Multiple factors can lead to impaired wound healing. In general terms, the factors that influence repair can be categorized into local and systemic. Local factors are those that directly influence the characteristics of the wound itself, while systemic factors are the overall health or disease state of the individual that affect his or her ability to heal. About local factors, it seems important include oxygenation, infection, foreign body, venous sufficiency, about systemic factors it seem relevant to mention age and gender, sex hormones, stress, ischemia, diseases as diabetes for example, obesity, medications, alcoholism and smoking, immunocompromised conditions, nutritions [
12]
3.1. Chronic Skin Lesion
According to international literature, a skin lesion is considered a chronic wound if it doesn't healed after six to eight weeks. These lesions have an inflammatory response that persists over time, maintaining an imbalance between productive and degenerative processes, but not following the traditional, orderly, and timely sequence of the repair process, or progressing through these phases without regaining the anatomical and functional integrity of the tissue [
7,
13,
14,
15].
Many factors cause the process to be delayed, which impedes it and makes it chronic in the first place. Based on probability, 1406 clinical presentations may occur [
3,
7,
11,
13,
14], with 140 pathologies thought to be involved and an average comorbidity of approximately 6 for people over 65 (85% of the population having at least one chronic illness and 30% having three or more chronic conditions). The main causes of CW include venous ulcers (VU), diabetic ulcers (DU) and pressure ulcers (PU), all of them influenced by systemic factors such as metabolic, hormonal, venous circulation or disability [
3,
12].
The causes of skin ulcers include a wide range of clinical symptoms and syndromes that have been well identified and documented in the literature; however, the treatment of these conditions is outside the purview of this investigation. During the protracted inflammatory phase of ulcers, protease activity increases, leading to the breakdown of growth factors and other molecular signals that help the reparative phase. The dominance of reparative processes over destructive ones in chronic ulcers is hampered by the overproduction of proinflammatory cytokines and hydrolytic enzymes [
4,
13,
15]. It has been suggested that intervention to lower protease activity can preserve endogenous growth factors and maintain the regular healing process. For the body to heal, the synthesis of new tissue and the breakdown of old tissue must happen in balance. Fibrinolytic systems and MMPs work together to remove fibrin and damaged ECM from acute wounds. However, it facilitates cell motility and makes the macromolecules trapped in the matrix accessible, which leads to remodeling. Chronic skin lesions have been found to have lower TIMP concentrations and higher MMP levels. This leads to a subsequent reorganization of the matrix and a worsening of its degradation [
4,
13,
15].
Further emphasis has been placed on the formation of pericapillary fibrin sheaths, especially in diabetic lesions, and on a decrease in tissue concentrations of nitric oxide (NO), especially in conditions like malnutrition, diabetes mellitus, corticosteroid therapy, ischemia, and smoking exposure. Additionally, tissue ischemia and an increased presence of neutrophils, which leads to enhanced extracellular matrix degradation, have been highlighted [
4,
13,
15].
The procedures involved in healing chronic wounds are similar to those involved in healing acute wounds. Chronic wounds are strongly linked to the dysregulation of MMP production, which prolongs the inflammatory phase. The primary cause of chronic wound (CW) persistent inflammation is mostly attributed to the presence of many cell types inside the cellular infiltration [
4,
13,
15,
16,
17,
18]. Neutrophils are widely distributed throughout the wound and release a substantial amount of MMPs These enzymes deactivate vital proteins essential to the healing process, such as PDGF and TGFbeta, in addition to impairing the connective tissue matrix and elastase [
4,
13,
15,
16,
17,
18].
However, it is imperative to investigate the connections between keratinocytes and immune cells. Different signaling chemicals are secreted during this process, albeit it is still unclear exactly what role these cells have in the formation of cell walls. Genes associated with partial proliferative activation are expressed by keratinocytes in chronic wounds, which may account for the epidermal hyperproliferation surrounding the ulcer's borders. Moreover, the fibroblasts are not sensitive to TGFbeta, a migratory stimulator. This is reflected in much lower TGFbetaR levels as well as lower levels of the subsequent components of the TGFbetaR signaling cascade [
4,
13,
15,
16,
17,
18,
19].
Notable is the significance of the immune system's interactions with the nervous system in controlling wound healing processes [
20]. Recent research suggests that interactions between mast cells and nerve cells transmit neurotransmitters important for wound healing, including substance P (SP), protein gene product 9.5 (PGP 9.5), nitric oxide (NO), nerve growth factor (NGF), neurokinin A (NKA), neuropeptide Y (NPY), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) [
21,
22]. The release of ECM by fibroblasts, the elevation of TGFbeta levels, and the reaction of infiltrating cells are responsible for this outcome [
17,
18].
