2.1. Results
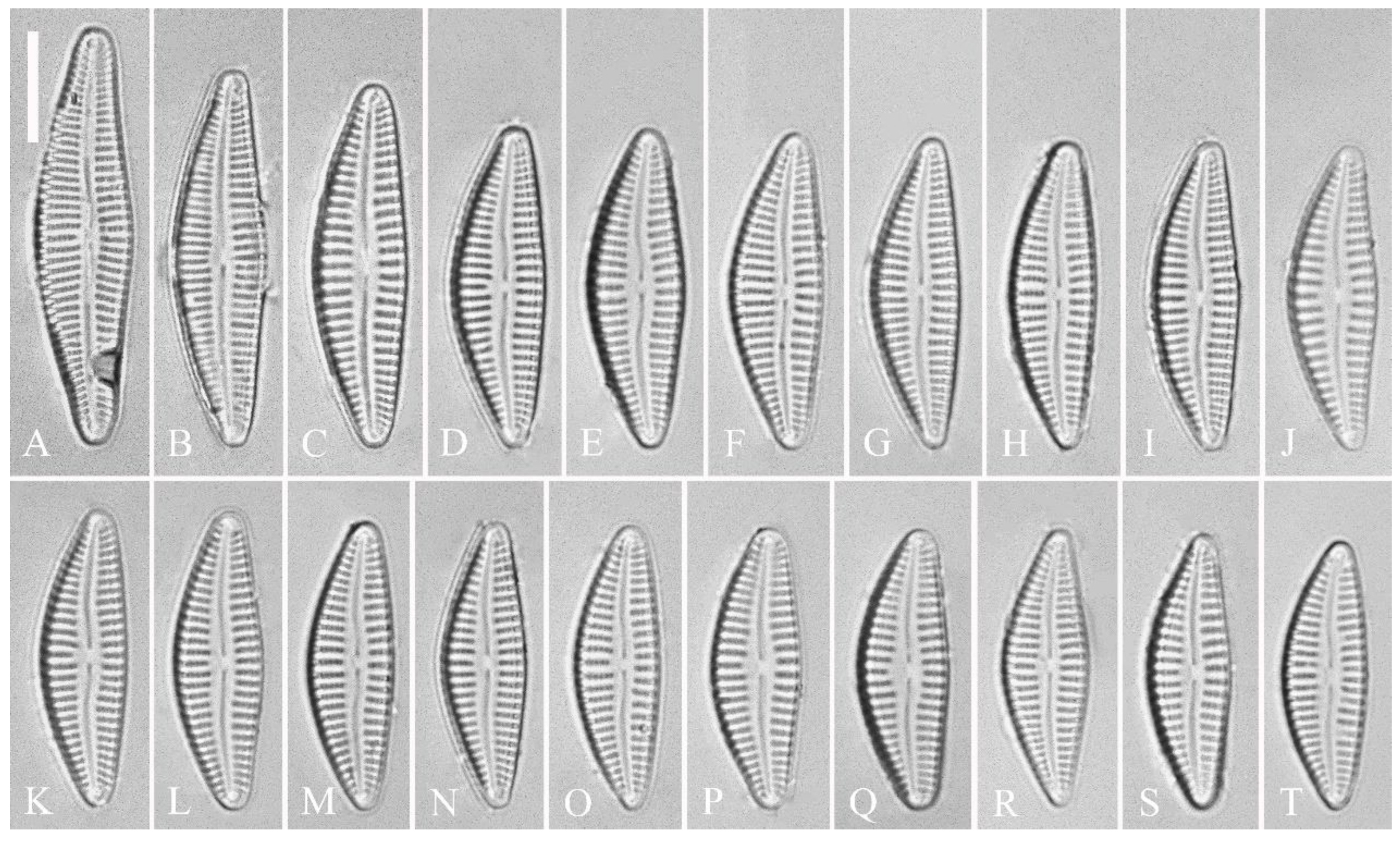
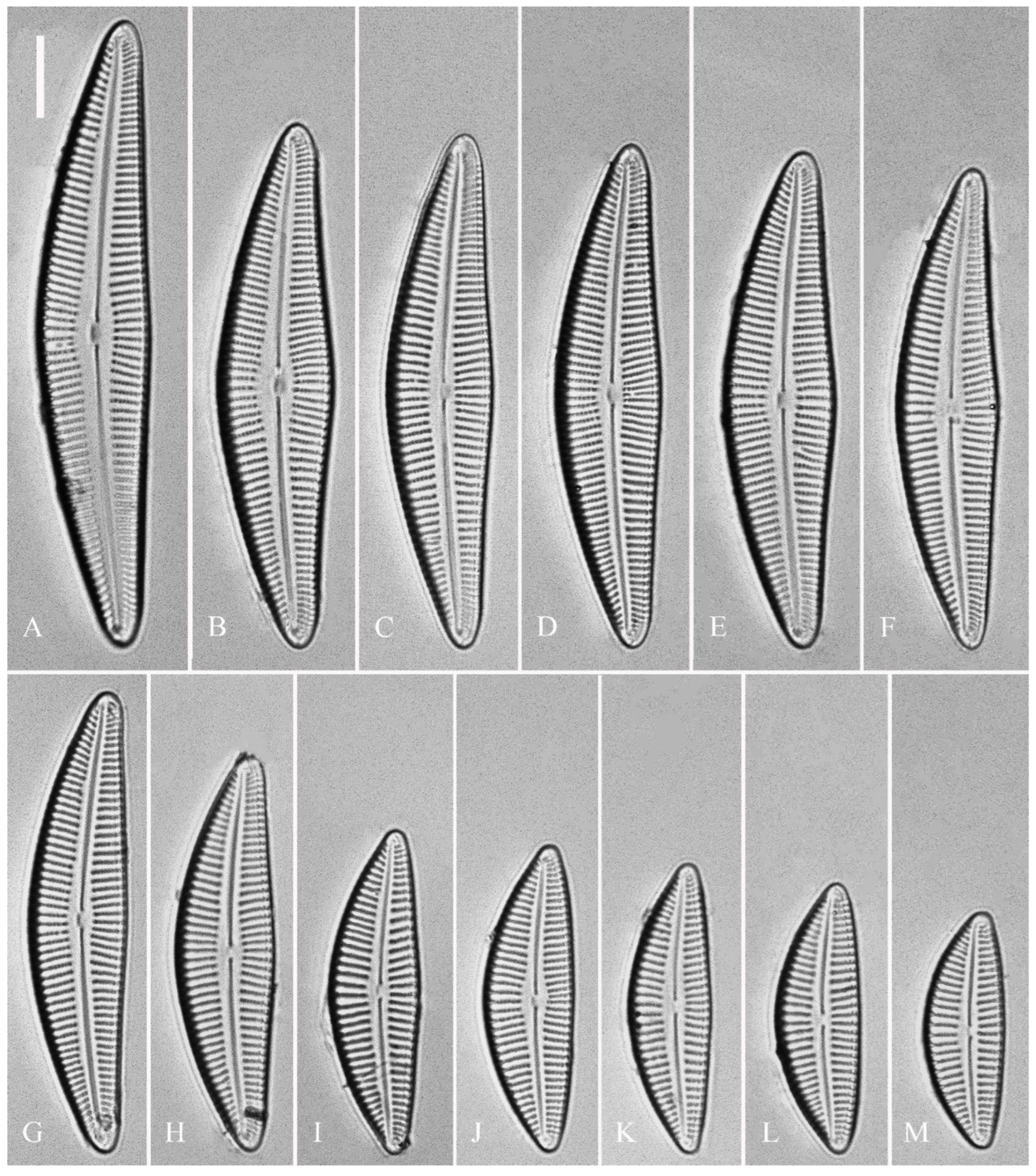
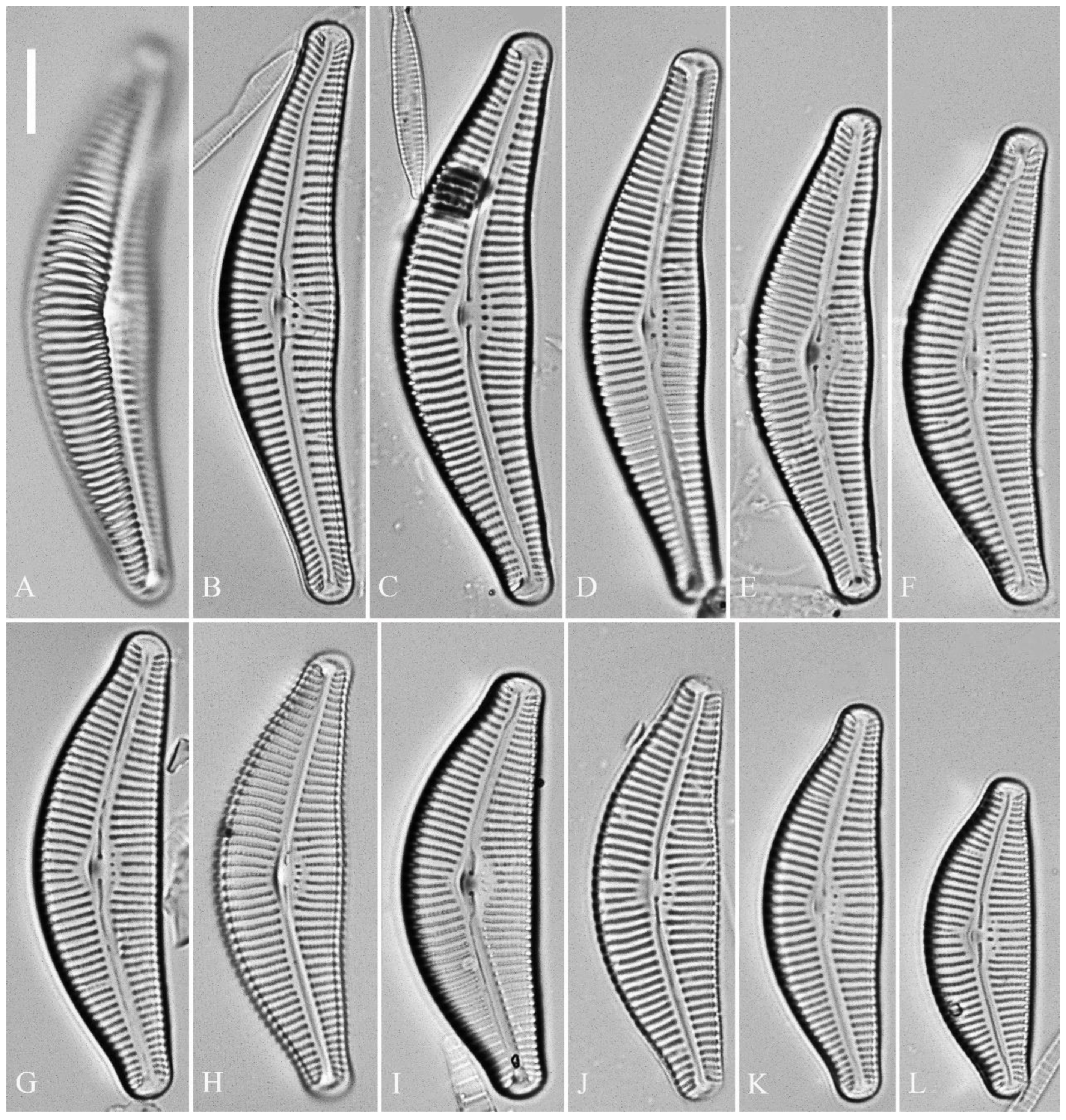
Description. LM (
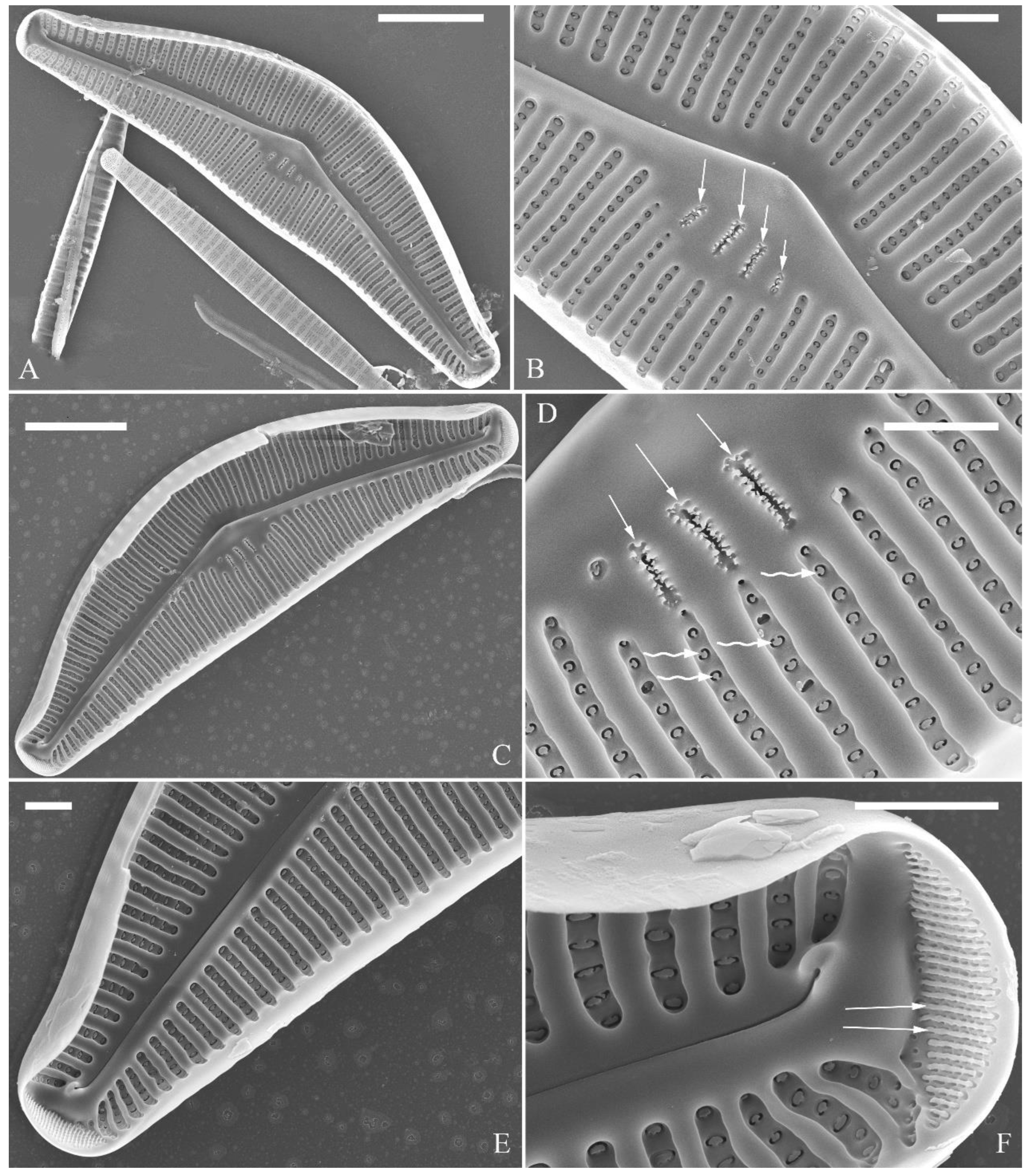
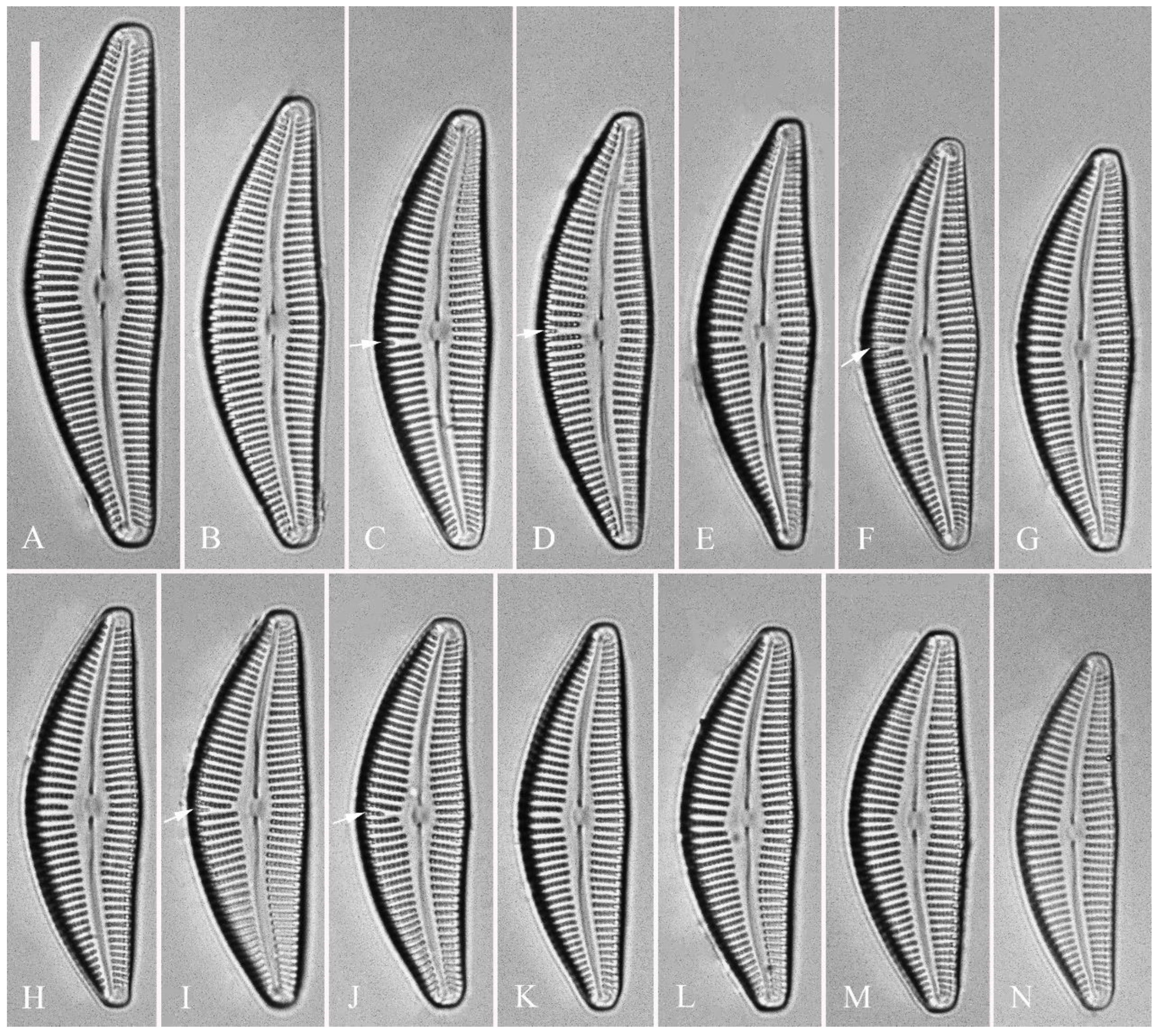
Figure 1). Living cells in valve view have same outlines as valves (
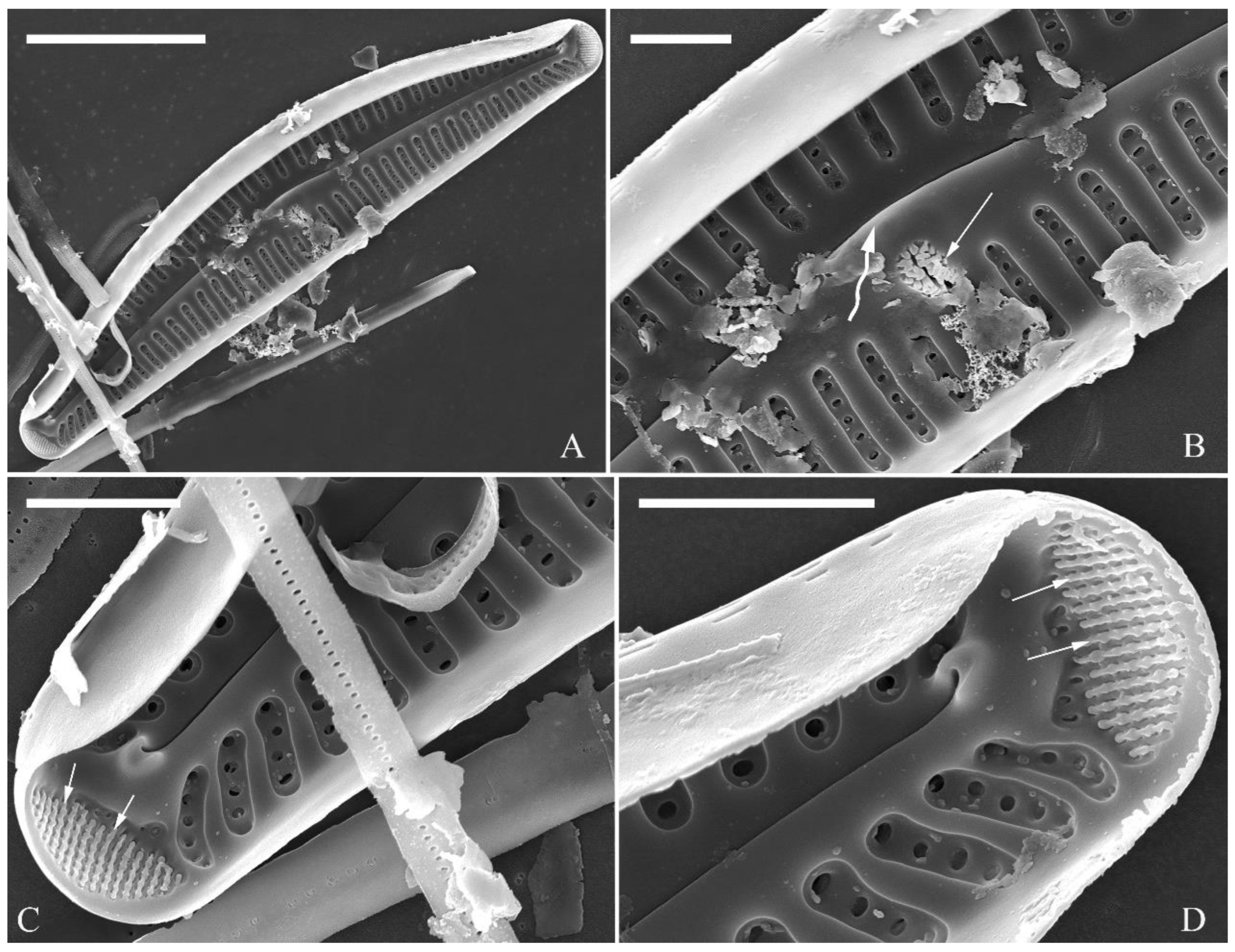
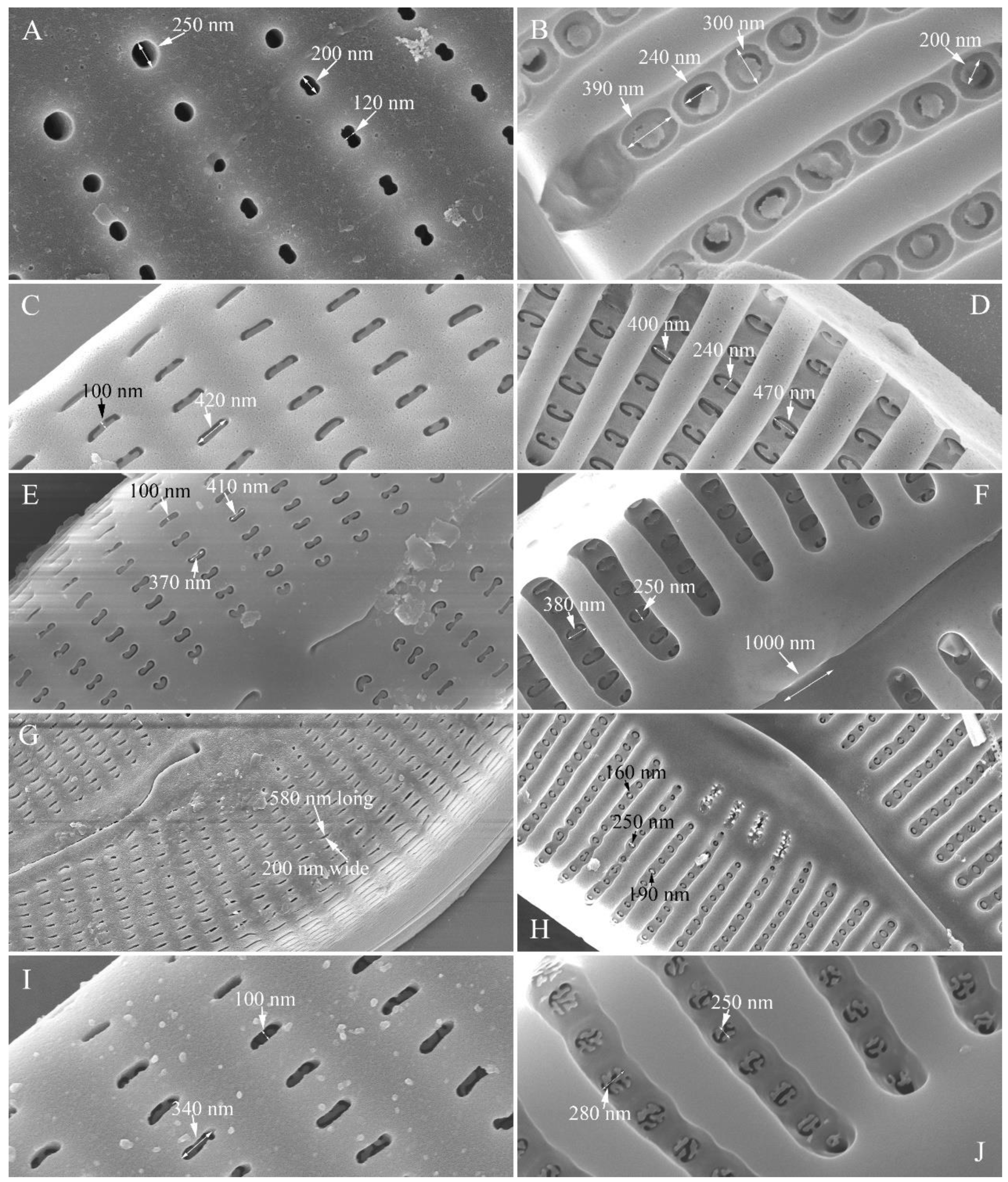
Figure 1A, B). Valves moderately dorsiventral with convex dorsal margin and slightly tumid ventral margin. Valve apices apiculate. Valve dimensions (n = 23): length 60–66 μm, width 15.5–18.5 μm, length/width ratio range 3.5–4.5. Axial area lanceolate. Central area trapezoid, more developed on dorsal side. Raphe slightly lateral, becoming filiform near distal and proximal ends. Central pores