3. Results
Genera are arranged in systematic order according to Cox (2015), updated from AlgaeBase (Guiry & Guiry 2024) and within them species generally in alphabetical order. Over 200 taxa are included in this report, including 165 records new for Yap and 31 records new for Micronesia (
Table 2). In addition, 43 Yap records of
Mastogloia taxa published in Lobban (in press) and included in
Table 2. Diagnostics and comparison with other species are written out if not previously published for the region.
Systematic List
MELOSIRALES R.M.Crawford
Hyalodiscaceae R.M.Crawford
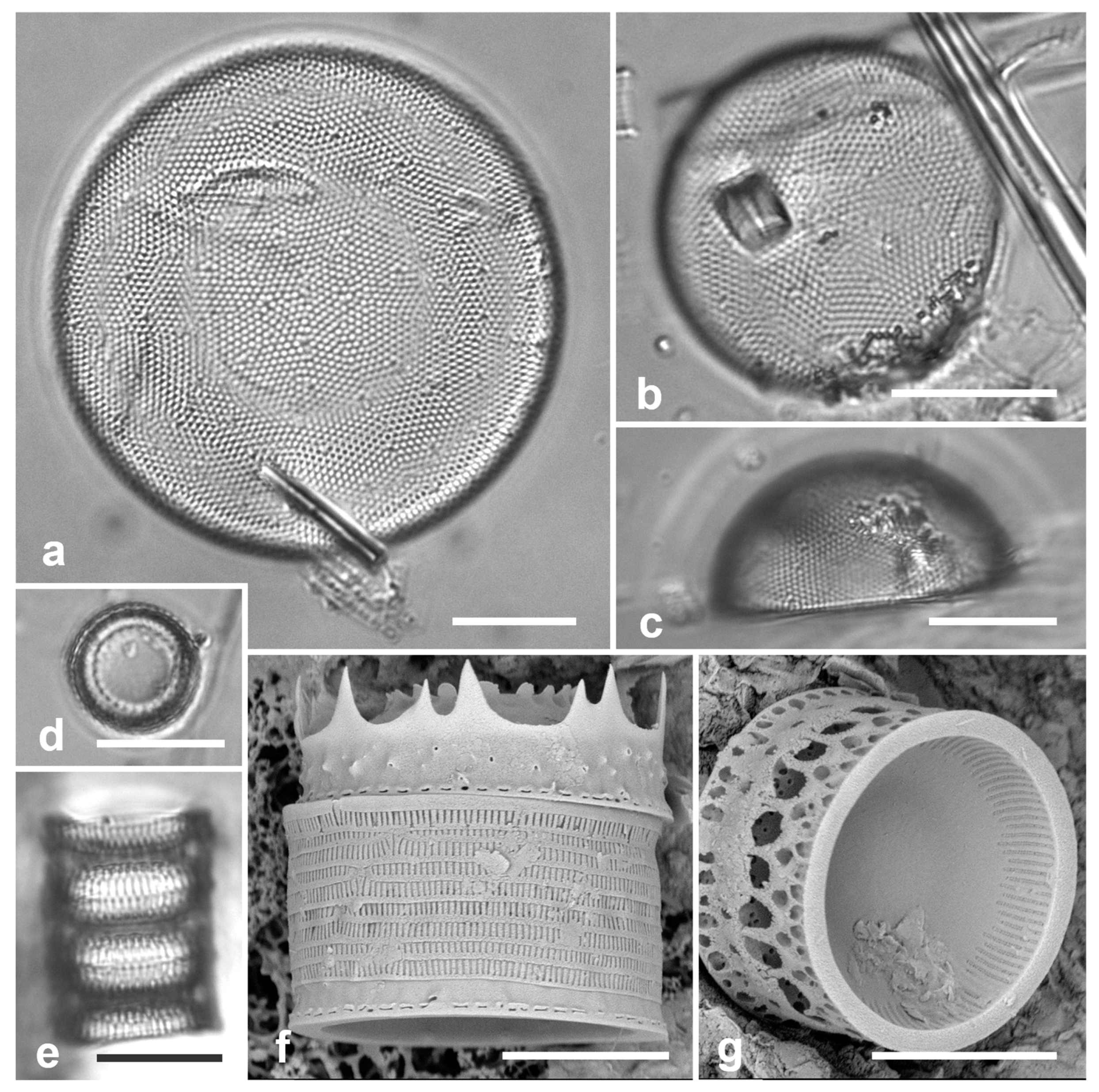
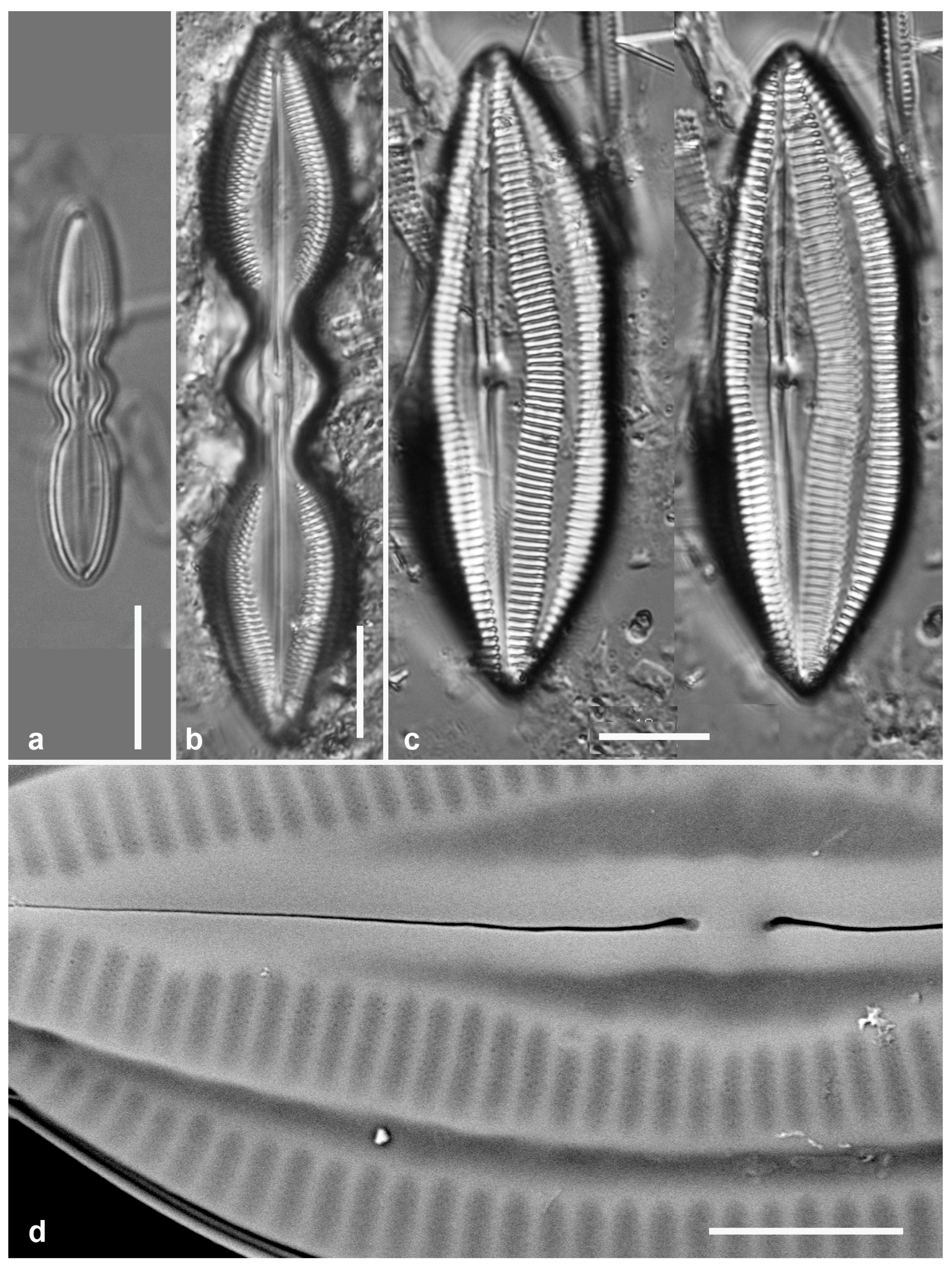
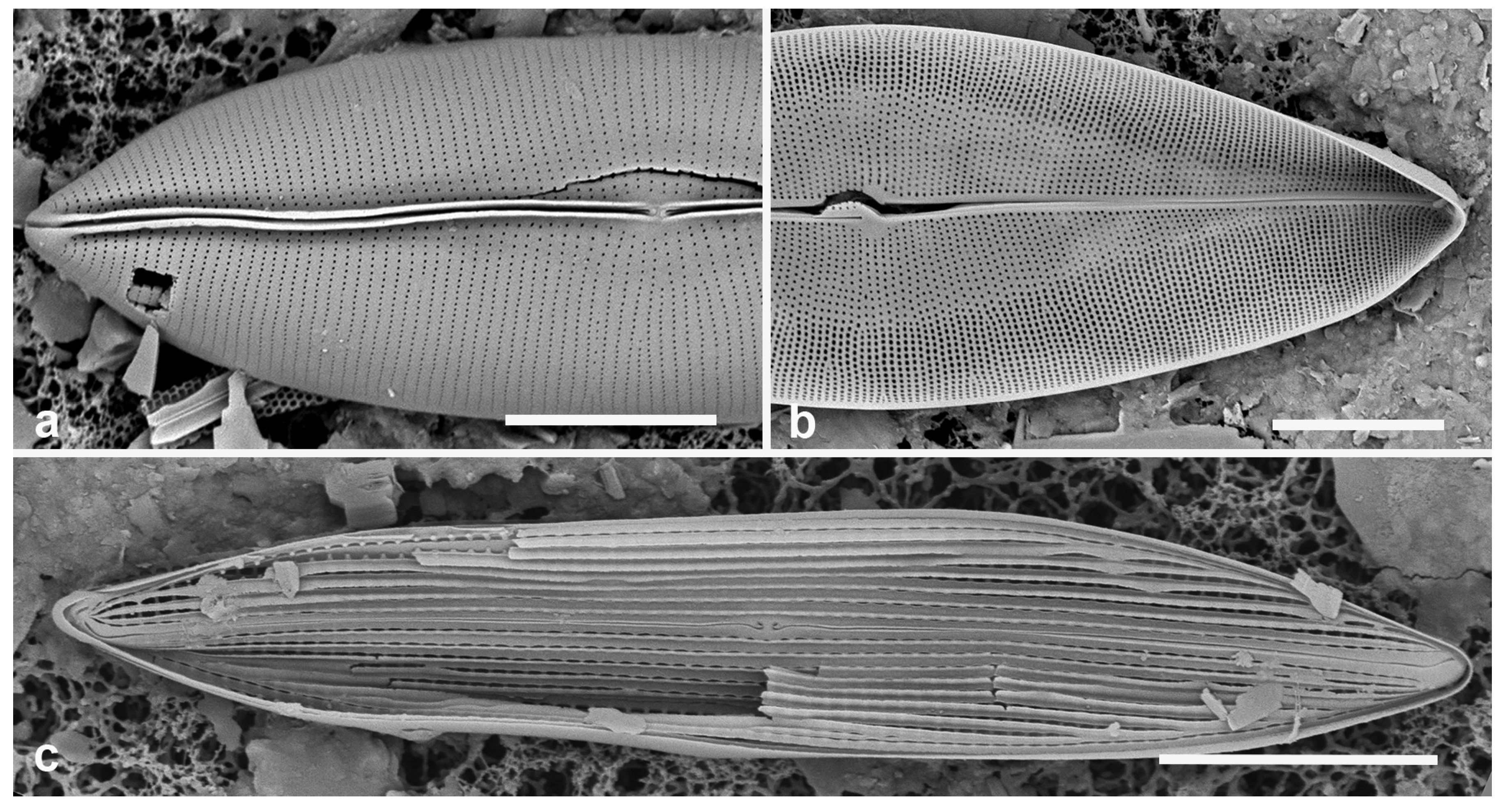
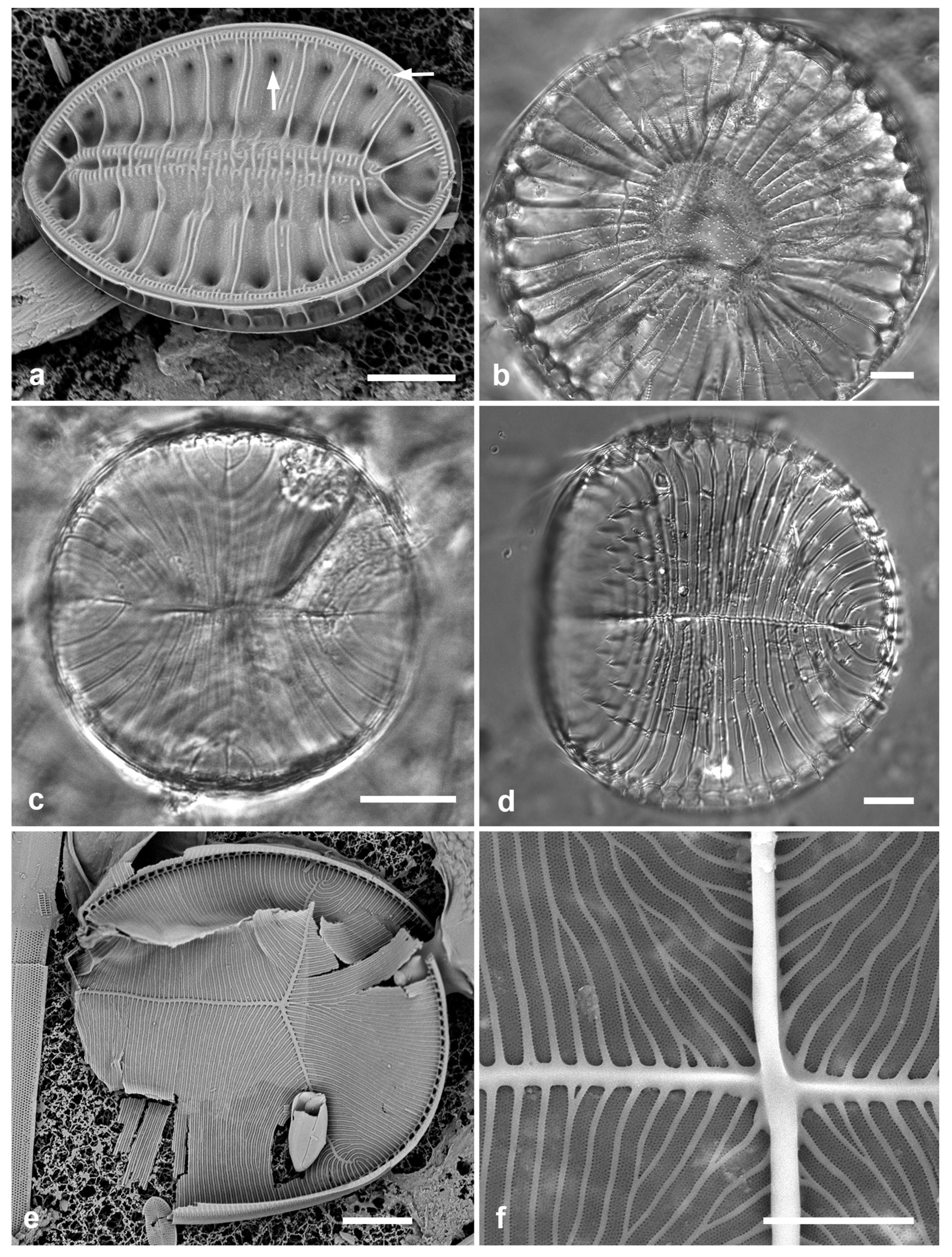
3.1. Podosira hormoides (Montagne) Kützing 1844 Figure 2a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 248, pl. 4, figs 1, 2); Chuuk (Park et al. 2022, p. 28, figs 2a, b, 72); 585758
Yap samples: Y33A, Y37-8
Dimensions: Diam. 31–41 µm., areolae 15–16 in 10 µm.
Comments:
P. hormoides is distinguished from
P. montagnei in girdle view as the valve is lenticular, whereas
P. montagnei valves are hemispherical (
Figure 2b). In valve view we use the areola densities to distinguish them,
P. montagnei having 20–24 in 10 µm vs 15–18 in 10 µm in
P. hormoides (Hustedt 1927–1930, p. 282–283). However, the profile views in Hustedt do not match the specimens of our two species; rather, his figure for
P. hormoides resembles our
Figure 4, even though frustules of
P. hormoides in Guam are spherical, his key character for this species. For the sake of consistency, we have used the names applied to Guam specimens, which may have been force-fitted into the European taxa. There are few taxonomic characters available but careful SEM observations (as MacGillivary & Kaczmarska 2012 did for
Paralia –see below) or molecular analysis in both regions may show that our species of
Podosira are different from those reported for Europe.
3.2. Podosira montagnei Kützing 1844 Figure 2b, c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 248, pl. 4, figs 3, 4); 585759
Yap samples: Y36-2, Y41-8
Dimensions: Diam. 20 µm., areolae 20 in 10 µm.
Comments: See P. hormoides. These specimens are small compared to the ranges given in Hustedt (1927–1930) and Lobban et al. (2012).
