1. Introduction
Duchenne Muscular Dystrophy (DMD) is an X-linked inherited disorder characterized by progressive muscle weakness and degeneration, primarily observed in male children. The etiology of DMD is linked to a genetic defect resulting in absent or dysfunctional production of the protein dystrophin. This deficiency leads to muscle fiber damage and subsequent loss of muscle function. However, DMD is not limited to skeletal muscle involvement; its systemic effects also contribute to impairment of the cardiovascular system. In particular, cardiac function in individuals with DMD typically deteriorates in parallel with muscle weakness, significantly affecting patients’ quality of life and prognosis [
1].
Cardiovascular involvement is a frequently overlooked but critical component of DMD. Most children with DMD develop cardiac muscle weakness (cardiomyopathy) that becomes more pronounced with advancing age. This cardiomyopathy mainly manifests as left ventricular dysfunction, which can eventually progress to heart failure [
2]. Additionally, arrhythmias and other electrophysiological abnormalities play a significant role in disease progression and contribute to increased mortality rates in these patients [
3].
Non-invasive diagnostic tools such as electrocardiography (ECG) enable early detection of cardiovascular involvement in DMD. ECG provides valuable information on cardiac status; commonly observed abnormalities in children with DMD include QRS complex widening, PR interval prolongation, T-wave inversions, and pathological Q waves [
4].
Recently, systemic inflammatory indices have gained prominence as novel markers for predicting cardiovascular disease prognosis and quantifying inflammation. Indices such as neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory response index (SIRI), systemic immune-inflammation index (SII), and pan-immune inflammation value (PIV) are simple, low-cost parameters derived from routine blood tests that reflect the extent of systemic inflammation. Their prognostic values have been demonstrated in various cardiovascular, neurovascular, oncological, and metabolic disorders [
5,
6,
7].
Early identification and management of cardiovascular complications in children with Duchenne Muscular Dystrophy may improve disease prognosis. In this context, further research is required to clarify whether systemic inflammatory indices can serve as predictors of cardiovascular involvement in this population. This study aims to investigate the cardiovascular effects and electrocardiographic findings of DMD and to evaluate the potential utility of novel inflammatory indices in guiding cardiac monitoring and treatment strategies.
2. Materials and Methods
This retrospective study evaluated data from 25 patients diagnosed with Duchenne Muscular Dystrophy between January 1, 2021, and July 1, 2024. Sample size calculations were performed to ensure a minimum statistical power of 80% and a Type I error rate of 5% for each variable analyzed. The control group consisted of an equal number of healthy male children aged 2 to 18 years, randomly selected from individuals presenting to the pediatric outpatient clinic who had undergone complete blood counts.
The variables assessed included patient age, age at diagnosis, wheelchair dependence, tracheostomy status, treatment regimens, hematological parameters, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory response index (SIRI: neutrophils × monocytes / lymphocytes), systemic immune-inflammation index (SII: neutrophils × platelets / lymphocytes), pan-immune inflammation value (PIV: neutrophils × monocytes × platelets / lymphocytes), and electrocardiographic (ECG) and echocardiographic (ECHO) parameters.
Statistical Analysis
All statistical analyses were performed using SPSS for Windows, version 26 (IBM Corp., Armonk, NY, USA), with statistical significance set at p < 0.05. The normality of continuous variables was assessed using the Kolmogorov-Smirnov test and skewness-kurtosis measures. Since the data were normally distributed, parametric tests were applied.
Descriptive statistics were presented as mean, standard deviation, count (n), and percentage (%). Independent samples t-test was used for comparisons between groups. Receiver operating characteristic (ROC) curve analysis was conducted to determine optimal cut-off values for variables within the patient group, calculating area under the curve (AUC), sensitivity, and specificity.
Pearson correlation coefficient was employed to evaluate relationships between continuous variables.
