Submitted:
28 October 2024
Posted:
30 October 2024
You are already at the latest version
Abstract
Keywords:
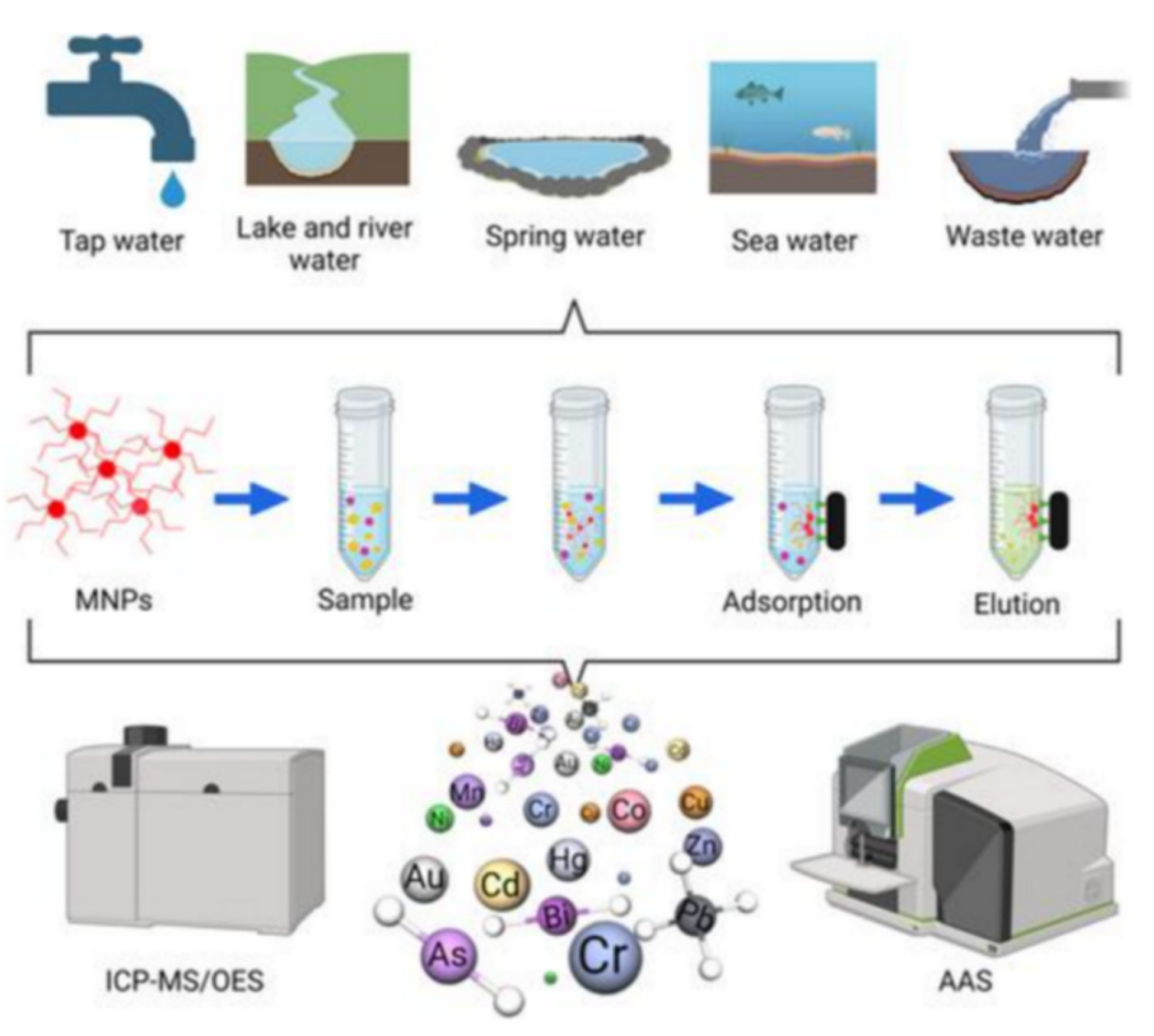
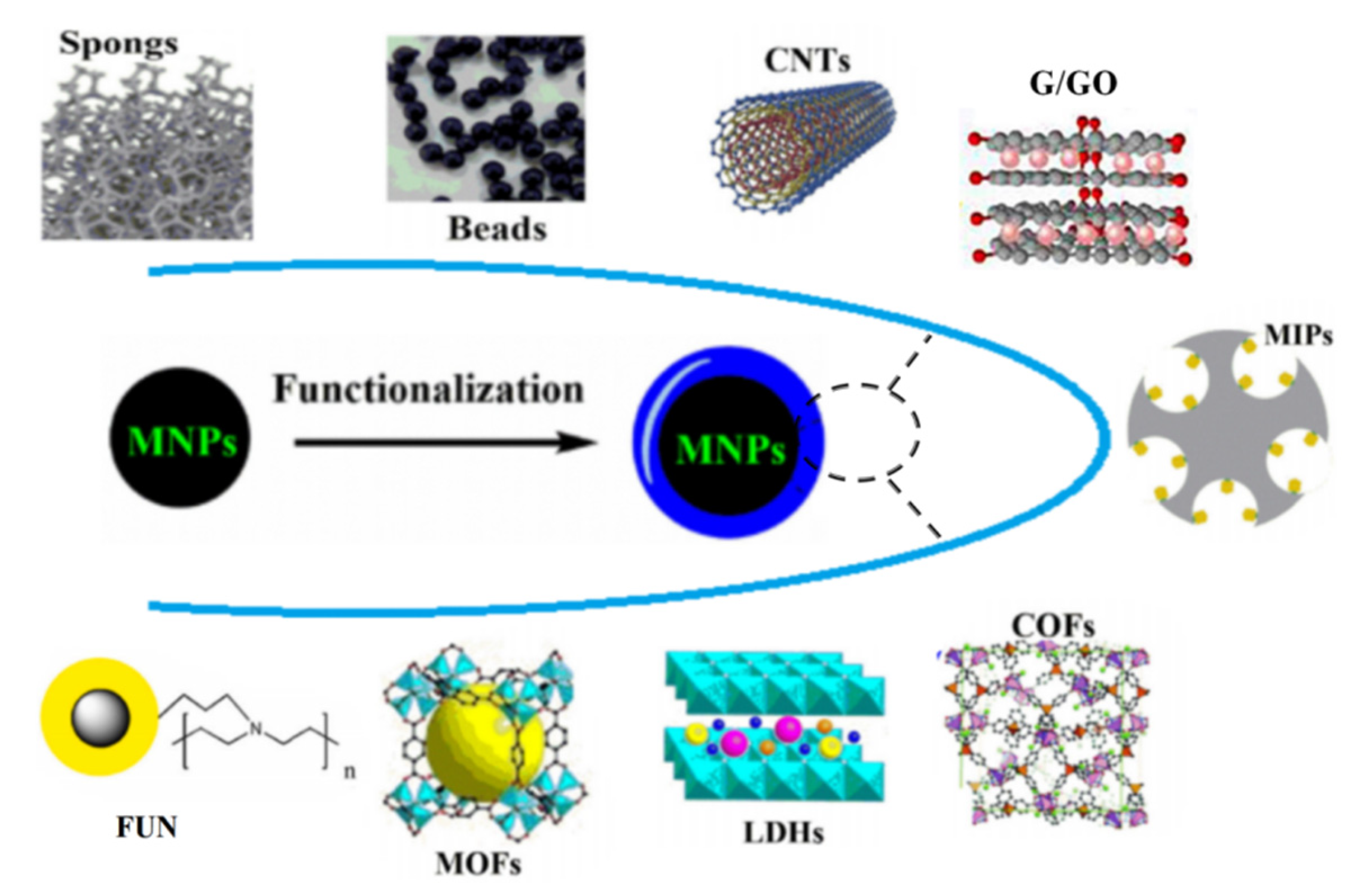
1. Introduce
2. Experimental
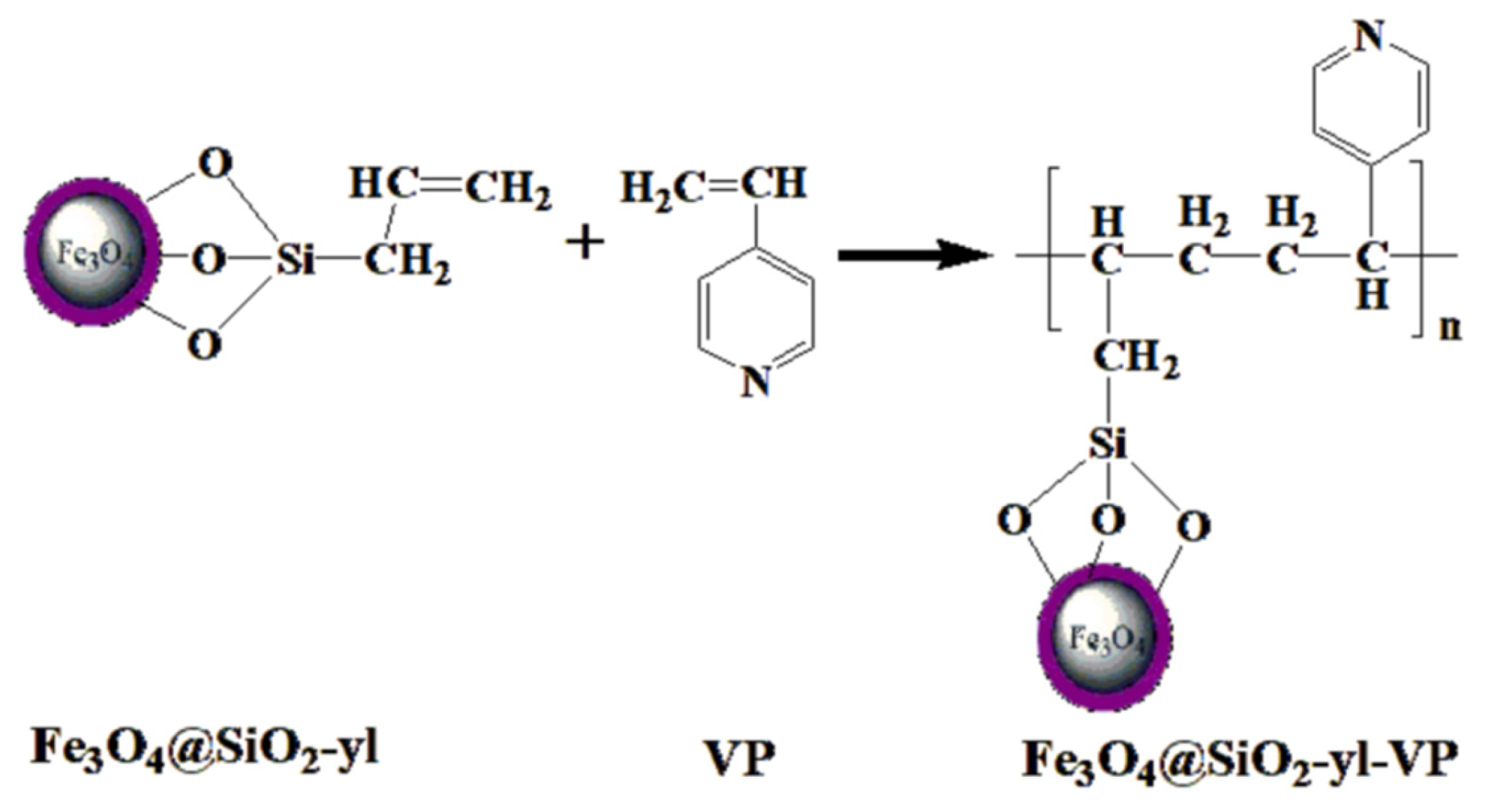
2.1. Synthesis of Fe3O4@SiO2-yl
2.2. Synthesis of Fe3O4@SiO2-yl-VP
3. Results and Discussion
3.1. Characterization
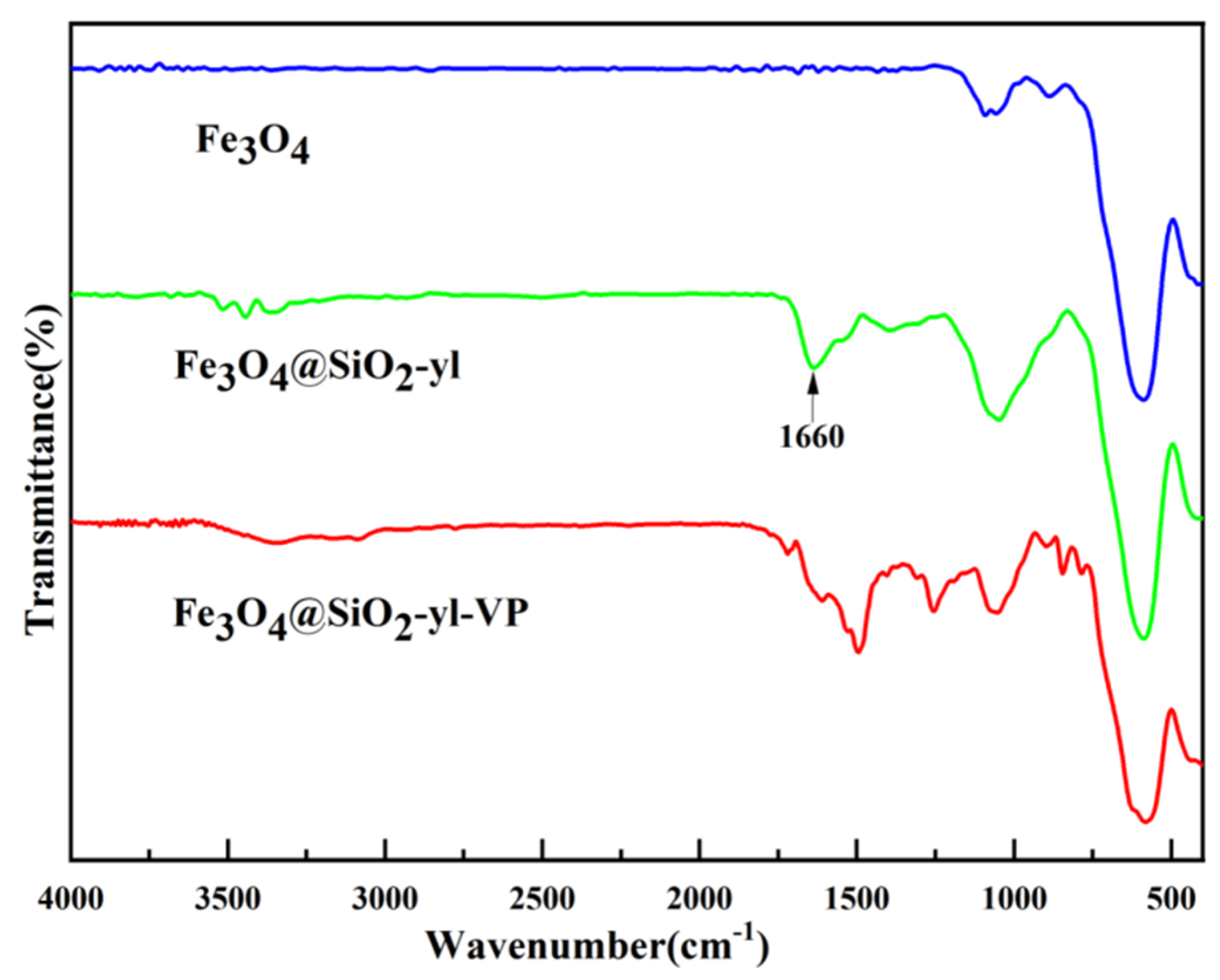
