1. Introduction
Endometrial macrophages play key roles in mediating the menstrual cycle through the initiation of the breakdown of endometrium during menstruation [
1] or contribution to tissue remodeling during the establishment of pregnancy [
2]. Macrophages exhibit extensive plasticity, and their phenotype and function are highly specific to the local tissue milieu. Dysregulation of their activity occurs in abnormalities and pathologies of the uterus including endometriosis. It is evident that macrophages are abundant in endometrial lesions and are critical for their establishment and growth. Their enhanced ability to produce pro-inflammatory cytokines contributes to a microenvironment that favors pelvic inflammation and promotes other immune cells’ recruitment, endometriotic cells’ proliferation, angiogenesis, and innervation as well as leads to the generation of endometriosis-associated pain [
3,
4]. Therefore, the reciprocal communication between macrophages and endometriotic cells appears, and their intercellular dialogue sustains the formation of endometriosis lesions. As macrophages are the predominant cellular component in endometriotic tissue, the targeting of macrophage alternation could be critical in developing potential therapeutic candidates.
The pathological processes associated with endometriosis like altered inflammatory microenvironment and oxidative stress, can be targets of natural bioactive compounds [
5]. Numerous studies suggest that resveratrol (Res) demonstrated an immunomodulatory role in immunologic disorders, like cancers, autoimmune, neurodegenerative, metabolic, cardiovascular, and infectious diseases [
6,
7]. Regarding the immune cells, it has been found that resveratrol affects the anti-inflammatory profile in macrophages, exhibit inhibitory function on T cells, reduces the suppressive function of CD4+CD25+ regulatory T cells, and stimulates the killing activity of NK. Studies on macrophages revealed that the resveratrol regulates TLR-mediated inflammatory responses, NF-κB activation and COX-2 expression and attenuates TLR4-TRAF6, mitogen-activated protein kinase (MAPK), and AKT pathways in LPS-induced macrophages. Resveratrol reduced the production of granulocyte-macrophage colony-stimulating factor (GM-CSF), decreased LPS-induced pro-oxidant effect and modulated the immune response linked to prostaglandin E2 (PGE2) and Sirtuin 1 (Sirt1) level [
6,
8]. In searching for potential health-beneficial agents with increased bioavailability, it is necessary to investigate the biological mechanisms for which resveratrol analogs and metabolites can reach the target tissues and exert biological activity. Experiments utilizing analogs demonstrated that hydroxylation of resveratrol to piceatannol (3,5,3′,4′-tetrahydroxy-trans-stilbene) attributed the antioxidant and growth-inhibitory effects of the compound [
9].Whereas the structure of pterostilbene (3,5-dimethoxy-4′-hydroxystilbene) with two extra methoxy groups showed more lipophilicity than resveratrol and thus possesses higher intestinal permeability and cellular uptake and enhanced stability [
10]. Polydatin (3,4′,5-trihydroxystilbene-3-β-D-glucoside) as a glycoside form of resveratrol resulted in different biological characteristics like greater bioavailability and anti-inflammatory and antioxidative properties [
11,