visible, bulbous. Striae radiate throughout valve surface. One shortened stria, widely spaced from each adjacent stria, less than half-length of adjacent stria, always present on dorsal middle part of valve (e.g.,
Figure 1C–F, arrows; see also
Figure 2A and
Figure 3A,B). Striae 10–12 in 10 μm in ventral middle part of valve. Areolae discernible, 18–24 in 10 μm. Stigmata four to six, present on ventral side of central nodule, very close to ends of corresponding ventral striae.
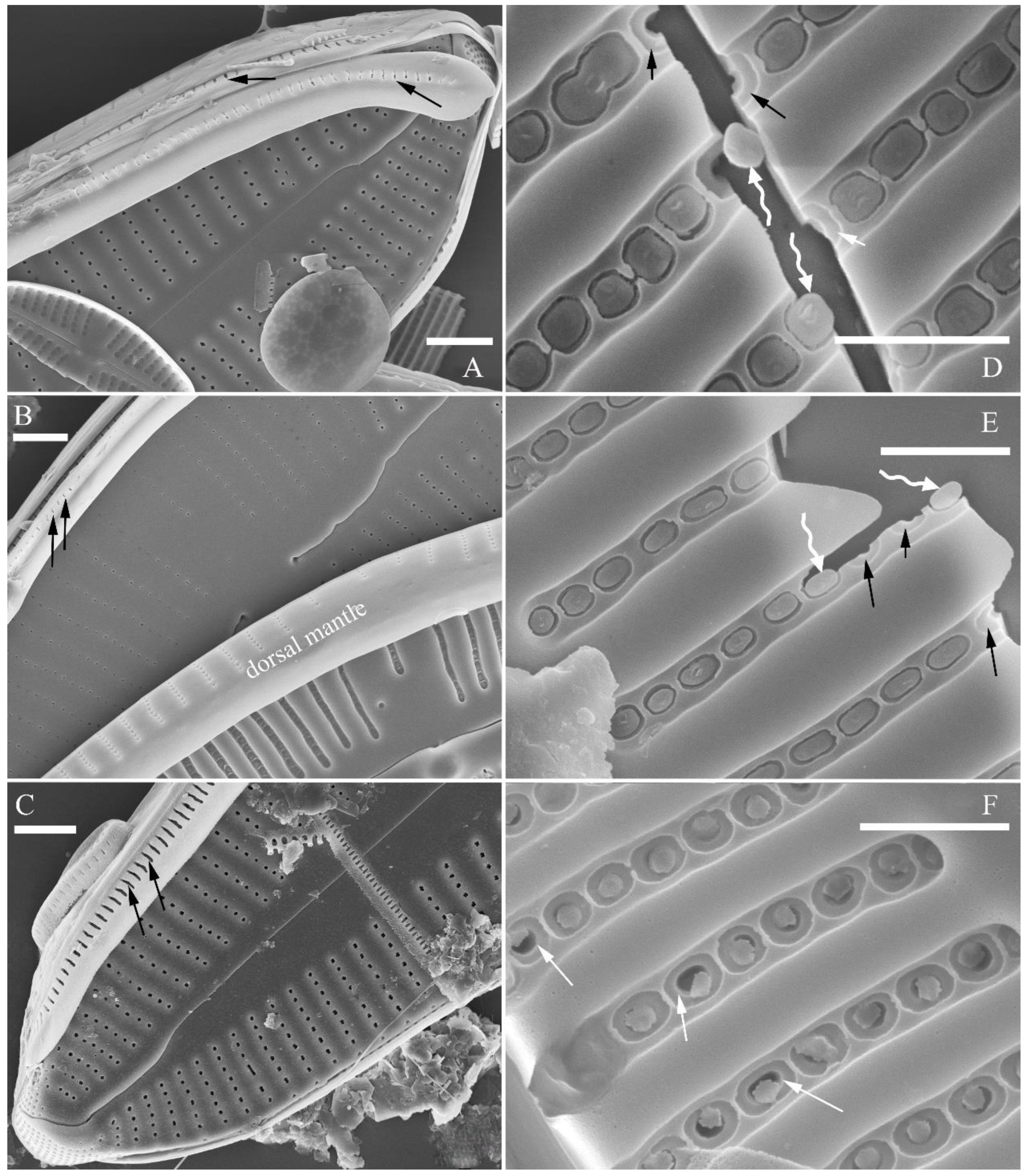
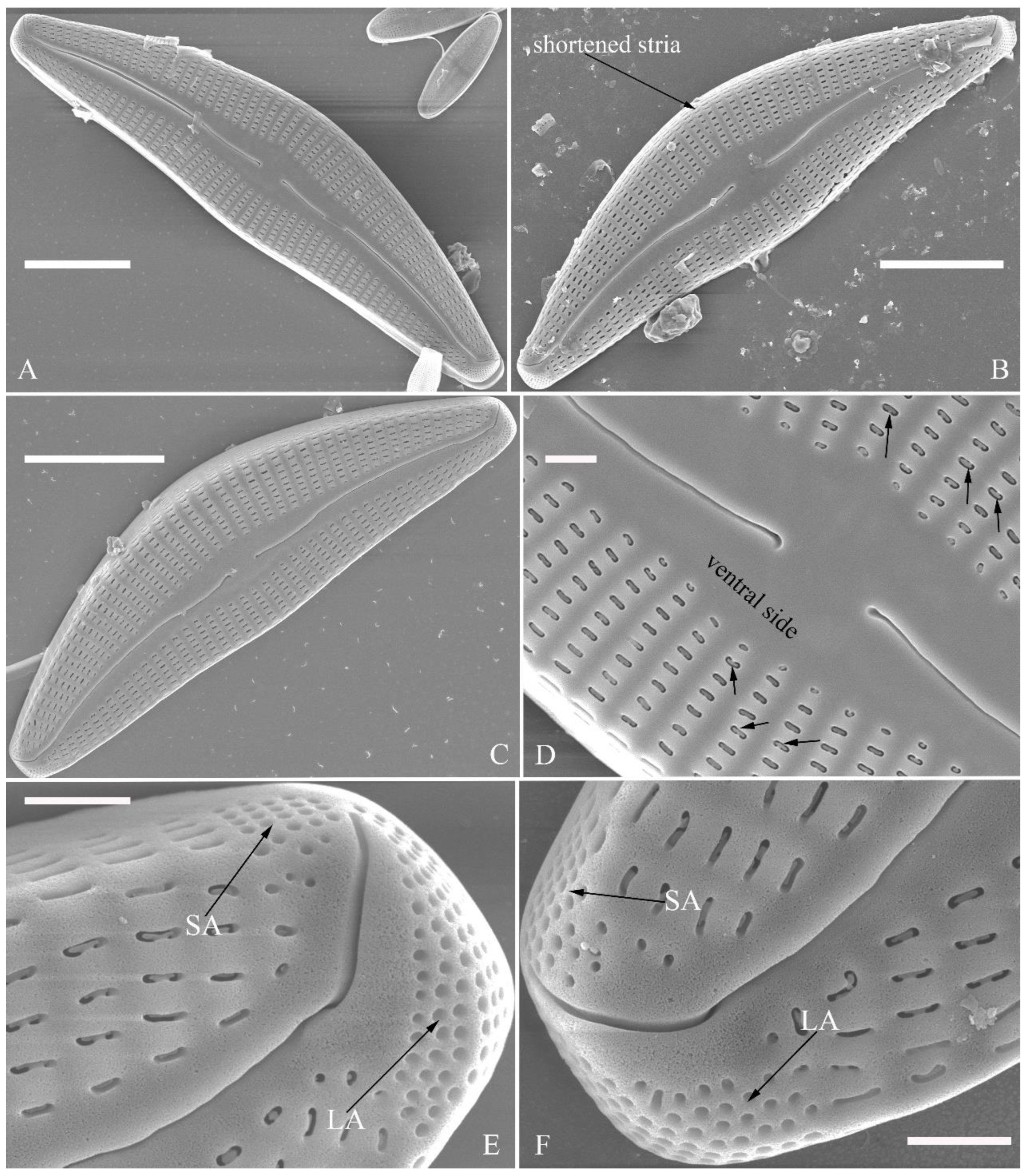
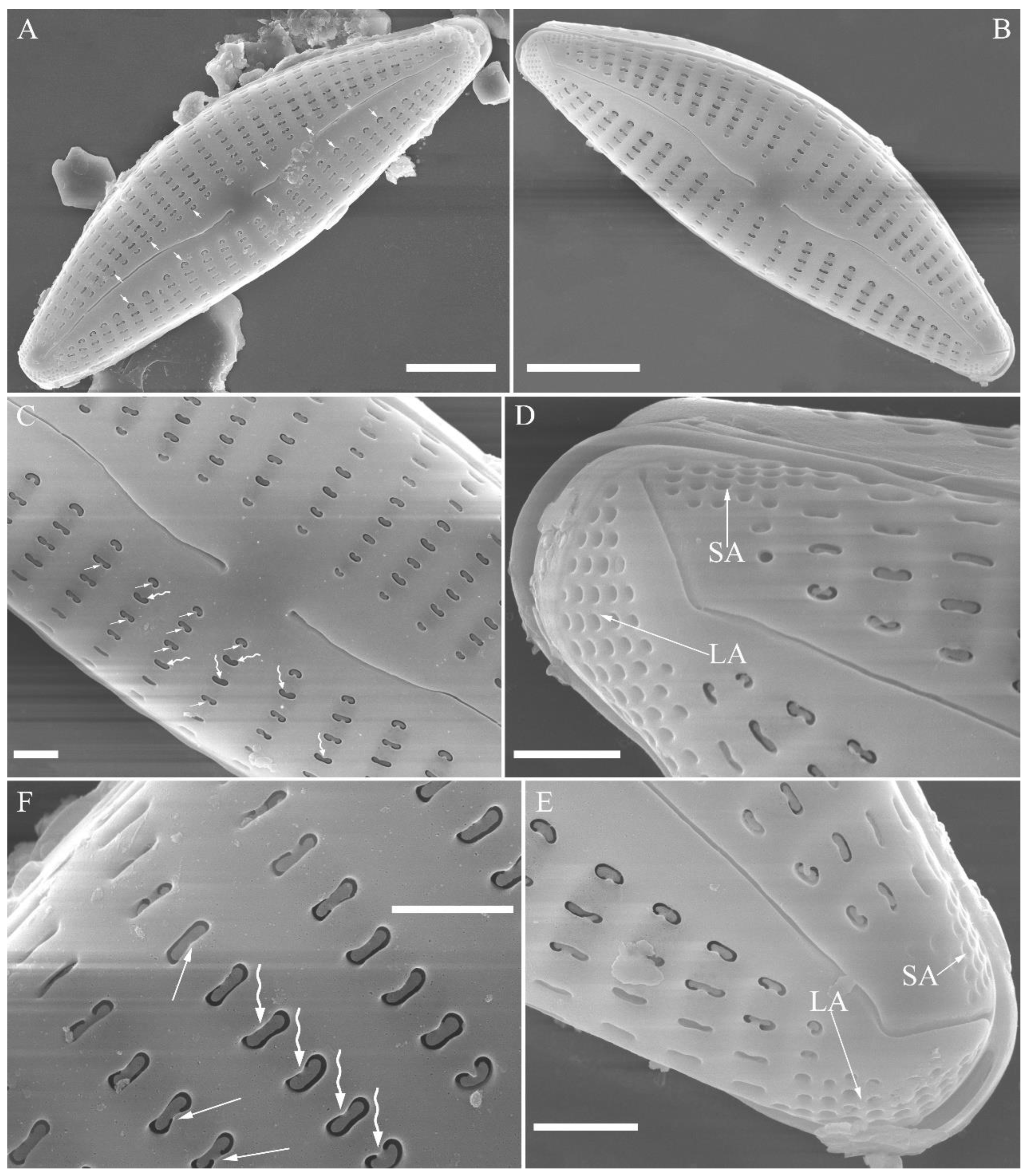
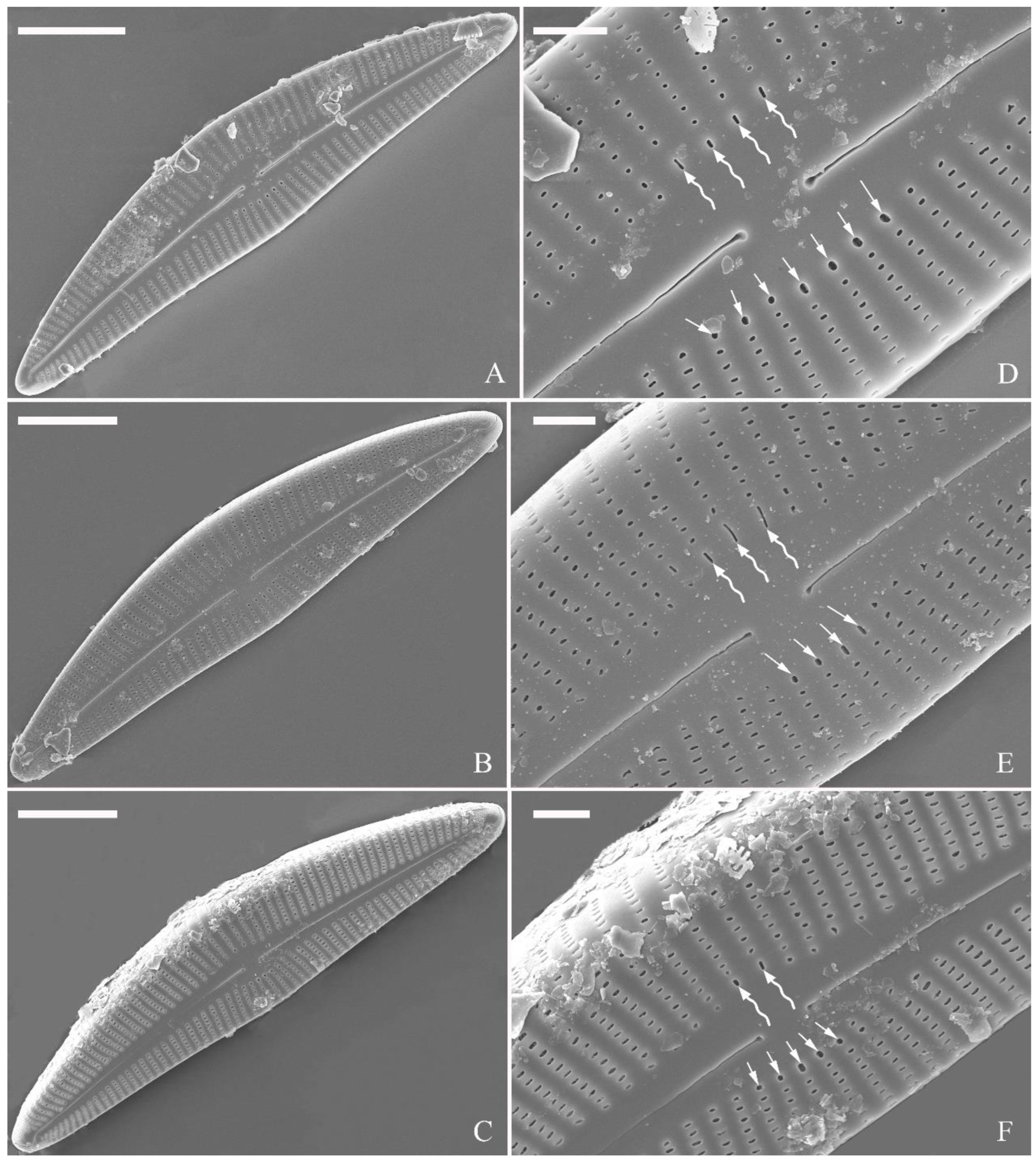
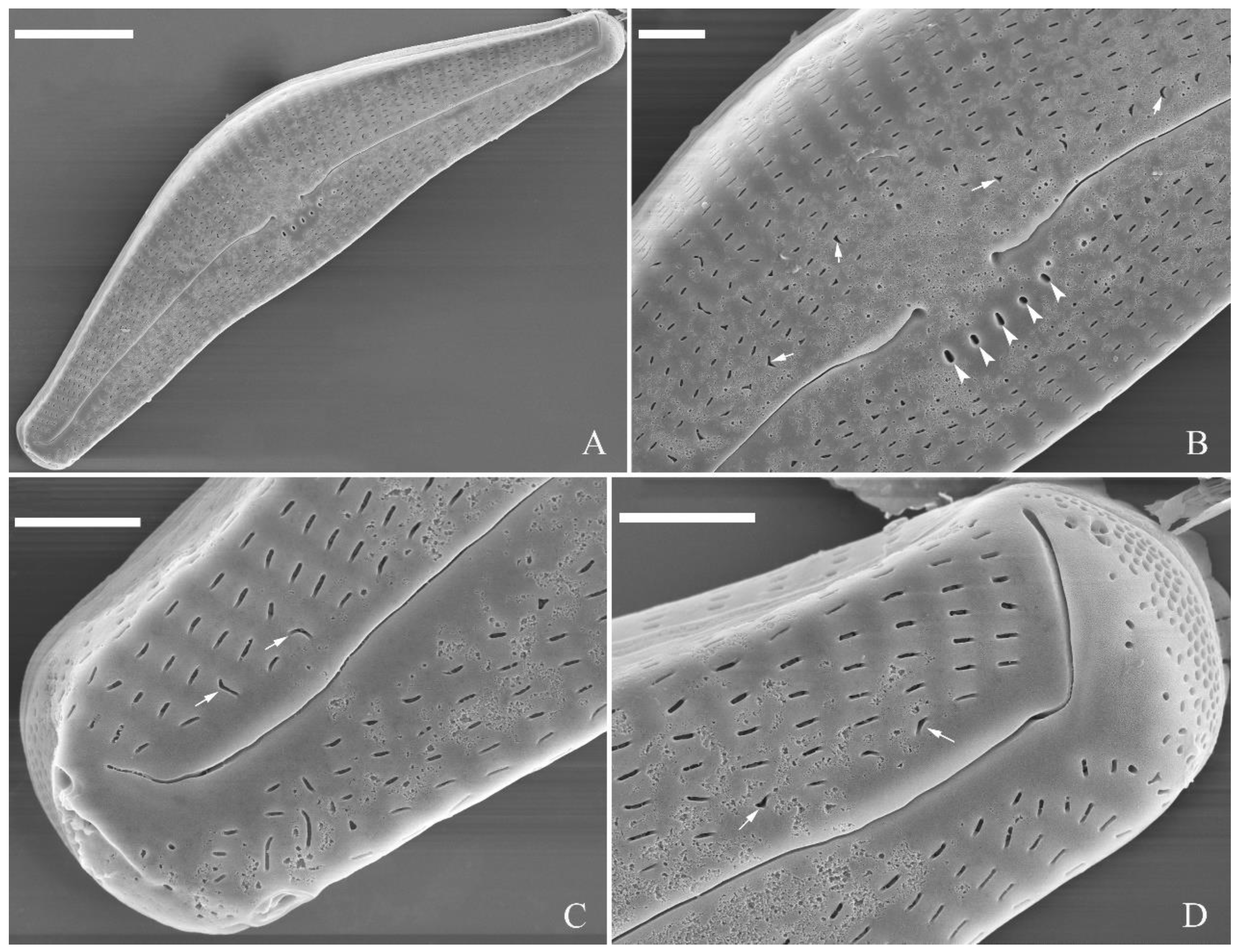
SEM, external view (
Figure 2 and
Figure 4A–C). Proximal raphe endings expanded (
Figure 2A, B, E, F), distal raphe fissures deflected towards dorsal side of valve and dividing each apical pore field into two unequal areas: a larger ventral one composed of ca. 14–18 pervalvar columns of rounded porelli (each column comprising ca. 2–8 porelli) and a smaller dorsal one composed of ca. 6–9 pervalvar columns of rounded porelli (each column comprising ca. 2–8 porelli) (
Figure 2C,D and
Figure 4CFour to six stigmata located on the ventral side of central nodule with rounded to oblong outer openings (
Figure 2B, E, F). Areola openings rounded near apex, similar to porelli of apical pore field (
Figure 2C, D) or dumbbell-like (
Figure 2B, E, arrows). Girdle bands open with a row of large, elongated pores located along midline of copula (
Figure 4A–C, two arrows respectively).
