Submitted:
11 January 2025
Posted:
14 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
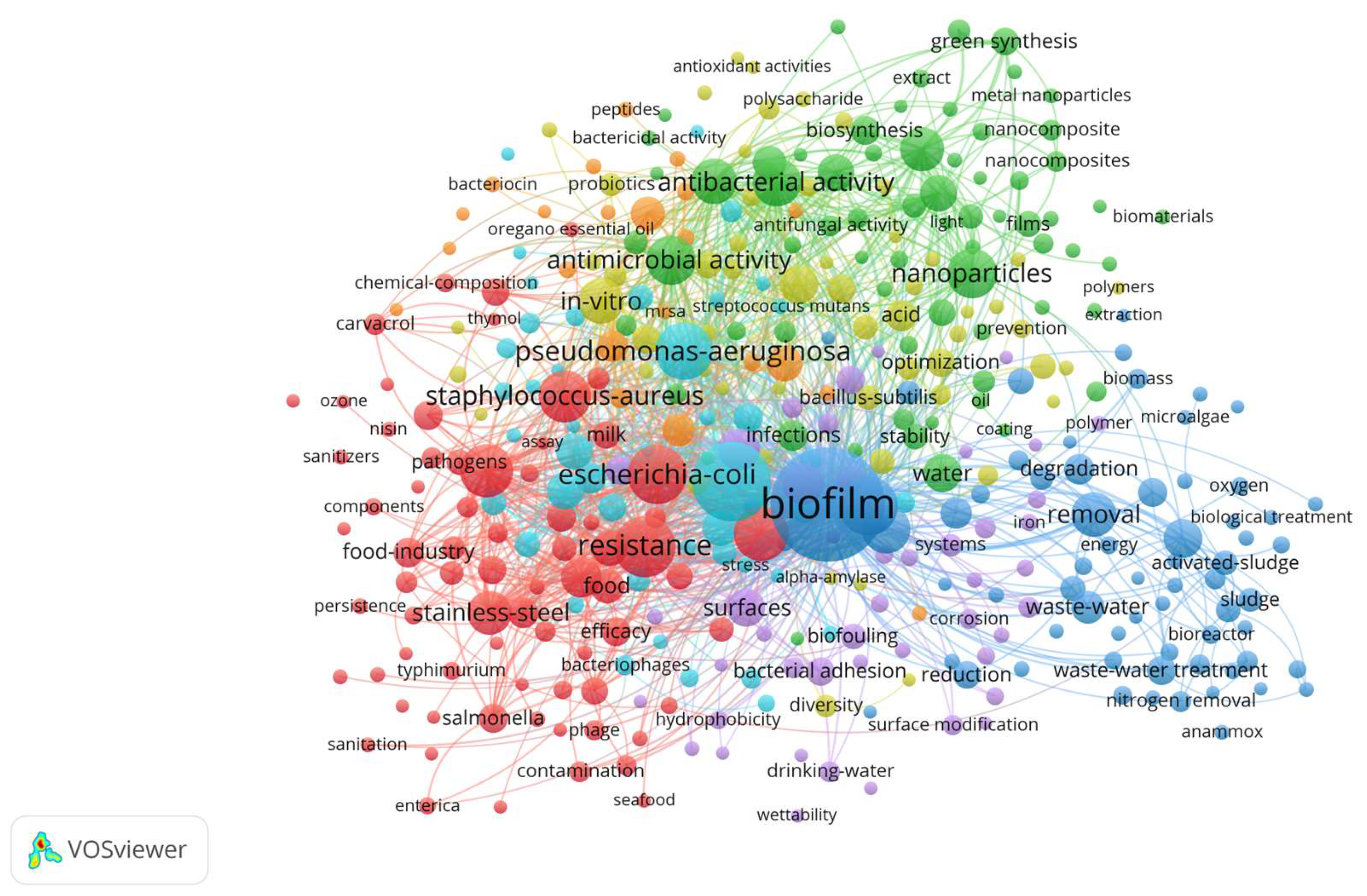
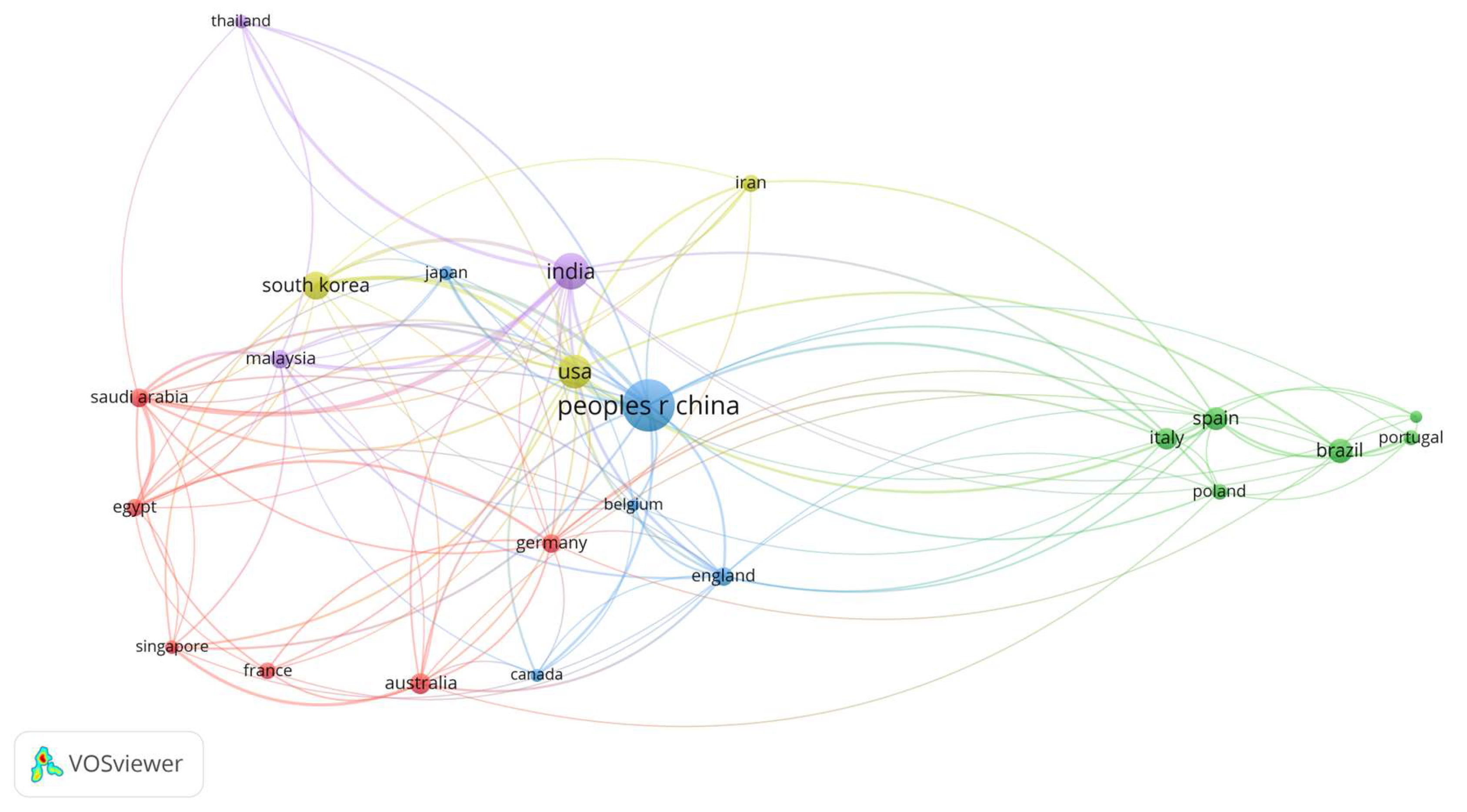
3. Results
4. Discussion
4.1. Recent Biofilm Industry Application Summary
4.2. Future Opportunities with Big Data and Machine Learning
5. Conclusions
References
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: the “house of biofilm cells”. Journal of bacteriology 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nature reviews microbiology 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Liu, Y.; Tay, J.-H. The effects of extracellular polymeric substances on the formation and stability of biogranules. Applied Microbiology and Biotechnology 2004, 65, 143–148. [Google Scholar] [CrossRef]
- Flemming, H.-C. EPS—then and now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Izadi, P.; Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Buhlin, K.; Dufrêne, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Åkerlund, B. Biofilm formation–what we can learn from recent developments. 2018, 284, 332–345. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annual Reviews in Microbiology 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Martin, M.; Hölscher, T.; Dragoš, A.; Cooper, V.S.; Kovács, Á.T. Laboratory evolution of microbial interactions in bacterial biofilms. Journal of bacteriology 2016, 198, 2564–2571. [Google Scholar] [CrossRef]
- de Vos, M.G.J.; Zagorski, M.; McNally, A.; Bollenbach, T. Interaction networks, ecological stability, and collective antibiotic tolerance in polymicrobial infections. Proceedings of the National Academy of Sciences 2017, 114, 10666–10671. [Google Scholar] [CrossRef]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm formation, and related impacts on healthcare, food processing and packaging, industrial manufacturing, marine industries, and sanitation–a review. Applied Microbiology 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Vishwakarma, V. Impact of environmental biofilms: Industrial components and its remediation. Journal of basic microbiology 2020, 60, 198–206. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Advanced drug delivery reviews 2015, 85, 7–23. [Google Scholar] [CrossRef]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef]
- Tenke, P.; Köves, B.; Nagy, K.; Hultgren, S.J.; Mendling, W.; Wullt, B.; Grabe, M.; Wagenlehner, F.M.E.; Cek, M.; Pickard, R. Update on biofilm infections in the urinary tract. World journal of urology 2012, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Importance of biofilms in urinary tract infections: new therapeutic approaches. Advances in biology 2014, 2014, 543974. [Google Scholar] [CrossRef]
