1. Drought Stress & Climate Change
Drought is a natural disaster that occurs when there is a prolonged period of time with insufficient precipitation, resulting in a shortage of water resources. The impacts of drought on agriculture are significant, and one of the most critical consequences is the alteration of soil habitat, which can have a significant impact on the microbial structure and function. Microbes play a vital role in maintaining the health and productivity of soil ecosystems, and drought conditions can negatively affect their populations and diversity. Changes in microbial populations can alter the availability of nutrients, impact the growth of plants, and ultimately affect crop yields. Therefore, it is essential to understand how drought affects microbiomes in agriculture and how we can manage these impacts to improve crop productivity and sustainability. In this context, studying the effects of drought on soil microbiomes can provide valuable insights into the ecological mechanisms underlying drought response in agricultural systems, which can help us develop effective strategies for managing these impacts.
Climate change is increasing the frequency, duration, and severity of droughts. One of the reasons that acknowledged to the drought severity and occurrence is global warming. The higher temperature of global surface was found to increase over 0.99 and 1.09 C between 19 years from 2001 and 2011 to 2020, respectively (IPCC, 2021). Berg & Sheffield (2018) explained that the higher temperatures and changes of precipitation patterns are contributing to increase the evapotranspiration rates and higher average aridity scores for many landscapes. The water limitation condition share pronounced impacts in semi-arid and arid regions across the world including South America, Asia, Africa, and India (Mukherjee et al., 2018). The increment of temperature globally and the following effects have been the major reasons why the climate change scenarios are important to predict and evaluate where the worst drought stress occurrence, especially during dry season.
Drought stress is acknowledged as a significant abiotic stress that exacerbates profound alteration in physio-chemical properties of soil due to water limitation and performs worse influences during the growing season. On drier season, where the water availability is decreasing, the most prevalent stress by drought will corresponds to direct and indirect influence to the microbial communities and habitat, microbiome resiliency under drought stress, legacies that contributing to crop growth, health, yield, plant and microbes interaction, and ecosystem services (Ochoa-Hueso et al., 2018; Schimel, J.P., 2018; Bogati and Walczak, 2022). If severe drought stress occurs in a long period of time, this will need further research to investigate the continuous worsening effects that follow. Also, whether the condition of post-drought can be restored to the first initial conditions or not. As such, understanding the effects of drought on the soil habitat is essential for managing and conserving soil resources in the face of climate change. In the next sections we will identify overarching patterns in microbial community structure and highlight key functional shifts in agricultural microbiomes experiencing drought stress.
2. Impact of Drought on Soil Microbiome
Drought is one of the most significant consequences of climate change that affects agriculture, and it can have a significant impact on the soil microbiome. When soil moisture is limited, it affects the composition and function of soil microbial communities, leading to changes in soil nutrient availability, soil structure, and plant-microbe interactions. These stress conditions decrease microbial abundance, disturb microbial structure, decline microbial activity, including enzyme production (e.g., such as oxidoreductases, hydrolases, dehydrogenase, catalase, urease, phosphatases, β-glucosidase) and nutrient cycling, leading to a decrease in soil fertility followed by lower plant productivity and loss in economy. These changes can have a cascading effect on crop yield, soil health, and the overall productivity of the agroecosystem (Bogati & Walczak, 2022).
Also, drought can exacerbate the impact of other environmental stressors on the agriculture microbiome, such as high temperatures, soil salinity, and pests and diseases. For instance, drought stress can weaken the plant's defense mechanisms, making it more susceptible to pest and disease
infestations, which in turn, can alter the composition and function of the soil microbiome. Similarly, high temperatures can affect the growth and activity of soil microbes, leading to changes in the soil microbial community structure and function (Khan et al., 2019). Drought directly affects plant morphology, anatomy, physiology, and biochemistry. Its effect on plants can also be observed by changes at the transcriptomic and metabolomic levels. However, in plants, it can be mitigated by rhizosphere microbial communities, especially by plant growth-promoting bacteria (PGPB) and fungi (PGPF) that adapt their structural and functional compositions to water scarcity (Bogati & Walczak, 2022).
Overall, studying drought is crucial for understanding the impacts of climate change on the agriculture microbiome. By elucidating the mechanisms by which drought affects soil microbial communities and their interactions with plants, we can develop strategies to mitigate the adverse effects of drought on crop productivity and soil health.
3. Key Knowledge Gaps
While there has been prior work characterizing changes in microbiome structure under drought conditions, the effects of prolonged stress (press disturbance) and increasing frequency of drought events (repeated pulse disturbances) is not well understood. Evaluating changes in community composition and diversity following disturbance events is essential for identifying characteristics of resilient microbiomes (Bogati et al., 2022; Kristy et al., 2022; Hanaka et al., 2021; Preece et al., 2019; Kaurin et al., 2018), that may be managed under real-world conditions to ameliorate drought stress in cropping systems. Additionally, the link between changes in microbiome structure and microbial community function are poorly understood, particularly in response to drought stress in agricultural systems across diverse regions and with differing host crops. Elucidating patterns of microbiome function and not only microbial community structure will be essential for understanding how drought events may mediate plant- soil feedback to shape crop health and soil fertility. This will be particularly important for addressing the role of climate change in shaping spatio-temporal variability in plant-microbe interactions that may de-stabilize beneficial associations or heighten the risk of pathogenicity and disease (Singh, et al., 2023). Similarly, there is a need to understand how drought stress impacts the abundance, diversity, and functional roles of specific microbial groups that support crop health, including plant growth promoting bacteria and mycorrhizae. Illuminating how microbe-microbe interactions are mediated by drought stress can provide unique insight into how beneficial functional traits may be promoted in agroecosystems (Bogati et al., 2022; Kristy et al., 2022).
4. Effect of Drought on Soil Habitat
Drought can have a significant impact on the soil habitat, which encompasses the physical, chemical, and biological environment in which soil organisms live and interact. Soil habitat is essential for supporting plant growth and ecosystem functioning, as it provides a source of nutrients, water, and other resources necessary for life. During a drought, soil water content decreases, which can lead to changes in soil structure, nutrient availability, and microbial activity. These changes can negatively impact the soil habitat, making it less hospitable for many soil organisms (Sun et al., 2020, Berg & Sheffield, 2018). Drought stress can have a direct effect on soil habitats by altering soil moisture content and soil temperature, as well as indirect effects by modifying soil carbon and nitrogen availability, soil pH, and influencing plant growth dynamics. Changes in soil temperature during drought conditions can affect soil organic matter (SOM) decomposition and increase the release of carbon dioxide. Also, during this process additional mineral N, mostly in the form of nitrate, will be released in the soil system. Recognizing how drought shapes soil environments is important for understanding how this stressor may drive changes in microbiome structure and function.
4.1. Soil Moisture
Droughts typically occur when there is a prolonged period of low precipitation or when there is high evapotranspiration due to hot and dry weather conditions (Baik et al., 2019). As a result, the soil becomes increasingly dry, and soil moisture is depleted, which can lead to changes in the structure and composition of soils (Baik et al., 2019). As soil moisture decreases, the water potential of the soil becomes more negative, creating a gradient that draws water away from plant roots and into the atmosphere through evaporation and transpiration (Berg & Sheffield, 2018). Reduction of soil moisture can indeed lead to soil compaction. When soil moisture levels decrease, the soil particles are more likely to come into close contact with each other, reducing the amount of pore space between them. This can result in a reduction in soil volume and an increase in soil density, which is referred to as soil compaction. In addition, when the soil becomes drier, it can become more difficult for roots to penetrate and grow, which can further contribute to soil compaction. This is because the roots are not able to break up the soil as effectively when it is dry, and so the soil particles remain tightly packed together. The soil can become more compact, reducing soil porosity and oxygen availability, which can negatively impact the growth and activity of aerobic soil microbes (Indoria et al., 2020). When soil moisture is too high, it can reduce the amount of oxygen available to soil organisms, leading to anaerobic conditions that can be harmful or even lethal to many types of soil organisms. Excess moisture can also increase the risk of fungal and bacterial diseases, which can further harm soil organisms. Conversely, when soil moisture is too low, soil organisms may struggle to obtain the water they need for survival, growth, and reproduction. This can lead to dehydration and reduced activity, and in severe cases, mortality. Further, when the soil is dry, the forces that hold water in the soil, such as capillary forces and the attraction between water and soil particles, become stronger (Berg & Sheffield, 2018). This makes it more difficult for plants to access water (AghaKouchak et al.,2015), which can negatively feedback to hinder microbial growth and activity in soils and the rhizosphere (AghaKouchak et al.,2015), Soil moisture content is the primary driver of differences in soil habitat following drought stress and can alter nutrient availability across plant-soil compartments.
