1. Introduction
Immunohistochemistry (IHC) is a technique that uses antibodies to detect specific antigens within samples. Combining antigen-antibody reactions with a color reaction enables the presence and localization of an antigen within a tissue sample to be observed under a microscope, thereby confirming the expression of specific genes or proteins. For cancer treatment, IHC is helpful for making differential diagnoses of benign and malignant tumors, for visualizing the extent of cancers, and for selection of drug therapies.
We have developed an innovative device that enables rapid completion of IHC analyses within about 20 minutes. With this device, we apply a high-voltage, low-frequency alternating current (AC) electric field to tissue sections. The resultant coulomb force stirs the antibody solution within droplets on the sections without increasing the temperature of the droplets. We previously reported the utility of this method for staining frozen sections for intraoperative detection lymph node metastasis in non-small cell lung cancer [
1]; differential diagnosis of brain [
2] and lung tumors [
3]; and detection of sentinel lymph node metastasis in breast cancer [
4]. The activated antigen-antibody reaction induced with this method also showed the potential to reduce the amount of expensive antibody needed for analysis [
5].
As originally developed, our rapid IHC (R-IHC) method was largely manual. Only the antibody reaction is carried out in a staining machine; the application of reagents and washing out of antibodies had to be carried out manually. To streamline the protocol and make it more reproducible, we have developed a fully automated R-IHC staining device (R-Auto) that automates all steps in the IHC protocol. By filling the cartridge with the necessary reagents, registering them in the instrument and setting the protocol, the entire staining process can be carried out automatically. Moreover, the AC electric field is now applied to the process of washing out the antibody solutions, which was not done when washouts were done manually.
We previously reported that R-IHC is a time-saving utility useful for intraoperative frozen sections. On the other hand, formalin fixed paraffin embedded (FFPE) sections are crucial for diagnosis and treatment decision-making in nearly all cancer patients, regardless of disease stage, and large numbers of diagnoses are made using FFPE sections in daily practice. Against that background, in this report we evaluated the utility of R-Auto for use with FFPE specimens of breast cancer, the most commonly occurring cancer in women worldwide [
6]. IHC analyses of estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor type 2 (HER2) and the Ki-67 index are used as surrogates for genetic profiling to determine breast cancer treatment strategies, which include neoadjuvant or adjuvant systemic therapy to reduce the risk of recurrence, to prolong life and to maintain quality of life. In recent years, improved diagnostic accuracy and the development of multidisciplinary treatments combining surgery, pharmacotherapy and radiotherapy have improved prognoses. For instance, clinical trials have shown that use of CDK4/6 inhibitors in combination with aromatase inhibitors and fulvestrant in patients with hormone receptor-positive, HER2-negative metastatic or recurrent breast cancer prolonged both progression-free and overall survival [7-10]. Other trials showed that abemaciclib or ribociclib administration contributed to the better prognosis in patients with hormone receptor-positive, HER2-negative early breast cancer at high risk of recurrence [
11,
12]. In addition, administration of trastuzumab deruxtecan (T-DXd) as a second-line treatment prolonged progression-free survival and improved overall survival in patients with HER2-positive metastatic or recurrent breast cancer [
13]. IHC has contributed to this remarkable progress in breast cancer therapeutics by enabling assembly of the most appropriate treatments against specific forms of the disease.
The aim of the present study is to establish a staining protocol and evaluate the staining performance of R-Auto against ERs as a prototype for breast cancer FFPE specimens.