3. Results
3.1. Demographic and Clinical Characteristics
In this retrospective study, clinical data from 25 patients diagnosed with Duchenne Muscular Dystrophy (DMD) were analyzed. All patients were male. The median age was 12 years (range: 5–18), with a mean age of 11.2 ± 4.3 years. The mean age at diagnosis was 4.8 ± 2.6 years, with a median age of 4 years (range: 2–10 years). Wheelchair dependence was observed in 16% of the patients. None of the patients required home mechanical ventilation or tracheostomy during the study period.
Among the DMD patients, three cases were receiving cardiac treatment due to cardiovascular involvement. Of these, one patient was treated with an angiotensin-converting enzyme (ACE) inhibitor, one with a combination of beta-blocker and ACE inhibitor, and one with digoxin, diuretics, and ACE inhibitor. Descriptive statistics for other measured parameters are presented in
Table 1.
3.2. Electrocardiographic and Echocardiographic Findings
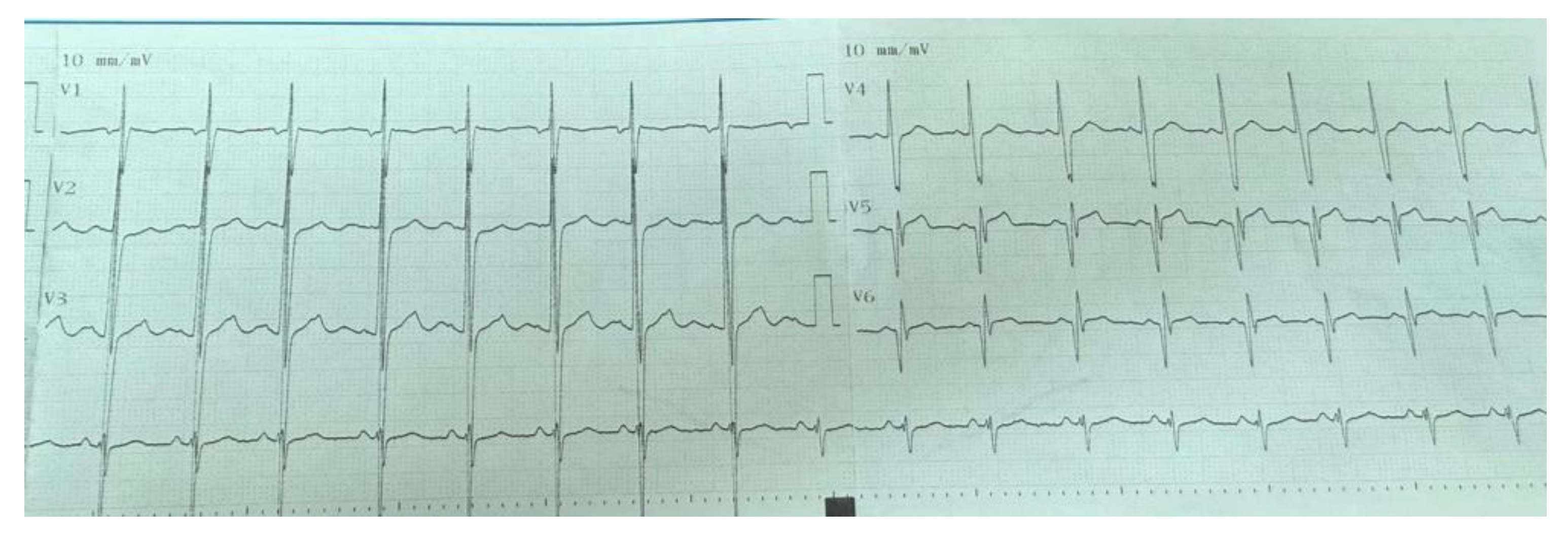
