Submitted:
18 November 2024
Posted:
18 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Canine Monocytic Ehrlichiosis
1.1.1. Background
1.1.2. Hosts
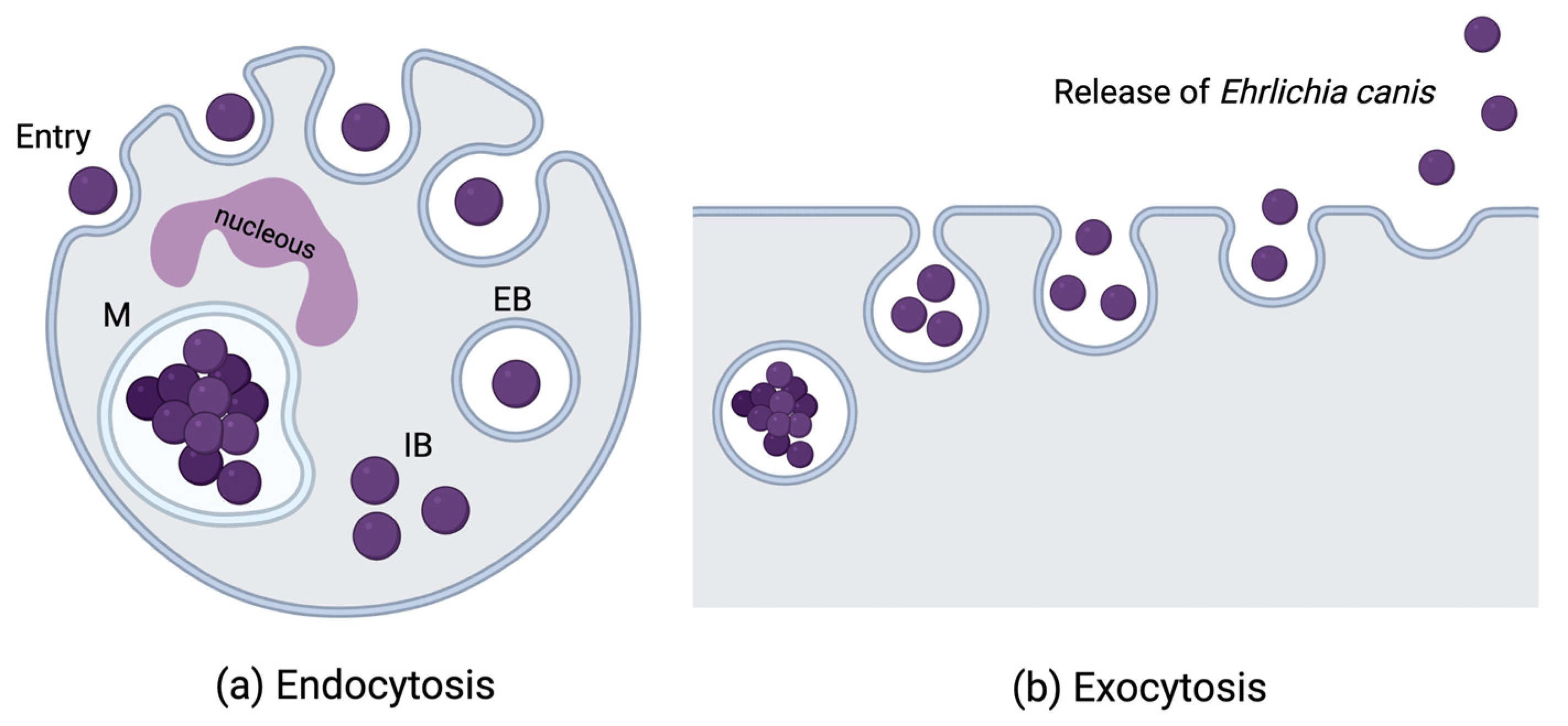
1.1.3. Ehrlichia canis Development in the Host
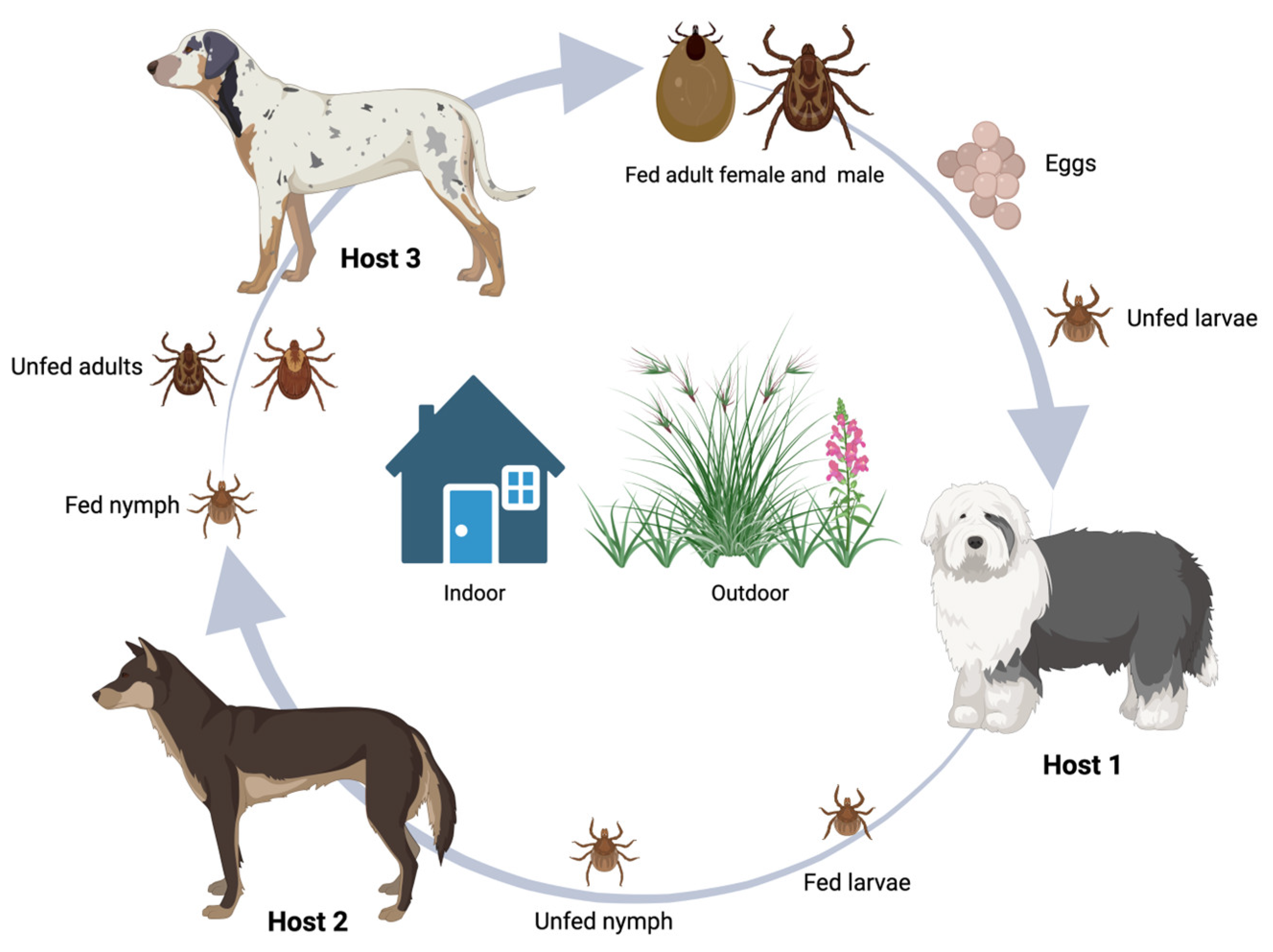
1.1.4. Vectors
1.1.5. Ehrlichia canis Development in the Vector
1.1.6. Pathogenesis
1.1.7. Diagnosis
1.1.8. Treatment, Control and Prevention
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bremer, W.G.; Schaefer, J.J.; Wagner, E.R.; Ewing, S.A.; Rikihisa, Y.; Needham, G.R.; Jittapalapong, S.; Moore, D.L.; Stich, R.W. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet. Parasitol. 2005, 131, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Cao, W.; Pan, H. Ehrlichiae and Ehrlichial Diseases in China. Ann. New York Acad. Sci. 2003, 990, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 2008, 152, 173–185. [Google Scholar] [CrossRef]
- Maeda, K.; Markowitz, N.; Hawley, R.C.; Ristic, M.; Cox, D.; McDade, J.E. Human Infection withEhrlichia canis, a Leukocytic Rickettsia. N. Engl. J. Med. 1987, 316, 853–856. [Google Scholar] [CrossRef]
