Submitted:
17 December 2024
Posted:
17 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Materials
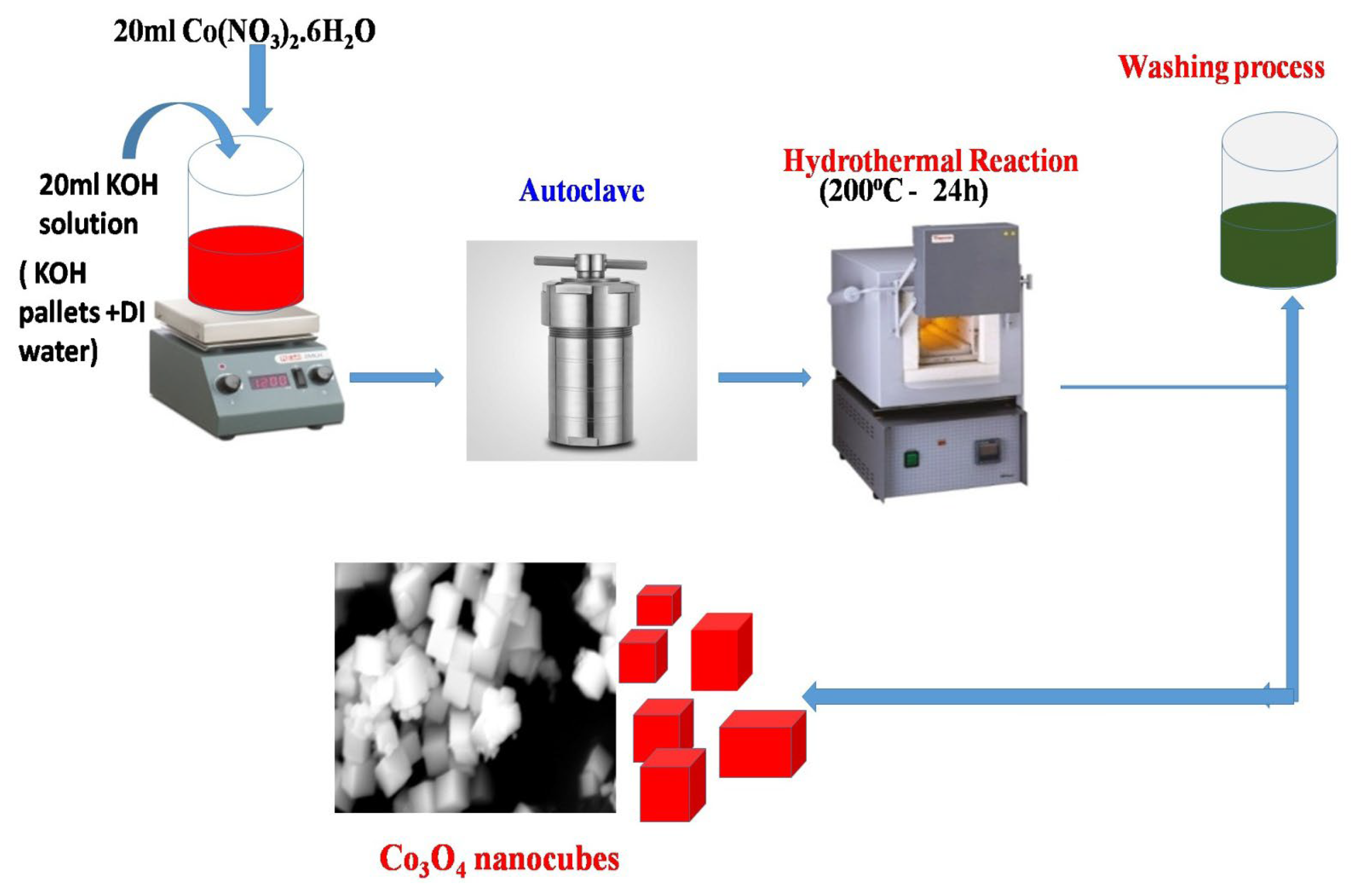
2.2. Synthesis Procedure
2.3. Microstructural and Electrochemical Measurements
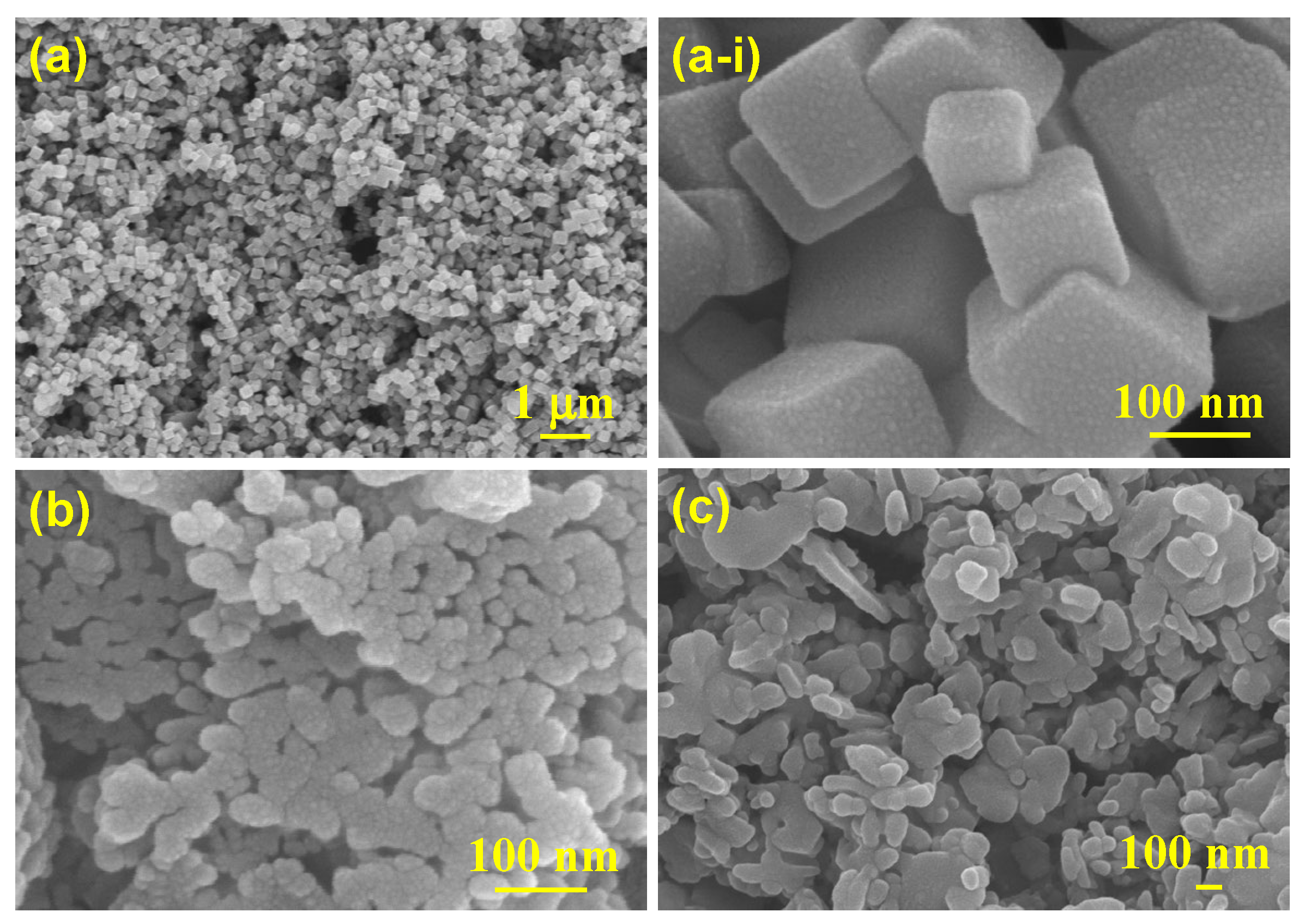
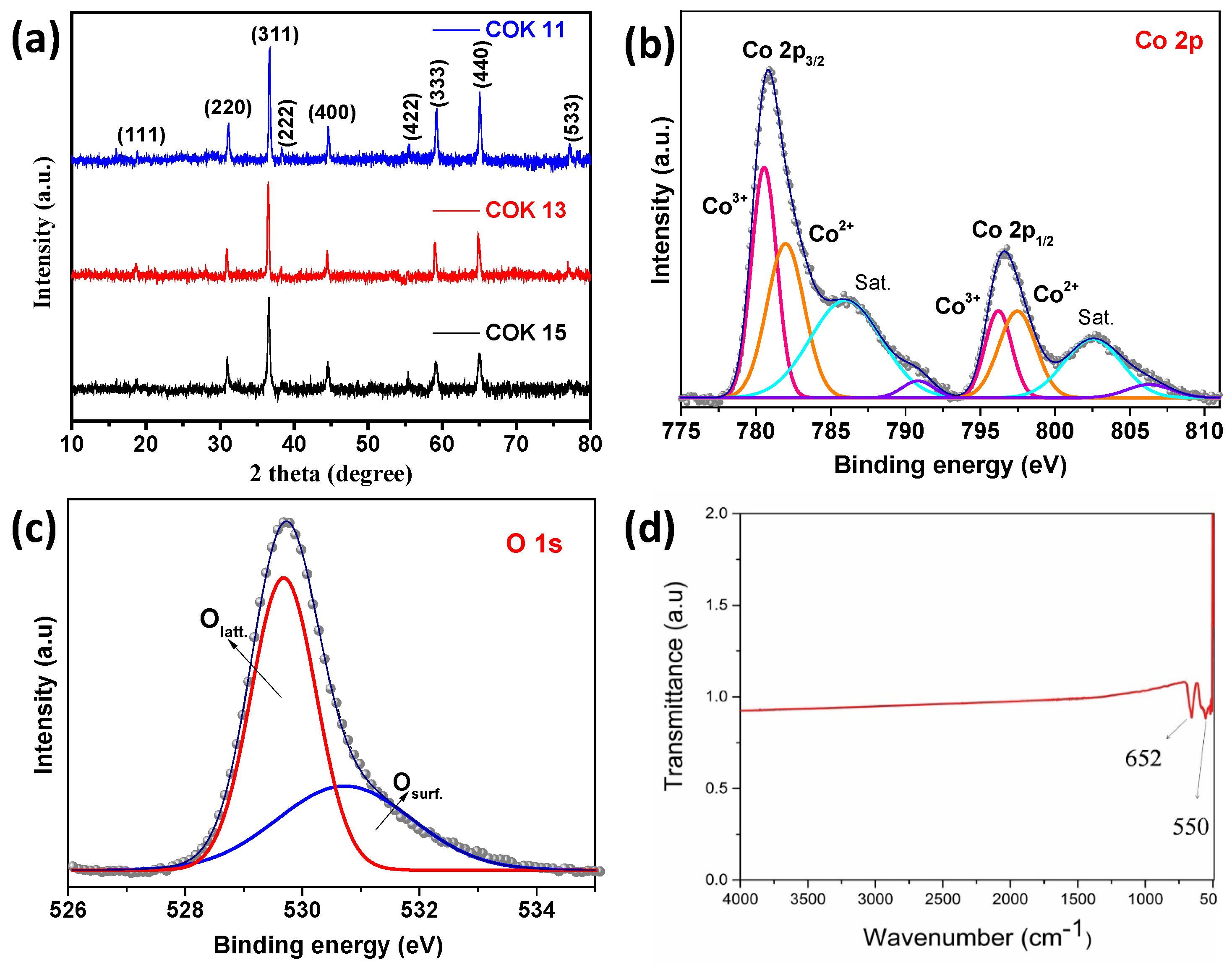
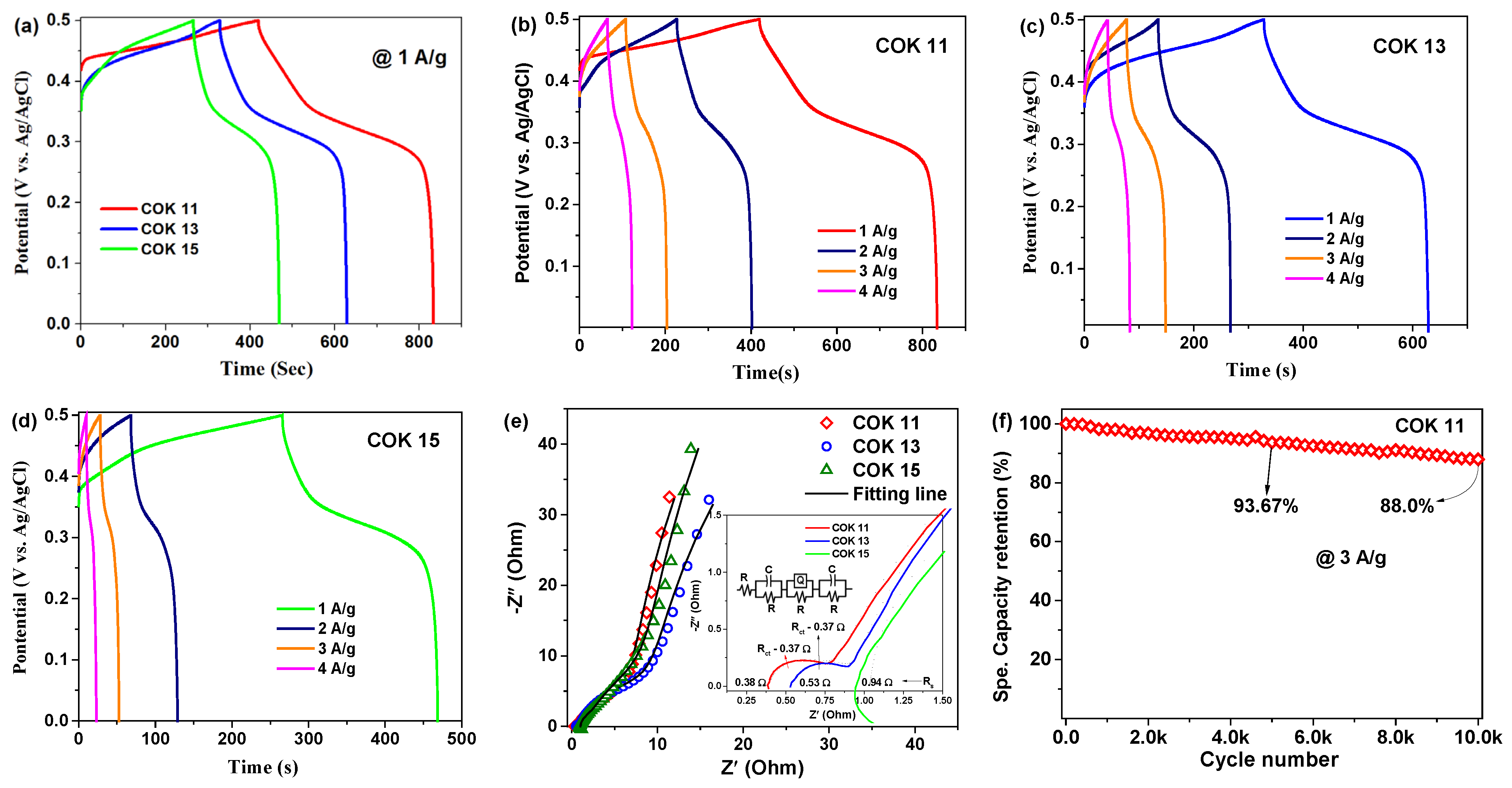
3. Results and Discussion
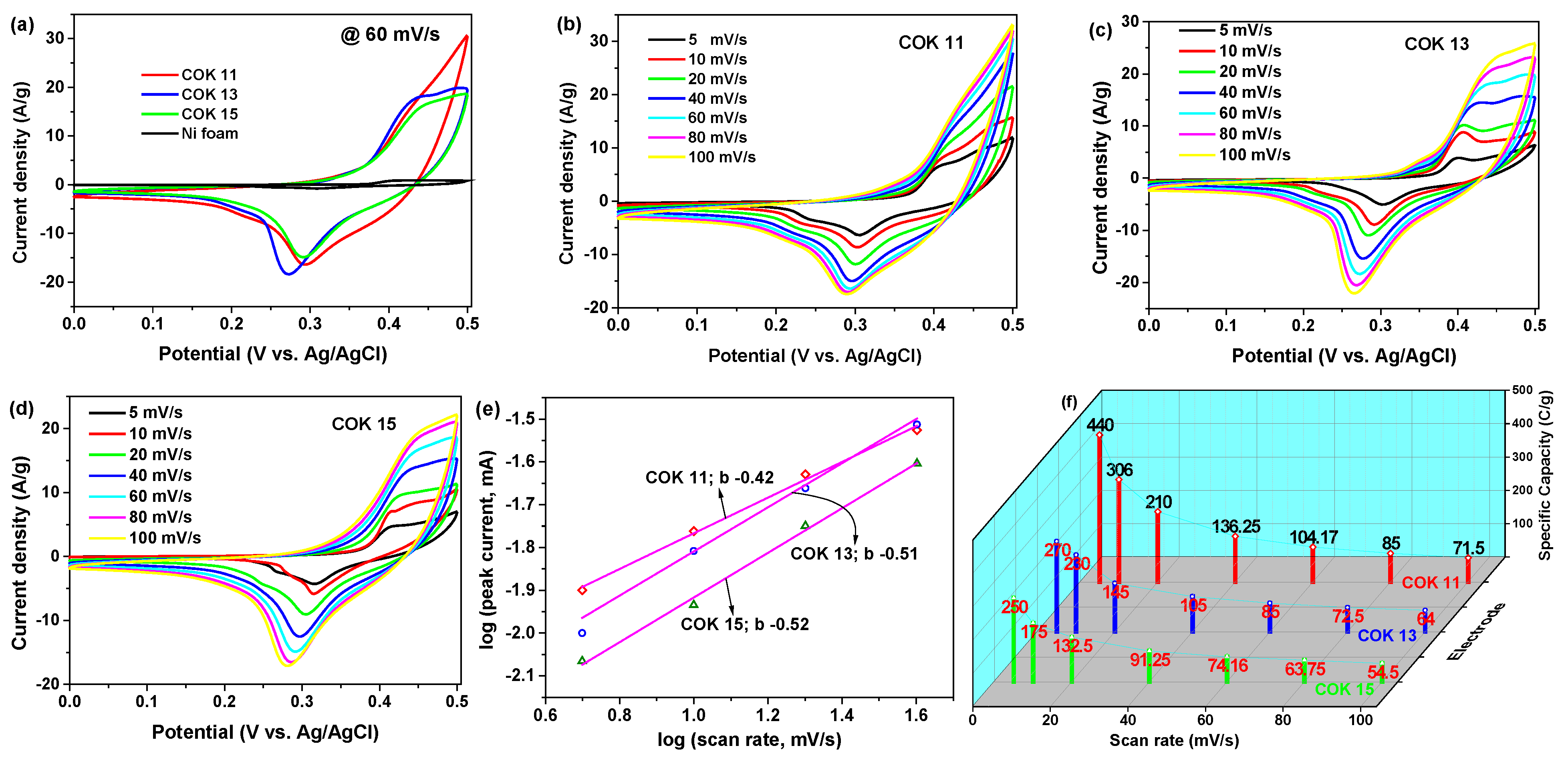
- where represents the integral area of the CV curve, ‘m’ is the mass of the active electrode material (in mg), and ‘ν’ is the scan rate (in mV/s). The calculated specific capacities of the COK 11 electrode were 440, 306, 210, 136, 104, 85, and 71.5 C/g at a scan rate of 5, 10, 20, 40, 60, 80, and 100 mV/s, respectively, as shown in Figure 4f. In comparison, the COK 13 and COK 15 electrodes exhibited lower specific capacities of 270, 230, 145, 105, 85, 72, and 64 C/g, and 250, 175, 132, 91, 74, 63, and 54 C/g, respectively, under the same scan rates.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-oxide Nanomaterials Synthesis and applications in flexible and wearable sensors. ACS Nanoscience Au 2022, 2, 64–92. [CrossRef]
- Goyal, M.; Singh, K.; Bhatnagar, N. Applications of nanomaterials for enhanced performance, and sustainability in energy storage devices: A review. ChemistrySelect 2024, 9, e202400543. [CrossRef]
- Singh, P.K.; Kaur, G.A.; Shandilya, M.; Rana, P.; Rai, R.; Mishra, Y.K.; Syväjärvi, M.; Tiwari, A. Trends in piezoelectric nanomaterials towards green energy scavenging nanodevices. Mater. Today Sustain. 2023, 24, 100583. [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and characterization of nanomaterials for application in cost-effective electrochemical devices. Sustainability 2023, 15, 10891. [CrossRef]
- Al-Mahmud, M.Z. A Concise Review of nanoparticles utilized energy storage and conservation. J. Nanomaterials 2023, 2023, 1–14. [CrossRef]