4.2. Plant Growth
Drought can help us identify the microbial mechanisms that enable some plants to tolerate and adapt to water stress better than others. Understanding the microbial communities associated with drought-tolerant plants can provide insights into the microbial traits that confer drought tolerance, which can be harnessed to improve crop productivity under drought conditions (Bogati & Walczak, 2022, Khan et al., 2019, You et al., 2019)
Soils experiencing water stress directly influence plant growth contributing to changes in plant physiology, morphology and biochemistry (Hanaka et al., 2021). The impact of drought stress on plants encompasses various morphological features, including limited seed germination and seedling growth, reduced leaf size, area, and number, fewer stomata, decreased flower production, disrupted stem and root elongation, impaired plant height, growth, development, and yield, as well as decreased fresh and dry biomass (Khan et al., 2021; Abdelaal et al., 2021).
To adapt to these adverse conditions and enhance water availability, plants respond by increasing the length and number of roots (Shao et al., 2008). Drought significantly affects cell elongation, division, overall growth, and development, primarily due to reduced cellular differentiation, plant growth, and yield (Abdelaal et al., 2021). The reduction in leaf area under drought conditions may be attributed to decreased leaf number, size, and longevity, in conjunction with factors like temperature, leaf turgor pressure, and assimilation rate (Reddy et al., 2004). The decrease in plant height and shoot dry weight adversely affects yield quality (Shao et al., 2008).
Morphological responses are often accompanied by anatomical changes in drought- stressed plants, such as cell wall thickening, increased cuticle layer on leaf surfaces, and improved development of vascular tissues (Ullah et al., 2019). Drought stress induces anatomical modifications in the lower and upper epidermis, mesophyll tissue, and vascular bundle diameter of leaves (Hafez et al., 2020). These anatomical changes are driven by inadequate water supply from the soil, nutrient uptake limitations, and reduced photosynthetic rates. Drought stress modulates plant hydraulic conductivity, leading to disrupted water flow in xylem vessels (embolism) or alterations in vessel size and function (Hargrave et al., 1994). Consequently, reduced water movement from roots to shoots causes stomatal closure and disruption of transpiration (Abdelaal et al., 2021).
Drought also impacts physiological traits, including leaf relative water content, water potential, stomatal conductance, transpiration, and photosynthetic rates (Liu et al., 2005). Decreased water content and conductivity result in loss of turgidity and limited stomatal conductance, restricting gaseous exchange (carbon assimilation rates (Ullah et al., 2019). Additionally, climatic conditions like higher temperatures, drought, and soil aeration impede nutrient movement in the soil, nutrient uptake by roots, and nutrient transport in plant tissues (Ragel et al., 2019).
4.3. Soil Carbon Availability
Drought can have a significant impact on soil carbon storage, as it affects the balance between carbon inputs and outputs in the soil ecosystem.
During drought periods, plant productivity is often reduced. Deng et al. (2021) discovered that drought significantly decreased the soil organic carbon content by 3.3 primarily, because of a decline in plant litter input by 8.7% and reduced litter decomposition by 13% across all three ecosystem types (forest, shrubs, and grassland) worldwide. Globally, significant reductions in C of leaves, shoots, roots, and bulk soil were seen during drought conditions. During drought periods, plants decrease the carbon inputs into the soil through photosynthesis and root exudation. Furthermore, soil carbon storage also decreases in drought period through increment the rate of microbial/soil respiration, which releases carbon dioxide from the soil into the atmosphere. This decline may have been caused by lowered stomatal conductance and decreased activity of vital photosynthesis-related enzymes, such as ribulose-1,5-bisphosphate carboxylase/oxygenase. These elements probably decreased net primary production (NPP) and plant C concentrations, both of which affected the amount of organic matter added to the soil (Sun et al., 2020)
In addition, drought can increase the rate of microbial/soil respiration, which releases carbon dioxide from the soil into the atmosphere, further reducing soil carbon storage (Wang et al., 2021). Based on previous meta-analysis data which includes 107 studies, drought stress significantly decreased soil C by an average of 12.8 % and range between 18.4 % to 7.2 % (Wang et al., 2021). Measured extractable organic carbon was significantly reduced by drought stress in 0-15 cm, 15- 30 cm, and 30-45 cm depths the whole year from November 2018 to August 2019 in the Poplar plantation field in China. The effect of drought on soil respiration, however, was found to be dependent on the severity and duration of the drought. Drought induced changes in soil moisture and temperature are the primary drivers of the observed changes in soil organic carbon (SOC) concentrations and turnover rates. Specifically, drought reduces soil moisture, which limits microbial activity and reduces plant growth, resulting in lower inputs of organic matter into the soil. Drought also increases soil temperature, which accelerates microbial decomposition of organic matter and reduces the stability of soil aggregates, further contributing to the loss of SOC.
4.4. Soil Nitrogen Storage
Drought can have a significant impact on the soil nitrogen pool. Nitrogen is an essential nutrient for plant growth and is present in the soil in various forms, including organic nitrogen, ammonium (NH4+), and nitrate (NO3-). Drought conditions can reduce microbial activity in the soil, which can decrease the rate of organic matter decomposition and nitrogen mineralization. Drought stress can also affect plant growth and reduce their ability to take up nitrogen from the soil. This can lead to a buildup of available nitrogen in the soil, as plants are not using it for growth. (Deng et al., 2021).
Drought increased mineral N (NH4+ and NO3−) content (31%) since decreased plant mineral N uptake, less litter and root productions, and reduced NO3− leaching in drought. Plant growth is restricted under decreased water availability in ecosystem types (forest, shrubs, and grassland), leading to less litter and root production (Deng et al., 2021). Furthermore, less roots inevitably decreased the uptake of mineral N (Homyak et al., 2017). Fewer roots absorbed less available N (e.g., NH4+ and NO3−) from the soil (Hartmann et al., 2013). Drought reduced N mineralization rate (5.7%) and nitrification rate (13.8%), and thus left total N unchanged across all the three ecosystem types (forest, shrubs, and grassland) in the world. The reason for that is drought limited microbial activities leading to lower N mineralization and nitrification rates (Deng et al., 2021). Sean et al. (2017) found that summer drought in a California annual grassland increased exchangeable NH4+ and which is explained by reduced N uptake by plants, as grasses were dead and microbial death and subsequent mineralization of the microbial N.
Also, drought reduce the soil C:N ratio since the changes of soil carbon and soil nitrogen pools (Sun et al., 2020, Wang et al., 2021). Drought stress can accelerate the decomposition of organic matter in soils and plant tissues. As moisture becomes limited, microbial activity may slow down, reducing the rate of decomposition. However, drought can also lead to the death of microbial organisms that are responsible for decomposing organic matter. Consequently, the decomposition process can become imbalanced, resulting in a relatively greater loss of carbon compared to nitrogen. This can lead to a decrease in the C:N ratio.
