Submitted:
30 November 2024
Posted:
02 December 2024
You are already at the latest version
Abstract
Introduction: solid organs transplanted recipients (OTR) have been recently involved in exercise prescription programs in order to reduce the high prevalence of cardiovascular diseases. The normal systolic and diastolic cardiac function is fundamental to personalize the prescription. Diastolic dysfunction can be associated to an higher risk of cardiovascular events and left atrial (LA) strain is an emerging parameter in the evaluation of diastolic compromising, especially in subjects with preserved ejection fraction. Left atrial (LA) strain has never been explored in this category. The study aimed to evaluate the contribution of the LA strain in the assessment of diastolic function of OTR and its potential contribution in the exercise program. Materials and Methods: 54 solid OTR (liver and kidney transplanted) regularly trained for at least 12 months in an home based partially supervised model at moderate intensity estimated by cardiopulmonary exercise test, underwent a complete echocardiographic analysis. Measured variables included left ventricle systolic function (ejection fraction: EF), diastolic function (E/A and E/E’), LA indexed volumes, LA peak atrial longitudinal strain (PALS) and LA peak atrial contraction strain (PACS). The data were compared to 44 healthy subjects (HS). Results: OTR showed an overweight condition (BMI: 25.79 ±2.92 vs 22.25±2.95). Both groups showed a preserved systolic function (EF: OTR 63.1±3.5% vs HS 66.9±6.1; p<0,001) while diastolic standard parameters were significantly different (E/A: 1.01±0.4 vs 1.960.74; p<0.001; E/E’: 9.2±2.7 vs 6.91.3; p<0.001, in OTR and HS respectively) despite normal. LA strain was significantly lower in OTR vs HS (4C PALS: 33.7±9.7 vs 45.4; p<0.001 and 4C PACS: 15.9±6.7 vs 11.67.5; p=0.006; 2C PALS: 35.3±11.1 vs 47.614.9; p<0.001; 2C PALS: 17.4±4.9 vs 13.2; p=0.001, in OTR and HS respectively). A specific correlation of 2 and 4 chamber PACs and PALs with BMI has been observed (R for 4C PALS -0.406** and 2C PALS -0.276*). Conclusions: These findings suggest that the coexistence of increased body weight in asymptomatic OTR patients can exacerbate the impairment of LA strains. LA strain detection could be useful in the personalized exercise program for OTR especially if asymptomatic subjects and with elevated cardiovascular risk profile, to potentially manage the exercise program in the long term. Larger studies will confirm the role in an eventual structured clinical score index.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol Study
2.2. Echocardiographic Exam
2.3. Strain Analysis
2.4. Cardiopulmonary Test
2.5. Statistical Analysis
3. Results
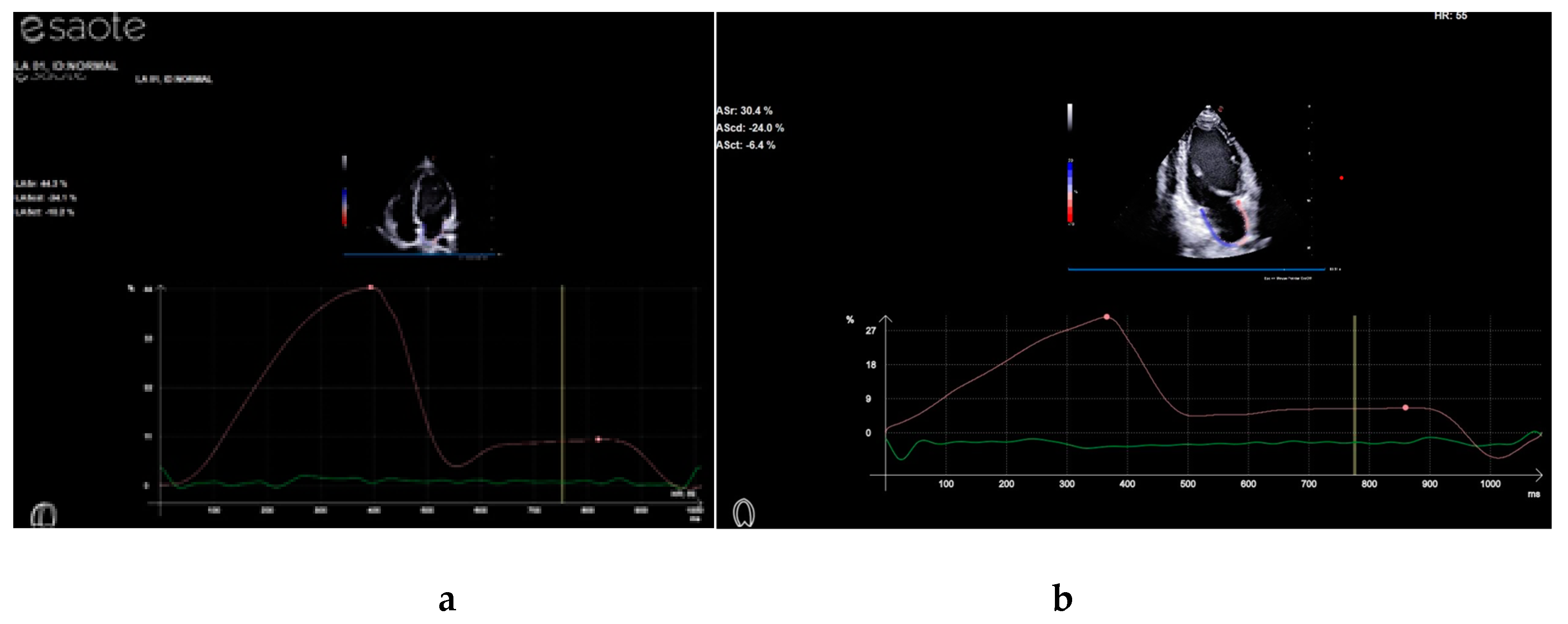