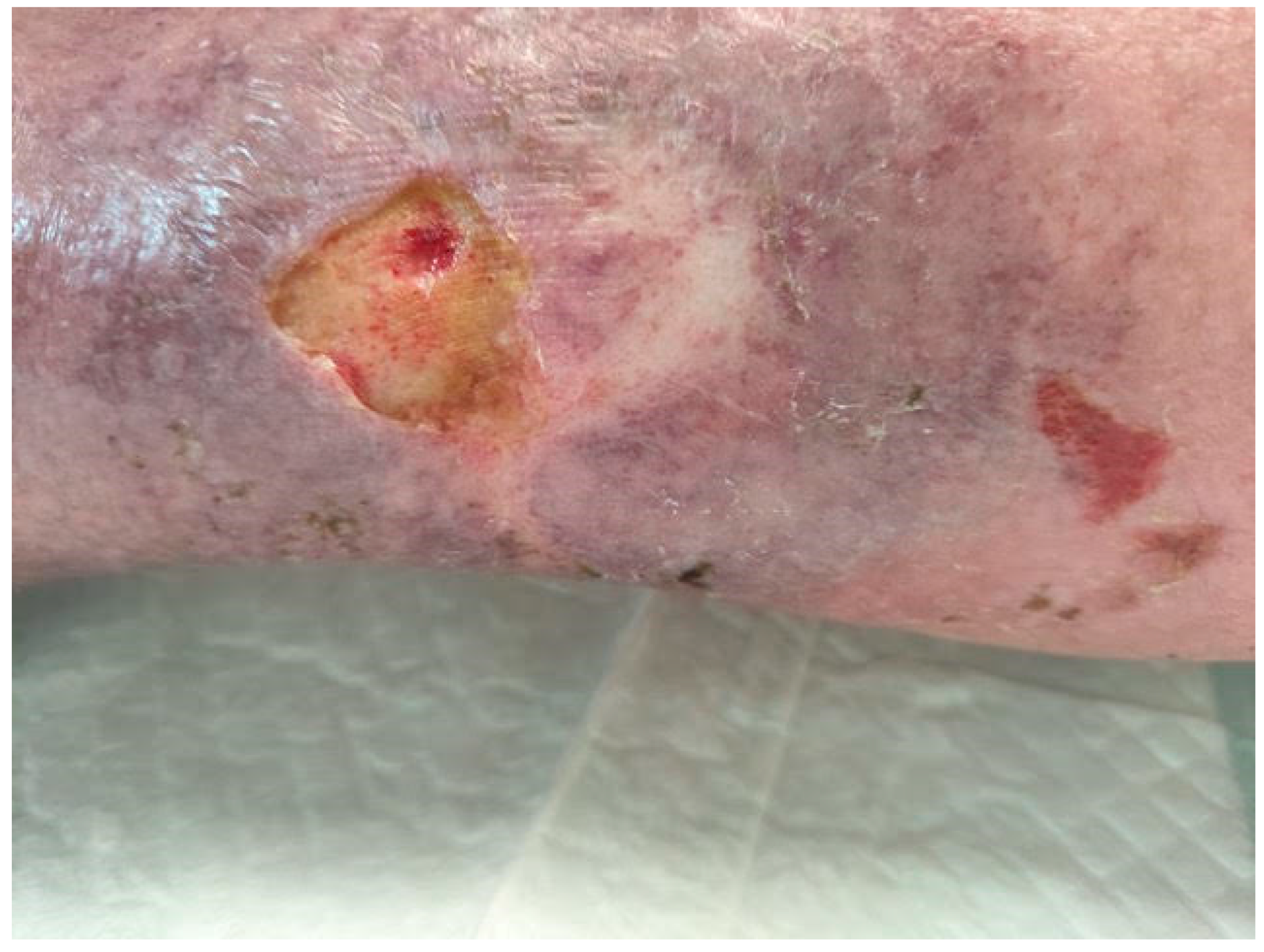
In addition, infections represent a substantial and frequent source of hindrance in the healing process: an increase in the bacterial load extends the inflammatory phase, which is marked by increased MMP levels and a subsequent escalation of the damaging processes affecting the extracellular matrix. Some bacterial species form a polysaccharide biofilm that is rich in proteins and other metabolic byproducts when they adhere to a substrate; within this biofilm, the bacteria clump together to form micro-colonies, which are protected from antibiotics by reduced penetration through the biofilm matrix as well as genetic mutations that alter their susceptibility to antibiotics [
23,
24].
Periodically, biofilms release single bacterial cells that can colonize new surfaces or break down the collagen matrix in healed ulcers, a process known as "re-ulceration." Within the biofilm, bacteria establish themselves, prevent the colonization of new bacteria, store energy in the polysaccharide matrix, and interact through the transfer of genetic material (a process known as quorum sensing). Variations in the conditions within the biofilm can also affect which cells remain in the colony, die, or leave. Furthermore, skin lesions are typically more subtle when they are infected. This is because the release of endotoxins and proteases by the microorganisms breaks down the extracellular matrix, which triggers a significant release of mediators that worsen the local inflammatory response. This is one way that infection and increased exudate are related. Increased levels of macromolecules including fibrin and albumin, as well as pro-inflammatory cytokines and MMPs inhibit growth factors in the exudate [
23,
24] (See
Figure 1).
Therapies in Chronic Wounds
CW represent a significant challenge in the field of medicine due to their prolonged healing time and the complex factors involved in their management. Typically, a wound is classified as chronic if fails to progress through the normal stages of healing within six weeks. CW encompass a variety of pathologies, including venous, arterial, and mixed ulcers, diabetic foot ulcers (DFU), and pressure injuries (also known as pressure ulcers). These wounds are often the result of underlying conditions such as vascular insufficiency, diabetes, or prolonged pressure, which inhibit normal tissue repair [
4,
5,
6].
The impact of CW is substantial, both for healthcare systems and patients. In addition to physical discomfort, CW often led to reduced mobility, increased healthcare costs, and decreased quality of life due to the prolonged and treatment. Multiple factors, including patient-specific variables like age, skin health, and the presence of comorbidities, contribute to the development and persistence of CW. Trauma or infection can further complicate the healing process, making CW particularly resistant to conventional treatment strategies [
25,
26].
Effective management of CW requires a complex approach that addresses not only the wound itself but also the underlying conditions and patient care. Emerging therapies aim to modulate the wound environment, control infection, and promote healing, but a standardized approach remains elusive [
26].
Venous leg ulcers (VLUs) are the most common type of chronic wound, often associated with chronic venous insufficiency. The primary treatment for VLUs is compression therapy, which remains the gold standard. However, successful management of VLUs often requires a comprehensive, multidisciplinary approach. This includes regular wound assessments, proper dressing choices, and sometimes surgical interventions like venous ablation or debridement [
27]. Additionally, addressing comorbid conditions such as diabetes or arterial disease is necessary for optimizing wound healing outcomes [
26].
