Submitted:
06 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
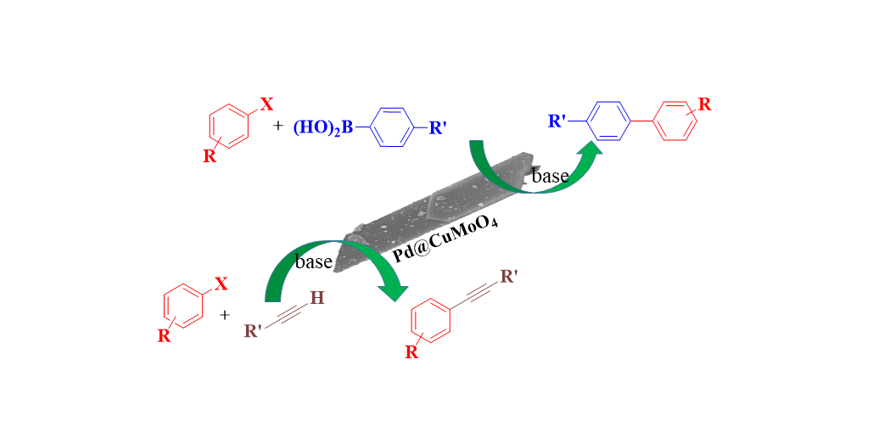
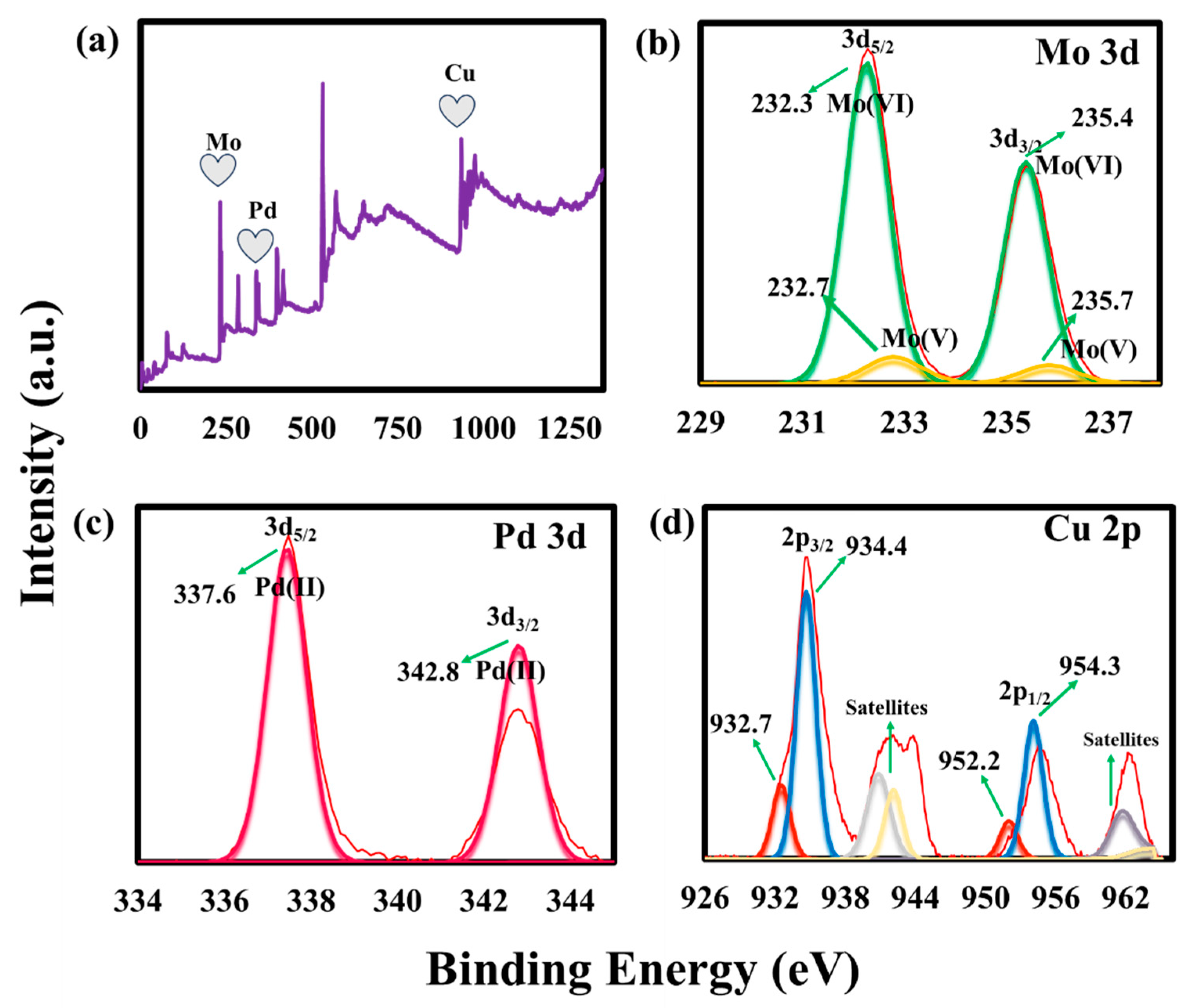
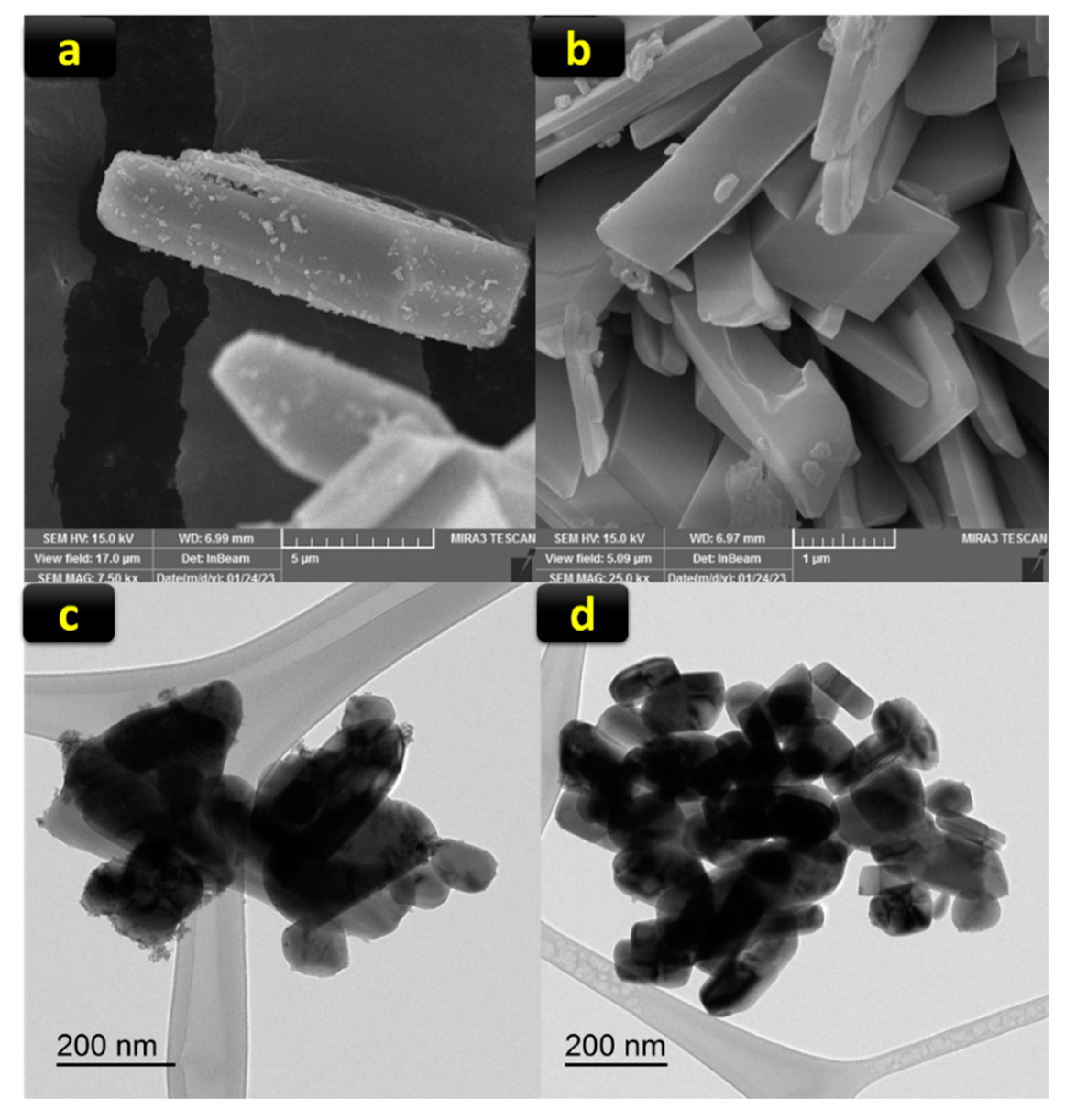
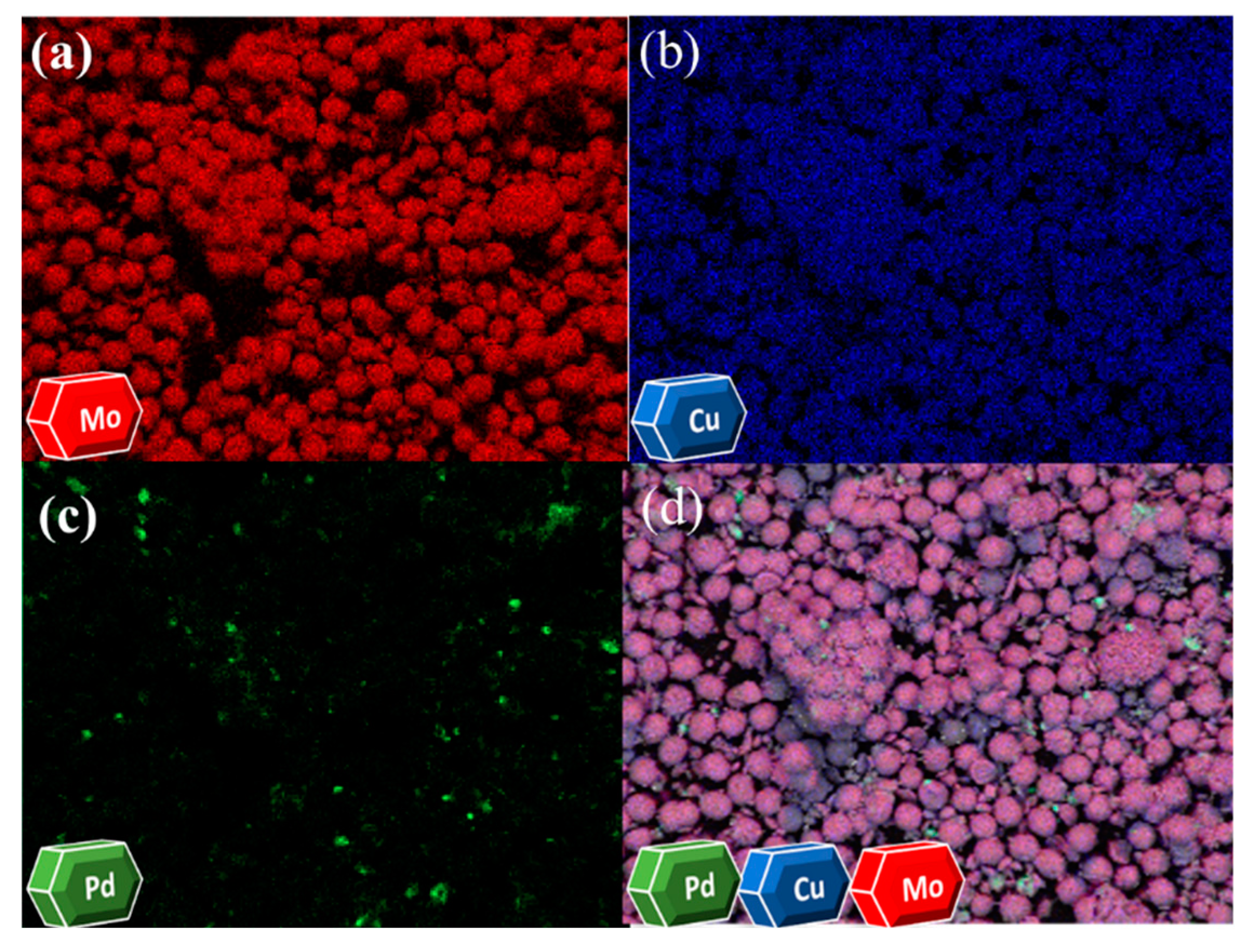
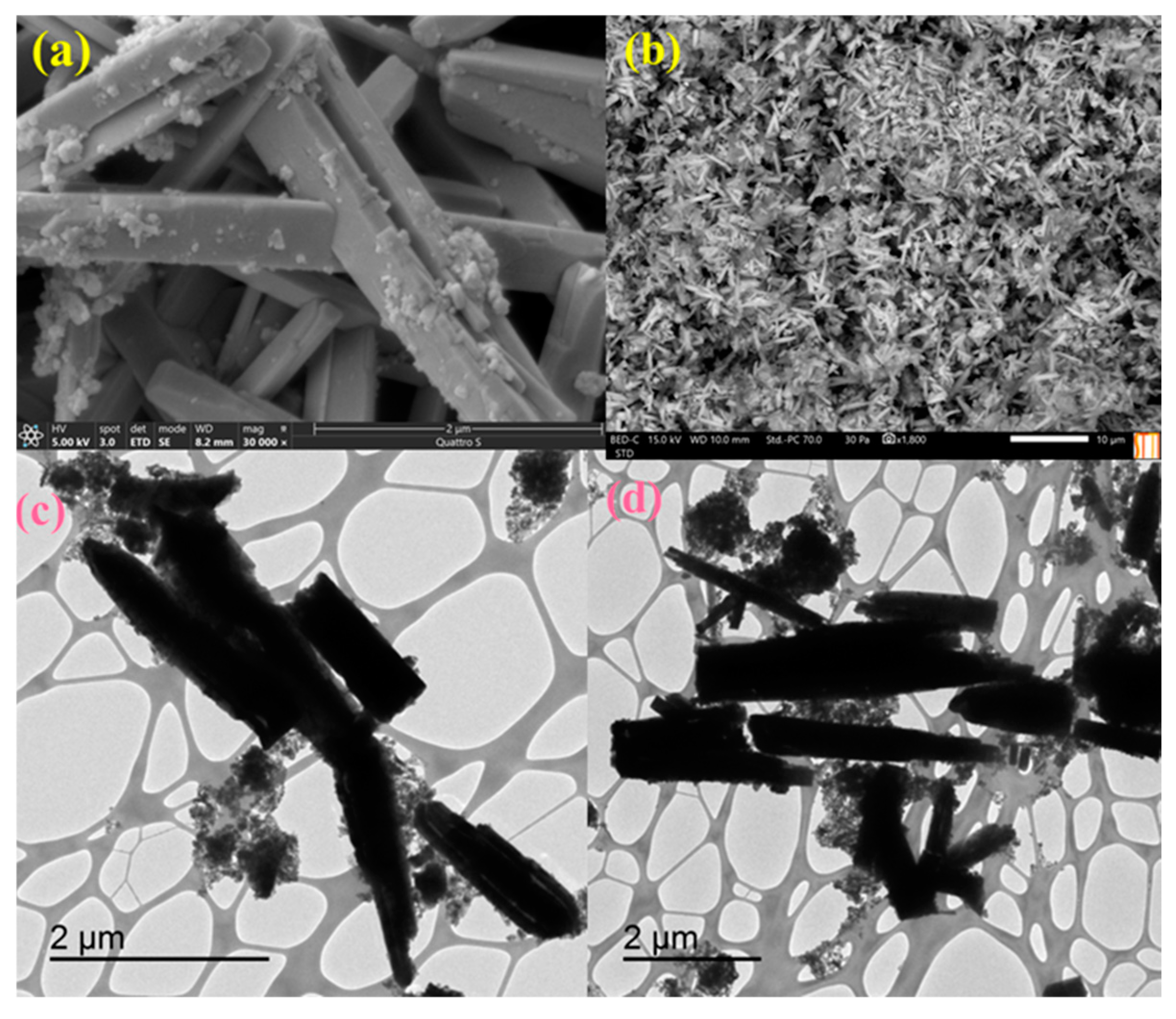
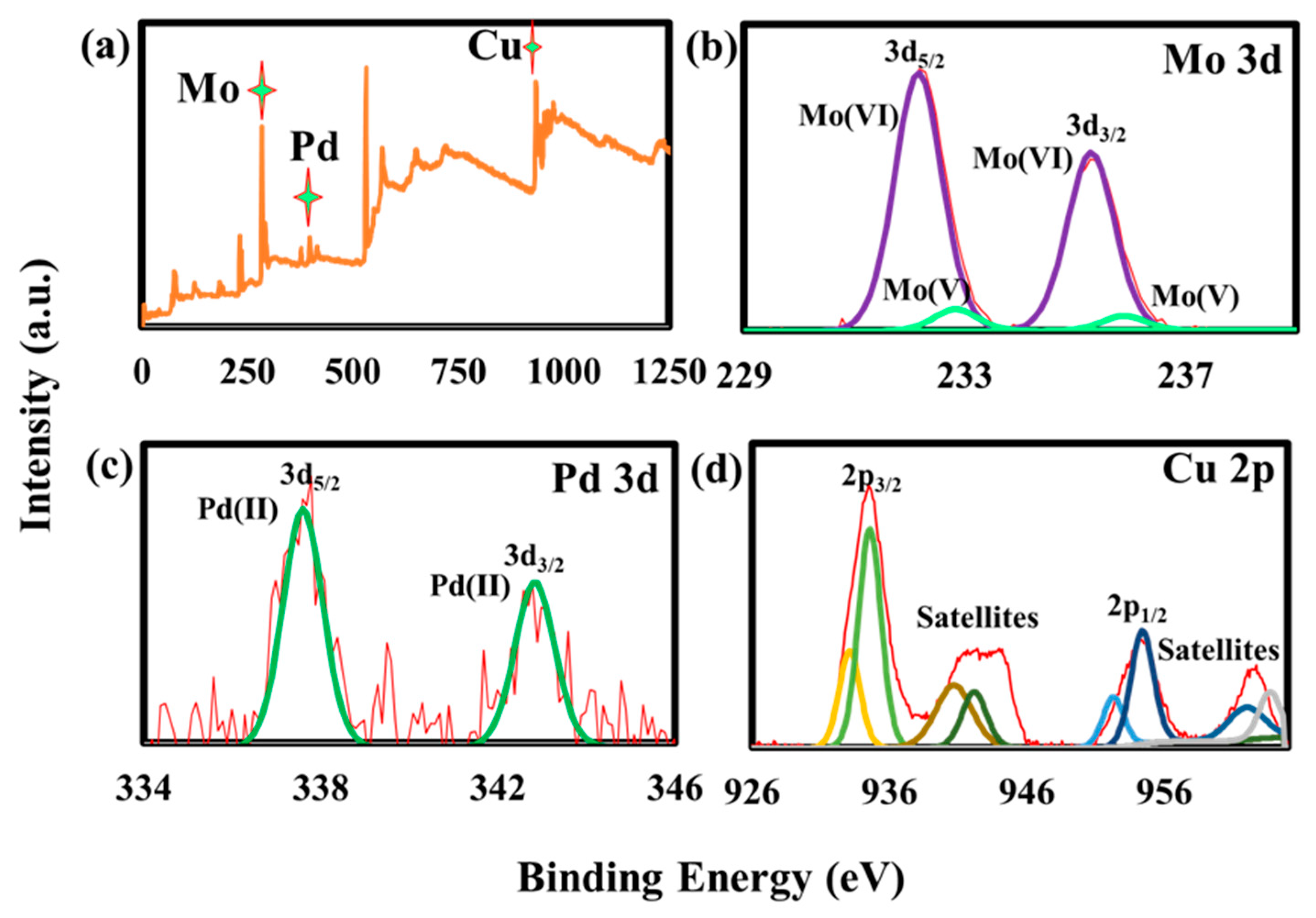
2.1. Preparation and Characterization of Pd@CuMoO4
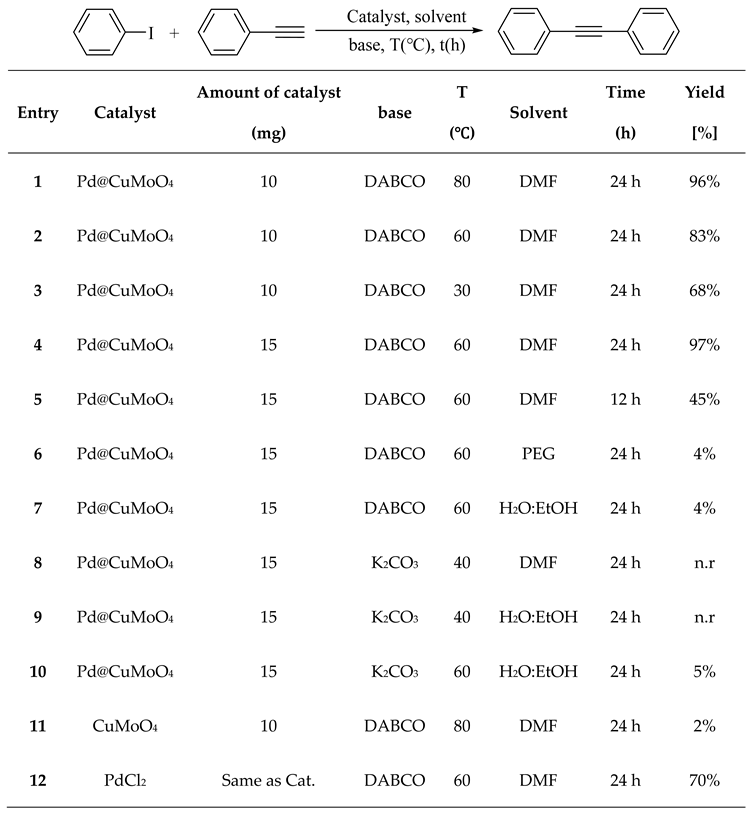
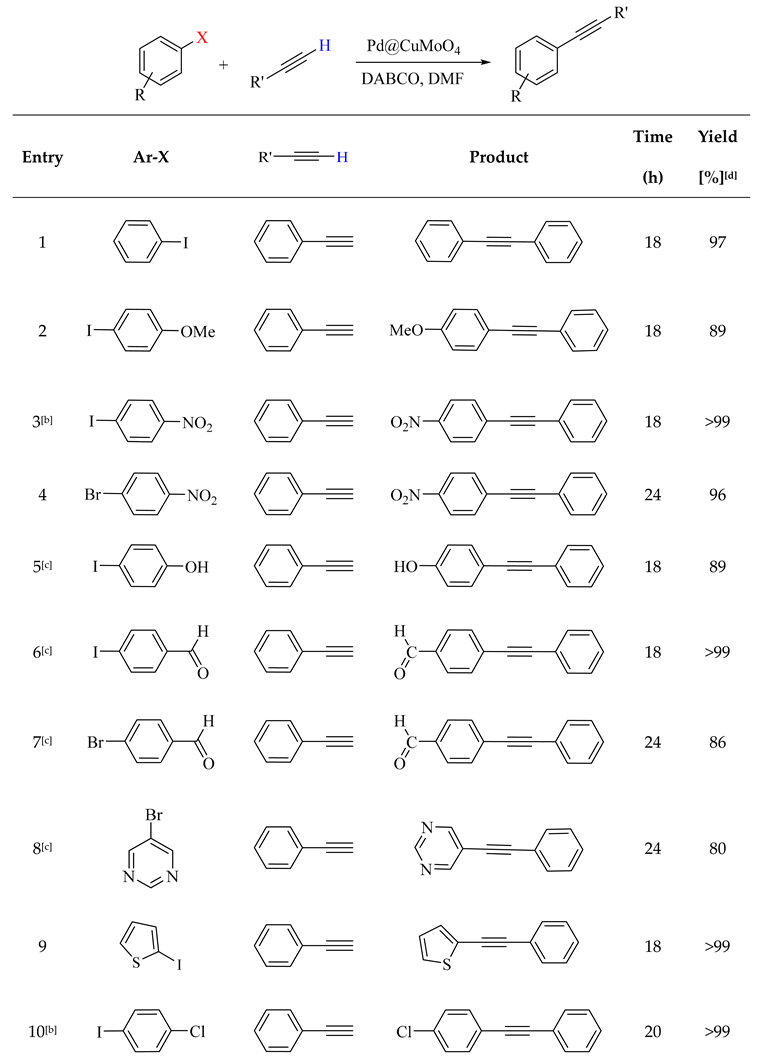
