Submitted:
16 November 2024
Posted:
20 November 2024
You are already at the latest version
Abstract

Keywords:
Introduction
Methodology:
Discussion:
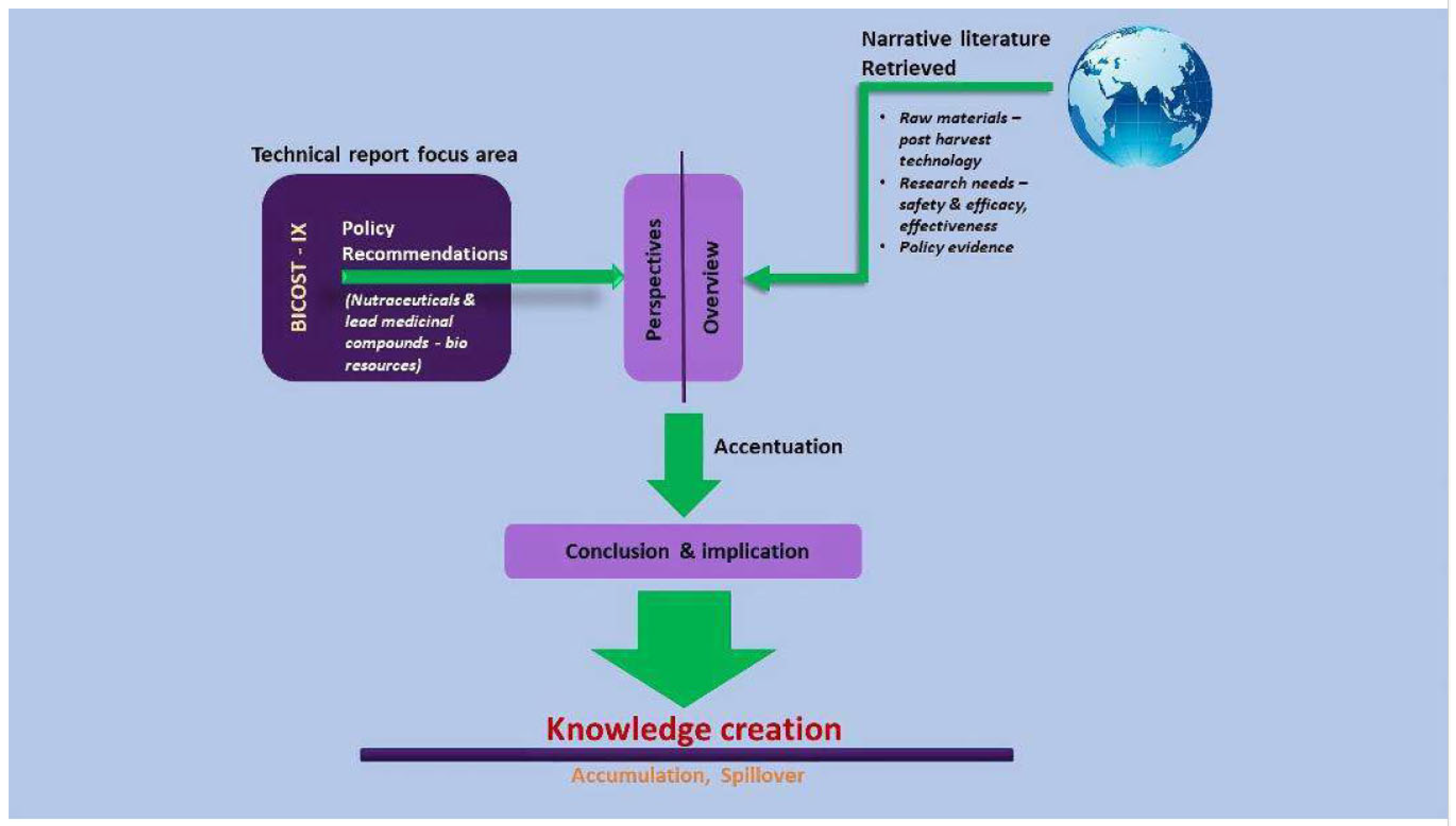
I. Cultivation and Postharvest Technology of Herbal Raw Materials
II. An overview of Research Needs and Clinical Trials in the Nutraceutical Industry
Conclusion and Implications:
Limitations
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
References
- Asase, A. J. F. i. P. (2023). Ghana’s herbal medicine industry: prospects, challenges and ways forward from a developing country perspective. 14, 1267398.
- Bulathgama, U., Lakshman, N., & Bulugahapitiya, V. P. (2022). Recent Trends in Functional Foods and Nutraceuticals as Health-Promotive Measures: A Review. Applied Bio-Systems Technology.
