Submitted:
15 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Complex Interplay between Plant Roots and Soil Microbes under Drought and Desiccation Stresses
2.1. Microbial Community Structure Affected by Drought
2.2. Rhizobacteria-Plants Interactions Affected by Drought
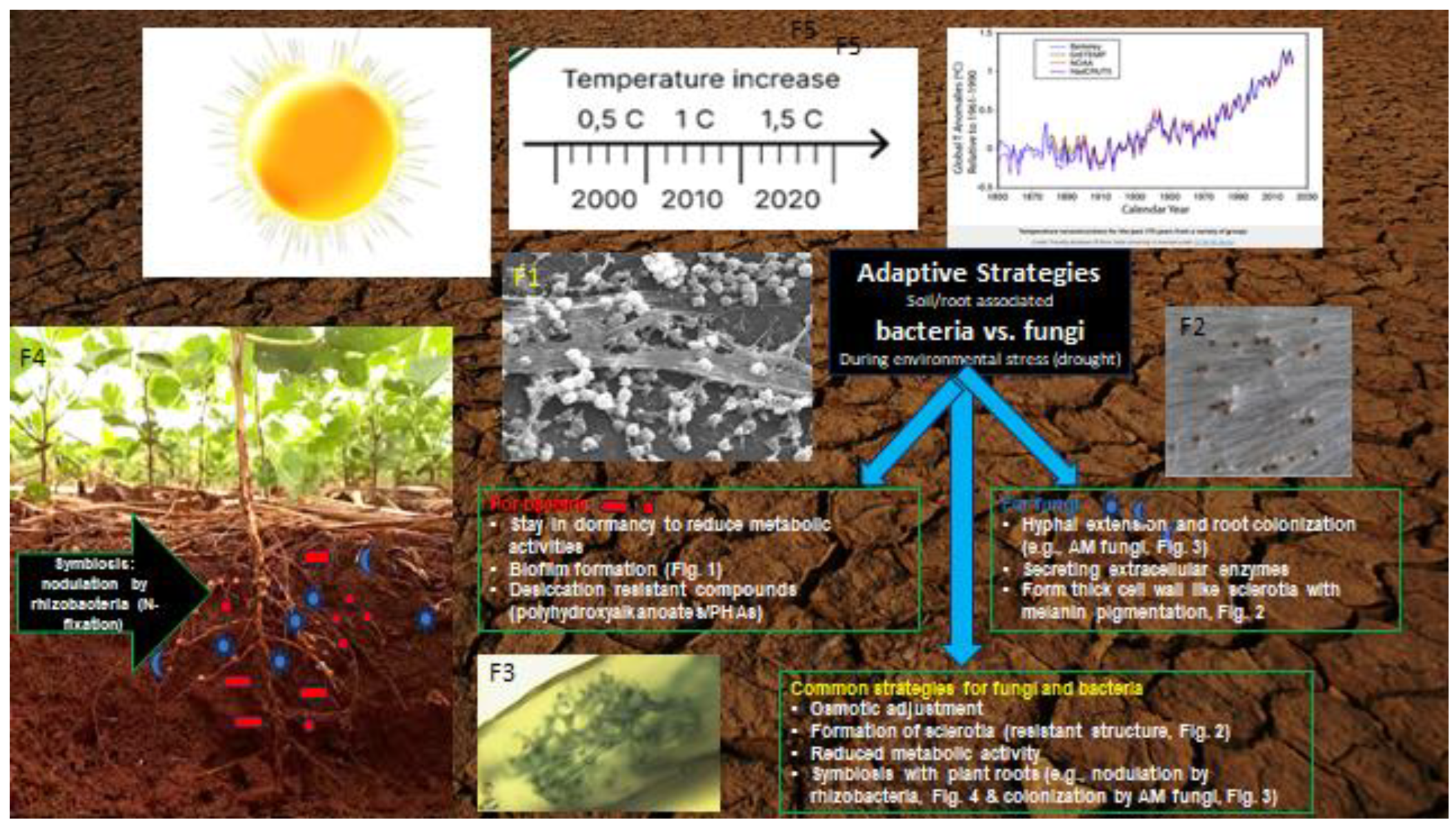
3. Adaptive Strategies Employed by Pathogenic and Beneficial Microbes under Drought
3.1. Adaptive Mechanisms of Rhizobacteria under Drought
3.2. Sensitivity of Rhizobacterial Species to Desiccation
3.3. Adaptive Mechanisms of AMFs under Drought
3.4. Alteration of Taxonomic Structure of AMFs under Drought
3.5. Distinct Adaptation Strategies in Rhizobacteria and AMFs to Drought
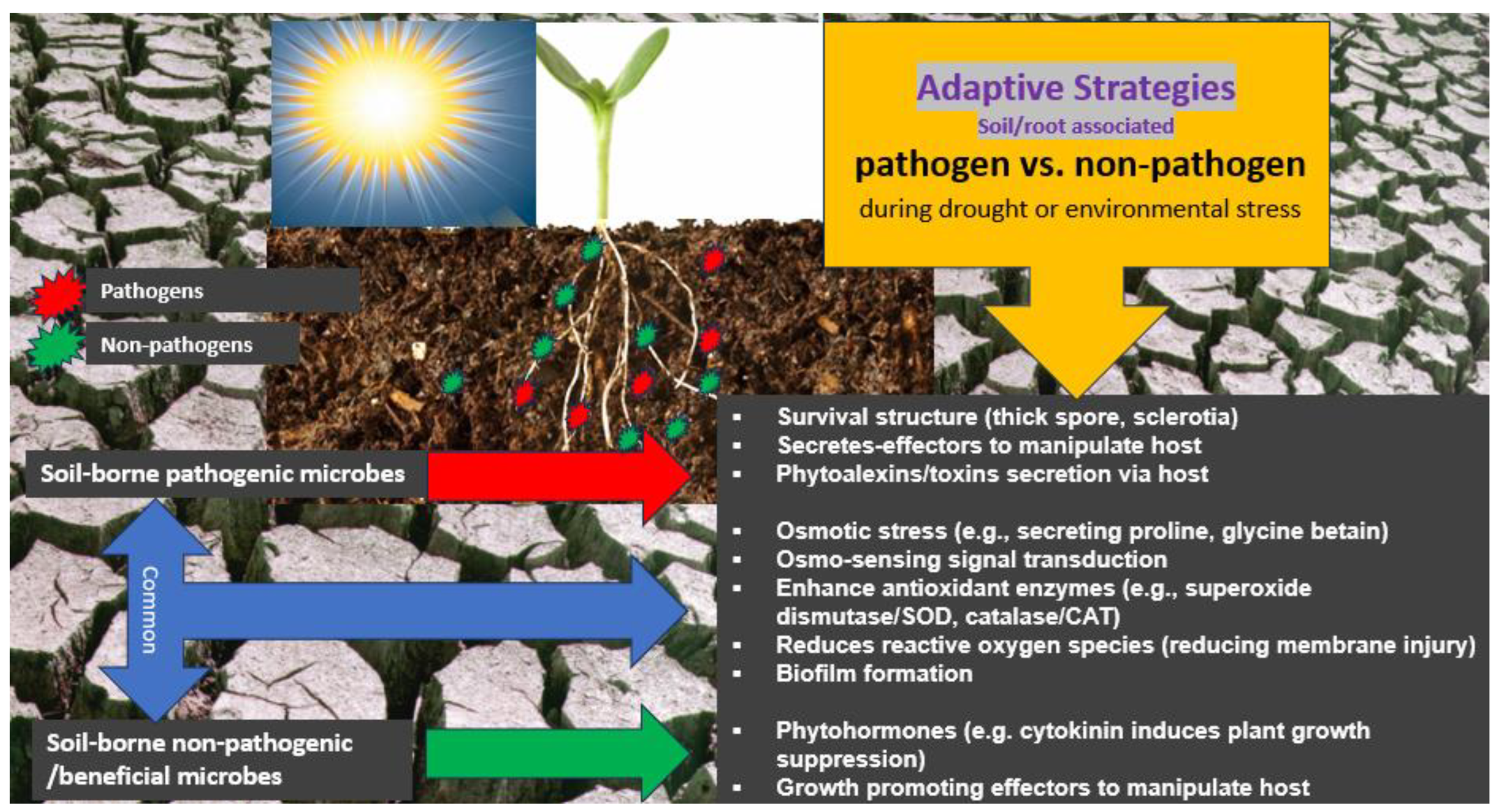
3.6. Distinct Adaptive Strategies of Pathogenic and Beneficial Microbes to Drought
3.7. Adaptation of Phytopathogenic Fungi and Bacteria to Drought
4. Soil Microbiomes Mediate Plant Defence Mechanism under Drought and Desiccation Stresses
4.1. Rhizobacteria-Mediated Plant Defense Mechanisms under Drought Conditions
4.2. Arbuscular Mycorrhizal Fungal-Mediated Plant Defense Mechanisms under Drought
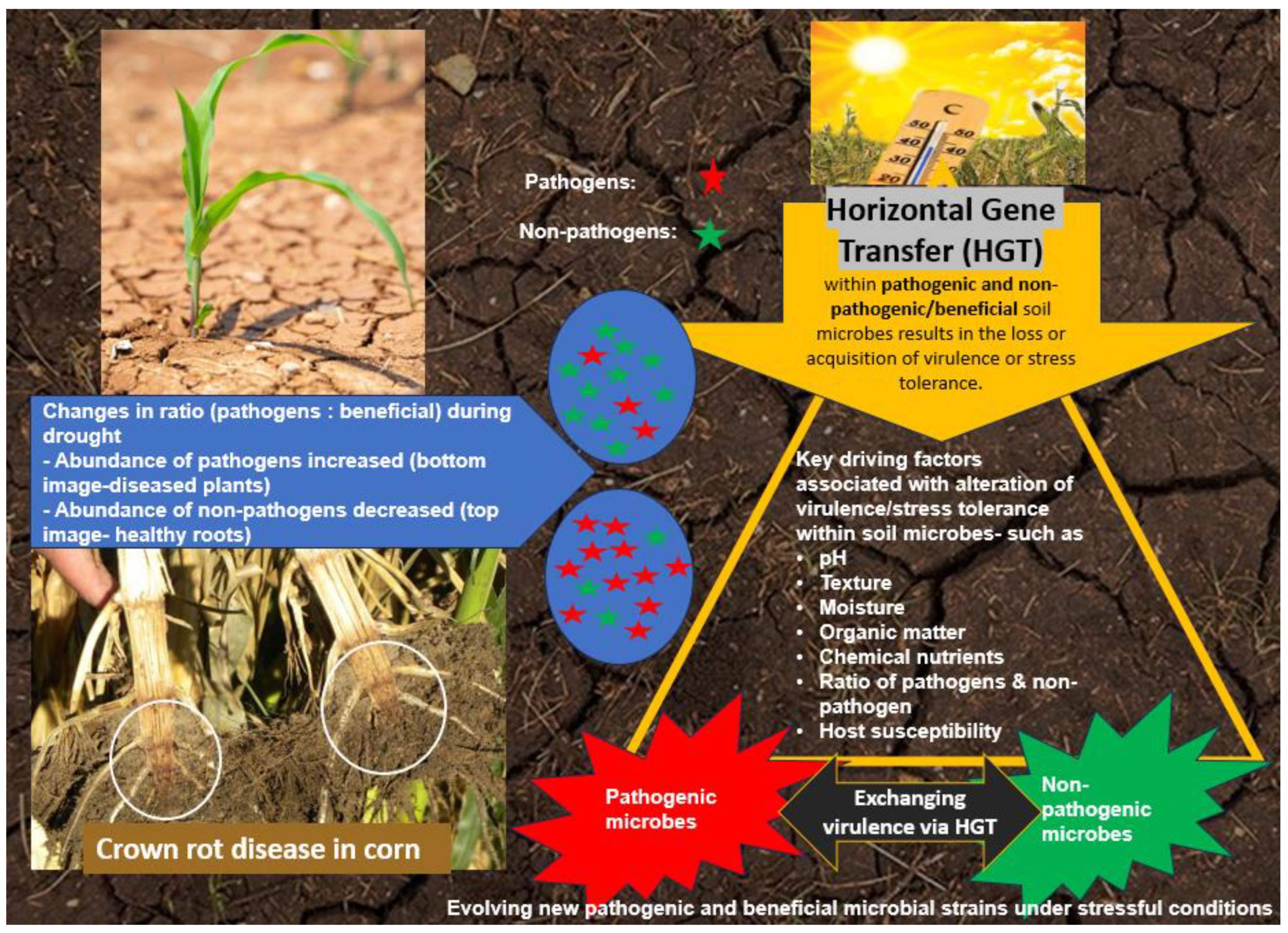
5. Horizontal Gene Transfer in the Adaptation of Plant Pathogenic and Non-Pathogenic Beneficial Microbes under Drought