12]. Since endometriosis is an inflammatory pathology, the study aims to investigate the comparative anti-inflammatory effects of resveratrol, pterostilbene, piceatannol and polydatin on endometriosis. It is suggested that macrophage influence on endometriotic cells may be fundamentally important for endometriosis development; however, the cell interactions have not been fully understood. Therefore, in this study, we explored the effect of stilbenes in the co-culture model of macrophages and endometriotic cells. We investigated the effects of compounds on the expression of key inflammatory and oxidative stress markers, production of cytokines, and radical scavenging activity. Whether the co-culture system could mediate the inflammatory niche in endometriosis was also addressed and discussed.
2. Materials and Methods
2.1. Chemicals
Unless stated otherwise, all chemicals were purchased from Merck KGaA (Darmstadt, Germany).
2.2. Cell Culture
The immortalized human endometriotic epithelial cells (12Z) were obtained from Applied Biological Materials Inc. (Richmond, Canada) and maintained at 37°C in a containing 5% CO2 and cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) with 10% fetal bovine serum (FBS) and 50 mg/L gentamycin. The human monocyte THP-1 cells (ATCC TIB-202) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) containing FBS 10%, 50 mg/L gentamycin and 2-mercaptoethanol (0.05 mM). THP-1 cells were kept at a minimum density of 2 × 105 cells/ml and were subcultured when reaching 8 × 105 cells/ml. They were passaged every 48 h by adding a fresh medium or complete medium replacement, until they reached the above-mentioned maximum density. Both cell lines were tested regularly for mycoplasma contamination.
2.3. Macrophages Differentiation and Co-Culture Setup
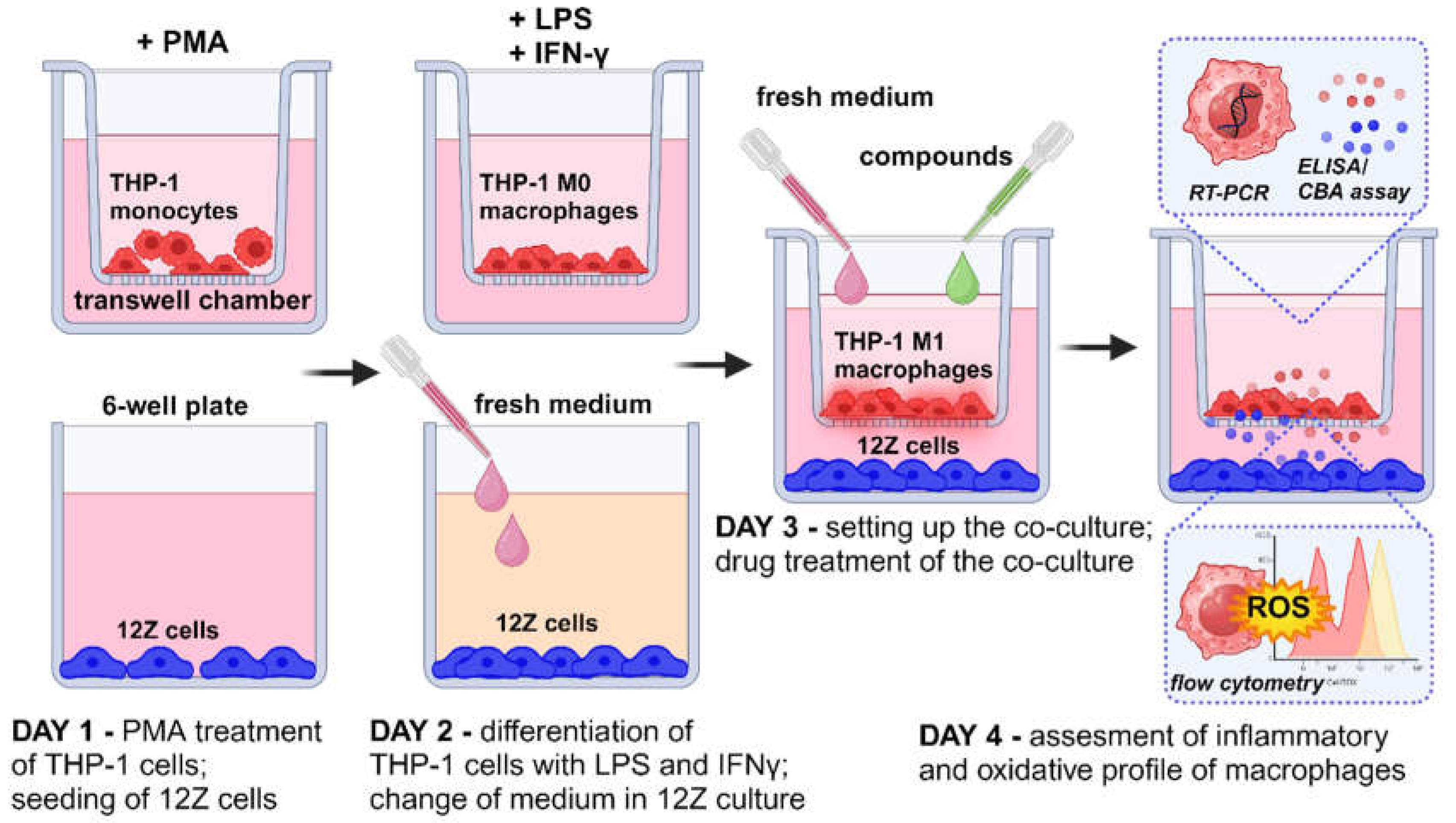
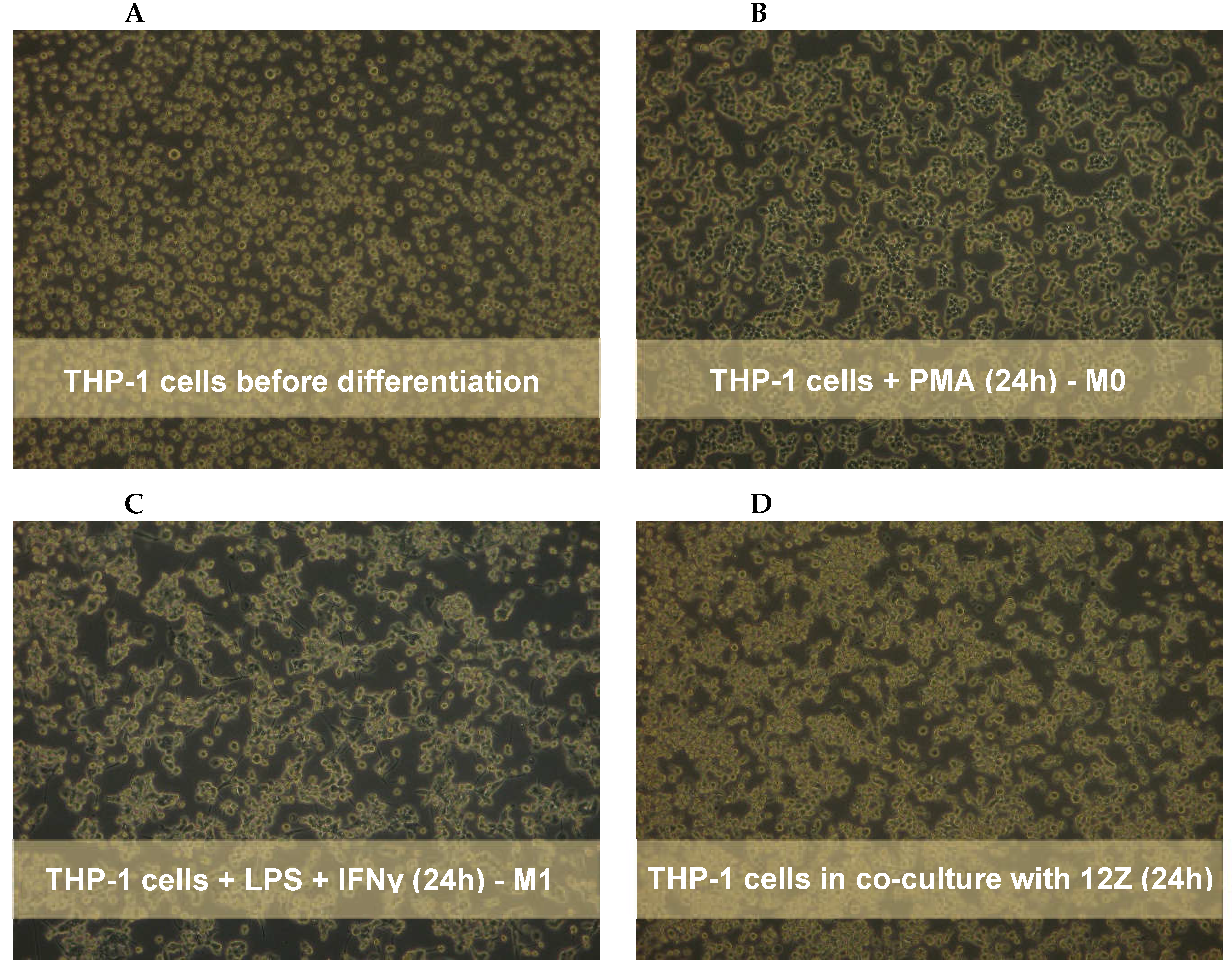
Monocytes were stimulated by 24 h incubation with 10 ng/ml with phorbol-12 myristate 13-acetate (PMA) followed by washing with RPMI 1640 medium to eliminate the effect of PMA as described by Smith
et al. [
13]. Morphological changes, such as an increase in cell size, formation of pseudopodia, and adhesion, were observed to assess the acquisition of a macrophage-like phenotype. The co-culture system was performed using a 6-well hanging cell culture inserts (0.4-µm porous; Millicell, Billerica, MA, USA). First, the THP-1 monocytes were seeded (2×10
5 cells/cm
2) into the upper chamber of the transwell system in 2 ml of RPMI 1640 medium and 10% of FBS and placed into 6- well plate containing the 3 ml of medium. Once seeded THP-1 cells were treated immediately with 10 ng/ml of PMA for 24 h to stimulate differentiation. A new 6-well plate was prepared the same day with 12Z cells, which were seeded at 0.3×10
5 cells/cm
2 in DMEM/F12 media with 10% FBS. The THP-1 cells placed into the insert and activated with PMA as well as 12Z on the 6-well plate were incubated separately for 24 h to allow for attachment. The next day, differentiated THP-1 cells on the inserts were washed with the medium. Macrophages were polarized in M1 macrophages by incubation with 50 ng/ml of IFN-γ (PeproTech, USA) and 10 pg/ml of LPS (PeproTech, USA) in RPMI 1640 medium and 10% of FBS according to the method described by Smith
et al. and Genin
et al. [
13,
14]. Simultaneously, medium on the 6-well plate with 12Z was changed to RPMI 1640 and 2% of FBS. After 24 h the chambers containing the THP-1-derived macrophages were directly placed on top of the 6-well plates with the 12Z cells, and the resulting co-culture system cells. Macrophages and 12Z cells were co-cultured in RPMI 1640 supplemented with 2% of FBS treated with resveratrol and its derivatives for 24 h.
