Submitted:
29 December 2024
Posted:
01 January 2025
You are already at the latest version
Abstract
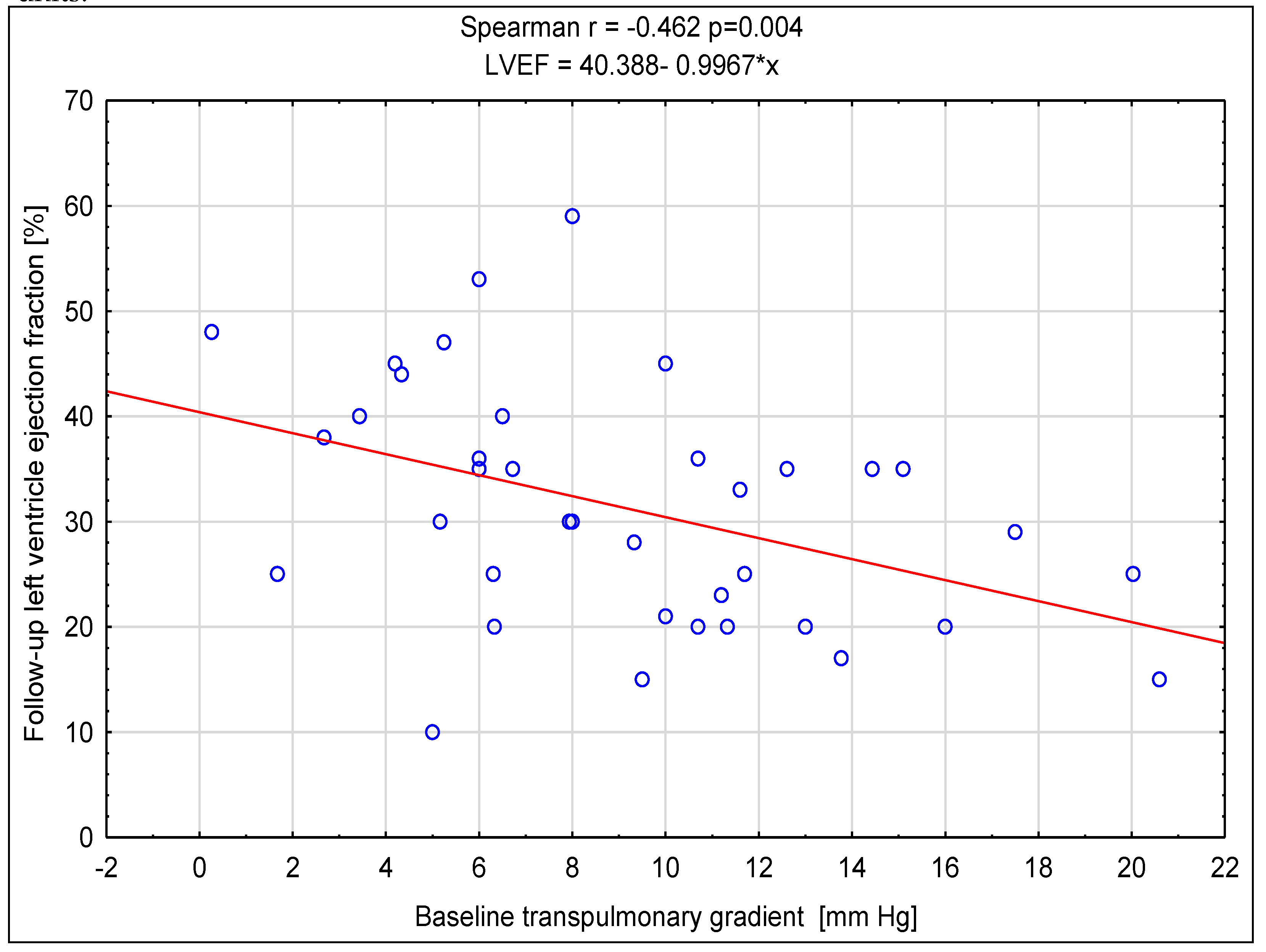
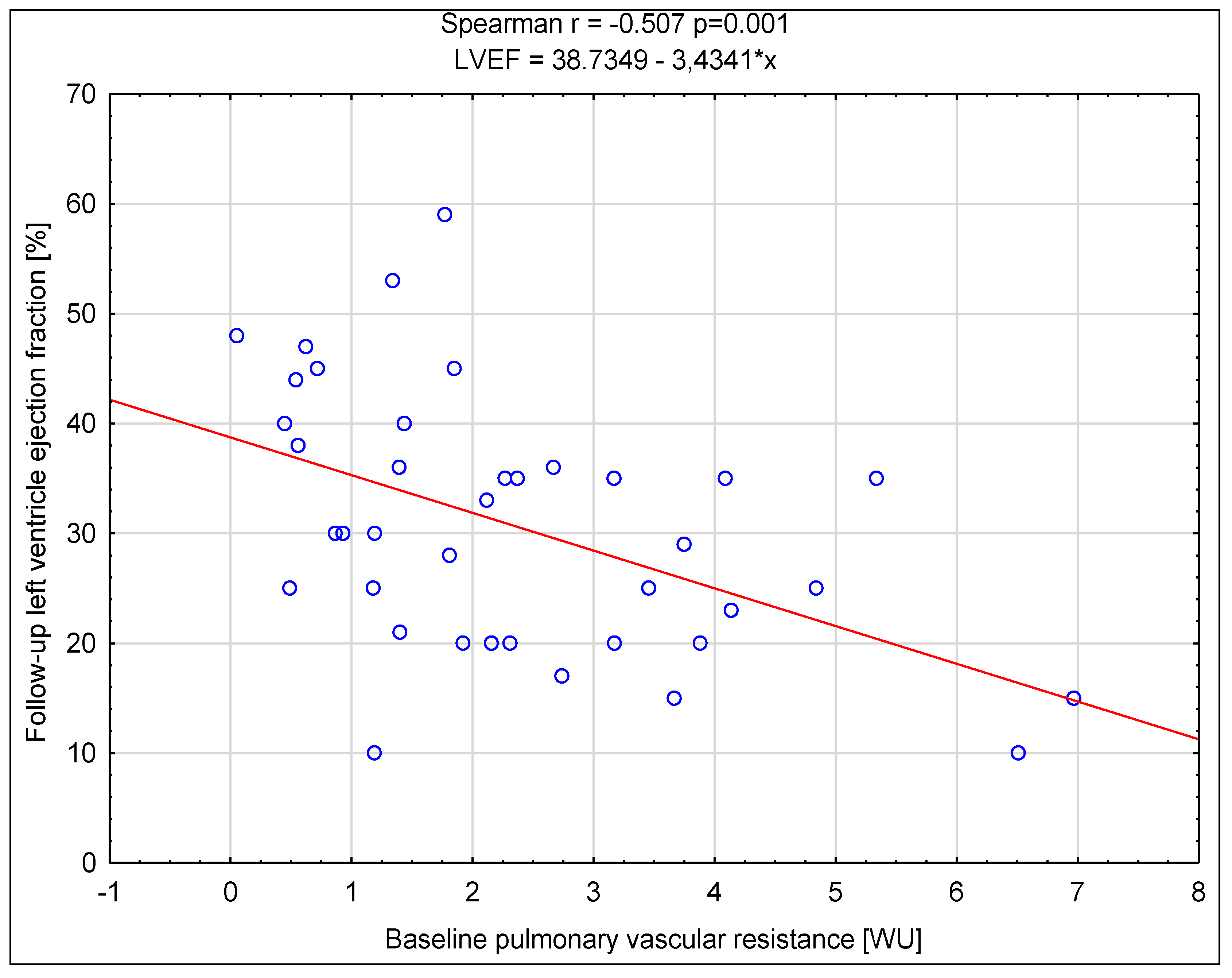
Background/Objectives: Cardiac resynchronization therapy (CRT) is one of the interventional methods of heart failure (HF) treating, with the criteria for CRT device implantation based on the value of left ventricular ejection fraction, New York Heart Association (NYHA) functional class, QRS duration and electrocardiographic morphology. Pulmonary hypertension is an important factor influencing the prognosis of patients with heart failure, but its influence on CRT is not fully understood. Aim: The main aim of the study was to determine the prognostic value of baseline right heart catheterization (RHC) derived parameters on the response to CRT. Methods: It was a single centre study with retrospective analysis of data of 39 non-ischemic HF patients. Clinical, biochemical, echocardiographic, electrocardiographic and hemodynamic data were obtained before the CRT device implantation, and after 6 months of follow-up non-invasive re-assessment was performed. Various criteria for the response to CRT were assessed along with the correlation between the baseline parameters. Results: After follow-up a significant difference was found in the reduction of symptoms associated with heart failure, an increase in the distance achieved in the six-minute walk test distance (6MWT) and a reduction of N-terminal pro-brain natriuretic peptide (NT-proBNP) concentration as well as improvement of left ventricular function assessed in echocardiographic examination. Among all parameters assessed, the baseline higher value of transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR) most often had a significant negative impact on meeting the criteria of response to CRT. Conclusions: The results of the analyses show that the initial assessment of pulmonary hemodynamics may be crucial in predicting the response to CRT in patients with non-ischemic cardiomyopathy.