5.1. Genetic Exchange Employed by Pathogens through Horizontal Gene Transfer (HGT)
5.2. Virulence and Pathogenicity Gene Exchange under Drought
5.3. Implication of Genomic Changes in the Plant-Soil System under Drought
5.4. Shift of Pathogens and Non-Pathogens through HGT under Drought
6. Summary
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Bach, E.M.; Ramirez, K.S.; Fraser, T.D.; Wall, D.H. Soil biodiversity integrates solutions for a sustainable future. Sustainability 2020, 12(7), 2662. [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat Rev Microbiol 2023, 22, 226-239. [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecological Indicators 2019, 105, 601-612. [CrossRef]
- Sarsan, S.; Pandiyan, A.; Rodhe, A.V.; Jagavati, S. Synergistic interactions among microbial communities. Microbes in Microbial Communities: Ecological and Applied Perspectives 2022, 1-37. [CrossRef]
- Kumar, I.; Mondal, M.; Gurusamy, R.; Balakrishnan, S.; Natarajan S. Plant-microbiome interaction and the effects of biotic and abiotic components in agroecosystem. Microb. Interv. Agric. Environ. 2019, 2, 517-546. [CrossRef]
- Ault, T. R. On the essentials of drought in a changing climate. Science 2020, 368(6488), 256-260.