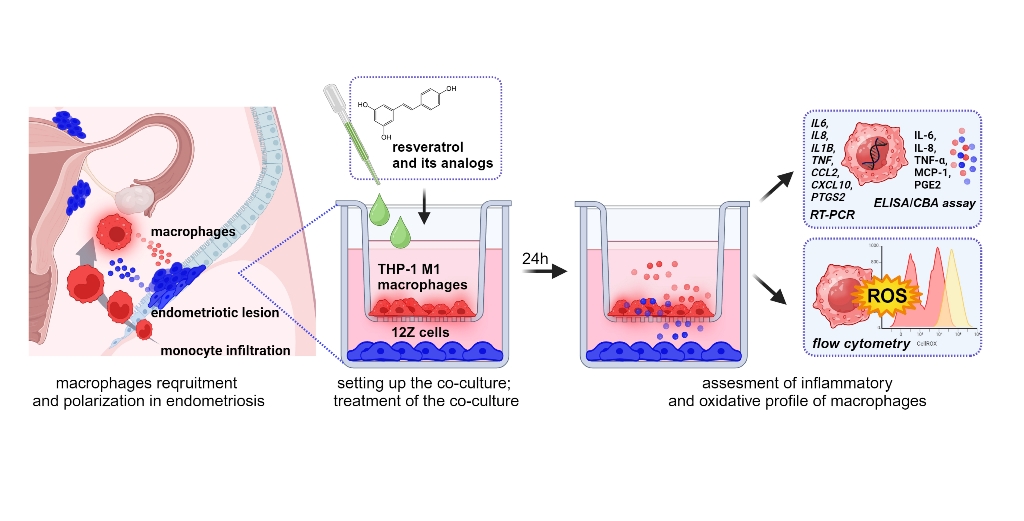
Figure 1 provides a schematic representation of the experimental design consisting of macrophage differentiation, macrophage polarization, co-culture setup, treatment with analyzed compounds and further analysis.
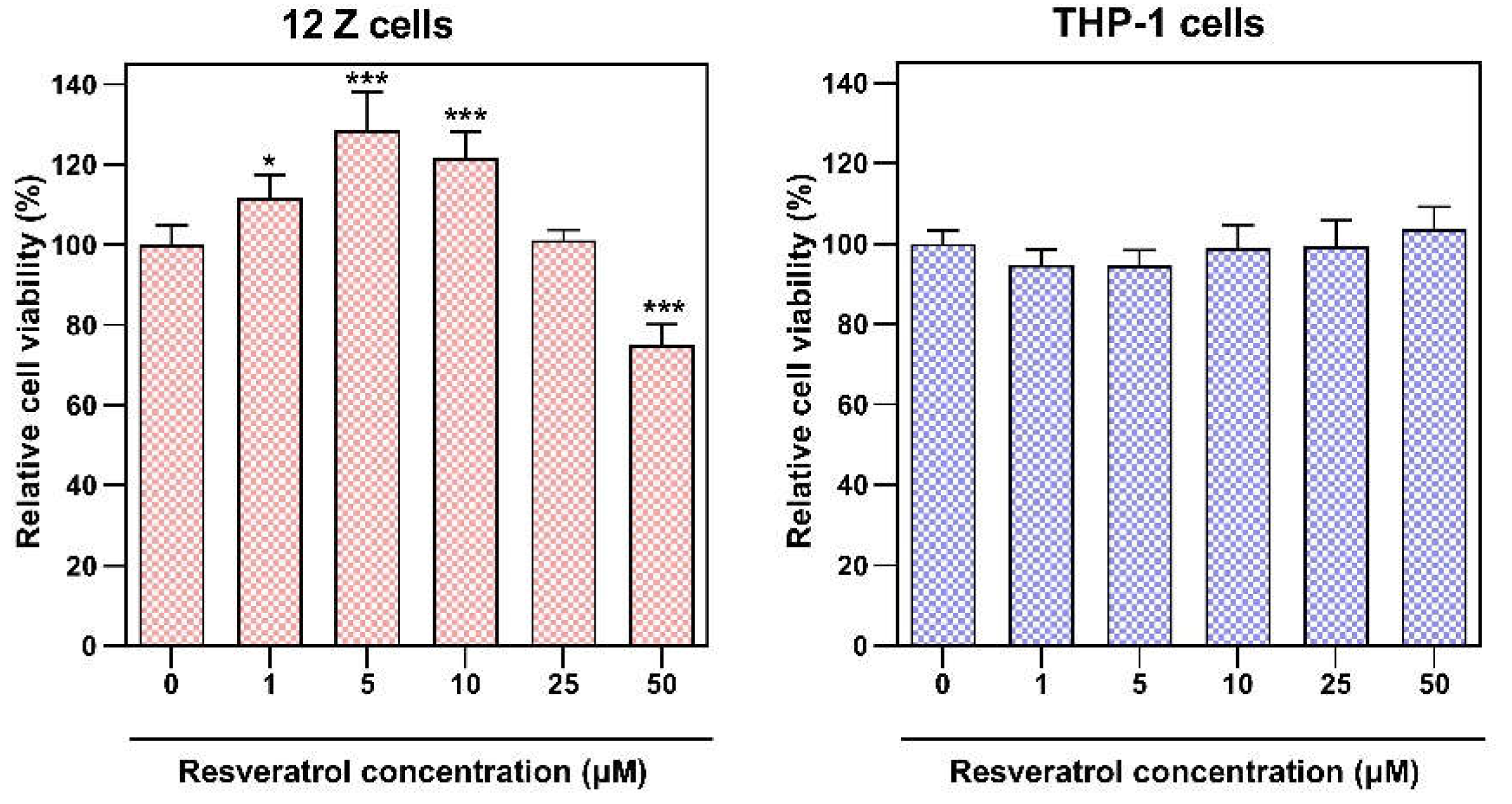
2.4. MTT Assay
THP-1 cells were grown in 24-well plates at an initial density of 1×10
5/cm
2 and stimulated by 24-hour incubation with 10 ng/ml PMA. Cultures were then treated with resveratrol at concentrations ranging from 1 µM to 50 µM and incubated for 24 h under standard culture conditions in RPMI 1640 medium and 2% of FBS. 12Z cells were grown in 24-well plates at an initial density of 0.25 ×10
5/cm
2 and were treated with resveratrol at the same range concentrations under standard culture conditions in RPMI 1640 medium and 2% of FBS for 24 h. We applied the same culture conditions for both cell lines as for the co-cultures, such as the same cell media, concentration of FBS in the media and time of incubation. Cell viability and metabolic activity in both cultures were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay according to the protocol described in previous studies [
15]. The absorbance was measured using a Tecan M200 Infinite microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
2.5. RNA Extraction and Real-Time PCR
Total RNA from the THP-1-derived macrophages co-cultured with 12Z was extracted using Trizol reagent (Invitrogen, USA) according to the previously described protocol [
16]. Complementary deoxyribonucleic acids (cDNAs) were synthesized using a cDNA Transcriptor First-Strand kit (Roche Diagnostics GmbH, Mannheim, Germany). Quantitative real-time polymerase chain reaction (qRT-PCR) reactions were carried out using SYBR® Select Master Mix (Life Technologies, Carlsbad, CA, USA). The relative expression was calculated using the 2
−ΔΔCt method, where Ct represents the threshold cycle. GAPDH was used as the internal control. The primers used for the amplification of cDNAs are listed in
Table 1.