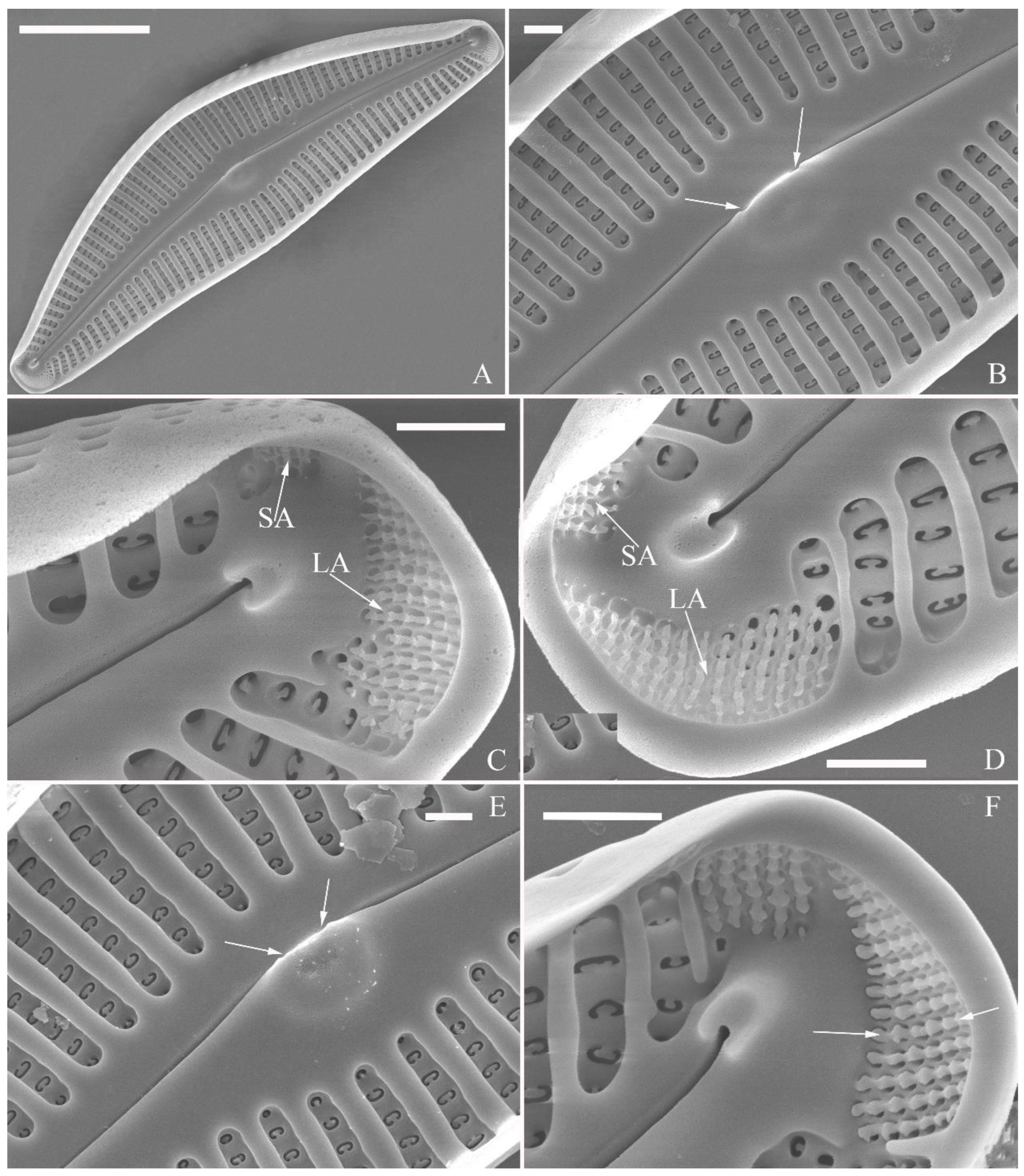
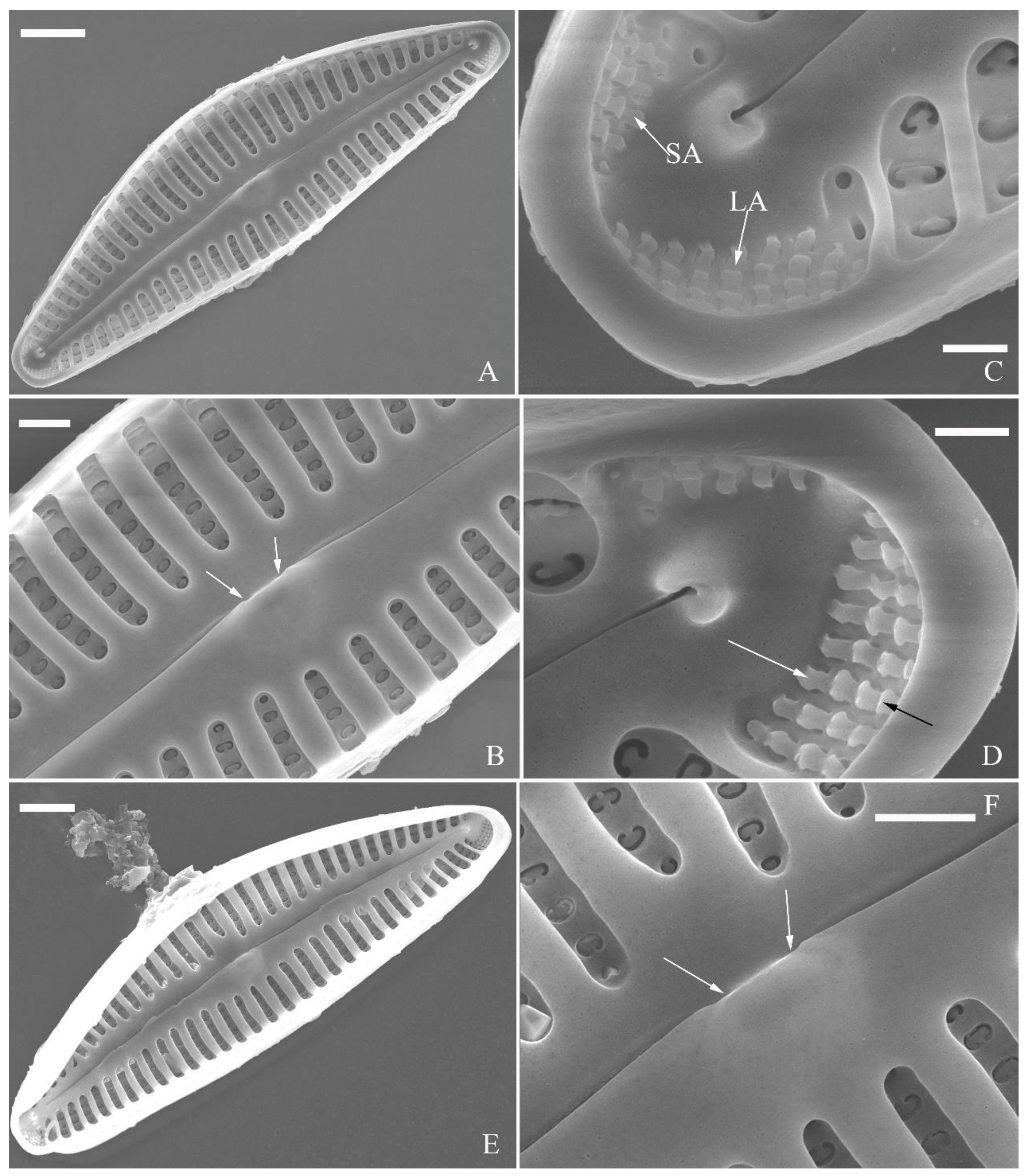
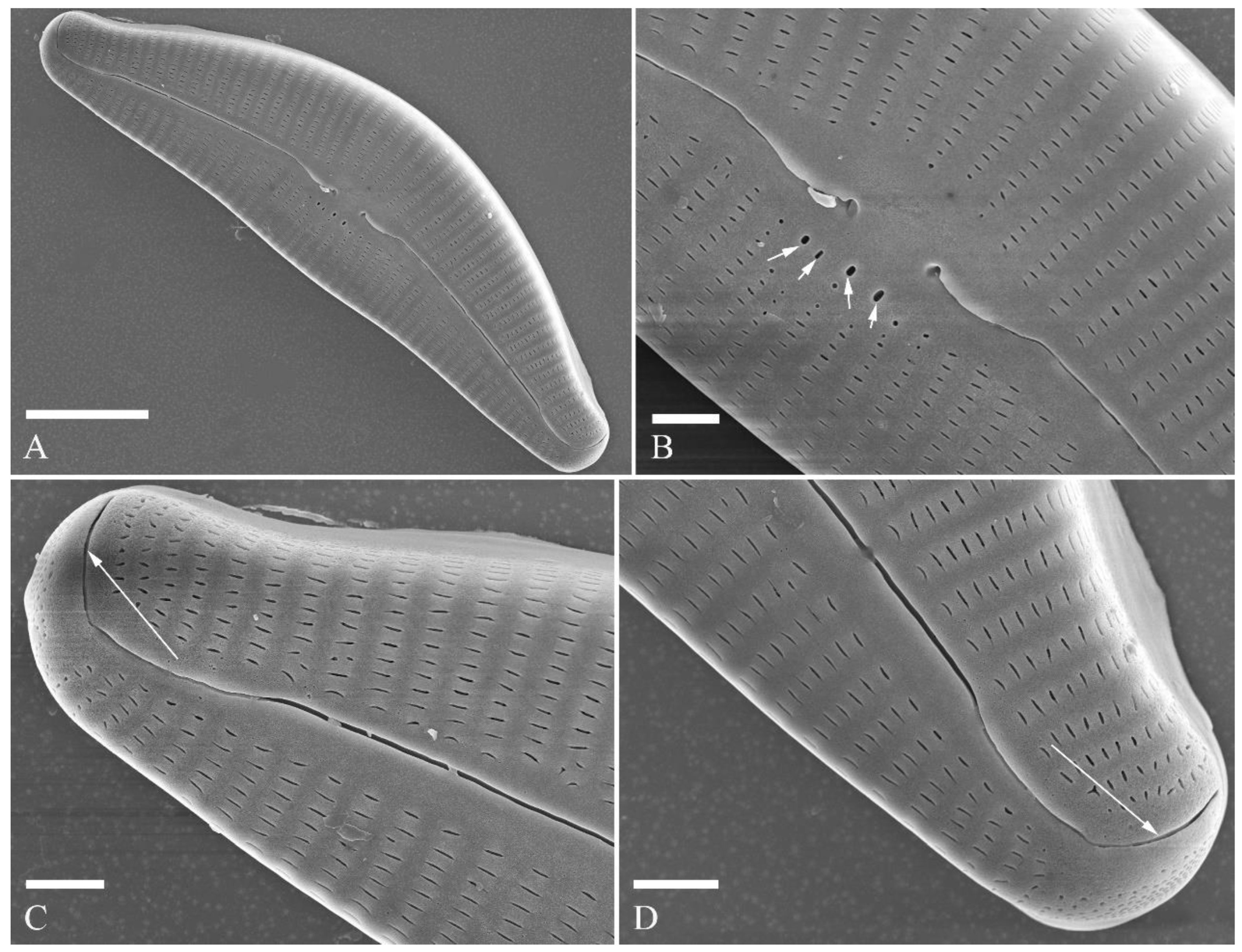
SEM, internal view (
Figure 3; 4D–F). Raphe straight, almost along valve midline, proximal raphe endings hidden, i.e., intermissio invisible due to being covered by siliceous hood (
Figure 3A–D), distal raphe fissures terminating in raised bilabiate helictoglossae (
Figure 3E, F). Four to six stigmata located on ventral side of central nodule with convoluted internal occlusions (
Figure 3C, D, arrowheads). Structure of areolar inner openings similar to manhole covers, i.e., areolar inner openings located in the middle of rounded depression that is completely covered by rounded to oblong solid silica plates (
Figure 4D–F). Apical pore fields composed of a larger ventral area and a smaller dorsal area (
Figure 3E). Porellus openings of apical pore fields covered by columns of silica strips composed of V-shaped plates (
Figure 3F, arrows).
Holotype designated here. Slide DIA2024004, specimen circled on the slide, illustrated here as
Figure 1I, deposited in the herbarium of Jishou University (JIU), China. Registration:
http://phycobank.org/(pending)
Type locality. China. Hunan Province, Shimen County, Huping Town, Xie River. A specific sampling location (29°57′6” N, 110°45′37” E, 230 m a.s.l.) in a riffle of the Xie River, collected by Bing Liu, 14 March, 2021.
Etymology. The epithet apiculatophora refers to the abrupt, short, pointed valve apices of this new species.
Ecology and distribution. The samples that included this species were scraped off the surface of stones collected in the Xie River. Hence, this is an epilithic species. The following environmental parameters were measured in the field with three replications: Conductivity = 236.3 ± 1.2 μS∙cm-1; pH = 8.49 ± 0.02; Water temperature = 13.6 ± 0.1 °C. Known only from the type locality so far.
Comments. Cymbella apiculatophora sp. nov. is characterized by its dorsiventral valve outline, one shortened stria located on the dorsal middle part of valve, large dorsal central area, and its apiculate apices. The most similar species to
C. apiculatophora is
C. neuquina Frenguelli and its variety
C. neuquina var.
fastigata (Krasske) Krammer, Maidana & Villanueva. All three taxa have similar valve outlines and apices, but
C. apiculatophora bears one distinctly shortened stria on the dorsal middle part whereas
C. neuquina and the variety
fastigata do not have this character (
Table 3). The morphometric data, such as stria and areola densities, are also noticeably different (see
Table 3). Four low resolution SEM images for
C. neuquina were provided in Maidana et al. [
18]. From their
Figure 23, we can see
C. neuquina having an APF composed of a complete area, thus differing from that composed of two unequal areas in
C. apiculatophora (
Table 3).
Cymbella orientalis and its variety
C. orientalis var.
delicatula Stancheva & Ivanov also have a large dorsal central area, but these two taxa differ from
C. apiculatophora by their weakly dorsiventral valve outline, narrowly rounded apices, and by lacking stigma (
Table 3). Interestingly, the apical pore fields of
C. apiculatophora and
C. orientalis are very similar in structure and their areola internal openings are completely covered by solid closing plates. For both species, the porelli have similar size and shape as the areolae, such that they could be classified as undifferentiated [
19], i.e., their APFs are not clearly physically separated and morphologically differentiated from the striae.