- Pérez Mendoza, A.E.; Schmidt, A.; Zarbin, A.J.G.; Winnischofer, H. Review of nanoscale approaches for tailoring electrode materials for advanced energy storage systems. ACS Appl. Nano Mater. 2024, 7, 23295–23320. [CrossRef]
- Kumar, N.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Recent advanced supercapacitor: A review of storage mechanisms, electrode materials, modification, and perspectives. Nanomaterials 2022, 12, 3708. [CrossRef]
- Ngidi, N.P.D.; Koekemoer, A.F.; Ndlela, S.S. Recent advancement in the electrochemical performance of electrochemical capacitors based on biomass-derived porous carbon: A review. J. Energy Storage 2024, 89, 111638. [CrossRef]
- Waris, M.S.; Chaudhary, A.H.; Anwer, S.; Sultana, P.P.; Ingole, S.A.A.; Nami, M.Z.; Khan, A. Review on development of carbon-based nanomaterials for energy storage devices: opportunities and challenges. Energy & Fuels 2023, 37, 19433–19460. [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M. Ramadass, K. Joseph, S. Benzigar, M. Karakoti, A. Yi, J. Kumar, P. Vinu, A. Recent advances in carbon-based electrodes for energy storage and conversion. Adv. Sci. 2023, 10, 2301045. [CrossRef]
- Jayakumar, S.; Santhosh, P.C.; Mohideen, M.M.; Radhamani, A.V. A comprehensive review of metal oxides (RuO2, Co3O4, MnO2 and NiO) for supercapacitor applications and global market trends. J. Alloys Compd. 2024, 976, 173170. [CrossRef]
- Pan, J.; Li, C.; Peng, Y.; Wang, L.; Li, B.; Zheng, G.; Song, M. Application of transition metal (Ni, Co and Zn) oxides based electrode materials for ion-batteries and supercapacitors. Int. J. Electrochem. Sci. 2023, 18, 100233. [CrossRef]
- Quispe-Garrido, V.; Cerron-Calle, G.A.; Bazan-Aguilar, A.; Ruiz-Montoya, J.G.; López, E.O.; Baena-Moncada, A.M. Advances in the design and application of transition metal oxide-based supercapacitors. Open Chem. 2021, 19, 709–725. [CrossRef]
- Tadesse, M.G.; Ahmmed, A.S.; Lübben, J.F. Review on conductive polymer composites for supercapacitor applications. J. Composites Sci. 2024, 8, 53. [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [CrossRef]