2.6. Quantitation of Cytokine Levels by Cytometric Bead Array
The conditioned media (CM) from macrophages and 12Z cells co-culture were collected 24 h after treatment with compounds, centrifuged to remove cellular debris, and applied for subsequent experiment or stored at −80°C until use. The concentrations of Interleukin-6 (IL-6) and Interleukin 8 (IL-8) in the conditioned media were quantitatively determined immediately with the CBA Human Inflammatory Cytokine Cytometric Bead Array (BD Biosciences, San Jose, CA, USA), as previously described [
17]. A standard calibration curve was established for each kit with a maximum and minimum detection limit (1.0-5000 pg/mL). Samples were analyzed using a FACSAriaII flow cytometer (BD Biosciences) and FACSDiva v6.1.2 software (BD Biosciences).
2.7. Quantitation of Cytokine Levels by ELISA
The monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor alpha (TNF-α) levels were determined using enzyme-linked immunosorbent assay (ELISA) according to the standard protocols of the supplier (R&D Systems, Minneapolis, MN, USA). The prostaglandin E2 (PGE2) concentration was detected using respective ELISA kits (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Results are expressed in pg of cytokine normalized per μg of proteins quantified using Pierce® BCA Protein Assay Kit (Thermo Fisher Scientific).
2.8. Assessment of Intracellular Reactive Oxygen Species (ROS) by Flow Cytometry
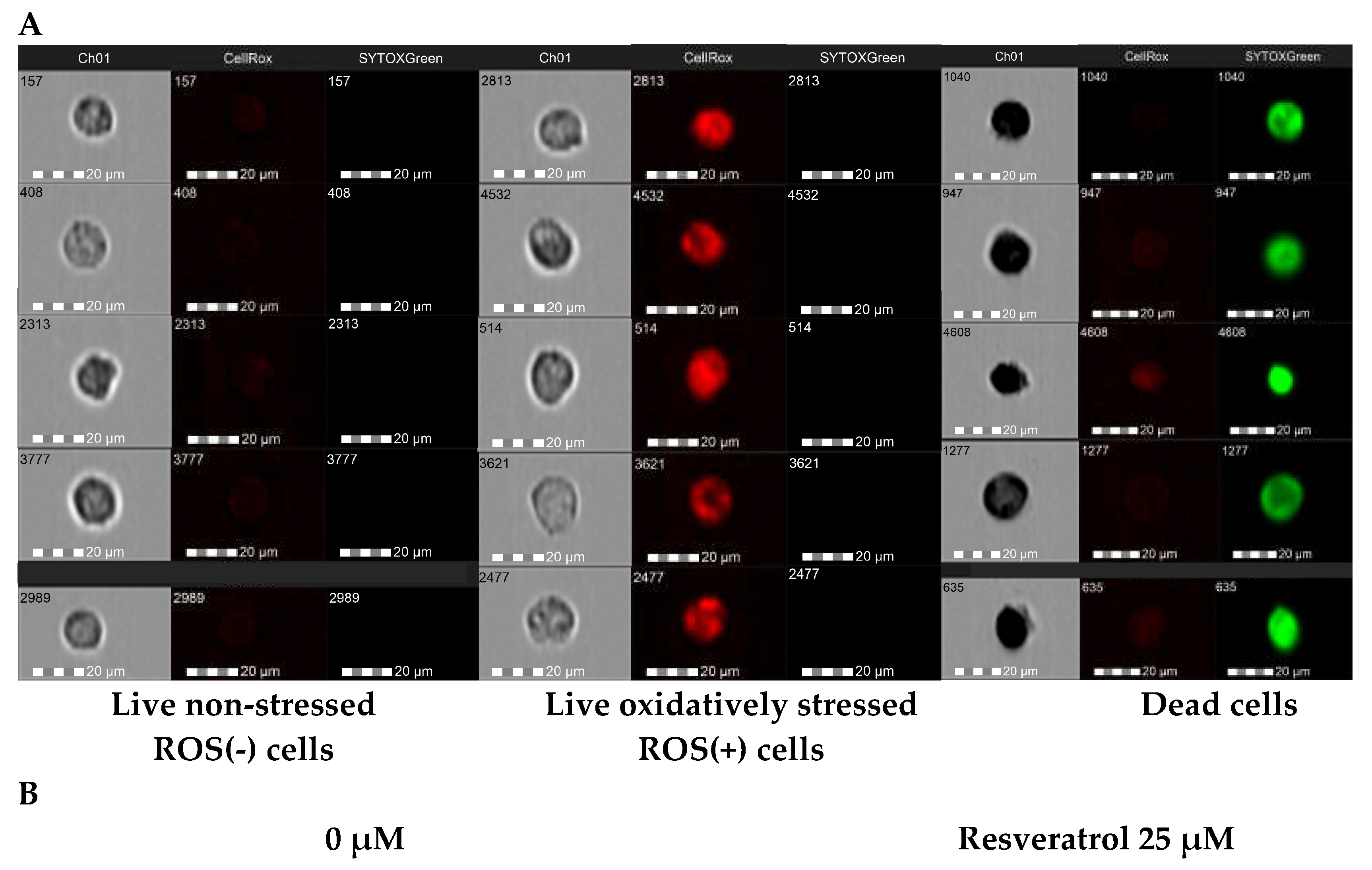
Reactive oxygen species (ROS) generation was determined using a CellROX® Deep Red Flow Cytometry Assay Kit (Life Technologies, Carlsbad, CA, USA). The cell-permeable CellROX Deep Reagent is non-fluorescent in a reduced state and exhibits a strong fluorogenic signal upon oxidation. The fluorescence intensity of CellROX® Deep Red reflects the ROS levels in live cells. Dead cells were measured in each sample using the Sytox® Green Dead Cell Stain.
Macrophages were co-cultured with 12Z cells according to standard protocol. After treatment of the co-cultures with resveratrol or its derivatives, macrophages were gently detached from the surface of the flask, rinsed, and centrifuged. Cells were resuspended in phenol red-free RPMI medium, and CellROX® Deep Red reagent was added to the samples at a final concentration of 500 nM and incubated for 60 min at 37 °C in the dark. During the final 15 minutes of staining, Sytox Green Dead Stain in an optimized concentration of 45 nM was added. Cells were subjected to imaging Amnis™ FlowSight™ flow cytometer (Luminex Corp., Austin, TX, USA) equipped with three lasers for excitation (405 nm, 488 nm, and 642 nm), five fluorescence channels (acquisition by a multi-channel CCD camera), and side scatter detector (SSC). Post-acquisition data analysis was performed using ImageStream Data Exploration and Analysis Software (IDEAS® 6.2.187, Luminex, Austin, TX, USA). Approximately 0.5 × 104 cells were analyzed in each of the samples. Single-color compensation controls for CellROX® Deep Reagent and Sytox® Green Dead Stain were also acquired using the integrated software INSPIRE® (Luminex, Austin, TX, USA) for data collection. The analysis detected of oxidatively stressed (live cells), non-stressed cells (live cells), and dead cells representing the discrete subpopulations.
2.9. Statistical Analysis
The obtained results were expressed as means ± SD from three independent replications. The Shapiro–Wilk test was used to assess distributional assumptions, and the equality of variances hypothesis was verified with Levene’s test. A Student’s t-test was used to compare two groups of data. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was performed to determine the differences between the mean values of multiple groups. p<0.05 was considered to be statistically significant and asterisks were used to indicate different levels of significance (*p<0.05, **p<0.01, ***p<0.001). Statistical analysis was performed using STATISTICA version 13.3 software (Statsoft, Inc., Tulsa, OK, USA).