Electrocardiographic (ECG) abnormalities were detected in 9 (36%) of the patients. The most common ECG abnormality was pathological Q waves observed in the V5–V6 leads in 6 patients (24%) (
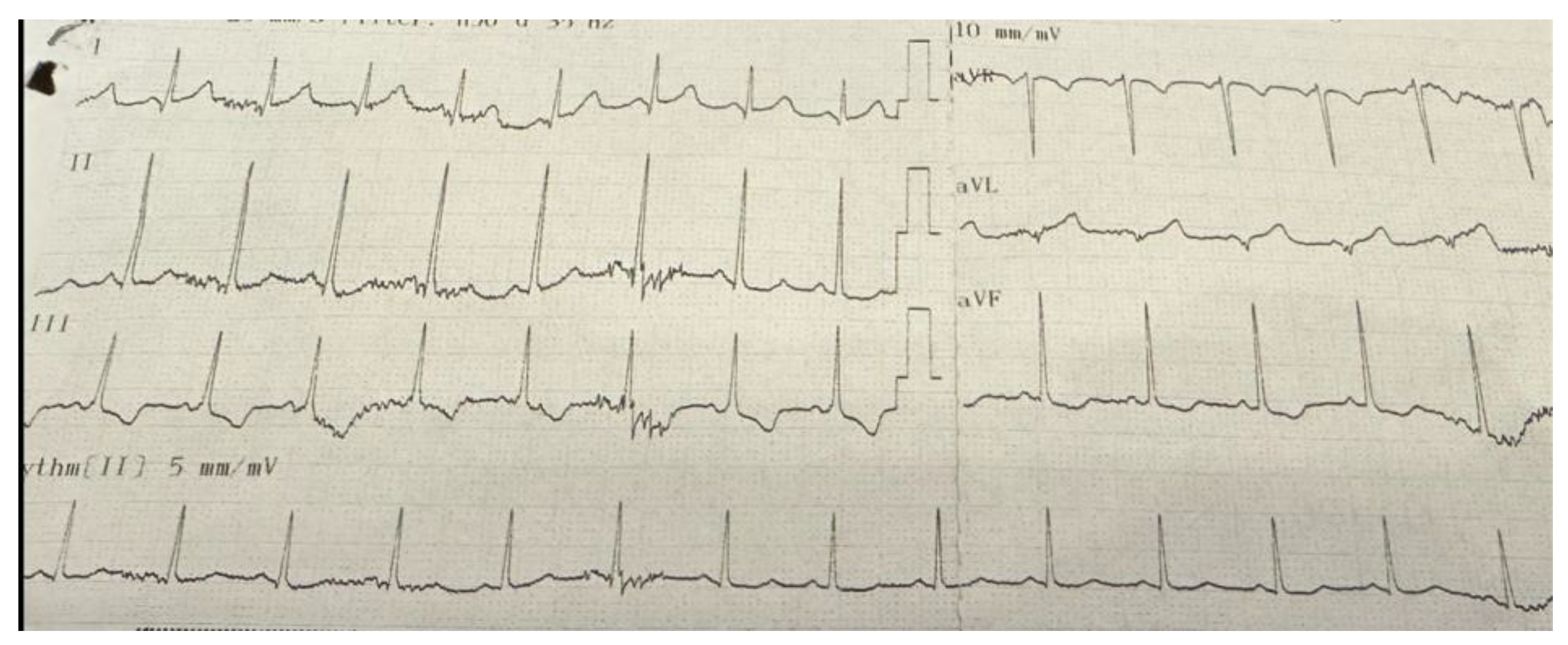
Figure 1). Other abnormalities included T-wave inversion in the inferior leads in one patient (
Figure 2), right bundle branch block in another, and supraventricular tachycardia in a third patient. Among these 9 patients with ECG abnormalities, transthoracic echocardiography (TTE) was normal in 6 (66.7%). Of the remaining three patients, one had left ventricular systolic dysfunction, another presented with both left ventricular dysfunction and dilated cardiomyopathy, and the third was diagnosed with mitral valve prolapse accompanied by mitral regurgitation. All three patients who received cardiac treatment were among those with echocardiographic abnormalities. Two of these patients exhibited pathological Q waves on ECG, while one patient (25%) showed T-wave inversion. Echocardiographic and electrocardiographic abnormalities of the patients are summarized in
Table 2.