- Alcaraz-Espinoza, J.J.; de Melo, C.P.; de Oliveira, H.P. Fabrication of highly flexible hierarchical polypyrrole/carbon nanotube on eggshell membranes for supercapacitors. ACS Omega 2017, 2, 2866–2877. [CrossRef]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 2015, 9, 5310–5317. [CrossRef]
- Xu, W.; Li, T.-T.; Zheng, Y.-Q. Porous Co3O4 nanoparticles derived from a Co( ii )-cyclohexanehexacarboxylate metal–organic framework and used in a supercapacitor with good cycling stability. RSC Adv. 2016, 6, 86447–86454. [CrossRef]
- Wiegmann, T.; Pacheco, I.; Reikowski, F.; Stettner, J.; Qiu, C.; Bouvier, M.; Bertram, M.; Faisal, F.; Brummel, O.; Libuda, J.; Drnec, J.; Allongue, P.; Maroun, F.; Magnussen, O.M. Operando identification of the reversible skin layer on Co3O4 as a three-dimensional reaction zone for oxygen evolution. ACS Catalysis 2022, 12, 3256–3268. [CrossRef]
- Velhal, N.B. Yun, T.H. Ahn, J. Kim, T. Kim, J. Yim, C. Tailoring cobalt oxide nanostructures for stable and high-performance energy storage applications. Ceram. Int. 2023, 49, 4889–4897. [CrossRef]
- Chen, M.; Ge, Q.; Qi, M.; Liang, X.; Wang, F.; Chen, Q. Cobalt oxides nanorods arrays as advanced electrode for high performance supercapacitor. Surf. Coatings Technol. 2019, 360, 73–77. [CrossRef]
- Kunhikrishnan, L.; Shanmugham, R. High electrochemical performance of morphologically controlled cobalt oxide for supercapacitor application. Mater. Character. 177 2021, 177, 111160. [CrossRef]
- Xu, J.; Gao, L.; Cao, J.; Wang, W.; Chen, Z. Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 2010, 56, 732–736. [CrossRef]
- More, S.; Joshi, B.; Khadka, A.; Samuel, E.; Il Kim, Y.; Aldalbahi, A.; El-Newehy, M.; Gurav, K.; Lee, H.-S.; Yoon, S.S. Oriented attachment of carbon/cobalt-cobalt oxide nanotubes on manganese-doped carbon nanofibers for flexible symmetric supercapacitors, Appl. Surf. Sci. 2023, 615, 156386. [CrossRef]