Paraliaceae R.M.Crawford
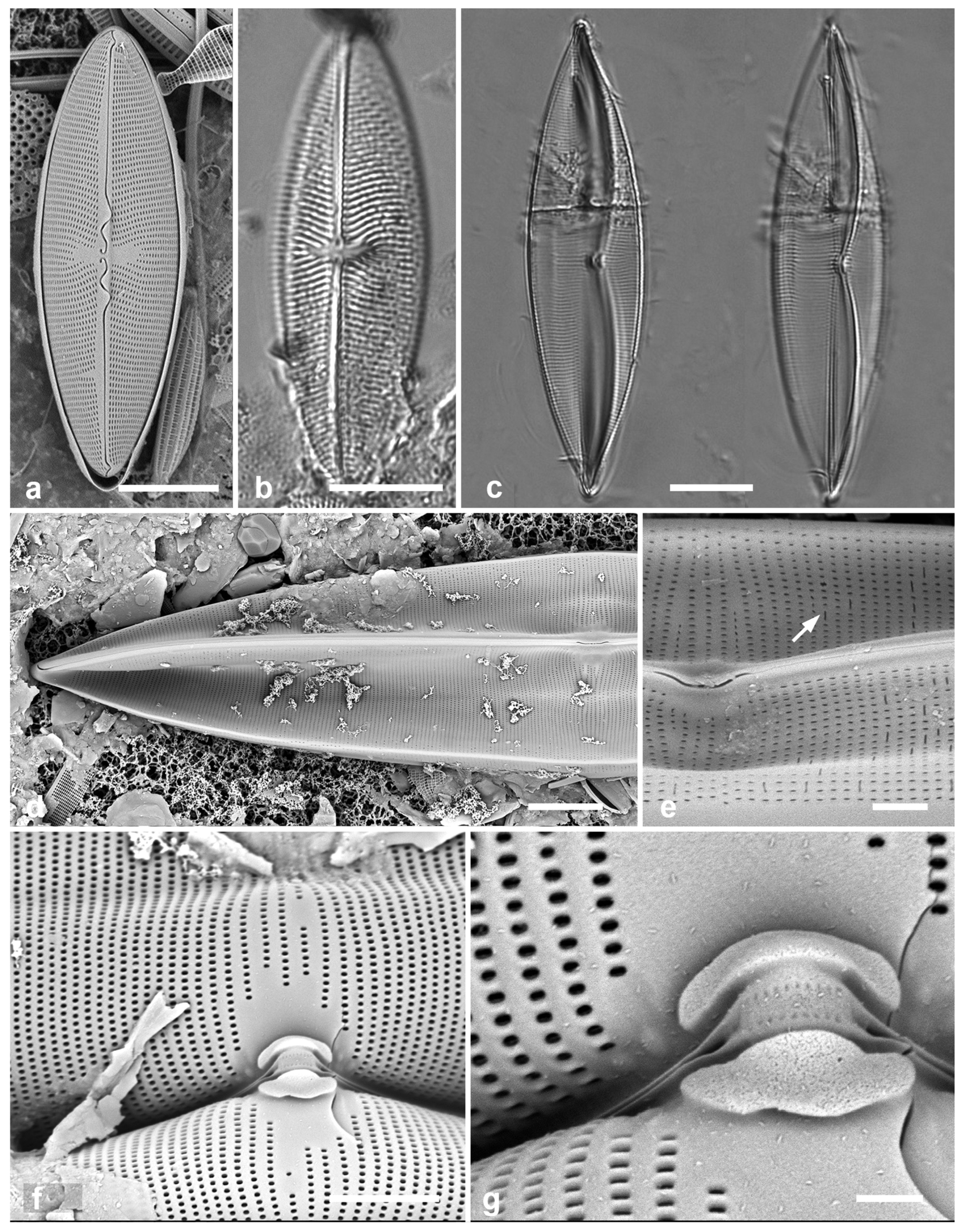
3.3. Paralia longispina Konno & Jordan 2008 Figure 2d–g
Previous Micronesia records: Guam (Lobban et al. 2012, p. 249, pl. 4, figs 6–8); Chuuk (Park et al. 2022, p. 28,
Figure 3); 585489
Yap samples: Y25H-1, Y25H-2, Y26B, Y41-7, Y41-8, Y18B
Dimensions: 10–13 µm diam.
Comments: Although this resembles the common
P. sulcata (Ehrenberg) Cleve, all our specimens are thought to conform to
P. longispina. However, MacGillivary & Kaczmarska (2012) separated several new species in a
P. longispina complex on east and west coasts of North America. A key difference in those new species is lower internal pore density in the striae, but this requires high resolution SEM and
Figure 2g is uninformative. MacGillivary & Kaczmarska (2012) also noted that
P. longispina-like specimens had been reported (with LM and therefore indistinguishable within the complex) from the Philippines (Podzorski & Håkanssen 1987, pl. 1, figs 14–17, 21; Martinez-Goss & Lopez 2011, both as
P. sulcata), Fiji (Foged 1987, pl. 4, figs 13, 14, as
Melosira sulcata), and two locations in Florida.
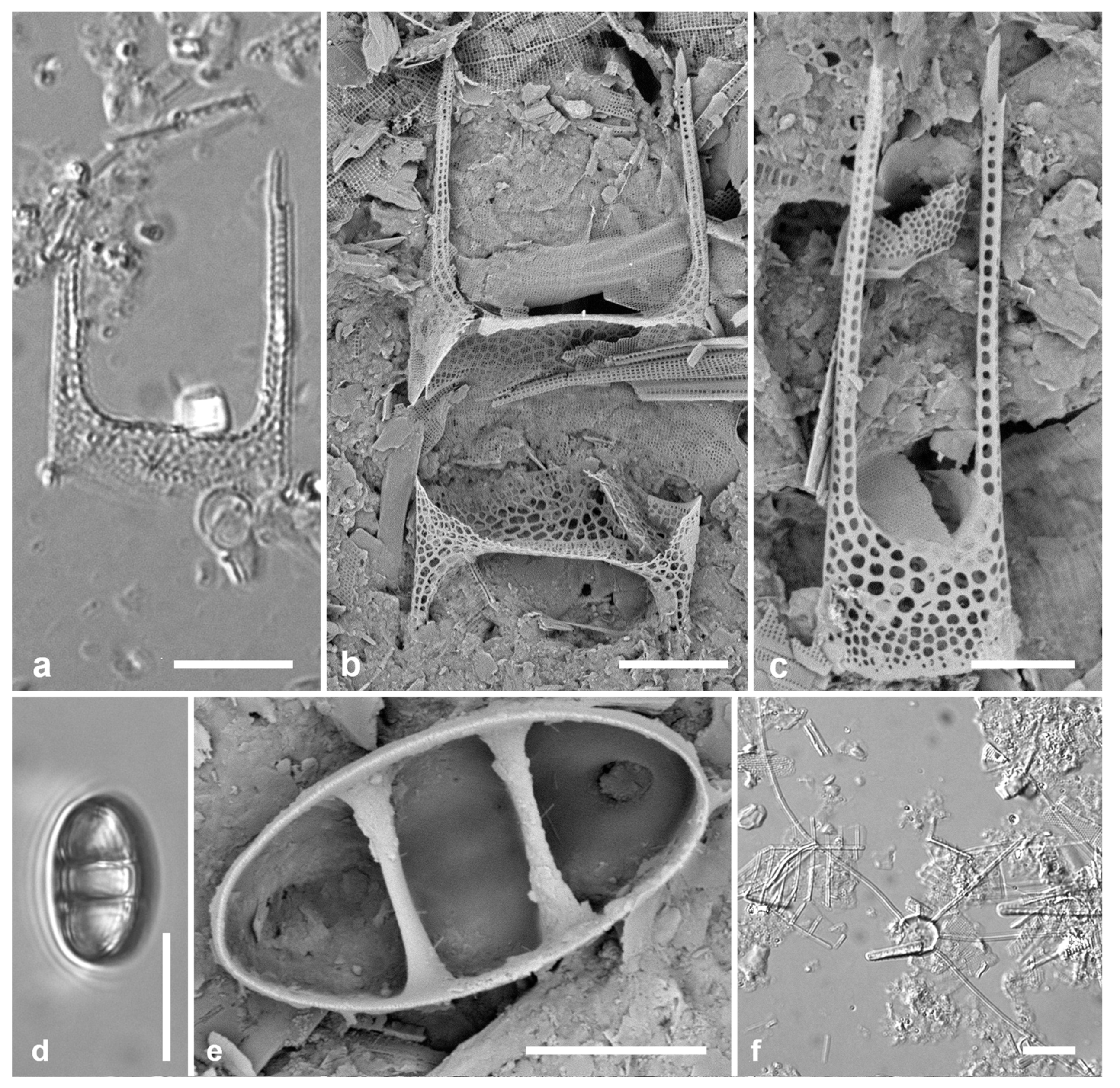
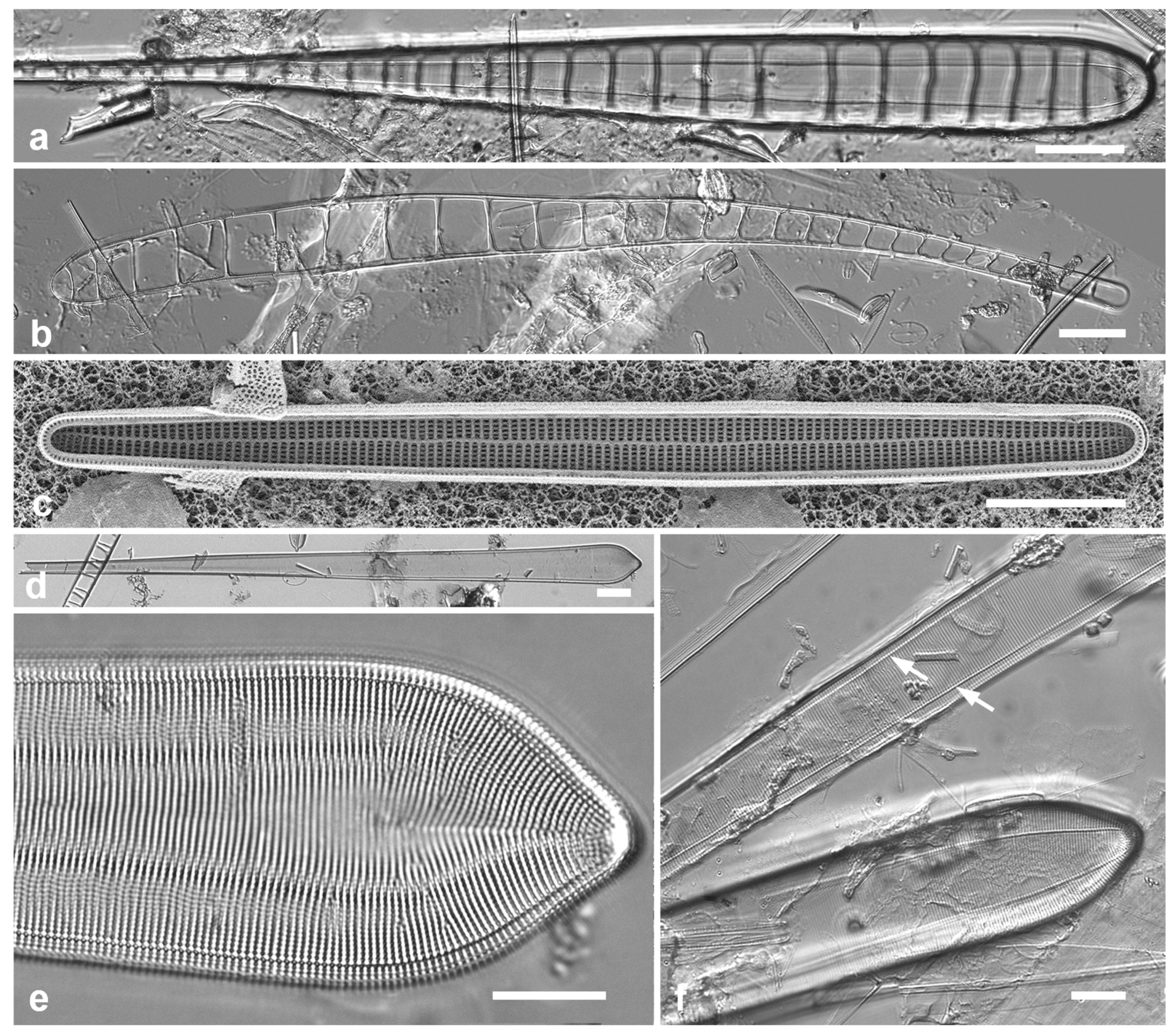