3.1.1. Infrared Spectrum Analysis of Fe3O4@SiO2-yl-VP
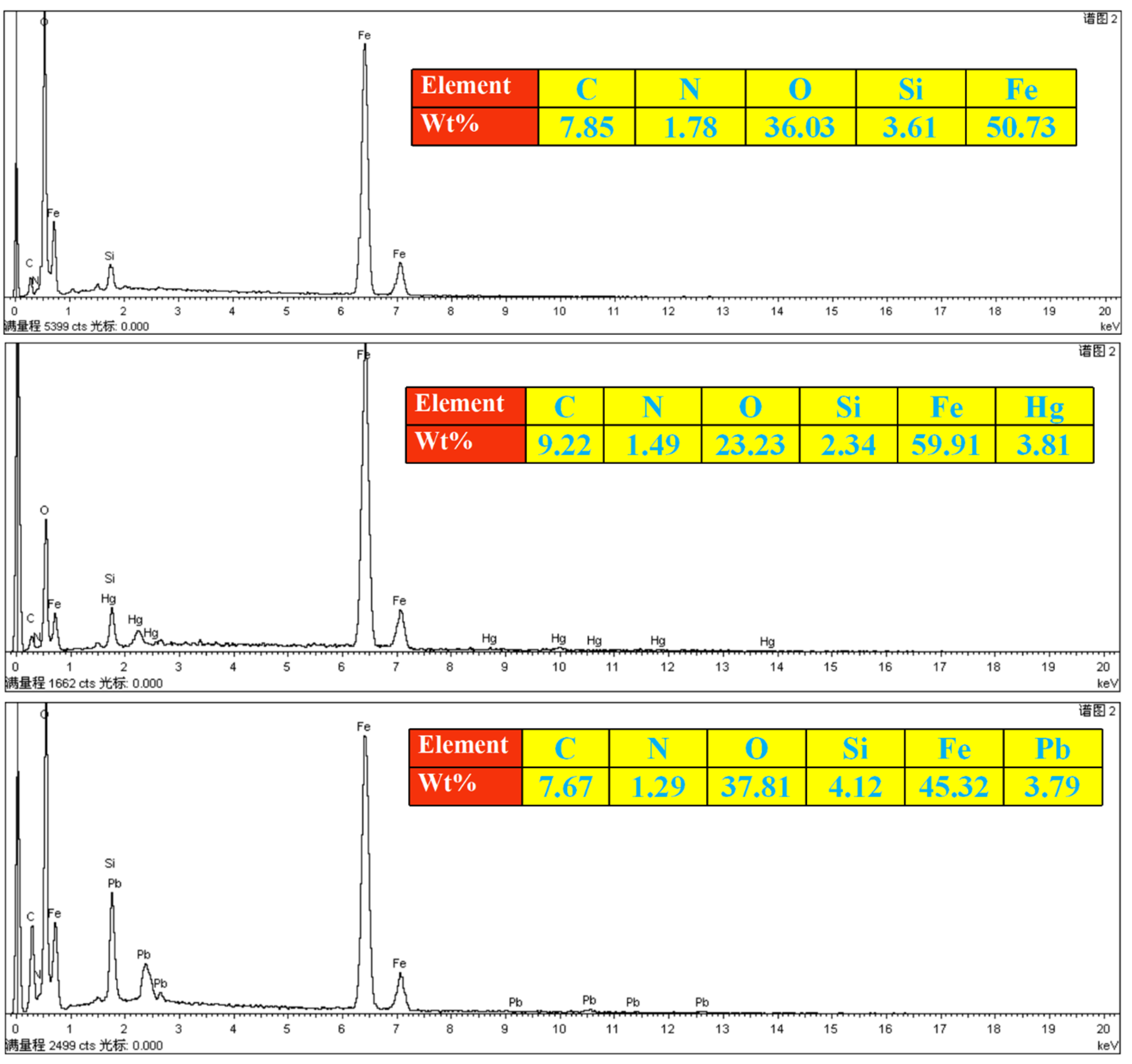
3.1.2. EDS Spectrum Analysis of Fe3O4@SiO2-yl-VP
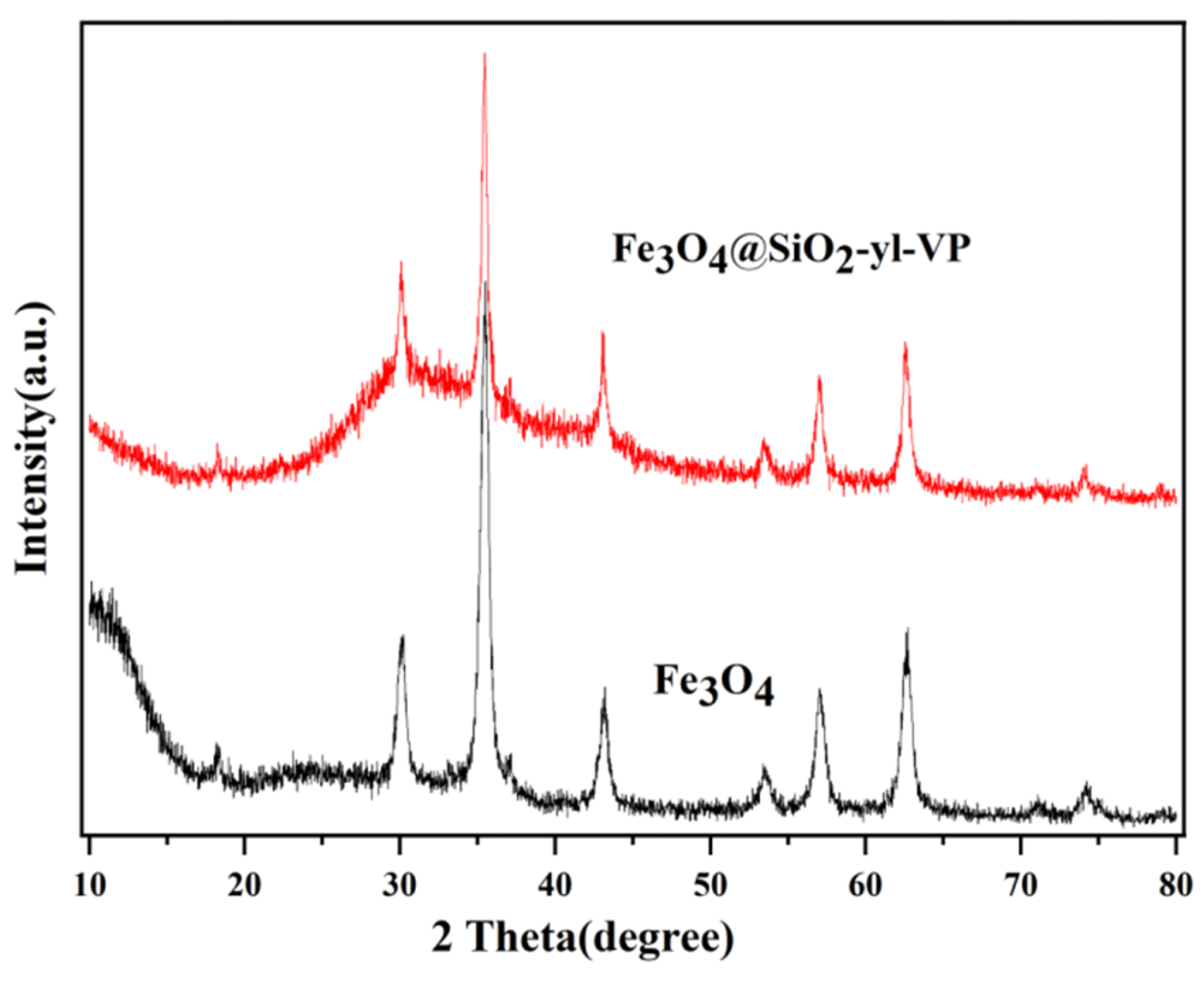
3.1.3. XRD Pattern Analysis of Fe3O4@SiO2-yl-VP
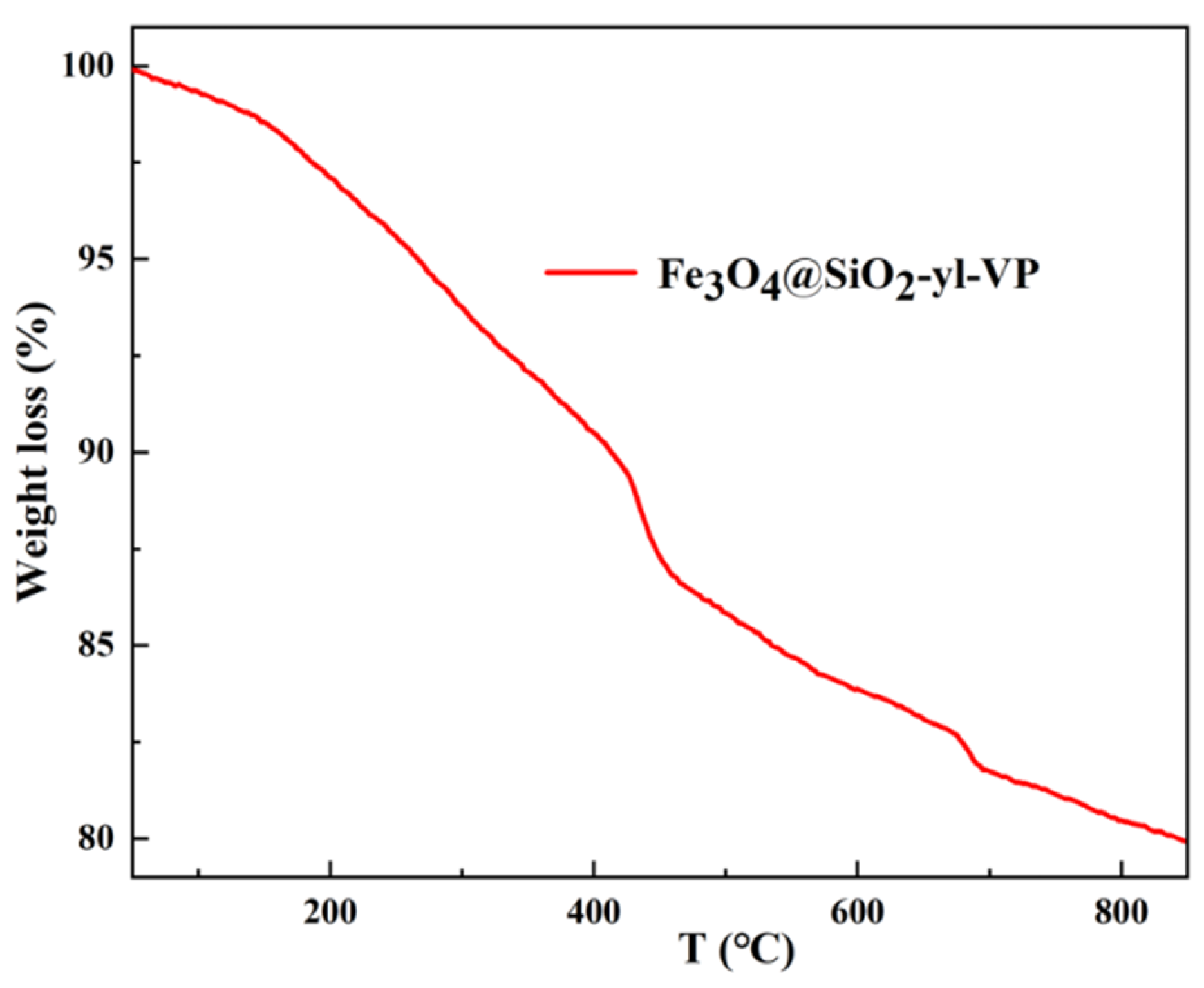
3.1.4. Thermogravimetric (TG) Analysis
3.1.5. SEM Analysis of Fe3O4@SiO2-yl-VP
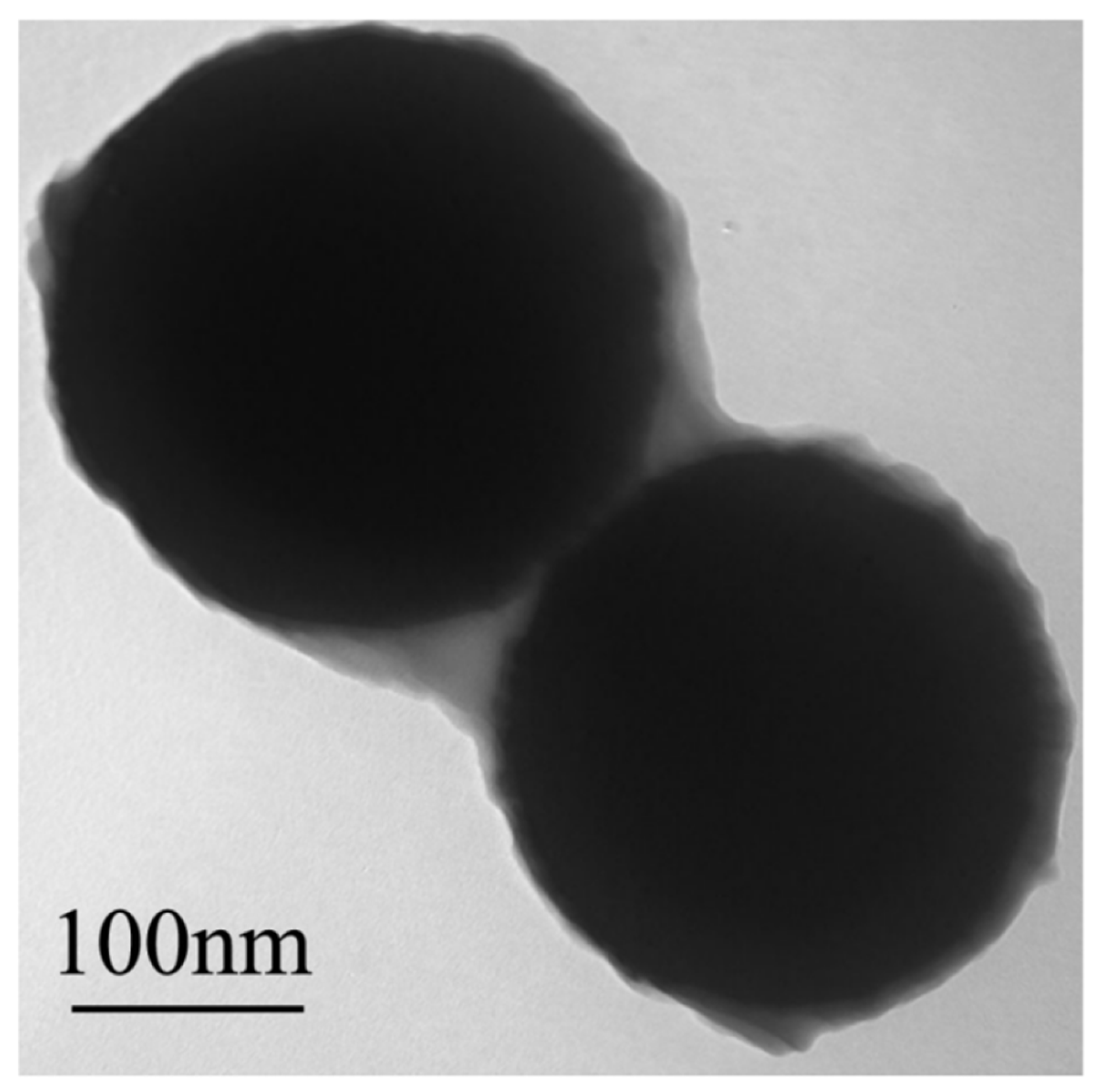
3.1.6. TEM Analysis of Fe3O4@SiO2-yl-VP