2. Methods
2.1. Ethical Condition and Patients Sample
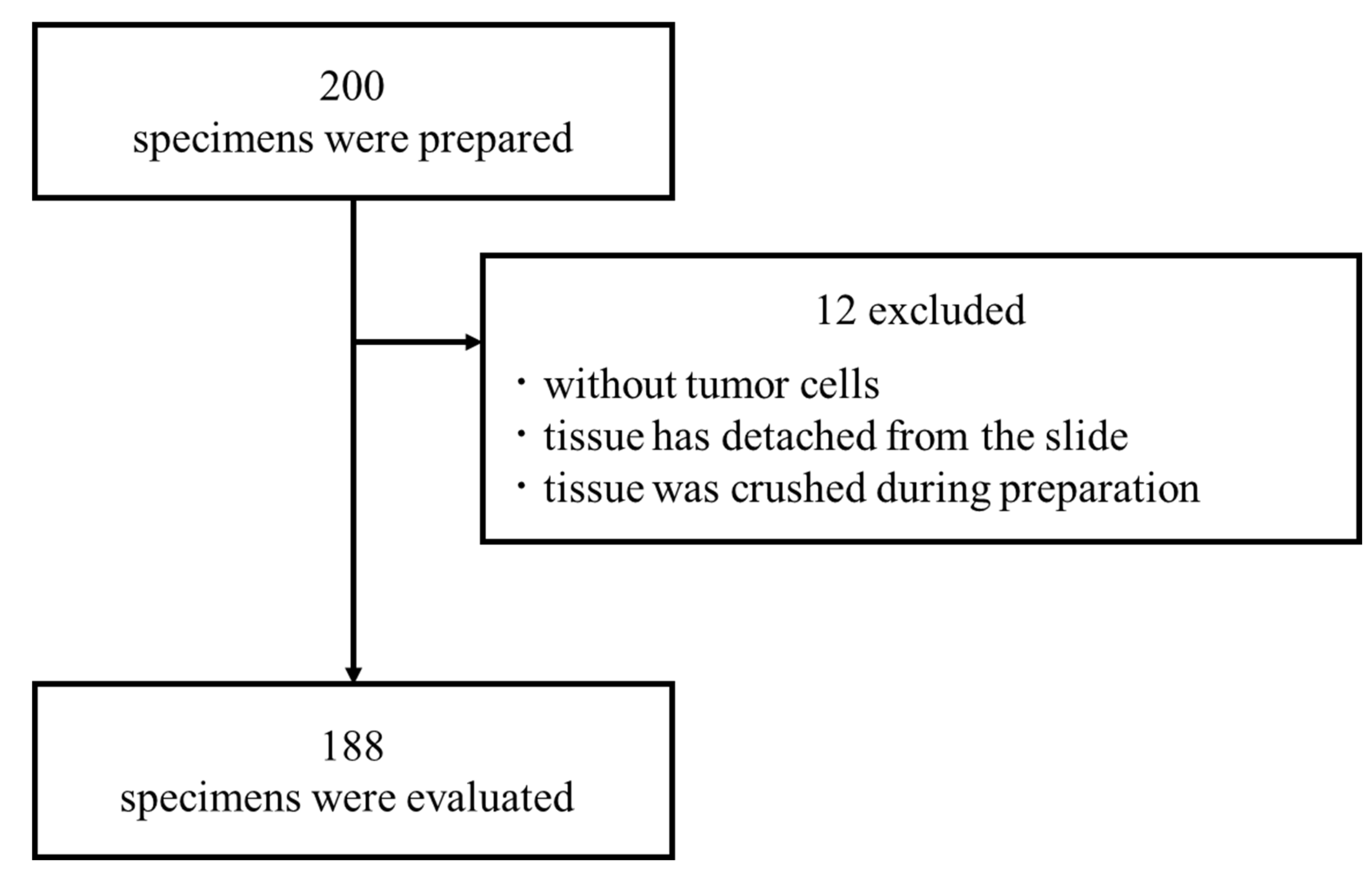
The medical records from 200 patients who underwent surgery for breast cancer at our hospital between July 2014 and January 2017 were serially extracted. From among them, 188 eligible patients with appropriate pathological specimens were enrolled in the study. This retrospective study was approved by the Institutional Review Board at Akita University School of Medicine and University Hospital (Permit number: 896, 929 & 3105) and all samples were collected under IRB Protocol No. 3105. A diagram of the process by which cases were selected for study is shown in Supplementary
Figure 1.
2.2. IHC Procedures
The breast cancer specimens were fixed in 10% buffered formalin and embedded in paraffin. Four-micrometer-thick sections from the FFPE samples were incubated with Paraffin Stretcher (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) at 50 ℃ overnight, then stained using IHC. At the time of their surgery, each patient’s FFPE specimen was stained for ER using IHC performed with a commercially available autostainer (Ventana BenchMark ULTRA (Ventana Medical Systems, Tucson, AZ)). In the present study, staining for ER was also carried out using R-Auto and conventional manual (hereafter refered to as conventional) IHC.
