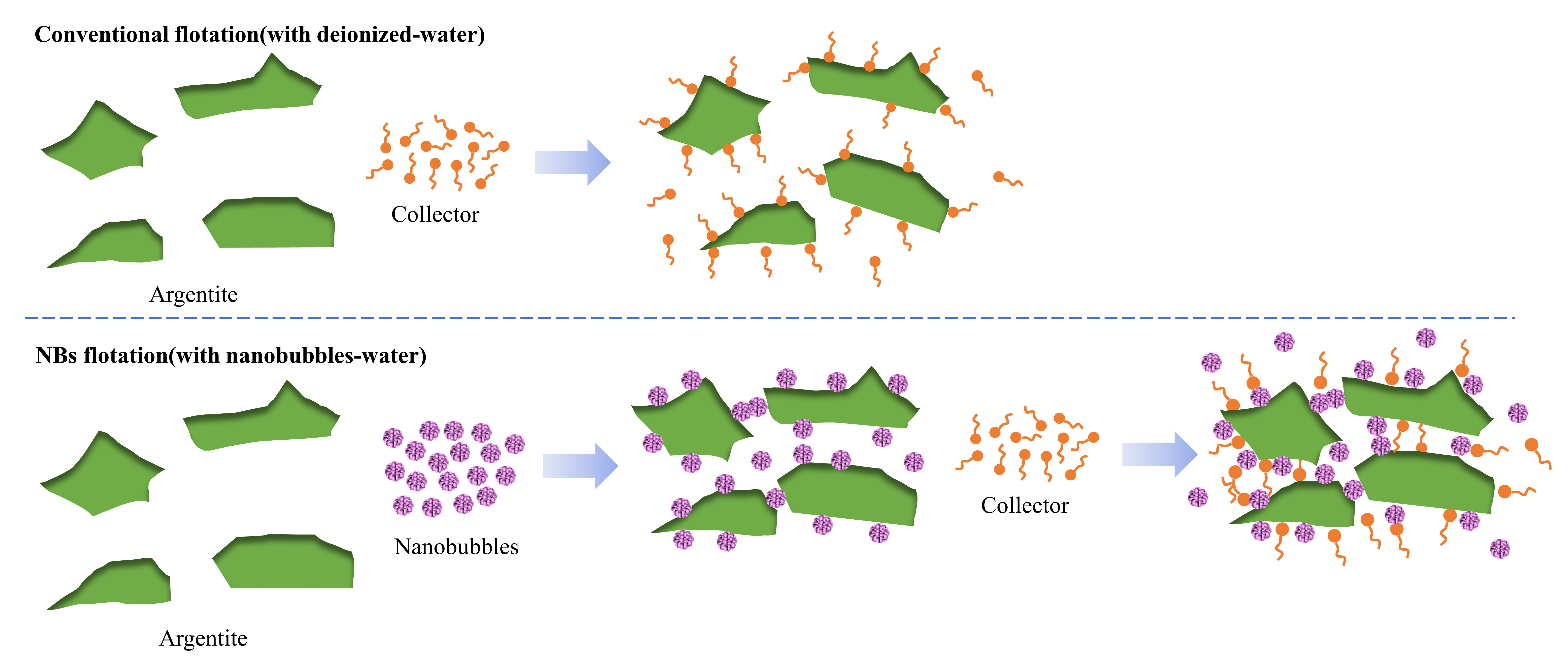
1. Introduction
Silver (Ag) is an important precious metal, approximately one-third of global resources occur as independent deposits, while the remaining two-thirds are associated with other metal deposits. Silver exhibits strong affinities for sulfur (S), iron (Fe), and copper (Cu), commonly found in copper-lead-zinc ores both domestically and internationally [
1,
2]. Despite over 200 identified Ag-containing minerals in nature, only about ten are industrially viable [
3]. In flotation processes, priority is typically given to the recovery of main metals such as Cu, lead (Pb), and zinc (Zn) [
4], often resulting in adequate enrichment of Ag-bearing resources and their subsequent loss in tailings. Therefore, enhancing the efficient recovery of Ag-bearing minerals remains a critical research focus.
Furthermore, Ag-bearing minerals generally exhibit the characteristics of fine dissemination size and low grade, ensuring recovery of these minerals is highly significant. Several studies have employed grinding techniques, reagent optimization, and other methodologies. For instance, Jiang et al. [
5] addressed low recovery of associated Ag in Pb concentrates by regrinding Pb middling, achieving a 5.59% increase in Ag recovery. Zhang et al. [
6] substituted a ceramic-medium stirred mill (SCSM) instead of a ball mill (BM) to enhance Ag flotation recovery by ~ 2%. Zhang et al. [
7] developed a highly efficient collector A11 to enhance the recovery of associated gold (Au) and Ag, thereby increasing Au recovery in Pb concentrate by 5.78% and Ag recovery by 8.61% in industrial production. The small particle size, small mass, and large specific surface area of fine Ag mineral particles contributed to low flotation efficiency and insufficient recovery [
8,
9].
Furthermore, NBs are ultrafine bubbles with a diameter of < 1 micron ( 1,000 nanometers) [
10], which possess special properties such as small size [
11,
12], high stability [
13,
14,
15], and large contact angles [
16,
17]. Recently, researchers have increasingly investigated NB applications in mineral flotation. Notably, NBs increase the probability of collision between bubbles and mineral particles, facilitating agglomeration of microfine-grained minerals, and significantly enhancing recovery rates. Wang et al. [
18] utilized NBs for flotation of ultrafine molybdenite, demonstrating reduced particle repulsion, facilitating particle aggregation, and enhancing mineral surface hydrophobicity. Zhang et al. [
19] found that NBs competed with benzohydroxamic acid (BHA) collector for adsorption on the rutile surface, indicating that NBs adhered to the surface of the mineral particles and reduced the BHA adsorption on the rutile. The flotation of minerals was enhanced by the corroboration of NBs and BHA, with NBs serving an auxiliary role during collection. Despite some studies on NB mechanism in enhancing the coal flotation [
20], metal ores [
21], oxidized ores [
22], and other minerals, there are very few studies on the characteristics of NBs themselves and their combined effects on associated Ag minerals and reagents, with underlying mechanisms remaining unclear.
Argentite, second only to natural silver, is the most common silver mineral in sulfide ores. Thus, this study focused on a representative Ag-bearing mineral. It investigated the introduction of NBs into fine argentite flotation systems in the presence of DDTC, aiming to elucidate NB characteristics, their adsorption mechanisms on fine argentite particles, and their impact on argentite recovery. The findings offered critical insights for the enrichment of fine Ag-bearing minerals.
2. Experimental
2.1. Materials and Reagents
Pure argentite mineral samples utilized in the tests were sourced from Henan Province, China. The samples were initially crushed and then ground in an agate jar until achieving a particle size of less than 38 μm. In addition, some samples ranging from 38μm to 74μm were prepared. The final samples were stored at low temperatures.
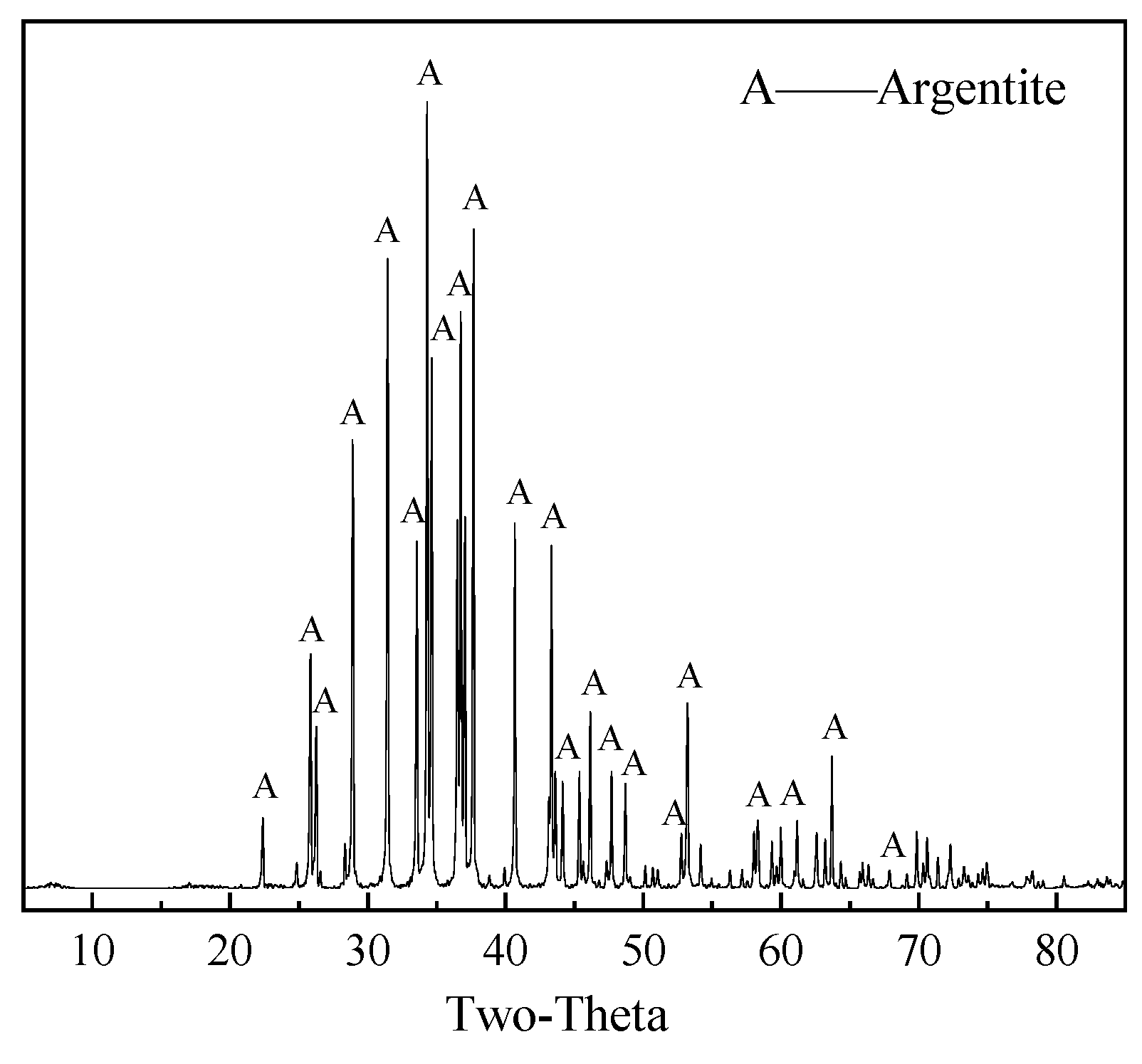
Figure 1 shows the results of the XRD analysis, indicating that argentite was of high purity. Chemical analysis results (
Table 1) indicated a silver content of 78.88% and argentite purity of 90.56%, meeting the requirements for pure mineral flotation.
