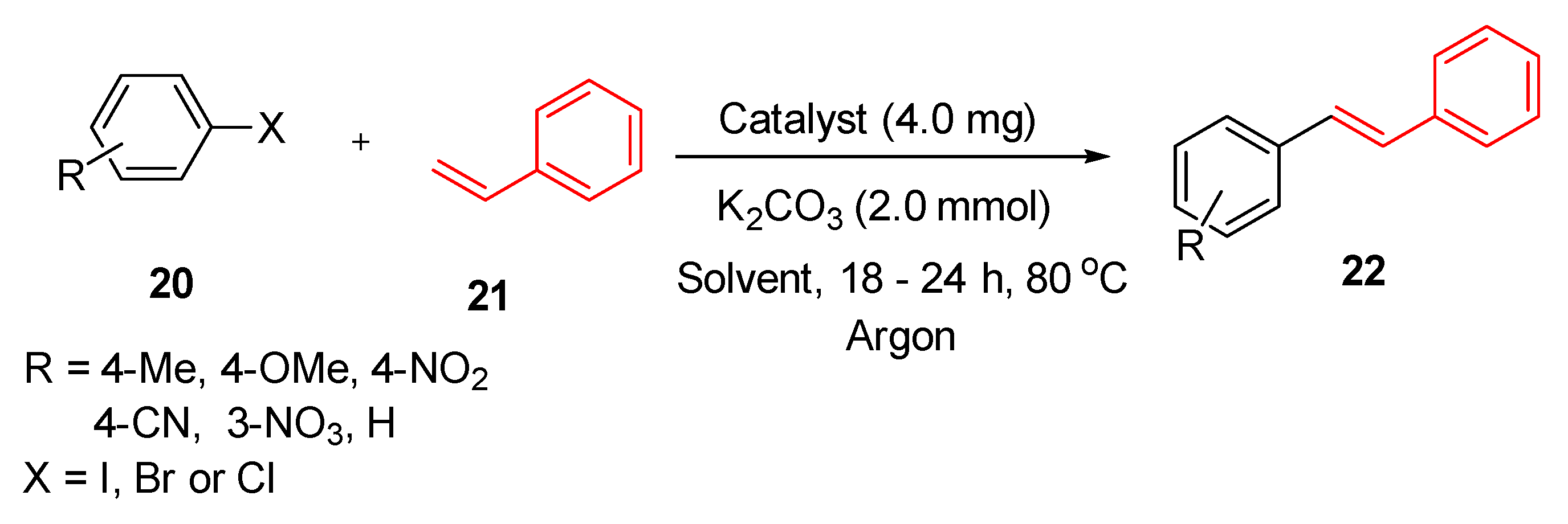
9.1. Immobilised Palladium Nanoparticles on Schiff Base-Modified ZnO Particles (Pd–ZnO–Scb)
Baran and co-workers employed nanoparticles immobilized on Schiff base-modified ZnO particles (Pd–ZnO–Scb) (
Figure 2) as catalysts for the Heck coupling reactions [
124].
This study highlights the effectiveness of this catalyst in the coupling of various aryl halides (
20) with styrene (
21) and the yield was up to 98% (
Scheme 20). Compared to other methods, the authors found that the catalyst was also highly efficient in reduction of 4-nitrophenol due to the formation of merely one product and this was achieved in 135 s at room temperature [
124].
9.2. Pd Nanoparticles Supported by Atomically Dispersed Metal Oxide
Atomically dispersed palladium (Pd) catalysts stabilized by metal oxides represent a breakthrough in cross-coupling catalysis, offering enhanced catalytic performance, stability and sustainability. These catalysts were synthesized through oxidative fragmentation, where metallic Pd nanoparticles were converted to PdO at 400 °C and subsequently fragmented into atomically dispersed Pd cations at temperatures above 500 °C. These atomically dispersed species, stabilized by metal oxide supports, exhibit high surface accessibility and unique electronic properties that enhance their catalytic efficiency and selectivity in reactions such as Suzuki-Miyaura and Heck coupling [
127].
Among metal oxides, magnesium oxide (MgO) has demonstrated exceptional efficacy in stabilizing Pd cations during these transformations. The surface-dispersed Pd species on MgO show superior catalytic activity because of their optimized coordination environments which facilitate effective substrate interactions and accelerate reaction rates. The strong metal-support interactions provided by MgO prevent nanoparticle agglomeration and ensure consistent catalytic performance across diverse conditions, highlighting its critical role in enabling efficient cross-coupling reactions [
127].
Eco-friendly synthesis methods are integral to the development of these catalysts. Green chemistry principles, such as the use of ethanol as a solvent, energy-efficient ultrasonic cavitation techniquesand recycling strategies to minimize Pd leaching, enhance the sustainability and durability of these systems. These approaches align with the industrial demands for environmentally responsible catalysis. [
127] Magnetic supports, like Fe₃O₄ nanoparticles, further improve catalyst recovery and reusability, making them practical for repeated applications without significant loss of activity [
127].
Atomically dispersed Pd catalysts excel in stabilizing intermediates and provide uniform active sites, leading to high turnover frequencies (TOFs), superior selectivity and energy-efficient operation at lower temperatures. Alternative supports such as Al₂O₃ and TiO₂ present opportunities to diversify catalytic properties while computational modelling and experimental studies will deepen the mechanistic understanding. These advancements will drive the rational design of next-generation catalytic systems, thereby solidifying the role of atomically dispersed Pd catalysts in sustainable industrial catalysis [
127].
9.3. Palladium Nanoparticles Supported on Solid Matrices
Solid matrices play an important role in enhancing the performance of palladium nanoparticles (Pd NPs) in cross-coupling reactions by addressing challenges such as nanoparticle agglomeration, leaching and deactivation. These matrices significantly improve the stability, dispersion and catalytic efficiency of Pd NPs, thereby extending their applicability in Suzuki-Miyaura, Heck and Sonogashira reactions. By leveraging a wide array of support materials, from graphene-based platforms to hybrid organic-inorganic systems, researchers have established solid matrices as indispensable tools in modern catalysis [
46,
128].
The inclusion of functionalized organic and inorganic supports such as dendritic organosilica (DON), aminobenzamide-modified silica-coated superparamagnetic iron oxide and chitosan-MWCNT hybrids, has proven to be particularly effective in enhancing the recyclability and catalytic efficiency of Pd NPs [
129]. These supports mitigate nanoparticle aggregation while promoting improved substrate interactions. Furthermore, materials such as biochar, carbon nanohorns and mesoporous silica provide high surface areas, tailored pore structures and functionalized surfaces, contributing to their superior catalytic performance [
46].
