Submitted:
14 February 2025
Posted:
17 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Definition
2.2. Data Collection
2.3. Echocardiography Procedure
2.4. Statistical Analysis
3. Results
3.1. Overall Patients’ Characteristics.
3.2. Subclinical Echocardiographic Findings
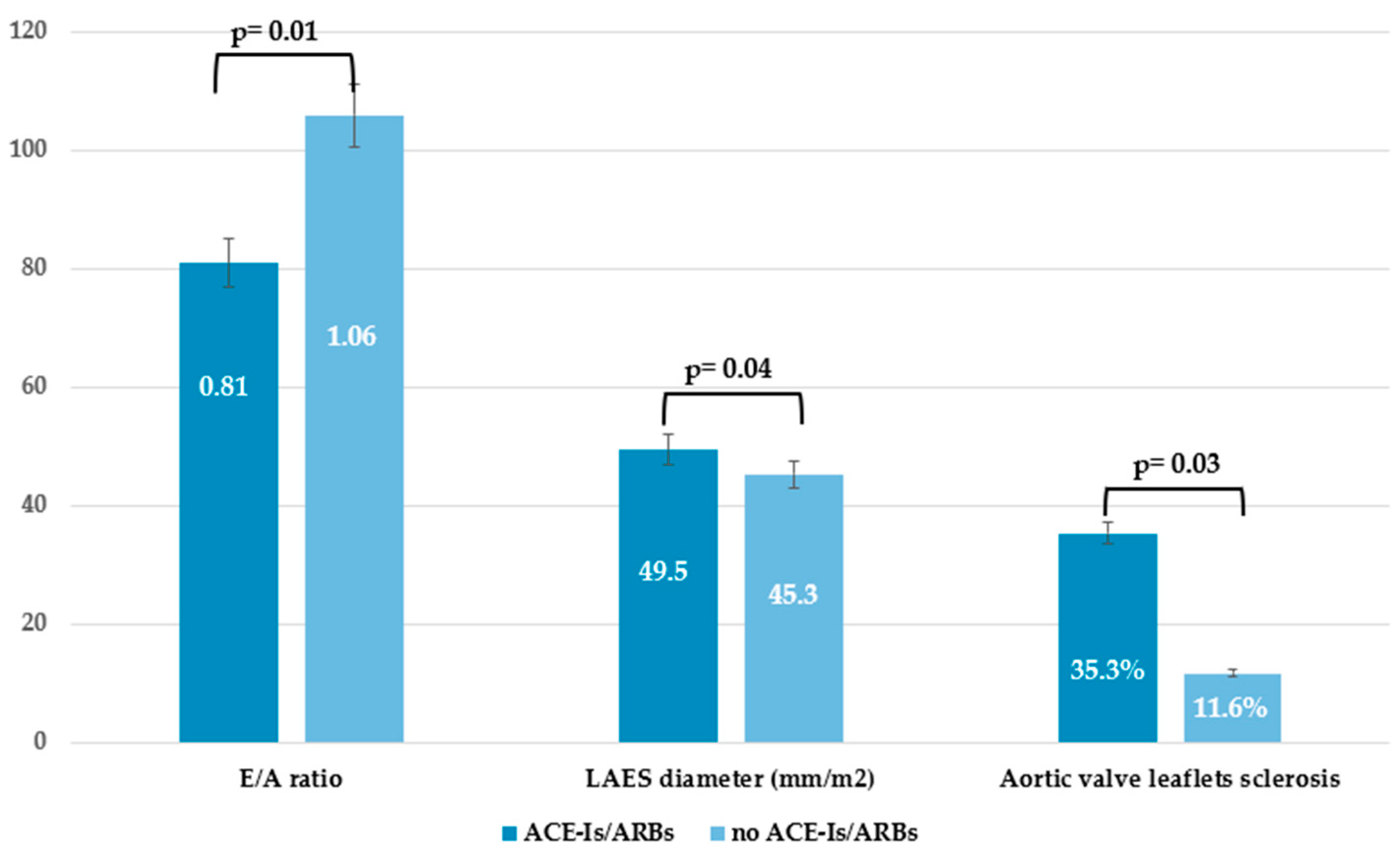
3.3. Subclinical Echocardiographic Findings by Comparing Patients Receiving Current Therapy with Vasodilators
3.4. General Multivariable Regression Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko J, Noviani M, Chellamuthu VR, Albani S, Low AHL. The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity. Int J Mol Sci. 2023 Sep 19;24(18):14287. [CrossRef]
- Rosa I, Romano E, Fioretto BS, Manetti M. The contribution of mesenchymal transitions to the pathogenesis of systemic sclerosis. Eur J Rheumatol. 2020 Oct;7(Suppl 3):S157-S164. [CrossRef]
- Herrick AL, Assassi S, Denton CP. Skin involvement in early diffuse cutaneous systemic sclerosis: an unmet clinical need. Nat Rev Rheumatol. 2022 May;18(5):276-285. [CrossRef]
- LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001 Jul;28(7):1573-6.
