Submitted:
12 December 2024
Posted:
13 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
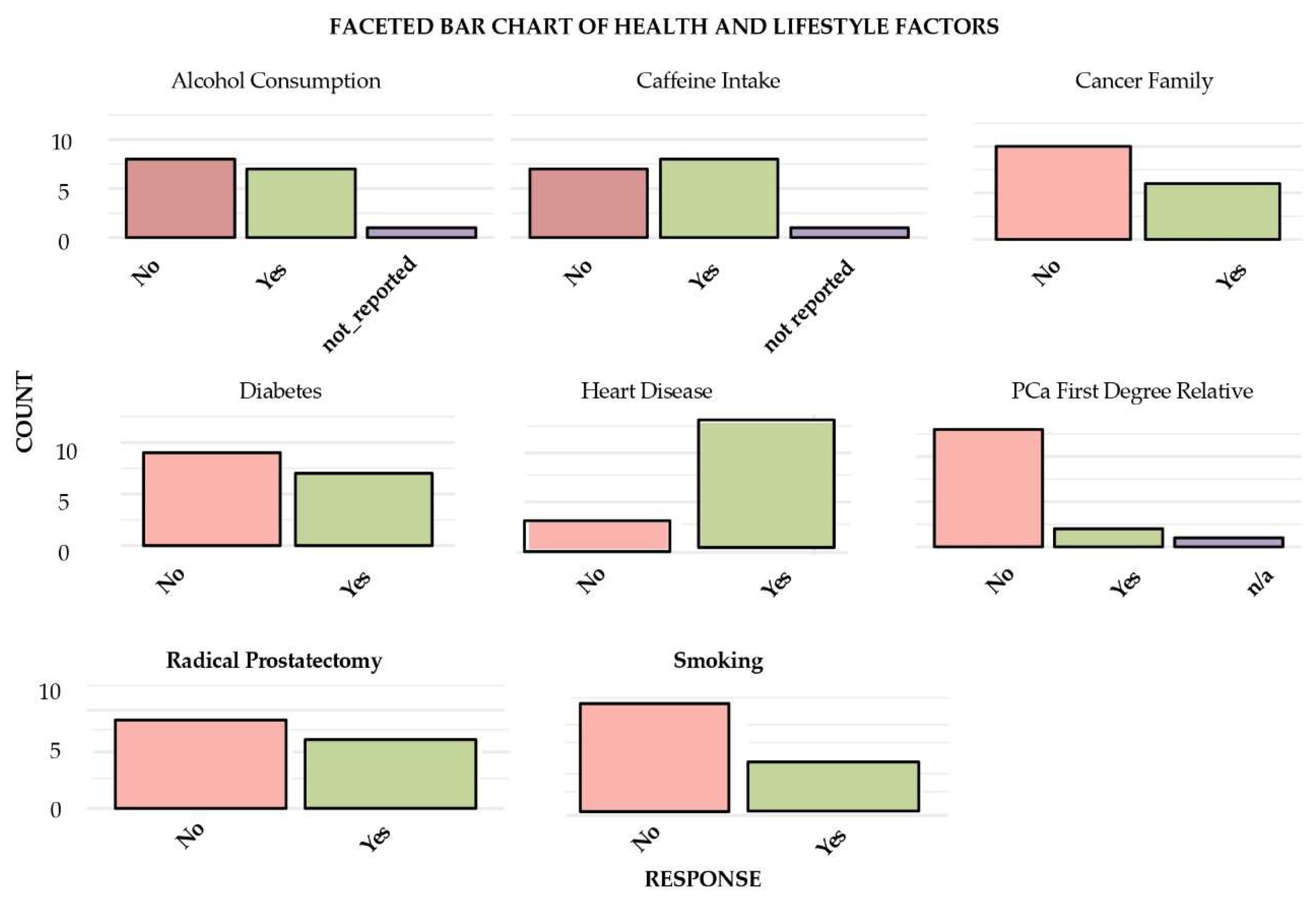
3.1. Clinicopathological Characteristics of Patients with mCRPCa
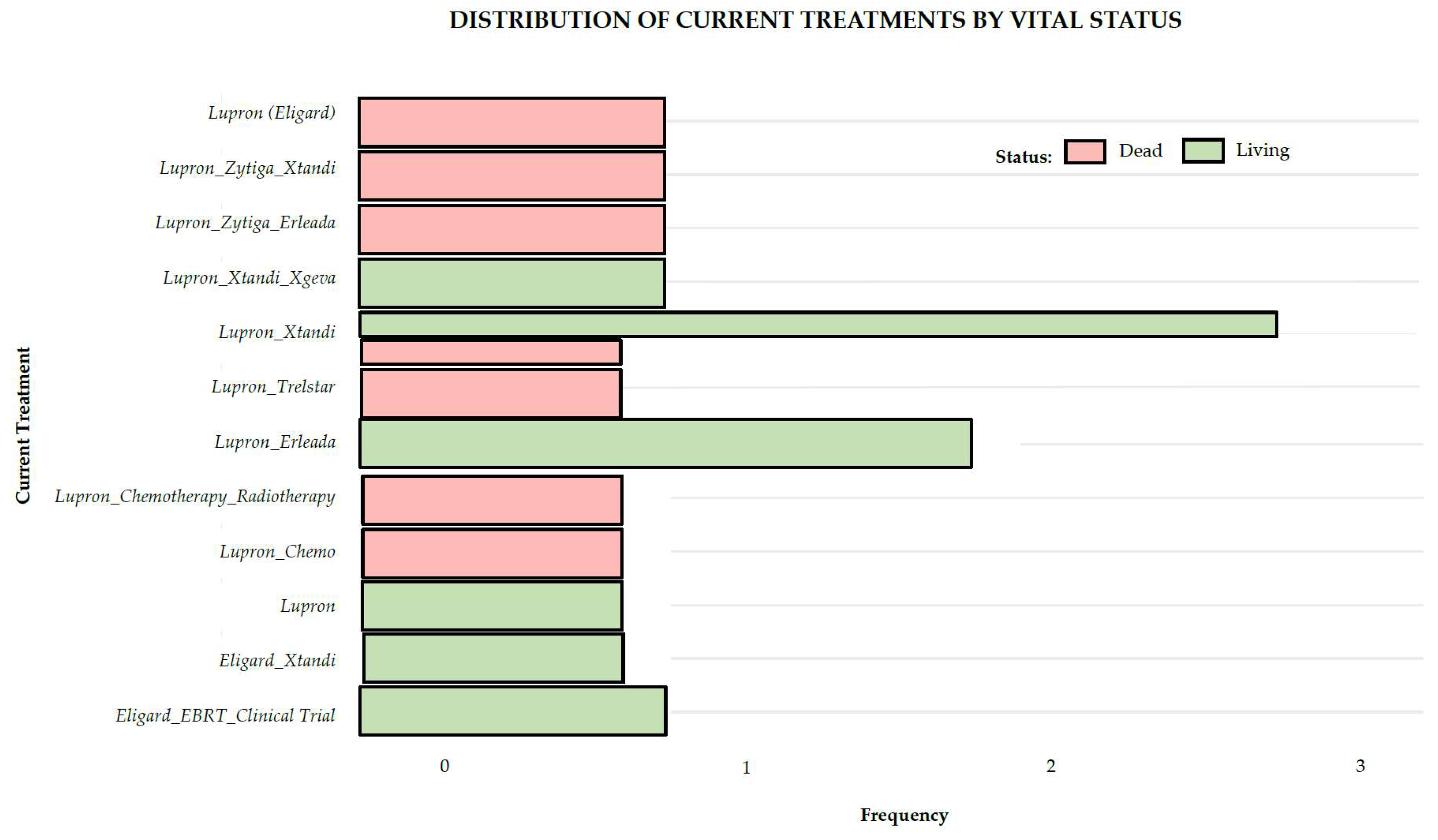
3.2. Treatment
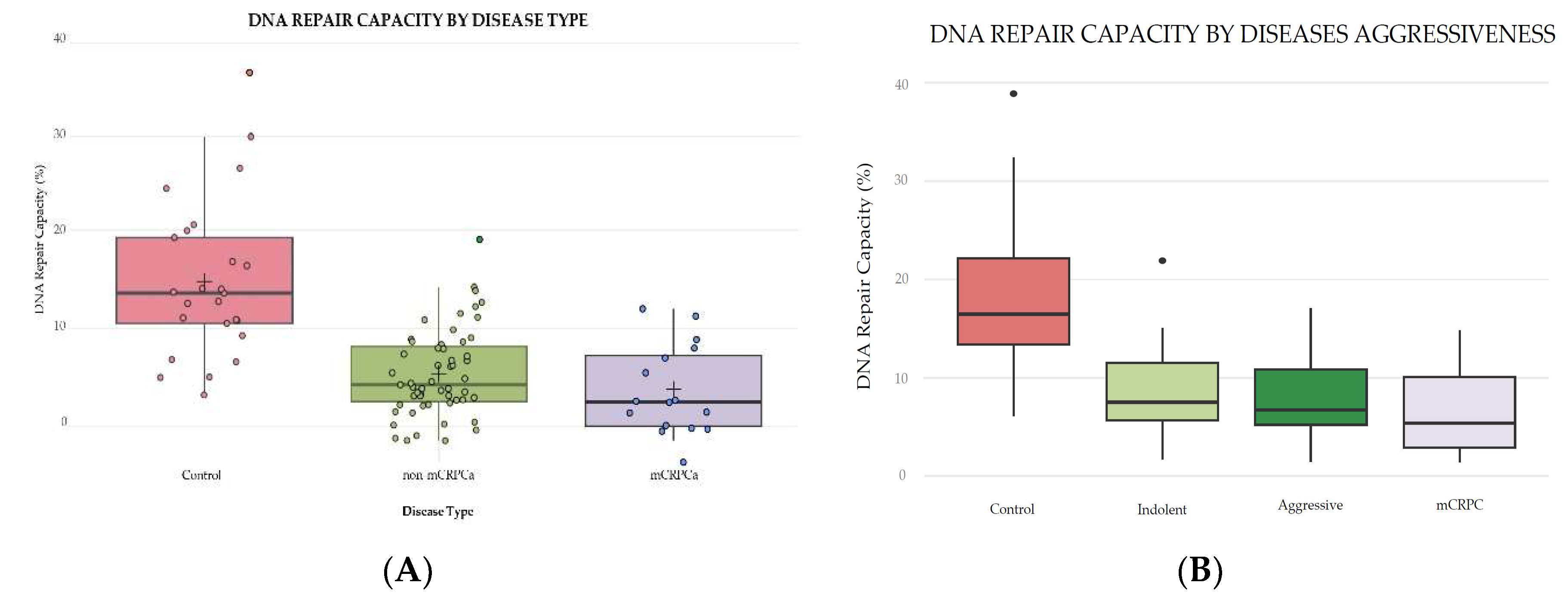
3.3. DNA Repair Analysis
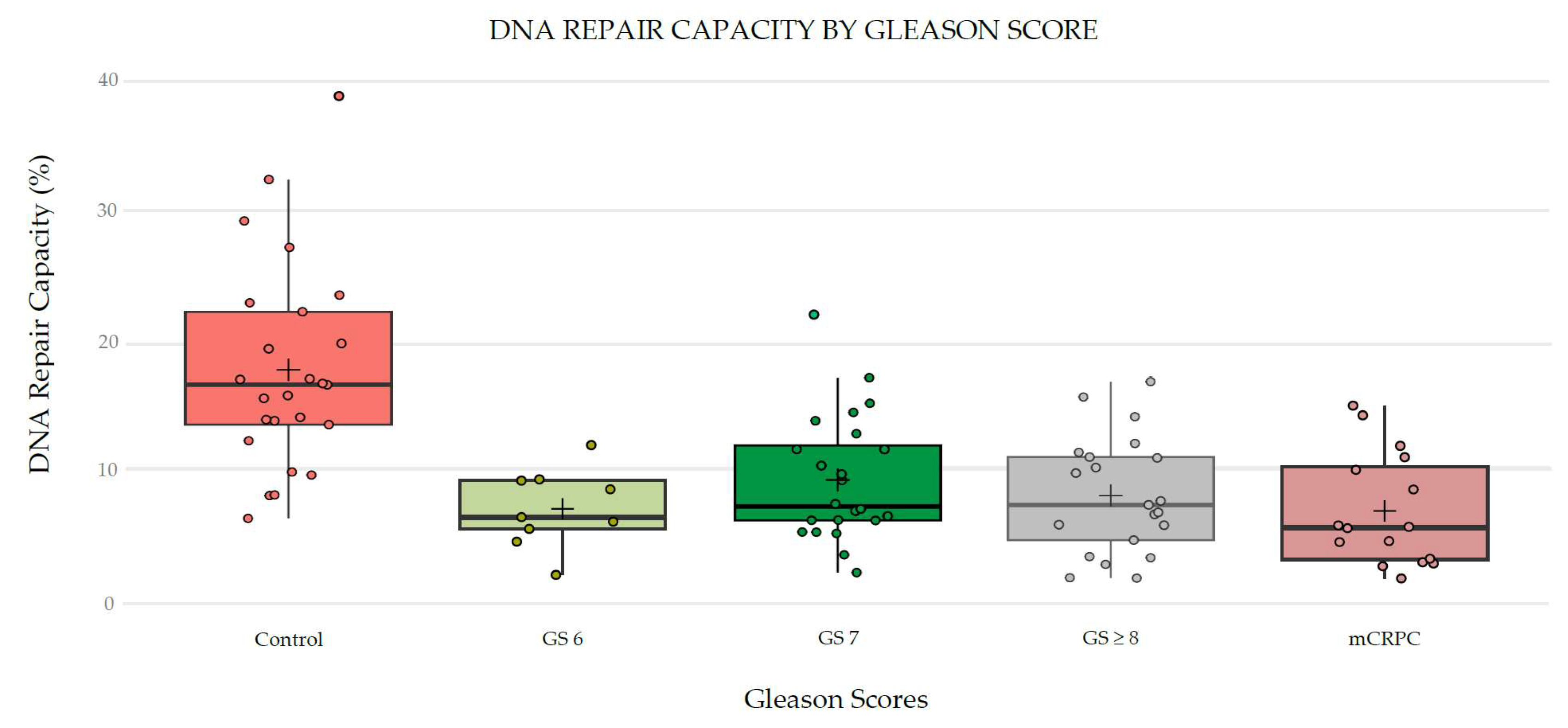
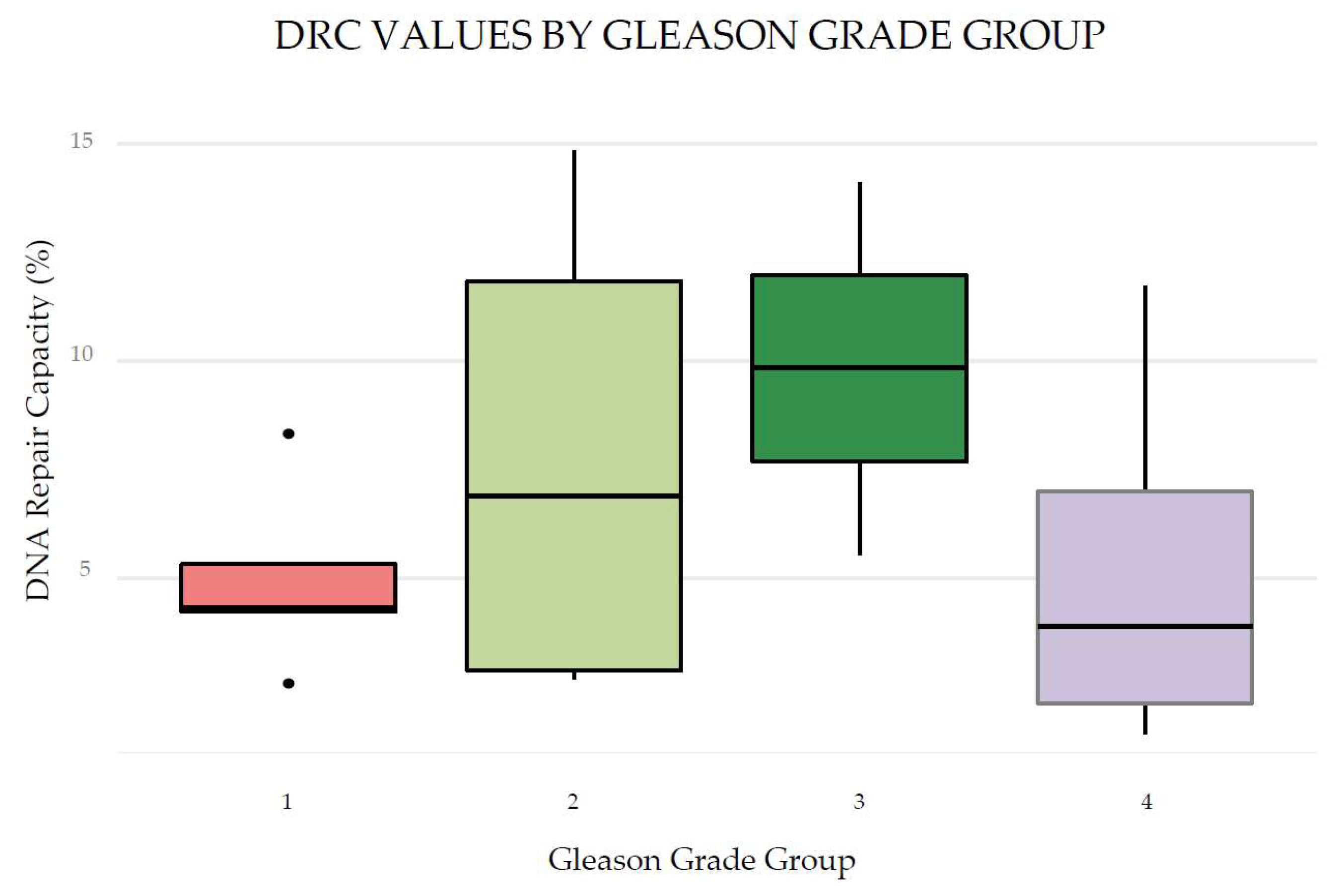
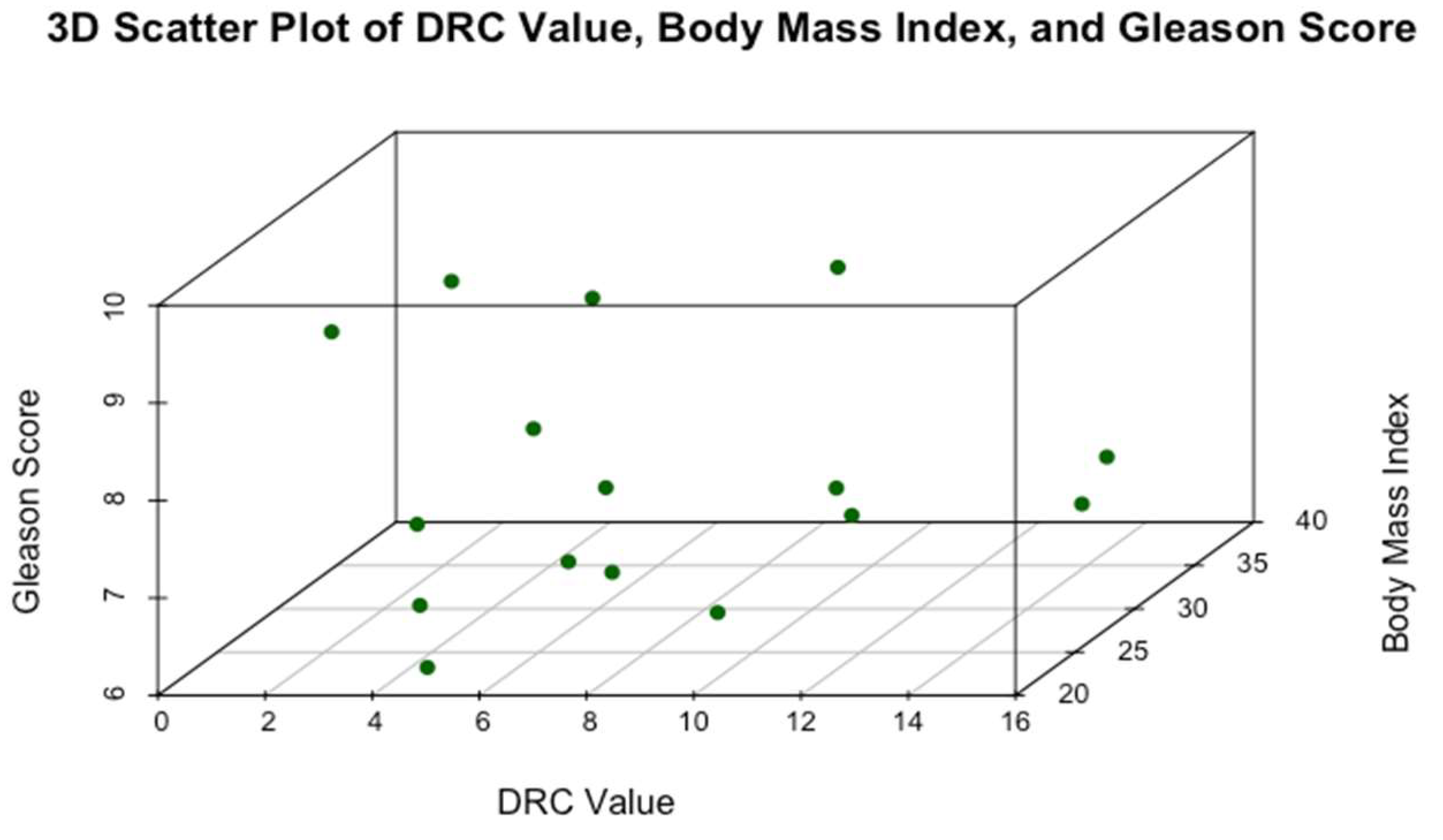
3.4. Distribution of DRC Levels and Correlation with Clinicopathological Variables
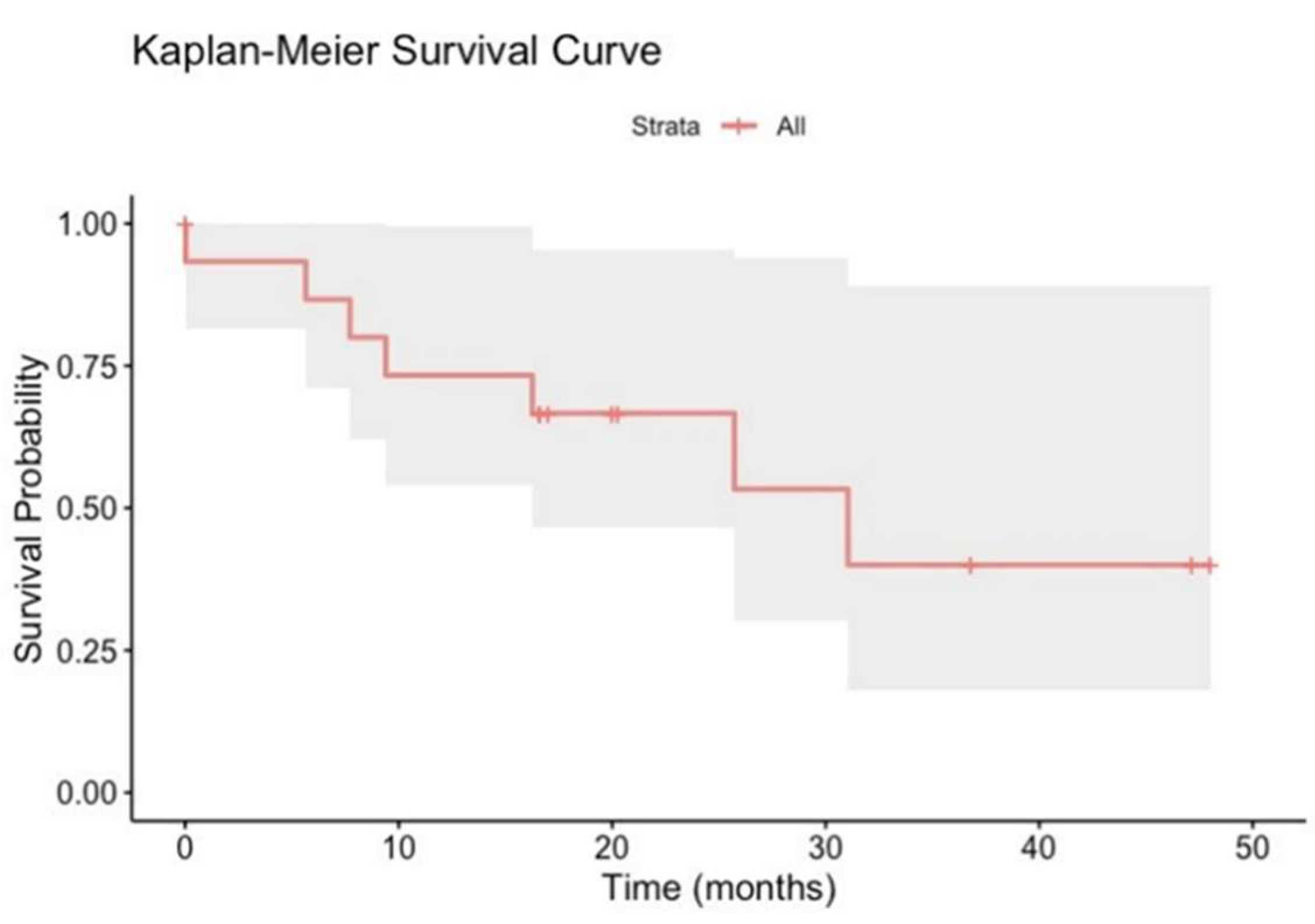
3.5. Kaplan-Meier Survival Curves of mCRPCa Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
Appendix A
References
- Gatsinga, R.; Tan, Y.G.; Chen, W.; Yang, X.; Tuan, J.K.L.; Chua, M.L.K.; Chan, J.; Kanesvaran, R.; Tay, K.J.; Chen, K.; et al. Lost opportunities: the underutilization of castrate-resistant prostate cancer treatment in real-world settings. Translational Andrology and Urology 2024, 13, 1786–1794. [Google Scholar] [CrossRef]
- Broderick, J.M. Incidence of Metastatic Prostate Cancer On the Rise. Oncology (Williston Park) 2020, 34, 460. [Google Scholar] [CrossRef] [PubMed]
- Posdzich, P.; Darr, C.; Hilser, T.; Wahl, M.; Herrmann, K.; Hadaschik, B.; Grünwald, V. Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nature Reviews Disease Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J Oncol 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Almeeri, M.N.E.; Awies, M.; Constantinou, C. Prostate Cancer, Pathophysiology and Recent Developments in Management: A Narrative Review. Curr Oncol Rep 2024. [Google Scholar] [CrossRef] [PubMed]
- Stopsack, K.H.; Vijai, J.; Conry, M.; Berchuck, J.E.; Kemel, Y.; Vasselman, S.E.; Freeman, D.A.; Lee, G.-S.M.; Mandelker, D.; Solit, D.B.; et al. Germline DNA damage repair variants and prognosis of patients with high-risk or metastatic prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2024. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; Henry de Villeneuve, M.; Rocchi, P. The hallmarks of castration-resistant prostate cancers. Cancer Treat Rev 2015, 41, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Kulasegaran, T.; Oliveira, N. Metastatic Castration-Resistant Prostate Cancer: Advances in Treatment and Symptom Management. Curr Treat Options Oncol 2024, 25, 914–931. [Google Scholar] [CrossRef]
