Submitted:
12 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. B1 B Cells
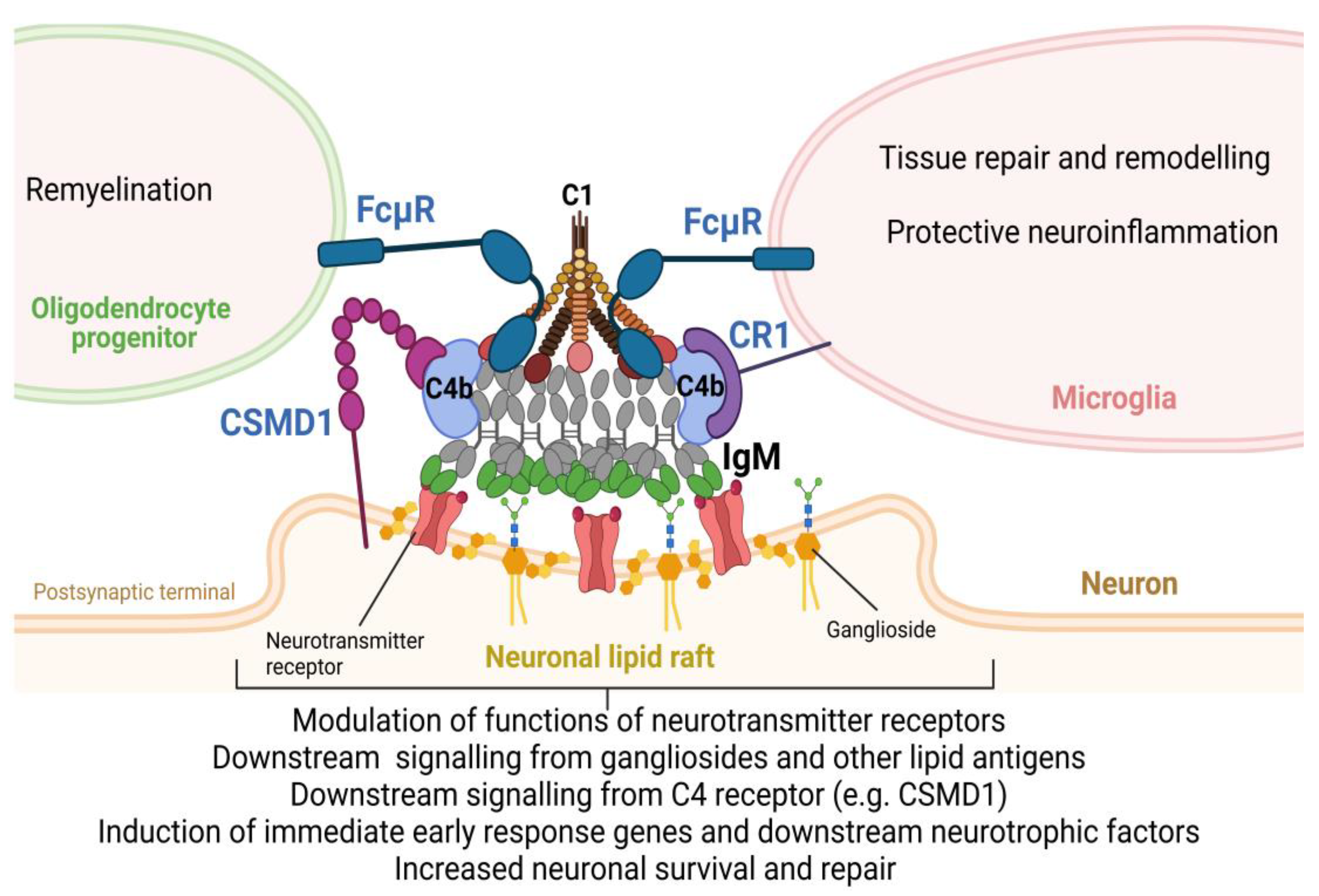
2.1. Mouse B1 B Cells
2.2. Human B1 B Cells
2.3. Function of B1 B Cells and Their Antibodies
| Antigen | Location in the CNS | Isotype (Clone) |
Species (Source of Ab1) |
Relation to pathology and/or repair |
References | |
|---|---|---|---|---|---|---|
| AQP43 | Astrocytes (limitans) | IgG | Human | Neuromyelitis Optica | [29] | |
| NMDAR3 | Neurons (NLR4) | IgM, IgA | Human | Encephalitis, psychosis, seizures | [30] | |
| AMPAR5 | Neurons (NLR) IgG, IgM | Human | Encephalitis, seizures | [30] | ||
| GABAAR6 | Neurons (NLR) IgG1, IgG3, IgM | Human | Encephalitis, psychosis, seizures | [31] | ||
| D2R7 | Neurons (NLR) IgG, IgM | Human | Parkinson's disease, psychosis | [32] | ||
| MOG8 | Oligodendrocytes IgM | Mice | Multiple sclerosis | [33] | ||
| Sulfatide | Oligodendrocytes IgM (hIgM22) | Human | Remyelination | [34] | ||
| Gangliosides | Neurons (NLR) IgM (sHIgM12) | Human | Axonal outgrowth | [35] | ||
| Amyloid | Neurons (NLR) IgM | Human | Alzheimer's disease, neuroprotection | [36] | ||
| MLD9 | Neurons, astrocytes IgM | Human | Schizophrenia | [37] | ||
| PC10 | CNS (including NLR) IgM | Human | Stroke, Alzheimer’s disease | [38,39] | ||
3. Traumatic Brain Injury
4. Multiple Sclerosis
5. Alzheimer’s Disease
6. Epilepsy
7. Emerging Role of B1 B Cells and NAAs in Neurological Diseases
8. New Role of Complement in Neurologic Diseases
9. Conclusion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arumugham, V.B.; Rayi, A. Intravenous Immunoglobulin (IVIG). StatPearls 2023. [Google Scholar]
- Jolles, S.; Sewell, W.A.C.; Misbah, S.A. Clinical Uses of Intravenous Immunoglobulin. Clin Exp Immunol 2005, 142, 1–11. [Google Scholar] [CrossRef]
- Thom, V.; Arumugam, T. V.; Magnus, T.; Gelderblom, M. Therapeutic Potential of Intravenous Immunoglobulin in Acute Brain Injury. Front Immunol 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Soelberg Sorensen, P. Intravenous Polyclonal Human Immunoglobulins in Multiple Sclerosis. Neurodegener Dis 2007, 5, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Relkin, N.R.; Thomas, R.G.; Rissman, R.A.; Brewer, J.B.; Rafii, M.S.; Van Dyck, C.H.; Jack, C.R.; Sano, M.; Knopman, D.S.; Raman, R.; et al. A Phase 3 Trial of IV Immunoglobulin for Alzheimer Disease. Neurology 2017, 88, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Pourfathollah, A.A.; Rasaee, M.J.; Porpak, Z.; Jafari, M.E. The Concentration of Total Serum IgG and IgM in Sera of Healthy Individuals Varies at Different Age Intervals. Biomedicine & Aging Pathology 2013, 3, 241–245. [Google Scholar] [CrossRef]