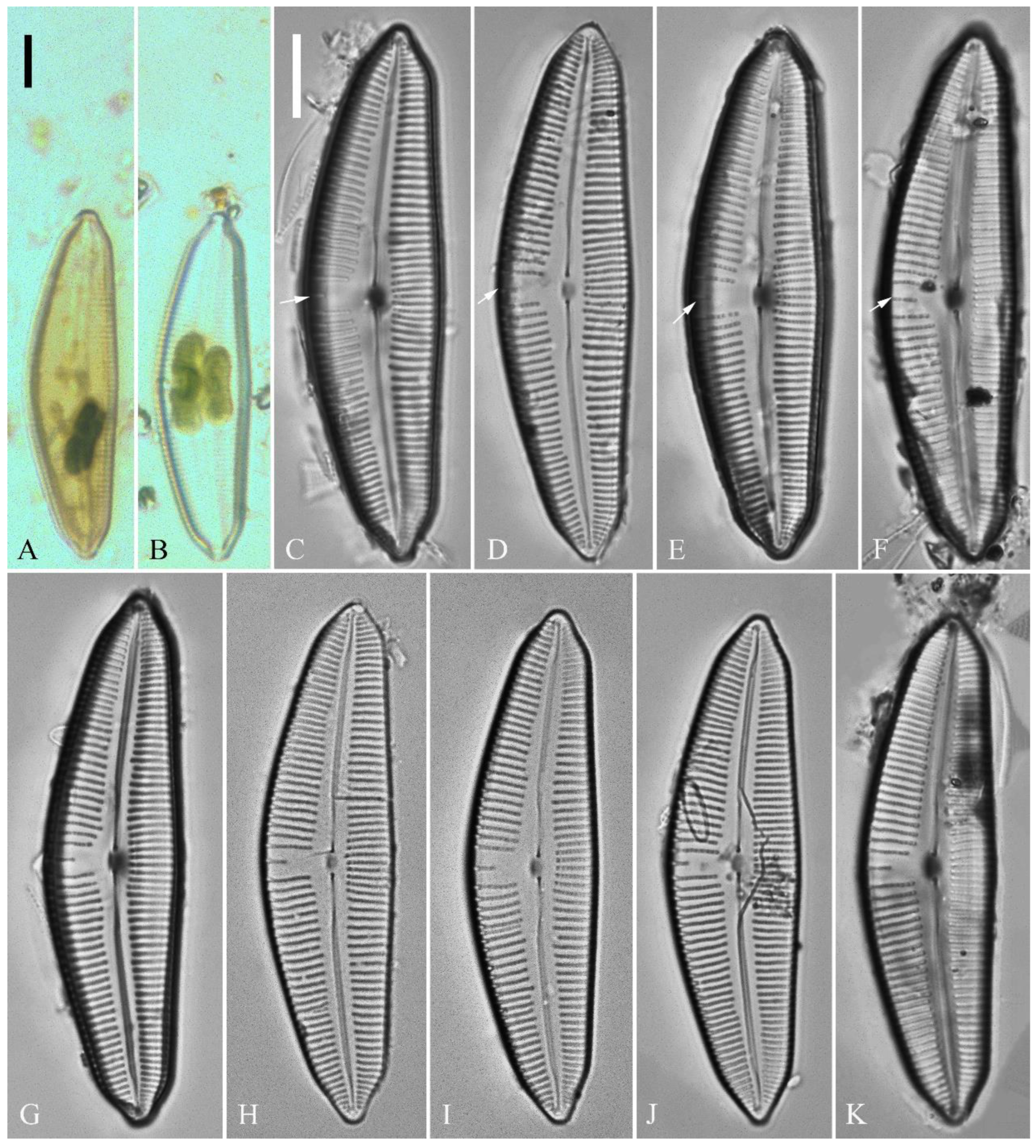
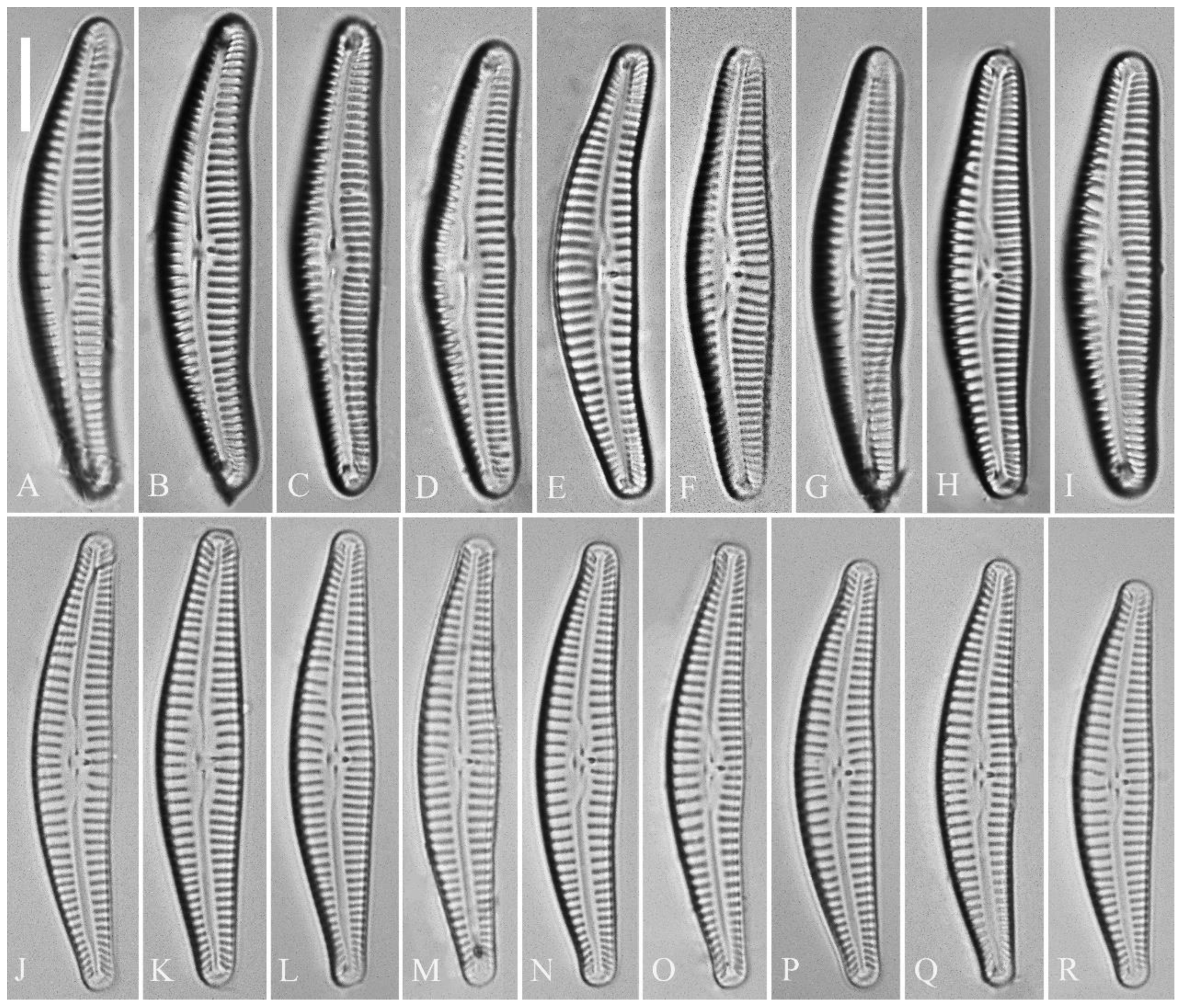
Description. LM (
Figure 5). Pre-normal valves somewhat vaulted (
Figure 5A–I). Normal valves dorsiventral, dorsal margin strongly convex, ventral margin slightly convex. Apices subrostrate to subcapitate. Valve dimensions (n = 36): length 44–53 μm, width 8–9.5 μm. Axial area narrow. Central area present only in pre-normal valves (
Figure 1A–I), in normal valves nearly absent (
Figure 1J–R). Raphe lateral, slightly reverse-lateral towards valve central part. Striae slightly radiate in valve middle, radiate towards apices. Areolae difficult to discern under LM. An isolated stigma located on ventral side of central nodule. Striae in dorsal middle part 8–10 in 10 μm, in ventral middle part 9–12 in 10 μm. Punctae 28–32 in 10 μm.
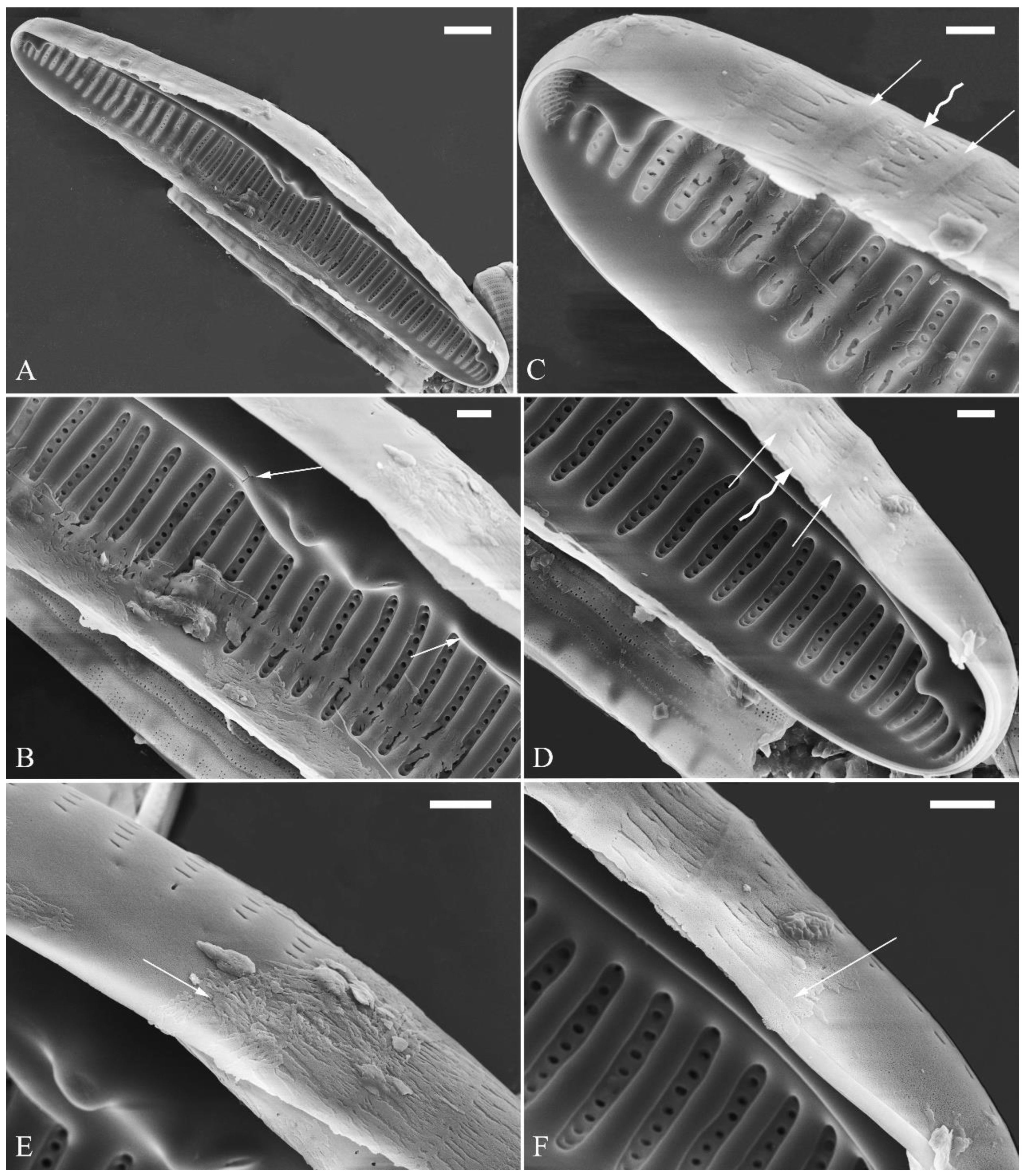
SEM, external view (
Figure 6 and
Figure 7): Pre-normal valves more vaulted and having lineolate areola openings oriented more transapically or at an angle relative to apical axis than in normal vegetative valves (
Figure 6A–D). Proximal raphe fissures reverse-lateral (
Figure 7A, B), dorsal raphe fissures deflected towards dorsal side (
Figure 7C, D). External opening of stigma rounded (
Figure 7B, arrow). Most areola openings lineolate, apically oriented, some not. Apical pore fields composed of a single area, not divided by distal raphe into two unequal areas (
Figure 7C, D, arrows, respectively).
SEM, internal view (
Figure 8). Proximal raphe endings obscured by a silica hood so that the intermissio is invisible (
Figure 8A, B, wavy arrow). Distal raphe fissures terminating in raised, bilabiate helictoglossae. Internal opening of stigma with convoluted occlusions (
Figure 8B, arrow). Internal areola openings located in shallow depressions between two adjacent virgae, rounded, no occlusion present. APFs composed of a single area. An undulate silica strip covering each column of foramina but not completely occluding (
Figure 8C, D, two arrows respectively).
Comments.Cymbella cf.
excisiformis was commonly found with
C. menyuanensis sp. nov. in an unnamed river (37°27’28”N, 101°23’15”E, 2940 m a.s.l.) in Menyuan County, Qinghai Province, China. It lives on the stone surfaces of a plateau river. In the original description of
C. excisiformis, Krammer [
1] reported that its valve length range is 18–44 μm and its puncta density is 24–30 in 10 μm. Our population has larger cells than Krammer’s (44–53 vs. 18–44 μm) and has a higher puncta density (28–32 vs. 24–30 in 10 μm). In our population we found an initial valve and many pre-normal valves but did not find specimens smaller than 44 µm in length. Our population is similar to the larger specimens of
C. excisiformis illustrated by Krammer [
1], so we identified it as
C. cf.
excisiformis. Below, we will describe in detail its initial valve.
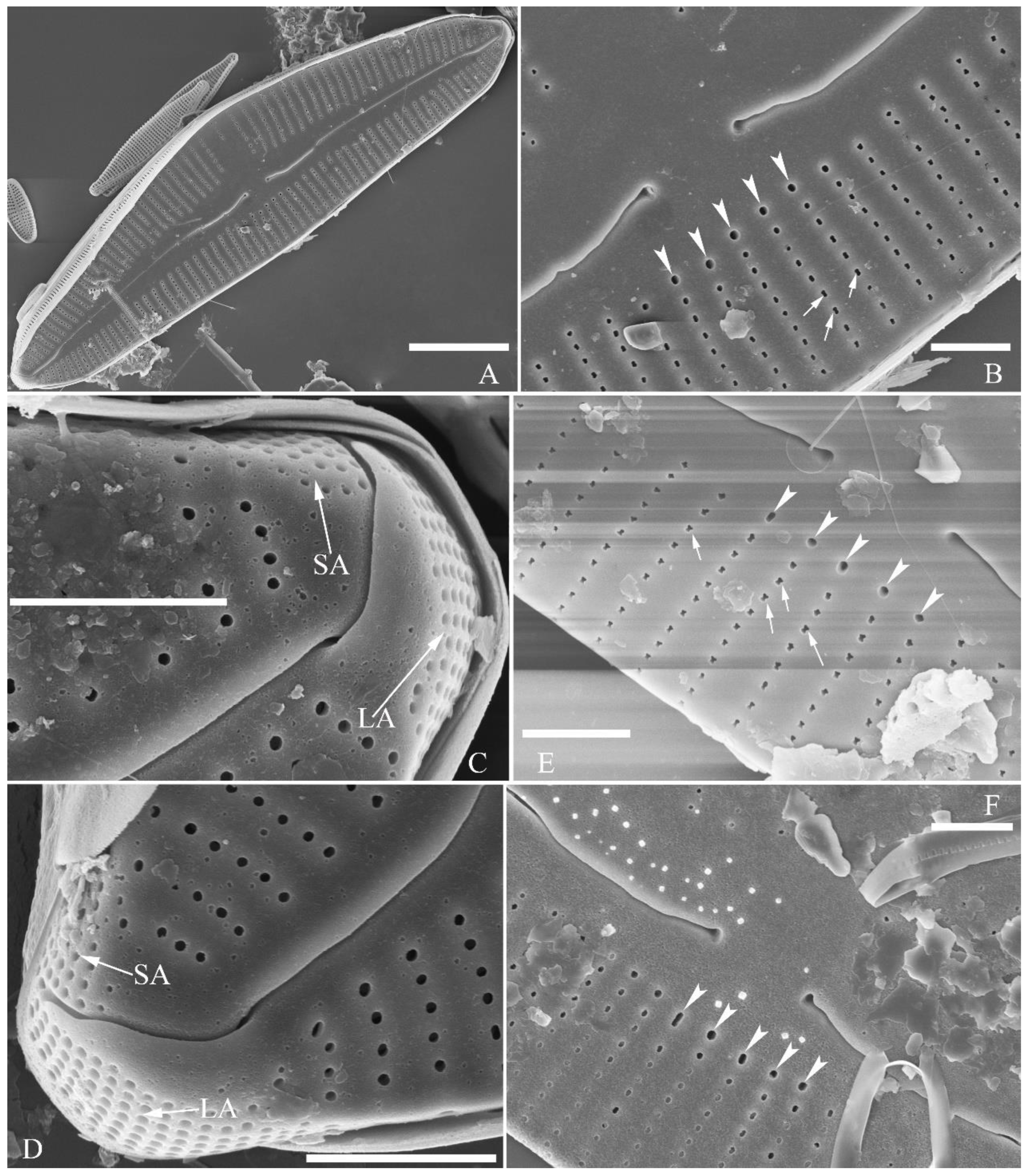
Description. LM (
Figure 9). Valves slightly dorsiventral, almost rhombic-lanceolate, dorsal margin highly arched, ventral margin slightly convex due to presence of a slightly gibbous central portion. Valve apices cuneate, obtuse, not protracted. Valve dimensions (n = 42): length 32–56 μm, width 8.5–12.5 μm. Axial area lanceolate. Central area elliptical. Raphe slightly lateral, proximal raphe fissures almost straight with small central pores. Stigmata absent. Striae radiate throughout valve surface, 10–12 in 10 μm in dorsal middle part, 11–13 in 10 μm in ventral middle part. A shortened stria sometimes produced on dorsal middle part (
Figure 9C, D, F, I, J, arrow respectively). Areolae discernible, 20–25 in 10 μm.
