Submitted:
22 July 2024
Posted:
24 July 2024
Read the latest preprint version here
Abstract
Keywords:
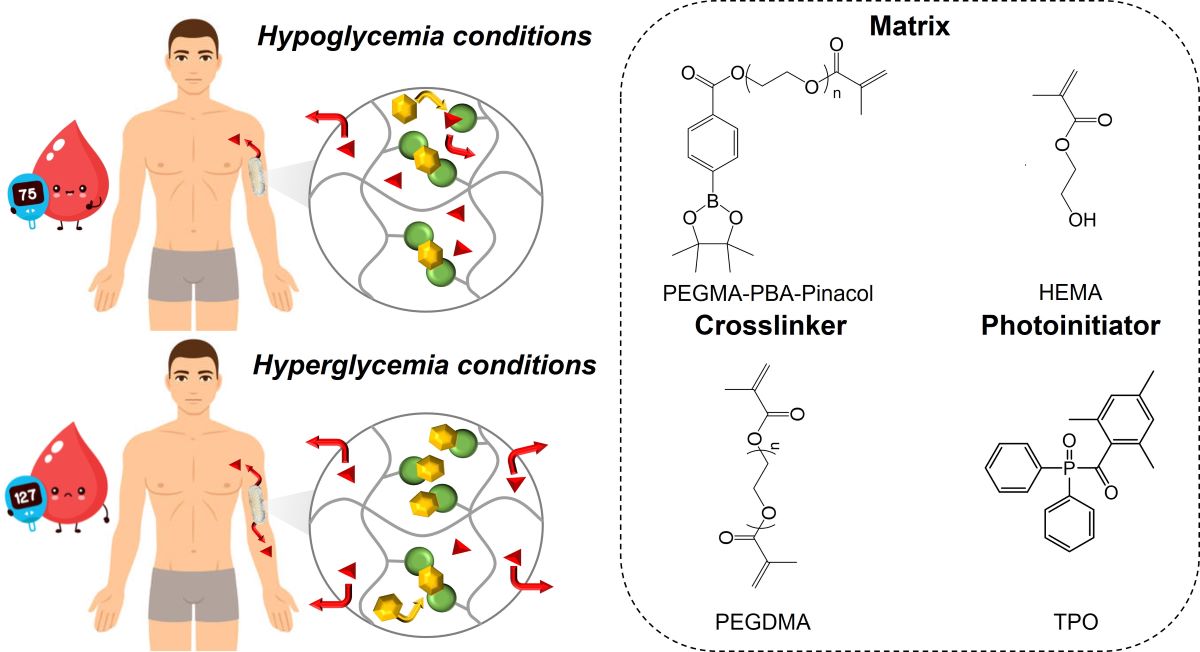
Introduction
Experimental
Materials
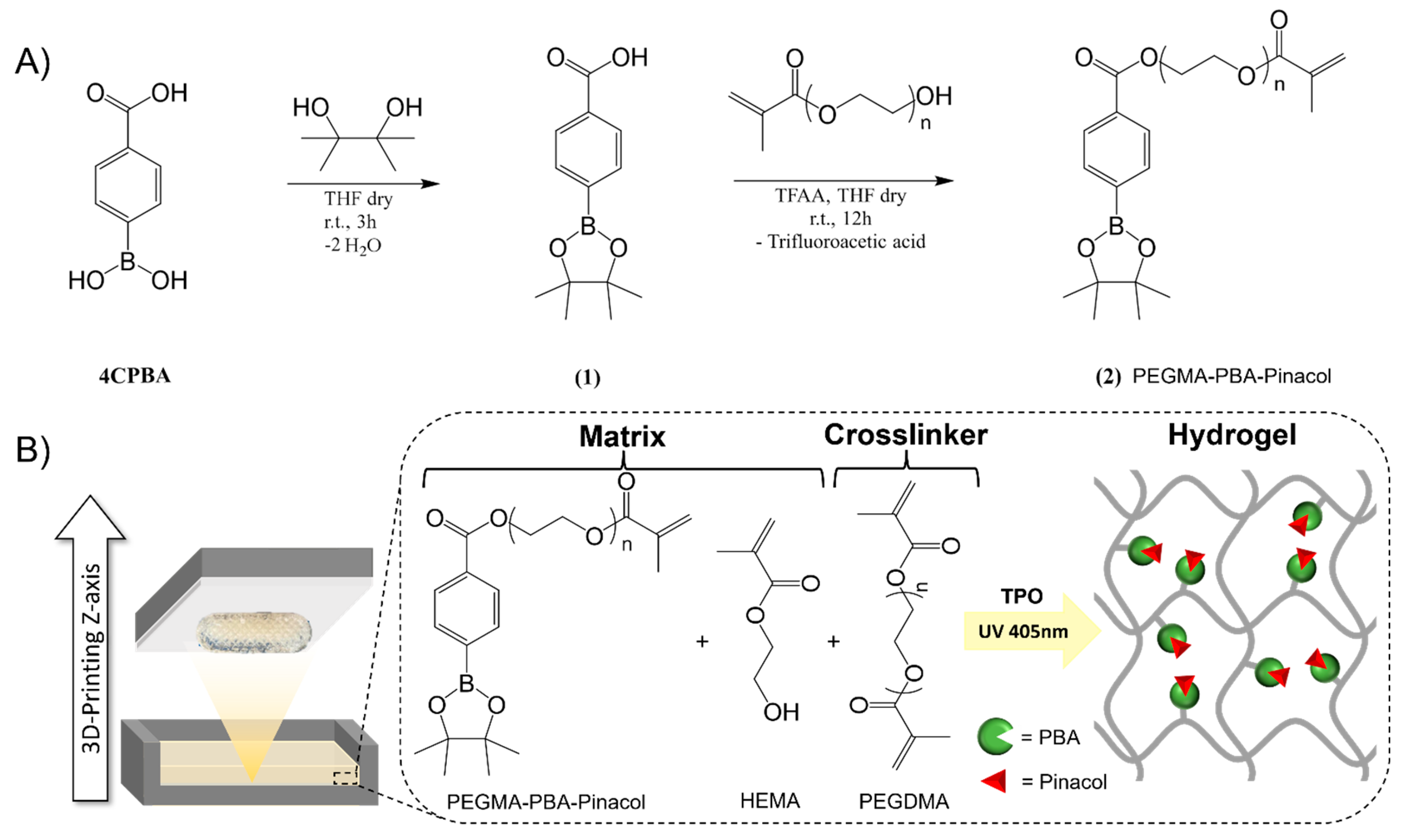
Synthesis of Phenylboronic Acid-Based Methacrylate
3D Printing of Bioinert Phenylboronic Acid-Containing Hydrogels
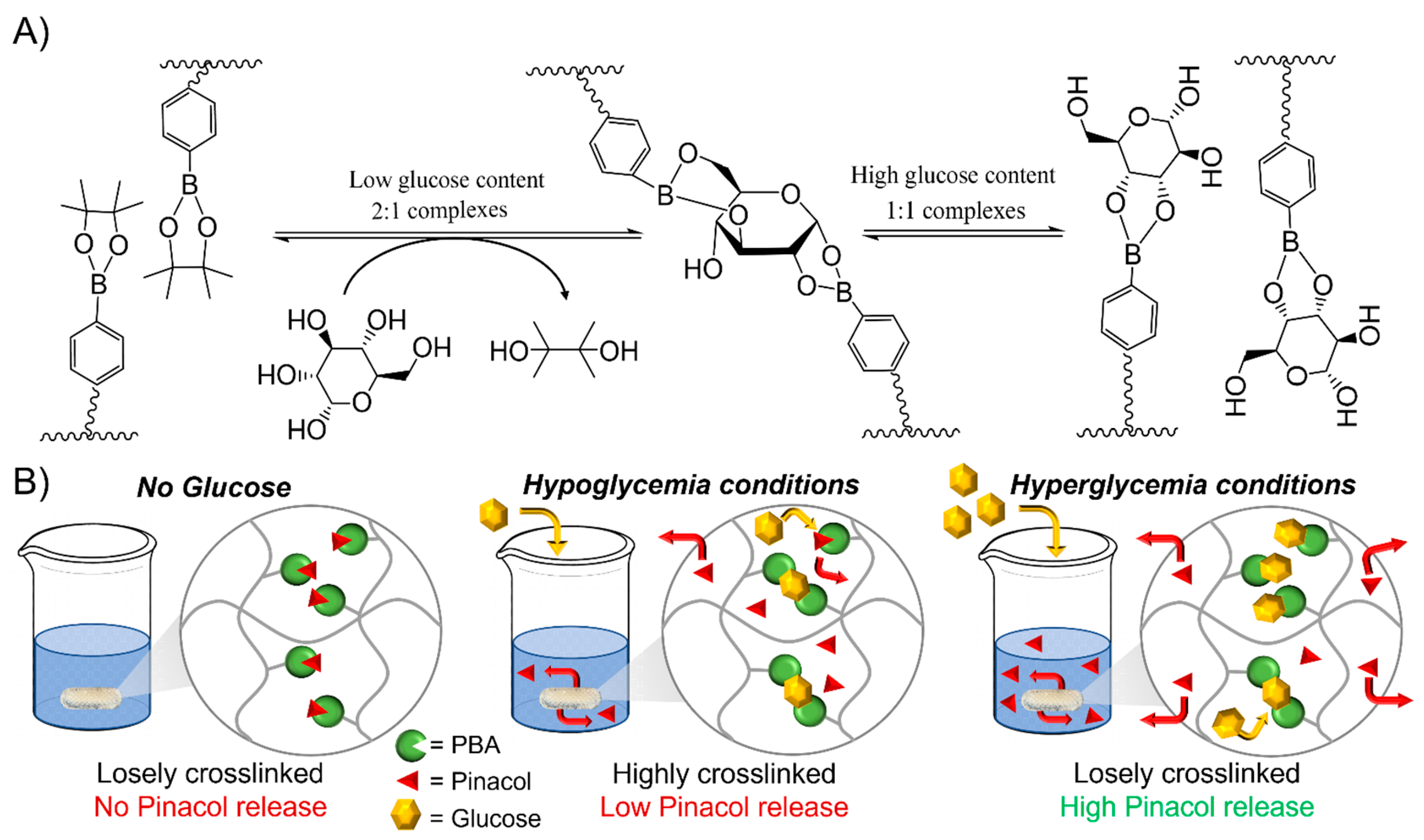