- Li, Y.; Shen, H.; Zhang, R.; Ji, C.; Wang, Y.; Su, C.; Xiao, J. Immunoglobulin M Perception by FcμR. Nature 2023 615:7954 2023, 615, 907–912. [Google Scholar] [CrossRef]
- Trebst, C.; Stangel, M. Promotion of Remyelination by Immunoglobulins: Implications for the Treatment of Multiple Sclerosis. Curr Pharm Des 2005, 12, 241–249. [Google Scholar] [CrossRef]
- Palma, J.; Tokarz-Deptuła, B.; Deptuła, J.; Deptuła, W. Natural Antibodies – Facts Known and Unknown. Cent Eur J Immunol 2018, 43, 466. [Google Scholar] [CrossRef]
- Rodriguez-Zhurbenko, N.; Quach, T.D.; Hopkins, T.J.; Rothstein, T.L.; Hernandez, A.M. Human B-1 Cells and B-1 Cell Antibodies Change with Advancing Age. Front Immunol 2019, 10, 430461. [Google Scholar] [CrossRef]
- Xu, X.; Ng, S.M.; Hassouna, E.; Warrington, A.; Oh, S.H.; Rodriguez, M. Human-Derived Natural Antibodies: Biomarkers and Potential Therapeutics. 2015; 10, 25–39. [Google Scholar] [CrossRef]
- Halperin, S.T.; ’T Hart, B.A.; Luchicchi, A.; Schenk, G.J. The Forgotten Brother: The Innate-like B1 Cell in Multiple Sclerosis. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Prieto, J.M.B.; Felippe, M.J.B. Development, Phenotype, and Function of Non-Conventional B Cells. Comp Immunol Microbiol Infect Dis 2017, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, T.L. Natural Antibodies as Rheostats for Susceptibility to Chronic Diseases in the Aged. Front Immunol 2016, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Rakhmanov, M.; Keller, B.; Gutenberger, S.; Foerster, C.; Hoenig, M.; Driessen, G.; Van Der Burg, M.; Van Dongen, J.J.; Wiech, E.; Visentini, M.; et al. Circulating CD21low B Cells in Common Variable Immunodeficiency Resemble Tissue Homing, Innate-like B Cells. Proc Natl Acad Sci U S A 2009, 106, 13451–13456. [Google Scholar] [CrossRef]
- Popi, A.F.; Longo-Maugéri, I.M.; Mariano, M. An Overview of B-1 Cells as Antigen-Presenting Cells. Front Immunol 2016, 7, 177941. [Google Scholar] [CrossRef]
- Valeff, N.J.; Ventimiglia, M.S.; Dibo, M.; Markert, U.R.; Jensen, F. Splenic B1 B Cells Acquire a Proliferative and Anti-Inflamatory Profile During Pregnancy in Mice. Front Immunol 2022, 13, 873493. [Google Scholar] [CrossRef]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol 2016, 7, 324. [Google Scholar] [CrossRef]
- Benboubetra, M.; Nicholl, D.; Wagner, E.R.; Bowes, T.; Willison, H.J.; Cochrane, L.; Conner, J.; Furukawa, K.; Boffey, J. Tolerance to Self Gangliosides Is the Major Factor Restricting the Antibody Response to Lipopolysaccharide Core Oligosaccharides in Campylobacter Jejuni Strains Associated with Guillain-Barre Syndrome. Infect Immun 2002, 70, 5008–5018. [Google Scholar] [CrossRef]