Keywords:
1. Introduction
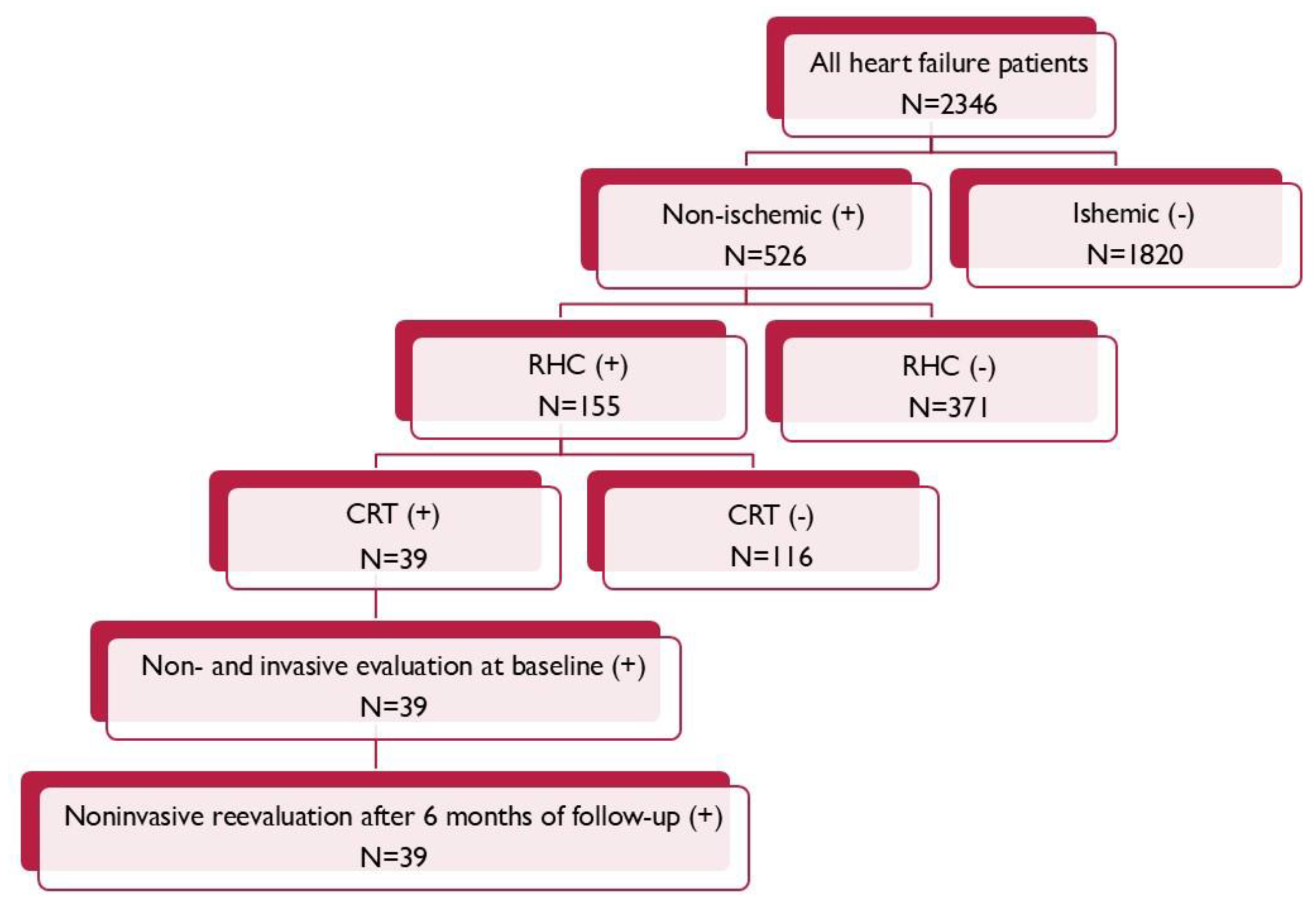
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Right Heart Catheterization
2.4. CRT Device Implantation and Optimization
2.5. The Responder Criteria
2.6. End of Study
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Authors/Task Force Members:; McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022 Jan;24(1):4-131. [CrossRef] [PubMed]
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM. Corrigendum to: 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC): With the special contribution of the European Heart Rhythm Association (EHRA). Europace. 2022 Apr 5;24(4):699. Erratum for: Europace. 2022 Jan 4;24(1):71-16. [CrossRef] [PubMed]
- Georgiopoulou VV, Kalogeropoulos AP, Borlaug BA, Gheorghiade M, Butler J. Left ventricular dysfunction with pulmonary hypertension: Part 1: epidemiology, pathophysiology, and definitions. Circ Heart Fail. 2013 Mar;6(2):344-54. [CrossRef] [PubMed]
- Kalogeropoulos AP, Georgiopoulou VV, Borlaug BA, Gheorghiade M, Butler J. Left ventricular dysfunction with pulmonary hypertension: part 2: prognosis, noninvasive evaluation, treatment, and future research. Circ Heart Fail. 2013 May;6(3):584-93. PMCID: PMC3662027. [CrossRef] [PubMed]
- Shalaby A, Voigt A, El-Saed A, Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008 Jan 15;101(2):238-41. [CrossRef] [PubMed]
- Wang D, Han Y, Zang H, Yu H, Wang S, Wang Z, Jing Q. Prognostic effects of pulmonary hypertension in patients undergoing cardiac resynchronization therapy. J Thorac Dis. 2010 Jun;2(2):71-5. PMCID: PMC3256451. [PubMed]
- Stern J, Heist EK, Murray L, Alabiad C, Chung J, Picard MH, Semigran MJ, Ruskin JN, Singh JP. Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007 May;30(5):603-7. [CrossRef] [PubMed]
- Callan P, Clark AL. Right heart catheterisation: indications and interpretation. Heart. 2016 Jan;102(2):147-57. Epub 2015 Dec 23. [CrossRef] [PubMed]
- Sharma A, Bax JJ, Vallakati A, Goel S, Lavie CJ, Kassotis J, Mukherjee D, Einstein A, Warrier N, Lazar JM. Meta-Analysis of the Relation of Baseline Right Ventricular Function to Response to Cardiac Resynchronization Therapy. Am J Cardiol. 2016 Apr 15;117(8):1315-21. Epub 2016 Jan 28. [CrossRef] [PubMed]
- Aimo A, Fabiani I, Vergaro G, Arzilli C, Chubuchny V, Pasanisi EM, Petersen C, Poggianti E, Taddei C, Pugliese NR, Bayes-Genis A, Lupón J, Giannoni A, Ripoli A, Georgiopoulos G, Passino C, Emdin M. Prognostic value of reverse remodelling criteria in heart failure with reduced or mid-range ejection fraction. ESC Heart Fail. 2021 Aug;8(4):3014-3025. Epub 2021 May 18. PMCID: PMC8318429. [CrossRef] [PubMed]