- J. E. Dawson et al., "Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis," J Clin Microbiol, vol. 29, no. 12, pp. 2741-5, Dec 1991.
- Brouqui, P.; Le Cam, C.; Kelly, P.J.; Laurens, R.; Tounkara, A.; Sawadogo, S.; Lo-Marcel, V.; Gondao, L.; Faugere, B.; Delmont, J.; et al. Serologic evidence for human ehrlichiosis in Africa. Eur. J. Epidemiology 1994, 10, 695–698. [Google Scholar] [CrossRef]
- Perez, M.; Rikihisa, Y.; Wen, B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J. Clin. Microbiol. 1996, 34, 2133–9. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human Infection with Ehrlichia Canis Accompanied by Clinical Signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- S. A. Ewing, "Canine ehrlichiosis," Adv Vet Sci Comp Med, vol. 13, pp. 331-53, 1969.
- J. S. Dumler et al., "Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila," Int J Syst Evol Microbiol, vol. 51, no. Pt 6, pp. 2145-65, Nov 2001.
- Donatien and, F. Lestoquard, "Existence in Algerie d'une Rickettsia du chien.," Bull. Soc. Pathol. Exot., vol. 28, pp. 418-419, 1935.
- M. G. Groves, G. L. Dennis, H. L. Amyx, and D. L. Huxsoll, "Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus)," Am J Vet Res, vol. 36, no. 7, pp. 937-40, Jul 1975.