- Fokkink, W.J.R.; Selman, M.H.J.; Dortland, J.R.; Durmuş, B.; Kuitwaard, K.; Huizinga, R.; Van Rijs, W.; Tio-Gillen, A.P.; Van Doorn, P.A.; Deelder, A.M.; et al. IgG Fc N-Glycosylation in Guillain-Barré Syndrome Treated with Immunoglobulins. J Proteome Res 2014, 13, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; Van Doorn, P.A. Guillain–Barré Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis. Nature Reviews Neurology 2014 10:8 2014, 10, 469–482. [Google Scholar] [CrossRef]
- Kumar, S.; Rodriguez, M.; Watzlawik, J.O.; Jordan, L.R.; Wittenberg, N.J.; Warrington, A.E.; Xu, X.; Oh, S.-H. A Patterned Recombinant Human IgM Guides Neurite Outgrowth of CNS Neurons. Sci Rep 2013, 3, 2267. [Google Scholar] [CrossRef]
- Ponomarev, E.D. Fresh Evidence for Platelets as Neuronal and Innate Immune Cells: Their Role in the Activation, Differentiation, and Deactivation of Th1, Th17, and Tregs during Tissue Inflammation. Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Dukhinova, M.; Kuznetsova, I.; Kopeikina, E.; Veniaminova, E.; Yung, A.W.Y.; Veremeyko, T.; Levchuk, K.; Barteneva, N.S.; Wing-Ho, K.K.; Yung, W.H.; et al. Platelets Mediate Protective Neuroinflammation and Promote Neuronal Plasticity at the Site of Neuronal Injury. Brain Behav Immun 2018, 74, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Dukhinova, M.; Veremeyko, T.; Yung, A.W.Y.; Kuznetsova, I.S.; Lau, T.Y.B.; Kopeikina, E.; Chan, A.M.L.; Ponomarev, E.D. Fresh Evidence for Major Brain Gangliosides as a Target for the Treatment of Alzheimer’s Disease. Neurobiol Aging 2019, 77, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Starossom, S.C.; Veremeyko, T.; Yung, A.W.Y.; Dukhinova, M.; Au, C.; Lau, A.Y.; Weiner, H.L.; Ponomarev, E.D. Platelets Play Differential Role during the Initiation and Progression of Autoimmune Neuroinflammation. Circ Res 2015, 117, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Kopeikina, E.; Dukhinova, M.; Yung, A.W.Y.; Veremeyko, T.; Kuznetsova, I.S.; Lau, T.Y.B.; Levchuk, K.; Ponomarev, E.D. Platelets Promote Epileptic Seizures by Modulating Brain Serotonin Level, Enhancing Neuronal Electric Activity, and Contributing to Neuroinflammation and Oxidative Stress. Prog Neurobiol 2020, 188. [Google Scholar] [CrossRef] [PubMed]
- Sotnikov, I.; Veremeyko, T.; Starossom, S.C.; Barteneva, N.; Weiner, H.L.; Ponomarev, E.D. Platelets Recognize Brain-Specific Glycolipid Structures, Respond to Neurovascular Damage and Promote Neuroinflammation. PLoS One 2013, 8, e58979. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Franciotta, D.; Bergamaschi, R.; Wildemann, B.; Wandinger, K.P. Immunoglobulin M Antibodies to Aquaporin-4 in Neuromyelitis Optica and Related Disorders. Clin Chem Lab Med 2010, 48, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Olivero, G.; Roggeri, A.; Pittaluga, A. Anti-NMDA and Anti-AMPA Receptor Antibodies in Central Disorders: Preclinical Approaches to Assess Their Pathological Role and Translatability to Clinic. International Journal of Molecular Sciences 2023, Vol. 24, Page 14905 2023, 24, 14905. [Google Scholar] [CrossRef]
