Submitted:
01 November 2024
Posted:
04 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Characterization of Accession Prospecting Sites/Provenances
2.2. Methodology for Sugar Contents Analysis
2.3. Data Processing and Statistical Analysis
3. Results
3.1. Analysis of Fruit Sugar Content of Accessions from Different Provenances
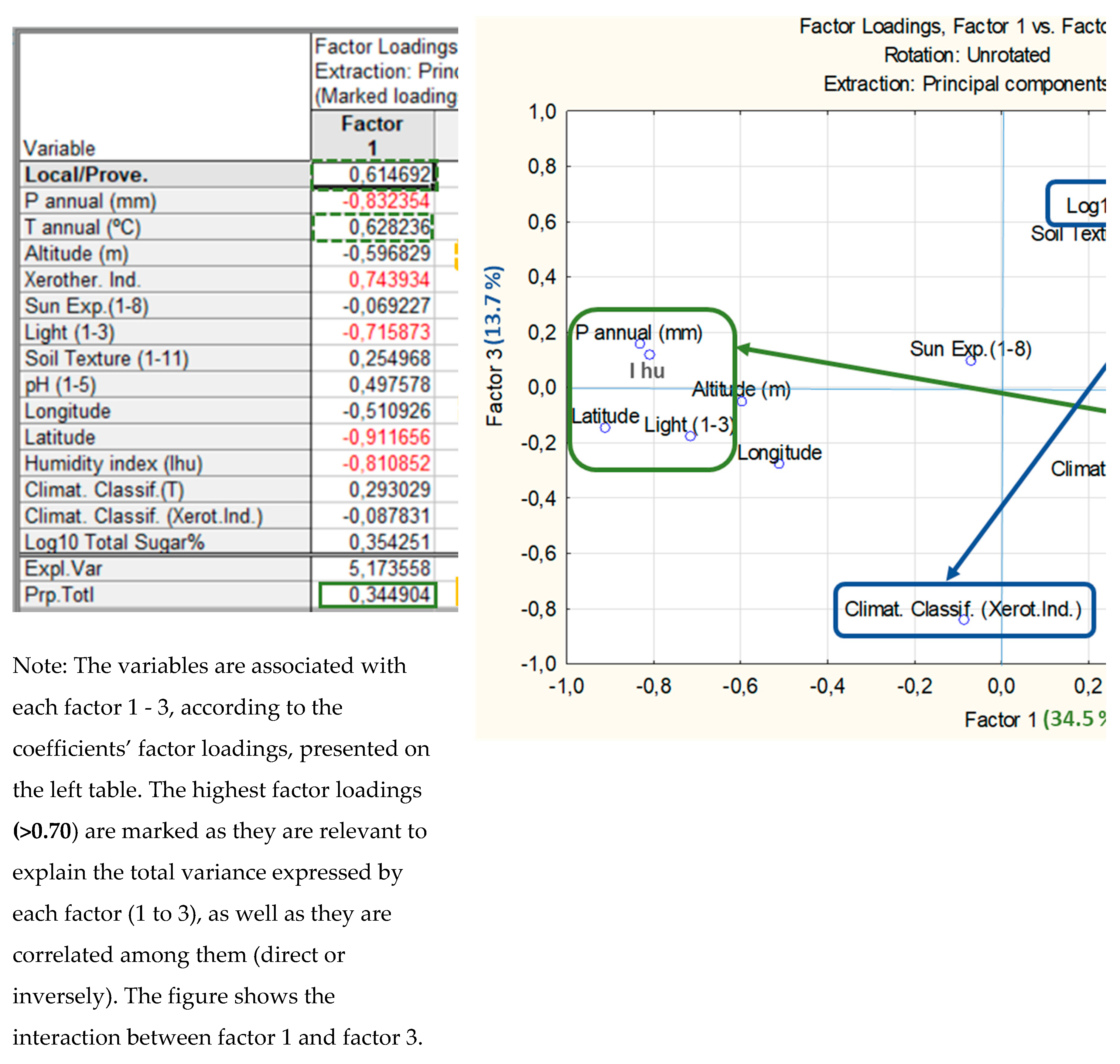
3.2. Sugar Content and Its Relation to Climatic Classifications and Edaphic Characteristics
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus Unedo L.: Chemical and Biological Properties. Molecules 2014, 19, 15799–15823. [CrossRef]
- Oliveira, I.; Baptista, P.; Bento, A.; Pereira, J.A. Arbutus Unedo L. and Its Benefits on Human Health. J. Food Nutr. Res 2011, 50, 73–85.