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013 Nov;65(11):2737-47.
- Yang M, Goh V, Lee J, Espinoza M, Yuan Y, Carns M et al. Clinical Phenotypes of Patients with Systemic Sclerosis With Distinct Molecular Signatures in Skin. Arthritis Care Res (Hoboken). 2023 Jul;75(7):1469-1480. [CrossRef]
- Bellando-Randone S, Galdo FD, Lepri G, et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS). The Lancet Rheumatology 2021; 3: e834–43. [CrossRef]
- Avouac J, Fransen J, Walker UA, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis 2011; 70: 476–81. [CrossRef]
- Blaja E, Jordan S, Mihai CM, Dobrota R, Becker MO et al. The Challenge of Very Early Systemic Sclerosis: A Combination of Mild and Early Disease? J Rheumatol. 2021 Jan 1;48(1):82-86. [CrossRef]
- Ross RL, Caballero-Ruiz B, Clarke EL, Kakkar V, Wasson CW, Mulipa P et al. Biological hallmarks of systemic sclerosis are present in the skin and serum of patients with Very Early Diagnosis of SSc (VEDOSS). Rheumatology (Oxford). 2024 Dec 19:keae698. Epub ahead of print. [CrossRef]
- El Aoufy K, Melis MR, Bandini G, et al POS1593-HPR GASTROINTESTINAL INVOLVEMENT IS ALREADY REPORTED IN AN ITALIAN VEDOSS COHORT: RESULTS FROM A RHEUMATOLOGICAL NURSE ASSESSMENT Annals of the Rheumatic Diseases 2023;82:1172-1173. [CrossRef]
- Zhu L, Wang Y, Zhao S, Lu M. Detection of myocardial fibrosis: Where we stand. Front Cardiovasc Med. 2022 Sep 29;9:926378. [CrossRef]
- Cusmà Piccione M, Zito C, Bagnato G, Oreto G, Di Bella G, Bagnato G, Carerj S. Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovasc Ultrasound. 2013 Feb 3;11:6. [CrossRef]
- Lee SW, Choi EY, Jung SY, Choi ST, Lee SK, Park YB. E/E’ ratio is more sensitive than E/A ratio for detection of left ventricular diastolic dysfunction in patients with systemic sclerosis. Clin Exp Rheumatol. 2010 Mar-Apr;28(2 Suppl 58):S12-7.
- Pokeerbux, M.R.; Giovannelli, J.; Dauchet, L.; Mouthon, L.; Agard, C.; Lega, J.C.; Allanore, Y.; Jego, P.; Bienvenu, B.; Berthier, S.; et al. Survival and prognosis factors in systemic sclerosis: Data of a French multicenter cohort, systematic review, and meta-analysis of the literature. Arthritis Res. Ther. 2019, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Avouac J, Fransen J, Walker UA, Riccieri V, Smith V, Muller C, Miniati I, Tarner IH, Randone SB, Cutolo M, Allanore Y, Distler O, Valentini G, Czirjak L, Müller-Ladner U, Furst DE, Tyndall A, Matucci-Cerinic M; EUSTAR Group. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis. 2011 Mar;70(3):476-81. [CrossRef]
- Ruaro B, Smith V, Sulli A, Decuman S, Pizzorni C, Cutolo M. Methods for the morphological and functional evaluation of microvascular damage in systemic sclerosis. Korean J Intern Med. 2015 Jan;30(1):1-5. [CrossRef]
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019 Jan;32(1):1-64. [CrossRef]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology Journal of the American Society of Echocardiography, Volume 18, Issue 12, 1440 – 1463.
- Devereux RB, Alonso DR, Lutas EM,Gottlieb GJ, Campo E, Sachs I, Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458.
- Pyxaras SA, Pinamonti B, Barbati G, Santangelo S, Valentincic M, Cettolo F, Secoli G, Magnani S, Merlo M, Lo Giudice F, Perkan A, Sinagra G. Echocardiographic evaluation of systolic and mean pulmonary artery pressure in the follow-up of patients with pulmonary hypertension. Eur J Echocardiogr. 2011 Sep;12(9):696-701. [CrossRef]
- Skinner H, Kamaruddin H, Mathew T. Tricuspid Annular Plane Systolic Excursion: Comparing Transthoracic to Transesophageal Echocardiography. J Cardiothorac Vasc Anesth. 2017 Apr;31(2):590-594. [CrossRef]
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA et al. (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10(2):165–193. [CrossRef]
- Schwarz ER, Dashti R. The clinical quandary of left and right ventricular diastolic dysfunction and diastolic heart failure. Cardiovasc J Afr. 2010 Jul-Aug;21(4):212-20. [CrossRef]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e35-e71. Erratum in: Circulation. 2021 Feb 2;143(5):e228. Erratum in: Circulation. 2021 Mar 9;143(10):e784.