Transesterification of Phenylboronic Pinacol Ester with Glucose
In Vitro Drug Release Testing
Additional Techniques
3D-Printed Bioinert Phenylboronic Acid-Containing Hydrogels
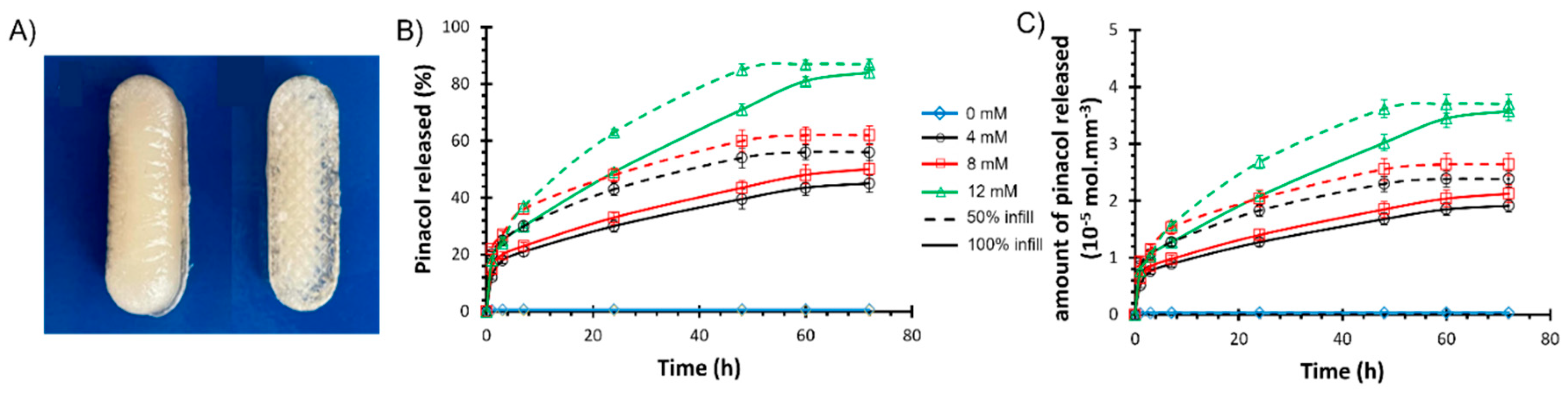
Glucose Responsiveness Features
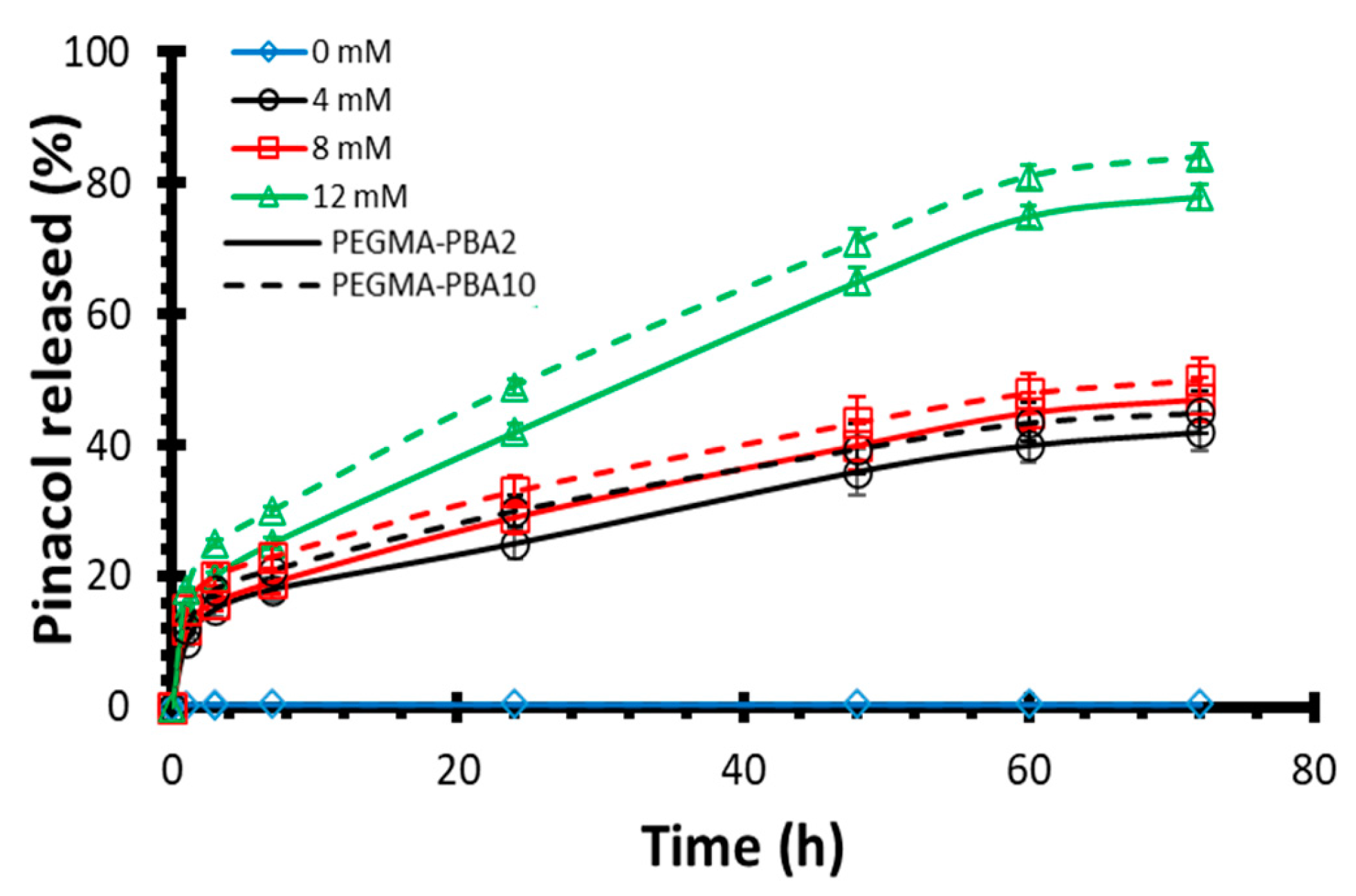
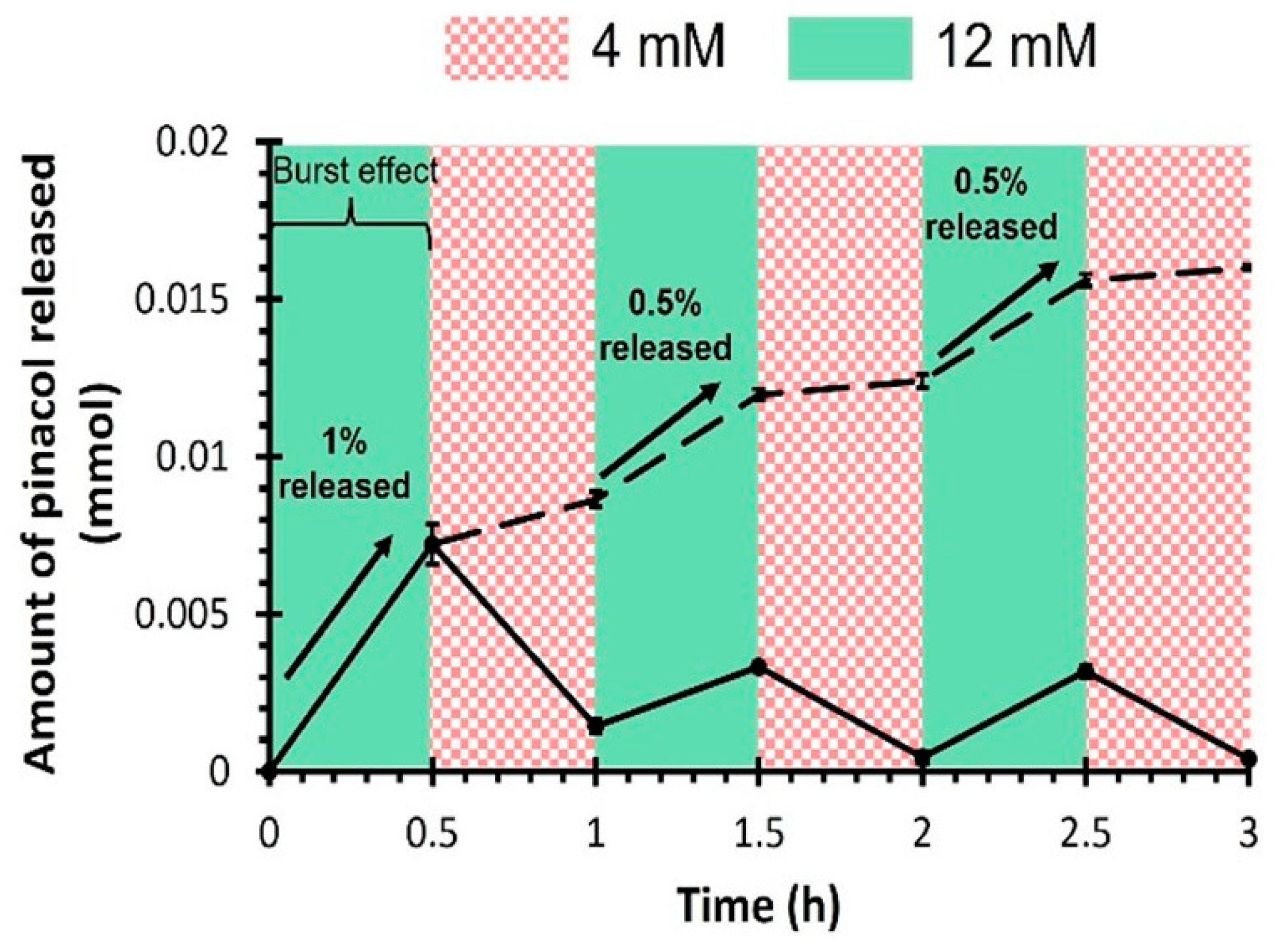
Drug Release Kinetics
Conclusions
Conflicts of interest:
Supplementary Materials
Acknowledgements