- Kalpana, S.; Bhat, V.S.; Hegde, G.; Niranjana Prabhu, T. Anantharamaiah, P.N.Morphology-dependent supercapacitive properties of Co3O4 nanomaterials synthesized via coprecipitation and hydrothermal methods. Inorg. Chem. Commun. 2023, 158, 111458. [CrossRef]
- Desai, R.S.; Jadhav, V.S.; Morankar, P.J.; Patil, S.B.; Sadale, S.B.; Pardeshi, S.R.; Lad, D.D.; Patil, P.S.; Jeon, C.-W.; Dalavi, D.S. Hydrothermal synthesis of self-supported hierarchical microflowers of Co3O4 nanowires for potential supercapacitor application. J. Electroanal. Chem. 2025, 976, 118800. [CrossRef]
- Wei, G.; Yan, L.; Huang, H.; Yan, F.; Liang, X.; Xu, S.; Lan, Z.; Zhou, W.; Guo, J. The hetero-structured nanoarray construction of Co3O4 nanowires anchored on nanoflakes as a high-performance electrode for supercapacitors. Appl. Surf. Sci. 2021, 538, 147932. [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [CrossRef]
- Lu, C.; Liu, L.; Yang, Y.; Ma, Y.; Luo, Q.; Zhu, M. Recent progress in Co3O4-based nanomaterials for supercapacitors. ChemNanoMat 2023, 9, e202200537. [CrossRef]
- Deori, K.; Ujjain, S.K.; Sharma, R.K.; Deka, S. Morphology Controlled Synthesis of Nanoporous Co3O4 nanostructures and their charge storage characteristics in Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10665–10672. [CrossRef]
- Shwetha, K.P.; Manjunatha, C.; Sudha Kamath, M.K.; Vinaykumar; Radhika, M.G.R.; Khosla, A. Morphology-controlled synthesis and structural features of ultrafine nanoparticles of Co3O4 : An active electrode material for a supercapacitor. Appl. Res. 2022, 1, e202200031. [CrossRef]
- Babu, C.R.; Avani, A.V.; Shaji, S.; Anila, E.I. Electrochemical characteristics of Co3O4 nanoparticles synthesized via the hydrothermal approach for supercapacitor applications, J. Solid State Electrochem. 2024, 28, 2203–2210. [CrossRef]
- Nare, R.K. Ramesh, S. Basavi, P.K. Kakani, V. Bathula, C. Yadav, H.M. Dhanapal, P.B. Kotanka, R.K.R. Pasupuleti, V.R. Sonication-supported synthesis of cobalt oxide assembled on an N-MWCNT composite for electrochemical supercapacitors via three-electrode configuration. Sci. Rep. 2022, 12, 1998. [CrossRef]