The treatment of DFU involves a multiple approach designed to promote healing, prevent infection, and reduce complications such as amputations. Among the most effective therapies, negative pressure wound therapy (NPWT) and platelet-rich plasma (PRP) stand out. NPWT accelerates healing by applying controlled suction to the wound, removing exudate, promoting granulation tissue formation, and improving circulation. When combined with PRP, these therapies demonstrate even greater efficacy of CW healing rate and reduced healing time. Ultrasonic debridement (UD) is another key technique that removes necrotic tissue while promoting a clean wound environment, which enhances the effects of other therapies such as NPWT. Other innovative approaches include hyperbaric oxygen therapy (HBOT), which improves oxygenation in hypoxic tissues, thus promoting angiogenesis and reducing infection rates [
28].
Arterial ulcers (AU), though less common than VLUs, are particularly challenging to treat due to their association with poor blood flow caused by peripheral arterial disease. The primary treatment goal is to restore blood circulation, typically through revascularization procedures like angioplasty or bypass surgery. In cases where revascularization is not possible, the focus shifts to wound care and pain management, avoiding aggressive debridement due to the risk of further tissue damage from poor perfusion. Moisture balance is crucial, with specialized dressings used to maintain an optimal healing environment. Adjunct therapies such HBOT may also be employed to enhance oxygenation in ischemic tissues and promote healing [
29].
Recent advances in the management of CW have significantly expanded, ranging from emerging technologies to novel therapies. Smart hydrogels, which respond to stimuli like pH, temperature, and glucose levels, enable controlled drug release and enhance the healing of hard-to-treat wounds such as diabetic ulcers [
30]. Additionally, electrical stimulation (ES) has emerged as a non-invasive strategy to promote tissue regeneration and reduce inflammation by modulating cellular migration and proliferation [
31]. The integration of nanotechnology into wound dressings has also improved infection control in CW by nanoparticles for controlled antimicrobial release and enhanced tissue regeneration [
32]. Growth factors and gene therapies are also under investigation to correct disrupted cellular signaling in CW, with recent studies highlighting their potential when administered in combination [
33] Finally, advanced drug delivery systems directed to the complex microenvironment of CW continue to be a focus, allowing for more precise and effective therapy for treating infections and promote healing [
34].
Physical therapies have become an option as adjunct in the management of CW. Techniques such as laser therapy, photobiomodulation (PBM), and ES have been used for their ability to enhance the wound healing process. PBM, using low-level light therapy, stimulates cellular activity and nitric oxide production, helping to reduce inflammation and enhance tissue regeneration. Similarly, ES mimics the body’s natural electrical fields to promote angiogenesis, reduce bacterial colonization, and accelerate healing by stimulating fibroblast proliferation [
6].
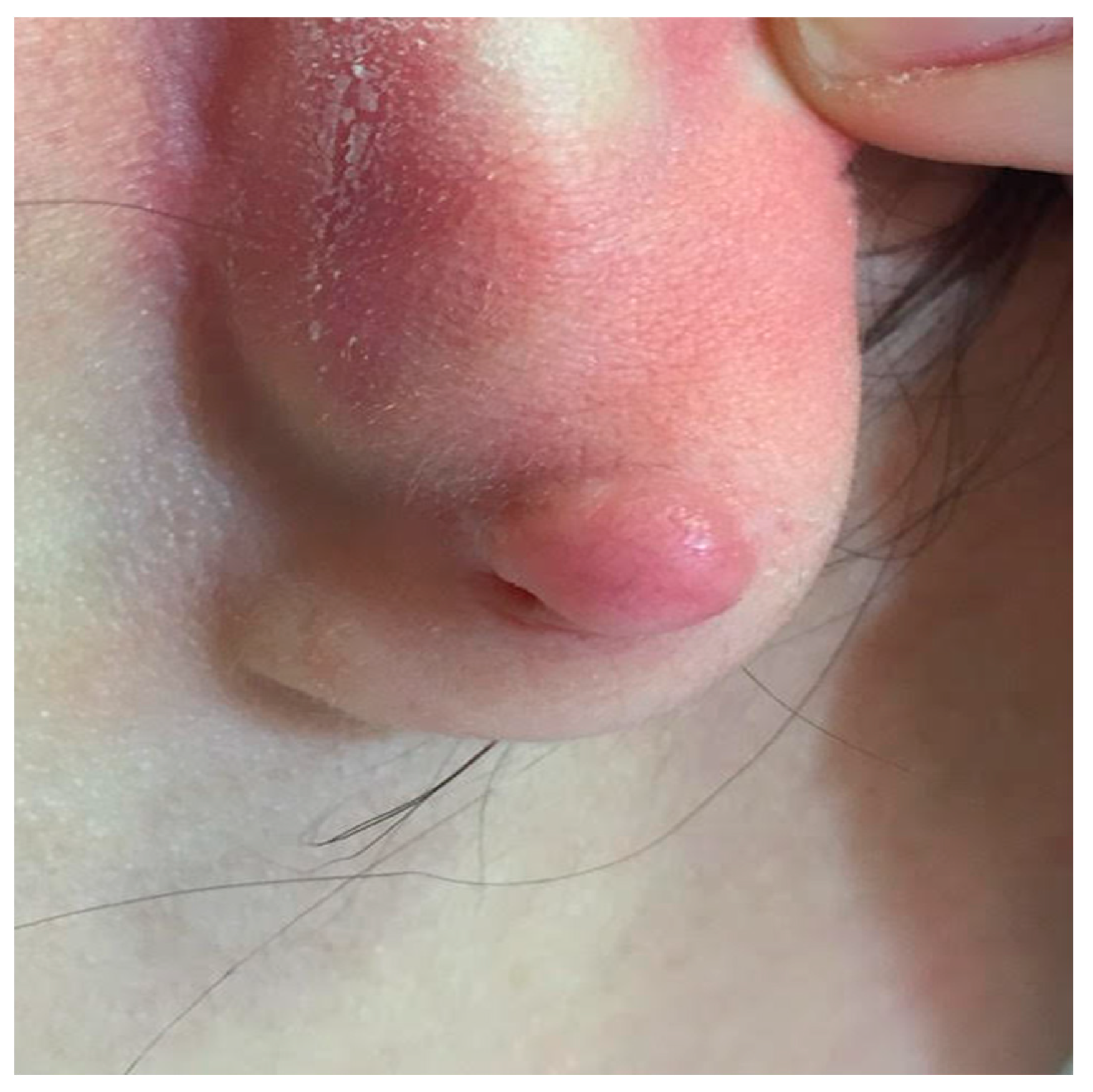
3.2. Keloids
Around 11 million cases of keloid, a pathological condition with distinct clinical symptoms, are reported annually in wealthy countries [
35]. Around 70% of cases occur in younger groups [
36]. Clinically, keloid scars appear as hard, rough papules and plaques with an uneven surface. They have a wide range of colors, including pigments that are red, purple, and brown. Unlike hypertrophic scars, keloids extend beyond the original wound's boundaries and do not fade with time [
37]. Because of their disruptive properties, keloids often cause symptoms like pruritus, pain, and discomfort. These symptoms can appear months or years after the initial damage. People who have keloids frequently experience psychological distress linked to negative self-perception, negative experiences, and body dysmorphic disorder [
38].
Numerous investigations have demonstrated that a mix of systemic and local variables, including mechanical strain, hormonal imbalances, metabolic changes, and inflammatory processes, initiate the pathogenesis of keloids [
36,
39]. Collagen strands, specifically kinds one and three collagen, are primarily disorganized in these atypical scars. Keloids are fibrous, proliferative lesions that resemble noticeable scars. They are distinguished by their gradual, ongoing growth into the surrounding healthy skin beyond the boundaries of the incision. Patients with keloids may have severe pain, ongoing itching, psychological anguish, and motor limitation, all of which add significantly to their burden [