4. Discussion
The clinical presentation of endometriosis is largely identified as chronic inflammation in the pelvic cavity [
18]. The increased number of monocytes and macrophages in the peritoneal fluid of endometriosis compared to normal women has been well documented in the last decades. They may release a wide list of components, such as prostaglandins, cytokines, growth factors, iron, hormones and other enzymes, presumed to be the essence of disease initiation and progression [
19]. Endometriosis shares a common inflammatory pathogenesis with cancers, involving TNF-α, IL-1, IL-6, IL-8, and PGE2, associated with the activation of macrophages, retrograde menstruation, iron overload, and triggering aberrant inflammatory signaling [
20]. In actuality, the dualistic concept of macrophage polarization has been discussed because it may not represent the dynamic
in vivo immune environment [
21]. Mixtures of recruited resident and differentiated macrophages exist in the peritoneum lesions and present a wide spectrum of phenotypes, thus the classification of M1/M2 macrophages may be over simplified. As it is challenging to replicate the complexity of macrophages using an
in vitro cell-line-based culture tool, proinflammatory phenotypes of monocyte-derived macrophages were used for the present study. Regarding the disease’s inflammatory hallmark, the proinflammatory cytokines’ pivotal role, and oxidative stress in its pathophysiology, the study aimed to investigate the effect of stilbenes in the co-culture inflamed model of endometriosis. To mimic the condition of the inflamed state, a co-culture of endometriotic 12Z cells and macrophages derived from THP-1 was established. Within this study, our objective was to assess potential differences between resveratrol and its analogs concerning the anti-inflammatory curative potential and resolution of oxidative stress.
Immune cells are the key cellular components of the microenvironment in endometriotic tissue with diverse functions. Thus, the focus on the crosstalk and interaction between endometriotic cells and macrophages should be included in evaluating the effect of anti-inflammatory and antioxidant therapy. So far, only several
in vitro co-culture models have been developed to mimic endometriosis with representation of the immune system. For the establishment of these immuno-active co-cultures, human endometrial epithelial and stromal cells (HES, HESC), human endometriotic epithelial and stromal cells (12Z, 22B), as well as THP-1 cells differentiated into macrophages have been used. The endometriosis-associated macrophages were the macrophages stimulated by the conditioned medium of human endometriotic cells [
22]. To represent the macrophage–epithelial cell interaction studies, THP-1 cells were differentiated, polarized and then 12Z cells were cultured in the conditioned media from M0, M1, M2 macrophages [
23]. The experimental design based on the
in vitro direct co-culture of proinflammatory macrophages and endometriotic cells, which was applied in this work, allowed for the creation of a reciprocal activation of macrophages and endometriotic cells via the soluble factors and the ability to obtain more physiologically relevant findings regarding the response of endometriotic microenvironment to anti-inflammatory substances.
The differentiation and polarization protocol for macrophages was optimized, and favorable growth conditions were selected to establish their co-culture with endometriotic cells. The co-culture setup was chosen depending on the doubling rate of cell line used and the planned length of the experiment, according to the recommendations previously reported [
13]. As highlighted, several factors that can impact the outcomes of co-cultures with macrophages between research groups like PMA concentration and differentiation period, LPS and IFN-γ concentration, polarization time, cells’ seeding density and time of simultaneous co-culture [
14,