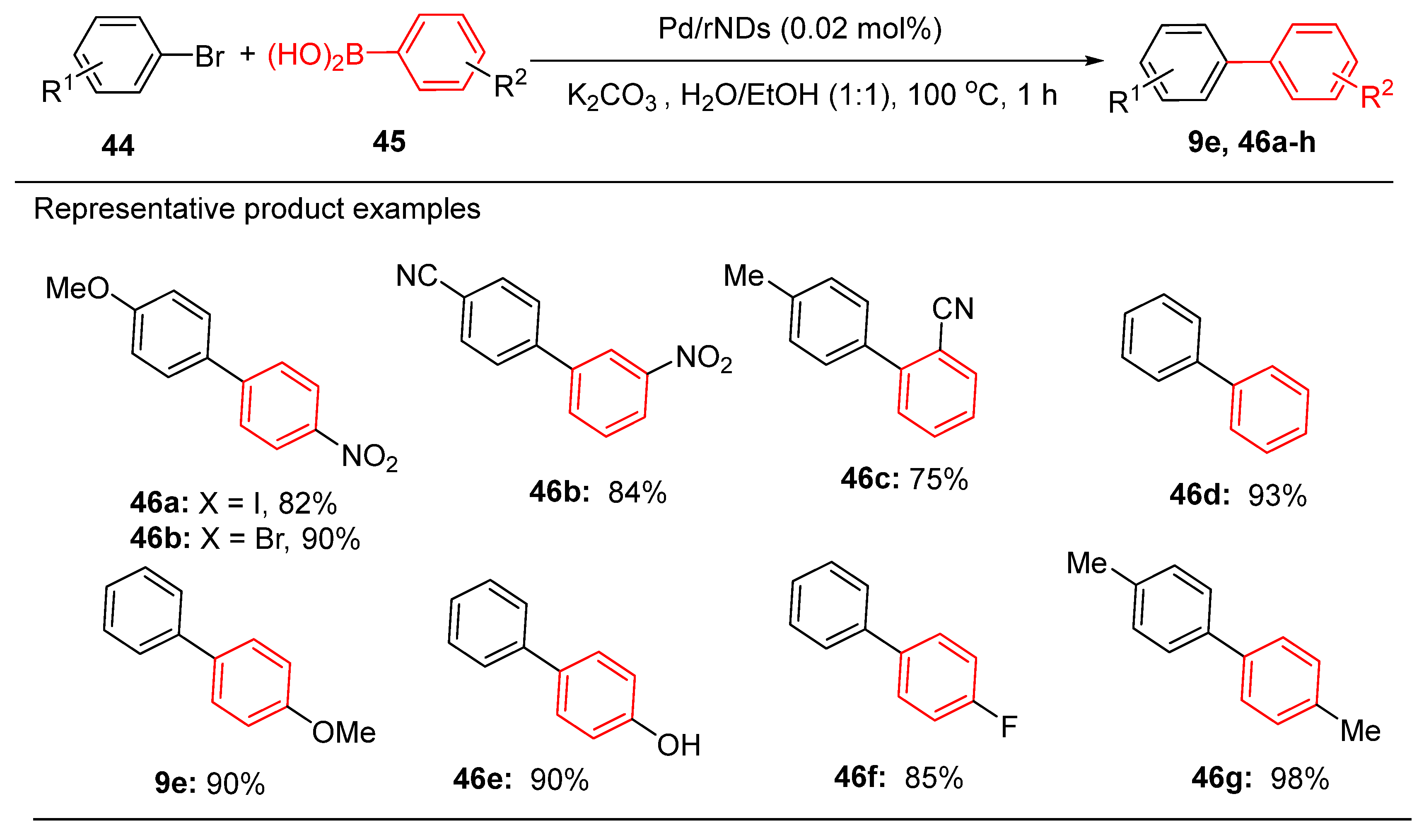
Graphene oxide (GO) has emerged as a widely studied support due to its high surface area, electronic conductivity and chemical stability, which enable effective stabilization of Pd NPs [
130]. Kumar et al. demonstrated the exceptional catalytic activity of Pd NPs immobilized on GO in Suzuki-Miyaura and Mizoroki-Heck reactions, highlighting their environmentally friendly design with minimal reliance on toxic reagents. [
128] Similarly, nanodiamond-based supports, such as Pd/rNDs, synthesised by Pocklanová et al., achieved yields of up to 98% for biaryl products in Suzuki reactions under mild solvent-free conditions. These catalysts also exhibited excellent stability and retained activity over multiple reuse cycles. (
Scheme 21) [
126].
Notably, the substrate functional groups strongly influence catalytic efficiency. In Suzuki reactions catalyzed by Pd/rNDs, electron-withdrawing groups such as nitro- and hydroxy-substituted substrates showed slightly lower yields and required longer reaction times than electron-donating groups such as methyl- and methoxy-substituted substrates. High yields were consistently obtained for all substrates bearing electron-donating or electron-withdrawing groups at various ortho and para positions. Aryl bromides also demonstrated excellent interactions with p-tolylboronic acid, yielding the desired products with remarkable efficiency. [
126]
Inorganic supports, such as mesoporous graphitic carbon nitride (mpg-CN), offer tailored pore structures and high thermal stability, enabling the efficient dispersion of Pd NPs and robust catalytic activity under mild conditions [
77]. Biochar, derived from renewable resources, is a cost-effective and sustainable alternative. Functionalized biochars, such as Pd(0)-TBA@biochar, demonstrate high turnover frequencies and recyclability, aligning well with green chemistry principles [
46].
Hybrid support systems further enhance Pd NP catalysis by combining the strengths of organic and inorganic materials [
127]. Metal-organic frameworks (MOFs) with tunable porosity and functional surfaces create highly stable environments for Pd NPs, optimizing their performance in cross-coupling reactions [
131]. Additionally, Zn-Al mixed metal oxides modified with polydopamine have shown high recyclability and catalytic efficiency in Suzuki-Miyaura reactions [
89].
Magnetic supports, such as Fe₃O₄ nanoparticles, offer an innovative approach by enabling straightforward recovery and reuse through magnetic separation. For example, phosphonated polyethylenimine-grafted Fe₃O₄ nanoparticles retain high catalytic efficiency over multiple cycles in the Heck and Suzuki-Miyaura reactions, reducing waste and promoting sustainable catalytic practices [
132].
Although solid matrices have significantly enhanced the performance of palladium nanoparticles in cross-coupling reactions, ongoing efforts to optimize these supports highlight the dynamic interplay between material innovation and catalytic efficiency. Advances in hybrid systems, scalable synthesis and sustainable practices will continue to expand the applicability of solid matrices, thereby setting the stage for broader discussions on future challenges and directions in the field.
10. Future Directions and Challenges
The catalytic applications of palladium nanoparticles (PdNPs) have made substantial advancements; however, challenges such as leaching, support degradation and scalability persist, limiting their industrial adoption. Addressing Pd leaching is critical for ensuring catalyst recyclability and preventing the contamination of reaction products. Studies have shown that nitrogen-doped carbon materials, such as Pd/N-CNS and biochar-based supports effectively reduce Pd leaching owing to strong metal-support interactions, enabling consistent catalytic performance over multiple cycles [
46,
59].
Support degradation under harsh conditions such as elevated temperatures or prolonged reaction cycles presents another significant challenge. Robust materials such as mesoporous graphitic carbon nitride (mpg-CN) and biochar have demonstrated enhanced durability, while hybrid supports, such as polydopamine-modified Zn-Al mixed metal oxides, exhibit superior thermal and chemical stabilities [
77,
89]. However, these systems require further optimization to achieve widespread application across various reaction conditions.
Scalability remains a barrier particularly for complex hybrid designs or systems that are dependent on expensive materials. Simplified synthetic approaches, such as using renewable biopolymers and natural templates, could facilitate the development of cost-effective catalytic systems. Techniques such as 3D-printed supports and sol-gel synthesis have shown promise for creating uniform scalable catalysts without compromising performance [
46,
131].
Future advancements will rely heavily on computational modelling integrated with experimental studies to predict reaction mechanisms and optimize catalyst design. Understanding the interplay between heterogeneous and homogeneous catalytic pathways, particularly in atomically dispersed Pd systems, can reveal new efficiencies [
127]. Additionally, eco-friendly synthesis methods, including solvent-free processes and the use of renewable starting materials, will align catalyst development with green chemistry principles, ensuring sustainability and performance.
