Submitted:
23 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of Microbial Diversity
2.3. Analysis of Secondary Metabolites
2.4. Analysis of the Volatile Flavor Compounds Using HS-SPME-GC-MS
3. Results and Discussion
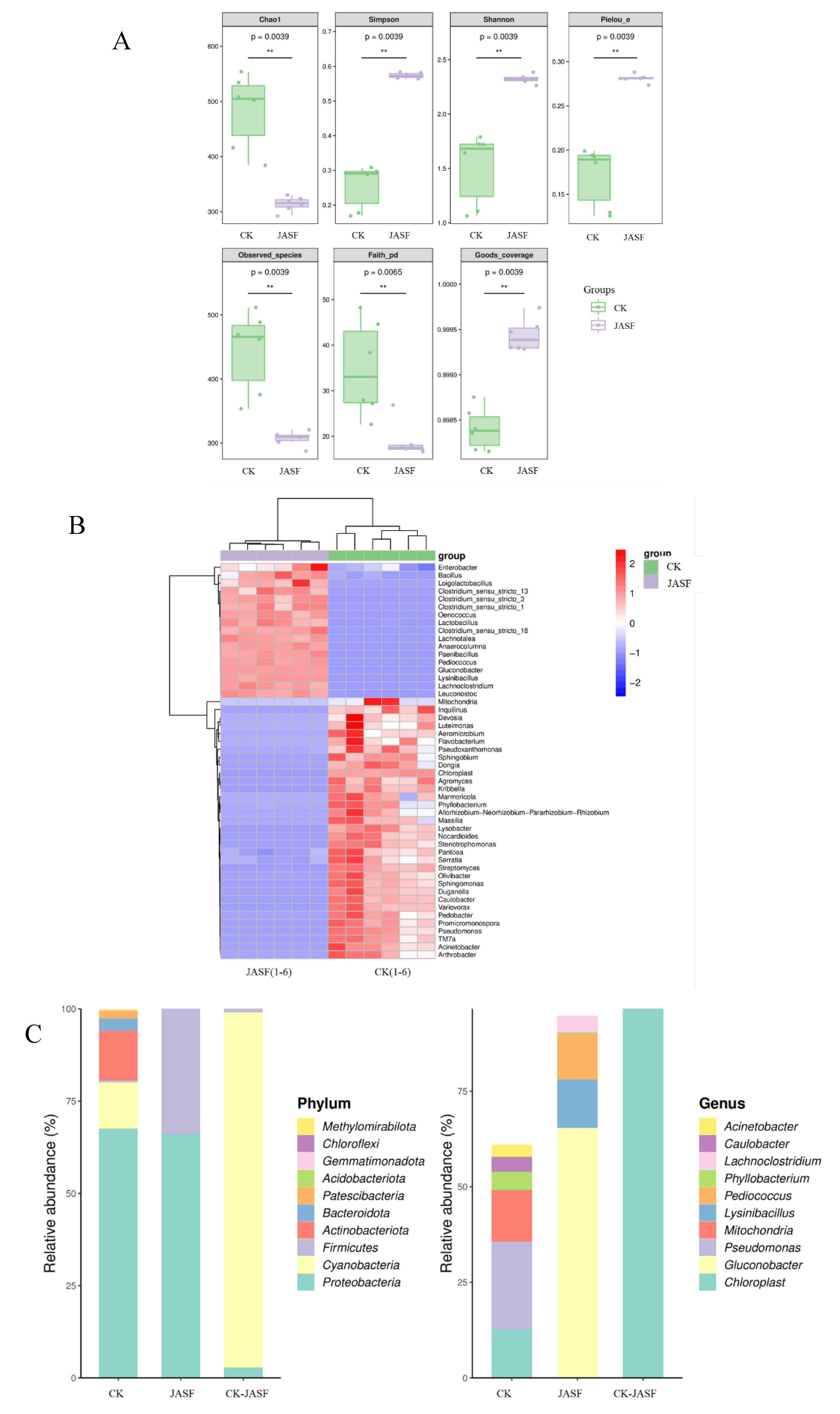
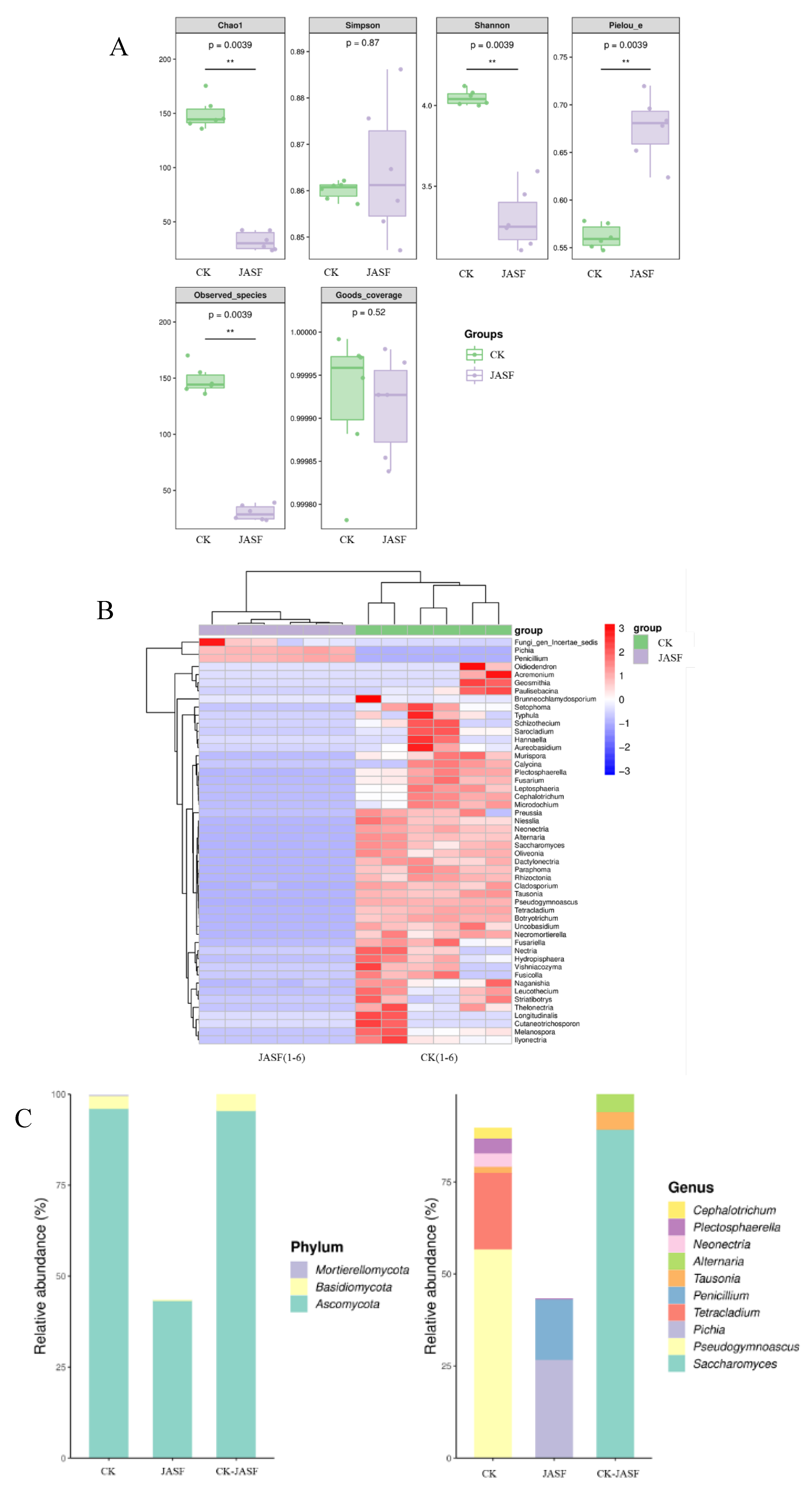
3.1. Changes of Microbial Diversity during Fermentation
3.1.1. Bacterial Diversity
3.1.2. Fungal Diversity
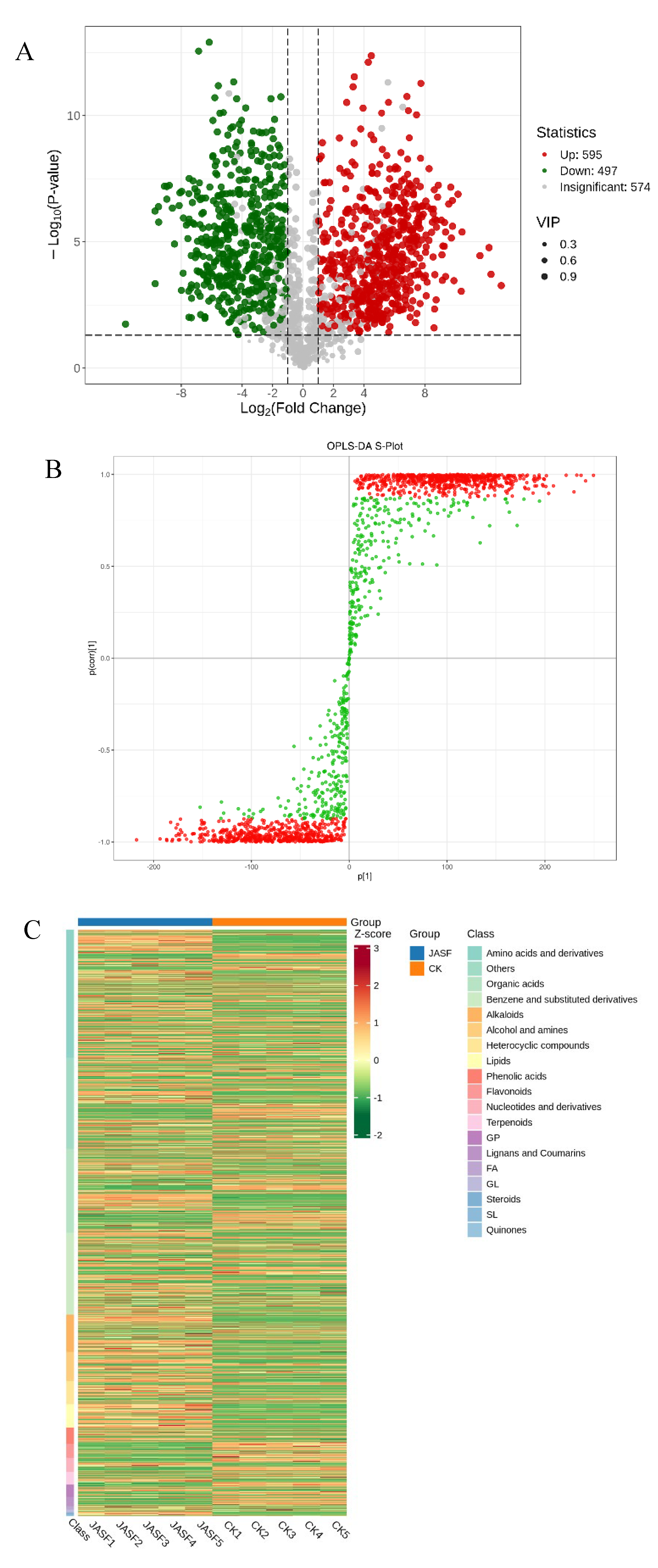
3.2. Dynamic Changes of Secondary Metabolites during Fermentation
3.3. Dynamic Changes of Volatile Flavor Compounds during Fermentation
| Volatile Compounds | RI | CAS | rOAV | Content(μg/L) | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| CK | JASF | CK | JASF | |||||
| Alcohols | ||||||||
| 1 | cis-2-Furanmethanol, 5-Ethenyltetrahydro-α,α,5-Trimethyl | 1074 | 5989-33-3 | 0–1 | 0–1 | 0.28±0.02 | 5.30±0.60 | 0.0142 |
| 2 | 3-Undecanol | 1400 | 6929-08-4 | 0–1 | >1 | 0.68±0.02 | 24.29±0.30 | 0.0002 |
| 3 | 2,3-Dimethyl-2-Butanol | 720 | 594-60-5 | 0–1 | 0–1 | 1.36±0.05 | 7.58±0.57 | 0.0097 |
| 4 | 4,4-Dimethyl-2-Pentanol | 812 | 6144-93-0 | 0–1 | 0–1 | 1.36±0.03 | 7.58±0.50 | 0.0058 |
| 5 | α,α,4-Trimethyl-3-Cyclohexene-1-Methanethiol | 1283 | 71159-90-5 | >1 | >1 | 2.83±0.12 | 15.75±1.09 | 0.0056 |
| 6 | 2-Ethyl-1-Hexanol | 1029 | 104-76-7 | 0–1 | 0–1 | 3.59±0.24 | 20.01±1.18 | 0.0058 |
| 7 | 1-Octanol | 1070 | 111-87-5 | 0–1 | >1 | 3.79±0.06 | 170.37±14.04 | 0.0070 |
| 8 | 1-Undecanol | 1371 | 112-42-5 | 0–1 | 0–1 | 4.13±0.11 | 7.73±0.40 | 0.0188 |
| 9 | 2-Mercaptoethanol | 723 | 60-24-2 | 0–1 | 0–1 | 6.70±0.38 | 37.32±1.46 | 0.0035 |
| 10 | 1-Decanol | 1272 | 112-30-1 | 0–1 | >1 | 8.36±0.60 | 46.58±3.68 | 0.0119 |
| 11 | 6-Undecanol | 1281 | 23708-56-7 | 0–1 | >1 | 8.43±0.49 | 46.95±2.60 | 0.0030 |
| 12 | 2-Nonanol | 1099 | 628-99-9 | 0–1 | 0–1 | 10.32±0.60 | 4.20±0.21 | 0.0045 |
| 13 | 3-Methyl-4-Heptanol | 997 | 1838-73-9 | 0–1 | >1 | 14.79±0.59 | 207.21±9.49 | 0.0027 |
| 14 | 5-Hexen-1-ol | 868 | 821-41-0 | 0–1 | 0–1 | 17.70±0.85 | 15.85±0.93 | 0.4025 |
| 15 | 2-Butoxyethanol | 905 | 111-76-2 | 0–1 | 0–1 | 18.04±0.76 | 100.47±5.98 | 0.0044 |
| 16 | 2-Heptanol | 900 | 543-49-7 | 0–1 | >1 | 23.58±1.58 | 131.35±13.45 | 0.0189 |
| 17 | 6-Ethenyltetrahydro-2,2,6-Trimethyl-2H-Pyran-3-ol | 1173 | 14049-11-7 | 0–1 | 0–1 | 25.69±0.26 | 154.34±13.49 | 0.0112 |
| 18 | 2-Undecanol | 1301 | 1653-30-1 | >1 | >1 | 27.97±1.81 | 155.82±12.67 | 0.0081 |
| 19 | trans,cis-2,6-Nonadien-1-ol | 1170 | 28069-72-9 | >1 | >1 | 31.48±1.00 | 126.11±9.14 | 0.0078 |
| 20 | Phenylethyl Alcohol | 1116 | 60-12-8 | 0–1 | >1 | 113.08±10.17 | 15675.14±834.64 | 0.0028 |
| 21 | Hotrienol | 1106 | 20053-88-7 | >1 | >1 | 227.97±7.85 | 195.16±9.53 | 0.1997 |
| Aldehydes | ||||||||
| 22 | 3-Cyclohexene-1-Carboxaldehyde | 958 | 100-50-5 | 0–1 | 0–1 | 0.39±0.02 | 2.16±0.01 | 0.0002 |
| 23 | (Z)-3-Phenylacrylaldehyde | 1219 | 57194-69-1 | 0–1 | 0–1 | 0.50±0.02 | 2.79±0.05 | 0.0002 |
| 24 | 5-Methyl-2-Thiophenecarboxaldehyde | 1118 | 13679-70-4 | 0–1 | >1 | 0.99±0.05 | 5.49±0.17 | 0.0014 |
| 25 | 10-Undecenal | 1297 | 112-45-8 | 0–1 | >1 | 1.15±0.03 | 15.20±0.46 | 0.0011 |
| 26 | (Z,Z)-3,6-Nonadienal | 1100 | 21944-83-2 | >1 | >1 | 1.18±0.13 | 6.60±0.27 | 0.0029 |
| 27 | 2-Ethyl-2-Hexenal | 999 | 645-62-5 | 0–1 | 0–1 | 1.97±0.10 | 2.59±0.09 | 0.0450 |
| 28 | Glutaraldehyde | 895 | 111-30-8 | >1 | >1 | 4.65±0.34 | 25.91±0.48 | 0.0001 |
| 29 | Piperonal | 1334 | 120-57-0 | 0–1 | 0–1 | 5.07±0.22 | 3.17±0.22 | 0.0020 |
| 30 | (E)-4-Decenal | 1198 | 65405-70-1 | 0–1 | >1 | 6.00±0.30 | 39.83±4.79 | 0.0177 |