Actual ore samples were obtained from Ag-bearing Pb–Zn ores in Guangdong, China. Furthermore, it was crushed, thoroughly mixed, and stored in sealed bags for later use. The results of multielement analysis results are presented in
Table 2.
All chemical reagents used were of analytically purity. Subsequently, DDTC served as the collector, Zn sulfate as a depressant in actual ore flotation, methylisobutyl carbinol (MIBC) as the frother, and NaOH as well as H2SO4 were used as pH modifiers. The test water used was deionized (DI) water with a resistivity of 18.25 MΩ⋅cm.
2.2. Preparation of NBs
Air Nanobubbles were generated using the ZJC-NM-200L micro-nano bubbles generator produced by Shanghai Zongjie Environmental Protection Technology Co. Ltd., China (Fig. 2). The working principle of this equipment functioned by mixing water and air intently, creating NBs through cavitation. Before the operation, the inlet tube was immersed in a beaker of DI water. Upon activation, the equipment was filled with DI water and air through inlet holes, achieving a high-speed gas–liquid mixture. Under appropriate pressure, this mixture was ejected through a specially designed nozzle, undergoing hydrodynamic cavitation to form numerous NBs. The resultant solution appeared milky white. The equipment allowed for self-adjustment of DI water circulation time and inlet flow rate. After incubating for 2 min, the milky white appearance disappeared as larger bubbles ruptured, leaving a clear solution suitable for testing.
Figure 2.
ZJC-NM-200L Micro-Nano Bubble Generator.
Figure 2.
ZJC-NM-200L Micro-Nano Bubble Generator.
2.3. Effect of Cavitation Time on NBs Size
To ensure stable NBs and investigate the effect of different cavitation times on NB size, equipment pressure was set to 0.35 Mpa with an air inlet volume of 150 mL/min. The experiments were conducted using a nanoparticle tracking analyzer (Malvern Nanosight NS300) to assess the size, concentration, and stability of NBs across different cavitation times, thereby determining optimal conditions. Nanoparticle tracking analysis (NTA) employs light scattering and measures Brownian motion to determine particle sizes ranging from 30 nm to 1000 nm in solution [
23]. Thus, NBs were measured through NTA to determine the size distribution of the bubbles, the average diameter of the bubbles, and the density of the bubbles. Each test set was conducted in triplicate to obtain averaged values.
2.4. Zeta Potential Measurements
Ions adsorbed on the surface of mineral particles will change the potential on the surface of mineral particles. Zeta potential is used to detect the potential change on the surface of mineral particles, and then the influence on flotation is judged. The zeta potential of argentite particles under different pH conditions was measured using a NanoBrook 90Plus PALS potential analyzer. Each group weighed 50 mg of samples with particle sizes less than 5 μm, and added 40 mL of deionized water or NBs water with KCl concentration of 1mM. The slurry was stirred on a magnetic stirrer while adjusting the pH. After 10 min of stirring, about 10 mL of supernatant was collected for zeta potential testing. Additionally, to study the effect of DDTC on the zeta potential of argentite, the pH of the slurry was adjusted according to the previous procedure, and followed by 5 mg/L of DDTC addition. The slurry was magnetically stirred for 10 min and then left to settle before collecting the supernatant for zeta potential measurement. Each test set was repeated thrice, and the average value was taken as the final result.
2.5. Turbidity Tests and Microscope Tests
Turbidity was used to characterize the dispersion and aggregation of mineral particles. When turbidity is large, the amount of mineral particles in the suspension is large, and the pulp is in a dispersed state. When the turbidity is small, the amount of mineral particles in the suspension is small, and the pulp is in the aggregation state. The effect of NBs on the aggregation and dispersion of argentite particles was investigated through turbidity and microscope tests. Subsequently, 1g of argentite sample was weighed into a beaker and mixed with 40 ml of DI water and NBs water. The pH of the slurry was adjusted to the desired value, and the slurry was transferred into a 100 ml settling cylinder for 3 min. Furthermore, 20 ml of the upper suspension at a fixed position was collected and analyzed using a WZS-188 type turbidimeter for three measurements to obtain an average value.
Additionally, for the microscopy test, 0.5 g of argentite was weighed and added to 100 mL of DI water and NB water. No collector was added to the first two groups, and 5 mg/L DDTC was added to the last two groups. The slurry was stirred for 5 min and the suspension was taken at a fixed position on a slide. The aggregation and dispersion of the argentite under different conditions was observed using a polarized light microscope (Leica M165C).
2.6. Contact Angle Tests
The shaped regular argentite samples were cut out of the plane using a Unitom-type cutter, placed in the abrasive tool, infused with the appropriate amount of epoxy resin mixed with curing agent, and the samples were fixed and molded after 24 h of resting. The samples were polished to create fresh contact surfaces for testing.
The contact angle of argentite samples was measured using a DSA30 contact angle meter produced by KRUSS company in German, employing the sessile drop method. The natural contact angle of the mineral surface was measured by injecting DI water (2 μL) and NBs water (2 μL) into the mineral surface through a micro-syringe. The argentite samples were additionally immersed in 5 mg/L DDTC solution for 30 min. Subsequently, the surfaces were rinsed with DI water to clean the residual DDTC on the surfaces, and the samples were left to dry. Additionally, DI water and NBs water were injected on the DDTC-modified argentite surface to measure the contact angle.
2.7. Adsorption Capacity Measurements
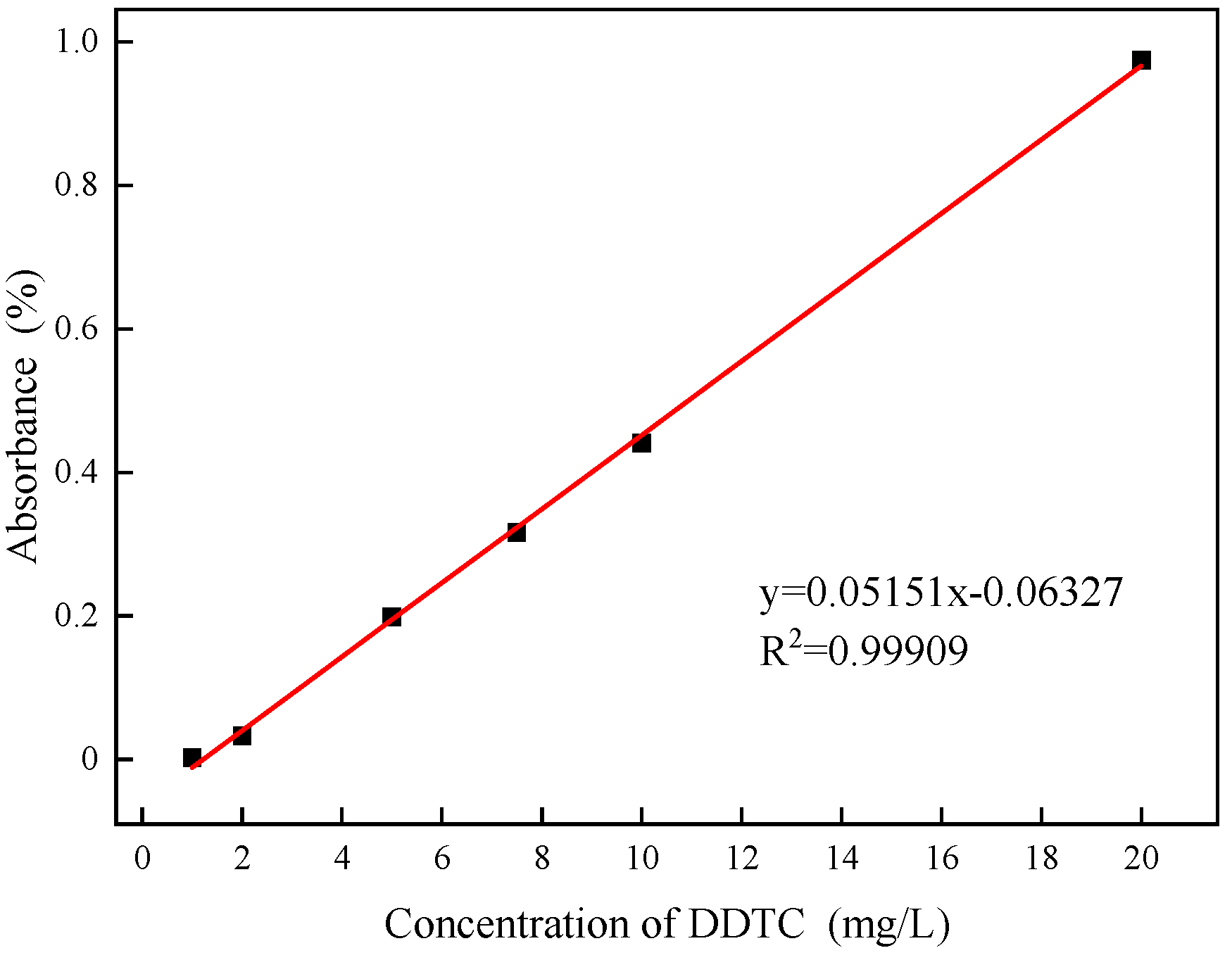
The adsorption of DDTC on the surface of argentite was measured using a T6 UV-visible spectrophotometer manufactured by Beijing PuXi General Instrument Co., China. Standard DDTC solutions of 5 mg/L, 10 mg/L, 20 mg/L, and 30 mg/L were configured, and the absorbance of DDTC at different concentrations was measured to establish a standard curve (Fig. 3). A linear formula y = 0.05151 x -0.06327 was derived from this curve.