By addressing these challenges through innovations in material design, process scalability, and eco-conscious practices, PdNP catalysis will continue to evolve as a cornerstone of industrial chemistry.
11. Mechanism
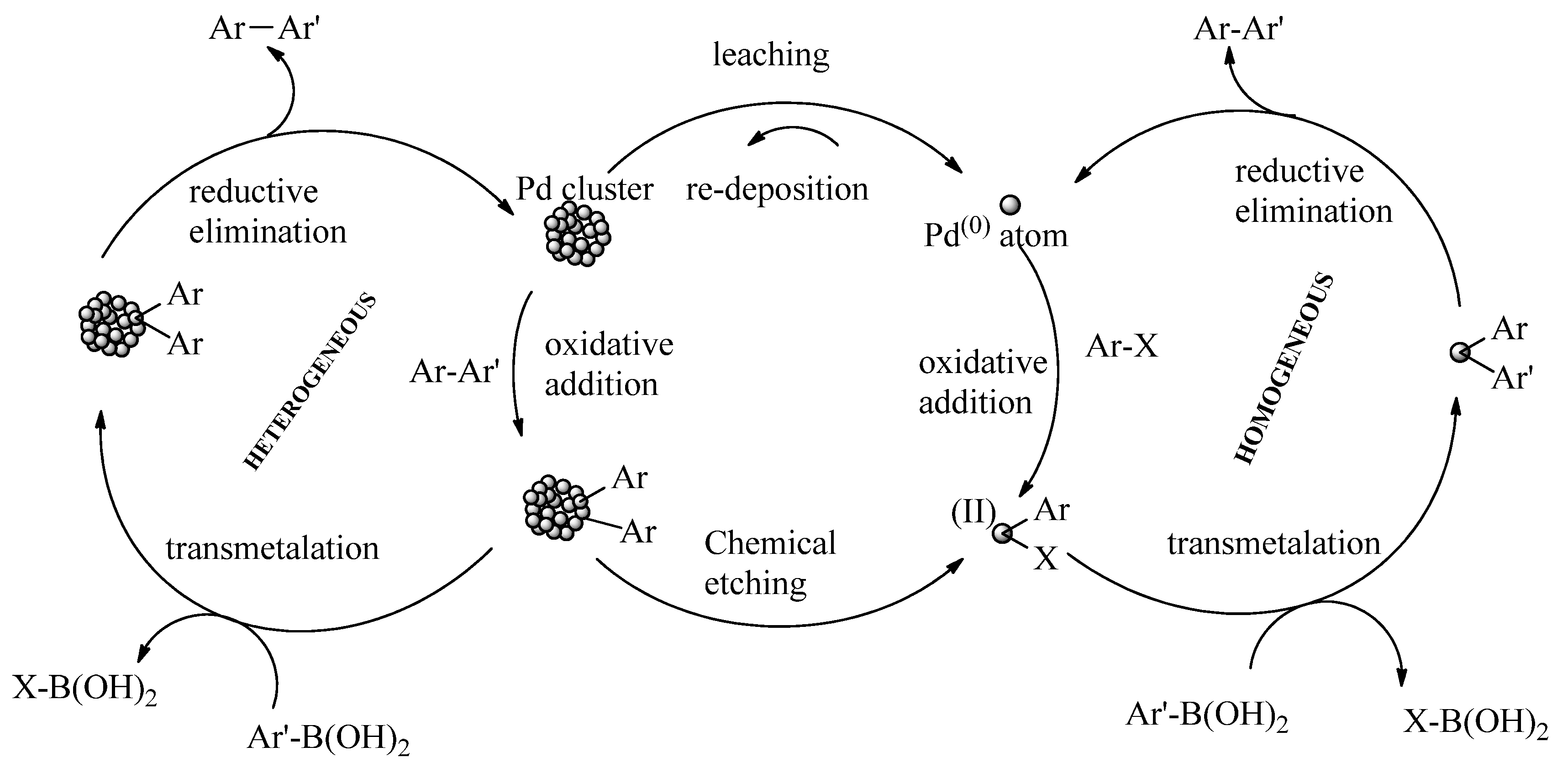
The mechanistic underpinnings of PdNP-catalyzed C–C cross-coupling reactions continue to be the subject of significant debate, particularly regarding the interplay between the homogeneous and heterogeneous pathways. A prominent hypothesis, the dissolution-redeposition theory, posits that PdNPs act as reservoirs, releasing active palladium species to catalyze the reaction, which subsequently redeposit on the nanoparticle surface (
Figure 3).
Real-time fluorescence imaging by Costa et al. confirmed the migration of Pd species during the Suzuki–Miyaura reaction, with catalysis occurring primarily on the surface, consistent with a heterogeneous mechanism [
134].
Despite these findings, some evidence suggests that homogeneous pathways can also play a role, depending on the reaction conditions and extent of Pd leaching [
135]. Trzeciak and Augustyniak discussed how active Pd species leach into solution but ultimately re-adsorb onto the solid support, highlighting a dynamic equilibrium between the two mechanisms [
135]. This interplay is not unique to Suzuki–Miyaura reactions but extends to other Pd-catalyzed cross-couplings, such as Heck and Sonogashira reactions. Greener approaches to catalysis, including the use of biogenic and mild conditions, have further demonstrated the potential of environmentally friendly catalytic processes [
136].
Building on these mechanistic insights, computational modelling has offered transformative approaches to elucidate reaction pathways in organic synthesis. The development of density functional theory (DFT) and the increasing accessibility of high-performance computing (HPC) centres have enabled accurate modelling of reactions involving organometallic compounds. However, a simplified approach rooted in fundamental chemical principles may also aid experimentalists in predicting reaction mechanisms. This involves employing Lewis structures, Valence Shell Electron Pair Repulsion (VSEPR) theory and kinetic modelling to mentally visualize and rationalize mechanistic steps. In this framework, electropositive centres react with electronegative centers to maintain the octet rule, while the preferred pathways are those with fewer reaction steps due to their kinetic efficiency.