Figure 1.
The conceptual framework illustrating the impacts of drought on carbon (C) (a) and nitrogen (N) (b) pools and processes in soil is depicted. The observed directions are indicated by red and blue arrows, while conclusions and inferences from previous publications are represented by grey arrows. The red "↑" denotes an increase, while the blue "↓" signifies a decrease in pools or fluxes under drought conditions compared to non-drought soil. The sections in black within the C and N cycles signify pools or fluxes that were not considered in the previous meta-analysis (Cregger et al., 2014; Manzoni et al., 2014). Noteworthy components include SOC (soil organic C), TN (soil total N), TON (total organic N), DOC (dissolved organic C), DON (dissolved organic N), MBC (microbial biomass C), MBN (microbial biomass N), CM (C mineralization), Root-CO2 (root respiration), MicrCO2 (microbial respiration), Total-CO2 (total CO2 efflux from soil), NM (N mineralization), NN (nitrification), NDN (denitrification), and N2 + N2O (total N emission as N2 and N2O) (Deng et al., 2021).
Figure 1.
The conceptual framework illustrating the impacts of drought on carbon (C) (a) and nitrogen (N) (b) pools and processes in soil is depicted. The observed directions are indicated by red and blue arrows, while conclusions and inferences from previous publications are represented by grey arrows. The red "↑" denotes an increase, while the blue "↓" signifies a decrease in pools or fluxes under drought conditions compared to non-drought soil. The sections in black within the C and N cycles signify pools or fluxes that were not considered in the previous meta-analysis (Cregger et al., 2014; Manzoni et al., 2014). Noteworthy components include SOC (soil organic C), TN (soil total N), TON (total organic N), DOC (dissolved organic C), DON (dissolved organic N), MBC (microbial biomass C), MBN (microbial biomass N), CM (C mineralization), Root-CO2 (root respiration), MicrCO2 (microbial respiration), Total-CO2 (total CO2 efflux from soil), NM (N mineralization), NN (nitrification), NDN (denitrification), and N2 + N2O (total N emission as N2 and N2O) (Deng et al., 2021).
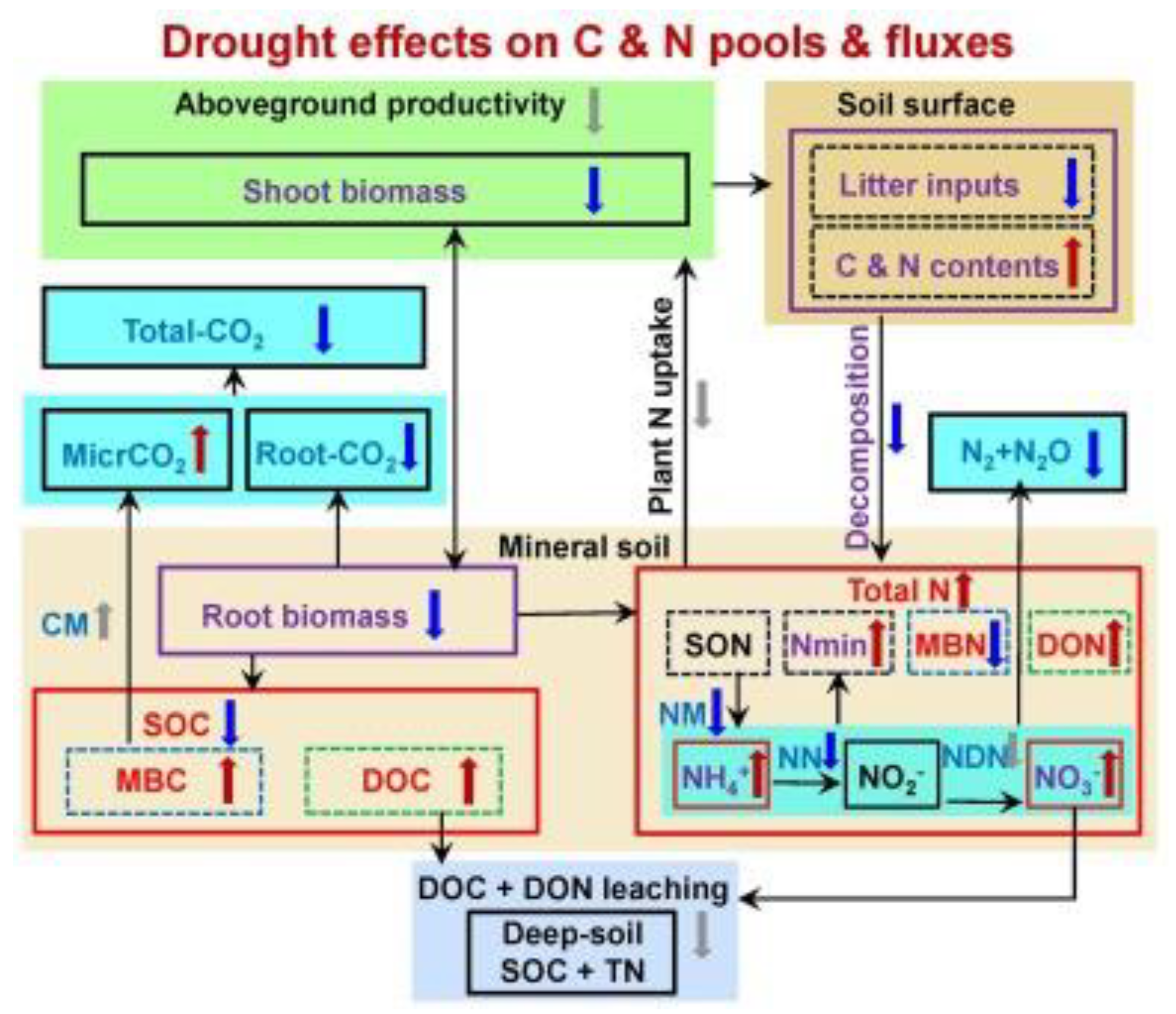
Soil N pools, plant N inputs from litter and roots into soils, soil N turnover processes, including mineralization, ammonification, nitrification and denitrification, N leaching, and N uptake by plants and microorganisms.
4.5. Soil pH
Drought can have a significant impact on soil pH, although the exact effects can vary depending on the soil type and the severity and duration of the drought. Drought can lead to buildup of salt in the soil since soil dries out via evapotranspiration, which can increase soil pH levels, making the soil more alkaline (Corwin, 2021). Also, during drought season, soil pH can be decreased in fertilized agricultural land since unavailability of up taken of nutrients by plants.
In dry soils, pH is high, due to drought stress in forest and Tundra of Western Sayan Mountains, Southern Siberia (Chytry et al., 2007). When the soil is unable to hold onto water, these organic acids are not retained in the soil and are instead leached out. This causes a decrease in the concentration of organic acids in the soil, which in turn reduces the acidity of the soil. Song et at. (2012) found that decrease in pH under water stress in the rhizosphere for drought tolerant crops still produce the exudates and increase the nutrient availability. DesRochers et al. (2007) found that the drought treatment only slightly reduced growth and rarely interacted with N source or soil pH.
5. Effect of Drought on Soil Microbial Structure
Drought can directly affect microbes by desiccation on resource limitation because substrate diffusion is reduced at low levels of soil moisture (Naylor and Coleman-Derr,2017). Drought experiments have reported decreases in microbial biomass and activity, reduction in carbon and nitrogen mineralization (Hueso et al.,2012), and accumulation of solutes, such as amino acids (in bacteria) and polyols (in fungi), which help prevent dehydration but are energetically expensive (Schimel et al.,2007). Drought may also have indirect effects, through interactions with plants, because plants can have species specific effects on rhizosphere microbiota mediated by rhizodeposits (Lareen et al., 2016). Severe drought conditions modify the microbial community structure, biomass, and activity in soils. Understanding how different fractions of the microbiome respond to drought stress is important for addressing how abiotic stress may shape crop-microbe interactions to influence agroecosystems resiliency. Evaluating how microbial community structure shifts immediately following drought events and tracking the amount of time it takes for communities to recover is important for improving our understanding of microbiome rescue.
