1. Introduction
Endometriosis remains an intriguing and complex gynecological condition that continues to raise significant challenges in both diagnosis and treatment. Ascertained as the presence of ectopic endometrial tissue outside the uterus, this pathology is retrieved in millions of women worldwide, leading to chronic pelvic pain, infertility, and a kaleidoscope of other clinical signs and symptoms [
1]. Numerous theories attempt to explain the origins of endometriosis. However, the most widely accepted theory attributes it to retrograde menstruation [
2]. While this theory is compelling and enjoys broad recognition, it falls short of elucidating why merely 3.7% of the 90% of women experiencing menstrual retrograde flow are affected by the condition. This drives us to suspect the involvement of additional pathogenic mechanisms in the appearance of these ectopic endometrial foci. The literature describes many genetic, epigenetic, and immunological factors that interfere with the normal signaling pathways, altering the microenvironment and the immune response of the endometriosis affected individual. Researchers continue the race to uncover the molecular factors that contribute to this disease development and progression [
3,
4,
5,
6]. A combination of surgery and medical treatments are available for these women, but there is ongoing research that focuses especially on molecular targeted therapies. Despite its disputed etiopathogenesis, there seems to be a consensus about the involvement of dysfunctional cell adhesion molecules. Although the precise cause of the disease remains unclear, growing evidence indicates that dysregulated cell adhesion molecules may play a role [
2].
Such an adhesion molecule, with many functions beyond the standard attributed role, is CD44. This molecule is a cell surface glycoprotein highly involved in cellular dynamics, modulating the local immune response and tissue homeostasis [
7]. It has numerous isoforms resulting from its alternative splicing. These isoforms entail cell-to-cell and cell-to-matrix adhesion, maintaining tissue integrity, cell signaling; and extracellular matrix remodeling. They are also involved in processes such as embryonic development, lymphocyte homing, and wound healing [
2]. CD44 is a hyaluronic acid (HA) receptor, the ligand representing an essential component for tissue organization [
2]. By their interaction, CD44 and HA promote essential processes such as angiogenesis and immune evasion, emphasizing also the complexity of their functions [
8]. When focusing on endometriosis, CD44 emerges as one of the key players. It is involved in the progression of this disease by governing cell adhesion, attachment, invasion, and migration of the endometrial-like cells into the ectopic sites [
9,
10]. The adhesion capacity explains the establishment and persistence of endometriotic lesions, furthering the understanding of endometriosis’s invasive nature [
11].
Only a few studies in the existing literature have explored the potential therapeutic advantages of vitamin D in addressing endometriosis. Beyond its well-known role as a secosteroid that governs calcium metabolism and bone mineralization, vitamin D and its receptor also wield significant influence over the immune system, which aids in reducing bodily inflammation. These dual functions aroused the interest of scientists, who have ventured into studying its implications in endometriosis [
12,
13]. Several studies within the literature have indicated that vitamin D can act as an inhibitor of cellular proliferation [
14]. There is a very limited percentage of literature studies that focus on the possibility and benefits of using vitamin D supplementation for endometriosis treatment. With its immunomodulatory, anti-inflammatory, anti-proliferative, and anti-invasive properties, vitamin D is speculated to play a potentially vital role in the multifaceted pathogenesis of endometriosis [
15,
16]. But, this promising frontier remains relatively uncharted, with a multitude of ongoing research efforts concentrated on the attempt to elucidate the precise mechanisms of action and therapeutic implications of vitamin D in the context of endometriosis.
Due to the chronic characteristic inflammation that characterizes endometriosis, cyclooxygenase-2 (COX-2), with its inflammatory molecules synthesis, appears to play a key part in its pathogenesis [
17,
18,
19,
20]. Literature reports elevated levels of COX-2 in the ectopic endometrial-like tissue, with Bulum et al. suggesting a potential connection with pain related symptoms [
21,
22,
23]. Research suggests that COX-2 contributes to various aspects of endometriosis, including implantation and growth of the ectopic tissue, angiogenesis, and modulation of the immune response [
24], potentially leading to either pro-inflammatory or anti-inflammatory effects [
25].
Driven by our inquisitiveness regarding this controversial pathology, our research efforts to unravel endometriosis physio-pathogenesis converged in studying these three molecule expressions (CD44, VDR, and COX-2) in the endometriotic tissue samples of progestin-treated and untreated women. The purpose of our research is to serve as a foundation for the development of new diagnostic tools and therapeutic strategies that might ease the burden for endometriosis-affected women.
2. Results
The age of our 60 included patients varied between 18 and 45 years, with a mean age of 31.92 ± 4.706 (95% CI 30.70/33.13, Std Error 0.608). Before undergoing surgery, 24 (40%) patients underwent progestin treatment with dienogest for three months, while 36 (60%) patients did not receive any treatment.