24]. These variabilities might explain different results in the co-culture establishment and systemic response to the treatment.
Multiple lines of evidence from research on
in vivo and
in vitro have shown that the anti-inflammatory properties of resveratrol may be explained through the regulation of a variety of signaling pathways, such as arachidonic pathway, nuclear factor kappa B (NF-κb), mitogen-activated protein kinase (MAPK), and activator protein-1 (AP-1) [
7]. In studies on tumors, resveratrol has been proven to downregulate the production of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β. Mitigating the inflammatory milieu within the tumor microenvironment hinders the development of angiogenesis, tissue invasion, and metastasis processes [
25]. In addition, resveratrol promotes antioxidant defense pathways in several cancer cell lines by eliminating free radicals, enhancing the activities of SOD, CAT, and GPX [
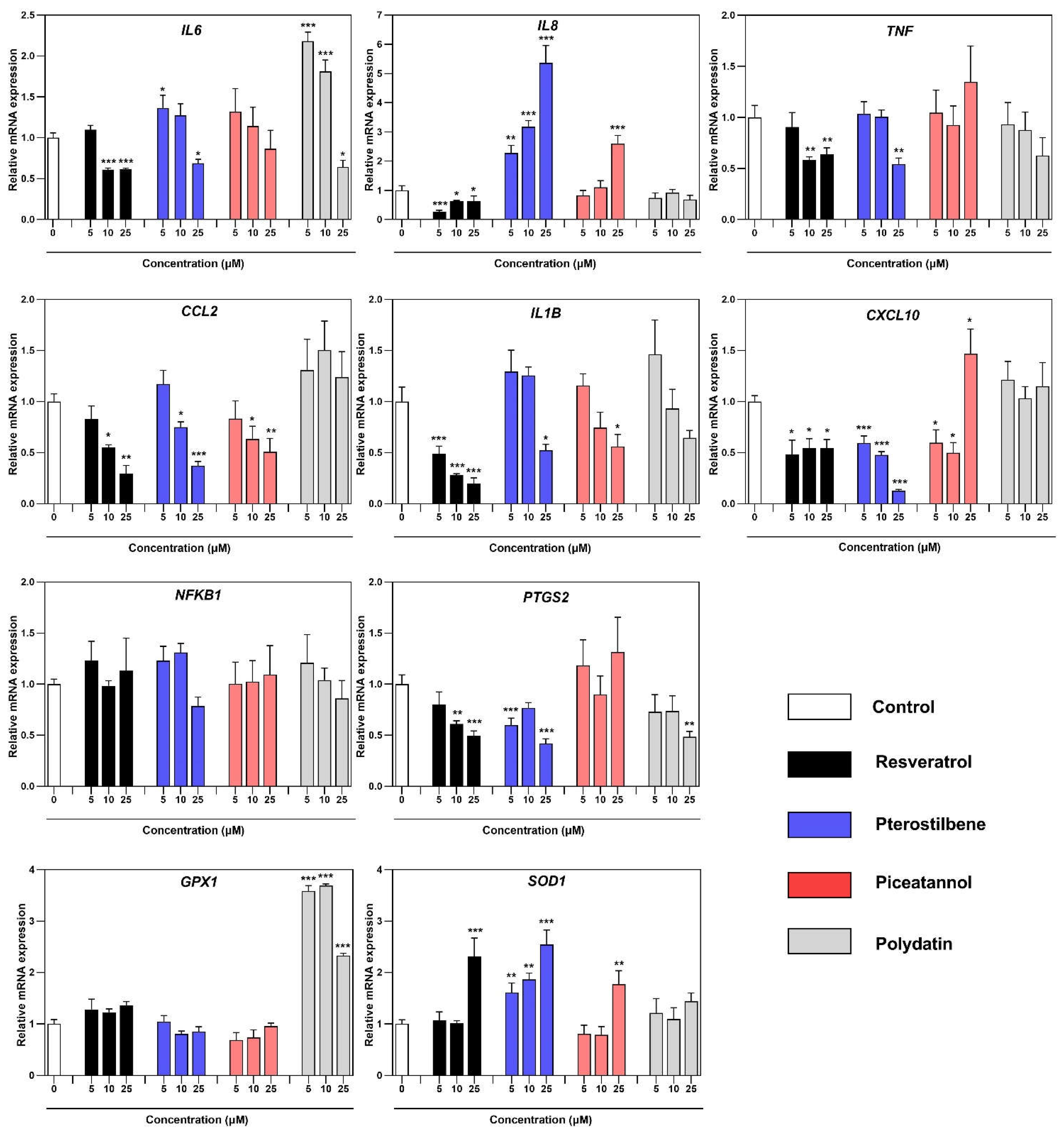
7]. As endometriosis behaves malignantly, the study was undertaken to unravel the effect of resveratrol and its analogs in the inflamed model of this disease. The results demonstrate that resveratrol and its analogs can suppress the expression of transcript copies of key pro-inflammatory regulators. As already mentioned, not all induced genes followed the same pattern of downregulation depending on the analyzed compound. While some genes, such as
IL6,
IL1B, and
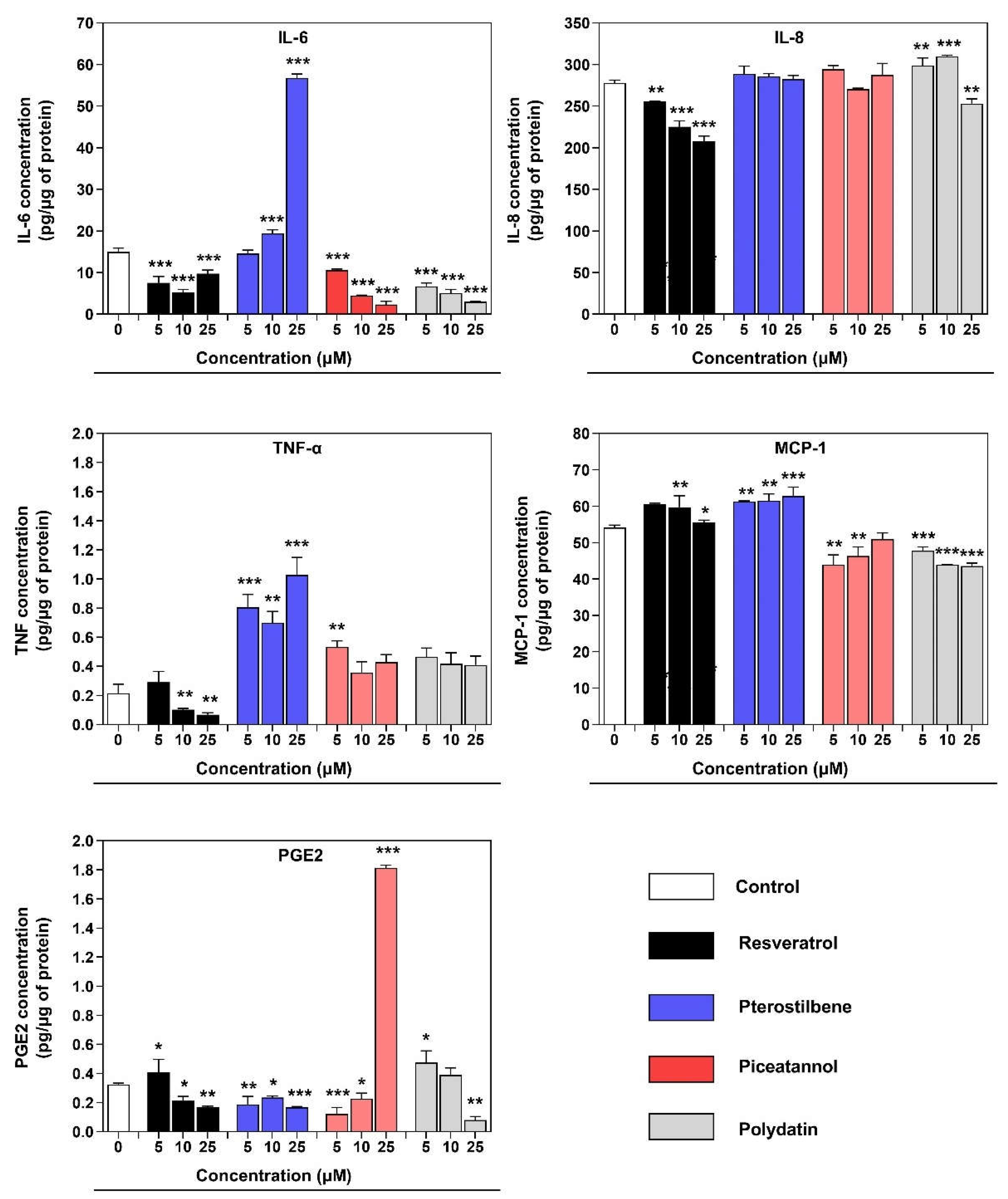
CCL2, displayed particular responsiveness to resveratrol, pterostilbene, and piceatannol. IL-6 is the most widely studied pro-inflammatory cytokine in the endometriosis. Macrophages in endometriosis produced a significantly higher level of IL-6, which is implicated in monocyte recruitment and, together with its receptor (IL-6R), regulates endometrial stromal cells (ESC) growth
in vitro [
26]. The inhibitory effect on the IL-6 concentration in the co-culture supernatant was observed after treatment with resveratrol, piceatannol, and polydatin. The standing out result of the IL-6 concentration after treatment with pterostilbene may be associated with the stimulation effect of endometriotic cells to produce high amount of IL-6 by 12Z-associated macrophages, described in the similar model based on the conditioned media experiments [
22]. The immunological role of IL-1β is to stimulate macrophages for the synthesis of Il-6 and activation of COX2 and enhancement of PGE2 levels, contributing to the synthesis of estradiol by binding of steroidogenic factor 1 (SF-1) to promoters of steroidogenic and aromatase genes [
27]. However, the content of this cytokine in the peritoneal fluid of women with endometriosis is still under discussion [
26]. The exposure of co-culture to the resveratrol has a prominent effect on reducing IL-1β expression. The general response of
CCL2 expression remained the same for the resveratrol, pterostilbene, and piceatannol, whereas the piceatannol and polydatin noticeably reduced its concentration. MCP-1 (CCL2) is a well-known chemoattractant cytokine for monocytes/macrophages. It is elevated in the peritoneal fluid in women with endometriosis, which can act in a paracrine and autocrine on macrophages [
26]. The elevated level of MCP-1 in the co-culture supernatant is probably related to its secretion by epithelial endometriotic cells 12Z. It was previously reported that MCP-1 secreted from endometriotic epithelial cells (12Z) recruits macrophages in endometriotic lesions [
22]. Another key chemoattractant in endometriosis is IL-8, which has been proven by many observations of elevated levels of this chemokine in patients’ peritoneal fluid and sera. There was also a significantly high correlation between the Il-8 concentration and angiogenic pathophysiology because it stimulates the adhesion of endometrial cells to fibronectin [
27]. In our study,