When comparing hematological parameters, hemoglobin levels were found to be significantly higher in patients with Duchenne Muscular Dystrophy (DMD) compared to the control group. The mean hemoglobin level in the DMD group was 13.46 ± 1.11 g/dL, whereas in the control group it was 12.65 ± 0.84 g/dL. This difference between the two groups was statistically significant (p = 0.006), indicating a notable increase in hemoglobin levels in DMD patients relative to controls.
No statistically significant differences were observed between groups for other hematological parameters. Although white blood cell (WBC), lymphocyte, neutrophil, monocyte, and platelet (PLT) counts appeared slightly elevated in the patient group compared to controls, these differences did not reach statistical significance (
Table 3).
3.4. Inflammatory Marker Levels
Key inflammatory markers were examined in patients with Duchenne Muscular Dystrophy. The mean neutrophil-to-lymphocyte ratio (NLR) was 2.00 ± 2.28, showing high inter-individual variability. Platelet-to-lymphocyte ratio (PLR) was measured at 102.63 ± 46.92, while monocyte-to-lymphocyte ratio (MLR) was low at 0.21 ± 0.15. The systemic inflammatory response index (SIRI) and systemic immune-inflammation index (SII) demonstrated considerable variation within the patient group, with mean values of 0.14 ± 0.19 and 592.8 ± 659.16, respectively. The pan-immune inflammation value (PIV) was 502.84 ± 103.66.
Comparison of these inflammatory markers between the patient and control groups revealed no statistically significant differences for NLR, PLR, MLR, SIRI, SII, and PIV. The results are presented in
Table 4.
3.5. Correlation Analysis
Correlation analysis was performed among the measured parameters within the patient group. A statistically significant positive correlation was identified between Pro-BNP levels and PLR values, with a correlation coefficient of 0.86 (p < 0.05). This indicates that as Pro-BNP levels increase, PLR values also tend to increase. In contrast, no statistically significant correlations were found among other paired variables outside of those mentioned (p > 0.05) (
