Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
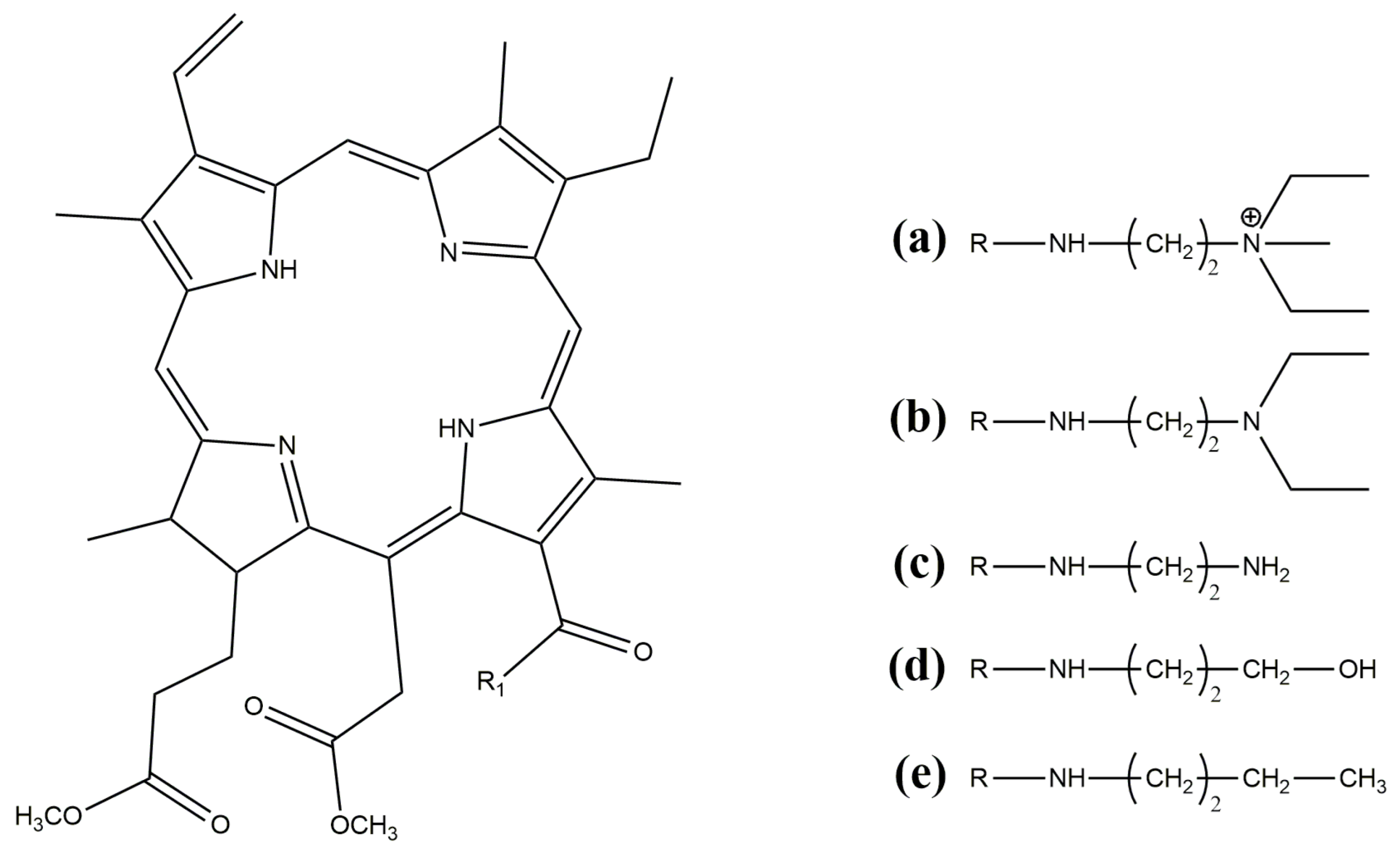
2.1. Materials
2.2. Methods
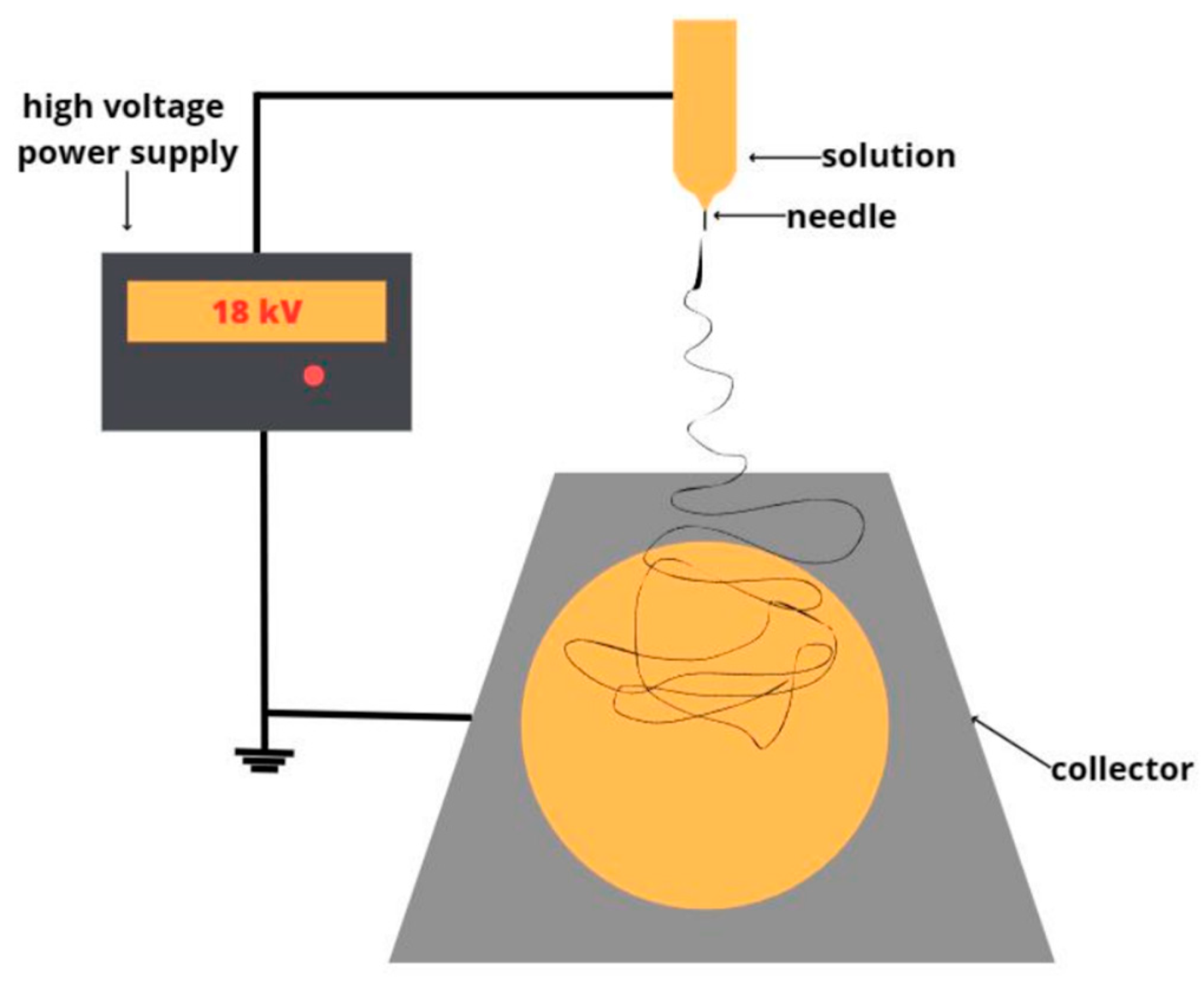
2.2.1. Obtaining of nonwoven fibrous materials
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.5. Wettability
2.2.6. Air Permeability
2.2.7. Antimicrobial Properties
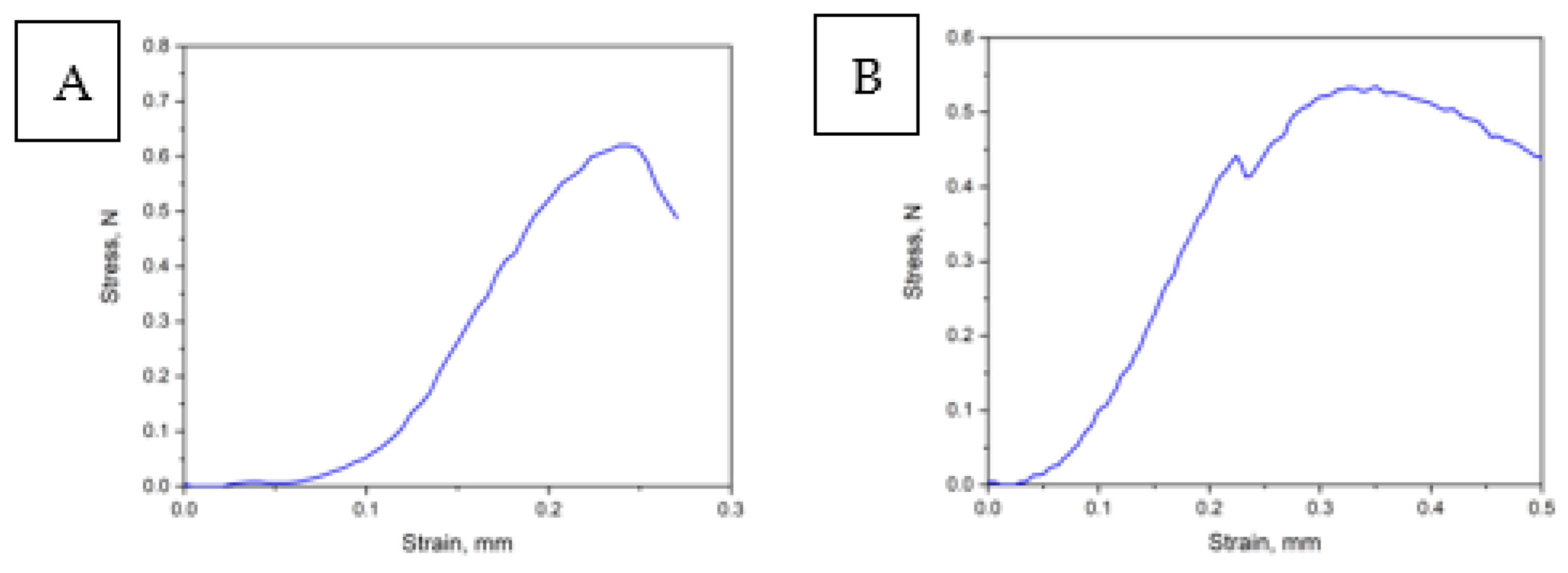
2.2.8. Mechanical Properties
3. Results
3.1. Morphology of PHB/chlorophyll derivatives electrospun materials
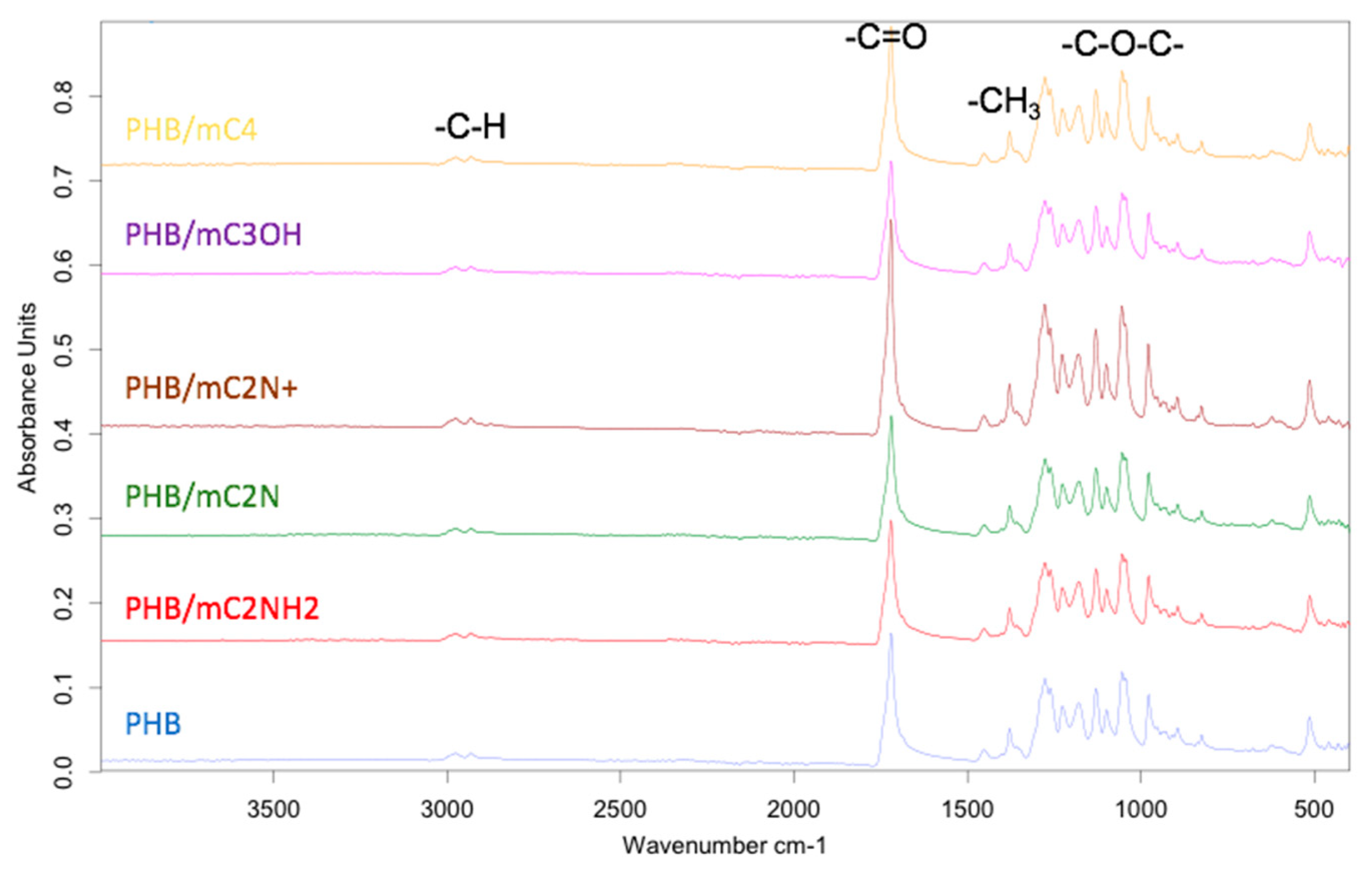
3.2. Chemical structure PHB/chlorophyll derivatives electrospun materials
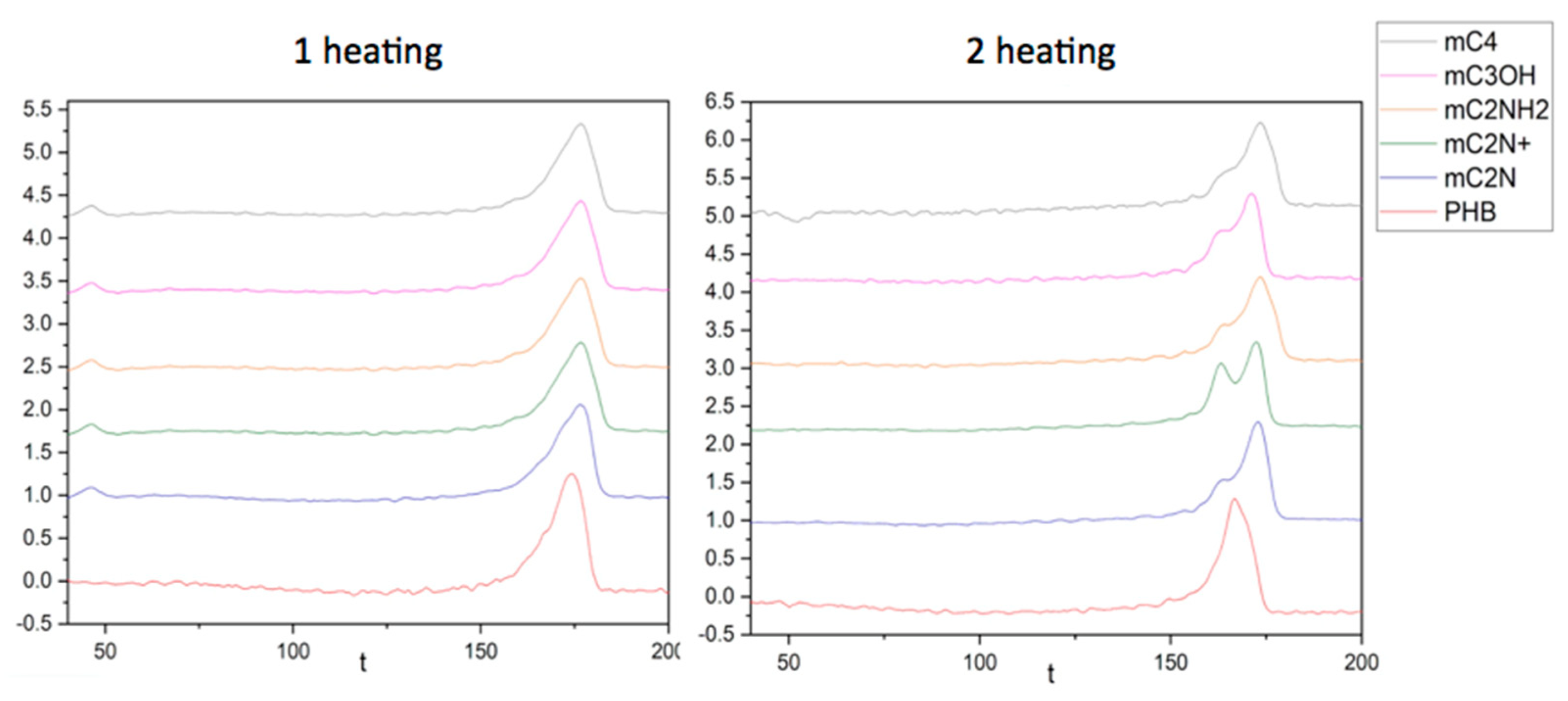
3.3. Thermophysical characteristics of PHB/chlorophyll derivatives electrospun materials
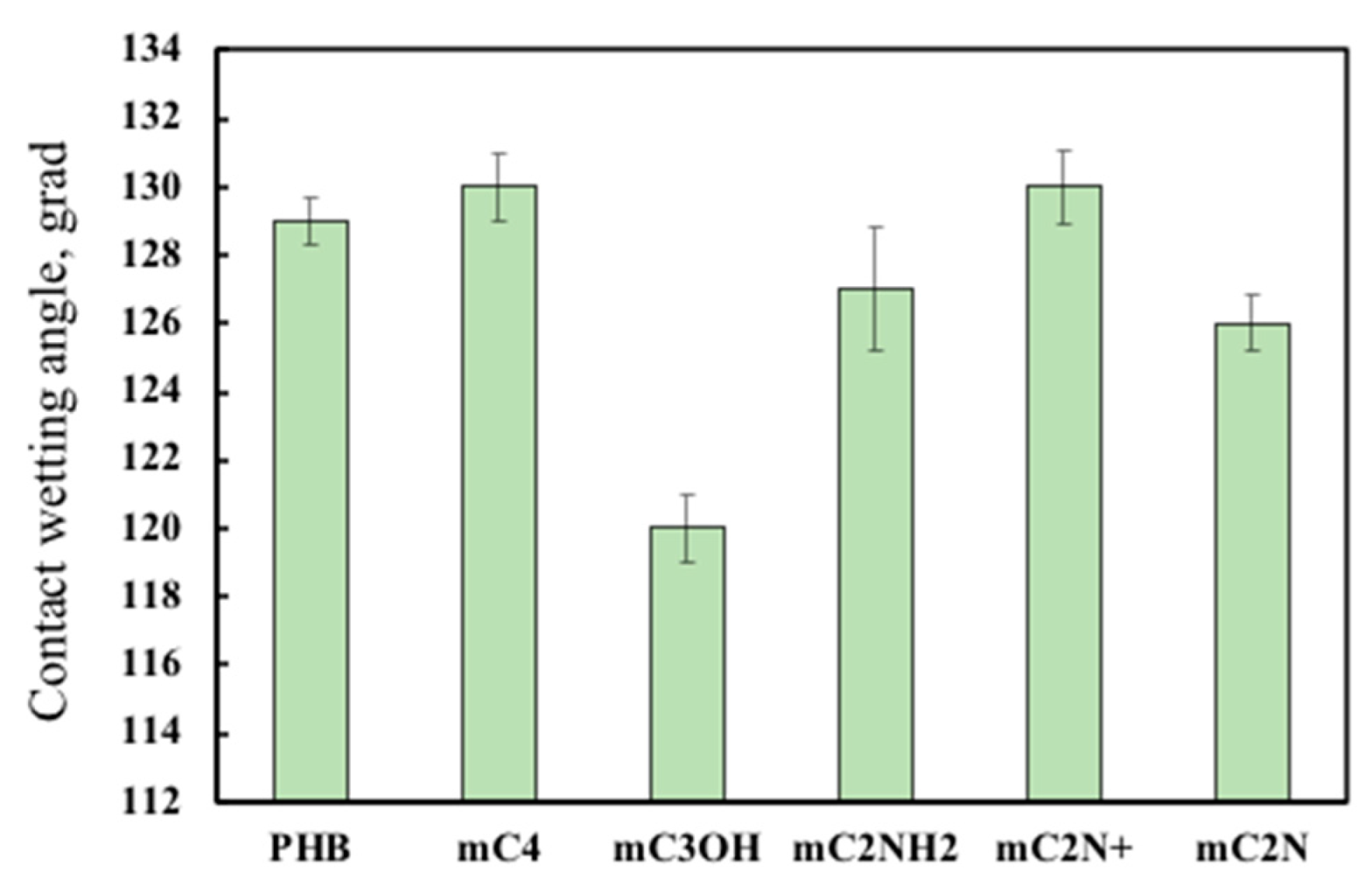
3.4. Wettability of PHB/chlorophyll derivatives electrospun materials
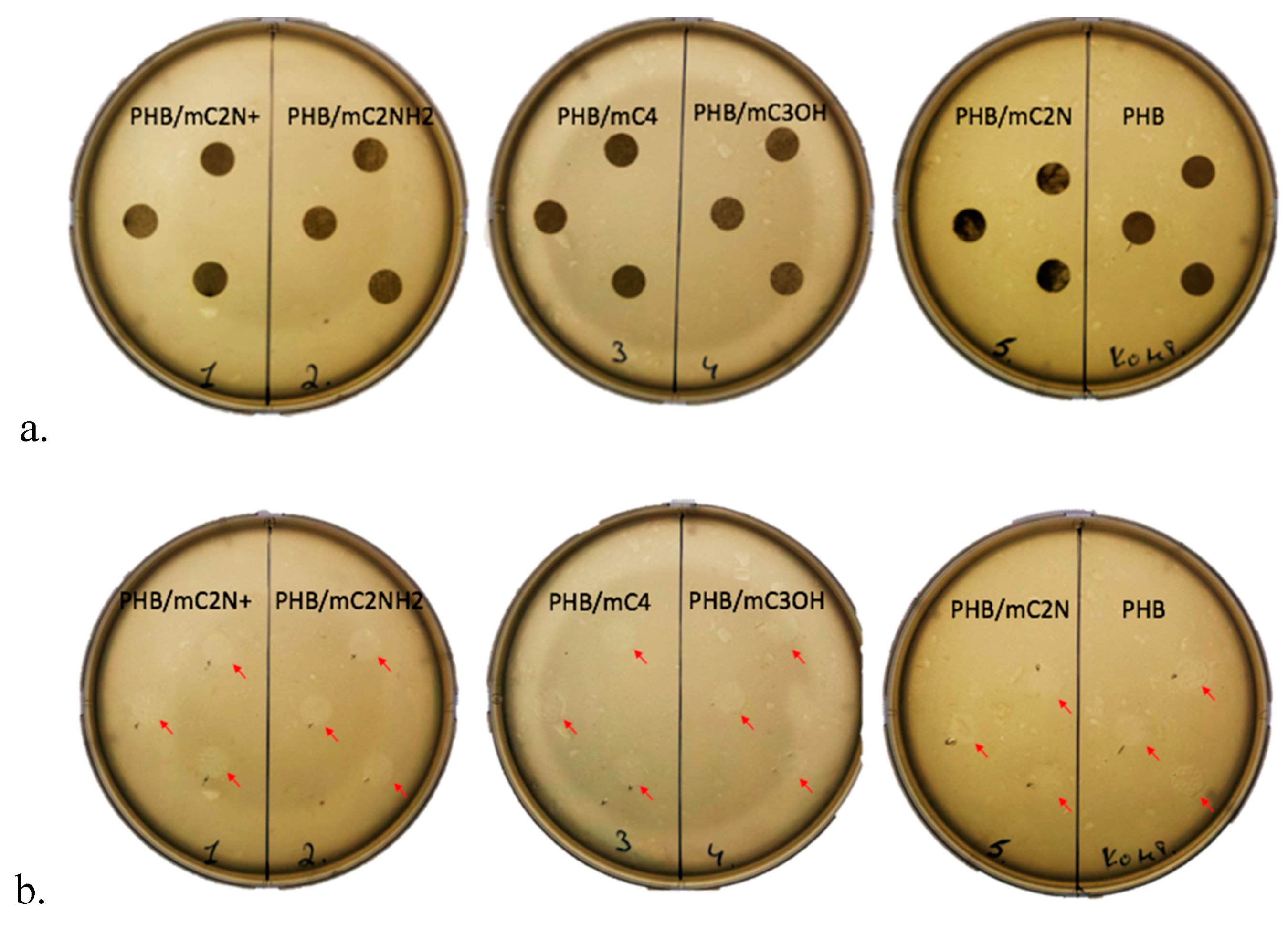
3.5. Antimicrobial properties of PHB/chlorophyll derivatives electrospun materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pucci, C.; Martinelli, C.; Degl’Innocenti, A.; Desii, A.; De Pasquale, D.; Ciofani, G. Light-Activated Biomedical Applications of Chlorophyll Derivatives. Macromol. Biosci. 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]
- Lukyanets, E.A. The key role of peripheral substituents in the chemistry of phthalocyanines and their analogs. J. Porphyr. Phthalocyanines 2010, 14, 40. [Google Scholar] [CrossRef]
- Staron, J.; Boron, B.; Karcz, D.; Szczygieł, M.; Fiedor, L. Recent Progress in Chemical Modifications of Chlorophylls and Bacteriochlorophylls for the Applications in Photodynamic Therapy. Curr. Med. Chem. 2015, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu. Rev. Biochem. 2014, 83, 40. [Google Scholar] [CrossRef] [PubMed]
- Perez-Galvez, A.; Viera, I.; Roca, M. Chemistry in the Bioactivity of Chlorophylls: An Overview. Curr. Med. Chem. 2017, 24, 4515–4536. [Google Scholar] [CrossRef]