- Walker, J.S.; Rundquist, J.D.; Taylor, R.; Wilson, B.L.; Andrews, M.R.; Barck, J.; Hogge, A.L.; Huxsoll, D.L.; Hildebrandt, P.K.; Nims, R.M. Clinical and clinicopathologic findings in tropical canine pancytopenia. J Am Vet Med Assoc 1970, 157, 43–55. [Google Scholar]
- J. W. Harvey, C. F. Simpson, J. M. Gaskin, and J. H. Sameck, "Ehrlichiosis in wolves, dogs, and wolf-dog crosses," J Am Vet Med Assoc, vol. 175, no. 9, pp. 901-5, Nov 1 1979.
- Ewing, S.A.; Buckner, R.G.; Stringer, B.G. The Coyote, a Potential Host for Babesia canis and Ehrlichia sp. J. Parasitol. 1964, 50, 704. [Google Scholar] [CrossRef]
- Amyx, H.L.; Huxsoll, D.L. RED AND GRAY FOXES — POTENTIAL RESERVOIR HOSTS FOR Ehrlichia canis. J. Wildl. Dis. 1973, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Gilad, M.; Cortes, H.C.; Nachum-Biala, Y.; Lopes, A.P.; Vila-Viçosa, M.J.; Simões, M.; A Rodrigues, P.; Baneth, G. First report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasites Vectors 2015, 8, 144–144. [Google Scholar] [CrossRef] [PubMed]
- Bouloy, R.P.; Lappin, M.R.; Holland, C.H.; Thrall, M.A.; Baker, D.; O'Neil, S. Clinical ehrlichiosis in a cat. J. Am. Veter- Med Assoc. 1994, 204, 1475–1478. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Abrams-Ogg, A.C.; Lappin, M.R.; Bienzle, D.; Hancock, S.I.; Cowan, S.M.; Clooten, J.K.; Hegarty, B.C.; Hawkins, E.C. Molecular Evidence Supporting Ehrlichia canis–Like Infection in Cats. J. Veter- Intern. Med. 2002, 16, 642–9. [Google Scholar] [CrossRef]
- Stich, R.; Schaefer, J.J.; Bremer, W.G.; Needham, G.R.; Jittapalapong, S. Host surveys, ixodid tick biology and transmission scenarios as related to the tick-borne pathogen, Ehrlichia canis. Veter- Parasitol. 2008, 158, 256–273. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Murphy, S.M.; Luttrell, M.P.; Little, S.E.; Massung, R.F.; Stallknecht, D.E.; Conti, L.A.; Blackmore, C.G.; Durden, L.A. Experimental and Field Studies on the Suitability of Raccoons (Procyon lotor) as Hosts for Tick-Borne Pathogens. Vector-Borne Zoonotic Dis. 2008, 8, 491–504. [Google Scholar] [CrossRef]
- Y. Rikihisa, "The tribe Ehrlichieae and ehrlichial diseases," Clin Microbiol Rev, vol. 4, no. 3, pp. 286-308, Jul 1991.
- Z. Woldehiwet and M. Ristic, Rickettsial and chlamydial diseases of domestic animals, 1st ed. Oxford, England ; New York: Pergamon Press, 1993, pp. xiii, 427 p.
- M. Ristic and C. J. Holland, "Canine ehrlichiosis," Pergamon Press - Oxford, 1993.
- Popov, V.L.; Han, V.C.; Chen, S.-M.; Dumler, J.S.; Feng, H.-M.; Andreadis, T.G.; Tesh, R.B.; Walker, D.H. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J. Med Microbiol. 1998, 47, 235–251. [Google Scholar] [CrossRef]
- McQuiston, J.H.; McCall, C.L.; Nicholson, W.L. Ehrlichiosis and related infections. J. Am. Veter- Med Assoc. 2003, 223, 1750–1756. [Google Scholar] [CrossRef]
- Bell-Sakyi, L.; Zweygarth, E.; Blouin, E.F.; Gould, E.A.; Jongejan, F. Tick cell lines: tools for tick and tick-borne disease research. Trends Parasitol. 2007, 23, 450–457. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasites Vectors 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Ewing, S.A.; Harkess, J.R.; Kocan, K.M.; Barker, R.W.; Fox, J.C.; Tyler, R.D.; Cowell, R.L.; Morton, R.B. Failure to Transmit Ehrlichia canis (Rickettsiales: Ehrlichieae) with Otobius megnini (Acari: Argasidae). J. Med Èntomol. 1990, 27, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Ewing, S.; Barker, R.; Fox, J.; Crow, D.; Kocan, K. Experimental transmission of Ehrlichia canis (Rickettsiales: Ehrlichieae) by Dermacentor variabilis (Acari: Ixodidae). Veter- Parasitol. 1998, 74, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Estrada-Peña, A.; Petney, T.; Beati, L.; Labruna, M.B.; Szabó, M.P.; Venzal, J.M.; Mastropaolo, M.; Mangold, A.J.; Guglielmone, A.A. The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Veter- Parasitol. 2015, 208, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.P.; Mangold, A.J.; João, C.F.; Bechara, G.H.; Guglielmone, A.A. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Veter- Parasitol. 2005, 130, 131–140. [Google Scholar] [CrossRef]
- Burlini, L.; Teixeira, K.R.S.; Szabó, M.P.J.; Famadas, K.M. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: is there a geographical pattern? Exp. Appl. Acarol. 2009, 50, 361–374. [Google Scholar] [CrossRef]