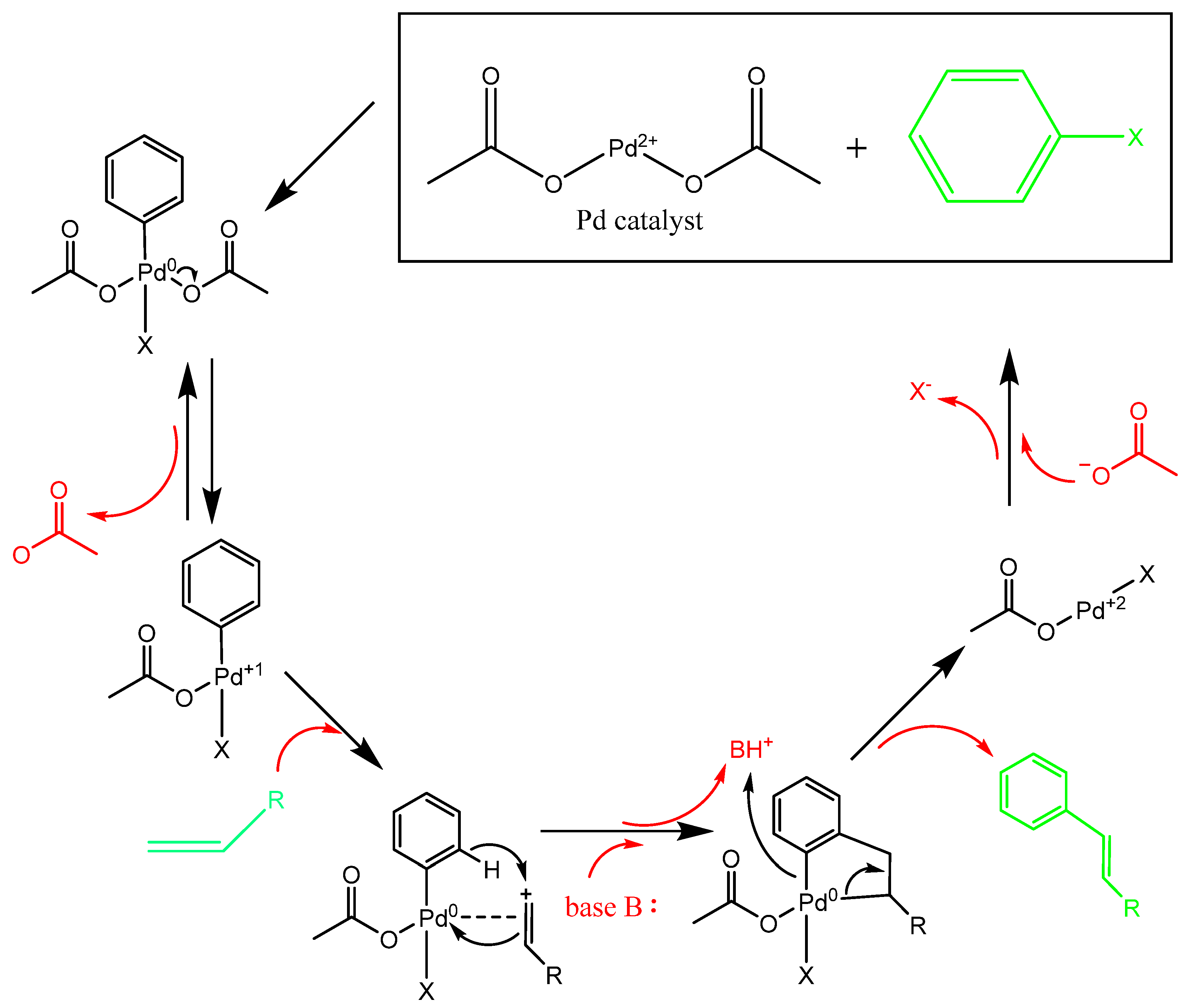
To illustrate this concept, a proposed mechanism for the Heck reaction is shown in
Figure 4. This model, based on the interaction of ligands achieving maximal separation (as per VSEPR) and the stepwise addition and elimination of intermediates, aligns well with computational and experimental data. Computational verification remains essential to validate such heuristic models, particularly in complex systems where deviations from idealized rules may occur.
Recent advances in spectroscopic and mechanistic research have further strengthened the field of heterogeneous catalysis. Wang et al. demonstrated that PdNPs supported on zeolites achieve remarkable stability and recyclability, with negligible palladium leaching, ensuring that the catalytic activity is localized on the surface [
137]. Furthermore, enhanced electronic interactions between the nanoparticle surface and substrate molecules have been shown to significantly increase the turnover frequency (TOF), providing additional evidence for surface-driven catalysis [
138].
The evolving consensus now leans toward a predominantly heterogeneous mechanism, where the direct interaction between the palladium surface and aryl halides drives catalytic transformations. However, the negligible activity of the leached Pd species under certain conditions suggests that homogeneous catalysis might be excluded in well-optimized systems [
134]. As catalytic processes continue to evolve, the integration of advanced support materials and real-time mechanistic studies will be critical for resolving these debates and optimizing PdNP-based catalysis for industrial applications.
12. Conclusions
The integration of Pd nanoparticles (PdNPs) into sustainable catalysis represents a transformative advancement in chemical synthesis, offering unparalleled catalytic efficiency, selectivity and reusability. The high surface-to-volume ratio of PdNPs allows their application across diverse cross-coupling reactions, thereby addressing critical challenges in traditional catalysis, including leaching, agglomeration and environmental toxicity [
55,
139,
140]. These attributes make PdNPs an important tool for advancing greener and more efficient chemical processes [
9].
Recent innovations have emphasized the importance of advanced support materials such as biopolymers and nitrogen-doped carbon nanosheets to further enhance the performance of PdNPs. Nitrogen-doped carbon nanosheets, for example, optimize the electronic environment and improve turnover frequencies and catalytic activity by up to nine-fold compared to conventional catalysts [
59]. Biopolymer supports, including kenaf-cellulose and chitosan derivatives, introduce a biodegradable dimension to catalysis, aligning with green chemistry principles [
61]. These advances mitigate the ecological impacts while maintaining high yields and catalyst stability, demonstrating significant progress toward scalable and environmentally friendly catalytic systems.
Despite these achievements, challenges remain, particularly in scaling up the PdNP systems for industrial applications. Issues related to cost efficiency, catalyst recovery, and long-term stability must be addressed to fully realize their potential [
141]. Future research should prioritize integrating PdNPs into industrial workflows by leveraging renewable supports and innovative synthetic approaches. These efforts will be instrumental in overcoming existing limitations, while fostering economic and environmental sustainability.
This review highlights the transformative potential of PdNPs as catalysts for bridging the gap between traditional systems and the demands of sustainable industrial chemistry. By addressing critical challenges and leveraging cutting-edge innovations, PdNPs can significantly advance catalytic science, contribute to global sustainability goals, and establish new standards for efficiency and environmental responsibility [
142].
Funding
This research received no internal or external funding
Acknowledgements
The authors thank the catalysts for covering the APC charge for this manuscript.
Conflict of Interest
The authors declare no conflict of interest
References
- Sheldon, R.A. , Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, P.; et al. Nanomaterials and catalysis for green chemistry. Curr. Opin. Green Sustain. Chem. 2020, 24, 48–55. [Google Scholar] [CrossRef]
- Centi, G. and S. Perathoner, Catalysis and sustainable (green) chemistry. Catal. Today 2003, 77, 287–297. [Google Scholar] [CrossRef]
- Turner, N.J. , Sustainable catalysis. 2016, Beilstein-Institut. p. 1778-1779.