- Negishi, T.; Rai, H.; Hayatsu, H. Antigenotoxic activity of natural chlorophylls. Mutat. Res. 1997, 12, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their Derivatives Used in Food Industry and Medicine. Mini Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef]
- Hoober, J.K.; Eggink, L.L.; Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 2007, 94, 387–400. [Google Scholar] [CrossRef]
- Pogorilyy, V.; Ostroverkhov, P.; Efimova, V.; Plotnikova, E.; Bezborodova, O.; Diachkova, E.; Vasil’ev, Y.; Pankratov, A.; Grin, M. Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties. Molecules 2023, 20, 4215. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 28, 261–280. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-infrared photodynamic therapy. Mol. Pharm. 2012, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. Photosensitizers: Therapy and detection of malignant tumors. Photochem. Photobiol. 1987, 45, 89. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Chu, C.H.; Wang, S.B.; Chen, K.Y.; Gao, H.D.; Lee, M.M.; Sun, S.S.; Li, J.P.; Chen, J.Y.; Chen, J.K.; et al. Lipid-Wrapped Upconversion Nanoconstruct/Photosensitizer Complex for Near-Infrared Light-Mediated Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 9, 95. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Conceição, D.S.; Calhelha, R.C.; Sousa, T.; Socoteanu, R.; Ferreira, I.C.F.R.; Vieira Ferreira, L.F. Porphyrin dye into biopolymeric chitosan films for localized photodynamic therapy of cancer. Carbohydr. Polym. 2016, 20, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.V.; Machado, I.F.; Gama, A.; Socoteanu, R.P.; Boscencu, R.; Manda, G.; Calhelha, R.C.; Ferreira, I.C.F.R. Photochemical/Photocytotoxicity Studies of New Tetrapyrrolic Structures as Potential Candidates for Cancer Theranostics. Curr. Drug Discov. Technol. 