- Tao, Y.; Wu, Y.; Chen, H.; Chen, W.; Wang, J.; Tong, Y.; Pei, G.; Shen, Z.; Guan, C. Synthesis of amorphous hydroxyl-rich Co3O4 for flexible high-rate supercapacitor. Chem. Eng. J. 2020, 396, 125364. [CrossRef]
- Hao, P.; Peng, B.; Shan, B.-Q.; Yang, T.-Q.; Zhang, K. Comprehensive understanding of the synthesis and formation mechanism of dendritic mesoporous silica nanospheres. Nanoscale Adv. 2020, 2, 1792–1810. [CrossRef]
- Quirk, J.; Rothmann, M.; Li, W.; Abou-Ras, D.; McKenna, K.P. Grain boundaries in polycrystalline materials for energy applications: First principles modeling and electron microscopy. Appl. Phys. Rev. 2024, 11, 011308. [CrossRef]
- Sun, J.; Wang, H.; Li, Y.; Zhao, M. Porous Co3O4 column as a high-performance Lithium anode material. J. Porous Mater. 2021, 28, 889–894. [CrossRef]
- Wei, Z.; Xia, T.; Ma, J.; Feng, W.; Dai, J.; Wang, Q.; Yan, P. Investigation of the lattice expansion for Ni nanoparticles. Mater. Character. 2007, 58, 1019–1024. [CrossRef]
- Merum, D.; Nallapureddy, R.R.; Pallavolu, M.R.; Mandal, T.K.; Gutturu, R.R.; Parvin, N.; Banerjee, A.N.; Joo, S.W. Pseudocapacitive performance of freestanding Ni3V2O8 nanosheets for high energy and power density asymmetric supercapacitors. ACS Appl. Energy Mater. 2022, 5, 5561–5578. [CrossRef]
- Nasiri, S.; Rabiei, M.; Palevicius, A.; Janusas, G.; Vilkauskas, A.; Nutalapati, V.; Monshi, A. Modified Scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano Trends 2023, 3, 100015. [CrossRef]
- Supriya, S.; Das, S.; Senapati, S.; Naik, R. Cu2Te/CoTe nanoparticles with tuneable bandgaps: Implications for photovoltaic and optoelectronic devices. Surfaces and Interfaces 2024, 44, 103823. [CrossRef]
- Adesuji, E.T.; Guardado-Villegas, E.; Fuentes, K.M.; Sánchez-Domínguez, M.; Videa, M. Pt-Co3O4 superstructures by one-pot reduction/precipitation in bicontinuous microemulsion for electrocatalytic oxygen evolution reaction. Catalysts 2020, 10, 1311. [CrossRef]