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen D, Niebauer J, Cornelissen V, Barna O, Neunhäuserer D, Stettler C, Tonoli C, Greco E, Fagard R, Coninx K, Vanhees L, Piepoli MF, Pedretti R, Ruiz GR, Corrà U, Schmid JP, Davos CH, Edelmann F, Abreu A, Rauch B, Ambrosetti M, Braga SS, Beckers P, Bussotti M, Faggiano P, Garcia-Porrero E, Kouidi E, Lamotte M, Reibis R, Spruit MA, Takken T, Vigorito C, Völler H, Doherty P, Dendale P. Exercise Prescription in Patients with Different Combinations of Cardiovascular Disease Risk Factors: A Consensus Statement from the EXPERT Working Group. Sports Med. 2018 Aug;48(8):1781-1797. [CrossRef] [PubMed]
- Hansen D, Abreu A, Ambrosetti M, Cornelissen V, Gevaert A, Kemps H, Laukkanen JA, Pedretti R, Simonenko M, Wilhelm M, Davos CH, Doehner W, Iliou MC, Kränkel N, Völler H, Piepoli M. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: why and how: a position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2022 Feb 19;29(1):230-245, Erratum in: Eur J Prev Cardiol. 2024 Sep 20;31(13):e102. [CrossRef] [PubMed]
- Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, Wang R. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019 Sep;8(5):422-441. Epub 2019 Apr 18. [CrossRef] [PubMed]
- Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015 Dec;25 Suppl 3:1-72. [CrossRef] [PubMed]
- Gitto S, Golfieri L, Gabrielli F, Falcini M, Sofi F, Tamè MR, De Maria N, Marzi L, Mega A, Valente G, Borghi A, Forte P, Cescon M, Di Benedetto F, Andreone P, Petranelli M, Morelli MC, De Simone P, Lau C, Stefani L, Vizzutti F, Chiesi F, Marra F; MEDITRA Research Group. Physical activity in liver transplant recipients: a large multicenter study. Intern Emerg Med. 2024 Mar;19(2):343-352. Epub 2023 Nov 20. [CrossRef] [PubMed]
- Polara G, Montagnoli A, Palazzo R, Orlandi M, Mascherini G, Corsi M, Falconi E, Stefani L. Cardiorespiratory Performance in Kidney and Liver Transplant Recipients: The Dilemma to Combine Lifestyle and Fitness. J Funct Morphol Kinesiol. 2024 Feb 29;9(1):44. [CrossRef] [PubMed]
- Orlandi M, Bini V, Leone B, Zappelli E, Pedrizzetti G, Stefani L. Home-based exercise program improves normal right ventricle function in renal transplant recipients. J Sports Med Phys Fitness. 2022 Mar;62(3):412-417. Epub 2021 Oct 15. [CrossRef] [PubMed]
- Carvalho MVH, Kroll PC, Kroll RTM, Carvalho VN. Cirrhotic cardiomyopathy: the liver affects the heart. Brazilian Journal of Medical and Biological Research 2019;52. Yoon KT, Liu H, Lee SS. Cirrhotic Cardiomyopathy. Curr Gastroenterol Rep 2020;22:45. [CrossRef]
- Diastolic dysfunction and preserved Ejection Fraction JACC Cardiovasc Imaging. 2020 Jan;13(1 Pt 2):245-257. [CrossRef]
- G.Di Salvo. Atrial myocardial deformation Circulation. 2005;112:387-395. [CrossRef]
- Silva MR, Sampaio F, Braga J, Ribeiro J, Fontes-Carvalho R. Left atrial strain evaluation to assess left ventricle diastolic dysfunction and heart failure with preserved ejection fraction: a guide to clinical practice : Left atrial strain and diastolic function. Int J Cardiovasc Imaging. 2023 Jun;39(6):1083-1096. Epub 2023 Feb 24. [CrossRef] [PubMed]
