Background/Objectives: To compare the lumbopelvic muscle mechanical properties (MMPs) of women with and without multiple sclerosis (MS) and explore relationships between these properties and sociodemographic/clinical characteristics. Methods: This cross-sectional observational study included 22 women with MS and 22 age- and BMI-matched women without MS. MMPs (frequency, stiffness, decrement, relaxation, and creep) of pelvic floor and lumbar paravertebral muscles were assessed using a MyotonPRO device. Sociodemographic and clinical data related to pelvic floor health were also collected. Results: Women with MS showed significant differences in pelvic floor MMPs, including higher frequency (3.26 Hz; 95% CI [2.12, 4.41]), stiffness (90 N/m; 95% CI [55.09, 124.91]), and decrement (0.2; 95% CI [0.09, 0.31]) and lower relaxation (6.15 ms; 95% CI [8.26, 4.05]) and creep (0.24; 95% CI [0.34, 0.13]) compared to women without MS. For lumbar paravertebral muscles, differences were observed only on the right side, with lower frequency (2.15 Hz; 95% CI [0.28, 4.02]) and stiffness (62.17 N/m; 95% CI [10.7, 113.65]) in women with MS. Correlation patterns between MMPs and clinical characteristics differed by group, with moderate correlations found only in the MS group (e.g., EDSS: r = 0.57; p = 0.006; PFDI-20: r = 0.47; p = 0.026). Conclusions: Women with MS exhibit altered pelvic floor MMPs, characterized by reduced tone and stiffness and increased elasticity and viscoelasticity, while lumbar paravertebral differences are minimal. These findings highlight the need for objective MMP assessments in women with MS to guide preventive and therapeutic interventions.
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease that affects the central nervous system (CNS) [
1] and causes demyelination which is diagnosed in young adults (aged 20–40 years), predominantly in female patients [
2,
3]. MS causes alteration of the function of the pelvic floor (PF) which, although common in women [
4], is aggravated by deterioration of the motor innervation of the external anal sphincter [
5]. In fact, up to 80% of patients with MS are affected by lower urinary tract symptoms, manifesting as problems with urine storage, evacuation, or both [
6]. Although these symptoms are not life-threatening, they have a significant negative impact on the quality of life of those affected [
7].
The PF muscles (PFM) play an important role in maintaining continence [
8] and in lumbopelvic stability because increased PF tension stabilises the sacroiliac joint, positions the sacrum, and improves the ability of the lumbar spine and pelvis to withstand mechanical stress resulting from increased pressure [
9]. Furthermore, studies suggest that the correct configuration of the spine and its curvatures protect the pelvis and PF from direct intra-abdominal pressures, allowing for more efficient contraction of the PFMs [
10,
11]. This can be explained by the regional interdependence of these structures and allows us to describe existing clinical observations between different regions of the body [
12]. However, to date, there is no specific knowledge available regarding the behaviour of lumbopelvic muscle mechanical properties (MMPs) in MS, nor about their relationships with factors such as sociodemographic characteristics, type and degree of incontinence, or quality of life.
In this context, myotonometry represents a novel and non-invasive method to characterise MMPs. In this approach, a brief superficial mechanical impulse is applied to record different oscillation parameters of the muscle response in terms of tone, stiffness, elasticity, relaxation time, and fluence [
13]. This technology is now being applied in different research fields including physiotherapy, oncology, rheumatology, neurology, and musculoskeletal disorders, among others [
14]. From among the studies published using myotonometry on the musculoskeletal system, much of the work to date has focussed on the paravertebral muscles of the lumbar spine [
15], with good to excellent absolute and relative reliability results. Indeed, the intrarater and interrater reliability of PFMs with this device is good to very good in healthy women and in those with urinary incontinence (UI), thereby supporting the use of this device both in women with and without UI [
18]. Furthermore, myotonometry has been shown to be clinically applicable, valid, and sufficiently reliable for measuring tone and other properties such as stiffness according to a phantom tissue model [
16]. Finally, recent studies use of myotonometry showing changes in muscle tone and elasticity of the superior orbitalis muscle in patients with MS when compared to healthy ones [
17].