- Pettingill, P.; Kramer, H.B.; Coebergh, J.A.; Pettingill, R.; Maxwell, S.; Nibber, A.; Malaspina, A.; Jacob, A.; Irani, S.R.; Buckley, C.; et al. Antibodies to GABAA Receptor A1 and Γ2 Subunits: Clinical and Serologic Characterization. Neurology 2015, 84, 1233–1241. [Google Scholar] [CrossRef]
- Dale, R.C.; Merheb, V.; Pillai, S.; Wang, D.; Cantrill, L.; Murphy, T.K.; Ben-Pazi, H.; Varadkar, S.; Aumann, T.D.; Horne, M.K.; et al. Antibodies to Surface Dopamine-2 Receptor in Autoimmune Movement and Psychiatric Disorders. Brain 2012, 135, 3453–3468. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Wheeler, S.E. Clinical Significance of Uncommon, Non-Clinical, and Novel Autoantibodies. Immunotargets Ther 2024, 13, 215. [Google Scholar] [CrossRef]
- Warrington, A.E.; Rodriguez, M. Method of Identifying Natural Antibodies for Remyelination. J Clin Immunol, 2010; 30 Suppl. [Google Scholar] [CrossRef]
- Denic, A.; Macura, S.I.; Warrington, A.E.; Pirko, I.; Grossardt, B.R.; Pease, L.R.; Rodriguez, M. A Single Dose of Neuron-Binding Human Monoclonal Antibody Improves Spontaneous Activity in a Murine Model of Demyelination. PLoS One 2011, 6, e26001. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, M.; Olin, C.E.; Johns, H.T.; Takeda-Uchimura, Y.; Lemieux, M.C.; Rufibach, K.; Rajadas, J.; Zhang, H.; Tomooka, B.; Robinson, W.H.; et al. Neuroprotective Natural Antibodies to Assemblies of Amyloidogenic Peptides Decrease with Normal Aging and Advancing Alzheimer’s Disease. Proc Natl Acad Sci U S A 2009, 106, 12145–12150. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F. In Schizophrenia, Deficits in Natural IgM Isotype Antibodies Including Those Directed to Malondialdehyde and Azelaic Acid Strongly Predict Negative Symptoms, Neurocognitive Impairments, and the Deficit Syndrome. Mol Neurobiol 2019, 56, 5122–5135. [Google Scholar] [CrossRef]
- Fiskesund, R.; Stegmayr, B.; Hallmans, G.; Vikström, M.; Weinehall, L.; De Faire, U.; Frostegård, J. Low Levels of Antibodies Against Phosphorylcholine Predict Development of Stroke in a Population-Based Study From Northern Sweden. Stroke 2010, 41, 607–612. [Google Scholar] [CrossRef]
- Eriksson, U.K.; Sjöberg, B.G.; Bennet, A.M.; De Faire, U.; Pedersen, N.L.; Frostegrd, J. Low Levels of Antibodies against Phosphorylcholine in Alzheimer’s Disease. J Alzheimers Dis 2010, 21, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Mckee, A.C.; Daneshvar, D.H. The Neuropathology of Traumatic Brain Injury. In Handbook of Clinical Neurology; 2015; Vol. 127, pp. 45–66 ISBN 1857364570.