Each group weighed 2 g of argentite sample in a 100 ml beaker and added 40 ml of DI water and NBs water. The pH was adjusted to 8, and different concentrations of DDTC were added to each group. The slurry was stirred for 20 min to ensure sufficient reaction between the agent and minerals and then followed by centrifugation. The supernatant was taken into the sample pool and the absorbance was measured. All tests were repeated thrice and the final results were averaged. Absorbance values of the supernatant at different DDTC concentrations were calculated. The concentration of DDTC in the supernatant was determined using the linear formula. The adsorption capacity of the reagent on the mineral surface was calculated by subtracting the concentration of DDTC in the supernatant from the initial DDTC concentration in the solution before the reaction.
Figure 3.
Adsorption standard curve of DDTC.
Figure 3.
Adsorption standard curve of DDTC.
2.8. Micro Flotation Tests
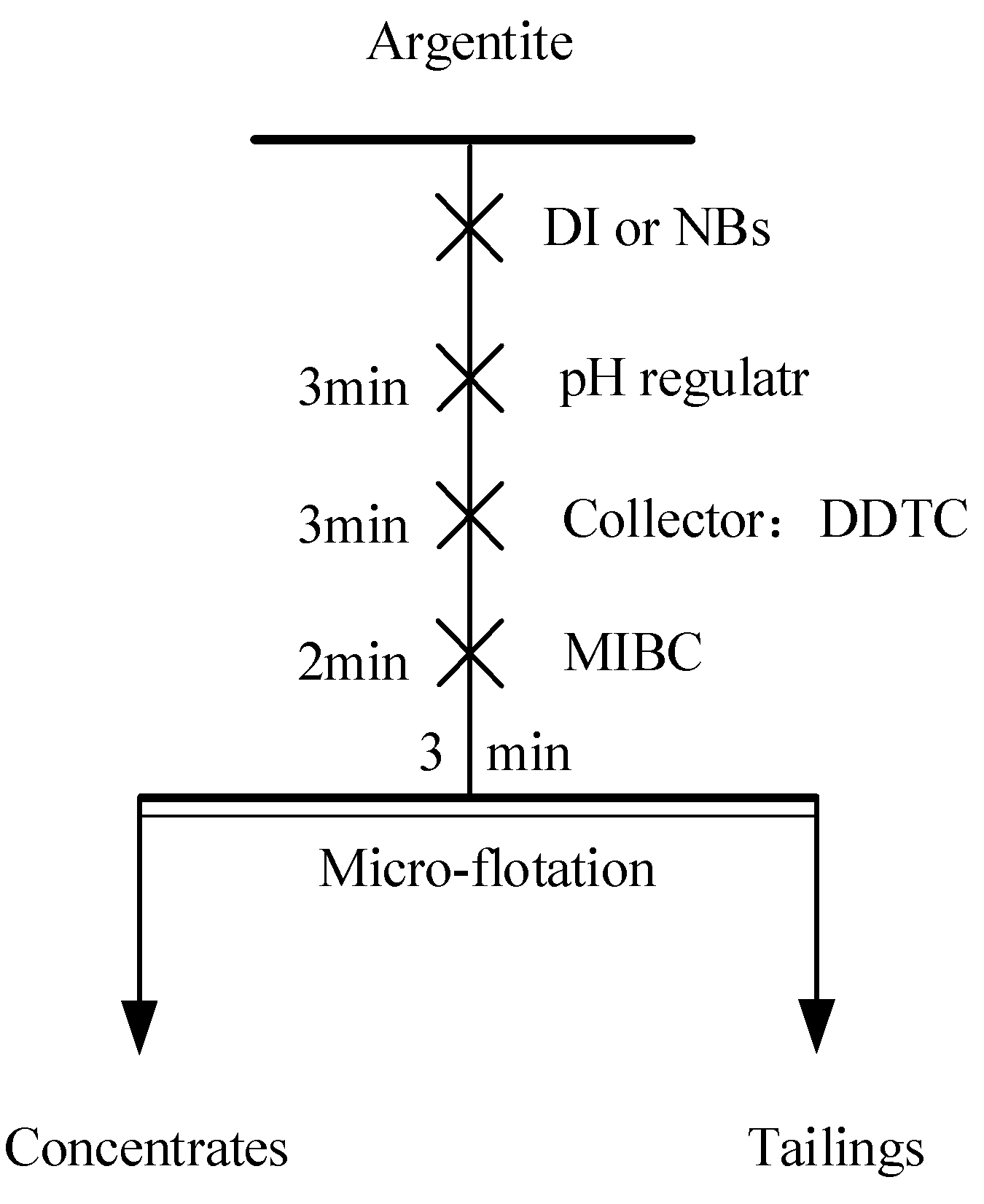
Micro-flotation tests were conducted using an XFGCII type flotation machine (Jilin Prospecting Machinery Factory, China) with a stirring speed set at 1500 r/min and a flotation tank volume of 40 mL (Fig. 4). Before each test, 2 g of argentite was weighed and placed into a clean flotation tank, and DI water and NBs water was added to adjust the liquid level. After stirring for 3min to ensure thorough mineral dispersion, pH regulators, collectors, and frothers were added according to the test protocol. This mixture was stirred for a specified duration before commencing flotation. The froth product was manually obtained through scraping. Upon completion of flotation, the froth product and residual material in the flotation tank are filtered, dried, weighed, and used to calculate the recovery rate.
Figure 4.
The flowsheet of micro flotation test.
Figure 4.
The flowsheet of micro flotation test.
2.9. Actual Ore Flotation Tests
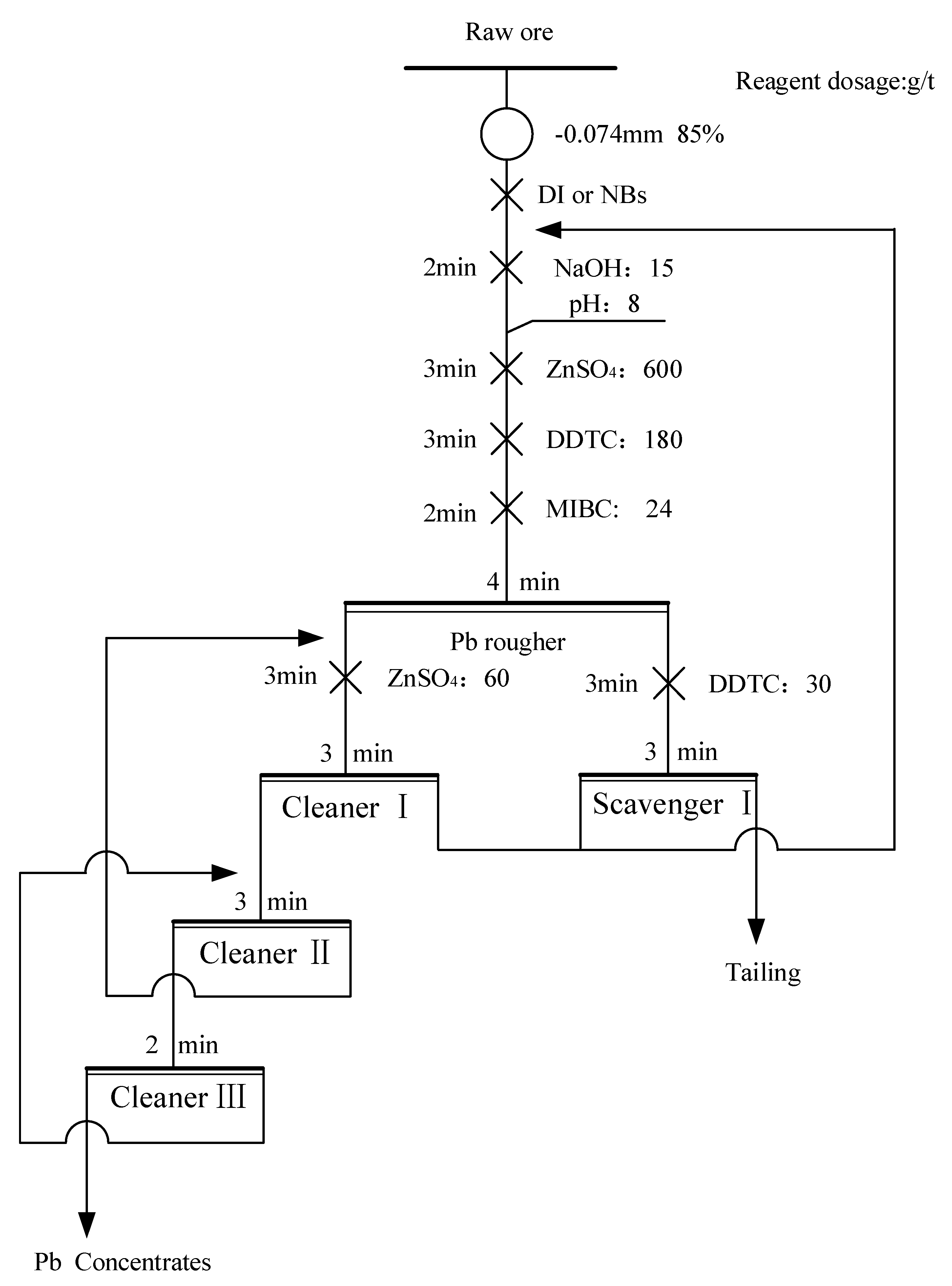
A total of 500 g of raw ore was ground to achieve 85% passing of 74 μm using a wet BM. Conventional flotation and NBs flotation were conducted using an XFD series flotation machine (Jilin Prospecting Machinery Factory, Jilin, China). The ground sample was placed in a 1.5 L flotation cell, and the pulp was conditioned with DI water and NBs water. Subsequently, pH modifiers, depressants, collectors, and frothers were added to the flotation cell (Fig. 5). Closed-circuit tests were conducted using 1.5 L flotation cells for rougher and scavenger flotation, and 0.3 L flotation cells for cleaner flotation. In NBs flotation, NBs water served as the supplementary water. Concentrates and tailings were subjected to filtration drying, weighing, and chemical analysis, followed by recovery calculation. The effect of NBs on associated Ag in Pb concentrate was specifically studied during actual ore tests.
Figure 5.
Flowsheet of closed-circuit flotation tests of lead separation.
Figure 5.
Flowsheet of closed-circuit flotation tests of lead separation.