2020, 7, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Xu, X.; Zhang, X.; Yu, Y.; Wang, X. Photodynamic Treatment of Colorectal Cancer Using Chlorin e6-Loaded Poly(lactide-co-glycolide)- Based Nanoparticles. J. Biomed. Nanotechnol. 2021, 1, 1939–1950. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Nikolskaya, E.; Beganovskaya, V.; Sokol, M.; Chirkina, M.; Obydennyi, S.; Belykh, D.; Startseva, O.; Mollaev, M.D.; Yabbarov, N. Oxidative Damage Induced by Phototoxic Pheophorbide a 17-Diethylene Glycol Ester Encapsulated in PLGA Nanoparticles. Antioxidants 2021, 10, 1985. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 4, 2908. [Google Scholar] [CrossRef]
- Tyubaeva, P.M.; Varyan, I.A.; Zykova, A.K.; Yarysheva, A.Y.; Ivchenko, P.V.; Olkhov, A.A.; Arzhakova, O.V. Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering. Polymers 2022, 12, 4878. [Google Scholar] [CrossRef] [PubMed]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Tyubaeva, P.M.; Varyan, I.A.; Nikolskaya, E.D.; Mollaeva, M.R.; Yabbarov, N.G.; Sokol, M.B.; Chirkina, M.V.; Popov, A.A. Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin. Nanomaterials 2023, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Woods, I.; Flanagan, T.C. Electrospinning of biomimetic scaffolds for tissue-engineered vascular grafts: Threading the path. Expert. Rev. Cardiovasc. Ther. 2014, 12, 32. [Google Scholar] [CrossRef]
- Pramual, S.; Assavanig, A.; Bergkvist, M.; Batt, C.A.; Sunintaboon, P.; Lirdprapamongkol, K.; Svasti, J.; Niamsiri, N. Development and characterization of bio-derived polyhydroxyalkanoate nanoparticles as a delivery system for hydrophobic photodynamic therapy agents. J. Mater. Sci. Mater. Med. 2016, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Scarascia-Mugnozza, G.; Macagnano, A. Electrospinning of Polystyrene/Polyhydroxybutyrate Nanofibers Doped with Porphyrin and Graphene for Chemiresistor Gas Sensors. Nanomaterials 2019, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Tyubaeva, P.; Varyan, I.; Lobanov, A.; Olkhov, A.; Popov, A. Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate). Polymers 2021, 21, 4024. [Google Scholar] [CrossRef] [PubMed]
- Ol’khov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Kurnosov, A.S.; Karpova, S.G.; Iordanskii, A.L. Structure and Properties of Biopolymeric Fibrous Materials Based on Polyhydroxybutyrate-Metalloporphyrin Complexes. Russ. J. Gen. Chem. 2021, 91, 546–553. [Google Scholar] [CrossRef]
- Gushchina, O.I.; Grishina, M.Y.; Larkina, E.A.; Lebedeva, V.S.; Mironov, A.F. Synthesis of Hydroxyl Derivatives of Chlorin e6. Macroheterocycles 2017, 10, 81–83. [Google Scholar] [CrossRef]
- Belykh, D.V.; Kopylov, E.A.; Gruzdev, I.V.; Kuchin, A.V. Opening of the extra ring in pheophorbide a methyl ester by the action of amines as a one-step method for introduction of additional fragments at the periphery of chlorin macroring. Russ. J. Org. Chem. 2010, 46, 577–585. [Google Scholar] [CrossRef]
- Rybkin, A.Y.; Belik, A.Y.; Goryachev, N.S.; Mikhaylov, P.A.; Kraevaya, O.A.; Filatova, N.V.; Parkhomenko, I.I.; Peregudov, A.S.; Terent’ev, A.A.; Larkina, E.A. Self-assembling nanostructures of water-soluble fullerene [60]–chlorin e6 dyads: Synthesis, photophysical properties, and photodynamic activity // Dyes and Pigments. – 2020. – V. 180. P. 108411 ISSN 0143-7208. (Scopus, http://www.sciencedirect.com/science/article/pii/S0143720820304265). [CrossRef]
- Gushchina, O.I.; Larkina, E.A.; Mironov, A.F. Makrogeterotsikly. Macroheterocycles 2014, 7, 414. (in Russian). [Google Scholar] [CrossRef]
- Hantel, M.M.; Armstrong, M.J.; DaRosa, F.; l’Abee, R. Characterization of Tortuosity in Polyetherimide Membranes Based on Gurley and Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2016, 164, 334–339. [Google Scholar] [CrossRef]