- González, M.J.; Robino, L.; Iribarnegaray, V.; Zunino, P.; Scavone, P. Effect of different antibiotics on biofilm produced by uropathogenic Escherichia coli isolated from children with urinary tract infection. Pathogens and disease 2017, 75, ftx053. [Google Scholar] [CrossRef]
- Pinto, H.; Simões, M.; Borges, A. Prevalence and impact of biofilms on bloodstream and urinary tract infections: a systematic review and meta-analysis. Antibiotics 2021, 10, 825. [Google Scholar] [CrossRef]
- Philipp, L.-A.; Bühler, K.; Ulber, R.; Gescher, J. Beneficial applications of biofilms. Nature Reviews Microbiology 2023, 1–15. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, J.; Yuan, X.; Jiang, X.; Wang, Y.; Zhong, C.; Xu, D.; Gu, T.; Wang, F. Bacterial biofilms as platforms engineered for diverse applications. Biotechnology advances 2022, 57, 107932. [Google Scholar] [CrossRef]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef]
- Philips, J.; Verbeeck, K.; Rabaey, K.; Arends, J.B.A. Electron transfer mechanisms in biofilms. In Microbial electrochemical and fuel cells; Elsevier, 2016; pp. 67–113. [Google Scholar]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. npj Biofilms and Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, Y. From bibliography to understanding: water microbiology and human health. Journal of Water and Health 2024, 22, 1911–1921. [Google Scholar] [CrossRef]
- Chen, W.; Fu, B.; Ma, F.; He, Z.; Li, M. Hot spots and trends in microbial disease research on cultural heritage: a bibliometric analysis. Environmental Science and Pollution Research 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, Y. A bibliography study of Shewanella oneidensis biofilm. FEMS Microbiology Ecology 2023, 99, fiad124. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, Y. Tackling heavy metal pollution: evaluating governance models and frameworks. Sustainability 2023, 15, 15863. [Google Scholar] [CrossRef]
- Wang, B.; Pan, S.-Y.; Ke, R.-Y.; Wang, K.; Wei, Y.-M. An overview of climate change vulnerability: a bibliometric analysis based on Web of Science database. Natural hazards 2014, 74, 1649–1666. [Google Scholar] [CrossRef]
- Mongeon, P.; Paul-Hus, A. The journal coverage of Web of Science and Scopus: a comparative analysis. Scientometrics 2016, 106, 213–228. [Google Scholar] [CrossRef]
- Saiz-Alvarez, J.M. Innovation Management: A Bibliometric Analysis of 50 Years of Research Using VOSviewer® and Scopus. World 2024, 5, 901–928. [Google Scholar] [CrossRef]