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Rabbett, B.; Seaquist, E.R. Hypoglycemia in diabetes: The dark side of diabetes treatment. A patient-centered review. J. Diabetes 2019, 11, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Gordijo, C.R.; Koulajian, K.; Shuhendler, A.J.; Bonifacio, L.D.; Huang, H.Y.; Chiang, S.; Ozin, G.A.; Giacca, A.; Wu, X.Y. Nanotechnology-Enabled Closed Loop Insulin Delivery Device: In Vitro and In Vivo Evaluation of Glucose-Regulated Insulin Release for Diabetes Control. Adv. Funct. Mater. 2011, 21, 73–82. [Google Scholar] [CrossRef]
- Chu, M.K.L.; Chen, J.; Gordijo, C.R.; Chiang, S.; Ivovic, A.; Koulajian, K.; Giacca, A.; Wu, X.Y.; Sun, Y. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab a Chip 2012, 12, 2533–2539. [Google Scholar] [CrossRef]
- Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375(9733):2215–22.
- Brownlee, M.; Cerami, A. A Glucose-Controlled Insulin-Delivery System: Semisynthetic Insulin Bound to Lectin. Science 1979, 206, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Brownlee M, Cerami A. Glycosylated Insulin Complexed to Concanavalin A: Biochemical Basis for a Closed-Loop Insulin Delivery System. Diabetes. 1983;32(6):499–504.