- Lim, L.T.; Mendes, A.C.; Chronakis, I.S. Electrospinning and electrospraying technologies for food applications. Adv. Food Nutr. Res. 2019, 88, 167–234. [Google Scholar]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The effect of polarity in the electrospinning process on PCL/chitosan nanofibres’ structure, properties and efficiency of surface modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Markin, V.S.; Kosenko, R.; Filatova, A.G.; Gasparyan, K.G.; Iordanskii, A.L. The Influence of Technological Factors and Polar Molecules on the Structure of Fibrillar Matrices Based on Ultrafine Poly-3-hydroxybutyrate Fibers Obtained via Electrospinning. Technologies 2023, 11, 118. [Google Scholar] [CrossRef]
- Behere, I.; Ingavle, G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J. Biomed. Mater. Res. A 2022, 110, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.H.; Olekhnovich, R.; Sitnikova, V.; Kremleva, A.; Snetkov, P.; Uspenskaya, M. PHB/PEG Nanofiber Mat Obtained by Electrospinning and Their Performances. Technologies 2023, 11, 48. [Google Scholar] [CrossRef]
- Borisova, I.; Stoilova, O.; Manolova, N.; Rashkov, I. Modulating the Mechanical Properties of Electrospun PHB/PCL Materials by Using Different Types of Collectors and Heat Sealing. Polymers 2020, 20, 693. [Google Scholar] [CrossRef] [PubMed]
- El-hadi, A.M.; Al-Jabri, F.Y. Influence of Electrospinning Parameters on Fiber Diameter and Mechanical Properties of Poly(3-Hydroxybutyrate) (PHB) and Polyanilines (PANI) Blends. Polymers 2016, 8, 97. [Google Scholar] [CrossRef]