IL8 gene expression and IL-8 protein secretion into co-culture supernatant were reduced only after incubation with resveratrol. The cyclooxygenase-2 (COX-2)/prostaglandin E
2 (PGE2) pathway is showing its direct involvement in the pathophysiology of endometriosis. COX-2, a rate-limiting enzyme in the PGE2 compound, is overexpressed in endometriotic tissues inducible by inflammation and other pathogenic stimuli. PGE2 is a master modulator in estrogen biosynthesis and the promotion of cellular proliferation, angiogenesis, chronic pain generation, and immune surveillance [
28,
29]. The therapeutic inhibitors of COX-2, PGE2, and their receptors may contribute to inhibiting the proliferative potential, migration and invasion of endometriotic cells. COX-2 is an essential therapeutic target for anti-inflammatory drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, called COXIBS, used in treating endometriosis-affected women. Indeed, both of the inhibitors represent severe side effects and must be used cautiously, as gastrointestinal, renal, disorders and cardiovascular toxicity were reported. In light of this, polyphenols may provide alternative NSAIDs because they often are recognized as COX-2 selective [
30,
31]. The obtained results demonstrate that the resveratrol, pterostilbene, and polydatin notably reduced expression of COX-2 (
PTGS2) in macrophages co-cultured with endometriotic cells, and a qualitatively similar response was observed for the PGE2 concentration in co-culture supernatant. TNF-α, as a product of macrophages, was transcriptionally decreased by the treatment with resveratrol and pterostilbene in our co-culture model. The release of this cytokine to the media was blocked by a maternity compound – resveratrol. Even though TNF-α is a physiological cytokine in proliferative- and secretory-phase endometrium, scientific data indicates the correlation between the concentration of TNF-α in peritoneal fluid and the stage of endometriosis [
32]. The scientific data confirmed a statistically significant higher proportion of NF-κB nuclear translocation in peritoneal macrophages from women with endometriosis than non-affected group [
33]. There is also reported crosstalk between peritoneal macrophages and endometriotic cells, which secrete CCL17 on JNK signaling, induce NF-κB activation in macrophages, and then promote IL-6 production by them, and form a positive loop [
34]. We did not observe the effect of stilbenes on the expression of NF-κB transcription factor, a central regulator of the LPS, cytokine, and stress responses in many cell types, including macrophages.
In vitro studies on the cell line THP-1 indicated that LPS rapidly induces NF-κB DNA-binding, persisting until 12 h of incubation and being lost entirely after 24 hours [
35]; thus, the effect of analyzed compounds was not noticeable.
Several promising, consistent
in vitro studies reported the beneficial effects of resveratrol on the mono-culture of endometriotic cells in the context of anti-inflammatory properties. Kolahdouz-Mohammadi
et al. were the first to describe the impact of resveratrol treatment on MCP-1, IL-6, and IL-8 gene expression and protein production and RANTES protein expression in ectopic and eutopic endometrial stromal cells of endometriotic women. Their studies have found that resveratrol effectively diminished the expression of MCP-1, IL-6, IL-8, and RANTES in ectopic endometrial stromal cells [
36]. Similar studies with endometriotic stromal cells demonstrated that resveratrol treatment significantly suppressed TNF-α -induced IL-8 release through endogenous SIRT1 activation [
37]. However, the significant effects of resveratrol and its analogs on the inflammatory pathology in endometriosis have been described for the first time in the present work. The use of endometriotic cells together with macrophages underlined the importance of establishing a complex
in vitro model, which addresses interactions between cells in the endometriotic microenvironment. Obtained results expand current knowledge about the anti-inflammatory properties of the stilbenes, exanimated on the mono-culture of endometriotic cells or immune cells.