| 31 | 3-Methylbenzaldehyde | 1070 | 620-23-5 | 0–1 | 0–1 | 9.27±0.73 | 51.65±0.22 | 0.0005 |
| 32 | Heptanal | 901 | 111-71-7 | >1 | >1 | 9.50±0.43 | 52.90±3.14 | 0.0067 |
| 33 | 2-Nonenal | 1161 | 2463-53-8 | >1 | >1 | 10.29±0.71 | 57.32±3.67 | 0.0056 |
| 34 | (E,E)-2,4-Octadienal | 1115 | 30361-28-5 | 0–1 | 0–1 | 10.71±0.80 | 59.63±4.57 | 0.0096 |
| 35 | 4-(1-Methylethenyl)-1-Cyclohexene-1-Carboxaldehyde | 1274 | 2111-75-3 | 0–1 | >1 | 11.52±0.75 | 64.19±3.76 | 0.0063 |
| 36 | (Z)-6-Nonenal | 1104 | 2277-19-2 | >1 | >1 | 14.56±0.33 | 7.88±0.67 | 0.0056 |
| 37 | 2,5-Dimethylbenzaldehyde | 1154 | 5779-94-2 | 0–1 | 0–1 | 17.47±0.29 | 3.89±0.36 | 0.0002 |
| 38 | (E,E)-2,4-Nonadienal | 1216 | 5910-87-2 | >1 | >1 | 21.22±1.28 | 118.21±11.23 | 0.0161 |
| 39 | Tridecanal | 1513 | 10486-19-8 | 0–1 | 0–1 | 45.38±2.90 | 53.60±0.37 | 0.0887 |
| 40 | (S)-4-(1-Methylethenyl)-1-Cyclohexene-1-Carboxaldehyde | 1243 | 18031-40-8 | >1 | >1 | 49.49±2.36 | 34.55±0.13 | 0.0240 |
| 41 | Benzeneacetaldehyde | 1046 | 122-78-1 | >1 | >1 | 98.40±2.14 | 36.35±1.20 | 0.0026 |
| 42 | Nonanal | 1105 | 124-19-6 | >1 | >1 | 117.08±2.46 | 90.79±4.02 | 0.0526 |
| 43 | (Z)-2-Decenal | 1252 | 2497-25-8 | >1 | >1 | 170.39±13.31 | 128.60±2.42 | 0.0627 |
| 44 | 4-(1,1-Dimethylethyl)benzenepropanal | 1521 | 18127-01-0 | >1 | >1 | 258.20±14.06 | 309.48±23.63 | 0.2868 |
| Acids | ||||||||
| 45 | Hexanoic Acid | 987 | 142-62-1 | 0–1 | 0–1 | 0.23±0.01 | 177.36±8.39 | 0.0022 |
| 46 | 9-Decenoic Acid | 1360 | 14436-32-9 | 0–1 | 0–1 | 0.64±0.06 | 43.76±4.64 | 0.0111 |
| 47 | 4-Methyloctanoic Acid | 1232 | 54947-74-9 | 0–1 | 0–1 | 2.35±0.03 | 13.06±0.81 | 0.0054 |
| 48 | (E)-2-Hexenoic Acid | 1045 | 13419-69-7 | 0–1 | 0–1 | 12.48±0.95 | 69.51±1.08 | 0.0001 |
| 49 | 4-Aminobutanoic Acid | 1190 | 56-12-2 | 0–1 | 0–1 | 17.57±1.88 | 78.55±2.82 | 0.0010 |
| Ketones | ||||||||
| 50 | 2-Undecanone | 1295 | 112-12-9 | 0–1 | >1 | 0.58±0.03 | 10.70±0.42 | 0.0020 |
| 51 | Isophorone | 1123 | 78-59-1 | 0–1 | 0–1 | 1.24±0.10 | 6.92±0.39 | 0.0029 |
| 52 | 1-(4-Methylphenyl)ethanone | 1183 | 122-00-9 | 0–1 | 0–1 | 1.25±0.05 | 6.00±0.09 | 0.0001 |
| 53 | Acetophenone | 1068 | 98-86-2 | 0–1 | >1 | 1.28±0.13 | 86.86±10.04 | 0.0131 |
| 54 | 2-Dodecanone | 1395 | 6175-49-1 | 0–1 | 0–1 | 1.47±0.10 | 4.93±0.23 | 0.0022 |
| 55 | (E,E)-3,5-Octadien-2-one | 1073 | 30086-02-3 | >1 | >1 | 2.31±0.04 | 12.87±1.30 | 0.0150 |
| 56 | (E)-5,9-Undecadien-2-one, 6,10-Dimethyl | 1453 | 3796-70-1 | 0–1 | 0–1 | 3.35±0.11 | 2.62±0.06 | 0.0502 |
| 57 | 2-Octanone | 991 | 111-13-7 | 0–1 | 0–1 | 3.50±0.28 | 19.48±1.15 | 0.0030 |
| 58 | 2-Methylcyclohexanone | 953 | 583-60-8 | 0–1 | 0–1 | 3.56±0.09 | 41.88±2.23 | 0.0036 |
| 59 | 5-Ethyl-3-Hydroxy-4-Methyl-2(5H)-Furanone | 1195 | 698-10-2 | >1 | >1 | 4.13±0.33 | 303.74±23.36 | 0.0060 |
| 60 | 1-(4,5-Dihydro-2-Thiazolyl)ethanone | 1106 | 29926-41-8 | >1 | >1 | 4.64±0.06 | 25.86±2.12 | 0.0096 |
| 61 | 3-Butylisobenzofuran-1(3H)-one | 1656 | 6066-49-5 | 0–1 | 0–1 | 5.33±0.39 | 3.17±0.21 | 0.0427 |
| 62 | 1-Nonen-3-one | 1076 | 24415-26-7 | >1 | >1 | 8.76±0.79 | 216.16±8.62 | 0.0015 |
| 63 | 3-Octen-2-one | 1016 | 1669-44-9 | >1 | >1 | 11.08±0.28 | 61.71±4.26 | 0.0079 |
| 64 | 3-Decanone | 1187 | 928-80-3 | 0–1 | >1 | 13.10±0.32 | 72.95±2.82 | 0.0022 |
| 65 | 4-(2,6,6-Trimethylcyclohexa-1,3-Dienyl)but-3-en-2-one | 1485 | 1203-08-3 | >1 | >1 | 14.06±1.51 | 6.96±0.06 | 0.0403 |
| 66 | 1-(2,6,6-Trimethyl-1,3-Cyclohexadien-1-yl)-2-Buten-1-one | 1362 | 23696-85-7 | >1 | >1 | 17.67±0.33 | 17.37±1.11 | 0.8407 |
| 67 | 1-(2-Thienyl)ethanone | 1092 | 88-15-3 | >1 | >1 | 17.95±0.88 | 43.99±0.52 | 0.0010 |