Thus, we aimed to examine whether the lumbopelvic MMPs of women with MS differ from those without MS. As a secondary objective, we aimed to identify possible relationships between the status of the MMPs and the sociodemographic and clinical characteristics in each group of women. Investigating MMPs of the PFM in women with MS and healthy controls is crucial to understanding how this neurological disease affects muscle tone, stiffness, and elasticity, contributing to issues such as incontinence and sexual dysfunction. These comparisons can identify specific alterations, guide personalized therapeutic interventions and provide evidence to improve treatments and quality of life for MS patients.
This study hypothesizes that women with MS exhibit altered mechanical properties in the PFM and lumbar muscles, including reduced strength, elasticity, and coordination, due to neurological impairment. These changes may lead to increased prevalence of pelvic dysfunctions, such as urinary and fecal incontinence, compared to healthy women.
2. Materials and Methods
2.1. Design
This was a cross-sectional observational study of cases (women with MS with PF involvement) and controls (healthy women), carried out at the Reina Sofía University Hospital in the province of Cordoba (Spain). Before starting the work, the patients were informed about the procedure, duration, and objective of the study and signed the corresponding written informed consent document.
2.2. Participants
Women from the specialised MS clinic who met the following inclusion criteria were recruited through a non-probabilistic sampling of consecutive cases: (1) female patients of legal age (2) with MS diagnosed according to McDonald’s criteria (2017) [
19], (3) with an Expanded Disability Status Scale (EDSS) score between 0.0 and 6.5, (4) receiving a disease-modifying treatment (DMT), (5) whose pharmacological treatment dose for spasticity had been stable in the 30 days prior, (6) who had undergone a previous neurological evaluation that had determined the EDSS score and defined its associated symptoms, and (7) were able to understand the objective of the work, complete the study procedures, and sign the informed consent document. The exclusion criteria were: (1) being in a disease flare-up phase or having had a flare-up in the 30 days prior to the start of the study, (2) participating in another clinical trial with a drug or intervention, (3) being in the first three days of menstruation or pregnant, (4) a body mass index (BMI) exceeding 40 kg/m
2, (5) carrying a metallic device such as a pacemaker or intrauterine device (IUD) that could interfere with the MyotonPRO result, (6) consumption of any substance that alters muscle tone, (7) patients with myopathies or concomitant peripheral nervous system diseases, and (8) an inability to collaborate or any other situation in which the evaluation could be altered. The control group also comprised women who met both the inclusion and exclusion criteria, except they did not have a diagnosis of MS. In addition, one-to-one matching was performed for each case according to age (± 3 years) and BMI (± 3 Kg/m
2), also considering whether the participants had given birth.
2.3. Data Collection
After providing informed consent, participants completed a registration form (age, BMI, vaginal deliveries) and the following validated questionnaires: the Spanish version of the Pelvic Floor Distress Inventory (PFDI-20) [
20], assessing dysfunction across genital prolapse (6 items), colorectal–anal (8 items), and urinary (6 items) domains, with scores ranging from 0 to 300; the Spanish version of the Pelvic Floor Impact Questionnaire–Short Form (PFIQ-7) [
20], evaluating the impact of urinary, colorectal–anal, and genital prolapse symptoms on daily activities, with scores from 0 to 300; the Spanish version of the Global Physical Activity Questionnaire (GPAQ) [
21], measuring activity intensity, frequency, and duration; and a Visual Analogue Scale (VAS), scoring pain intensity from 0 to 10.
At the end of data collection, the PF and lumbar paravertebral musculature of the participants was evaluated. A MyotonPRO (Myoton AS, Estonia) [
22,
23], a small, non-invasive, and portable handheld device to measure the MMPs was used in this study. MyotonPRO was used to assess superficial musculature because it is designed to evaluate biomechanical properties through non-invasive mechanical vibrations, which only penetrate tissues close to the surface. This makes it ideal for measuring muscles accessible via the skin, as its technology does not reach deeper layers, providing precise data for clinical and research applications related to muscle monitoring and rehabilitation. The measurement consists of 3 main components: mechanical impulse stress, co-oscillation recording, and parameter calculation. The tip of the probe is 3 mm in diameter and is applied perpendicular to the skin surface, above the muscle being measured. A constant pressure (0.18 N) is applied, so that the superficial subcutaneous tissues are slightly compressed. Next, a brief (15 ms) low-force (0.4 N) mechanical impulse is transmitted to the underlying muscle. The muscle's damped oscillation, recorded via accelerometer, quantifies key properties: tone, reflecting intrinsic tension at rest (Hz); biomechanical properties, including stiffness (N/m), resistance to deformation, decrement (logarithmic damped oscillation decay, inverse of elasticity), and recovery ability; and viscoelastic properties, such as stress relaxation time (ms), the time to recover shape post-deformation, and creep (Deborah number), representing gradual elongation under sustained tensile stress [
24].