- Kalogeropoulos AP, Vega JD, Smith AL, Georgiopoulou VV. Pulmonary hypertension and right ventricular function in advanced heart failure. Congest Heart Fail. 2011 Jul-Aug;17(4):189-98. Epub 2011 Jul 21. [CrossRef] [PubMed]
- Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013 Jan;41(1):217-23. Epub 2012 Aug 30. [CrossRef] [PubMed]
- Chatterjee NA, Upadhyay GA, Singal G, Parks KA, Dec GW, Singh JP, Lewis GD. Pre-capillary pulmonary hypertension and right ventricular dilation predict clinical outcome in cardiac resynchronization therapy. JACC Heart Fail. 2014 Jun;2(3):230-7. Epub 2014 Apr 30. [CrossRef] [PubMed]
- Li J, Qian Z, Qiu H, Jiang Z, Wang Y, Zhao H, Zhang H, Zhou Y, Hou X, Li X, Zou J. Correlation between cardiac resynchronization response and pulmonary artery hemodynamic parameters. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020 Jun 28;45(6):715-721. English, Chinese. [CrossRef] [PubMed]
- Schmeisser A, Rauwolf T, Ghanem A, Groscheck T, Adolf D, Grothues F, Fischbach K, Kosiek O, Huth C, Kropf S, Lange S, Luani B, Smid J, Schäfer MH, Schreiber J, Tanev I, Wengler F, Yeritsyan NB, Steendijk P, Braun-Dullaeus RC. Right heart function interacts with left ventricular remodeling after CRT: A pressure volume loop study. Int J Cardiol. 2018 Oct 1;268:156-161. Epub 2018 Mar 8. [CrossRef] [PubMed]
- Martens P, Verbrugge FH, Bertrand PB, Verhaert D, Vandervoort P, Dupont M, Tang WHW, Janssens S, Mullens W. Effect of Cardiac Resynchronization Therapy on Exercise-Induced Pulmonary Hypertension and Right Ventricular-Arterial Coupling. Circ Cardiovasc Imaging. 2018 Sep;11(9):e007813. [CrossRef] [PubMed]
- Rasmussen JT, Thenappan T, Benditt DG, Weir EK, Pritzker MR. Is cardiac resynchronization therapy for right ventricular failure in pulmonary arterial hypertension of benefit? Pulm Circ. 2014 Dec;4(4):552-9. PMCID: PMC4278617. [CrossRef] [PubMed]
- Oktay AA, Mandras SA, Shah S, Kancharla K, Shams OF, Pascual MI, Morin DP. First in human: the effects of biventricular pacing on cardiac output in severe pulmonary arterial hypertension. Heart Vessels. 2020 Jun;35(6):852-858. Epub 2019 Dec 2. [CrossRef] [PubMed]
- Varma N, Bourge RC, Stevenson LW, Costanzo MR, Shavelle D, Adamson PB, Ginn G, Henderson J, Abraham WT; CHAMPION Investigator Group. Remote Hemodynamic-Guided Therapy of Patients With Recurrent Heart Failure Following Cardiac Resynchronization Therapy. J Am Heart Assoc. 2021 Feb;10(5):e017619. Epub 2021 Feb 25. PMCID: PMC8174266. [CrossRef] [PubMed]
| Evaluated Parameters |
Median (Q1–Q3) n (%) |