| 68 | 4-Undecanone | 1208 | 14476-37-0 | 0–1 | >1 | 28.48±2.43 | 170.27±9.34 | 0.0024 |
| 69 | 2-Sec-Butylcyclohexanone | 1220 | 14765-30-1 | 0–1 | >1 | 75.95±3.34 | 423.10±13.08 | 0.0022 |
| 70 | 2-Hydroxy-3,4-Dimethyl-2-Cyclopenten-1-one | 1075 | 21835-00-7 | >1 | >1 | 141.02±4.04 | 136.87±10.86 | 0.8055 |
| 71 | 3-Methyl-4-Heptanone | 928 | 15726-15-5 | >1 | >1 | 170.38±17.84 | 72.88±1.21 | 0.0287 |
| 72 | 3,4-Dimethyl-1,2-Cyclopentadione | 1109 | 13494-06-9 | >1 | >1 | 181.10±9.76 | 281.39±4.42 | 0.0192 |
| 73 | 6-Methyl-3,5-Heptadien-2-one | 1107 | 1604-28-0 | >1 | >1 | 214.76±8.41 | 167.15±14.82 | 0.1383 |
| Esters | ||||||||
| 74 | Ethyl Decanoate | 1396 | 110-38-3 | 0–1 | >1 | 0.06±0.00 | 162.31±13.54 | 0.0069 |
| 75 | Ethyl Hexanoate | 999 | 123-66-0 | 0–1 | >1 | 0.10±0.01 | 160.55±3.85 | 0.0006 |
| 76 | Octyl Acetate | 1210 | 112-14-1 | 0–1 | 0–1 | 0.35±0.05 | 61.37±5.29 | 0.0074 |
| 77 | 1-Methylbutyl Butanoate | 970 | 60415-61-4 | 0–1 | >1 | 0.38±0.02 | 69.98±5.37 | 0.0059 |
| 78 | Ethyl Hexadecanoate | 1993 | 628-97-7 | 0–1 | 0–1 | 0.56±0.01 | 67.52±4.04 | 0.0036 |
| 79 | Ethyl 4-Methylpentanoate | 969 | 25415-67-2 | 0–1 | >1 | 0.59±0.05 | 492.01±27.89 | 0.0032 |
| 80 | 1-Methylpropyl 2-Methylbutanoate | 971 | 869-08-9 | 0–1 | 0–1 | 0.78±0.05 | 4.32±0.12 | 0.0021 |
| 81 | Ethyl Benzenepropanoate | 1353 | 2021-28-5 | 0–1 | >1 | 0.86±0.05 | 2088.29±67.40 | 0.0010 |
| 82 | 4-tert-Butylcyclohexyl Acetate | 1368 | 32210-23-4 | 0–1 | 0–1 | 0.90±0.08 | 23.44±1.30 | 0.0037 |
| 83 | Ethyl 9-Decenoate | 1388 | 67233-91-4 | 0–1 | 0–1 | 1.00±0.00 | 34.92±3.26 | 0.0091 |
| 84 | 2-Ethylhexyl Acrylate | 1220 | 103-11-7 | 0–1 | 0–1 | 1.14±0.08 | 14.04±0.53 | 0.0019 |
| 85 | Geranyl Formate | 1301 | 105-86-2 | 0–1 | 0–1 | 1.16±0.09 | 138.01±2.15 | 0.0002 |
| 86 | Pentyl Butanoate | 1077 | 540-18-1 | 0–1 | 0–1 | 1.22±0.07 | 279.13±7.96 | 0.0008 |
| 87 | Hexyl Acetate | 1013 | 142-92-7 | 0–1 | 0–1 | 1.24±0.06 | 6.90±0.09 | 0.0002 |
| 88 | Pentyl 2-Methylbutanoate | 1142 | 68039-26-9 | 0–1 | >1 | 1.25±0.06 | 24.67±1.29 | 0.0028 |
| 89 | Methyl 4-Methoxybenzoate | 1373 | 121-98-2 | 0–1 | 0–1 | 1.58±0.08 | 3.45±0.11 | 0.0096 |
| 90 | trans-3-Methyl-4-Octanolide | 1288 | 39638-67-0 | 0–1 | 0–1 | 1.86±0.07 | 0.41±0.02 | 0.0034 |
| 91 | Methyl Anthranilate | 1349 | 134-20-3 | 0–1 | >1 | 1.92±0.12 | 100.47±0.93 | 0.0001 |
| 92 | Butyl 2-Hydroxybenzoate | 1436 | 2052-14-4 | 0–1 | 0–1 | 1.93±0.11 | 2.13±0.13 | 0.4387 |
| 93 | Hexyl 2-Methylbutanoate | 1236 | 10032-15-2 | 0–1 | 0–1 | 2.00±0.11 | 1.62±0.04 | 0.0323 |
| 94 | 1,2-Ethanediol, Diacetate | 991 | 111-55-7 | 0–1 | 0–1 | 2.03±0.14 | 11.28±1.06 | 0.0145 |
| 95 | 2-Ethylhexyl Methacrylate | 1296 | 688-84-6 | 0–1 | 0–1 | 2.15±0.17 | 28.94±1.30 | 0.0029 |
| 96 | 1-Isothiocyanato-2-Butene | 1070 | 2253-93-2 | 0–1 | 0–1 | 2.56±0.03 | 311.28±18.03 | 0.0034 |
| 97 | Phenyl Acetate | 1062 | 122-79-2 | 0–1 | 0–1 | 2.61±0.12 | 10.34±0.86 | 0.0091 |
| 98 | 2-Ethylhexyl Acetate | 1185 | 103-09-3 | 0–1 | 0–1 | 2.94±0.11 | 16.37±0.50 | 0.0009 |
| 99 | 1-Ethylpropyl Acetate | 793 | 620-11-1 | 0–1 | >1 | 3.10±0.34 | 194.85±4.87 | 0.0006 |
| 100 | δ-Dodecalactone | 1720 | 713-95-1 | 0–1 | 0–1 | 3.45±0.17 | 4.04±0.04 | 0.0464 |
| 101 | Methyl Heptanoate | 1024 | 106-73-0 | 0–1 | >1 | 3.56±0.12 | 19.85±0.98 | 0.0044 |
| 102 | Ethyl Dodecanoate | 1595 | 106-33-2 | 0–1 | 0–1 | 4.27±0.20 | 23.78±1.75 | 0.0095 |
| 103 | 3-Phenylpropyl Acetate | 1373 | 122-72-5 | 0–1 | 0–1 | 4.28±0.07 | 6.10±0.56 | 0.0701 |