- Innes, N.; Johnson, I.G.; Al-Yaseen, W.; Harris, R.; Jones, R.; Kc, S.; McGregor, S.; Robertson, M.; Wade, W.G.; Gallagher, J.E. A systematic review of droplet and aerosol generation in dentistry. J. Dent. 2021, 105, 103556. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Ledezma, A.; Romero, J.; Saldívar, R.; Langlois, V.; Renard, E.; Grande, D. Electrospinning and electrospraying techniques for designing novel antibacterial poly(3-hydroxybutyrate)/zinc oxide nanofibrous composites. J. Mater. Sci. 2016, 51, 8593–8609. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibers by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Wagner, A.; Poursorkhabi, V.; Mohanty, A.K.; Misra, M. Analysis of Porous Electrospun Fibers from Poly(l-lactic acid)/Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Blends. Chem. Eng. 2014, 2, 1976–1982. [Google Scholar] [CrossRef]
- Shamala, T.R.; Divyashree, M.S.; Davis, R.; Kumari, K.S.L.; Vijayendra, S.V.N.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by Fourier transform infrared spectroscopy and scanning electron microscopy. Indian J. Microbiol. 2009, 49, 251–258. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications, 2nd ed.; University of Technology: Sydney, Australia, 2004; p. 208. [Google Scholar]
- Luo, S.; Netravali, A.N. A study of physical and mechanical properties of poly(hydroxybutyrate-co-hydroxyvalerate) during composting. Polym. Degrad. Stab. 2003, 80, 59–66. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.; Jakesova, M.; Sobkova, M.; Marova, I. Enzymatic Hydrolysis of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Scaffolds. Materials 2020, 13, 2992. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H.; Yang, R.; Wu, Q.; Chen, G.Q.; Zhang, Z.M. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Berezin, D.B.; Makarov, V.V.; Guseinov, S.S.; Romanenko, Y.V.; Khudyaeva, I.S.; Startseva, O.M.; Belykh, D.V.; Kustov, A.V. Thermal stability of chlorophyll a derivatives containing hydrophilic groups. Russ. J. Gen. Chem. 2017, 87, 1557–1561. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages, and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Walle, G.A.; Koning, G.J.; Weusthuis, R.A.; Eggink, G. Properties, modifications and applications of biopolyesters. Adv. Biochem. Eng. Biotechnol. 2001, 71, 263–291. [Google Scholar] [CrossRef]
