1. Background and Significance of UHS
By 2050, it is anticipated that there will be 10 billion people on earth and there will be an increasing need for energy to meet this growth and demand [
1]. To be able to address this need, many countries are turning to renewable energy sources as a supplement to fossil fuels. The limited resource of fossil fuels necessitates the usage of alternative energy sources as the need for energy rises [
2]. Consuming fossil fuels without extreme environmental controls substantially impacts the environment, including greenhouse gas emissions leading to global warming [
3]. Scientists have emphasized that if global warming continues unchecked, the consequences will be severe. However, immediate action is still needed to mitigate global warming such as reducing greenhouse gas emissions by integrating more sustainable energy into our energy mix, advancing carbon capture and storage (CCS), and increasing climate resilience and adaptation. The world energy outlook 2024 reports that renewable energy is outpacing electricity demand growth reducing fossils fuel dependency. In 2023, renewables supplied 30% of the global electricity, which fossil fuels dropped to 60%their lowest share in 50 years.
In 2020, carbon emissions from energy use dropped by over 6%, making the most significant decline since 1945, driven by a sharp reduction in global primary energy demand due to impacts of the global COVID-19 pandemic had on energy markets. Thus, the decline in the energy demand primarily impacted fossil fuels, while renewables especially hydroelectricity, wind and solar continued to grow because of their competitive costs and supportive policies. This reduction aligns with the increasing adoption of renewables such as wind, solar and hydroelectric power, which continued to grow despite the overall energy downturn [
3]. These energy sources depend on seasonal changes in sunlight, wind strength, and other geographical factors. The interplay and changing yearly energy demands can result in either excess or shortages of renewable energy. Because of the unpredictability of these renewable sources, hydrogen offers a reliable alternative that is not dependent on seasons. It can be stored and used when demand increases. Hydrogen can be derived from both renewable and non-renewable sources. It has the highest energy content and is the most common and lightest element [
4]. Hydrogen can be seen as a key replacement for fossil fuels because it can be burned, stored and used similarly. This makes hydrogen an attractive alternative for decarbonizing high-demand industries such as shipping, aviation, and steel and iron production [
5,
6,
7]. The smooth operation of large-scale hydrogen value chains requires adequate storage capacity and performance. Geological storage is considered the most effective method for large-scale, long -term storage. Hydrogen can be stored in underground geological formations such as aquifers, rock caverns, and depleted oil and gas reservoirs [
8]. Subsurface hydrogen storage is less advanced than gas storage methods like CO
2 and CH
4. Large -scale underground hydrogen storage is crucial for managing seasonal energy supply and driving the hydrogen economy. Hydrogen storage plays an important role in enhancing electricity system stability, integrating renewable energy sources, and advancing decarbonisation efforts. It provides long-term energy storage, enhances energy security, and powers industries and transportation [
9]. Underground natural gas (UGS) has several similarities to underground hydrogen storage (UHS). The knowledge gained in UGS can often be applied to UHS projects such as site selections, storage methods, monitoring, and managing injection and withdrawal cycles. The key fundamental difference is that Hydrogen has a higher chemical reactivity compared to natural gas, meaning that hydrogen can participate in biological, microbial, and chemical activities [
10]. In addition, unique hydrogen properties such as mobility and low viscosity result in fingering, gravity segregation, and overriding [
10].
Hydrogen may become a major energy vector by 2050, supplementing or replacing coal and natural gas by 2050 [
11]. It is’ predicted to be widely employed in aviation, shipping, transportation, steelmaking and chemical. H
2 plays a crucial role in efforts to establish a carbon-neutral economy thereby supporting goals outlined in the Paris Climate Agreement and the European Green Deal.
This paper reviews the potential of underground hydrogen storage to meet the growing global energy demand by 2050. Its highlights technological advancements, environmental benefits, and economic feasibility, emphasizing its role in reducing greenhouse gas emissions. Addressing gaps in existing research, the study covers storage capacity, efficiency, safety. And sustainability, along with integration into renewable energy systems. It also evaluates policy frameworks and real-world applications, offering insights for enhancing energy security and promoting the adoption of hydrogen storage.
2. Sources of Hydrogen
Hydrogen production related CO
2 emissions vary depending on the energy source, including wind, solar, biomass, natural gas, and nuclear. Electrolysis, which employs water to produce H
2, has evolved greatly to produce low-emission options. Most of the hydrogen produced today comes from natural gas (76%) and coal (23%), while some are produced via electrolysis [
11]. The EU used 7.94 million metric tons of Hydrogen in 2024, largely in refineries (57%) and the chemical industry, where it is used to manufacture products like ammonia (25%) and methanol (12%), which account for 8% of global production. However, most EU-produced hydrogen is still “gray”, meaning it comes from fossil fuels rather than renewable resources [
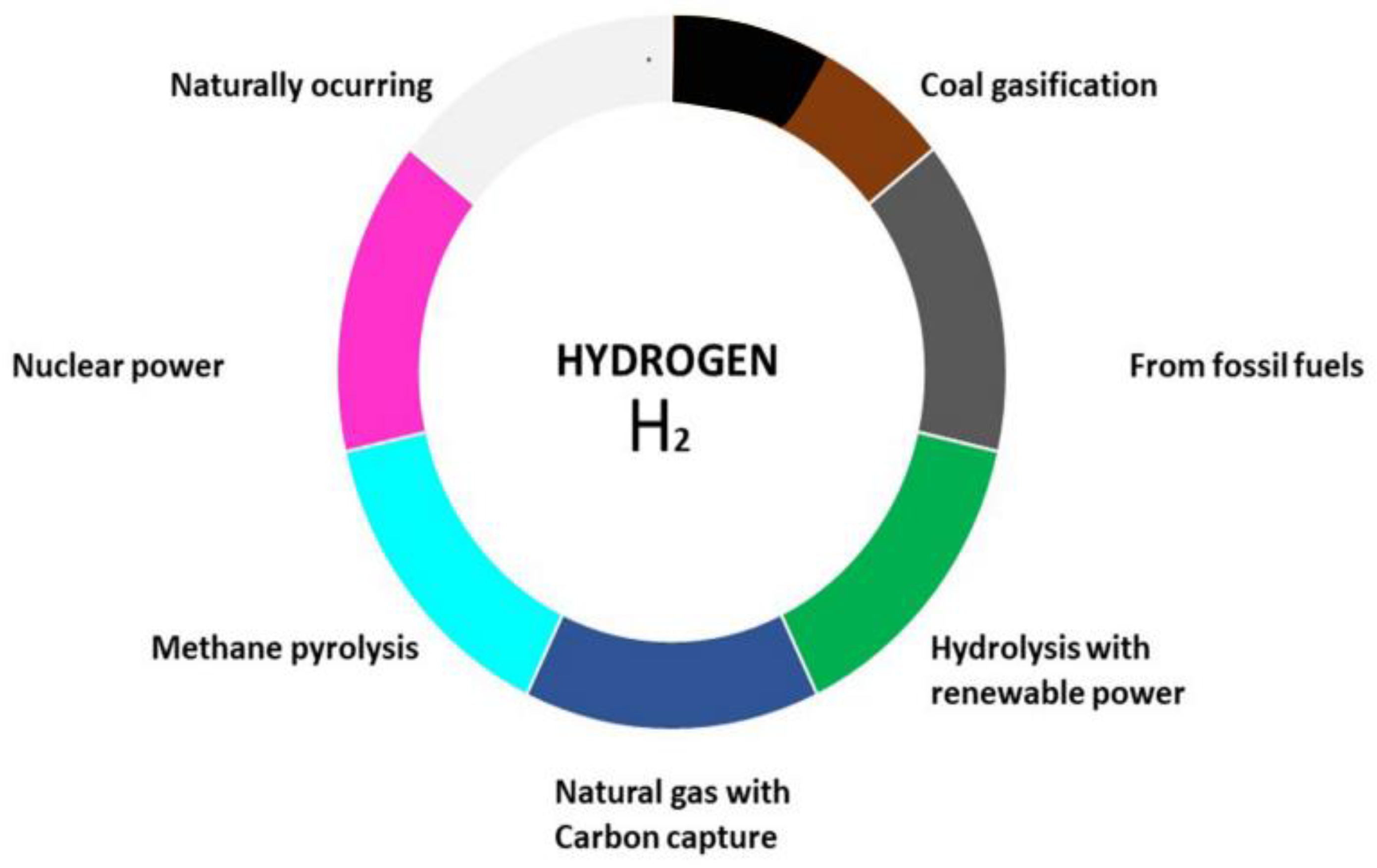
11]. The various processes that produce hydrogen are represented by the color code blue, green, black, brown, turquoise, yellow, pink, gray, and white. These colors also symbolize how each process affects the environment as shown in (
Figure 1). The three most abundant forms of hydrogen are green, gray, and blue [
11].
Hydrogen derived from fossil fuels is known as gray hydrogen, produced through the steam methane reforming process, which releases CO₂ emissions. At about
$1 per kilogram, this method remains the most cost-effective way to produce hydrogen, accounting for around 6% of the world's natural gas. Carbon capture and storage (CCS) can help mitigate emissions from gray hydrogen, but as clean energy alternatives expand, green hydrogen is expected to gradually grow [
12]. Endothermic steam reformation uses steam that is between 700
oC and 1000
o C.
The cost of producing gray hydrogen is influenced by several technical and financial factors, with capital expenditures and gas costs being the most significant. Between 45% and 75% of production costs are attributed to gas expenses. Additionally, the production of hydrogen from this process generates 9 -12 tons of CO
2 per unit of hydrogen produced [
13]. The production of blue hydrogen involves steam methane reforming, with carbon capture and storage (CCS) technologies used to capture the CO
2 emissions produced during its generation [
14]. The most common technique for generating hydrogen today is using this fossil fuel-based process. In 2024, the price for producing hydrogen using fossil fuels, ranges from
$0.98-
$2.93 per kilogram, according to the IEA, about three times cheaper than the peak pricing for hydrogen generated from renewable electricity [
12]. Although this price disparity is predicted to shrink by 2050, blue hydrogen will likely remain the more economical. Future costs will depend on factors such as technological advancements, production scale, availability of CO₂ storage, and price of natural gas. However, blue hydrogen synthesis is energy - intensive; only 70 – 75% of the initial natural gas heat is retained in the final hydrogen product. Additionally, in order to reach carbon neutrality, adding carbon capture and storage (CCS) technology raises production costs by roughly
$0.5 per kilogram, with overall costs varying between
$1.5 to
$2.5 per kilogram, depending on natural gas prices [
12].
In contrast, green hydrogen is generated through zero-emission methods like water electrolysis powered by renewable energy sources like solar, wind, biomass gasification [
15]. Green hydrogen is costly so it currently makes up only a portion of the hydrogen mix. According to a study conducted in 2020, called “Path to Hydrogen Competitiveness”, the present cost of green hydrogen was about
$6/kg, which makes it costlier than blue and gray hydrogen [
12]. Aiming to promote hydrogen to facilitate the clean energy transition, the worldwide CEO-led Hydrogen Council, composed of top companies, is pushing for
$2/kg to be the tipping price point for green hydrogen to become a desirable option for use in a variety of industries [
16]. Influenced by global goals, trends, and sector-specific actions, Price waterhouse coopers(PwC) forecasts that green hydrogen demand would rise modestly until 2030 and reach 150 – 500 million tons annually by 2050.
Black hydrogen is produced by gasifying coal to create syngas, a mixture of carbon dioxide, hydrogen, carbondioxide and methane. The composition of the syngas can vary based on the feedstock and gasification method used. This process involves heating coal at high temperatures with steam and oxygen. Brown hydrogen, on the other hand, is produced directly from coal through a more basic method of extraction, typically without the additional step of gasification. In both processes, the hydrogen output can be enhanced by further treatment of the syngas. However, black hydrogen is typically associated with a more complex gasification process, while brown hydrogen comes from simpler, less processed coal [
17].
The cost of producing brown hydrogen is between $1.20 and $2.20 per kilogram. However, each year, it emits a considerable amount of CO2, approximately 830 million tonnes which is not environmentally desirable. The market for brown hydrogen is projected to reach $48.9 billion by 2030, driven by its economic benefits. However, over time, environmental concerns regarding emissions may reduce its appeal.
Pink hydrogen, also known as purple or red hydrogen, is produced by using nuclear energy to electrolyze water. Initiatives in the United States and the United Kingdom are advancing the use of nuclear energy for hydrogen production, with costs currently between
$2 -
$6 per kilogram. In the next decade, the US Department of Energy (DOE) aims to reduce the cost of clean hydrogen generated from hybrid nuclear systems, which use leftover heat and electricity, down to
$1 per kilogram [
12]. Nuclear hydrogen generation costs anywhere from
$2 -
$6 depending on the production process. For example, the Japan Atomic Energy Agency has successfully tested this technique on a small scale and intends to produce hydrogen for under
$3 per kilogram using high-temperature reactors [
18].