- Gotschy A, Jordan S, Stoeck CT, von Deuster C, Peer T, Gastl M, Vishnevskiy V, Wissmann L, Dobrota R, Mihai C, Becker MO, Maurer B, Kozerke S, Ruschitzka F, Distler O, Manka R. Diffuse myocardial fibrosis precedes subclinical functional myocardial impairment and provides prognostic information in systemic sclerosis. Eur Heart J Cardiovasc Imaging. 2023 Feb 17;24(3):373-382. [CrossRef]
- De Luca G, Campochiaro C, Peretto G, Busnardo E, Matucci-Cerinic M, Dagna L. Cardiac involvement, a threatening very early manifestation of systemic sclerosis: evidence from VEDOSS patients. Clin Exp Rheumatol. 2023 Aug;41(8):1723-1724. [CrossRef]
- Volkmann ER, Fischer A. Update on Morbidity and Mortality in Systemic Sclerosis-Related Interstitial Lung Disease. J Scleroderma Relat Disord. 2021 Feb;6(1):11-20. [CrossRef]
- Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, Jones SR, Toth PP. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am J Prev Cardiol. 2020 Nov 21;4:100130. [CrossRef]
- Rangarajan, V.; Matiasz, R.; Freed, B.H. Cardiac complications of systemic sclerosis and management: Recent progress. Curr. Opin. Rheumatol. 2017, 29, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Giunta A, Tirri E, Maione S et al.: Right ventricular diastolic abnormalities in systemic sclerosis. Relation to left ventricular involvement and pulmonary hypertension. Ann Rheum Dis 2000; 59: 94-8. [CrossRef]
- Markusse IM, Meijs J, de Boer B, Bakker JA, Schippers HPC, Schouffoer AA, Ajmone Marsan N, Kroft LJM, Ninaber MK, Huizinga TWJ, de Vries-Bouwstra JK. Predicting cardiopulmonary involvement in patients with systemic sclerosis: complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology (Oxford). 2017 Jul 1;56(7):1081-1088. [CrossRef]
- Pagkopoulou E, Soulaidopoulos S, Triantafyllidou E, Arvanitaki A, Katsiki N, Loutradis C, Karagiannis A, Doumas M, Garyfallos A, Kitas GD, Dimitroulas T. Peripheral microcirculatory abnormalities are associated with cardiovascular risk in systemic sclerosis: a nailfold video capillaroscopy study. Clin Rheumatol. 2021 Dec;40(12):4957-4968. [CrossRef]
- Szucs, G.; Tímár, O.; Szekanecz, Z.; Dér, H.; Kerekes, G.; Szamosi, S.; Shoenfeld, Y.; Szegedi, G.; Soltész, P. Endothelial Dysfunction Precedes Atherosclerosis in Systemic Sclerosis–Relevance for Prevention of Vascular Complications. Rheumatology 2007, 46, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Zanatta E, Famoso G, Boscain F, Montisci R, Pigatto E, Polito P, Schiavon F, Iliceto S, Cozzi F, Doria A, Tona F. Nailfold avascular score and coronary microvascular dysfunction in systemic sclerosis: A newsworthy association. Autoimmun Rev. 2019 Feb;18(2):177-183. [CrossRef]
- Tsai CY, Hsieh SC, Wu TH, Li KJ, Shen CY, Liao HT, Wu CH, Kuo YM, Lu CS, Yu CL. Pathogenic Roles of Autoantibodies and Aberrant Epigenetic Regulation of Immune and Connective Tissue Cells in the Tissue Fibrosis of Patients with Systemic Sclerosis. Int J Mol Sci. 2020 Apr 27;21(9):3069. [CrossRef]
- Hénault J, Robitaille G, Senécal JL, Raymond Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Rheum. 2006 Mar;54(3):963-73. [CrossRef]
- Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM, Hsieh SC, Yu CL. Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis. Cells. 2021 Dec 2;10(12):3402. [CrossRef]
- Bellando-Randone S, Galdo FD, Lepri G, et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS). Lancet Rheumatol 2021; 3: e834–843.