2.1. CD44
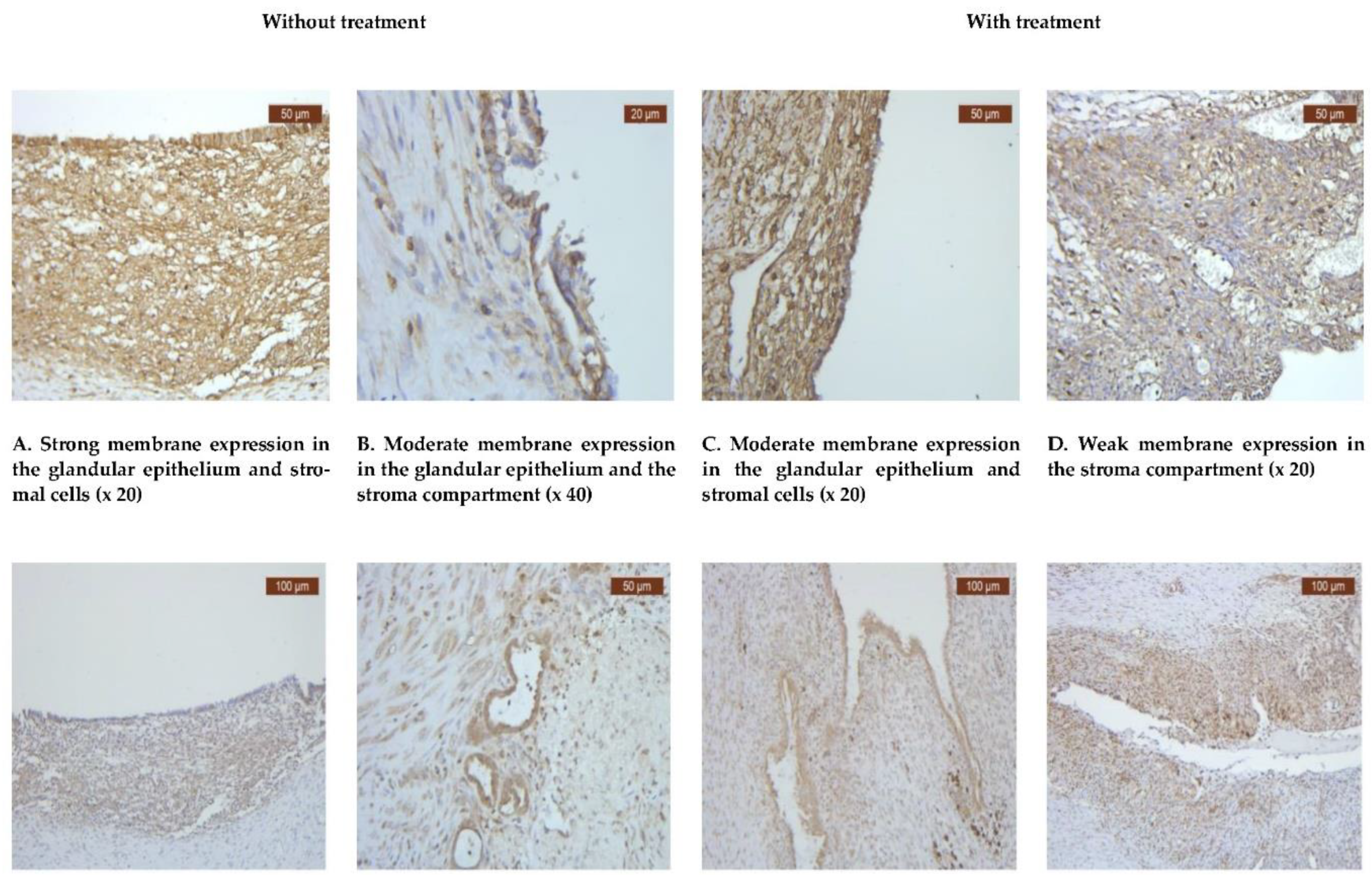
All instances of endometriosis, regardless of treatment status, exhibited a notably strong positive reaction for CD44. Despite the widespread expression of CD44 observed in all cases of endometriosis, a positive score was detected in 32 (60%) of the untreated cases and 21 (40%) of the treated ones (
Table 1). Immunohistochemistry examination revealed high expression levels of CD44 in both the stroma and endometriotic cells (
Figure 1A-D).
Upon scrutinizing the tissue expression of CD44, no statistically significant difference was observed between the progestin-treated and untreated groups, with a p-value of 0.99 (
Table 1). When examining the CD44 expression in the epithelial and stromal compartments separately, no notable variation was detected either (
Table 2).
