Submitted:
31 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
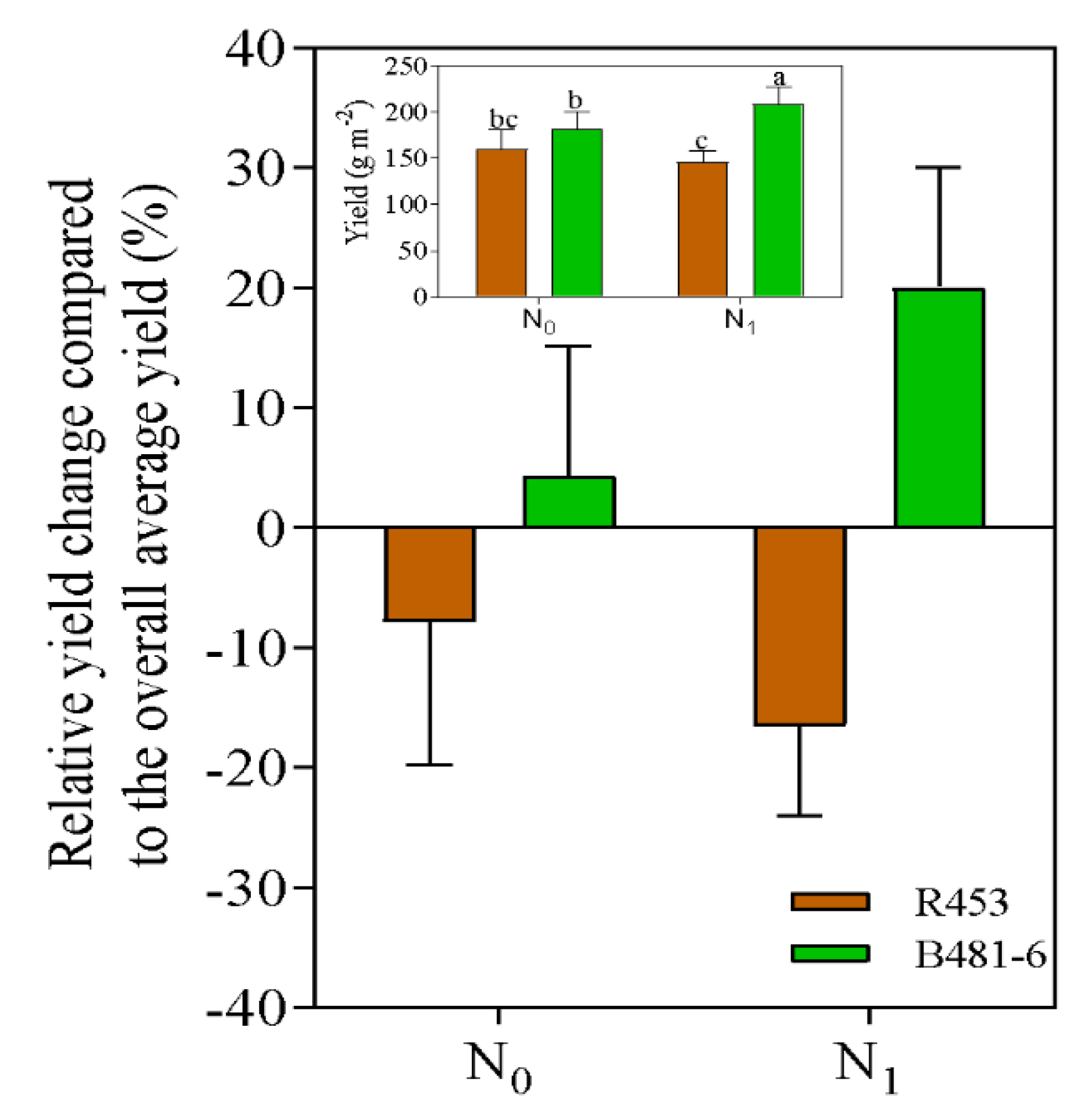
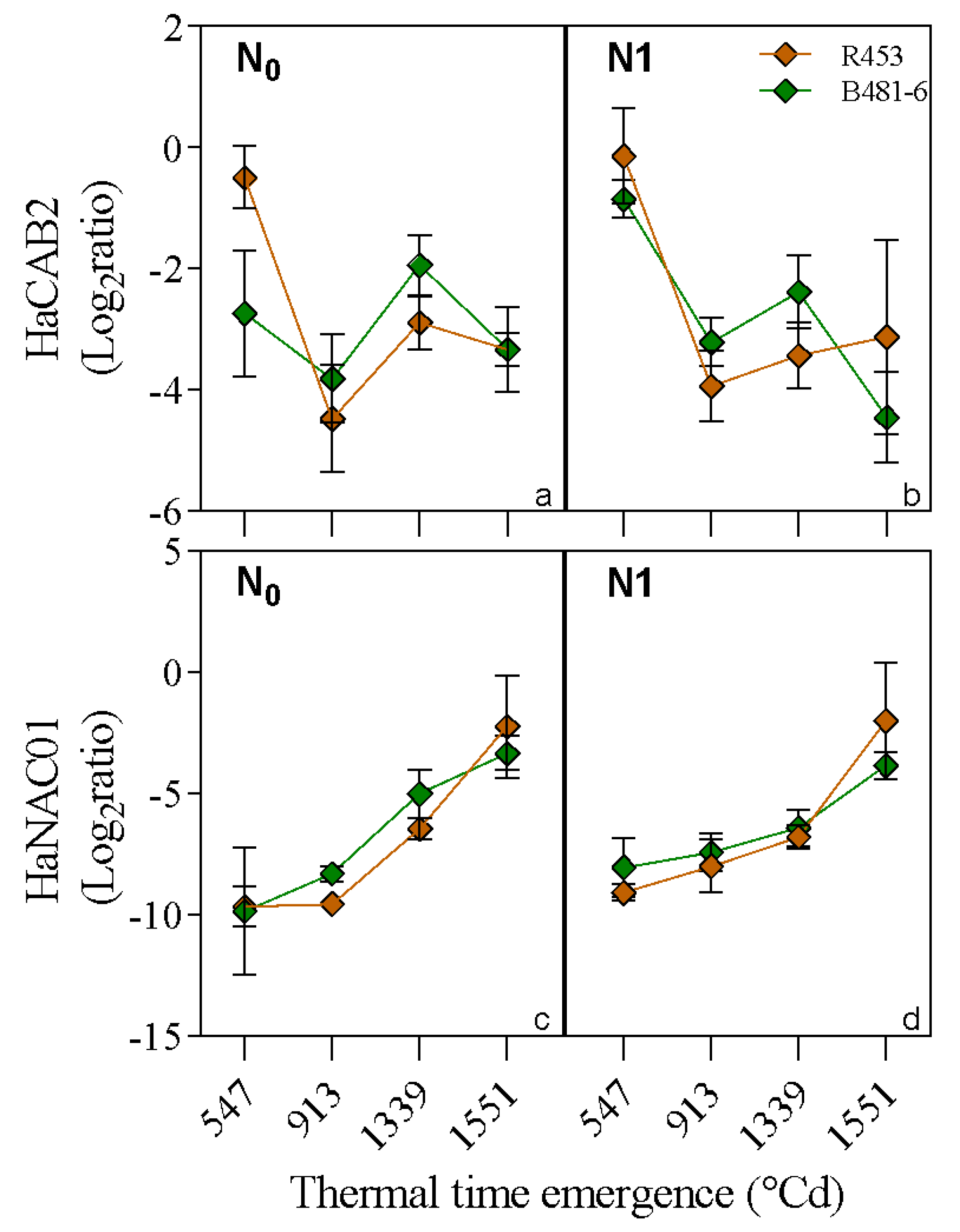
Leaf senescence in plants is the last stage of leaf development that is characterized by a decline in photosynthetic activity, an active degeneration of cellular structures and the recycling of accumulated nutrients to areas of active growth such as buds, young leaves, flowers, fruits and seeds. This process holds economic significance as it can impact yield, influencing the plant´s ability to maintain an active photosynthetic system during prolonged periods, especially during the grain filling stage, which affects plant weight and oil content. It can be associated with different stresses or environmental conditions, manifesting itself widely in the context of climate change and limiting yield, especially in crops of agronomic relevance. In this work we study the stability of two widely described sunflower genotypes belonging to the INTA Breeding Program against differential N conditions, to verify their yield stability in control conditions and under N supply. Two inbred lines were utilized, namely R453 (early senescence) and B481–6 (late senescence), with contrasting nitrogen availability in the soil but sharing the same ontogeny cycle length. It was observed that, starting from R5.5, the B481-6 genotype not only delayed senescence but also exhibited a positive response to increased nitrogen availability in the soil. This response included an increase in intercepted radiation, resulting in a statistically significant enhancement in grain yield. Conversely, the R453 genotype did not show significant differences under varying nitrogen availability and exhibited a tendency to decrease grain yield when nitrogen availability was increased. The response to nitrogen can vary depending on the specific genotype.