- Guédon AF, Carrat F, Mouthon L, Launay D, Chaigne B, Pugnet G, Lega JC, Hot A, Cottin V, Agard C, Allanore Y, Fauchais AL, Lescoat A, Dhote R, Papo T, Chatelus E, Bonnotte B, Kahn JE, Diot E, Aouba A, Magy-Bertrand N, Queyrel V, Le Quellec A, Kieffer P, Amoura Z, Granel B, Gaultier JB, Balquet MH, Wahl D, Lidove O, Espitia O, Cohen A, Fain O, Hachulla E, Mekinian A, Rivière S. Vasodilator drugs and heart-related outcomes in systemic sclerosis: an exploratory analysis. RMD Open. 2024 Dec 9;10(4):e004918. [CrossRef]
- Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019 Apr 1;18(1):45. [CrossRef] [PubMed] [PubMed Central]
- Alcocer LA, Bryce A, De Padua Brasil D, Lara J, Cortes JM, Quesada D, Rodriguez P. The Pivotal Role of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Hypertension Management and Cardiovascular and Renal Protection: A Critical Appraisal and Comparison of International Guidelines. Am J Cardiovasc Drugs. 2023 Nov;23(6):663-682. [CrossRef]
- Bütikofer L, Varisco PA, Distler O, Kowal-Bielecka O, Allanore Y, Riemekasten G, Villiger PM, Adler S; EUSTAR collaborators. ACE inhibitors in SSc patients display a risk factor for scleroderma renal crisis-a EUSTAR analysis. Arthritis Res Ther. 2020 Mar 24;22(1):59. [CrossRef]
- He H, Tong X, Ning Z, Zhou J, Du C, Wang Y, Wang Q, Xu D, Zeng X, He ZX, Zhao X. Diffusing capacity of lungs for carbon monoxide associated with subclinical myocardial impairment in systemic sclerosis: A cardiac MR study. RMD Open. 2023 Dec 7;9(4):e003391. [CrossRef]
| TOTAL n=61 |
e-VEDOSS n=37 |
ml-VEDOSS n=24 |
p-Value | |
|---|---|---|---|---|
| Female, n(%) | 58 (95.1) | 36 (97.3) | 22 (91.7) | 0.56 |
| Male, n(%) | 3 (4.9) | 1 (2.7) | 2 (8.3) | 0.56 |
| Age at enrollment, mean±SD | 58.7 ± 14.5 | 55.2 ± 14.6 | 64.0 ± 12.8 | 0.01 |
| Age at VEDOSS diagnosis, mean±SD | 49.8 ± 14.7 | 51.7 ± 15.1 | 47.3 ±14.1 | 0.27 |
| Age at RP onset, mean±SD | 43.2 ±17.1 | 44.2 ± 17.4 | 41.8 ± 16.9 | 0.62 |
| Disease Duration of VEDOSS, mean±SD | 9.9 ± 7.9 | 4.9 ± 2.7 | 16.8 ± 7.7 | <0.001 |
| Disease duration of RP, mean±SD | 16.1 ± 11.6 | 12.3 ± 10.9 | 21.6 ± 10.4 | 0.002 |
| Diagnostic delay, mean±SD | 6.4 ± 9.5 | 7.5 ± 10.6 | 4.7 ± 7.4 | 0.28 |
| Body Mass Index (Kg/m2), mean±SD | 23.5 ± 5.9 | 22.4 ± 5.7 | 25.2 ± 5.9 | 0.07 |
| Smoking habits, n(%) | 10 (16.4) | 8 (21.6) | 2 (8.3) | 0.29 |
| Alcohol Consumption, n(%) | 13 (21.3) | 2 (5.3) | 1 (4.2) | 1.0 |
| Puffy hands, n(%) | 18 (29.5) | 10 (27.0) | 8 (33.3) | 1.0 |
| ANA (positive) only, n(%) | 21 (34.4) | 16 (43.2) | 5 (20.8) | 0.09 |
| Anticentromere (positive), n(%) | 21 (34.4) | 9 (24.3) | 12 (50) | 0.05 |
| Anti-topoisomerase I (positive), n(%) | 3 (4.9) | 2 (5.3) | 1 (4.2) | 1.0 |
| Gastrointestinal tract symptoms, n(%) | 30 (49.2) | 15 (40.5) | 15 (62.5) | 0.12 |
| COMORBIDITIES, n(%) | ||||
| Cardiovascular diseases, | 29 (47.5) | 13 (35.1) | 16 (66.7) | 0.02 |