2.2. VDR
To elucidate the involvement of VDR in ovarian endometriotic pathology, we aimed to characterize its expression patterns and distribution. Among the 24 women included in the treatment group, 20 (83.3%) tested positive for VDR, while only 4 (16.7%) were negative. Conversely, in the group of women with endometriosis but without any treatment, 32 (88.9%) were positive for VDR, with only 4 (11.1%) testing negative (
Table 1).
The expression of VDR was observed to be higher in the group without treatment, with 32 individuals (88.9%) showing positive expression, compared to 20 individuals (83.33%) in the treatment group. Conversely, the occurrence of negative VDR expression was consistent across both groups (
Table 1).
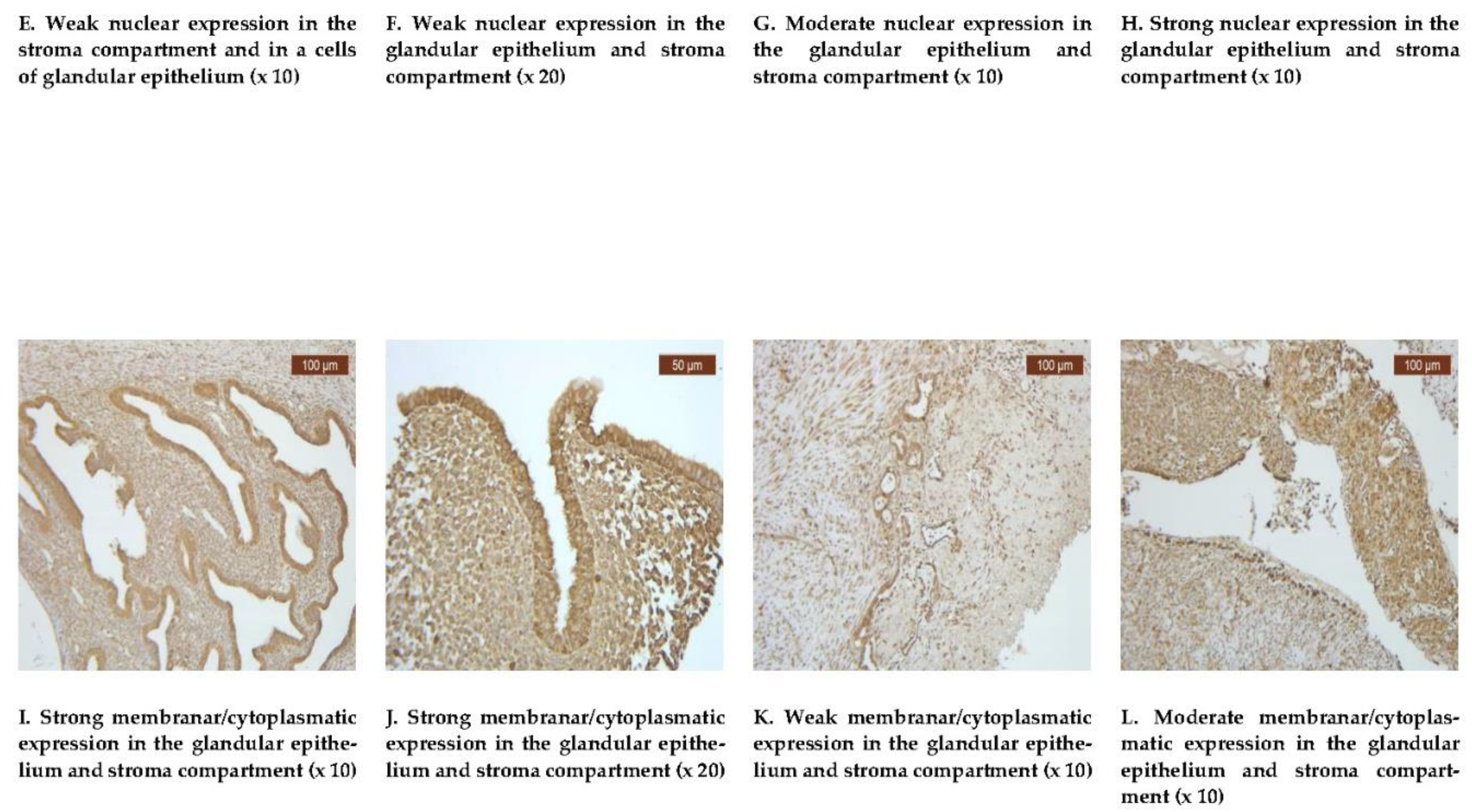
VDR exhibited a diffuse positive nuclear expression in both the epithelial and stromal compartments (
Figure 1E-H). However, the analysis revealed that the expression levels of VDR in the epithelial compartment were lower in the treated group, with 20 individuals (35.71%), compared to the untreated group, with 36 individuals that proved to be positive (64.28%) (
Table 2). Comparative images of the immunohistochemical staining in the two groups (with or without treatment) are illustrated in
Figure 1E-H.