- Moraes-Filho, J.; Marcili, A.; Nieri-Bastos, F.A.; Richtzenhain, L.J.; Labruna, M.B. Genetic analysis of ticks belonging to the Rhipicephalus sanguineus group in Latin America. Acta Trop. 2011, 117, 51–55. [Google Scholar] [CrossRef]
- Levin, M.L.; Studer, E.; Killmaster, L.; Zemtsova, G.; Mumcuoglu, K.Y. Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). Exp. Appl. Acarol. 2012, 58, 51–68. [Google Scholar] [CrossRef]
- Nava, S.; Mastropaolo, M.; Venzal, J.M.; Mangold, A.J.; Guglielmone, A.A. Mitochondrial DNA analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in the Southern Cone of South America. Veter- Parasitol. 2012, 190, 547–555. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Latrofa, M.S.; Annoscia, G.; Giannelli, A.; Parisi, A.; Otranto, D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasites Vectors 2013, 6, 213–213. [Google Scholar] [CrossRef]
- Liu, G.-H.; Chen, F.; Chen, Y.-Z.; Song, H.-Q.; Lin, R.-Q.; Zhou, D.-H.; Zhu, X.-Q. Complete Mitochondrial Genome Sequence Data Provides Genetic Evidence That the Brown Dog Tick Rhipicephalus sanguineus (Acari: Ixodidae) Represents a Species Complex. Int. J. Biol. Sci. 2013, 9, 361–369. [Google Scholar] [CrossRef]
- Sanches, G.S.; Évora, P.M.; Mangold, A.J.; Jittapalapong, S.; Rodriguez-Mallon, A.; Guzmán, P.E.; Bechara, G.H.; Camargo-Mathias, M.I. Molecular, biological, and morphometric comparisons between different geographical populations of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Veter- Parasitol. 2016, 215, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.O.; Gruntmeir, J.M.; Hamer, S.A.; Little, S.E. Temperate and tropical lineages of brown dog ticks in North America. Veter- Parasitol. Reg. Stud. Rep. 2017, 7, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Latrofa, M.S.; Ramos, R.A.N.; Lia, R.P.; Capelli, G.; Parisi, A.; Porretta, D.; Urbanelli, S.; Otranto, D. Biological compatibility between two temperate lineages of brown dog ticks, Rhipicephalus sanguineus (sensu lato). Parasites Vectors 2018, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.R.; Bechara, G.H.; Denardi, S.E.; Saito, K.C.; Nunes, E.T.; Szabó, M.P.J.; Mathias, M.I.C. Comparison of the external morphology of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks from Brazil and Argentina. Veter- Parasitol. 2005, 129, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Gerardi, M.; Krawczak, F.S.; Moraes-Filho, J. Comparative biology of the tropical and temperate species of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) under different laboratory conditions. Ticks Tick-borne Dis. 2017, 8, 146–156. [Google Scholar] [CrossRef]
- Moraes-Filho, J.; Krawczak, F.S.; Costa, F.B.; Soares, J.F.; Labruna, M.B. Comparative Evaluation of the Vector Competence of Four South American Populations of the Rhipicephalus sanguineus Group for the Bacterium Ehrlichia canis, the Agent of Canine Monocytic Ehrlichiosis. PLOS ONE 2015, 10, e0139386. [Google Scholar] [CrossRef]
- Cicuttin, G.L.; Tarragona, E.L.; De Salvo, M.N.; Mangold, A.J.; Nava, S. Infection with Ehrlichia canis and Anaplasma platys (Rickettsiales: Anaplasmataceae) in two lineages of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) from Argentina. Ticks Tick-borne Dis. 2015, 6, 724–729. [Google Scholar] [CrossRef]
- D. M. Aguiar, G. T. Cavalcante, A. Pinter, S. M. Gennari, L. M. Camargo, and M. B. Labruna, "Prevalence of Ehrlichia canis (Rickettsiales: Anaplasmataceae) in dogs and Rhipicephalus sanguineus (Acari: Ixodidae) ticks from Brazil," J Med Entomol, vol. 44, no. 1, pp. 126-32, Jan 2007. [CrossRef]
- Souza, B.M.P.d.S.; Leal, D.C.; Barboza, D.C.P.M.; Uzêda, R.S.; De Alcântara, A.C.; Ferreira, F.; Labruna, M.B.; Gondim, L.F.P.; Franke, C.R. Prevalence of ehrlichial infection among dogs and ticks in Northeastern Brazil. Rev. Bras. De Parasitol. Veter- 2010, 19, 89–93. [Google Scholar] [CrossRef]
- J. M. Venzal, A. Estrada-Peña, O. Castro, C. G. De Souza, A. Portillo, and O. J.A., "Study on seasonal activity in dogs and ehrlichial infection in Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) from southern Uruguay," Parasitol. Latinoam., vol. 62, pp. 23-26, 2007.