- Salem, I. Ben; Ouesleti, S.; Mabrouk, Y.; Landolsi, A.; Saidi, M.; Boulilla, A. Exploring the Nutraceutical Potential and Biological Activities of Arbutus Unedo L. (Ericaceae) Fruits. Ind Crops Prod 2018, 122, 726–731. [CrossRef]
- Fortalezas, S.; Tavares, L.; Pimpão, R.; Tyagi, M.; Pontes, V.; Alves, P.M.; Mcdougall, G.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant Properties and Neuroprotective Capacity of Strawberry Tree Fruit (Arbutus Unedo). Nutrients 2010, 2, 214–229. [CrossRef]
- Hileman, L.C.; Vasey, M.C.; Thomas Parker, V. Phylogeny and Biogeography of the Arbutoideae (Ericaceae): Implications for the Madrean-Tethyan Hypothesis. Syst Bot 2001, 26, 131–143. [CrossRef]
- Torres, J.A.; Valle, F.; Pinto, C.; Garcia-Fuentes, A.; Salazar, C.; Cano, E. Arbutus Unedo Communities in Southern Iberian Peninsula Mountains. Plant Ecol. 2002, 160, 207–223. [CrossRef]
- Konstantinidis, P.; Tsiourlis, G.; Xofis, P. Effect of Fire Season, Aspect and Pre-Fire Plant Size on the Growth of Arbutus Unedo L. (Strawberry Tree) Resprouts. For Ecol Manage 2006, 225, 359–367. [CrossRef]
- Gomes, B.; Castro, F.; Santos, R.; Figueiredo, P.; Silva, M.; Vidal, M.; Ferreira, I.; Nunes, J.; Machado, H.; Gomes, F. Effect of Quercetin on Mycorrhizal Synthesis between Tuber Borchii and Arbutus Unedo L. In Vitro Plants. Microbiology Research 2021, 12.
- Richard, F.; Selosse, M.-A.; Gardes, M. Facilitated Establishment of Quercus Ilex in Shrub-Dominated Communities within a Mediterranean Ecosystem: Do Mycorrhizal Partners Matter? . FEMS Microbiol Ecol 2009, 68, 14–24. [CrossRef]
- Azcón-Aguilar, C.; Palenzuela, J.; Roldán, A.; Bautista, S.; Vallejo, R.; Barea, J.M. Analysis of the Mycorrhizal Potential in the Rhizosphere of Representative Plant Species from Desertification-Threatened Mediterranean Shrublands; 2003; Vol. 22;.
- Arnan, X.; Quevedo, L.; Rodrigo, A. Forest Fire Occurrence Increases the Distribution of a Scarce Forest Type in the Mediterranean Basin. Acta Oecologica 2013, 46, 39–47. [CrossRef]
- Oliveira, A.S.; Silva, J.S.; Guiomar, N.; Fernandes, P.; Nereu, M.; Gaspar, J.; Lopes, R.F.R.; Rodrigues, J.P.C. The Effect of Broadleaf Forests in Wildfire Mitigation in the WUI – A Simulation Study. International Journal of Disaster Risk Reduction 2023, 93. [CrossRef]
- Floris, I.; Satta, A.; Ruiu, L. Honeys of Sardinia (Italy). J Apic Res 2007, 46, 198–209. [CrossRef]
- Botelho, G.; Gomes, F.; Ferreira, F.M.; Caldeira, I. Influence of Maturation Degree of Arbutus (Arbutus Unedo L.) Fruits in Spirit Composition and Quality. International Scholarly and Scientific Research & Innovation 2015, 9 (6), 615–620.
- Ulloa, P.A.; Maia, M.; Brigas, A.F. Physicochemical Parameters and Bioactive Compounds of Strawberry Tree (Arbutus Unedo L.) Honey. J Chem 2015, 2015. [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; Del Castillo, M.D.; Cano, M.P.; Pascual-Teresa, S. Characterization of the Antioxidant Composition of Strawberry Tree (Arbutus Unedo L.) Fruits. J. Food Compos. Anal. 2008, 21, 273–281. [CrossRef]
- Tavares, L.; Fortalezaa, S.; Carrilho, C.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C. Antioxidant and Antiproliferative Properties of Strawberry Tree Tissues. Journal Berry Research 2010, 1, 3–12. [CrossRef]
- Scarano, P.; Guida, R.; Zuzolo, D.; Tartaglia, M.; Prigioniero, A.; Postiglione, A.; Pinto, G.; Illiano, A.; Amoresano, A.; Schicchi, R.; et al. An Endemic Plant of the Mediterranean Area: Phytochemical Characterization of Strawberry Tree (Arbutus Unedo L.) Fruits Extracts at Different Ripening Stages. Front Nutr 2022, 9. [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; Casado, N.; Sierra, I. Study of the Phenolic Compound Profile of Arbutus Unedo L. Fruits at Different Ripening Stages by HPLC-TQ-MS/MS. Applied Sciences (Switzerland) 2021, 11. [CrossRef]
- Fonseca, D.F.S.; Salvador, Â.C.; Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Silvestre, A.J.D.; Rocha, S.M. Bioactive Phytochemicals from Wild Arbutus Unedo L. Berries from Different Locations in Portugal: Quantification of Lipophilic Components. Int J Mol Sci 2015, 16. [CrossRef]
- Selen Isbilir, S.; Hulya Orak, H.; Yagar, H.; Ekinci, N. Determination of Antioxidant Activities of Strawberry Tree (Arbutus Unedo L.) Flowers and Fruits at Different Ripening Satges; 2012; Vol. 11;.