- Vásquez-Dean, J.; Maza, F.; Morel, I.; Pulgar R.; González M. Microbial communities from arid environments on a global scale. A systematic review. Biol Res 2020, 53(1), 29. [CrossRef]
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil Constraints in an Arid Environment—Challenges, Prospects, and Implications. Agronomy 2023, 13, 220. [CrossRef]
- Alpert, P. The limits and frontiers of desiccation-tolerant life. Integrative and comparative biology 2005, 45(5), 685-695. [CrossRef]
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Science Reviews 2020, 201, 102953. [CrossRef]
- Esbelin, J.; Santos, T.; Hébraud, M. Desiccation: An environmental and food industry stress that bacteria commonly face. Food Microbiology 2018, 69, 82–88. [CrossRef]
- Brettner, L.; Ho W.C.; Schmidlin K.; Apodaca, S.; Eder, R.; Geiler-Samerotte, K. Challenges and potential solutions for studying the genetic and phenotypic architecture of adaptation in microbes. Curr Opin Genet Dev. 2022, 75, 101951. [CrossRef]
- Meisner, A.; Jacquiod, S.; Snoek, B.L.; Ten Hooven, F.C.; van der Putten, W.H. Drought legacy effects on the composition of soil fungal and prokaryote communities. Front Microbiol. 2018, 9, 294. [CrossRef]
- Thompson, S.; Levin, S.; Rodriguez-Iturbe, I. Linking plant disease risk and precipitation drivers: A dynamical systems framework. Am Nat. 2013, 181(1), E1-16. [CrossRef]
- Garbeva, P.; Hol, W.H.; Termorshuizen, A. J.; Kowalchuk, G.A.; de Boer, W. Fungistasis and general soil biostasis – A new synthesis. Soil Biology and Biochemistry 2011, 43, 469-477. [CrossRef]
- Durán, P.; Tortella, G.; Sadowsky, M.J.; Viscardi, S.; Barra, P.J.; Mora, M.L. Engineering Multigenerational Host-Modulated Microbiota against Soilborne Pathogens in Response to Global Climate Change. Biology 2021, 10(9), 865. [CrossRef]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the phytobiome. Cell 2017, 169, 587–596. [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [CrossRef]
- Manzanera, M. Dealing with water stress and microbial preservation. Environ Microbiol. 2021, 23(7), 3351-3359. [CrossRef]
- Loiko, N.; Tereshkina, K.; Kovalenko, V.; Moiseenko, A.; Tereshkin, E.; Sokolova, O.S.; Krupyanskii, Y. DNA-binding protein Dps protects Escherichia coli cells against multiple stresses during desiccation. Biology, 2023, 12(6), 853. [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [CrossRef]
- Matthews, K.E.; Facelli, J.M.; Cavagnaro, T.R. Response of soil microbial community structure, carbon and nitrogen cycling to drying and rewetting. Applied Soil Ecology 2023, 192, 105099. [CrossRef]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annual Review of Ecology, Evolution, and Systematics 2018, 49(1), 409-4321. [CrossRef]
- Evans, S.E.; Allison, D.S.; Hawkes, V.C. Microbes, memory and moisture: Predicting microbial moisture responses and their impact on carbon cycling. Functional Ecology 2022, 36(6), 1430-1441. [CrossRef]
- Gillespie, L.M.; Prada-Salcedo, L.D.; Shihan, A.; Fromin, N.; Goldmann, K.; Milcu, A.; Buscot, F.; Buatois, B.; Hättenschwiler, S. Taxonomical and functional responses of microbial communities from forest soils of differing tree species diversity to drying-rewetting cycles. Pedobiologia 2023, 97–98, 150875. [CrossRef]
- Maestre, F.T., Delgado-Baquerizo, M., Jeffries, T.C., Eldridge, D.J., Ochoa, V., Gozalo, B., ... & Singh, B.K. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proceedings of the National Academy of Sciences 2015, 112(51), 15684-15689. [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [CrossRef]
- Lebre, P., De Maayer, P.; Cowan, D. Xerotolerant bacteria: Surviving through a dry spell. Nat Rev Microbiol. 2017, 15, 285–296. [CrossRef]
- Scales, N.C.; Huynh, K.T.; Weihe, C.; Martiny, J.B.H. Desiccation induces varied responses within a soil bacterial genus. Environ Microbiol. 2023, 25(12), 3075-3086. [CrossRef]
- Breitkreuz, C.; Herzig, L.; Buscot, F.; Reitz, T.; Tarkka, M. Interactions between soil properties, agricultural management and cultivar type drive structural and functional adaptations of the wheat rhizosphere microbiome to drought. Environ. Microbiol. 2021, 23, 5866–5882. [CrossRef]
- Chodak, M.; Gołębiewsk, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Soil chemical properties affect the reaction of forest soil bacteria to drought and rewetting stress. Ann Microbiol. 2015, 65(3),1627-1637. [CrossRef]
- Maisnam, P.; Jeffries, T.C.; Szejgis, J.; Bristol, D.; Singh, B.K.; Eldridge, D.J.; Horn, S.; Chieppa, J.; Nielsen, U.N. Severe prolonged drought favours stress-tolerant microbes in australian drylands. Microb Ecol. 2023, 86(4), 3097-3110. [CrossRef]
- Metze, D.; Schnecker, J.; Canarini, A.; Fuchslueger, L.; Koch, B.J.; Stone, B.W.; Hungate, B.A.; Hausmann, B.; Schmidt, H.; Schaumberger, A.; Bahn, M.; Kaiser, C.; Richter, A. Microbial growth under drought is confined to distinct taxa and modified by potential future climate conditions. Nat Commun. 2023,14(1), 5895. [CrossRef]
- Jaeger, A.C., Hartmann, M.; Six, J.; Solly, E.F. Contrasting sensitivity of soil bacterial and fungal community composition to one year of water limitation in Scots pine mesocosms. FEMS Microbiology Ecology 2023, 99(6), fiad051. [CrossRef]
- Rosinger, C.; Rousk, J.; Bonkowski, M.; Rethemeyer, J.; Jaeschke, A. Rewetting the hyper-arid Atacama Desert soil reactivates a carbon-starved microbial decomposer community and also triggers archaeal metabolism. Sci Total Environ. 2023, 892, 164785. [CrossRef]
- Chilakala, A. R.; Pandey, P.; Durgadevi, A.; Kandpal, M.; Patil, B. S.; Rangappa, K.; Reddy, P.C.O.; Ramegowda, V.; Senthil-Kumar, M. Drought attenuates plant responses to multiple rhizospheric pathogens: A study on a dry root rot-associated Disease complex in chickpea fields. Field Crops Research 2023, 298, 108965. [CrossRef]
- Sinha, R.; Irulappan, V.; Mohan-Raju, B.; Suganthi, A.; Senthil-Kumar, M. Impact of drought stress on simultaneously occurring pathogen infection in field-grown chickpea. Scientific Reports 2019, 9, 5577. [CrossRef]
- Kaisermann, A.; Maron, P.A.; Beaumelle, L.; Lata, J.C. Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Applied Soil Ecology 2015, 86, 158-164. [CrossRef]