| Dyslipidemia | 17 (27.9) | 9 (24.3) | 8 (33.3) | 0.56 |
| Hyperuricemia | 2 (3.3) | 0 (0) | 2 (8.3) | 0.15 |
| Type 2 Diabetes Mellitus | 4 (6.6) | 2 (5.3) | 2 (8.3) | 0.64 |
| Thyroid disorders | 13 (21.3) | 7 (18.9) | 6 (25.0) | 0.75 |
| Lung Diseases | 12 (19.6) | 6 (16.2) | 6 (25.0) | 0.51 |
| Kidney Diseases | 8 (13.1) | 4 (10.8) | 4 (16.7) | 0.7 |
| Gastrointestinal diseases | 35 (57.4) | 17 (45.9) | 18 (75.0) | 0.03 |
| Hematological disorders | 8 (13.1) | 2 (5.3) | 6 (25.0) | 0.04 |
| Malignancies | 10 (16.4) | 5 (13.5) | 5 (20.8) | 0.49 |
| Psychiatric disorders | 3 (4.9) | 2 (5.3) | 1 (4.2) | 1.0 |
| Neurological disorders | 11 (18.0) | 5 (13.5) | 6 (25.0) | 0.32 |
| Comorbidities count, mean±SD | 2.7 ± 2.1 | 2.1 ±1.9 | 3.6 ± 2.0 | 0.005 |
| Comorbidities count ≥ 3, n(%) | 31 (50.8) | 15 (40.5) | 16 (66.7) | 0.01 |
|
NVC PATTERN, n(%) Aspecific alterations Early pattern Active pattern Late pattern |
7 (11.5) 34 (55.7) 14 (23.0) 6 (9.8) |
5 (13.5) 23 (62.2) 9 (24.3) 0 (0) |
2 (8.3) 11 (45.8) 5 (20.8) 6 (25.0) |
0.69 0.29 1.0 0.002 |
| TREATMENT, n(%) | ||||
| Iloprost | 44 (72.1) | 23 (62.2) | 21 (87.5) | 0.04 |
| Calcium Channel Blockers | 20 (32.7) | 8 (21.6) | 12 (50.0) | 0.03 |
| Low-dose Aspirin | 39 (63.9) | 23 (62.2) | 16 (66.7) | 0.78 |
| ACE-I/ARBs | 16 (26.2) | 6 (16.2) | 10 (41.7) | 0.04 |
| Beta-Blockers | 4 (6.6) | 2 (5.3) | 2 (8.3) | 0.64 |
| Diuretics | 1 (1.6) | 1 (2.7) | 0 | 1.0 |
| Hydroxychloroquine | 20 (32.7) | 9 (24.3) | 11 (45.8) | 0.09 |
| Immunosuppressants | 3 (4.9) | 1 (2.7) | 2 (8.3) | 0.56 |
| ECHOCARDIOGRAPHIC PARAMETERS | TOTAL n=61 |
|---|---|
| E deceleration time (m/s), mean±SD | 244.3±281.7 |
| E/E’ ratio, mean±SD | 6.9±1.9 |
| E wave, (m/s), mean±SD | 0.69±0.17 |
| A wave, (m/s), mean±SD | 0.75±0.19 |
| E/A ratio, mean±SD | 0.99±0.39 |
| E/A ratio < 1.0, n (%) | 37 (60.7) |
| LVED diameter, (mm), mean±SD | 40.3±4.8 |
| PWED thickness, (mm), mean±SD | 8.4±1.6 |
| PWED > 9 mm, n(%) | 19 (31.1) |
| IVS thckness, (mm), mean±SD | 9.3±1.8 |
| IVS > 10 mm, n (%) | 17 (27.9) |
| LVED volume 4CH Simpson, (ml), mean±SD | 67.7±16.6 |
| LVES volume 4CH Simpson, (ml), mean±SD | 25.5±11.8 |
| LVED volume 4CH AL, (ml/m2), mean±SD | 43.4±8.8 |
| LVES volume 4CH AL, (ml/m2), mean±SD | 14.7±4.3 |
| EF%, mean±SD | 64.5±4.8 |
| EF%<55% | 3 (4.9) |
| Mass ASE, (g), mean±SD | 123.2±123.9 |
| Mass/BSA, (g/m2), mean±SD | 66.3±17.3 |
| Relative wall thickness, mean±SD | 0.44±0.10 |
| Mass/height, (g/m), mean±SD | 65.8±18.2 |
| Aortic diameter, (mm2), mean±SD | 29.7±3.9 |
| LAES area, (cm2), mean±SD | 15.6±3.6 |
| LAES 4CH Simpson, (ml), mean±SD | 40.4±13.7 |
| LAES 4CH ind, (ml/m2), mean±SD | 24.4±7.5 |
| LAES diameter sup-inf 4CH, (mm/m2), mean±SD | 46.5±7.6 |
| RAES diameter AL, (mm), mean±SD | 46.1±5.8 |
| RAES 4CH Simpson, (ml), mean±SD | 30.3±8.9 |
| RAES 4CH ind, (ml/m2), mean±SD | 18.5±5.0 |
