Submitted:
13 November 2024
Posted:
14 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
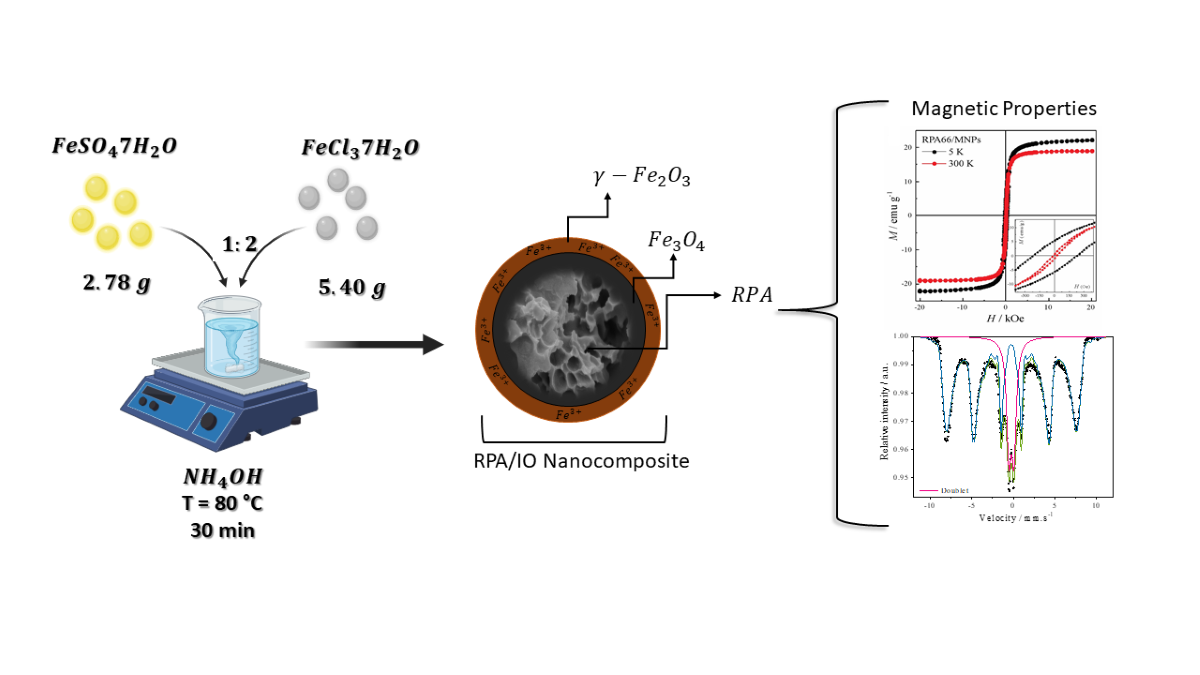
2.2. Synthesis of IO Nanoparticles
2.3. Synthesis of RPA/IO Nanocomposites
3. Materials Characterization
4. Results and Discussion
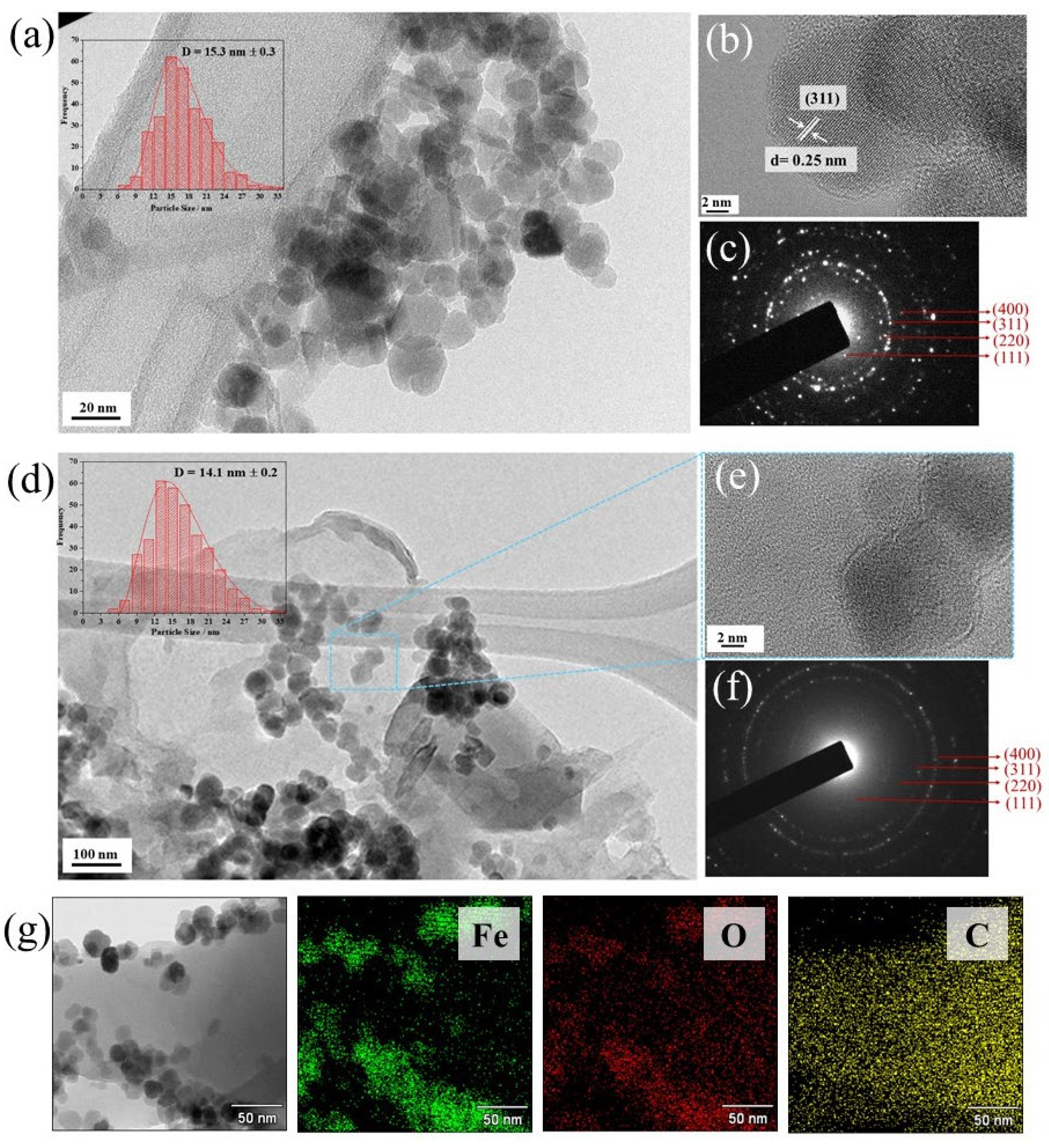
4.1. Microstructural Properties
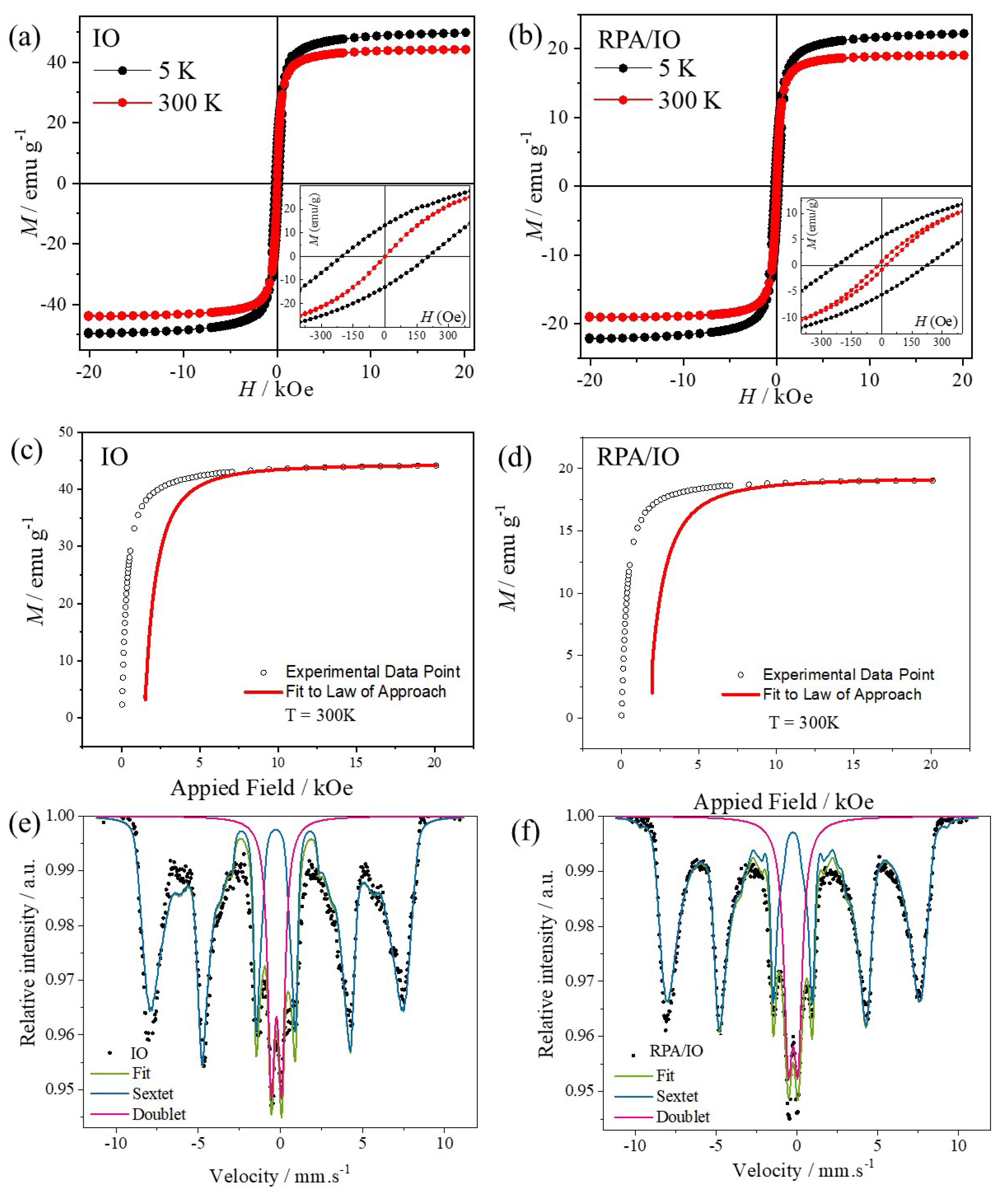
4.2. Magnetic Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koszewska, M. Circular Economy - Challenges for the Textile and Clothing Industry. Autex Res. J. 2018, 18, 337–347. [CrossRef]
- Roy Choudhury, A.K. Environmental Impacts of the Textile Industry and Its Assessment Through Life Cycle Assessment. 2014, 1–39. [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Text. 2022, Vol. 2, Pages 174-188 2022, 2, 174–188. [CrossRef]
- Wang, Y. Fiber and Textile Waste Utilization. Waste and Biomass Valorization 2010, 1, 135–143. [CrossRef]
- Tang, K.H.D. State of the Art in Textile Waste Management: A Review. Text. 2023, Vol. 3, Pages 454-467 2023, 3, 454–467. [CrossRef]
- Patwary, S. Clothing and Textile Sustainability: Current State of Environmental Challenges and the Ways Forward. Text. Leather Rev. 2020, 3, 158–173. [CrossRef]
- Markovičová, L.; Zatkalíková, V.; Kojnoková, T. Environmental Impact on the Life of a Polymeric Composite with Polyamide Matrix and Glass Fibres. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1178, 012043. [CrossRef]
- Hirschberg, V.; Rodrigue, D. Recycling of Polyamides: Processes and Conditions. J. Polym. Sci. 2023, 61, 1937–1958. [CrossRef]
- Al-Shawabkeh, A.F. Optoelectronic Investigation and Spectroscopic Characteristics of Polyamide-66 Polymer. E-Polymers 2022, 22, 858–869. [CrossRef]
- Hashemi Sanatgar, R.; Campagne, C.; Nierstrasz, V. Investigation of the Adhesion Properties of Direct 3D Printing of Polymers and Nanocomposites on Textiles: Effect of FDM Printing Process Parameters. Appl. Surf. Sci. 2017, 403, 551–563. [CrossRef]
