Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotypic Evaluation
2.3. Genome-Wide Association Mapping
2.4. Haplotype Analysis for Candidate Genes
3. Results
3.1. Phenotypic Variations in the Cold Tolerance (CT) of Rice at the Seedling Stage
3.2. GWAS for CT
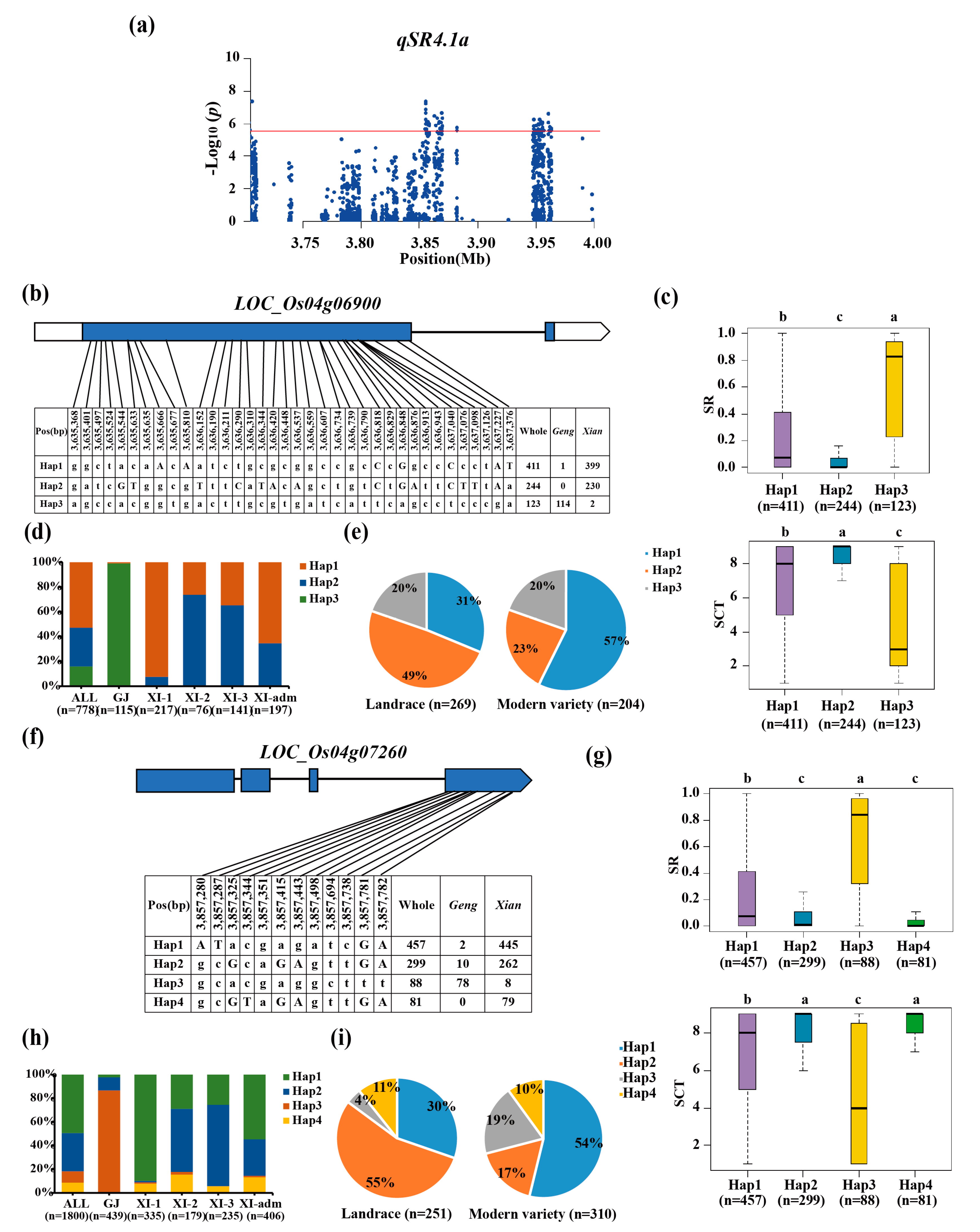
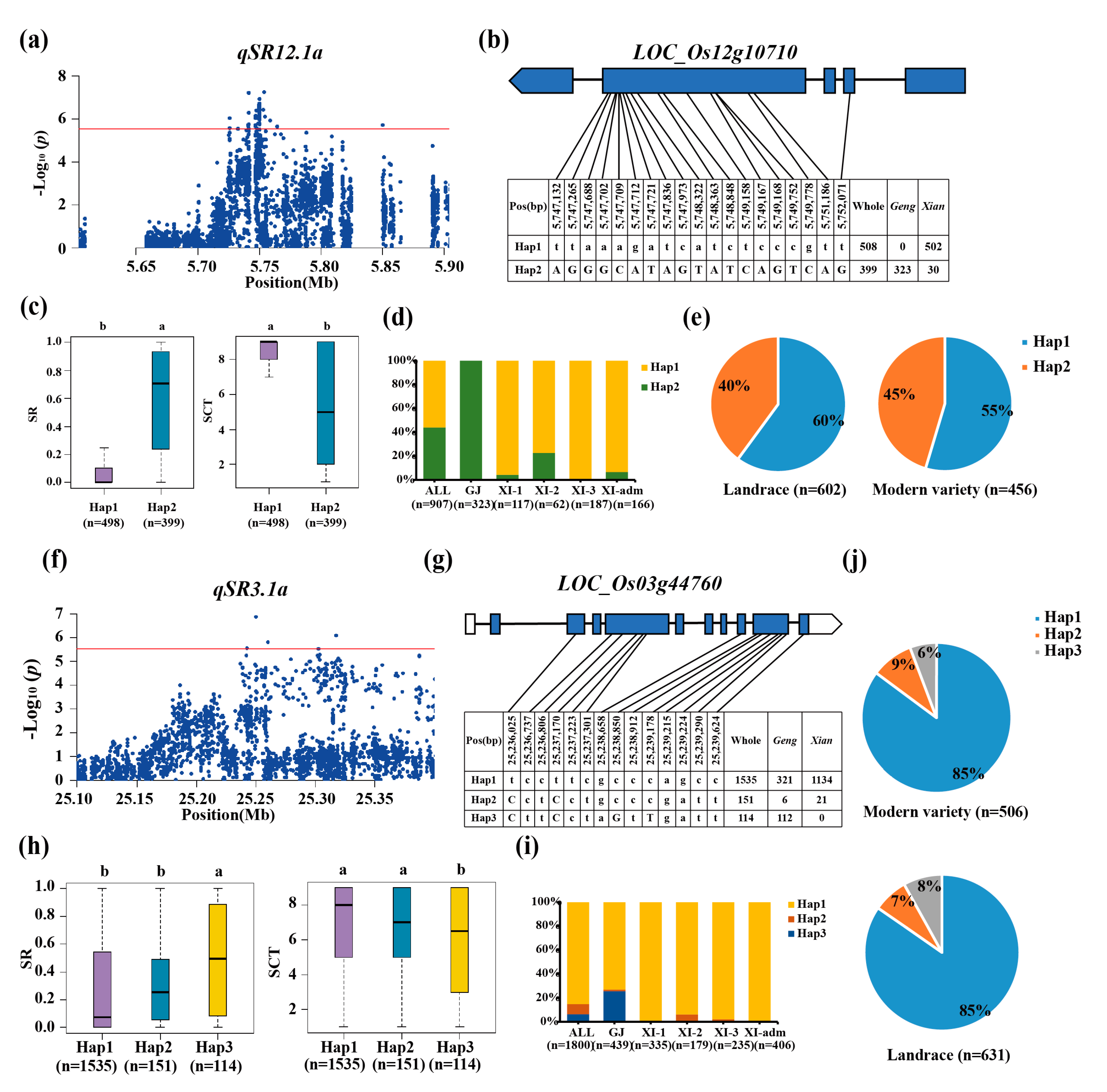
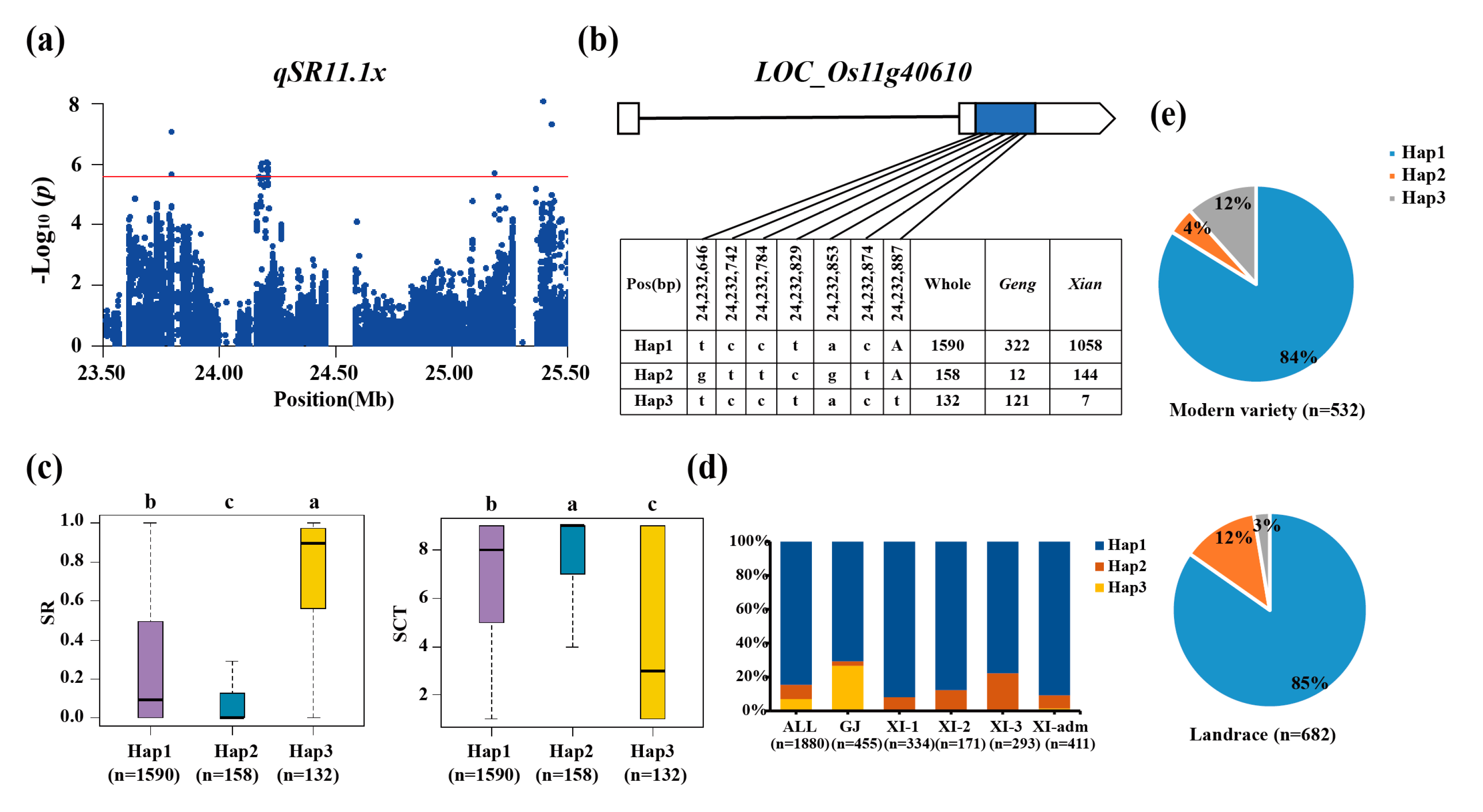
3.3. Haplotype Analyses of the Candidate Genes
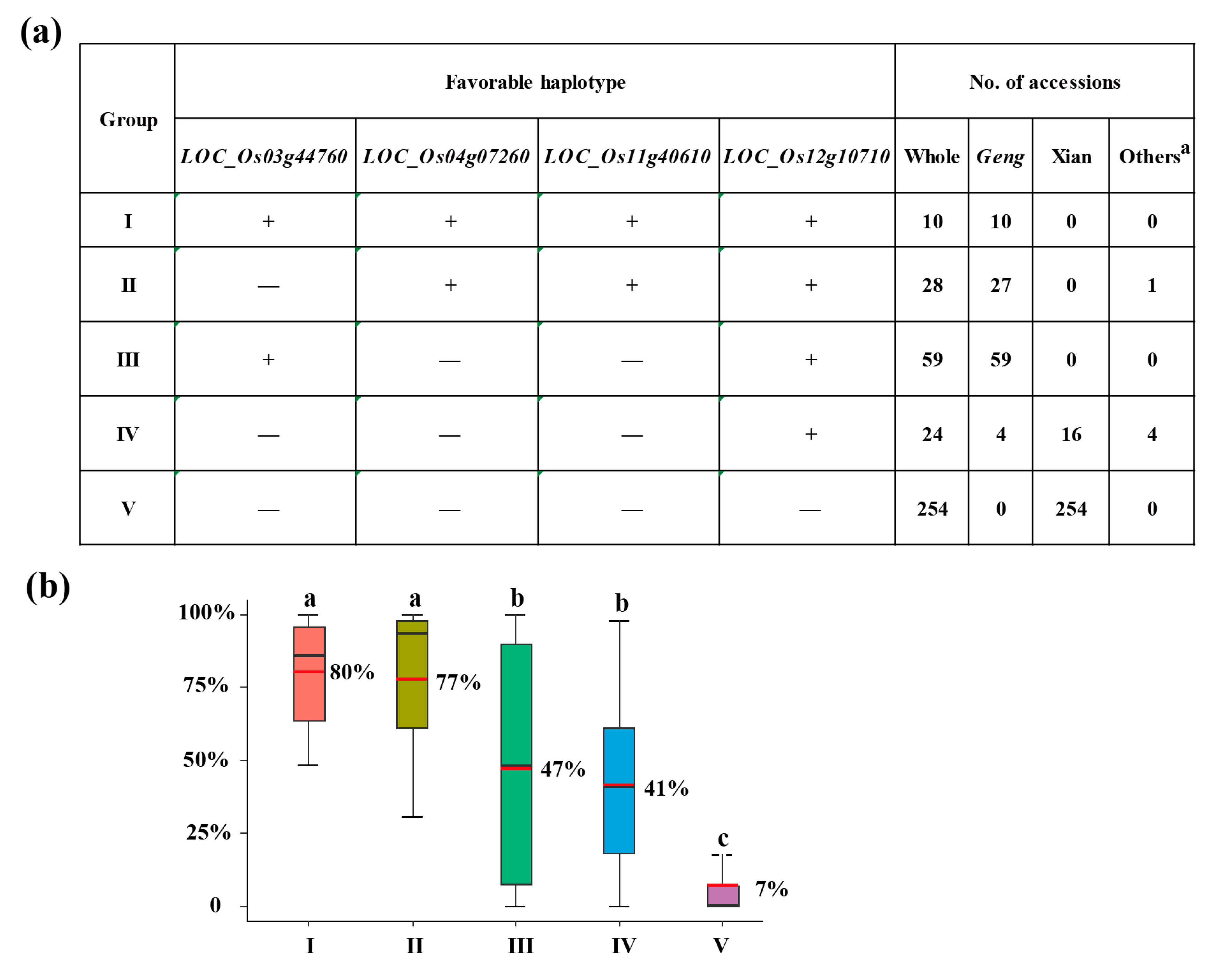
3.4. Optimal Combination of CT-Haplotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Rational design of high-yield and superior-quality rice. Nature Plants 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Pan, Y.; Li, J.; Zhou, L.; Shi, H.; Zeng, Y.; Guo, H.; Yang, S.; Zheng, W.; et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nature Communications 2017, 8, 1. [Google Scholar] [CrossRef]
- Li, L.; Mao, D.; Prasad, M. Deployment of cold tolerance loci from Oryza sativa ssp. Japonica cv. 'Nipponbare' in a highyielding Indica rice cultivar '93-11'. Plant Breeding 2018, 137, 553–560. [Google Scholar]
- Fujino, K.; Matsuda, Y. Genome-wide analysis of genes targeted by qLTG3-1 controlling low-temperature germinability in rice. Plant Molecular Biology 2009, 72, 137–152. [Google Scholar] [CrossRef]
- Mao, D.; Xin, Y.; Tan, Y.; Hu, X.; Bai, J.; Liu, Z.; Yu, Y.; Li, L.; Peng, C.; Fan, T.; et al. Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proceedings of the National Academy of Sciences 2019, 116, 3494–3501. [Google Scholar] [CrossRef]