- Luzzi, M.d.C.; de Carvalho, L.A.L.; Pinheiro, D.G.; Lima-Duarte, L.; Camargo, J.V.; Kishi, L.T.; Fernandes, C.C.; Machado, R.Z.; Soares, J.F.; André, M.R.; et al. Analysis on the prokaryotic microbiome in females and embryonic cell cultures of Rhipicephalus sanguineus tropical and temperate lineages from two specific localities in Brazil. Rev. Bras. De Parasitol. Veter- 2021, 30, e005721. [Google Scholar] [CrossRef]
- T. M. Neer, E. B. Breitschwerdt, R. T. Greene, and M. R. Lappin, "Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. American College of Veterinary Internal Medicine," J Vet Intern Med, vol. 16, no. 3, pp. 309-15, May-Jun 2002.
- Keefe, T.J.; Holland, C.J.; E Salyer, P.; Ristic, M. Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United States. . 1982, 181, 236–8. [Google Scholar]
- Estrada-Peña, A.; Jongejan, F. Ticks Feeding on Humans: A Review of Records on Human-Biting Ixodoidea with Special Reference to Pathogen Transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Yuhai, M.; Shide, T.; Yang, S.; Bohai, W.; Xiangrui, C. Canine Ehrlichiosis Caused Simultaneously by Ehrlichia canis and Ehrlichia platys. Microbiol. Immunol. 2000, 44, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, E.; Sainz, A.; Dunner, S.; Amusategui, I.; López, L.; Rodríguez-Franco, F.; Luaces, I.; Cortés, O.; Tesouro, M.A. First isolation and molecular characterization of Ehrlichia canis in Spain. Veter- Parasitol. 2004, 125, 365–372. [Google Scholar] [CrossRef]
- Maxwell, I. C. O. C. G. O. "Environmental and multi-host infestation of the brown dog tick, Rhipicephalus sanguineus in Owerri, South-east Nigeria- a case report," Veterinarski arhiv, vol. 76, no. 1, 2006.
- D. E. Sonenshine, Biology of ticks. New York: Oxford University Press, 1991.
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Rhipicephalus sanguineus on dogs: relationships between attachment sites and tick developmental stages. Exp. Appl. Acarol. 2010, 53, 389–397. [Google Scholar] [CrossRef]
- H. G. Koch, "Oviposition of the Brown Dog Tick (Acari, Ixodidae) in the Laboratory," (in English), Annals of the Entomological Society of America, vol. 75, no. 5, pp. 583-586, 1982.
- Regendanz, P.; Muniz, J. O Rhipicephalus sanguineus como transmissor da Piroplasmose canina no Brasil. Mem. Do Inst. Oswaldo Cruz 1936, 31, 81–84. [Google Scholar] [CrossRef]
- S. K. Sen, "The vector of canine piroplasmosis due to Piroplasma gibsoni," Ind. J. Vet. Sci. Anim. Husband, vol. 3, pp. 356-363, 1933.
- Mantovani, A.; Benazzi, P. THE ISOLATION OF COXIELLA BURNETII FROM RHIPICEPHALUS-SANGUINEUS ON NATURALLY INFECTED DOGS. 1953, 122, 117–118.
- Nordgren, R.; Craig, T. Experimental transmission of the texas strain of Hepatozoon canis. Veter- Parasitol. 1984, 16, 207–214. [Google Scholar] [CrossRef]
- E. Brumpt, "Longevite du virus de la fievre boutonneuse chez la tique, Rhipicephalus sanguineus.," Compt Rend Soc Biol, vol. 8, pp. 1199-1202, 1932.
- R. R. Parker, C. B. Philip, and W. L. Jellison, "Rocky Mountain spotted fever: potentialities of tick transmission in relation to geographical occurence in the United States," Am. J. Trop. Med. Hyg., vol. 13, pp. 341-379, 1933.
- Coutinho, M.T.Z.; Bueno, L.L.; Sterzik, A.; Fujiwara, R.T.; Botelho, J.R.; De Maria, M.; Genaro, O.; Linardi, P.M. Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Veter- Parasitol. 2005, 128, 149–155. [Google Scholar] [CrossRef]
- R. D. Smith, D. M. Sells, E. H. Stephenson, M. R. Ristic, and D. L. Huxsoll, "Development of Ehrlichia canis, causative agent of canine ehrlichiosis, in the tick Rhipicephalus sanguineus and its differentiation from a symbiotic Rickettsia," Am J Vet Res, vol. 37, no. 2, pp. 119-26, Feb 1976.
- Huxsoll, D.L.; Hildebrandt, P.K.; Nims, R.M.; Amyx, H.L.; Ferguson, J.A. Epizootiology of Tropical Canine Pancytopenia. J. Wildl. Dis. 1970, 6, 220–225. [Google Scholar] [CrossRef]
- Harrus, S.; Kass, P.H.; Klement, E.; Waner, T. Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the disease. Veter- Rec. 1997, 141, 360–363. [Google Scholar] [CrossRef]
- Harrus, S.; Waner, T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): An overview. Veter- J. 2011, 187, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Leiva, M.; Naranjo, C.; Peña, M.T. Ocular signs of canine monocytic ehrlichiosis: a retrospective study in dogs from Barcelona, Spain. Veter- Ophthalmol. 2005, 8, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Waner, T.; Aizenberg, I.; Foley, J.E.; Poland, A.M.; Bark, H. Amplification of Ehrlichial DNA from Dogs 34 Months after Infection with Ehrlichia canis. J. Clin. Microbiol. 1998, 36, 73–76. [Google Scholar] [CrossRef] [PubMed]
- W. C. Buhles, Jr., D. L. Huxsoll, and M. Ristic, "Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation," J Infect Dis, vol. 130, no. 4, pp. 357-67, Oct 1974.