- Habib, U.; et al. Sustainable Catalysis: Navigating Challenges and Embracing Opportunities for a Greener Future. J. Chem. Environ. 2023, 2, 14–53. [Google Scholar] [CrossRef]
- McCarthy, S. , D. C. Braddock, and J.D. Wilton-Ely, Strategies for sustainable palladium catalysis. Coord. Chem. Rev. 2021, 442, 213925. [Google Scholar] [CrossRef]
- Fiorio, J.L.; et al. Recent advances in the use of nitrogen-doped carbon materials for the design of noble metal catalysts. Coord. Chem. Rev. 2023, 481, 215053. [Google Scholar] [CrossRef]
- Ashraf, M.; et al. Transition metal nanoparticles as nanocatalysts for Suzuki, Heck and Sonogashira cross-coupling reactions. Coord. Chem. Rev. 2023, 476, 214928. [Google Scholar] [CrossRef]
- Liu, L. and A. Corma, Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Hong, K.; et al. Palladium nanoparticles on assorted nanostructured supports: applications for Suzuki, Heck, and Sonogashira cross-coupling reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Swain, S.; et al. Computational and experimental design of the octahedral PdFe alloy nanocatalyst for hiyama cross-coupling and environmental pollutant degradation. ACS Appl. Nano Mater. 2023, 6, 3254–3267. [Google Scholar] [CrossRef]
- Wang, J.; et al. Facile Assembly of C–N Bond-Containing Polymer Electrolytes Enabled by Lithium Salt-Catalyzed Aza-Michael Addition. Macromolecules 2023, 56, 2484–2493. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A. , B. Tahmasbi, and P. Moradi, Palladium–S-propyl-2-aminobenzothioate immobilized on Fe3O4 magnetic nanoparticles as catalyst for Suzuki and Heck reactions in water or poly (ethylene glycol). Appl. Organomet. Chem. 2016, 30, 422–430. [Google Scholar] [CrossRef]
- Aryanasab, F.; et al. Immobilizing palladium on melamine-functionalized magnetic nanoparticles: An efficient and reusable phosphine-free catalyst for Mizoroki–Heck reaction. Appl. Organomet. Chem. 2021, 35, e6198. [Google Scholar] [CrossRef]
- Wolfson, A. and O. Levy-Ontman, Development and application of palladium nanoparticles on renewable polysaccharides as catalysts for the Suzuki cross-coupling of halobenzenes and phenylboronic acids. Mol. Catal. 2020, 493, 111048. [Google Scholar] [CrossRef]
- Heravi, M.M. and E. Hashemi, Recent applications of the Suzuki reaction in total synthesis. Tetrahedron 2012, 68, 9145–9178. [Google Scholar] [CrossRef]
- Maity, P.; et al. Development of a scalable synthesis of BMS-978587 featuring a stereospecific Suzuki coupling of a cyclopropane carboxylic acid. Org. Process Res. Dev. 2018, 22, 888–897. [Google Scholar] [CrossRef]
- Sirindil, F.; et al. Total synthesis of Rhazinilam through gold-catalyzed cycloisomerization–sulfonyl migration and palladium-catalyzed Suzuki–Miyaura coupling of pyrrolyl sulfonates. Org. Lett. 2019, 21, 5542–5546. [Google Scholar] [CrossRef]
- Otte, F. and B. Schmidt, Matsuda–Heck arylation of glycals for the stereoselective synthesis of aryl C-glycosides. J. Org. Chem. 2019, 84, 14816–14829. [Google Scholar] [CrossRef]
- Ashworth, I.W.; et al. Process development of a Suzuki reaction used in the manufacture of lanabecestat. Org. Process Res. Dev. 2018, 22, 1801–1808. [Google Scholar] [CrossRef]
- Luo, J. and J.A. May, Enantioselective Total Synthesis of Cannabinoids via a Tandem Conjugate Addition/Enolate Alkylation Annulation with Ambiphilic Organoboronates. Org. Lett. 2023, 25, 708–713. [Google Scholar] [CrossRef]
- Qin, Y.; et al. Silacyclization through palladium-catalyzed intermolecular silicon-based C (sp 2)–C (sp 3) cross-coupling. Chem. Sci. 2021, 12, 14224–14229. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; et al. Intramolecular heck reaction in total synthesis of natural products: an update. Eur. J. Org. Chem. 2021, 2021, 2057–2076. [Google Scholar] [CrossRef]
- Stanway-Gordon, H.A. J.A. Odger, and M.J. Waring, Development of a micellar-promoted heck reaction for the synthesis of DNA-encoded libraries. Bioconjugate Chem. 2023, 34, 756–763. [Google Scholar]
- Hayama, N. , Y. Kobayashi, and Y. Takemoto, Asymmetric hetero-Michael addition to α, β-unsaturated carboxylic acids using thiourea–boronic acid hybrid catalysts. Tetrahedron 2021, 89, 132089. [Google Scholar] [CrossRef]
- Evano, G. , J. Wang, and A. Nitelet, Metal-mediated C–O bond forming reactions in natural product synthesis. Org. Chem. Front. 2017, 4, 2480–2499. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; et al. Heterogeneous catalysis for cross-coupling reactions: an underutilized powerful and sustainable tool in the fine chemical industry? Org. Process Res. Dev. 2021, 25, 740–753. [Google Scholar] [CrossRef]
- Hegedus, L.S. , Transition metals in the synthesis of complex organic molecules. 1999: University Science Books.
- Wu, T.-C. , Process for preparing olefins. 1996, Google Patents.
- Beller, M. , A. Zapf, and W. Mägerlein, Efficient Synthesis of Fine Chemicals and Organic Building Blocks Applying Palladium-Catalyzed Coupling Reactions. Chemical Engineering & Technology: Industrial Chemistry-Plant Equipment-Process Engineering-Biotechnology 2001, 24, 575–582. [Google Scholar]
- Shinkai, I. , A. King, and R. Larsen, A practical asymmetric synthesis of LTD4 antagonist. Pure Appl, Chem. 1994, 66, 1551–1556. [Google Scholar] [CrossRef]
- Macor, J.E. and M.J. Wythes, Indole derivatives. 1998, Google Patents.
- Shen, H.C. , Selected Applications of Transition Metal-Catalyzed Carbon–Carbon Cross-Coupling Reactions in the Pharmaceutical Industry. 2012, Wiley Online Library. p. 25-43.
- Perkins, J., Eur. Patent 1088817 (2001); JE Macor and MJ Wythes, Int. Patent WO 9206973 (1992); CQ Meng. Curr. Opin. Cent. Peripher. Nerv. Syst. Invest. Drugs 2000, 2, 186.
- King, A.; et al. An efficient synthesis of LTD4 antagonist L-699,392. J. Org. Chem. 1993, 58, 3731–3735. [Google Scholar] [CrossRef]
- Hervé, G. and C. Len, First ligand-free, microwave-assisted, Heck cross-coupling reaction in pure water on a nucleoside–Application to the synthesis of antiviral BVDU. RSC Adv. 2014, 4, 46926–46929. [Google Scholar] [CrossRef]
- Shen, C.; et al. A novel D-glucosamine-derived pyridyl-triazole@ palladium catalyst for solvent-free Mizoroki–Heck reactions and its application in the synthesis of Axitinib. Green Chem. 2015, 17, 225–230. [Google Scholar] [CrossRef]
- Herkes, F.E. , Catalysis of organic reactions. Vol. 75. 1998: CRC Press.
- Polshettiwar, V. and T.N. Asefa, Synthesis and Applications. 2013: Wiley Online Library.