The Turquoise Hydrogen method uses a process of methane pyrolysis, thus splitting natural gas into solid carbon and Hydrogen without emitting CO₂. When powered by renewable energy, turquoise hydrogen is carbon-neutral. Companies like Mitsubishi are investing in turquoise hydrogen as an appealing option that bridges traditional and renewable hydrogen production, leveraging existing gas infrastructure with minimal emissions. The process becomes carbon neutral if the energy required to produce heat for the breakdown of natural gas comes from renewable sources [
19]. The process may be carbon-negative if the natural gas is derived from biogenic sources. The goal is to increase the appeal of turquoise hydrogen to consumers by lowering its price to conventionally produced (gray) hydrogen.
White hydrogen is a naturally occurring substance that occurs in rock formations and geological zones of the Earth's crust without the help of humans. A potentially carbon-neutral resource, it has been found in regions like Australia and Mali, though it has not been widely exploited so far [
20]. Early modelling assessment by the US Geological Survey (USGS) indicate that the United States has at least two key regions with promising geology for significant natural hydrogen accumulations: The Atlantic Coastal Plain and Central area such as the Great plains and the upper Midwest. Like natural gas production, hydrogen can become a clean, affordable energy source. There will probably be more exploration and extraction projects as the field develops. Hydrogen, produced from geologic sources deep underground, holds significant potential as the most cost-effective and competitive alternative to fossil fuels. While estimates of its global production vary, research suggests that hydrogen may be present in large quantities in the Earth’s crust. Recently, large hydrogen reservoirs have been identified, driving new exploration efforts. This finding has generated a wave of rivalry among businesses seeking exploration rights, with 18 white hydrogen drilling licenses already issued or filed in South Australia. Despite these advancements, the white hydrogen market remains in the initial stages of development [
12].
3. General Consideration of Underground Hydrogen Storage
The small molecular size, low density, and viscosity of hydrogen pose a major concern for storage. A good reservoir for a hydrogen storage system should have suitable permeability and porosity with a great caprock seal. Geological storage formation approaches vary in terms of depth deposits, lithology of the site storage, storage capacity, geological tightness, recent experience, availability of structures, and existing infrastructure. Geological, technical, environmental, and economic factors are critical in site selection [
20]. Hydrogen exhibits low solubility in water and argillaceous rocks, with a correspondingly low diffusion coefficient. During storage cycles, 1% is lost due to operational procedures and 0.4% due to hydrogen dissolving in brine, causing undesired hydrodynamic behavior. The hydrodynamic behavior during cyclic processes shows unwanted behaviors such as viscous fingering, cushioning gas mixing and lateral spreading [
21]. These events have led to the development of numerically simulated strategies to mitigate the adverse subsurface hydrodynamic behavior of hydrogen. Key mitigation techniques employed are selective technology, increasing the number of storage cycles, optimizing the arrangement of extracted wells and controlling the injection rate [
22]. Hydrogen can also catalyze subsurface settings and induce bacterial activity which may lead to several adverse effects, including hydrogen loss, compromised geological integrity, and reduced permeability. The impact of the subsurface hydrodynamic behavior of hydrogen is a universal concern for all underground hydrogen storage (UHS) methods, but it is particularly significant for depleted reservoirs and aquifers [
21,
22]. Fluid management in underground hydrogen storage must address challenges such as environmental concerns and rheological performance [
23]. The distinct physical characteristics of hydrogen have major relevance in the effectiveness of underground hydrogen storage (UHS). Hydrogen, is significantly less dense as compared to methane and carbon dioxide which have been extensively researched and stored underground for years. Hydrogen's lower viscosity and solubility enhance the efficiency in storage and retrieval cycles, preventing fluid coning problems and reducing losses in saline aquifers or depleted reservoirs, particularly in water, hydrogen, and salt systems [
24].
Table 1 illustrate the difference in physiochemical properties of H
2, CO
2 and CH
4 that’s are relevant for consideration during storage purposes.
4. Key Drivers for Utility-Scale UHS
To overcome the problem of energy curtailment that emerges with renewable sources, utility -scale UHS, is crucial. Because of fluctuating weather, shifting demand, sluggish transmission networks, fluctuating fuel prices, varying consumption patterns, and geographic constraints, extra energy frequently goes underutilized during peak output periods. To augment the total dependability and efficiency of renewable resources, UHS offers an effective solution for storing excess energy for later use. Effectively capturing and storing excess renewable energy, such as hydrogen (H
2) can help mitigate seasonal fluctuations in renewable energy generation. When renewable energy production is low, utilizing this storage method minimizes and optimizes the use of clean energy resources. [
25,
26,
27]. Leveraging natural gas infrastructure for UHS can significantly boost H
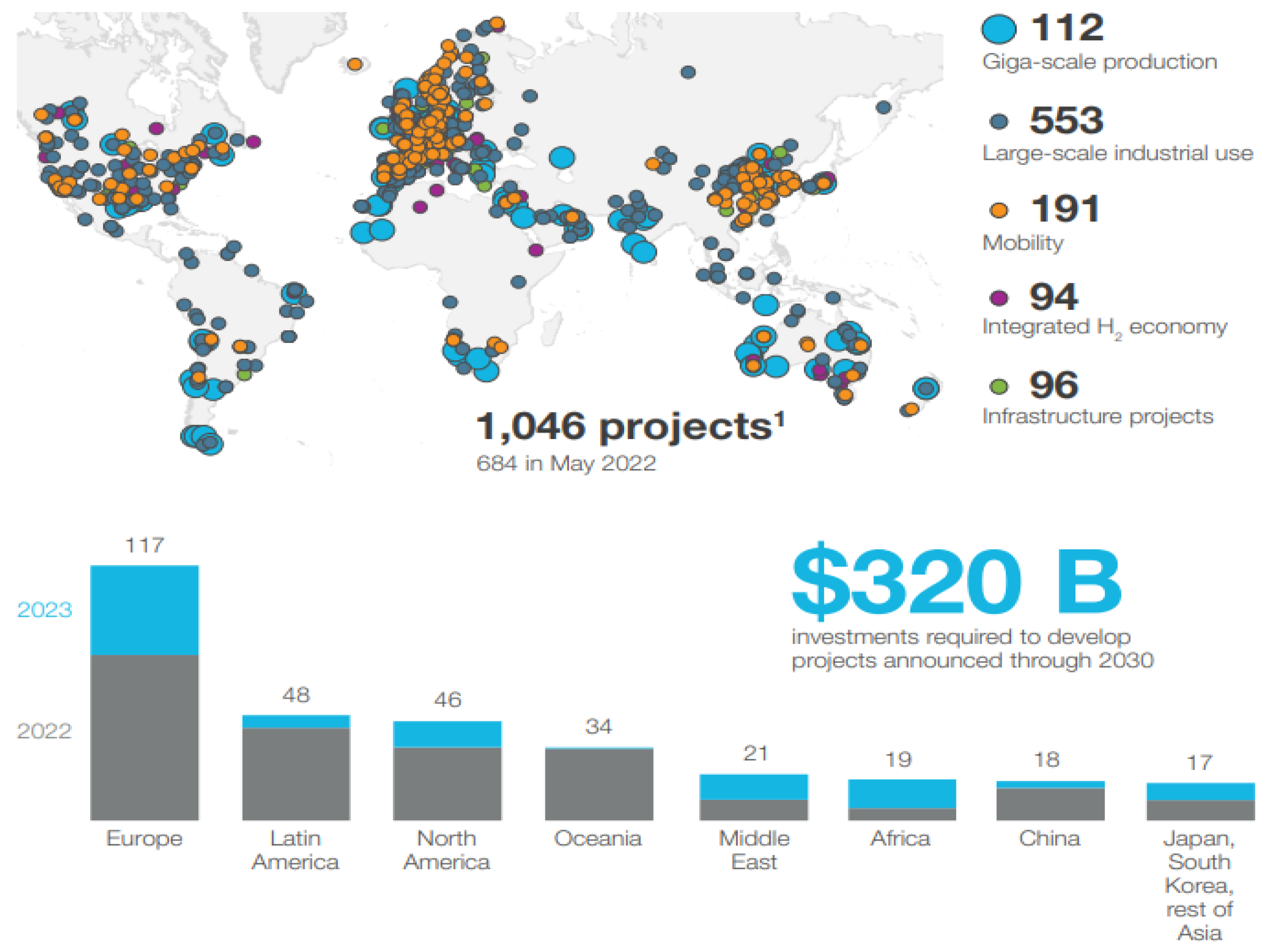
2 storage capacity quickly and affordably, reducing additional investments and expediting utility-scale deployment, thereby facilitating a low-carbon energy future. Global interest in H
2 production projects and political actions for their widespread use has increased, with national and international projects aiming to increase H
2 production, as shown in (
Figure 2). As of December 2023, national H
2 strategy road maps and, first drafts have been published in more than 40 countries. More than 1000 project plans have been revealed by the global H
2 Sector, and since 2022, more than 350 additional plans have been added.
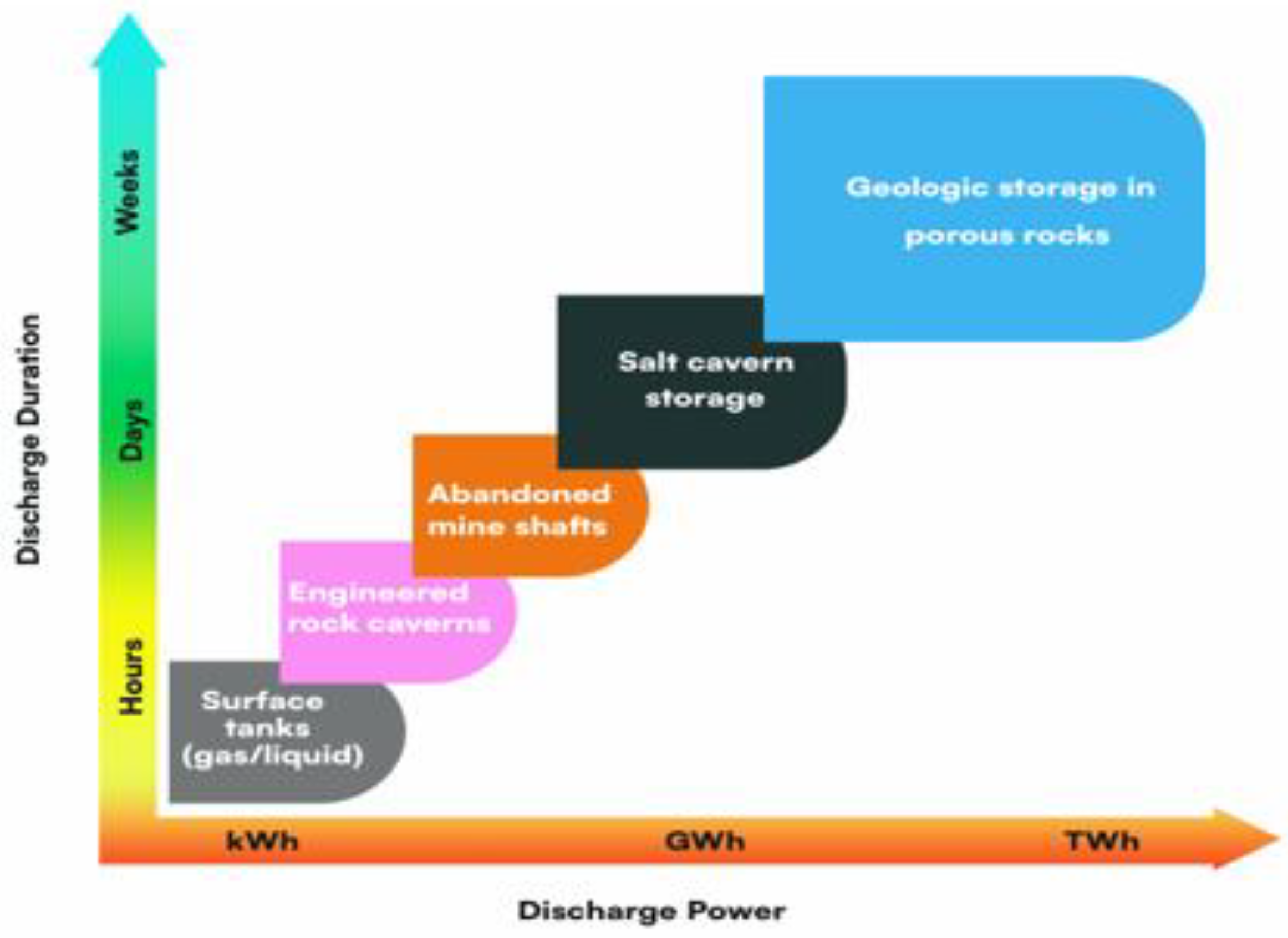
The Storage of large volumes of energy above the earth appears unworkable due to pressure limitations and the need for specialized materials. Long-term use and extensive energy output make UHS more feasible. UHS technology improves the feasibility for extended duration and large-scale energy generation.
Figure 3 data encompasses gigawatts-hours to terawatt-hours over timeframes ranging from weeks to seasons in salt caverns and porous media such as saline aquifers and depleted reservoirs [
26,
29].