- Cole, K.M.; Kirk, D.W.; Thorpe, S.J. Co3O4 nanoparticles characterized by XPS and UPS. Surf. Sci. Spectra 2021, 28, 014001. [CrossRef]
- Daza-Gómez, L.; Pérez Salas, K.Y.; Ruiz-Huerta, L.; García Peña, N.G.; Maturano Rojas, V.; Redón, R. Co3O4 @SiO2 3D monolith catalysts, additive manufactured structures for propane oxidation reaction. ChemistrySelect 2024, 9, e202304849. [CrossRef]
- Murugesan, R.A.; Chandar Nagamuthu Raja, K.; Devi, N.; Lin, H.-T.; Huang, C.-C.; Jiang, X.-Y.; Li, Y.-Y.; Arthanareeswaran, G.; Ponvijayakanthan, L.; Jaiswal, N.K.; Chen, Y.-S. Development of Ni-doped Co3O4 oxygen evolution catalysts for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 2024, 72, 677–686. [CrossRef]
- Urgunde, A.B.; Kamboj, V.; Kannattil, H.P.; Gupta, R. Layer-by-layer coating of cobalt-based ink for large-scale fabrication of OER electrocatalyst. Energy Technol. 2019, 7, 1900603. [CrossRef]
- Makhlouf, S.A.; Bakr, Z.H.; Aly, K.I.; Moustafa, M.S. Structural, electrical and optical properties of Co3O4 nanoparticles. Superlattices and Microstructures 2013, 64, 107–117. [CrossRef]
- Al-Senani, G.M.; Deraz, N.M.; Abd-Elkader, O.H. Magnetic and characterization studies of CoO/Co3O4 nanocomposite. Processes 2020, 8, 844. [CrossRef]
- Guragain, D.; Zequine, C.; Gupta, R.K.; Mishra, S.R. Facile synthesis of bio-template tubular MCo2O4 (M = Cr, Mn, Ni) microstructure and its electrochemical performance in aqueous electrolyte. Processes 2020, 8, 343. [CrossRef]
- Yamada, H.; Yoshii, K.; Asahi, M.; Chiku, M.; Kitazumi, Y. Cyclic voltammetry Part 2: Surface adsorption, electric double layer, and diffusion layer. Electrochemistry 2022, 90, 22–66084. [CrossRef]
- Merum, D.; Ambadi, L.N.; Mahammad, H.O.; Pallavolu, M.R.; Goddati, M.; Lee, J.; Al-Asbahi, B.A.; Pitcheri, R.; Banerjee, A.N.; Joo, S.W. Direct growth of cobalt-doped nickel vanadate shelf-like architectures on Ni foam electrodes for solid-state alkaline battery. J. Alloys Compd. 2023, 950, 169771. [CrossRef]