2.3. COX-2
Endometriotic tissue consistently demonstrated uniformly strong membrane positivity for COX-2 in all of our 60 cases, this includes epithelial cells and endometrial stroma, regardless of therapeutic status. COX-2 was uniformly positive in both our groups (24 cases with dienogest treatment and 36 cases without treatment), with a slight tendency to decrease in intensity in the treated group (
Table 1).
Comparative images of the immunohistochemical staining in the two groups, with or without treatment, are depicted in
Figure 1I-L.
3. Discussion
Vitamin D levels are classified as follows: deficiency (<20 ng/ml), insufficiency (20–30 ng/ml), and sufficiency (>30 ng/ml) [
26]. The vitamin D nuclear receptor (VDR), which participates in the transcription of over 900 genes, facilitates the biological effects of vitamin D. This receptor is involved in various immunological processes, acting in conjunction with the active form of vitamin D, known as 1,25 (OH)2 vitamin D. Upon binding with the active vitamin D, the receptor relocates from the cytoplasm into the nucleus to initiate gene transcription. Emerging endometriosis research evidence is beginning to outline a potential therapeutic direction in the form of vitamin D, whose immunomodulatory and anti-inflammatory properties have caught considerable attention [
27,
28].
The literature is abundant with ongoing research into potential markers for endometriosis and treatment options, as no specific marker has yet proven highly sensitive and specific, and current treatments are still limited, especially by side effects. Owing to its incompletely understood pathophysiology, there are limited therapy options available, most of which involve progestin treatments and painkillers with less severe side effects. To treat endometriosis, progestin therapy has typically been used either alone or in combination with other drugs [
11,
29,
30,
31,
32]. As an enhancement of an already complicated to manage pathology, as stated by Guidice et al., the presence of progesterone inhibitory isoform A receptor results in progesterone treatment resistant endometriotic implants [
31].
The present study investigated the expression of CD44, VDR, and COX-2 in endometriotic tissues, comparing cases with and without progestin (desogestrel) treatment, with the hope of offering insights into the molecular pathophysiology of endometriosis and describing potential diagnosis and target-treatment markers.
There was no notable difference in CD44 levels between the two groups in our study. This cell-adhesion and migration molecule was expressed at similar levels in both the progestin-treated group (32 cases/60%) and the untreated ovarian cyst tissue samples (21 cases/40%). Although the difference was not statistically significant (p=0.99), further research could explore the observed trend of increased CD44 expression in progestin-treated women. In our study, we have noticed that CD44's membranar expression is typically elevated in the endometriotic tissue of women without treatment, compared to the treatment group, supporting endometriotic ectopic implant cell adhesion and lesion persistence in untreated patients. This trend is noteworthy due to CD44 implications in adhesion, migration, and invasion, underlining its potential as a therapeutic target for future interventions [
2]. We need also to emphasize that the lack of statistical significance may be attributed to the limited progestin treatment time-frame, which was restricted to just three months. Additionally, patient-specific characteristics could have influenced the outcomes as noted by Guidice et al., the presence of a specific progesterone receptor isoform can modulate an individual’s response to progestin therapy [
32].
Literature data about CD44 levels in endometriotic ectopic lesions is scarce and controversial [
33,
34,
35,
36], motivating our decision to further study its dynamics in progestin treated endometriosis compared to untreated ones. A 2007 research by Kim et al. detected high levels of CD44 expression in both epithelial and stroma compartments of various endometriotic lesions [
37]. Conversely, a 2016 animal study by Knudtson et al. yielded intriguing results, showing that endometriotic lesions could still manifest themselves in knockout mice, even in the absence of CD44 [
38]. Sancakli et al., Poncelet et al., and Nothnick et al., all found lower levels of CD44 in the tissue samples from women with endometriosis compared to controls [
2,
39,
40], while Pazhohan et al. and Matsuzaki et al. detected exactly the opposite [
10,
41]. These discrepancies underline the need for expanded further research to clarify the role and implications of CD44 in endometriosis.