The global spread of subterranean hydrogen storage projects is being fueled by the switch to renewable energy sources, advancements in subsurface storage technologies, and increased investments in sustainable energy infrastructure.
Table 2 gives an overview and summary of worldwide underground hydrogen storage (UHS) demonstrated projects and feasibility studies conducted in many nations. Germany leads with the biggest number of projects, mostly focused on feasibility studies for porous formations. Other notable countries, such as the Netherlands and the United States, also show significant interest in cavern and porous storage planning. Operational projects remain restricted, illustrating the early–stage development of UHS technology worldwide.
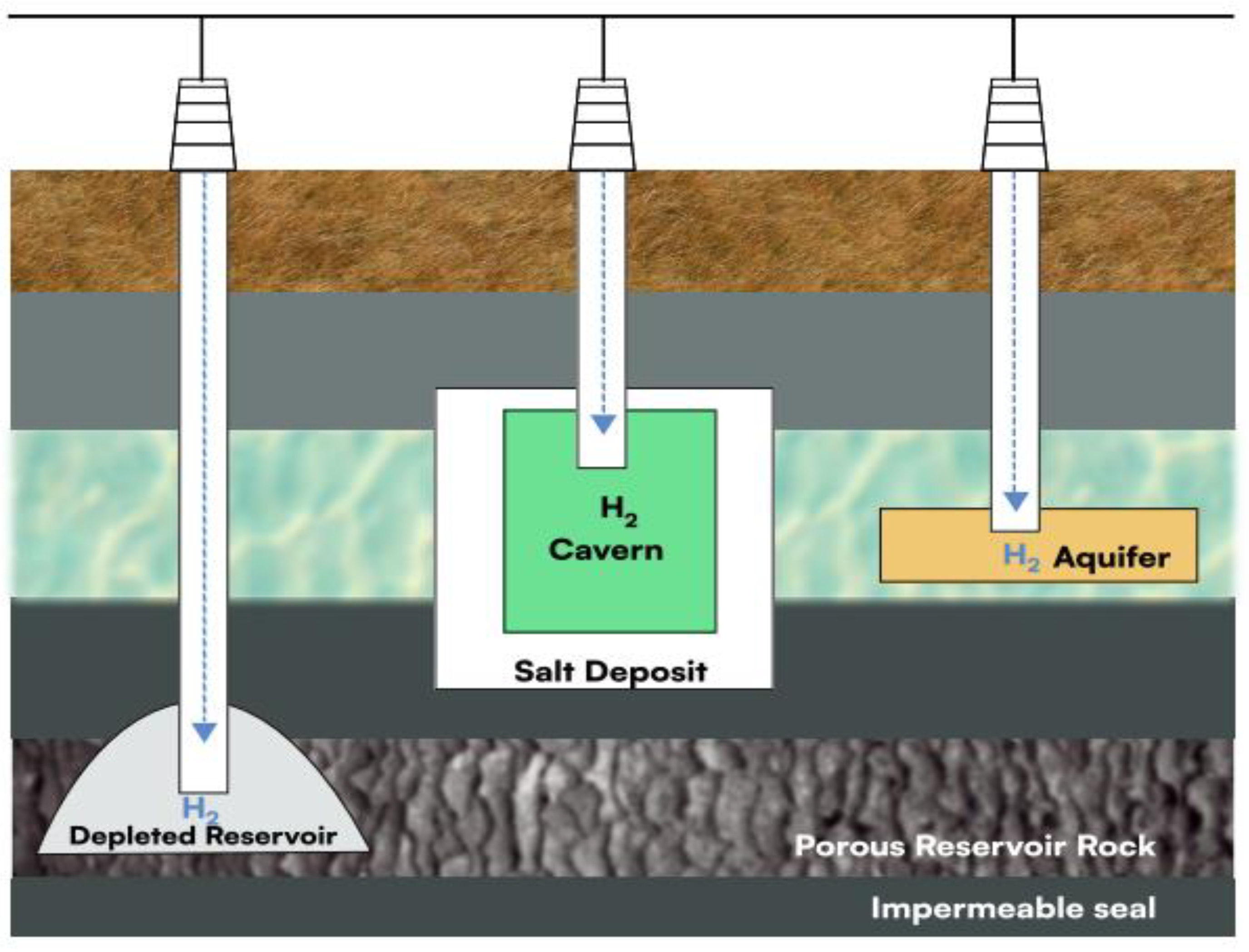
5. Subsurface Geological Formations for UHS
There are several types of UHS options currently under consideration: aquifers, which are natural underground water reservoirs; (b) depleted hydrocarbon deposits, such as those in natural gas or oil reservoirs; (c) salt caverns, created by dissolving rock salt; (d) Subterranean mining areas, including abandoned salt or limestone mines along with rock caverns. Each offers unique characteristics that influence the hydrogen storage efficiency and suitability.
Figure 4.
Hydrogen storage in porous reservoir rocks.
Figure 4.
Hydrogen storage in porous reservoir rocks.
5.1. Depleted Oil and Gas Reservoirs
Depleted oil and gas fields have suitable infrastructure and clearly defined geological formations, making them a good candidate for hydrogen storage. Formation storage capacity, storage depth and caprock formation thickness are essential considerations when choosing a storage location [
20]. Depleted Gas offers advantages, particularly the residual gas and well-characterized underlying structures. Hydrogen is preserved in geological formations by mostly reversible trapping mechanisms such structural/ stratigraphic, capillary trapping and solubility /dissolution in fluids. A number of parameters must be satisfied for UHS in depleted reservoirs to operate reliably over the long term. A comprehensive assessment of a reservoir’s structural integrity, economic feasibility, and safety is important. The demonstrated tightness of depleted hydrocarbon reservoirs, filled with gas confined for millions of years, is a major benefit of using them. Residual gases might persist in the reservoirs chosen for UHS, and they might serve as cushion gases and aid in pressure maintenance. A cushion gas volume of 50% - 60% is typically required [
33,
34]. Notwithstanding this economic advantage, hydrogen may mix with the reservoir’s residual native gases. The extent of interactions between several phases and the components in the reservoir may induce contamination of the hydrogen stored. The reservoir must be clean of contaminants to ensure safe UHS operation. Significant hydrogen loss can occur when contaminants like methanogenic and sulfate-reducing bacteria are present [
35]. Among the most researched subsurface geological porous media are oil and gas reservoirs. Depleted gas reservoirs are particularly easy to handle due to their extensive history of development, management, and operation; their stable caprock and well-documented geological properties make them suitable for UHS. However, before storing hydrogen in these reservoirs, a number of important issues must be resolved, including controlling bacterial hydrogen loss, reducing biological reactions [
36] impacted by hydrogen, avoiding cushion gas mixing, and guaranteeing hydrogen gas’s purity during retrieval [
33]. A depleted hydrocarbon reservoir offers hydrogen storage, with cushion gas likely natural gas. Processes like mixing, bacterial activity, and leakage may occur. The reservoir's depth ranges between 1 - 3 kilometers, and the hydrogen plume extends several kilometers [
37].
Table 3.
Overview of key considerations and specifications for underground energy storage (UES) in depleted gas and oil [
38].
Table 3.
Overview of key considerations and specifications for underground energy storage (UES) in depleted gas and oil [
38].
| Criteria |
Requirements |
Geology of Rock Type
Structure
|
Large sedimentary rock
Homogeneous, isotropic rocks, no significant
tectonic deformations, rocks poorly faulted,
fissured, jointed and folded, no discontinuities |
Depth
Porosity
Permeability
Hydraulic Fracture
Thermal Stability
Lifetime of Storage |
70m - 200m and or depth where ground water
hydrostatic pressure is equal or marginally greater
than the stored product (H2, C02, Natural gas) pressure
Low Porosity
Low Permeability
< 10^-8 ms-1 for H20
Between 4 and 80oC
>30 years |
5.2. Aquifers
Aquifers are subsurface rocks or soil layers containing pore-filled water. Their widespread distribution makes them useful for hydrogen storage. Aquifers are also capable of successfully storing various gases; as multiple studies have demonstrated [
39,
40]. A low-permeability cap rock must exist above the aquifer to halt gas leakage, and the rock must have high porosity and permeability to let gas flow in order to store hydrogen in the aquifers. However, faulting, mineral interactions, and chemical or biological activities can cause issues with hydrogen storage in aquifers and decrease storage performance. Furthermore, aquifers are frequently poorly studied compared to depleted oil and gas fields, necessitating extra testing and drilling to minimize risks and uncertainties. An important contrast between depleted fields and aquifers is the cushion gas quantity needed to maintain steady pressure. Aquifers require 80% of Cushing gas, whereas depleted fields only require 50% to 60%. Therefore, for aquifer storage projects, selecting a suitable gas type for cushioning is critical. Considering the physiochemical properties of hydrogen, a significant funding for subsurface infrastructure, including wells, and injection systems, is required to switch from underground gas storage to hydrogen storage in aquifers, enhancing its economic feasibility [
39]. Aquifer water can also generate problems such as excessive hydrogen and water generation and alterations to the liquid-gas interface during injection and withdrawal, which can raise prices. Underground hydrogen storage in aquifers must be practical and cost-effective. To achieve this, it is necessary to address the challenges similar to those faced when using depleted reservoirs for storage. Detailed hydrodynamic analysis during storage and recovery cycles is essential to ensure the efficiency of UHS in aquifers. Key factors include the reservoir’s ability to store and transmit hydrogen effectively and the presence of a non-permeable barrier to prevent gas leakage.
Table 4.
Overview of key consideration and specifications for UES in Saline Aquifer [
38].
Table 4.
Overview of key consideration and specifications for UES in Saline Aquifer [
38].
| Criteria |
Requirements |
Geology of Rock Type
Caprock
Minimum Caprock Thickness
Depth
Porosity
Permeability
Thickness
Vertical closure
Discovery Pressure |
Sandstones, and preferably conglomerates
Shale, Siltstone and Carbonate rocks
6m
200-2000m
>10%
300m
10m
>10m
2- 8 MPa |
5.3. Salt Caverns
Caverns can be formed in two types of salt deposits: bedded salts and salt domes. Salt domes are thick and uniform masses of salt, making it easy to create a stable cavern for typical operations. Bedded salts are thinner layers found closer to the surface and mixed with other rocks like anhydrite, dolomite, and shale. Nonetheless, if the depth exceeds 6000ft beneath the ground, the salt may deform due to high pressure and temperature, even if the cavern is well-designed [
38]. In addition, underground hydrogen storage caverns in formations like bedded salts are less stable and thinner because of the different types of rocks. Salt caverns have advantages and disadvantages compared to porous formations (aquifers and depleted reservoirs) for gas storage. Some disadvantages are: their limited availability, the need to manage and dispose of water, the possibility of irregular-shaped caverns, and thermal and mechanical stability challenges. Some advantages are: natural impermeability that prevents leakages, rapid injection and extraction, ensuring efficient energy management, maintains hydrogen purity and operate flexibly at varying pressure. Another factor that affects the injection rate is the amount of water left at the bottom of the cavern, which needs to be considered carefully. Cavern lithology varies across different layers, each with specific properties that influencing creep rates, deformation, and sliding behavior along the bedding planes. Caverns can extend to depths of around 2 km and store up to 1 million cubic meters of working gas [
37]. Stability and safety concerns are critical variables that constrain the shape, size, spacing, and target pressure levels for both the working and cushion gases. In addition, the creep behavior of creep in rock salt must be assessed before beginning operations. For underground rock formations such as caverns, initial stress is required before construction. [
37]. Standard operating procedures, conventional practices, and geomechanical evaluations of the location-controlled gas operating pressures. To prevent structural loss from salt hydraulic fracturing and possible failure of the cemented well casing, the maximum pressure is generally kept 75-85% of the initial vertical starting stress component. [
37,
38].
Table 5 below gives an overview of considerations for underground energy storage in salt caverns.
Underground hydrogen storage is possible in depleted reservoirs, aquifers, and salt caverns, each with advantages and disadvantages.
Table 6 compares the underground storage types.