36,
39].
In keloid investigations, the healthy skin next to keloid scars is often ignored; yet, it has been noted to exhibit properties more in line with keloid tissue than with normal skin. This is evident in the nearby normal skin's pruritus and the increased blood flow compared to the unaffected normal skin. Heat shock protein 70 and the hematopoietic stem cell marker c-KIT are more strongly stained in the surrounding normal skin, while keloid-derived keratinocytes and fibroblasts exhibit aberrant gene expression. The epidermal appendages that are lacking from the keloid tissue reappear in the surrounding normal skin, however the extracellular matrix of the normal skin may become infiltrated by thick and excessive collagen deposition. Moreover, it has been noticed that portions of the adjacent keloid's nodules penetrate the nearby normal skin [
40].
The proliferation and death of the surrounding normal skin differs from that of keloids. Only the nearby normal skin showed enhanced dermal proliferation and raised apoptotic keratinocyte counts; the healthy skin did not exhibit these features. Additionally, compared to keloid scars, the surrounding normal skin has more blood vessels and has higher expression of the proliferative PCNA marker in the dermis [
39,
40].
Further inquiry is necessary since in vitro tests reveal that the normal skin in the vicinity has certain properties with the surrounding keloid. Adjacent normal skin that had been in vitro rebuilt displayed a phenotype that was more noticeable than real normal skin but less severe than the peripheral keloid models. Between genuinely unaffected normal skin and keloid scar, the surrounding normal skin showed intermediate amounts of α-SMA staining, HGF production, and collagen type IV α2 gene expression [
39,
40].
Most studies support the observation that keloid scars have thicker epidermis, which is supported by micrometer measurements and the number of living cell layers. Regarding rete ridge formation, there are conflicting results from research that show normal, reduced, or nonexistent ridge creation. Several studies, including histological examinations, show a progressively thicker epidermis that has flattened. Although there appears to be little change in epidermal differentiation, there have been observations of increased activation and proliferation of the epidermis. The larger keloid epidermis underwent immunohistochemical examination, which showed normal levels of epidermal proliferation and differentiation despite the early expression of the terminal differentiation marker involucrin. Instead of epidermal hyperproliferation, the research indicates that higher epidermal thickness associated with atypical differentiation. The expression of EMT markers is higher in keloid keratinocytes [
39,
40,
41,
42].
Since keloids are primarily defined by increased fibroblast proliferation, excessive production of extracellular matrix components, and a subsequent decrease in MMPs, research on keloids has primarily focused on the role of fibroblasts in their development [
39,
40,
41,
42,
43]. The formation of keloids, may be influenced by inflammation-induced EGF development. Studies have shown that keloids show decreased expression of E-cadherin, ferroptosis suppressor protein 1 (FSP1), vimentin, α-SMA, TGF-β1, and Smad3 phosphorylation. In vitro experiments have confirmed the role of keratinocytes in keloids. In vitro TGF-β1-induced culture of human hair follicle outersheath and epidermal keratinocytes showed decreased E-cadherin expression and increased FSP1 and vimentin expression. The expression of ECM-related genes, including hyaluronan synthase 2, vimentin, cadherin-11, wingless-type MMTV integration site family, member 5A, frizzled 7, ADAM metallopeptidase domain 19, and interleukin-6, was significantly higher in keratinocytes of keloids than in normal skin keratinocytes. Inhibition of the TGFβ1 signaling pathway resulted in the downregulation of ECM-related genes in keloid keratinocytes, while stimulation of normal skin keratinocytes led to the upregulation of ECM related genes. Inhibition of the ERK1/2 or p38 MAPK pathway in keloid keratinocytes reversed these changes in gene expression [
43,
44]
In the context of keloid formation, cytokines, including interleukins, tumor necrosis factors, and growth factors, have been found to accelerate the formation of scar tissue by promoting fibroblast proliferation and collagen synthesis (9). The formation of pathological scars is significantly influenced by dysregulated inflammation, which involves immune cells, inflammatory cytokines, and intracellular signaling pathways. Current and prospective therapies are designed to target these processes in order to reduce scarring [
39,
40,
41,
42,
43].