- Luo, S.; et al. Perspectives on palladium-based nanomaterials: green synthesis, ecotoxicity, and risk assessment. Environ. Sci. Nano 2021, 8, 20–36. [Google Scholar] [CrossRef]
- Piermatti, O. , Green synthesis of Pd nanoparticles for sustainable and environmentally benign processes. Catalysts 2021, 11, 1258. [Google Scholar] [CrossRef]
- Law, C.K.Y.; et al. Biogenic synthesis of palladium nanoparticles: New production methods and applications. Nanotechnol. Rev. 2022, 11, 3104–3124. [Google Scholar] [CrossRef]
- Kamel, S. and T.A. Khattab, Recent advances in cellulose supported metal nanoparticles as green and sustainable catalysis for organic synthesis. Cellulose 2021, 28, 4545–4574. [Google Scholar] [CrossRef]
- Ayogu, J.I. and E.A. Onoabedje, Prospects and Applications of Palladium Nanoparticles in the Cross-coupling of (hetero) aryl Halides and Related Analogues. Chem. Open 2021, 10, 430–450. [Google Scholar]
- Aksoy, M.; et al. Recent advances in the development of palladium nanocatalysts for sustainable organic transformations. Inorg. Chem. Front. 2021, 8, 499–545. [Google Scholar] [CrossRef]
- Srivastava, A.; et al. Optimal exploitation of supported heterogenized Pd nanoparticles for CC cross-coupling reactions. Coord. Chem. Rev. 2024, 507, 215763. [Google Scholar] [CrossRef]
- Palem, R.R.; et al. Biogenic palladium nanoparticles: An effectual environmental benign catalyst for organic coupling reactions. J. Ind. Eng. Chem. 2022, 106, 52–68. [Google Scholar] [CrossRef]
- Dong, Z.; et al. Palladium supported on urea-containing porous organic polymers as heterogeneous catalysts for C–C cross coupling reactions and reduction of nitroarenes. J. Saudi Chem. Soc. 2021, 25, 101317. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; et al. Towards high-performance heterogeneous palladium nanoparticle catalysts for sustainable liquid-phase reactions. React. Chem. Eng. 2020, 5, 1556–1618. [Google Scholar] [CrossRef]
- Xiao, Q.; et al. Visible light-driven cross-coupling reactions at lower temperatures using a photocatalyst of palladium and gold alloy nanoparticles. ACS Catal. 2014, 4, 1725–1734. [Google Scholar] [CrossRef]
- Hamdi, J.; et al. Room-temperature aqueous Suzuki–Miyaura cross-coupling reactions catalyzed via a recyclable palladium@ halloysite nanocomposite. Org. Lett. 2019, 21, 3471–3475. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; et al. KCC-1 supported palladium nanoparticles as an efficient and sustainable nanocatalyst for carbonylative Suzuki–Miyaura cross-coupling. Green Chem. 2016, 18, 5890–5899. [Google Scholar] [CrossRef]
- Doherty, S.; et al. Heteroatom donor-decorated polymer-immobilized ionic liquid stabilized palladium nanoparticles: efficient catalysts for room-temperature Suzuki-Miyaura cross-coupling in aqueous media. Adv. Synth. Catal. 2018, 360, 3716–3731. [Google Scholar] [CrossRef]
- Rezapour, E. , M. Jafarpour, and A. Rezaeifard, Palladium niacin complex immobilized on starch-coated maghemite nanoparticles as an efficient homo-and cross-coupling catalyst for the synthesis of symmetrical and unsymmetrical biaryls. Catal. Lett. 2018, 148, 3165–3177. [Google Scholar] [CrossRef]
- Astruc, D. , Palladium nanoparticles as efficient green homogeneous and heterogeneous carbon− carbon coupling precatalysts: A unifying view. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef]
- Ricciardi, R. , J. Huskens, and W. Verboom, Dendrimer-encapsulated Pd nanoparticles as catalysts for C–C cross-couplings in flow microreactors. Org. Biomol. Chem. 2015, 13, 4953–4959. [Google Scholar] [CrossRef]
- Ricciardi, R.; et al. Dendrimer-Encapsulated Palladium Nanoparticles for Continuous-Flow Suzuki–Miyaura Cross-Coupling Reactions. ChemCatChem 2015, 7, 936–942. [Google Scholar] [CrossRef]
- Benyettou, F.; et al. Palladium-Loaded Cucurbit [7] uril-Modified Iron Oxide Nanoparticles for C− C Cross-Coupling Reactions. Chem. Eur. J. 2018, 24, 2349–2353. [Google Scholar] [CrossRef]
- Cui, S.; et al. Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions. Nanomater. 2024, 14, 1690. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekharan, B.; et al. Palladium nanoparticle immobilized on coconut coir extract coated boron carbon nitride: A green and sustainable nanocatalyst for cross-coupling reactions and HER studies. Diamond Relat. Mater. 2024, 111261. [Google Scholar] [CrossRef]
- Islam, M.S.; et al. Applications of bio-resource based sustainable heterogeneous Pd-Nanocatalyst for cross-coupling and michael addition reactions. Chem. Eng. J. 2024, 483, 149271. [Google Scholar] [CrossRef]
- Rybochkin, P.V.; et al. Aerobic bacteria-supported biohybrid palladium catalysts for efficient cross-coupling reactions. J. Catal. 2024, 429, 115238. [Google Scholar] [CrossRef]
- Campelo, J.M.; et al. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; et al. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Naghipour, A. and A. Fakhri, Heterogeneous Fe3O4@ chitosan-Schiff base Pd nanocatalyst: Fabrication, characterization and application as highly efficient and magnetically-recoverable catalyst for Suzuki–Miyaura and Heck–Mizoroki C–C coupling reactions. Catal. Commun. 2016, 73, 39–45. [Google Scholar] [CrossRef]
- Sinha, V.; et al. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Bao, Y.; et al. Cobalt, nickel and iron embedded chitosan microparticles as efficient and reusable catalysts for Heck cross-coupling reactions. Int. J. Biol. Macromol. 2019, 130, 203–212. [Google Scholar] [CrossRef]
- Hajipour, A.R. and Z. Tavangar-Rizi, Methionine-functionalized chitosan–Pd (0) complex: A novel magnetically separable catalyst for Heck reaction of aryl iodides and aryl bromides at room temperature in water as only solvent. Appl. Organomet. Chem. 2017, 31, e3638. [Google Scholar] [CrossRef]
- Jadhav, S. , A. Kumbhar, and R. Salunkhe, Palladium supported on silica–chitosan hybrid material (Pd-CS@ SiO2) for Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions. Appl. Organomet. Chem. 2015, 29, 339–345. [Google Scholar] [CrossRef]
- Orooji, Y.; et al. Polysaccharide-based (nano) materials for Cr (VI) removal. Int. J. Biol. Macromol. 2021, 188, 950–973. [Google Scholar] [CrossRef] [PubMed]
- Baran, T. and M. Nasrollahzadeh, Facile synthesis of palladium nanoparticles immobilized on magnetic biodegradable microcapsules used as effective and recyclable catalyst in Suzuki-Miyaura reaction and p-nitrophenol reduction. Carbohydr. Polym. 2019, 222, 115029. [Google Scholar] [CrossRef]
- Guibal, E. , Heterogeneous Catalysis on Chitosan-Based Materials. ChemInform 2006, 37, no–no. [Google Scholar] [CrossRef]
- Leonhardt, S.E.; et al. Chitosan as a support for heterogeneous Pd catalysts in liquid phase catalysis. Appl Catal., A 2010, 379, 30–37. [Google Scholar] [CrossRef]