2.3. R-IHC Method and R-Auto Protocol
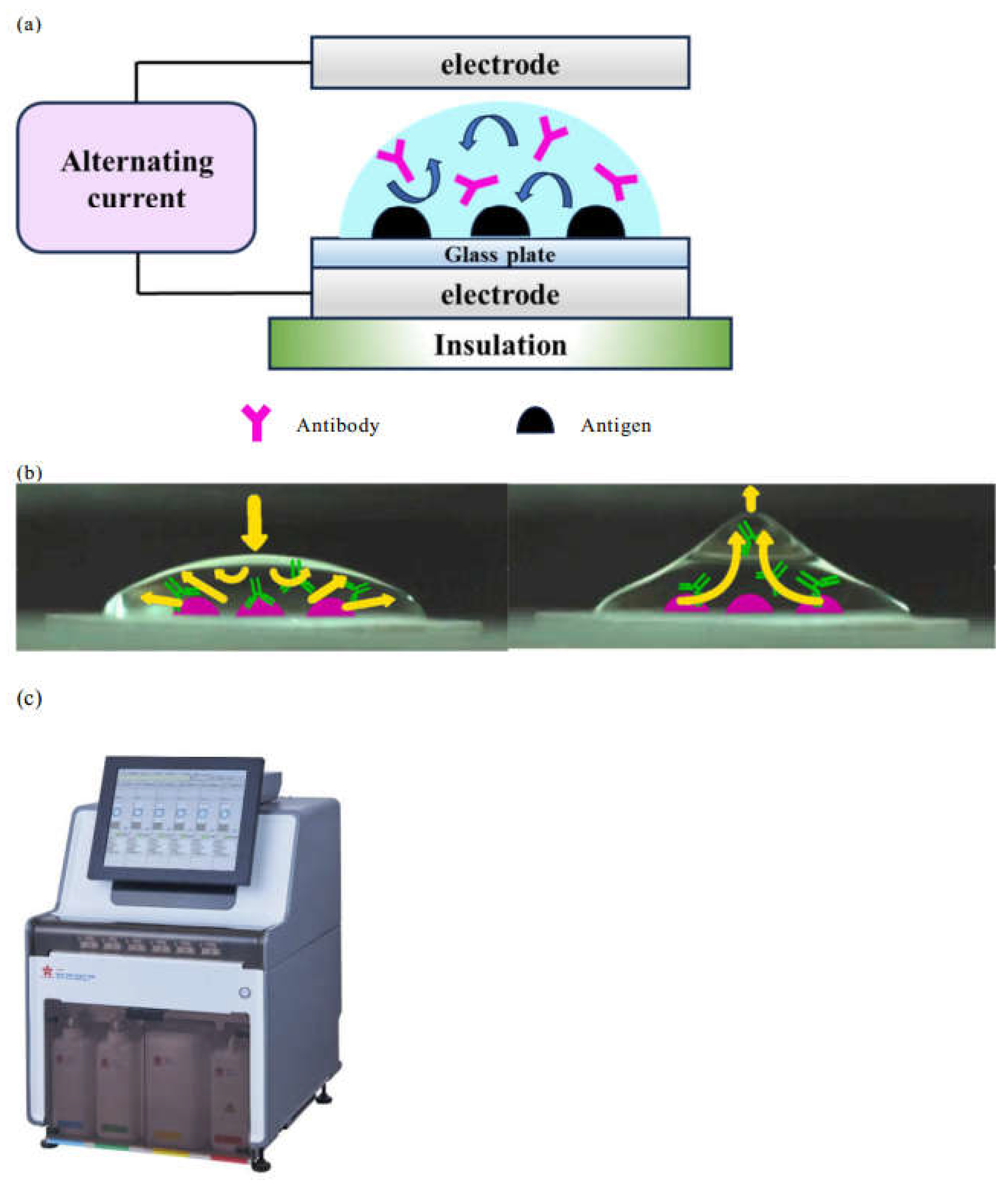
We have developed a device that reduces the time required for IHC analysis. Its mechanism was previously described in detail [
6,
14]. Briefly, with this R-IHC system, we apply a high-voltage, low-frequency (4KV, 10Hz) AC electric field to tissue sections while they are incubating with the antibodies (
Figure 2a). The resultant coulomb force transforms the shape of the microdroplets, stirring the antibody solution on the sections, which increases the opportunity for contact between the antibody and antigen (
Figure 2b). This greatly reduces the time required for the antigen-antibody reaction. The AC electric field is an ideal technique for IHC as it is effective for stirring liquids in volumes on the order of µL and does not increase the temperature of the droplets. Furthermore, in R-Auto (
Figure 2c) the AC electric field was applied to the process of washing out the antibody solutions, which had previously been carried out manually.