| General characteristic | |
| Population size | 39 (100%) |
| Female – n (%) | 8 (20,50%) |
| Age at HF onset [years] | 52.46 (43.16; 55.75) |
| NYHA II | 17 (43.59%) |
| NYHA III | 21 (53.85%) |
| NYHA IV | 1 (2.56%) |
| NT-proBNP [pg/ml] | 1041 (275.0; 1863) |
| 6MWT [m] | 465.0 (409.0; 547.0) |
| Hypertension | 14 (35.89%) |
| Diabetes mellitus | 6 (15,38%) |
| Electrocardiographic and echocardiographic data | |
| PQ interval duration[ms] | 200.0 (180.0; 220.0) |
| QRS complex duration[ms] | 160.0 (160.0; 180.0) |
| DFT / RR [%] | 44.00 (38.00; 47.00) |
| IVMD [ms] | 67,00 (45,00; 75,00) |
| LVEDD [mm] | 69.00 (64.00; 78.00) |
| LVESD [mm] | 61.00 (55.00; 70.00) |
| LVEDV [ml] | 230.0 (189.0; 280.0) |
| LVESV [ml] | 170.0 (140.0; 220.0) |
| LVEF [%] | 22.00 (17.00; 25.00) |
| Hemodynamic data | |
| sPAP [mmHg] | 39.00 (32.00; 54.75) |
| dPAP [mmHg] | 23.33 (16.00; 31.60) |
| mPAP [mmHg] | 28.60 (20.33; 40.00) |
| sAP [mmHg] | 125.0 (112.0; 135.0) |
| dAP [mmHg] | 78.00 (71.67; 90.0) |
| mAP [mmHg] | 92.00 (85.50; 103.3) |
| HR [min] | 78.00 (70.00; 8.00) |
| SV [ml] | 60.88 (42.47; 80.40) |
| PAWP [mmHg] | 20.00 (14.00; 28.50) |
| RVs [mmHg] | 40.00 (33.00; 56.00) |
| RVd [mmHg] | 8.00 (5.00; 10.00) |
| PVR [WU] | 1.92 (1.18; 3.46) |
| TPG [WU] | 9.33 (6.00; 12.61) |
| TPR [WU] | 6.20 (3.75; 10.68) |
| SVR [WU] | 19.45 (13.97; 22.42) |
| RAP [mmHg] | 8.00 (5.00; 12.00) |
| CO [l/min] | 4.51 (3.40; 5.40) |
|
Baseline Median (Q1-Q3) n% |
Follow-Up Median (Q1-Q3) n% |
Wilcoxon Test Chi-Square Test P |
|
| NYHA I/II | 17 (43.59%) | 34 (87.18%) | <0.001 |
| NYHA III/IV | 22 (56.41%) | 5 (12.82%) | <0.001 |
| 6MWT [m] | 465.0 (409.0 – 547.0) | 515.5 (462.0 – 567.5) | <0.001 |
| NT-proBNP [pg/ml] | 1041 (275.0 – 1863) | 587 (104 – 1420) | 0.018 |
| QRS complex [ms] | 160.0 (160.0 – 180.0) | 160.0 (140.0 – 160.0) | <0.001 |
| AVD [ms] | 200.0 (180.0 – 220.0) | 95.00 (70.00 – 120.0) | <0.001 |
| DFT / RR [%] | 44.00 (38.00 – 47.00) | 48.00 (44.00 – 51.00) | 0.002 |
| IVMD [ms] | 67.00 (45.00 – 75.00) | 15.00 (8.00 – 35.00) | <0.001 |
| LVEDV [ml] | 230.0 (189.0 – 280.0) | 170.0 (137.0 – 240.0) | <0.001 |
| LVESV [ml] | 170.0 (140.0 – 220.0) | 117.0 (80.00 – 170.0) | <0.001 |
| LVEDD [mm] | 69.00 (64.00 – 78.00) | 63.00 (57.00 – 74.00) | 0.004 |
| LVESD [mm] | 61.00 (55.00 – 70.00) | 53.00 (46.00 – 67.00) | <0.001 |
| LVEF [%] | 22.00 (17.00 – 25.00) | 30.00 (21.00 – 38.00) | <0.001 |
| Responder | Nonresponder | |
| 6MWT ↑ ≥10% | 10 (25,64%) | 29 (74,36%) |
| NT-proBNP ↓ ≥30% | 16 (41,03%) | 23 (58,97) |
| NYHA ↓ by I | 13 (33,33%) | 26 (66,67%) |