- Marasco, R., Rolli, E., Ettoumi, B., Vigani, G., Mapelli, F., Borin, S., Abou-Hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; Zocchi, G.; Daffonchio, D. A A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE 2012, 7(10), e48479. [CrossRef]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Gałązka, A.; Grządziel, J.; Stuczyński, T. Impact of Water Stress on Microbial Community and Activity in Sandy and Loamy Soils. Agronomy 2020, 10, 1429. [CrossRef]
- Barnard, R.L.; Blazewicz, S.J.; Firestone, M.K. Rewetting of soil: Revisiting the origin of soil CO2 emissions. Soil Biology and Biochemistry 2020, 147, 107819. [CrossRef]
- Liao, Z.; Junliang, F., Zhenlin, L.; Zhentao, B.; Haidong, W.; Minghui, C.; Fucang, Z.; Zhijun, L. Chapter Three - Response network and regulatory measures of plant-soil-rhizosphere environment to drought stress. Advances in Agronomy, 2023, 180, 93-196. [CrossRef]
- Xu, L.; Dong, Z.; Chiniquy, D.; Pierroz, G.; Deng, S.; Gao, C.; Diamond, S.; Simmons, T.; Wipf, H.M.; Caddell, D.; Varoquaux, N.; Madera, M.A.; Hutmacher, R.; Deutschbauer, A.; Dahlberg, J.A; Guerinot, M.L.; Purdom, E.; Banfield, J.F.; Taylor, J.W.; Lemaux, P.G.; Coleman-Derr, D. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat Commun. 2021, 12(1), 3209. [CrossRef]
- Estrada-González, Á.J.; Medina-De la Rosa, G.; Bautista, E.; Flores, J.; López-Lozano, N.E. Physiological regulations of a highly tolerant cactus to dry season modify its rhizospheric microbial communities. Rhizosphere, 2023, 25, 100655. [CrossRef]
- Hestrin, R.; Kan, M.; Lafler, M.; Wollard, J.; Kimbrel, J.A.; Ray, P.; Blazewicz, S.J.; Stuart, R.; Craven, K.; Firestone, M.; Nuccio, E.E.; Pett-Ridge, J. Plant-associated fungi support bacterial resilience following water limitation. ISME J. 2022, 16(12), 2752-2762. [CrossRef]
- Milošević, N.A.; Marinković, J.B.; Tintor, B.B. Mitigating abiotic stress in crop plants by microorganisms. Proc. Nat. Sci. Matica Serpska Novi. Sad 2012, 123, 17–26. [CrossRef]
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W.; Dar, T.U.H. The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J Appl Microbiol. 2022, 133(5), 2717-2741. [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front Plant Sci. 2018, 9, 1473. [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23(1), 407. [CrossRef]
- Berendsen, Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478-486. [CrossRef]
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; Liira, J.; Metsis, M.; Neshataeva, V.; Paal, J.; Phosri, C.; Põlme, S.; Reier, Ü.; Saks, Ü.; Schimann, H.; Thiéry, O.; Vasa, M.; Moora, M. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23(5),411-30. [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbiosis. Nat. Rev. Microbiol. 2008, 6, 763-775. [CrossRef]
- Bennett, A.E.; Groten, K. The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annual Review of Plant Biology 2022, 73, 649-672. [CrossRef]
- Bittencourt, P.P.; Alves, A.F.; Ferreira, M.B.; da Silva Irineu, L.E.S.; Pinto, V.B.; Olivares, F.L. Mechanisms and Applications of Bacterial Inoculants in Plant Drought Stress Tolerance. Microorganisms 2023, 11, 502. [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L. K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. PGPR amelioration in sustainable agriculture 2019, 129-157. [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S. P. P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12(17), 3102. [CrossRef]
- Bao, X.; Zou, J.; Zhang, B.; Wu, L.; Yang, T.;Huang, Q. Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere. Agronomy 2022, 12(6), 1277. [CrossRef]
- Agudelo, M.G.; Ruiz, B., Capela, D.; Remigi P. The role of microbial interactions on rhizobial fitness. Front Plant Sci. 2023, 14,1277262. [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9(1), 205. [CrossRef]
- Fred, E.B.; Baldwin, I.L.; McCoy, E. Some factors which influence the growth and longevity of the nodule bacteria. In Root nodule bacteria and leguminous plants; Baldwin, I. L.; McCoy, E.; Fred, E. B.; Publisher: University of Wisconsin, Madison, WI, 1932, Volume 5, pp. 104-117.
- Massa, F.; Defez, R.; Bianco, C. Exploitation of Plant Growth Promoting Bacteria for Sustainable Agriculture: Hierarchical Approach to Link Laboratory and Field Experiments. Microorganisms 2022, 10(5), 865. [CrossRef]
- Kloepper, J.; Leong, J.; Teintze, M.; Milton N.S. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [CrossRef]
- Amaresan, N.; Kumar, M.S.; Annapurna, K.; Kumar, K.; Sankaranaryanan, N. Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Academic Press: Cambridge, MA, USA, 2020.
- Santos L.F.; Olivares, F. L. Plant microbiome structure and benefits for sustainable agriculture. Current Plant Biology 2021, 26, 100198. ISSN 2214-6628. [CrossRef]
- Lehmann, J.; Bossio D.A.; Kögel-Knabner I., Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544-553. [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture. Horticulture 2021, 7, 390. [CrossRef]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [CrossRef]
- Igiehon, O.N.; Babalola, O.O. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Current Microbiology 2021, 78(4), 1615-1627. [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. N. Phytol. 2022, 234, 1951–1959. [CrossRef]
- Suryanarayanan, T.S.; Shaanker, R.U. Can fungal endophytes fast-track plant adaptations to climate change? Fungal Ecol. 2021, 50, 101039. [CrossRef]
- Marasco, H.I.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant and Soil 2016, 399(1-2), 219-229. [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Frontiers in Plant Science 2018, 8, 2223. [CrossRef]
- Maryani, Y.; Dewi, W.S.; Yunus, A. Study on osmoprotectant rhizobacteria to improve mung bean growth under drought stress. In IOP Conference Series: Earth and Environmental Science 2018, 129(1), 012014. [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17(3), 392-403. [CrossRef]
- Cytryn, E.J.; Sangurdekar, D.P.; Streeter, J.G.; Franck, W.L.; Chang, W.S.; Stacey, G.; Emerich, D.W.; Joshi, T.; Xu, D.; Sadowsky, M.J. Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation-induced stress. J Bacteriol. 2007, 189(19), 6751-6762. [CrossRef]
- Zhu, J.; Jiang, X.; Guan, D.; Kang, Y.; Li, L.; Cao, F.; Zhao, B.; Ma, M.; Zhao, J.; Li, J. Effects of rehydration on physiological and transcriptional responses of a water-stressed rhizobium. J Microbiol. 2022, 60(1), 31-46. [CrossRef]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tol-erance enhancement by trehalose production of desiccation-tolerant microorganisms. Frontiers in Microbiology 2016, 7, 1577.