- Marques, M.P.; Martin, D.; Bosch, M.; Martins, J.; Biswal, A.; Zuzarte, M.; de Carvalho, L.B.; Canhoto, J.; da Costa, R. Unveiling the Compositional Remodelling of Arbutus Unedo L. Fruits during Ripening. Sci Hortic 2022, 303. [CrossRef]
- Özcan, M.M.; Haciseferogullan, H. The Strawberry (Arbutus Unedo L.) Fruits: Chemical Composition, Physical Properties and Mineral Contents. J Food Eng 2007, 78, 1022–1028. [CrossRef]
- Ruiz-Rodríguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of Wild Strawberry-Tree Fruits (Arbutus Unedo L.) through Nutritional Assessment and Natural Production Data. Food Research International 2011, 44, 1244–1253. [CrossRef]
- Oliveira, I.; Pinho, P.G.; Malheiro, R.; Baptista, P.; Pereira, J.A. Volatile Profile of Arbutus Unedo L. Fruits through Ripening Stage. Food Chem 2011, 128, 667–673. [CrossRef]
- Celikel, G.; Demirsoy, L.; Demirsoy, H. The Strawberry Tree (Arbutus Unedo L.) Selection in Turkey. Sci. Hort. 2008, 118, 115–119. [CrossRef]
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Influence of Strawberry Tree (Arbutus Unedo L.) Fruit Ripening Stage on Chemical Composition and Antioxidant Activity. Food Research International 2011, 44, 1401–1407. [CrossRef]
- Morales, D. Use of Strawberry Tree (Arbutus Unedo) as a Source of Functional Fractions with Biological Activities. Foods 2022, 11. [CrossRef]
- Botelho, G.; Anjos, O.; Estevinho, L.M.; Caldeira, I. Methanol in Grape Derived, Fruit and Honey Spirits: A Critical Review on Source, Quality Control, and Legal Limits. Processes 2020, 8, 1–21. [CrossRef]
- Galego, L.; Botelho, G.; Da Silva, J.P. Arbutus Unedo L. Fruit Distillates and the Requirement for Further Quality Specifications. In Proceedings of the 12th Meeting on Food Chemistry; Lisboa, 2014; p. 191.
- Caldeira, I.; Gomes, F.; Mira, H.; Botelho, G. Distillates Composition Obtained of Fermented Arbutus Unedo L. Fruits from Different Seedlings and Clonal Plants. Annals of Agricultural Sciences 2019, 64, 21–28. [CrossRef]
- Franco, J. O Medronho - Da Planta Ao Fruto, as Práticas Culturais. Actas Portuguesas de Horticultura - Jornadas do Medronho 2012, 22, 18–25.
- Šic Žlabur, J.; Bogdanović, S.; Voća, S.; Skendrović Babojelić, M. Biological Potential of Fruit and Leaves of Strawberry Tree (Arbutus Unedo L.) from Croatia. Molecules 2020, 25, 5102. [CrossRef]
- Pato, R.L.; Botelho, G.; Franco, J.; Santos, S.; Ressurreição, S.; Figueiredo, P.; Gama, J.; Gomes, F. Interaction between Farming Type, Nutrient Uptake and Plant Material in Strawberry Tree Fruit Production and Quality. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, January 31 2022; pp. 275–284.
- INIAV Plano Nacional Para Os Recursos Genéticos Vegetais; Ministério da Agricultura e do Mar, Ed.; INIAV: Lisboa, 2015;
- Ribeiro, M.M.; Piotti, A.; Ricardo, A.; Gaspar, D.; Costa, R.; Parducci, L.; Vendramin, G.G. Genetic Diversity and Divergence at the Arbutus Unedo L. (Ericaceae) Westernmost Distribution Limit. PLoS One 2017, 1–15.
- Gomes, F.; Costa, R.; Ribeiro, M.M.; Figueiredo, E.; Canhoto, J.M. Analysis of Genetic Relationship among Arbutus Unedo L. Genotypes Using RAPD and SSR Markers. J For Res (Harbin) 2013, 24, 227–236. [CrossRef]
- Gomes, F.; Simões, M.; Lopes, M.L.; Canhoto, J.M. Effect of Plant Growth Regulators and Genotype on the Micropropagation of Adult Trees of Arbutus Unedo L. (Strawberry Tree). N Biotechnol 2010, 27, 882–892. [CrossRef]
- Gomes, F.; Gama, J.; Figueiredo, P.; Clemente, M.; Plácito, F.; Pato, R.L.; Botelho, G.; Franco, J.; Nazaré, N.; Guilherme, R.; et al. Avaliação de Clones de Arbutus Unedo L.: Apresentação de Resultados. In Proceedings of the II Jornadas do Medronho; Gomes, F., Sousa, R.M., Guilherme, R., Eds.; Actas Portuguesas de Horticultura: Coimbra, 2015; Vol. 24, pp. 15–23.
- Soares da Silva, A.M. Carta Litológica – Notícia Explicativa I.13; Atlas do Ambiente, Estação Agronómica Nacional, C.N. do A., Ed.; Lisboa, 1983;
- IUSS Working Group WRB World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; International Union of Soil Sciences (IUSS), Ed.; 4th ed.; Vienna, 2022;
- Cardoso, J.C.; Bessa, M.T.; Marado, M.B. Carta Dos Solos de Portugal (1: 1 000 000). Sep. da Agronomia Lusitana 1973, 33, 481–602.
- INMG O Clima de Portugal. Normais Climatológicas Correspondentes a 1951-1980 (1a, 2a, 3a e 4a Regiões).; INMG: Lisboa, 1991;
- Alcoforado, M.J.; Alegria, M.F.; Ramos-Pereira, A.; Sirgado, C. Domínios Bioclimáticos Em Portugal Definidos Por Comparação Dos Índices de Gaussen e de Emberger; Centro de Estudos Geográficos Universidade de Lisboa, Ed.; 3aEd.; Lisboa, 2009; ISBN 9789726361930.