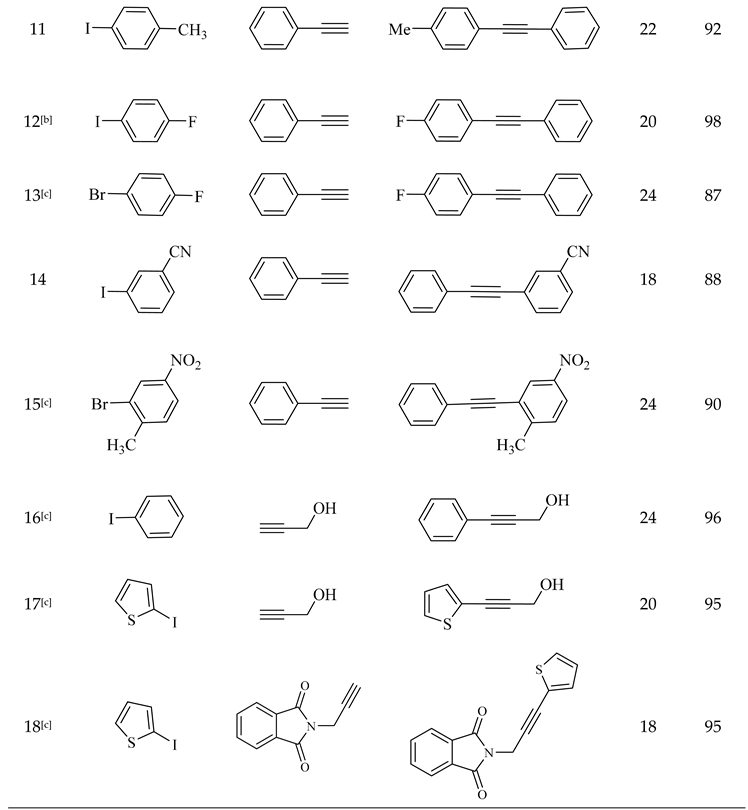
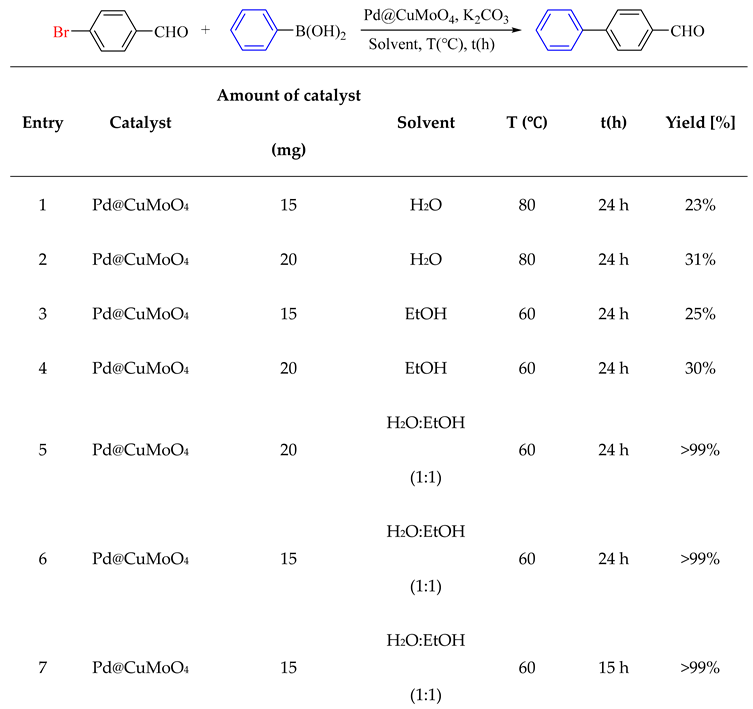
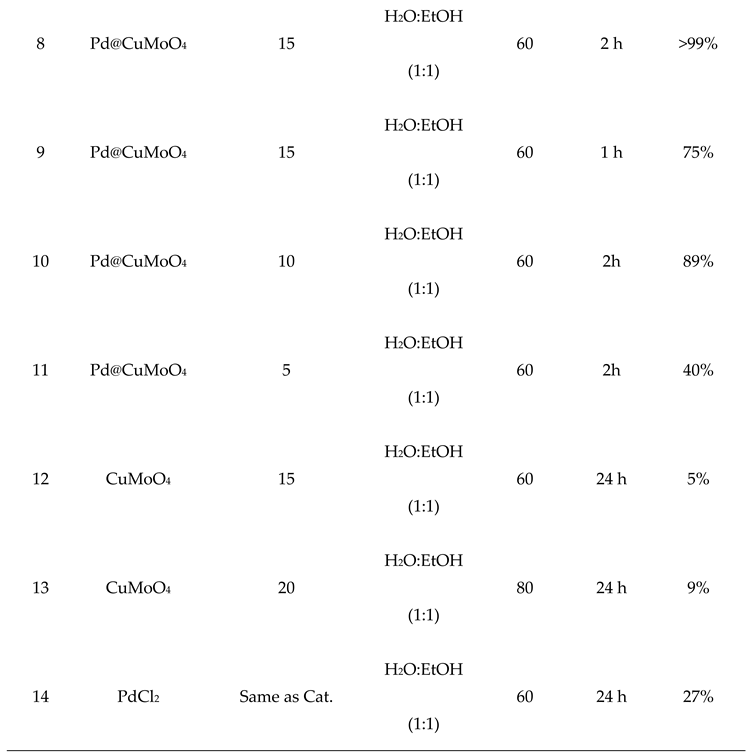
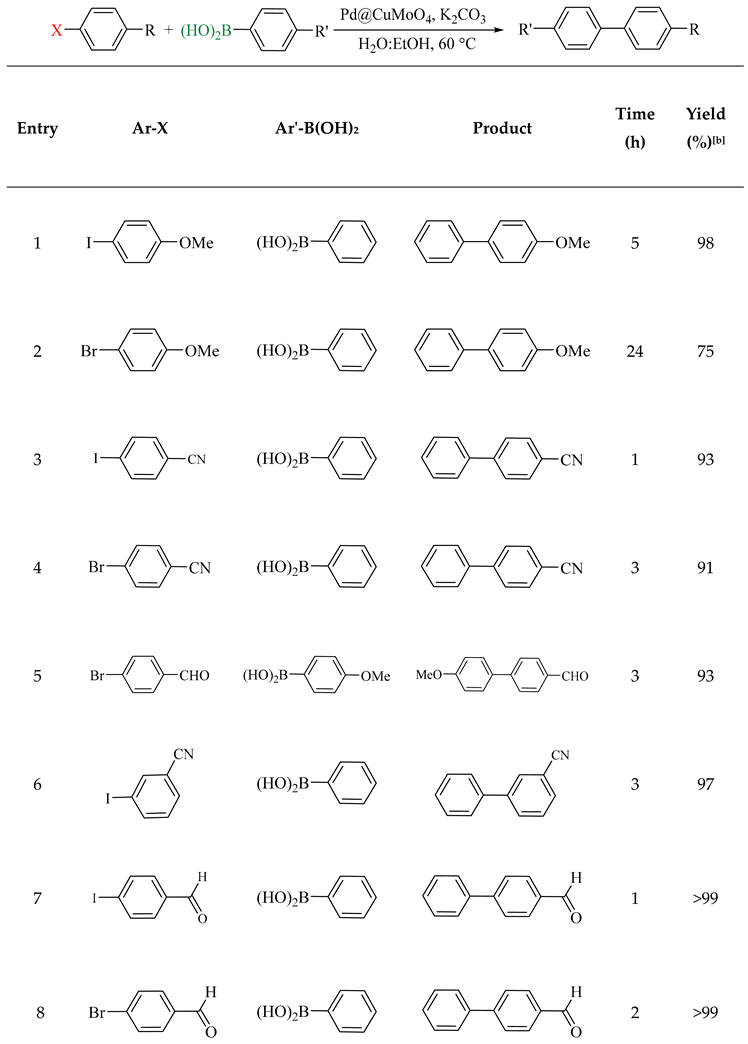
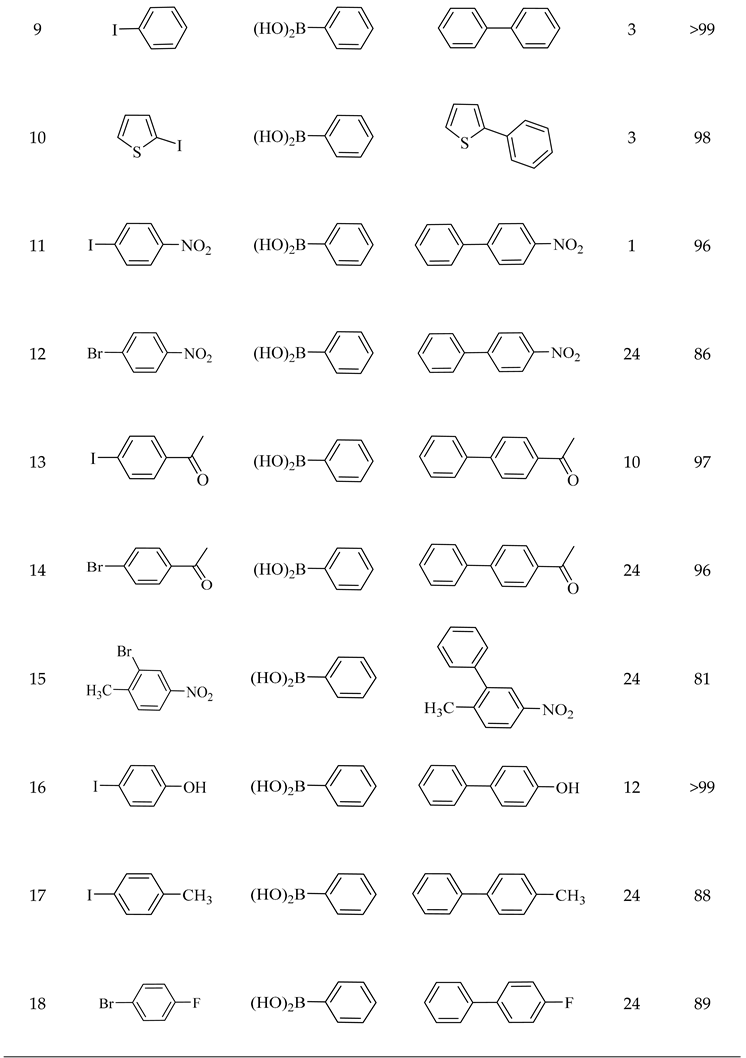
2.2. Catalytic Performance of Pd@CuMoO4
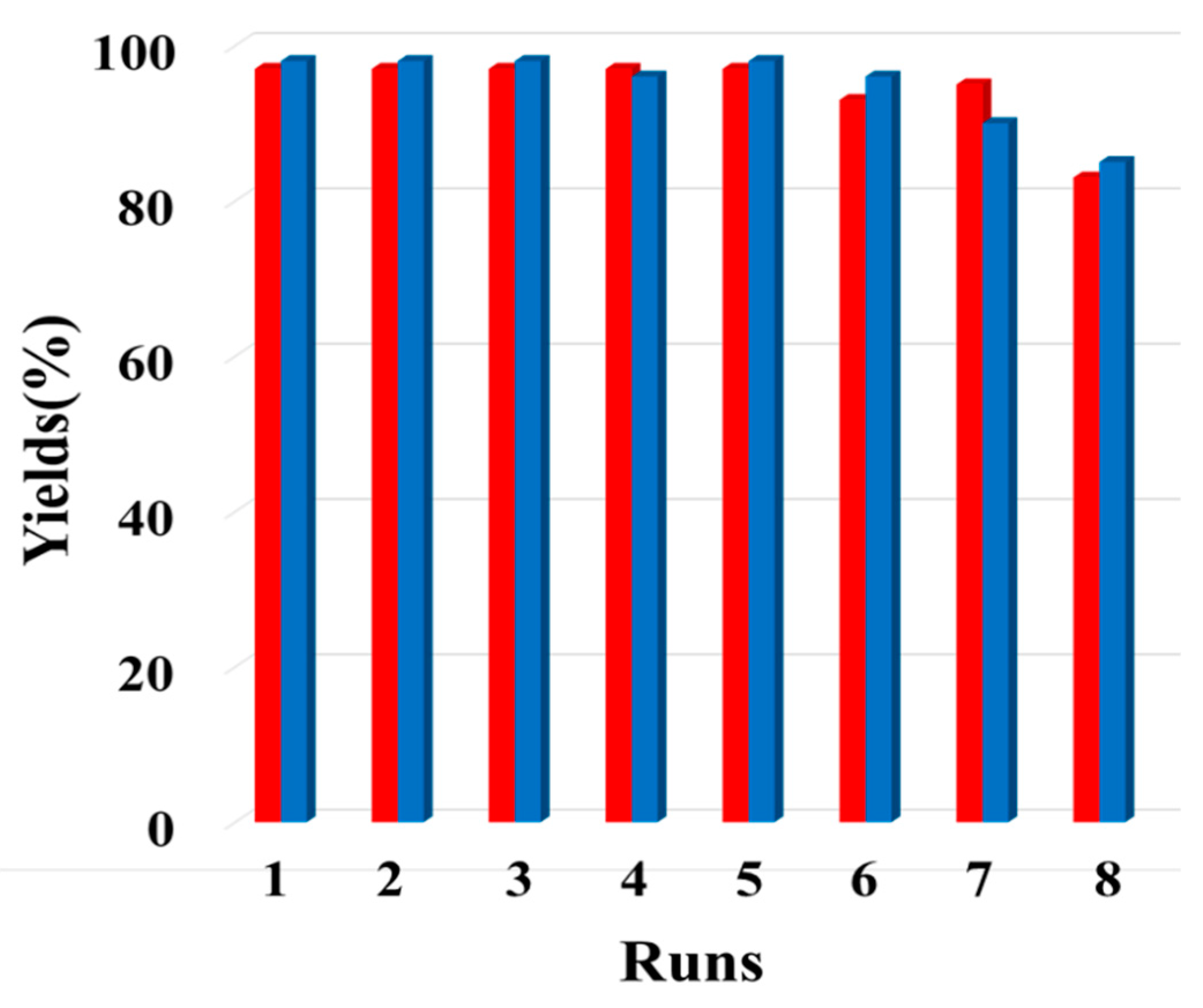
3.3. Recyclability of Pd@CuMoO4
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pd@CuMoO4 Nanostructures
3.3. General Procedure for the Suzuki-Miyaura Reaction