As a disturbance event in ecological systems drought effects can be categorized as either a press or pulse disturbance, depending on its duration and frequency. Press disturbance can result in a prolonged water shortage, which can lead to selective mortality of certain plants and microorganisms that are unable to tolerate the prolonged water scarcity. The effects of drought on selective mortality and demographic and/or adaptive rescue depend on the severity, duration, and frequency of the drought. However, some species may be more adapted to survive under drought conditions and may be able to reproduce more successfully during periods of drought. This can lead to changes in the population structure of the ecosystem, with the more drought-tolerant species becoming more dominant. Additionally, some species may adapt to drought over time through adaptive rescue. This involves the evolution of traits that allow species to cope with prolonged drought conditions, such as the ability to store water, deeper roots, or the ability to reduce water loss through transpiration. Over time, such adaptations can increase the resilience of the ecosystem to future drought events.
Understanding how different fractions of the microbiome respond to drought stress is important for addressing how abiotic stress may shape crop-microbe interactions to influence agroecosystem resiliency. Evaluating how microbial community structure shifts immediately following drought events and tracking the amount of time it takes for communities to recover is important for improving our understanding of microbiome rescue.
Soil moisture is a main aspect that regulating microbe survival and activity, drought conditions have detrimental effects on soil biochemical properties since microorganisms that are not adapted to high water tension may survive under these unfavorable conditions. (Van Meeteren et al., 2008). The soil subjected to severe water stress could be responsible for the low microbial biomass due to the mechanisms of reduced diffusion of soluble substrates and/or reduced microbial mobility and consequent access to substrates. Not only physiology but the soil microbial community structure is also affected by the changing of water status. Several types of microorganisms being affected differently by changing the water potential due to the drought. Fungi were not affected by the drought compared to the bacteria (Williams and Rice,2007).
Because in some cases, changes in the soil moisture may have a minor impact on the microbial community composition. Dissimilar types of microorganisms are differentially affected by various and changing amounts of water potential (Drenovsky et al.,2004). For example, gram-negative type bacteria are thought to be more sensitive to dramatic changes in water potential, while fungi have been implicated as more tolerant of low water availability (Soderberg et al.,2004). While many management, plant and soil factors are important mediators of soil microbial community structure (Drenovsky et al.,2004).
The effect of drought stress on microbial communities can vary in several types of soils or under soil modifications. Severe drought conditions modify the microbial community structure in soils. However, their effect on the microbial structure is more significant in soils with low organic matter content. For example, controlled conditions of drought stress-induced changes in the relative abundances of phyla present in sandy and loamy soils which content low organic matters. Among six phyla, namely Actinobacteria, Bacteroidetes, Firmicutes, Planctomycetes, Protecobacteria, and Verrucomicribia that accounted for >95% of the total bacteria abundance, the Actinobacteria (especially genera Gaiella and Nocarioides) were most prevalent in analyzed samples. While relative abundance of Proteobacteria, Corticotrophs, and Verrucomicrobia decreased significantly (Siebielec et al.,2020).
Carbon and nitrogen availability is more important in relation to microbial growth. Microbial phyla such as Verrucomicrobia, Proteobacteria and Acidobacteria, which are sensitive to nitrogen ratios, and they are lost during drought because drought induced the reductions in labile carbon and nitrogen entering the rhizosphere.
Bacteria may be more sensitive due to their lifestyle strategies. Bacteria are less motile and more reliant on water availability to create channels and connect spaces for access to nutrients or exchange genetics (Sokol et al., 2022). Gram-positive bacterial lineages are drought resistant than Gram-negative lineages, due to their thicker cell walls (Schimel et al.,2007). This linked to the increase in root exudation by Q.ilex during increasing drought because Gram-negative bacteria preferentially consume this type of labile carbon source, where Gram-positive bacteria tend to consume more recalcitrant C sources (Naylor and Coleman-Derr,2017).
The impacts of drought on soil microbial biomass can be explained through direct effects mediated by the lower soil moisture in drought-affected plots, as observed here, but also by plant-mediated effects. Some studies indicated that drought reduced belowground C allocation by plants, which coincides with the lower survival of P. Other studies observed a decrease in microbial biomass because of drought (Bastida et al.,2017). Moreover, reduced soil moisture and pore connectivity in drought exposed plots would have limited the transfer of organic matter from plants to soil, and the formation of organo-mineral complexes. The combined analysis of the total and active microbial community composition allowed the ecological adaptations of phyla to climate change to be deciphered. A reduction in the C allocation in soils submitted to drought can limit the growth and activity of Proteobacteria, including copiotrophic taxa that are strongly related to ecosystem multifunctionality (Bastida et al., 2017). Actinobacteria is one of the most enriched bacterial taxa in drought -treated soils across a range of environments (Bouskill et al., 2013, 2016) and in drought-treated rhizospheres for several plant species (Nessner Kavamura et al., 2013). Actinobacteria can be enriched due to their spore-forming ability which allows them to enter a stable and inactive state during periods of environmental stress, which would lead them to persist under drought conditions (Nessner Kavamura et al., 2013). Some studies were carried out to find how Actinobacteria enriched within the plant root under drought and their enrichment patterns depend on the hosts. The study showed that they observed enrichment of Actinobacteria under drought are in more pronounced in root endosphere than the surrounding soil and further they provide evidence selection of different microorganisms under drought conditions correlated with the plant host evolutionary histories also (Dan Naylor et al., 2017).
Drought can decrease microbial bacterial biomass, and the magnitude of this effect can be intensified under nutrient-poor soils. In a study evaluating soils receiving organic amendments compared to soils receiving no amendments, the total PLFAs for gram-positive bacteria were significantly lower under drought stress, and this decline was more pronounced in unamended soils (Kohler et al.,2009). Similarly, gram-negative bacteria of the wheat rhizosphere were significantly lower under drought conditions, and the effect was more prominent in rhizospheric communities from Luvisol compared to Chenozem soils (Breitkreuz et al., 2021). Drought stress is contributing to decreases in bacterial biomass and persistence. This is due to soils with higher organic matter buffering declines in bacterial biomass.
Drought duration had a significant effect on the fungal: bacterial ratio, which increased with increasing drought. The evidence suggests that fungi are more relevant than bacteria to water stress
(Barnard et al.,2015). It is attributable to the chitinous cell walls of fungi, which should increase their resistance to environmental fluctuations, such as water stress (Holland and Coleman) and fungal hypal growth allowing them to cross small areas of dry soil. Fungi are thought to be more resilient and/or resistant to drought conditions than bacteria. In cases of spores that are produced by the fungi spent a dormancy period to tolerate the adverse climatic conditions like drought. Some evidence suggests that among fungi, yeasts may have a high tolerance to drought, because they tend to be more common in more extreme environments and tend to reproduce by building, which is generally a more stress tolerant strategy of reproduction (Treseder and Lennon,2015) Analysis of microbial diversity through co-occurrence networks highlights distinct patterns among bacterial communities compared to fungal communities (Franciska et al.,2018). Bacterial networks contain central or hub OTUs sensitive to drought conditions and serve as indicators for recovery after drought stress. In contrast, fungal communities have much more stable networks between drought conditions showing less sensitivity to drought stress and higher resiliency (Franciska et al.,2018).