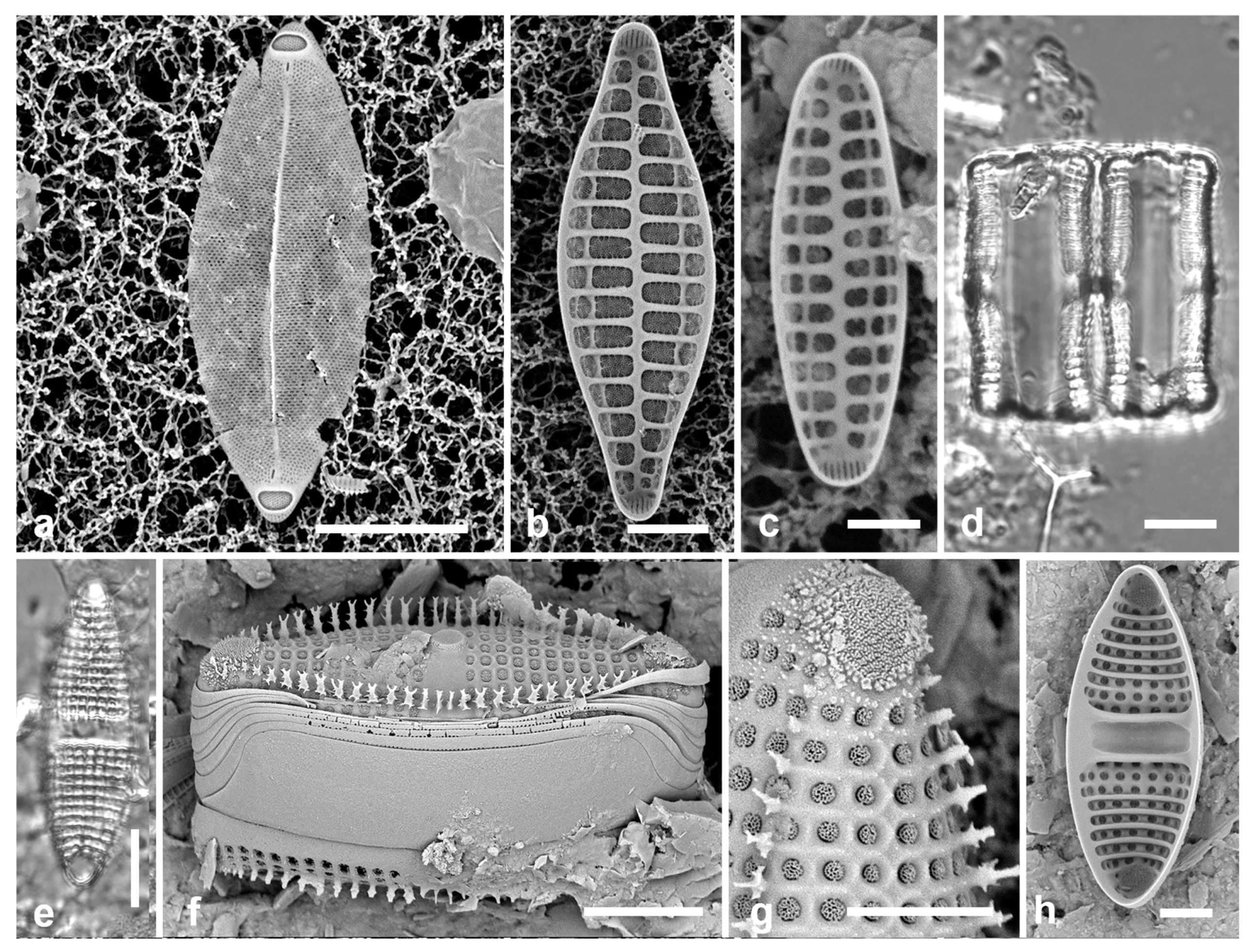
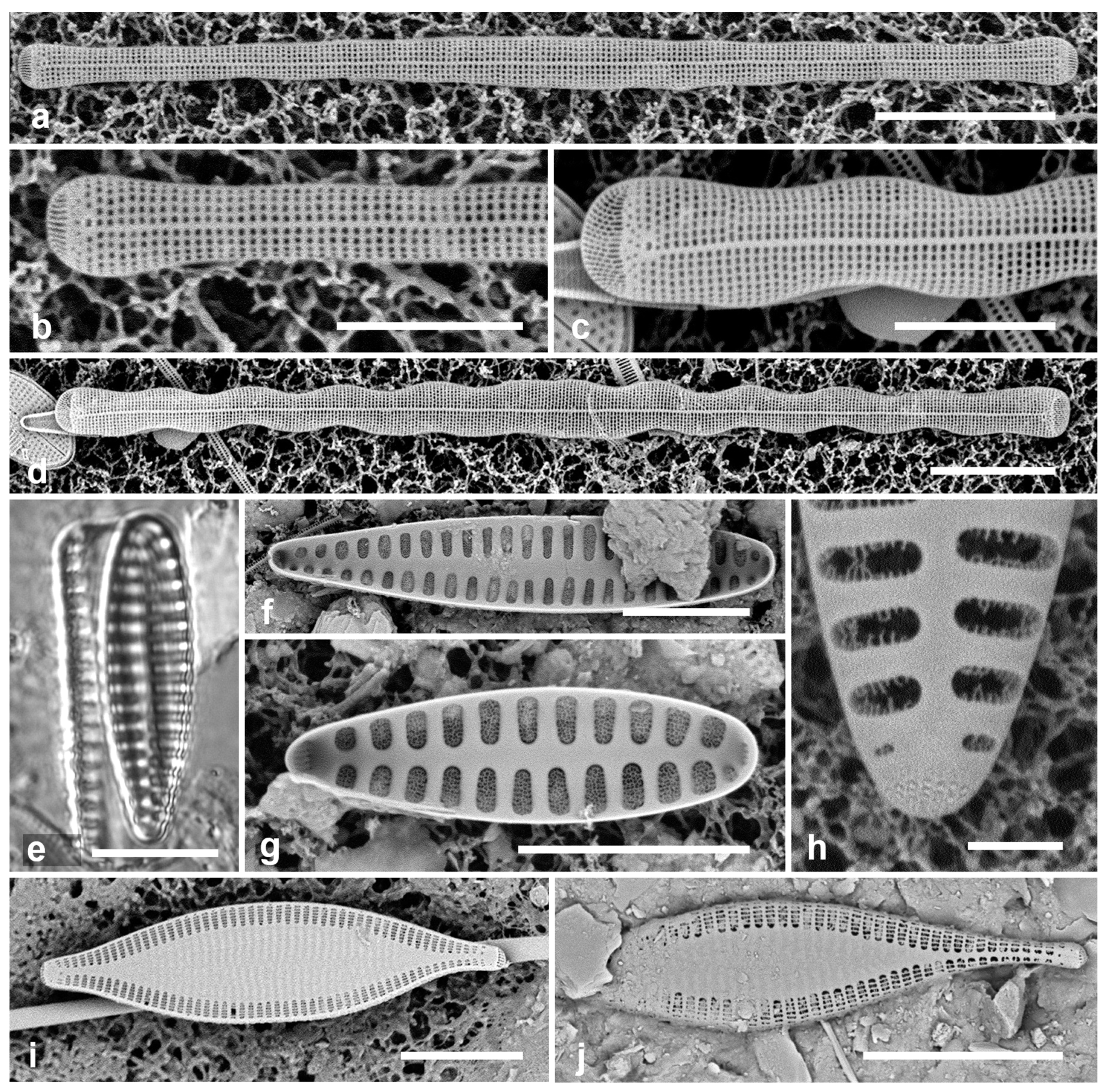
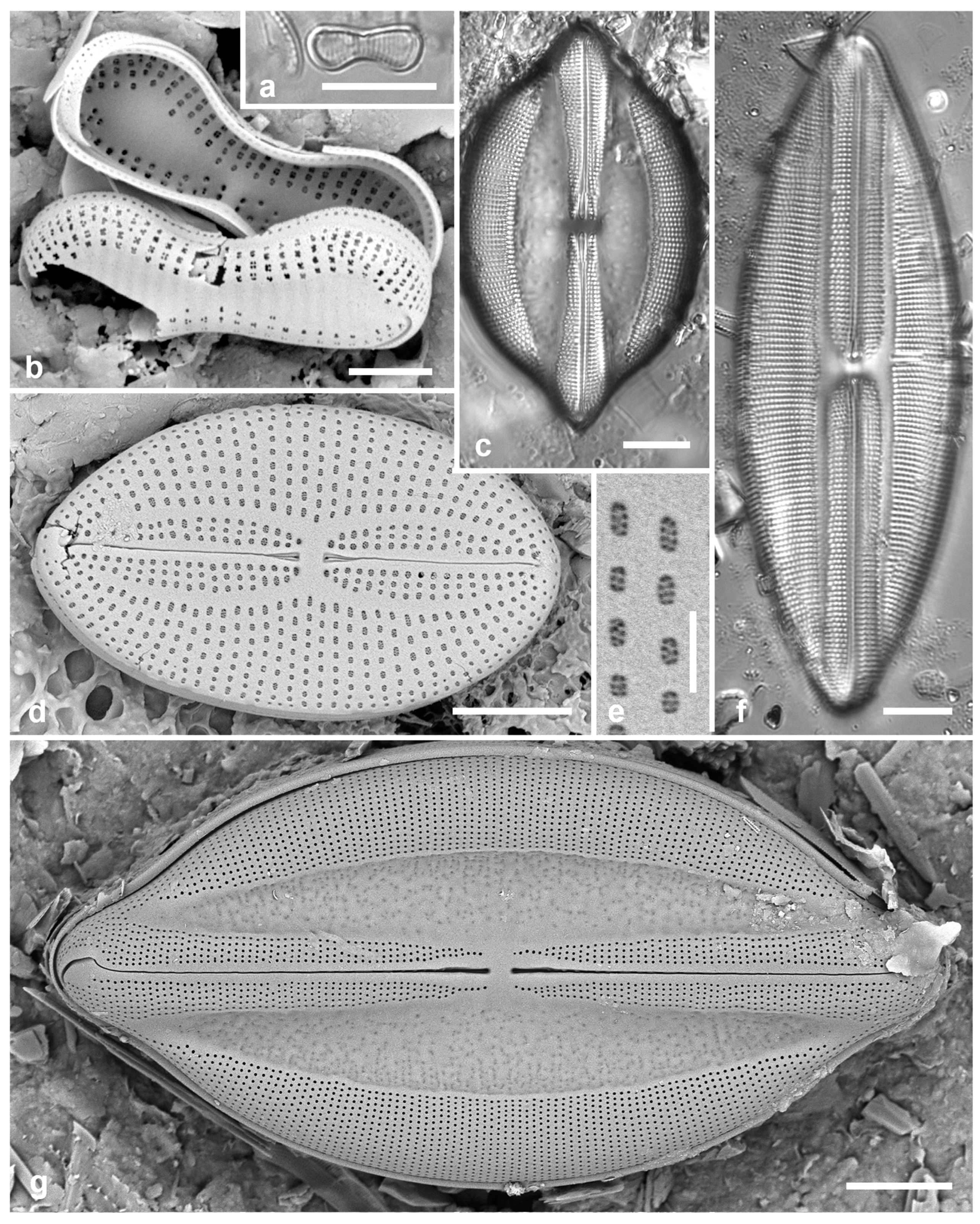
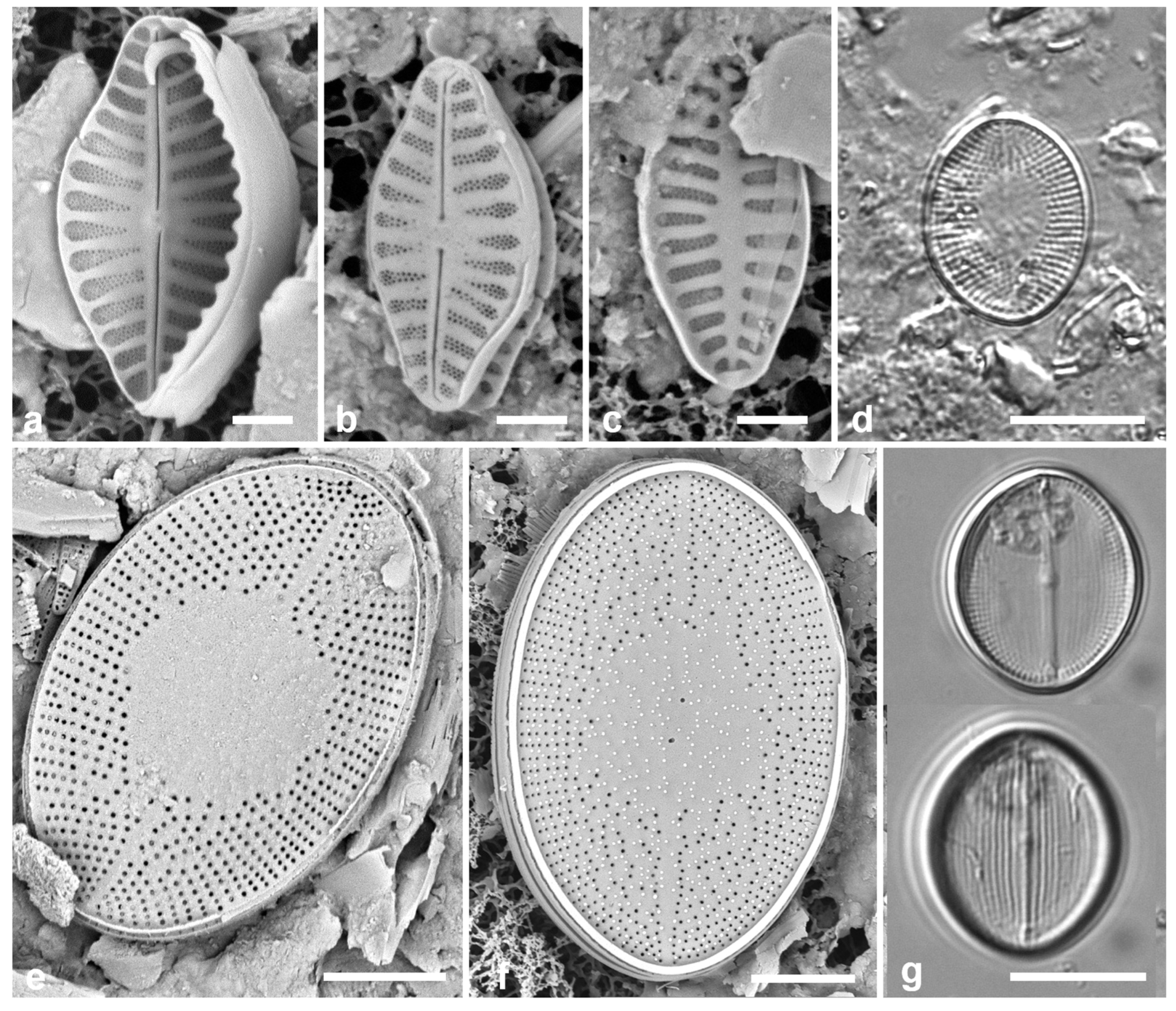
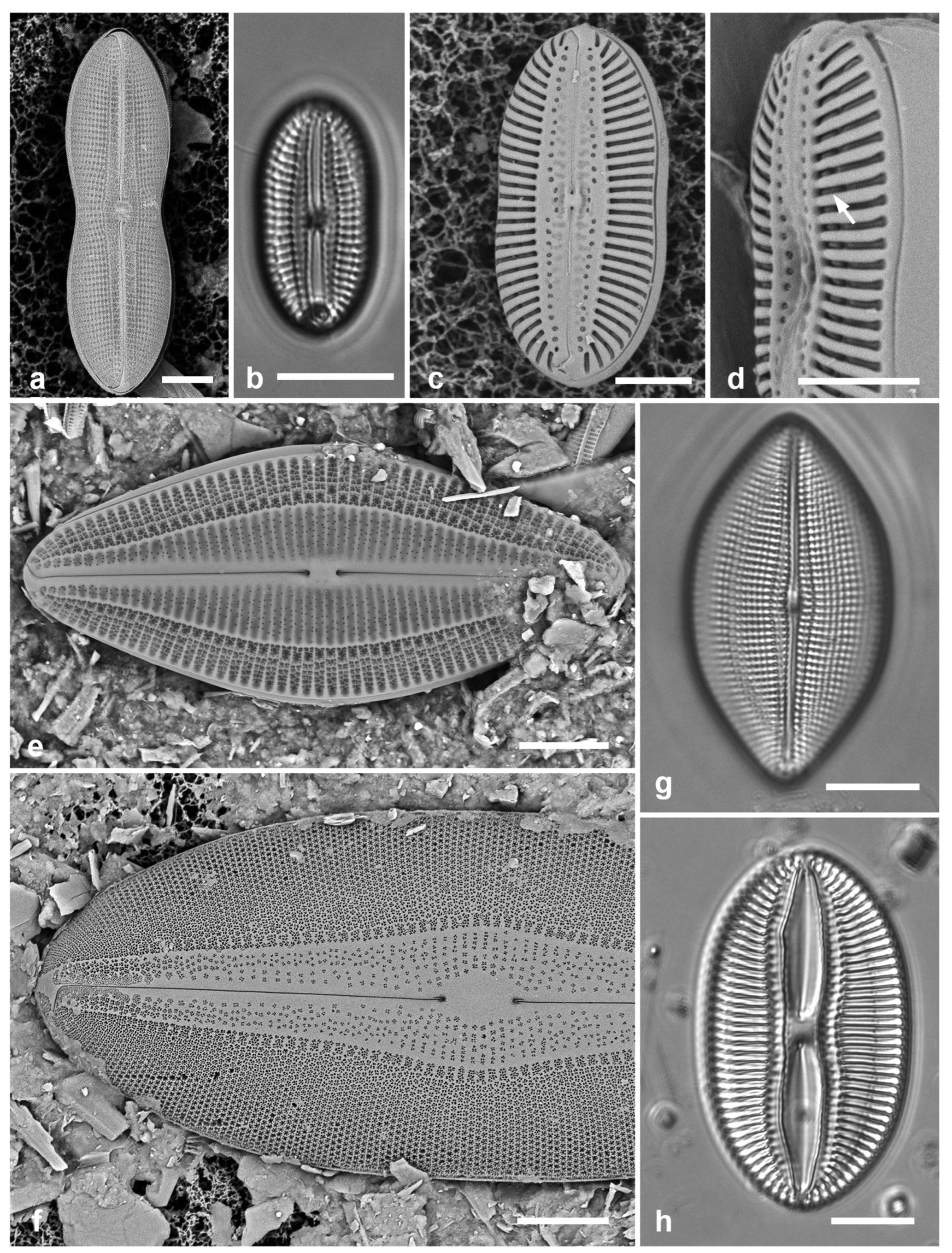
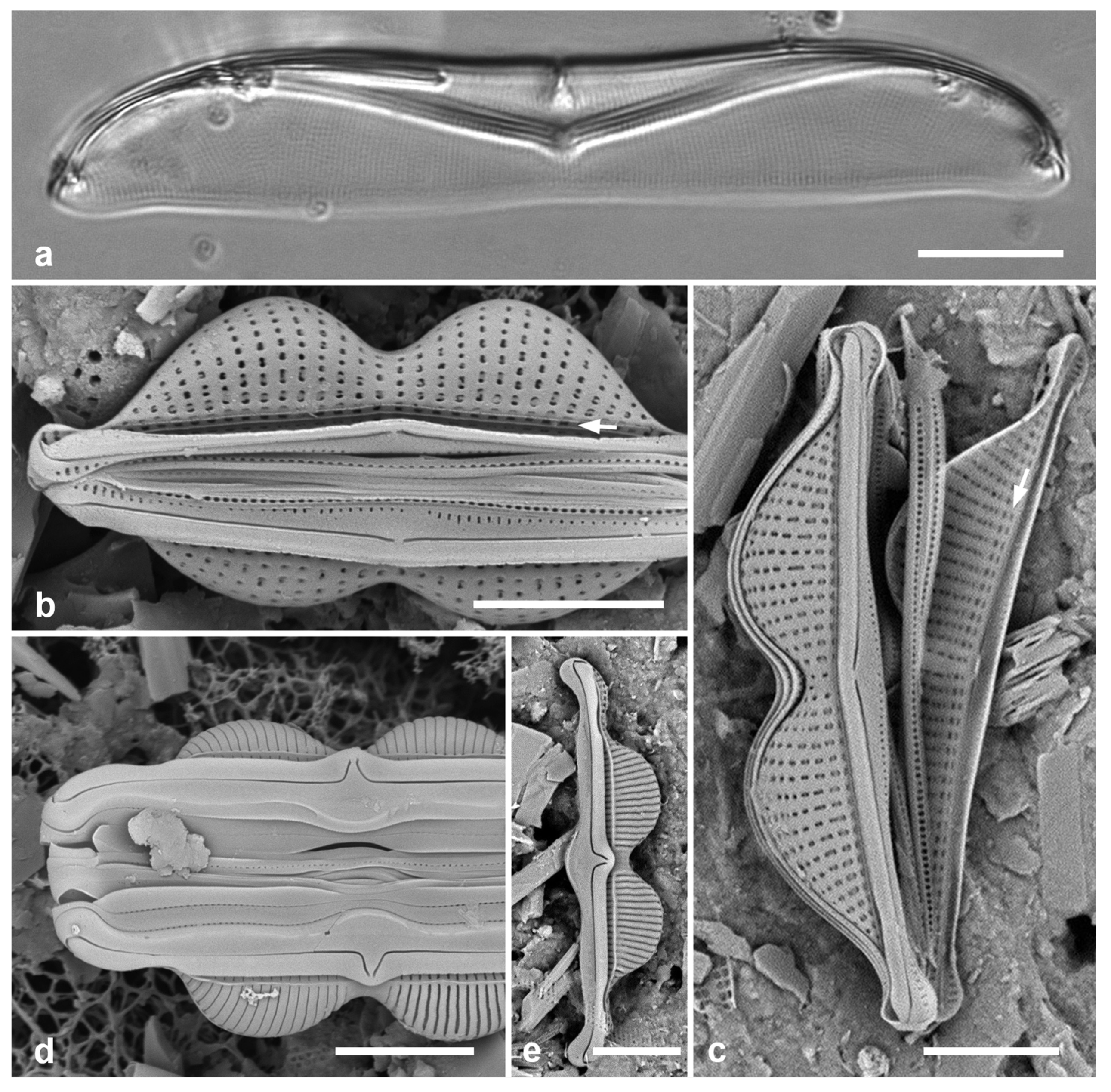
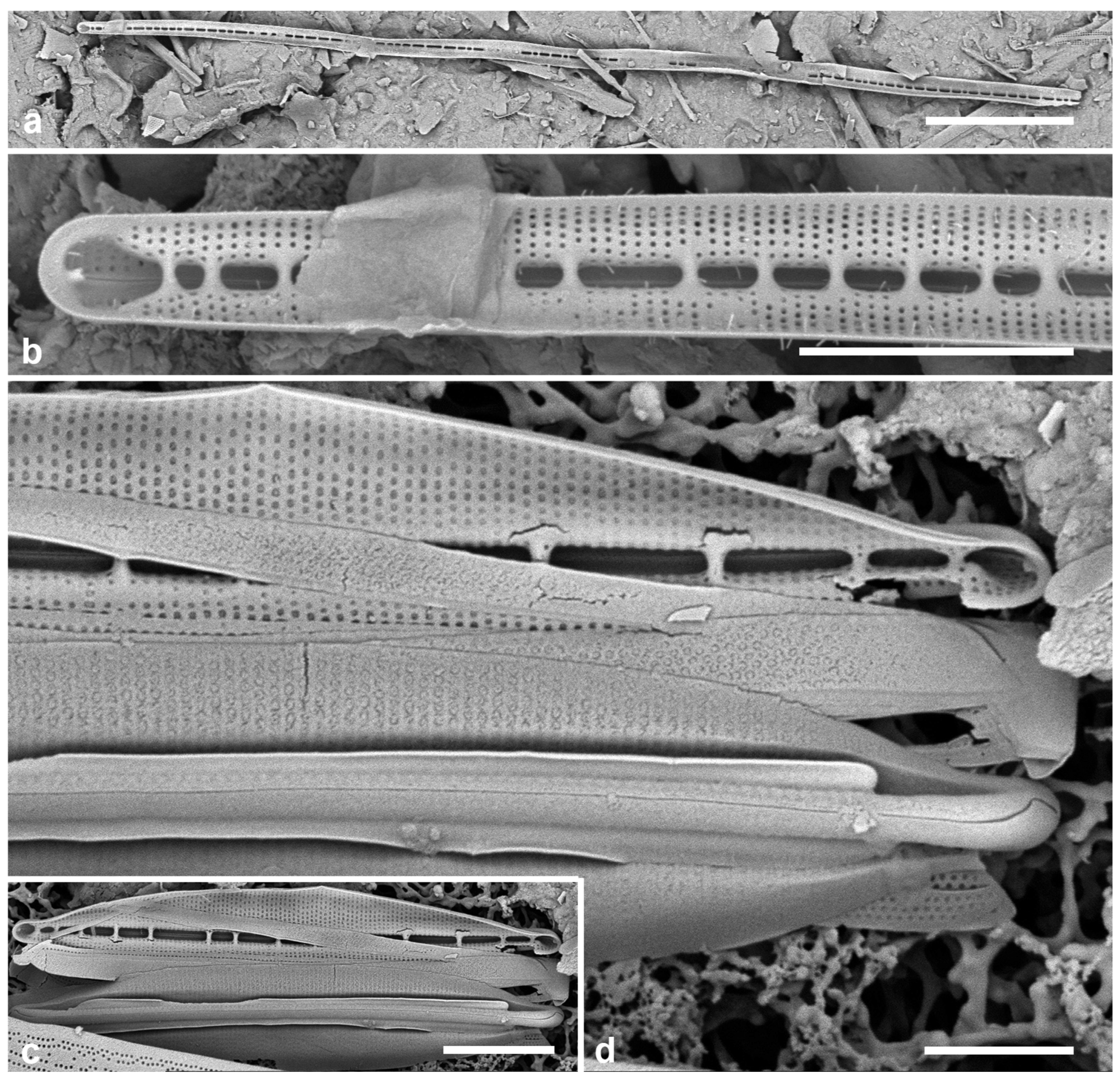
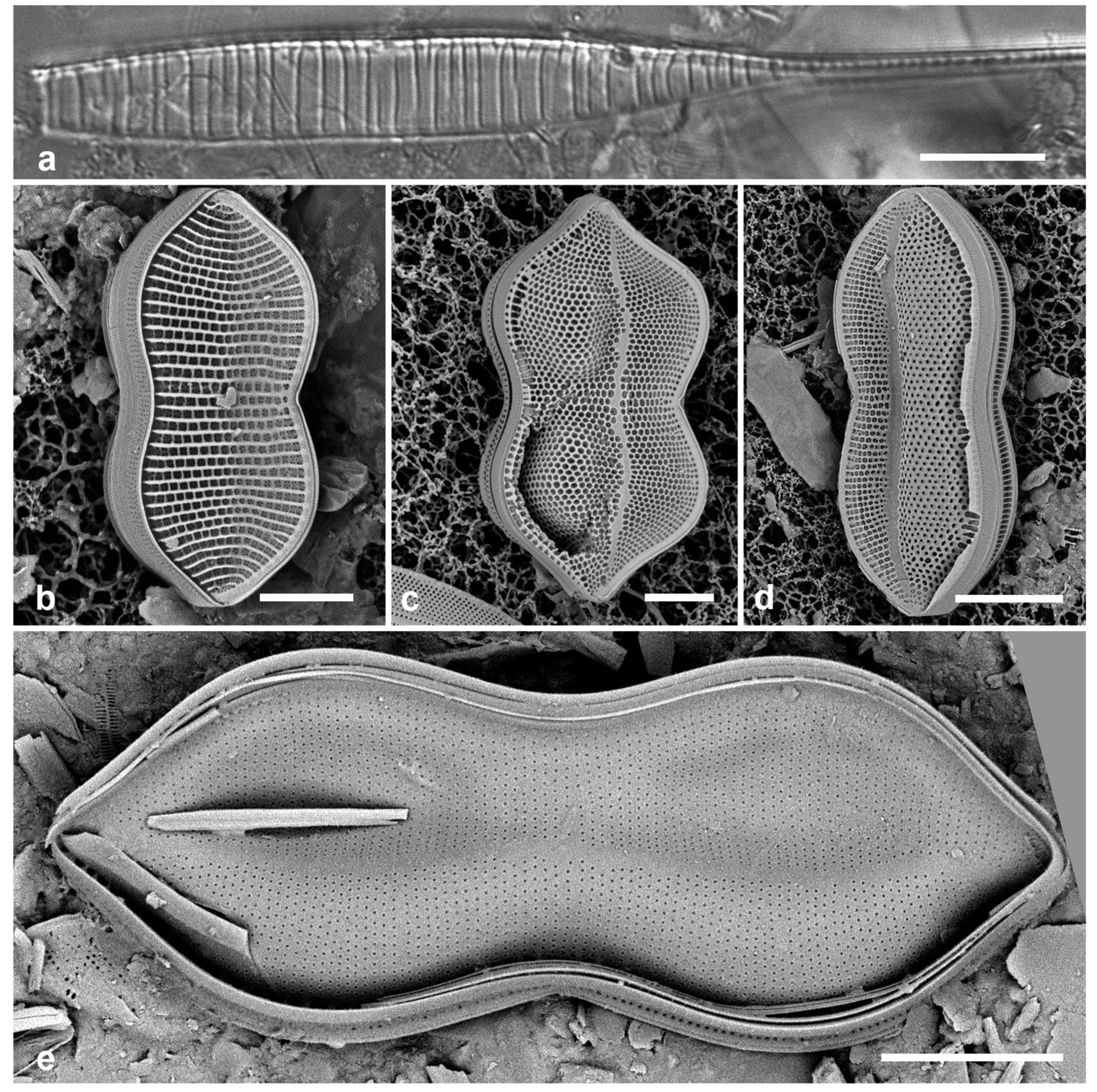
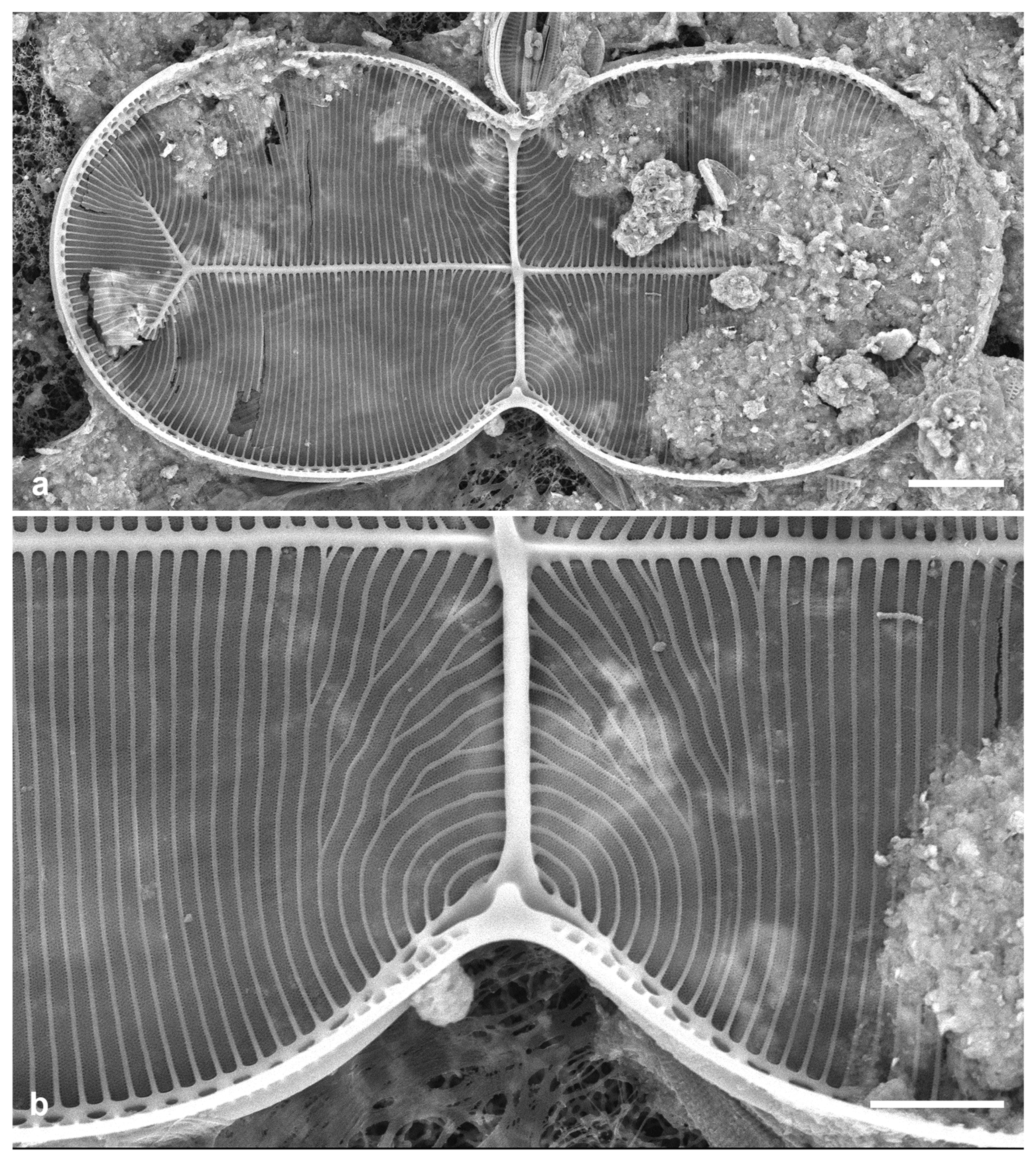
Figure 2.
Podosira and Paralia. (a) Podosira hormoides, valve view, LM. (b, c) Podosira montagnei in valve and girdle views, LM. (d–g) Paralia longispina. (d, e) Frustules in valve and girdle views, LM. (f, g) SEM images of frustules. (f) Separating valve and girdle bands in girdle view. (g) Separating valve and linking valve, the latter showing interior aspect with internal striae. Scale bars: (a–e) = 10 µm, (f, g) = 5 µm.
Figure 2.
Podosira and Paralia. (a) Podosira hormoides, valve view, LM. (b, c) Podosira montagnei in valve and girdle views, LM. (d–g) Paralia longispina. (d, e) Frustules in valve and girdle views, LM. (f, g) SEM images of frustules. (f) Separating valve and girdle bands in girdle view. (g) Separating valve and linking valve, the latter showing interior aspect with internal striae. Scale bars: (a–e) = 10 µm, (f, g) = 5 µm.