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell Mol Neurobiol 2017, 37, 571–585. [Google Scholar] [CrossRef]
- Shah, M.; Garvin, R.; Shakir, A.; Jackson, C.; Carr, K.R. Post-Traumatic Brain Injury (TBI) Presenting with Guillain-Barre Syndrome and Elevated Anti-Ganglioside Antibodies: A Case Report and Review of the Literature. International Journal of Neuroscience 2014, 125, 486–492. [Google Scholar] [CrossRef]
- Dukhinova, M.; Kuznetsova, I.; Kopeikina, E.; Veniaminova, E.; Yung, A.W.Y.Y.; Veremeyko, T.; Levchuk, K.; Barteneva, N.S.; Wing-Ho, K.K.; Yung, W.-H.H.; et al. Platelets Mediate Protective Neuroinflammation and Promote Neuronal Plasticity at the Site of Neuronal Injury. Brain Behav Immun 2018. [Google Scholar] [CrossRef] [PubMed]
- Kelso, M.L.; Gendelman, H.E. Bridge between Neuroimmunity and Traumatic Brain Injury. Curr Pharm Des 2014, 20, 4284–4298. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Popovich, P.G. B Cells and Autoantibodies: Complex Roles in CNS Injury. Trends Immunol 2010, 31, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Veremeyko, T.; Yung, A.W.Y.; Dukhinova, M.; Kuznetsova, I.S.; Pomytkin, I.; Lyundup, A.; Strekalova, T.; Barteneva, N.S.; Ponomarev, E.D. Cyclic AMP Pathway Suppress Autoimmune Neuroinflammation by Inhibiting Functions of Encephalitogenic CD4 T Cells and Enhancing M2 Macrophage Polarization at the Site of Inflammation. Front Immunol 2018, 9, 50. [Google Scholar] [CrossRef]
- Rangachari, M.; Kerfoot, S.M.; Arbour, N.; Alvarez, J.I. Editorial: Lymphocytes in MS and EAE: More Than Just a CD4+ World. Front Immunol 2017, 8, 133. [Google Scholar] [CrossRef]
- van de Veen, W.; Stanic, B.; Wirz, O.F.; Jansen, K.; Globinska, A.; Akdis, M. Role of Regulatory B Cells in Immune Tolerance to Allergens and Beyond. Journal of Allergy and Clinical Immunology 2016, 138, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Maaser-Hecker, A.; Tanzi, R.E. The Neuroimmune Axis of Alzheimer’s Disease. Genome Med 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, Y.; Ding, S.; Chen, S.; Wang, T.; Wang, Z.; Zou, Y.; Sheng, C.; Chen, Y.; Pang, Y.; et al. B Lymphocytes Ameliorate Alzheimer’s Disease-like Neuropathology via Interleukin-35. Brain Behav Immun 2023, 108, 16–31. [Google Scholar] [CrossRef]
- Kim, K.; Wang, X.; Ragonnaud, E.; Bodogai, M.; Illouz, T.; DeLuca, M.; McDevitt, R.A.; Gusev, F.; Okun, E.; Rogaev, E.; et al. Therapeutic B-Cell Depletion Reverses Progression of Alzheimer’s Disease. Nature Communications 2021 12:1 2021, 12, 1–11. [Google Scholar] [CrossRef]
- de Mol, J.; Kuiper, J.; Tsiantoulas, D.; Foks, A.C. The Dynamics of B Cell Aging in Health and Disease. Front Immunol 2021, 12, 733566. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol Rev 2020, 72, 606. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Lang, B.; Aronica, E. Immunity and Inflammation in Epilepsy. Cold Spring Harb Perspect Med 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Sanli, E.; Sirin, N.G.; Kucukali, C.I.; Baykan, B.; Ulusoy, C.A.; Bebek, N.; Yilmaz, V.; Tuzun, E. Peripheral Blood Regulatory B and T Cells Are Decreased in Patients with Focal Epilepsy. J Neuroimmunol 2024, 387, 578287. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, G.; Morichi, S.; Takamatsu, T.; Watanabe, Y.; Suzuki, S.; Ishida, Y.; Oana, S.; Yamazaki, T.; Takata, F.; Kawashima, H. Links between Immune Cells from the Periphery and the Brain in the Pathogenesis of Epilepsy: A Narrative Review. International Journal of Molecular Sciences 2021, Vol. 22, Page 4395 2021, 22, 4395. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Yamashita, T. B-1a Lymphocytes Promote Oligodendrogenesis during Brain Development. Nat Neurosci 2018, 21, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I €“ Molecular Mechanisms of Activation and Regulation. Front Immunol 2015, 6, 262. [Google Scholar] [CrossRef]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular Intercommunication between the Complement and Coagulation Systems. The Journal of Immunology 2010, 185, 5628–5636. [Google Scholar] [CrossRef]