- Mandoli GE, Sisti N, Mondillo S, Cameli M. Left atrial strain in left ventricular diastolic dysfunction: have we finally found the missing piece of the puzzle? Heart Fail Rev. 2020 May;25(3):409-417. Orlandi G, Sofi F, Moscarelli L, Cirami L, Mancini S, Stefani L. Exercise Prescription in Renal Transplant Recipients: From Sports Medicine Toward Multidisciplinary Aspects: A Pilot Study. J Funct Morphol Kinesiol 2020;5:10. [CrossRef] [PubMed]
- Orlandi G, Sofi F, Moscarelli L, Cirami L, Mancini S, Stefani L. Exercise Prescription in Renal Transplant Recipients: From Sports Medicine Toward Multidisciplinary Aspects: A Pilot Study. J Funct Morphol Kinesiol 2020;5:10. [CrossRef]
- M Alonso Gómez Left atrial strain improves echocardiographic classification of diastolic function in patients with metabolic syndrome and overweight-obesity,Angel, Int J Cardiol. 2022 February 01; 348: 169–174. [CrossRef]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29:277–314. [CrossRef] [PubMed]
- Gary Liguori, ACSM's Guidelines for Exercise Testing and Prescription, American College of Sports Medicine (ACSM), ISBN/ISSN: 9781975150181, Publication Date: April 8, 2021.
- ATS/ACCP Statement on Cardiopulmonary Exercise Testing This Joint Statement of the American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP), March 1, 2002 and by the ACCP Health Science Policy Committee, November 1, 2001.
- Hallal, P.C.; Victora, C.G. Reliability and validity of the international physical activity questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [CrossRef]
- Yabe H, Kono K, Onoyama A, Kiyota A, Moriyama Y, Okada K, et al. Predicting a target exercise heart rate that reflects the anaerobic threshold in non beta-blocked hemodialysis patients: The Karvonen and heart rate reserve formulas. Therapeutic Apheresis and Dialysis 2021;25:884–9. [CrossRef]
- Goodyear MDE, Krleza-Jeric K, Lemmens T. The declaration of Helsinki. BMJ. 2007;335:624–625. [CrossRef]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2015;28:1-39.e14. [CrossRef]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St. John Sutton M, Stewart W; American Society of Echocardiography’s No-menclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [CrossRef]
- Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU; Industry representatives; Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018 Jun 1;19(6):591-600; Erratum in: Eur Heart J Cardiovasc Imaging. 2018 Jul 1;19(7):830-833. [CrossRef] [PubMed]
- D’Ascenzi F, Cameli M, Padeletti M, Lisi M, Zacà V, Natali B, Malandrino A, Alvino F, Morelli M, Vassallo GM, Meniconi C, Bonifazi M, Causarano A, Mondillo S. Characterization of right atrial function and dimension in top-level athletes: a speckle tracking study. Int J Cardiovasc Imaging. 2013;29:87–94. [CrossRef]
- Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15.;Appleton CP, Kovács SJ. The role of left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:6–9. [CrossRef]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. Focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274.