6. Mechanisms of Underground Hydrogen Storage
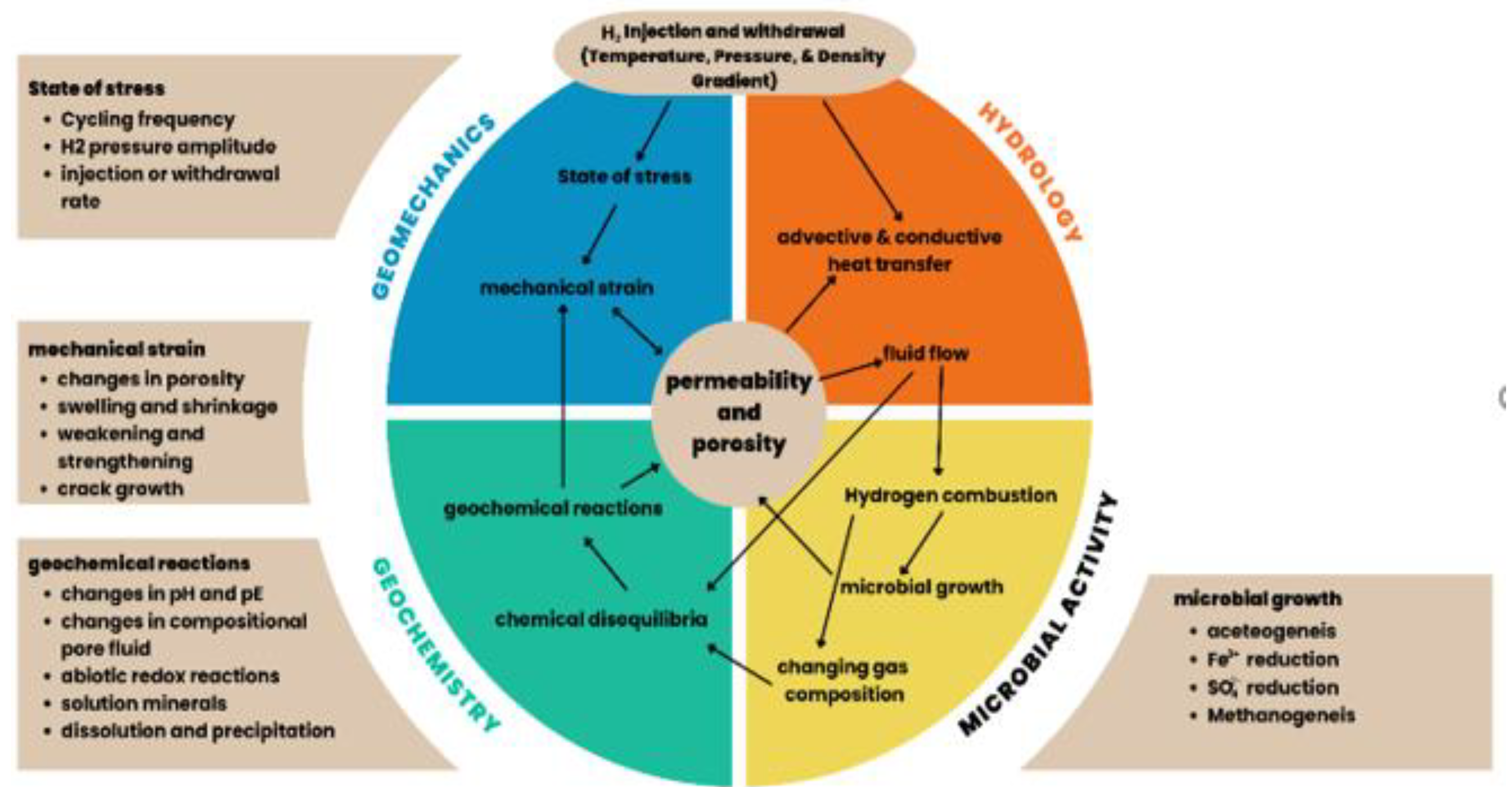
UHS involves a complex interplay of geochemical, physicochemical, hydrodynamic, microbial, and biological factors as indicated in
Figure 5. The advancement of UHS technology requires a strong understanding of the mechanisms at work throughout time and operational cycles. Many important problems hinder UHS development: controlling hydrogen flow in the porous reservoirs, understanding the possible geochemical reactions during and after injection, addressing microbial interactions leading to hydrogen consumption, and determining how storage affects the geomechanical stability of the formation. Further challenges are limited storage capacity, geological integrity maintenance, and inadequate sites. Ultimately, the viability and potential of UHS depend heavily on the features of the geological formation.
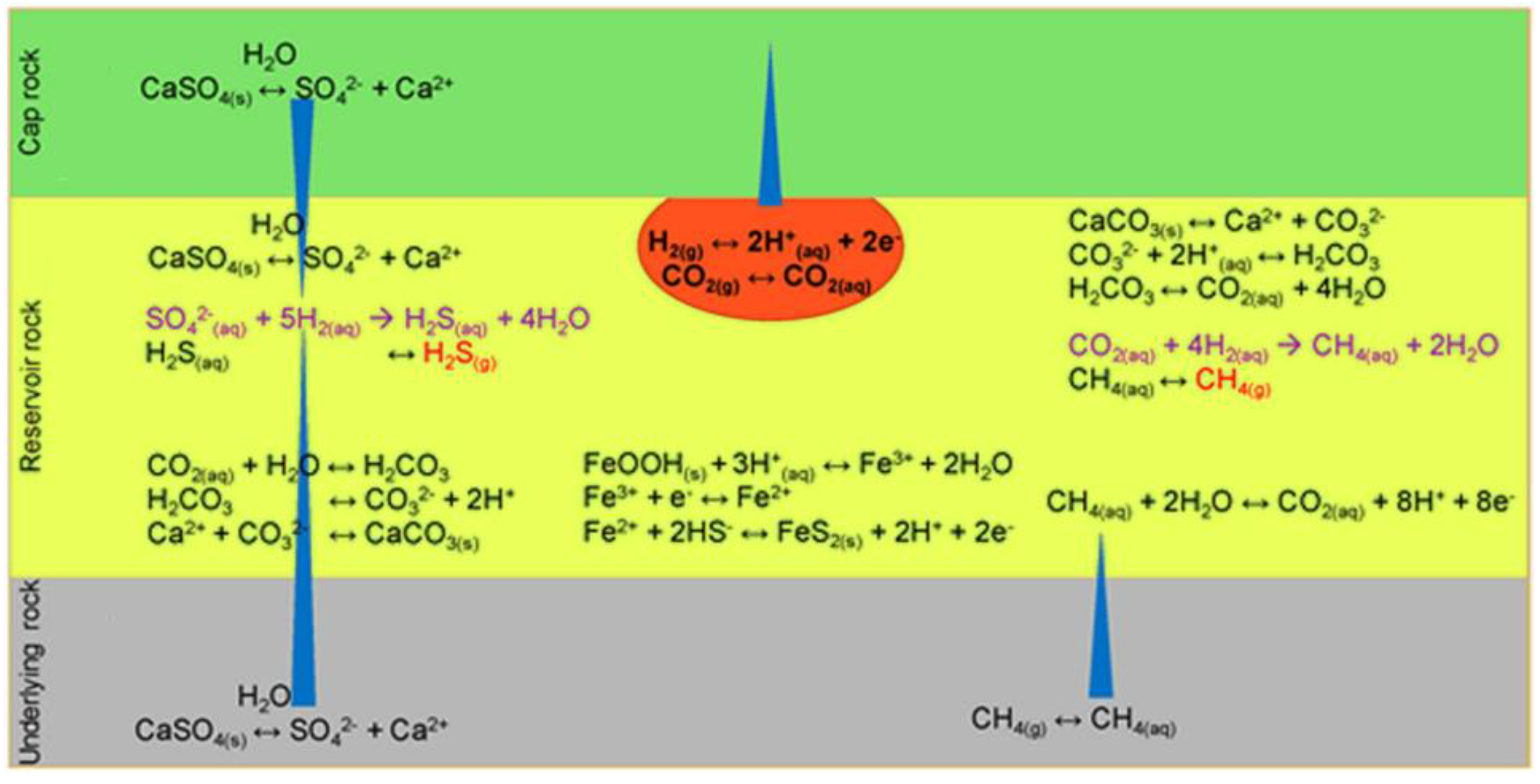
6.1. Geochemical Activities
Hydrogen reactivity can produce harmful gases, that impact; the wellbore, soil, and the atmosphere, affecting UHS systems sustainability and long-term material lifespan. During UHS, geochemical interactions between reservoir fluids, cushion gas, hydrogen gas, and reservoir minerals may occur. These reactions deplete hydrogen by converting it to hydrogen sulfide (H₂S) and methane (CH₄). In addition, geochemical reactions can lower the hydrogen purity by combining it with other reservoir gases. The quality of hydrogen may be affected by residual gases in depleted reservoirs or gases produced over time via hydrogen-related reactions, regardless of storage conditions. Geochemical activity during UHS can lead to mineral precipitation or dissolution, altering permeability and porosity, and potentially causing structural collapse or faults in the cap rock integrity [
26]. Geochemical processes involve both biotic and abiotic reactions, with abiotic reactions involving interactions between non-living molecules like oil, gas, brine, rock minerals, and hydrogen [
41]. Abiotic reactions, including mineral precipitation, dissolution, equilibrium, and ion exchange, can significantly influence the mineral makeup of formation fluids over time, as highlighted by [
42]. The study reveals that hydrogen may slightly dissolve in fluids upon injection, which could lead to hydrogen contamination with water vapor due to chemical disequilibrium. Additionally, hydrogen trapping due to capillary forces and hydrogen adsorption by clay minerals can progressively degrade the reservoir and caprock integrity [
40]. In high-temperature conditions with hydrogen present, undesirable geochemical reactions can release toxic gases and changes in the water pH, further accelerating mineral dissolution. Studies [
43,
44] reveals hydrogen solubility decreases under high-temperature and high-salinity conditions, indirectly influencing mineral dissolution but not significantly altering the native chemical makeup of formation fluids. These findings underscore the need for further investigation into the hydrogeochemical interactions within UHS to ensure safe and efficient storage. [
45] work reveals that hydrogen losses due to bacterial and hydrogeochemical interactions are influenced by factors such as co-injected CO2 and sulfate in the reservoir. They noted that extended storage periods increased the risk of hydrogen loss, particularly because of the higher propensity for methanogenesis when CO2 is coinjected. However, over time, mineralogical attenuation through the consumption of anhydrite and calcite reduces the impact of bacterial processes like sulfate reduction and methanogenesis, leading to safer storage conditions [
46].
Table 7 indicates possible geochemical reactions in UHS.
The current experimental findings indicate that geochemical effects are probably negligible when microbial activity is absent. The study [
44] exposed sandstones to hydrogen gas in a batch experiment; however, they found no indication of reactions or significant changes in the petrophysical characteristics of the samples, such as their porosity or specific surface area. Moreover, recent study comprising 250 batch trials on diverse sandstone samples indicated that pure geochemical reactions impact underground hydrogen storage (UHS) reservoirs less. This was because fluid chemistry revealed minor variations between hydrogen-rich and nitrogen-rich environments over two-months [
51].
Figure 6 shows the background of main reactions and processes involved in bacterial sulfate reduction and methanogenesis (marked in purple). Kinetically controlled reactions are represented by single arrows, whereas equilibrium reactions are shown with double arrows. The blue triangles indicate the diffusion of aqueous components over time, while bold text highlights the injected gas used for storage.
6.2. Microbial Activity
Hydrogen can cause embrittlement in the auxiliary parts of the rock caverns such as compressors, pipes, and steel linings. Finding glaring flaws at every location is crucial, particularly for hydrogen consumption through homoacetogenesis, sulfate reduction, and methanogenesis. The fact that certain microbes can consume hydrogen at rates of up to 4533 nM per hour emphasizes the need for proper site selection. Iron-rich strata are more appropriate for UHS, and the current storage locations in rock caves may need to be modified. Generally, sulfate, carbonate, or sulfide rocks should be avoided [
26]. Many features of microbial interactions are currently poorly understood and understuied. Therefore, more research is required to build effective prediction tools for hydrogen consumption and microbial growth in various geological hydrogen storage systems. In particular, studies aimed at establishing crucial parameters—such as salinity, temperature, and pressure—may yield significant new insights into the dynamics of microbes. Early research indicated that sulfate reducers, homoacetogens, methanogens, and bacteria that reduce iron (III) grow in the pH range of 6 - 7.5. These bacteria thrive in salinity ranges below 60 <100 < 40, and 40 g/L-1 respectively. Optimal temperature ranges for these microorganisms vary: sulfate reducers and homoacetogens thrive at 20°C –30°C, methanogens prefer 30–40°C, while iron(III)-reducing bacteria perform best at 0–30°C [
52]. When H₂ is stored underground, increased H₂ concentrations can stimulate microbial activity in subsurface environments, leading to adverse effects like reservoir weakening, infrastructure corrosion, H₂ losses, reduced permeability, and potential fault activation. These interactions vary with the geological storage type, where depleted hydrocarbon reservoirs and saline aquifers are more prone to these effects than salt caverns. Research has emphasized the importance of microbial activity but has not provided numerical insights into the impacts of higher H₂ levels, in saline and alkaline environments [
26]. Consequently, the biological consumption of H₂ in hydrocarbon reservoirs and aquifers becomes inevitable and undesirable. It is advised that H₂-driven reduction of acetate and sulfate occur gradually in gas reservoirs and acidic, high-salinity aquifers owing to the reaction patterns. However, H₂ may also react with hydrocarbons in the same medium, which could result in H₂ depletion. The presence of sulfur further exacerbates this issue, as H₂ can form hydrogen sulfide (H₂S), resulting in economic losses and posing safety risks for storage operations. Generally, four main categories of microbial activity must be considered in geological H₂ storage; methanogenesis, Acetogenesis, Sulfate Reduction and Iron Reduction [
28,
52].
Table 8.
Microbial Activity for underground hydrogen storage [
53,
54].