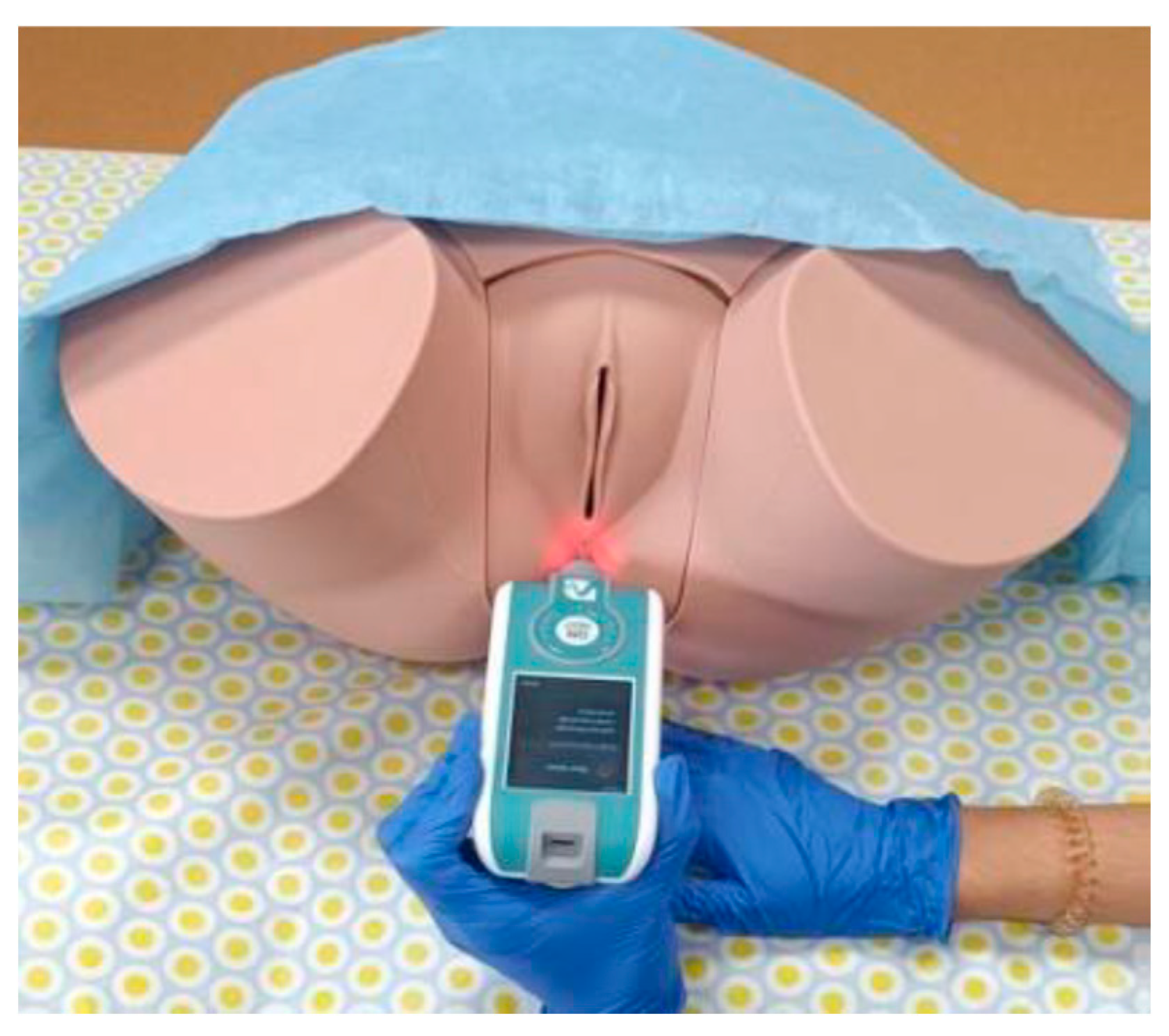
The PF MMPs evaluation (
Figure 1) was subsequently carried out, as described in detail and supported by different study protocols elsewhere [
18,
25]. For this, the volunteers were placed in a supine position with modified lithotomy, and a single measurement was taken on both the right and left sides of the central perineal core. The device was also used to measure lumbar muscle tone [
26], where the volunteers were placed in a prone position, and a single measurement was taken on both the right and left sides of the lumbar paravertebral area at the L5-S1 level.