| RAES area, (cm2), mean±SD | 13.5±3.1 |
| TAPSE, (mmHg), mean±SD | 21.5±3.0 |
| TAPSE < 22 mmHg, n (%) | 36 (59.0) |
| TAPSE < 16 mmHg, n (%) | 1 (1.6) |
| TAPSE/sPAP, mean±SD | 0.72±0.32 |
| TAPSE/sPAP < 0.55, n (%) | 1 (1.6) |
| sPAP, (mmHg), mean±SD | 27.2±5.3 |
| Tricuspid maximum regurgitation gradient, (mmHg), mean±SD | 22.2±6.5 |
| Mitral Valve Insufficiency, n (%) | 42 (68.9) |
| Mitral Valve Sclerosis, n (%) | 25 (40.9) |
| Tricuspid Valve Insufficiency, n (%) | 33 (54.1) |
| Aortic Valve Insufficiency, n (%) | 13 (21.3) |
| Aortic Valve Sclerosis, n (%) | 11 (18.0) |
| Pericardial Effusion, n (%) | 4 (6.6) |
| Late pattern N=6 |
No late pattern N=55 |
p-value |
ACA/ATA+ N=24 |
ACA/ATA- N=37 |
p-value |
Puffy Hands N=18 |
No Puffy hands N=43 |
p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| E deceleration time (m/s), mean±SD | 225-2±46-1 | 246.7±298.9 | 0.86 | 213.4±56.2 | 261.6±349.9 | 0.55 | 230.5±52.8 | 194.4±47.9 | 0.02 |
| E/E’ ratio, mean±SD | 7.55±1.37 | 6.92±1.90 | 0.52 | 7.6±1.8 | 6.7±1.8 | 0.12 | 7.0±1.3 | 6.8±1.9 | 0.77 |
| E wave, (m/s), mean±SD | 0.70±0.22 | 0.68±0.16 | 0.86 | 0.71±0.17 | 0.65±0.17 | 0.27 | 0.68±0.19 | 0.69±0.16 | 0.78 |
| A wave, (m/s), mean±SD | 0.81±0.17 | 0.75±0.19 | 0.46 | 0.84±0.20 | 0.70±0.15 | 0.006 | 0.79±0.20 | 0.74±0.18 | 0.38 |
| E/A ratio, mean±SD | 0.92±0.40 | 1.0±0.39 | 0.61 | 0.84±0.27 | 1.08±042 | 0.02 | 0.92±0.4 | 1.02±0.39 | 0.39 |
| E/A ratio < 1.0, n (%) | 3 (50) | 34 (61.8) | 0.12 | 18 (75) | 19 (51.4) | 0.11 | 10 (55.6) | 27 (62.8) | 0.77 |
| LVED diameter, (mm), mean±SD | 38.5±3.6 | 40.5±4.9 | 0.34 | 39.4±5.6 | 40.8±4.4 | 0.45 | 40.2±6.2 | 40.4±4.07 | 0.86 |
| PWED thickness, (mm), mean±SD | 8.4±1.5 | 8.3±1.6 | 0.89 | 8.6±1.2 | 8.2±1.8 | 0.31 | 8.5±1.5 | 8.3±1.7 | 0.63 |
| PWED > 9 mm, n(%) | 4 (66.7) | 13 (23.6) | 0.04 | 9 (37.5) | 8 (21.6) | 0.24 | 8 (44.4) | 9 (20.9) | 0.11 |
| IVS thckness, (mm), mean±SD | 9.9±1.6 | 9.3±1.8 | 0.37 | 9.9±1.3 | 8.9±1.9 | 0.03 | 9.4±1.6 | 9.4±2.0 | 0.90 |
| IVS > 10 mm, n (%) | 2 (33.3) | 20 (36.4) | 0.39 | 10 (41.7) | 12 (32.4) | 0.58 | 6 (33.3) | 16 (37.2) | 1.0 |
| LVED volume 4CH Simpson, (ml), mean±SD | 69.7±22.2 | 67.5±16.1 | 0.76 | 66.9±17.3 | 68.3±16.4 | 0.75 | 24.8±3.5 | 85.3±363.2 | 0.49 |
| LVES volume 4CH Simpson, (ml), mean±SD | 24.8±9.7 | 25.6±12.1 | 0.88 | 24.0±9.9 | 26.4±12.8 | 0.46 | 68.3±17.8 | 68.7±16.3 | 0.93 |
| LVED volume 4CH AL, (ml/m2), mean±SD | 41.3±12.4 | 43.6±8.4 | 0.88 | 41.7±10.3 | 44.3±7.8 | 0.29 | 25.7±10.9 | 26.0±12.7 | 0.92 |
| LVES volume 4CH AL, (ml/m2), mean±SD | 14.8±5.6 | 14.7±4.2 | 0.54 | 14.7±6.2 | 14.7±2.9 | 0.92 | 43.1±10.5 | 44.3±8.2 | 0.64 |
| EF%, mean±SD | 64.5±6.8 | 64.6±4.6 | 0.92 | 64.9±5.7 | 64.4±4.3 | 0.64 | 15.6±6.2 | 14.6±3.2 | 0.42 |
| EF%<55% | 1 (16.7) | 2 (3.6) | 0.27 | 2 (8.3) | 1 (2.7) | 0.55 | 2 (11.1) | 1 (2.3) | 0.21 |
| Mass ASE, (g), mean±SD | 108.3±30.9 | 124.9±130.8 | 0.96 | 129.1±153.4 | 112±30.3 | 0.63 | 63.5±5.7 | 65.1±4.5 | 0.26 |