COSCINODISCALES Round & R.M.Crawford
Coscinodiscaceae Ehrenberg
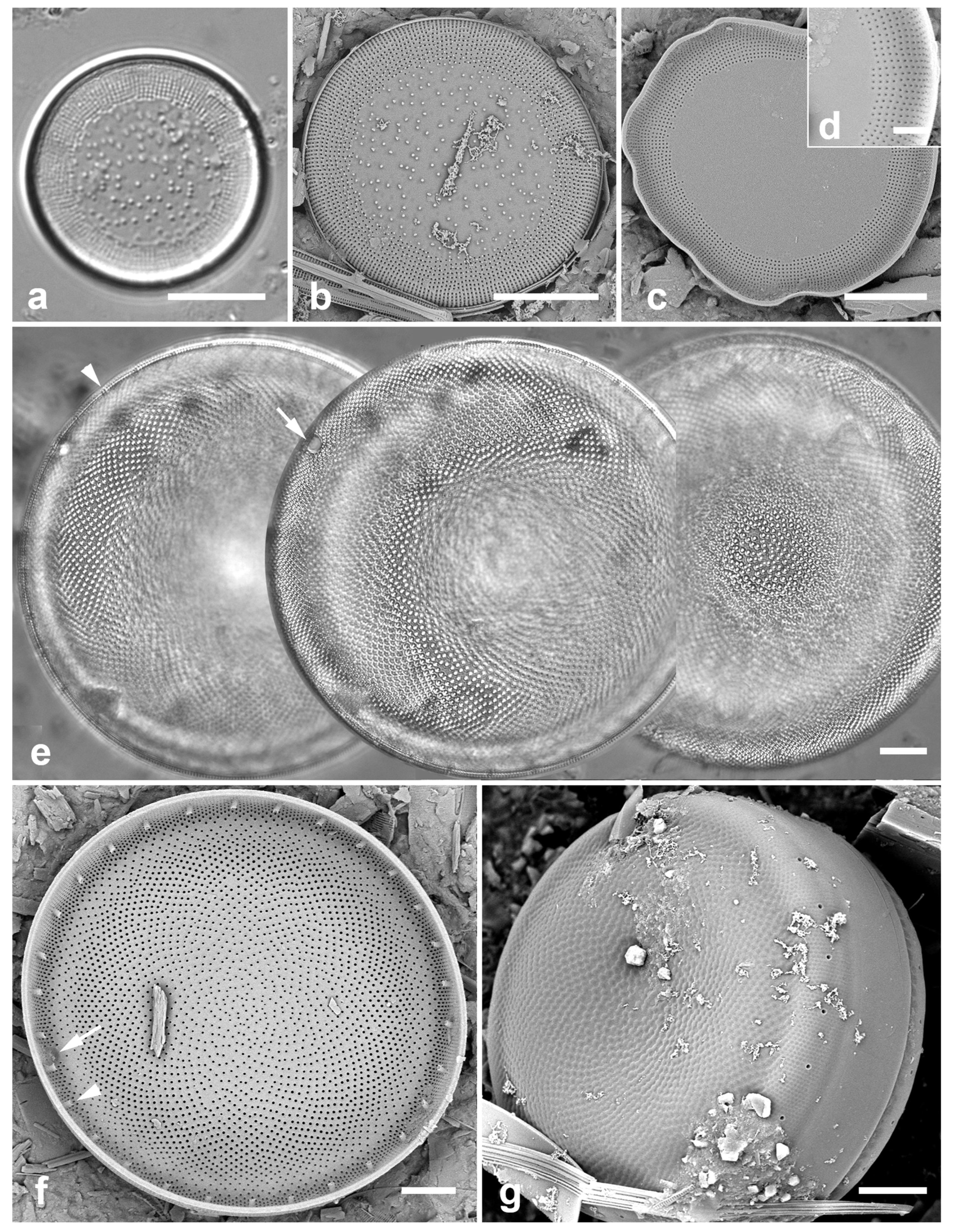
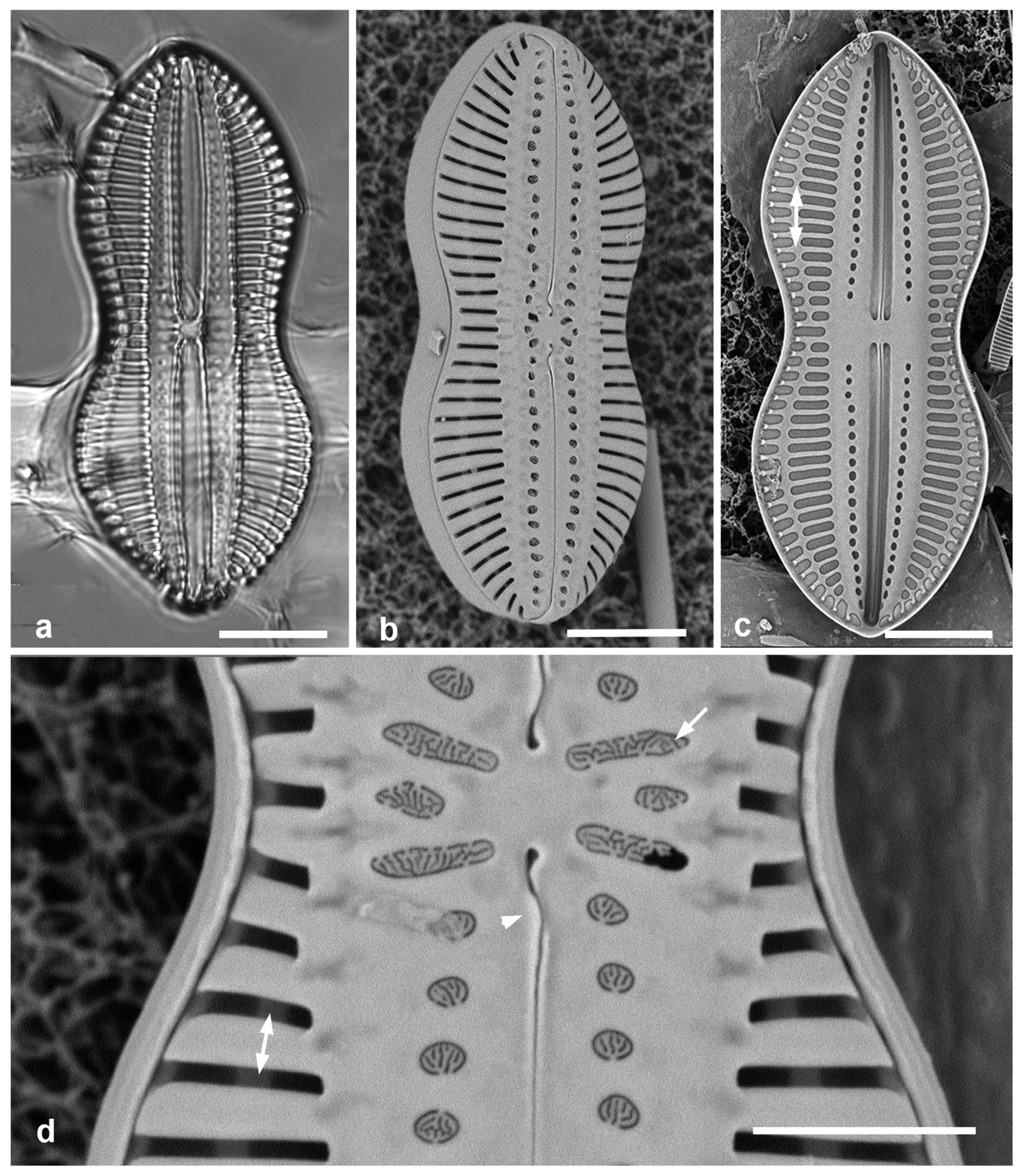
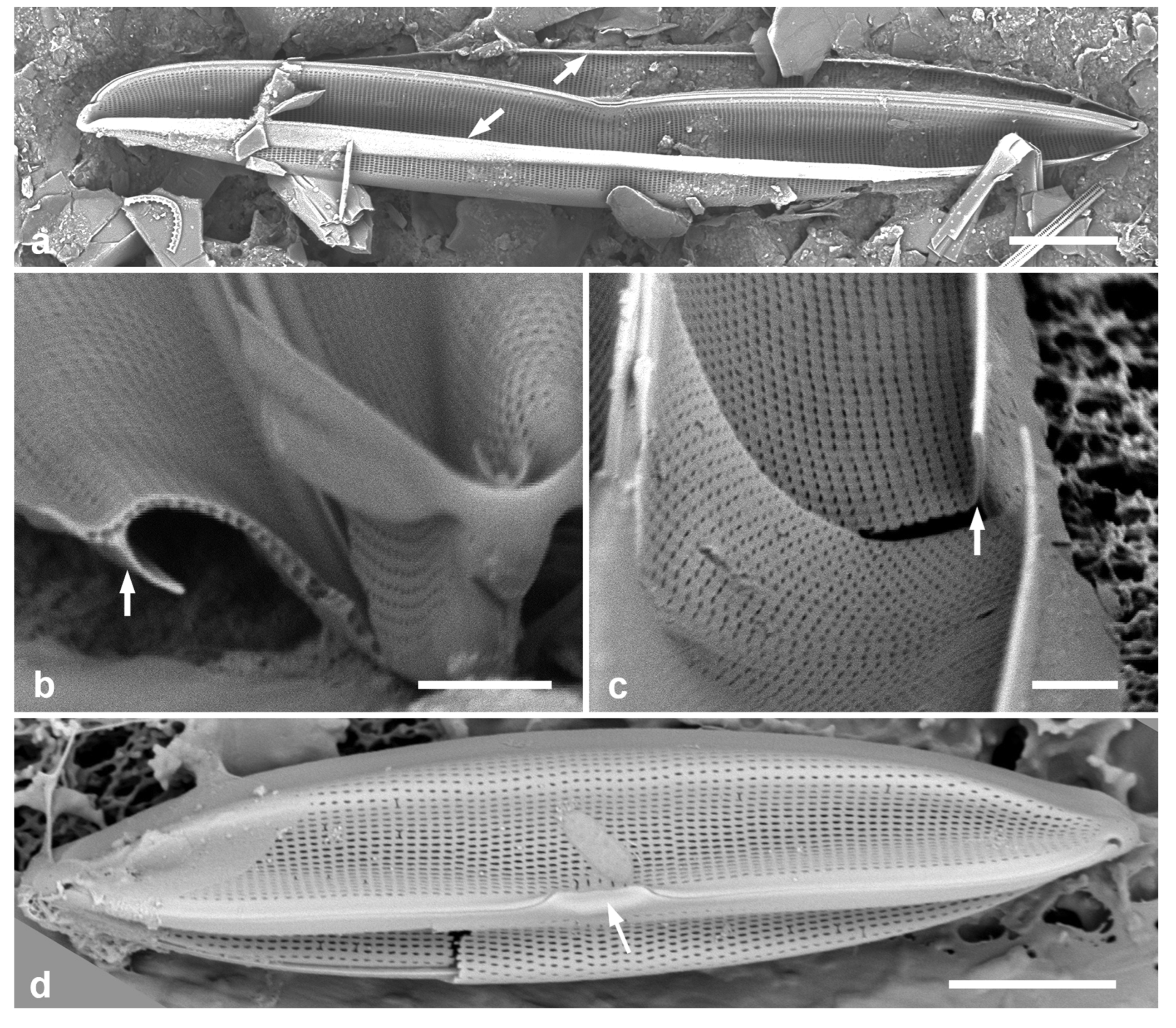
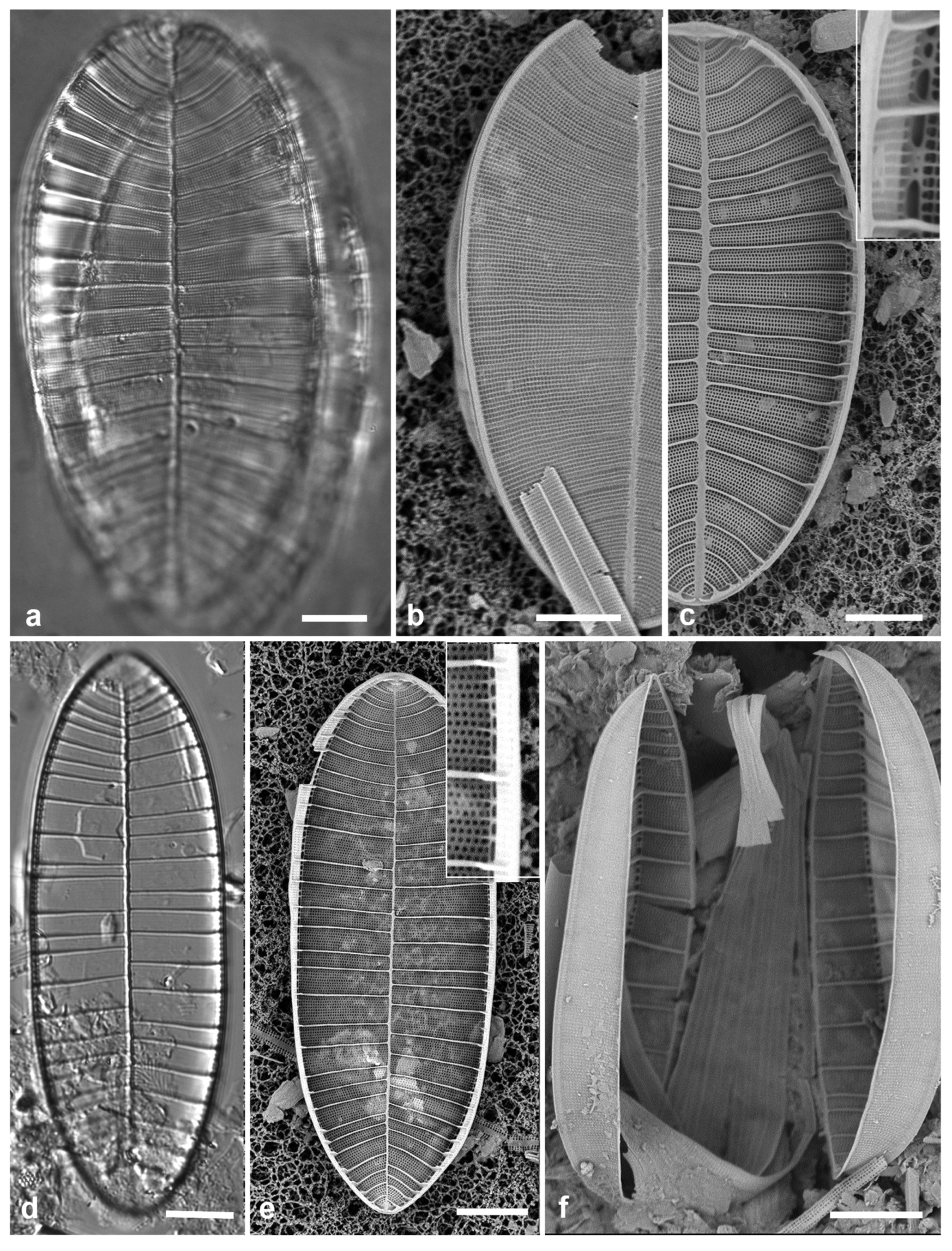
3.4. Ehrenbergiopsis Lobban, gen. nov.
Diagnostics: Circular valves with margin of radiating short striae, no processes of any kind.