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. In Geographical Review; 1948; Vol. 38, pp. 55–94. [CrossRef]
- Nunes, A.S.; Castro, F.; Simões, M.; Botelho, G.; Gomes, F.; Franco, J. Caracterização Dos Estados Fenológicos e Da Produção de Clones de Medronheiro. Actas Portuguesas de Horticultura 2022, 35, 27–34. [CrossRef]
- Bento, V.A.; Russo, A.; Vieira, I.; Gouveia, C.M. Identification of Forest Vulnerability to Droughts in the Iberian Peninsula. Theor Appl Climatol 2023, 152, 559–579. [CrossRef]
- Jafari, M.; Tavili, A.; Panahi, F.; Zandi Esfahan, E.; Ghorbani, M. Chapter 1 Introduction. In Reclamation of Arid Lands; Jafari, M., Tavili, A., Panahi, F., Zandi Esfahan, E., Ghorbani, M., Eds.; Springer International Publishing: Cham, 2018; pp. 1–19 ISBN 978-3-319-54828-9.
- Thornthwaite, C.W.; Mather, J.R. The Water Balance. In Publications in climatology; Drexel Institute of Technology, 1955; Vol. 8, pp. 5–86.
- Nunes, A.S.; Castro, F.; Simões, M.; Gomes, F.; Botelho, G.; Franco, J. AGROTEC Revista técnico-científica agrícola No 34. Suplemento Pequenos Frutos. Porto March 2020, pp. 4–6.
- Fleckinger, J. Notations Phénologiques et Représentations Graphiques Du Dévelopment Des Bourgeons de Poirier. In Proceedings of the Congrès de Paris de L ’Association française pour l’avancement des Sciences; Paris, 1945; p. 118.
- Baggiolini, M. Les Stades Repères Dans Le Développement Annuel de La Vigne et Leur Utilisation Pratique. Rev. Rom. Agric. 1952, 8–10.
- Anastácio, J. Contributo Para o Estudo Do Medronheiro (Arbutus Unedo L.): Caracterização Morfológica de Clones e Fisiologia Pós-Colheita Do Fruto. Master’s in Agricultural Engineering, Instituto Superior de Agronomia: Lisboa, 2014.
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Fifth edit.; Pearson Education Limited: Edinburgh, 2005; ISBN 0 131 29192 0.
- Caldeira, I.; Gomes, F.; Botelho, G. Arbutus Unedo L. Spirit: Does the Water Addition Before Fermentation Matters? In INCREaSE; Mortal, A. et al., Ed.; Springer International Publishing: Cham, 2018; pp. 206–215 ISBN 978-3-319-70271-1.
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, Non-Volatile and Phenolic Acids Composition of Strawberry Tree (Arbutus Unedo L. Var.Ellipsoidea ) Fruits. J. Food Compos. Anal. 2000, 13, 171–177. [CrossRef]
- Seker, M.; Toplu, C. Determination and Comparison of Chemical Characteristics of Arbutus Unedo L. and Arbutus Andrachnae L. (Family Ericaceae) Fruits. J Med Food 2010, 13, 1013–1018. [CrossRef]
- Vidrih, R.; Hribar, J.; Prgomet, Ž.; Ulrih, N.P. The Physico-Chemical Properties of Strawberry Tree (Arbutus Unedo L.) Fruits. Croatian Journal of Food Science and Technology 2013, 5, 29–33.
- Doukani, K.; Tabak, S. Profil Physicochimique Du Fruit “Lendj” (Arbutus Unedo L.). Nature & Technologie».B-Sciences Agronomiques et Biologiques 2015, 12, 51–64.
- Alarcão e Silva, M.L.; Leitão, A.E.; Azinheira, H.G.; Leitão, M.C. The Arbutus Berry: Studies on Its Color and Chemical Characteristics at Two Mature Stages. J. Food Comp. Anal. 2001, 14, 27–35. [CrossRef]
- Boussalah, N.; Boussalah, D.; Cebadera-Miranda, L.; Fernández-Ruiz, V.; Barros, L.; Ferreira, I.C.F.R.; Cortes Sanchez Mata, M.; Madani, K. Nutrient Composition of Algerian Strawberry-Tree Fruits (Arbutus Unedo L.). Fruits 2018, 73, 283–297. [CrossRef]
- Ait Lhaj, Z.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan Strawberry Tree (Arbutus Unedo L.) Fruits: Nutritional Value and Mineral Composition. Foods 2021, 10. [CrossRef]
- Molina, M.; Pardo-De-Santayana, M.; Aceituno, L.; Morales, R.; Tardio, J. Fruit Production of Strawberry Tree (Arbutus Unedo L.) in Two Spanish Forests. Forestry 2011, 84, 419–429. [CrossRef]
- Duarte, R.; Castro, F.; Ramos, I.; Antunes, C.; Figueiredo, P.; Franco, J.; Gama, J.D.; Silva, J.P.; Balseiro, M.; Chá, L.C.; et al. Apresentação de Resultados de Ensaios Instalados No Campo Com Plantas Clonais e Seminais de Medronheiro. In Proceedings of the Congresso Nacional dos Recursos Silvestres 2023; Escola Superior Agrária de Bragança: Bragança, October 18 2023; p. 37.