- Dutt-Ballerstadt, R.; Evans, C.; McNichols, R.; Gowda, A. Concanavalin A for in vivo glucose sensing: A biotoxicity review. Biosens. Bioelectron. 2006, 22, 275–284. [Google Scholar] [CrossRef]
- Yin, R.; Tong, Z.; Yang, D.; Nie, J. Glucose-responsive microhydrogels based on methacrylate modified dextran/concanavalin A for insulin delivery. J. Control. Release 2011, 152, e163–e165. [Google Scholar] [CrossRef]
- Xu, M.; Huang, J.; Jiang, S.; He, J.; Wang, Z.; Qin, H.; Guan, Y.-Q. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. Int. J. Biol. Macromol. 2022, 202, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Luo FQ, Chen G, Xu W, Zhou D, Li JX, Huang YC, et al. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021;14(8):2689–96.
- Wang, Y.; Fan, Y.; Zhang, M.; Zhou, W.; Chai, Z.; Wang, H.; Sun, C.; Huang, F. Glycopolypeptide Nanocarriers Based on Dynamic Covalent Bonds for Glucose Dual-Responsiveness and Self-Regulated Release of Insulin in Diabetic Rats. Biomacromolecules 2020, 21, 1507–1515. [Google Scholar] [CrossRef]
- Liu, Q.; Rauth, A.M.; Wu, X.Y. Immobilization and bioactivity of glucose oxidase in hydrogel microspheres formulated by an emulsification–internal gelation–adsorption–polyelectrolyte coating method. Int. J. Pharm. 2007, 339, 148–156. [Google Scholar] [CrossRef]
- Wen, J.; Anderson, S.M.; Du, J.; Yan, M.; Wang, J.; Shen, M.; Lu, Y.; Segura, T. Controlled Protein Delivery Based on Enzyme-Responsive Nanocapsules. Adv. Mater. 2011, 23, 4549–4553. [Google Scholar] [CrossRef]
- Dutta, K.; Hu, D.; Zhao, B.; Ribbe, A.E.; Zhuang, J.; Thayumanavan, S. Templated Self-Assembly of a Covalent Polymer Network for Intracellular Protein Delivery and Traceless Release. J. Am. Chem. Soc. 2017, 139, 5676–5679. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2020, 32, 1902004–e1902004. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Nam, J.-S.; Doss, G.P.C.; Lee, S.-S.; Chakraborty, C. Nanoparticle based insulin delivery system: the next generation efficient therapy for Type 1 diabetes. J. Nanobiotechnology 2015, 13, 1–13. [Google Scholar] [CrossRef]
- Horgan, A.M.; Marshall, A.J.; Kew, S.J.; Dean, K.E.; Creasey, C.D.; Kabilan, S. Crosslinking of phenylboronic acid receptors as a means of glucose selective holographic detection. Biosens. Bioelectron. 2006, 21, 1838–1845. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Choi, W.M.; Chung, J.S.; Hur, S.H. Highly biocompatible phenylboronic acid-functionalized graphitic carbon nitride quantum dots for the selective glucose sensor. Sensors Actuators B: Chem. 2018, 282, 36–44. [Google Scholar] [CrossRef]
- Tang, Z.; Guan, Y.; Zhang, Y. Contraction-type glucose-sensitive microgel functionalized with a 2-substituted phenylboronic acid ligand. Polym. Chem. 2014, 5, 1782–1790. [Google Scholar] [CrossRef]
- Meng, H.; Zheng, J.; Wen, X.; Cai, Z.; Zhang, J.; Chen, T. pH- and Sugar-Induced Shape Memory Hydrogel Based on Reversible Phenylboronic Acid-Diol Ester Bonds. Macromol. Rapid Commun. 2015, 36, 533–537. [Google Scholar] [CrossRef]
- Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A Synthetic Approach Toward a Self-Regulated Insulin Delivery System. Angew Chem Int Ed. 2012;51(9):2124–8.
- Yin W, Wang Y, Xiao Y, Mao A, Lang M. Phenylboronic acid conjugated mPEG-b-PCL micelles as DOX carriers for enhanced drug encapsulation and controlled drug release. Eur Polym J. 2022;173:111235.