Keywords:
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Measurements
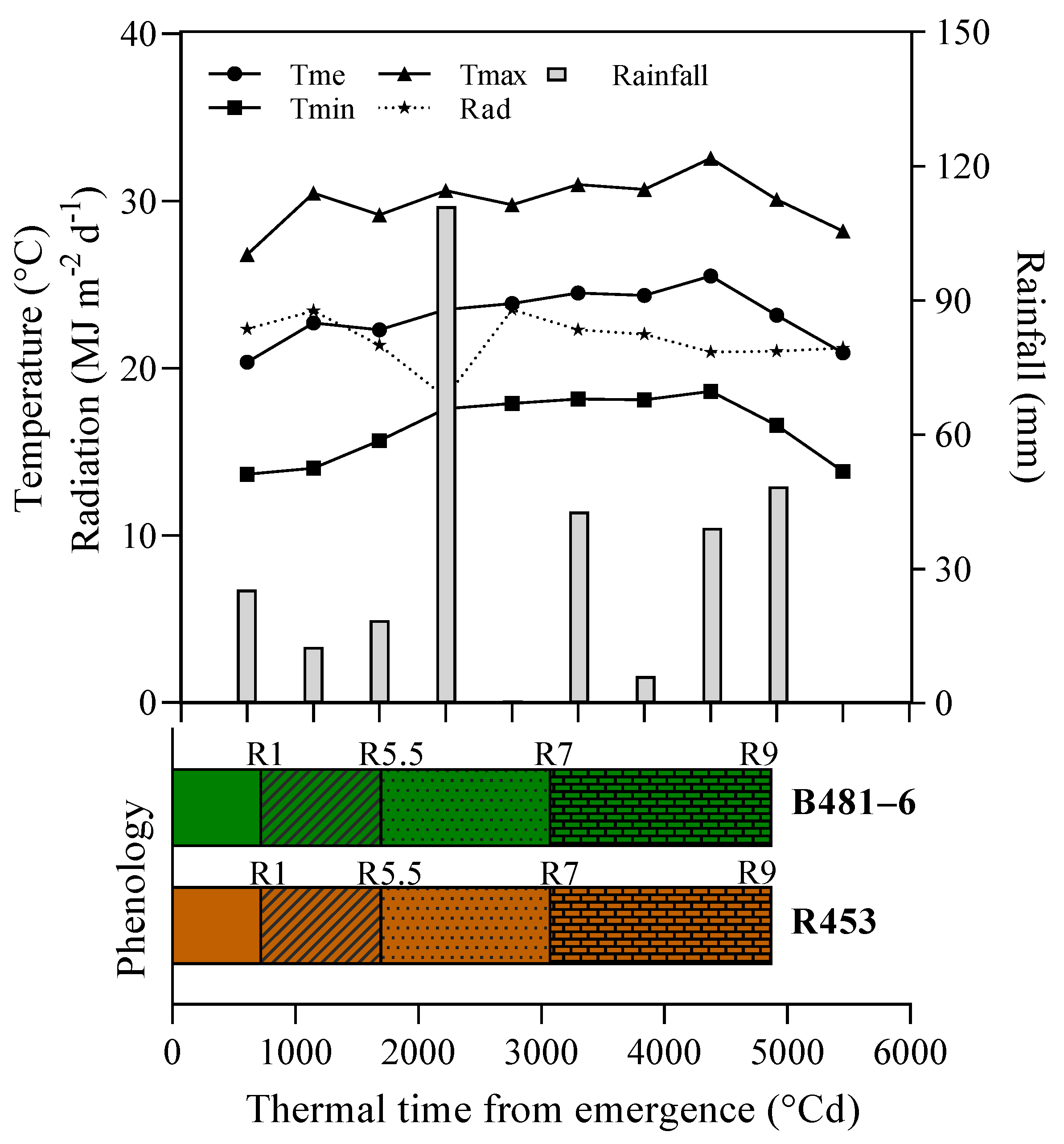
4.2.1. Phenology
4.2.2. Climate Conditions
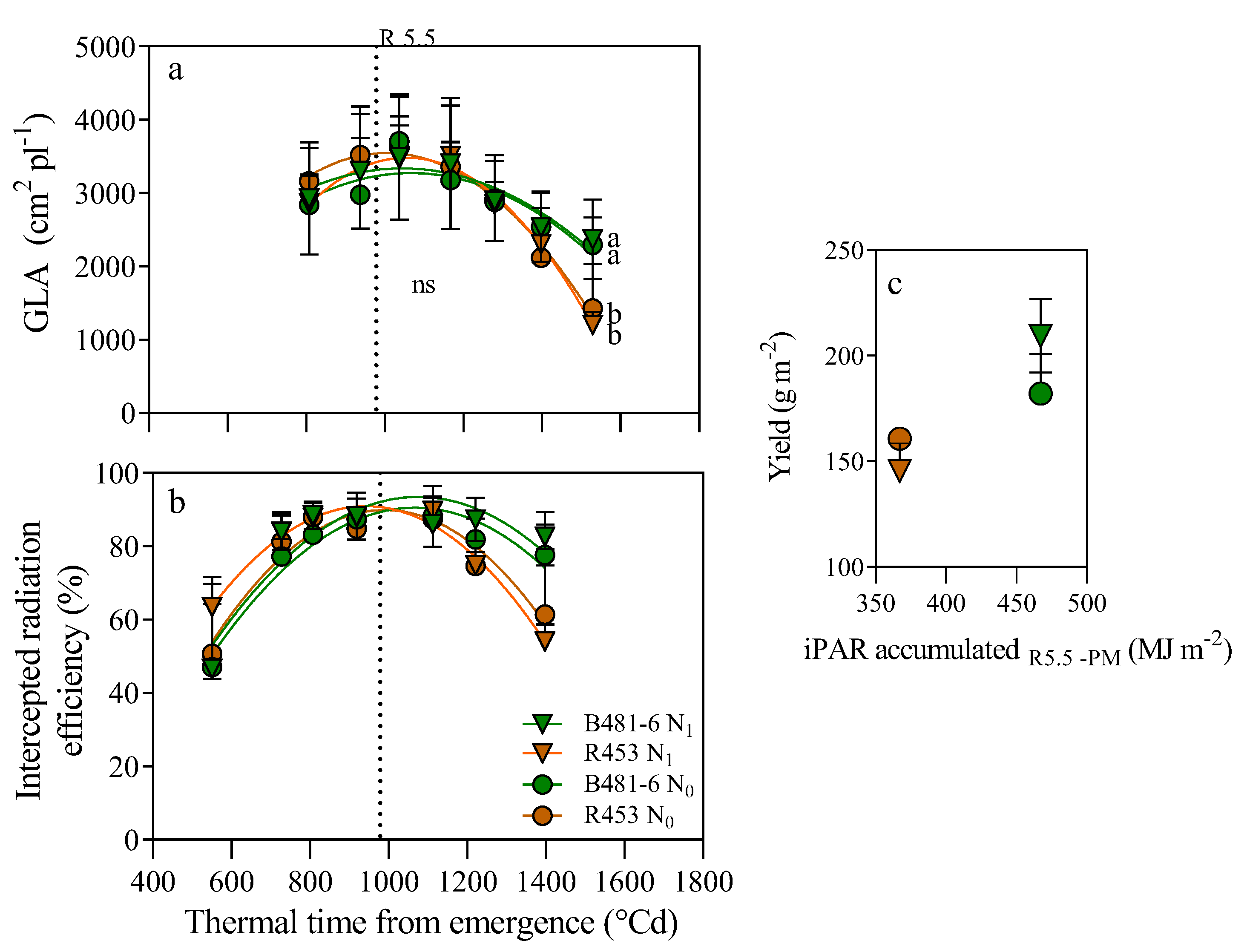
4.2.3. Radiation Interception
4.2.4. Green Leaf Area (GLA)
4.2.5. Dry Weight, Yield and Its Components
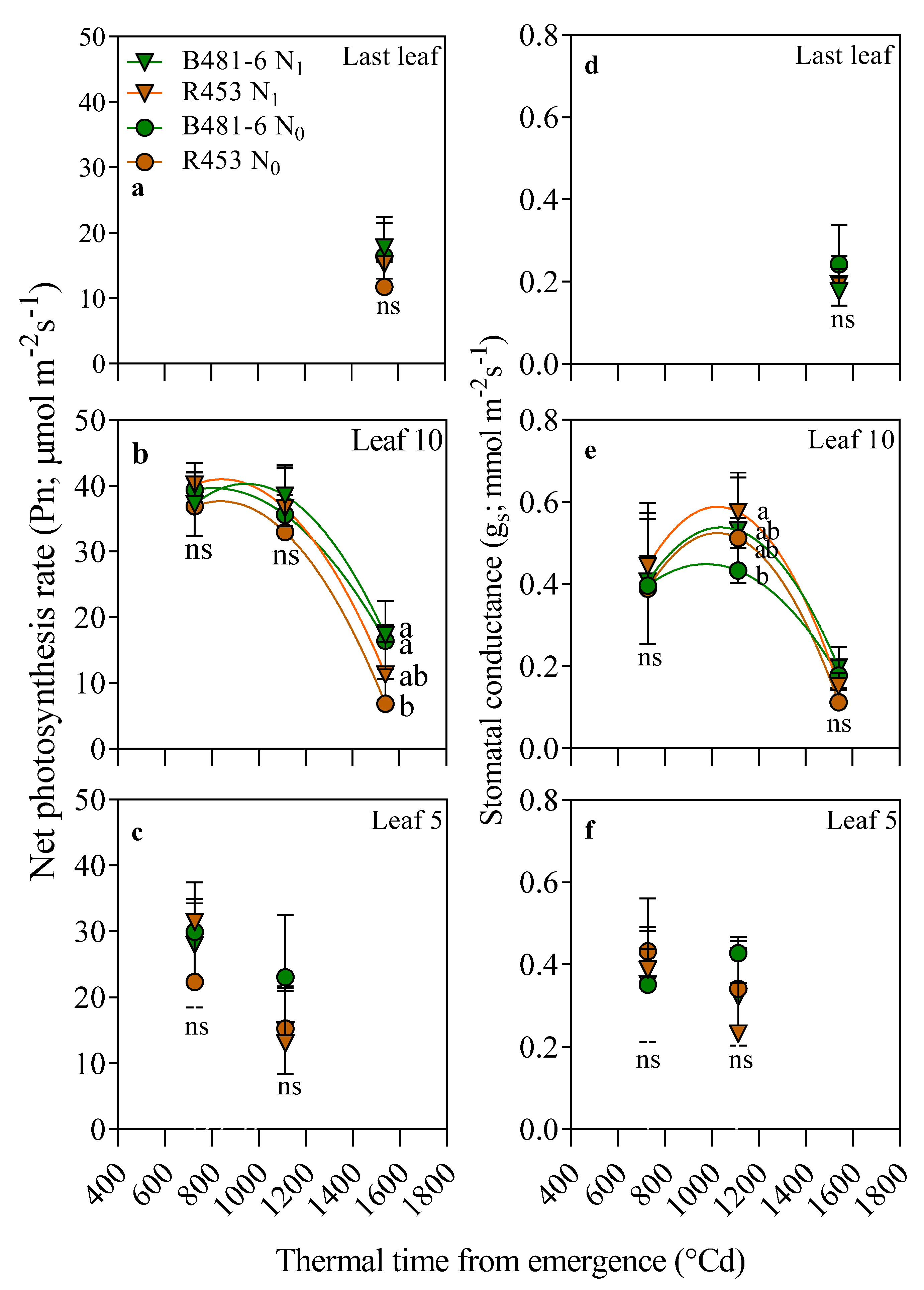