3.2. Research on Adsorption Performance
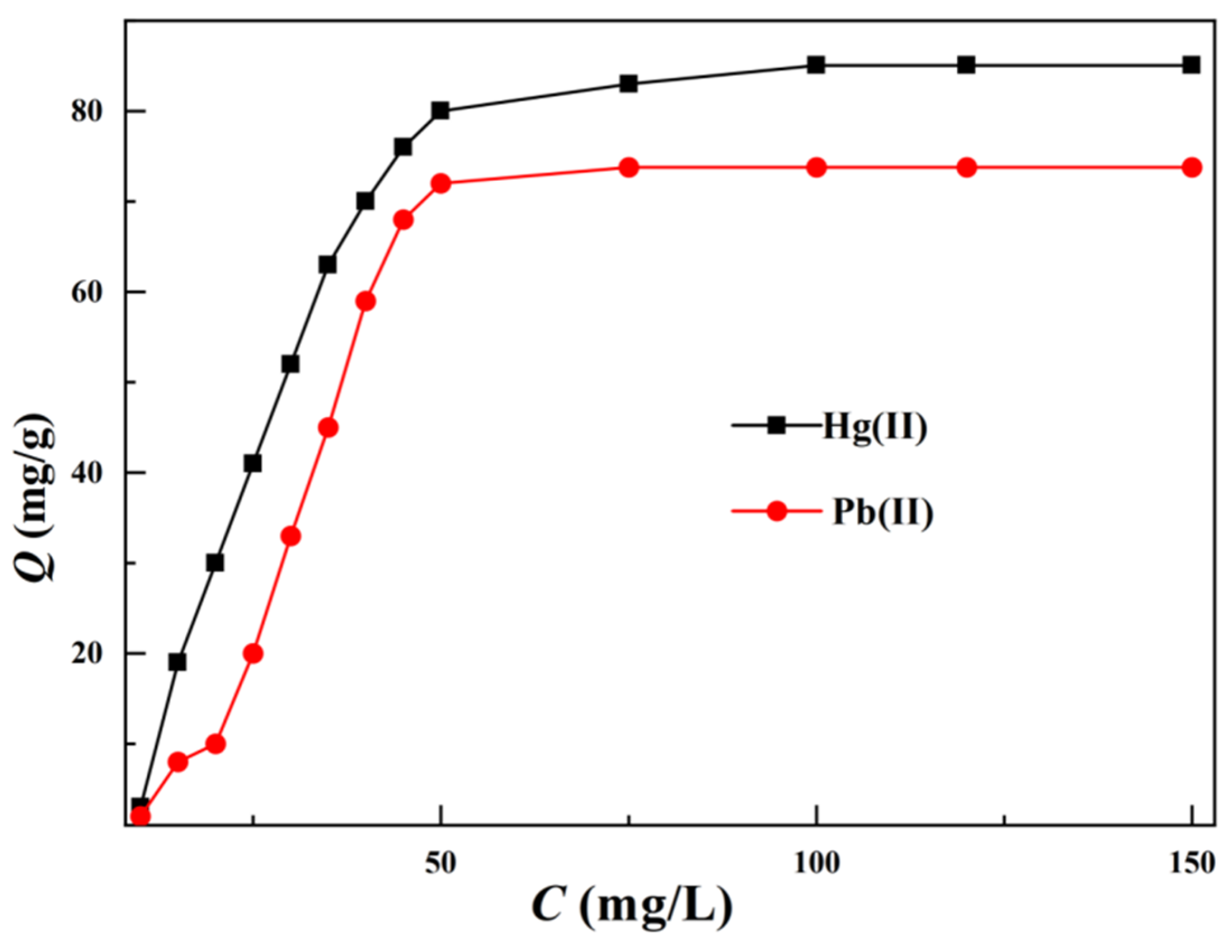
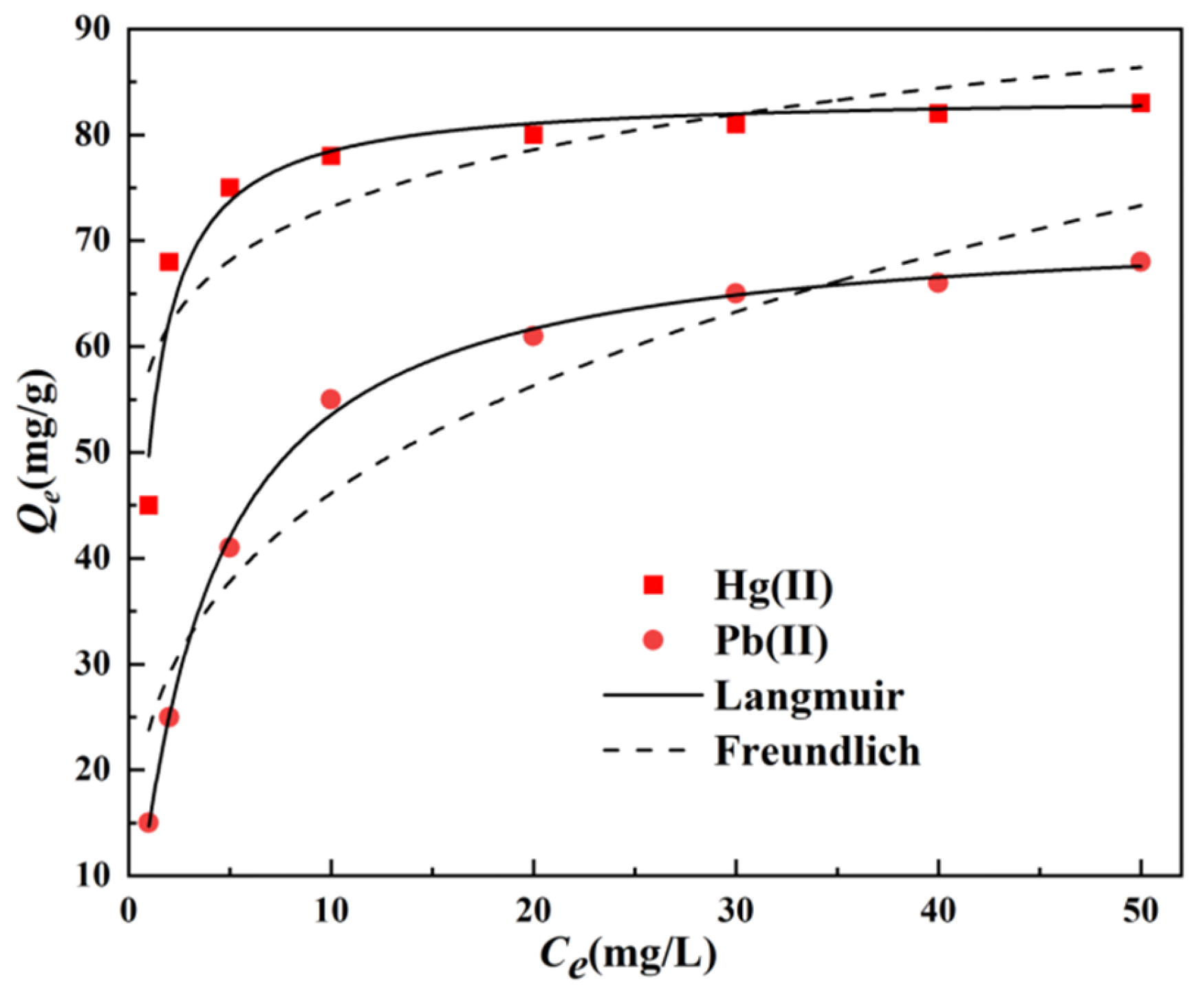
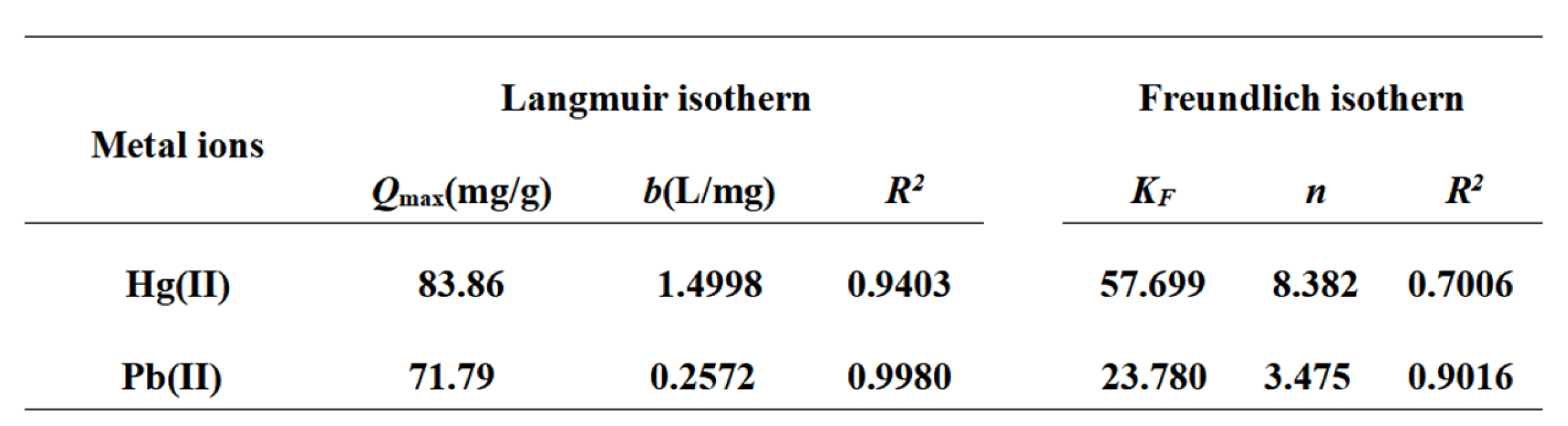
3.2.1. Saturated Adsorption Capacity and Thermodynamic Analysis
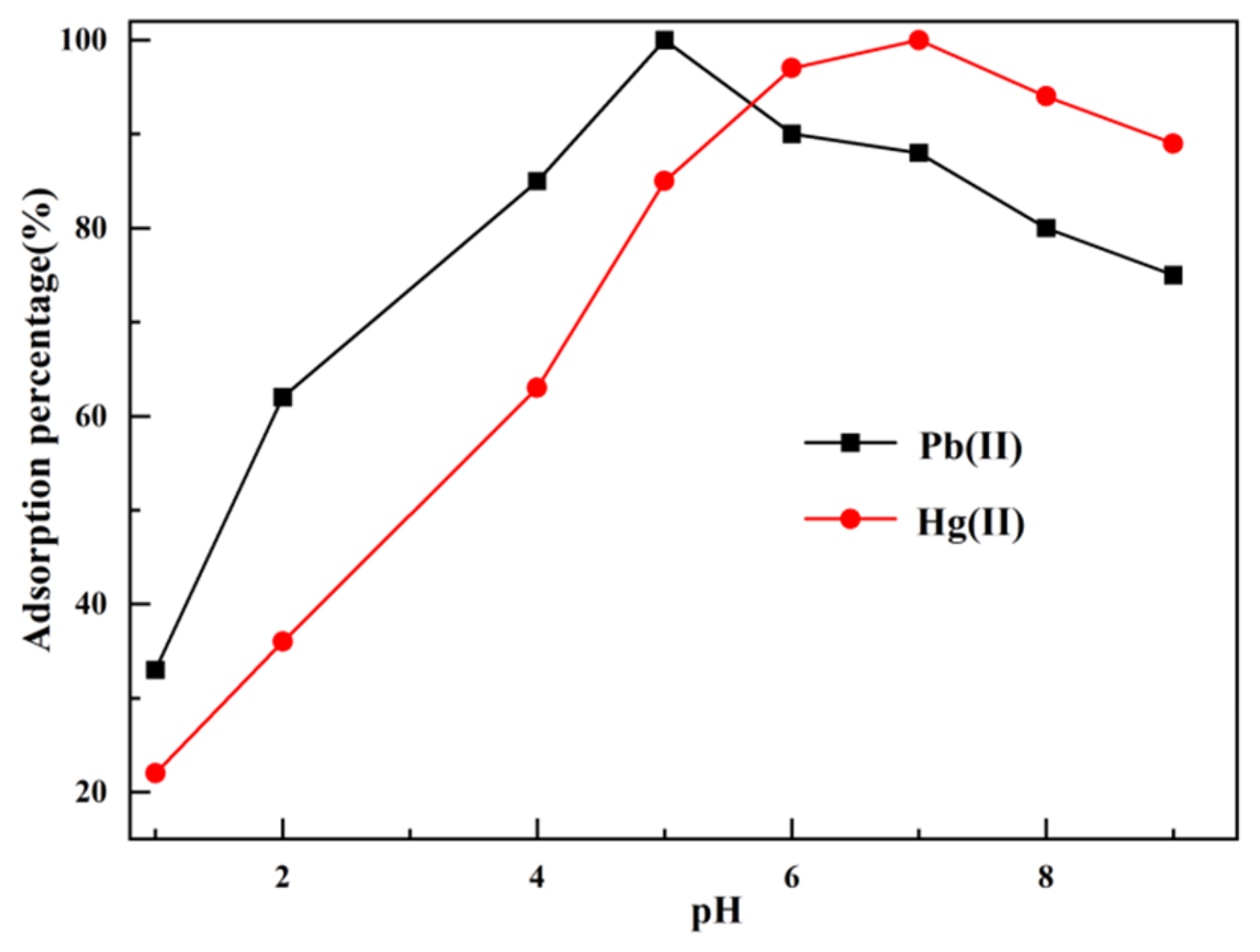
3.2.2. Effect of pH
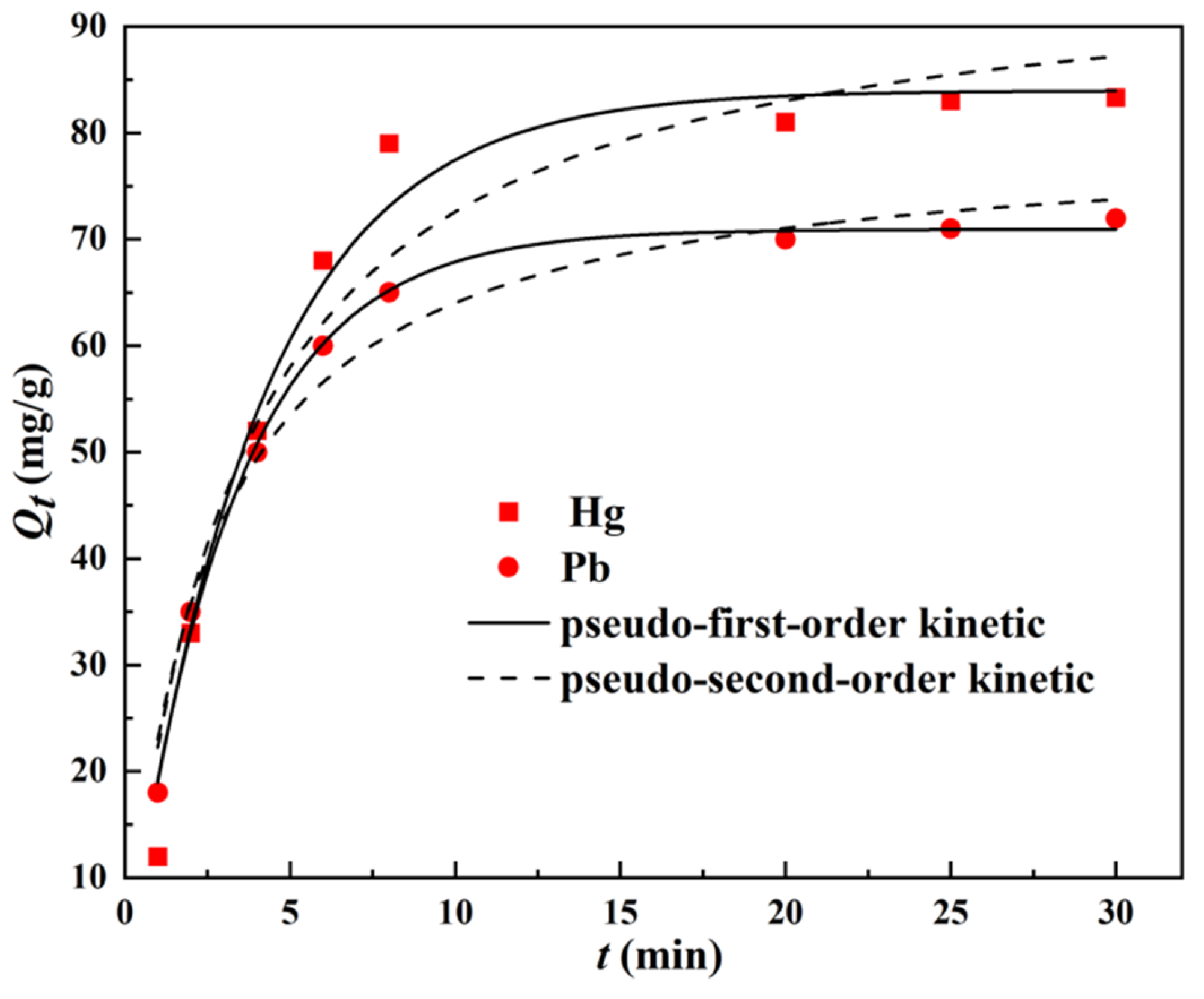
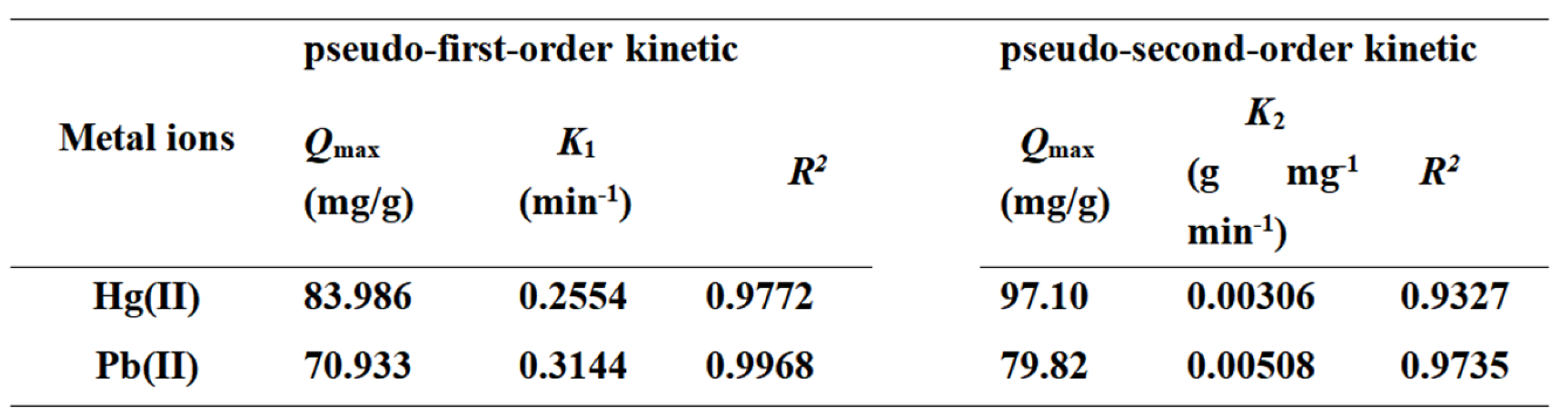
3.2.3. Research on Adsorption Kinetics
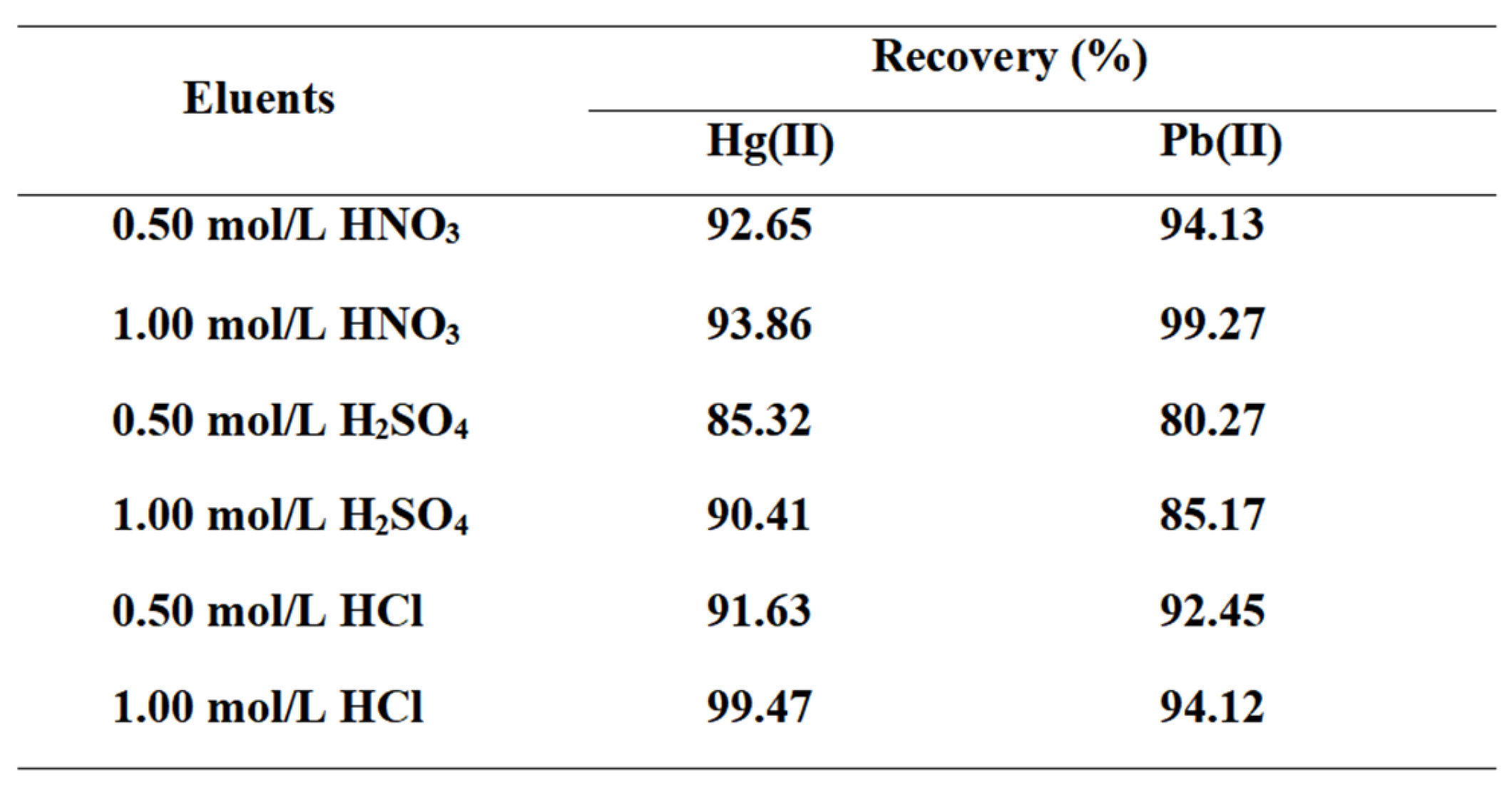
