1. Introduction
The term Chronic Musculoskeletal Pain (CMP) identifies a rather heterogeneous group of myofascial and joint problems that range from localized pathologies such as Low Back Pain or Neck Pain to more generalized pathologies such as Osteoarthritis and Fibromyalgia [
1]. It is rather complex to determine an exact prevalence of CMP, due to the variability of the prevalence of individual problems converging within the pathology. However, it is estimated that a percentage ranging from 13.5 to 47% of the general population has experienced at least once in their life a pain that can be classified as CMP [
2].
Howsoever, CMP can also be determined and perpetuated by a very wide range of factors. Although the cause is in most cases mechanical, i.e. attributable to injuries or physical overuse syndromes [
2], CMP can also manifest itself following systemic and/or rheumatic pathologies, as in the case of fibromyalgia [
3] and various forms of arthritis [
4], which also involve persistent states of an inflammatory/oxidative nature and phenomena of dysregulation of the pain perception mechanisms [
4].
Furthermore, CMP would appear to be strongly influenced by psycho-emotional [
5] and nutritional factors [
6], which can determine a predisposition to the genesis of the problem and at the same time be important elements perpetuating it.
Given the extremely complex nature of CMP, it is very important to research all possible methods of evaluating the progress of the pathology, even beyond the predominant aspect of the presence of pain. In fact, although pain represents the dominant symptom of CMP, it is clear that the cause of the problem can be extremely complex and linked to a series of organic, psychological and habitual factors, which deserve to be investigated and monitored with dedicated instruments.
Among the least invasive and potentially most interesting evaluation methods in the clinical field, body Bioimpedance Analysis (BIA) is attracting growing interest in literature [
7]. This method is based on the detection of the behavior of a low intensity current that is made to flow through the patient's body [
7]. This passage of current allows to calculate two fundamental quantities such as Resistance (determined by the state of hydration of the body) and Reactance (due to the capacitance of cells membrane), relating them to each other in a single quantity defined as Phase Angle (PA), thus allowing to estimate the patient's body composition and his state of hydration [
7].
Although BIA is intended primarily as a method for evaluating body composition, several authors have used this instrument, in particular through the PA parameter, as a method for monitoring various clinical conditions [
8] ranging from metabolic syndrome [
9] to diabetic polyneuropathy [
10], up to the boundaries of the field of musculoskeletal pain [
11,
12,
13].
The use of BIA as a monitoring tool in musculoskeletal pain has already proven useful in our previous research experience [
14]. In that context, it was observed how the therapy consisting in the administration of Extremely Low-Frequency Electromagnetic Fields (ELF-MFs), falling within the scope of Bioresonance Therapy (BT) and Quantum Medicine (QM) [
14,
15,
16,
17,
18], was effective in improving musculoskeletal pain in the patients studied and, at the same time, it normalized the values of Resistance and Reactance detected in the sample through BIA [
14]. BT, also applied in the form of Ion Cyclotron Resonance (ICR), is essentially based on the use of ELF-MFs, with frequencies typically in the order of a few Hz or kHz and intensities in the order of a few µT which, in pathological situations, positively interfere with the altered magnetic biofield of the human body [
19], determining a balancing of the suffering physiological and metabolic patterns. These therapeutic effects of QM techniques such as the ICR-BT seems to be mediated by its ability to counteract oxidative stress, reduce inflammation levels and increase cellular metabolism and the production of mitochondrial ATP [
14,
15,
16,
17,
18].
Compared to our previous experience [
14], an even faster and more intuitive way to monitor the health status of patients suffering from musculoskeletal pain could be to use the single parameter of the PA, calculated according to the formula {arctangent (Reactance/Resistance) × (180°/π)}, for a current typically of about 50 kHz that is made to flow through the human body [
20]. This measurement method has proven to be the most reliable and repeatable in the bioelectrical evaluation of the human body [
21]. This is particularly true in the field of the assessment of so-called "cellular health" although this application is still controversial and unclear in terms of mechanisms justifying its use [
21]. Thus, more data and research are required on the reliability of BIA and, specifically, of the PA as tools for assessing health status in pathological contexts [
21].
Therefore, in light of all these considerations we decided to conduct an observation of previously collected data to determine the potential reliability of PA as a health status monitoring tool in a CMP context.
2. Materials and Methods
This research is a pilot retrospective analytical observational study carried out at the Gemelli Molise Hospital (Campobasso, Italy) cooperating with Ce.Fi.R.R. Organization (Center for Physiotherapy, Rehabilitation and Re-education) staff from January to June 2023.