- C. E. Greene, Clinical microbiology and infectious diseases of the dog and cat. Philadelphia: Saunders, 1984, pp. xviii, 967 p.
- Gal, A.; Harrus, S.; Arcoh, I.; Lavy, E.; Aizenberg, I.; Mekuzas-Yisaschar, Y.; Baneth, G. Coinfection with multiple tick-borne and intestinal parasites in a 6-week-old dog. Can Vet J 2007, 48, 619–22. [Google Scholar]
- Hildebrandt, P.K.; Huxsoll, D.L.; Walker, J.S.; Nims, R.M.; Taylor, R.; Andrews, M. Pathology of canine ehrlichiosis (tropical canine pancytopenia). Am J Vet Res 1973, 34, 1309–20. [Google Scholar]
- Nyindo, M.; Huxsoll, D.; Ristic, M.; Kakoma, I.; Brown, J.; Carson, C.; Stephenson, E. CELL-MEDIATED AND HUMORAL IMMUNE-RESPONSES OF GERMAN SHEPHERD DOGS AND BEAGLES TO EXPERIMENTAL-INFECTION WITH EHRLICHIA-CANIS. 1980, 41, 250–254.
- Harrus, S.; Waner, T.; Bark, H.; Jongejan, F.; Cornelissen, A.W.C.A. Recent Advances in Determining the Pathogenesis of Canine Monocytic Ehrlichiosis. J. Clin. Microbiol. 1999, 37, 2745–2749. [Google Scholar] [CrossRef]
- Waner, T.; Harrus, S.; Bark, H.; Bogin, E.; Avidar, Y.; Keysary, A. Characterization of the subclinical phase of canine ehrlichiosis in experimentally infected beagle dogs. Veter- Parasitol. 1997, 69, 307–317. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.-L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; Aikawa, E.; Mempel, T.R.; Libby, P.; Weissleder, R.; Pittet, M.J. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Villaescusa, A.; Tesouro, M.A.; García-Sancho, M.; Ayllón, T.; Rodríguez-Franco, F.; Sainz, A. Evaluation of lymphocyte populations in dogs naturally infected by Ehrlichia canis with and without clinical signs. Ticks Tick-borne Dis. 2012, 3, 279–282. [Google Scholar] [CrossRef]
- Gianopoulos, A.; Mylonakis, M.E.; Theodorou, K.; Christopher, M.M. Quantitative and qualitative leukocyte abnormalities in dogs with experimental and naturally occurring acute canine monocytic ehrlichiosis. Veter- Clin. Pathol. 2016, 45, 281–290. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. 2019, 40, 565–583. [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.J.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.; Ceron, J.; Leontides, L.; Siarkou, V.; Martinez, S.; Tvarijonaviciute, A.; Koutinas, A.; Harrus, S. Serum Acute Phase Proteins as Clinical Phase Indicators and Outcome Predictors in Naturally Occurring Canine Monocytic Ehrlichiosis. J. Veter- Intern. Med. 2011, 25, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Levenhagen, M.; Rieck, S.; Labruna, M.; Beletti, M. The spreading process of Ehrlichia canis in macrophages is dependent on actin cytoskeleton, calcium and iron influx and lysosomal evasion. Veter- Microbiol. 2013, 168, 442–446. [Google Scholar] [CrossRef]
- Mavromatis, K.; Doyle, C.K.; Lykidis, A.; Ivanova, N.; Francino, M.P.; Chain, P.; Shin, M.; Malfatti, S.; Larimer, F.; Copeland, A.; et al. The Genome of the Obligately Intracellular Bacterium Ehrlichia canis Reveals Themes of Complex Membrane Structure and Immune Evasion Strategies. J. Bacteriol. 2006, 188, 4015–4023. [Google Scholar] [CrossRef]
- Felek, S.; Huang, H.; Rikihisa, Y. Sequence and Expression Analysis of virB9 of the Type IV Secretion System of Ehrlichia canis Strains in Ticks, Dogs, and Cultured Cells. Infect. Immun. 2003, 71, 6063–6067. [Google Scholar] [CrossRef]
- Harrus, S.; Waner, T.; Friedmann-Morvinski, D.; Fishman, Z.; Bark, H.; Harmelin, A. Down-regulation of MHC class II receptors of DH82 cells, following infection with Ehrlichia canis. Veter- Immunol. Immunopathol. 2003, 96, 239–243. [Google Scholar] [CrossRef]
- Boschiroli, M.L.; Ouahrani-Bettache, S.; Foulongne, V.; Michaux-Charachon, S.; Bourg, G.; Allardet-Servent, A.; Cazevieille, C.; Lavigne, J.-P.; Liautard, J.P.; Ramuz, M.; et al. Type IV secretion and Brucella virulence. Veter- Microbiol. 2002, 90, 341–348. [Google Scholar] [CrossRef]
- Ohashi, N.; Zhi, N.; Lin, Q.; Rikihisa, Y. Characterization and Transcriptional Analysis of Gene Clusters for a Type IV Secretion Machinery in Human Granulocytic and Monocytic Ehrlichiosis Agents. Infect. Immun. 2002, 70, 2128–2138. [Google Scholar] [CrossRef]