Details of the staining method are listed in
Table 1. For deparaffinization and antigen activation, the samples were first immersed three times for 3 minutes each in a solution of 85% xylene and 10% ethylbenzene. This was followed by three immersions for 3 minutes each in 99.5% ethanol. Thereafter, the samples were immersed in distilled water for 5 minutes and then treated with a heated antigen activation solution (ULTRA Cell Conditioning Solution (ULTRA CC2) Roche Diagnostics Inc., Tokyo, Japan) at 98°C for 40 minutes. Finally, the samples were then left at room temperature for 20 minutes to complete the antigen activation process.
For the IHC antigen-antibody reaction, the slides were first incubated for 6 min with anti-ER antibody (CONFIRM anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody, Roche Diagnostics Inc., Tokyo, Japan) in the R-Auto. The primary antibody was then detected by incubating the slides for 4 min with a secondary antibody (ultraView Universal DAB Detection Kit, Roche Diagnostics Inc., Tokyo, Japan), after which the slides were developed using 3,3′diaminobenzidine (Dako liquid DAB+ substate chromogen System, Dako, Tokyo, Japan) and counterstained with hematoxylin. The slides were incubated in the Bluing Reagent (Roche Diagnostics Inc., Tokyo, Japan) and Ventana univerasal DAB copper (included in ultraView Universal DAB Detection Kit) before and after counterstaining. The R-Auto protocol was established by setting the reaction times of the primary and secondary antibodies in 1-minute increments and performing test staining. The protocol subsequently judged by a pathologist to produce the best diagnostic performance was employed. With the previously reported equipment for R-IHC, only the primary and secondary antibody reactions were performed in the automated equipment, but with R-Auto all steps in the protocol, including washing out of the antibody solution, were automated.
2.4. Histopathological Evaluation
Assigning Allred scores, which combines five levels of positive cell occupancy (Proportion Score, PS) and three levels of staining intensity (Intensity Score, IS), is a typical scoring method [
15] for assessing the ER status of breast cancers. A total Allred score (TS) of 0-2 was classified as ER negative, 3-6 weakly positive, and 7-8 strongly positive [
16]. Pathological diagnoses were made by two certified pathologists.
2.5. Statistical Analysis
The diagnostic concordance between R-Auto and conventional IHC and between R-Auto and a commercial autostainer were evaluated. The number of required cases was calculated to be 214 (192 cases excluding specimen deficiencies) for 80% power when tested at a significance level of 0.05, assuming an expected concordance rate of 0.8 and a dropout rate of 10% due to incomplete specimens. Because evaluation bias is expected, the AC
1 statistic [
17] in addition to the weighted kappa coefficient was used to evaluate the degree of agreement. The cutoffs for the weighted kappa coefficient and the AC
1 statistic were defined as poor for less than 0.40, moderate for 0.40 to 0.60, and substantive agreement for 0.60 or more. Statistical analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
3. Results
The R-Auto protocol is shown in
Table 1. After deparaffinization and antigen activation as pretreatment (total 83 minutes), IHC was carried out using R-Auto or a conventional method. As in previous reports [1-5,14], the application of an AC electric field reduced the reaction time needed for the primary antibody from 32 minutes to 6 minutes as compared to the conventional IHC and commercial autostainer. Similarly, the reaction time of the secondary antibody was reduced from 32 minutes to 4 minutes compared to the conventional IHC and from 12 minutes to 4 minutes compared to the commercial autostainer. An additional unique feature of R-Auto is that an AC electric field is also applied for the washing process, which reduced the time required for washing out the antibody from 10 minutes needed for the conventional method to 5 minites. As a result, the total time required for IHC was significantly reduced from 209 minutes for the conventional IHC and 210 minutes for the commercial autostainer to 121 minutes for the R-Auto method.