- Saito, K.; Hayano, S.Y.; Kuroki, M.; Sato, Y. Map-based cloning of the rice cold tolerance gene Ctb1. Plant Science 2010, 179, 97–102. [Google Scholar] [CrossRef]
- Saito, K.; Miura, K.; Nagano, K.; Hayano, S.Y.; Araki, H.; Kato, A. Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theoretical and Applied Genetics 2001, 103, 862–868. [Google Scholar] [CrossRef]
- Saito, K.; Hayano, S.Y.; Maruyama-Funatsuki, W.; Sato, Y.; Kato, A. Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice. Theoretical and Applied Genetics 2004, 109, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jorde, L.B. Linkage Disequilibrium and the Search for Complex Disease Genes. Genome Research 2000, 10, 1435–1444. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Li, C.; Kang, H.; Wang, Y.; Tan, X.; Liu, M.; Deng, Y.; Wang, Z.; Liu, Y.; et al. Genome-wide Association Mapping of Cold Tolerance Genes at the Seedling Stage in Rice. Rice 2016, 9, 1. [Google Scholar] [CrossRef]
- Lv, Y.; Guo, Z.; Li, X.; Ye, H.; Li, X.; Xiong, L. New insights into the genetic basis of natural chilling and cold shock tolerance in rice by genome-wide association analysis. Plant, Cell & Environment 2015, 39, 556–570. [Google Scholar]
- Wang, X.; Zou, B.; Shao, Q.; Cui, Y.; Lu, S.; Zhang, Y.; Huang, Q.; Huang, J.; Hua, J. Natural variation reveals that OsSAP16 controls low-temperature germination in rice. Journal of Experimental Botany 2018, 69, 413–421. [Google Scholar] [CrossRef]