- Baeg, K.-J.; Lee, J.; Baeg, -J K.; Lee, J. Flexible Electronic Systems on Plastic Substrates and Textiles for Smart Wearable Technologies. Adv. Mater. Technol. 2020, 5, 2000071. [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green Composites: A Review of Adequate Materials for Automotive Applications. Compos. Part B Eng. 2013, 44, 120–127. [CrossRef]
- Mahalik, N.P.; Nambiar, A.N. Trends in Food Packaging and Manufacturing Systems and Technology. Trends Food Sci. Technol. 2010, 21, 117–128. [CrossRef]
- Singh, R.; Kumar, R.; Ranjan, N.; Penna, R.; Fraternali, F. On the Recyclability of Polyamide for Sustainable Composite Structures in Civil Engineering. Compos. Struct. 2018, 184, 704–713. [CrossRef]
- Yao, W.H. The Preparation of Modified Polyamide Clay Nanocomposite/Recycled Maleic Anhydride Polyamide 6 and Blending with Low Density Polyethylene for Film Blowing Application. Polym. Polym. Compos. 2021, 29 (9_suppl), S631–S643. [CrossRef]
- Medeiros, D.G.; Jardim, P.M.; De Tatagiba, M.K.V.; D’Almeida, J.R.M. Composites of Recycled Nylon 11 and Titanium Based Nanofillers. Polym. Test. 2015, 42, 108–114. [CrossRef]
- Benaducci, D.; de Oliveira, V.; Yin Tze, W.T.; Hafez, I.; Branciforti, M.C. Nanocomposites of Recycled and of Virgin Polyamide 6.6 with Cellulose Nanofibers. Hybrid Adv. 2024, 6, 100261. [CrossRef]
- Korkees, F.; Aldrees, A.; Barsoum, I.; Alshammari, D. Functionalised Graphene Effect on the Mechanical and Thermal Properties of Recycled PA6/PA6,6 Blends. J. Compos. Mater. 2021, 55, 2211–2224. [CrossRef]
- Bassyouni, D.; Mohamed, M.; El-Ashtoukhy, E.S.; El-Latif, M.A.; Zaatout, A.; Hamad, H. Fabrication and Characterization of Electrospun Fe3O4/o-MWCNTs/Polyamide 6 Hybrid Nanofibrous Membrane Composite as an Efficient and Recoverable Adsorbent for Removal of Pb (II). Microchem. J. 2019, 149, 103998. [CrossRef]
- Liang, Y.; Xia, X.; Luo, Y.; Jia, Z. Synthesis and Performances of Fe2O3/PA-6 Nanocomposite Fiber. Mater. Lett. 2007, 61 (14–15), 3269–3272. [CrossRef]
- Cornell, Rochelle M., and U. S. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses.; Weinheim: Wiley-vch, Ed.; De Gruyter, 2003; Vol. 664. [CrossRef]