Several lines of evidence support the potential connection between keloid formation and inflammatory cytokines. Keloid tissues have been found to contain elevated levels of specific cytokines in comparison to normal skin. Additionally, genetic research has identified polymorphisms in cytokine genes that are linked to an elevated risk of keloid formation. These results indicate that cytokines may have a dual function in the fibrotic response in keloids, as they may both initiate and perpetuate it. Nevertheless, the causal relationship between inflammatory cytokines and keloid formation has not been definitively established, indicating the need for additional research [
39,
40,
41,
42,
43].
In vivo, hyperbaric oxygen therapy administered to patients with keloids has been shown to reduce blood perfusion and the expression of hypoxia inducible factor-1α and vascular endothelial growth factor in keloid tissues, possibly due to the induced EMT phenomenon in keloid tissue. It has recently come to light that the immune milieu surrounding keloids plays a major role in their formation [
39,
40,
41,
42,
43]. Skin fibrosis is associated with increased T cell, MC, Langerhans cell, and macrophage counts in keloids relative to normal tissues. Interactions between immune cells and keloid fibroblasts can result in a complex and synergistic regulation of the molecules and mechanisms that underlie keloid development. Macrophages play a crucial role in the healing of cutaneous wounds by controlling the microenvironment during the various stages of the healing process. M2 macrophages are found in keloids and are specifically associated with fibrosis and scarring [
39,
40,
41,
42,
43,
44]. (
Figure 2)
Genetically Induced Keloids
Genetics plays a major role in the incidence of keloids, with varying prevalence rates among ethnic groups. People with darker skin tones appear to be more likely than people with lighter skin to be affected, and African Americans have the greatest incidence rate. Asian and Hispanic people are less likely to be affected, and white people are least likely to experience it. Furthermore, the presence of familial inheritance suggests that genetics contributes to the incidence of keloids [
45,
46,
47,
48]. Keloids are more common among twins, and families with several affected individuals over several generations frequently show a genetic predisposition to scarring that follows an autosomal dominant inheritance pattern. There are reports of X-linked and autosomal recessive inheritance, which suggests that more than one gene may be involved in the development of keloid formation. People who have a genetic susceptibility to keloids may acquire them at multiple anatomical sites; instances of multiple family members presenting with identical patches of keloids are common. Similarities in phenotypic expression within the same family point to a genetic inclination rather than just environmental influences. Although it has proven difficult to identify predisposing genes in familial keloids, linkage research has identified keloid susceptibility loci in an African-American and Japanese family [
45,
46,
47,
48]. Potential genes within these particular sites have been found, including the EGFR gene at chromosome band 7p11 and the TNFAIP6 gene at chromosomal band 2q23. Rubinstein-Taybi syndrome, Ehlers-Danlos syndrome Type III, X-linked Goeminne syndrome, and FLNA mutations that cause keloids, joint contractures, an elevated optic cup-to-disc ratio, and renal calculi are among the syndromes associated with keloids. To determine if these genes are really susceptibility genes for keloid formation, more investigation is needed. Four single-nucleotide polymorphisms (SNPs), covering three loci connected to keloid formation, have been found by two genome-wide association studies. The chromosomal bands 1q41, 3q22.3–23, and 15q21.3 include these SNPs [
45,
46,
47,
48].There is just a single SNP found inside a gene, rs8032158, which is found in the NEDD4 gene's intron. Animal development and survival depend on the NEDD4 protein via the insulin-like growth factor-1 signaling pathway. On the other hand, SNPs in the MYO1E gene, close to NEDD4 on chromosome bands 15q21.2–22.3, were connected to keloid formation in admixture mapping done on an African American cohort. TGF-β, or transforming growth factor beta, plays a critical role in many fibrotic illnesses, including keloids. Compared to normal fibroblasts, keloid fibroblasts exhibit higher ratios of TGF-β1 to TGF-β2 receptors, lower amounts of TGF-β3, and higher levels of TGF-β1 and TGF-β2. However, neither the previously mentioned TGFB genes nor the downstream signaling components SMAD3, SMAD6, and SMAD7 have shown any mutations or polymorphisms associated with keloids, according to the studies conducted to far [
45,
46,
47,
48]. This suggests the existence of either long-range enhancer/repressor variants of TGF-β pathway components that affect the expression of TGFB genes in keloids, or upstream genes regulating TGFB genes. Tumor suppressor p53 plays a crucial role in both cell division and death; its potential dysregulation in the development of keloid tumors has been studied. It is still unclear what the TP53 gene's function is, particularly in relation to the codon 72 polymorphism. Two important studies have looked at the relationship between keloids and variations in the human leukocyte antigen (HLA). Further studies could corroborate these initial findings, identify novel or similar HLA variants in a range of ethnic groups, and elucidate the effects of these polymorphisms on the wound-healing process. In summary, keloid pathogenesis susceptibility is influenced by a number of genetic loci, albeit it is unclear which specific gene variants are involved. Keloid susceptibility is made more complex by the possibility of epigenetic control and the unclear processes underlying these genome-wide related SNPs [
45,
46,
47,
48].