Type species: Ehrenbergiopsis hauckii (Grunow) Lobban, comb. nov.
Etymology: Named for the similarity to Ehrenbergiulva.
Phycobank registration: [pending]
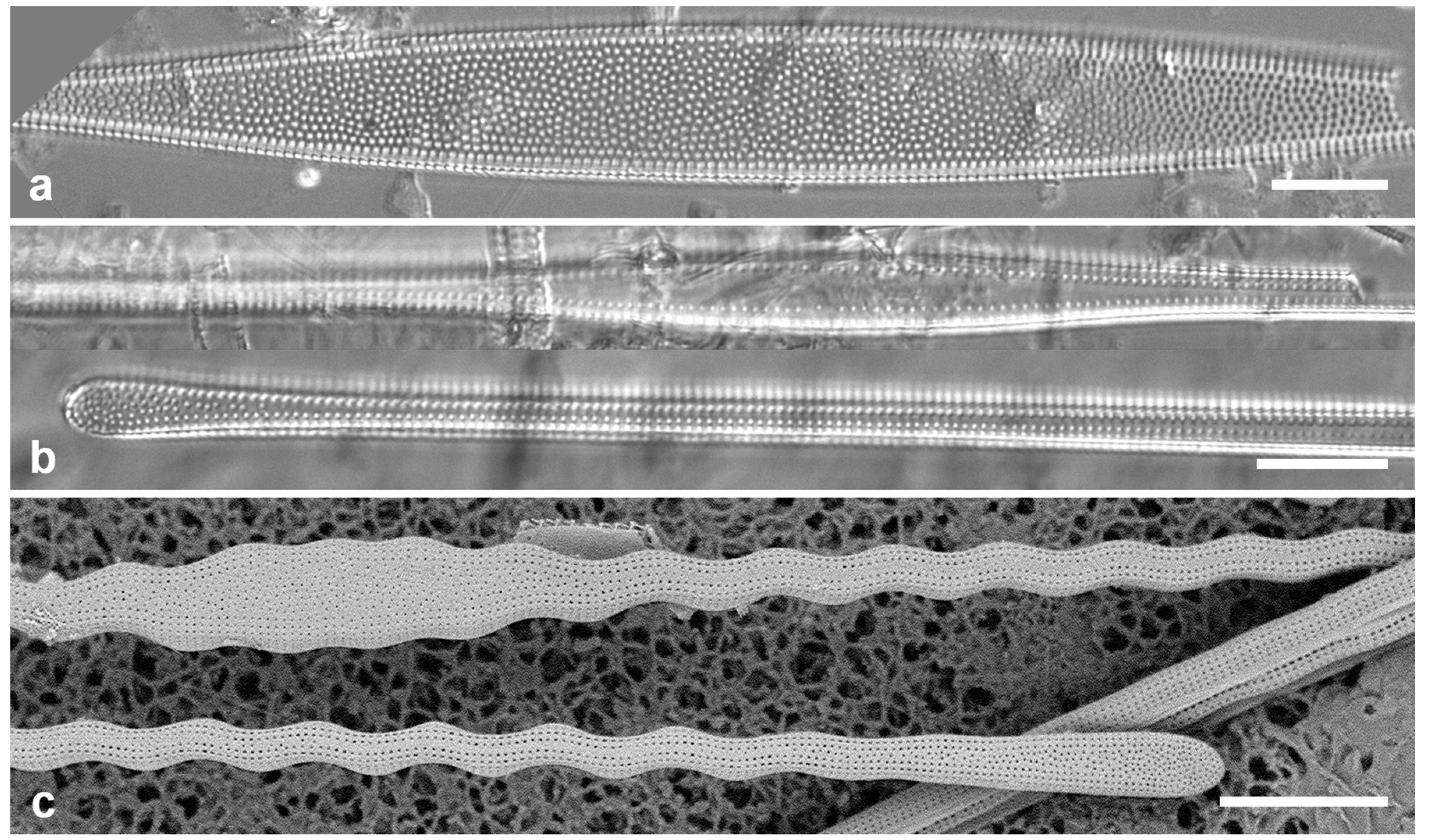
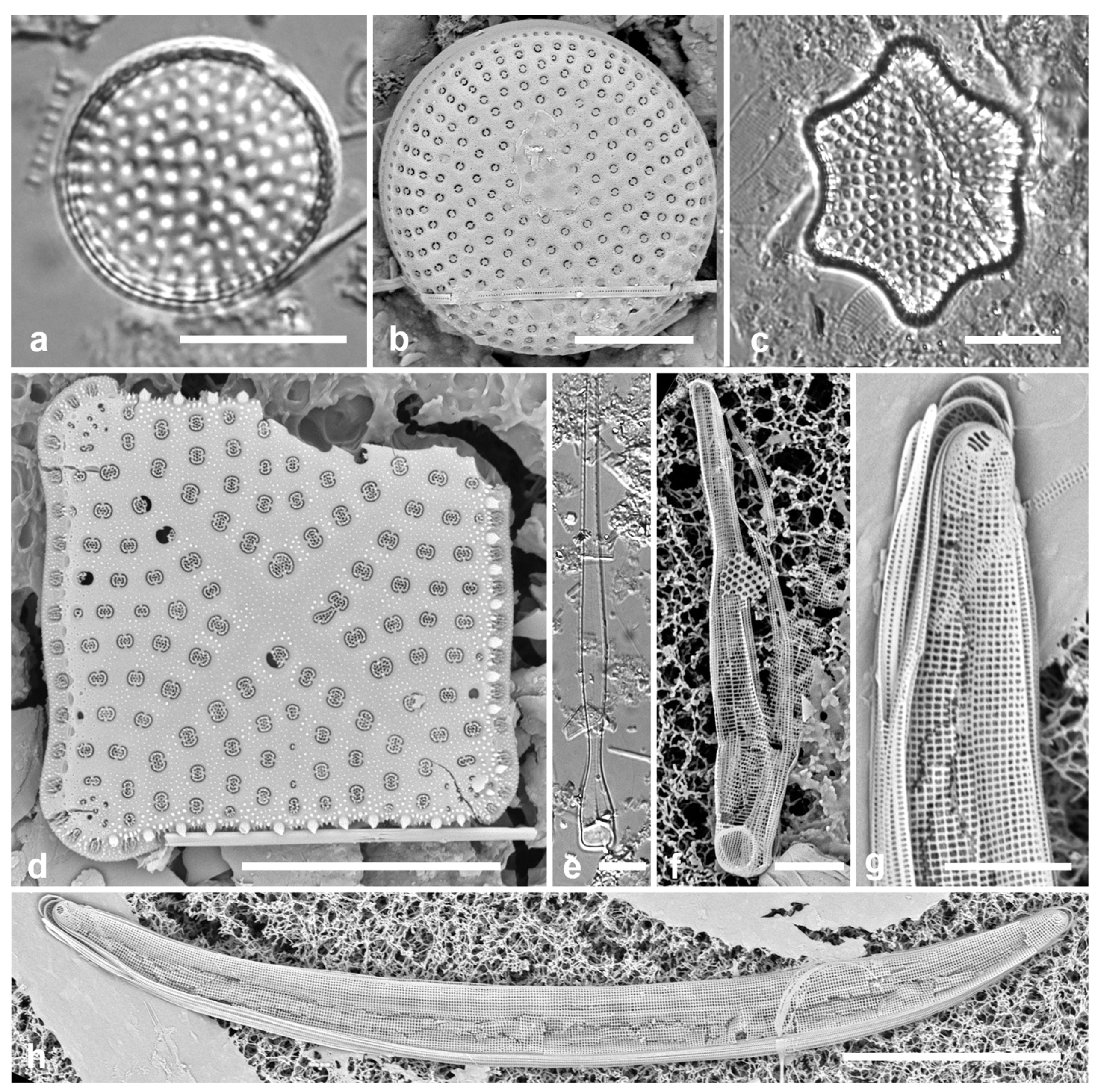
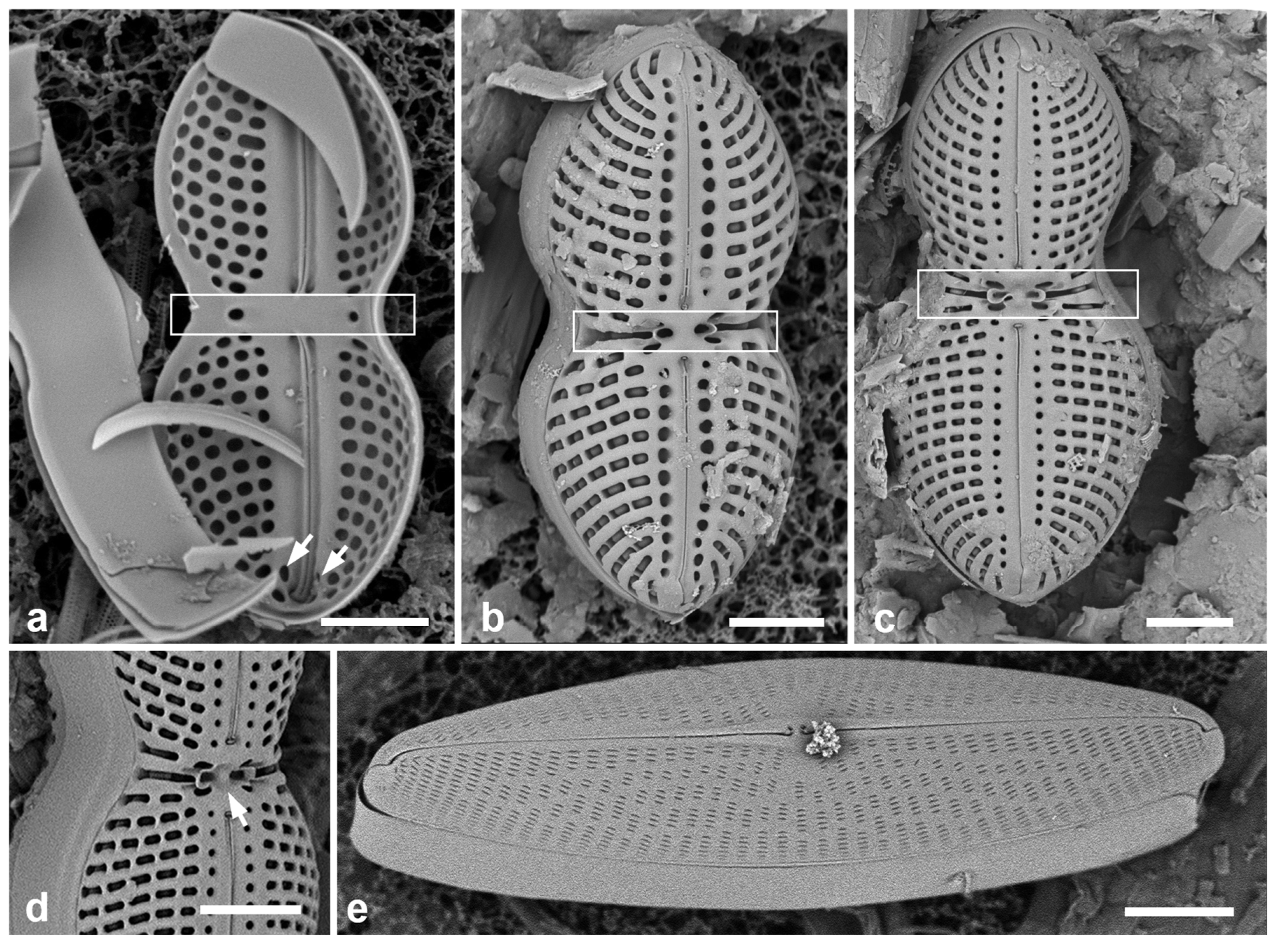
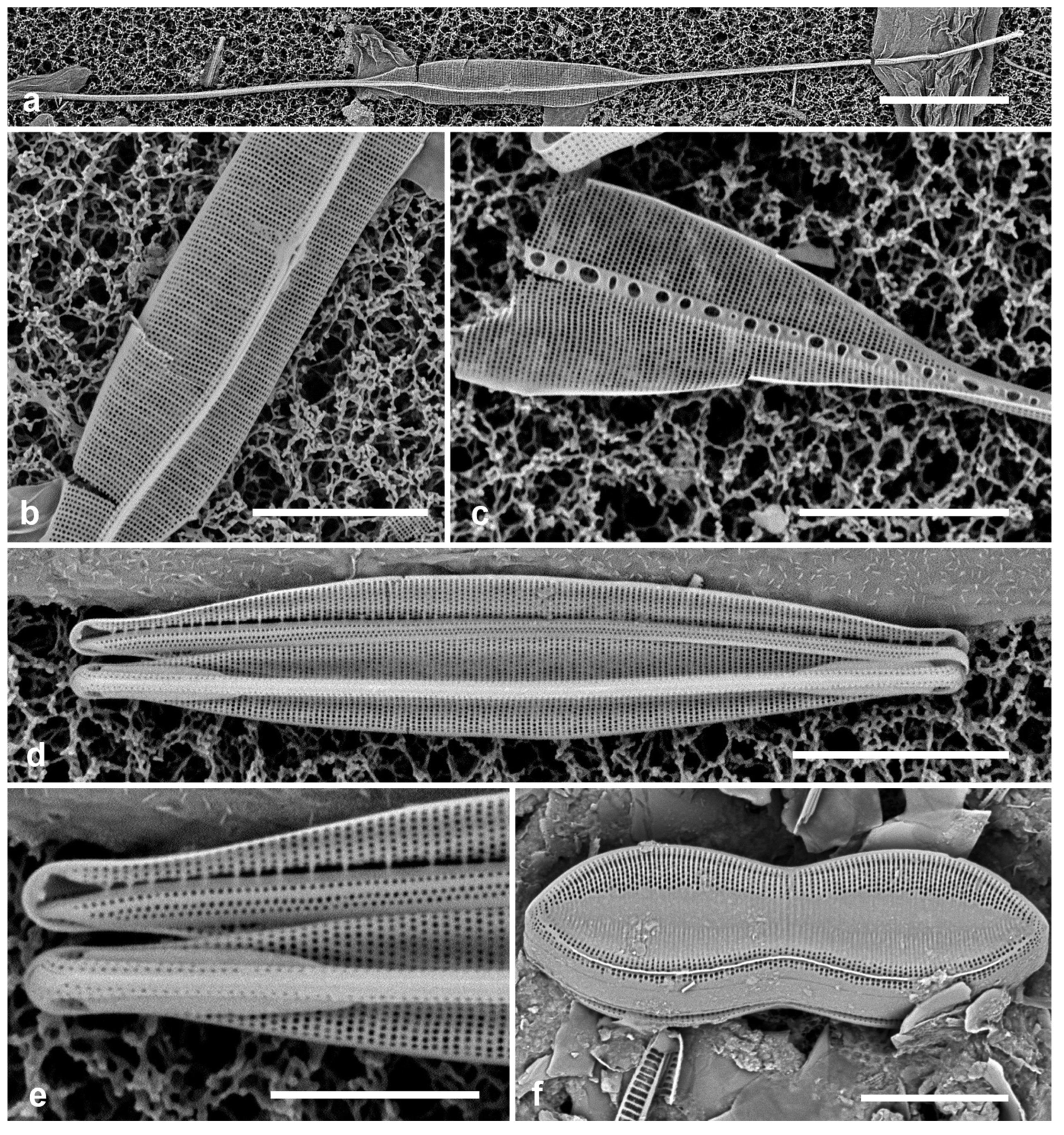
Figure 3.