- Gutierrez, F.V.; De Falco, A.; Yokoyama, E.; Mendoza, L.A.F.; Luz-Lima, C.; Perez, G.; Loreto, R.P.; Pottker, W.E.; La Porta, F.A.; Solorzano, G.; Arsalani, S.; Baffa, O.; Araujo, J.F.D.F. Magnetic Characterization by Scanning Microscopy of Functionalized Iron Oxide Nanoparticles. Nanomater. 2021, Vol. 11, Page 2197 2021, 11, 2197. [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis and Applications of Nano-Structured Iron Oxides/Hydroxides – a Review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [CrossRef]
- Hamed, M.H.; Mueller, D.N.; Müller, M. Thermal Phase Design of Ultrathin Magnetic Iron Oxide Films: From Fe3O4 to γ-Fe2O3 and FeO. J. Mater. Chem. C 2020, 8, 1335–1343. [CrossRef]
- Li, Z.; Chanéac, C.; Berger, G.; Delaunay, S.; Graff, A.; Lefèvre, G. Mechanism and Kinetics of Magnetite Oxidation under Hydrothermal Conditions. RSC Adv. 2019, 9, 33633–33642. [CrossRef]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative Determination of Magnetite and Maghemite in Iron Oxide Nanoparticles Using Mössbauer Spectroscopy. SN Appl. Sci. 2019, 1, 1–8. [CrossRef]
- Buelvas, D.D.A.; Camargo, L.P.; Valezi, D.F.; Tupan, L.F.S.; Dall’Antonia, L.H.; Rocha, C.M.M.; Lopez, D.A.S.; Urbano, A.; Vicentin, B.L.S. Impact of Varying Magnetite Nanoparticle Concentrations on the Structural, Electrical, and Magnetic Properties of Polyaniline-Based Magnetic Nanocomposites. Synth. Met. 2024, 117703. [CrossRef]
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nat. 1915 952386 1915, 95, 561–561. [CrossRef]
- Wareppam, B.; Kuzmann, E.; Garg, V.K.; Singh, L.H. Mössbauer Spectroscopic Investigations on Iron Oxides and Modified Nanostructures: A Review. J. Mater. Res. 2022 384 2022, 38, 937–957. [CrossRef]
- Petrychuk, M.; Kovalenko, V.; Pud, A.; Ogurtsov, N.; Gubin, A. Ternary Magnetic Nanocomposites Based on Core–Shell Fe3O4/Polyaniline Nanoparticles Distributed in PVDF Matrix. Phys. status solidi 2010, 207, 442–447. [CrossRef]