Therapies for Keloids
The treatment of keloid scars remains a significant challenge due to their high recurrence rates and resistance to many traditional therapies. Various treatment strategies have been explored, each with its own advantages and limitations.
Intralesional corticosteroid injections, primarily with triamcinolone acetonide, are a first-line treatment for keloid scars due to their ability to reduce collagen synthesis, scar inflammation, and abnormal fibroblast activity. Corticosteroids are effective in reducing the size and symptoms of keloids, although recurrence remains a significant issue post-treatment. Combining triamcinolone with 5-fluorouracil (5-FU) has demonstrated improved outcomes [
49]
While surgical excision can remove the keloid mass, recurrence rates are notoriously high, with over 50% of excised keloids regrowing without adjuvant therapy [
50]. Therefore, excision is often combined with other therapies such as corticosteroids, silicone gel sheeting, or radiation to mitigate recurrence. Silicone gel sheeting, in particular, is widely used postoperatively and has been shown to reduce recurrence to 9% when combined with compression devices like pressure earrings for earlobe keloids [
51]. The use of surgical techniques such as Z-plasty or W-plasty can also help redistribute tension across the scar, which may help in reducing the likelihood of recurrence.
Cryotherapy, the controlled freezing of keloid tissue, has been employed as a treatment modality either alone or in combination with other treatments. Cryotherapy can effectively reduce the size of keloids by destroying the scar tissue. When combined with corticosteroids, cryotherapy has been shown to enhance outcomes, but care must be taken due to the risk of hypopigmentation, especially in darker skin types, which limits its widespread applicability [
49]
Laser therapy, particularly pulsed dye laser (PDL), targets the vascular component of keloid scars, reducing their redness and overall visibility. Previous articles demonstrated that PDL is effective in reducing keloid height and erythema when used in combination with other treatments like intralesional corticosteroids [
52]. Non-ablative fractional lasers (NAFL) have also been shown to be beneficial in improving the texture and pliability of keloid scars [
53]. However, laser treatments alone are often insufficient, with most studies recommending their use as part of a multi-modality approach [
54,
55,
56].
Radiotherapy is typically reserved for more severe cases of keloids, particularly post-excision. It is effective in reducing recurrence but carries a potential risk of long-term complications such as malignancy. Some studies reported that low-dose radiation therapy following keloid excision can significantly reduce recurrence rates, although its use remains controversial due to these potential risks [
57].
Botulinum toxin type A (BTA) has recently gained attention as a treatment option for keloids, primarily for its ability to reduce muscle tension and thereby lessen mechanical forces on scars. Several studies showed that botulinum toxin not only improved keloid appearance but also reduced symptoms such as itching and pain, making it an attractive option for patients who suffer from symptomatic keloids [
58,
59,
60]. Moreover, a randomized clinical trial comparing botulinum toxin with intralesional corticosteroids demonstrated fewer side effects and better patient satisfaction in the botulinum toxin group suggesting it may offer an advantage in long-term management [
61].
Intralesional chemotherapy agents such as 5-fluorouracil (5-FU) and bleomycin have also been explored as alternatives to corticosteroids for keloid treatment. A study demonstrated that the combination of 5-FU with corticosteroids significantly enhances treatment efficacy while reducing adverse effects like skin atrophy [
49]. Bleomycin, though effective, is limited by its potential side effects, including hyperpigmentation and systemic toxicity [
49,
62].
As previously mentioned, keloids frequently require combined therapies of the detailed above in order to obtain satisfactory outcomes.
3.3. Hypertrophic Scars
One common outcome of the healing process is the scar. A fibrotic hypertrophic scar may form in burn victims as a result of their wound healing processes. This scar form is characterized by a red, elevated, and stiff appearance that causes significant problems with both function and appearance [
39,
40]. In areas of the body with tight skin, such as the back, chest, shoulders, upper arms, elbows, and other joints, hypertrophic scars are common. Anywhere there is a wound or injury on the skin, hypertrophic scars may form [
63,
64,
65,
66].
Hypertrophic scars (HTS) are associated with a number of different mechanisms, such as abnormal extracellular matrix synthesis, increased neovascularization, irregular extracellular matrix remodeling, prolonged inflammation, and extended reepithelialization. Fibroblasts are stimulated both directly and indirectly by platelets, macrophages, T-lymphocytes, mast cells, Langerhans cells, and keratinocytes, which leads to an excess of extracellular matrix formation. Although the exact relationship between these processes and the development of atypical scar tissue is yet unknown, studies suggest that immune responses that occur shortly after damage may have a substantial impact on this result [
36,
43,
65,
66,
67].
A large amount of evidence suggests that acute inflammation generates pro-fibrotic substances, which in turn stimulate fibroblasts and cause HTS. Prolonged re-epithelialization and excessive angiogenesis may also delay the release of pro-fibrotic growth factors. Many biomolecules have been linked to HTS in recent years, but the exact mechanisms underlying these associations are still not fully understood, in part due to the complex and overlapping nature of wound-healing processes. Many factors play a role in the development of a hypertrophic scar while the lesion heals. HTS and myofibroblast activation are linked to the fibrin provisional matrix that forms following hemostasis. High-density fibrin clot deposition during the first healing phase may be predictive of the production of hypertrophic scars, although more investigation is needed to validate this relationship. During hemostasis, platelets release growth factors that promote fibrosis, such as PDGF, VEGF, TGF-β1, and CTGF, which are linked to the development of HTS. Because it inhibits the synthesis of pro-fibrotic molecules, PRP, which is obtained from peripheral blood platelets, is thought to be an alternate treatment for hypertrophic scars HTS [
36,
43,
65,
66,
67].