- Molina, R. Pure Culture Synthesis and Host Specificity of Red Alder Mycorrhizae - :’’: In Future Forest Management ( Tarrant and Found Only Six Types Associated with Three Japanese Alder Species ; Mejstrik and Benecke ( 1969 ) Report Only Three Types on Alnus Viridi. 1979, 1223–1228.
- Gomes, F.; Franco, J.; Pato, R.L.; Botelho, G.; Rodrigues, I.; Figueiredo, P.; Casau, F. Produção de Medronho Para Destilar. In Medronheiro, Caderno Técnico; Pestana, M., Ed.; INIAV, Silva Lusitana: Lisboa, 2017; pp. 5–33 ISBN 978-972-579-045.
- ICNF 6o. Inventário Florestal Nacional. 2015 Relatório Final; ICNF, Ed.; ICNF: Lisboa, 2019;
- ICNF 6o Inventário Florestal Nacional, Áreas Dos Usos Do Solo e Das Espécies Florestais de Portugal Continental. Resultados Preliminares; Instituto da Conservação da Natureza e das Florestas: Lisboa, 2013;
- Oliveira, A.S. De; Silva, J.S. Is Native Forest an Alternative to Prevent Wildfire in the WUI in Central Is Native Forest an Alternative to Prevent Wildfire in the WUI in Central Portugal ? 2021.
- Martins, J.; Pinto, G.; Canhoto, J. Biotechnology of the Multipurpose Tree Species Arbutus Unedo: A Review. J For Res (Harbin) 2022, 33, 377–390. [CrossRef]
- Martins, J.; Correia, S.; Pinto, G.; Canhoto, J. Cloning Adult Trees of Arbutus Unedo L. through Somatic Embryogenesis. Plant Cell Tissue Organ Cult 2022, 150, 611–626. [CrossRef]
- Gomes, F.; Canhoto, J.M. Micropropagation of Strawberry Tree (Arbutus Unedo L.) from Adult Plants. In Vitro Cell. Dev. Biol.-Plant 2009, 45, 72–82. [CrossRef]
- Gomes, F.; Botelho, G.; Franco, J.; Gama, J.; João, C.; Santos, R.; Figueiredo, P. Assessment of Arbutus Unedo L. Clonal Plants in a Field Clonal Trial. In Proceedings of the IUFRO Forest Tree Breeding Conference. Program and Abstract Book; IUFRO, Ed.; Prague, Czech Republic, 25-29 August, 2014; pp. 29, ISBN: 978-80-213-2471–2478.
- Figueiredo, P.; Gomes, F.; Santos, R.; Pop, R.L. Rapid Propagation of Arbutus Unedo L. Adult Selected Plants Using Ex Vitro Rooting. In Proceedings of the 8th International Symposium on In Vitro Culture and Horticultural Breeding. Program and Abstract Book; Canhoto, J., Correia, S., Eds.; Coimbra, Portugal, June 2-7, 2013; p. 157.
- Guerreiro, A.C.; Gago, C.M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Temperature and Film Covers on the Storage Ability of Arbutus Unedo L. Fresh Fruit. Sci Hortic 2013, 159, 96–102. [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Alginate Based Edible Coatings Enriched with Essential Oils Constituents on Arbutus Unedo L. Fresh Fruit Storage. Postharvest Biol Technol 2015, 100, 226–233. [CrossRef]
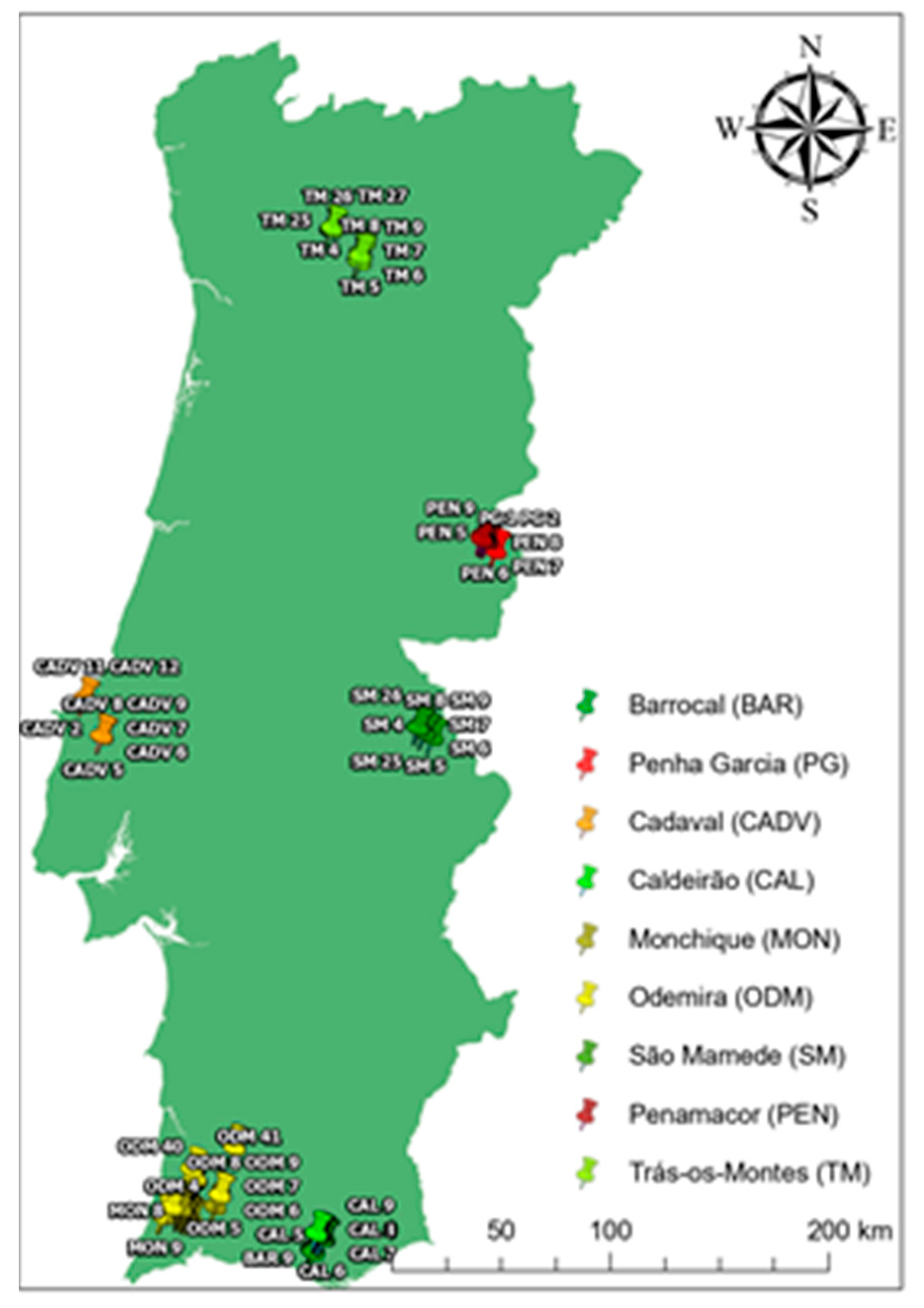