2.4. Sample Size
To identify a large effect size (Cohen
d index = 0.87) based on a minimum detectable change of 41.48 N/m and a pooled standard deviation of 47.95 N/m for muscle stiffness [
18], a type I error of 0.05, and power of 0.80, we required 22 women per group (G*power 3.1.9.2 software,
t-test for differences between two independent groups).
2.5. Statistical Analysis
Frequencies and percentages were used to describe the qualitative data. All the MMP and BMI variables showed a normal data distribution (Kolmogorov–Smirnov test
P > 0.05) and were described by their means and standard deviations or 95% confidence intervals (95% CI). Age and questionnaire variables were not normally distributed and were described by their medians and interquartile ranges. To assess the between-group comparability, sociodemographic and clinical data were compared with unrepeated Student
t-tests and Mann–Whitney U tests, according to their distributions. Fisher and χ
2 tests were used for the qualitative data. For the study’s primary aim, unpaired Student
t-tests were applied to identify differences in the MMPs of the PF and lumbar muscles between groups. To identify intra-group associations between the MMPs and sociodemographic and clinical features, Pearson
r and Spearman
p (
rs) coefficients were calculated. Correlations were considered negligible (0.0 to 0.19), fair (0.20 to 0.39), moderate (0.40 to 0.69), strong (0.70 to 0.89), or almost perfect (0.0 to 1.00) [
28]. All the comparisons were bilateral, and probabilities exceeding 95% (alpha
p-values less than 0.05) were considered significant. The statistical analysis was performed using SPSS software (version 28, IBM Corp., Armonk, NY).
3. Results
3.1. Sociodemographic and Clinical Variables
For this study, 22 women with MS were recruited and formed the case group, while 22 nulliparous women without MS formed the control group. There were no differences in the mean age of the women with MS compared to those in the control group. In reference to the number of births, 45.45% of patients with MS were nulliparous compared to 50% in control group, with no significant differences between the two. In turn, the average score on the PFDI-20 and PFIQ-7 questionnaires was notably higher in the patient cases than in the controls. For the GPAQ questionnaire, the cases indicated that the patients engaged in less intense physical activity than the controls, with statistically significant differences. Regarding UI, as set out in
Table 1, 36.36% of the patients with MS presented urgency UI (UUI) and 31.82% of the controls reported stress UI (SUI). Finally, in women with MS the average time from diagnosis of the disease had been approximately 10.77 years; their average score on the EDSS scale was 2.89 points; 100% were on a DMT; and a high percentage had not received treatment for spasticity, nor neurorehabilitation or botulinum toxin treatment. None of the women indicated the presence of any pain during the evaluations (VAS = 0).
3.2. Comparisons of Lumbopelvic Muscle Mechanical Properties Between the Groups
Significant differences between the cases and controls were identified for all the MMPs of the PF. We found differences on the right side of the PF, with some of the variables being higher in women without MS, with a frequency of 3.26 Hz (95% CI [2.12, 4.41]), stiffness of 90 N/m (95% CI [55.09, 124.91]), and decrement of 0.2 (95% CI [0.09, 0.31]), compared to patients with MS. Likewise, the relaxation and creep values were lower in the controls by 6.15 ms (95% CI [8.26, 4.05]) and 0.24 ms (95% CI [0.34, 0.13]) compared to the cases. There were also significant differences in the variables for the left side of the central nucleus of the perineal muscle between women with and without MS, with the cases presenting a frequency of 2.82 Hz (95% CI [1.53, 4.12]), stiffness of 77.5 N/m (95% CI [36.27, 118.73]), and decrement of 0.19 (95% CI [0.07, 0.31]) more than women without the disease. The relaxation and creep variables were also significantly different between women with and without MS, with women without MS showing values of 5.55 ms (95% CI [7.9, 3.21]) and 0.21 (95% CI [0.3, 0.11]) lower than those with MS. Thus, it is worth highlighting that the frequency, stiffness, and decrement variables tended to be higher and the relaxation and creep variables trended to be lower in women without MS compared to those with the disease (
Table 2).