- Sharma, M.P.; Grover, M.; Chourasiya, D., Bharti, A., Agnihotri, R., Maheshwari, H.S.; Pareek, A.; Buyer, J.S.; Sharma, S.K.; Schütz, L.; Mathhimaran, N.; Singla-Pareek, S.L.; Grossman, J.M.; Bagyaraj, D. J. Deciphering the role of trehalose in tripartite symbiosis among rhizobia, arbuscular mycorrhizal fungi, and legumes for enhancing abiotic stress tolerance in crop plants. Frontiers in microbiology 2020, 11, 509919. [CrossRef]
- McIntyre, H.J.; Davies, H.; Hore, T.A.; Miller, S.H.; Dufour, J.P.; Ronson, C.W. Trehalose biosynthesis in Rhizobium leguminosarum bv. trifolii and its role in desiccation tolerance. Appl Environ Microbiol. 2007, 73(12), 3984-3992. [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10(9), 3793-3810. [CrossRef]
- Nawaz, M.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Shah, A.N.; Hashem, M.; Alamri, S.; Batool, M.; Rasheed, A.; Thabit, M.A.; Alhaithloul, H.A.S.; Qari, S.H. Trehalose: A promising osmo-protectant against salinity stress-physiological and molecular mechanisms and future prospective. Mol. Biol. Rep. 2022, 49(12), 11255-11271. [CrossRef]
- Dukare, A.; Mhatre, P.; Maheshwari, H.S.; Delineation of mechanistic approaches of rhizosphere microorganisms facilitated plant health and resilience under challenging conditions. 3 Biotech 2022, 12, 57. [CrossRef]
- Manzanera, M.; Garcia de Castro, A.; Tondervik, A.; Rayner-Brandes,M.; Strom, A.R.; Tunnacliffe, A. Hydroxyectoine is superior to trehalose for anhydrobiotic engineering of Pseudomonas putida KT2440. Appl. Environ. Microbiol. 2002, 68, 4328-4333. [CrossRef]
- Narváez-Reinaldo, J.J.; Barba, I.; González-López J.; Tunnacliffe, A.; Manzanera, M. Rapid method for isolation of desiccation-tolerant strains and xeroprotectants. Appl Environ Microbiol. 2010, 76(15), 5254-62. [CrossRef]
- SantaCruz-Calvo, L.; González-López, J.; Manzanera, M. Arthrobacter siccitolerans sp. nov., a highly desiccation-tolerant, xeroprotectant-producing strain isolated from dry soil. Int J Syst Evol Microbiol. 2013, 63(Pt11), 4174-4180. [CrossRef]
- Peterson, C.; Niraula, S.; Parks, D.; Chang, W.S. Draft Genome Sequences of Two Desiccation-Tolerant Strains, Bradyrhizobium japonicum TXVA and TXEA, Isolated from the Root Nodules of Soybean Grown in Texas. Microbiol Resour Announc. 2022, 11(8), e0046722. [CrossRef]
- Pazos-Rojas, L.A.; Cuellar-Sánchez, A.; Romero-Cerón, A.L.; Rivera-Urbalejo, A.; Van Dillewijn, P.; Luna-Vital, D.A.; Muñoz-Rojas, J.; Morales-García, Y.E.;Bustillos-Cristales,M.D.R. The Viable but Non-Culturable (VBNC) State, a Poorly Explored Aspect of Beneficial Bacteria. Microorganisms 2023, 12(1), 39. [CrossRef]
- Muñoz-Rojas, J. Desiccation-tolerant rhizobacteria maintain their plant growth-promoting capability after experiencing extreme water stress. SciFed J. Appl. Microbiol 2018, 1, 15-17.
- Shankar, A.; Prasad, V. Potential of desiccation-tolerant plant growth-promoting rhizobacteria in growth augmentation of wheat (Triticum aestivum L.) under drought stress. Front Microbiol. 2023, 14, 1017167. [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H.; Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20(17), 4199. [CrossRef]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [CrossRef]
- Zhang, F.; Ying-Ning, Z.; Wu, Q. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Scientia Horticulturae 2018, 229, 132-136. [CrossRef]
- Liu, X. J. A.; Han, S.; Frey, S. D.; Melillo, J. M.; Zhou, J.; DeAngelis, K. M. Microbial responses to long-term warming differ across soil microenvironments. ISME Commun 2024, 4(1), ycae051. [CrossRef]
- Symanczik, S.; Courty, P.E.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the intro-duction of a non-native AMF species. Mycorrhiza 2015, 25, 639-647. [CrossRef]
- Lumini, E.; Vallino, M.; Alguacil, M.M.; Romani, M.; Bianciotto, V. Different farming and water regimes in Italian rice fields affect arbuscular mycorrhizal fungal soil communities. Ecological Applications 2011, 21(5), 1696-1707. [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63(11), 4033-44. [CrossRef]
- Auge, R. M. Mycorrhiza and drought resistance. In Mycorrhiza - Function, Diversity, State of the Art, 2020. 207-226. IntechOpen.
- Balestrini, R.; Nerva, L.; Sillo, F.; Girlanda, M.; Perotto, S.; Plant and fungal gene expression in mycorrhizal protocorms of the orchid Serapias vomeracea colonized by Tulasnella calospora. Plant Signal Behav 2014, 9(11), e977707. [CrossRef]
- Miransari, M.; Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl Microbiol Biotechnol. 2011, 89(4), 917-930. [CrossRef]
- Zadworny, M., Górska, A., Politycka, B. Arbuscular mycorrhizal fungi alter enzymatic activities in phosphorus-transforming soils. Mycorrhiza, 2015, 25(4), 243-251.
- Chourasiya, D.; Gupta, M.M.; Sahni, S.; Oehl, F.; Agnihotri, R.; Buade, R.; Maheshwari, H.S.; Prakash, A.; Sharma, M. P. Unraveling the AM fungal community for understanding its ecosystem resilience to changed climate in agroecosystems. Symbiosis 2021, 84(3), 295-310. [CrossRef]
- Stahl, P.D.; Christensen, M. Population variation in the mycorrhizal fungus Glomus mosseae: Breadth of environmental tolerance. Mycological Research 1991, 95(3), 300-307. [CrossRef]
- Mahmoudi, N.; Cruz, C.; Mahdhi, M.; Mars, M.; Caeiro, M.F. Arbuscular mycorrhizal fungi in soil, roots and rhizosphere of Medicago truncatula: Diversity and heterogeneity under semi-arid conditions. PeerJ 2019, 7, e6401. [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25(1), 13-24. [CrossRef]
- Veresoglou, S.D.; Chen, B.; Rillig, M.C. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biology and Biochemistry 2012, 46, 53-62. [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Frontiers in Microbiology 2016, 6, 1559. [CrossRef]
- Moreira-Souza, M.; Trufem, S. F. B.; Gomes-Da-Costa, S. M.; Cardoso, E. J. B. N. Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza 2003, 13(4), 211-5. [CrossRef]
- Lozano, Y. M.; Aguilar-Trigueros, C.A.; Roy, J.; Rillig, M.C. Drought induces shifts in soil fungal communities that can be linked to root traits across 24 plant species. New Phytologist 2021, 232, 1917-1929. [CrossRef]
- Kuyper, T.W.; Jansa J. Arbuscular mycorrhiza: Advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses. Plant and Soil 2023, 489(1), 41-88. [CrossRef]
- Safari, M.M.R.; Farokhzad, M.; Kaviani, B.; Kulus, D. Endophytic Fungi as Potential Biocontrol Agents against Sclerotium rolfsii Sacc. - The Causal Agent of Peanut White Stem Rot Disease. Cells 2022, 11(17), 2643. [CrossRef]
- Grenville-Briggs, L.J.; Avrova, A.O.; Bruce, C. R.; Williams, A.; Whisson, S.C.; Birch, P.R.J.; West, P.V. Elevated temperature and CO2 levels affect the interactions between potato and the root pathogen Phytophthora infestans. Global Change Biology 2005, 11(9), 1714–1722. [CrossRef]
- El-Abyad, M.S.; Attaby, H.; Abu-Taleb, A.M. Impact of salinity stress on the free amino acid pools of some phytopathogenic fungi. Microbiological research 1994, 149(3), 309-315. [CrossRef]
- Rossier, O.; Vorhölter, F. J. New Insights into the Extracellular Secretion of Xanthomonads. Trends in Microbiology 2019, 27(7), 605–614.