- Fenfen, Z.; Guoshuang, Z.; Jiali, C.; Jianhong, Z.; Sihui, D.; Shaomin, C. Current status and trends in the modernization of pulse diagnosis research: a bibliometric analysis based on Citespace and VOSviewer. Digital Chinese Medicine 2023, 6, 405–415. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Y.; Qian, S. Influence of bacterial incorporation on mechanical properties of engineered cementitious composites (ECC). Construction and Building Materials 2019, 196, 195–203. [Google Scholar] [CrossRef]
- Zhang, Z.; Weng, Y.; Ding, Y.; Qian, S. Use of genetically modified bacteria to repair cracks in concrete. Materials 2019, 12, 3912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Ding, Y.; Wang, S. Mechanical performance of strain-hardening cementitious composites (SHCC) with bacterial addition. Journal of Infrastructure Preservation and Resilience 2022, 3, 3. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Cohen, Y.; Cao, B. Elevated level of the second messenger c-di-GMP in Comamonas testosteroni enhances biofilm formation and biofilm-based biodegradation of 3-chloroaniline. Applied microbiology and biotechnology 2015, 99, 1967–1976. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Ding, Y.; Qian, S. Mechanical and antibacterial behavior of photocatalytic lightweight engineered cementitious composites. Journal of Materials in Civil Engineering 2021, 33, 04021262. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Ding, Y.; Qian, S. Cementitious Composite Materials for Self-Sterilization Surfaces. ACI Materials Journal 2022, 119, 197–210. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Ding, Y.; Qian, S. Visible light antibacterial potential of graphene-TiO2 cementitious composites for self-sterilization surface. Journal of Sustainable Cement-Based Materials 2023, 12, 972–982. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Ding, Y.; Qian, S. Graphene-Based TiO2 Cement Composites to Enhance the Antibacterial Effect of Self-Disinfecting Surfaces. Catalysts 2023, 13, 1313. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, N.; Du, Y.; Ji, L.; Cao, B. Disruption of putrescine biosynthesis in Shewanella oneidensis enhances biofilm cohesiveness and performance in Cr (VI) immobilization. Applied and environmental microbiology 2014, 80, 1498–1506. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Yao, J.; Szymanski, C.; Fredrickson, J.; Shi, L.; Cao, B.; Zhu, Z.; Yu, X.-Y. In situ molecular imaging of the biofilm and its matrix. Analytical chemistry 2016, 88, 11244–11252. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Yao, J.; Xiong, Y.; Zhu, Z.; Yu, X.-Y. Molecular evidence of a toxic effect on a biofilm and its matrix. Analyst 2019, 144, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.e.; Wu, J.; Ding, Y.; Wang, V.B.; Zhang, Y.; Kjelleberg, S.; Loo, J.S.C.; Cao, B.; Zhang, Q. Hybrid conducting biofilm with built-in bacteria for high-performance microbial fuel cells. ChemElectroChem 2015, 2, 654–658. [Google Scholar] [CrossRef]