- II(I), 11-33.
- Cameron, A. (2022). Building an Innovation Hotspot: Approaches and Policies to Stimulating New Industry: CSIRO PUBLISHING.
- Chandra, S., Stanford, D., Fletcher, E., Walker, L. A. J. T. S., & Drugs, R. o. N. D. C. (2019). Raw Materials Production and Manufacturing Process Control Strategies. 175-190.
- Chaurasia, S., Pati, R. K., Padhi, S. S., Jensen, J. M., & Gavirneni, N. J. D. S. (2022). Achieving the United Nations Sustainable Development Goals-2030 through the nutraceutical industry: A review of managerial research and the role of operations management. 53(4), 630-645.
- Chopra, A. S., Lordan, R., Horbańczuk, O. K., Atanasov, A. G., Chopra, I., Horbańczuk, J. O.,... Banach, M. J. P. R. (2022). The current use and evolving landscape of nutraceuticals. 175, 106001.
- de Vrueh, R. L., & Crommelin, D. J. J. P. r. (2017). Reflections on the future of pharmaceutical public‒private partnerships: from input to impact. 34(10), 1985-1999.
- DeFelice, S. L. J. R. o. f. f., & perspective, n. a. g. (2005). The nutraceutical health sector: a Point of view. 201-212.
- Dissanayake, H. N. K. (2015). Medicinal plant research in Sri Lanka : A scientometric study based on Scopus database. Collnet Journal of Scientometrics and Information Management, 9(2), 225-234.
- Echeverría King, L. F., González, D. A., Andrade-Sastoque, E. J. F. i. R. M., & Analytics. (2021). Science diplomacy in emerging economies: a phenomenological analysis of the colombian case. 6, 636538.
- Evans, N., Miklosik, A., & Du, J. T. J. H. (2023). University-industry collaboration as a driver of digital transformation: Types, benefits and enablers. 9(10).
- Galvez, E. (2022). Scaling up inclusive innovations in agrifood chains in Asia and the Pacific: Food & Agriculture Org.
- Gluckman, P. D., Bardsley, A., Kaiser, M. J. H., & Communications, S. S. (2021). Brokerage at the science–policy interface: from conceptual framework to practical guidance. 8(1), 1-10.
- Science and Technology Development Act 1994/11, (1994).
- Govindaraghavan, S., Sucher, N. J. J. E., & Behavior. (2015). Quality assessment of medicinal herbs and their extracts: Criteria and prerequisites for consistent safety and efficacy of herbal medicines. 52, 363-371.
- Green, B. N., Johnson, C. D., & Adams, A. J. J. o. c. m. (2006). Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. 5(3), 101-117.
- Gulati, O. P., Ottaway, P. B., Jennings, S., Coppens, P., & Gulati, N. (2019). Botanical nutraceuticals (food supplements and fortified and functional foods) and novel foods in the EU, with a main focus on legislative controls on safety aspects. In Nutraceutical and functional food regulations in the United States and around the World (pp. 277-321): Elsevier.
- Haji, M., Kerbache, L., & Al-Ansari, T. J. P. (2022). Food quality, drug safety, and increasing public health measures in supply chain management. 10(9), 1715.
- ITI. (2015). Annual Report of Industrial Technology Institute of Sri Lanka Retrieved from ITI: http://www.iti.lk/images/isc/publication/Annual%20Report%202015.pdf (date accessed March 20th, 2024).
- Jain, N., & Ramawat, K. G. J. N. p. (2013). Nutraceuticals and antioxidants in prevention of diseases. 2559-2580.
- Jayaweera, J. (2023). Current trends and technologies in nutraceutical industry. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods (pp. 347-360): Elsevier.
- Jost, S., Birringer, M., & Herzig, C. J. J. o. F. F. (2022). Brokers, prestige and information exchange in the European Union’s functional food sector–A policy network analysis. 99, 105309.
- Kamal, N., & Dir, Z. C. J. J. o. C. B. (2015). Accelerating the growth of the bioeconomy in Malaysia. 21(2).
- Kano, H., Hayashi, T. I. J. E. S., & Policy. (2021). A framework for implementing evidence in policymaking: perspectives and phases of evidence evaluation in the science-policy interaction. 116, 86-95.
- Karunaratne, V. J. V. J. o. S. (2021). Potential pharmaceutical applications of endemic plants: When will Sri Lanka understand the economic value chain? , 24(02).
- Komala, M. G., Ong, S. G., Qadri, M. U., Elshafie, L. M., Pollock, C. A., & Saad, S. J. F. (2023). Investigating the Regulatory Process, Safety, Efficacy and Product Transparency for Nutraceuticals in the USA, Europe and Australia. 12(2), 427.