3.4. General Procedure for the Sonogashira-Hagihara Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Guczi, L. Mechanism of reactions on multimetallic catalysts. J. Mol. Catal. 1984, 25, 13–29. [Google Scholar] [CrossRef]
- Gholinejad, M.; Oftadeh, E.; Shojafar, M.; Sansano, J.M.; Lipshutz, B.H. Synergistic effects of ppm levels of palladium on natural clinochlore for reduction of nitroarenes. ChemSusChem. 2019, 12, 4240–4248. [Google Scholar] [CrossRef] [PubMed]
- Davies, I.W.; Matty, L.; Hughes, D.L.; Reider, P.J. Are heterogeneous catalysts precursors to homogeneous catalysts? J. Am. Chem. Soc. 2001, 123, 10139–10140. [Google Scholar] [CrossRef]
- Kalz, K.F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; ... Grunwaldt, J.D. Future challenges in heterogeneous catalysis: Understanding catalysts under dynamic reaction conditions. ChemCatChem 2017, 9, 17–29. [CrossRef]
- Samaniyan, M.; Mirzaei, M.; Khajavian, R.; Eshtiagh-Hosseini, H.; Streb, C. Heterogeneous catalysis by polyoxometalates in metal–organic frameworks. ACS Catal. 2019, 9, 10174–10191. [Google Scholar] [CrossRef]
- Stamate, A.E.; Pavel, O.D.; Zavoianu, R.; Marcu, I.C. Highlights on the catalytic properties of polyoxometalate-intercalated layered double hydroxides: Rev. Catal. 2020, 10, 57. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mehrvarz, E.; Taghipour, A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: A critical review. Rev. Chem. Eng. 2020, 36, 831–858. [Google Scholar] [CrossRef]
- Hill, C.L. Progress and challenges in polyoxometalate-based catalysis and catalytic materials chemistry. J. Mol. Catal. A Chem. 2007, 262, 2–6. [Google Scholar] [CrossRef]
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of polyoxometalates: Towards advanced applications in catalysis and materials science. Chem. Comm. 2008, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, V.; Satyanarayana, V.S.V.; Singh, V.; Pradeep, C.P.; Ghosh, S.; Sharma, S.K.; Gonsalves, K.E. New Polyoxometalates Containing Hybrid Polymers and Their Potential for Nano-Patterning. Chem. Eur. J. 2015, 21, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Organically encapsulated polyoxometalate catalysts: Supramolecular composition and synergistic catalysis. In Encapsulated Catalysts. 2017, (pp. 1-33). Academic Press.