3. Results and Discussion
3.1. Characteristics of NBs
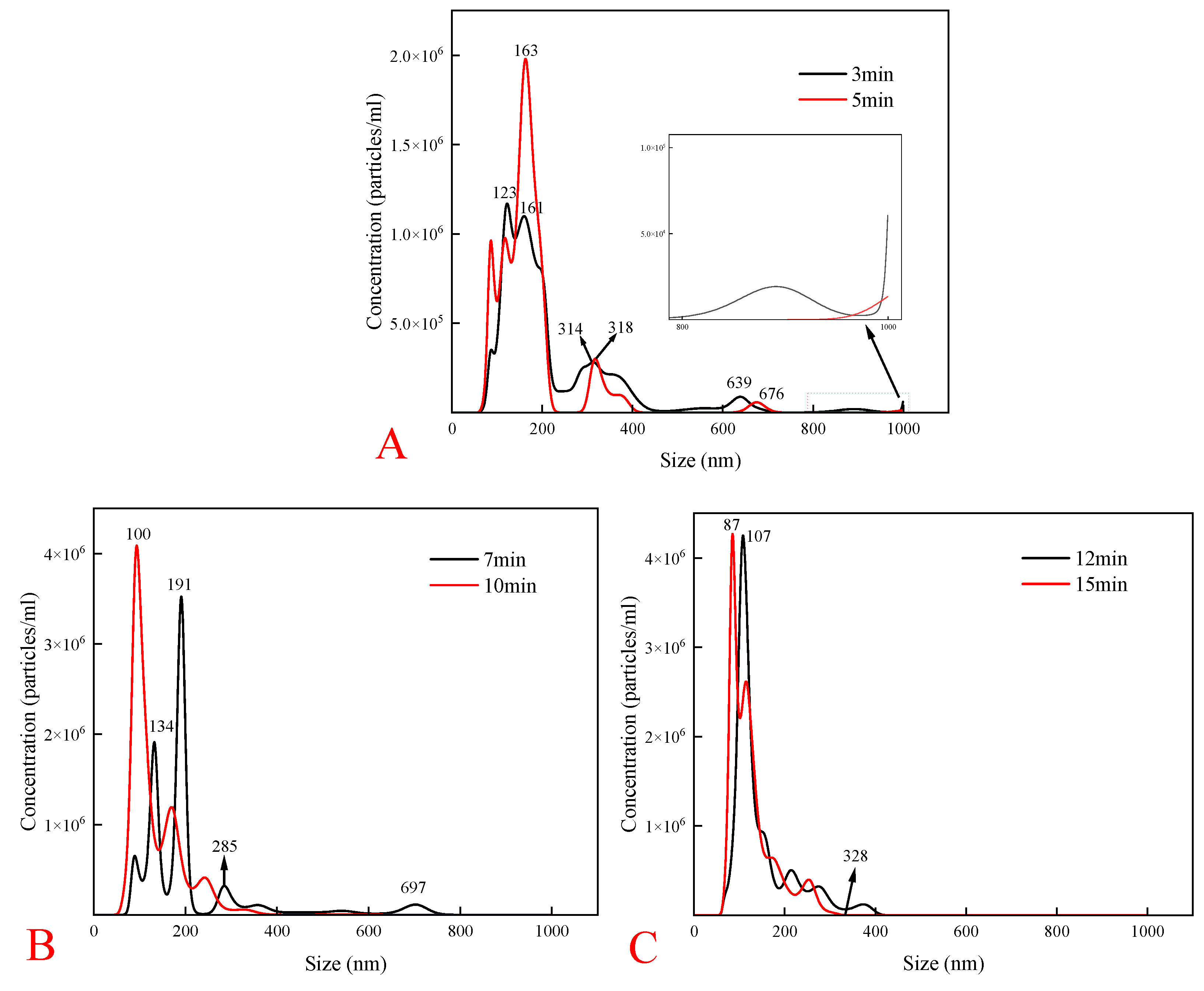
To validate the presence of NBs and investigate the effect of cavitation time on NB characteristics, an investigation using a nanoparticle tracking analyzer was conducted. The study varied cavitation operation times to observe changes in NB size distribution and concentration at intervals of 3 min, 5 min, 7 min, 10 min, 12 min, and 15 min.
As shown in
Figure 6-A, the overall concentration of NBs at the beginning of cavitation was low, and the NBs were ~ 1000 nm in size during the early cavitation stages. It indicated initial instability and turbulence in the solution, correlating with findings by Feng et al. [
24]. As the cavitation time was extended from 3 min to 10 min (Fig. 6-B), the NB concentration was gradually increased, and the unstable bubbles broke up and disappeared. The bubbles around the particle size of 1000 nm disappeared, forming more stable NBs capable of surviving in highly turbulent environments [
25]. Statistical analysis indicated average NB sizes of 211 nm at 7 min of cavitation and 135 nm at 10 min of cavitation, demonstrating a reduction in NB sizes with increased cavitation time.
Figure 6-C illustrates further size reduction with average sizes of 144 nm at 12 min and 127 nm at 15 min of cavitation, indicating enhanced bubble distribution and stability with longer cavitation time. Zhang et al. [
26] similarly measured the average size of the NBs after 10 min of standing was 192 nm, using a similar bubble generator using the DLS technique. It was found that the cavitation time was determined to be 10 min.
3.2. Effect of Nanobubbles on the Zeta Potential of Argentite
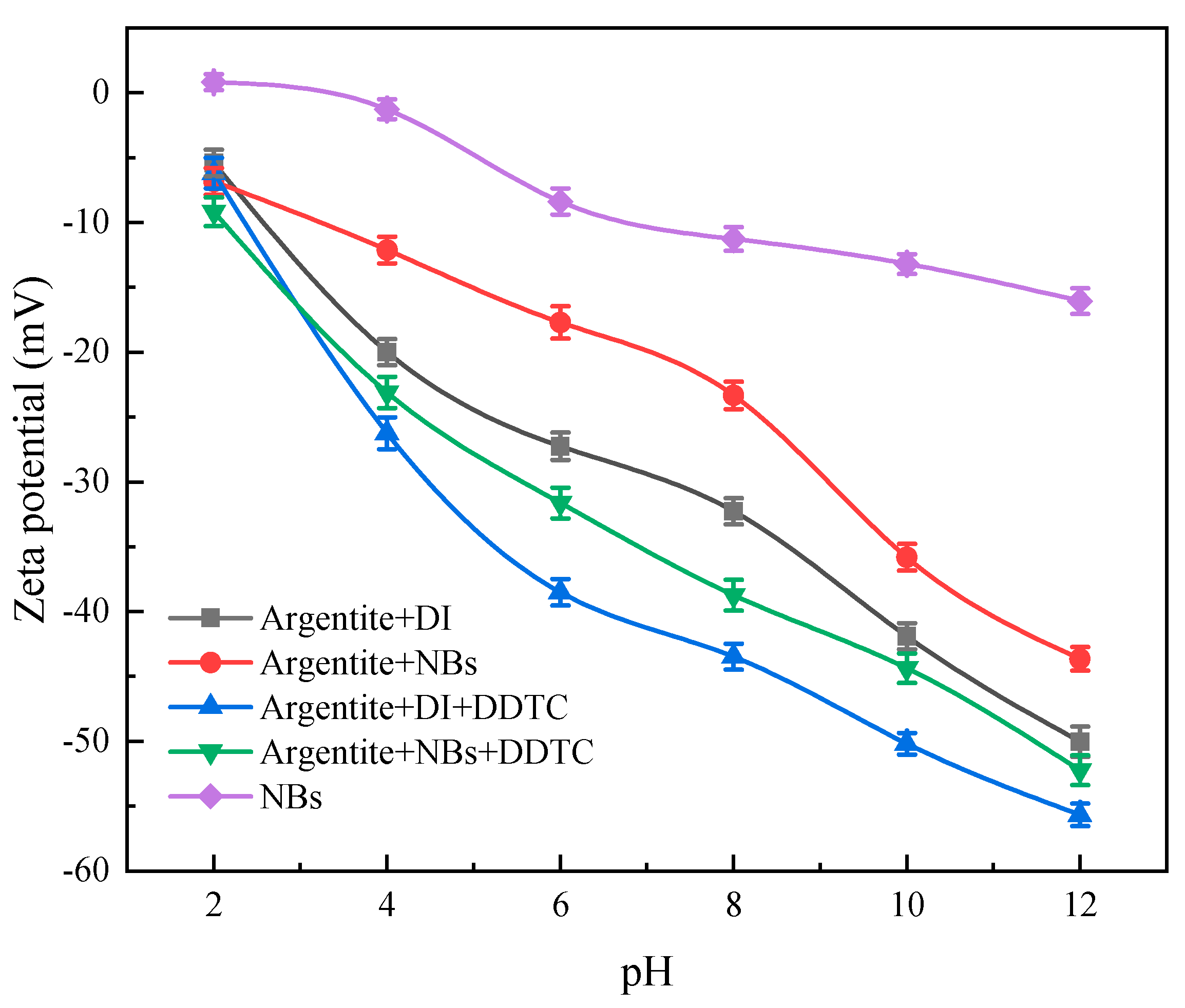
Figure 7 shows the variation in zeta potential of argentite particles with pH under different conditions. The zeta potential of the argentite surface in both DI water and NBs water exhibited a negative shift with increasing pH, primarily owing to increasing concentration of OH
− ions in the solution as the pH of the slurry increases. However, the absolute zeta potential value of argentite decreased in NB water, primarily owing to the attachment of NBs to the argentite surface, thereby affecting the dispersion stability of argentite particles. This decrease in the absolute zeta potential value of argentite indicated that the electrostatic repulsion between argentite was weakened, thereby facilitating their aggregation between fine argentite. Thus, such aggregation enhanced the upward flotation of fine minerals during the flotation process. Zhou et al. [
27] investigated the effect of NBs on the zeta potential of scheelite particles, and the absolute zeta of scheelite particle values decreased, owing to the attachment of NBs on scheelite particles. Wang et al. [
18] investigated the effect of NBs on the zeta potential of molybdenite under the conditions of kerosene as a collector and similar results were also obtained.
In the pH range of 4–12, the zeta potential of the argentite surface after the reaction of the collector DDTC shifted significantly in the negative direction. This shift occurred due to the adsorption of negatively charged DDTC colloidal particles onto the argentite surface. DDTC is adsorbed on the argentite by chemisorption, the argentite surface become negative charge. Additionally, the presence of NBs reduced the negative shifts in zeta potential after DDTC reaction compared with conventional flotation. It indicated that the NBs adsorbed on the surface of argentite, and this adsorption hindered the adsorption of DDTC on the argentite surface.