3.2.4. Selection of Eluents
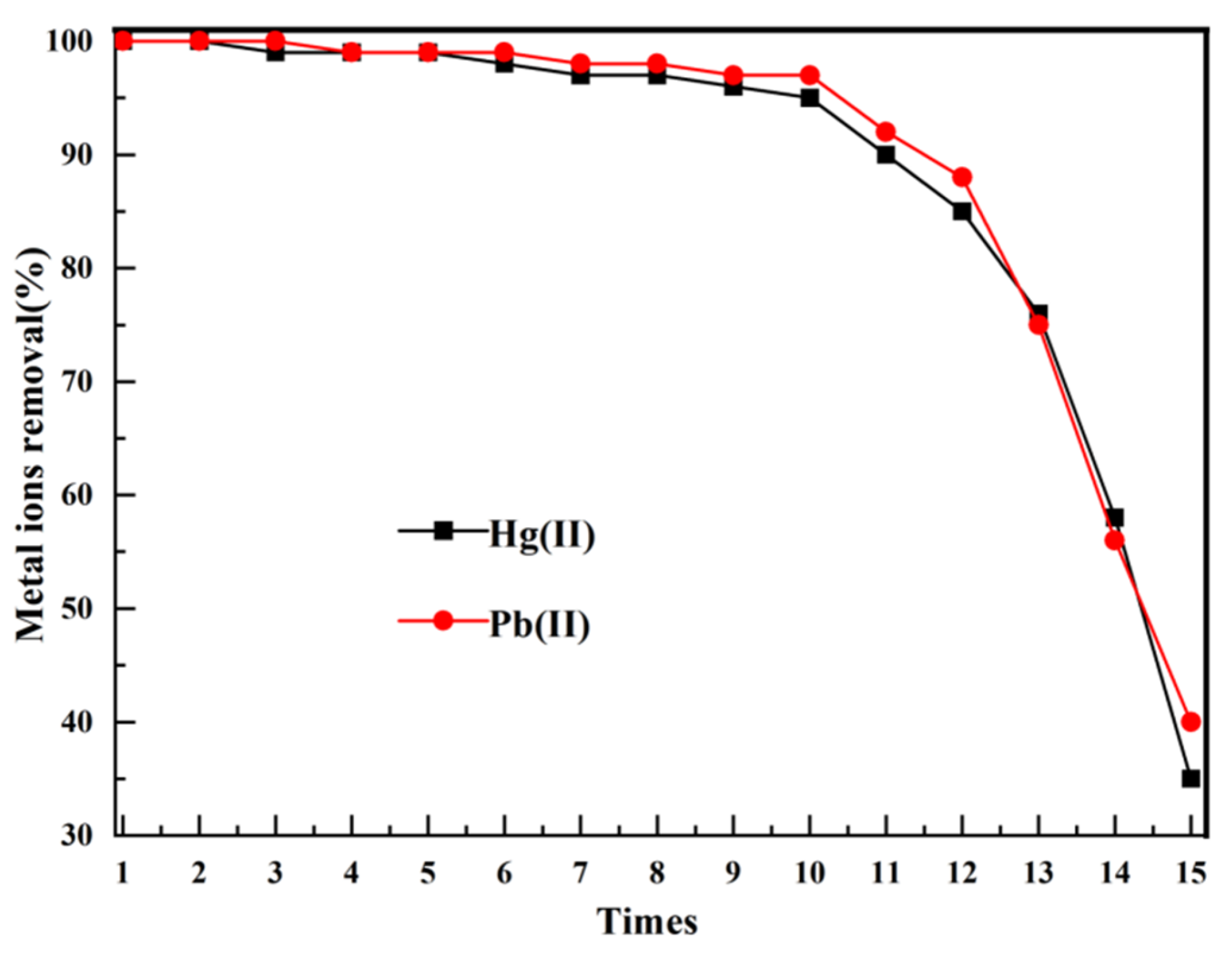
3.2.5. Reusability of Adsorbents
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Kotova, I.B.; Taktarova, Y.V.; Tsavkelova, E.A.; Egorova, M.A.; Bubnov, I.A.; Malakhova, D.V.; Shirinkina, L.I.; Sokolova, T.G.; Bonch-Osmolovskaya, E.A. Microbial Degradation of Plastics and Approaches to Make it More Efficient. Microbiology 2021, 90, 671–701. [CrossRef]