- Zhang, J.; Xu, J.; Lim, J.; Nolan, J.K.; Lee, H.; Lee, C.H. Wearable Glucose Monitoring and Implantable Drug Delivery Systems for Diabetes Management. Adv. Heal. Mater. 2021, 10, 2100194. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Sharma, A.C.; Goponenko, A.V.; Das, S.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N.; Asher, S.A. High Ionic Strength Glucose-Sensing Photonic Crystal. Anal. Chem. 2003, 75, 2316–2323. [Google Scholar] [CrossRef]
- Banach. ; Williams, G.T.; Fossey, J.S. Insulin Delivery Using Dynamic Covalent Boronic Acid/Ester-Controlled Release. Adv. Ther. 2021, 4, 2100118. [Google Scholar] [CrossRef]
- Yang T, Ji R, Deng XX, Du FS, Li ZC. Glucose-responsive hydrogels based on dynamic covalent chemistry and inclusion complexation. Soft Matter. 2014;10(15):2671.
- Yesilyurt V, Webber MJ, Appel EA, Godwin C, Langer R, Anderson DG. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv Mater. 2016;28(1):86–91.
- Dong, Y.; Wang, W.; Veiseh, O.; Appel, E.A.; Xue, K.; Webber, M.J.; Tang, B.C.; Yang, X.-W.; Weir, G.C.; Langer, R.; et al. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid–Glucose Complexation. Langmuir 2016, 32, 8743–8747. [Google Scholar] [CrossRef]
- Yu J, Wang J, Zhang Y, Chen G, Mao W, Ye Y, et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng. 2020;4(5):499–506.
- Marco-Dufort, B.; Willi, J.; Vielba-Gomez, F.; Gatti, F.; Tibbitt, M. Environment Controls Biomolecule Release from Dynamic Covalent Hydrogels. Biomacromolecules. 2021, 22, 146–157. [Google Scholar] [CrossRef]
- Xiang, Y.; Xian, S.; Ollier, R.C.; Yu, S.; Su, B.; Pramudya, I.; Webber, M.J. Diboronate crosslinking: Introducing glucose specificity in glucose-responsive dynamic-covalent networks. J. Control. Release 2022, 348, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Losego, M.D.; Braun, P.V. Hydrogel-Based Glucose Sensors: Effects of Phenylboronic Acid Chemical Structure on Response. Chem. Mater. 2013, 25, 3239–3250. [Google Scholar] [CrossRef]
- Achilli, C.; Ciana, A.; Fagnoni, M.; Balduini, C.; Minetti, G. Susceptibility to hydrolysis of phenylboronic pinacol esters at physiological pH. Open Chem. 2013, 11, 137–139. [Google Scholar] [CrossRef]
- Trivedi, M.; Jee, J.; Silva, S.; Blomgren, C.; Pontinha, V.M.; Dixon, D.L.; Van Tassel, B.; Bortner, M.J.; Williams, C.; Gilmer, E.; et al. Additive manufacturing of pharmaceuticals for precision medicine applications: A review of the promises and perils in implementation. Addit. Manuf. 2018, 23, 319–328. [Google Scholar] [CrossRef]
- Zhang, J.; Vo, A.Q.; Feng, X.; Bandari, S.; Repka, M.A. Pharmaceutical Additive Manufacturing: a Novel Tool for Complex and Personalized Drug Delivery Systems. Aaps Pharmscitech 2018, 19, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Domsta, V.; Seidlitz, A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, L.; Rasoulianboroujeni, M.; Moharamzadeh, K.; Almela, T.K.D.; Cui, Z.; Ye, H. 3D-printed membrane for guided tissue regeneration. Mater. Sci. Eng. C 2018, 84, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Visscher, L.E.; Dang, H.P.; Knackstedt, M.A.; Hutmacher, D.W.; Tran, P.A. 3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics. Mater. Sci. Eng. C 2018, 87, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef]
- Mizuno, Y.; Takasawa, K.; Hanada, T.; Nakamura, K.; Yamada, K.; Tsubaki, H.; Hara, M.; Tashiro, Y.; Matsuo, M.; Ito, T.; et al. Fabrication of novel-shaped microneedles to overcome the disadvantages of solid microneedles for the transdermal delivery of insulin. Biomed. Microdevices 2021, 23, 38. [Google Scholar] [CrossRef]
- Xenikakis, I.; Tsongas, K.; Tzimtzimis, E.K.; Katsamenis, O.L.; Demiri, E.; Zacharis, C.K.; Georgiou, D.; Kalogianni, E.P.; Tzetzis, D.; Fatouros, D.G. Transdermal delivery of insulin across human skin in vitro with 3D printed hollow microneedles. J. Drug Deliv. Sci. Technol. 2022, 67, 102891. [Google Scholar] [CrossRef]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, H.; Shi, D.; Liu, Z.; Yuan, W. Microneedles As a Delivery System for Gene Therapy. Front. Pharmacol. 2016, 7, 137. [Google Scholar] [CrossRef]
- Dorsey, P.J.; Rubanov, M.; Wang, W.; Schulman, R. Digital Maskless Photolithographic Patterning of DNA-Functionalized Poly(ethylene glycol) Diacrylate Hydrogels with Visible Light Enabling Photodirected Release of Oligonucleotides. ACS Macro Lett. 2019, 8, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Liu H, Li W, Liu C, Tan J, Wang H, Hai B, et al. Incorporating simvastatin/poloxamer 407 hydrogel into 3D-printed porous Ti 6 Al 4 V scaffolds for the promotion of angiogenesis, osseointegration and bone ingrowth. Biofabrication. 2016;8(4):045012.