- Moreira, J.B.; Kuntzler, S.G.; Vaz, B.S.; da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate (PHB)-based blends and composites. Biodegrad. Polym. Blends Compos. 2022, 389–413. [Google Scholar]
- Pantani, R.; Turng, L.S. Manufacturing of advanced biodegradable polymeric components. J. Appl. Polym. Sci. 2015, 132, 48. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef] [PubMed]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: An in vitro study on Gram-negative and Gram-positive bacteria. J. Photochem. Photobiol. B Biol. 2006, 85, 28–38. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ladan, H.; Gozansky, S.; Malik, Z. Characterization of hemin antibacterial action on Staphylococcus aureus. FEMS Microbiol. Lett. 1987, 48, 401–406. [Google Scholar] [CrossRef]
- Beyene, B.B.; Wassie, G.A. Antibacterial activity of Cu(II) and Co(II) porphyrins: Role of ligand modification. BMC Chemistry 2020, 14, 51. [Google Scholar] [CrossRef]
- Gerola, A.P.; Santana, A.; França, P.B.; Tsubone, T.M.; de Oliveira, H.P.M.; Caetano, W.; Kimura, E.; Hioka, N. Effects of Metal and the Phytyl Chain on Chlorophyll Derivatives: Physicochemical Evaluation for Photodynamic Inactivation of Microorganisms. Photochem. Photobiol. 2011, 87, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Indrawati, R.; Zubaidah, E.; Sutrisno, A.; Limantara, L.; Yusuf, M.M.; Brotosudarmo, T.H.P. Visible Light-Induced Antibacterial Activity of Pigments Extracted from Dregs of Green and Black Teas. Scientifica 2021, 12, 5524468. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef] [PubMed]