- Malinović, B.N.; Markelj, J.; Gotvajn, A..; Cigić, I.K.; Prosen, H. Electrochemical treatment of wastewater to remove contaminants from the production and disposal of plastics: a review. Environ. Chem. Lett. 2022, 20, 3765–3787. [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics – A review. Sci. Total. Environ. 2021, 797, 149140. [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption behaviour of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685. [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [CrossRef]
- Wang, J.; Li, J.; Gao, M.; Zhang, X. Recent advances in covalent organic frameworks for separation and analysis of complex samples. TrAC Trends Anal. Chem. 2018, 108, 98–109. [CrossRef]
- Chen, L.; Wu, Q.; Gao, J.; Li, H.; Dong, S.; Shi, X.; Zhao, L. Applications of covalent organic frameworks in analytical chemistry. TrAC Trends Anal. Chem. 2019, 113, 182–193. [CrossRef]
- Faraji, M.; Shirani, M.; Rashidi-Nodeh, H. The recent advances in magnetic sorbents and their applications. TrAC Trends Anal. Chem. 2021, 141, 116302. [CrossRef]
- Wang, Q. G. , Tian, H. , Lin, G. , Ya, L. , Wei, G. , Li, W. , Chun, W. , Zhi, W. , Qiu, H. Advances in magnetic porous organic frameworks for analysis and adsorption applications. Trends Anal. Chem. 2020, 132, 116048.