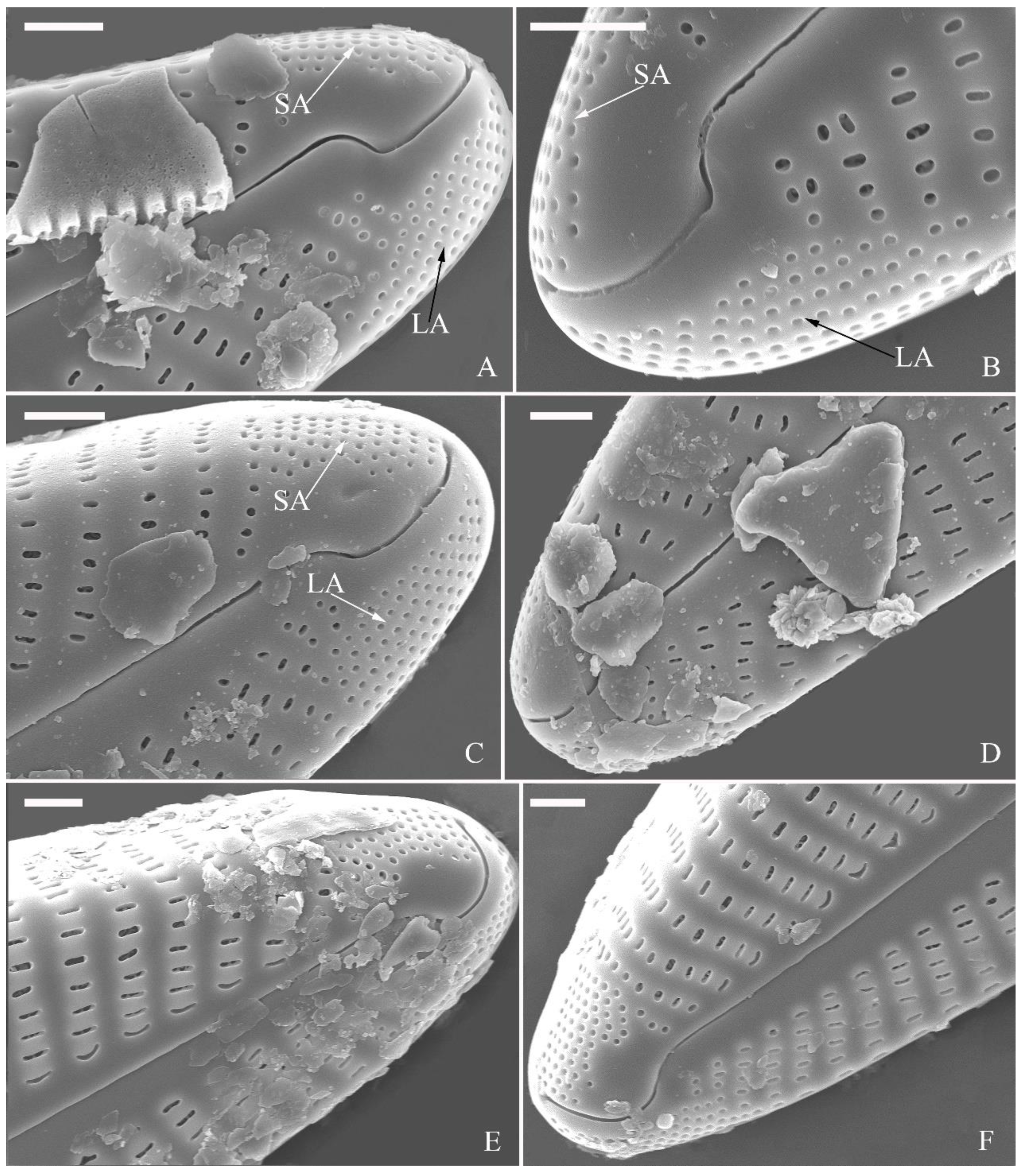
SEM, external view (
Figure 10). Proximal raphe endings expanded (
Figure 10A–D), distal raphe fissures dorsally deflected, divided apical pore fields into two unequal areas: a larger ventral area (LA) composed of ca. 14–18 pervalvar columns of porelli (each column composed of ca. 1–7 porelli) and smaller dorsal area (SA) composed of 4–7 pervalvar columns of porelli (each column composed of ca. 1–7 porelli) (
Figure 10 E, F). Areola outer openings reniform; areolar occlusions (closing plates) also reniform, with strut affixed to areolar wall, produced below valve surface, partially occluding areolae (
Figure 10D, arrows).
SEM, internal view (
Figure 11). Raphe straight, proximal raphe endings interrupted by central nodule, intermissio clearly visible (
ca. 1.5 μm long), i.e., no silica hood obscuring intermissio (
Figure 11A, B, E), distal raphe fissure terminating in raised bilabiate helictoglossa (
Figure 11C, F). Areolae internal openings oblong, located in depression between two adjacent virgae, occluded by reniform closing plates. APFs composed of a larger ventral area and a smaller dorsal area (
Figure 11C, F). An undulate flap-like silica strip covering apertures of each pervalvar column of porelli, but not completely occluding (
Figure 11F, two arrows).
Holotype. Slide DIA2024005, specimen circled on the slide, illustrated here as
Figure 9A, deposited in the herbarium of Jishou University (JIU), China.
Type locality. China. Hunan Province, Yuanling County, Shenxi River. A specific sampling location (28°44′48” N, 110°25′27” E, 200 m asl.) in a riffle of the Shenxi River, collected by Bing Liu, 17 March, 2017.
Etymology. The epithet hunanensis is named after Hunan Province where this new species was found.
Ecology and distribution. The diatom samples were scraped off of stone surfaces. Hence, this is an epilithic species. The following environmental parameters were measured in the field. pH = 8.3 ± 0.1, conductivity = 215.7 ± 2.6 μS∙cm-1, water temperature = 12.2 ± 0.1°C. Known from the type locality and the Li River, Sangzhi County, Hunan Province, China.
Comments. Cymbella hunanensis sp. nov. is characterized by its slightly dorsiventral and almost rhombic-lanceolate valve outline, lack of stigma, clearly visible intermissio, areoale occluded by reniform closing plates, and apical pore fields divided by the distal raphe fissure into two unequal areas. The two most similar species to
C. hunanensis are
C. stigmaphora and
C. subleptoceros (
Table 4). However, unlike these two species which do not possess a central area,
C. hunanensis has an elliptical central area (
Table 4). Moreover, the apices of
C. hunanensis are more obtuse than those of
C. stigmaphora and
C. subleptoceros.
Description. LM (
Figure 12). Valves slightly dorsiventral, both valve margins convex, but dorsal margin markedly more arched than ventral one. Valve apices cuneate to acute. Valve dimensions (n = 45): length 23–37 μm, breadth 5.5–7.5 μm. Axial area narrow, central area absent or in some specimens not well expressed on dorsal side. Raphe nearly along valve midline except approaching valve center, where raphe ventrally displaced. Central pores absent. Striae slightly radiate in middle part, radiate towards apices. Striae in dorsal middle part 10–14 in 10 μm, in ventral middle part 10–12 in 10 μm. Stigmata absent. Areolae difficult to discern under LM, 25–31 in 10 μm.
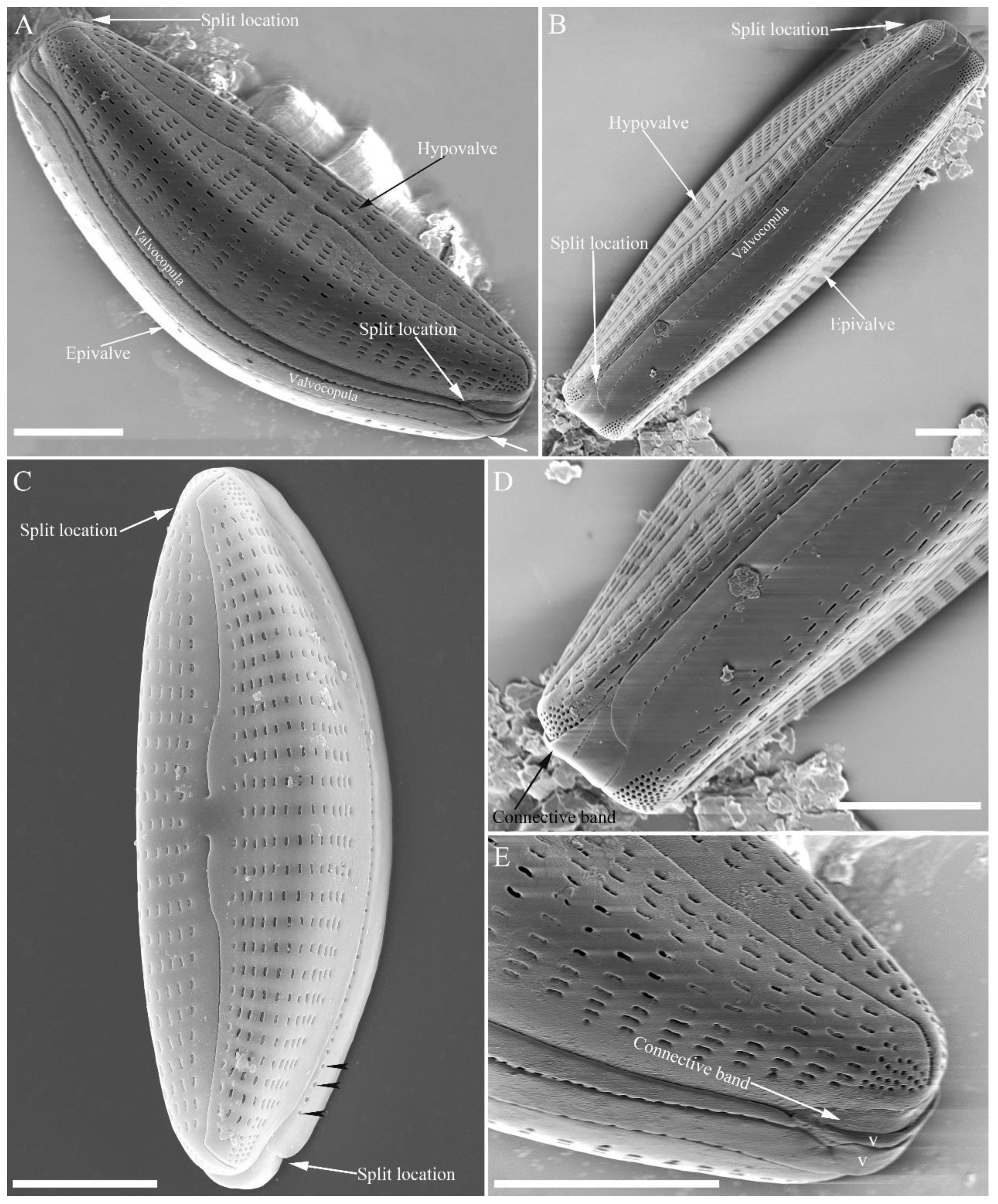
SEM, external view (
Figure 13 and
Figure 14). Frustule with deeper dorsal mantle than ventral mantle (
Figure 13A, B). Epicingulum comprising a single valvocopula composed of two parts: one part inserting dorsal mantle margin (
Figure 13A), another part inserting ventral mantle margin (
Figure 13B). Hypocingulum comprising valvocopula and two connective bands. Valvocopulae of hypocingulum and epicingulum identical in structure and position. Connective bands short, surrounding and inserting each apex of hypovalve (
Figure 13D, E, arrow respectively). For each frustule, there are two split locations for all girdle bands, each split location situated near apex (
Figure 13A, B, C, labelled split location). A row of rounded to oblong poroids dividing valvocopula into pars exterior and pars interior (
Figure 13C, three black arrowheads). Proximal raphe endings slightly displaced towards ventral side, distal raphe fissures dorsally deflected, dividing apical pore fields into two unequal areas: a larger ventral area (LA) composed of ca. 9–12 pervalvar columns of porelli (each column composed of ca. 1–5 porelli) and a smaller dorsal area (SA) composed of 4–7 pervalvar columns of porelli (each column composed of ca. 1–5 porelli) (
Figure 14 A, B, D, E). Areola outer openings reniform, areolae close to axial area smaller than most other areolae (
Figure 14A, arrows). Areola occlusions (closing plates) also reniform, developed from strut affixed to areola wall on either its dorsal or ventral side (
Figure 14C, D, arrows and wavy arrows, respectively). Reniform closing plates projecting in areola lumens, partially occluding each areolar opening below valve surface (
Figure 14F).
SEM, internal view (
Figure 15). Proximal raphe endings interrupted by central nodule, intermissio clearly visible (
ca. 1 μm long), not hidden by a silica hood (
Figure 15A, B, E, F). Areolae internal openings oblong, located in depression between two adjacent virgae, occluded by reniform closing plates.
Distal raphe fissures terminating in raised bilabiate helictoglossae. Apical pore fields composed a larger area and a smaller area (
Figure 15C, D). An undulate flap-like silica strip covering internal apertures of each column of porelli, but not completely occluding (
Figure 15D, two arrows).
Comments. Cymbella hustedtii was commonly found with
C. hunanensis sp. nov. (see above).
Cymbella hustedtii differs from most
Cymbella species by its divided APFs, clearly visible intermissio, and reniform areola outer openings and closing plates. Five
Cymbella species have this combination of characters:
C. bourrellyi Maillard ex Moser, Steindorf & Lange-Bertalot [
17],
C. cognata [
4],
C. hustedtii Krasske [
1],
C. subleptoceros Krammer [
1], and
C. hunanensis sp. nov. (see above). The lectotype species
C. cymbiformis shares dorsal deflected distal raphe fissures, APFs and internal areolae occlusions with
C. hustedtii. The intermissio in
C. cymbiformis is hidden by a silica hood whereas the intermissio in
C. hustedtii is clearly visible. These characteristics make C.
hustedtii an interesting species. Its transfer to the genus
Cymbopleura by Novelo et al. [
20] is in our opinion unjustified. On the other hand, Liu et al. [
21] did not mention this species when they described the genus
Qinia Y. Liu, Kociolek & Kulikovskiy as they only focused their discussion at the generic level.
Description. LM (Figure 16). Valves slightly dorsiventral, almost lanceolate, dorsal margin arched, ventral side slightly convex except in smaller specimens where ventral side almost straight (
Figure 16L, M). Valve apices acuminate. Valve dimensions (n = 39): length 28–75 μm, width 8–12 μm. Axial area variable, from narrow in smaller specimens to moderately wide in larger specimens, broadening gradually towards valve centre. Central area elliptical in larger specimens, indistinct in smaller specimens. Raphe located along midline, straight (filiform). Central pores small. Stigmata absent. Striae radiate throughout valve surface, 10–12 in 10 μm in dorsal middle part, 10–13 in 10 μm in ventral middle part. Areolae discernible under LM, 22–27 in 10 μm.
SEM, external view (
Figure 17 and
Figure 18). Raphe straight (
Figure 17A–C), proximal raphe endings slightly expanded (
Figure 17D–F), distal raphe fissures deflected towards dorsal side (
Figure 18). External openings of areolae mostly slit-like, some rounded. Particularly, a few of the openings bordering dorsal central area are slit-like, transapically oriented (
Figure 17D–F, wavy arrows); while a few bordering ventral central area are rounded, separate from ventral striae (
Figure 17D–F, arrows). Distal raphe fissures dividing apical pore fields into two unequal areas: a larger ventral area (LA) composed of ca. 14–16 pervalvar columns of porelli (each column composed of ca. 1–7 porelli) and a smaller dorsal area (SA) composed of 9–11 pervalvar columns of porelli (each column composed of ca. 1–7 porelli) (
Figure 18). Occlusions produced below valve surface, partially occluding areolae (
Figure 18B, D).
SEM, internal view (
Figure 19). Raphe straight, proximal raphe endings obscured by silica hood, i.e., intermissio invisible (
Figure 19A, B, E), distal raphe fissures terminating in raised bilabiate helictoglossae. Internal view confirms absence of stigmata (
Figure 19B, E, F). Internal openings of areolae located in depressions between adjacent virgae, rounded, elliptical, or oblong; areola occlusions (volae) shaped as walnut-kernels, developed from two or more struts that are affixed to areolar wall (
Figure 19F, arrows). Apical pore fields composed of two unequal areas (
Figure 19C). An undulate silica strip covering each column of porelli but not completely occluding (
Figure 19D, two arrows).
Holotype. Slide DIA2024006, specimen circled on the slide, illustrated here as
Figure 16A, deposited in the herbarium of Jishou University (JIU), China. Registration:
http://phycobank.org/(pending).
Type locality. China. Hunan Province, Suining County, Changpu Town, Wu River. A specific sampling location (26°34.59’N, 110°09.19’E, 300 m a.s.l.) in a riffle of the Wu River, collected by Bing Liu, 22 March 2021.
Etymology. The epithet juglandis refers to the areola occlusions that resemble walnut kernels.
Ecology and distribution. Epilithic in a mountain river with oligotrophic waters. The following environmental parameters were measured in the field. Conductivity was 99.7 ± 0.3 μS∙cm–1, pH was 7.9 ± 0.1 and water temperature was 13.2 ± 0.2 °C. Known only from the type locality so far.