Total fungal community and active microbiome were impacted by drought (). This is because that fungal community was more sensitive to the lowered soil moisture than bacterial community (Kaisermann et al., 2015). Different mechanisms can explain the distinct sesitivity of total bacterial and fungal communities to drought. For instance, Kaisermann et al. (2015) suggested that fungal community composition depends on non-extreme moisture conditions. As an example, the study Bastida et al. (2017) proved drought negatively affected the activity of Eurotiales and Hypocreales and the reduction in their activity was related to a lower MF. By indicating that some fungal populations could coexist by occupying different moisture niches (Kaisermann et al., 2015).
Microbial effects towards the plants can be neutral or positive, although soil feedback effects are negative, depending on the different microbial groups involved. In fact, plant species responses to environmental changes such as drought may also be controlled by soil microorganisms. For example, mycorrhizal fungi may be benefit to water-stressed plants by increasing access to soil water, improving plant hydraulics and gas exchange, whereas fungal pathogens may exacerbate plant vulnerability to drought (Aroca and RuizLozano, 2009; Kannenberg and Phillips, 2017).
Microbial structure can be varied with the different regions under drought condition. Below table present key microbial groups that can be found in the different regions.
| Region |
Bacterial Communities |
Fungal Communities |
References |
| Brazil (Caatinga semi-arid biome) |
Higher proportion of Gram-positive bacteria, represented by the phylum Actinobacteria and the genus Bacillus
|
|
Vanessa et al., 2013 |
| North America and Australia (similar climatic and edaphic conditions) |
Drought induced the changes in soil microbial community composition and structure rather than the changes in abundance and diversity. |
Lower abundance of a widely symbiotic, mycorrhizal-forming fungal class Glomeromycotan |
Ochoa et al., 2018 |
| |
Greater abundance of drought resistant bacterial taxa Actinobacteria and Chloroflexi |
|
|
6. Effect of Drought on Microbiome Functions
Changes in Growth, Activity, and Potential Nutrient Cycling
Microbiome structural changes are linked to shifts in microbial community function. Characterizing shifts in nutrient cycling, abundance of key genes or functional traits, and metabolic signature (e.g., phytohormones) is important for understanding how microbial communities modify their environment and influence crop growth under drought conditions.
Microorganisms decompose dead plant and microbe material, taking some material in for nutritional value for themselves, but also releasing what they do not need into the microbiome, where other microbes or plants can then uptake it. During droughts there is less labile carbon and nitrogen being released to the environment due to the microbial community as a whole being less active, as bacterial growth and decomposition of litter slows and some microbes become dormant or produce spores that will grow when water is available again (Kaurin et al. 2018). In studies of inoculation of sugarcane with Bacillus subtilis (Cassia da Fonseca, 2022) during drought the inoculated host plant had increased nitrogen (N), phosphorus (P), calcium (Ca), magnesium (Mg), and sulfur (S) along with chlorophyll a and b, total chlorophyl, and total cartenoids. Internal carbon dioxide concentration and carboxylation was double that of the non-inoculated plants under water restrictions. Synergism between B subtilis and the host improves the use of soil and fertilizer by plants, by inducing expression of stress response genes, phytohormones, and related metabolites, explaining the increased uptake of N, P, Mg, and S. Increased phosphorus solubilization was increased via acidification, chelation, and organic acid production. B subtilis was able to decrease chlorophyll degradation during drought possibly due to mitigating the drought stress on the plant and increasing the activity of antioxidant enzymes. Photosynthesis rates were increased in the inoculated plant possibly due to the increased leaf width (more surface area for absorbing light) and stomatal efficiency of carbon dioxide (increasing carboxylation efficiency). Having a larger photosynthetic active area may have contributed to the increased N, P, S, and Mg as B Subtilis stimulated plant hormone productions.
Drought causes microbiomes to biosynthesize secondary metabolites and sugars and increase pathways for osmolyte production. Drying soil has also shown shifts in microbial functional genes which increase transcripts, that increase nucleotide metabolism, adenylyl transferase, polyribonucleotide nucleotransferase, DNA-directed DNA polymerase, and DNA topoisomerase. An example of this is that transcription levels increase for trehalose gene production after soil drying, which includes transcripting for genes that encode trehalose phosphatase. Most taxa had higher levels of gene expression under dry conditions. Microbial metabolite profiles also shift with drought as there is an increase in sugars (simple and carboxyl acids) and sugar alcohols. Along with increased sugar metabolites, carbon metabolites are also increases which increases carbon fixation (Chowdhury, 2019).
In Bastida (2017), drought soils later in the growing season had higher abundances of Proteobacteria, Actinobacteria, Chloroflexi, and Nitrospirae than non-drought soils that early in the growing season were dominated by Acidobacteria. There were also changes in the fungal community.
Plant growth promoting rhizobacteria (PGPR) form symbiotic relationships with plant hosts and these relationships can help mitigate drought stress to the host. PGPR helps with bacterial N fixation (BNF), increases nitrate reductase, synthesis of phytohormones (auxins, cytokinins, gibberellins, ethylene), solubilizing phosphate, systematic resistance to drought, and tolerance to abiotic stress. Inoculating legumes with both Azospirillium and Rhizobia leads to early nodulation, which increases BNF rates, promoting plant growth and yield. This leads to protection against abiotic and biotic stresses, as the Azospirillium produced phytohormones that extended the root system of the plant and increased the root architecture. Improved root architecture increased the plants' ability for nutrient and water uptake and increased the enzyme expression, which is necessary for detoxing against reactive oxygen species (ROS). These microbes also produced secondary metabolites, which act on defense hormones and signals symbiosis for plant and microbe growth promotion (including rhizobia’s Nod factor, which establishes symbiosis with legumes) (Frietas, 2021).
Silva et al. (2023) studied inoculating soil with arbuscular mycorrhizal fungi (AMF), PGPR Bacillus subtilus, or both. AMF only inoculated soils with plants, showed increased AMF hyphae in severe drought and increased AMF vesicles in moderate drought soils, increasing root colonization. Phosphatase activity was changed during severe and moderate drought. Acidic phosphatase increased only in AMF only samples and decreased in PGPR only and AMF + PGPR samples. Alkaline phosphatase activity increased with PGPR only samples, although only slightly. But this phosphatase decreased drastically with AMF only and AMF + PGPR samples. All inoculants showed increased P uptake and nutrient acquisition due to the synergistic plant-microbe relationships.
AMF are recruited by plants through phytohormones and signaling molecules, like strigolactone, that are released into the rhizosphere. AMF will then grow to the plant roots and can modulate the plant roots to become highly plastic like AMF, which enhances water uptake and can help minimize water loss. The reduction of water lost during drought stress helps the plant to continue to grow. AMF’s increased hyphal network and glomalin secretion assists with water and nutrient uptake. Extra-radical hyphae increase the plant’s root architecture, root hydraulic conductivity, and photosynthetic rate (helps regulate stomatal conductance) (Badahur, 2019).
7. Future Needs and Approaches
Drought is one of the most significant climate hazards affecting agriculture worldwide. Its impact on crop yields can be severe, leading to food insecurity and economic losses for farmers. The effects of drought vary according to the different climatic zones around the world. For future needs, it is important to understand the variation of drought stress impacts on plant-soil relation within different climatic zones. However, the information about it is still limited and not available largely in all climatic zones. As such, it will be important to gather additional data on the structure and function of microbiomes associated with different crops in regions expected to experience heightened drought stress. For instance, in the United States, drought can cause significant yield losses for maize, soybeans, and wheat, which are the main crops grown in the country (Zipper et al., 2016; Manning et al., 2021). In Africa, drought can reduce the yield of staple crops such as maize, sorghum, and millet, which are crucial for food security in the region (IPCC, 2014; Sultan et al., 2019). In Asia, specifically in tropical countries like India and China drought linked to a decline yield of the most essential crops there like rice, maize, and soybean (Geng et al., 2014; Shew, 2016; Saud et al., 2022). In scale of global study for maize and wheat, Daryanto et al (2016) explained that the global yield production is reduced by 39.3% for maize and 20.6% for wheat at 40% water reduction. The sensitivity towards drought were found higher in corn either under dryland and non-dryland region. Meanwhile, wheat cultivation in the dryland region is more prone to the drought effects.