All the procedures applied during the data collection comply with the safety regulations of the country where the study was made; the protocol is accessible to anyone who does not highlight specific contraindications during the initial clinical evaluation necessary for all patients who access the study facility. The protocol does not constitute an experimental practice, as it applies the same standard treatment procedures used for all patients who do not present the above-mentioned contraindications. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained at enrolment from participants who were willing and able.
Furthermore, the Ce.Fi.R.R. Organization, as the main company conducting the study, enjoys ISO 9001:2015 accreditation for the implementation of observational clinical studies in the rehabilitation field (ACCREDIA Certificate no. IT15/0304). Due to all these considerations and the lack of incontrovertible national legislation regarding the need for the submission of retrospective and/or non-pharmacological observational studies to an ethics committee [
22], normal ethics committee clearance was not required [
23].
Data were observed from a total of 53 Caucasian patients (37 women and 16 men) affected by CMP, mean age 60.3±15.3 years old, who underwent ICR-BT treatment at the study site during the reference period.
All patients were diagnosed with CMP by physicians specialized in musculoskeletal disorders, following the criterion recognized in the literature that defines CMP as a persistent or recurrent pain condition deriving from musculoskeletal structures such as muscles, joints or bones that lasts for more than 3 months [
24]. After the initial evaluation, patients received the medical prescription to perform a cycle of ICR-BT treatments.
All patients presenting other pathologies in addition to the diagnosed CMP were excluded from the observation. In particular, all the patients presenting the following conditions were excluded: pregnancy, epilepsy, electrical implants, tumors, infections, tuberculosis, serious heart disease, neurological pathologies and an age under 18 years old.
All observed patients were evaluated before the start (T0) of the first ICR-BT session and after the end (T1) of the last ICR-BT session, through the following two assessment methods:
- The Numeric Pain Rating Scale (NPRS): it is one of the most widespreaded instruments for measuring the pain perceived by patients. It derives from the Visual-Analogue Scale (VAS) and it is divided into ten levels, usually evenly distributed on a 10 cm long strip, with each number written on the strip corresponding to the level of pain perceived by the patient at the time of the evaluation, where 0 is the total absence of pain and 10 is the maximum level of pain imaginable and/or ever experienced by the patient [
25]. This scale, reliable and easy to apply, proved to be effective even in the presence of chronic dysfunctions of the musculoskeletal system [
25]. In the case of the present study, patients were asked to express a value from 0 to 10 corresponding to the maximum level of pain perceived at the most insidious musculoskeletal level.