Table 5).
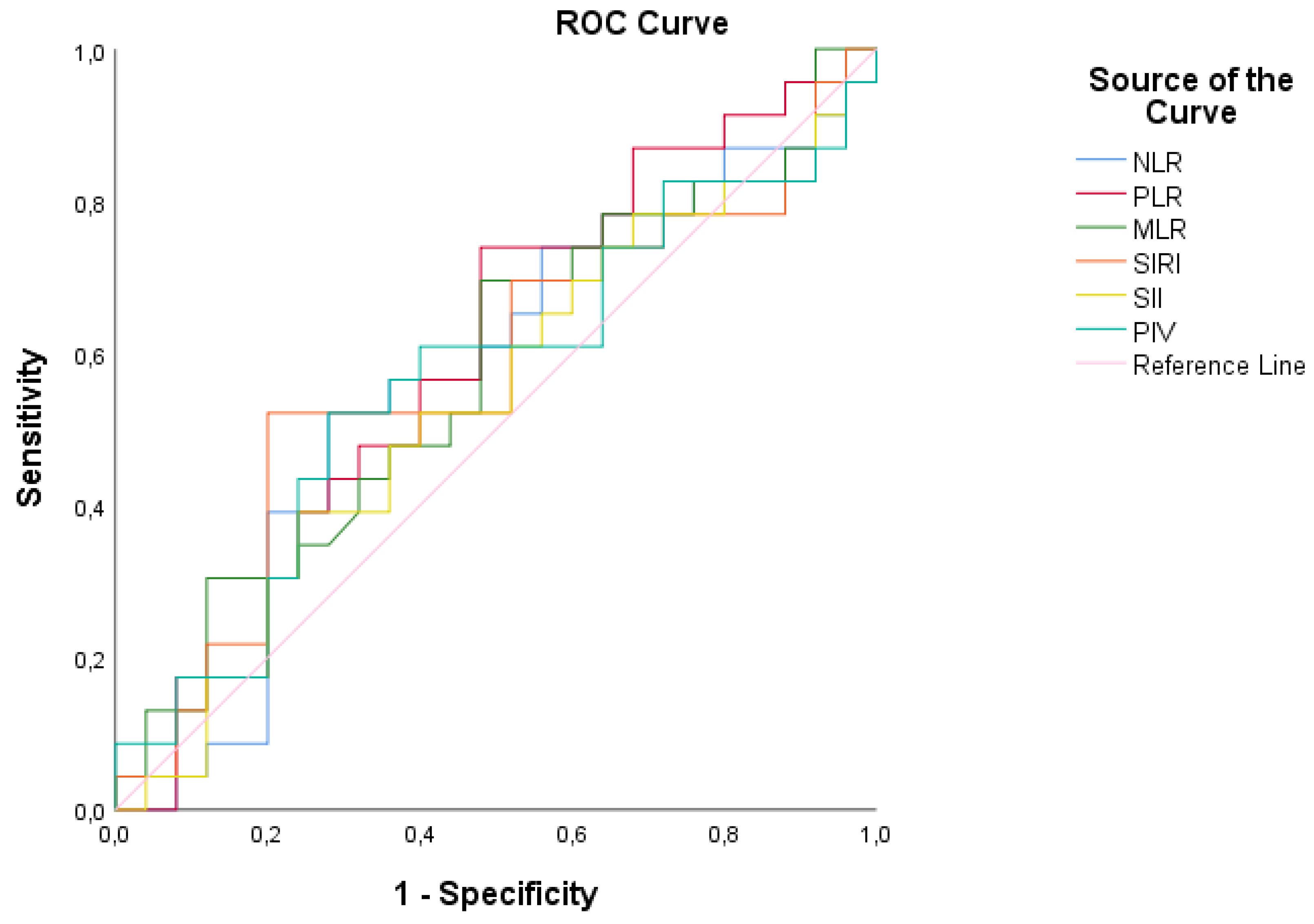
3.6. ROC Analysis of Inflammatory Markers
Receiver Operating Characteristic (ROC) analysis revealed that the neutrophil-to-lymphocyte ratio (NLR) had a cut-off value of 1.21, with an area under the curve (AUC) of 56.5%, indicating its discriminative ability between patient and control groups. However, based on this calculation, the sensitivity (56.5%) and specificity (60.0%) values demonstrate that the NLR measurement does not provide statistically adequate discrimination between the patient and control groups (p < 0.05). Similarly, the platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic inflammatory response index (SIRI), systemic immune-inflammation index (SII), and pan-immune inflammation value (PIV) also showed insufficient discriminative power between the patient and control groups (p < 0.05) (
Figure 3,
Table 6).
3.7. Comparison by ECG/ECHO Abnormalities
Comparisons between patients with and without ECG/ECHO abnormalities revealed no statistically significant differences in any of the measured parameters (p > 0.05) (
Table 7).
4. Discussion
This study examined the clinical, biochemical, and cardiological data of 25 patients diagnosed with Duchenne Muscular Dystrophy (DMD). The findings provide valuable insights into the clinical course, treatment strategies, and cardiac complications associated with DMD.
All patients included in the study were male, which is consistent with the expected inheritance pattern of DMD as an X-linked genetic disorder [
8]. The median age of patients was 12 years (range: 5–18), and the mean age at diagnosis was 4.8 ± 2.6 years. This finding emphasizes that DMD is typically diagnosed during childhood, highlighting the importance of early diagnosis in monitoring disease progression [
9]. The diagnosis of DMD is commonly established between ages 3 and 5, and early diagnosis can shorten the time to treatment initiation, aiding in the preservation of motor functions [
10].