| Mass/BSA, (g/m2), mean±SD | 65.5±17.0 | 66.4±17.0 | 0.90 | 68.8±17.4 | 64.9±17.4 | 0.42 | 163.3±217.5 | 107.3±36.9 | 0.14 |
| Relative wall thickness, mean±SD | 0.48±0.08 | 0.44±0.1 | 0.35 | 0.48±0.09 | 0.42±0.10 | 0.04 | 68.3±19.5 | 66.5±17.0 | 0.73 |
| Mass/height, (g/m), mean±SD | 69.2±21.8 | 65.5±17.9 | 0.64 | 68.1±20.3 | 64.7±17.2 | 0.51 | 0.45±0.12 | 0.44±0.09 | 0.65 |
| Aortic diameter, (mm2), mean±SD | 29.7±2.4 | 29.6±4.1 | 0.96 | 29.7±3.7 | 29.6±4.1 | 0.94 | 65.3+20.6 | 67.5±17.8 | 0.68 |
| LAES area, (cm2), mean±SD | 16.1±2.7 | 15.6±3.7 | 0.75 | 16.7±4.1 | 15.1±3.2 | 0.11 | 28.5±2.5 | 30.0±4.3 | 0.19 |
| LAES 4CH Simpson, (ml), mean±SD | 41.6±10.5 | 40.3±14.3 | 0.83 | 46.7±18.7 | 37.2±8.9 | 0.01 | 15.4±3.3 | 16.1±3.8 | 0.52 |
| LAES 4CH ind, (ml/m2), mean±SD | 24.6±5.7 | 24.4±7.8 | 0.95 | 27.7±10.2 | 22.6±5.1 | 0.01 | 42.3±13.2 | 40.8±14.2 | 0.73 |
| LAES diameter 4CH, (mm/m2), mean±SD | 50.3±5.8 | 46.1±8.1 | 0.27 | 49.7±5.9 | 45.0±8.4 | 0.04 | 25.6±8.3 | 24.6±7.3 | 0.68 |
| RAES diameter AL, (mm), mean±SD | 47.2±1.7 | 46.0±6.1 | 0.74 | 47.8±45.4 | 45.4±5.8 | 0.22 | 46.9±6.0 | 46.7±9.1 | 0.91 |
| RAES 4CH Simpson, (ml), mean±SD | 32.9±9.6 | 30.0±8.9 | 0.55 | 30.1±8.1 | 30.5±9.5 | 0.91 | 44.8±4.5 | 47.0±6.2 | 0.27 |
| RAES 4CH ind, (ml/m2), mean±SD | 19.1±5.4 | 18.4±5.1 | 0.78 | 18.5±3.6 | 18.5±5.6 | 0.98 | 28.4±7.9 | 31.7±9.5 | 0.33 |
| RAES area, (cm2), mean±SD | 13.8±2.5 | 13.5±3.2 | 0.83 | 14.4±2.9 | 13.0±3.2 | 0.12 | 17.9±4.8 | 18.9±5.4 | 0.59 |
| TAPSE, (mmHg), mean±SD | 22.0±4.9 | 21.5±2.9 | 0.70 | 21.9±3.7 | 21.4±2.7 | 0.61 | 13.1±2.2 | 13.8±3.6 | 0.43 |
| TAPSE < 22 mmHg, n (%) | 4 (66.7) | 32 (58.2) | 1.0 | 14 (58.3) | 22 (59.4) | 1.0 | 12 (66.7) | 23 (53.5) | 0.40 |
| TAPSE < 16 mmHg, n (%) | 0 (0) | 1 (1.8) | 1.0 | 1 (4.2) | 0 (0) | 0.39 | 0 (0) | 1 (2.3) | 1.0 |
| TAPSE/sPAP, mean±SD | 0.98±0.31 | 0.69±0.32 | 0.24 | 0.71±0.38 | 0.72±0.29 | 0.92 | 21.8±3.3 | 21.3±2.8 | 0.59 |
| TAPSE/sPAP < 0.55, n (%) | 1 (16.7) | 3 (5.5) | 0.35 | 1 (4.2) | 3 (8.1) | 1.0 | 1 (5.6) | 3 (7.0) | 1.0 |
| sPAP, (mmHg), mean±SD | 28.0±4.2 | 27.1±5.5 | 0.83 | 26.7±4.4 | 27.7±6.2 | 0.63 | 0.7+±.36 | 0.69±0.33 | 0.29 |
| Tricuspid maximum regurgitation gradient, (mmHg), mean±SD | 21.9±3.4 | 22.2±6.7 | 0.93 | 22.4±4.8 | 22.1±7.4 | 0.88 | 27.7±3.9 | 26.8±6.3 | 0.75 |
| Mitral Valve Insufficiency, n (%) | 5 (83.3) | 37 (67.3) | 0.66 | 21 (87.5) | 21 (56.8) | 0.01 | 12 (66.7) | 30 (69.8) | 1.0 |
| Mitral Valve Sclerosis, n (%) | 3 (50) | 22 (40) | 0.68 | 17 (70.8) | 8 (21.6) | <0.001 | 9 (50) | 16 (37.2) | 0.40 |
| Tricuspid Valve Insufficiency, n (%) | 3 (50) | 30 (54.5) | 1.0 | 15 (62.5) | 18 (48.6) | 0.31 | 10 (55.6) | 23 (53.5) | 1.0 |
| Aortic Valve Insufficiency, n (%) | 4 (66.7) | 10 (18.2) | 0.02 | 10 (41.7) | 4 (10.8) | 0.01 | 5 (27.8) | 9 (20.9) | 0.74 |