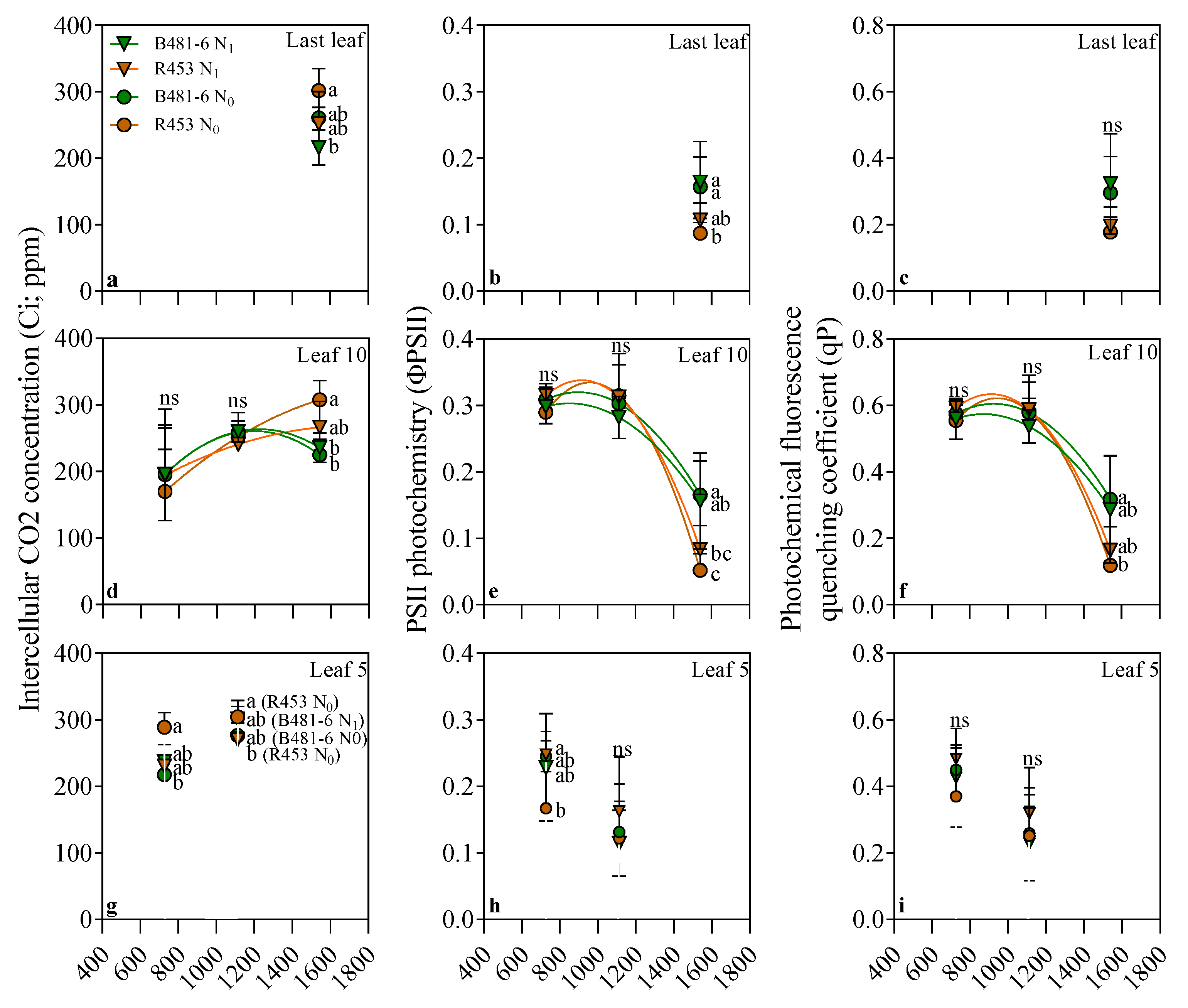
4.2.6. Leaf Physiological Measurements
4.2.7. Quantitative RT-PCR Analysis
4.3. Data Analysis
Author Contributions
Funding
References
- Haj Sghaier, H. Khaeim, Á. Tarnawa, G. P. Kovács, C. Gyuricza, y Z. Kende, «Germination and Seedling Development Responses of Sunflower (Helianthus annuus L.) Seeds to Temperature and Different Levels of Water Availability», Agriculture, vol. 13, n.o 3, Art. n.o 3, mar. 2023. [Google Scholar] [CrossRef]
- «Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits - Adeleke - 2020 - Food Science & Nutrition - Wiley Online Library». Accedido: 15 de marzo de 2024. [En línea]. Disponible en: https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.1783.