- Zheng, X.; et al. Preparation of porous chitosan/reduced graphene oxide microspheres supported Pd nanoparticles catalysts for Heck coupling reactions. Carbohydr. Polym. 2020, 230, 115583. [Google Scholar] [CrossRef]
- Dohendou, M. , M. G. Dekamin, and D. Namaki, Pd@ l-asparagine–EDTA–chitosan: a highly effective and reusable bio-based and biodegradable catalyst for the Heck cross-coupling reaction under mild conditions. Nanoscale Adv. 2023, 5, 2621–2638. [Google Scholar] [CrossRef]
- Çalışkan, M. and T. Baran, Nanoscaled reusable palladium catalyst supported on chitosan hybrid composite microcapsules reinforced with ZnO nanoparticles for Heck coupling reactions. Cellulose 2022, 29, 7789–7802. [Google Scholar] [CrossRef]
- Albano, G. , A. Petri, and L.A. Aronica, Palladium Supported on Bioinspired Materials as Catalysts for C–C Coupling Reactions. Catalysts 2023, 13, 210. [Google Scholar] [CrossRef]
- Dahl, J.A. , B. L. Maddux, and J.E. Hutchison, Toward greener nanosynthesis. Chemical reviews 2007, 107, 2228–2269. [Google Scholar] [CrossRef]
- Astruc, D. , F. Lu, and J.R. Aranzaes, Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Intl Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Panziera, N.; et al. MVS-derived palladium nanoparticles deposited on polydimethylphosphazene as recyclable catalysts for Heck-type reactions: Preparation, structural study, and catalytic activity. J. Catal. 2007, 246, 351–361. [Google Scholar] [CrossRef]
- Caporusso, A.M.; et al. Functional resins in palladium catalysis: promising materials for Heck reaction in aprotic polar solvents. J. Catal. 2005, 234, 1–13. [Google Scholar] [CrossRef]
- Boisselier, E.; et al. Encapsulation and stabilization of gold nanoparticles with “click” polyethyleneglycol dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar] [CrossRef]
- Wu, Z.; et al. Immobilization of Pd (0) nanoparticles on polyaza-ligand-functionalized polyacrylonitrile fiber as highly active catalyst for the Heck reaction. J. Catal. 2023, 427, 115121. [Google Scholar] [CrossRef]
- Cao, Y.; et al. Metal/porous carbon composites for heterogeneous catalysis: old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Xu, X.; et al. Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade. J. Am. Chem. Soc. 2012, 134, 16987–16990. [Google Scholar] [CrossRef]
- Gao, Y.; et al. Nitrogen-Doped sp 2-Hybridized Carbon as a Superior Catalyst for Selective Oxidation. Angew. Chem. Intl. Ed. 2013, 52. [Google Scholar] [CrossRef]
- Chen, S.; et al. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution. RSC Adv. 2015, 5, 31566–31574. [Google Scholar] [CrossRef]
- Zhang, L.; et al. N-Doped porous carbon supported palladium nanoparticles as a highly efficient and recyclable catalyst for the Suzuki coupling reaction. Chin. Chem. Lett. 2016, 27, 149–154. [Google Scholar] [CrossRef]
- Joshani, Z.; et al. Palladium nanoparticles (Pd NPs) immobilized over polydopamine-functionalized Zn-Al mixed metal oxide as a novel nanocatalyst for synthesis of CC bond via Suzuki-Miyaura coupling reactions. Inorg. Chem. Commun. 2024, 163, 112308. [Google Scholar] [CrossRef]
- Dory, H.; et al. Monolith Catalyst Design by Combining 3D Printing and Atomic Layer Deposition: Toward Green Palladium-Catalyzed Cross-Coupling Reactions. Adv. Eng. Mater. Adv. Eng. Mater., 2024, 2401546. [Google Scholar] [CrossRef]
- Abdellah, A.R.; et al. Palladium nanocrystals-embedded covalent organic framework as an efficient catalyst for Heck cross-coupling reaction. Microporous and Mesoporous Mater. 2022, 339, 111961. [Google Scholar] [CrossRef]
- Zhou, J. and D. Astruc, Recent Trends and Perspectives in Palladium Nanocatalysis: From Nanoparticles to Frameworks, Atomically Precise Nanoclusters and Single-Atom Catalysts. J. Inorg. Organomet. Polym. Mater. 2024, 1–23. [Google Scholar]
- Salemi, H.; et al. Covalent organic framework supported palladium catalysts. J. Mate. Chem., A 2022, 10, 20707–20729. [Google Scholar] [CrossRef]
- Dong, Z.; et al. Palladium Immobilized on a Polyimide Covalent Organic Framework: An Efficient and Recyclable Heterogeneous Catalyst for the Suzuki–Miyaura Coupling Reaction and Nitroarene Reduction in Water. Catal. Lett. 2022, 152, 299–306. [Google Scholar] [CrossRef]
- Shukla, F.; et al. Palladium nanoparticles-confined pore-engineered urethane-linked thiol-functionalized covalent organic frameworks: a high-performance catalyst for the Suzuki Miyaura cross-coupling reaction. Dalton Trans. 2023, 52, 2518–2532. [Google Scholar] [CrossRef]
- Xiao, S.; et al. Ultrahigh stable covalent organic framework-derived carbon–nitrogen-supported palladium nanoparticles for highly efficient electrocatalytic methanol and ethanol oxidation reactions. Green Chem. 2022, 24, 5813–5821. [Google Scholar] [CrossRef]
- Zhu, Q.-L. and Q. Xu, Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Luo, S.; et al. Metal organic frameworks as robust host of palladium nanoparticles in heterogeneous catalysis: synthesis, application, and prospect. ACS Appl. Mater. Interfaces 2019, 11, 32579–32598. [Google Scholar] [CrossRef]
- Hajek, J.; et al. Mechanistic studies of aldol condensations in UiO-66 and UiO-66-NH2 metal organic frameworks. J. Catal. 2015, 331, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; et al. Covalent construction of sustainable hybrid UiO-66-NH2@ Tb-CP material for selective removal of dyes and detection of metal ions. ACS Sustainable chem. Eng. 2019, 7, 3203–3212. [Google Scholar] [CrossRef]
- Mohammadi, L. and M.R. Vaezi, Palladium Nanoparticle-Decorated Porous Metal–Organic-Framework (Zr)@ Guanidine: Novel Efficient Catalyst in Cross-Coupling (Suzuki, Heck, and Sonogashira) Reactions and Carbonylative Sonogashira under Mild Conditions. ACS Omega 2023, 8, 16395–16410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; et al. Stable metal–organic frameworks with group 4 metals: current status and trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. and D. Astruc, State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2019, 120, 1438–1511. [Google Scholar] [CrossRef]
- Stock, N. and S. Biswas, Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Bai, Y.; et al. Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- D’Amato, R.; et al. “Shake ‘n Bake” Route to Functionalized Zr-UiO-66 Metal–Organic Frameworks. Inorg. Chem. 2021, 60, 14294–14301. [Google Scholar] [CrossRef]
- Hendrickx, K.; et al. Understanding intrinsic light absorption properties of UiO-66 frameworks: a combined theoretical and experimental study. Inorg. Chem. 2015, 54, 10701–10710. [Google Scholar] [CrossRef]
- Katz, M.J.; et al. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Hajighasemi, Z.; et al. Efficient and biocompatible new palladium-supported boehmite nanoparticles: synthesis, characterization and application in Suzuki–Miura and Mizoroki–Heck coupling reactions. Nanoscale Adv. 2023, 5, 4925–4933. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; et al. Palladium nanoparticles on polydimethylsiloxane film for C− C coupling reactions, and catalytic reduction of organic pollutants in water. ChemistrySelect 2023, 8, e202303057. [Google Scholar] [CrossRef]