- Luo, T.; Patel, J.G.; Zhang, X.; Walker, D.H.; McBride, J.W. Immunoreactive Protein Repertoires of Ehrlichia chaffeensis and E. canis Reveal the Dominance of Hypothetical Proteins and Conformation-Dependent Antibody Epitopes. Infect. Immun. 2021, 89, e0022421. [Google Scholar] [CrossRef]
- Luo, T.; Patel, J.G.; Zhang, X.; Walker, D.H.; McBride, J.W. Ehrlichia chaffeensis and E. canis hypothetical protein immunoanalysis reveals small secreted immunodominant proteins and conformation-dependent antibody epitopes. npj Vaccines 2020, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Chaichanasiriwithaya, W.; Rikihisa, Y. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J. Clin. Microbiol. 1994, 32, 1658–62. [Google Scholar] [CrossRef] [PubMed]
- Kakoma, I.; Hansen, R.D.; E Anderson, B.; A Hanley, T.; Sims, K.G.; Liu, L.; Bellamy, C.; Long, M.T.; Baek, B.K. Cultural, molecular, and immunological characterization of the etiologic agent for atypical canine ehrlichiosis. J. Clin. Microbiol. 1994, 32, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Matthewman, L.; Mahan, S.; Semu, S.; Peter, T.; Mason, P.; Brouqui, P.; Raoult, D. Serological evidence for antigenic relationships between Ehrlichia canis and Cowdria ruminantium. Res. Veter- Sci. 1994, 56, 170–174. [Google Scholar] [CrossRef]
- E. B. Breitschwerdt, B. C. Hegarty, and S. I. Hancock, "Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii," J Clin Microbiol, vol. 36, no. 9, pp. 2645-51, Sep 1998.
- B. J. Woody and J. D. Hoskins, "Ehrlichial diseases of dogs," Vet Clin North Am Small Anim Pract, vol. 21, no. 1, pp. 75-98, Jan 1991.
- Cadman, H.; Kelly, P.; Matthewman, L.; Zhou, R.; Mason, P. Comparison of the dot-blot enzyme linked immunoassay with immunofluorescence for detecting antibodies to Ehrlichia canis. Vet Rec 1994, 135, 362–362. [Google Scholar] [CrossRef]
- Waner, T.; Strenger, C.; Keysary, A.; Harrus, S. Kinetics of serologic cross-reactions between Ehrlichia canis and the Ehrlichia phagocytophila genogroups in experimental E. canis infection in dogs. Veter- Immunol. Immunopathol. 1998, 66, 237–243. [Google Scholar] [CrossRef]
- Rikihisa, Y.; A Ewing, S.; Fox, J.C.; Siregar, A.G.; Pasaribu, F.H.; Malole, M.B. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J. Clin. Microbiol. 1992, 30, 143–148. [Google Scholar] [CrossRef]
- Harrus, S.; Alleman, A.; Bark, H.; Mahan, S.M.; Waner, T. Comparison of three enzyme-linked immunosorbant assays with the indirect immunofluorescent antibody test for the diagnosis of canine infection with Ehrlichia canis. Veter- Microbiol. 2002, 86, 361–368. [Google Scholar] [CrossRef]
- G. L. Murphy, S. A. Ewing, L. C. Whitworth, J. C. Fox, and A. A. Kocan, "A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma," Vet Parasitol, vol. 79, no. 4, pp. 325-39, Nov 27 1998.
- Doyle, C.K.; Nethery, K.A.; Popov, V.L.; McBride, J.W. Differentially Expressed and Secreted Major Immunoreactive Protein Orthologs of Ehrlichia canis and E. chaffeensis Elicit Early Antibody Responses to Epitopes on Glycosylated Tandem Repeats. Infect. Immun. 2006, 74, 711–720. [Google Scholar] [CrossRef]
- McBride, J.W.; Doyle, C.K.; Zhang, X.; Cardenas, A.M.; Popov, V.L.; Nethery, K.A.; Woods, M.E. Identification of a Glycosylated Ehrlichia canis 19-Kilodalton Major Immunoreactive Protein with a Species-Specific Serine-Rich Glycopeptide Epitope. Infect. Immun. 2007, 75, 74–82. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Doyle, C.K.; Zhang, X.; Nethery, K.; Corstvet, R.E.; Walker, D.H.; McBride, J.W. Enzyme-Linked Immunosorbent Assay with Conserved Immunoreactive Glycoproteins gp36 and gp19 Has Enhanced Sensitivity and Provides Species-Specific Immunodiagnosis ofEhrlichia canisInfection. Clin. Vaccine Immunol. 2007, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.M.; Zhang, X.; Braga, I.A.; Taques, I.I.G.G.; McBride, J.W. Detection of genotype-specific Ehrlichia canis exposure in Brazilian dogs by TRP36 peptide ELISA. Ticks Tick-borne Dis. 2016, 7, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Lim, S.Y.; Sharma, R.S.K.; Cheng, N.A.B.Y.; Watanabe, M. Molecular Detection of Ehrlichia canis in Dogs in Malaysia. PLOS Neglected Trop. Dis. 2013, 7, e1982. [Google Scholar] [CrossRef] [PubMed]