- The BIA: as previously explained, this method is based on the detection of the behavior of a small current, typically at a fixed frequency of 50 kHz, which is made to flow through the body of the patient to then derive physical quantities such as Resistance, Reactance and PA [
21]. This method allows to evaluate the body composition of the patient, in terms of lean mass and fat mass, as well as the state of body hydration and the distribution of liquids between the intracellular and extracellular compartments, potentially indicating metabolic and functional alterations in the presence of various types of pathologies [
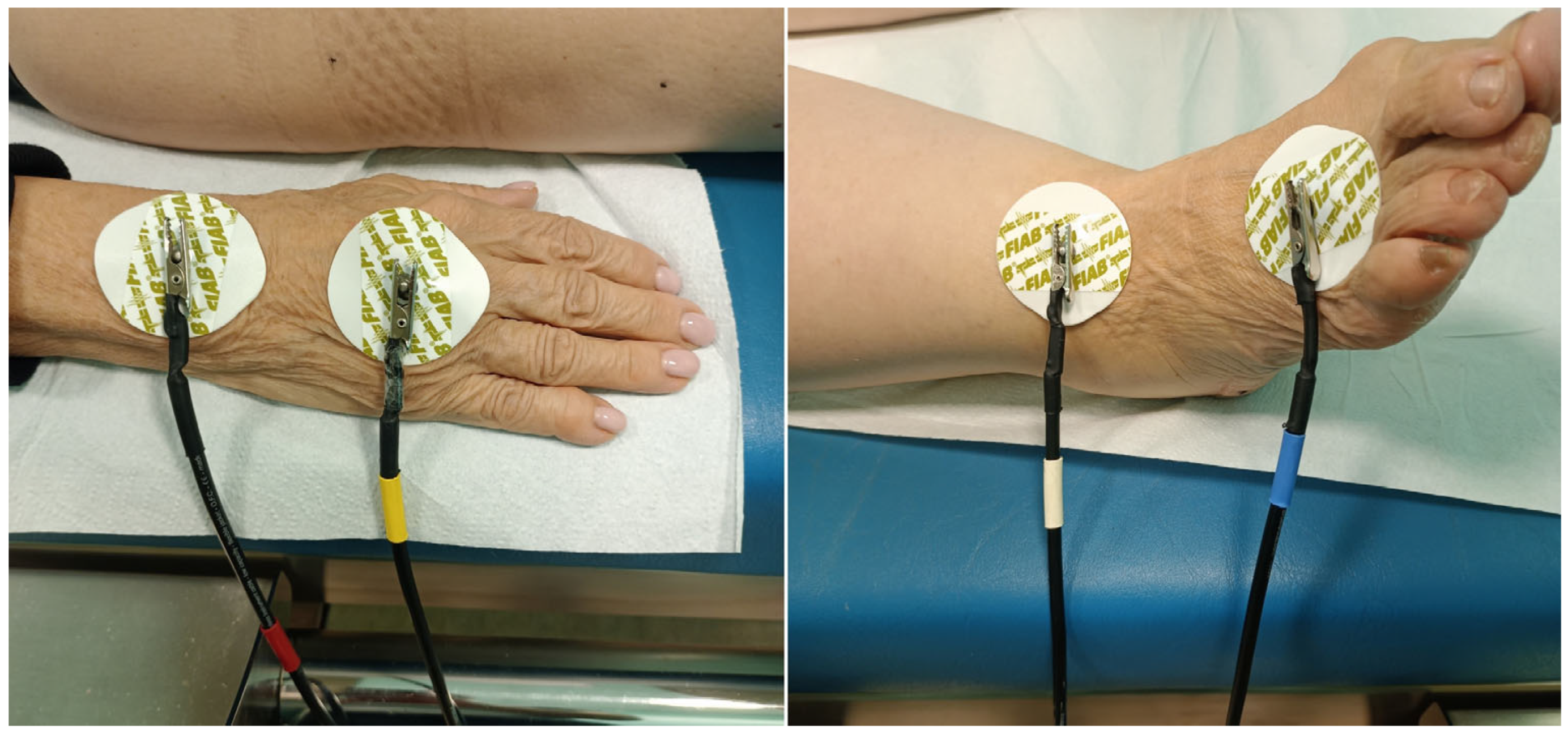
21]. For this study, the BIA data were collected through the bioimpedance system integrated into the same instrumentation used for the ICR-BT sessions, called Quec Phisis 1 (Prometeo S.r.l., Padova, Italy). The measurement was performed as usual on the right side of the patient's body, lying on the same treatment bed in a supine and relaxed position. The 4 detection channels of the instrumentation were connected to the patient's body using disposable 48x50 mm F9060 ECG electrodes (FIAB SpA, Firenze, Italy), arranged in pairs at the level of the hand-wrist and ankle-foot areas, as shown in
Figure 1.
To limit the influence of environmental factors on NPRS and BIA measurements, both evaluations and treatments were always carried out in the same room, with constant artificial lighting and a fixed temperature of 21°C. The BIA evaluation at T0 was always performed after a period of adaptation to the supine position for the patient of at least 5 minutes.
All patients underwent a total of 10 sessions of ICR-BT over a period of one month, with a treatment frequency of one session every 48-72 hours. Each therapeutic session, with a fixed duration of 45 minutes, consisted of the application of ELF-MFs emitted by the same Quec Phisis 1 equipment used for the BIA assessment. The ICR-BT device used consists of a flat diamagnetic bed surrounded by 4 resonant coils based on the principles of the Helmholtz resonator [
26], connected to a generator of ultra-weak magnetic fields that can be modulated in both frequency and intensity and to a sensor for detecting the local geomagnetic field. The control of the instrumentation is carried out by means of a common portable PC equipped with a proprietary software dedicated to the operation of the instrumentation and the BIA assessment. The emitted magnetic field is automatically set by the proprietary software of the equipment, with frequency values up to 100 Hz and intensity between 5 and 15 µT. These automatically modulated emission parameters are based on the answers given by the patient to a questionnaire about his general health status and on the items checked by the operator in a list of pathologies that could affect the patient.
Both the collection of the assessment data and the treatment setting and execution were carried out by the same therapist, adequately trained, for all the patients studied.
For data analysis, longitudinal linear mixed models were applied using time since baseline as the time scale to identify factors associated with change over time of PA and NRS. Age and PA were considered as confounders; second order interactions were explored and reported if statistically significant.