Based on the background and effects of drought stress that disturb the ecosystem stability and yield, it is crucial for understanding the process for maintaining the health and productivity of ecosystems in the long term. Drought can lead to a decrease in soil moisture, which can affect the microbial communities that are essential for nutrient cycling and plant growth. In addition, drought can alter the structure and diversity of microbial communities, which can have cascading effects on the entire ecosystem. Subsequently, the drought also affects the plant-soil feedback where each crop in certain region may have different coping mechanism during the extreme water stress condition. Lastly, to alter this issue, it is important to find out the effective agronomic strategies to reduce the negative impact of drought (Schimel et al., 2018; Seleiman et al., 2021).
Currently, while there is growing research on the influence of drought on microbiome structure, the impact of this stress on microbiome resiliency is poorly characterized. Studies that consider drought stress as pulse or press disturbances and aim to elucidate the responses of microbiomes across temporal scales are needed to better understand how abiotic stress can alter plant-soil-microbe interactions. This is particularly important in the context of plant-soil feedback, where the frequency, duration, and intensity of drought events contribute to changes in plant growth, soil characteristics, and microbial community dynamics which can feedback to shape crop growth and microbial ecology across spatio-temporal scale (Veresoglou et al., 2022; de Vries et al., 2023). Plant-soil feedback plays a crucial role in determining the ecosystem's resilience to drought stress. Drought stress can alter the composition of soil microbial communities, leading to changes in soil structure, nutrient cycling, and organic matter decomposition. These changes can, in turn, affect plant growth and development (Schimel et al., 2018). One of the rooms of improvement is to understand the specific mechanism on each crop, and further research is needed to elucidate the molecular and biochemical processes involved in plant-soil feedback (PSF) under drought stress. The information about the PSF on different soil types and different agricultural management practices is still limited, and it is deeper study to understand whether the PSF undergo similar complex and multiple mechanisms or not.
One of the key mechanisms of PSF involves the modulation of plant exudates, which can change the composition and function of the soil microbial community (Chen et al., 2022). Under drought stress, plants may release specific exudates that promote the growth of drought-tolerant microbial communities or inhibit the growth of pathogenic microbes, which can enhance plant growth and stress tolerance. Studying PSFs under drought conditions is critical for improving our understanding of ecosystem resiliency. Ecosystem resilience refers to the capacity of an ecosystem to recover from disturbances and maintain its structure and function. Understanding the mechanisms driving PSFs under drought stress can inform strategies for enhancing ecosystem resilience and productivity under water-limited conditions, which are expected to become more frequent and severe under climate change (Veresoglou et al., 2022; de Vries et al., 2023).
Under the changing environment due to water stress conditions, recent studies have identified specific microbial taxa that are adapted to drought conditions (Preece et al., 2019; Kristy et al., 2022). Despite these promising results, there is still a significant knowledge gap regarding the mechanisms underlying microbial adaptation to drought conditions. Further research is needed to determine the specific genetic and biochemical adaptations that enable these microbes to survive and thrive in water-limited environments. Understanding these mechanisms could help inform the development of strategies to enhance the resilience of soil microbial communities and maintain soil health under drought stress. Another critical knowledge gap is the need to study the long-term effects of drought on soil microbial communities. Most studies on the topic have focused on short- term drought stress, but in reality, drought conditions can persist for years or even decades in some regions. The long-term effects of drought on soil microbial communities, and how these effects may impact plant growth and productivity, remain poorly understood. Addressing this knowledge gap is essential for developing effective strategies to mitigate the impacts of drought on agricultural productivity and food security.
As the continuous effects of global warming will still occur and correspond to the drought stress, it is important to find the future directions and next steps to answer this issue. Previous research suggests that breeding and integrating drought tolerant varieties will be a key answer to address the drought stress issues. Drought tolerant crops have special coping mechanisms that able to enhance their growth and development, even under the scarcity water conditions. Moreover, there have been some agronomic practices that were done to reduce the negative effects of drought on soil, some of which are application of additional organic matter like by- products (sewage clugs, compost, biofertilizer) that help to increases soil C, soil enzymes activities, which later will be beneficial to improve, water holding capacity and aggregate stability in the soil. The organic matter that contains rhizosphere microbial communities like plant growth- promoting bacteria and fungi (PGPB and PGPF) will be more helpful to enhance the retain water in the soil. The technology of biomolecular, and DNA sequencing for the soil sample from the rhizosphere of crops under the healthy plants can be used to identify any potential microorganisms that help under the drought conditions (Solh and Ginkel, 2014; Mansoor et al., 2022).
References
- Abdelaal, K., AlKahtani, M., Attia, K., Hafez, Y., Király, L., & Künstler, A. (2021). The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology, 10(6), 520. [CrossRef]
- AghaKouchak, A., Farahmand, A., Melton, F. S., Teixeira, J., Anderson, M. C., Wardlow, B. D., & Hain, C. R. (2015). Remote sensing of drought: Progress, challenges and opportunities. Reviews of Geophysics, 53(2), 452-480. [CrossRef]
- Badahur, Ali. Batool, Asfa. Nasir, Fahad. Jiang, Shengjin. Mingsen, Qin. Zhang, Qi. Pan, Jianbin. Liu, Yongjun. Feng, Huyuan. (2019). Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. [CrossRef]
- Baik, J., Zohaib, M., Kim, U., Aadil, M., & Choi, M. (2019). Agricultural drought assessment based on multiple soil moisture products. Journal of Arid Environments, 167, 43-55. [CrossRef]
- Baldrian, P., Merhautová, V., Petránková, M., Cajthaml, T., & Šnajdr, J. (2010). Distribution of microbial biomass and activity of extracellular enzymes in a hardwood forest soil reflect soil moisture content. Applied Soil Ecology, 46(2), 177-182. [CrossRef]
- Barnard, R. L., Osborne, C. A., & Firestone, M. K. (2015). Changing precipitation pattern alters soil microbial community response to wet-up under a Mediterranean-type climate. The ISME journal, 9(4), 946-957.