Ehrenbergiopsis and Actinocyclus. (a–d) Ehrenbergiopsis hauckii. (a) LM. (b) SEM external of papillate valve. (c, d) SEM of internal valve faces showing absence of fultoportulae and rimoportulae. (e–g) Actinocyclus decussatus. (e) Series of focal planes from low to high of a slightly tilted valve. Arrow points to pseudonodulus, arrowhead to one of the many rimoportulae. (f) Interior aspect in SEM showing pseudonodulus (arrow) and rimoportulae (arrowhead). (g) Frustule in oblique view showing valve contours, SEM. Scale bars: (a–c, e–g) = 10 µm, (d) = 2 µm.
Figure 3.
Ehrenbergiopsis and Actinocyclus. (a–d) Ehrenbergiopsis hauckii. (a) LM. (b) SEM external of papillate valve. (c, d) SEM of internal valve faces showing absence of fultoportulae and rimoportulae. (e–g) Actinocyclus decussatus. (e) Series of focal planes from low to high of a slightly tilted valve. Arrow points to pseudonodulus, arrowhead to one of the many rimoportulae. (f) Interior aspect in SEM showing pseudonodulus (arrow) and rimoportulae (arrowhead). (g) Frustule in oblique view showing valve contours, SEM. Scale bars: (a–c, e–g) = 10 µm, (d) = 2 µm.
Ehrenbergiopsis hauckii (Grunow) Lobban, comb. nov.
Figure 3a–c
Basionym:
Coscinodiscus hauckii Grunow in Van Heurck 1881,
Synopsis, pl. 94,
Figure 29
Synonyms: Coscinodiscus (Hauckii var.?) mesoleia Grunow in Van Heurck 1881
Coscinodiscus mesoleius (Grunow) Rattray 1890
Ehrenbergia hauckii (Grunow) Witkowski et al. 2000
Ehrenbergiulva hauckii (Grunow) Witkowski et al. 2004
References: Hustedt 1927–1930, p. 387, figs 200a, b
Previous Micronesia records: Guam (Lobban & Witkowski 2024b, figs 46, 47, as Coscinodiscus hauckii)
Yap samples: Y26C
Dimensions: Diam.: 24–29, striae 22–23 in 10 µm (at margin)
Phycobank registration: [pending]
Comments: In Yap we found several specimens, one valve without papillae, most with papillae shown in
Figure 3b. Those could be classified as
C. hauckii var.
mesoleia, but Lobban in Lobban & Witkowski (2024b) made a case against the variety, while missing Witkowski’s earlier new genus names. Unfortunately, the nomenclature is not resolved yet, since Witkowski et al. (2000, p. 30, pl. 2, figs 12–18) described the new genus (as
Ehrenbergia) based on
E. granulosa, noting a ring of strutted processes seen in SEM, whereas our specimens, which well match the description in Hustedt (1927–1930), entirely lack processes (new specimens shown in
Figure 3c and inset). Hustedt noted it as a warm-water species. The LM in Witkowski et al. 2000 (pl. 2,
Figure 11), from San Francisco, has no visible margin of striae and is unrecognizable as this species. The problem can be resolved by transferring the species to yet another new genus, with one species.
Hemidiscaceae (Hendey) Simonsen
3.5. Actinocyclus decussatus A.Mann 1925 Figure 3e–g