3. Results
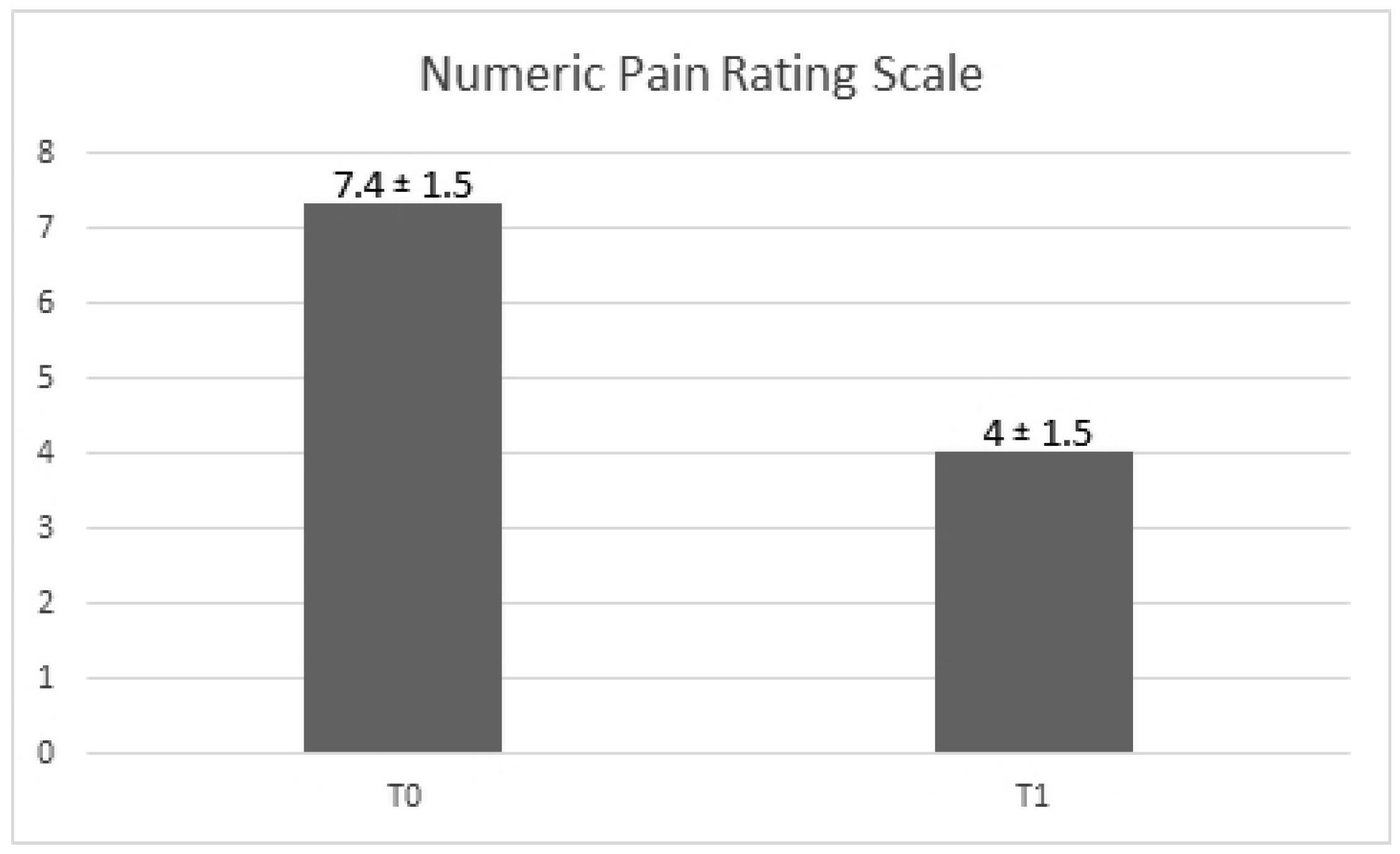
We observe a decrease of the NPRS value between the two times of the study. As matter of fact, the score change is -3.35 ± 0.26, with a
p-value <0.001 (
Figure 2). The change in the NPRS values took place independently from that of the PA and age, that did not reach a statistically significant role (
p-value = 0.69;
p-value = 0.25).