- Gupta, P.K. , N. Kumar, and K.V. Ranganath, Asymmetric Suzuki Cross-Coupling Reactions Catalyzed by Chiral Surfactant-Stabilized Palladium Nanoparticles. Asian J. Org. Chem. 2022, 11, e202200362. [Google Scholar] [CrossRef]
- Wang, J.; et al. Pd nanoparticles supported in PDMAEMA-b-PLMA micelles: A superb catalytic platform for Suzuki-Miyaura cross-coupling in water. Eur. Polym. J. 2024, 202, 112650. [Google Scholar] [CrossRef]
- Shi, W.; et al. Nano-sized alumina supported palladium catalysts for methane combustion with excellent thermal stability. J. Environ. Sci. 2023, 126, 333–347. [Google Scholar] [CrossRef]
- Kalishyn, Y.Y.; et al. Synthesis and Thermal Stability of Palladium Nanoparticles Supported on γ-Al2O3. Curr. Nanomater.s 2020, 5, 79–90. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A. , B. Tahmasbi, and P. Moradi, Synthesis of a new Pd (0)-complex supported on boehmite nanoparticles and study of its catalytic activity for Suzuki and Heck reactions in H 2 O or PEG. RSC Adv. 2016, 6, 43205–43216. [Google Scholar] [CrossRef]
- Liu, J. X. Liao, and B. Shi, Pd nanoparticles immobilized on boehmite by using tannic acid as structure-directing agent and stabilizer: a high performance catalyst for hydrogenation of olefins. Res. Chem. Intermed. 2014, 40, 249–258. [Google Scholar] [CrossRef]
- Tahmasbi, B. , M. Darabi, and M. Nikoorazm, A new Schiff-base complex of palladium nanoparticles on modified boehmite with di (pyridin-2-yl) methanone as a robust, reusable, and selective nanocatalyst in the C-C coupling reaction. Appl. Organomet. Chem. 2024, 38, e7348. [Google Scholar] [CrossRef]
- Wu, S.; et al. Micelle-Derived palladium nanoparticles for suzuki–miyaura coupling reactions in water at room temperature. ACS Appl. Nano Mater. 2023, 6, 1592–1602. [Google Scholar] [CrossRef]
- Nalakath, T.A.A. , Sustainable nanocatalysis in water for C–C and C–N Cross-Couplings. [Doctoral Dissertation, The Univerdsity of Louisville’s] 2022, 2022. [Google Scholar]
- Mastalir, Á. and Á. Molnár, Palladium Nanoparticles Supported on Porous Silica Materials as Heterogeneous Catalysts of C− C Coupling and Cross-Coupling Reactions. ChemCatChem 2023, 15, e202300643. [Google Scholar] [CrossRef]
- Kumbhar, A. , Palladium catalyst supported on zeolite for cross-coupling reactions: an overview of recent advances. Top. Curr. Chem. 2017, 375, 2. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P. and A. Sarkar, Palladium Nanoparticles Immobilized on Chemically Modified Silica Gel: Efficient Heterogeneous Catalyst for Suzuki, Stille and Sonogashira Cross-Coupling Reactions. Advanced Synthesis & Catalysis 2011, 353, 2814–2822. [Google Scholar]
- Saleh, M.Y.; et al. Palladium fabricated on Fe3O4 as an organic-inorganic hybrid nanocatalyst for the Suzuki and Stille coupling reactions. Adv. Synth. Catal. 2025, 1321, 139597. [Google Scholar] [CrossRef]
- Baran, N.Y., T. Baran, and M. Nasrollahzadeh, Synthesis of palladium nanoparticles stabilized on Schiff base-modified ZnO particles as a nanoscale catalyst for the phosphine-free Heck coupling reaction and 4-nitrophenol reduction. Sci. Rep. 2023, 13, 12008.
- Wilson, K.A. , L. A. Picinich, and A.R. Siamaki, Nickel–palladium bimetallic nanoparticles supported on multi-walled carbon nanotubes; versatile catalyst for Sonogashira cross-coupling reactions. RSC Adv. 2023, 13, 7818–7827. [Google Scholar] [CrossRef]
- Pocklanová, R.; et al. Nanodiamond Supported Ultra-Small Palladium Nanoparticles as an Efficient Catalyst for Suzuki Cross-Coupling Reactions. Catalysts 2024, 14, 53. [Google Scholar] [CrossRef]
- Chen, Y.; et al. Dynamic structural evolution of MgO-supported palladium catalysts: from metal to metal oxide nanoparticles to surface then subsurface atomically dispersed cations. Chem. Sci. 2024, 15, 6454–6464. [Google Scholar] [CrossRef]
- Kumar, M., M. S. Solanki, and S. Sharma, Graphene Supported Palladium Nanocatalyst for Cross-Coupling Reactions, in Graphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1. 2023, Bentham Science Publishers. p. 263-296.
- Fatahi, Y.; et al. Palladium supported aminobenzamide modified silica coated superparamagnetic iron oxide as an applicable nanocatalyst for Heck cross-coupling reaction. J. Organomet. Chem. 2021, 936, 121711. [Google Scholar] [CrossRef]
- Tran, T.P.N.; et al. Tailoring graphene oxide framework with N-and S-containing organic ligands for the confinement of Pd nanoparticles towards recyclable catalyst systems. Catalysis Letters 2021, 151, 247–254. [Google Scholar] [CrossRef]
- Lawrence, A.S.; et al. Palladium-Based Metal Organic Frameworks as Heterogeneous Catalysts for C− C Couplings. ChemCatChem 2022, 14, e202200403. [Google Scholar] [CrossRef]
- Monteil, C.; et al. Phosphonated Polyethylenimine Maghemite Nanoparticles: A Convenient Support of Palladium for Cross-Coupling Reactions. Organics 2022, 3, 491–501. [Google Scholar] [CrossRef]
- Pérez-Lorenzo, M. , Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Costa, P. , D. Sandrin, and J.C. Scaiano, Real-time fluorescence imaging of a heterogeneously catalysed Suzuki–Miyaura reaction. Nat. Catal. 2020, 3, 427–437. [Google Scholar] [CrossRef]
- Trzeciak, A. and A. Augustyniak, The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Kempasiddaiah, M.; et al. Palladium-catalyzed denitrogenative cross-coupling of aryl halides with arylhydrazines under mild reaction conditions. Transition Met. Chem. 2021, 46, 273–281. [Google Scholar] [CrossRef]
- Wang, Y.; et al. Zeolite-enhanced sustainable Pd-catalyzed C–C cross-coupling reaction: Controlled release and capture of palladium. ACS Appl. Mater. Interfaces 2020, 12, 11419–11427. [Google Scholar] [CrossRef]
- Zhou, T. and M. Szostak, Palladium-catalyzed cross-couplings by C–O bond activation. Catal. Sci. Technol. 2020, 10, 5702–5739. [Google Scholar] [CrossRef]
- Sengupta, T.; et al. Experimental and computational study of the catalytic activity of Pd and PdCu nanoparticle catalysts and clusters supported on reduced graphene oxide and graphene acid for the suzuki cross-coupling reaction. Appl. Catal., A 2023, 667, 119448.
- Choudhary, A.; et al. Pd nanoparticles supported on magnetic CoTiMgLDH with enhanced catalytic activity in Suzuki cross-coupling and reductive degradation of dyes. Appl. Organomet. Chem. 2023, 37, e7112. [Google Scholar] [CrossRef]
- Balanta, A. , C. Godard, and C. Claver, Pd nanoparticles for C–C coupling reactions. Chem. Soc. Rev. 2011, 40, 4973–4985. [Google Scholar] [CrossRef]
- Mpungose, P.P.; et al. The current status of heterogeneous palladium catalysed Heck and Suzuki cross-coupling reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).