References: Mann 1925 p. 12; pl. 2,
Figure 1, 2; Witkowski et al. 2000, p. 21, pl. 4,
Figure 4, pl. 5, figs 2, 3 (as
A. gallicus Meister)
Yap samples: Y16B, Y18C, Y25H-1, -2, Y36-3
Dimensions: Diam. 76–94 µm, areolar density varying across the surface: 7–9 in 10 µm in the main part of the valve, fewer on the valve–mantle junction and across the dome, 15 in 10 µm in the mantle
Diagnostics: Large cells with concentric waves on the valve face (i.e., depressed ring inside the periphery/mantle, followed by an elevated ring surrounding a central depression—
Figure 3g) and curving decussate striae. Segments not obvious but marked by 30 or more rimoportulae on the mantle.
Comments: Specimens were abundant in Y36-3 and Y25H-1, along with
Actinocyclus subtilis (see below). Meister (1937, p. 262, pl. 6, figs 3, 4) described
A. gallicus as very rare in a fossil sample from France, and refered to Lefébure’s (1935) report on the diatoms in that material, which was reported to be Aquitanian Stage, lower Miocene Epoch, ca. 20 Mya. The valve was said to be strongly domed with a flat central part about 20 µm diameter, and a 4-µm hyaline center. Although Witkowski et al. (2000) mention a distinct central area, it is not apparent in their image. They reported it common in samples from New Caledonia and the Caribbean. The elevations in the valve face of our specimens are hard to determine from serial focal planes but are clearly shown in an oblique SEM of a frustule (
Figure 3g). We propose
A. decussatus as a more probable hypothesis for the identity of the Yap and New Caledonia specimens, since Mann’s samples were recent sediments in the same region. We judge
A. gallicus less likely given its geological age, location, and presence of a flat-topped dome with central hyaline area. New record for Micronesia.
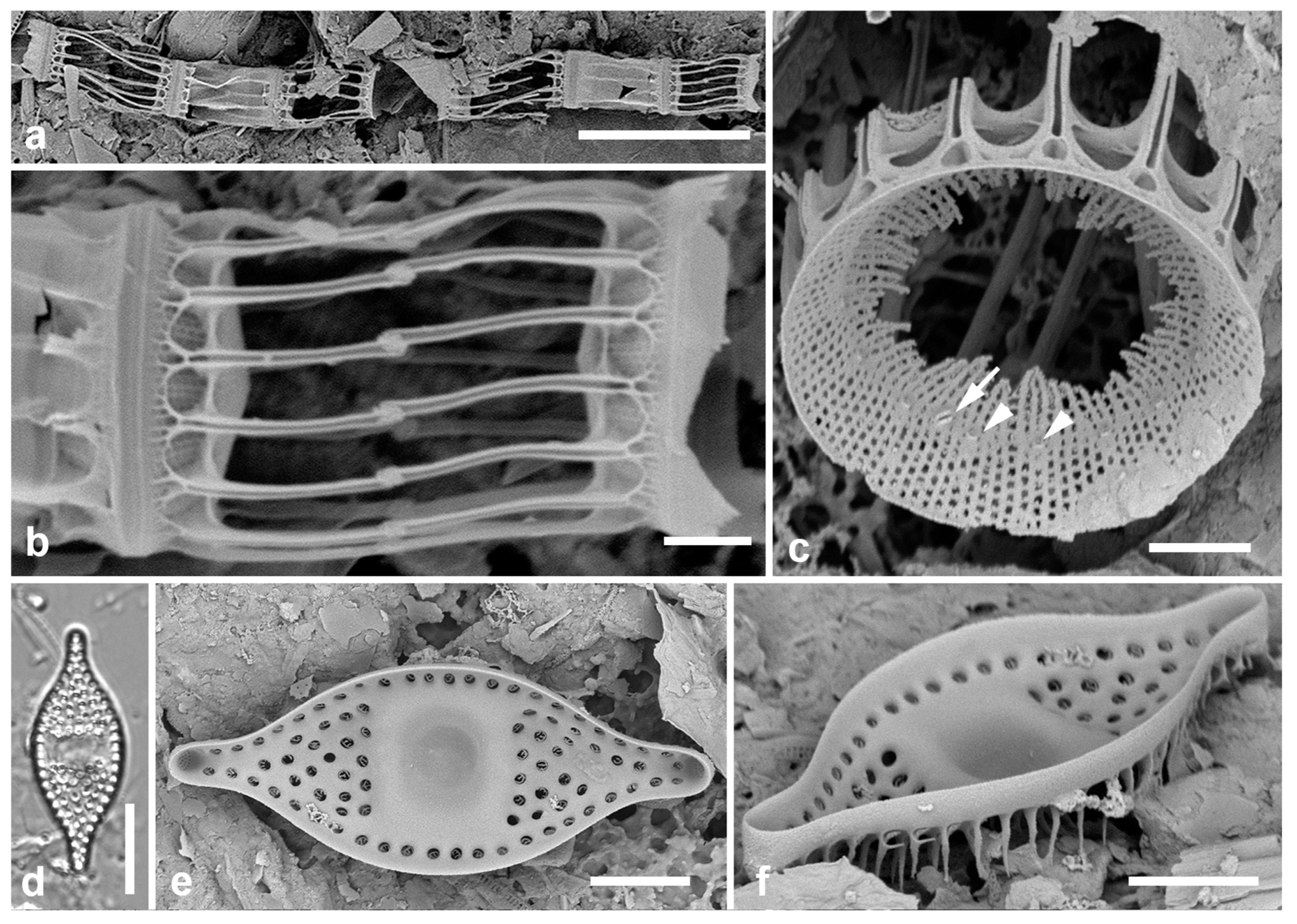
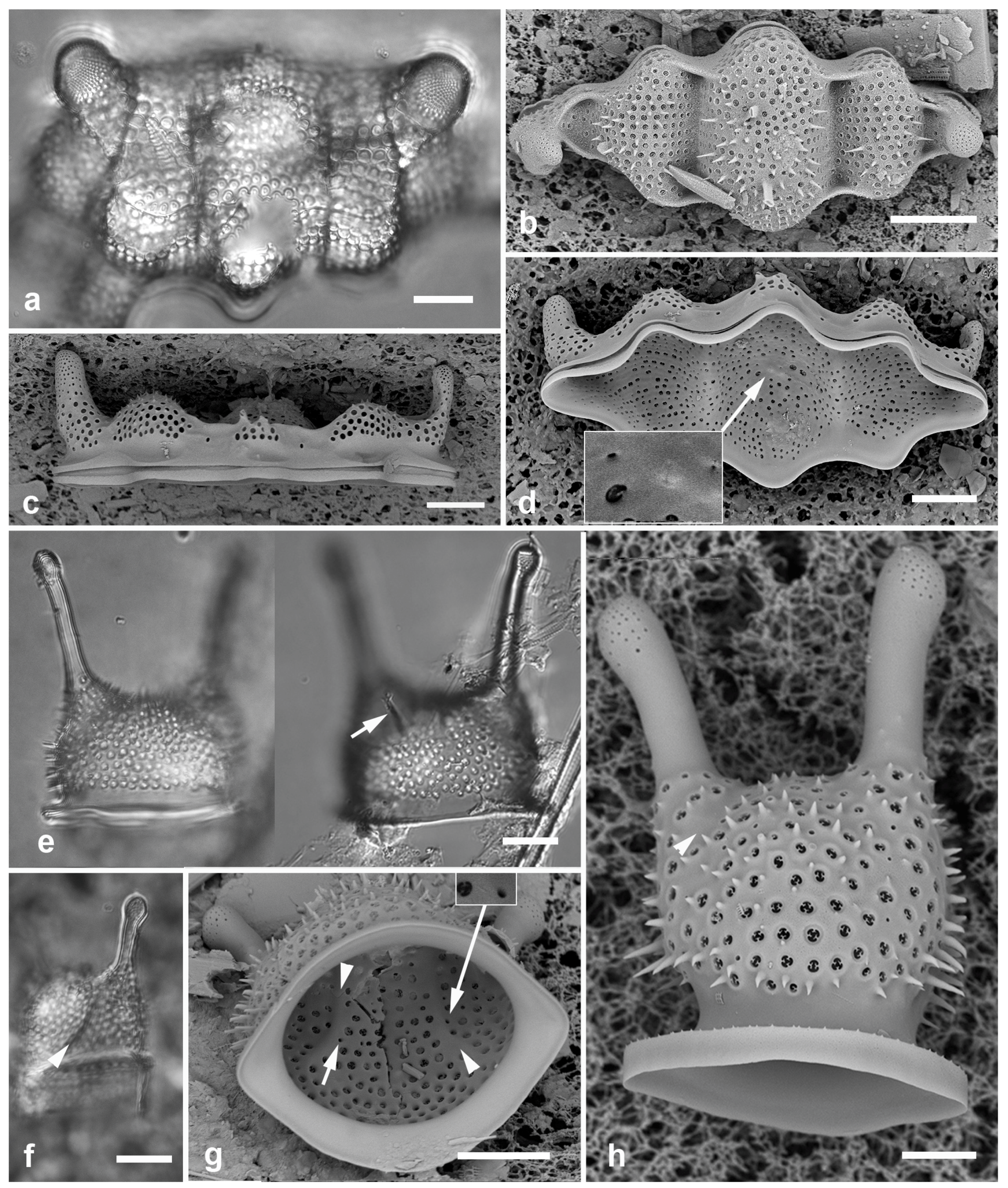
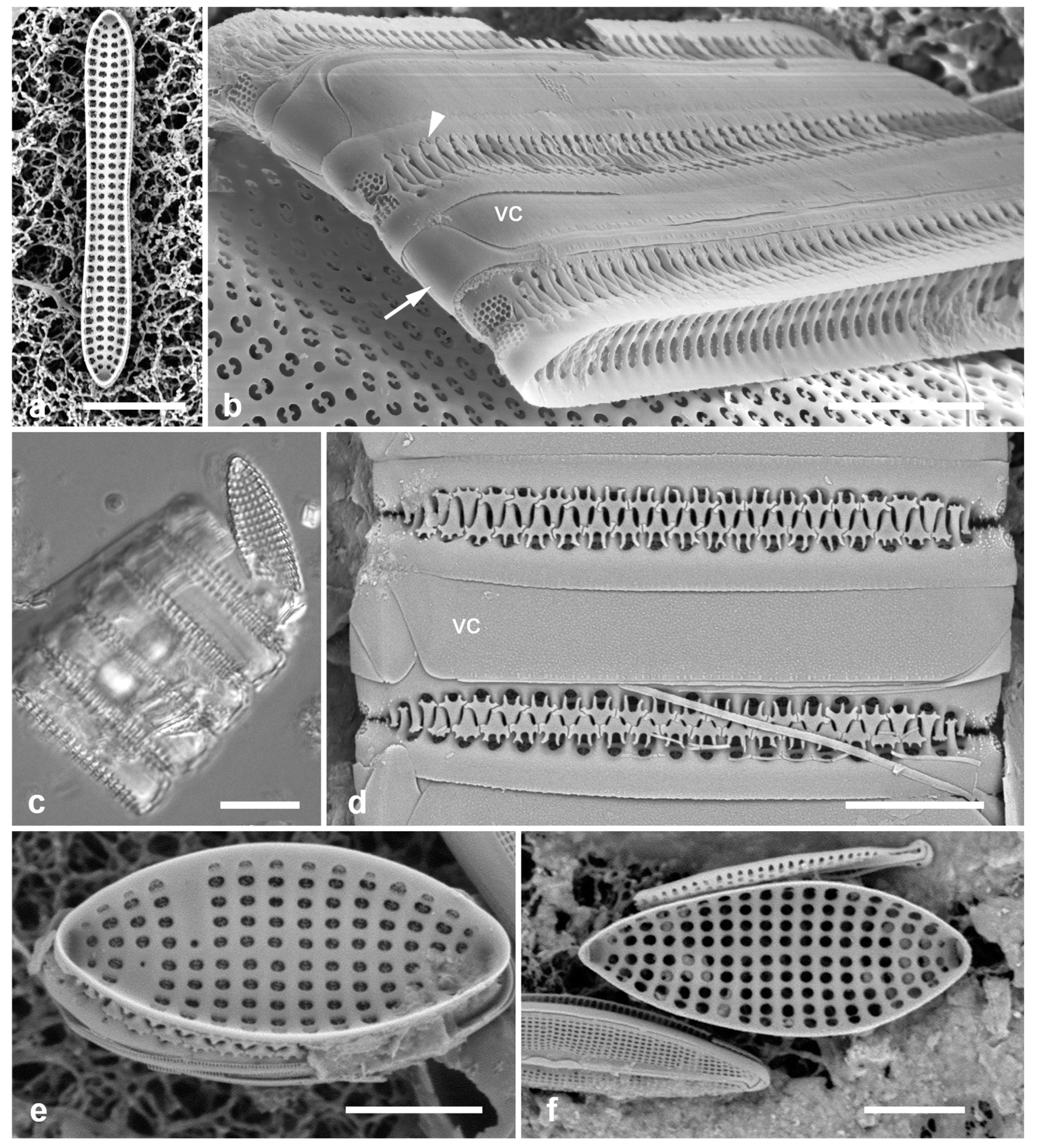
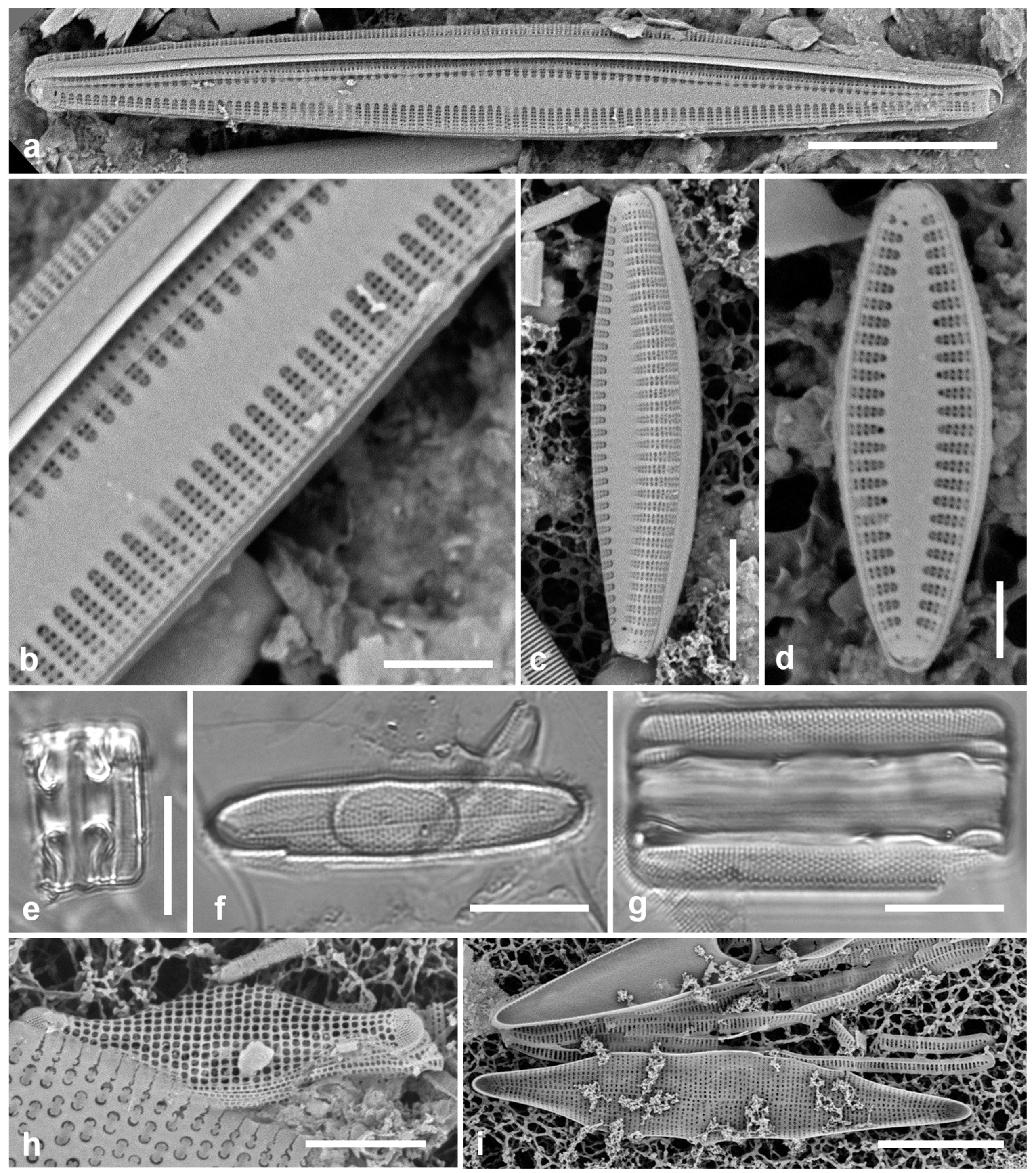
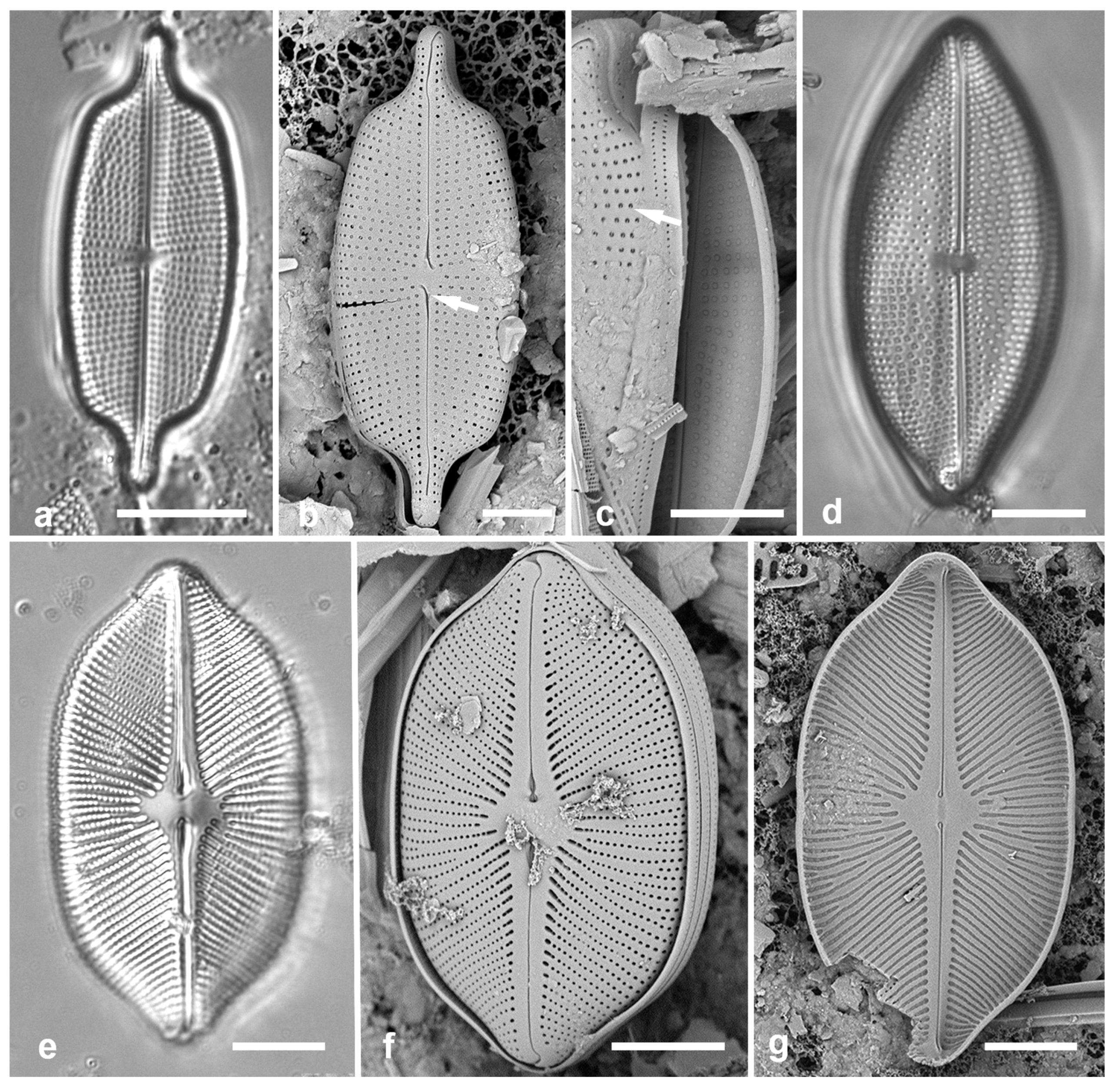
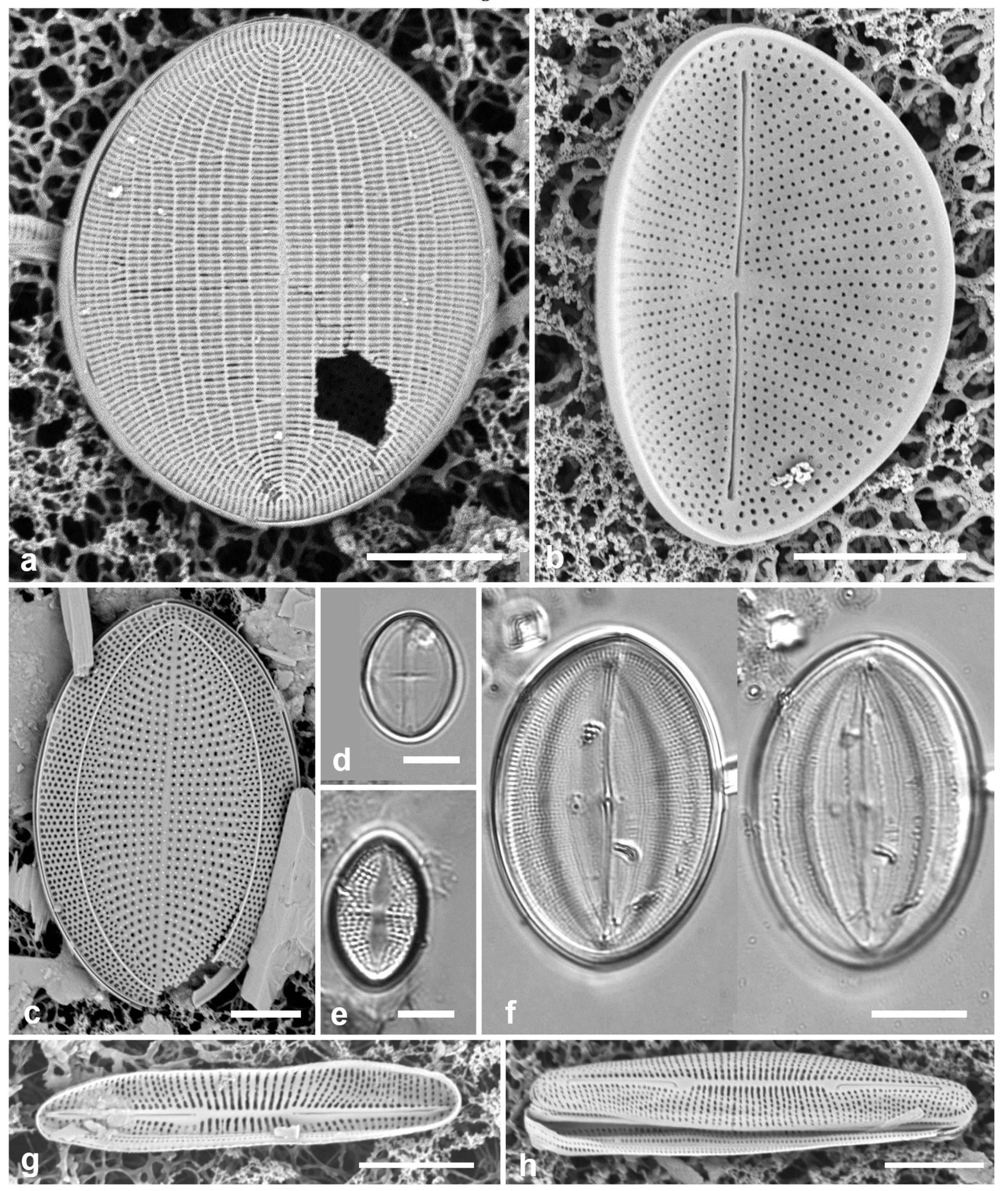
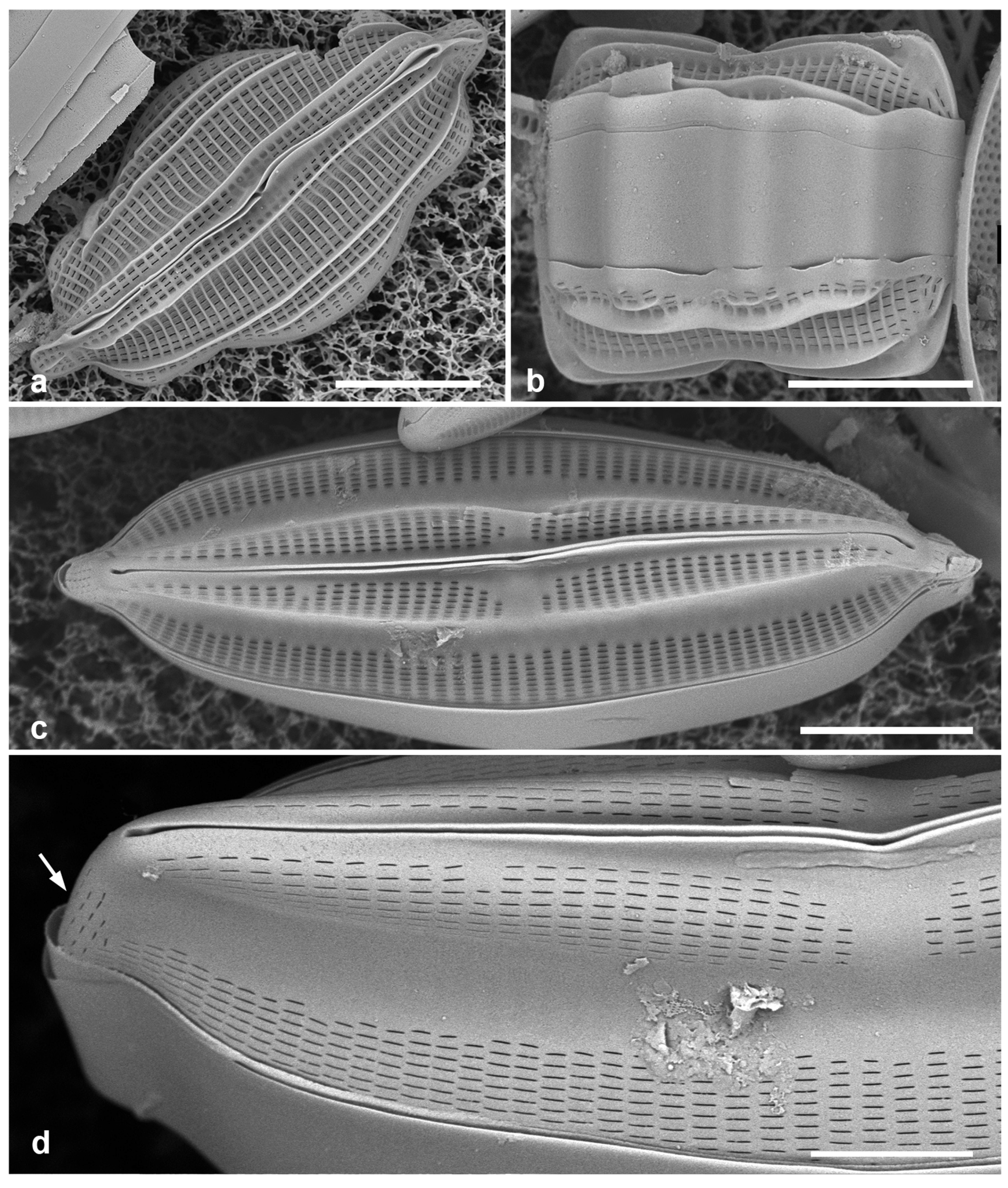
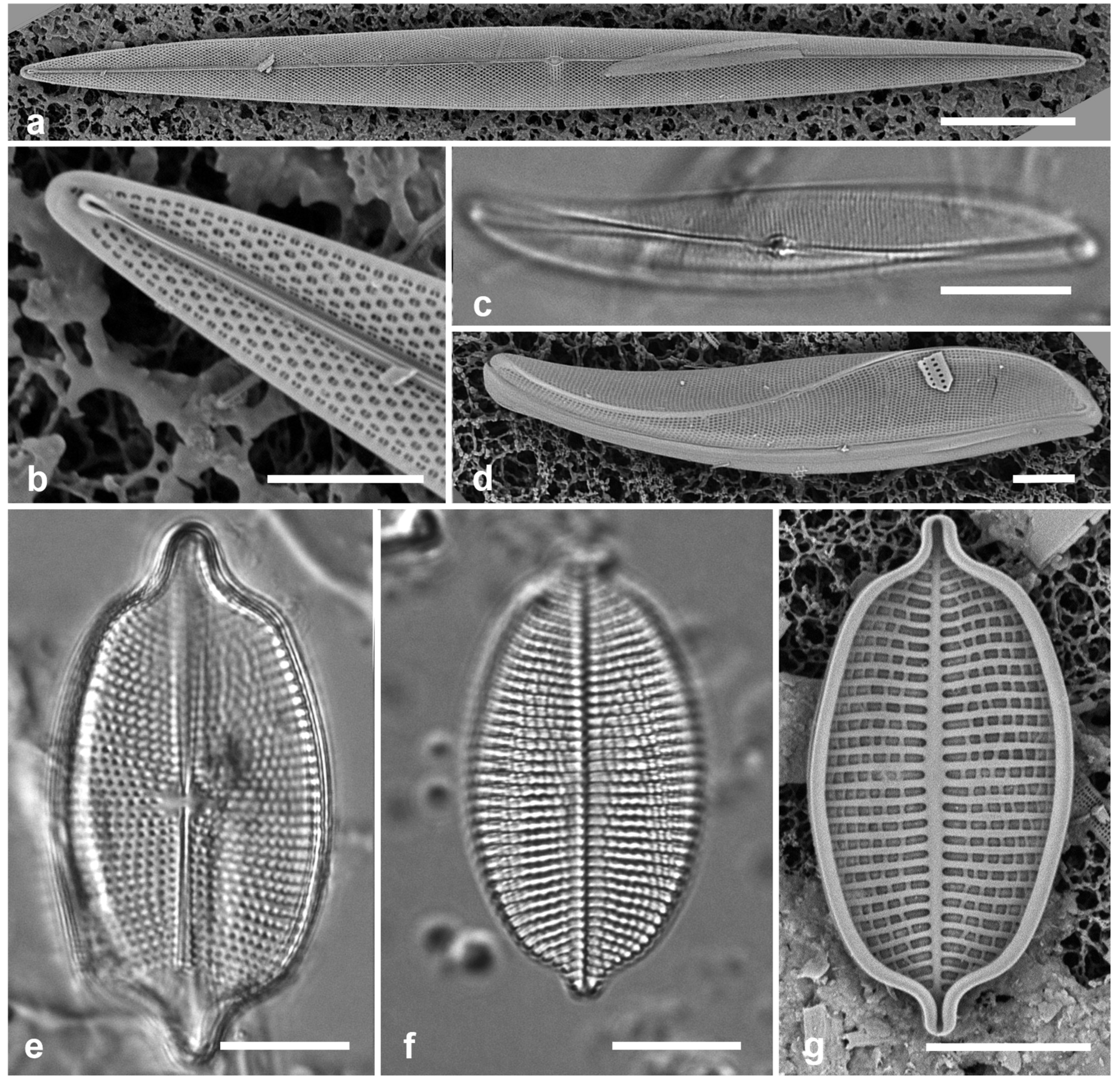
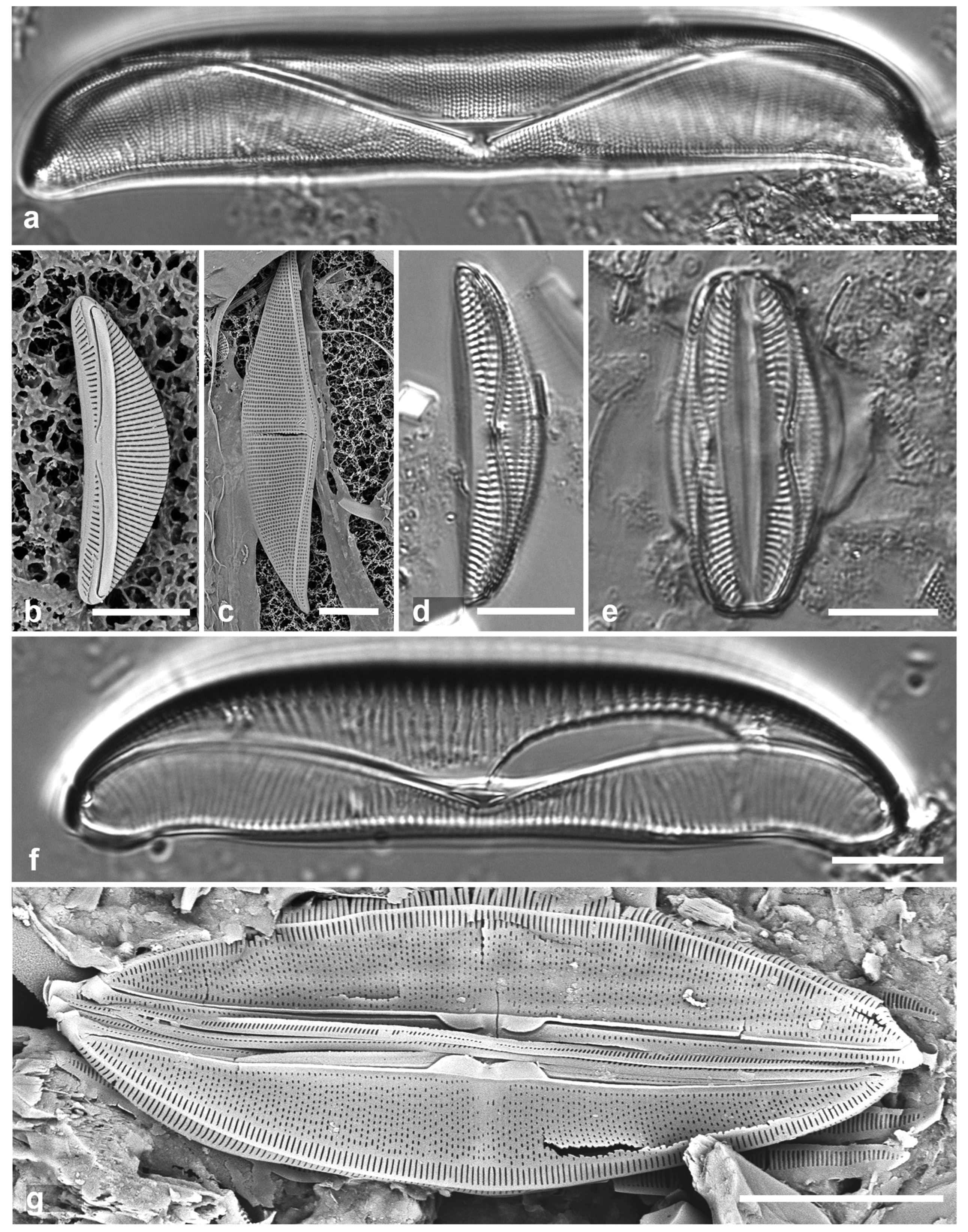
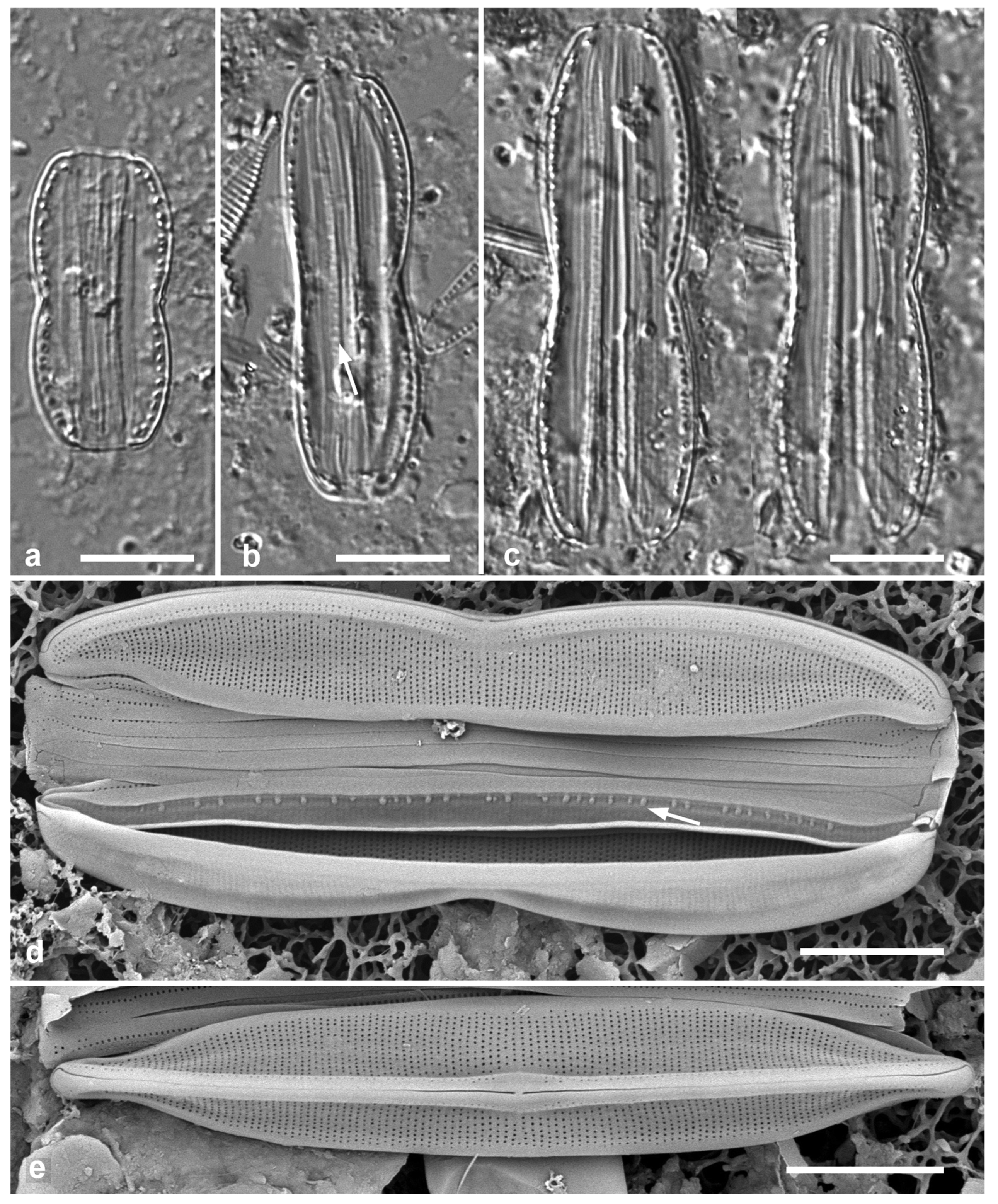
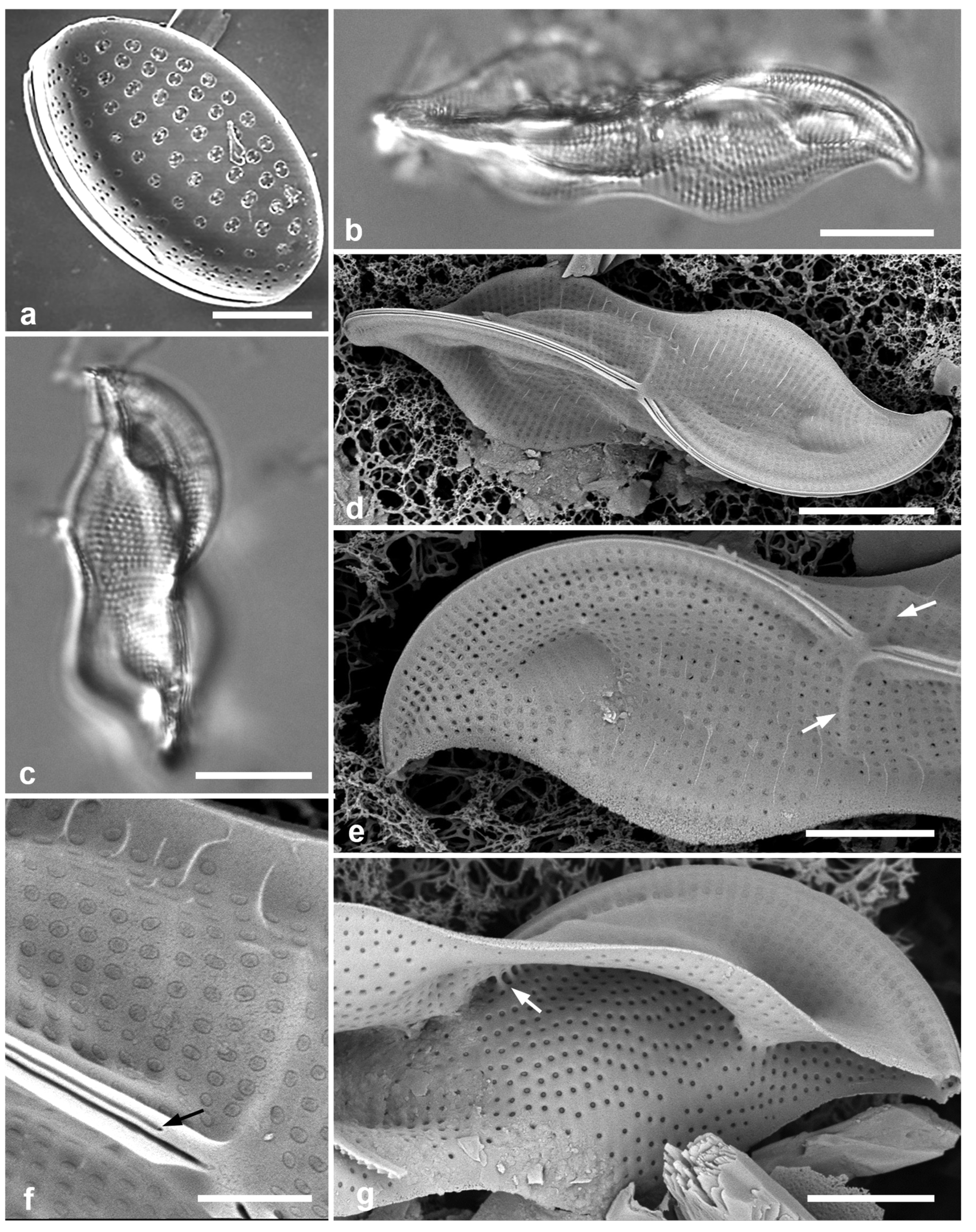
Figure 4.
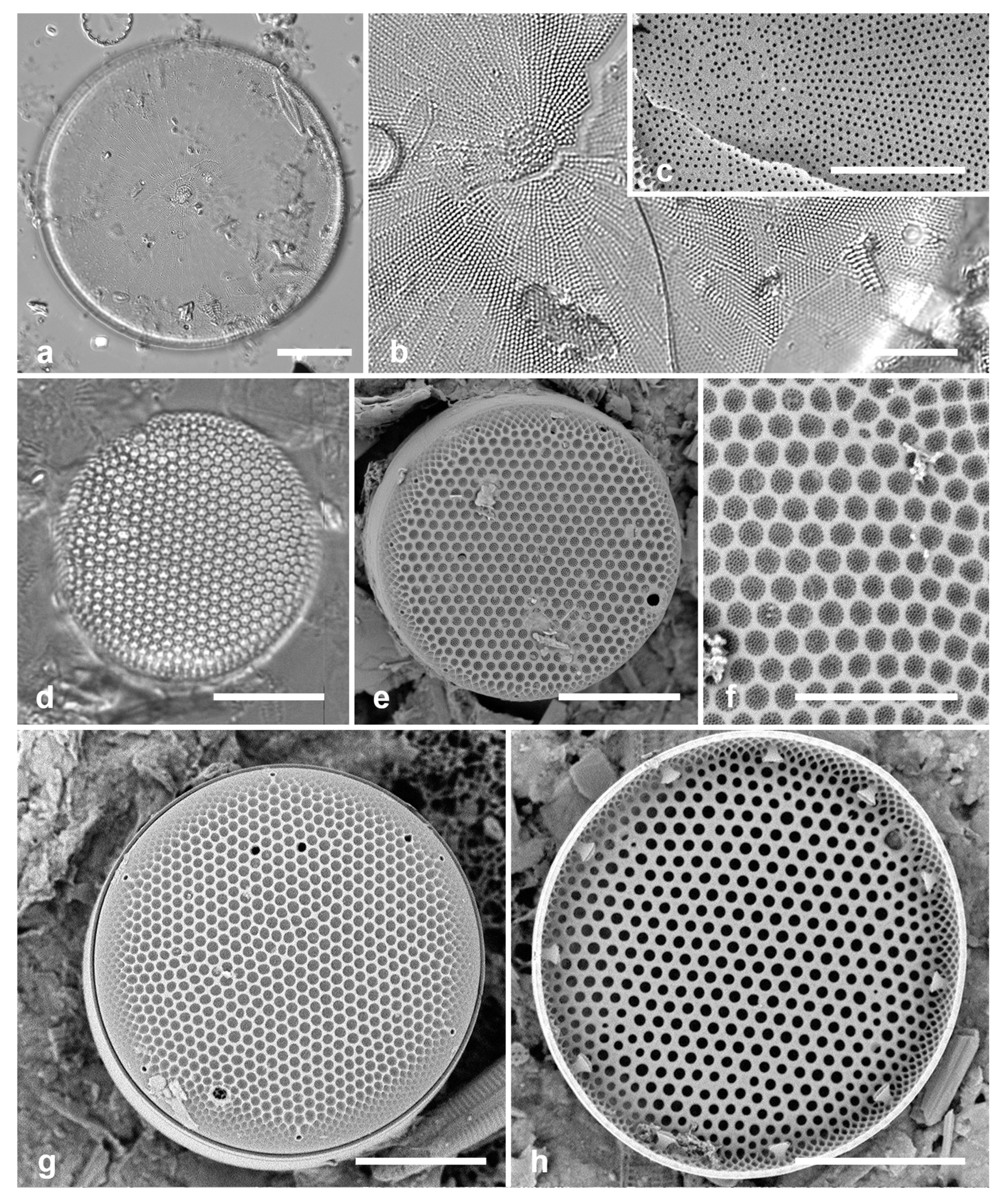
Actinocyclus and Roperia. (a–c) Actinocyclus subtilis. (a) Whole valve, LM. (b, c) Portions of valves to show areolae without clear interfascicular rows and central area with numerous areolae separated by a hyaline ring, LM and SEM (internal), respectively. (d–h) Roperia tesselata. (d, e) Valves with decussate areola pattern in LM and SEM (external). (f) Detail of cribrate areolae in external view, SEM. (g) Valve with less regular decussate pattern, external SEM. (h) Internal aspect, SEM, showing rimoportulae. Scale bars: (a) = 25 µm, (b–e, g, h) = 10 µm, (f) = 5 µm.