Additionally, the absolute zeta potential value on the argentite surface was lesser in the presence of both NBs and DDTC compared with the conventional flotation system. This reduction in zeta potential value enhanced particle attraction, leading to the formation of stable hydrophobic flocs under the action of the reagent, which increased the likelihood of fine argentite attachment and reduced detachment during flotation.
3.3. The Flocculation Effect of NBs on Fine Argentite Particle
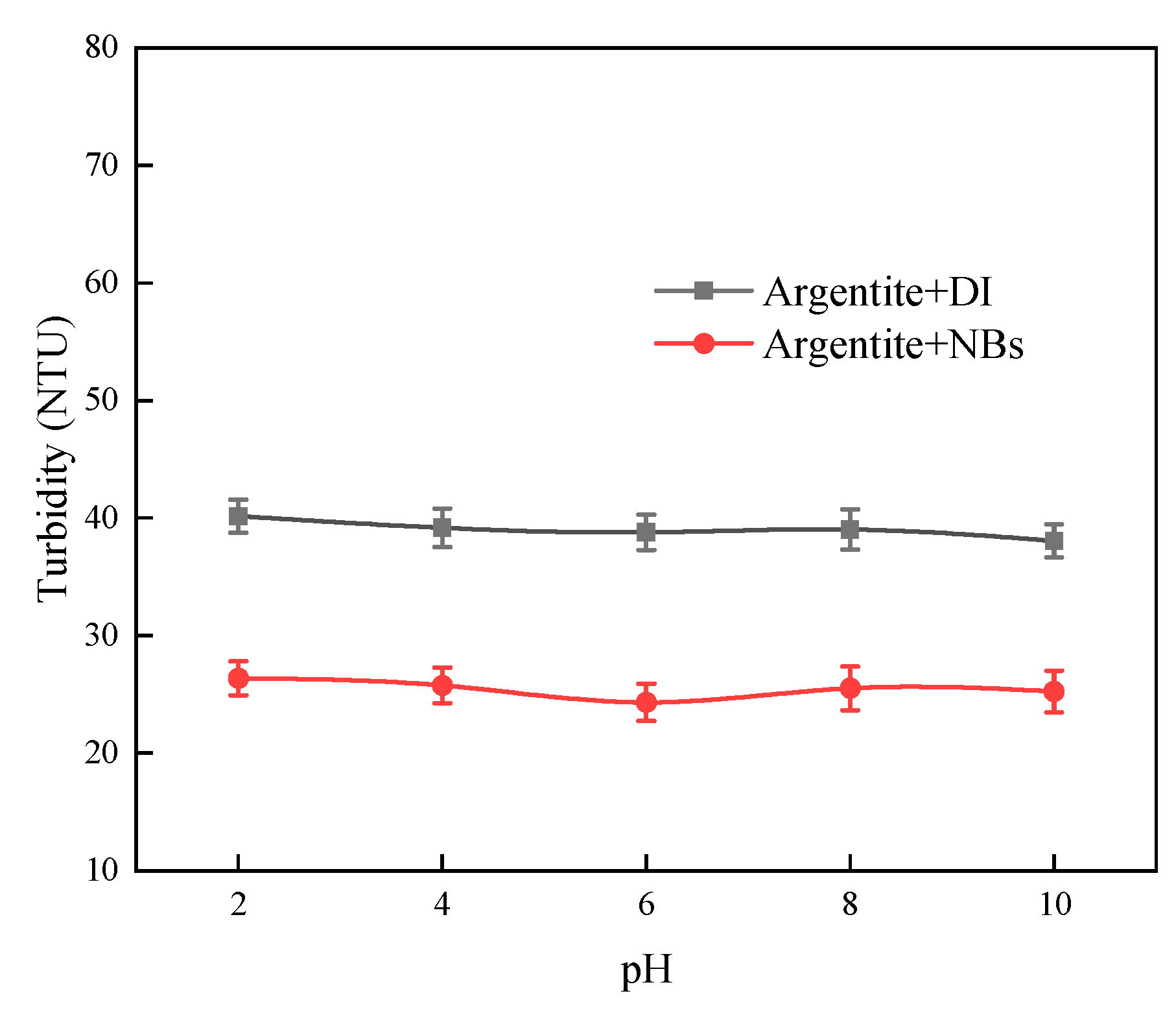
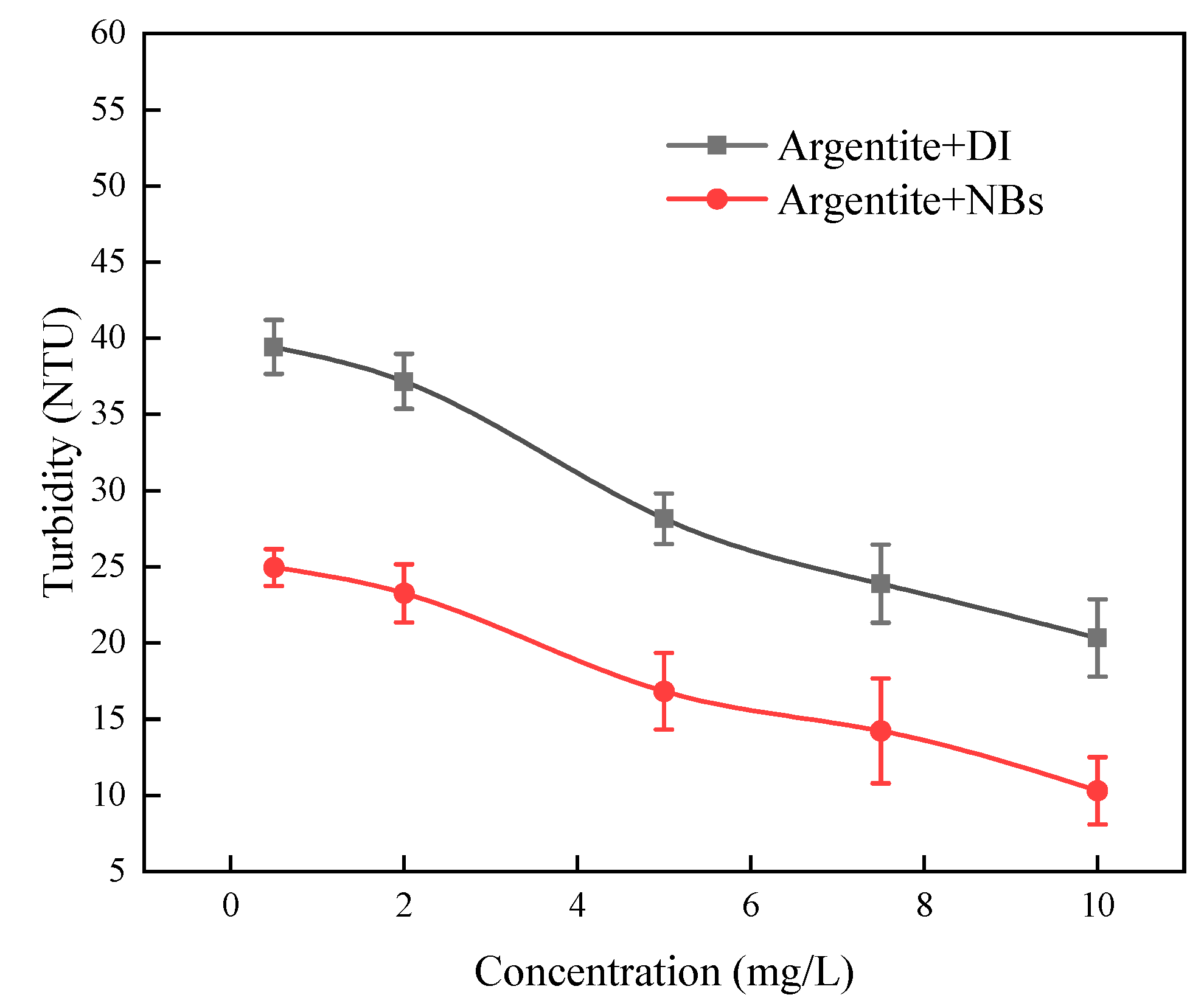
To elucidate the effect of NBs on argentite particles, turbidity tests were conducted.
Figure 8 shows the variation of slurry turbidity with pH for argentite in DI and NBs water. The test results indicated that the dispersion effect of argentite particles was not affected by pH. However, the turbidity value of argentite in NB water was smaller compared with DI water. It indicated that the aggregation of argentite occurred in the presence of NBs, and the aggregation between mineral particles led to a reduction of particles in the slurry as well as a decreased dispersion effect of mineral particles.
Figure 9 shows the variation of slurry turbidity with concentration of DDTC for argentite in DI and NBs water. The test results showed that the turbidity value of argentite decreased with the increase of DDTC concentration, indicating that the collector enhanced the agglomeration of argentite. In addition, in the presence of NBs, the aggregation of argentite particles is more obvious.