Fewer statistically significant differences were identified between the groups for the MMPs of the lumbar paravertebral muscles. Only the
frequency and
stiffness variables for the right area differed between women with and without MS. At 2.15 Hz (95% CI [0.28, 4.02]), the right
frequency values were higher in women without MS compared to women with MS. Higher values were also evident for right-side
stiffness in healthy women compared to those with MS, at 62.17 N/m (95% CI [10.7, 113.65]). These results are shown in
Table 3.
3.3. Intragroup Relationships Between the Lumbopelvic Muscle Mechanical Properties and Sociodemographic and Clinical Data
Very few correlations were identified for the lumbopelvic MMPs, with different patterns in each group. Women with MS only presented a moderate positive correlation between the PF MMPs and the PFDI-20 questionnaire and EDSS scale results for relaxation and creep on the right side (EDSS: r = 0.50; p = 0.017 and r = 0.57; p = 0.006, respectively) and a moderate positive correlation on the left side for stiffness and decrement (PFDI-20: r = 0.43; p = 0.049 and r = 0.47; p = 0.026, respectively). Regarding the correlations between the PMMs of the lumbar paravertebral muscles, a moderate positive correlation has been shown for the variables frequency and stiffness of the left side with age (r = 0.45; p = 0.042 and r = 0.47; p = 0.033, respectively). However, there were no correlations between the MMPs of the lumbar paravertebral muscles and the clinical variables, the EDSS scale, or the questionnaires.
In women without MS, no correlations were identified between the MMPs of the PF and the sociodemographic and clinical variables or with the questionnaires. In this population group, the MMPs of the lumbar paravertebral muscles were only moderately correlated with age and the number of births. Age was positively related to the right zone for frequency, stiffness, decrement, relaxation, and creep, and with relaxation and decrement in the left zone. Regarding the number of births, a moderate interaction with the lumbar MMPs of the left zone was observed for frequency (r = 0.57; p = 0.006), stiffness (r = 0.51; p = 0.015), relaxation (r = 0.54; p = 0.009) and creep (r = 0.54; p = 0.01).
4. Discussion
This present study identified differences in the MMPs of the PF between women with and without MS, meaning that this disease could influence the MMPs of the lumbopelvic region. Furthermore, there were some specific differences in the MMPs of the lumbar paravertebral muscles in healthy women compared to women with MS. It was also possible to identify different correlation patterns between MMPs and clinical characteristics, depending on whether the participant had MS or not. Finally, it is worth noting that none of the women withdrew from the study or reported pain or discomfort as a result of the study evaluation techniques.
Compared to women without MS, there was less muscle tone, stiffness and the relaxation time was lower on both sides of the central nucleus of the perineal area in the women with MS, with the differences found in this present study being slightly higher than these values (a minimal detectable change (MDC) of less than 2 Hz and 52.34 N/m [
18]). Furthermore, the elasticity and creep of both sides was greater in healthy women compared to those with MS. In this sense, there is evidence suggesting that more than a third of patients with MS experience signs of PF weakness [
28] along with preservation of peripheral muscle function caused by CNS lesions [
5] and the presence of plasticity in antigravity muscles [
29] such as the triceps, quadriceps femoris, gluteus medius, and hip adductor, among others [
30]. The spinal cord is a key part of the CNS and is the main source of neurogenic dysfunction of the lower urinary tract, which occurs in 75–80% of patients with MS [
30] (even reaching almost 100% 10 years after diagnosis of the disease [
32]) and which correlates with increased EDSS scores [
33]. Furthermore, MMP involvement has previously been described for other PF dysfunctions such as UI [
7], although in this present work we identified an increase in PF tone and stiffness.