| 104 | 3-Methylphenylmethyl Butanoate | 1396 | 103-38-8 | 0–1 | >1 | 5.49±0.50 | 30.60±1.68 | 0.0027 |
| 105 | cis-2-Methyl-5-(1-Methylethenyl)-2-Cyclohexen-1-ol Acetate | 1362 | 1205-42-1 | 0–1 | >1 | 5.63±0.24 | 31.37±5.71 | 0.0463 |
| 106 | Butyl Hexanoate | 1189 | 626-82-4 | 0–1 | 0–1 | 6.39±0.30 | 32.25±0.62 | 0.0004 |
| 107 | Pentyl Acetate | 916 | 628-63-7 | 0–1 | 0–1 | 6.69±0.32 | 37.27±2.74 | 0.0072 |
| 108 | 4-Methylphenyl Acetate | 1171 | 140-39-6 | 0–1 | >1 | 8.00±0.33 | 36.87±1.79 | 0.0051 |
| 109 | 3-Methylbutyl Butanoate | 1046 | 109-19-3 | 0–1 | 0–1 | 8.41±0.45 | 46.84±1.05 | 0.0015 |
| 110 | Ethyl Nonanoate | 1295 | 123-29-5 | 0–1 | >1 | 9.00±0.58 | 50.16±2.23 | 0.0038 |
| 111 | Methyl Thiocyanate | 702 | 556-64-9 | 0–1 | >1 | 9.51±0.92 | 52.95±1.39 | 0.0026 |
| 112 | Ethyl 3-Methylpentanoate | 960 | 5870-68-8 | >1 | >1 | 9.52±0.93 | 295.96±2.07 | 0.0000 |
| 113 | Tetrahydro-6-Pentyl-2H-Pyran-2-one | 1502 | 705-86-2 | 0–1 | 0–1 | 10.89±0.36 | 43.16±2.44 | 0.0069 |
| 114 | Methyl Decanoate | 1326 | 110-42-9 | >1 | >1 | 11.03±0.30 | 17.51±0.57 | 0.0172 |
| 115 | 2-Methylbutyl 2-Methylbutanoate | 1105 | 2445-78-5 | 0–1 | >1 | 11.63±1.03 | 148.48±6.08 | 0.0023 |
| 116 | 2-Phenylethyl 3-Methylbutanoate | 1491 | 140-26-1 | >1 | >1 | 11.65±1.16 | 64.87±3.29 | 0.0043 |
| 117 | Butyl Butanoate | 996 | 109-21-7 | 0–1 | >1 | 12.14±0.26 | 233.19±15.59 | 0.0049 |
| 118 | Ethyl Pentanoate | 902 | 539-82-2 | 0–1 | >1 | 12.16±0.18 | 67.76±3.27 | 0.0033 |
| 119 | Ethyl Benzeneacetate | 1247 | 101-97-3 | 0–1 | 0–1 | 12.52±1.44 | 71.91±3.06 | 0.0016 |
| 120 | Dihydro-5-Propyl-2(3H)-Furanone | 1156 | 105-21-5 | 0–1 | 0–1 | 12.71±0.46 | 70.78±3.50 | 0.0028 |
| 121 | 3-Methylbutyl Butanoate | 1056 | 106-27-4 | 0–1 | >1 | 12.74±0.12 | 1406.55±18.28 | 0.0002 |
| 122 | n-Amyl Isovalerate | 1110 | 25415-62-7 | 0–1 | 0–1 | 18.84±0.91 | 473.98±30.74 | 0.0044 |
| 123 | Isothiocyanatoethane | 796 | 542-85-8 | 0–1 | 0–1 | 20.83±0.96 | 205.69±30.54 | 0.0247 |
| 124 | Propyl Propanoate | 810 | 106-36-5 | 0–1 | >1 | 22.72±0.54 | 126.54±7.70 | 0.0051 |
| 125 | 3-Methyl-1-Butanol Acetate | 878 | 123-92-2 | >1 | >1 | 24.93±0.67 | 471.94±35.83 | 0.0066 |
| 126 | Propyl Butanoate | 899 | 105-66-8 | >1 | >1 | 30.20±0.85 | 168.23±4.58 | 0.0010 |
| 127 | (E)-Methyl 3-Hexenoate | 920 | 13894-61-6 | 0–1 | 0–1 | 34.56±1.43 | 7.70±0.67 | 0.0009 |
| 128 | n-Butyl Tiglate | 1134 | 7785-66-2 | >1 | >1 | 51.65±2.22 | 287.71±2.48 | 0.0001 |
| 129 | Isopentyl Hexanoate | 1250 | 2198-61-0 | 0–1 | 0–1 | 59.91±1.01 | 39.01±0.71 | 0.0023 |
| 130 | Butyl Acetate | 815 | 123-86-4 | >1 | >1 | 99.48±5.70 | 554.18±17.65 | 0.0017 |
| 131 | 2-Butoxyethyl Acetate | 1090 | 112-07-2 | 0–1 | 0–1 | 102.40±4.71 | 199.72±6.11 | 0.0118 |
| 132 | Ethyl Tiglate | 939 | 5837-78-5 | >1 | >1 | 150.65±11.45 | 66.08±2.19 | 0.0223 |
| 133 | Methyl 2-Octynoate | 1202 | 111-12-6 | >1 | >1 | 156.55±12.81 | 91.76±6.52 | 0.0758 |
| 134 | Ethyl Butanoate | 802 | 105-54-4 | >1 | >1 | 189.73±15.80 | 1056.87±79.69 | 0.0109 |
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chyc, M.; Ogonowski, J. Jerusalem artichoke as a prospective raw material for industry. Przemysl Chemiczny 2015, 94, 578–582. [Google Scholar]
- Qin, Y.-Q.; Wang, L.-Y.; Yang, X.-Y.; Xu, Y.-J.; Fan, G.; Fan, Y.-G.; Ren, J.-N.; An, Q.; Li, X. Inulin: properties and health benefits. Food & Function 2023, 14, 2948–2968. [Google Scholar] [CrossRef]