| Local / Provenance | N° | Lithology1 | pH2 | T annual (°C)3 | P annual (mm)3 | Humidity index | Xeroth. Index | Climatic classification4 | Climatic Classif.5 |
|---|---|---|---|---|---|---|---|---|---|
| TM-Fisgas Ermelo/FE | 6 | Shales, Quarzites | 2 | 13.4 | 1390.5 | 111.51 | 42.40 | Sub-Mediterranean | B4 B´2 s a´ |
| TM -Mosteiró | 19 | Shales | 3 | 13.4 | 1128.1 | 84.69 | 60.96 | Attenuated Mesomed4.1 | B3 B´2 s2 a´ |
| São Mamede | 26 | Shales, Sandst.1.1 | 2 | 15.2 | 852.4 | 45.41 | 71.38 | Attenuated Mesomed4.1 | B1 B´2 s2 a´ |
| Penamacor | 24 | Shales, Quarzites | 2 | 14.4 | 838.2 | 47.80 | 98.64 | Accentuated Mesomed4.1 | B1 B´2 s2 a´ |
| Penha Garcia* | 2 | Shales | 3 | 14.4 | 838.2 | 47.80 | 98.64 | Accentuated Mesomed4.1 | B1 B´2 s2 a´ |
| Cadaval* | 4 | Gonglomerates1.2 Sand1.1 | 3 | 15.0 | 777.6 | 40.20 | 86.64 | Accentuated Mesomed4.1 | C2 B´2 s a´ |
| Monchique* | 25 | Syenite & Shale | 2 | 15.1 | 949.0 | 60.22 | 87.36 | Accentuated Mesomed4.1 | B1 B´2 s2 a´ |
| ODM -Pomba* | 16 | Shale, graywacke | 3 | 15.8 | 715.7 | 34.27 | 87.52 | Accentuated Mesomed4.1 | C2 B´2 s2 a´ |
| ODM -Cerca Pomares* | 3 | Shale, graywacke | 3 | 15.0 | 760.5 | 35.69 | 88.96 | Accentuated Mesomed4.1 | C2 B´2 s a´ |
| ODM -Trancão* | 9 | Shale, graywacke | 3 | 15.0 | 571.9 | 15.73 | 85.04 | Accentuated Mesomed4.1 | C1 B´2 s a´ |
| ODM -Nave Redondo* | 10 | Shale, graywacke | 3 | 15.1 | 748.9 | 40.03 | 90.56 | Accentuated Mesomed4.1 | C2 B´2 s2 a´ |
| ODM -Cortes Pereira* | 15 | Shale, graywacke | 3 | 15.8 | 623.1 | 22.08 | 83.52 | Accentuated Mesomed4.1 | C1 B´2 s2 a´ |
| Caldeirão | 25 | Shale, graywacke | 3 | 15.9 | 866.7 | 51.40 | 95.90 | Accentuated Mesomed4.1 | B1 B´2 s2 a´ |
| Barrocal | 20 | Limestone | 4 | 15.9 | 697.0 | 33.96 | 118.18 | Attenuated Thermomed4.2 | C2 B´2 s2 a´ |
| *Cultivated and wild plants identified with the Producers' support. 1Lithology according to lithological map [40] associated, in general (according to Soil Classification WRB [41]), to Leptosols and Cambisols [42]. 1.1Shales and sandstones; 1.2Gonglomerates and sandstones. 2pH soil classification mean per provenance: 1- very low (<4); 2- low (4-5.4); 3- medium (5.5-7.4); 4- hight (7.5-9); 5- very hight (>9). 3Annual averages of temperature (°C) and precipitation (mm), corresponding to 1951-1980 period [43]. 4Climatic classification according to xerothermic index [44]. 4.1Mesomediterranean; 4.2Thermomediterranean. 5Climatic classification according to Thornthwaite [45] present in the table: 1st letter (Hydric index - IH): i) humid and rainy climates B4, B3 e C2 – respectively very humid, humid and subhumid vs. ii) dry climates C1 - Dry subhumid. 