- Kotagama, S. (2008). Biodiversity conservation in Sri Lanka. Paper presented at the Proceedings of International Forestry and Environment Symposium.
- Kumar, A., P, N., Kumar, M., Jose, A., Tomer, V., Oz, E.,... K, S. J. M. (2023). Major phytochemicals: Recent advances in health benefits and extraction method. 28(2), 887.
- Kumari, R., & Kotecha, M. (2016). A review on the Standardization of herbal medicines. International Journal of Pharma Sciences and Research, 07(02), 97-106.
- Kunle, Oluyemisi, F., Egharevba, Henry, O., Ahmadu, Peter, O. J. I. j. o. b., & conservation. (2012). Standardization of herbal medicines-A review. 4(3), 101-112.
- Lathrop, D., & Ruma, L. (2010). Open government: Collaboration, transparency, and participation in practice: " O'Reilly Media, Inc.".
- Mendoza, R. L. J. H. M. Q. (2020). Cross-country experiences and lessons in nutraceutical health marketing in the United States, China, and India. 37(4), 281-299.
- MIDA. (2020, 09-2020). Nutraceutical, Health Supplements and Traditional Medicines in Malaysia. MIDA.
- MoF. (2023). National Budget for What for Whom 2023. Retrieved from Treasury GoSL: https://www.treasury.gov.lk/api/file/bcb4fc90-96ea-4edc-8f69-a097b85c1a32 (date accessed March 20th, 2024).
- Mtewa, A. G., Chikowe, I., Kumar, S., Ngwira, K. J., Lampiao, F. J. F. F., Nutraceuticals: Bioactive Components, F., & Innovations. (2020). Good manufacturing practices and safety issues in functional food industries. 613-628.
- Muhammad, B. Y., Awaisu, A. J. A. J. o. T., Complementary,, & Medicines, A. (2008). The need for enhancement of research, development, and commercialization of natural medicinal products in Nigeria: Lessons from the Malaysian experience. 5(2), 120.
- NASTEC. (2016). National Research and Development Framework -NRDF 2016-2020. Colombo: NASTEC Retrieved from https://www.nastec.gov.lk/reports/nrdf (date accessed March 20th, 2024).
- NASTEC. (2023). Nutraceuticals and Lead meedical compounds derived from Sri Lankan bioresources (ISBN978-955-8630-29-7). Retrieved from https://osf.io/r5u9p (date accessed March 20th, 2024).
- NIPHM. (2020). Annual Report 2020. Retrieved from https://www.parliament.lk/uploads/documents/paperspresented/1674035955033416.pdf (date accessed March 20th, 2024).
- NRC. (2023-2024). PRIVATE-PUBLIC PARTNERSHIP PROGRAME. Retrieved from https://www.nrc.gov.lk/index.php/private-public-partnership-programe/ (date accessed March 20th, 2024).
- NSF. (2022). Technology Grant schemes. NSF Grants. Retrieved from http://www.nsf.ac.lk/index.php/technology-grant-schemes (date accessed March 20th, 2024).
- NSF (Producer). (2023, 03 10). Overseas Special Training Programme (OSTP): Special Call. National Science Foundation. Retrieved from http://www.nsf.ac.lk/index.php/grant-support/capacity-building/short-term-training (date accessed March 20th, 2024).
- Parveen, A., Parveen, B., Parveen, R., Ahmad, S. J. J. o. p., & sciences, b. (2015). Challenges and guidelines for clinical trial of herbal drugs. 7(4), 329.
- Patel, D., Dufour, Y., & Domigan, N. J. J. P. P. S. (2008). Functional food and nutraceutical registration process in Japan and China. Similarities and differences. 11(4), 1-11.
- Peduruhewa, P., Jayathunge, K., Liyanage, R., & Manatunga, D. J. M. A. t. A. E. T. i. t. F. o. C. i. S. L. (2023). Creating Opportunities for Rural Economic Development and Food Security in Sri Lanka by Contributing Under utilized Plants to the Global Food System. 50.
- Perera, P., Jhalani, M., Kim, S., & Grant, S. (2021). A review of traditional medicine research in Sri Lanka: 2015–2019. World Health Organization.
- Perera, P. K. Invited Guest talk: Current Scenario of Ayurveda and Traditional Medicine in Sri Lanka.
- promotion, S. m. o. i. m. (2021). Performance report for 2021. Retrieved from.
- Puri, V., Nagpal, M., Singh, I., Singh, M., Dhingra, G. A., Huanbutta, K.,... Sangnim, T. J. N. (2022). A comprehensive review on nutraceuticals: therapy support and formulation challenges. 14(21), 4637.