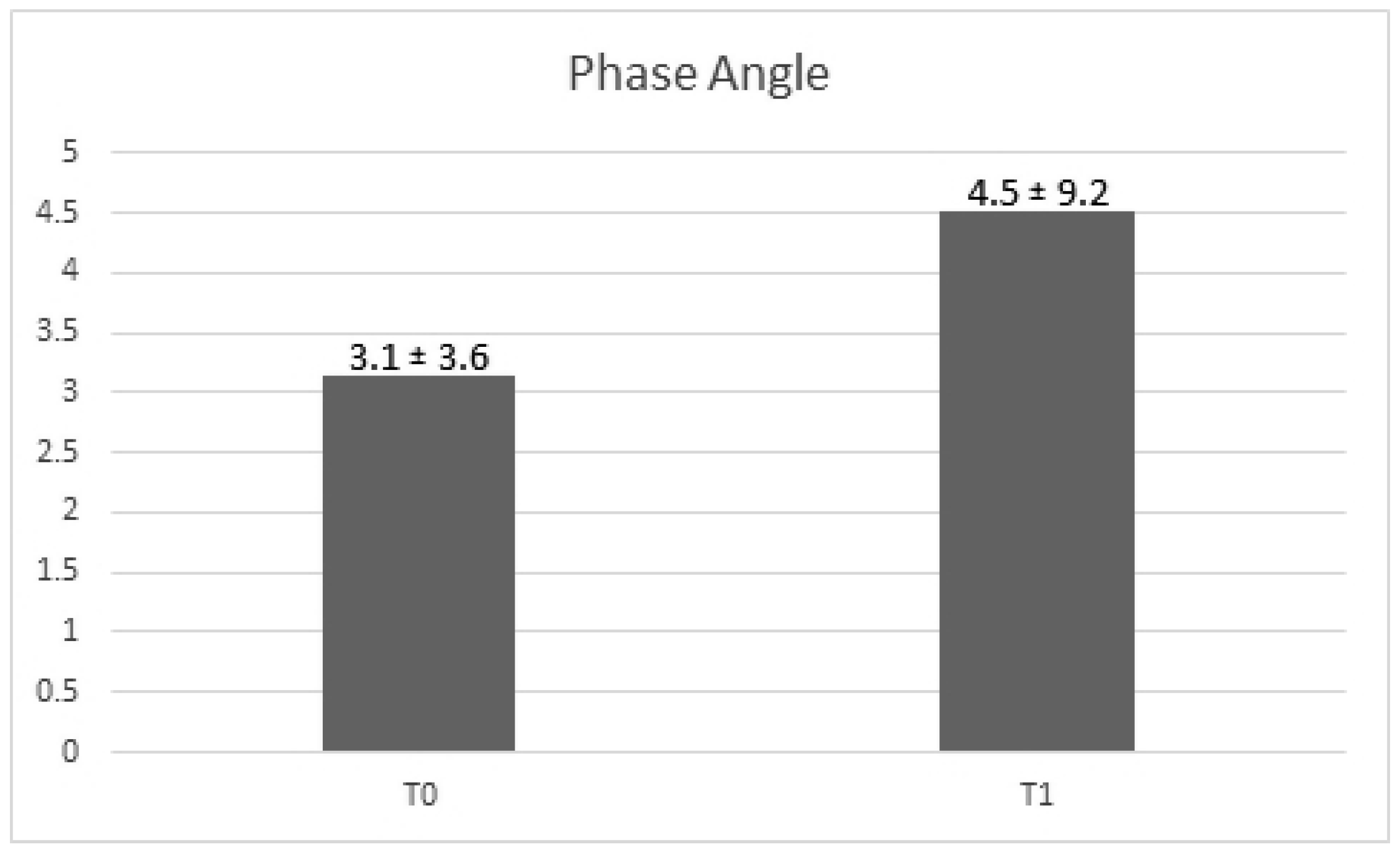
Regarding the PA value, it is possible to observe a slight increase of it between times T0 and T1. However, this increase does not reach statistical significance over the two time of the study (beta ± SE: 1.37 ± 1.21,
p-value =0.26) (
Figure 3). The model considers also age (
p-value = 0.21).
4. Discussion
Observing the data collected between T0 and T1 during this study, it is possible to highlight how the treatment with ICR-BT has significantly reduced the level of pain perceived by the patients according to the NPRS scale. Furthermore, a positive although not significant PA increase is highlighted.
The first finding of the present research is the confirmation of the analgesic properties of ICR-BT. It has already been highlighted in the past by our experience and that of other authors that BT is able to reduce painful symptoms in pathological conditions that include, among others, chronic pain [
14], visceral and somatic pain [
27], oncological pain [
28] and phantom limb pain [
29].