2nd letter (Thermal efficiency (∑ ETP): B’2 – mesothermic. 3rd letter for mediterranean climate: i) Humid and subhumid climate group (1st Letter A, B or C2): - s (moderate water deficiency in summer); s2 (severe scarcity of water in the summer); ii) Dry climate group (1st letter C1 or D): s (moderate excess water in winter); s2 (high water excesses in the winter season). 4th letter (concentration of thermal efficiency in the hot season): a’ - small concentration of thermal efficiency during summer. | |||||||||
| Local / Provenance | N° | Maltose | Sucrose | Glucose | Fructose | Reducing sugar | Total sugar |
|---|---|---|---|---|---|---|---|
| (Mean ± SE g/100g, fresh pulp fruit) | |||||||
| TM-Fisgas Ermelo | 6 | 0.87 ± 0.30 d | 0.00 ± 0.00 c | 4.33 ± 0.10 b-d | 8.66 ± 0.43 d-f | 13.86 ± 0.71 c-e | 13.86 ± 0.71 de |
| TM-Mosteiró | 19 | 1.24 ± 0.23 cd | 0.12 ± 0.07 b | 3.98 ± 0.11 cd | 8.54 ± 0.32 ef | 13.77 ± 0.55 de | 13.89 ± 0.58 e |
| São Mamede | 26 | 0.63 ± 0.13 d | 0.85 ± 0.11 b | 5.13 ± 0.55 b-d | 10.42 ± 1.14 c-f | 16.18 ± 1.76 de | 17.04 ± 1.81 de |
| Penamacor | 24 | 0.61 ± 0.14 d | 0.74 ± 0.12 b | 5.14 ± 0.22 bc | 11.70 ± 0.39 b-d | 17.45 ± 0.58 cd | 18.19 ± 0.63 cd |
| Penha Garcia* | 2 | 0.00 ± 0.00 de | 0.00 ± 0.00 c | 4.13 ± 0.04 b-d | 12.26 ± 0.20 a-d | 16.39 ± 0.16 b-d | 16.39 ± 0.16 a-d |
| Cadaval* | 4 | 0.81 ± 0.27 d | 0.45 ± 0.45 ab | 4.72 ± 0.29 b-d | 12.16 ± 0.61 a-d | 17.70 ± 1.03 a-d | 18.15 ± 0.73 a-d |
| Monchique* | 25 | 0.67 ± 0.13 d | 1.58 ± 0.15 a | 6.80 ± 0.26 a | 12.29 ± 0.61 a-d | 19.77 ± 0.88 a-c | 21.34 ± 0.97 ac |
| ODM-Pomba* | 16 | 2.08 ± 0.16 bc | 0.16 ± 0.09 b | 4.91 ± 0.28 b-d | 12.66 ± 0.60 a-c | 19.65 ± 0.86 a-c | 19.81 ± 0.89 a-d |
| ODM-Cerca Pomares* | 3 | 0.96 ± 0.50 cd | 0.32 ± 0.32 b | 3.77 ± 0.11 b-d | 10.82 ± 0.06 b-d | 15.54 ± 0.43 b-d | 15.86 ± 0.61 b-d |
| ODM-Trancão* | 9 | 2.06 ± 0.30 bc | 0.32 ± 0.16 b | 4.16 ± 0.30 b-d | 11.31 ± 0.61 b-d | 17.54 ± 0.85 b-d | 17.85 ± 0.88 b-d |
| ODM-Nave Redondo* | 10 | 3.30 ± 0.96 ab | 0.66 ± 0.36 ab | 6.03 ± 0.40 ab | 15.33 ± 1.28 ab | 24.66 ± 1.63 ab | 25.31 ± 1.57 ab |
| ODM - Cortes Pereira* | 15 | 3.73 ± 0.53 a | 0.51 ± 0.17 b | 5.82 ± 0.39 ab | 17.01 ± 1.46 a | 26.56 ± 2.31 a | 27.07 ± 2.30 a |
| Caldeirão | 25 | 0.41 ± 0.12 d | 0.97 ± 0.14 ab | 4.93 ± 0.11 b-d | 11.78 ± 0.33 b-d | 17.12 ± 0.43 cd | 18.08 ± 0.45 cd |
| Barrocal | 20 | 1.02 ± 0.15 d | 0.39 ± 0.14 b | 4.03 ± 0.25 cd | 7.89 ± 0.55 f | 12.95 ± 0.79 e | 13.34 ± 0.84 e |
| Total / Global mean | 204 | 1.25 ± 0.10 | 0.67 ± 0.05 | 5.07 ± 0.11 | 11.49 ±0.28 | 17.81 ± 0.43 | 18.47 ± 0.44 |