- Buelvas, D.D.A.; Camargo, L.P.; Salgado, I.K.I.; Vicentin, B.L.S.; Valezi, D.F.; Dall’Antonia, L.H.; Tarley, C.R.T.; Mauro, E. Di. Study and Optimization of the Adsorption Process of Methylene Blue Dye in Reusable Polyaniline-Magnetite Composites. Synth. Met. 2023, 292, 117232. [CrossRef]
- Gupta, R.; Pancholi, K.; De Sa, R.; Murray, D.; Huo, D.; Droubi, G.; White, M.; Njuguna, J. Effect of Oleic Acid Coating of Iron Oxide Nanoparticles on Properties of Magnetic Polyamide-6 Nanocomposite. JOM 2019, 71, 3119–3128. [CrossRef]
- Muhazeli, N.S.; Nordin, N.A.; Mazlan, S.A.; Abdul Aziz, S.A.; Ubaidillah; Nazmi, N. Mini Review: An Insight on the Fabrication Methods of Smart Magnetic Polymer Foam. J. Magn. Magn. Mater. 2021, 534, 168038. [CrossRef]
- Buelvas, D.D.A.; Camargo, L.P.; Salgado, I.K.I.; Vicentin, B.L.S.; Valezi, D.F.; Dall’Antonia, L.H.; Tarley, C.R.T.; Mauro, E. Di. Study and Optimization of the Adsorption Process of Methylene Blue Dye in Reusable Polyaniline-Magnetite Composites. Synth. Met. 2023, 292, 117232. [CrossRef]
- Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W.; Norfarhana, A.S.; Azami, M.S.M. The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. Gels 2023, 9, 121. [CrossRef]
- Li, X.; Li, Z.; Shen, J.; Zheng, Z.; Liu, J. Role of a Nanoparticle Network in Polymer Mechanical Reinforcement: Insights from Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2021, 23, 21797–21807. [CrossRef]
- Li, X.; Huang, B.; Liu, J.; Hu, X.; Zheng, Z.J. Revealing the Reinforcing Effect of a Nanorod Network on a Polymer Matrix through Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2023, 25, 18757–18765. [CrossRef]
- Wenlei, X.; Ning, M. Immobilized Lipase on Fe3O4 Nanoparticles as Biocatalyst for Biodiesel Production. Energy and Fuels 2009, 23, 1347–1353. [CrossRef]
- Rietveld, H.M. Line Profiles of Neutron Powder-Diffraction Peaks for Structure Refinement. urn:issn:0365-110X 1967, 22, 151–152. [CrossRef]
- A., G. X-Ray Diffraction in Crystals. Imperfect Crystals, Amorph. Bodies. Dorer 1963.