The most well-known pathophysiological reason of HTS formation is excessive inflammation, and several current interventions are designed to reduce inflammation. Increased levels of pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), toll-like receptor (TLR) activation, and the infiltration of inflammatory cells at the wound site are the outcomes of severe burn wounds with severe infection and tissue necrosis [
36,
43,
65,
66,
67].
Chemokine expression during HTS has been inconsistently reported in research; some studies found enhanced expression for 21–35 days, while others found reduced expression for 21–56 days. Interleukins, interferon, and growth factors are among the compounds secreted by inflammatory cells. These substances activate fibroblasts and are linked to the development of HTS. Shortly after damage, hypofibrotic diabetic wounds have been shown to have insufficient pro-inflammatory responses, which makes them prone to infection and impairs healing. More research is needed to understand why inflammatory responses are delayed during the initial healing phase after damage and how this affects the formation of hypertrophic scars [
36,
43,
65,
66,
67]. One important cytokine that impacts the intermediate and later phases of healing, including hypertrophic scarring is inflammation. Since IL-6 is highly expressed in HTS, treating HTS may involve targeting it as a therapeutic target. Other inflammatory cytokines that are being investigated as possible HTS treatment targets include IL-1β, IL-4, IL-8, IL-17, IL-13, and IL-22 [
36,
43,
65,
66,
67].
Inflammation is impacted by alternative lipid metabolism, which can cause severe scarring. In sick HTS and HTS-derived fibroblasts, there is a reduction in the mRNA and protein expression of fatty acid synthase (FASN) and sterol regulatory element-binding protein-1 (SREBP1). It has been demonstrated that TNF-stimulated gene 6 (TSG-6) prevents scarring by suppressing the IRE1α/TRAF2/NF-κB signaling cascade [
36,
43,
65,
66,
67].
In HTS, the proliferative phase processes, including as angiogenesis and extracellular matrix deposition, are notably active. During high-throughput screening (HTS), pro-fibrotic growth factors such TGF-β, PDGF, VEGF, and CTGF are secreted by cells such as fibroblasts, endothelial cells, and keratinocytes. Fibroblasts are stimulated to produce more extracellular matrix proteins, such as collagen, fibronectin, laminin, periostin, fibrillin, and tenascin, when the environment is pro-fibrotic. However, there have been reports of altered or decreased expression of certain extracellular matrix proteins, such as decorin, dermatopontin, and hyaluronic acid [
36,
43,
65,
66,
67].
HTS may be exacerbated by endothelial dysfunction and altered expression of angiogenic genes, such as endothelin-1, angiopoietin-1, angiopoietin-2, and angiogenin, according to endothelial cells obtained from pig burn wounds. Hypertrophic scarring may also result from keratinocytes' dysregulation of Notch signaling. Patients with hypertrophic scars had significantly higher levels of expression in their epidermis for Notch 1 signaling and intracellular domains, such as Hes1 and Jagged1 [
36,
43,
65,
66,
67].
The balance between ECM synthesis and remodeling is upset in HTS. In HTS, fibroblasts and myofibroblasts continue to deposit collagen I and III. Scarring results from the accumulation of excess fibrous collagen I brought on by the irregularities in apoptosis, which maintain the presence of myofibroblasts over time. Hypertrophic scars are characterized by nodules that include myofibroblasts HTS. HTS development can be aided by the differentiation of fibroblasts into myofibroblasts, which can be induced by mechanical strain and TGF-β [
36,
43,
65,
66,
67].
During the formation of HTS, MMPs—including MMP1 and MMP7—are downregulated. This results in a reduced breakdown of ECM components such collagen I, collagen III, and fibronectin. It has been shown that MMP1 injection is effective in improving the appearance of scars. TIMP1 and TIMP2, two TIMPs, reduce the activity of matrix MMPs during the development of hypertensive heart disease. In HTS, there is an increase in MMP2, MMP9, and MMP13 expression. The delivery of decorin, a matricellular protein involved in the structure of collagen fibers, has been effective in reducing the formation of HTS. Furthermore, via modifying TGF-β1 activity, blocking the lysyl hydroxylase enzyme, which is involved in the formation of pyridinoline cross-links, reduces fibroblast proliferation [
36,
43,
65,
66,
67].
HTS is caused by the activation of many genes required for the formation of extracellular matrix and fibroblast proliferation by the IL-6/STAT3 pathway. Those with hypertrophic scarring had lower levels of IL-10, an anti-inflammatory cytokine and possible therapeutic agent, than those without hypertrophic scarring. Based on certain research, fibroblasts are directly impacted by IL-10 through stimulation of the AKT or STAT3 signaling pathways. It has been shown that IL-10 inhibits the TLR4/NF-kB pathway in dermal fibroblasts, which reduces the production of scars. To fully understand IL-10's function in inhibiting the formation of HTS, more research is required [
47,
68].
One theory that is put up to explain the genesis of keloids is genetic causes. Mutations and polymorphisms in genes can interfere with the signaling pathways that are necessary for the later stages of the wound healing process. The pathophysiology of abnormal scarring may involve multiple pathways, including the TGF-B1/Smad pathway, MAPK kinase pathway, IGF-I and its receptor, plasminogen activator inhibitor-1, urokinase plasminogen activator, gene polymorphisms of the vitamin D receptor and the ADAM33 gene, and aberrant expression of suppressor genes. Additionally, the effect on type 2 hyaluronidase synthase and heat shock protein expression was shown. Clarifying the genetic basis of hypertrophic scar formation may contribute to a thorough comprehension of their pathophysiology and provide important therapeutic benefits, ultimately leading to the development of effective treatment approaches [
47,
68].
Therapies for Hypertrophic Scars
Hypertrophic scars are a common concern in dermatology, and several treatments have emerged to address their development, severity, and aesthetic impact. The main differences between keloids and hypertrophic scars are detailed on
Table 1.