| Aortic Valve Sclerosis, n (%) | 4 (66.7) | 9 (16.4) | 0.01 | 9 (37.5) | 4 (10.8) | 0.02 | 5 (27.8) | 8 (18.6) | 0.49 |
| Pericardial Effusion, n (%) | 0 (0) | 4 (7.3) | 1.0 | 1 (4.2) | 3 (8.1) | 1.0 | 2 (11.1) | 2 (4.7) | 0.57 |
| Echocardiographic parameters | Iloprost iv N=25 |
CCBs N=8 |
Iloprost+CCBs N=18 |
No Vasodilators N=13 |
|
|---|---|---|---|---|---|
| E deceleration time (m/s), mean±SD | 295.0±422.8 | 176.7±40.7 | 203.6±62.8 | 220.9±49.7 | 0.74 |
| E/E’ ratio, mean±SD | 7.3±2.1 | 7.8±2.8 | 6.8±1.8 | 6.6±1.1 | 0.71 |
| E wave, (m/s), mean±SD | 0.67±0.18 | 0.68±0.22 | 0.68±0.15 | 0.77±0.23 | 0.59 |
| E/A ratio, mean±SD | 1.08±0.44 | 0.92±0.35 | 0.85±0.21 | 1.0±0.46 | 0.32 |
| LVED diameter, (mm), mean±SD | 40.6±5.1 | 42.5±7.4 | 39.6±4.4 | 40.4±5.0 | 0.72 |
| PWED thickness, (mm), mean±SD | 8.2±1.9 | 8.7±0.8 | 8.2±0.9 | 8.7±2.1 | 0.75 |
| IVS thckness, (mm), mean±SD | 9.3±1.8 | 8.9±1.4 | 9.5±1.4 | 9.3±2.6 | 0.93 |
| LVED volume 4CH Simpson, (ml), mean±SD | 67.5±13.8 | 54.1±31.5 | 67.8±15.3 | 73.8±14.6 | 0.17 |
| LVES volume 4CH Simpson, (ml), mean±SD | 23.7±6.5 | 47.2±35.1 | 23.8±6.0 | 24.6±7.4 | 0.001 |
| LVED volume 4CH AL, (ml/m2), mean±SD | 42.8±9.8 | 41.4±8.4 | 42.1±8.7 | 46.9±7.1 | 0.46 |
| LVES volume 4CH AL, (ml/m2), mean±SD | 14.5±3.6 | 16.5±12.9 | 14.4±3.5 | 15.3±4.0 | 0.84 |
| EF%, mean±SD | 64.9±4.4 | 59.6±6.7 | 65.1±5.0 | 65.2±4.2 | 0.12 |
| Mass ASE, (g), mean±SD | 109.3±33.5 | 95.9±60.3 | 160.1±217.4 | 109.1±42.2 | 0.56 |
| Mass/BSA, (g/m2), mean±SD | 66.1±16.8 | 71.9±21.4 | 65.0±13.5 | 68.1±23.4 | 0.89 |
| Relative wall thickness, mean±SD | 0.44±0.11 | 0.45±0.10 | 0.46±0.08 | 0.44±0.13 | 0.93 |
| Mass/height, (g/m), mean±SD | 67.4±20.8 | 57.0±21.1 | 66.9±14.4 | 66.7±18.9 | 0.78 |
| Aortic diameter, (mm2), mean±SD | 30.8±2.9 | 26.9±4.8 | 28.9±4.7 | 29.3±4.1 | 0.27 |
| LAES area, (cm2), mean±SD | 14.4±2.4 | 15.5±4.1 | 16.3±3.8 | 17.0±4.7 | 0.16 |
| LAES 4CH Simpson, (ml), mean±SD | 36.1±8.1 | 41.0±19.8 | 44.7±16.3 | 42.1±16.3 | 0.26 |
| LAES 4CH ind, (ml/m2), mean±SD | 21.6±4.3 | 25.5±11.4 | 26.9±8.3 | 26.0±9.8 | 0.14 |
| LAES diameter 4CH, (mm/m2), mean±SD | 45.8±5.9 | 46.9±5.8 | 48.5±6.2 | 44.0±13.7 | 0.54 |
| RAES diameter AL, (mm), mean±SD | 45.1±5.9 | 40.5±6.1 | 48.7±5.3 | 46.2±5.4 | 0.12 |
| RAES 4CH Simpson, (ml), mean±SD | 31.2±8.4 | 14.1±6.5 | 26.5±5.4 | 30.2±9.1 | 0.005 |
| RAES 4CH ind, (ml/m2), mean±SD | 18.1±5.1 | 12.4±6.7 | 21.7±4.5 | 16.6±2.1 | 0.01 |
| RAES area, (cm2), mean±SD | 13.4±3.6 | 12.1±3.0 | 14.8±3.1 | 12.6±1.9 | 0.24 |
| TAPSE, (mmHg), mean±SD | 20.8±2.9 | 20.9±3.1 | 21.3±2.7 | 22.9±3.3 | 0.29 |
| TAPSE/sPAP, mean±SD | 0.79±0.33 | 0.31±0.44 | 0.76±0.10 | 0.65±0.39 | 0.28 |
| sPAP, (mmHg), mean±SD | 25.1±4.8 | 24.5±6.4 | 29.5±6.1 | 29.4±3.6 | 0.21 |
| Tricuspid maximum regurgitation gradient, (mmHg), mean±SD | 21.0±4.6 | 20.4±6.2 | 24.5±4.9 | 21.6±11.3 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