- Henríquez, I.; Roach, M.; Morgan, T.M.; Bossi, A.; Gómez, J.A.; Abuchaibe, O.; Couñago, F. Current and Emerging Therapies for Metastatic Castration-Resistant Prostate Cancer (mCRPC). Biomedicines 2021, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Stein, C.A.; Sartor, O. Enzalutamide for the treatment of prostate cancer. Expert Opin Pharmacother 2013, 14, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Antonarakis, E.S. Prognostic and therapeutic implications of DNA repair gene mutations in advanced prostate cancer. Clin Adv Hematol Oncol 2017, 15, 785–795. [Google Scholar]
- Ferretti, S.; Mercinelli, C.; Marandino, L.; Litterio, G.; Marchioni, M.; Schips, L. Metastatic Castration-Resistant Prostate Cancer: Insights on Current Therapy and Promising Experimental Drugs. Res Rep Urol 2023, 15, 243–259. [Google Scholar] [CrossRef]
- Amin Al Olama, A.; Kote-Jarai, Z.; Schumacher, F.R.; Wiklund, F.; Berndt, S.I.; Benlloch, S.; Giles, G.G.; Severi, G.; Neal, D.E.; Hamdy, F.C.; et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet 2013, 22, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Bieńkowski, M.; Tomasik, B.; Braun, M.; Jassem, J. PARP inhibitors for metastatic castration-resistant prostate cancer: Biological rationale and current evidence. Cancer Treat Rev 2022, 104, 102359. [Google Scholar] [CrossRef]
- Leongamornlert, D.; Mahmud, N.; Tymrakiewicz, M.; Saunders, E.; Dadaev, T.; Castro, E.; Goh, C.; Govindasami, K.; Guy, M.; O'Brien, L.; et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer 2012, 106, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Francini, E.; Agarwal, N.; Castro, E.; Cheng, H.H.; Chi, K.N.; Clarke, N.; Mateo, J.; Rathkopf, D.; Saad, F.; Tombal, B. Intensification Approaches and Treatment Sequencing in Metastatic Castration-resistant Prostate Cancer: A Systematic Review. Eur Urol 2024. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, D.J.; Hussain, M. Management Decisions for Metastatic Castration-resistant Prostate Cancer in 2024. European urology 2024. [Google Scholar] [CrossRef] [PubMed]