The pain modulation induced by BT would appear to be determined by directly anti-inflammatory and immunomodulatory mechanisms [
14,
27,
28,
30] as well as by the possible release of analgesic neurotransmitters [
29].
Regarding the PA values detected between T0 and T1, it is possible to observe that, although the increase recorded is not significant, the trend observed for the value is still consistent with an improvement in the clinical picture of the observed patients.
The definition of “normal” PA values is still a deeply debated topic in the clinical and diagnostic field. Some studies tend to classify as “normal” PA values that are approximately higher than 6° or 7° [
31,
32], with a tendency to greater values in males and in young subjects (approximately 18-39 years old) [
32]. However, more recent evidence tends to lower these “normal” reference values, bringing them to around 4.5° and 5° [
33,
34] depending on sex-age and taking also into account the patient's health status in hospitalization situations [
34]. Since in our study, following the ICR-BT treatments, the PA values increased from an average of 3.14° to a mean of 4.51°, it is possible to assert that the treatment induced a general, although statistically not significant, improvement of the PA values, which at the end of the observation were found to be closer to the normal healthy values indicated in the literature [
31,
32,
33,
34].
In any case, the data analysis highlighted that at the end of the treatment cycle it was not possible to detect a correlation between the trend of pain values recorded with the NPRS and that of the PA recorded with BIA. This result appears to be in contrast with the scientific literature, which attributes an increasing diagnostic and, above all, predictive value to PA in the presence of various types of pathologies [
8,
9,
10,
11,
12,
13,
14,
34].
Although at first glance this lack of correlation could suggest a poor value of PA as a diagnostic tool in the presence of CMP, the fact that both values tend to improve following the application of ICR-BT treatment protocol, albeit significantly only for pain, would seem to suggest that this misalignment could depend on intrinsic factors of the protocol.
It is possible that many of the discrepancies observed in the present study are attributable to some structural limitations of the same. First, the observational nature of the study has placed clear limits in terms of sample selection and duration of the research. The sample size was objectively limited to the number of patients who turned to the study center and who received, from the evaluating physicians, the prescription of a therapeutic path with ICR-BT, alternative to other classic CMP management protocols. Furthermore, since the data collection took place on patients with an evaluation and therapeutic path already defined, it was not possible to define a control group or follow-up assessments. However, the observational nature of the protocol allowed us to obtain data in a real clinical setting, possibly less artificial than an experimental setting, allowing at least to obtain preliminary data useful for designing specific experimental studies on the topic.
5. Conclusions
This study confirmed the effectiveness of ICR-BT in reducing pain perceived by patients with CMP. Furthermore, it was possible to observe a positive but non-significant modification of the PA value detected by BIA. This misalignment in the significance of the trend of pain and PA did not allow to confirm a direct correlation between the two factors, making it necessary to further clarify the diagnostic role of BIA, and in particular of PA, in the clinical and diagnostic field. Since the usefulness of BIA as an element of evaluation in the presence of pathologies is still widely studied, this brief observational research lays the foundation for future studies on the topic of the use of BIA as an assessment tool for musculoskeletal pathologies such as CMP. In light of our results, a more extensive and structured setting would be required to draw clear conclusions on the topic. In fact, despite our observations, the PA remains a promising but still not easily interpretable tool as a diagnostic element in the musculoskeletal field, which could benefit from observations conducted on larger samples and over a longer period of time, to clearly identify its potential and limits.