Table 8.
Microbial Activity for underground hydrogen storage [
53,
54].
Variables
Primary |
Reservoir, caprock, and Wellbore |
|
Free Energy(kJ/mol) |
Methanogenesis
Acetogenesis
Iron reduction
Sulfate reduction |
CO2 + 4H2 ↔ CH4 + 2H2O
2CO2 + 4H2 ↔CH3COOH + 2H2O
2Fe3+ + H2 ↔ 2Fe2+ + 2H+
SO42- + 5H2 ↔ H2S + 2H2O |
−33.9
− 26.1
−228.3
−38.0 |
| Secondary |
|
|
Denitrification
|
2NO3- +5H2 +2H+ ↔N2 +11H2O
7C6H12O6 + 6NO3- + 6H+ ↔ 6C5H7O2N + 12CO2 + 24H2O |
|
Heterotrophic
Biomass synthesis |
2C3H8O3 +NO3- + H+ ↔ C5H7O2N + CO2 + 5H2O
7CH3COOH + 2NO3- + 2H+ ↔ 2C5H7O2N + 4CO2 + 8H2O
14H(CH2) nCOOH + (3n + 1) NO3-+ (3n + 1) H+ ↔ (3n + 1) C5H7O2N+ (9 - n) CO2 + (5n + 11) H2O |
|
|
Microorganisms catalyze H
2 conversion, and are influenced by microbial concentration, equilibrium constant, enthalpy, and water chemistry, such as temperature and salinity. Key parameters impacting microbial development during methanogenesis include temperature (optimal: 30–40°C, critical: up to 122°C), salinity (optimal: <60 g/L, critical: up to 200 g/L), pH (optimal: 6.0 –7.5, critical range: 4.5 – 9.0), low redox potential, and medium CO₂/carbonate content. In UHS, microbial methanogenesis can affect operations through hydrogen consumption at rates of 0.0008–0.58 mM/h, leading to hydrogen loss as compounds like CH₄, CH₃COOH, H₂S, or Fe(II) are produced. This process can also result in altered permeability, pH reduction, corrosion, hydrogen embrittlement, pore-clogging, mineral dissolution, and precipitation, ultimately impacting the injection and retrieval capacity. Monitoring these factors is crucial for maintaining storage efficiency and infrastructure integrity [
55]. In underground hydrogen storage, acetogenesis can consume hydrogen at rates of 0.02–0.5 mM/h, posing risks of hydrogen loss as acetate is produced. The three main variables affecting microbial development during acetogenesis are pH (optimal: 6.0 –7.5, critical range: 3.6 –10.7), salinity (optimal: <40 g/L, critical: up to 300 g/L), and temperature (optimal: 20–30°C, critical: up to 72°C) [
52]. Acetogenesis increases the risks of leakage and water saturation, which may affect storage conditions. Managing these parameters is essential for reducing hydrogen loss and maintaining stable storage conditions. Iron reduction in underground hydrogen storage can consume hydrogen at 0.0005–0.22 mM/h, contributing to hydrogen loss as iron is reduced. Microbial growth during iron reduction is influenced by temperature (optimal: 20 –30°C, critical: up to 113°C), salinity (optimal: <100 g/L, critical: up to 240 g/L), pH (optimal: 6.0 –7.5, critical range: 0.8 –11.5), and sulfate mineral content. Key factors affecting microbial activity in iron reduction include temperature (optimal: 0–30°C, critical: up to 90°C), salinity (optimal: <40 g/L, critical: up to 200 g/L), pH (optimal: 6.0–7.5, critical range: 1.6–9.0), and the presence of iron minerals. Monitoring and controlling these conditions are vital to reduce hydrogen loss and ensure stable storage conditions [
52,
53,
54,
55,
56].
6.3. Hydrodynamic Phenomena
Hydrogen storage in porous reservoirs involves complex fluid interactions, with the injected hydrogen pushing out existing brine or hydrocarbons. These interactions are influenced by fluid properties, reservoir rock characteristics and phase interactions [
51]. However, there is still a limited understanding of how H₂ behaves in these environments, as there has been minimal experimental research under actual reservoir conditions. In contrast to gases such as methane (CH₄) or carbon dioxide (CO₂), H₂ presents distinct challenges in porous reservoirs due to its low density and viscosity [
51]. These properties can lead to issues like gravity segregation, unstable flow, and lateral spreading, making recovering some of the injected hydrogen difficult. Hydrogen is also prone to mixing with cushion gases and any remaining hydrocarbons in depleted oil and gas reservoirs. These mixing effects, and how H₂ moves in the reservoir are critical for maintaining hydrogen purity, storage efficiency, and reliable recovery over long periods and multiple injection and withdrawal cycles. Whether H₂ flow is miscible or immiscible depends on the reservoir type. In aquifers, for example, the absence of a cushion gas can create conditions where capillary trapping and viscous fingering occur, which can trap hydrogen and reduce the efficiency during withdrawal. In depleted oil and gas fields, where miscible flow is more likely, hydrogen can mix with existing gases, reducing the purity of the hydrogen recovered. These flow dynamics are key factors to examine the viability and efficiency of underground hydrogen storage [
21,
22].
6.3.1. Interfacial Tension Hydrogen–Fluid
A clear understanding of interfacial tension (IFT) between hydrogen and other fluids is essential for analyzing the flow behavior of hydrogen in subsurface storage. For hydrogen-brine systems, IFT has been measured experimentally as 72.3 mN·m⁻¹ under pressure of 0.5 MPa and room temperature as against Hydrogen-Carbondioxide-brine which is 72.0 mN·m⁻¹ at the same conditions, but decreases with higher pressure and temperature, reaching 66.8 mN·m⁻¹ at 25 MPa and 50 °C. Similarly, in hydrogen–C0
2–brine systems, IFT drops significantly from 72.0 to 33.3 mN·m⁻¹ at 44.7 MPa and 73 °C [
51]. Unlike C0
2 –Brine systems, increased brine salinity does not raise hydrogen-brine IFT. IFT influences multiphase flow by controlling capillary forces, which affect flow patterns like stable flows or fingering phenomena [
57,
58].
6.3.2. Wettability Behavior Under Hydrogen Conditions
The wetting properties of reservoir rock significantly affect fluid displacement during hydrogen storage, especially as different fluids interact within the reservoir. Hashemi et al. examined hydrogen-methane interaction involving sandstone and brine at various salinities, pressures, and temperatures and he found that sandstone wettability remains largely unchanged by hydrogen [
58]. Other studies have shown that brine shows a stronger wetting phase than hydrogen, particularly in sandstones, where injected H₂ tends to flow through larger pores whereas brine occupies smaller pores under higher capillary pressures. Additionally, studies by Iglauer et al. demonstrated that hydrogen on quartz surfaces exhibits lower wettability than CO
2, a trait beneficial for hydrogen storage, as it allows H₂ to stay as a mobile, easily retrievable phase within the reservoir [
51,
59].
6.3.3. Relative Permeability
Relative permeability studies for hydrogen-brine systems highlight how fluid interactions and rock properties affect hydrogen flow efficiency in subsurface storage. Drainage and imbibition curves at room temperature and pressures reveal hysteresis effects that reduce hydrogen withdrawal efficiency. Additionally, minimal crossover points indicate flow interference between the relative permeabilities of brine and hydrogen when both fluids move simultaneously [
51]. Study by [
60] conducted unsteady-state research and showed that the relative permeability of the hydrogen decreases at high pressures (10.34MPa and 20.68 MPa) due to the increase in hydrogen viscosity. The study found that increased rock porosity increases hydrogen permeability indicating that more porous rocks improve hydrogen flow. Although these insights are valuable for hydrogen storage in saline aquifers, research on how hydrogen interacts with cushion gases and residual hydrocarbons in depleted oil and gas reservoirs requires extensive research [
51,
58]. [
51,
61] study found that adding 50% CH₄ as a cushion gas to H
2 improves gas relative permeability by 70.5%. This indicates better displacement efficiency because of the decreased gas-brine interfacial tension and increased gas viscosity. Studies on other cushion gases like nitrogen and CO₂ would further inform optimal cushion gas choices for efficient hydrogen storage.
6.3.4. Displacement and Fingering Phenomena
Porous hydrogen storage faces challenges due to unstable displacement, this instability known as density fingering occurs due to variations in fluid viscosity. Viscous fingering, a phenomenon where hydrogen moves unevenly, increases the risk of residual trapping and hydrogen dissolution in the brine, thereby reducing hydrogen recovery efficiency. Early research on UHS in 1982 revealed fingering [
62]. Direct experiments are crucial for understanding multiphase flow in porous reservoirs. Recent hydrogen-brine displacement experiments for water-wet reservoirs on sandstone samples, confirmed that residual brine occupies pore corners and throats, whereas hydrogen flows centrally within pores. The findings affirm that capillary fingering patterns are dominant in multiphase H₂-brine systems, mainly due to the significant interfacial tension between hydrogen and brine at low flow rates in the experiments. High H₂-brine interfacial tension favors capillary fingering, which occurs when capillary forces exceed viscous forces, specifically when the capillary number falls below a critical threshold, generally between 10⁻⁶ and 10⁻⁴ [
51,
63]. This unstable displacement forms finger-like paths that lead, to hydrogen loss. The interaction between hydrogen and brine is influenced by the flow rate; higher flow rates reduce capillary fingering but increases viscous fingering. Experiments and simulations on multiphase hydrogen-brine displacements provide valuable insights into improving underground hydrogen storage (UHS) by optimizing recovery and minimizing hydrogen loss [
51].
6.3.5. Dissolution of Hydrogen in Brine
Carden and Paterson theorized that hydrogen dissolution in brine during underground storage is minimal, with less than 2% of injected hydrogen dissolving [
64]. However, experiments have highlighted the significant impact on phase behavior and hydrogen recovery. Jangda et al. found that injecting hydrogen into non-equilibrated brine could result in up to 7% dissolution, reducing residual saturation compared to equilibrated brine [
65]. Boon and Hajibeygi also revealed that hydrogen dissolution into unequilibrated brine could lead to miscible displacement [
63]. Lysyy et al. observed nonequilibrium dissolution through imaging, with dissolved hydrogen averaging 16%, below the equilibrium solubility of 28.3%, which could limit hydrogen loss [
66]. Meanwhile, Amiri et al. showed that cushion gas choice impacts recovery, with CO₂’s high solubility lowering recovery efficiency compared to CH₄ or N₂. Further studies, especially core flooding experiments, are needed to better understand and optimize hydrogen recovery in brine systems [
67].
6.3.6. Residual Trapping
Experimental investigations have identified residual hydrogen trapping in rock due to fluid redistribution after drainage and shut-in phases. This results in hydrogen clusters in the pore spaces, limiting hydrogen recovery and potentially causing loss. Since 1915, the idea of gas storage in depleted reservoirs has been around, and the behavior of H
2 in subterranean formations has been understood through comparisons with natural gas and CO
2 geo-storage. Four techniques have been identified in maintaining gas in subsurface formations: mineral trapping, solubility/dissolution, residual/capillary, and structural/stratigraphic. Whereas residual/capillary trapping is typical in sedimentary formations, structural/stratigraphic trapping is common in caprock and sedimentary formations [
62,
63]. The trapping mechanism in sedimentary rocks is based on wettability, where rocks prefer a specific phase. This wettability affects residual trapping and helps identify the interaction of H
2 with brine in reservoir and storage rock. The Young –Laplace equation can be used to estimate the capillary forces that retain the buoyant H
2 gas within the capillary pores. Hysteresis is a fundamental feature of multiphase flow and forms the basis for the trapping mechanism. This mechanism significantly assists in quantifying the amount of gas migration as well as the distribution in the formation, thereby influencing the efficiency of other trapping mechanisms [
51,
63]. Hydrogen trapping in water-wet reservoirs is affected by various factors such as capillary number and flow rates. Higher injection rates increase the initial gas saturation, whereas higher imbibition rates reduce the residual gas saturation, promoting hydrogen recovery. Multiple injection-withdrawal cycles enhance gas recovery, with secondary drainage improving recovery. Larger injection and withdrawal flow rates improve recovery, but residual trapping can cause losses of up to 40% loss during withdrawal. Optimizing the flow rates and cycles significantly enhances the hydrogen extraction efficiency.