- Zhao, C.-e.; Chen, J.; Ding, Y.; Wang, V.B.; Bao, B.; Kjelleberg, S.; Cao, B.; Loo, S.C.J.; Wang, L.; Huang, W. Chemically functionalized conjugated oligoelectrolyte nanoparticles for enhancement of current generation in microbial fuel cells. ACS Applied Materials & Interfaces 2015, 7, 14501–14505. [Google Scholar]
- Yang, Y.; Ding, Y.; Hu, Y.; Cao, B.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS synthetic biology 2015, 4, 815–823. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Wei, Y.; Wang, W.; Wang, F.; Yang, Y.; Song, H.; Yuan, Q. Multiplexed identification of bacterial biofilm infections based on machine-learning-aided lanthanide encoding. ACS nano 2022, 16, 3300–3310. [Google Scholar] [CrossRef]
- Papa, R.; Garzoli, S.; Vrenna, G.; Sabatino, M.; Sapienza, F.; Relucenti, M.; Donfrancesco, O.; Fiscarelli, E.V.; Artini, M.; Selan, L. Essential oils biofilm modulation activity, chemical and machine learning analysis—Application on Staphylococcus aureus isolates from cystic fibrosis patients. International journal of molecular sciences 2020, 21, 9258. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: nutrient, organics removal and bioenergy production. Chemical Engineering Journal 2018, 332, 277–285. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. Journal of Chemical Technology & Biotechnology 2013, 88, 508–518. [Google Scholar]
- Sousa, A.M.; Ferreira, A.; Azevedo, N.F.; Pereira, M.O.; Lourenço, A. Computational approaches to standard-compliant biofilm data for reliable analysis and integration. Journal of Integrative Bioinformatics 2012, 9, 57–68. [Google Scholar] [CrossRef]
- Martins, F.G.; Melo, A.; Sousa, S.F. Databases for the study of biofilms: current status and potential applications. Biofouling 2021, 37, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.P.; Vieira, T.F.; Fernandes, H.S.; Melo, A.; Simões, M.; Sousa, S.F. The biofilms structural database. Trends in biotechnology 2020, 38, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pan, Y.; Hussain, W.; Chen, G.; Li, E. BBSdb, an open resource for bacterial biofilm-associated proteins. Frontiers in Cellular and Infection Microbiology 2024, 14, 1428784. [Google Scholar] [CrossRef]
- Chen, J.; Jenkins, W.K. Facial recognition with PCA and machine learning methods. 2017; pp. 973–976.