- Ranasinghe, P., Galappaththy, P., Constantine, G. R., Jayawardena, R., Sirimal Premakumara, S., Premakumara, S., & Katulanda, P. (2017). Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial. Trials, 18, 446.
- Rungsung, W., Dutta, S., & Hazra, J. (2013). Pharmacognostical Approach towards Authentication and Quality Evaluation of Medicinal Plants-A Compendious Description. Research Journal of Pharmacognosy.
- Phytochemistry, 5(2), 77-83.
- Rybnicek, R., & Königsgruber, R. J. J. o. b. e. (2019). What makes industry–university collaboration succeed? A systematic review of the literature. 89(2), 221-250.
- Saini, A., Malik, A., Kumar, M., Bhatt, S. J. I. J. o. S., & Research, T. (2020). Nutraceuticals are for healthy life. 9(2), 150-162.
- Saliya, C. A. J. A. a. S. (2023). Budget, Taxation Turmoil and Policy Blunders: Case of Sri Lanka.
- Sandhu, H. J. A.-A. G. C. D., & Indo-Pacific, C. i. (2020). Health Sector Cooperation in Asia-Africa Growth Corridor. 153-163.
- Selmar, D., Kleinwächter, M. J. I. C., & Products. (2013). Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. 42, 558-566.
- Shahmy, S. (2023). Sri Lanka’s 9th Biennial Conference on Science and Technology (BICOST IX)—2023 Technical Outputs [Press release]. Retrieved from https://vidyaenews.mostr.gov.lk/editors-picks/sri-lankas-9th-biennial-conference-on-science-and-technology-bicost-ix-2023-technical-outputs/ (date accessed March 20th, 2024).
- Shahmy, S., Munagamage, T., Perera, W., & Fernando, S. (2022). A proposed National Science Advice System to the Government of an Emerging Economy in South Asia. Research Gate - Preprint. [CrossRef]
- Shahmy, S., Perera, W. R. T. S., Munagamage, T., & Fernando, S. (2021). New horizon for data-driven Science for Policy -A proposed system to generate robust scientific evidence. Paper presented at the 4th International Conference- INGSA 2021, Canada. https://www.researchgate.net/publication/361791859_New_horizon_for_data-driven_Science_for_Policy_-A_proposed_system_to_generate_robust_scientific_evidence (date accessed March 20th, 2024).
- Siddiqui, R. A., & Moghadasian, M. H. J. N. (2020). Nutraceuticals and nutrition supplements: Challenges and opportunities. 12(6), 1593.
- Silva, S., Piyumali, K., Perera, R. T. S., Kaluarachchi, K., Munagamage, T., Ahammed, R.,... Karunaratne, V. J. C. E. (2024). Assessing the biennial conference on science and technology (BICOST IX) 2023 technical output on renewable energy, energy storage, and green hydrogen in line with UN SDG commitments. 8(2), 85-88.
- Siriwardena, A., Wijesundara, S., & Karunarathna, V. (2015). A review of studies on bioactive compounds isolated from Sri Lankan flora. J.Natn.Sci.Foundation Sri Lanka, 43 (41): 11-33.
- Tilburt, J. C., & Kaptchuk, T. J. J. B. o. t. W. H. O. (2008). Herbal medicine research and global health: an ethical analysis. 86, 594-599.
- UNCTAD. (2015). Policies to promote collaboration in science, technology and.
- innovation for development: The role of science, technology .
- and innovation parks (TD/B/C.II/30). Retrieved from Geneva: https://unctad.org/system/files/official-document/ciid30_en.pdf (date accessed March 20th, 2024).
- Weerakoon, D., & Perera, N. (2014). The role of Sri Lanka in enhancing connectivity between South Asia and Southeast Asia.
- Weerakoon, W. A. S. S., Perera, P. K., Samarasinghe, K., Gunasekera, D., & Suresh, T. S. (2020). Acute and Subchronic Oral Safety Profiles of the Sudarshana Suspension. Evidence-based complementary and alternative medicine.
- WHO. (2013). WHO traditional medicine strategy: 2014-2023: World Health Organization.
- WHO. (2021). A review of traditional medicine research in Sri Lanka: 2015–2019. doi:https://iris.who.int/handle/10665/347359 (date accessed March 20th, 2024).
- WHO, G. (2003). WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants.
- WHO, W. H. O.-. (2003). WHO guidelines on good agricultural and collection practices [GACP] for medicinal plants: World Health Organization.
- WIckramarathne, M., Sudasinghe, S. R. S. N., Wanigasekara, T., Perera, K., & Ranasinghe, P. (2020). Institutional Review on Bandaranayake Memorial Ayurveda Research Institute (BMARI). Retrieved from.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