Regarding the MMPs of the lumbar paravertebral muscles, the tone and stiffness of the right side was lower in women who had MS compared to those who did not. This may be because of the fact that patients with MS tend be less physically active than healthy people [
34] and are therefore more oriented towards greater resistance and muscle strengthening [
35]. Furthermore, 97% of patients with MS present spinal cord involvement, with 55% reporting low back pain and gait disorders as possible causes of physical inactivity [
36].
In relation to clinical characteristics, there was evidence of a moderate relationship between the MMPs of the PF of the right zone and the EDSS score in women with MS, probably partly because the gut/bladder system is one of the seven functional systems used to calculate the EDSS score and so this assessment also includes symptoms such as UI. In turn, the tone and speed of recovery of the PF tissue of the left zone in women with MS has also previously been related to the results of the PFDI-20 questionnaire because the state of the PF MMPs is used as a reference to assess the clinical status of this musculature [
18].
A positive relationship was also identified between age and lumbar MMPs in both women with and without MS because lumbar spine degeneration caused by biomechanical stress and the consequent effects this has on the paravertebral musculature is a general artefact of the aging process [
36]. This muscle involvement has been evidenced as an increased degree of trunk flexor and extensor muscle co-contraction [
37] which thereby results in the previously described increased tension and stiffness. Furthermore, age-related degenerative disorders of the lumbar spine have not been previously studied in patients with MS while MS lesions may be misdiagnosed as lumbar spine disease [
36]. Finally, in healthy women, there is a positive correlation between the tone of the PF in the left zone and the number of births. This asymmetry of behaviour between the left and right sides could be related with the presence, in up to 80% of healthy individuals, of muscle fascial tension, with multiparous women presenting a greater tendency towards asymmetries that, in turn, alters PFM contraction and biomechanics of the spine and lower extremities [
25]. Musculoskeletal dysfunction of these muscles also results in a loss of support from the PFM, which contributes to UI in 46% of cases [
33].
Finally, the strengths of this current study were that no previous work published in the scientific literature has described lumbopelvic MMPs in patients with MS, making our work pioneering in its evaluation of these variables in these patients. The main limitation of this work was the heterogeneity in the evolution of MS seen in different participants, as well as the subjectivity of the questionnaires used, with the latter depending on the truthfulness of each of the volunteers. Therefore, future studies, which should include more longitudinal studies, should consider this limitation as well as the presence of other factors, such as the presence of comorbid disorders that may influence the study results.
5. Conclusions
The MMPs of the PF on both sides of women with MS have lower tone and stiffness and increased elasticity and viscoelastic properties than women without MS. There were fewer differences in the MMPs of the lumbar paravertebral muscles in these women, with greater tone and stiffness on the right side in women who did not have MS. Finally, there was a relationship between the MMPs of the PF of women with MS and clinical data related to pelvic floor dysfunction (which is not seen in healthy women) and the EDSS score, as well as a relationship between age and the MMPs of the lumbar paravertebral musculature in both groups. Thus, it is important to conduct an objective evaluation of the pelvic floor muscle properties in women with multiple sclerosis. This would enable the development of tailored preventive treatments and the ongoing assessment of their effectiveness in improving muscle function and overall quality of life.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Inés Cruz Medel, María Ángeles Peña Toledo, Francisco Alburquerque Sendín and Eduardo Agüera Morales. The first draft of the manuscript was written by Inés Cruz Medel and Maria Ángeles Peña Toledo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Bioethics Committee of Cordoba (reference 5199).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yamout, B.; Alroughani, R. Multiple Sclerosis. Semin Neurol 2018, 38, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Pérez Carmona, N.; Fernández Jover, E.; Pérez Sempere, Á. Epidemiología de la esclerosis múltiple en España. Rev Neurol 2019, 69, 32. [Google Scholar] [CrossRef] [PubMed]