The biological effects of vitamin D are mediated through the vitamin D receptor (VDR), which participates in the transcription process of more than 900 genes. Acting in conjunction with the active form of vitamin D, known as 1,25 (OH) vitamin D, VDR is involved in a multitude of immunological processes, initiating gene transcription by relocating from the cytoplasm into cells nucleus. Endometriosis research results begin to underline the potential therapeutic benefits of vitamin D administration in affected women due to its immunomodulatory and anti-inflammatory properties [
27,
28]. Our research comes as an effort to take a further step in the direction suggested by Agic et al. 2007 that vitamin D might have more endometriosis-contributing autocrine and/or paracrine local effects [
42]. Our results underline that VDR expression was detected in the majority of cases, regardless of the women’s progestin treatment status (83.3% from the progestin-treated cases and 88.9% from the untreated ones). Our result also has shown weak nuclear VDR expression in untreated women, potentially contributing to the persistent inflammation associated with this condition. This ascertainment fuses with the rest of the results from literature that suggest potential VDR key roles in the modulation of the immune response and inflammation in endometriosis [
1]. However, therapeutic interventions might interfere with VDR expression. This aspect comes as a result of the fact that progestin-treated women had a lower VDR expression in the epithelial compartment. Vitamin D supplementation or other treatments targeting VDR may upregulate VDR expression, leading to decreased inflammatory responses. In treated group, VDR expression often showed moderate and strong nuclear expression. However, the exact mechanism and its clinical practice implications are still to be discovered.
The well-known COX-2 pathway, which produces pro-inflammatory molecules, is considered to play a central role in endometriosis pathophysiology [
3,
4,
43]. Our research detected strong membranous positivity for COX-2 in the epithelial cells and endometrial stroma of both treated and untreated patients. Regarding the IHC intensity, we noticed strong COX-2 expression in endometriotic lesions of women without treatment, fact that is often correlated with increased inflammatory response, while reduced COX-2 expression in treated endometriosis samples might explained its weak and moderate expression.This similitude suggests that COX-2 expression is not influenced by progestin treatment, making it a suitable diagnostic marker and a target for future therapeutic interventions. The overexpression of COX-2, regardless of progestin treatment, similar to the observations of Lai et al., implies that it may substantially contribute to the chronic inflammatory state observed in women affected by endometriosis [
44].
We hope that the results of our study will contribute to the growing body of evidence regarding the molecular pathways in this remaining enigmatic disease. The patterns of our three analyzed molecular markers - VDR, CD44, and COX-2 - indicate their potential as targets for novel therapeutic strategies. Specifically, VDR may provide a new direction in managing the immune response in endometriosis by modulating it, while inhibitors targeting CD44 and COX-2 could be exploited therapeutically due to their roles in reducing invasiveness and inflammation. A more comprehensive understanding of endometriosis pathophysiology might be achieved by exploring the interplay of these markers.
The impact of our research limitations must be carefully considered for an accurate interpretation of our results. The first limitation resides in our small sample size and in the short 3 months progestin treatment time-frame. To validate our observations, we need to expand our research to a broader population and extend the progestin treatment time-frame. The second limitation arises from our group selection criteria, as we included only women with endometriotic ovarian cysts (stages III or IV of the disease) from a diverse phenotypic pool. To be able to generalize our findings, we need to validate them in a population that includes all stages of endometriosis. Despite these obvious limitations, we are confident that our research will contribute to advancing the understanding of this complex disease.
4. Materials and Methods
4.1. Patients and Tissue Samples
The study period was from January 2021 to January 2022. Prior to the enrollment in the study all of the patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Medicine and Pharmacy “Gr. T. Popa” from Iasi (No. 10/28.09.2020) and the University of Medicine and Pharmacy “Carol Davila” from Bucharest (No. 6/21.12.2021).
Tissue samples were collected during ovarian cyst laparoscopic interventions from 60 women diagnosed with endometriosis. These women underwent surgery at the Obstetrics and Gynecology Hospital "Cuza-Voda" in Iasi, and the Obstetrics and Gynecology Hospital "Panait Sirbu" in Bucharest. We have divided them into two groups. The first group of women diagnosed with endometriosis decided to undergo progestin (dienogest) treatment for three months before the surgical interventions. The second group of women chose not to undergo dienogest treatment before surgery.
Inclusion criteria:
We included only Caucasian women with the histological examination confirming the suspected endometriosis diagnosis in every case we included. All of the enrolled women were diagnosed during the surgical procedure with endometriosis classified as stages III or IV, according to the American Society for Reproductive Medicine (ASRM) criteria [
29,
30]. Tissue samples were collected from all of the included patients. The women who underwent hormone therapy had 2 mg of dienogest administered daily for three months prior to surgery. The selection of untreated endometriosis patients was done by matching their age and body mass index (BMI) with those receiving dienogest therapy. All the tissue samples were obtained during the proliferative phase of the menstrual cycle.