- M. Ijaz et al., «Citric acid assisted phytoextraction of nickle from soil helps to tolerate oxidative stress and expression profile of NRAMP genes in sunflower at different growth stages», Front. Plant Sci., vol. 13, dic. 2022. [CrossRef]
- H. Ahmad et al., «Evaluation of Genetic Diversity in Maintainer and Restorer Inbreds of Helianthus annus L. using Multivariate Techniques», Pakistan Journal of Agricultural Research, vol. 34, jun. 2021. [CrossRef]
- D. Noodén, J. J. Guiamét, y I. John, «Senescence mechanisms», Physiologia Plantarum, vol. 101, n.o 4, pp. 746-753, 1997. [CrossRef]
- S. Nehe, S. Misra, E. H. Murchie, K. Chinnathambi, B. Singh Tyagi, y M. J. Foulkes, «Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and protein concentration in Indian wheat cultivars», Field Crops Research, vol. 251, p. 107778, jun. 2020. [CrossRef]
- Ostrowska-Mazurek, P. Kasprzak, S. Kubala, M. Zaborowska, y E. Sobieszczuk-Nowicka, «Epigenetic Landmarks of Leaf Senescence and Crop Improvement», International Journal of Molecular Sciences, vol. 21, n.o 14, Art. n.o 14, ene. 2020. [Google Scholar] [CrossRef]
- S. Moschen et al., «Exploring gene networks in two sunflower lines with contrasting leaf senescence phenotype using a system biology approach», BMC Plant Biol, vol. 19, n.o 1, p. 446, oct. 2019. [CrossRef]
- E. dos S. Coêlho et al., «Efficiency of Nitrogen Use in Sunflower», Plants (Basel), vol. 11, n.o 18, p. 2390, sep. 2022. [CrossRef]
- Bono, J. Montoya, y F. Babinec, «FERTILIZACION EN GIRASOL Resultados obtenidos en tres años de estudio».
- R. Melgar y M. Diaz Zorita, La fertilizacion de cultivos y pasturas. 1997. Accedido: 15 de marzo de 2024. [En línea]. Disponible en: https://agris.fao. 1226.
- Sosa, L.J. , Echeverria, H.E., Dosia, G.A.A, y Aguirrezabal, L.A.N., «Nitrogen nutrition of sunflower cultivated in Balcare (Buenos Aires, Argentina)», Ciencia del Suelo, vol. Vol. 17, n.o No. 1, pp. 20-26, 1999.
- S. J. Whitcomb, E. Heyneke, F. Aarabi, M. Watanabe, y R. Hoefgen, «Mineral Nutrient Depletion Affects Plant Development and Crop Yield», en Nutrient Use Efficiency in Plants: Concepts and Approaches, M. J. Hawkesford, S. Kopriva, y L. J. De Kok, Eds., Cham: Springer International Publishing, 2014, pp. 205-228. [CrossRef]
- G. A. Dosio et al., «A whole-plant analysis of the dynamics of expansion of individual leaves of two sunflower hybrids», J Exp Bot, vol. 54, n.o 392, Art. n.o 392, 2003. [CrossRef]
- J. De la Vega, M. a. Cantore, M. M. Sposaro, N. Trápani, M. López Pereira, y A. J. Hall, «Canopy stay-green and yield in non-stressed sunflower», Field Crops Research, vol. 121, pp. 175-185, feb. 2011. [CrossRef]
- E. Agüera, P. Cabello, L. de la Mata, E. Molina, y P. de la Haba, «Metabolic regulation of leaf senescence in sunflower (Helianthus annuus L.) plants», en Senescence, InTech Open Access Publisher, 2012.