- Merum, D.; Arla, S.K.; Radhalayam, D.; Tighezza, A.M.; Mooni, S.P.; Joo, S.W. Garland-structured Bi2O2CO3@Ni(OH)2 as a battery-type electrode for high-performance electrochemical energy storage device applications. J. Energy Storage 2024, 99, 113189. [CrossRef]
- Thonge, P.N.; Dhas, S.D.; Waghmare, S.D.; Patil, A.H.; Patil, T.M.; Yewale, M.A.; Mendhe, A.C.; Kim, D. Facile hydrothermal synthesis of NiMn2O4/C nanosheets for solid-state asymmetric supercapacitor and electrocatalytic oxygen evolution reaction. ACS Appl. Nano Mater. 2024, 7, 18579–18589. [CrossRef]
- Sanayee, M.; Arvand, M. Synthesis and electrochemical properties of nanocubes Mn2SnS3 for high-performance supercapacitors. Sci. Rep. 2023, 13, 20838. [CrossRef]
- Merum, D.; Parvin, N.; Vattikuti, S.V.P.; Nallapureddy, R.R.; Pitcheri, R.; Shkir, M.; Manthrammel, M.A.; Banerjee, A.N.; Joo, S.W. Impact of Co-doping on the microstructural and electrochemical features of mesoporous 3D oval–shaped Ni3-xCoxV2O8 electrodes for high-performance hybrid supercapacitors. J. Energy Storage 2023, 61, 106674. [CrossRef]
- Chen, X.; Zhuang, Y. Sacrificial template synthesis of hollow-structured NiCoP microcubes as novel electrode materials for asymmetric supercapacitors. Dalton Trans. 2022, 51, 16017–16026. [CrossRef]
- Sethi, M.; Shenoy, U.S.; Bhat, D.K. A porous graphene–NiFe2O4 nanocomposite with high electrochemical performance and high cycling stability for energy storage applications. Nanoscale Adv. 2020, 2, 4229–4241. [CrossRef]
- Dhananjaya, M.; Lakshmi Narayana, A.; Guru Prakash, N.; Rosaiah, P.; Hussain, O.M. Intertwining network structured VnO2n+1-CNT/GO nanocomposite electrodes for supercapacitors. Mater. Chem. Phys. 2019, 237, 121825. [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy─A tutorial. ACS Measurement Sci. Au. 2023, 3, 162–193. [CrossRef]
- Dhananjaya, M.; Guru Prakash, N.; Lakshmi Narayana, A.; Hussain, O.M. Electrochemical performance of nanocrystalline vanadium pentoxide thin films grown by RF magnetron sputtering. J. Electronic Mater. 2020, 49, 1922–1934. [CrossRef]