- Bohnert, H. J.; Jensen, R. G.; Strategies for engineering water-stress tolerance in plants. Trends in Biotechnology 1996, 14, 89-97. [CrossRef]
- Peng, Y.; Li, S. J.; Yan, J.; Tang, Y.; Cheng, J. P.; Gao, A. J.; Yao, X.; Ruan, J. J.; Xu, B. L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Frontiers in microbiology 2021, 12, 670135. [CrossRef]
- van der Wolf, J., De Boer, S.H. Phytopathogenic bacteria. In Principles of plant-microbe interactions: Microbes for Sustainable Agriculture. 2014. pp. 65-77. Cham: Springer International Publishing.
- Delgado-Baquerizo, M.; Guerra, C. A.; Cano-Díaz, C.; Egidi, E.; Wang, J. T.; Eisenhauer, N.; Singh, B.; Maestre, F. The proportion of soil-borne pathogens increases with warming at the global scale. Nature Climate Change 2020. 10, 550-554. https://www.nature.com/articles/s41558-020-0759-3.
- Wakelin, S. A.; Gomez-Gallego, M.; Jones, E.; Smaill, S.; Lear, G.; Lambie, S. Climate change induced drought impacts on plant diseases in New Zealand. Australasian Plant Pathology 2018, 47, 101-114. [CrossRef]
- Rai, A.; Irulappan, V.; Muthappa, S. K. Dry root rot of chickpea: A disease favored by drought. Plant Disease 2021. [CrossRef]
- Sharath Chandran, U.S.; Tarafdar, A.; Mahesha, H. S.; Sharma, M. Temperature and Soil Moisture Stress Modulate the Host Defense Response in Chickpea During Dry Root Rot Incidence. Frontiers in plant science 2021. 12, 653265. [CrossRef]
- Chilakala, A.R.; Mali, K. V.; Irulappan, V.; Patil, B. S.; Pandey, P.; Rangappa, K.; Ramegowda, V.; Kumar, M. N.; Puli, C.O.R.; Mohan-Raju, B.; Senthil-Kumar, M. Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes. Frontiers in plant science 2022, 13, 890551. [CrossRef]
- Batista, E.; Lopes, A.; Miranda, P.; Alves, A. Can species distribution models be used for risk assessment analyses of fungal plant pathogens? A case study with three Botryosphaeriaceae species. European Journal of Plant Pathology 2023, 165(1), 41-56. [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytologist 2014, 203: 1028-1035. [CrossRef]
- Al-Turki, A.; Murali, M.; Omar, A. F.; Rehan, M.; Sayyed, R.Z.; Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front Microbiol. 2023, 27(14), 1214845. [CrossRef]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water Conservation and Plant Survival Strategies of Rhizobacteria under Drought Stress. Agronomy 2020, 10, 1683. [CrossRef]
- Jajoo, A.; Mathur, S. Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiology and Molecular Biology of Plants 2021, 27(11), 2589-2603. [CrossRef]
- Newman, E.D.; Rowland, J.B.; Hammer, T.G. Frost, L.A.; Lumibao, C.Y.; Henning, J. A. Trade-Offs in Arbuscular Mycorrhizal Fungal Responses to Drought and Salinity Stress in Panicum amarum (United States Gulf Coast). Journal of Coastal Research 2024, 40(1), 51-63. [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiological Research 2021, 242, 126640. [CrossRef]
- Vaishnav, A., Kasotia, A., Choudhary, D.K. Role of Functional Bacterial Phylum Proteobacteria in Glycine max Growth Promotion Under Abiotic Stress: A Glimpse on Case Study. In Silico Approach for Sustainable Agriculture; Choudhary, D., Kumar, M., Prasad, R., Kumar, V.; Publisher: Springer, Singapore, 2018; pp. 17-49. [CrossRef]
- Nawaz, M.; Ishaq, S.; Ishaq, H.; Khan, N.; Iqbal, N.; Ali, S.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Salicylic Acid Improves Boron Toxicity Tolerance by Modulating the Physio-Biochemical Characteristics of Maize (Zea mays L.) at an Early Growth Stage. Agronomy 2020, 10, 2013. [CrossRef]
- Lin, Y.; Watts, D.B.; Kloepper, J.W.; Feng, Y.; Torbert, H.A. Influence of plant growth-promoting rhizobacteria on corn growth under drought stress. Communications in Soil Science and Plant Analysis 2020, 51(2), 250-264. [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Frontiers in Microbiology 2022, 13, 962427. [CrossRef]
- Admassie, M.; Woldehawariat, Y.; Alemu, T.; Gonzalez, E.; Jimenez, J.F. The role of plant growth-promoting bacteria in alleviating drought stress on pepper plants. Agricultural Water Management 2022, 272, 107831. [CrossRef]
- Kohler, J.; Hernández, J. A.; Caravaca, F.; Roldán, A.; Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol. 2008, 35(2), 141-151. [CrossRef]
- Amini, R.; Zafarani-Moattar, P.; Shakiba, M.R.; Hasanfard, A. Inoculating moldavian balm (Dracocephalum moldavica L.) with mycorrhizal fungi and bacteria may mitigate the adverse effects of water stress. Scientific Reports 2023, 13(1), 16176. [CrossRef]
- Mondani, F.; Khani, K.; Honarmand, S.J.; Saeidi, M. Evaluating effects of plant growth-promoting rhizobacteria on the radiation use efficiency and yield of soybean (Glycine max) under water deficit stress condition. Agricultural water management 2019, 213, 707-713. [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14(9), e0222302. [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M. F.; Erinle, K.O.; Iqbal, R.; Ahmad, S. Effect of rhizobacteria and cytokinins application on wheat growth and yield under normal vs drought conditions. Communications in Soil Science and Plant Analysis 2019, 50(20), 2521-2533. [CrossRef]
- Muhammad, F.; Raza, M. A. S;. Iqbal, R.; Zulfiqar, F.; Aslam, M.U.; Yong, J.W.H.; Altaf, M.A.; Zulfiqar, F.; Amin, J.; Ibrahim, M. A. Ameliorating drought effects in wheat using an exclusive or co-applied rhizobacteria and ZnO nanoparticles. Biology 2022, 11(11), 1564. [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L. M. P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015, 38(4), 401-19. [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D. W.; Kannepalli, A.; Kumar, S.; Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9(11), 1596. [CrossRef]
- Zandi, P.; Schnug, E.; Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11(2), 155. [CrossRef]
- Tiepo, A. N.; Hertel, M. F.; Rocha, S. S.; Calzavara, A. K.; Oliveira, A. L. M. D.; Pimenta, J. A.; Oliveira, H. C.; Bianchini, E.; Stolf-Moreira, R.; Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol Biochem 2018, 130, 277-288. [CrossRef]