Exclusion criteria:
To mitigate potential confounding variables, we excluded individuals with a body mass index (BMI) exceeding 30, as well as those diagnosed with malignancy or other tumoral lesions, diabetes, depression, genetic syndromes, any infectious or autoimmune diseases, smokers, pregnant women, individuals receiving hormonal therapy other than dienogest, or any other treatment known to interfere with bone and mineral metabolism.
4.2. Immunohistochemistry
Routinely prepared hematoxylin and eosin (H&E) sections have been examined, and IHC has been independently evaluated by two pathologists to confirm the diagnosis.
Monoclonal antibodies targeting CD44 were employed for analysis. Prior to analysis, the samples underwent fixation with 10% neutral formalin, followed by embedding in paraffin and sectioning to achieve a thickness of 4-5 micrometers. IHC was utilized to assess the expressions of CD44, employing specific (1:250) dilutions provided by Abcam Company. For CD44 the membrane immunostaining pattern was considered positive (
Table 3).
Formalin-fixed, paraffin-embedded tissue sections were deparaffinized, and immunohistochemical staining was performed using protocols optimized for COX-2 antibody. The antibody clone name, source, dilution, and pattern of expression are listed in
Table 3. The membrane pattern of COX-2 immunostaining was considered positive, however, if a cytoplasmic and/or membranous pattern was present, it was considered positive. The expression levels of CD44, VDR, and COX-2 were quantified by assessing the percentage of positively stained cells and the staining intensity in each section. For overall positivity, immunostaining in >5% of cells were considered positive, and < 5% positive cells was considered negative. The expression of intensity was evaluated as weak, moderate, and strong both at the epithelial and stromal compartments.
Vitamin D anti-receptor dilution 1:3000 (Abcam, Cambridge, UK) was incubated for an overnight period at 4°C. The sections were washed, exposed to the secondary antibody for 45 minutes at 37 degrees, and then thoroughly cleaned with
phosphate-buffered saline (PBS). Hematoxylin was used as a counterstain in the standard avidin-biotin-peroxidase technique, which used a liquid DAB (diaminobenzidine) substrate and chromogen system for viewing. As a negative control, primary antibodies were left out. For VDR the nuclear immunostaining pattern was considered positive. Human jejunum was utilized as a positive reference for vitamin D receptor (
Table 3).
4.3. Statistical Analysis
Medical data were imported and verified in Microsoft Excel and then analyzed in SPSS 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). The information was in the form of measurable numerical values (age), respectively categorical variables. Within the descriptive statistics, we calculated the values of the following statistical measures: sample size (N), mean, standard deviation, standard error, and 95% confidence interval for mean, min, max, and absolute and relative frequencies, respectively. Statistical hypothesis tests were done by Chi-square or Fisher exact tests (for categorical type). The standard cut-off of 5% or 0.05 significance was used to decide on the conclusion of the hypothesis.
5. Conclusions
The current study concludes by highlighting the importance of VDR, CD44, and COX-2 as major participants in endometriosis pathogenesis. The tissue expression of CD44 reavealed no statistically significant difference between the progestin-treated and untreated groups, with a p-value of 0.99. Our results indicated a lower expression of VDR in the epithelial compartment of the treated cases, compared to untreated ones, with 40% of treated and 60% of untreated cases showing positive expression. COX-2 was highly expressed across all endometriotic tissue samples, irrespective of treatment status. Even though the precise mechanisms are still not fully understood, our findings provide a basis for further investigation and possible clinical uses targeted at enhancing endometriosis treatment.
Author Contributions
Conceptualization, D.R.M. and L.L.; methodology, C.E.M. and M.O.; software, I.E.B and L.V.B.; validation, I.E.B., and M.O.; formal analysis, L.L. and M.G.; investigation, D.R.M.; resources, M.G. and A.U.; data curation, A.U.; writing—original draft preparation, D.R.M.; writing—review and editing, A.U.; D.R.M. and L.L.; visualization, I.E.B.; supervision, A.U.; project administration, D.R.M.; funding acquisition, D.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the UNIVERSITY OF MEDICINE AND PHARMACY “GR. T. POPA”, IASI, Romania, grant number 10310/29.06.2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the UNIVERSITY OF MEDICINE AND PHARMACY “GR. T. POPA” IASI (10/28.09.2020) and of the UNIVERSITY OF MEDICINE AND PHARMACY “CAROL DAVILA, BUCHAREST (06/21.12.2021).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data used to support the findings of this study are available upon request to the corresponding author.