6.4. Geo-Mechanic Considerations
The geomechanical elements affecting underground hydrogen storage (UHS) are described in this section. The reaction of the reservoir rock to UHS conditions is primarily influenced by the rate of injection and production, structure of the wellbore, rock tensile strength, and the existence of fractures or faults [
26]. Repeated cycles of gas injection and withdrawal during hydrogen storage affect the formation's effective stress because of fluctuations in pore pressure, which threatens the reservoir and caprocks integrity. Geomechanical modeling considers geomechanical repercussions such as fault reactivation, wellbore and cap rock stability, and probable reservoir fracture. Geochemical interactions may be the source of this degradation, as the dissolution of minerals in the rock increases the probability of gas escape. Furthermore, cyclic stress variations caused by H
2 injection and withdrawal may reactivate fractures and flaws, enhancing the risk of leaks and losses even more [
41,
51]. Therefore, it is vital to understand changes in rock strength, elasticity, and strain under different loads to evaluate how the reservoir formation might geomechanically behave in response to UHS. Because of this knowledge, geomechanical changes can be modeled more precisely, to ensure reservoir integrity throughout UHS operations. During underground hydrogen storage, injection and withdrawal cycles cause reservoir pore pressure and temperature to vary, which can introduce significant risks during and after operations. These risks include potential reductions in rock strength, ground subsidence or uplift, compromised well integrity, persistent hydrogen leakage, reduced effectiveness of the caprock seal, and seismicity due to fault reactivation. Storing hydrogen in salt caverns poses additional hazards, such as excessive cavern shrinkage (which reduces storage capacity), roof failure, fluid leakage, and other unforeseen issues. Caverns with intricate structures, thick heterogeneous layers, or interactions among multiple caverns within the same geological area present additional challenges for UHS. Because both depleted reservoirs and salt caverns rely on wells for hydrogen injection and withdrawal, maintaining wellbore integrity is essential to prevent hydrogen leakage and ensure the safety and efficiency of the storage system [
68].
Table 9 compares natural gas, CO2 and Hydrogen storage’s subsurface mechanical responses, highlighting the importance of geomechanical study in predicting stress distribution, deformation, and sealing integrity for safe and effective storage across all gases.
7. Modelling Strategies for Underground Hydrogen Storage
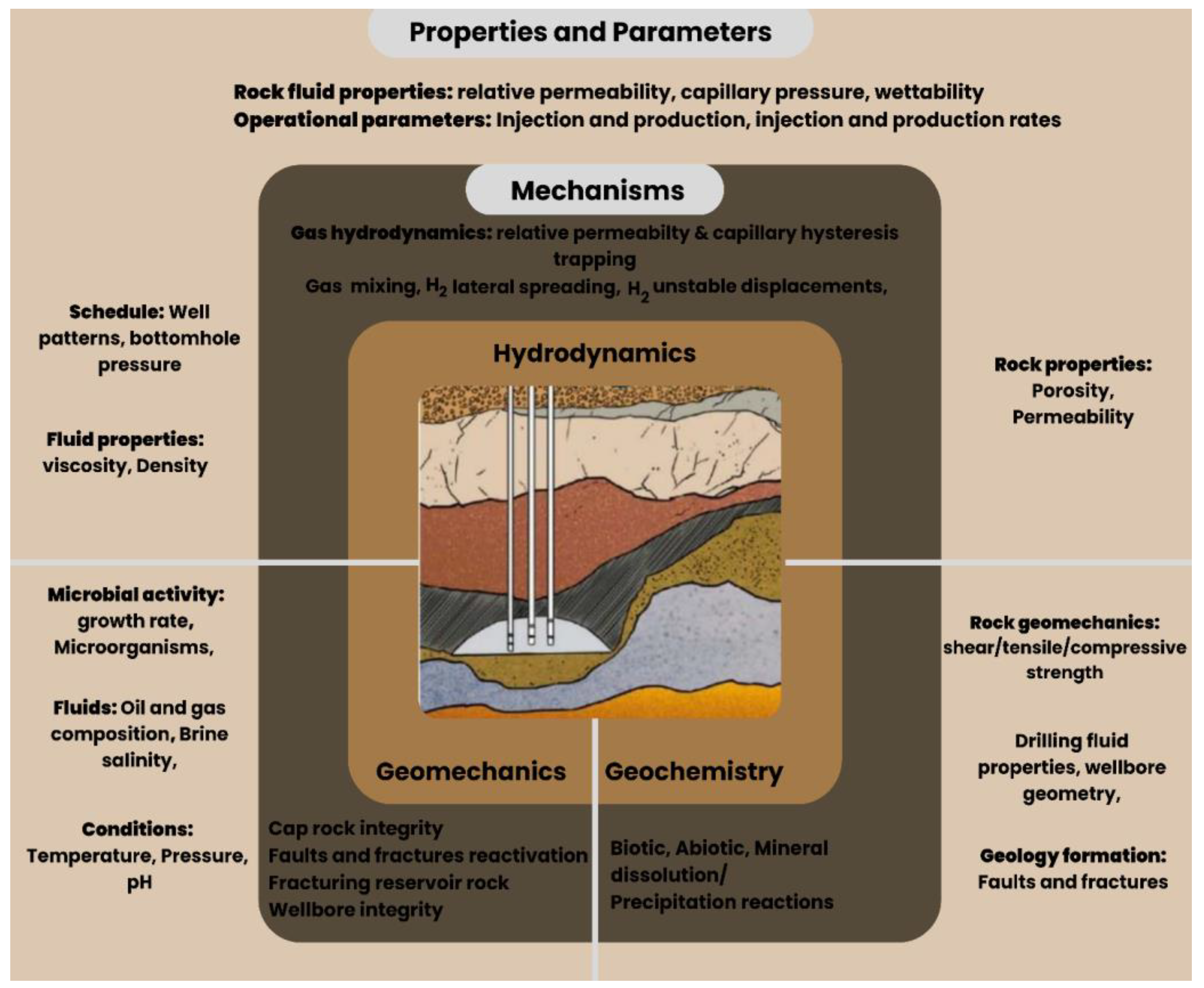
Geochemical, geomechanical, and hydrodynamic interactions influence UHS, a complicated process affecting the injection, storage, and generation of hydrogen gas. Forecasting UHS performance requires both knowledge of these pathways. Important qualities and considerations include rock, fluid, operational, geomechanical, reservoir, and microbiological environments. Many modeling approaches have been presented to evaluate these implications;
Figure 7 below presents the key features and related factors.
Geochemical modeling is important to comprehending and mimicking the numerous processes involved in underground hydrogen storage (UHS). The models must capture equilibrium processes and precipitation and mineral dissolution reactions. Among the elements impacting the rates of these kinetic reactions are the temperature, pressure and salinity [
45]. Because of this, modeling methodologies that apply constant reaction rates could result in erroneous predictions. Additionally, conditions are necessary for validating and calibrating reaction rates and eventually enhancing the precision of geochemical models for UHS. Geochemical research investigates how hydrogen interacts with different minerals in reservoirs and the brine composition of the reservoir.
Table 10 summarizes geochemical modeling strategies undertaken for underground hydrogen storage.
Predicting geomechanical changes during UHS requires determining hydrogen gas injection and production stresses on reservoir rock, and establishing rock behavior in terms of deformation, strength, and elasticity. The fluid conservation of momentum equation is used mostly in modeling the geomechanical differences in our formation.
The fluid velocity is represented by
v in this conservation of momentum equation accounting for the pressure gradient,
∇p, which can be used to describe the fluid flow behavior of the fluid flow during H
2 injection and production [
73]. Biot equation correlates between rock and fluid and is essential for computing the pressure gradient [
26]. The Biot equation is given by
where σ′, σ,
ρ (pore pressure), and
β (Biot coefficient) represent the effective and total stresses, respectively. The main equation used to analyze UHS geomechanical effects is the Hooks law, which describes rock elasticity and matrix deformation by stress and strain.
Geomechanical modeling systems are crucial for assessing stability, sealing integrity and mechanical behavior of underground hydrogen storage reservoirs under cyclic injection and withdrawal.
Table 11 summarizing selected geomechanical models for UHS.
A 3D hydromechanical model was created to assess the practicality of underground hydrogen storage (UHS) in Wyoming's Powder River Basin by Bai and Tahmasebi. They employed a one-way coupling technique to predict how rock stress would react to changes in pore pressure and computed hydrogen properties using the Peng-Robinson equation. They found that 75% of the hydrogen could be recovered by the third cycle, and the geomechanical stability was confirmed using the Mohr-Coulomb criterion [
78]. [
76] demonstrated that short-term storage at the Anning salt mine in China met safety requirements without issues like surface subsidence, creep, or spalling [
74] proposed fewer storage cycles to limit the tensile stress, which can be increased by large temperature variations at the cavern margins. [
75] researched the effects of salt cavern heterogeneity on elasticity and found that while impurities cause localized deformation, cavern creep is not affected by short-term hydrogen storage. Their stability study of shallow and deep caves, [
77] found that deeper caverns suit intense green hydrogen storage because they have more stability issues but less gas losses. Considering hydrodynamic modelling in (UHS) as indicated in Figure 16, it is necessary to understand the fluid flow within storage formations. Rock factors such as porosity and permeability, fluid properties such as density, viscosity, interfacial tension, solubility, and diffusivity, and rock-fluid interactions under reservoir conditions all influence the behavior of fluid flow in storage [
79]. It is vital to incorporate these parameters into hydrodynamic models to appropriately estimate UHS performance.
Software tools like Eclipse, DuMux, CMG-GEM, TOUGH, and COMSOL are commonly applied in underground hydrogen storage to model hydrogen flow and apply fluid flow concepts [
41]. An open-source simulator called DuMux can be modified with various equations of state to control the transport of multiple phases and components within the porous media [
80,
81,
82,
83]. In oil and gas reservoir modeling, SLB's Eclipse software (100 and 300) is extensively used; Eclipse 100 employs a black oil model, while CMG-GEM and Eclipse 300 use compositional models to account for phase behavior differences in fluid compositions. TOUGH is ideal for hydrogen and cushion gas injection since it is made to reflect gas injection and reactive transport [
82]. Finally, COMSOL Multiphysics is a versatile simulation tool for chemical reactions and fluid flow [
81].
Table 12 reviews a summary of selected hydrodynamics models for underground hydrogen storage.
A review of UHS modeling research reveals an uneven focus across key areas. Hydrodynamics dominates, emphasizing the need to understand subsurface hydrogen flow and distribution for safety and feasibility. In contrast, geochemical interactions, geomechanical effects, and microbial activities critical for storage integrity and hydrogen quality remain underexplored. Modeling tools like CMG software align with current priorities, whereas machine learning (ML) gains traction for enhancing model accuracy and efficiency. Tools such as PHREEQC underscore the importance of diverse methodologies in addressing UHS challenges.
8. Knowledge Gaps and Future Research
The current study on UHS has revealed various concerns and challenges that need to be addressed in future studies: a) Optimizing Hydrogen Storage Efficiency and Reducing Costs: Current studies lack standardized cost-effectiveness metrics and data on hydrogen recovery efficiency across geological formations. Further research is needed to understand the impact of cushion gases, cycling frequency and injection/withdrawal rate. b) Ensure Long-Term Storage Security and Stability: Research gaps in underground hydrogen storage, such as geomechanical effects, geochemical interactions, and real-time leakage detection, necessitate comprehensive improvement for long-term storage security and stability. c) Impact Assessment of Hydrogen Injection on Subsurface and Surface Stability: Research to assess the long-term impacts of hydrogen injection on subsurface stability and surface conditions, including potential ground deformation and seismicity. It seeks to establish safe injection guidelines and regulatory frameworks for long-term monitoring and risk management of hydrogen storage projects. d) Optimizing Cushion Gas Injection for Enhanced Hydrogen Recovery: The selection of cushion gas mixtures, injection strategies, and well placement should be optimized to maximize hydrogen recovery while minimizing contamination and reducing operational costs in hydrogen storage systems. e) Evaluating the Methanation Potential in CO₂-Buffered Hydrogen Storage: The Methanation potential in CO₂-buffered hydrogen storage systems is under-explored, with limited research on controlling methane production, optimizing hydrogen recovery, and managing risks. f) Commercial-Scale Hydrogen Storage Facility Modeling: The development of advanced modeling techniques like Co-Flow and Co-FlowX for commercial hydrogen storage facility assessment, economic feasibility studies, and scalable designs is insufficiently researched. g) Comprehensive Environmental Impact and Risk Assessment: Research gaps in hydrogen storage, including potential impacts on groundwater quality and ecosystems, and the need for comprehensive life cycle assessments, highlight the need for further investigation for safe, sustainable, and environmentally responsible practices. h) Development of Digital and Smart Monitoring Systems: The application of AI and machine learning to optimize hydrogen storage systems, enabling early leak detection, real-time anomaly detection, and environmental impact assessments are areas that needs further research.
9. Conclusions
Underground hydrogen storage (UHS) in geological formations is a pivotal technology for achieving global energy security and supporting the transition to renewable energy systems. Hydrogen, with its high efficiency, environmental benefits, and sustainability, is poised to become a cornerstone of future energy systems. However, the deployment of UHS faces multifaceted challenges that require careful consideration:
Depleted gas reservoirs are a promising option for storage due to their pre-existing infrastructure, favorable economics, and extensive storage capacity. The cost advantage of aquifers and salt caverns arises from reduced site characterization requirements and the reuse of existing petrochemical facilities. However, challenges such as hydrogen’s high mobility, geochemical reactivity with reservoir fluids and rock matrix, and potential issues with gas containment requires further studies. In aquifers, higher costs are driven by extensive site characterization needs, whereas salt caverns, though efficient for cyclic injection and withdrawal, involve substantial upfront leaching and brine disposal costs. Key considerations for UHS include the thermophysical properties of hydrogen-such as density, viscosity, diffusivity, and solubility, which influence injectivity, withdrawal efficiency, and gas mobilization.