- Riva, A.; Rasoulimehrabani, H.; Cruz-Rubio, J.M.; Schnorr, S.L.; von Baeckmann, C.; Inan, D.; Nikolov, G.; Herbold, C.W.; Hausmann, B.; Pjevac, P.; et al. Identification of inulin-responsive bacteria in the gut microbiota via multi-modal activity-based sorting. Nature Communications 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. International Journal of Biological Macromolecules 2022, 208, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Ponder, M.A.; McMillan, R.P.; Hughes, M.D.; Hulver, M.W.; Neilson, A.P.; Davy, K.P. Prebiotic Inulin Supplementation and Peripheral Insulin Sensitivity in adults at Elevated Risk for Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, J.B.; Rodrigues, V.F.; Pereira, I.S.; Manso, G.M.d.C.; Elias-Oliveira, J.; Leite, J.A.; Waldetario, M.C.G.M.; de Oliveira, S.; Gomes, A.B.d.S.P.; Faria, A.M.C.; et al. Inulin prebiotic ameliorates type 1 diabetes dictating regulatory T cell homing via CCR4 to pancreatic islets and butyrogenic gut microbiota in murine model. Journal of Leukocyte Biology 2024, 115, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Li, J.; Yu, B.; Li, M.; Cui, B. Improvement of myocardial injury and gut microbiota disturbance in type 2 diabetic mice by inulin with various degrees of polymerization. Food Bioscience 2023, 51. [Google Scholar] [CrossRef]
- Tang, Z.; Shao, T.; Gao, L.; Yuan, P.; Ren, Z.; Tian, L.; Liu, W.; Liu, C.; Xu, X.; Zhou, X.; et al. Structural elucidation and hypoglycemic effect of an inulin-type fructan extracted from Stevia rebaudiana roots. Food & Function 2023, 14, 2518–2529. [Google Scholar] [CrossRef]
- Bakirhan, H.; Karabudak, E. Effects of inulin on calcium metabolism and bone health. International Journal for Vitamin and Nutrition Research 2023, 93, 85–96. [Google Scholar] [CrossRef]
- Gomez-Betancur, A.M.; Carmona-Tamayo, R.; Martinez-Alyarez, O.L.; Casanova-Yepes, H.; Torres-Oquendo, J.D. Effect of fat substitution using long-chain inulin and fortification with microencapsulated calcium in the rheological and sensory properties of yogurt mousse. Journal of Food Process Engineering 2022, 45. [Google Scholar] [CrossRef]
- Sulejmani, E.; Boran, O.S.; Huppertz, T.; Hayaloglu, A.A. Rheology, microstructure and sensory properties of low-fat milk jam: Influence of inulin type, sucrose content, sodium bicarbonate and calcium chloride. International Dairy Journal 2021, 123. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Znamirowska-Piotrowska, A.; Buniowska-Olejnik, M.; Zagula, G.; Pawlos, M. Bioavailability of Macroelements from Synbiotic Sheep’s Milk Ice Cream. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Ichikawa, N.; Sasaki, H.; Shibata, S. The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.; Nagashima, T. Preparation of dried chips from Jerusalem artichoke (Helianthus tuberosus) tubers and analysis of their functional properties. Food Chemistry 2011, 126, 922–926. [Google Scholar] [CrossRef]
- Showkat, M.M.; Falck-Ytter, A.B.; Straetkvern, K.O. Phenolic Acids in Jerusalem Artichoke (Helianthus tuberosus L.): Plant Organ Dependent Antioxidant Activity and Optimized Extraction from Leaves. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Jerusalem artichoke (Helianthus tuberosus L.) maintains high inulin, tuber yield, and antioxidant capacity under moderately-saline irrigation waters. Industrial Crops and Products 2016, 94, 1009–1024. [Google Scholar] [CrossRef]
- Mu, Y.; Gao, W.; Lv, S.; Li, F.; Lu, Y.; Zhao, C. The antioxidant capacity and antioxidant system of Jerusalem artichoke (Helianthus tuberosus L.) tubers in relation to inulin during storage at different low temperatures. Industrial Crops and Products 2021, 161. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kita, A.; Peksa, A.; Wawrzyniak, A.; Hamulka, J.; Jeznach, M.; Danilcenko, H.; Jariene, E. Analysis of the content of bioactive compounds in selected flours and enriched extruded corn products. Journal of Food Composition and Analysis 2017, 64, 147–155. [Google Scholar] [CrossRef]