- Siddikee, M. A.; Glick, B. R.; Chauhan, P. S.; jong Yim, W.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol Biochem 2011, 49(4), 427-434. [CrossRef]
- Carezzano, M.E.; Alvarez Strazzi, F.B.; Pérez, V.; Bogino, P.; Giordano, W. Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress. Appl. Microbiol. 2023, 3, 1249-1261. [CrossRef]
- Bhargavi, G.; Arya, M.; Jambhulkar, P.P.; Singh, A.; Rout, A. K.; Behera, B.K.; Chaturvedi, S.K.; Singh, A.K. Evaluation of biocontrol efficacy of rhizosphere dwelling bacteria for management of Fusarium wilt and Botrytis gray mold of chickpea. BMC Genom Data 2024, 25(1), 7. [CrossRef]
- Huang, T.; Zhang, Y.; Yu Z.; Zhuang, W.; Zeng, Z. Bacillus velezensis BV01 Has Broad-Spectrum Biocontrol Potential and the Ability to Promote Plant Growth. Microorganisms 2023, 11(11), 2627. [CrossRef]
- Xie, J.; Singh, P.; Qi, Y.; Singh, R.K.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. Pseudomonas aeruginosa Strain 91: A Multifaceted Biocontrol Agent against Banana Fusarium Wilt. Fungi 2023, 9(11), 1047. [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N., Baranov, O.; Belbahri, L. Plant growth-promoting rhizobacteria (PGPR): A rampart against the adverse effects of drought stress. Water 2023, 15(3), 418. [CrossRef]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agriculture, Ecosystems & Environment, 2016, 231, 68-78. [CrossRef]
- Etesami, H.; Maheshwari, D. K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol Environ Saf 2018, 30(156), 225-246. [CrossRef]
- Vu, B.; Chen, M.; Crawford, R. J.; Ivanova, E. P.; Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14(7), 2535-54. [CrossRef]
- Sato, Y.; Miwa, T.; Inaba, T.; Akachi, T.; Tanaka, E.; Hori, T.; Murofushi, K.; Takagi, H.; Futamata, H.; Aoyagi, T.; Habe, H. Microbially produced fertilizer provides rhizobacteria to hydroponic tomato roots by forming beneficial biofilms. Appl Microbiol Biotechnol. 2023, 107(23), 7365-7374. [CrossRef]
- Fadiji, A.E.; Orozco-Mosqueda, M.d.C.; Santos-Villalobos, S.d.l.; Santoyo, G.; Babalola, O.O. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants 2022, 11, 3090. [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M. D.; Kitouni, M.; Barik, D.P.; Panneerselvam, P.; Mohapatra, P.K.D. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. Journal of Plant Nutrition 2021, 44(13), 1993-2028. [CrossRef]
- Liu, R.C.; Ding, Y.E.; Wu, Q.S.; Zou, Y.N. Mycorrhizae enhance drought tolerance of trifoliate orange by regulating circadian clock response patterns. Scientia Horticulturae 2022, 305, 111426. [CrossRef]
- Dowarah, B.; Gill, S. S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. Journal of Plant Growth Regulation 2022, 41(4), 1429-1444. [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. Journal of plant biology 2016, 59, 407-426. [CrossRef]
- Wang, Y.; Zou, Y.N.; Shu, B.; Wu, Q.S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Scientia Horticulturae 2022, 308, 111591. [CrossRef]
- Zou, Y.; Wu, Q.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biology 2021, 23, 50-57. [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agricultural Water Management 2013, 117, 106-114. [CrossRef]
- Zhang, X.; Yan, J.; Khashi u Rahman, M.; Wu, F. The impact of root exudates, volatile organic compounds, and common mycorrhizal networks on root system architecture in root-root interactions. Journal of Plant Interactions 2022, 17(1), 685-694. [CrossRef]
- Cheng, H.Q.; Zou, Y. N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Frontiers in Plant Science 2021, 12, 659694. [CrossRef]
- Zou, Y.; Qin, Q.; Ma, W.; Zhou, L.; Wu, Q.; Xu, Y.; Kuča, K.; Hashem, A.; Al-Arjani, A. F.; Almutairi, K. F.; Abd-Allah, E. F. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23(1), 118. [CrossRef]
- Sheteiwy, M.S.; Elgawad, H.A.; Xiong, Y.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamoud, Y. A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol Plant. 2021, 172(4), 2153-2169. [CrossRef]
- Sheteiwy, M.S.; Ali, D. F. I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; Sharma, A.; El-Sawah, A. M. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC plant biology 2021, 21(1), 195. [CrossRef]
- Xu, L.; Li, T.; Wu, Z.; Feng, H.; Yu, M.; Zhang, X.; Chen, B. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Applied soil ecology 2018, 125, 213-221. [CrossRef]
- Drogue, B.; Doré, H.; Borland, S.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Research in microbiology 2012, 163(8), 500–510. [CrossRef]
- Matilla, M. A.; Ramos, J. L. Biosurfactant produced by a Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Applied and Environmental Microbiology 2007, 73(4), 1423–1429. [CrossRef]
- Newman, K. L.; Almeida, R. P. P.; Purcell, A. H.; Lindow, S. E. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc Natl Acad Sci USA 2004, 101(6), 1737-42. [CrossRef]
- Drogue, B.; Sanguin, H.; Chamam, A.; Mozar, M.; Llauro, C.; Panaud, O.; Prigent-Combaret, C.; Picault, N.; Wisniewski-Dyé, F. Plant root transcriptome profiling reveals a strain-dependent response during Azospirillum–rice cooperation. Frontiers in Plant Science 2014, 5, 607. [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol 2009, 10(3), 311-24. [CrossRef]
- Elad, Y.; Yunis, H.; Katan, J. Multiple resistance mechanisms to benzimidazole fungicides in Botrytis cinerea field isolates. Phytopathology 2007, 97(6), 686–695. [CrossRef]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 2010, 34(2), 107-33. [CrossRef]
- Shoresh, M.; Harman, G. E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 2010, 48, 21-43. [CrossRef]
- Flemming, H.C.; Wingender, J.; The biofilm matrix. Nature Reviews Microbiology 2010, 8(9), 623–633. [CrossRef]
- de Pontes, J.G.M.; Fernandes, L.S.; Santos, R.V.D.; Tasic, L.; Fill, T.P. Virulence Factors in the Phytopathogen-Host Interactions: An Overview J Agric Food Chem. 2020, 68(29), 7555-7570. [CrossRef]
- Garbelotto, M. Host and environmental feed-backs on invasive plant pathogens: Ecological and evolutionary consequences on novel plant pathogen interactions. Journal of Plant Pathology 2012, 94(4), S41-S84.