- Czerniecka-Kubicka, A.; Frącz, W.; Jasiorski, M.; Błażejewski, W.; Pilch-Pitera, B.; Pyda, M.; Zarzyka, I. Thermal properties of poly(3-hydroxybutyrate) modified by nanoclay. J. Therm. Anal. Calorim. 2017, 128, 1513–1526. [Google Scholar] [CrossRef]
- Iordanskii, A.; Fomin, S.; Burkov, A.; Pankova, Y.; Zaikov, G. Characterization of the structure and properties of the polymer blends based on poly-3-hydroxybutyrate and polyisobutylene. J. Charact. Dev. Nov. Mater. 2015, 1937, 7975. [Google Scholar]
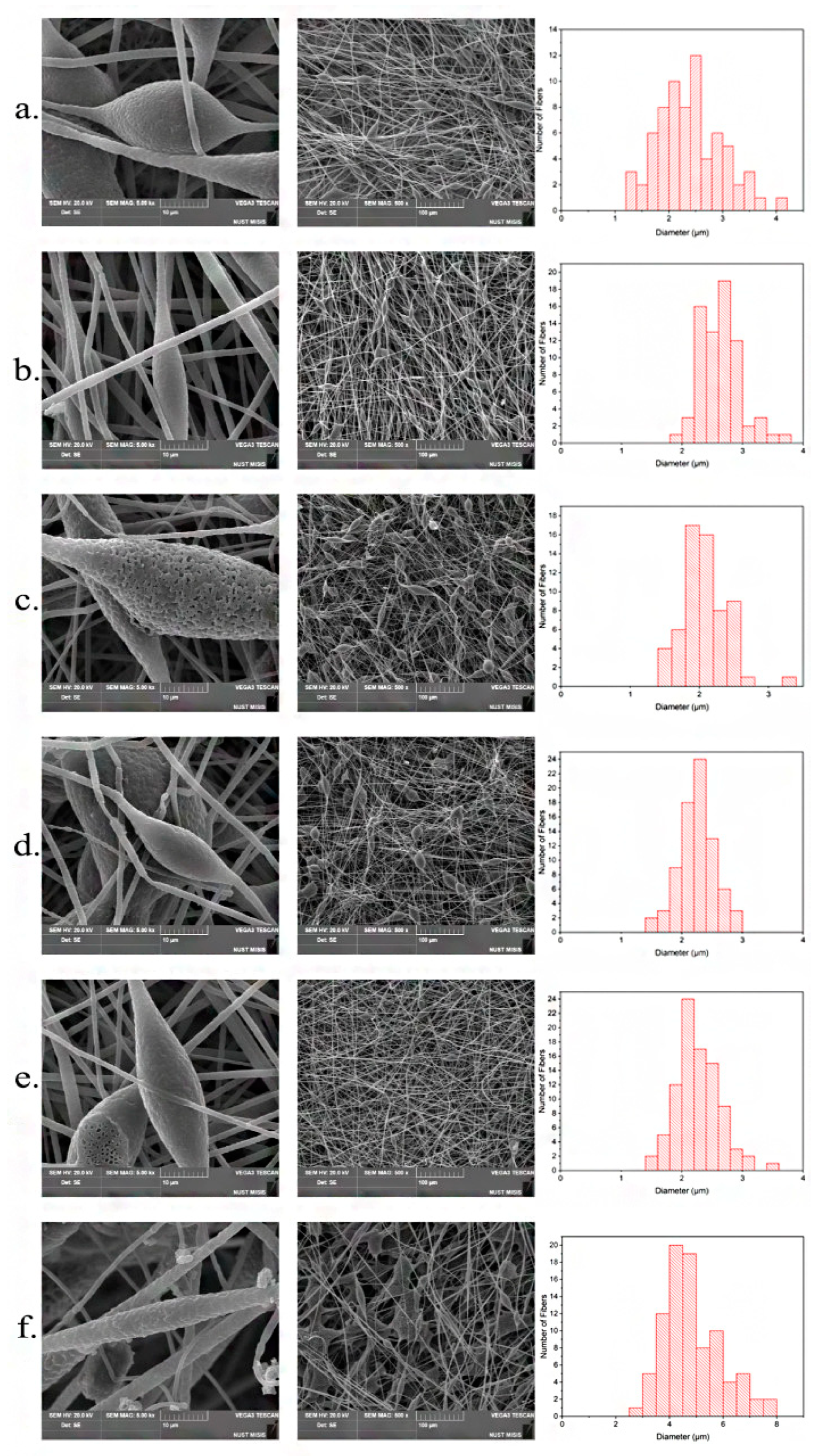
| Sample | Diameter of capillary, mm | Distance between the electrodes, mm | Gas pressure on the solution, kg(f)/cm2 | Voltage, Kv | Viscosity, Pa s |
|---|---|---|---|---|---|
| PHB | 0.1 | 210 | 10 | 18 | 1.0 |
| PHB/mC2NH2 | 220 | 10 | 17-18 | 1.2 | |
| PHB/mC2N | 220 | 10 | 17-18 | 1.2 | |
| PHB/mC2N+ | 220 | 10 | 17-18 | 1.2 | |
| PHB/mС4 | 220 | 10 | 17-18 | 1.2 | |
| PHB/mC3OH | 220 | 10 | 17-18 | 1.2 |
| Sample | Average diameter, µm | Bulk density, g/cm3 | Air permeability, mL | Time by Guerly method, s |
|---|---|---|---|---|
| PHB | 3.5 | 0.30 | 0.38 | 50.0 |
| PHB/mC2NH2 | 2.24 | 0.18 | 3.75 | 26.7 |
| PHB/mC2N | 4.86 | - | - | - |
| PHB/mC2N+ | 2.27 | 0.13 | 2.78 | 36.0 |
| PHB/mС4 | 2.63 | 0.16 | 2.35 | 42.6 |
| PHB/mC3OH | 2.08 | 0.16 | 4.35 | 23.0 |
| Sample | First heating run | Second heating run | ||
|---|---|---|---|---|
| Tm, ⁰C | ΔH, J/g | Tm, ⁰C | ΔH, J/g | |
| PHB | 175 | 93.1 | 170 | 90.8 |
| PHB/mC2NH2 | 176 | 83.3 | 174 | 78.7 |
| PHB/mC2N | 176 | 85.8 | 173 | 81.2 |
| PHB/mC2N+ | 177 | 80.6 | 173 | 79.8 |
| PHB/mС4 | 176 | 82.0 | 174 | 78.9 |
| PHB/mC3OH | 175 | 77.4 | 171 | 74.9 |
| Sample | Tensile strength, MPa Δ ±0.02 MPa |
Elongation at break, % Δ ±0.2 % |
|---|---|---|
| PHB | 1.7 | 3.6 |
| PHB/mC2NH2 | - | - |
| PHB/mC2N | - | - |
| PHB/mC2N+ | 1.5 | 1.3 |
| PHB/mС4 | 1.5 | 0.6 |
| PHB/mC3OH | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