- McManus, H.D.; Dorff, T.; Morgans, A.K.; Sartor, O.; Shore, N.; Armstrong, A.J. Navigating therapeutic sequencing in the metastatic castration-resistant prostate cancer patient journey. Prostate Cancer Prostatic Dis 2024. [Google Scholar] [CrossRef]
- Antonarakis, E.S. Germline DNA Repair Mutations and Response to Hormonal Therapy in Advanced Prostate Cancer. Eur Urol 2017, 72, 43–44. [Google Scholar] [CrossRef]
- Arce, S.; Athie, A.; Pritchard, C.C.; Mateo, J. Germline and Somatic Defects in DNA Repair Pathways in Prostate Cancer. Adv Exp Med Biol 2019, 1210, 279–300. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.A.; McPherson, J.D.; Gao, A.C.; DeVere White, R.W.; Gregg, J.P.; Lara Jr, P.N. Germline and somatic DNA repair gene alterations in prostate cancer. Cancer 2020, 126, 2980–2985. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Ryan, C.J.; Ashworth, A. DNA Repair Deficiency Is Common in Advanced Prostate Cancer: New Therapeutic Opportunities. Oncologist 2016, 21, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Boysen, G.; Barbieri, C.E.; Bryant, H.E.; Castro, E.; Nelson, P.S.; Olmos, D.; Pritchard, C.C.; Rubin, M.A.; de Bono, J.S. DNA Repair in Prostate Cancer: Biology and Clinical Implications. European urology 2017, 71, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. New England Journal of Medicine 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Burdak-Rothkamm, S.; Mansour, W.Y.; Rothkamm, K. DNA Damage Repair Deficiency in Prostate Cancer. Trends in Cancer 2020, 6, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Bugoye, F.C.; Torrorey-Sawe, R.; Biegon, R.; Dharsee, N.; Mafumiko, F.M.S.; Patel, K.; Mining, S.K. Mutational spectrum of DNA damage and mismatch repair genes in prostate cancer. Front Genet 2023, 14, 1231536. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Sánchez, C.; Encarnación-Medina, J.; Park, J.Y.; Moreno, N.; Ruiz-Deya, G.; Matta, J. Reduced DNA Repair Capacity in Prostate Cancer Patients: A Phenotypic Approach Using the CometChip. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Conteduca, V.; Zoubeidi, A.; Beltran, H. Biological Evolution of Castration-resistant Prostate Cancer. European Urology Focus 2019, 5, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Plym, A.; Dióssy, M.; Szallasi, Z.; Sartor, O.; Silberstein, J.; Powell, I.J.; Rebbeck, T.R.; Penney, K.L.; Mucci, L.A.; Pomerantz, M.M.; et al. DNA Repair Pathways and Their Association With Lethal Prostate Cancer in African American and European American Men. JNCI Cancer Spectr 2022, 6. [Google Scholar] [CrossRef] [PubMed]
- Bourlon, M.T.; Valdez, P.; Castro, E. Development of PARP inhibitors in advanced prostate cancer. Ther Adv Med Oncol 2024, 16, 17588359231221337. [Google Scholar] [CrossRef]
- Nizialek, E.; Antonarakis, E.S. PARP Inhibitors in Metastatic Prostate Cancer: Evidence to Date. Cancer Manag Res 2020, 12, 8105–8114. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018, 174, 758–769.e759. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Antonarakis, E.S. PARP inhibitors in prostate cancer: time to narrow patient selection? Expert Rev Anticancer Ther 2020, 20, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, G.; Mangaldzhiev, R.; Slavov, C.; Popov, E. Precision Medicine in Castration-Resistant Prostate Cancer: Advances, Challenges, and the Landscape of PARPi Therapy-A Narrative Review. Int J Mol Sci 2024, 25, 2184. [Google Scholar] [CrossRef]
- Matta, J.; Echenique, M.; Negron, E.; Morales, L.; Vargas, W.; Gaetan, F.S.; Lizardi, E.R.; Torres, A.; Rosado, J.O.; Bolanos, G.; et al. The association of DNA Repair with breast cancer risk in women. A comparative observational study. BMC cancer 2012, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Matanoski, G.M.; Farmer, E.R.; Hedayati, M.A.; Grossman, L. DNA repair and susceptibility to basal cell carcinoma: a case-control study. Am J Epidemiol 1994, 140, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Spitz, M.R.; Gu, J.; Cheng, L.; Xu, X.; Strom, S.S.; Kripke, M.L.; Hsu, T.C. DNA repair capacity correlates with mutagen sensitivity in lymphoblastoid cell lines. Cancer Epidemiol Biomarkers Prev 1996, 5, 199–204. [Google Scholar]