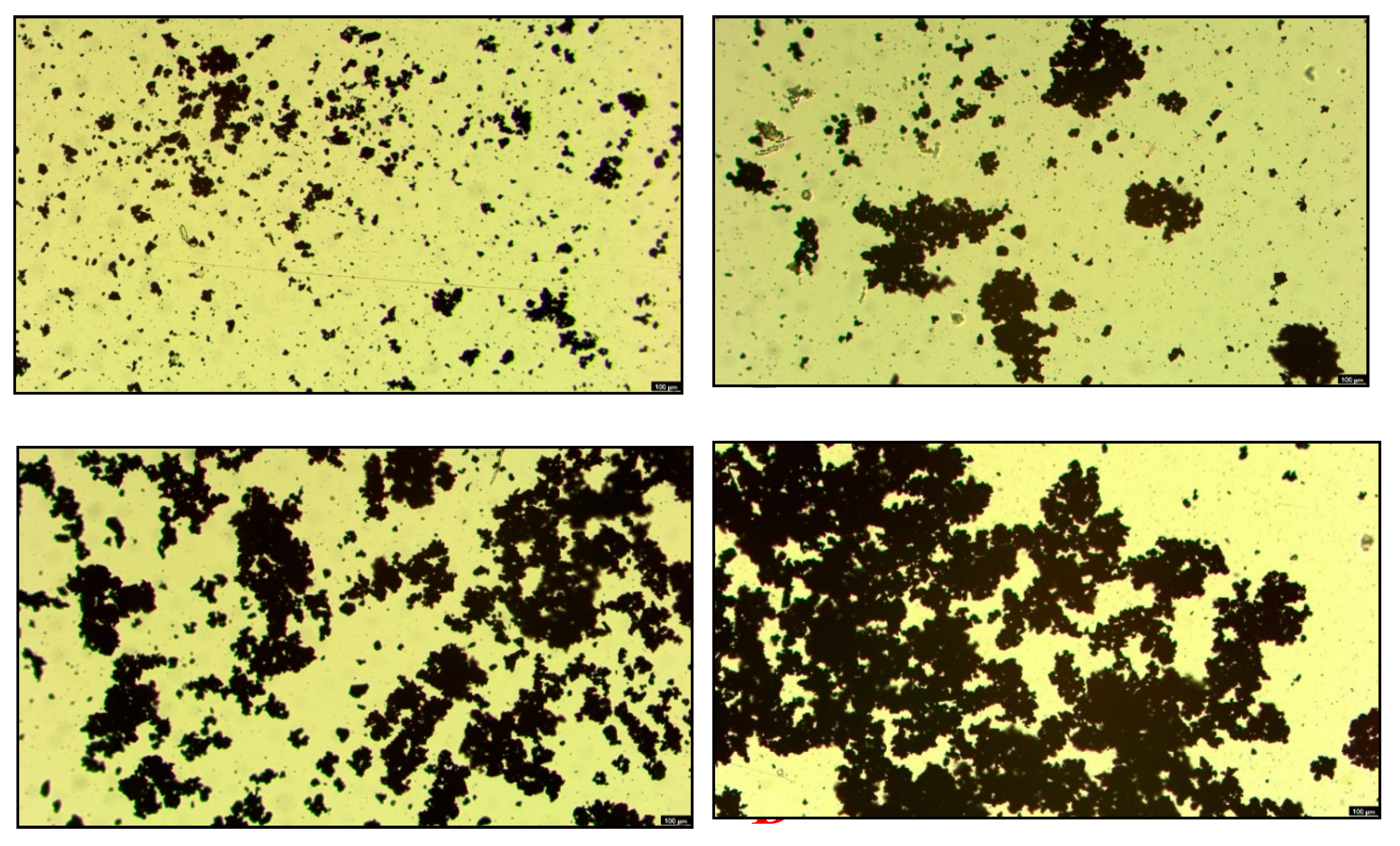
To further elucidate the effect of NBs on the aggregation behavior of argentite particles, the aggregation/dispersion of argentite particles was observed using an optical microscope. As shown in
Figure 10, without the addition of the collector DDTC, fewer aggregates were formed by argentite particles in DI water (Fig. 10. A). Notably, the presence of NBs facilitated the aggregation of argentite particles (Fig. 10. B), thereby resulting in significantly larger particle size. Subsequently, 5 mg/L of DDTC was added to both systems, owing to the hydrophobic coalescence of DDTC. Irregular aggregates were formed between particles in DI water (Fig. 10. C), while a trace amount of dispersed fine particles remained. Subsequently, under the combined effect of both NBs and DDTC, the induced aggregation of fine minerals was significantly enhanced (Fig. 10.D), which resulted in the formation of larger-sized aggregates. It was observed that the introduction of NBs overcame the electrostatic repulsion between particles and formed aggregates of larger size, thereby resulting in higher flotation recoveries of fine argentite.
In summary, it was observed that the introduction of NBs led to the aggregation of mineral particles. The primary mechanism of influence was that NBs formed bridges between particles, inducing capillary forces between particles [
28]. Previous studies [
29,
30] underscored the substantial impact on bubble-particle interactions, reducing interparticle distances and enhancing the likelihood of particle-bubble collisions during flotation processes.
3.4. Effect of NBs on the Surface Wettability of Argentite
The contact angle is a crucial indicator of mineral surface wettability, where an increased angle signifies higher hydrophobicity. Several studies have found that NBs exhibit significantly higher contact angles. Ishida et al. [
31] observed NBs on hydrophobic silicon wafers with the aid of atomic force microscopy measuring their dimensions and deducing contact angles up to 160°. Additionally, NBs changed the hydrophobicity of the mineral surface, enhancing macroscopic bubble contact angles, thus increasing the likelihood of mineral particles- air bubble collision and facilitating particle flotation [
32]. Zhou et al. [
27] investigated the effects of the collector DDA (dodecylamine) and NBs on the wettability of muscovite surfaces, indicating that the contact angle of the muscovite gradually increased with increasing DDA concentration. Furthermore, upon increasing the presence of NBs, adsorption of NBs enhanced the hydrophobicity of the muscovite surface.
Figure 11 shows the contact angle of the argentite surface under different conditions, The aqueous solution used to measure the contact angle has a pH of 7, and the results indicated that the contact angle measured using NB water exceeded compared with that under conventional DI water conditions. The introduction of NBs increased the contact angle on the argentite surface by 9.7°. Additionally, the hydrophobicity of the argentite surface was further enhanced after DDTC action, with a contact angle measuring 85.9° with DI water and 93.1° with NBs water, thereby increasing the surface contact angle of argentite. The above results indicated that the contact angle of the mineral surface increased when NBs were adsorbed on the mineral surface, thereby enhancing the hydrophobicity of the mineral surface.
3.5. Adsorption Capacity Measurement Results
To investigate the effect of varying DDTC concentrations on its adsorption capacity on argentite surface, measurements were conducted in both DI water and NBs water.
As shown in
Figure 12, the adsorption capacity of the collector on the surface of argentite initially increased with increasing DDTC dosage in both systems. Furthermore, when the amount of DDTC exceeded 5mg/L, the adsorption capacity amount tended to level off, indicating that the collector adsorption on the mineral surface was saturated. Upon comparing conventional flotation with NB flotation, the adsorption capacity of DDTC on the surface of argentite under NB flotation was lower compared with conventional flotation.
Wang et al. [
33] investigated the adsorption capacity of sodium oleate on the calcite surface in the presence of NBs and concluded that the generation of NBs led to the formation of calcite flocs, and the formation of flocs led to a decreased specific surface area of calcite particles, thus reducing the adsorption capacity of sodium oleate on the calcite surface. Ren et al. [
34] investigated the effect of NBs on the flotation of fine cassiterite, and it was observed that under NB flotation conditions, the adsorption of the collector on the cassiterite surface was always lower compared with that under conventional flotation conditions. However, NBs enhanced the recovery of cassiterite.
In the presence of NBs, the adsorption capacity of the reagents on the mineral surface was lower compared with that of conventional flotation. This phenomenon was attributable to the introduction of NBs occupying reagent adsorption sites on mineral surfaces, thereby reducing the effective contact area between minerals and collectors. Thus, NBs served as bridges between fine minerals and facilitated aggregation [
30]. The adsorption capacity of DDTC on the surface of argentite was affected by the aggregation between fine minerals, which resulted in a decreased specific surface area of the particles.
3.6. Micro Flotation Tests Results
Given the aforementioned studies, this section focused on the effect of NBs on the flotation behavior of fine argentite. Single-mineral flotation tests were conducted to investigate the effect of NB introduction on the recovery of fine argentite. Subsequently, DDTC was selected as the collector, with MIBC serving as a frother. Initially, pH variable tests were conducted to investigate the relationship between NB flotation and pH, aiming to determine the optimum pH for argentite flotation. Additionally, a DDTC dosage test was conducted under optimal pH conditions to examine the effect of DDTC dosage on argentite flotation behavior and to compare flotation outcomes in the presence of NBs.
A fixed DDTC dosage of 5 mg/L and an MIBC dosage of 5 mg/L were maintained. NaOH and H2SO4 were used to adjust the pH of the slurry, and the tests were conducted under conditions of DI and NB water, respectively, thereby exploring the effect of pH on the flotation of argentite in two systems.
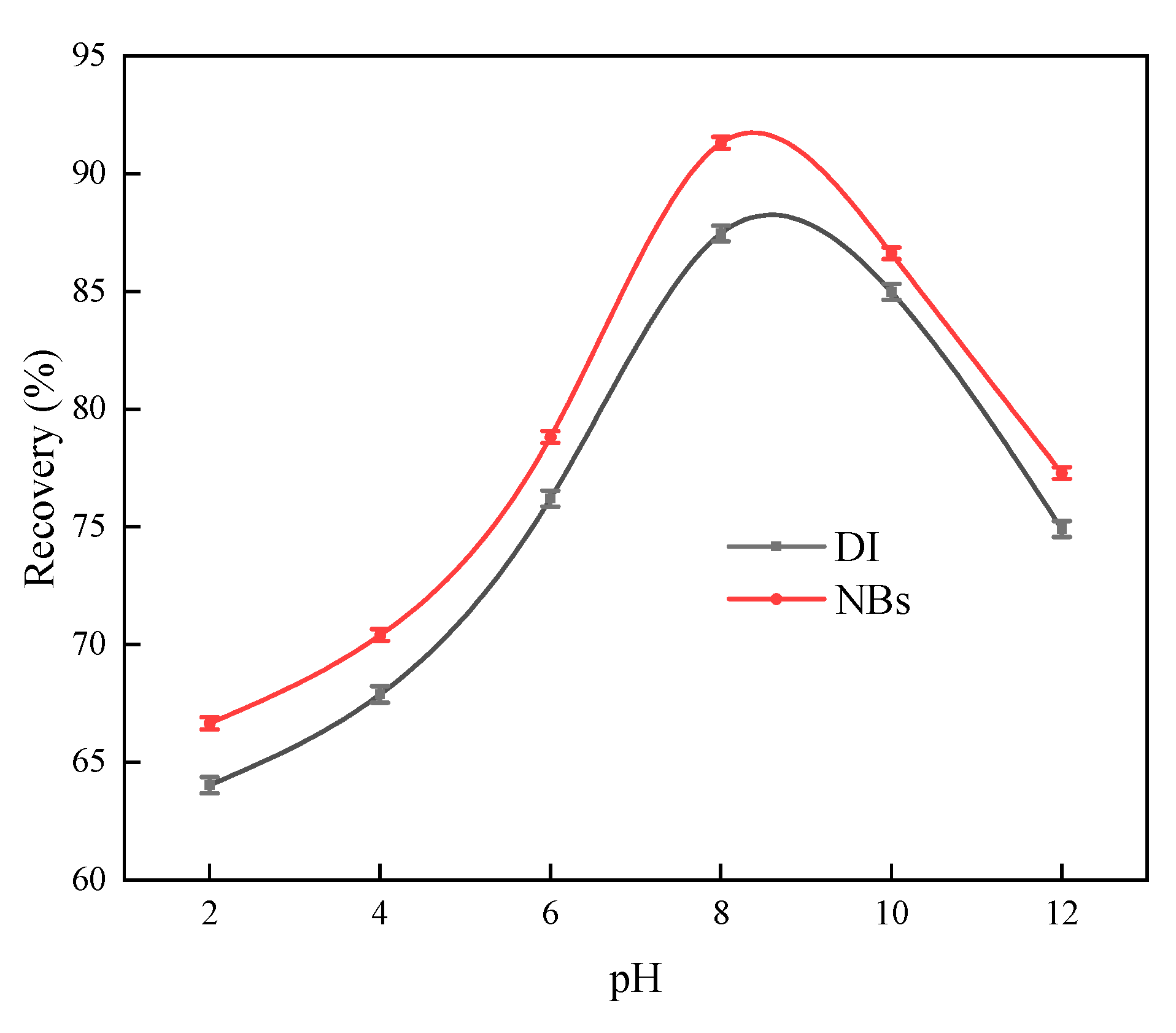
In
Figure 13, it was observed that as the pH increased, argentite recovery initially increased and then decreased rapidly in an alkaline environment. Optimal flotation of argentite occurred under weakly alkaline conditions, achieving 87.9% recovery in conventional flotation and 91.23% recovery in NBs flotation at pH 8. At pH > 10, the floatability of argentite significantly decreased. Throughout the pH range tested, the recovery of NB flotation exceeded conventional flotation, thereby facilitating the flotation of fine argentite under similar pH conditions. However, NBs exhibited a minimal effect on determining the optimal pH for flotation.