| LVEDV↓ ≥15% | 19 (48,72%) | 20 (51,28%) |
| LVESV↓ ≥10% | 23 (58,97%) | 16 (41,03%) |
| LVESV↓ ≥15% | 21 (53,85%) | 18 (46,15%) |
| LVESV ↓ ≥ 30% | 16 (41,03%) | 23 (58,97%) |
| LVEF ↑ ≥5% | 27 (69,23%) | 12 (30,77%) |
| LVEF ↑ ≥10% | 25 (64,10%) | 14 (35,90%) |
| LVEF ↑ ≥15% | 25 (64,10%) | 14 (35,90%) |
|
6MWT ↑ ≥10% OR, 95% CI, p |
NT-proBNP ↓ ≥30% OR, 95% CI, p |
NYHA ↓by I OR, 95% CI, p |
LVEDV ↓ ≥15% OR, 95% CI, p |
LVESV ↓ ≥10% OR, 95% CI, p |
LVESV ↓ ≥15% OR, 95% CI, p |
LVESV ↓ ≥30% OR, 95% CI, p |
LVEF ↑ ≥5% OR, 95% CI, p |
LVEF ↑ ≥10% OR, 95% CI, p |
LVEF ↑ ≥15% OR, 95% CI, p |
|
| Female | 0.786 0.126-4.885 p=0.789 |
0.438 0.087-2.196 p=0.299 |
1.000 0.195-5.125 p=1.000 |
0.218 0.037-1.306 p=0.085 |
0.250 0.049-1.281 p=0.086 |
0.333 0.066-1.684 p=0.169 |
0.327 0.055-1.948 p=0.204 |
0.857 0.166-4.438 p=0.849 |
1.158 0.228-5.882 p=0.855 |
1.158 0.228-5.883 p=0.855 |
| Age [years] |
0.989 0.902-1.085 p=0.812 |
0.930 0.851-1.016 p=0.098 |
0.999 0.917-1.088 p=0.973 |
0.981 0.904-1.064 p=0.626 |
0.952 0.873-1.039 p=0.256 |
0.956 0.879-1.041 p=0.284 |
0.921 0.841-1.008 p=0.064 |
0.942 0.854-1.038 p=0.212 |
0.952 0.869-1.042 p=0.267 |
0.943 0.860-1.035 p=0.202 |
| NYHA | 1.342 0.294-6.136 p=0.695 |
0.788 0.201-3.099 p=0.725 |
1.784 0.427-7.456 p=0.412 |
0.491 0.117-2.052 p=0.313 |
0.326 0.074-1.446 p=0.127 |
0.311 0.069-1.402 p=0.116 |
0.565 0.132-2.416 p=0.426 |
0.435 0.099-1.899 p=0.252 |
0.251 0.053-1.193 p=0.072 |
0.556 0.135-2.289 p=0.400 |
| 6MWT [m] |
0.882 0.785-0.991 p=0.028 |
1.061 0.981-1.146 p=0.124 |
1.057 0.977-1.143 p=0.156 |
1.103 1.008-1.207 p=0.028 |
1.102 1.004-1.211 p=0.035 |
1.123 1.017-1.239 p=0.018 |
1.094 1.003-1.193 p=0.035 |
1.045 0.962-1.134 p=0.282 |
1.079 0.988-1.179 p=0.078 |
1.047 0.967-1.133 p=0.243 |
| NT-proBNP [pg/ml] |
1.005 0.938-1.077 p=0.890 |
0.962 0.903-1.025 p=0.218 |
0.976 0.914-1.043 p=0.461 |
0.938 0.873-1.009 p=0.074 |
0.946 0.884-1.012 p=0.093 |
0.951 0.890-1.017 p=0.126 |
0.949 0.884-1.020 p=0.141 |
0.951 0.891-1.015 p=0.118 |
0.943 0.881-1.009 p=0.078 |
0.974 0.917-1.035 p=0.378 |
| QRS [ms] |
0.998 0.961-1.036 p=0.907 |
1.002 0.970-1.036 p=0.888 |
0.982 0.947-1.019 p=0.320 |
1.009 0.976-1.043 p=0.592 |
0.992 0.958-1.026 p=0.623 |
1.006 0.973-1.040 p=0.722 |
1.008 0.975-1.043 p=0.623 |
1.014 0.978-1.051 p=0.448 |
1.026 0.988-1.065 p=0.165 |
1.016 0.981-1.053 p=0.360 |
| PQ [ms] |
1.005 0.977-1.033 p=0.741 |
0.965 0.932-0.998 p=0.029 |
0.993 0.967-1.020 p=0.590 |
0.980 0.952-1.008 p=0.134 |
0.992 0.966-1.019 p=0.526 |
0.994 0.970-1.020 p=0.654 |
0.981 0.955-1.008 p=0.152 |
0.980 0.950-1.011 p=0.179 |
0.988 0.962-1.015 p=0.369 |