- Sicras-Mainar, A.; Ruíz-Beato, E.; Navarro-Artieda R, M.J. Impact on healthcare resource utilization of multiple sclerosis in Spain. BMC Health Serv Res 2017, 17, 854. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, V.C.; White, A.B.; Rogers, R.G. Updates on the diagnostic tools for evaluation of pelvic floor disorders. Curr Opin Obstet Gynecol 2017, 29, 458–464. [Google Scholar] [CrossRef]
- Jameson, J.S.; Rogers, J.; Chia, Y.W.; Misiewicz, J.; Henry, M.; Swash, M. Pelvic floor function in multiple sclerosis. Gut 1994, 35, 388–390. [Google Scholar] [CrossRef]
- Pérez, D.C.; Chao, C.W.; Jiménez, L.L.; Fernández, I.; de la Llave Rincón, A. Pelvic floor muscle training adapted for urinary incontinence in multiple sclerosis: A randomized clinical trial. Int Urogynecol J 2020, 31, 267–275. [Google Scholar] [CrossRef]
- Lúcio, A.C.; Perissinoto, M.C.; Natalin, R.A.; Prudente, A.; Damasceno, B.; D’ancona, C. A comparative study of pelvic floor muscle training in women with multiple sclerosis: Its impact on lower urinary tract symptoms and quality of life. Clinics 2011, 66, 1563–1568. [Google Scholar] [CrossRef]
- Khorasani, F.; Ghaderi, F.; Bastani, P.; Sarbakhsh, P.; Berghmans, B. The Effects of home-based stabilization exercises focusing on the pelvic floor on postnatal stress urinary incontinence and low back pain: A randomized controlled trial. Int Urogynecol J 2020, 31, 2301–2307. [Google Scholar] [CrossRef]
- Welk, B.; Baverstock, R. Is there a link between back pain and urinary symptoms? Neurourol Urodyn 2020, 39, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Bertuit, J.; Bakker, E.; Rejano-Campo, M. Relationship between urinary incontinence and back or pelvic girdle pain: A systematic review with meta-analysis. Int Urogynecol J 2021, 32, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Çelenay, T.; Kaya, Ö. Relationship of spinal curvature, mobility, and low back pain in womenwith and without urinary incontinence. Turk J Med Sci 2017, 47, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Sueki, D.G.; Cleland, J.A.; Wainner, R.S. A regional interdependence model of musculoskeletal dysfunction: Research, mechanisms, and clinical implications. J Man Manip Ther 2013, 21, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bernal, M.I.; Heredia-Rizo, A.M.; Gonzalez-Garcia, P.; Cortés-Vega, M.; Casuso-Holgado, M. Validity and reliability of myotonometry for assessing muscle viscoelastic properties in patients with stroke: A systematic review and meta-analysis. Sci Rep 2021, 11, 5062. [Google Scholar] [CrossRef]
- Myoton, A.S. Aplicaciones del Myoton 2022. https://www.myoton.com/applications/.
- Lohr, C.; Braumann, K.M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of tensiomyography and myotonometry in detecting mechanical and contractile characteristics of the lumbar erector spinae in healthy volunteers. Eur J Appl Physiol 2018, 118, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.; Bauermeister, W.; Klingler, W.; Schleip, R. Assessing reliability and validity of different stiffness measurement tools on a multi-layered phantom tissue model. Sci Rep 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Turhan, B.; Maden, T.; Maden, C. The comparison of tone and viscoelastic properties of superior orbicularis oris muscle in multiple sclerosis patients to healthy individuals. Mult Scler Relat Disord 2022, 65, 103983. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-de-Souza, D.P.; Alcaraz-Clariana, S.; García-Luque, L.; Carmona-Pérez, C.; Garrido-Castro, J.; Cruz-Medel, I.; et al. Absolute and Relative Reliability of the Assessment of the Muscle Mechanical Properties of Pelvic Floor Muscles in Women with and without Urinary Incontinence. Diagnostics 2021, 11, 2315. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.; Coetzee, T.; Comi, G.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.S.; Torres Lacomba, M.; Navarro Brazález, B.; Cerezo Téllez, E.; Pacheco Da Costa, S.; Gutiérrez Ortega, C. Responsiveness of the Spanish Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaires Short Forms (PFDI-20 and PFIQ-7) in women with pelvic floor disorders. Eur J Obstet Gynecol Reprod Biol 2015, 190, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Escalante, Y. Physical Activity, Exercise, and Fitness in the Public Health Field. Rev Esp Salud Publica 2011, 84, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dellalana, L.E.; Gandelman, J.S.; Vain, A.; Jagasia, M.; Tkaczyk, E. Non-invasive measurement of sclerosis in cutaneous cGVHD patients with the handheld device Myoton: A cross-sectional study. Bone Marrow Transplant 2019, 54, 616–619. [Google Scholar] [CrossRef]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; van der Schans, C.; Hobbelen, H. Psychometric Properties of the MyotonPRO in Dementia Patients with Paratonia. Gerontology 2018, 64, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Frawley, H.; Shelly, B.; Morin, M.; Bernard, S.; B∅, K.; Digesu, G.; et al. An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol Urodyn 2021, 40, 1217–1260. [Google Scholar] [CrossRef]
- Rodrigues-de-Souza, D.P.; Beleza, A.C.S.; Garcia-Luque, L.; Alcaraz-Clariana, S.; Carmona-Pérez, C.; De Miguel-Rubio, A.; et al. Asymmetries of the Muscle Mechanical Properties of the Pelvic Floor in Nulliparous and Multiparous Women, and Men: A CrossSectional Study. Symmetry (Basel) 2022, 14, 2124. [Google Scholar] [CrossRef]
- Alcaraz-Clariana, S.; Garcia-Luque, L.; Garrido-Castro, J.L.; Aranda-Valera, I.; Ladehesa-Pineda, L.; Puche-Larrubia, M.; et al. Paravertebral Muscle Mechanical Properties in Patients with Axial Spondyloarthritis or Low Back Pain: A Case-Control Study. Diagnostics 2021, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.J.; Bryant, A.L.; Bower, W.F.; Frawley, H.C. Myotonometry Reliably Measures Muscle Stiffness in the Thenar and Perineal Muscles. Physiotherapy Canada 2017, 69, 104–112. [Google Scholar] [CrossRef]
- Mosalanejad, F.; Afrasiabifar, A.; Zoladl, M. Investigating the combined effect of pelvic floor muscle exercise and mindfulness on sexual function in women with multiple sclerosis: A randomized controlled trial. Clin Rehabil 2018, 32, 1340–1347. [Google Scholar] [CrossRef]
- Masani, K.; Sayenko, D.G.; Vette, A.H. What triggers the continuous muscle activity during upright standing? Gait Posture 2013, 37, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.P.S.; Marsden, J. The management of spasticity in adults. BMJ 2014, 349, g4737. [Google Scholar] [CrossRef] [PubMed]
- Medina-Polo, J.; Adot, J.M.; Allue, M.; Arlandis, S.; Blasco, P.; Casanova, B.; et al. Consensus document on the multidisciplinary management of neurogenic lower urinary tract dysfunction in patients with multiple sclerosis. Neurourol Urodyn 2020, 39, 762770. [Google Scholar] [CrossRef]
- Tornic, J.; Panicker, J.N. The Management of Lower Urinary Tract Dysfunction in Multiple Sclerosis. Curr Neurol Neurosci Rep 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, T.B.; Gopal, A.; Block, V.J.; Suskind, A.; Zhao, C.; Polgar-Turcsanyi, M.; et al. Challenges to Longitudinal Characterization of Lower Urinary Tract Dysfunction in Multiple Sclerosis. Mult Scler Relat Disord 2022, 62, 103793. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Multiple Sclerosis Journal 2020, 26, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Miko, H.C.; Zillmann, N.; Ring-Dimitriou, S.; Dorner, T.; Titze, S.; Bauer, R. Auswirkungen von Bewegung auf die Gesundheit. Das Gesundheitswesen 2020, 82, S184–S195. [Google Scholar] [CrossRef]
- McGrath, K.; Lee, J.; Steinmetz, M.; Lonser, R.; Resnick, D. Degenerative Spine Disorders and Multiple Sclerosis. Neurol Clin 2022, 40, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.M.H.; Szeto, G.P.Y.; Li, L.M.K.; Wong, D.; Yip, M.; Lee, R. The effects of bending speed on the lumbo-pelvic kinematics and movement pattern during forward bending in people with and without low back pain. BMC Musculoskelet Disord 2017, 18, 157. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).