- Stern, P.; Polymer, E.S.-; 1968, undefined. On the Structure of Polypropylene Fibres. Elsevier.
- Buelvas, D.D.A.; Vicentin, B.L.S.; Urbano, A.; Besegato, J.F.; Hoeppner, M.G.; Galvão, T.D.; Parreira, P.S.; Di Mauro, E. Crystalline Properties and Morphology of Bulk-Fill Dental Resin Composites as Function of Light-Cure Protocol and Composition. Polym. Bull. 2023, 80, 2349–2366. [CrossRef]
- Sasaki, S. Radial Distribution of Electron Density in Magnetite, Fe3O4. urn:issn:0108-7681 1997, 53, 762–766. [CrossRef]
- Jørgensen, J.E.; Mosegaard, L.; Thomsen, L.E.; Jensen, T.R.; Hanson, J.C. Formation of γ-Fe2O3 Nanoparticles and Vacancy Ordering: An in Situ X-Ray Powder Diffraction Study. J. Solid State Chem. 2007, 180, 180–185. [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [CrossRef]
- Xia, H.; Zhao, X.; Yang, G. Double in Situ Synthesis of Fe3O4/Polyamide 6 Magnetic Nanocomposite. Mater. Lett. 2013, 98, 90–93. [CrossRef]
- Zhang, R.; Zhang, B.; Dou, W.; Wu, Y.; Luo, L. Preparation of Nano- Fe3O4/Nylon Composite Fabric with Magnetic Properties by Post Finishing Method. Fibers Polym. 2019, 20, 1396–1403. [CrossRef]
- Ranganathan, P.; Mutharani, B.; Tseng, C.-H.; Tsai, P.-S. The Isothermal and Nonisothermal Crystallization Kinetics and Morphology of Solvent-Precipitated Nylon 66. Polym. 2022, Vol. 14, Page 442 2022, 14, 442. [CrossRef]
- Qianhui, X.; Hongmei, H.; Ruishu, Z.; Lina, S.; Jianyong, Y.; Xueli, W. A Non-Destructive, Environment-Friendly Method for Separating and Recycling Polyamide 6 from Waste and Scrap Polyamide 6 Blended Textiles. Text. Res. J. 2023, 93 (13–14), 3327–3340. [CrossRef]
- Colucci, G.; Ostrovskaya, O.; Frache, A.; Martorana, B.; Badini, C. The Effect of Mechanical Recycling on the Microstructure and Properties of PA66 Composites Reinforced with Carbon Fibers. J. Appl. Polym. Sci. 2015, 132. [CrossRef]
- Morales-Luckie, R.A.; Sánchez-Mendieta, V.; Olea-Mejia, O.; Vilchis-Nestor, A.R.; López-Téllez, G.; Varela-Guerrero, V.; Huerta, L.; Arenas-Alatorre, J. Facile Solventless Synthesis of a Nylon-6,6/Silver Nanoparticles Composite and Its XPS Study. Int. J. Polym. Sci. 2013, 2013, 235850. [CrossRef]
- Sadeghi-Kiakhani, M.; Safapour, S.; Mirnezhad, S. Thermodynamic and Kinetic Studies of the Adsorption Behaviour of the Natural Dye Cochineal on Polyamide 66. Color. Technol. 2018, 134, 308–314. [CrossRef]
- Cheval, N.; Gindy, N.; Flowkes, C.; Fahmi, A. Polyamide 66 Microspheres Metallised with in Situ Synthesised Gold Nanoparticles for a Catalytic Application. Nanoscale Res. Lett. 2012, 7, 1–9. [CrossRef]
- Zakaria, Z.; Izzah, Z.; Tarmizi, A.; Jawaid, M.; Hassan, A. Effect of Degree of Deacetylation of Chitosan on Thermal Stability and Compatibility of Chitosan-Polyamide Blend. [CrossRef]
- Abdel-Rahman, M.A.; El-Said, W.A.; Sayed, E.M.; Abdel-Wahab, A.M.A. Synthesis, Characterization of Some Conductive Aromatic Polyamides/ Fe3O4 NPs/ITO, and Their Utilization for Methotrexate Sensing. Surfaces 2023, 6, 83–96. [CrossRef]
- Yu, D.; Ni, H.; Wang, L.; Wu, M.; Yang, X. Nanoscale-Confined Precursor of CuFe2O4 Mediated by Hyperbranched Polyamide as an Unusual Heterogeneous Fenton Catalyst for Efficient Dye Degradation. J. Clean. Prod. 2018, 186, 146–154. [CrossRef]
- Mohan, A.; Singhal, R.; Ramanan, S.R. A Study on the Effect of the Collector Properties on the Fabrication of Magnetic Polystyrene Nanocomposite Fibers Using the Electrospinning Technique. J. Appl. Polym. Sci. 2023, 140, e53461. [CrossRef]
- , R. Modern Magnetic Materials: Principles and Applications. IEEE Electrical Insulation Magazine. 1999, 13, 768.