- Yuen, J., Collinge, D.B., Djurle, A. and Tronsmo, A.M. Plant Pathology in a Changing World 23. In Plant Pathology and Plant Diseases; Tronsmo, A.M.; Collinge, D.B.; Djurle, A.; Munk, L.; Yuen, J.; Tronsmo A., 2020, pp. 379-386. [CrossRef]
- Yao, S.; Hao, L.; Zhou, R.; Jin, Y.; Huang, J.; Wu, C. Multispecies biofilms in fermentation: Biofilm for-mation, microbial interactions, and communication. Comprehensive reviews in food science and food safety 2022, 21(4), 3346-3375. [CrossRef]
- Plett, J.M.; Martin, F.M. Know your enemy, embrace your friend: Using omics to understand how plants respond differently to pathogenic and mutualistic microorganisms. The Plant Journal 2018, 93(4), 729-746. [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annual Review of Phytopathology 2017, 55(1), 61-83. [CrossRef]
- Saini, A.; Mani, I.; Rawal, M.K.; Verma, C.; Singh, V.; Mishra, S.K. An introduction to microbial genomic islands for evolutionary adaptation and pathogenicity. In Microbial Genomic Islands in Adaptation and Pathogenicity; Mani, I., Singh, V., Alzahrani, K.J., Chu, D.T., Eds.; Springer Nature, Singapore, 2023; pp. 1-15. [CrossRef]
- Bailey-Serres, J.; Parker, J. E.; Ainsworth, E. A.; Oldroyd, G. E.; Schroeder, J. I. Genetic strategies for improving crop yields. Nature 2019, 575(7781), 109-118. [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Frontiers in microbiology 2021, 12, 743512. [CrossRef]
- Keeling, P.J; Palmer, J.D.; Horizontal gene transfer in eukaryotic evolution. Nature Reviews Genetics 2008 9, 605–618. [CrossRef]
- Kloesges, T.; Popa, O.; Martin, W.; Dagan, T. Networks of gene sharing among 329 proteobacterial genomes reveal differences in lateral gene transfer frequency at different phylogenetic depths. Mol Biol Evol 2011, 28(2), 1057-1074. [CrossRef]
- McDonald, M.C.; Taranto, A.P.; Hill, E.; Schwessinger, B.; Liu, Z.; Simpfendorfer, S.; Milgate, A.; Solomon, P. S.; Transposon-Mediated Horizontal Transfer of the Host-Specific Virulence Protein ToxA between Three Fungal Wheat Pathogens. mBio 2019, 10(5), e01515-19. [CrossRef]
- Wintersdorff, C.J. H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P. H.M.; Wolffs P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol 2016, 19(7),173. [CrossRef]
- Walton, J.D. Horizontal gene transfer and the evolution of secondary metabolite gene clusters in fungi: Anhypothesis. Fungal Genet. Biol. 2000, 30(3), 167–171. [CrossRef]
- Friesen, T.; Stukenbrock, E.; Liu, Z.; Meinhardt, S.; Ling, H.; Faris, J. D.; Rasmussen J. B.; Solomon, P. S.; McDonald, B. A.; Oliver, R. P. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet 2006, 38(8), 953-6. [CrossRef]
- Kobayashi, N.; Dang, T.A.; Pham, K.T.M.; Gómez Luciano, L.B.; Van, V.B.; Izumitsu, K.; Shimizu, M.; Ikeda, K.I.; Li, W.H.; Nakayashiki H. Horizontally Transferred DNA in the Genome of the Fungus Pyricularia oryzae is Associated with Repressive Histone Modifications. Mol Biol Evol. 2023, 40(9), msad186. [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Aravind L. Horizontal gene transfer in prokaryotes: Quantification and classification. Ann Rev Microbiol 2001, 55, 709–742. [CrossRef]
- Fitzpatrick, D.A. Horizontal gene transfer in fungi. FEMS Microbiol Lett 2012, 329(1), 1–8. [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiological Research 2016, 184, 13–24. [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol 2023, 21(10), 640-656. [CrossRef]
- Chen, L.; Huang, X.; Zhang, F.; Zhao, D.). The effect of climate change on soilborne diseases: Trends, methods, and challenges. Phytopathology, 2020, 110(7), 1120-1131.
- Cubeta, M.A.; Thomas, E.; Dean, R.A. Neotyphodium/Epichloë species endophytes of grasses: Tapping into a rich source of biodiversity. In Advances in endophytic research; Publisher: Springer, New York, USA, 2014; pp. 205-227.
- Maheshwari, M.; Abulreesh, H.H.; Khan, M.S.; Ahmad, I.; Pichtel, J. Horizontal gene transfer in soil and the rhizosphere: Impact on ecological fitness of bacteria. In Agriculturally Important Microbes for Sustainable Agriculture: Volume I: Plant-Soil-Microbe Nexus; Meena, V.S., Mishra, P.K., Bisht, J.K., Pattanayak, A., Eds.; Springer: Singapore, 2017; pp. 111–130. ISBN 978-981-10-5589-8.
- Jang, H.; Gopinath, G.R.; Eshwar, A.; Srikumar, S.; Nguyen, S.; Gangiredla, J.; Patel, I.R.; Finkelstein, S.B.; Negrete, F.; Woo, J.; Lee, Y. The secretion of toxins and other exoproteins of Cronobacter: Role in virulence, adaption, and persistence. Microorganisms 2020, 8(2), 229. [CrossRef]
- Aminov, R.I. Horizontal gene exchange in environmental microbiota. Frontiers in microbiology 2011, 2, 158. [CrossRef]
- Mehrabi, R.; Bahkali, A.H.; Abd-Elsalam, K.A.; Moslem, M.; Ben M’Barek, S.; Gohari, A.M.; Jashni, M.K.; Stergiopoulos, I.; Kema, G.H.; de Wit, P.J. Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS microbiology reviews 2011, 35(3), 542-554. [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiological research 2021, 242, 126626. [CrossRef]
- Nielsen, K.M.; Van Elsas, J.D. Horizontal gene transfer and microevolution in soil. In Modern Soil Microbiology, 3rd Ed., CRC Press, 2019, pp. 105-123.
- Zibo, L.; Yuan, T.; Zhou, L.; Sen, C.; Qu, X.; Lu, P.; Qiyan, F. Impact factors of the accumulation, migration and spread of antibiotic resistance in the environment. Environmental Geochemistry and Health 2021, 43(5), 1741-1758. [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; Lemanceau, P. Soil bacterial networks are less stable under drought than fungal networks. Nature communications 2018, 9(1), 3033. [CrossRef]
- Pritchard, S.G. Soil organisms and global climate change. Plant Pathology 2011, 60(1), 82-99. [CrossRef]
- Ruiz-Gómez, F.J.; Pérez-de-Luque, A.; Navarro-Cerrillo, R.M. The involvement of Phytophthora root rot and drought stress in holm oak decline: From ecophysiology to microbiome influence. Current Forestry Reports, 2019, 5(4), 251-266. [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.M.; Nayak S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS microbiology reviews 2016, 40(2), 182-207. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