- Berg, A., & Sheffield, J. (2018). Climate change and drought: the soil moisture perspective. Current Climate Change Reports, 4, 180-191. [CrossRef]
- Bogati, K., & Walczak, M. (2022). The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy, 12(1), 189. [CrossRef]
- Bouskill, N. J., Lim, H. C., Borglin, S., Salve, R., Wood, T. E., Silver, W. L., & Brodie, E.L. (2013). Pre-exposure to drought increases the resistance of tropical forest soil bacterial communities to extended drought. The ISME journal, 7(2), 384-394. [CrossRef]
- Breitkreuz, C., Herzig, L., Buscot, F., Reitz, T., & Tarkka, M. (2021). Interactions between soil properties, agricultural management and cultivar type drive structural and functional adaptations of the wheat rhizosphere microbiome to drought. Environmental Microbiology, 23(10), 5866-5882. [CrossRef]
- Cassia da Fonseca, Mariley de. Bossolani, Joao William. de Oliveira, Sirlene Lopes. Moretti, Luiz Gustavo. Portugal, Jose Roberto. Scudeletti, Daniele. de Oliveira, Elisa Fidencio. Cruscio, Carlos Alexandre Costa. (2022). Bacillus Subtilis Inoculation Improves Nutrient Uptake and Physiological Activity in Sugarcane under Drought Stress. [CrossRef]
- Chen, Y., Yao, Z., Sun, Y., Wang, E., Tian, C., Sun, Y., Liu, J., Sun, C., & Tian, L. (2022). Current Studies of the Effects of Drought Stress on Root Exudates and Rhizosphere Microbiomes of Crop Plant Species. International journal of molecular sciences, 23(4), 2374. [CrossRef]
- Chowdhury,Taniya Roy. Lee, Joon-Young. Bottos, Eric M. Brislawn, Colin J. White III, Richard Allen. Bramer, Lisa M. Brown, Joseph. Zucker, Jeremy D. Kim, Young-Mo. Jumponen, Ari. Rice, Charles W. Fansler, Sarah J. Metz, Thomas O. McCue, Lee Ann. Callister, Stephen J. Song, Hyun-Seob. Jansson, Janet K. (2019). Metaphenomic Responses of a Native Prairie Soil Microbiome to Moisture Perturbations. Msystems 4:e00061-19. [CrossRef]
- Chytrý, M., Danihelka, J., Ermakov, N., Hájek, M., Hájková, P., Kočí, M., ... & Valachovič, M. (2007). Plant species richness in continental southern Siberia: effects of pH and climate in the context of the species pool hypothesis. Global Ecology and Biogeography, 16(5), 668-678. [CrossRef]
- Corwin, D. L. (2021). Climate change impacts on soil salinity in agricultural areas. European Journal of Soil Science, 72(2), 842-862. [CrossRef]
- Daryanto, S., Wang, L., & Jacinthe, P. A. (2016). Global Synthesis of Drought Effects on Maize and Wheat Production. PloS one, 11(5), e0156362. [CrossRef]
- de Vries, F. T., Griffiths, R. I., Bailey, M., Craig, H., Girlanda, M., Gweon, H. S., & Bardgett, R. D. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nature communications, 9(1), 3033. https://www.nature.com/articles/s41467-018-05516-7. [CrossRef]
- de Vries, F., Lau, J., Hawkes, C., & Semchenko, M. (2023). Plant–soil feedback under drought: Does history shape the future? Trends in Ecology & Evolution. [CrossRef]
- Deng, L., Peng, C., Kim, D. G., Li, J., Liu, Y., Hai, X., ... & Kuzyakov, Y. (2021). Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Science Reviews, 214, 103501. [CrossRef]
- DesRochers, A., Van Den Driessche, R., & Thomas, B. R. (2007). The interaction between nitrogen source, soil pH, and drought in the growth and physiology of three poplar clones. Botany, 85(11), 1046-1057. [CrossRef]
- Drenovsky, R. E., Vo, D., Graham, K. J., & Scow, K. M. (2004). Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microbial ecology, 48, 424-430. [CrossRef]
- Freitas, Vanessa Fogaca de. Cerezini, Paula. Hungria, Mariangela. Nogueira, Marco Antonio. (2021). Strategies to deal with drought-stress in biological nitrogen fixation with soybean. Applied soil ecology, 172. https://doi.org/10.1016/j.apsoil.2021.104352. eng, S.M., Yan, D.H., Zhang, T.X., Weng, B.S., Zhang, Z.B., and Qin, T.L. 2014. Effect of drought stress on agriculture soil. Nat Hazards. 73. [CrossRef]
- Hanaka, A., Ozimek, E., Reszczy´ nska, E., Jaroszuk-´Sciseł, J., & Stolarz, M. (2021). Plant tolerance to drought stress in the presence of supporting bacteria and fungi: An efficient strategy in horticulture. Horticulturae 2021, 7, 390. [CrossRef]
- Hargrave, K. R., Kolb, K. J., Ewers, F. W., & Davis, S. D. (1994). Conduit diameter and drought-induced embolism in Salvia mellifera Greene (Labiatae). New Phytologist, 126(4), 695-705. [CrossRef]
- Hartmann, A. A., Barnard, R. L., Marhan, S., & Niklaus, P. A. (2013). Effects of drought and N-fertilization on N cycling in two grassland soils. Oecologia, 171, 705-717. [CrossRef]
- Holland, E. A., & Coleman, D. C. (1987). Litter placement effects on microbial and organic matter dynamics in an agroecosystem. Ecology, 68(2), 425-433. [CrossRef]
- Homyak, P. M., Allison, S. D., Huxman, T. E., Goulden, M. L., & Treseder, K. K. (2017). Effects of drought manipulation on soil nitrogen cycling: A meta-analysis. Journal of Geophysical Research: Biogeosciences, 122(12), 3260-3272. [CrossRef]
- Hueso, S., García, C., & Hernández, T. (2012). Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biology and Biochemistry, 50, 167-173. [CrossRef]
- Indoria, A. K., Sharma, K. L., & Reddy, K. S. (2020). Hydraulic properties of soil under warming climate. In Climate change and soil interactions (pp. 473-508). Elsevier.
- Intergovernmental Panel on Climate Change (IPCC). (2014). Climate Change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
- Intergovernmental Panel on Climate Change (IPCC). (2021) Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; MassonDelmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C.,Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021 in press.
- Kaisermann, A., Maron, P. A., Beaumelle, L., & Lata, J. C. (2015). Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Applied Soil Ecology, 86, 158-164. [CrossRef]
- Kaurin, A., Kihelic, R., Kastelec, D., Greman, H., Bru, D., Philippot, L., Suhadolc, M. 2018. Resilience of bacteria, archaea, fungi, and N-cycling microbial guilds under plough and conservation tillage, to agricultural drought. Soil biology and biochemistry, 120, 233-245. [CrossRef]
- Khan, M. N., Zhang, J., Luo, T., Liu, J., Ni, F., Rizwan, M., & Hu, L. (2019). Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiologiae Plantarum, 41, 1-13. [CrossRef]
- Khan, N., Ali, S., Shahid, M. A., Mustafa, A., Sayyed, R. Z., & Curá, J. A. (2021). Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells, 10(6), 1551. [CrossRef]
- Kristy, B., Carrell, A. A., Johnston, E., Cumming, J. R., Klingeman, D. M., Gwinn, K., Syring, K. C., Skalla, C., Emrich, S., & Cregger, M. A. (2022). Chronic drought differentially alters the belowground microbiome of drought-tolerant and drought- susceptible genotypes of populus trichocarpa. Phytobiomes Journal, 6(4), 317–330. [CrossRef]
- Landesman, W. J., & Dighton, J. (2010). Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biology and Biochemistry, 42(10), 1751-1758. [CrossRef]
- Lareen, A., Burton, F., & Schäfer, P. (2016). Plant root-microbe communication in shaping root microbiomes. Plant molecular biology, 90, 575-587. [CrossRef]
- Liu, F., Jensen, C. R., Shahanzari, A., Andersen, M. N., & Jacobsen, S. E. (2005). ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Science, 168(3), 831-836. [CrossRef]
- Manning, D., Burkhardt, J., Goemans, C., and Maas, A. 2021. An Analysis of the Impact of Drought on Agriculture, Local Economies, Public Health, and Crime Across the Western United States. Documents Retrieved April 26, 2023 from https://www.drought.gov/documents/analysis-impact-drought-agriculture-local- economies-public-health-and-crime-across.