Author Contributions
Conceptualization, G.B., M.P. and R.P.; methodology, G.B.; software, A.d.I.; validation, P.E.G. and V.G.; formal analysis, A.d.I.; investigation, G.B.; resources, M.P.; data curation, A.d.I.; writing—original draft preparation, G.B. and L.P.; writing—review and editing, L.P. and P.E.G.; visualization, P.S.; supervision, P.S.; project administration, G.B. and M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All the procedures applied during the data collection comply with the safety regulations of the country where the study was made; the protocol is accessible to anyone who does not highlight specific contraindications during the initial clinical evaluation necessary for all patients who access the study facility. The protocol does not constitute an experimental practice, as it applies the same standard treatment procedures used for all patients who do not present the above-mentioned contraindications. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Furthermore, the Ce.Fi.R.R. Organization, as the main company conducting the study, enjoys ISO 9001:2015 accreditation for the implementation of observational clinical studies in the rehabilitation field (ACCREDIA Certificate no. IT15/0304). Due to all these considerations and the lack of incontrovertible national legislation regarding the need for the submission of retrospective and/or non-pharmacological observational studies to an ethics committee, normal ethics committee clearance was not required.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study, due to the preservation of the privacy of interested parties, could be made available upon motivated and reasonable private request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flynn, D.M. Chronic musculoskeletal pain: nonpharmacologic, noninvasive treatments. American family physician 2020, 102(8), 465–477. [Google Scholar] [PubMed]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.; Pergolizzi, J.V.; Christo, P.J. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain and therapy 2021, 10, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic pain in musculoskeletal diseases: Do you know your enemy? Journal of clinical medicine 2022, 11(9), 2609. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.A.; Celik, O.F.; Ayhan, F.F. The important role of central sensitization in chronic musculoskeletal pain seen in different rheumatic diseases. Clinical rheumatology 2020, 39, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calderon, J.; Flores-Cortes, M.; Morales-Asencio, J.M.; Luque-Suarez, A. Which psychological factors are involved in the onset and/or persistence of musculoskeletal pain? An umbrella review of systematic reviews and meta-analyses of prospective cohort studies. The clinical journal of pain 2020, 36(8), 626–637. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do nutritional factors interact with chronic musculoskeletal pain? A systematic review. Journal of clinical medicine 2020, 9(3), 702. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14(6), 10895–10928. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Garcia-Almeida, J.M. Phase angle in applications of bioimpedance in health and disease. Reviews in Endocrine and Metabolic Disorders 2023, 24(3), 367–370. [Google Scholar] [CrossRef]
- Praget-Bracamontes, S.; González-Arellanes, R.; Aguilar-Salinas, C.A.; Martagón, A.J. Phase angle as a potential screening tool in adults with metabolic diseases in clinical practice: a systematic review. International Journal of Environmental Research and Public Health 2023, 20(2), 1608. [Google Scholar] [CrossRef]
- Schimpfle, L.; Tsilingiris, D.; Mooshage, C.M.; Kender, Z.; Sulaj, A.; von Rauchhaupt, E.; Szendroedi, J.; Herzig, S.; Goepfert, J.; Groener, J.; Nawroth, P.P. Phase angle of bioelectrical impedance analysis as an indicator for diabetic polyneuropathy in type 2 diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism 2024, dgad737. [Google Scholar]
- Pais, S.R.; Guerreiro, P.M.; Guerreiro, C.S.; Botelho, M.C. Phase angle in osteoarthritis patients, relations with pain, muscular mass and strength. Osteoarthritis and Cartilage 2020, 28, S395–396. [Google Scholar] [CrossRef]
- Otsubo, R.; Hashida, R.; Murotani, K.; Iwanaga, S.; Hirota, K.; Koya, S.; Tsukada, Y.; Ogata, Y.; Yokosuka, K.; Yoshida, T.; Nakae, I. Phase angle is related to physical function and quality of life in preoperative patients with lumbar spinal stenosis. Scientific Reports 2023, 13(1), 13909. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Tetsunaga, T.; Misawa, H.; Nishida, K.; Ozaki, T. Association of phase angle with sarcopenia in chronic musculoskeletal pain patients: a retrospective study. Journal of Orthopaedic Surgery and Research 2023, 18(1), 87. [Google Scholar] [CrossRef] [PubMed]