The characteristics of the 188 patients evaluated are shown in
Table 2. Based on the pathological diagnostics at surgery, most (70.2%) were diagnosed were invasive carcinoma NST. Staging revealed that 53.2% were stage I, 21.3% were stage II and 10.2% were stage III. ER immunostaining results (synonymous with commercial autostainer staining results) at the time of postoperative diagnosis were TS0-2 in 28 specimens (14.9%), TS3-6 in 11 specimens (5.9%) and TS7-8 in 149 specimens (79.2%).
The staining profile obtained with R-Auto was TS0-2 in 28, TS3-6 in 13 and TS7-8 in 147 specimens. With conventional IHC, the corresponding staining profile was 28, 5 and 155 specimens, and for postoperative diagnosis with a commercial autostainer it was 28, 11 and 149 specimens, respectively (
Table 3 and
Table 4).The AC
1 statistic for comparison between R-Auto and conventional IHC was 0.9490 (0.9139-0.9841) with a 95.7% agreement rate. For comparison between R-Auto and the commercial autostainer, the AC
1 statistic was 0.9095 (0.8620-0.9570) with a 92.6% agreement rate. Thus, the AC
1 statistics showed substantial agreement between results obtained using R-Auto and both the conventional staining method and staining using a commercial autostainer. Moreover, when we separately compared the agreement rate for each score from TS0 to TS8 instead of the 3-level scoring, the AC
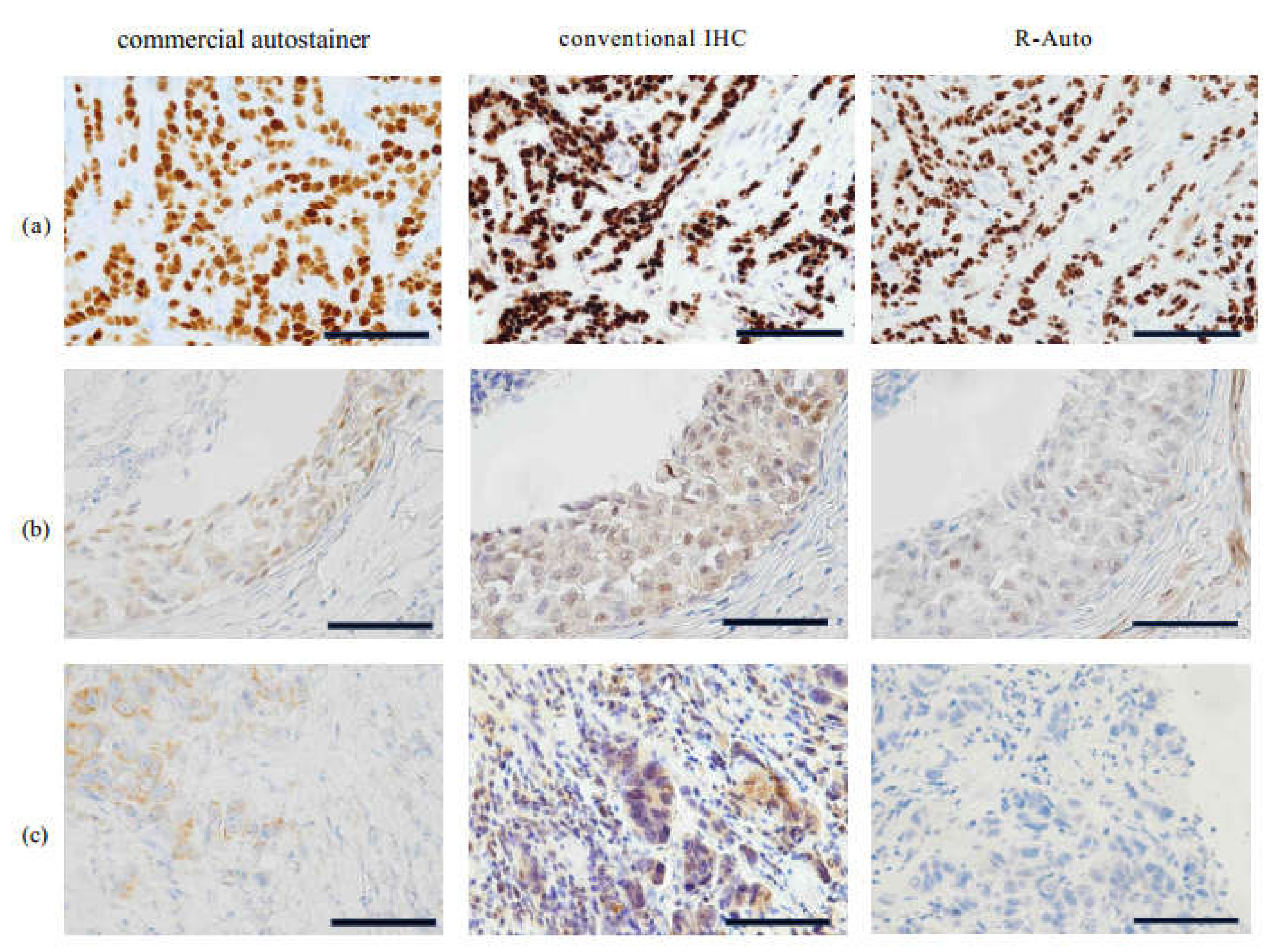
1 statistic was 0.9160 (0.8749-0.9572) for R-Auto vs. the conventional IHC and 0.8372 (0.7816-0.8927) for R-Auto vs. the commercial autostainer. There was substantial agreement. As the microscopic images show, the samples were stained very well and clear in R-Auto and nearly equally (
Figure 3).
4. Discussion
In this study, there was strong agreement between the Allred scores obtained using R-Auto, conventional IHC and a commercial autostainer. However, R-Auto reduced the antigen-antibody reaction time from 64 and 44 minutes to 10 minutes, respectively, compared to conventional IHC and commercial autostainer, and R-Auto reduced the washing time from 28 minutes to 2 minutes compared to conventional IHC. The time reduction and good staining performance are attributable to activation of the antigen-antibody reaction using an AC electric field, which also sped up the washing process.