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Filipović VV, Božić Nedeljković BĐ, Vukomanović M, Tomić SLj. Biocompatible and degradable scaffolds based on 2-hydroxyethyl methacrylate, gelatin and poly(beta amino ester) crosslinkers. Polym Test. 2018;68:270–8.
- Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wallin, T.J.; Odent, J.; Yip, M.C.; Mosadegh, B.; Shepherd, R.F.; Giannelis, E.P. Optical stereolithography of antifouling zwitterionic hydrogels. J. Mater. Chem. B 2019, 7, 2855–2864. [Google Scholar] [CrossRef]
- Odent J, Wallin TJ, Pan W, Kruemplestaedter K, Shepherd RF, Giannelis EP. Highly Elastic, Transparent, and Conductive 3D-Printed Ionic Composite Hydrogels. Adv Funct Mater. 2017;27(33):1701807.
- Robinson, L.L.; Self, J.L.; Fusi, A.D.; Bates, M.W.; de Alaniz, J.R.; Hawker, C.J.; Bates, C.M.; Sample, C.S. Chemical and Mechanical Tunability of 3D-Printed Dynamic Covalent Networks Based on Boronate Esters. ACS Macro Lett. 2021, 10, 857–863. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Xue, L.; Liu, S.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.P.; Zhang, F.; DiMarchi, R.D. Insulin structure and function. Biopolym. 2007, 88, 687–713. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takada, K.; Kiso, Y.; Muranishi, S. Synthesis of Palmitoyl Derivatives of Insulin and Their Biological Activities. Pharm. Res. 1989, 06, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Korich, A.L.; Iovine, P.M. Boroxine chemistry and applications: A perspective. Dalton Trans. 2009, 39, 1423–1431. [Google Scholar] [CrossRef]
- Stacey, M.; Bourne, E.J.; Tatlow, J.C.; Tedder, J.M. A General Method of Esterification using Trifluoracetic Anhydride. Nature 1949, 164, 705–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, B.; Zhu, H.; Bi, F.; Xiao, S.; Wang, L.; Gai, G.; Zhao, L. Recent Advances in Phenylboronic Acid-Based Gels with Potential for Self-Regulated Drug Delivery. Molecules 2019, 24, 1089. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.E.; Baker, L.S.; Melock, T.L.; Yang, B. Ultraviolet cure kinetics of a low Tg polyurethane acrylate network under varying light intensity and exposure time. Prog. Org. Coatings 2021, 158, 106353. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coatings 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Schurz, J. Rheology of polymer solutions of the network type. Prog. Polym. Sci. 1991, 16, 1–53. [Google Scholar] [CrossRef]
- Jensen, S.S.; Jensen, H.; Cornett, C.; Møller, E.H.; Østergaard, J. Insulin diffusion and self-association characterized by real-time UV imaging and Taylor dispersion analysis. J. Pharm. Biomed. Anal. 2014, 92, 203–210. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Understanding the Burst Release Phenomenon: Toward Designing Effective Nanoparticulate Drug-Delivery Systems. Ther. Deliv. 2021, 12, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Bove, L.; D'Aniello, C.; Gorrasi, G.; Guadagno, L.; Vittoria, V. Transport properties of dichloromethane in glassy polymers. VI. Poly(ethylene terephthalate). J. Appl. Polym. Sci. 1996, 62, 1035–1041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