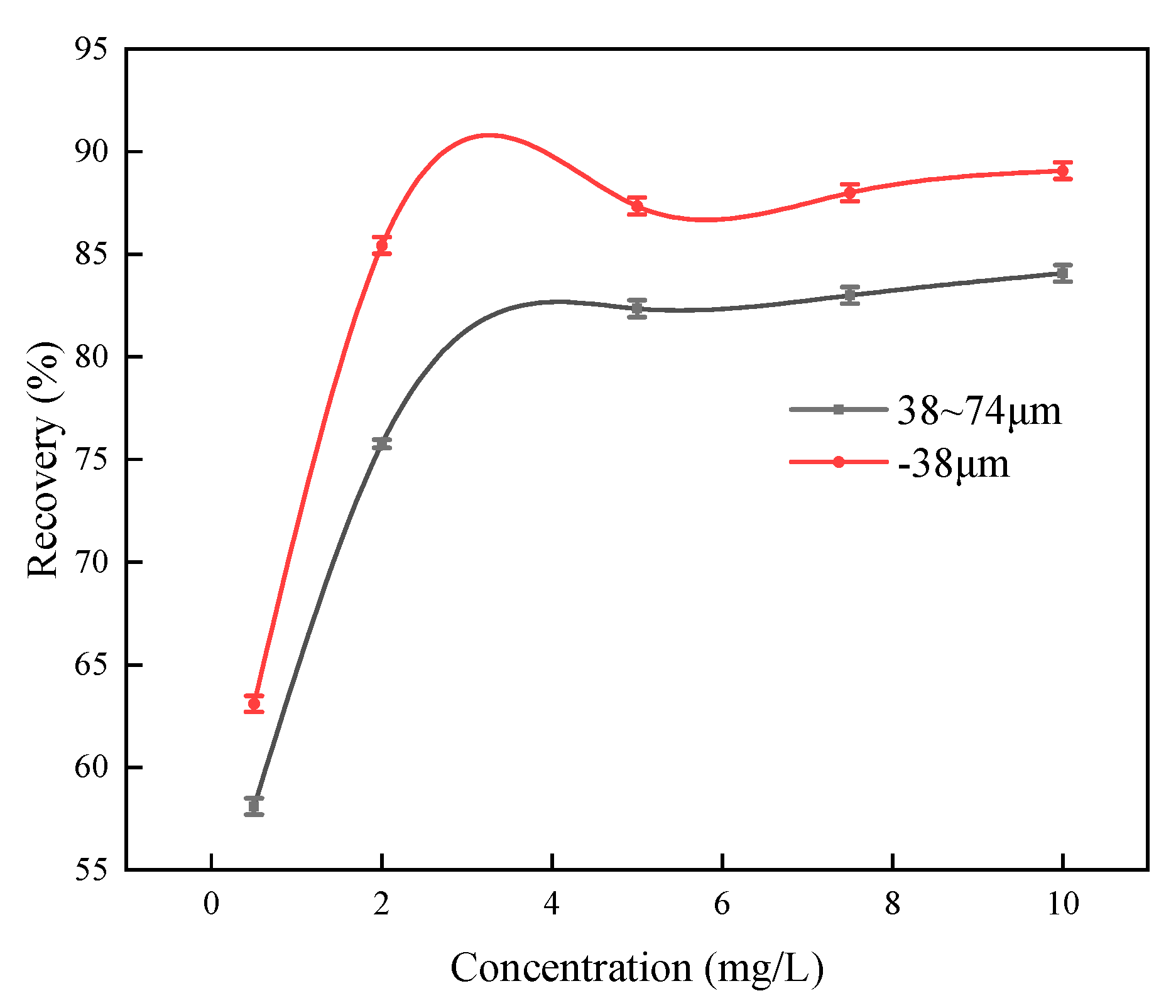
According to the pH condition tests, a pH value of 8 was the determined optimal argentite flotation. Under this condition, Firstly, the flotation test of 38μm ~ 74μm samples was carried out to study the relationship between the flotability of argentite and DDTC concentration in the range of particle size. The dosage of MIBC was 5mg/L(Fig. 14).In addition, the effect of DDTC concentration on argentite flotation was investigated in both conventional and NBs flotation systems, with a fixed MIBC frother dosage of 5 mg/L (Fig. 15).
As shown in
Figure 14, with the increase of DDTC concentration, the recovery rate of argentite gradually increased, and the maximum recovery rate of argentite was 84.07%. The main reason for the insufficient recovery of argentite is the large mineral particle size, which is also the main reason for the difficult separation of argentite. In actual production, silver minerals must be fine grinding to improve the recovery rate.
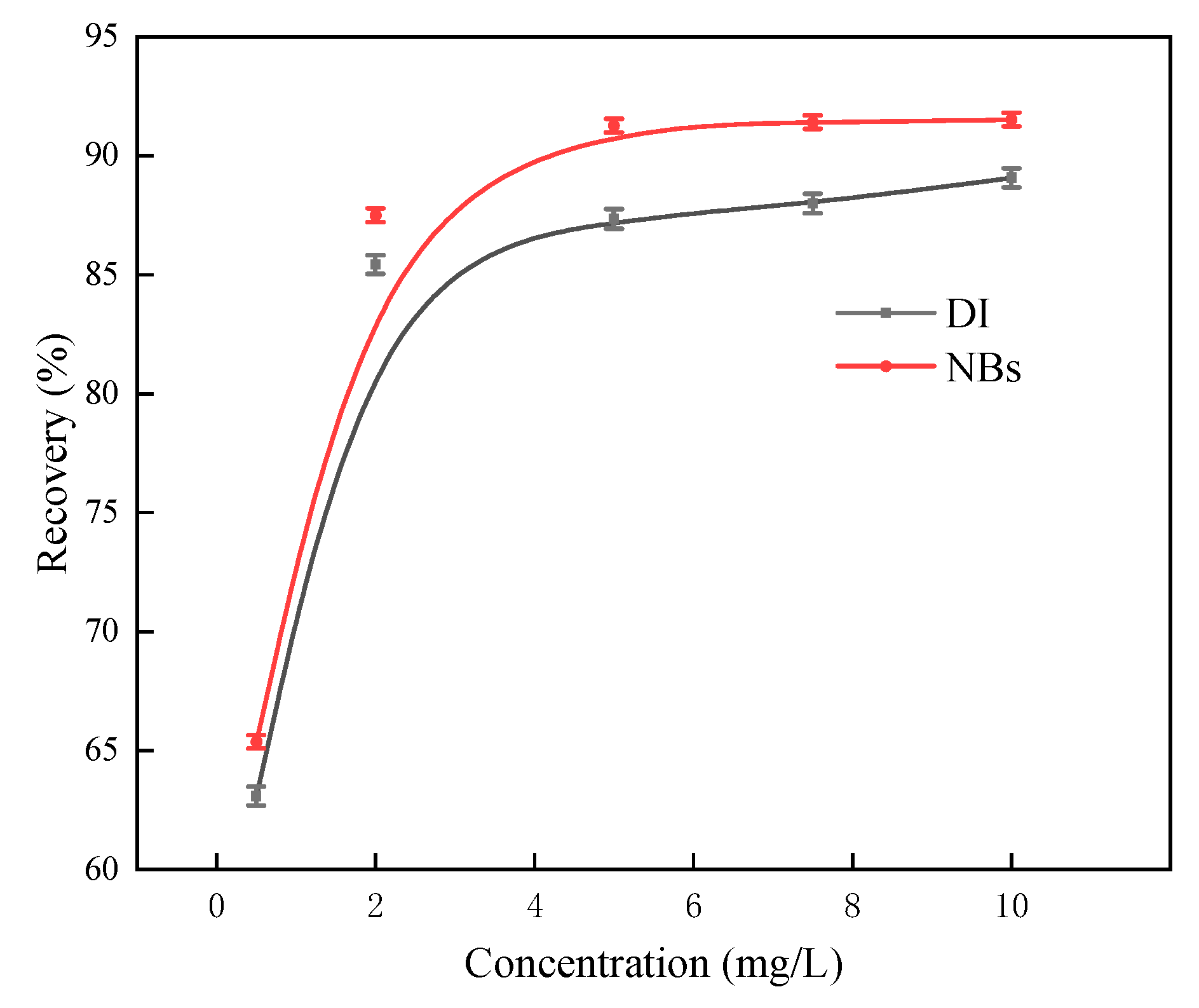
As shown in
Figure 15, DDTC dosage at 0.5 mg/L resulted in 63.65% recovery for conventional flotation and 65.29% recovery for NB flotation. Higher DDTC dosages significantly increased flotation recoveries, stabilizing at 0.5 mg/L where further increases exhibited marginal impact on recovery rates. Throughout the concentration range tested, NB flotation consistently achieved about 3% higher recoveries compared with conventional flotation.