Similarly, petrophysical properties such as porosity and permeability directly affect the reservoir’s storage capacity and containment integrity. The interplay of cushion gas requirements, depth and geological viability further adds to the complexity of site selection, and operational planning expenditures (OPEX) are highly site- dependent and influenced by surface infrastructure, depth, and reservoir –specific factors. Hydrogen production costs, particularly those from electrolysis, remain a bottleneck, underscoring the importance of technological advancements to reduce expenses. Cushion gas strategies, material costs, and maintenance also play significant roles in shaping the financial outlook of UHS projects.
The realization of UHS at an industrial scale requires overcoming geological, economic, technological, and regulatory barriers. A phased approach combining experimental studies, simulation analyses, and demonstrated projects is essential to address safety concerns, improve gas containment, and optimize operational strategies. Geochemical interactions, well integrity, and potential microbial reactions with stored hydrogen must also be carefully managed to ensure long-term viability.
In conclusion, while UHS offers unparalleled potential for large-scale energy storage, its success hinges on continued research, innovation, and investment. Addressing current challenges through interdisciplinary efforts will pave the way for hydrogen to play a transformative role in achieving a low-carbon energy future.
Author Contributions
Conceptualization, K.O.D, W.A., H.R; methodology, K.O.D., H.R., and W.A.; validation, K.O.D., W.A., H.R, and M.M.; formal analysis, K.O.D., W.A., and H.R.; investigation, K.O.D, W.A and H.R.; resources, W.A.; writing—original draft preparation, K.O.D., W.A., and H.R.; writing—review and editing, K.O.D, W.A., H.R. and M.M; visualization, K.O.D, W.A., and H.R.; supervision, W.A.; project administration, W.A.; funding acquisition, W.A. All authors have read and agreed to the published version of the manuscript.
Funding
“This work was supported by the U.S. Department of Energy (DOE) through the National Energy Technology Laboratory to New Mexico Institute of Mining and Technology under ward DE-FE0032363, CUSP Regional Initiative project. Additional support from New Mexico Consortium (subcontract No. AP-045578) through the US Department of Energy under Contract No. 89233218CNA000001”.
Acknowledgments
We thank the U.S. DOE for providing the funding support for this work and the Petroleum Recovery and Research Center, New Mexico Tech for providing additional support and resources. We also thank the MDPI editorial team and anonymous reviewers for their helpful comments on this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Bank. World Population Will Continue to Grow. World Development Indicators. https://datatopics.worldbank.org/world-development-indicators/stories/world-population-will-continue-to-grow.html [Accessed: Oct. 19, 2024].
- Najjar, Y.S.H. Hydrogen Safety: The Road Toward Green Technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728. [Google Scholar] [CrossRef]
- BP. Statistical Review of World Energy 2020, 69th ed.; BP p.l.c.: London, UK, 2020. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf [Accessed: Oct. 19, 2024]. [Google Scholar]
- Zhou, J.; Ji, W.; Cao, X.; He, W.; Fan, J.; Yuan, Y. A Current Perspective on the Renewable Energy Hydrogen Production Process. J. Therm. Sci. 2023, 32, 542–596. [Google Scholar] [CrossRef]
- Chandler, L. Making the Case for Hydrogen in a Zero-Carbon Economy. MIT News, Aug. 31, 2021. https://news.mit.edu/2021/making-case-hydrogen-zero-carbon-economy-0831 [Accessed: Oct. 19, 2024].
- IRENA. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency: Abu Dhabi, 2019. https://www.irena.org//media/Files/IRENA/Agency/Publication/2019/Sep/IRENA_Hydrogen_2019.pdf [Accessed: Oct. 19, 2024]. [Google Scholar]
- OECD. Hydrogen Production. In Technology Roadmap: Hydrogen and Fuel Cells; OECD Publishing: Paris, 2015. https://www.oecd-ilibrary.org/docserver/093f609e-en.pdf [Accessed: Oct. 19, 2024]. [Google Scholar]
- Ziobrowski, Z.; Rotkegel, A. Assessment of Hydrogen Energy Industry Chain Based on Hydrogen Production Methods, Storage, and Utilization. Energies 2024, 17, 1808. [Google Scholar] [CrossRef]
- Crotogino, F.; Donadei, S.; Bünger, U.; Landinger, H. Large-Scale Hydrogen Underground Storage for Securing Future Energy Supplies. In Proceedings of the 18th World Hydrogen Energy Conference (WHEC 2010), Essen, Germany, 2010, May 16-21; pp. 37–45.
- Kanaani, M.; Sedaee, B.; Asadian-Pakfar, M. Role of Cushion Gas on Underground Hydrogen Storage in Depleted Oil Reservoirs. Int. J. Hydrogen Energy 2022, 47, 18866–18880. [Google Scholar] [CrossRef]
- Clean Hydrogen Joint Undertaking. The European Hydrogen Market Landscape, /: at: https, 20 November 2024.
- The Color Palette of the Colorless Hydrogen. J. Pet. Technol. https://jpt.spe.org/twa/the-color-palette-of-the-colorless-hydrogen.
- Ziobrowski, Z.; Rotkegel, A. Assessment of Hydrogen Energy Industry Chain Based on Hydrogen Production Methods, Storage, and Utilization. Energies 2024, 17, 1808. [Google Scholar] [CrossRef]
- Department of Mineral Resources and Energy. Consultation Document on the Development of a Gas, Renewables and Hydrogen Partnership Strategy in SA.; Republic of South Africa, 2023. https://cer.org.za/wp-content/uploads/2023/02/Consultation-document-on-the-developmnet-of-a-gas-renewables-and-hydrogen-partnership-strategy-in-SA.
- Jeje, S.O.; Marazani, T.; Obiko, J.O.; Shongwe, M.B. Advancing the Hydrogen Production Economy: A Comprehensive Review of Technologies, Sustainability, and Future Prospects. Int. J. Hydrogen Energy 2024, 78, 642–661. [Google Scholar] [CrossRef]
- Hydrogen Council. Interview: Hydrogen Spot Markets a Decade Away, but Costs Falling Fast. S&P Global Commodity Insights, Apr. 12, 2021. https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/electric-power/041221-interview-hydrogen-spot-markets-a-decade-away-but-costs-falling-fast-hydrogen-council.
- World Nuclear Association. Hydrogen Production and Uses. https://world-nuclear.org/information-library/energy-and-the-environment/hydrogen-production-and-uses.
- World Nuclear Association. Hydrogen Production and Uses. https://world-nuclear.org/information-library/energy-and-the-environment/hydrogen-production-and-uses.
- Department of Mineral Resources and Energy. Consultation Document on the Development of a Gas, Renewables and Hydrogen Partnership Strategy in SA.; Republic of South Africa, Feb. 2023. https://cer.org.za/wp-content/uploads/2023/02/Consultation-document-on-the-developmnet-of-a-gas-renewables-and-hydrogen-partnership-strategy-in-SA.pdf.
- Taiwo, G.O.; Tomomewo, O.S.; Oni, B.A. A Comprehensive Review of Underground Hydrogen Storage: Insight into Geological Sites (Mechanisms), Economics, Barriers, and Future Outlook. J. Energy Storage 2024, 90 (Part B), 111844. [Google Scholar] [CrossRef]
- Navaid, H.B.; Emadi, H.; Watson, M. A Comprehensive Literature Review on the Challenges Associated with Underground Hydrogen Storage. J. Nat. Gas Sci. Eng. 2023, 109, 104952. [Google Scholar] [CrossRef]
- Okoroafor, E.R.; Saltzer, S.D.; Kovscek, A.R. Toward Underground Hydrogen Storage in Porous Media: Reservoir Engineering Insights. Int. J. Hydrogen Energy 2022, 47, 33781–33802. [Google Scholar] [CrossRef]
- Duartey, K.O.; Quainoo, A.K.; Darko, C.K. Evaluation Studies of KCl and Amino Acid Mixtures for Clay Stabilization and Rheological Enhancement of Water-Based Fracturing Fluids. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria; 2024. [Google Scholar]
- Fuentes, J.E.Q.; Santos, D.M.F. Technical and Economic Viability of Underground Hydrogen Storage. Hydrogen 2023, 4, 975–1000. [Google Scholar] [CrossRef]
- Panfilov, M. Hydrogenization of Underground Storage of Natural Gas: Impact of Hydrogen on the Hydrodynamic and Biochemical Behavior. Transp. Porous Media 2016, 20, 595–606. [Google Scholar] [CrossRef]
- Bade, S.O.; Taiwo, K.; Ndulue, U.F.; Tomomewo, O.S.; Oni, B.A. A Review of Underground Hydrogen Storage Systems: Current Status, Modeling Approaches, Challenges, and Future Prospective. Int. J. Hydrogen Energy 2024, 80, 449–474. [Google Scholar] [CrossRef]
- Shazad, A.; Uzair, M.; Tufail, M. Impact of Blending of Phase Change Material for Performance Enhancement of Solar Energy Storage. Renew. Energy 2024, 227, Art no 120530. [Google Scholar] [CrossRef]
- Hydrogen Council; McKinsey & Company. Hydrogen Insights 2023; May 2023. https://hydrogencouncil.com/wp-content/uploads/2023/05/Hydrogen-Insights-2023.pdf.
- Jahanbakhsh, A.; Potapov-Crighton, A.L.; Mosallanezhad, A.; Tohidi Kaloorazi, N.; Maroto-Valer, M.M. Underground Hydrogen Storage: A UK Perspective. Renew. Sustain. Energy Rev. 2024, 189, Art no 114001. [Google Scholar] [CrossRef]
- The Effects of Hydrogen Injection in Natural Gas Networks for the Dutch Underground Storages. 2017. https://www.scribd.com/document/507760964/The-Effects-of-Hydrogen-Injection-in-Natural-Gas-Networks-for-the-Dutch-Underground-Storages [Accessed: Aug. 15, 2023].
- Sambo, C.; Dudun, A.; Samuel, S.A.; Esenenjor, P.; Muhammed, N.S.; Haq, B. A Review on Worldwide Underground Hydrogen Storage Operating and Potential Fields. Int. J. Hydrogen Energy 2022, 47, 22840–22880. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground Hydrogen Storage: A Comprehensive Review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Navaid, H.B.; Emadi, H.; Watson, M. A Comprehensive Literature Review on the Challenges Associated with Underground Hydrogen Storage. J. Nat. Gas Sci. Eng. 2023, 109, 104952. [Google Scholar] [CrossRef]
- Gholami, R. Hydrogen Storage in Geological Porous Media: Solubility, Mineral Trapping, H₂S Generation and Salt Precipitation. Int. J. Hydrogen Energy 2022, 47, 18850–18865. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Carbonell, R.; et al. Enabling Large-Scale Hydrogen Storage in Porous Media – The Scientific Challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Ebrahimiyekta, A. Characterization of Geochemical Interactions and Migration of Hydrogen in Sandstone Sedimentary Formations: Application to Geological Storage; Ph.D. Dissertation, Université d'Orléans, 2017. https://theses.hal.science/tel-01713106v1/file/alireza-ebrahimiyekta_3466.pdf [accessed Oct. 19, 2024].