- Peng, M.; Zhu, Y.; Li, H.; He, K.; Zeng, G.; Chen, A.; Huang, Z.; Huang, T.; Yuan, L.; Chen, G. Synthesis and application of modified commercial sponges for oil-water separation. Chem. Eng. J. 2019, 373, 213–226. [CrossRef]
- Mohammadian, M. , Sahraei, R. , Ghaemy, M. Synthesis and fabrication of antibacterial hydrogel beads based on modified-gum tragacanth/poly (vinyl alcohol)/Ag0 highly efficient sorbent for hard water softening. Chemosphere 2019, 225, 259e269.
- Samadishadlou, M.; Farshbaf, M.; Annabi, N.; Kavetskyy, T.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A.; Mousavi, S. Magnetic carbon nanotubes: preparation, physical properties, and applications in biomedicine. Artif. Cells, Nanomedicine, Biotechnol. 2017, 46, 1314–1330. [CrossRef]
- Kui, L. U. , Guixia, Z. , Xiangke, W. A brief review of graphene-based material synthesis and its application in environmental pollution management. Chin. Sci. Bull. 2012, 57, 1223e1234.
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [CrossRef]
- Leandro, L.G. de Oliveira , F. A. C. , Suquila , E. , Costa de Figueiredo , M. G. , Segatelli , C. R.T. Restricted access material-ion imprinted polymer based method for on-line flow preconcentration of Cd2+ prior to flame atomic absorption spectrometry determination. Microchem. J. 2020, 157, 105022.
- Wang, J.; Li, J.; Gao, M.; Zhang, X. Recent advances in covalent organic frameworks for separation and analysis of complex samples. TrAC Trends Anal. Chem. 2018, 108, 98–109. [CrossRef]
- Chen, L.; Wu, Q.; Gao, J.; Li, H.; Dong, S.; Shi, X.; Zhao, L. Applications of covalent organic frameworks in analytical chemistry. TrAC Trends Anal. Chem. 2019, 113, 182–193. [CrossRef]
- Rasheed, T. Covalent organic frameworks as promising adsorbent paradigm for environmental pollutants from aqueous matrices: Perspective and challenges. Sci. Total. Environ. 2022, 833, 155279. [CrossRef]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent Progress on Layered Double Hydroxides and Their Derivatives for Electrocatalytic Water Splitting. Adv. Sci. 2018, 5, 1800064. [CrossRef]
- Silva, Ana Maria G.Pereira, Iaci M.Silva, Tamara, Ida Silva, Manoel R.Rocha, Renata A.Silva, Merces C. Magnetic foams from polyurethane and magnetite applied as attenuators of electromagnetic radiation in X band. J. Appl. Polym. Sci. 2021, 138, 49629.
- Malonzo, C. D. , Wang, Z. , Duan, J. , Zhao, W. , Webber, T. E. , Li, Z. Application and limitations of nanocasting in metaleorganic frameworks. Inorg. Chem. 2018, 57, 2782e2790.
- Bukhtiyarova, M. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2018, 269, 494–506. [CrossRef]
- Shen, Z.; Kuang, Y.; Zhou, S.; Zheng, J.; Ouyang, G. Preparation of magnetic adsorbent and its adsorption removal of pollutants: An overview. TrAC Trends Anal. Chem. 2023, 167. [CrossRef]
- Chen, D.; Sawut, A.; Wang, T. Synthesis of new functionalized magnetic nano adsorbents and adsorption performance for Hg(II) ions. Heliyon 2022, 8, e10528. [CrossRef]
- Chen, J.-K.; Wang, Y.-X.; Huang, C.-F.; Liu, H.-W. Acceleration of adsorption of heavy metal ion micelles onto iron/polyvinyltetrazole adsorbents with dielectrophoresis force under polarization. J. Water Process. Eng. 2024, 58. [CrossRef]
- Wang, Y.; Nakano, T.; Chen, X.; Xu, Y.-L.; He, Y.-J.; Wu, Y.-X.; Zhang, J.-Q.; Tian, W.; Zhou, M.-H.; Wang, S.-X. Studies on adsorption properties of magnetic composite prepared by one-pot method for Cd(II), Pb(II), Hg(II), and As(III): Mechanism and practical application in food. J. Hazard. Mater. 2024, 466, 133437. [CrossRef]
- Figueira, P.; Lopes, C.; Daniel-Da-Silva, A.; Pereira, E.; Duarte, A.; Trindade, T. Removal of mercury (II) by dithiocarbamate surface functionalized magnetite particles: Application to synthetic and natural spiked waters. Water Res. 2011, 45, 5773–5784. [CrossRef]
- Du, G. H. , Liu, Z. L. , Xia, X. , Chu, Q. , Zhang, S. M. Characterization and application of Fe3O4 /SiO2, nanocomposites. Journal of Sol-Gel Science and Technology. 2006, 39, 285-291.
- Shao, M. , Ning, F. , Zhao, J. , Wei, M. , Duan, X. Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins. Journal of the American Chemical Society. 2011, 134, 1071-1077.
- Chai, X. , Dong, H. , Zhang, Z. , Qi, Z. Y. , Chen, J. , Huang, Z. A novel Zr-MOF modified by 4,6-Diamino- 2-mercaptopyrimidine for exceptional Hg (II) removal. Journal of Water Process Engineering 2022, 46, 102606.
- Vajihe, N. , Islami, M. R. Adsorption capacity of heavy metal ions using sultonemodified magnetic activated carbon as a bio-adsorbent. Materials Science and Engineering 2019, 101, 42-52.
- Abdullah, N. , Mohd, F. A. , Jun, H. S. Recovery of waste cooking palm oil as a crosslinker for inverse vulcanized adsorbent to remove iron (Fe3+) ions. Journal of Environmental Chemical Engineering 2024, 12, 111853.
- Hania, A. , Ahmed, A. E. , Ahmed, E. , Hazim, Q. , Fares, A. A green route to the synthesis of highly porous activated carbon from walnut shells for mercury removal. Journal of Water Process Engineering 2024, 58, 104802.
- Hamouz, O. C. S. A. , Ali, S. A. Removal of heavy metal ions using a novel cross-linked polyzwitterionic phosphonate. Separation and Purification Technology 2012, 98, 94–101.
- Akar, T.; Alim, S.; Meltem, G.; Sayin, F.; Akar, S.T. A novel sustainable and eco-friendly biosourced hybrid sorbent for toxic Pb2+decontamination: Nano metal oxide functionalized salt-tolerant plant biomass. J. Clean. Prod. 2024, 439. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