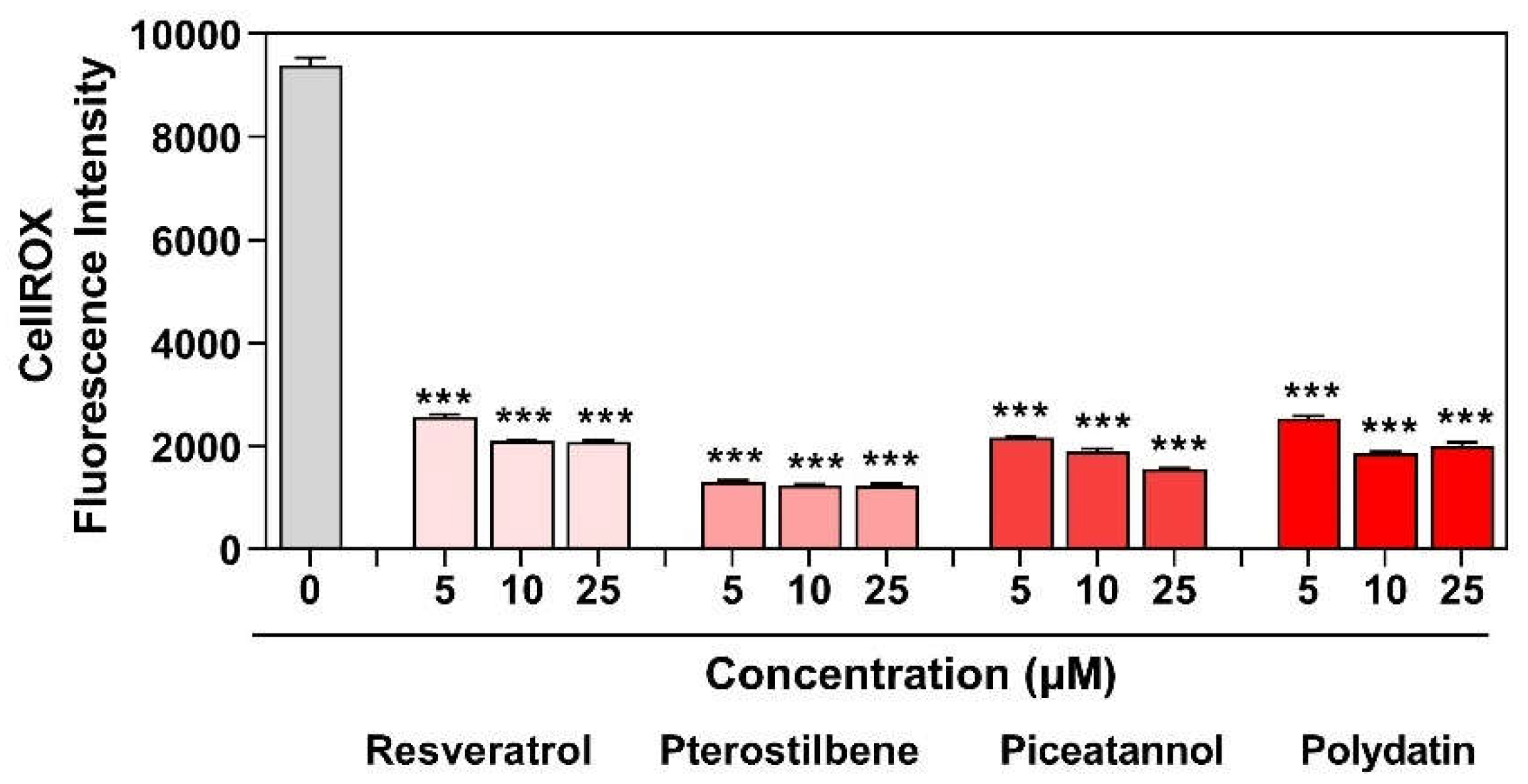
Macrophages, erythrocytes, and apoptotic endometriotic cells are found to be inducers of oxidative stress in endometriosis. There is a link between ROS and pro-inflammatory factors contributing to pain and the failure to detoxify lipid peroxidase products under oxidative stress. High levels of malondialdehyde (MDA), pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β), angiogenic factors (IL-8 and VEGF), monocyte chemoattractant proteins (MCP-1), and oxidized LDL were found in the peritoneal fluid of endometriosis patients. Pro-inflammatory and chemotactic cytokines play a significant role in the recruitment and activation of phagocytic cells, which are the leading producers of ROS [
38]. With the deepening of oxidative stress in the pelvic cavity, deleterious damage is beginning to appear, like tissue injury and chronic inflammation, leading to the proliferation and fibrosis of ectopic endometrial lesions [
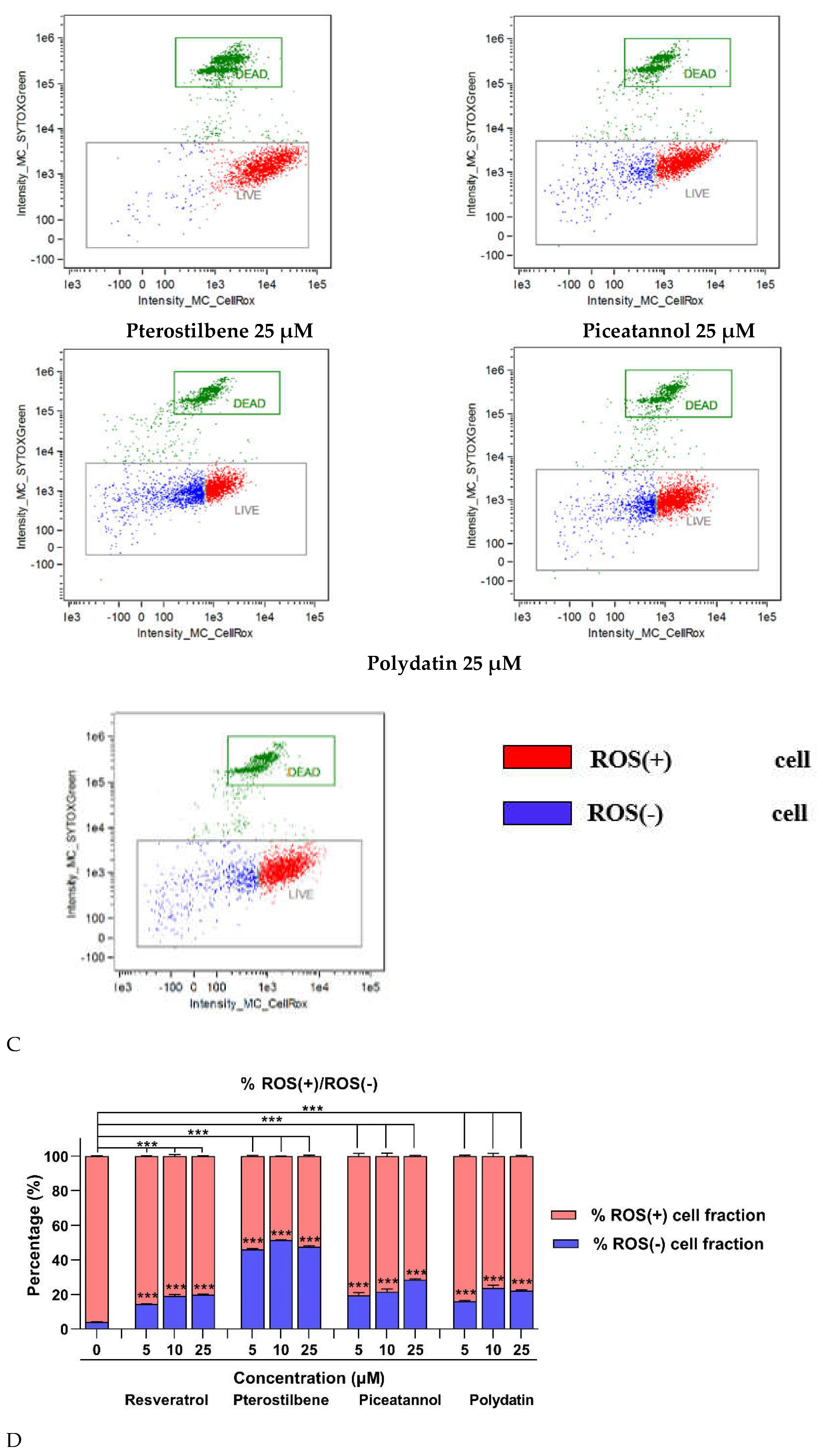
27]. Our findings indicate that all analyzed stilbenes significantly reduced ROS accumulation in macrophages co-cultured with 12Z cells. A reduced fraction of ROS-positive cells was detected even with the treatment of the lowest concentration of the stilbenes (5 µM). It is worth emphasizing that an excessive reduction of cells producing macrophages was observed under pterostilbene treatment by about 50%. These findings are consistent with the increasing expression of the antioxidant enzyme SOD1 in macrophages. According to this result, lines of evidence suggest that aberrant changes in endogenous antioxidant enzymes SOD as the first line of defense against oxygen free radicals and GPX – a major peroxide-scavenging enzyme, can contribute to the oxidative damage in endometriosis and be proposed as a biomarker for endometriosis [
39,
40].
Like other model
in vitro experiments, our study has some limitations, which should be considered when interpreting the findings. First, in this study, we included two types of cell lines. However, it is known that the endometriotic niche exists in a complex environment with a dynamic population of epithelial, stromal, immune, endothelial, and glandular cells [
41]. However, validating the specific pathology, like altered inflammatory processes in the disease, mostly requires the use of cells, which are most committed to. Secondly, only one phenotype of macrophages was used to form co-culture with endometriotic cells. For more significant validation of the present results, follow-up studies with naive (M0) and alternatively activated (M2) macrophage populations are needed to represent a more comprehensive immunodeficiency manifestation of the disease. Besides, which phenotype of macrophages is responsible for disease progression is still debated. Some analysis highlights that the pro-inflammatory population is typical of the early stages of the disease, whereas the switch to M2-polarization appears in advanced stages together with pro-fibrotic activity [
42,
43]. The point, which is often emphasized, is that many physiological or pathological macrophages do not show a clear M1 or M2 phenotype, and using the terms M1 and M2 with a specific phenotypic scoring criterion is confusing. Moreover, in that type of study, the verification of properties of natural compounds should be addressed to improve the bioaccessibility of the drug by considering their gastrointestinal digestion and metabolism by gut microorganisms [
5]. Therefore, in our experiments, we investigated the immunomodulatory effects of resveratrol and its derivatives at low physiologically attainable concentrations, based on the last work of Feng et al., who observed
in vitro and
in vivo physiological (5 µM) and pharmacological (>10 µM) effects of resveratrol on THP-1 cells and rodent model [
44,
45].