Regarding physical status and needs, the rate of wheelchair use was found to be 16%. Pane et al. reported that loss of ambulation in DMD patients generally begins around 12 years of age, coinciding with the initiation of wheelchair dependency [
11] This indicates significant motor function decline with advancing age, with wheelchair use typically required in later stages. None of the patients required home mechanical ventilation or tracheostomy, suggesting that early treatment may mitigate the impact of DMD on respiratory functions. Birnkrant et al. also stated that respiratory dysfunction in DMD patients usually becomes more prominent after loss of ambulation [
12].
Cardiological assessments revealed important findings. The most frequently observed ECG abnormality was pathological Q waves (24%), consistent with literature reports indicating a 30–40% prevalence in DMD patients. This finding is considered an early marker of silent myocardial fibrosis and is associated with cardiomyopathy [
13]. Another study linked frequent pathological Q waves in DMD patients with sudden cardiac death (SCD) [
14].
Cardiac complications in DMD are known to commence early and become more pronounced with age [
15]. In our study, there was no significant age difference between patients with or without cardiac pathology. Severe cardiac conditions such as left ventricular dysfunction and dilated cardiomyopathy were also observed. These results suggest that cardiac involvement in DMD may develop not only in advanced stages but also from early disease phases [
16]. Furthermore, the detection of severe cardiac pathologies such as dilated cardiomyopathy in echocardiography underscores the importance of ongoing cardiological monitoring. According to our echocardiographic data, 16% (4/25) of patients had left ventricular dysfunction or structural anomalies, consistent with the approximately 22% prevalence reported in a large cohort study by Ramaciotti et al. [
17]. The correlation between echocardiographic abnormalities and ECG findings was weak, further emphasizing the limited diagnostic value of ECG alone in DMD [
18].
Cardiac treatments employed included ACE inhibitors, beta blockers, and digoxin. These therapies are vital for managing cardiomyopathy and other cardiac complications, highlighting the critical role of cardiac monitoring and treatment in DMD patients [
19]. The need for cardiac therapy was confined to patients with ECG or echocardiographic abnormalities, and no significant relationship was found between clinical or biochemical parameters and cardiac treatment. This finding indicates that cardiac complications should be closely monitored through ECG and echocardiography, and that a multidisciplinary approach is necessary in managing these patients [
15].
Biochemically, hemoglobin, white blood cell, lymphocyte, and neutrophil counts varied widely among patients, generally remaining within normal ranges, possibly reflecting the effectiveness of current treatment regimens. Notably, hemoglobin levels were significantly higher in the patient group (p = 0.006). Although elevated hemoglobin is uncommon in DMD, some studies report increases secondary to corticosteroid therapy [
12].
The absence of significant differences in inflammatory markers between patient and control groups suggests a limited role for systemic inflammation biomarkers in routine monitoring of DMD [
20]. Conversely, Yükcü and Arslan demonstrated that MLR, SIRI, and PIV have acceptable diagnostic value for detecting ascending aortic dilation in children with bicuspid aortic valve [
7]. This association suggests inflammation may be an important factor in that patient group, whereas the lack of significant differences in inflammatory indices in DMD may indicate that inflammation is not directly related to cardiac complications in this population.
A notable finding in our study is the positive correlation between Pro-BNP and PLR, which may indicate a potential link between increased cardiovascular burden and inflammatory response. Pro-BNP is recognized as a useful biomarker for predicting left ventricular dysfunction and heart failure in DMD [
21]. Although several studies have examined the prognostic value of PLR in cardiovascular diseases, it is not considered an independent predictor of mortality. A 2019 cohort study reported that higher PLR levels in patients with acute heart failure (AHF) were associated with increased mortality rates [
22]. Studies directly evaluating the relationship between Pro-BNP and PLR are limited; however, both biomarkers have been shown to be important in assessing heart failure and acute coronary syndromes. For example, a study on chronic heart failure patients found a positive correlation between NT-proBNP levels and PLR [
23]. To our knowledge, no previous studies have investigated this correlation specifically in the DMD population.