Acknowledgments
The authors would like to acknowledge the contribution of the Department of Obstetrics and Gynecology, Women’s Hospital, Universities of Medicine and Pharmacy “Gr.T.Popa” Iasi and “Carol Davila” Bucharest, Romania, for supporting this study. We are also greatly indebted to all participants for donating their ectopic tissue and serum specimens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bulun, S.E. Endometriosis. N Engl J Med 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Sancakli Usta, C.; Turan, G.; Bulbul, C.B.; Usta, A.; Adali, E. Differential expression of Oct-4, CD44, and E-cadherin in eutopic and ectopic endometrium in ovarian endometriomas and their correlations with clinicopathological variables. Reprod Biol Endocrinol 2020, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, M.; Yu, Q.; Fei, W.; Li, T.; Zhu, L.; Yao, Y.; Zheng, C.; Zhang, X. Hyaluronic Acid-Modified Nanoplatforms as a Vector for Targeted Delivery of Autophagy-Related Gene to the Endometriotic Lesions in Mice. Front Bioeng Biotechnol 2022, 10, 918368. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Multinu, F.; Cosio, S.; Carinelli, S.; Ghioni, M.; Aletti, G.D. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol Oncol 2021, 162, 741–750. [Google Scholar] [CrossRef]
- Lin, S.; Xie, X.; Guo, Y.; Zhang, H.; Liu, C.; Yi, J.; Su, Y.; Deng, Q.; Zhu, W. Clinical characteristics and pregnancy outcomes of infertile patients with endometriosis and endometrial polyps: A retrospective cohort study. Taiwan J Obstet Gynecol 2020, 59, 916–921. [Google Scholar] [CrossRef]
- Mehdizadehkashi, A.; Tahermanesh, K.; Fazel Anvari-Yazdi, A.; Chaichian, S.; Azarpira, N.; Nobakht, M.; Abed, S.M.; Hashemi, N. Ultrastructural Investigation of Pelvic Peritoneum in Patients With Chronic Pelvic Pain and Subtle Endometriosis in Association With Chromoendoscopy. J Minim Invasive Gynecol 2017, 24, 114–123. [Google Scholar] [CrossRef]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J Nutr Biochem 2020, 86. [Google Scholar] [CrossRef]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Niiro, E.; Kawahara, N.; Yamada, Y.; Yoshimoto, C.; Shimada, K.; Sudo, T.; Kobayashi, H. Immunohistochemical expression of CD44v9 and 8-OHdG in ovarian endometrioma and the benign endometriotic lesions adjacent to clear cell carcinoma. J Obstet Gynaecol Res 2019, 45, 2260–2266. [Google Scholar] [CrossRef]
- Pazhohan, A.; Amidi, F.; Akbari-Asbagh, F.; Seyedrezazadeh, E.; Aftabi, Y.; Abdolalizadeh, J.; Khodarahmian, M.; Khanlarkhani, N.; Sobhani, A. Expression and shedding of CD44 in the endometrium of women with endometriosis and modulating effects of vitamin D: A randomized exploratory trial. J Steroid Biochem Mol Biol 2018, 178, 150–158. [Google Scholar] [CrossRef]
- Olivares, C.N.; Alaniz, L.D.; Menger, M.D.; Barañao, R.I.; Laschke, M.W.; Meresman, G.F. Inhibition of Hyaluronic Acid Synthesis Suppresses Angiogenesis in Developing Endometriotic Lesions. PLoS One 2016, 11, e0152302. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: a review with comparison of 8 guidelines. 2021; 21. [Google Scholar] [CrossRef]
- Lopes, V.M.; Lopes, J.R.; Brasileiro, J.P.; Oliveira, I.; Lacerda, R.P.; Andrade, M.R.; Tierno, N.I.; Souza, R.C.; Motta, L.A. Highly prevalence of vitamin D deficiency among Brazilian women of reproductive age. Arch Endocrinol Metab 2017, 61, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kurose, S.; Nakayama, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Sato, S.; Sakiyama, A.; Yoshioka, S.; Kobayashi, M.; Nakayama, S.; Otuski, Y.; Ishikawa, N.; Kyo, S. Whole-Exome Sequencing of Rare Site Endometriosis-Associated Cancer. Diseases 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- de Pascali, F.; Casarini, L.; Kuhn, C.; Simoni, M.; Mahner, S.; Jeschke, U.; von Schonfeldt, V. Nuclear expression of VDR and AHR is mutually exclusive in glandular cells in endometriosis. Histochem Cell Biol 2021, 156, 391–399. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Lempesis, I.G.; Athanasaki, F.; Schizas, D.; Samartzis, E.P.; Kolibianakis, E.M.; Goulis, D.G. Association between vitamin D and endometriosis: a systematic review. Hormones (Athens) 2020, 2020 19, 109–121. [Google Scholar] [CrossRef]
- Gazvani, R.; Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Lousse, J.C.; Van Langendonckt, A.; González-Ramos, R.; Defrère, S.; Renkin, E.; Donnez, J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil Steril 2008, 90, 217–220. [Google Scholar] [CrossRef]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed Res Int 2015, 2015, 795976. [Google Scholar] [CrossRef]