- V. Buchanan-Wollaston et al., «The molecular analysis of leaf senescence--a genomics approach.», Plant biotechnology journal, vol. 1, n.o 1, Art. n.o 1, 2003. [CrossRef]
- K. Pageau, M. Reisdorf-Cren, J.-F. Morot-Gaudry, y C. Masclaux-Daubresse, «The two senescence-related markers, GS1 (cytosolic glutamine synthetase) and GDH (glutamate dehydrogenase), involved in nitrogen mobilization, are differentially regulated during pathogen attack and by stress hormones and reactive oxygen species in Nicoti», Journal of experimental botany, vol. 57, n.o 3, Art. n.o 3, 2006. [CrossRef]
- E. Heyneke et al., «Characterization of the Wheat Leaf Metabolome during Grain Filling and under Varied N-Supply», Front. Plant Sci., vol. 8, nov. 2017. [CrossRef]
- D. R. Ort et al., «Redesigning photosynthesis to sustainably meet global food and bioenergy demand», Proceedings of the National Academy of Sciences, vol. 112, n.o 28, pp. 8529-8536, jul. 2015. [CrossRef]
- J. Burgess y P. Wang, «Not all Calvin’s are equal: Differential control of the Calvin cycle in C3 versus C4 plants», Plant Physiology, vol. 191, n.o 2, p. 817, nov. 2022. [CrossRef]
- S. Moschen, «Identificación y caracterización funcional de genes candidatos asociados a la senescencia foliar en girasol basado en perfiles transcripcionales y metabólicos», p. 183, 2014.
- S. Bengoa Luoni, «Análisis, identificación y validación de factores de transcripción asociados a senescencia foliar temprana en girasol (Helianthus annuus L.)», Universidad Nacional de San Martin, San Martin, Argentina, 2014.
- P. O. Lim, H. J. Kim, y H. G. Nam, «Leaf Senescence», Annual Rev. Plant Biol., vol. 58, pp. 115-136, 2007. [CrossRef]
- A. Schneiter y J. F. Miller, «Description of sunflower growth stages», Crop Science, vol. 21, pp. 901-903, 1981.
- I. López Gialdi et al., «Identification and characterization of contrasting sunflower genotypes to early leaf senescence process combining molecular and physiological studies (Helianthus annuus L.)», Plant Science, vol. 250, pp. 40-50, 2016. [CrossRef]
- P. L. Gregersen, A. Culetic, L. Boschian, y K. Krupinska, «Plant senescence and crop productivity.», Plant molecular biology, vol. 82, n.o 6, Art. n.o 6, 2013. [CrossRef]
- D. J. Connor, J. A. Palta, y T. R. Jones, «Response of sunflower to strategies of irrigation. III. Crop photosynthesis and transpiration», Field Crops Research, vol. 12, pp. 281-283, 1985.
- D. E. McCullough, A. Aguilera, y M. Tollenaar, «N uptake, N partitioning, and photosynthetic N-use efficiency of an old and a new maize hybrid», Can. J. Plant Sci., vol. 74, n.o 3, pp. 479-484, jul. 1994. [CrossRef]
- D. J. Connor, A. J. Hall, y V. O. Sadras, «Effect of Nitrogen Content on the Photosynthetic Characteristics of Sunflower Leaves», Functional Plant Biol., vol. 20, n.o 3, pp. 251-263, 1993. [CrossRef]
- M. R. Broadley, A. J. Escobar-Gutiérrez, A. Burns, y I. G. Burns, «What are the effects of nitrogen deficiency on growth components of lettuce?», The New Phytologist, vol. 147, n.o 3, pp. 519-526, sep. 2000. [CrossRef]
- Z. Shangguan, M. Z. Shangguan, M. Shao, y J. Dyckmans, «Effects of Nitrogen Nutrition and Water Deficit on Net Photosynthetic Rate and Chlorophyll Fluorescence in Winter Wheat», Journal of Plant Physiology, vol. 156, n.o 1, pp. 46-51, ene. 2000. [Google Scholar] [CrossRef]
- S. Ciompi, E. S. Ciompi, E. Gentili, L. Guidi, y G. F. Soldatini, «The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower», Plant Science, vol. 118, n.o 2, pp. 177-184, ago. 1996. [Google Scholar] [CrossRef]
- «Photosynthetic parameters in seedlings of Eucalyptus grandis as affected by rate of nitrogen supply - GRASSI - 2002 - Plant, Cell & Environment - Wiley Online Library». Accedido: 24 de octubre de 2024. [En línea]. Disponible en: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1365-3040.2002.00946.x.