The ROC analysis revealed that none of the inflammatory parameters achieved sufficient diagnostic accuracy, limiting their utility for routine screening in DMD. Furthermore, the impact of routine steroid use on inflammatory indices remains unclear and warrants further investigation. Prospective studies are needed to clarify these findings.
5. Conclusions
Our study comprehensively evaluated the clinical, cardiological, and biochemical characteristics of patients with Duchenne Muscular Dystrophy, notably highlighting the positive correlation between Pro-BNP and PLR. The findings suggest that DMD affects not only the muscular system but also the cardiovascular system from early stages, underscoring the importance of regular cardiac follow-up. However, larger prospective studies are necessary to elucidate the role of inflammatory markers in DMD.
Author Contributions
Conceptualization, E.I.A. and T.K.; methodology, T.K.; validation, E.I.A. and T.K. .; formal analysis, E.I.A.; investigation, X.X.; resources, T.K..; data curation, E.I.A.; writing—original draft preparation, E.I.A.; writing—review and editing, E.I.A.; visualization, T.K..; supervision, T.K. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Non-Interventional Research Ethics Committee of Ordu University (protocol code 2025/68; date of approval: 7 March 2025).
Informed Consent Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions and patient confidentiality.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DMD |
Duchenne Muscular Dystrophy |
| NLR |
Neutrophil-to-Lymphocyte Ratio |
| PLR |
Platelet-to-Lymphocyte Ratio |
| MLR |
Monocyte-to-Lymphocyte Ratio |
| SII |
Systemic Immune-Inflammation Index |
| SIRI |
Systemic Inflammatory Response Index |
| PIV |
Pan-Immune Inflammation Value |
| ECG |
Electrocardiography |
| Pro-BNP |
Pro-Brain Natriuretic Peptide |
| ROC |
Receiver Operating Characteristic |
| QRS |
QRS Complex |
| PR |
PR Interval |
| ECHO |
Echocardiography |
| SPSS |
Statistical Package for the Social Sciences |
| AUC |
Area Under the Curve |
| ACE |
Angiotensin-Converting Enzyme |
| TTE |
Transthoracic Echocardiography |
| RBB |
Right Bundle Branch |
| SVT |
Supraventricular Tachycardia |
| WBC |
White Blood Cell |
| PLT |
Platelet |
| CK-MB |
Creatine Kinase-MB Isoenzyme |
| AST |
Aspartate Aminotransferase |
| ALT |
Alanine Aminotransferase |
| ALP |
Alkaline Phosphatase |
| GGT |
Gamma-Glutamyl Transferase (Gama-Glutamil Transferaz) |
| CK |
Creatine Kinase |
| SCD |
Sudden Cardiac Death |
| |
|
References
- McNally, E.M.; Kaltman, J.R.; Benson, D.W.; Canter, C.E.; Cripe, L.H.; Duan, D.; Finder, J.D.; Groh, W.J.; Hoffman, T.M.; Judge, D.P.; et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Adorisio, R.; Mencarelli, E.; Cantarutti, N.; Calvieri, C.; Amato, L.; Cicenia, M.; Silvetti, M.; D’Amico, A.; Grandinetti, M.; Drago, F.; Amodeo, A. Duchenne dilated cardiomyopathy: Cardiac management from prevention to advanced cardiovascular Therapies. J. Clin. Med. 2020, 9, 3186. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Arcudi, A.; Narducci, M.L.; Novelli, V.; Canonico, F. Arrhythmic Risk stratification and sudden cardiac Death prevention in Duchenne muscular dystrophy: A Critical Appraisal. Rev. Cardiovasc. Med. 2025, 26, 27089. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Shao, S.; Wang, C. Electrocardiographic features of children with Duchenne muscular dystrophy. Orphanet J. Rare Dis. 2022, 17, 320. [CrossRef]