Comments.Cymbella juglandis has a unique suite of characters: acuminate apices, divided APFs, lack of stigma, and areola inner occlusions (volae) shaped as walnut-kernels.
Cymbella juglandis differs from
C. shii by morphometrics such as the valve width (the former having much narrower width than the latter,
Table 5).
Cymbella juglandis is distinguished from
C. subleptoceros by its obscured intermissio whereas the latter has clearly visible intermissio (
Table 5).
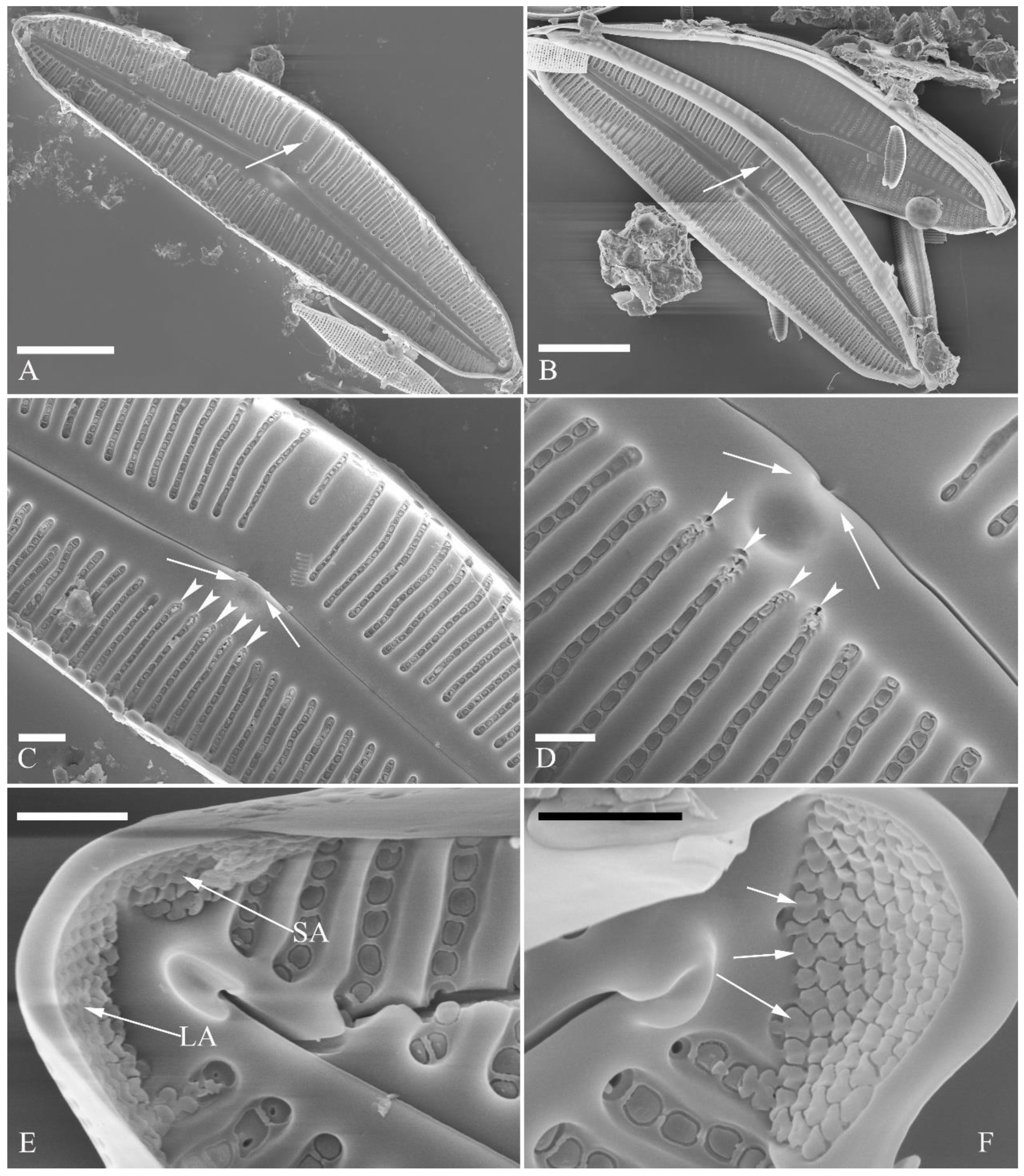
Description. LM (
Figure 20). Initial or pre-normal valves vaulted (
Figure 20A). Valves strongly dorsiventral, dorsal margin high, strongly arched, ventral margin concave with a swelling in the middle except in small valves which have a straight ventral margin (
Figure 20L). Apices rostrate to subcapitate, slightly turned towards dorsal side. Valve dimensions (n = 29): length 46–91 μm, width 12.5–20.5 μm. Axial area narrow, linear. Central area small. Raphe lateral, proximal raphe fissures relatively short. Central pores visible. 3–6 stigmata located on ventral side of central nodule, slightly detached from ventral striae. Striae radiate throughout valve surface, 8–11 in 10 μm in both dorsal and ventral middle parts. Areolae discernible under LM, 22–26 in 10 μm.
SEM, external view (
Figure 21 and
Figure 22). Areola openings of pre-normal valves have various shapes and orientations (
Figure 21, arrows). Apical pore fields not well-developed in pre-normal valves (
Figure 21C, D). Proximal raphe endings expanded; external openings of stigmata rounded to oblong (
Figure 22B). Distal raphe fissures deflected towards dorsal side, not dividing apical pore fields (
Figure 22C, D). Most external openings of areolae lineolate (
Figure 22B–D).
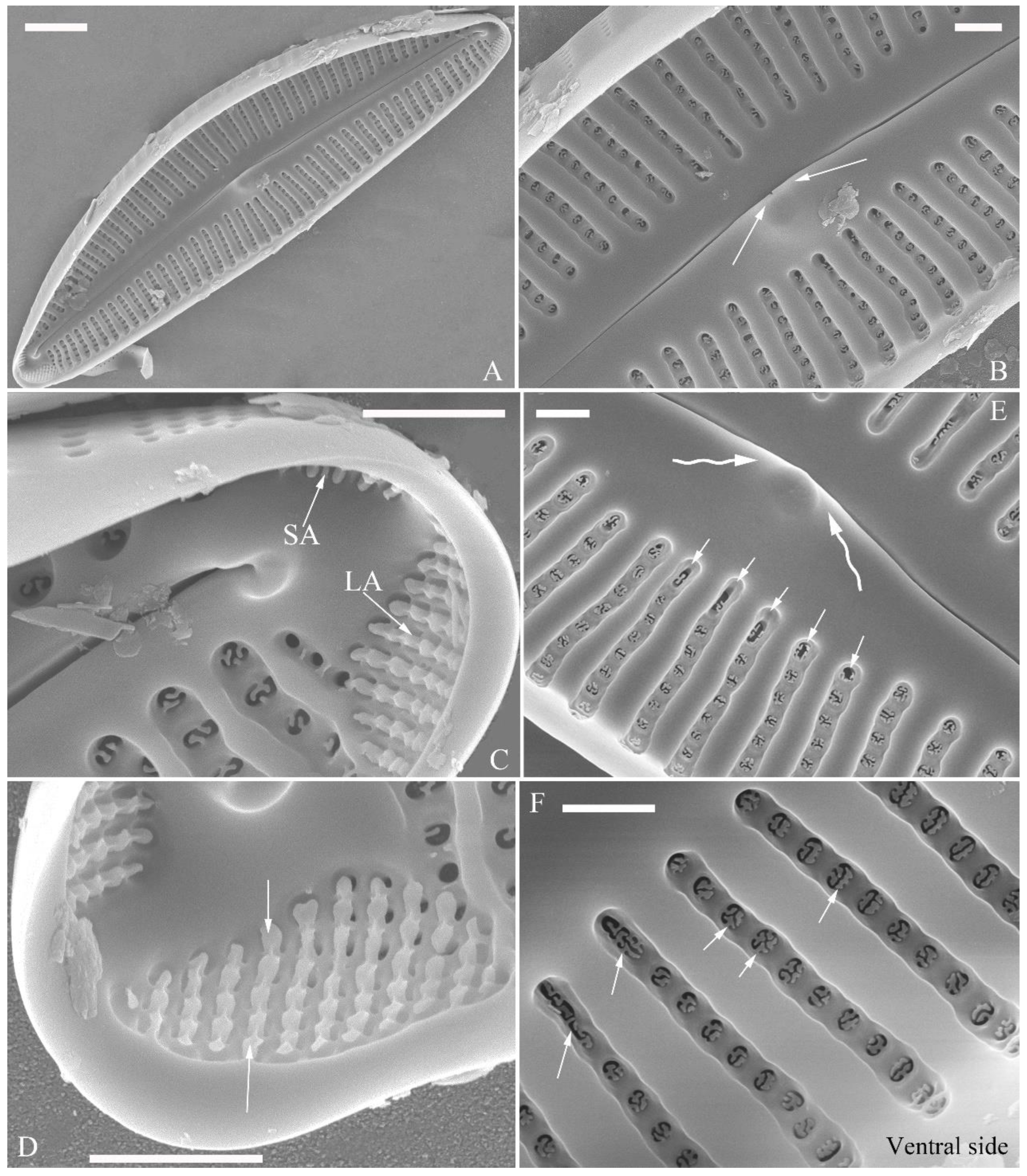
SEM, internal view (
Figure 23). Raphe straight, proximal raphe endings obscured by silica hood, i.e., intermissio invisible (
Figure 23A–C), distal raphe fissures terminating in raised bilabiate helictoglossae (
Figure 23E, F). Internal openings of stigmata with convoluted occlusions (
Figure 23B, D, arrows). Internal openings of areolae located in depressions between adjacent virgae, rounded, elliptical, or oblong, with mushroom-shaped closing plates developed from strut that is affixed to either dorsal or ventral areolar wall (
Figure 23D, wavy arrows). Apical pore fields composed of a single area, an undulate silica strip covering each column of foramina but not completely occluding (
Figure 23F, two arrows).
Holotype. Slide DIA2024007, specimen circled on the slide, illustrated here as
Figure 20B, deposited in the herbarium of Jishou University (JIU), China.
Figure 23.
Cymbella menyuanensis sp. nov., SEM, internal view. (A, C). Two complete valves. (B, D). Details of middle part, note the stigmata (arrows) and mushroom-shaped closing plates (wavy arrows). (E, F). Two apical details, note that an undulate flap-like silica strip above internal apertures of each row of foramina, but not occluding internal apertures completely (two arrows). Scale bars (A, C) = 10 μm, (B, D–F) = 2 μm.
Figure 23.
Cymbella menyuanensis sp. nov., SEM, internal view. (A, C). Two complete valves. (B, D). Details of middle part, note the stigmata (arrows) and mushroom-shaped closing plates (wavy arrows). (E, F). Two apical details, note that an undulate flap-like silica strip above internal apertures of each row of foramina, but not occluding internal apertures completely (two arrows). Scale bars (A, C) = 10 μm, (B, D–F) = 2 μm.
Type locality. China. Qinghai Province, Menyuan County, an unnamed river. A specific sampling location (37°27′28” N, 101°23′15” E, 2940 m a.s.l.) in a riffle of the unnamed river, collected by Bing Liu, July 18, 2019.
Etymology. The epithet menyuanensis is named after Menyuan County of Qinghai Province where this new species was found.
Ecology and distribution. The sampling site is located in the plateau which belongs to the highland continental climate zone. The diatom samples were scraped off of the stone surfaces, Cymbella menyuanensis is therefore epilithic. The following environmental parameters were measured in the field: Conductivity was 448.3 ± 0.5 μS∙cm-1, pH was 8.3 ± 0.1 and water temperature was 11.9 ± 0.5 °C. So far, its distribution is known from the type locality and a river in Huzhu County, Qinghai Province.
Comments. Cymbella menyuanensis sp. nov. is characterized by its strongly dorsiventral valve outline and rostrate to subcapitate apices, 3–6 stigmata, and areolae occluded internally by the mushroom-shaped closing plates. It is similar to
C. neocistula,
C. nepalensis and
C. proxima in morphometry. However, it differs from
C. neocistula and
C. nepalensis by its rostrate to subcapitate apices whereas the latter two have non-protracted, rounded apices (
Table 6). It also differs from
C. proxima by its much higher areola density (22–26 vs. 14–18 in 10 µm,
Table 6).
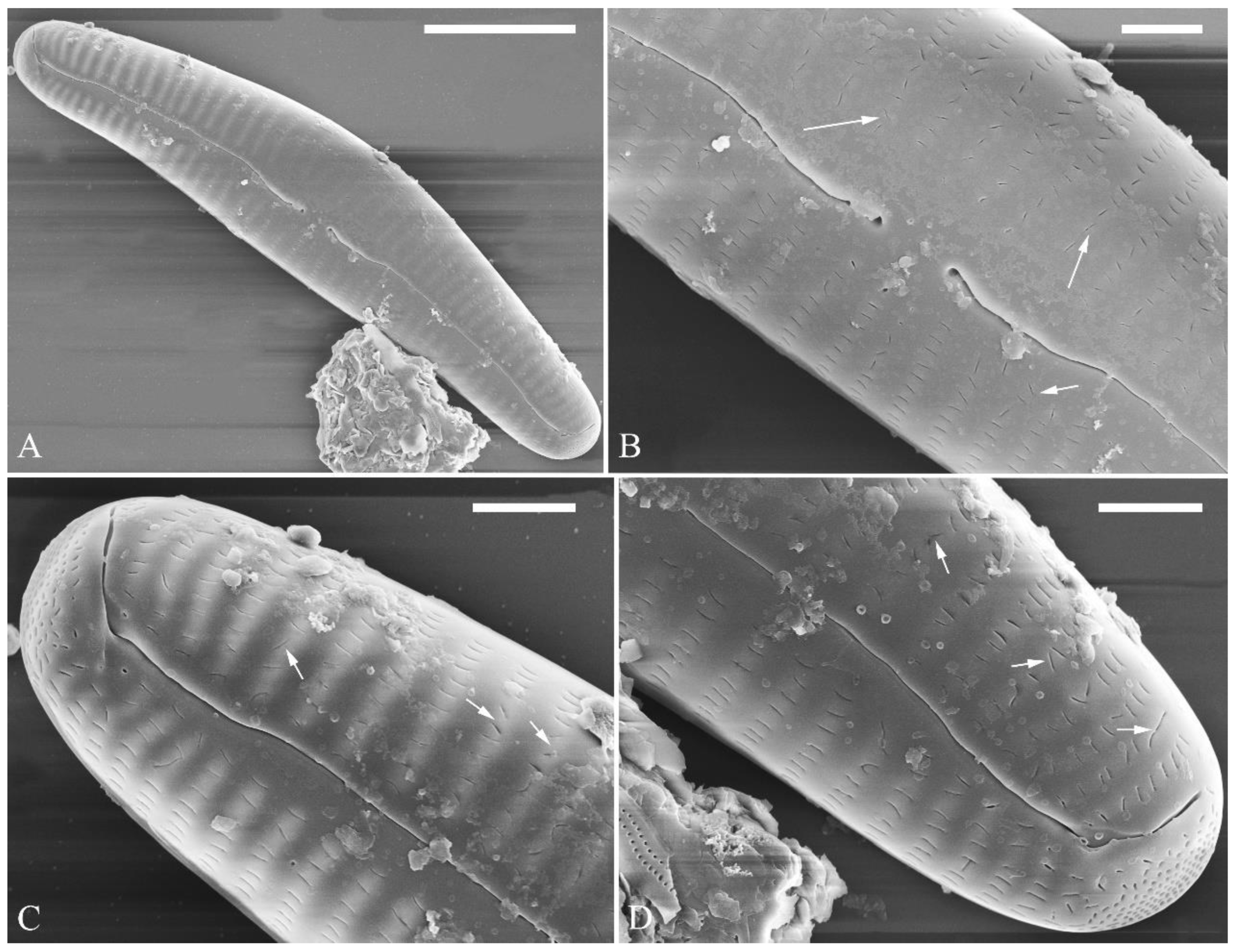
The perizonia of two Cymbella species
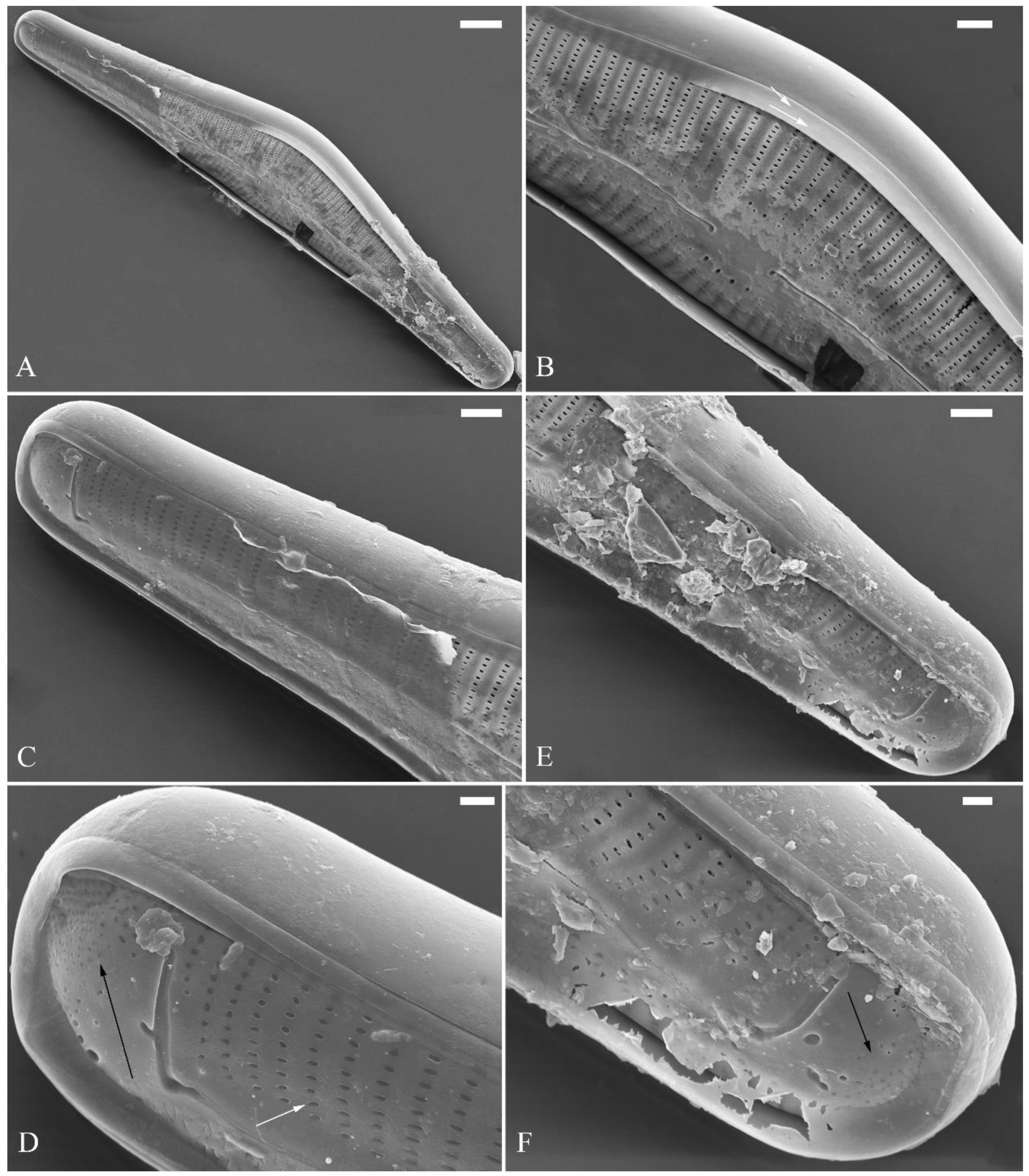
A pre-normal valve (
Figure 6) and an initial valve (
Figure 24 and
Figure 25) of
Cymbella cf.