- Zalan, Z.; Hudacek, J.; Toth-Markus, M.; Husova, E.; Solichova, K.; Hegyi, F.; Plockova, M.; Chumchalova, J.; Halasz, A. Sensorically and antimicrobially active metabolite production of Lactobacillus strains on Jerusalem artichoke juice. Journal of the Science of Food and Agriculture 2011, 91, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Isik, F.; Gursoy, O.; Yilmaz, Y. Use of Jerusalem artichoke (Helianthus Tuberosus) tubers as a natural source of inulin in cakes. Journal of Food Processing and Preservation 2013, 37, 483–488. [Google Scholar] [CrossRef]
- Radovanovic, A.; Stojceska, V.; Plunkett, A.; Jankovic, S.; Milovanovic, D.; Cupara, S. The use of dry Jerusalem artichoke as a functional nutrient in developing extruded food with low glycaemic index. Food Chemistry 2015, 177, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Bomben, R.; Dini, C.; Vina, S.Z.; Garcia, M.A.; Ponzi, M.; Comelli, N. Jerusalem artichoke tuber flour as a wheat flour substitute for biscuit elaboration. Lwt-Food Science and Technology 2019, 108, 361–369. [Google Scholar] [CrossRef]
- Diaz, A.; Garcia, M.A.; Dini, C. Jerusalem artichoke flour as food ingredient and as source of fructooligosaccharides and inulin. Journal of Food Composition and Analysis 2022, 114. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Dimitrovska, M.; Langerholc, T.; Winkelhausen, E. Synbiotic functional drink from Jerusalem artichoke juice fermented by probiotic Lactobacillus plantarum PCS26. Journal of Food Science and Technology-Mysore 2016, 53, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Sooresh, M.M.; Willing, B.P.; Bourrie, B.C.T. Opportunities and challenges of understanding community assembly in spontaneous food fermentation. Foods 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium ruthenicum Murr. (Qinghai-Tibet Plateau) natural vinegar. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhou, Q.; Chen, Q.; Gao, J.; Wu, Y.; Yang, F.; Zhong, K.; Gao, H. Improvement of physicochemical characteristics, flavor profiles and functional properties in Chinese radishes via spontaneous fermentation after drying. Journal of Food Science 2023, 88, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of spontaneous fermentation on the microorganisms diversity and volatile compounds during ‘Marselan’ from grape to wine. Lwt-Food Science and Technology 2020, 134. [Google Scholar] [CrossRef]
- Kumar, S.; Chhabra, V.; Shenoy, S.; Daksh, R.; Ravichandiran, V.; Swamy, R.S.; Kumar, N. Role of flavonoids in modulation of mitochondria dynamics during oxidative stress. Mini-Reviews in Medicinal Chemistry 2024, 24, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Wang, S.; Gao, X.; Zhang, X. Research progress on extraction and detection technologies of flavonoid compounds in foods. Foods 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xiumin, L.; Xiang, Z.; Bang-Ce, Y. Recent advances in microbial metabolic engineering for production of natural phenolic acids. Journal of Agricultural and Food Chemistry 2024, 72, 4538–4551. [Google Scholar]
- Yuan, G.; Shuai, M.; Min, D.; Meng, W.; Xiaoyuan, F. Progress in research on the biosynthesis pathway and metabolic regulation of phenolic acids. Food Science, China 2018, 39, 286–293. [Google Scholar]