- Sharma, S.; Bhatt, M.; Sharma, P. Face recognition system using machine learning algorithm. 2020; pp. 1162–1168.
- Bachute, M.R.; Subhedar, J.M. Autonomous driving architectures: insights of machine learning and deep learning algorithms. Machine Learning with Applications 2021, 6, 100164. [Google Scholar] [CrossRef]
- Dogan, Ü.; Edelbrunner, J.; Iossifidis, I. Autonomous driving: A comparison of machine learning techniques by means of the prediction of lane change behavior. 2011; pp. 1837–1843.
- Chen, S.; Ding, Y. Machine learning and its applications in studying the geographical distribution of ants. Diversity 2022, 14, 706. [Google Scholar] [CrossRef]
- Lesnik, K.L.; Liu, H. Predicting microbial fuel cell biofilm communities and bioreactor performance using artificial neural networks. Environmental science & technology 2017, 51, 10881–10892. [Google Scholar]
- Buetti-Dinh, A.; Galli, V.; Bellenberg, S.; Ilie, O.; Herold, M.; Christel, S.; Boretska, M.; Pivkin, I.V.; Wilmes, P.; Sand, W. Deep neural networks outperform human expert’s capacity in characterizing bioleaching bacterial biofilm composition. Biotechnology Reports 2019, 22, e00321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, G.; Hussain, W.; Pan, Y.; Yang, Z.; Liu, Y.; Li, E. Machine learning and network analysis with focus on the biofilm in Staphylococcus aureus. Computational and Structural Biotechnology Journal 2024, 23, 4148–4160. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.A.; Hayta, E.N.; Selle, D.M.; Kouroudis, I.; Harth, M.; Gagliardi, A.; Lieleg, O. Machine learning approach to analyze the surface properties of biological materials. ACS Biomaterials Science & Engineering 2021, 7, 4614–4625. [Google Scholar]
- Qamar, A.; Kerdi, S.; Amin, N.; Zhang, X.; Vrouwenvelder, J.; Ghaffour, N. A deep neural networks framework for in-situ biofilm thickness detection and hydrodynamics tracing for filtration systems. Separation and Purification Technology 2022, 301, 121959. [Google Scholar] [CrossRef]
- Dimauro, G.; Deperte, F.; Maglietta, R.; Bove, M.; La Gioia, F.; Renò, V.; Simone, L.; Gelardi, M. A novel approach for biofilm detection based on a convolutional neural network. Electronics 2020, 9, 881. [Google Scholar] [CrossRef]

| Model Species | Main Contribution | Reference |
|---|---|---|
| Bacillus halodurans | Bacillus halodurans bacteria improves crack healing and tensile strength in engineered cementitious composites. | [31] |
| Bacillus halodurans | Bacillus halodurans genetically modified for faster crack repair in concrete. | [32] |
| Bacillus halodurans | Bacillus halodurans enhances fracture toughness and strength in strain hardening cementitious composites. | [33] |
| Comamonas testosteroni | Comamonas testosteroni enhanced biofilm formation and biodegradation of 3-chloroaniline with c-di-GMP. | [34] |
| Escherichia coli | Escherichia coli used to test antibacterial behavior of photocatalytic lightweight engineered cementitious composites. | [35] |
| Escherichia coli | Escherichia coli testing shows fine-particulate TiO2’s superior antibacterial performance. | [36] |
| Escherichia coli | Escherichia coli inactivation enhanced by graphene oxide-titanium dioxide composite under visible light. | [37] |
| Escherichia coli | Escherichia coli decomposition improved using graphene-based TiO2 under visible light. | [38] |
| Shewanella oneidensis | Shewanella oneidensis shows improved Cr(VI) remediation with cohesive mutant biofilms developed | [39] |
| Shewanella oneidensis | Shewanella oneidensis biofilms analyzed for molecular responses to environmental Cr(VI) stress. | [40] |
| Shewanella oneidensis | Shewanella oneidensis biofilm responses to Cr(VI) analyzed via mass spectrometry imaging. | [41] |
| Shewanella oneidensis | Shewanella oneidensis hybrid biofilms improve microbial fuel cell efficiency and performance. | [42] |
| Shewanella oneidensis | Shewanella oneidensis achieves spontaneous nanoparticle accumulation, boosting biofilm-driven microbial fuel cell current densities. | [43] |
| Shewanella oneidensis | Shewanella oneidensis enhances extracellular electron transfer through synthetic flavin biosynthesis pathway expression. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