- Selvarajan, R.; Vadivel, S.; Saranya, A.; Baraneedharan, P.; Jayavel, R. Facile synthesis of rGO@ CoO nanocomposites electrode material for photocatalytic hydrogen generation and supercapacitor applications. Inorg. Chem. Commun. 2022, 139, 109345. [CrossRef]
- Zha, X.; Wu, Z.; Cheng, Z.; Yang, W.; Li, J.; Chen, Y.; He, L.; Zhou, E.; Yang, Y. High performance energy storage electrodes based on 3D Z-CoO/RGO nanostructures for supercapacitor applications. Energy 2021, 220, 119696. [CrossRef]
- Al-Jahdaly, B.A.; Abu-Rayyan, A.; Taher, M.M.; Shoueir, K. Phytosynthesis of Co3O4 nanoparticles as the high energy storage material of an activated carbon/Co3O4 symmetric supercapacitor device with excellent cyclic stability based on a Na2SO4 aqueous electrolyte. ACS Omega 2022, 7, 23673–23684. [CrossRef]

| Electrode material | Electrolyte | Specific capacity/capacitance (C/g or F/g) |
Current density (A/g) |
Cycling stability | Ref. |
|---|---|---|---|---|---|
| Co3O4 nanorod arrays | 3M KOH | 154.9 C/g (387.25 F/g) | 1 | 88% after 1000 cycles at 1 A/g | [21] |
| Hexagonal platelet Co3O4 particles | 2M KOH | 476 F/g | 0.5 | 82% after 2000 cycles at 2.5 A/g | [30] |
| Co3O4 nanoparticles | 2M KOH | 166 F/g | 0.5 | 90% after 5000 cycles at 5 A/g | [31] |
| Co3O4 nanoparticles | 3M KOH | 450 F/g | 1 | 88% over 10000 cycles at 20 A/g | [32] |
| Co3O4@N-MWCNT | 3M KOH | 225 F/g | 0.5 | 97.8% after 5000 cycles at 0.5 A/g | [33] |
| Hydroxyl-rich Co3O4 | 1M KOH | 226.1 C/g | 1.3 | 77% after 5000 cycles at 5 mA cm-2 | [34] |
| CoO/rGO nanocomposite | 1M KOH | 592 F/g | 2 | 90% after 3000 cycles at 5 A/g | [61] |
| ZIF-67-CoO/rGO | 6M KOH | 275 F/g | 1 | -- | [62] |
| AC/Co3O4 nanoparticles | 1M Na2SO4 | 182 F/g | 1 | 99.6% over 6000 cycles at 2.5 A/g | [63] |
| COK 11 or cobalt oxide nanocubes | 3M KOH | 412.8 C/g (825.6 F/g) | 1 | 88% after 10000 cycles @ 3 A/g | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