- Stich, R.W.; Rikihisa, Y.; Ewing, S.A.; Needham, G.R.; Grover, D.L.; Jittapalapong, S. Detection of Ehrlichia canis in Canine Carrier Blood and in Individual Experimentally Infected Ticks with a p30 -Based PCR Assay. J. Clin. Microbiol. 2002, 40, 540–546. [Google Scholar] [CrossRef]
- McClure, J.C.; Crothers, M.L.; Schaefer, J.J.; Stanley, P.D.; Needham, G.R.; Ewing, S.A.; Stich, R.W. Efficacy of a Doxycycline Treatment Regimen Initiated during Three Different Phases of Experimental Ehrlichiosis. Antimicrob. Agents Chemother. 2010, 54, 5012–5020. [Google Scholar] [CrossRef]
- Wen, B.; Rikihisa, Y.; Mott, J.M.; Greene, R.; Kim, H.Y.; Zhi, N.; Couto, G.C.; Unver, A.; Bartsch, R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 1997, 35, 1852–5. [Google Scholar] [CrossRef]
- Alexandre, N.; Santos, A.S.; Núncio, M.S.; de Sousa, R.; Boinas, F.; Bacellar, F. Detection of Ehrlichia canis by polymerase chain reaction in dogs from Portugal. Veter- J. 2009, 181, 343–344. [Google Scholar] [CrossRef]
- McBride, J.W.; Corstvet, R.E.; Gaunt, S.D.; Chinsangaram, J.; Akita, G.Y.; Osburn, B.I. PCR Detection of Acute Ehrlichia Canis Infection in Dogs. J. Veter- Diagn. Investig. 1996, 8, 441–447. [Google Scholar] [CrossRef]
- M. J. Homer, I. Aguilar-Delfin, S. R. Telford, 3rd, P. J. Krause, and D. H. Persing, "Babesiosis," Clin Microbiol Rev, vol. 13, no. 3, pp. 451-69, Jul 2000.
- Rodríguez-Alarcón, C.A.; Beristain-Ruiz, D.M.; Olivares-Muñoz, A.; Quezada-Casasola, A.; Pérez-Casio, F.; Álvarez-Martínez, J.A.; Tapia-Alanís, J.; Lira-Amaya, J.J.; Rivera-Barreno, R.; Cera-Hurtado, O.S.; et al. Demonstrating the presence of Ehrlichia canis DNA from different tissues of dogs with suspected subclinical ehrlichiosis. Parasites Vectors 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Sirigireddy, K.R.; Ganta, R.R. Multiplex Detection of Ehrlichia and Anaplasma Species Pathogens in Peripheral Blood by Real-Time Reverse Transcriptase-Polymerase Chain Reaction. J. Mol. Diagn. 2005, 7, 308–316. [Google Scholar] [CrossRef]
- Sirigireddy, K.R.; Mock, D.C.; Ganta, R.R. Multiplex Detection of Ehrlichia and Anaplasma Pathogens in Vertebrate and Tick Hosts by Real-Time RT-PCR. Ann. New York Acad. Sci. 2006, 1078, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.K.; Labruna, M.B.; Breitschwerdt, E.B.; Tang, Y.-W.; Corstvet, R.E.; Hegarty, B.C.; Bloch, K.C.; Li, P.; Walker, D.H.; McBride, J.W. Detection of Medically Important Ehrlichia by Quantitative Multicolor TaqMan Real-Time Polymerase Chain Reaction of the dsb Gene. J. Mol. Diagn. 2005, 7, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Peleg, O.; Baneth, G.; Eyal, O.; Inbar, J.; Harrus, S. Multiplex real-time qPCR for the detection of Ehrlichia canis and Babesia canis vogeli. Veter- Parasitol. 2010, 173, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, M.; Singh, H.; Panwar, H.; Jyoti, *!!! REPLACE !!!*; Singh, N.K. Development and validation of multiplex SYBR Green real-time PCR assays for detection and molecular surveillance of four tick-borne canine haemoparasites. Ticks Tick-borne Dis. 2022, 13, 101937. [Google Scholar] [CrossRef]
- Iqbal, Z.; Rikihisa, Y. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J. Clin. Microbiol. 1994, 32, 1644–1649. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Hegarty, B.C.; Hancock, S.I. Doxycycline Hyclate Treatment of Experimental Canine Ehrlichiosis Followed by Challenge Inoculation with TwoEhrlichia canisStrains. Antimicrob. Agents Chemother. 1998, 42, 362–368. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Harrus, S.; Breitschwerdt, E.B. An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Veter- J. 2019, 246, 45–53. [Google Scholar] [CrossRef]
- Jongejan, F.; Crafford, D.; Erasmus, H.; Fourie, J.J.; Schunack, B. Comparative efficacy of oral administrated afoxolaner (NexGard™) and fluralaner (Bravecto™) with topically applied permethrin/imidacloprid (Advantix®) against transmission of Ehrlichia canis by infected Rhipicephalus sanguineus ticks to dogs. Parasites Vectors 2016, 9, 348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