- Garris, A.J.; Tai, T.H.; Coburn, J.; Kresovich, S.; McCouch, S. Genetic Structure and Diversity in Oryza sativa L. Genetics 2005, 169, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; and Tanksley, S.D. Restriction fragment length polymorphism in Oryza sativa L. Genome 1989, 32, 1113–1118. [Google Scholar] [CrossRef]
- Wang, W.; Mauleon, R.; Hu, Z.; Chebotarov, D.; Tai, S.; Wu, Z.; Li, M.; Zheng, T.; Fuentes, R.R.; Zhang, F.; et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 2018, 557, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Yu, L.; Chen, D.; Li, L.; Zhu, Y.; Xiao, Y.; Zhang, D.; Chen, C. Multiple cold resistance loci confer the high cold tolerance adaptation of Dongxiang wild rice (Oryza rufipogon) to its high-latitude habitat. Theoretical and Applied Genetics 2015, 128, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.A.; Burgos, N.R.; Shivrain, V.K.; Murphy, B.; Gbur, E.E. Morphological and Physiological Responses of Weedy Red Rice (Oryza sativa L.) and Cultivated Rice (O. sativa) to N Supply. American Journal of Plant Sciences 2011, 2, 569–577. [Google Scholar] [CrossRef]
- Alexandrov, N.; Tai, S.; Wang, W.; Mansueto, L.; Palis, K.; Fuentes, R.R.; Ulat, V.J.; Chebotarov, D.; Zhang, G.; Li, Z.; et al. SNP-Seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Research 2015, 43, 1023–1027. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. The American Journal of Human Genetics 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Kang, H.M.; Sul, J.H.; Service, S.K.; Zaitlen, N.A.; Kong, S.; Freimer, N.B.; Sabatti, C.; Eskin, E. Variance component model to account for sample structure in genome-wide association studies. Nature Genetics 2010, 42, 348–354. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A Tool for Genome-wide Complex Trait Analysis. The American Journal of Human Genetics 2011, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Turner, S. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. Journal of Open Source Software 2018, 3, 25. [Google Scholar] [CrossRef]
- Yao, W.; Li, G.; Yu, Y.; Ou, Y. funRiceGenes dataset for comprehensive understanding and application of rice functional genes. GigaScience 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ou, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (New York, NY) 2013, 6, 4. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Li, M.; Cui, Y.; Shi, Y.; Wu, Z.; Hu, Z.; Wang, W.; Xu, J.; Li, Z. The landscape of gene-CDS-haplotype diversity in rice: Properties, population organization, footprints of domestication and breeding, and implications for genetic improvement. Molecular Plant 2021, 14, 787–804. [Google Scholar] [CrossRef]
- Huo, C.; Zhang, B.; Wang, H.; Wang, F.; Liu, M.; Gao, Y.; Zhang, W.; Deng, Z.; Sun, D.; Tang, W. Comparative Study of Early Cold-Regulated Proteins by Two-Dimensional Difference Gel Electrophoresis Reveals a Key Role for Phospholipase Dα1 in Mediating Cold Acclimation Signaling Pathway in Rice. Molecular & Cellular Proteomics 2016, 15, 1397–1411. [Google Scholar]
- Lou, Q.; Guo, H.; Li, J.; Han, S.; Khan, N.U.; Gu, Y.; Zhao, W.; Zhang, Z.; Zhang, H.; Li, Z.; et al. Cold-adaptive evolution at the reproductive stage in Geng/japonica subspecies reveals the role of OsMAPK3 and OsLEA9. The Plant Journal 2022, 111, 1032–1051. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Wei, H.; Wang, L. A clock regulatory module is required for salt tolerance and control of heading date in rice. Plant, Cell & Environment 2021, 44, 3283–3301. [Google Scholar]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 Phosphorylates OsbHLH002/OsICE1 and Inhibits Its Ubiquitination to Activate OsTPP1 and Enhances Rice Chilling Tolerance. Developmental Cell 2017, 43, 731–743. [Google Scholar] [CrossRef]
- Guo, H.; Zeng, Y.; Li, J.; Ma, X.; Zhang, Z.; Lou, Q.; Li, J.; Gu, Y.; Zhang, H.; Li, J.; Li, Z. Differentiation, evolution and utilization of natural alleles for cold adaptability at the reproductive stage in rice. Plant biotechnology journal 2020, 18, 2491–2503. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, X.; Gao, Y.; Hua, Z.; Ma, X.; Chen, W.; Xu, Z.; Zhu, L.; Li, Z. Improving seedling cold tolerance of japonica rice by using the "Hidden Diversity" in indica rice germplasm in backcross