- Ngo, L.P.; Kaushal, S.; Chaim, I.A.; Mazzucato, P.; Ricciardi, C.; Samson, L.D.; Nagel, Z.D.; Engelward, B.P. CometChip analysis of human primary lymphocytes enables quantification of inter-individual differences in the kinetics of repair of oxidative DNA damage. Free radical biology & medicine 2021, 174, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Ruiz, A.; Colen, R.; Lopez, I.D.; Grossman, L.; Matta, J.L. DNA repair and breast carcinoma susceptibility in women. Cancer 2004, 100, 1352–1357. [Google Scholar] [CrossRef]
- D'Errico, M.; Calcagnile, A.; Iavarone, I.; Sera, F.; Baliva, G.; Chinni, L.M.; Corona, R.; Pasquini, P.; Dogliotti, E. Factors that influence the DNA repair capacity of normal and skin cancer-affected individuals. Cancer Epidemiol Biomarkers Prev 1999, 8, 553–559. [Google Scholar]
- Landi, M.T.; Baccarelli, A.; Tarone, R.E.; Pesatori, A.; Tucker, M.A.; Hedayati, M.; Grossman, L. DNA repair, dysplastic nevi, and sunlight sensitivity in the development of cutaneous malignant melanoma. J Natl Cancer I 2002, 94, 94–101. [Google Scholar] [CrossRef]
- Matta, J.L.; Villa, J.L.; Ramos, J.M.; Sanchez, J.; Chompre, G.; Ruiz, A.; Grossman, L. DNA repair and nonmelanoma skin cancer in Puerto Rican populations. J Am Acad Dermatol 2003, 49, 433–439. [Google Scholar] [CrossRef]
- Matta, J.; Ortiz, C.; Encarnacion, J.; Dutil, J.; Suarez, E. Variability in DNA Repair Capacity Levels among Molecular Breast Cancer Subtypes: Triple Negative Breast Cancer Shows Lowest Repair. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Wu, H.C.; Kehm, R.; Santella, R.M.; Brenner, D.J.; Terry, M.B. DNA repair phenotype and cancer risk: a systematic review and meta-analysis of 55 case-control studies. Sci Rep 2022, 12, 022–07256. [Google Scholar] [CrossRef]
- Wei, Q.; Cheng, L.; Hong, W.K.; Spitz, M.R. Reduced DNA repair capacity in lung cancer patients. Cancer Res 1996, 56, 4103–4107. [Google Scholar]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013, 111, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Weingeist, D.M.; Ge, J.; Wood, D.K.; Mutamba, J.T.; Huang, Q.; Rowland, E.A.; Yaffe, M.B.; Floyd, S.; Engelward, B.P. Single-cell