- Mansoor, S., Khan, T., Farooq, I., Shah, L. R., Sharma, V., Sonne, C., Rinklebe, J., & Ahmad, P. (2022). Drought and global hunger: Biotechnological interventions in sustainability and management. Planta, 256(5). [CrossRef]
- Mukherjee, S., Mishra, A., & Trenberth, K. E. (2018). Climate change and drought: A perspective on drought indices. Current Climate Change Reports, 4(2), 145–163. [CrossRef]
- Naylor, D., DeGraaf, S., Purdom, E., & Coleman-Derr, D. (2017). Drought and host selection influence bacterial community dynamics in the grass root microbiome. The ISME journal, 11(12), 2691-2704. [CrossRef]
- Nessner Kavamura, V., Taketani, R. G., Lançoni, M. D., Andreote, F. D., Mendes, R., & Soares de Melo, I. (2013). Water regime influences bulk soil and rhizosphere of Cereus jamacaru bacterial communities in the Brazilian Caatinga biome. PloS one, 8(9), e73606. [CrossRef]
- Ochoa-Hueso, R., Collins, S. L., Delgado-Baquerizo, M., Hamonts, K., Pockman, W. T., Sinsabaugh, R. L., ... & Power, S. A. (2018). Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Global change biology, 24(7), 2818-2827. [CrossRef]
- Pérez Castro, S., Cleland, E. E., Wagner, R., Sawad, R. A., & Lipson, D. A. (2019). Soil microbial responses to drought and exotic plants shift carbon metabolism. The ISME journal, 13(7), 1776-1787. [CrossRef]
- Preece, C., Verbruggen, E., Liu, L., Weedon, J. T., & Peñuelas, J. (2019). Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biology and Biochemistry, 131, 28–39. [CrossRef]
- Preece, C., Verbruggen, E., Liu, L., Weedon, J. T., & Peñuelas, J. (2019). Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biology and Biochemistry, 131, 28-39. [CrossRef]
- Ragel, P., Raddatz, N., Leidi, E. O., Quintero, F. J., & Pardo, J. M. (2019). Regulation of K+ nutrition in plants. Frontiers in Plant Science, 10, 281.Hafez, Y., Attia, K., Alamery, S., Ghazy, A., Al-Doss, A., Ibrahim, E., ... & Abdelaal, K. (2020). Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy, 10(5), 630.
- Reddy, A. R., Chaitanya, K. V., & Vivekanandan, M. (2004). Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of plant physiology, 161(11), 1189-1202. [CrossRef]
- Saud, S., Wang, D., Fahad, S., Alharby, H. F., Bamagoos, A. A., Mjrashi, A., Alabdallah, N. M., AlZahrani, S. S., AbdElgawad, H., Adnan, M., Sayyed, R. Z., Ali, S., & Hassan, S. (2022). Comprehensive Impacts of Climate Change on Rice Production and Adaptive Strategies in China. Frontiers in microbiology, 13, 926059. [CrossRef]
- Schaeffer, S. M., Homyak, P. M., Boot, C. M., Roux-Michollet, D., & Schimel, J. P. (2017). Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biology and Biochemistry, 115, 54-62. [CrossRef]
- Schimel, J. P. (2018). Life in dry soils: Effects of drought on soil microbial communities and Processes. Annual Review of Ecology, Evolution, and Systematics, 49(1), 409–432. [CrossRef]
- Schimel, J., Balser, T. C., & Wallenstein, M. (2007). Microbial stress-response physiology and its implications for ecosystem function. Ecology, 88(6), 1386-1394. [CrossRef]
- Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., Dindaroglu, T., Abdul-Wajid, H. H., & Battaglia, M. L. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants, 10(2), 259. [CrossRef]
- Shao, H. B., Chu, L. Y., Jaleel, C. A., & Zhao, C. X. (2008). Water-deficit stress-induced anatomical changes in higher plants. Comptes rendus biologies, 331(3), 215-225. [CrossRef]
- Shew, A. M. (2016). Geospatial Analysis of Droughts, Rice and Wheat Production, and Agrarian Vulnerability: A District-Level Study of the self-calibrated Palmer Drought Severity Index in India. Graduate Theses and Dissertations Retrieved from https://scholarworks.uark.edu/etd/1458.
- Siebielec, S., Siebielec, G., Klimkowicz-Pawlas, A., Gałązka, A., Grządziel, J., & Stuczyński, T. (2020). Impact of water stress on microbial community and activity in sandy and loamy soils. Agronomy, 10(9), 1429. [CrossRef]
- Silva, Antonio M.M. Jones, Davey L. Chadwich, Dave R. Qi, Xue. Cotta, Simone R. Araujo, Victor L.V.P. Matteoli, Filipe P. Lacerda-Junior, Gileno V. Pereira, Arthur P.A. Fernandes-Junior, Paulo I. Cardoso, Elke J.B.N. (2023). Can arbuscular mycorrhizal fungi and rhizobacteria facilitate P uptake in maize plants under water stress. [CrossRef]
- Singh, B.K., Delgado-Baquerizo, M., Egidi, E., Guirado, E., Leach, J.E., Liu, H., and Trivedi, P. (2023). Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol, 1-17. [CrossRef]
- Söderberg, K.H., Probanza, A., Jumpponen, A., and Baath, E.. (2004). The microbial community in the rhizosphere determined by community-level physiological profiles (CLPP) and direct soil- and cfu PLFA techniques. Appl. Soil Ecol. 25, 135–145. [CrossRef]
- Sokol, N. W., Slessarev, E., Marschmann, G. L., Nicolas, A., Blazewicz, S. J., Brodie, E. L., ... & Pett-Ridge, J. (2022). Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nature Reviews Microbiology, 20(7), 415-430. [CrossRef]
- Solh, M., & van Ginkel, M. (2014). Drought preparedness and drought mitigation in the developing world׳s drylands. Weather and Climate Extremes, 3, 62–66. [CrossRef]
- Song, F., Han, X., Zhu, X., and Herbert, S. J. (2012). Response to water stress of soil enzymes and root exudates from drought and non-drought tolerant corn hybrids at different growth stages. Canadian Journal of Soil Science, 92(3), 501-507. [CrossRef]
- Sultan, B., Defrance, D., and Iizumi, T. (2019) Evidence of crop production losses in West Africa due to historical global warming in two crop models. Sci Rep, 9, 12834 (2019). [CrossRef]
- Sun, Y., Chen, H. Y., Jin, L., Wang, C., Zhang, R., Ruan, H., and Yang, J. (2020a). Drought stress induced increase of fungi: bacteria ratio in a poplar plantation. Catena, 193, 104607. [CrossRef]
- Sun, Y., Liao, J., Zou, X., Xu, X., Yang, J., Chen, H. Y., and Ruan, H. (2020b). Coherent responses of terrestrial C: N stoichiometry to drought across plants, soil, and microorganisms in forests and grasslands. Agricultural and Forest Meteorology, 292, 108104. [CrossRef]
- Treseder, K. K., & Lennon, J. T. (2015). Fungal traits that drive ecosystem dynamics on land. Microbiology and Molecular Biology Reviews, 79(2), 243-262. [CrossRef]
- Ullah, A., Nisar, M., Ali, H., Hazrat, A., Hayat, K., Keerio, A. A., ... & Yang, X. (2019). Drought tolerance improvement in plants: an endophytic bacterial approach. Applied Microbiology and Biotechnology, 103, 7385-7397. [CrossRef]
- Van Meeteren, M. J. M., Tietema, A., Van Loon, E. E., and Verstraten, J. M. (2008). Microbial dynamics and litter decomposition under a changed climate in a Dutch heathland. Applied Soil Ecology, 38(2), 119-127. [CrossRef]
- Wang, C., Sun, Y., Chen, H. Y., Yang, J., and Ruan, H. (2021). Meta-analysis shows non- uniform responses of above-and belowground productivity to drought. Science of the Total Environment, 782, 146901. [CrossRef]
- Williams, M. A., and Rice, C. W. (2007). Seven years of enhanced water availability influences the physiological, structural, and functional attributes of a soil microbial community. Applied Soil Ecology, 35(3), 535-545. [CrossRef]
- You, J., Zhang, Y., Liu, A., Li, D., Wang, X., Dossa, K., ... & Zhang, X. (2019). Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC plant biology, 19(1), 1-16. [CrossRef]
- Zipper, S. C., Qiu, J., and Kucharik, C. J. (2016). Drought effects on us maize and soybean production: Spatiotemporal patterns and historical changes. Environmental Research Letters, 11(9), 094021. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