- Hu, P.; Zhang, S.; Wang, H.; Pan, D.; Tian, J.; Tang, Z.; Volinsky, A.A. Heat Treatment Effects on Fe3O4 Nanoparticles Structure and Magnetic Properties Prepared by Carbothermal Reduction. J. Alloys Compd. 2011, 509, 2316–2319. [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of Magnetite Nanoparticles: Impact on Surface and Crystal Properties. CrystEngComm 2017, 19, 246–255. [CrossRef]
- Faaliyan, K.; Abdoos, H.; Borhani, E.; Afghahi, S.S.S. Magnetite-Silica Nanoparticles with Core-Shell Structure: Single-Step Synthesis, Characterization and Magnetic Behavior. J. Sol-Gel Sci. Technol. 2018, 88, 609–617. [CrossRef]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and Dynamic Magnetic Properties of Spherical Magnetite Nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [CrossRef]
- Chirita, M.; Bezergheanu, A.; Bazil Cizmas, C.; Ercuta, A. Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization. Magnetochemistry 2023, Vol. 9, Page 5 2022, 9, 5. [CrossRef]
- Zhang, H.; Zhu, G. One-Step Hydrothermal Synthesis of Magnetic Fe3O4 Nanoparticles Immobilized on Polyamide Fabric. Appl. Surf. Sci. 2012, 258, 4952–4959. [CrossRef]
- Gupta, R.; Pancholi, P.V.; Yu, X.; Gupta, L.; Stenning, G.B.G.; Bucknall, D.; Flynn, D.; Pancholi, K. Role of Interface in Optimisation of Polyamide-6/ Fe3O4 Nanocomposite Properties Suitable for Induction Heating. Nano-Structures & Nano-Objects 2023, 34, 100973. [CrossRef]
- Yoon, S. Determination of the Temperature Dependence of the Magnetic Anisotropy Constant in Magnetite Nanoparticles. J. Korean Phys. Soc. 2011, 59, 3069–3073. [CrossRef]
- Lee, J.S.; Cha, J.M.; Yoon, H.Y.; Lee, J.K.; Kim, Y.K. Magnetic Multi-Granule Nanoclusters: A Model System That Exhibits Universal Size Effect of Magnetic Coercivity. Sci. Reports 2015 51 2015, 5, 1–7. [CrossRef]
- Lima, E.; Brandl, A.L.; Arelaro, A.D.; Goya, G.F. Spin Disorder and Magnetic Anisotropy in Fe3 O4 Nanoparticles. J. Appl. Phys. 2006, 99. [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of Structure-Property Relationships in Nickel Ferrite Nanoparticles Annealed at Different Temperature. J. Phys. Chem. Solids 2021, 151, 109928. [CrossRef]
- Rondinone, A.J.; Samia, A.C.S.; Zhang, Z.J. Characterizing the Magnetic Anisotropy Constant of Spinel Cobalt Ferrite Nanoparticles. Appl. Phys. Lett. 2000, 76, 3624–3626. [CrossRef]
- Chikazumi, S. Physics of Ferromagnetism; Oxford University Press, Ed.; 1997.