- Yang, X.; Lan, W.; Sun, X. Antibacterial and antioxidant properties of phenolic acid grafted chitosan and its application in food preservation: A review. Food Chemistry 2023, 428. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Seni, K.; Chawla, P.A.; Chawla, V.; Ganti, S.S. An insight into recent updates on analytical techniques for bioactive alkaloids. Phytochemical Analysis 2024, 35, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, J.; Lyu, Y.; Chen, Y.; Han, S.; Wang, H. Efficacy of substances containing 3 types of active ingredientssaponins, flavones, and alkaloids in regulation of cytokines in autoimmune diseases a systematic review and Meta-analysis based on animal studies. Journal of Traditional Chinese Medicine 2024, 44, 417–426. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Paseephol, T.; Sherkat, F. Prebiotic and rheological effects of Jerusalem artichoke inulin in low-fat yogurt. Australian Journal of Dairy Technology 2009, 64, 159–163. [Google Scholar]
- Yi, H.; Zhang, L.; Hua, C.; Sun, K.; Zhang, L. Extraction and enzymatic hydrolysis of inulin from Jerusalem artichoke and their effects on textural and sensorial characteristics of yogurt. Food Bioprocess Tech 2010, 3, 315–319. [Google Scholar] [CrossRef]
- Canbulat, Z.; Ozcan, T. Effects of short-chain and long-chain inulin on the quality of probiotic yogurt containing Lactobacillus Rhamnosus. Journal of Food Processing and Preservation 2015, 39, 1251–1260. [Google Scholar]
- Guven, M.; Yasar, K.; Karaca, O.B.; Hayaloglu, A.A. The effect of inulin as a fat replacer on the quality of set-type low-fat yogurt manufacture. International Journal of Dairy Technology 2005, 58, 180–184. [Google Scholar] [CrossRef]
- Ehsani, J.; Mohsenzadeh, M.; Khomeiri, M.; Ghasemnezhad, A. Chemical characteristics, and effect of inulin extracted from artichoke (Cynara scolymus L.) root on biochemical properties of synbiotic yogurt at the end of fermentation. Iranian Journal of Chemistry & Chemical Engineering-International English Edition 2018, 37, 219–230. [Google Scholar]
- Balcazar-Zumaeta, C.R.; Castro-Alayo, E.M.; Cayo-Colca, I.S.; Idrogo-Vasquez, G.; Munoz-Astecker, L.D. Metabolomics during the spontaneous fermentation in cocoa (Theobroma cacao L.): An exploraty review. Food Research International 2023, 163. [Google Scholar] [CrossRef] [PubMed]
- Luis Navarrete-Bolanos, J. Improving traditional fermented beverages: How to evolve from spontaneous to directed fermentation. Engineering in Life Sciences 2012, 12, 410–418. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, Y.; Wu, Q.; Xu, Y. Challenges and perspectives of quantitative microbiome profiling in food fermentations. Critical Reviews in Food Science and Nutrition 2024, 64, 4995–5015. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Wong, J.; Heiner, C.; Oh, S.; Theriot, C.M.; Gulati, A.S.; McGill, S.K.; Dougherty, M.K. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Research 2019, 47. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Huang, L.; Zeng, Y.; Li, W.; Zhou, D.; Xie, J.; Xie, J.; Tu, Q.; Deng, D.; Yin, J. Pediococcus pentosaceus: screening and application as probiotics in food processing. Front Microbiol 2021, 12, 762467. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.B.; Qiao, Z.; Godspower, H.N.; Ajeje, S.B.; Xu, M.; Zhang, X.; Yang, T.; Rao, Z. Biotechnological innovations and therapeutic application of Pediococcus and lactic acid bacteria: the next-generation microorganism. Frontiers in Bioengineering and Biotechnology 2022, 9, 802031. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Pérez-Díaz, I.M.; Breidt, F.; Hayes, J.; Franco, W.; Butz, N.; Azcarate-Peril, M.A. Bacterial ecology of fermented cucumber rising pH spoilage as determined by nonculture-based methods. Journal of food science 2016, 81, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Doyle, M.P. Microbiology of fermented foods. In Acetic Acid Bacteria; CRC Press: 2017, pp. 213-260.