In summary, NBs enhanced the upwelling of fine argentite during flotation. For instance, under NB conditions, the flotation recovery was 87.42% when the DDTC dosage was 2 mg/L, compared with 87.89% requiring 5 mg/L under conventional flotation. The introduction of NBs reduced collector usage and was utilized as a secondary collector for mineral particles during flotation, thereby reducing reagent costs [
35]. Rahman et al. [
36] utilized NBs for the flotation of fine argentite and found that the introduction of NBs reduced collector and frother usage by 75% and 50%, respectively.
3.7. Actual Ore Flotation Tests
Micro-flotation tests indicated that the introduction of NBs enhanced the flotation recovery of fine argentite. To investigate the effect of NBs on associated Ag in actual ore, conventional flotation, and NBs flotation were conducted in the Pb separation area of Ag-bearing Pb–Zn ores in Guangdong, China, utilizing a known optimal reagent system and dosage. The test results are presented in
Table 3.
The results indicated that under conventional flotation conditions, the average grades of Pb and Ag in the Pb concentrate were 58.05% and 488.84 g/t. The recovery of Pb and Ag in the Pb concentrate was 60.28% and 45.57%, respectively. Additionally, under the flotation conditions with NBs, the average grades of Pb and Ag in the Pb concentrate were 62.13% and 560.92 g/t, respectively. The recovery rates for Pb and Ag in the Pb concentrate were 60.95% and 48.84%, respectively. The presence of NBs resulted in enhanced grades of Pb and Ag in the lead concentrate, with a 4.08% increase in Pb grade and a 71.88 g/t increase in Ag grade. Notably, the increase in Pb grade did not reduce Pb recovery in the Pb concentrate; instead, led to a slight increase in the recovery. Significantly, the recovery of Ag in the Pb concentrate increased by 3.27%. Actual ore tests further indicated that NBs enhanced the grade and recovery of concentrates, thereby facilitating the flotation of fine minerals.
4. Conclusions
- (1)
The prolonged cavitation time facilitated the formation of stable NBs, with average bubble sizes ranging from 120 nm to 150 nm when the cavitation time exceeded 10 min.
- (2)
The adsorbed NBs on argentite surfaces not only increased their hydrophobicity but also induced significant aggregation of fine argentite particles, thereby enhancing aggregation stability.
- (3)
The adsorbed NBs decrease the specific surface area of fine argentite particles, thus decreasing the adsorption amount of DDTC on the argentite surface.
- (4)
Both micro-flotation tests and actual ore flotation tests indicated that the presence of NBs enhanced the recovery of fine silver-bearing minerals.
- (5)
In view of the application of fine minerals in industry, NBs aqueous solution can be prepared for mineral flotation in the future, and the existence of NBs will improve the flotation effect of fine minerals.
Acknowledgements
The authors gratefully acknowledge the support from the National key research and development plan(No.2022YFC2904504). The Science and technology research project of Jiangxi Provincial Department of Education(No.GJJ2200864).
References
- J. Wang, D. Yu, Non-ferrous metal associated silver mineralogy, Beijing: Metallurgical Industry Press, 2018.
- L. Zhang, H. Yang, A. Feng, J. Zhao, X. Tan, Study on General Situation and Analysis of Supply and Demand of Global Sliver Resource, Conservation and Utilization of Mineral Resources, (2016) 44-48.
- B. Song, X. Qiu, J. Ran, Z. Hu, P. Li, Y. Yao, Behavior of Argentite in the Sulphide Flotation System, Precious Metals, 39 (2018) 24-28.
- S. Ao, Research progress on mineral processing of associated gold and silver in nonferrous metal mines, Precious Metals, (2023) 1-11.
- H. Jiang, Experimental Study on Occurrence and Recovery of Silver in a Lead-Zinc Mine, Nonferrous Metals(Mineral Processing Section), (2023) 116-123.
- X. Zhang, Y. Han, Y. Li, W. Li, J. He, J. Jin, Strengthening the flotation recovery of silver using a special ceramic-medium stirred mill, Powder Technology, 406 (2022).
- F. Zang, Y. Xu, H. Zhou, W. Guo, Y. Bai, G. Peng, Experimental Study and Production Practice on Improving the Recovery of Associated Gold and Silver in Xitieshan Lead-Zinc Mine, Nonferrous Metals(Mineral Processing Section), (2023) 100-105.
- L. Liu, S. Hu, C. WU, K. Liu, W. Li, W. Zhou, Aggregates characterizations of the ultra-fine coal particles induced by nanobubbles, Fuel, 297 (2021).
- D. Tao, Z. Wu, A. Sobhy, Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms, Powder Technology, 379 (2021) 12-25.
- D. Tao, Recent advances in fundamentals and applications of nanobubble enhanced froth flotation: A review, Minerals Engineering, 183 (2022).
- E. Favvas, G. Kyzas, E. Efthimiadou, A. Mitropoulos, Bulk nanobubbles, generation methods and potential applications, Current Opinion in Colloid & Interface Science, 54 (2021).
- A. Azevedo, R. Etchepare, S. Calgaroto, J. Rubio, Aqueous dispersions of nanobubbles: Generation, properties and features, Minerals Engineering, 94 (2016) 29-37.
- X. Zhang, A. Quinn, W. Ducker, Nanobubbles at the Interface between Water and a Hydrophobic Solid, Langmuir, 24 (2008) 4756-4764.
- K. Kikuchi, A. Ioka, T. Oku, Y. Tanaka, Y. Saihara, Z. Ogumi, Concentration determination of oxygen nanobubbles in electrolyzed water, Journal of Colloid and Interface Science, 329 (2009) 306-309.
- Y. Zhou, Z. Han, C. He, Q. Feng, K. Wang, Y. Wang, N. Luo, G. Dodbiba, Y. Wei, A. Otsuki, T. Fujita, Long-Term Stability of Different Kinds of Gas Nanobubbles in Deionized and Salt Water, Materials, 14 (2021).
- B. Song, W. Walczyk, H. Schönherr, Contact Angles of Surface Nanobubbles on Mixed Self-Assembled Monolayers with Systematically Varied Macroscopic Wettability by Atomic Force Microscopy, Langmuir, 27 (2011) 8223-8232.
- H. An, G. Liu, V.S.J. Craig, Wetting of nanophases: Nanobubbles, nanodroplets and micropancakes on hydrophobic surfaces, Advances in Colloid and Interface Science, 222 (2015) 9-17.
- X. Wang, S. Yuan, J. Liu, Y. Zhu, Y. Han, Nanobubble-enhanced flotation of ultrafine molybdenite and the associated mechanism, Journal of Molecular Liquids, 346 (2022).
- Z. Zhang, L. Ren, Y. Zhang, Role of nanobubbles in the flotation of fine rutile particles, Minerals Engineering, 172 (2021).
- C. Li, M. Xu, H. Zhang, The interactions between coal particles with different hydrophobicity and bulk nanobubbles in natural water, International Journal of Coal Preparation and Utilization, 42 (2019) 463-474.
- Z. Wu, D. Tao, Y. Tao, G. Ma, New insights into mechanisms of pyrite flotation enhancement by hydrodynamic cavitation nanobubbles, Minerals Engineering, 201 (2023).
- D. Tao, Z. Wu, S. Ahmed, Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms, Powder Technology, (2020).
- N. Nirmalkar, A.W. Pacek, M. Barigou, On the Existence and Stability of Bulk Nanobubbles, Langmuir, 34 (2018) 10964-10973.
- Q. Feng, W. Zhou, Q. Shi, Formation of nano-bubbles and their influences on ultrafine mineral flotation, Journal of Central South University(Science and Technology), 48 (2017) 9-15.
- A. Shekhar, K. Nomura, R.K. Kalia, A. Nakano, P. Vashishta, Nanobubble collapse on a silica surface in water: billion-atom reactive molecular dynamics simulations, Phys Rev Lett, 111 (2013) 184503.
- T. Zhang, Q. Zhang, Research of nanobubbles enhanced reverse anionic flotation of a mid-low grade phosphate ore, Physicochemical Problems of Mineral Processing, (2021).
- W. Zhou, J. Niu, W. Xiao, L. Ou, Adsorption of bulk nanobubbles on the chemically surface-modified muscovite minerals, Ultrason Sonochem, 51 (2019) 31-39.
- Z. Pourkarimi, B. Rezai, M. Noaparast, A.V. Nguyen, S. Chehreh Chelgani, Proving the existence of nanobubbles produced by hydrodynamic cavitation and their significant effects in powder flotation, Advanced Powder Technology, 32 (2021).
- N. Mishchuk, J. Ralston, D. Fornasiero, Influence of very small bubbles on particle/bubble heterocoagulation, J Colloid Interface Sci, 301 (2006) 168-175.
- M. Hampton, A. Nguyen, Nanobubbles and the nanobubble bridging capillary force, Adv Colloid Interface Sci, 154 (2010) 30-55.
- N. Ishida, T. Inoue, M. Miyahara, K. Higashitani, Nano Bubbles on a Hydrophobic Surface in Water Observed by Tapping-Mode Atomic Force Microscopy, Langmuir, 16 (2000) 6377-6380.
- C. Li, H. Zhang, A review of bulk nanobubbles and their roles in flotation of fine particles, Powder Technology, 395 (2022) 618-633.
- Y. Wang, Z. Pan, X. Luo, W. Qin, F. Jiao, Effect of nanobubbles on adsorption of sodium oleate on calcite surface, Minerals Engineering, 133 (2019) 127-137.
- L. Ren, Z. Zhang, W. Zeng, Y. Zhang, Adhesion between nanobubbles and fine cassiterite particles, International Journal of Mining Science and Technology, 33 (2023) 503-509.
- Y. Tao, J. Liu, S. Yu, D. Tao, Picobubble Enhanced Fine Coal Flotation, Separation Science and Technology, 41 (2007) 3597-3607.
- R. Ahmadi, D.A. Khodadadi, M. Abdollahy, M. Fan, Nano-microbubble flotation of fine and ultrafine chalcopyrite particles, International Journal of Mining Science and Technology, 24 (2014).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).