- Cechin y T. de Fátima Fumis, «Effect of nitrogen supply on growth and photosynthesis of sunflower plants grown in the greenhouse», Plant Science, vol. 166, n.o 5, pp. 1379-1385, may 2004. [CrossRef]
- H. Lambers y R. Oliveira, «Plant Physiological Ecology», Plant Physiological Ecology, ene. 2019. [CrossRef]
- Marc y J., H. Palmer, «Photoperiodic sensitivity of inflorescence initiation and development in sunflower», Field Crops Research, vol. 4, pp. 155-164, ene. 1981. [Google Scholar] [CrossRef]
- S. Moschen et al., «Integration of transcriptomic and metabolic data reveals hub transcription factors involved in drought stress response in sunflower (Helianthus annuus L.)», Plant Molecular Biology, vol. 94, n.o 4-5, Art. n.o 4-5, 2017. [CrossRef]
- S. Balazadeh, D. M. S. Balazadeh, D. M. Riaño-Pachón, y B. Mueller-Roeber, «Transcription factors regulating leaf senescence in Arabidopsis thaliana», Plant Biology, vol. 10, n.o (Suppl. 1), Art. n.o (Suppl. 1), sep. 2008. [Google Scholar] [CrossRef]
- Kim, H. R. Woo, y H. G. Nam, «Toward Systems Understanding of Leaf Senescence: An Integrated Multi-Omics Perspective on Leaf Senescence Research», Mol Plant, vol. 9, n.o 6, pp. 813-825, jun. 2016. [Google Scholar] [CrossRef]
- S. Bengoa Luoni, «Genome-wide and comparative phylogenetic analysis of senescence-associated NAC transcription factors in sunflower (Helianthus annuus)», BMC Genomics, 2021.
- J. Kim, K. J. Kim, K. Baek, y C.-M. Park, «Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis», Plant Cell Reports, vol. 28, pp. 1159-1167, 2009.
- H. J. Kim et al., «Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis.», Journal of experimental botany, vol. 65, n.o 14, Art. n.o 14, jul. 2014. [CrossRef]
- D. Zhao, a. P. Derkx, D.-C. Liu, P. Buchner, y M. J. Hawkesford, «Overexpression of a NAC transcription factor delays leaf senescence and increases grain nitrogen concentration in wheat», Plant Biology, p. n/a-n/a, 2015. [CrossRef]
- S. A. Trupkin et al., «Identification and expression analysis of NAC transcription factors potentially involved in leaf and petal senescence in Petunia hybrida», Plant Science, vol. 287, p. 110195, oct. 2019. [CrossRef]
- Wu, *!!! REPLACE !!!*; et al. , «JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis.», The Plant cell, vol. 24, n.o 2, Art. n.o 2, feb. 2012. [Google Scholar] [CrossRef]
- «Members of the barley NAC transcription factor gene family show differential co-regulation with senescence-associated genes during senescence of flag leaves | Journal of Experimental Botany | Oxford Academic». Accedido: 24 de octubre de 2024. [En línea]. Disponible en: https://academic.oup.com/jxb/article/65/14/4009/588262.
- J. R. Kiniry, R. Blanchet, J. R. Williams, V. Texier, K. Jones, y M. Cabelguenne, «Sunflower simulation using the EPIC and ALMANAC models», Field Crops Research, vol. 30, pp. 403-423, 1992.
- J. L. Monteith, «Radiation and Crops», Experimental Agriculture, vol. 1, n.o 4, pp. 241-251, oct. 1965. [CrossRef]
- G. Szeicz, «Solar Radiation for Plant Growth», Journal of Applied Ecology, vol. 11, n.o 2, pp. 617-636, 1974. [CrossRef]
- «librogirs.pdf». Accedido: 24 de octubre de 2024. [En línea]. Disponible en: https://www.criba.edu.ar/morfologiavegetal/producfiles/papers/librogirs.pdf.
- J. M. Ruijter et al., «Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data», Nucleic Acids Res, vol. 37, n.o 6, Art. n.o 6, 2009. [CrossRef]
- J. A. Di Rienzo, F. Casanoves, M. G. Balzarini, L. Gonzalez, y M. Tablada, «InfoStat». 2013.
| Phenological stages | Nitrogen | Genotypes | Flower head (g pl-1) |
Leaves (g pl-1) |
Stem (g pl-1) |
Total aerial biomass (g pl-1) |
|---|---|---|---|---|---|---|
| R5.5 stage | N0 | B481-6 | 95.7 a | 134.0 a | 256.3 a | 486.0 a |
| R453 | 171.3 a | 158.6 a | 376.5 a | 706.4 a | ||
| N1 | B481-6 | 162.0 a | 204.0 a | 437.3 a | 803.3 a | |
| R453 | 143.4 a | 169.9 a | 275.7 a | 710.3 a | ||
| G | ns | ns | ns | ns | ||
| N | ns | ns | ns | ns | ||
| G*N | * | ns | ns | ns | ||
| R8 stage | N0 | B481-6 | 334.3 a | 140.1 b | 299.1 a | 773.5 a |
| R453 | 406.9 a | 133.6 b | 296.9 a | 837.4 a | ||
| N1 | B481-6 | 439.7 a | 181.4 a | 313.7 a | 934.8 a | |
| R453 | 496.1 a | 123.4 b | 305.3 a | 924.7 a | ||
| G | ns | ** | ns | ns | ||
| N | ns | ns | ns | ns | ||
| G*N | ns | * | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