- Barassi, G.; Pokorski, M.; Pellegrino, R.; Supplizi, M.; Prosperi, L.; Marinucci, C.; Di Simone, E.; Mariani, C.; Younes, A.; Di Iorio, A. Quantum medicine: A role of extremely low-frequency magnetic fields in the management of chronic pain. In Integrative Clinical Research, Pokorski, M., Ed; Springer International Publishing: Cham, Switzerland, 2022; pp. 23–28. [Google Scholar]
- Rezaei, N. Quantum Medicine and the Immune System. In Handbook of Cancer and Immunology, 1st ed.; Rezaei, N. Springer International Publishing: New York, NY, USA, 2023; pp. 1–24. [Google Scholar]
- Gonzalez, M.J.; Sutherland, E.; Olalde, J. Quantum functional energy medicine: The next frontier of restorative medicine. J. Restor. Med. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Faramarzpour, M.; Ghaderinia, M.; Abadijoo, H.; Aghababa, H. The Possibility of Quantum Medicine in Cancer Research: A Review. Biophys. Rev. Lett. 2021, 16, 21–40. [Google Scholar] [CrossRef]
- Paolo, D.I. Quantum Physics between Matter, Information and Consciousness: The Case of Quantum Medicine. Turk. Online J. Educ. Technol. 2022, 187–193. [Google Scholar]
- Rubik, B. The biofield hypothesis: Its biophysical basis and role in medicine. J. Altern. Complement. Med. 2002, 8, 703–717. [Google Scholar] [CrossRef]
- Campa, F.; Coratella, G.; Cerullo, G.; Stagi, S.; Paoli, S.; Marini, S.; Grigoletto, A.; Moroni, A.; Petri, C.; Andreoli, A.; Ceolin, C. New bioelectrical impedance vector references and phase angle centile curves in 4,367 adults: the need for an urgent update after 30 years. Clinical Nutrition 2023, 42(9), 1749–1758. [Google Scholar] [CrossRef]
- Ward, L.C.; Brantlov, S. Bioimpedance basics and phase angle fundamentals. Reviews in Endocrine and Metabolic Disorders 2023, 24(3), 381–391. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Daar, S.; Tzoulis, P.; Fiscina, B.; Kattamis, C. Retrospective observational studies: Lights and shadows for medical writers. Acta Bio Medica Atenei Parmensis 2022, 93, e2022319. [Google Scholar]
- Winter, E.M.; Maughan, R.J. Requirements for ethics approvals. J. Sports Sci. 2009, 27, 985. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Mei, H.; Fang, F.; Ma, X. What is new in classification, diagnosis and management of chronic musculoskeletal pain: a narrative review. Frontiers in Pain Research 2022, 3, 937004. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, S.W.; Kolber, M.J.; Mokha, M.; Hanney, W.J. Concurrent validity of pain scales in individuals with myofascial pain and fibromyalgia. Journal of Bodywork and Movement Therapies 2018, 22(2), 355–360. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.K.; Sirignano, W.A. Theory of a generalized Helmholtz resonator. Journal of Sound and Vibration 1973, 26(2), 247–262. [Google Scholar] [CrossRef]
- Barassi, G.; Pirozzi, G.A.; Di Iorio, A.; Pellegrino, R.; Galasso, P.; Heimes, D.; Praitano, B.; Gallenga, P.E.; Prosperi, L.; Moccia, A.; Panunzio, M. Quantum Medicine and Irritable Bowel Syndrome-Associated Chronic Low-Back Pain: A Pilot Observational Study on the Clinical and Bio-Psycho-Social Effects of Bioresonance Therapy. Medicina 2024, 60(7), 1099. [Google Scholar] [CrossRef]
- Kirsever, E.; Kiziltan, H.S.; Yilmaz, R. Palliative effects of bioresonance therapy with or without radiotherapy or chemotherapy on cancer patients. International Journal of Radiation Research 2022, 20(1), 43–48. [Google Scholar] [CrossRef]
- Imanzade, M.; Shafaeizadeh, A.; Dadpay, M.; Yegane, H.T.; Keshvari, H.; Abadi, M.B.; Zeynalipour, M. Improvement of Phantom Pain by the Bioresonance Technology. Annals of Military and Health Sciences Research 2021, 19(2), e112871. [Google Scholar] [CrossRef]
- Sakharov, D.; Savtsova, Z.; Indyk, V.; Kovbasyuk, S.; Voicikova, I.; Zaritskaia, M.; Ortovsky, A.; Ja, S.; Kavetsky, R.E.; Lednyickzy, G. Bioresonance therapy corrects the immunodeficiency of Chernobyl mice. In BEMS 17th Annual Meeting, Boston, 18th June 1995, Vol. 22.
- Kumar, S.; Dutt, A.; Hemraj, S.; Bhat, S.; Manipadybhima, B. Phase angle measurement in healthy human subjects through bio-impedance analysis. Iranian journal of basic medical sciences 2012, 15(6), 1180. [Google Scholar]
- Barbosa-Silva, M.C.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson Jr, R.N. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. The American journal of clinical nutrition 2005, 82(1), 49–52. [Google Scholar] [CrossRef]
- Cimmino, F.; Petrella, L.; Cavaliere, G.; Ambrosio, K.; Trinchese, G.; Monda, V.; D’Angelo, M.; Di Giacomo, C.; Sacconi, A.; Messina, G.; Mollica, M.P. A bioelectrical impedance analysis in adult subjects: the relationship between phase angle and body cell mass. Journal of Functional Morphology and Kinesiology 2023, 8(3), 107. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. European journal of applied physiology 2002, 86, 509–516. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).