microarray enables high-throughput evaluation of DNA double-strand breaks and DNA repair inhibitors. Cell Cycle 2013, 12, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Sykora, P.; Chiari, Y.; Heaton, A.; Moreno, N.; Glaberman, S.; Sobol, R.W. Application of the CometChip platform to assess DNA damage in field-collected blood samples from turtles. Environ Mol Mutagen 2018, 59, 322–333. [Google Scholar] [CrossRef]
- Sykora, P.; Witt, K.L.; Revanna, P.; Smith-Roe, S.L.; Dismukes, J.; Lloyd, D.G.; Engelward, B.P.; Sobol, R.W. Next generation high throughput DNA damage detection platform for genotoxic compound screening. Scientific Reports 2018, 8, 2771. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.P.; Demokritou, P. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using CometChip technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef]
- Vande Loock, K.; Decordier, I.; Ciardelli, R.; Haumont, D.; Kirsch-Volders, M. An aphidicolin-block nucleotide excision repair assay measuring DNA incision and repair capacity. Mutagenesis 2010, 25, 25–32. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O'Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Wickman H, N.D. , Lin Pedersen T. In ggplot 2 Elegant Graphics for Data Analysis; Springer Verlag, 2016. [Google Scholar]
- Kaplan EL, M.P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 1958, 58, 457–481. [Google Scholar] [CrossRef]
- Guerrios-Rivera, L.; Howard, L.E.; Wiggins, E.K.; Hoyo, C.; Grant, D.J.; Erickson, T.R.; Ithisuphalap, J.; Freedland, A.R.; Vidal, A.C.; Fowke, J.H.; et al. Metabolic syndrome is associated with aggressive prostate cancer regardless of race. Cancer Causes Control 2023, 34, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Conteduca, V.; Zoubeidi, A.; Beltran, H. Biological Evolution of Castration-resistant Prostate Cancer. Eur Urol Focus 2019, 5, 147–154. [Google Scholar] [CrossRef]
- Ge, J.; Ngo, L.P.; Kaushal, S.; Tay, I.J.; Thadhani, E.; Kay, J.E.; Mazzucato, P.; Chow, D.; Fessler, J.; Weingeist, D.M.; et al. CometChip enables parallel analysis of multiple DNA repair activities [DNA repair 106 (2021) 103176-103202]. DNA Repair (Amst) 2024, 138, 103677. [Google Scholar] [CrossRef] [PubMed]