- Hammad, A.; Westacott, L.; Zaben, M. The Role of the Complement System in Traumatic Brain Injury: A Review. J Neuroinflammation 2018, 15, 24. [Google Scholar] [CrossRef]
- Lubbers, R.; van Essen, M.F.; van Kooten, C.; Trouw, L.A. Production of Complement Components by Cells of the Immune System. Clin Exp Immunol 2017, 188, 183–194. [Google Scholar] [CrossRef]
- Veerhuis, R.; Nielsen, H.M.; Tenner, A.J. Complement in the Brain. Mol Immunol 2011, 48, 1592–1603. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, J.M.T.; Chang, R.C.C.; Wong, G.T.C. The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration. Biomolecules 2022, Vol. 12, Page 337 2022, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.M.; Elliott, G.S.; Chute, H.; Horan, T.; Pfenninger, K.H.; Sanford, S.D.; Foster, S.; Scully, S.; Welcher, A.A.; Holers, V.M. CSMD1 Is a Novel Multiple Domain Complement-Regulatory Protein Highly Expressed in the Central Nervous System and Epithelial Tissues. The Journal of Immunology 2006, 176, 4419–4430. [Google Scholar] [CrossRef]
- Wu, T.; Dejanovic, B.; Gandham, V.D.; Gogineni, A.; Edmonds, R.; Schauer, S.; Srinivasan, K.; Huntley, M.A.; Wang, Y.; Wang, T.-M.; et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep 2019, 28, 2111–2123.e6. [Google Scholar] [CrossRef]
- Parker, S.E.; Hanton, A.M.; Stefanou, S.N.; Noakes, P.G.; Woodruff, T.M.; Lee, J.D. Revisiting the Role of the Innate Immune Complement System in ALS. Neurobiol Dis 2019, 127, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.; Hakobyan, S.; Robertson, N.P.; Morgan, B.P. Complement in Multiple Sclerosis: Its Role in Disease and Potential as a Biomarker. Clin Exp Immunol 2009, 155, 128–139. [Google Scholar] [CrossRef]
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia Risk from Complex Variation of Complement Component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef]
- Chu, Y.; Jin, X.; Parada, I.; Pesic, A.; Stevens, B.; Barres, B.; Prince, D.A. Enhanced Synaptic Connectivity and Epilepsy in C1q Knockout Mice. Proceedings of the National Academy of Sciences 2010, 107, 7975–7980. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat Rev Immunol 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Veremeyko, T.; Jiang, R.; He, M.; Ponomarev, E.D. Complement C4-Deficient Mice Have a High Mortality Rate during PTZ-Induced Epileptic Seizures, Which Correlates with Cognitive Problems and the Deficiency in the Expression of Egr1 and Other Immediate Early Genes. Front Cell Neurosci 2023, 17. [Google Scholar] [CrossRef]
- Schartz, N.D.; Aroor, A.; Li, Y.; Pinzón-Hoyos, N.; Brewster, A.L. Mice Deficient in Complement C3 Are Protected against Recognition Memory Deficits and Astrogliosis Induced by Status Epilepticus. Front Mol Neurosci 2023, 16, 1265944. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, T.; Bosco, D.B.; Xie, M.; Zheng, J.; Dheer, A.; Ying, Y.; Wu, Q.; Lennon, V.A.; Wu, L.J. The Complement C3-C3aR Pathway Mediates Microglia-Astrocyte Interaction Following Status Epilepticus. Glia 2021, 69, 1155. [Google Scholar] [CrossRef] [PubMed]
- Aspden, J.W.; Murphy, M.A.; Kashlan, R.D.; Xiong, Y.; Poznansky, M.C.; Sîrbulescu, R.F. Intruders or Protectors – the Multifaceted Role of B Cells in CNS Disorders. Front Cell Neurosci 2023, 17, 1329823. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Das, B.K. Decoding Intrathecal Immunoglobulins and B Cells in the CNS: Their Synthesis, Function, and Regulation. Int Rev Immunol 2020, 39, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.H.; Boyle, A.L.; Diebolder, C.A.; Kros, A.; Koster, A.J.; Gros, P. Insights into IgM-Mediated Complement Activation Based on in Situ Structures of IgM-C1-C4b. Proc Natl Acad Sci U S A 2019, 116, 11900–11905. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