- Muscas, G.; Peddis, D.; Cobianchi, M.; Lascialfari, A.; Cannas, C.; Musinu, A.; Omelyanchik, A.; Rodionova, V.; Fiorani, D.; Mameli, V. Magnetic Interactions Versus Magnetic Anisotropy in Spinel Ferrite Nanoparticles. IEEE Magn. Lett. 2019, 10. [CrossRef]
- Knobel, M.; Nunes, W.C.; Socolovsky, L.M.; De Biasi, E.; Vargas, J.M.; Denardin, J.C. Superparamagnetism and Other Magnetic Features in Granular Materials: A Review on Ideal and Real Systems. J. Nanosci. Nanotechnol. 2008, 8, 2836–2857. [CrossRef]
- Rumpf, K.; Granitzer, P.; Morales, P.M.; Poelt, P.; Reissner, M. Variable Blocking Temperature of a Porous Silicon/Fe3O4 Composite Due to Different Interactions of the Magnetic Nanoparticles. Nanoscale Res. Lett. 2012, 7, 1–4. [CrossRef]
- Mercante, L.A.; Melo, W.W.M.; Granada, M.; Troiani, H.E.; MacEdo, W.A.A.; Ardison, J.D.; Vaz, M.G.F.; Novak, M.A. Magnetic Properties of Nanoscale Crystalline Maghemite Obtained by a New Synthetic Route. J. Magn. Magn. Mater. 2012, 324, 3029–3033. [CrossRef]
- Gogoi, B.; Das, U. Enhanced Study of Magnetic Properties of Polyvinyl Alcohol-Coated Superparamagnetic Iron Oxide Nanoparticles Below Blocking Temperatures. Powder Metall. Met. Ceram. 2023, 62 (1–2), 41–57. [CrossRef]
- Correa, J.R.; Bordallo, E.; Canetti, D.; León, V.; Otero-Díaz, L.C.; Negro, C.; Gómez, A.; Sáez-Puche, R. Structure and Superparamagnetic Behaviour of Magnetite Nanoparticles in Cellulose Beads. Mater. Res. Bull. 2010, 45, 946–953. [CrossRef]
- Zheng, R.K.; Gu, H.; Xu, B.; Zhang, X.X. The Origin of the Non-Monotonic Field Dependence of the Blocking Temperature in Magneticnanoparticles. J. Phys. Condens. Matter 2006, 18, 5905. [CrossRef]
- Vértes, A.; Korecz, L. (László); Burger, K. (Kálmán). Moessbauer Spectroscopy. 1979, 432.
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative Determination of Magnetite and Maghemite in Iron Oxide Nanoparticles Using Mössbauer Spectroscopy. SN Appl. Sci. 2019, 1, 1–8. [CrossRef]
- Lavorato, G.C.; de Almeida, A.A.; Vericat, C.; Fonticelli, M.H. Redox Phase Transformations in Magnetite Nanoparticles: Impact on Their Composition, Structure and Biomedical Applications. Nanotechnology 2023, 34, 192001. [CrossRef]