- Weingeist, D.M.; Ge, J.; Wood, D.K.; Mutamba, J.T.; Huang, Q.; Rowland, E.A.; Yaffe, M.B.; Floyd, S.; Engelward, B.P. Single-cell microarray enables high-throughput evaluation of DNA double-strand breaks and DNA repair inhibitors. Cell Cycle 2013, 12, 907–915. [Google Scholar] [CrossRef]
- Li, L. Nucleotide Excision Repair. In DNA Repair, Genetic Instability and Cancer, Wei, Q., Lei, L., Chen, D.J., Ed.; World Scientific: Singapore, 2007; pp. 65–86. [Google Scholar]
- Turchi, J.J.; Patrick, S.M. Chapter 6 - Targeting the Nucleotide Excision Repair Pathway for Therapeutic Applications. In DNA Repair in Cancer Therapy, Kelley, M.R., Ed.; Academic Press: San Diego, 2012; pp. 109–117. [Google Scholar]
- Gillet, L.C.J.; Schärer, O.D. Molecular Mechanisms of Mammalian Global Genome Nucleotide Excision Repair. Chemical Reviews 2006, 106, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: from mechanisms to biological effects. Photochem Photobiol Sci 2018, 17, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Berwick, M.; Vineis, P. Measuring DNA repair capacity: Small steps. J Natl Cancer I 2005, 97, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; Generali, D.; Gatti, M.; Riboli, B.; Paganini, L.; Nesi, G.; Roviello, G. DNA repair deficiency as circulating biomarker in prostate cancer. Front Oncol 2023, 13, 1115241. [Google Scholar] [CrossRef]
- Chinea, F.M.; Patel, V.N.; Kwon, D.; Lamichhane, N.; Lopez, C.; Punnen, S.; Kobetz, E.N.; Abramowitz, M.C.; Pollack, A. Ethnic heterogeneity and prostate cancer mortality in Hispanic/Latino men: a population-based study. Oncotarget 2017, 8, 69709–69721. [Google Scholar] [CrossRef]
- Del Pino, M.; Abern, M.R.; Moreira, D.M. Prostate Cancer Disparities in Hispanics Using the National Cancer Database. Urology 2022, 165, 218–226. [Google Scholar] [CrossRef]