breeding program. Acta Agron Sin 2007, 33, 1618–1624. [Google Scholar]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; Xiao, J.; Guo, X.; Xu, S.; Niu, Y.; Jin, J.; Zhang, H.; Xu, X.; Li, L.; Wang, W.; Qian, Q.; Chong, K. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Liu, C.; Schläppi, M.R.; Mao, B.; Wang, W.; Wang, A.; Chu, C. The bZIP73 transcription factor controls rice cold tolerance at the reproductive stage. Plant biotechnology journal 2019, 17, 1834–1849. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Gao, Y.; Qian, H.; Gao, Q.; Wu, Y.; Zhang, D.; Zhang, X.; Yu, L.; Li, Y.; Pan, C.; Liu, G.; Zhou, C.; Jiang, M.; Huang, N.; Dai, Z.; Liang, C.; Chen, Z.; Chen, J.; Li, A. Identification of Genes Related to Cold Tolerance and a Functional Allele That Confers Cold Tolerance. Plant physiology 2018, 177, 1108–1123. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, L.; Zheng, J.; Chen, F.; Wang, T.; Wang, M.; Tao, Y.; Wang, H.; Hong, Z.; Huang, Y.; et al. A missense mutation in Large Grain Size 1 increases grain size and enhances cold tolerance in rice. Journal of Experimental Botany 2019, 70, 3851–3866. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Y.; Tang, W.; Chen, X.; Lin, C.; Liu, Y.; Ye, Y.; Wu, W.; Duan, Y. LUX ARRHYTHMO Interacts With ELF3a and ELF4a to Coordinate Vegetative Growth and Photoperiodic Flowering in Rice. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Tang, D.; Wang, K.; Wang, M.; Zhu, K.; Yu, H.; Gu, M.; Cheng, Z. OsAM1 is required for leptotene-zygotene transition in rice. Cell Research 2011, 21, 654–665. [Google Scholar] [CrossRef]
- Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant, Cell & Environment 2013, 37, 1–18. [Google Scholar]
- Tang, Z.; Zhang, L.; Yang, D.I.; Zhao, C.; Zheng, Y. Cold stress contributes to aberrant cytokinesis during male meiosis I in a wheat thermosensitive genic male sterile line. Plant, Cell & Environment 2010, 34, 389–405. [Google Scholar]
- Ali, J.; Pan, Y.; Zhang, H.; Zhang, D.; Li, J.; Xiong, H.; Yu, J.; Li, J.; Rashid, M.A.R.; Li, G.; et al. Genetic Analysis of Cold Tolerance at the Germination and Booting Stages in Rice by Association Mapping. Plos One 2015, 10, 3. [Google Scholar]
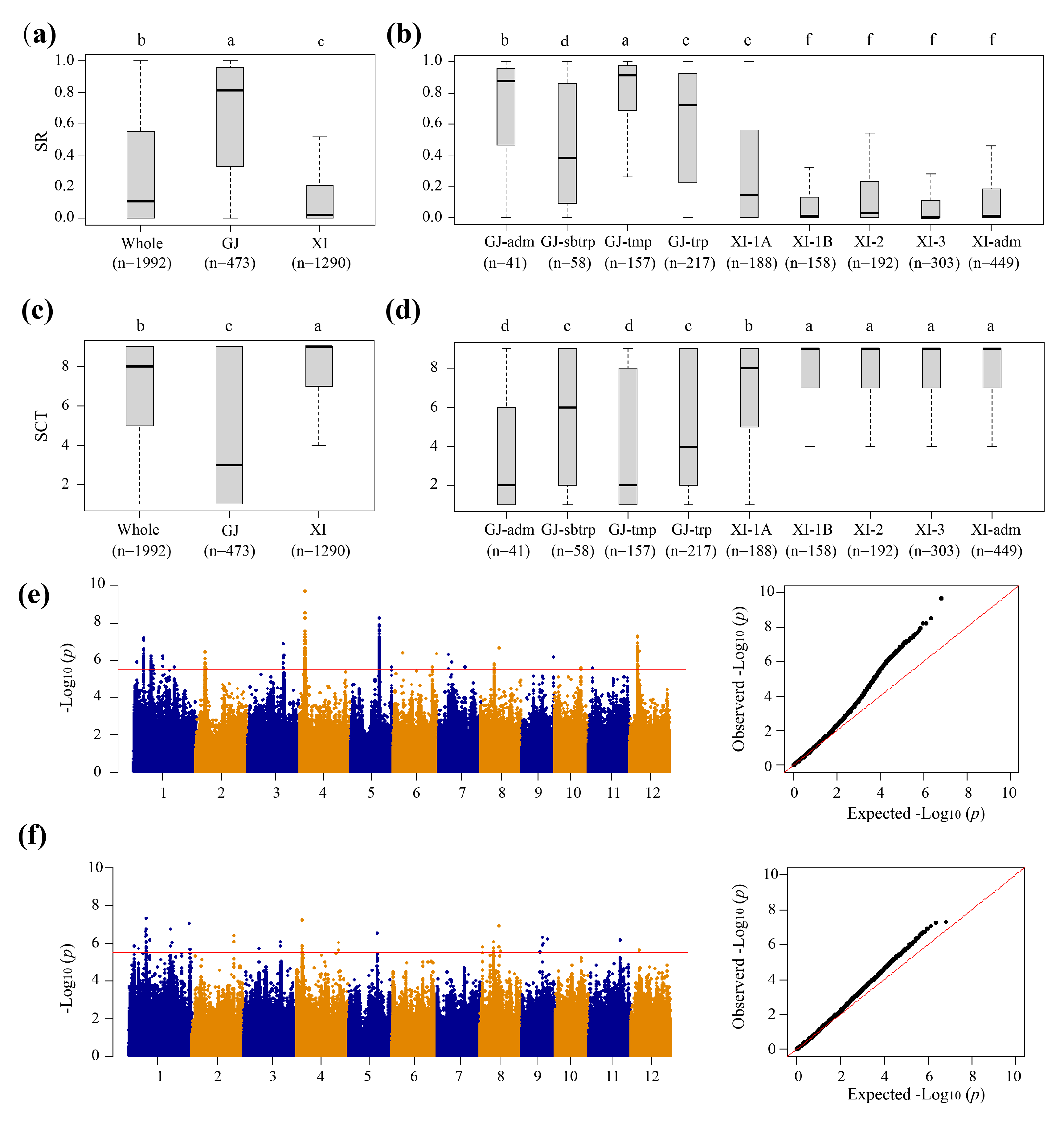
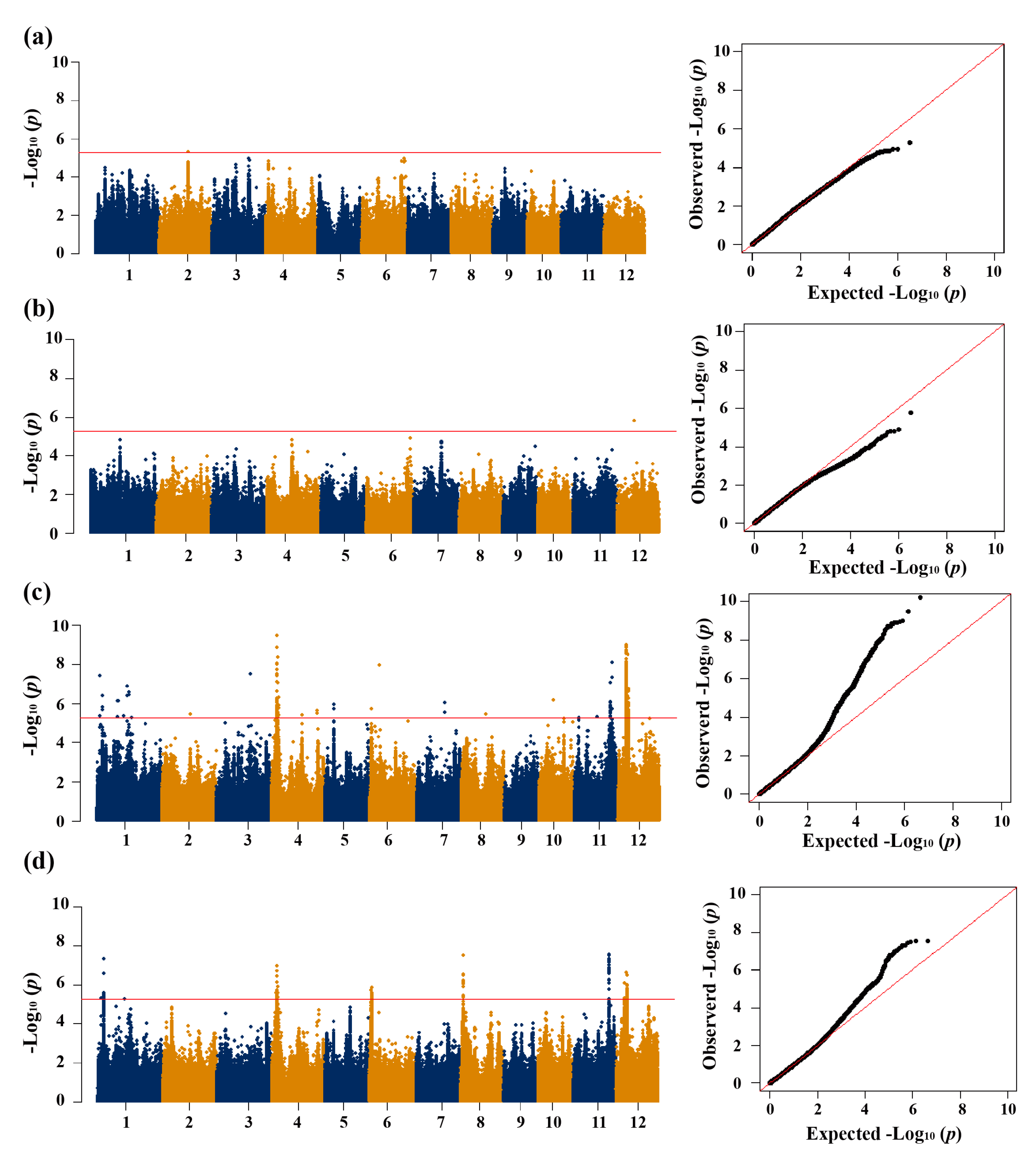
| Trait a | QTL | Chr | QTL region (Mb) | Lead SNP | P | Cloned gene |
|---|---|---|---|---|---|---|
| SR | qSR1.1a | 1 | 6.37-6.67 | 6,518,020 | 6.62E-08 | |
| qSR1.2a | 1 | 11.71-12.01 | 11,864,873 | 6.05E-07 | OsLEA9 [27] | |
| qSR1.3a | 1 | 19.98-20.27 | 20,125,327 | 6.39E-07 | ||
| qSR2.1a | 2 | 6.26-6.56 | 6,416,777 | 3.57E-07 | ||
| qSR3.1a | 3 | 25.10-25.55 | 25,249,852 | 1.36E-07 | ||
| qSR4.1a | 4 | 3.58-3.96 | 3,633,378 | 2.10E-10 | ||
| qSR5.1a | 5 | 19.63-19.93 | 19,779,633 | 5.72E-09 | ||
| qSR12.1a | 12 | 5.60-5.90 | 5,753,724 | 5.54E-08 | ||
| SCT | qSCT1.1a | 1 | 11.53-11.97 | 11,956,876 | 4.74E-08 | OsLEA9 [27] |