- Hematpur, H.; Abdollahi, R.; Rostami, S.; Haghighi, M. Review of Underground Hydrogen Storage: Concepts and Challenges. Adv. Geo-Energy Res. 2022, 7, 111–131. [Google Scholar] [CrossRef]
- Delshad, M.; Tavakoli, R.; Sepehrnoori, K. Advances in Hydrogen Storage in Porous Media: A Review of Geological and Engineering Aspects. Int. J. Hydrogen Energy 2022, 47, 2309–2330. [Google Scholar] [CrossRef]
- Katz, D.L.; Tek, M.R. Storage of Natural Gas in Saline Aquifers. Water Resources Research 1970, 6, 1515–1521. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Misiak, J. Underground Gas Storage in Saline Aquifers: Geological Aspects. Energies 2024, 17, 1666. [Google Scholar] [CrossRef]
- Saeed, M.; Jadhawar, P. Modelling Underground Hydrogen Storage: A State-of-the-Art Review of Fundamental Approaches and Findings. Gas Sci. Eng. 2024, 121, Art. [Google Scholar] [CrossRef]
- Rezaei, A.; Hassanpouryouzband, A.; Molnar, I.; Derikvand, Z.; Haszeldine, R.S.; Edlmann, K. Relative Permeability of Hydrogen and Aqueous Brines in Sandstones and Carbonates at Reservoir Conditions. Geophys. Res. Lett. 2022, 49, e2022GL099433. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Cowen, T.; Edlmann, K.; et al. Geological Hydrogen Storage: Geochemical Reactivity of Hydrogen with Sandstone Reservoirs. ACS Energy Lett. 2022, 7, 2405–2410. [Google Scholar] [CrossRef]
- Flesch, S.; Pudlo, D.; Albrecht, D.; Jacob, A.; Enzmann, F. Hydrogen Underground Storage—Petrographic and Petrophysical Variations in Reservoir Sandstones from Laboratory Experiments under Simulated Reservoir Conditions. Int. J. Hydrog. Energy 2018, 43, 20822–20835. [Google Scholar] [CrossRef]
- Hemme, C.; Van Berk, W. Hydrogeochemical Modeling to Identify Potential Risks of Underground Hydrogen Storage in Depleted Gas Fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Hemme, C. , 2019. Storage of gases in deep geological structures: spatial and temporal hydrogeochemical processes evaluated and predicted by the development and application of numerical modeling (Doctoral thesis). Clausthal University of Technology, Clausthal-Zellerfeld. [CrossRef]
- Hassannayebi, N.; Azizmohammadi, S.; De Lucia, M.; Ott, H. Underground Hydrogen Storage: Application of Geochemical Modelling in a Case Study in the Molasse Basin, Upper Austria. Environ. Earth Sci. 2019, 78, 177. [Google Scholar] [CrossRef]
- Zeng, L.; Sarmadivaleh, M.; Saeedi, A.; Chen, Y.; Zhong, Z.; Xie, Q. Storage Integrity During Underground Hydrogen Storage in Depleted Gas Reservoirs. Earth-Science Reviews 2023, 247, 104625. [Google Scholar] [CrossRef]
- Hemme, C.; van Berk, W. Change in Cap Rock Porosity Triggered by Pressure and Temperature Dependent CO₂–Water–Rock Interactions in CO₂ Storage Systems. Petroleum 2017, 3, 96–108. [Google Scholar] [CrossRef]
- Zhu, C. In Situ Feldspar Dissolution Rates in an Aquifer. Geochimica et Cosmochimica Acta 2005, 69, 1435–1453. [Google Scholar] [CrossRef]
- Gomez Mendez, I.; El-Sayed, W.M.M.; Menefee, A.H.; Karpyn, Z.T. Insights into Underground Hydrogen Storage Challenges: A Review on Hydrodynamic and Biogeochemical Experiments in Porous Media. Energy Fuels 2024, 38, 12345–12360. [Google Scholar] [CrossRef]
- Dopffel, N.; Jansen, S.; Gerritse, J. Microbial Side Effects of Underground Hydrogen Storage – Knowledge Gaps, Risks and Opportunities for Successful Implementation. Int. J. Hydrogen Energy 2021, 46, 8594–8606. [Google Scholar] [CrossRef]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical Reactions-Induced Hydrogen Loss During Underground Hydrogen Storage in Sandstone Reservoirs. Int. J. Hydrogen Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Zeng, L.; Sarmadivaleh, M.; Saeedi, A.; Chen, Y.; Zhong, Z.; Xie, Q. Storage Integrity During Underground Hydrogen Storage in Depleted Gas Reservoirs. Earth-Sci. Rev. 2023, 247, Art. [Google Scholar] [CrossRef]
- Malki, M.L.; Chellal, H.; Mao, S.; Rasouli, V.; Mehana, M. A Critical Review of Underground Hydrogen Storage: From Fundamentals to Applications, Unveiling Future Frontiers in Energy Storage. Int. J. Hydrogen Energy 2024, 79, 1365–1394. [Google Scholar] [CrossRef]
- Boon, M.; Buntic, I.; Ahmed, K.; Dopffel, N.; Peters, C.; Hajibeygi, H. Microbial Induced Wettability Alteration with Implications for Underground Hydrogen Storage. Sci. Rep. 2024, 14, Art. [Google Scholar] [CrossRef]
- Adam, A.M.; Bahamon, D.; Al Kobaisi, M.; Vega, L.F. Molecular Dynamics Simulations of the Interfacial Tension and the Solubility of Brine/H₂/CO₂ Systems: Implications for Underground Hydrogen Storage. Int. J. Hydrogen Energy 2024, 78, 1344–1354. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Ali, M.; Kalantariasl, A.; Sayyafzadeh, M.; You, Z.; Iglauer, S.; Keshavarz, A. A Review of Hydrogen/Rock/Brine Interaction: Implications for Hydrogen Geo-storage. Prog. Energy Combust. Sci. 2023, 95, Art. [Google Scholar] [CrossRef]
- Peters, E.J. Dispersion in Porous Media. In Advanced Petrophysics: Dispersion, Interfacial Phenomena/Wettability, Capillarity/Capillary Pressure, Relative Permeability; Live Oak Book Company: 2012; Vol. 2.
- Rezaei, A.; Hassanpouryouzband, A.; Molnar, I.; Derikvand, Z.; Haszeldine, R.S.; Edlmann, K. Relative Permeability of Hydrogen and Aqueous Brines in Sandstones and Carbonates at Reservoir Conditions. Geophys. Res. Lett. 2022, 49, e2022GL099433. [Google Scholar] [CrossRef]
- Mirchi, V.; Dejam, M.; Alvarado, V. Interfacial Tension and Contact Angle Measurements for Hydrogen-Methane Mixtures/Brine/Oil-Wet Rocks at Reservoir Conditions. Int. J. Hydrogen Energy 2022, 47, 34963–34975. [Google Scholar] [CrossRef]
- Thaysen, E.M.; Butler, I.B.; Hassanpouryouzband, A.; Freitas, D.; Alvarez-Borges, F.; Krevor, S.; Heinemann, N.; Atwood, R.; Edlmann, K. Pore-Scale Imaging of Hydrogen Displacement and Trapping in Porous Media. Int. J. Hydrogen Energy 2023, 48, 3091–3103. [Google Scholar] [CrossRef]
- Boon, M.; Hajibeygi, H. Experimental Characterization of H₂/Water Multiphase Flow in Heterogeneous Sandstone Rock at the Core Scale Relevant for Underground Hydrogen Storage (UHS). Sci. Rep. 2022, 12, Art. [Google Scholar] [CrossRef] [PubMed]
- Carden, P.O.; Paterson, L. Physical, Chemical and Energy Aspects of Underground Hydrogen Storage. Int. J. Hydrogen Energy 1979, 4, 559–569. [Google Scholar] [CrossRef]
- Jangda, Z.; Menke, H.; Busch, A.; Geiger, S.; Bultreys, T.; Lewis, H.; Singh, K. Pore-Scale Visualization of Hydrogen Storage in a Sandstone at Subsurface Pressure and Temperature Conditions: Trapping, Dissolution and Wettability. J. Colloid Interface Sci. 2023, 629, Part. [Google Scholar] [CrossRef]
- Lysyy, M.; Ersland, G. Pore-Scale Dynamics for Underground Porous Media Hydrogen Storage. Adv. Water Resour. 2022, 163, Art. [Google Scholar] [CrossRef]
- Amiri, I.I.; Zivar, D.; Ayatollahi, S.; Mahani, H. The Effect of Gas Solubility on the Selection of Cushion Gas for Underground Hydrogen Storage in Aquifers. J. Energy Storage 2024, 80, Art. [Google Scholar] [CrossRef]
- Shi, Z.; Driba, D.L.; Lopez Rivera, N.; Kariminasab, M.; Beckingham, L.E. A Review of Coupled Geochemical–Geomechanical Impacts in Subsurface CO₂, H₂, and Air Storage Systems. Energies 2024, 17, Art. [Google Scholar] [CrossRef]
- Kumar, K.R.; Honorio, H.; Chandra, D.; Lesueur, M.; Hajibeygi, H. Comprehensive Review of Geomechanics of Underground Hydrogen Storage in Depleted Reservoirs and Salt Caverns. Journal of Energy Storage 2023, 73, Art. [Google Scholar] [CrossRef]
- Zeng, L.; Vialle, S.; Ennis-King, J.; Esteban, L. Role of Geochemical Reactions on Caprock Integrity During Underground Hydrogen Storage. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Laban, M. Hydrogen Storage in Salt Caverns: Chemical Modelling and Analysis of Large-Scale Hydrogen Storage in Underground Salt Caverns. ResearchGate, https://www.researchgate.net/publication/371044665_Hydrogen_storage_in_salt_caverns_Chemical_modelling_and_analysis_of_large-scale_hydrogen_storage_in_underground_salt_caverns, 2020. [Google Scholar]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical Reactions-Induced Hydrogen Loss During Underground Hydrogen Storage in Sandstone Reservoirs. Int. J. Hydrogen Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Blyton, C.A.J.; Gala, D.P.; Sharma, M.M. A Comprehensive Study of Proppant Transport in a Hydraulic Fracture. In Proceedings - SPE Annual Technical Conference and Exhibition; 2015; pp. 3391–3411. [CrossRef]
- Böttcher, N.; Görke, U.-J.; Kolditz, O.; Nagel, T. Thermo-Mechanical Investigation of Salt Caverns for Short-Term Hydrogen Storage. Environ. Earth Sci. 2017, 76, 1–13. [Google Scholar] [CrossRef]
- Kumar, K.R.; Makhmutov, A.; Spiers, C.J.; Hajibeygi, H. Geomechanical Simulation of Energy Storage in Salt Formations. Sci. Rep. 2021, 11, 1–24. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Z.; Yue, Y.; Chen, Q.; Liu, J. Numerical Study of Hydrogen Storage Cavern in Thin-Bedded Rock Salt, Anning of China. In CRC Press, 2022; pp. 652–661.
- Coarita-Tintaya, E.-D.; Golfier, F.; Grgic, D.; Souley, M.; Cheng, L. Hydromechanical Modelling of Salt Caverns Subjected to Cyclic Hydrogen Injection and Withdrawal. Comput. Geotech. 2023, 162, Article–105690. [Google Scholar] [CrossRef]
- Bai, T.; Tahmasebi, P. Coupled Hydro-Mechanical Analysis of Seasonal Underground Hydrogen Storage in a Saline Aquifer. Journal of Energy Storage 2022, 50, Article–104308. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A Review on Underground Hydrogen Storage: Insight into Geological Sites, Influencing Factors and Future Outlook. Energy Rep. 2022, 8, 461–499. [Google Scholar] [CrossRef]
- Koch, T.; Gläser, D.; Weishaupt, K.; Ackermann, S.; Beck, M.; Becker, B.; Burbulla, S.; Class, H.; Coltman, E.; Emmert, S.; Fetzer, T.; Grüninger, C.; Heck, K.; Hommel, J.; Kurz, T.; Lipp, M.; Mohammadi, F.; Scherrer, S.; Schneider, M.; Seitz, G.; Stadler, L.; Utz, M.; Weinhardt, F.; Flemisch, B. DuMux 3 – An Open-Source Simulator for Solving Flow and Transport Problems in Porous Media with a Focus on Model Coupling. Computers & Mathematics with Applications 2021, 81, 423–443. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Ekström, H.; Fontes, E. COMSOL Multiphysics®: Finite Element Software for Electrochemical Analysis. A Mini-Review. Electrochem. Commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Pruess, K.; Oldenburg, C.M.; Moridis, G.J. TOUGH2 User's Guide Version 2. 1999. [Online]. Available. [CrossRef]
- Computer Modelling Group. CMG User Manual, Computer, Modelling Group, Calgary, Canada, 2022. [Online]. Available, 2022. [CrossRef]
- Kanaani, M.; Sedaee, B.; Asadian-Pakfar, M. Role of Cushion Gas on Underground Hydrogen Storage in Depleted Oil Reservoirs. J. Energy Storage 2022, 45. [Google Scholar] [CrossRef]
- Abdellatif, M.; Hashemi, M.; Azizmohammadi, S. Large-Scale Underground Hydrogen Storage: Integrated Modeling of Reservoir-Wellbore System. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Luboń, K.; Tarkowski, R. The Influence of the First Filling Period Length and Reservoir Level Depth on the Operation of Underground Hydrogen Storage in a Deep Aquifer. Int. J. Hydrogen Energy 2023, 48, 1024–1042. [Google Scholar] [CrossRef]
- Hagemann, B.; Rasoulzadeh, M.; Panfilov, M.; et al. Hydrogenization of Underground Storage of Natural Gas. Comput. Geosci. 2016, 20, 595–606. [Google Scholar] [CrossRef]
- Anderson, J.; Grönkvist, S. A Comparison of Two Hydrogen Storages in a Fossil-Free Direct Reduced Iron Process. Int. J. Hydrogen Energy 2021, 46, 28657–28674. [Google Scholar] [CrossRef]
- Wang, G.; Pickup, G.; Sorbie, K.; Mackay, E. Scaling Analysis of Hydrogen Flow with Carbon Dioxide Cushion Gas in Sub-Surface Heterogeneous Porous Media. Int. J. Hydrogen Energy 2022, 47, 1752–1764. [Google Scholar] [CrossRef]
- Pfeiffer, W.T.; Beyer, C.; Bauer, S. Hydrogen Storage in a Heterogeneous Sandstone Formation: Dimensioning and Induced Hydraulic Effects. Petrol. Geosci. 2017, 23, 315–326. [Google Scholar] [CrossRef]
- Feldmann, F.; Hagemann, B.; Ganzer, L.; Panfilov, M. Numerical Simulation of Hydrodynamic and Gas Mixing Processes in Underground Hydrogen Storages. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).