- Paramithiotis, S. Microorganisms associated with food fermentation. In Bioactive Compounds in Fermented Foods; CRC Press: 2021, pp. 3-47.
- Zhao, H.; Li, Y.; Liu, L.; Zheng, M.; Feng, Z.; Hu, K.; Tao, Y. Effects of inoculation timing and mixed fermentation with Pichia fermentans on Oenococcus oeni viability, fermentation duration and aroma production during wine malolactic fermentation. Food Research International 2022, 159, 111604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nguyen, T.T.H.; Jin, J.; Lim, J.; Lee, J.; Piao, M.; Mok, I.-K.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci Biotechnol 2021, 30, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Debeljak, P.; Baltar, F. Fungal diversity and community composition across ecosystems. Journal of Fungi 2023, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tang, J.; Zhang, Z.; Wu, Z.; Zhong, A.; Li, Z.; Wang, Y. Correlation between flavor compounds and microorganisms of Chaling natural fermented red sufu. Lwt 2022, 154, 112873. [Google Scholar] [CrossRef]
- Wang, X.; Schlatter, D.C.; Glawe, D.A.; Edwards, C.G.; Weller, D.M.; Paulitz, T.C.; Abatzoglou, J.T.; Okubara, P.A. Native yeast and non-yeast fungal communities of Cabernet Sauvignon berries from two Washington State vineyards, and persistence in spontaneous fermentation. International Journal of Food Microbiology 2021, 350, 109225. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, B.H.; Inbaraj, B.S.; Chen, L.; Alvarez-Rivera, G.; Cifuentes, A.; Zhang, N.; Yang, D.J.; Simal-Gandara, J.; Wang, M. Preventive potential and mechanism of dietary polyphenols on the formation of heterocyclic aromatic amines. Food Frontiers 2020, 1, 134–151. [Google Scholar] [CrossRef]
- Beygmoradi, A.; Homaei, A. Marine microbes as a valuable resource for brand new industrial biocatalysts. Biocatal Agr Biotech 2017, 11, 131–152. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Critical reviews in food science and nutrition 2022, 62, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; He, Z.; Wang, X.; Zhao, M.; Cao, X.; Lin, X.; Ji, C.; Zhang, S.; Liang, H. Improving the quality of Suancai by inoculating with Lactobacillus plantarum and Pediococcus pentosaceus. Food Research International 2021, 148, 110581. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Hassane, A.; Atta, O.; Song, Y. Deep learning strategies for active secondary metabolites biosynthesis from fungi: Harnessing artificial manipulation and application. Biocatal Agr Biotech 2021, 38, 102195. [Google Scholar] [CrossRef]
- Grewal, J. Gamma-aminobutyric acid (GABA): A versatile bioactive compound. Eur. J. Mol. Clin. Med 2020, 7, 3068–3075. [Google Scholar]
- Tang, M.; Wang, Z.; Luo, J.; Zhu, T.; Song, F.; Chen, H. Preparation, chemical profiles, antioxidative activities, and angiotensin-converting enzyme 2 inhibitory effect of date fruit vinegar. Journal of Food Science 2024, 89, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Keșa, A. L.; Pop, C.R.; Mudura, E.; Salanță, L.C.; Pasqualone, A.; Dărab, C.; Burja-Udrea, C.; Zhao, H.; Coldea, T.E. Strategies to improve the potential functionality of fruit-based fermented beverages. Plants 2021, 10, 2263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