excisiformis and an initial frustule (
Figure 26 and
Figure 27) of
C.
menyuanensis sp. nov. were investigated using SEM. The observed initial valve of
C. cf.
excisiformis was 52 µm long, 8 µm wide. It had slightly radiate striae, 10 in 10 μm in ventral middle part, and an areola density of 30–32 in 10 μm (
Figure 24). The initial frustule of
C. menyuanensis was 147 µm long, 24 µm wide, with radiate striae, 8 in 10 μm in both the dorsal and ventral middle parts and an areola density of 19–22 in 10 μm (
Figure 26).
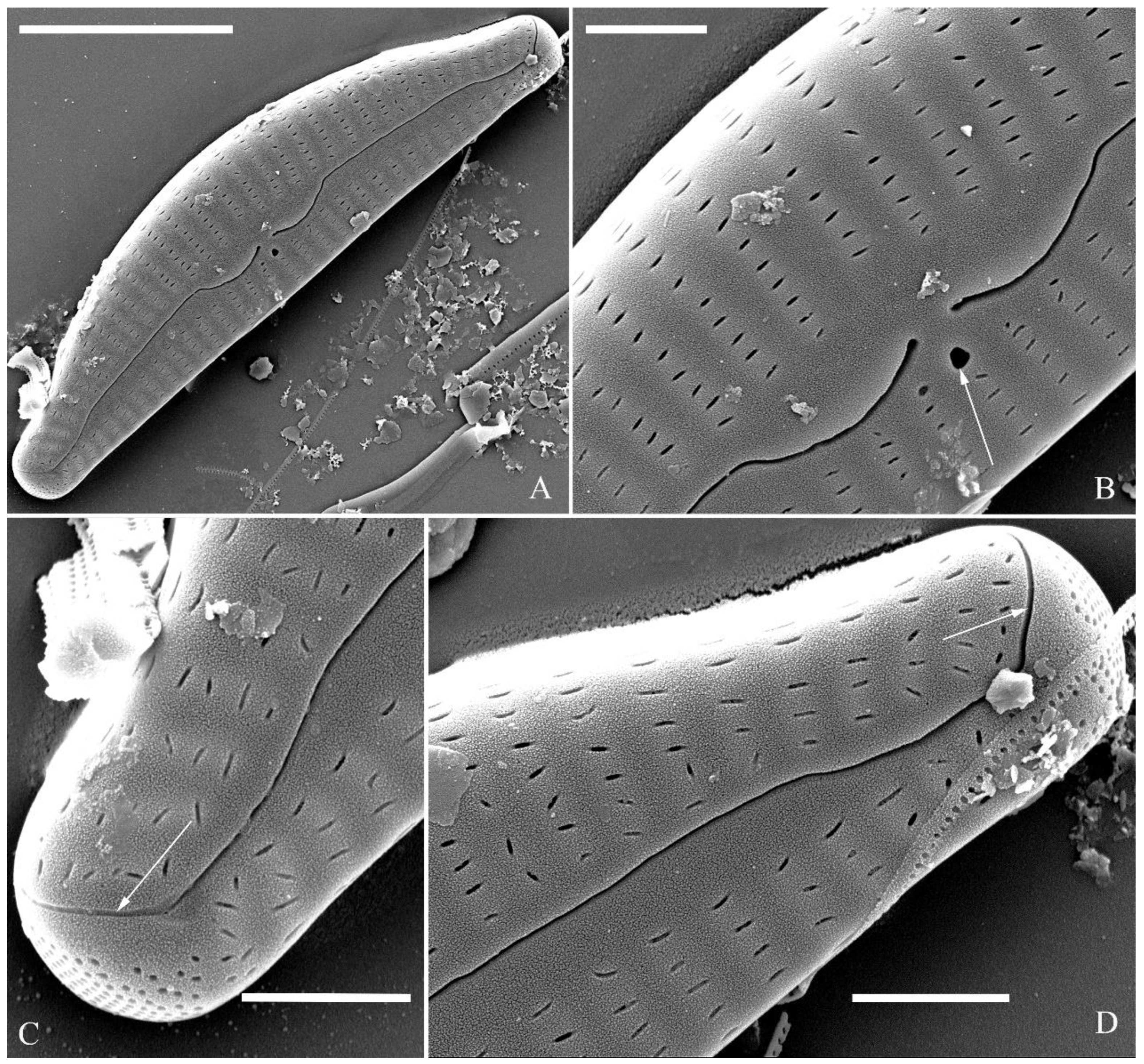
The perizonia in both
C. cf.
excisiformis and
C. menyuanensis are very similar in structure. They are composed of a whole silica sheet covering the surface of the initial frustule (
Figure 24,
Figure 25 and
Figure 26). The whole perizonium joins (overlap) at the girdle bands (
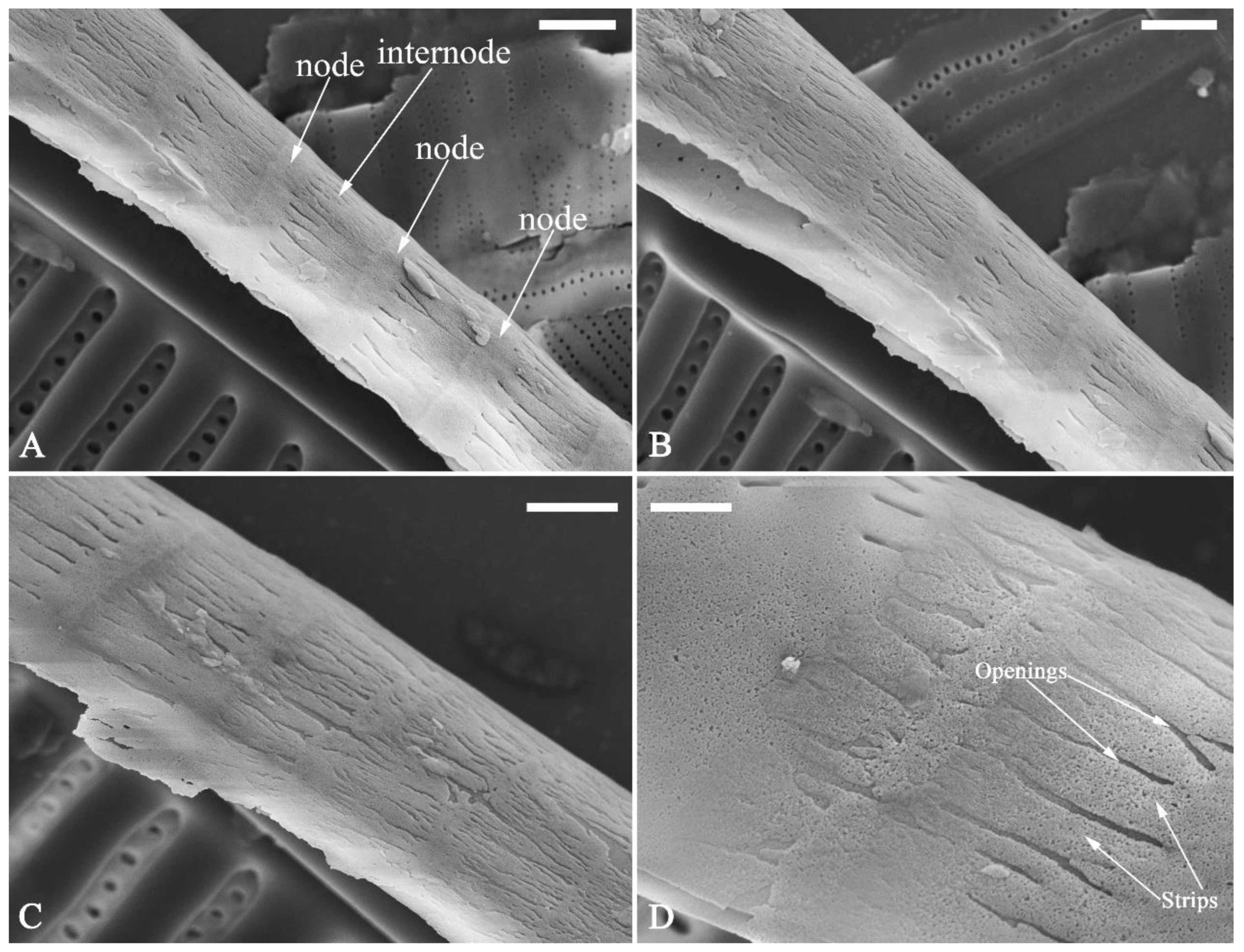
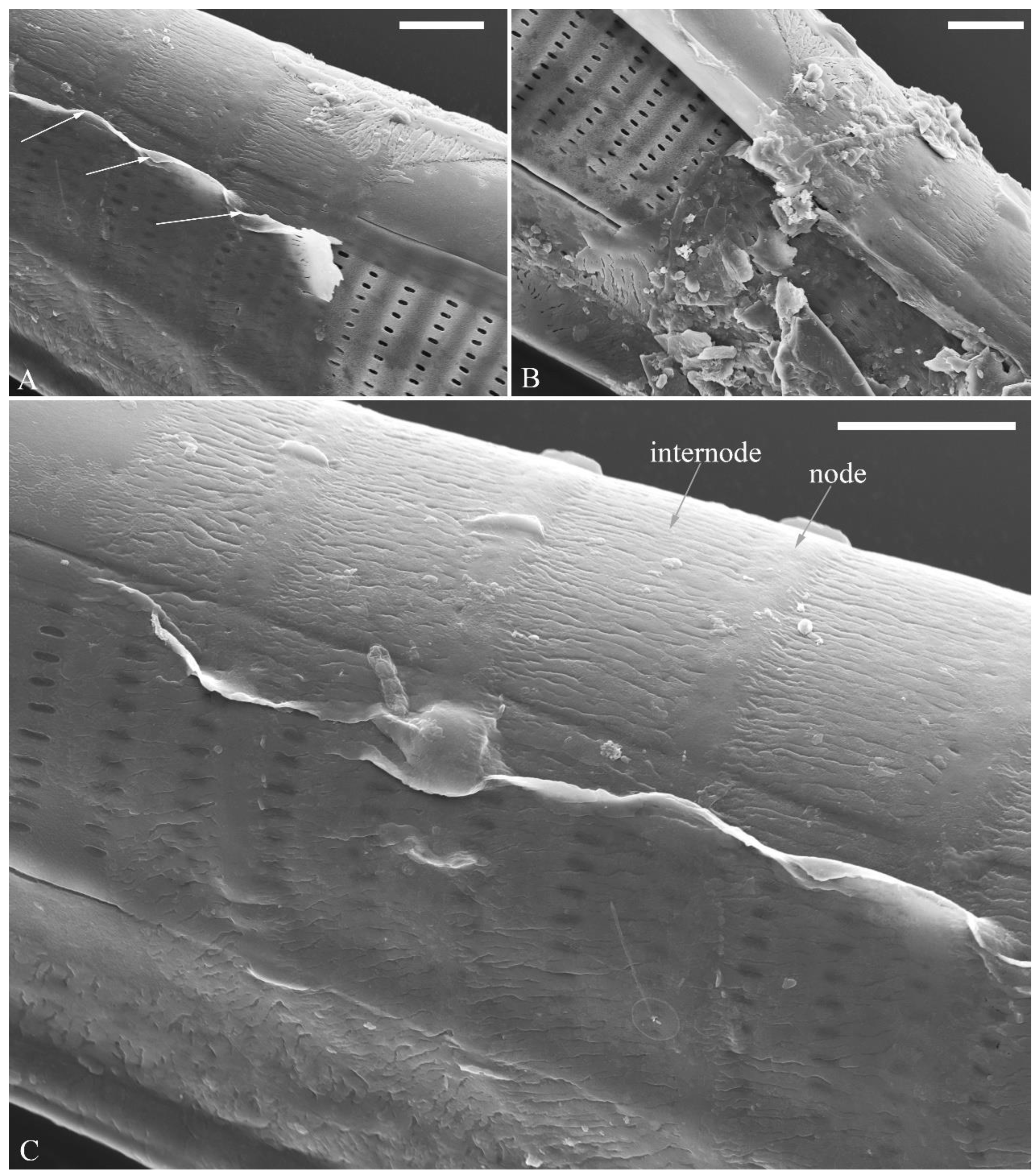
Figure 26C–F; 27A, C) and is composed of two parts: nodes and internodes (
Figure 25, 27C). The nodes are solid and thickened, resembling the transverse perizonium bands in other diatoms. The internodes are also composed of two parts: strips and openings between them (
Figure 25D, 27C). The nodes and internodes are fusing together and do not just overlap with each other. The perizonium is composed of alternate nodes and internodes (
Figure 25, 27C). No transverse perizonium bands were observed.