- Phan, M.H.; Alonso, J.; Khurshid, H.; Lampen-Kelley, P.; Chandra, S.; Repa, K.S.; Nemati, Z.; Das, R.; Iglesias, Ó.; Srikanth, H. Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems. Nanomater. 2016, Vol. 6, Page 221 2016, 6, 221. [CrossRef]
- Moacă, E.A.; Watz, C.G.; Socoliuc, V.; Racoviceanu, R.; Păcurariu, C.; Ianoş, R.; Cîntă-Pînzaru, S.; Tudoran, L.B.; Nekvapil, F.; Iurciuc, S.; Șoica, C.; Dehelean, C.A. Biocompatible Magnetic Colloidal Suspension Used as a Tool for Localized Hyperthermia in Human Breast Adenocarcinoma Cells: Physicochemical Analysis and Complex In Vitro Biological Profile. Nanomater. 2021, Vol. 11, Page 1189 2021, 11, 1189. [CrossRef]
- Kumar, P.; Kumar, P.; Khanduri, H.; Pathak, S.; Pathak, S.; Singh, A.; Singh, A.; Singh, A.; Basheed, G.A.; Basheed, G.A.; Pant, R.P. Temperature Selectivity for Single Phase Hydrothermal Synthesis of PEG-400 Coated Magnetite Nanoparticles. Dalt. Trans. 2020, 49, 8672–8683. [CrossRef]
- Singh, S.; Goswami, N. Structural, Optical, Magnetic and Dielectric Properties of Magnetite (Fe3O4) Nanoparticles Prepared by Exploding Wire Technique. J. Mater. Sci. Mater. Electron. 2021, 32, 26857–26870. [CrossRef]
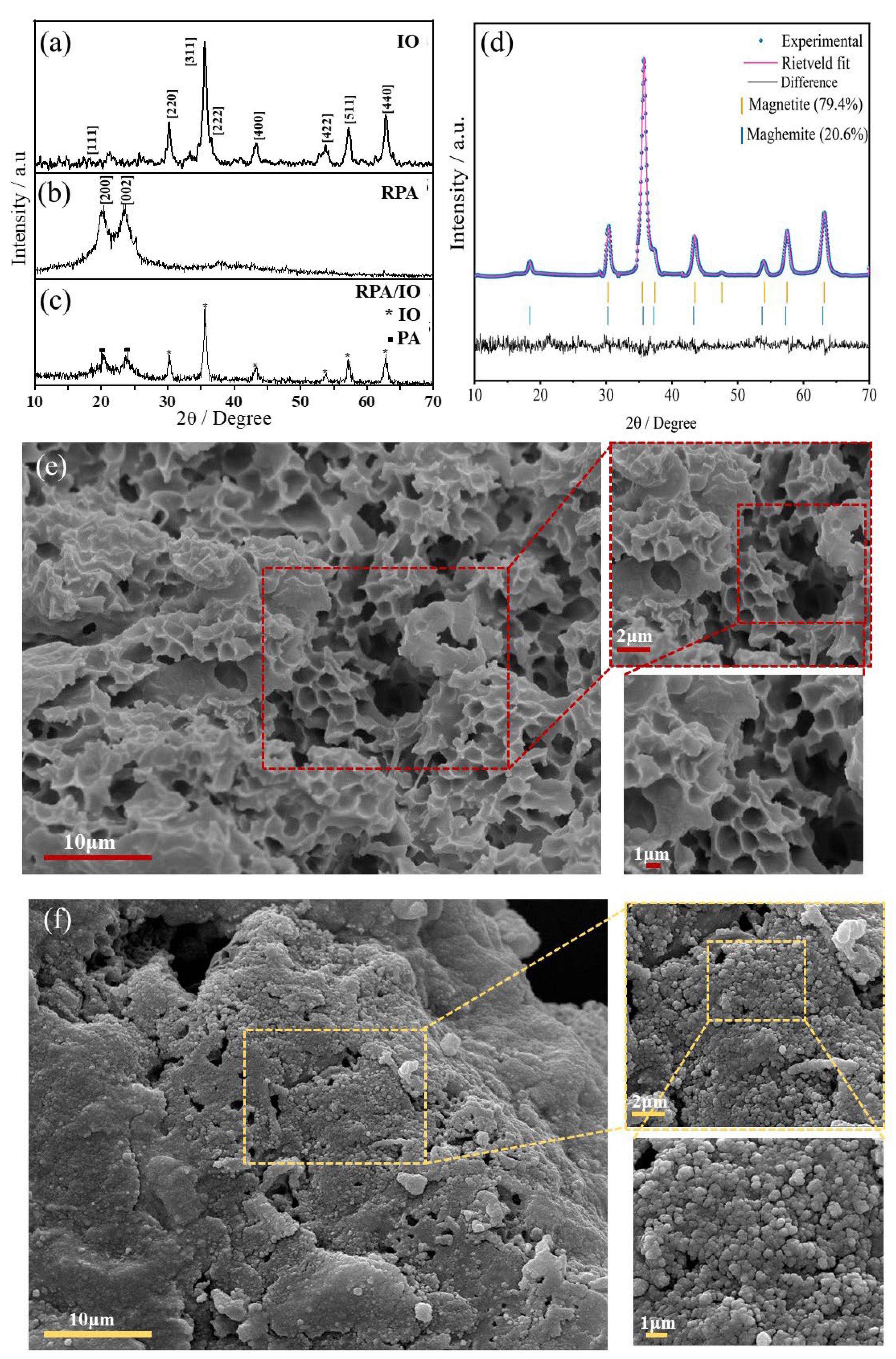
| Sample | Planes/ h k l | 2θ° | *β | **S / nm | ***%DOC |
| IO | 311 | 35.6 | 0.8341 | 10.0 | 69.2 |
| RPA | 200 | 20.1 | 2.6408 | 3.2 | 35.8 |
| 002 | 23.6 | 3.0403 | 2.9 | ||
| RPA / IO |
200 | 20.3 | 1.9383 | 4.4 | 11.5 |
| 002 | 23.9 | 1.8330 | 4.6 |
| Sample | Temperature / K | Ms/emu g–1 | Mr/emu g–1 | Mr/Ms | Hc / KOe | K1/erg cm–3 |
| IO | 5 | 50.0 | 13.2 | 0.26 | 0.2 | 4.77 ×105 |
| 300 | 44.1 | 0.7 | 0.02 | 2.5×10–4 | 2.90×105 | |
| RPA/IO | 5 | 22.2 | 5.5 | 0.25 | 0.2 | 3.53×105 |
| 300 | 19.1 | 0.8 | 0.04 | 0.02 | 1.26×105 |
| Sample | 57Fe Site | δ / mm s-1 | ΔEQ / mm s-1 | Bhf /T | Γ / mm s-1 | Area /% |
| IO | Distribution | 0.36 | 0.05 | 44.7 | - | 81.3 |
| Doublet | 0.35 | 0.62 | - | 0.52 | 18.7 | |
| RPA | - | - | - | - | - | - |
| RPA/IO | Distribution | 0.34 | 0.04 | 45.8 | - | 78.8 |
| Doublet | 0.35 | 0.60 | - | 0.66 | 21.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