- Ney, M.; Haykowsky, M.J.; Vandermeer, B.; Shah, A.; Ow, M.; Tandon, P. Systematic review: Pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment. Pharmacol. Ther. 2016, 44, 796–806. [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary exercise testing: What is its value? Am. Coll. Cardiol. 2017, 70, 1618–1636. [CrossRef]
- Shen MT, Guo YK, Liu X, Ren Y, Jiang L, Xie LJ, Gao Y, Zhang Y, Deng MY, Li Y, Yang ZG. Impact of BMI on Left Atrial Strain and Abnormal Atrioventricular Interaction in Patients With Type 2 Diabetes Mellitus: A Cardiac Magnetic Resonance Feature Tracking Study. J Magn Reson Imaging. 2022 May;55(5):1461-1475. Epub 2021 Sep 22. [CrossRef] [PubMed]
- Mascherini G, Corsi M, Falconi E, Cebrián-Ponce Á, Checcucci P, Pinazzi A, Russo D, Gitto S, Sofi F, Stefani L. Unsupervised Exercise Intervention vs. Adherence to a Mediterranean Diet Alone: The Role of Bioelectrical Impedance Vector Analysis and Cardiovascular Performance in Liver-Transplanted Recipients. Nutrients. 2024 Jan 5;16(2):190. [CrossRef]
- Minetti E, Klika R, Ingletto C, Mascherini G, Pedrizzetti G, Stefani L. Changes in global longitudinal strain in renal transplant recipients following 12 months of exercise. Intern Emerg Med. 2018 Aug;13(5):805-809. Epub 2018 Jun 20. [CrossRef]
| OTR (n=54) | HS (n=44) | P | |
|---|---|---|---|
| Age (yo) | 59.43±8.75 | 36.52±12.24 | <0.001 |
| BMI (kg/m2) | 25.79±2.92 | 22.25±2.45 | <0.001 |
| IPAQ (METs/week) | 1053.87±1024.30 | 1974.45±1438.87 | 0.02 |
| VO2 max (mL/kg/min) | 22.90±7.30 | 38.87±28.65 | 0.01 |
| VO2 max (%) | 85.3±23.62 | 91.74±12.43 | 0.02 |
| IVS (mm) | 9.81±1.1 | 9.49±0.94 | Ns |
| PW (mm) | 9.61±1.22 | 9.30±0.86 | Ns |
| LVEDD (mm) | 50.52±4.60 | 49.31±3.61 | Ns |
| EF (%) | 64.39±5.37 | 67.09±6.44 | 0.05 |
| LVMI (gr/m2) | 100±21.4 | 87.62±14 | Ns |
| E/A | 1.01±0.44 | 1.96±0.74 | <0.001 |
| IVRT (ms) | 85.67±20.26 | 71.83±26.60 | 0.01 |
| DTc (ms) | 219.15±79.00 | 195.86±38.47 | Ns |
| E/E’ septum | 9.93±3.06 | 6.91±1.31 | <0.001 |
| E/E’ lateral | 7.63±4.66 | 5.11±1.34 | 0.02 |
| LA Volume (ml) | 24.32±8.65 | 27.22±11.25 | Ns |
| 4C PALS (%) | 33.45±9.57 | 45.36±14.19 | <0.001 |
| 4C PACS (%) | 15.88±6.80 | 11.95±7.48 | 0.003 |
| 2C PALS (%) | 35.45±11.26 | 47.55±14.97 | <0.001 |
| 2CPACS (%) | 17.29±5.09 | 13.16±6.79 | 0.001 |
| 4c PALS | p | 4c PACS | p | 2c PALS | p | 2cPACS | p | |
|---|---|---|---|---|---|---|---|---|
| Age | -0.436** | 0.000 | 0.224* | 0.029 | -0.496** | 0.000 | 0.338** | 0.003 |
| BMI | -0.406** | 0.000 | 0.086 | 0.410 | -0.276* | 0.013 | 0.188 | 0.101 |
| IVS | -0.009 | 0.933 | 0.035 | 0.737 | 0.000 | 0.999 | 0.132 | 0.254 |
| PW | 0.113 | 0.266 | 0.228* | 0.026 | 0.097 | 0.394 | 0.187 | 0.103 |
| LVDD | 0.076 | 0.458 | 0.174 | 0.092 | 0.101 | 0.374 | 0.141 | 0.221 |
| EF | 0.184 | 0.069 | 0.025 | 0.808 | 0.080 | 0.478 | 0.118 | 0.305 |
| E | 0.040 | 0.696 | -0.190 | 0.066 | 0.287** | 0.010 | -0.198 | 0.085 |
| A | -0.463** | 0.000 | 0.286** | 0.005 | -0.362** | 0.001 | 0.295** | 0.009 |
| E/A | 0.378** | 0.000 | -0.315** | 0.002 | 0.415** | 0.000 | -0.346** | 0.002 |
| IVRT | -0.291** | 0.004 | 0.062 | 0.552 | -0.091 | 0.421 | 0.178 | 0.122 |