Although good agreement was obtained, some differences in the staining results btained with R-Auto the commercial autostainer and R-Auto were noted. In that regard, a study examining staining of old FFPE specimens reported no differences in IHC staining intensity of cytoplasmic antigen in old specimens but staining of membrane and nuclear antigens declined over time [
18]. It has also been reported that the intensity of ER staining differs depending on the storage method [
19]. In the present study, the staining intensity may have been affected by the use of thin slices of specimens that were prepared several years after the tissue blocks were made and were made and may have been a limitation in obtaining the accurate results possible.
The earlier version of the diagnostic equipment used for R-IHC was mainly used for rapid intraoperative differential diagnosis of lung tumors [
1] and brain tumors [
2] and diagnosis of sentinel node metastasis [
4]. However, because good results were obtained with R-Auto staining of FFPE specimens, we think it could also contribute to the final pathological diagnosis. A study of 5137 breast cancer patients requiring neoadjuvant chemotherapy reported that the estimated 5-year overall survival significantly worsened as the number of days from diagnosis to intervention increased [
20]. The speed of diagnosis, including subtype diagnosis, is very important not only for postoperative patients but also for patients prior to treatment.
In the present study, we showed that R-Auto is capable of performing IHC with sufficient staining accuracy in the same or less time than commercial autostainers. We suggest R-Auto could be used in both clinical practice and the laboratory to provide high-quality, reproducible IHC results. In addition, by activating antigen-antibody reaction with AC electric field agitation, R-Auto reduces the amount of expensive antibody needed [
1,
5], and we anticipate it will also be applicable to
in situ hybridization [
14,
21]. Although we stained only for ER in this study, a strength of this method is that it could also be applied to PgR, HER2 and Ki-67, which are important biomarkers in breast cancer treatment, and it could be used to expedite immunostaining in other types of cancer. Furthermore, with the potential spread of digital pathology in daily practice, it is essential to work toward standardizing and streamlining IHC, and we believe that our technology can contribute to that effort.