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Ali, N.U.H.L.; Manoharan, S.; Pazhamalai, P.; Kim, S.J. CuMoO4 nanostructures: A novel bifunctional material for supercapacitor and sensor applications. J. Energy Storage. 2022, 52, 104784. [Google Scholar] [CrossRef]
- Hassani, H.O.; Akouibaa, M.; Rakass, S.; Abboudi, M.; El Bali, B.; Lachkar, M.; Al Wadaani, F. A simple and cost-effective new synthesis method of copper molybdate CuMoO4 nanoparticles and their catalytic performance. J. Sci.: Adv. Mater. Devices. 2021, 6, 501–507. [Google Scholar] [CrossRef]
- Alabada, R.; Ayub, A.; Ajaj, Y.; Bhat, S.I.; Alshammari, R.H.; Abduldayeva, A.; ... Mohamed, R.M. A new approach to the synthesis of CuMoO4 nanoparticles with mechanistic insight into the sunlight-assisted degradation of textile pollutants and antibacterial activity evaluation. J. Alloys Compd. 2024, 977, 173400. [CrossRef]
- Ahmed, J.; Ahamad, T.; Alshehri, S.M. rGO supported CuMoO4 nanoparticles: Synthesis, characterization, and electrocatalytic oxygen evolution reaction. New. J. Chem. 2023, 47, 13903–13910. [Google Scholar] [CrossRef]
- Olekszyszen, D.N.; Albuquerque, B.L.; Silva, D.D.O.; Tripodi, G.L.; de Oliveira, D.C.; Domingos, J.B. Core–shell PdCu bimetallic colloidal nanoparticles in Sonogashira cross-coupling reaction: Mechanistic insights into the catalyst mode of action. Nanoscale. 2020, 12, 1171–1179. [Google Scholar] [CrossRef]
- Buskes, M.J.; Blanco, M.J. Impact of cross-coupling reactions in drug discovery and development. Molecules. 2020, 25, 3493. [Google Scholar] [CrossRef]
- González-Sebastián, L.; Morales-Morales, D. Cross-coupling reactions catalysed by palladium pincer complexes. A review of recent advances. J. Organomet. Chem. 2019, 893, 39–51. [Google Scholar] [CrossRef]
- Cheng, L.J.; Mankad, N.P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 2020, 49, 8036–8064. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ning, L.; Zeng, H.C. Confirmation of Suzuki–Miyaura cross-coupling reaction mechanism through synthetic architecture of nanocatalysts. J. Am. Chem. Soc. 2020, 142, 13823–13832. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Organoborane coupling reactions (Suzuki coupling). Proc. Jpn. Acad. Ser. B. 2004, 80, 359–371. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Kadu, B.S. Suzuki–Miyaura cross coupling reaction: Recent advancements in catalysis and organic synthesis. Catal. Sci. Technol. 2021, 11, 1186–1221. [Google Scholar] [CrossRef]
- D'Alterio, M.C.; Casals-Cruañas, È.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic aspects of the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Chem. Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef]
- Reina, A.; Dang-Bao, T.; Guerrero-Ríos, I.; Gómez, M. Palladium and copper: Advantageous nanocatalysts for multi-step transformations. Nanomater. 2021, 11, 1891. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Khosravi, F.; Gholinejad, M.; Sansano, J.M.; Luque, R. Low-amount palladium supported on Fe-Cu MOF: Synergetic effect between Pd, Cu and Fe in Sonogashira-Hagihara coupling reaction and reduction of organic dyes. Mol. Catal. 2022, 522, 112199. [Google Scholar] [CrossRef]
- Gholinejad, M.; Naghshbandi, Z.; Sansano, J.M. Zeolitic imidazolate frameworks-67 (ZIF-67) supported PdCu nanoparticles for enhanced catalytic activity in Sonogashira-Hagihara and nitro group reduction under mild conditions. Mol. Catal. 2022, 518, 112093. [Google Scholar] [CrossRef]
- Gholinejad, M.; Naghshbandi, Z.; Sansano, J.M. Co/Cu bimetallic ZIF as New heterogeneous catalyst for reduction of nitroarenes and dyes. Appl. Organomet. Chem. 2020, 34, e5522. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.X.; Han, J.; Hu, K.; Chen, J.S. Self-supported sheets-on-wire CuO@ Ni (OH) 2/Zn (OH) 2 nanoarrays for high-performance flexible quasi-solid-state supercapacitor. Processes. 2021, 9, 680. [Google Scholar] [CrossRef]
- Li, X.; Kong, W.; Qin, X.; Qu, F.; Lu, L. Self-powered cathodic photoelectrochemical aptasensor based on in situ-synthesized CuO-Cu2O nanowire array for detecting prostate-specific antigen. Mikrochim. Acta. 2020, 187, 325. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.U.H.L.; Manoharan, S.; Pazhamalai, P.; Kim, S.J. CuMoO4 nanostructures: A novel bifunctional material for supercapacitor and sensor applications. J. Energy Storage. 2022, 52, 104784. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Chemical synthesis of a microsphere-like copper molybdate electrode for oxygen evolution reaction. Surfaces and Interfaces. 2021, 26, 101425. [Google Scholar] [CrossRef]
- Khosravi, F.; Gholinejad, M.; Sansano, J.M. Carbon derived from orange peel waste for preparation of benign bimetallic catalyst in organic reactions. Inorg. Chem. Commun. 2024, 164, 112412. [Google Scholar] [CrossRef]
- Sadeghi, M. Investigation of the structural, optical and magnetic properties of CuMoO4 nanoparticles synthesized through a sonochemical method. J. Mater. Sci.: Mater. Electron. 2016, 27, 5796–5801. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.C.; Zhu, J.J. Green preparation and catalytic application of Pd nanoparticles. Nanotechnol. 2008, 19, 305603. [Google Scholar] [CrossRef]

 |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