- Zhao, Y.; Hao, C.; Bo, X.; et al. The prognostic value of admission lymphocyte-to-monocyte ratio in critically ill patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2022, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, M.; Liu, L.; et al. Systemic immune-inflammation index as a novel predictor of major adverse cardiovascular events in patients undergoing percutaneous coronary intervention: a meta-analysis of cohort studies. BMC Cardiovasc. Disord. 2024, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- Yükcü, B.; Arslan, H.F. New systemic inflammatory indices as predictors of ascending aortic dilation in children with bicuspid aortic valve: a retrospective cross-sectional study. Medicine 2024, 103, e40904. [Google Scholar] [CrossRef] [PubMed]
- Emery, A.E. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacologic management. Lancet 2010, 371, 945–953. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; et al. The 6-minute walk test as a functional endpoint in DMD clinical trials: a systematic review. J. Neurol. 2014, 261, 1–7. [Google Scholar] [CrossRef]
- Pane, M.; Mazzone, E.S.; Sivo, S.; et al. The 6 minute walk test and performance of upper limb in ambulant Duchenne muscular dystrophy boys. PLoS Curr. 2014, 6, ecurrents.md.a93d9904d57dcb08936f2ea89bca6fe6. [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacologic management. Lancet Neurol. 2018, 17, 299–312. [Google Scholar] [CrossRef]
- Hor KN; Wansapura J; Markham LW; et al. Evaluation of early cardiomyopathy in Duchenne muscular dystrophy using echocardiography and cardiac magnetic resonance imaging. Am J Cardiol. 2009;103(11):1623-1627. [CrossRef]
- Finsterer J; Stöllberger C. Cardiac involvement in patients with muscular dystrophies and myopathies. Expert Rev Cardiovasc Ther. 2015;13(10):1231-1240.
- Xu, R.; Xu, H.; Zhang, K.; et al. Prevalence and associated factors of myocardial involvement in Duchenne muscular dystrophy patients in the first decade of life. Chin. Med. J. (Engl.) 2023, 136, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil A; Abasse S; Silverston K. Cardiac involvement classification and therapeutic management in patients with Duchenne muscular dystrophy. J Neuromuscul Dis. 2017;4(1):17-23. [CrossRef]
- Ramaciotti C; Minicucci MF; Mattos LV; et al. Cardiomyopathy in Duchenne muscular dystrophy: echocardiographic and clinical findings in a large cohort. Orphanet J Rare Dis. 2021;16(1):297.
- Yamamoto, T.; Nambu, Y.; Bo, R.; Morichi, S.; Yanagiya, M.; Matsuo, M.; Awano, H. Electrocardiographic R wave amplitude in V6 lead as a predictive marker of cardiac dysfunction in Duchenne muscular dystrophy. J. Cardiol. 2023, 82, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Romfh, A.; McNally, E.M. Cardiac assessment in Duchenne and Becker muscular dystrophies. Curr. Heart Fail. Rep. 2010, 7, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guzmán, O.R.; Rodríguez-Cruz, M.; Escobar Cedillo, R.E. Systemic Inflammation in Duchenne muscular dystrophy: Association with muscle function and nutritional status. Biomed. Res. Int. 2015, 891972. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Fanelli, L.; Mazzone, E.S.; et al. Serum biomarkers and cardiac function in Duchenne muscular dystrophy. Neuromuscul. Disord. 2018, 28, 395–400. [Google Scholar] [CrossRef]
- Ye, G.L.; Chen, Q.; Chen, X.; et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: a cohort study. Sci. Rep. 2019, 9, 10639. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, L.; Tilea, I.; Iancu, D.G.; et al. Insights into the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio as predictors for the length of stay and readmission in chronic heart failure patients. Diagnostics 2024, 14, 2102. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).