| qSCT1.2a | 1 | 28.47-28.77 | 28,623,028 | 1.88E-07 | ||
| qSCT2.1a | 2 | 28.66-28.96 | 28,812,677 | 4.12E-07 | LGS1 [35] | |
| qSCT3.1a | 3 | 25.11-25.41 | 25,260,301 | 8.78E-07 | ||
| qSCT4.1a | 4 | 3.48-3.78 | 3,633,378 | 5.60E-08 | ||
| qSCT8.1a | 8 | 9.18-9.48 | 9,334,380 | 8.64E-07 | ||
| qSCT8.2a | 8 | 12.60-12.90 | 12,755,109 | 1.24E-07 | ||
| qSCT9.1a | 9 | 14.24-14.54 | 14,393,189 | 5.25E-07 |
| Population | Trait a | QTL | Chr. | QTL region (Mb) | lead SNP | P | Cloned gene |
|---|---|---|---|---|---|---|---|
| GJ | SR | qSR2.1g | 2 | 19.64-19.94 | 19,785,654 | 4.94E-06 | |
| GJ | SCT | qSCT12.1g | 12 | 11.01-11.31 | 11,161,160 | 1.64E-06 | |
| XI | SR | qSR1.1x | 1 | 3.45-3.75 | 3,604,713 | 4.19E-07 | OsPLDα1 [26] |
| qSR1.2x | 1 | 20.23-21.03 | 20,231,449 | 1.39E-07 | |||
| qSR4.1x | 4 | 3.63-4.80 | 3,855,187 | 3.33E-10 | |||
| qSR5.1x | 5 | 5.71-6.01 | 5,861,711 | 1.21E-06 | |||
| qSR11.1x | 11 | 23.79-25.42 | 25,393,345 | 8.00E-09 | |||
| qSR12.1x | 12 | 5.71-7.25 | 5,754,489 | 6.05E-11 | |||
| XI | SCT | qSCT1.1x | 1 | 3.45-3.75 | 3,604,730 | 4.93E-08 | OsPLDα1 [26] |
| qSCT4.1x | 4 | 3.63-4.23 | 3,855,187 | 1.09E-07 | |||
| qSCT6.1x | 6 | 1.21-1.51 | 1,361,687 | 1.48E-06 | |||
| qSCT8.1x | 8 | 1.42-1.72 | 1,578,048 | 3.12E-08 | |||
| qSCT11.1x | 11 | 24.03-24.33 | 24,179,477 | 2.76E-08 | |||
| qSCT12.1x | 12 | 5.71-7.25 | 6,765,874 | 2.59E-07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