| Climatic Classification | N | Maltose | Sucrose | Glucose | Fructose | Reducing sugar | Total sugar |
|---|---|---|---|---|---|---|---|
| (Mean ± SE g/100g, fresh pulp fruit) | |||||||
| Accentuated Mesomediterranean | 133 | 1.42 ± 0.15a | 0.78 ± 0.07 a | 5.40 ± 0.12 a | 12.79 ± 0.29a | 19.61 ± 0.46 a | 20.39 ± 0.47 a |
| Attenuated Mesomediterranean | 45 | 0.89 ± 0.13b | 0.55 ± 0.09 a | 4.64 ± 0.33 ab | 9.63 ± 0.68b | 15.16 ± 1.05 b | 15.71 ± 1.09 b |
| Attenuated Thermomediterranean | 20 | 1.02 ± 0.15ab | 0.39 ± 0.14 a | 4.03 ± 0.25 c | 7.89 ± 0.55c | 12.95 ± 0.79 b | 13.34 ± 0.84 b |
| Sub-Mediterranean | 6 | 0.87 ± 0.30b | 0.00 ± 0.00 a | 4.33 ± 0.10 bc | 8.66 ± 0.43bc | 13.86 ± 0.71 b | 13.86 ± 0.71 b |
| Total / Global mean | 204 | 1.25 ± 0.10 | 0.67 ± 0.05 | 5.07 ± 0.11 | 11.49 ± 0.28 | 17.81 ± 0.43 | 18.47 ± 0.44 |
| Values are expressed as Mean ± SE% (f.w.); different letters in columns indicate stastically significant differencesf (P<0.05) according to Tukey multiple range test. Bold values show the highest value (a - in columns) and those that are not significantly different from it. | |||||||
| Climatic classification | N | Maltose | Sucrose | Glucose | Fructose | Reducing sugar | Total sugar |
|---|---|---|---|---|---|---|---|
| Mean ± SE (g/100g, fresh pulp fruit) | |||||||
| B4 B´2 s a´ | 6 | 0.87 ± 0.30 c-e | 0.00 ± 0.00 ab | 4.33 ± 0.10 ab | 8.66 ± 0.43 bc | 13.86 ± 0.71 bc | 13.86 ± 0.71 bc |
| B3 B´2 s2 a´ | 19 | 1.24 ± 0.23 cd | 0.12 ± 0.07 b | 3.98 ± 0.11 b | 8.54 ± 0.32 c | 13.77 ± 0.55 c | 13.89 ± 0.58 c |
| B1 B´2 s2 a´ | 102 | 0.57 ± 0.06 e | 1.02 ± 0.07 a | 5.47 ± 0.18 a | 11.55 ± 0.35 b | 17.59 ± 0.54 b | 18.61 ± 0.57 b |
| C1 B´2 s a´ | 9 | 2.06 ± 0.30 b | 0.32 ± 0.16 ab | 4.16 ± 0.30 ab | 11.31 ± 0.61 bc | 17.54 ± 0.85 bc | 17.85 ± 0.88 bc |
| C1 B´2 s2 a´ | 15 | 3.73 ± 0.53 a | 0.51 ± 0.17 ab | 5.82 ± 0.39 a | 17.01 ± 1.46 a | 26.56 ± 2.31 a | 27.07 ± 2.30 a |
| C2 B´2 s a´ | 7 | 0.87 ± 0.24 de | 0.39 ± 0.27 ab | 4.31 ± 0.25 ab | 11.59 ± 0.43 a-c | 16.77 ± 0.72 bc | 17.17 ± 0.65 bc |
| C2 B´2 s2 a´ | 46 | 1.89 ± 0.25 bc | 0.37 ± 0.10 ab | 4.77 ± 0.20 ab | 11.17 ± 0.61 bc | 17.83 ± 0.89 bc | 18.19 ± 0.91 bc |
| Total / Global mean | 204 | 1.25 ± 0.10 | 0.67 ± 0.05 | 5.07 ± 0.11 | 11.49 ± 0.28 | 17.81 ± 0.43 | 18.47 ± 0.44 |
| Values are expressed as Mean ± SE% (f.w.); different letters in columns indicate stastically significant differences (P<0.05) according to Tukey multiple range test. Bold values show the highest value (a - in columns) and those that are not significantly different from it. | |||||||
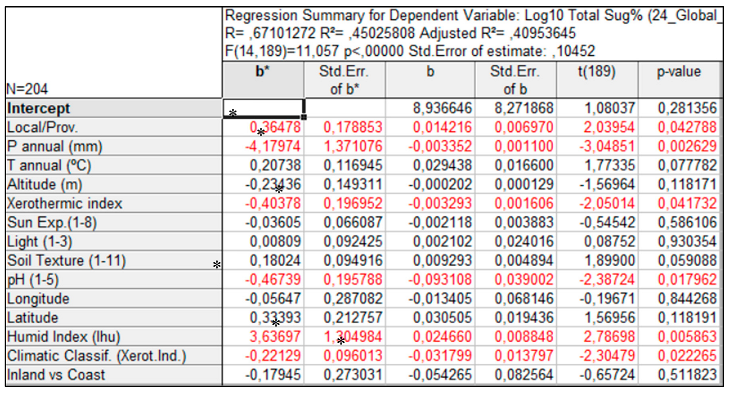 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