5. Conclusions
We have developed a fully automated R-IHC staining machine that achieves rapid, high quality IHC staining with little human effort through application of an AC electric field to enhance the antibody-antigen interaction and the washing process. This has enabled establishment of an ER immunostaining protocol in breast cancer that could serve as the basis for the use of R-IHC in many other cancers.
Authors' Contributions: (1) Conception and design: C.K. (2) Administrative support: K.I. (3) Provision of study materials or patients: C.K., K.T., E.T., A.Y., H.K., and M.O. (4) Collection and assembly of data: C.K., K.T. (5) Data analysis and interpretation: K.N., C.K., K.T. (6) Manuscript writing: All authors. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research was supported by AMED under Grant Number 21he0422008j0002. This R-IHC technology is supported by R-IHC Study Group.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Toda, H. et al. A Novel Immunohistochemical Staining Method Allows Ultrarapid Detection of Lymph Node Micrometastases While Conserving Antibody. Acta Histochem Cytochem. 2011; 44: pp. 133–139. [CrossRef]
- Tanino, M. et al. Erratum to: Rapid immunohistochemistry based on alternating current electric field for intraoperative diagnosis of brain tumors. Brain Tumor Pathol. 2015; 32: pp. 12–19. [CrossRef]
- Kuriyama, S. et al. Multiplex Intraoperative Rapid Immunohistochemistry with Noncontact Antibody Mixing for Distinguishing the Histologic Phenotype of Lung Cancer. Pathobiology. 2024. [CrossRef]
- Terata, K. et al. Novel rapid-immunohistochemistry using an alternating current electric field for intraoperative diagnosis of sentinel lymph nodes in breast cancer. Sci Rep. 2017; 7(1): pp. 2810. [CrossRef]
- Hoshino, I. et al. Reagent-saving immunohistochemistry for HER2 using non-contact alternating current electric field mixing. J Clin Pathol. 2019; 72(1): pp. 25-30. [CrossRef]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12): pp. 1749–1768. [CrossRef]
- Finn, R.S. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375(20): pp. 1925–1936. [CrossRef]
- Rugo, H.S. et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019; 174(3): pp. 719-729. [CrossRef]
- Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17(4): pp. 425-439. [CrossRef]
- Sledge, G.W. Jr et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017; 35(25): pp. 2875-2884. [CrossRef]
- Slamon D, Lipatov O, Nowecki Z, McAndrew N, Kukielka-Budny B, Stroyakovskiy D, Yardley DA, Hortobagyi G, et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N Engl J Med. 2024;390:pp.1080–1091. [CrossRef]
- Johnston, S.R.D. et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020; 38(34): pp. 3987-3998. [CrossRef]
- Cortés, J. et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med. 2022; 386(12): pp. 1143-1154. [CrossRef]
- Saito, Y. et al. Novel method for rapid in-situ hybridization of HER2 using noncontact alternating-current electric field mixing. Sci Rep. 2016; 6: pp. 30034. [CrossRef]
- Harvey, J.M. et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17(5): pp. 1474-1481. [CrossRef]
- Toyama, T. et al. New treatment strategy for triple-negative breast cancer targeting micro RNA. Grants-in-Aid for Scientific Research. Japan Society for the Promotion of Science; 2012-2015.
- Gwet, K.L. et al. Computing Inter-rater Reliability and Its Variance in the Presence of High Agreement. Br J Math Stat Psychol. 2008; 61: pp. 29–48. [CrossRef]
- Federica, G. et al. Immunohistochemistry on old archival paraffin blocks: is there an expiry date? J Clin Pathol. 2017; 70(11): pp. 988-993. [CrossRef]
- Federica, G. et al. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol. 2015; 144: pp. 93–99. [CrossRef]
- de Melo Gagliato, D. et al. Impact of Delayed Neoadjuvant Systemic Chemotherapy on Overall Survival Among Patients with Breast Cancer. Oncologist. 2020; 25(9): pp. 749-757. [CrossRef]
- Watanabe, S.; et al. Rapid HER2 cytologic fluorescence in situ hybridization for breast cancer using noncontact alternating current electric field mixing. Cancer Med. 2021, 10(2), pp. 586–594. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).