Silicone gel sheeting remains a cornerstone in the management of hypertrophic scars. It functions primarily by maintaining moisture and exerting pressure on the scar tissue, which helps reduce collagen proliferation [
69]. Studies have shown that silicone sheets effectively improve scar texture, pigmentation, and overall appearance. Shen et al. demonstrated that the use of silicone gel in combination with other treatments like elastic sleeves significantly reduced the severity of hypertrophic scars in pediatric patients with burn injuries [
70]. The Vancouver Scar Scale (VSS) scores in patients using silicone gel were significantly better than those who did not use the gel, supporting its role as a first-line treatment for hypertrophic scars.
Laser therapies, particularly fractional CO2 lasers and pulsed dye lasers (PDLs), have gained popularity for treating hypertrophic scars due to their ability to remodel collagen and improve scar appearance [
71]. Fractional CO2 laser therapy targets both the dermis and epidermis, promoting collagen remodeling and reducing scar height and thickness. In the study by Ge et al., patients with hypertrophic scars saw significant improvements in scar texture, pliability, and pigmentation after several sessions of fractional CO2 laser treatments. These lasers also proved effective in reducing postoperative pain and improving patient satisfaction, with most patients reporting smooth skin and reduced scar visibility [
72].
Similarly, Yang et al. highlighted the efficacy of PDLs in reducing vascularity and pigmentation in hypertrophic scars. The study demonstrated that patients treated with PDLs showed significant improvements in the visual appearance of their scars, particularly in reducing redness and scar height. The study also noted that PDL treatments were generally well-tolerated, with minimal side effects [
73].
Microneedling has emerged as a promising treatment for hypertrophic scars, especially when combined with other modalities like PRP or chemical peels. This technique involves using tiny needles to create controlled micro-injuries in the skin, stimulating collagen production and scar remodeling. A study by Ali et al. compared the effects of microneedling alone versus microneedling combined with Jessner's solution peeling in patients with hypertrophic scars. The combined treatment showed superior outcomes, with more significant improvements in scar texture and pigmentation [
74].
Microneedling is particularly favored for its low risk of post-inflammatory hyperpigmentation, making it suitable for patients with darker skin tones. An et al. further supported the efficacy of microneedling when combined with radiofrequency and topical poly-lactic acid. Their randomized controlled trial demonstrated that this combination significantly improved scar smoothness, size, and overall appearance compared to monotherapy. These findings suggest that microneedling, particularly in combination with other treatments, can offer significant improvements in the management of hypertrophic scars [
75].
PRP therapy, which utilizes concentrated platelets from the patient's own blood, is known for its regenerative properties and has been increasingly used to treat hypertrophic scars. PRP is rich in growth factors that promote collagen remodeling and tissue healing, making it a suitable adjunctive therapy for scar management [
76,
77,
78]. A systematic review by Mujahid et al. indicated that combining PRP with other treatments like microneedling or laser therapy enhanced scar reduction outcomes. Patients receiving PRP combined with fractional CO2 laser therapy reported faster recovery times, reduced erythema, and overall better clinical outcomes compared to those receiving laser treatment alone [
79]. However, while PRP shows promise, its efficacy as a standalone treatment for hypertrophic scars remains debated. Studies suggest that PRP is more effective when used in conjunction with other treatments, particularly those that target collagen remodeling at a deeper level [
80].
Corticosteroid injections, particularly intralesional triamcinolone acetonide, are a well-established treatment for hypertrophic scars. The mechanism involves reducing inflammation and inhibiting fibroblast proliferation, which in turn reduces collagen synthesis. This treatment is most effective when administered in the early stages of scar formation, helping to prevent excessive collagen buildup [
81]. According to some articles, corticosteroid injections were found to be equally effective as botulinum toxin injections in reducing hypertrophic scar volume and improving pliability, while being better tolerated by patients [
82].
However, corticosteroid therapy is often associated with side effects such as skin atrophy and telangiectasia, which limits its long-term use in some cases. The combination of corticosteroids with other therapies has also proven beneficial. Studies have demonstrated that combining corticosteroids with 5-fluorouracil (5-FU) or botulinum toxin can enhance treatment efficacy. For example, a study highlighted in the review found that the combination of triamcinolone with 5-FU resulted in a significant reduction in scar thickness and a decrease in recurrence rates after surgical excision [
49]. This combination therapy, while effective, requires multiple treatment sessions and careful monitoring for potential adverse effects, such as local skin irritation or allergic reactions.
Several emerging therapies hold promise for the future treatment of hypertrophic scars, highlighting botulinum toxin injections, stem cell therapy, and novel laser technologies.
BTA, commonly known for its use in cosmetic procedures, has been shown to reduce the mechanical forces on wounds, thus preventing the exacerbation of hypertrophic scars [
83]. Botulinum toxin’s ability to paralyze muscles surrounding the scarred area may reduce tension on the wound, leading to better healing outcomes [
84].
Stem cell therapy is another innovative treatment being explored for hypertrophic scar management. Mesenchymal stem cells (MSCs) have regenerative properties that can aid in tissue repair and reduce fibrosis. Preliminary studies have indicated that MSCs can decrease collagen production and improve the quality of scar tissue. However, this therapy is still in the experimental stages, and more research is required to understand its long-term efficacy and safety [
85].
Lastly, advancements in laser technology, such as fractional ablative lasers combined with growth factors, are showing promise in enhancing the remodeling of scar tissue. These treatments not only target the scar itself but also promote healing through the stimulation of surrounding healthy tissue. Research by Xi et al. has indicated that these advanced lasers, when used in conjunction with other treatments, can lead to significant improvements in the texture, thickness, and appearance of hypertrophic scars [
86].