- Guerrios-Rivera, L.; Howard, L.E.; Klaassen, Z.; Terris, M.K.; Cooperberg, M.R.; Amling, C.L.; Kane, C.J.; Aronson, W.J.; Freedland, S.J. Do Hispanic Men Have Worse Outcomes After Radical Prostatectomy? Results From SEARCH. Urology 2021, 149, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Bjartell, A.; Lumen, N.; Maroto, P.; Paiss, T.; Gomez-Veiga, F.; Birtle, A.; Kramer, G.; Kalinka, E.; Spaëth, D.; et al. Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry. Target Oncol 2020, 15, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Brewster, S.F. PSA Velocity and Doubling Time in Diagnosis and Prognosis of Prostate Cancer. Br J Med Surg Urol 2012, 5, 162–168. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat Rev Urol 2021, 18, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Cutruzzolà, F.; Giardina, G.; Marani, M.; Macone, A.; Paiardini, A.; Rinaldo, S.; Paone, A. Glucose Metabolism in the Progression of Prostate Cancer. Front Physiol 2017, 8, 97. [Google Scholar] [CrossRef]
- Simpson, K.; Allison, D.B.; He, D.; Liu, J.; Wang, C.; Liu, X. Metformin in Overcoming Enzalutamide Resistance in Castration-Resistant Prostate Cancer. J Pharmacol Exp Ther 2024. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.M.; Guzmán, M.; Ortiz, A.P.; Estrella, M.; Valle, Y.; Pérez, N.; Haddock, L.; Suárez, E. Prevalence of the metabolic syndrome in San Juan, Puerto Rico. Ethn Dis 2008, 18, 434–441. [Google Scholar]
- Buschemeyer, W.C., 3rd; Freedland, S.J. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol 2007, 52, 331–343. [Google Scholar] [CrossRef]
- Crespo-Orta, I.; Ortiz, C.; Encarnación, J.; Suárez, E.; Matta, J. Association between DNA repair capacity and body mass index in women. Mutat Res 2023, 826, 111813. [Google Scholar] [CrossRef]
- Murphy, D.G.; Risbridger, G.P.; Bristow, R.G.; Sandhu, S. The Evolving Narrative of DNA Repair Gene Defects: Distinguishing Indolent from Lethal Prostate Cancer. Eur Urol 2017, 71, 748–749. [Google Scholar] [CrossRef]
- Christenson, E.S.; Antonarakis, E.S. PARP inhibitors for homologous recombination-deficient prostate cancer. Expert Opin Emerg Drugs 2018, 23, 123–133. [Google Scholar] [CrossRef]
- Mahamud, O.; So, J.; Chua, M.L.K.; Bristow, R.G. Targeting DNA repair for precision radiotherapy: Balancing the therapeutic ratio. Curr Probl Cancer 2017, 41, 265–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