- Murakami, M.; Kudo, I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res 2004, 43, 3–35. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-β in endometriosis. Semin Reprod Med 2012, 30, 39–45. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; Li, M.Q. Cyclooxygenase-2 in Endometriosis. Int J Biol Sci 2019, 15, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Shoji, Y.; Chuang, P.C.; Tsai, S.J. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med 2007, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.K.; Lee, J.; Speights, V.O. Jr, Starzinski-Powitz, A.; Arosh, J.A. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology, 1180. [Google Scholar] [CrossRef]
- Park, G.Y.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 2006, 290, L797–805. [Google Scholar] [CrossRef] [PubMed]
- Siufi Neto, J.; Kho, R.M.; Siufi, D.F.; Baracat, E.C.; Anderson, K.S.; Abrão, M.S. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J Minim Invasive Gynecol 2014, 21, 55–63. [Google Scholar] [CrossRef]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin d receptor and T cell function. Front Immunol 2013, 4, 148. [Google Scholar] [CrossRef]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T.; Tan, J.; Zhang, H.; Wang, C. The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl Neurodegener 2020, 9, 34. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int J Mol Sci 2021, 22, 10554. [Google Scholar] [CrossRef]
- Szamatowicz, M. Endometriosis--still an enigmatic disease. What are the causes, how to diagnose it and how to treat successfully? Gynecol Endocrinol 2008, 24, 535–536. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Samartzis, N.; Daniilidis, A.; Leeners, B.; Makieva, S.; Nirgianakis, K.; Dedes, I.; Metzler, J.M.; Imesch, P.; Lempesis, I.G. Effects of vitamin D supplementation in endometriosis: a systematic review. Reprod Biol Endocrinol 2022, 20, 176. [Google Scholar] [CrossRef]
- Ho, N.T.; Lin, S.W.; Lee, Y.R.; Tzeng, C.R.; Kao, S.H. Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells. Int J Mol Sci 2022, 23, 15328. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.H.; Na, Y.J.; Ahn, M.Y.; Jeon, H.N.; Yeom, J.I.; Lee, K.S. Expression of CD44 in endometrial stromal cells from women with and without endometriosis and its effect on the adherence to peritoneal mesothelial cells. Obstet Gynecol Sci 2013, 56, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, C.; Chen, H.; Nian, Y.; Bai, Z.; Ha, C. The involvement of osteopontin and matrix metalloproteinase- 9 in the migration of endometrial epithelial cells in patients with endometriosis. Reprod Biol Endocrinol 2015, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016, 2016, CD012179. [Google Scholar] [CrossRef]
- Kim, H.O.; Yang, K.M.; Kang, I.S.; Koong, M.K.; Kim, H.S.; Zhang, X.; Kim, I. Expression of CD44s, vascular endothelial growth factor, matrix metalloproteinase-2 and Ki-67 in peritoneal, rectovaginal and ovarian endometriosis. J Reprod Med 2007, 52, 207–213. [Google Scholar]
- Knudtson, J.F.; Tekmal, R.R.; Santos, M.T.; Binkley, P.A.; Krishnegowda, N.; Valente, P.; Schenken, R.S. Impaired Development of Early Endometriotic Lesions in CD44 Knockout Mice. Reprod Sci 2016, 23, 87–91. [Google Scholar] [CrossRef]
- Poncelet, C.; Leblanc, M.; Walker-Combrouze, F.; Soriano, D.; Feldmann, G.; Madelenat, P.; Scoazec, J.Y.; Daraï, E. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet Gynecol Scand 2002, 81, 195–203. [Google Scholar] [CrossRef]
- Nothnick, W.B.; Fan, F.; Iczkowski, K.A.; Ashwell, R.; Thomas, P.; Tawfik, O.W. CD44s expression is reduced in endometriotic lesions compared to eutopic endometrium in women with endometriosis. Int J Gynecol Pathol 2001, 20, 140–146. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C.; Maleysson, E.; Canis, M.; Mage, G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metab 2010, 95, 3437–3445. [Google Scholar] [CrossRef]
- Agic, A.; Xu, H.; Altgassen, C.; Noack, F.; Wolfler, M.M.; Diedrich, K.; Friedrich, M.; Taylor, R.N.; Hornung, D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 2007, 14, 486–497. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. Int J Mol Sci, 9628. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; Li, M.Q. Cyclooxygenase-2 in Endometriosis. Int J Biol Sci 2019, 15, 2783–2797. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).