| DTc | 0.033 | 0.747 | 0.149 | 0.152 | -0.016 | 0.885 | 0.171 | 0.136 |
| E' septum | 0.267** | 0.008 | -0.245* | 0.017 | 0.396** | 0.000 | -0.376** | 0.001 |
| E/E' septum | -0.355** | 0.000 | 0.136 | 0.194 | -0.336** | 0.002 | 0.227* | 0.048 |
| E' lateral | 0.206 | 0.169 | -0.059 | 0.702 | 0.354* | 0.027 | 0.055 | 0.740 |
| E/E' lateral | -0.574** | 0.000 | -0.097 | 0.542 | -0.253 | 0.143 | 0.130 | 0.456 |
| LA Volume | -0.028 | 0.786 | -0.076 | 0.466 | 0.155 | 0.172 | 0.057 | 0.622 |
| 4c PALS | 1.000 | 0.170 | 0.100 | 0.446** | 0.000 | -0.094 | 0.416 | |
| c4c PACS | 0.170 | 0.100 | 10.000 | -0.066 | 0.561 | 0.527** | 0.000 | |
| 2c PALS | 0.446** | 0.000 | -0.066 | 0.561 | 10.000 | 0.183 | 0.111 | |
| 2c PACS | -0.094 | 0.416 | 0.527** | 0.000 | 0.183 | 0.111 | 10.000 |
| OTR | HS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4c PALS | 4c PACS | 2c PALS | 2c PACS | 4c PALS | 4c PACS | 2c PALS | 2c PACS | ||
| Age | R | ,000 | -,025 | -,233 | -,112 | -,347* | ,108 | -,213 | ,308 |
| p | ,999 | ,861 | ,138 | ,487 | ,021 | ,495 | ,200 | ,068 | |
| BMI | R | -,243 | -,133 | -,159 | -,312* | -,202 | ,092 | -,130 | ,315 |
| p | ,077 | ,341 | ,316 | ,047 | ,189 | ,561 | ,437 | ,061 | |
| IVS | R | ,056 | ,148 | ,187 | ,058 | ,058 | -,161 | -,107 | ,181 |
| p | ,687 | ,291 | ,237 | ,719 | ,708 | ,309 | ,523 | ,290 | |
| PW | R | ,048 | ,227 | ,236 | ,143 | ,311* | ,154 | -,011 | ,176 |
| p | ,730 | ,102 | ,132 | ,373 | ,040 | ,330 | ,948 | ,304 | |
| LVEDD | R | ,201 | ,246 | ,126 | ,013 | ,070 | ,121 | ,127 | ,293 |
| p | ,144 | ,076 | ,427 | ,933 | ,650 | ,444 | ,447 | ,083 | |
| EF | R | ,221 | -,024 | -,122 | ,148 | -,009 | ,220 | ,127 | ,257 |
| p | ,108 | ,866 | ,441 | ,357 | ,955 | ,162 | ,449 | ,130 | |
| E/A | R | -,057 | -,165 | -,002 | -,207 | ,406** | ,032 | ,375* | -,006 |
| p | ,682 | ,238 | ,990 | ,193 | ,007 | ,840 | ,020 | ,970 | |
| IVRT | R | -,090 | ,004 | ,015 | -,009 | -,247 | -,076 | ,017 | ,212 |
| p | ,517 | ,976 | ,925 | ,956 | ,111 | ,637 | ,918 | ,214 | |
| DTc | R | ,084 | ,106 | ,145 | ,221 | ,125 | ,017 | ,051 | -,067 |
| p | ,545 | ,449 | ,359 | ,164 | ,424 | ,914 | ,761 | ,700 | |
| E/E' set | R | -,119 | -,148 | -,169 | ,020 | -,081 | ,027 | ,190 | ,138 |
| p | ,393 | ,290 | ,286 | ,900 | ,609 | ,869 | ,261 | ,430 | |
| E/E' lat | R | -,459 | -,262 | -,159 | -,090 | -,414* | -,105 | ,060 | ,038 |
| p | ,055 | ,293 | ,588 | ,759 | ,035 | ,626 | ,797 | ,871 | |
| LA volume | R | -,263 | -,071 | ,130 | ,118 | ,088 | ,019 | ,131 | ,141 |
| p | ,057 | ,617 | ,418 | ,470 | ,572 | ,906 | ,431 | ,413 | |
| 4c PALS | R | 1,000 | ,488** | ,215 | ,376* | 1,000 | ,183 | ,338* | -,174 |
| p | ,000 | ,172 | ,016 | ,246 | ,038 | ,311 | |||
| 4c PACS | R | ,488** | 1,000 | ,128 | ,398* | ,183 | 1,000 | ,138 | ,433** |
| p | ,000 | ,418 | ,010 | ,246 | ,414 | ,009 | |||
| 2C PALS | R | ,215 | ,128 | 1,000 | ,629** | ,338* | ,138 | 1,000 | ,248 |
| p | ,172 | ,418 | ,000 | ,038 | ,414 | ,145 | |||
| 2C PACS | R | ,376* | ,398* | ,629** | 1,000 | -,174 | ,433** | ,248 | 1,000 |
| P | ,016 | ,010 | ,000 | ,311 | ,009 | ,145 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