0.988 0.962-1.015 p=0.369 |
| DFT/RR [%] |
0.959 0.859-1.071 p=0.444 |
0.946 0.859-1.043 p=0.249 |
0.984 0.890-1.087 p=0.738 |
0.998 0.915-1.090 p=0.971 |
0.989 0.902-1.084 p=0.803 |
0.963 0.877-1.057 p=0.410 |
1.009 0.921-1.107 p=0.838 |
1.003 0.910-1.104 p=0.955 |
0.985 0.898-1.082 p=0.750 |
0.977 0.890-1.073 p=0.617 |
| IVMD [ms] |
0.993 0.962-1.026 p=0.677 |
1.005 0.977-1.033 p=0.732 |
0.997 0.968-1.026 p=0.821 |
1.016 0.987-1.046 p=0.270 |
1.037 1.001-1.074 p=0.038 |
1.039 1.002-1.077 p=0.031 |
1.017 0.987-1.049 p=0.248 |
1.024 0.991-1.057 p=0.136 |
1.031 0.997-1.066 p=0.061 |
1.015 0.985-1.045 p=0.310 |
| LVEDD [mm] |
1.012 0.938-1.092 p=0.752 |
0.956 0.891-1.026 p=0.199 |
0.980 0.912-1.053 p=0.571 |
0.963 0.899-1.032 p=0.273 |
0.942 0.875-1.014 p=0.101 |
0.946 0.880-1.017 p=0.122 |
0.946 0.878-1.018 p=0.127 |
0.918 0.844-0.999 p=0.039 |
0.893 0.816-0.978 p=0.012 |
0.963 0.897-1.035 p=0.290 |
| LVEF [%] |
1.051 0.924-1.197 p=0.433 |
1.048 0.934-1.177 p=0.409 |
1.146 0.993-1.322 p=0.054 |
1.016 0.907-1.137 p=0.781 |
1.057 0.938-1.191 p=0.349 |
1.048 0.933-1.178 p=0.411 |
1.088 0.961-1.231 p=0.169 |
1.024 0.905-1.159 p=0.692 |
1.023 0.909-1.152 p=0.698 |
1.019 0.906-1.147 p=0.742 |
| sPAP [mmHg] |
0.966 0.914-1.021 p=0.207 |
0.956 0.910-1.004 p=0.060 |
0.951 0.900-1.005 p=0.063 |
0.970 0.926-1.016 p=0.182 |
0.954 0.909-1.002 p=0.053 |
0.952 0.906-1.000 p=0.044 |
0.913 0.854-0.975 p=0.005 |
0.944 0.894-0.996 p=0.029 |
0.951 0.904-1.000 p=0.044 |
0.962 0.916-1.009 p=0.100 |
| dPAP [mmHg] |
0.936 0.851-1.028 p=0.154 |
0.924 0.851-1.003 p=0.050 |
0.926 0.847-1.013 p=0.081 |
0.970 0.903-1.043 p=0.399 |
0.955 0.886-1.028 p=0.207 |
0.955 0.887-1.028 p=0.208 |
0.875 0.789-0.971 p=0.009 |
0.901 0.823-0.986 p=0.019 |
0.901 0.825-0.984 p=0.017 |
0.938 0.867-1.014 p=0.097 |
| mPAP [mmHg] |
0.946 0.874-1.024 p=0.156 |
0.935 0.873-1.002 p=0.048 |
0.933 0.865-1.007 p=0.067 |
0.968 0.910-1.030 p=0.294 |
0.950 0.891-1.014 p=0.113 |
0.949 0.890-1.013 p=0.106 |
0.887 0.811-0.970 p=0.007 |
0.917 0.850-0.989 p=0.020 |
0.922 0.857-0.991 p=0.023 |
0.946 0.885-1.012 p=0.093 |
| SV [ml] |
1.022 0.989-1.056 p=0.173 |
1.022 0.992-1.053 p=0.136 |
1.010 0.981-1.040 p=0.469 |
0.984 0.957-1.013 p=0.267 |
1.010 0.982-1.039 p=0.469 |
1.006 0.979-1.035 p=0.641 |
1.021 0.991-1.052 p=0.150 |
1.029 0.994-1.065 p=0.091 |
1.021 0.989-1.053 p=0.184 |
0.946 0.885-1.012 p=0.093 |
| PAWP [mmHg] |
0.954 0.872-1.043 p=0.284 |
0.959 0.888-1.035 p=0.269 |
0.961 0.886-1.043 p=0.329 |
1.022 0.949-1.101 p=0.546 |
0.983 0.913-1.060 p=0.648 |
0.991 0.921-1.066 p=0.800 |
0.939 0.865-1.020 p=0.124 |
0.934 0.857-1.019 p=0.112 |
0.947 0.874-1.027 p=0.176 |
1.016 0.986-1.047 p=0.295 |
| PVR [WU] |
0.597 0.314-1.132 p=0.102 |
0.534 0.308-0.924 p=0.020 |
0.561 0.307-1.025 p=0.052 |
0.689 0.437-1.088 p=0.098 |
0.657 0.417-1.034 p=0.060 |
0.625 0.388-1.007 p=0.046 |
0.329 0.146-0.741 p=0.006 |
0.537 0.320-0.902 p=0.015 |
0.543 0.324-0.908 p=0.016 |
0.587 0.361-0.955 p=0.026 |
| TPG [mmHg] |
0.924 0.788-1.084 p=0.314 |
0.860 0.738-1.001 p=0.044 |
0.840 0.704-1.002 p=0.046 |
0.769 0.632-0.936 p=0.007 |
0.844 0.723-0.985 p=0.026 |
0.802 0.673-0.956 p=0.011 |
0.688 0.532-0.890 p=0.003 |
0.830 0.706-0.976 p=0.020 |
0.805 0.678-0.956 p=0.011 |
0.838 0.716-0.981 p=0.023 |
| RAP [mmHg] |
0.812 0.659-1.000 p=0.043 |
0.863 0.726-1.025 p=0.083 |
0.754 0.603-0.943 p=0.010 |
0.817 0.676-0.986 p=0.030 |
0.934 0.797-1.094 p=0.381 |
0.907 0.771-1.067 p=0.224 |
0.863 0.725-1.027 p=0.087 |
0.939 0.795-1.110 p=0.447 |
0.964 0.822-1.130 p=0.638 |
0.943 0.801-1.110 p=0.466 |
| CO [l/min] |
1.486 0.925-2.385 p=0.090 |
1.520 0.946-2.445 p=0.074 |
1.216 0.792-1.867 p=0.354 |
0.923 0.613-1.390 p=0.692 |
1.365 0.860-2.167 p=0.172 |
1.360 0.864-2.143 p=0.169 |
1.502 0.947-2.382 p=0.074 |
1.637 0.923-2.903 p=0.081 |
1.383 0.853-2.244 p=0.174 |
1.515 0.894-2.567 p=0.110 |
| Cut Off Value | Specificity/Sensitivity [%] | AUC | 95% CI | |
| PVR [WU] | ||||
| 6MWT ↑ ≥10% | 1.42 | 70.0 | 0.703±0.098 | (0.511-0.896) |
| NT-proBNP ↓ ≥30% | 1.80 | 68.4 | 0.734±0.08 | (0.577-0.892) |
| NYHA ↓ by I | 1.81 | 61.5 | 0.683±0.086 | (0.514-0.853) |
| LVEDV↓ ≥15% | 1.81 | 66.7 | 0.692±0.088 | (0.520-0.864) |
| LVESV↓ ≥10% | 2.12 | 69.2 | 0.709±0.086 | (0.541-0.877) |
| LVESV↓ ≥15% | 1.92 | 71.8 | 0.73±0.082 | (0.569-0.891) |
| LVESV ↓ ≥ 30% | 1.80 | 75.0 | 0.823±0.069 | (0.688-0.959) |
| LVEF ↑ ≥5% | 2.16 | 66.7 | 0.765±0.081 | (0.606-0.925) |
| LVEF ↑ ≥10% | 2.16 | 68.0 | 0.757±0.081 | (0.599-0.915) |
| LVEF ↑ ≥15% | 2.12 | 64.2 | 0.737±0.08 | (0.580-0.894) |
| TPG [mmHg] | ||||
| 6MWT ↑ ≥10% | 7.93 | 61.0 | 0.619±0.11 | (0.404-0.834) |
| NT-proBNP ↓ ≥30% | 8.0 | 69.2 | 0.717±0.084 | (0.552-0.882) |
| NYHA ↓ by I | 7.93 | 69.2 | 0.701±0.086 | (0.533-0.869) |
| LVEDV↓ ≥15% | 8.0 | 79.5 | 0.821±0.074 | (0.676-0.966) |
| LVESV↓ ≥10% | 9.5 | 79.7 | 0.749±0.086 | (0.58-0.918) |
| LVESV↓ ≥15% | 9.33 | 77.0 | 0.784±0.08 | (0.628-0.941) |
| LVESV ↓ ≥ 30% | 7.93 | 81.9 | 0.856±0.065 | (0.729-0.983) |
| LVEF ↑ ≥5% | 10.0 | 74.5 | 0.765±0.083 | (0.603-0.928) |
| LVEF ↑ ≥10% | 9.8 | 72.0 | 0.783±0.077 | (0.632-0.933) |
| LVEF ↑ ≥15% | 9.78 | 70.0 | 0.753±0.08 | (0.597-0.909) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