Figure 4.
Actinocyclus and Roperia. (a–c) Actinocyclus subtilis. (a) Whole valve, LM. (b, c) Portions of valves to show areolae without clear interfascicular rows and central area with numerous areolae separated by a hyaline ring, LM and SEM (internal), respectively. (d–h) Roperia tesselata. (d, e) Valves with decussate areola pattern in LM and SEM (external). (f) Detail of cribrate areolae in external view, SEM. (g) Valve with less regular decussate pattern, external SEM. (h) Internal aspect, SEM, showing rimoportulae. Scale bars: (a) = 25 µm, (b–e, g, h) = 10 µm, (f) = 5 µm.
3.6. Actinocyclus subtilis (Gregory) Ralfs in Pritchard 1861 Figure 4a–c
Previous Micronesia records: Chuuk (Park et al. 2022, p. 34, figs 7a, b, 73); Guam (Lobban et al. 2012, as A. tenuissimus)
Yap samples: Y18E, Y41-8
Dimensions: Diam. 71–117 µm, areolae 18 in 10 µm
Diagnostics: Large valves with interfascicular rows indistinct and central area 7 µm with many areolae and a hyaline boundary.
Comments: Park et al. (2022) discussed the distinction between this species and A. tenuissimus Cleve. The areola density here is in the range for A. tenuissimus Cleve, rather than the 12–15 in 10 µm given by Hustedt (1927–1930) for A. subtilis. Interfascicular rows of areolae are distinct in A. tenuissimus, indistinct in A. subtilis. the central area of A. tenuissimus is only about 4 µm diameter with few areolae and the size range 28–80 µm vs. 40–160 µm in A. subtilis, according to Hustedt (1927–1930). Thus, despite the stria density, we interpret our specimens as A. subtilis, and reinterpret our Guam records.
3.7. Roperia cf. tesselata (Roper) Grunow ex Pelletan 1889 Figure 4d–h
Previous Micronesia records: Chuuk (Park et al. 2018, p. 103,
Figure 2); Guam (Lobban & Witkowski 2024b, Figs 109–114)
Yap samples: Y18B, Y26C, Y34B, E, F, Y42-1
Dimensions: Diam. 20–30 µm, areolae 9–10 in 10 µm.
Comments: Although the description in Round et al. (1990) includes special features of the rimoportulae (slitlike openings with flaps or a rim above them), Lee & Lee (1990) found specimens with circular openings without flaps. Although those were from among their cold-water samples and the usual ones from warmer water samples, these specimens seem to be within the scope of R. tesselata as presently understood. The large cribrate areolae on the valve face and diamond-shaped areolae on the mantle are consistent with R. tesselata. It is possible that there is more than one species being confounded here but we do not have the material to resolve them. Lobban & Witkowski (2024b) give the reasons for doubting an exact match with R. tesselata.
ASTEROLAMPRALES Round & R.M.Crawford
Asterolampraceae H.L.Smith
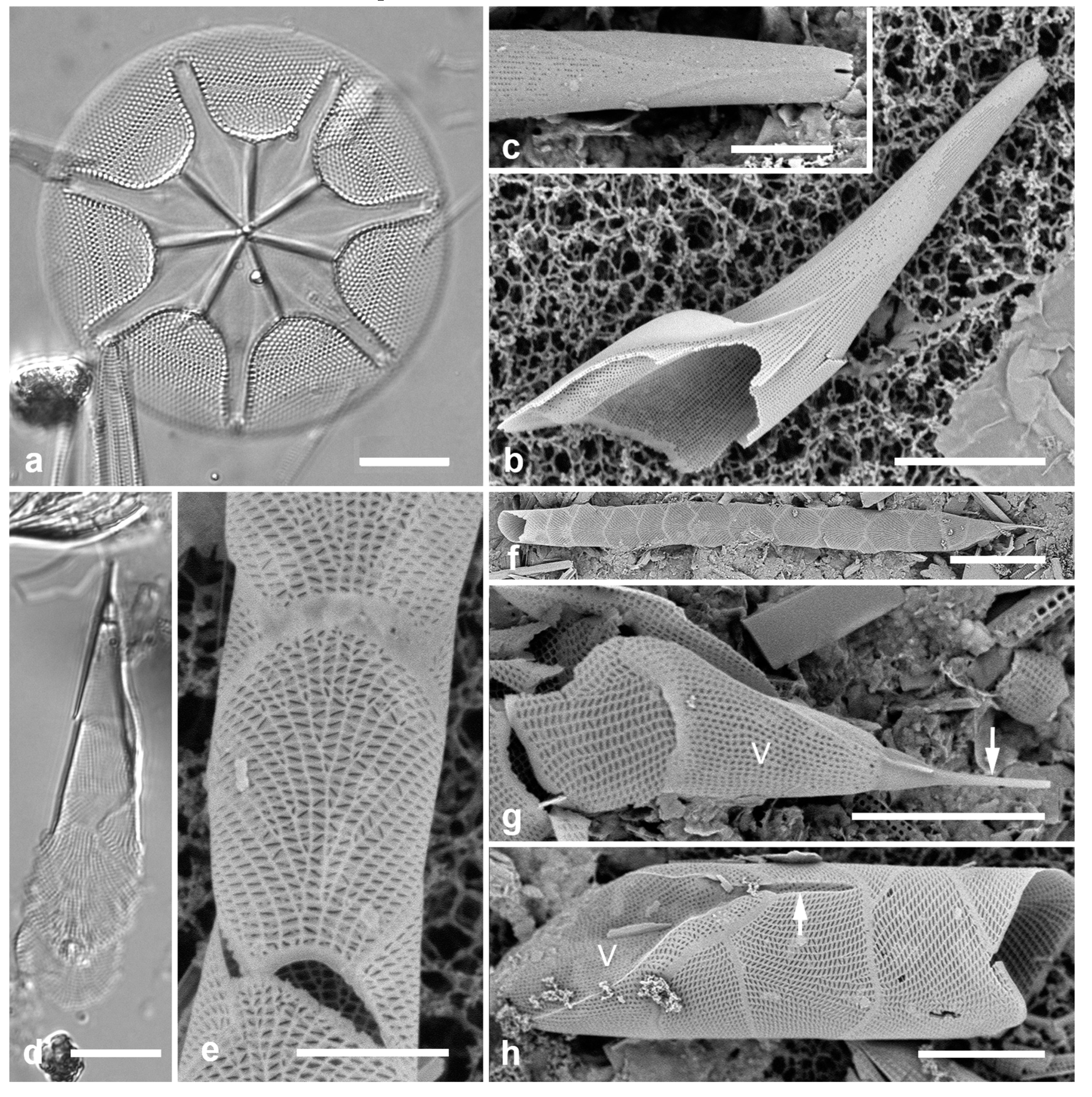
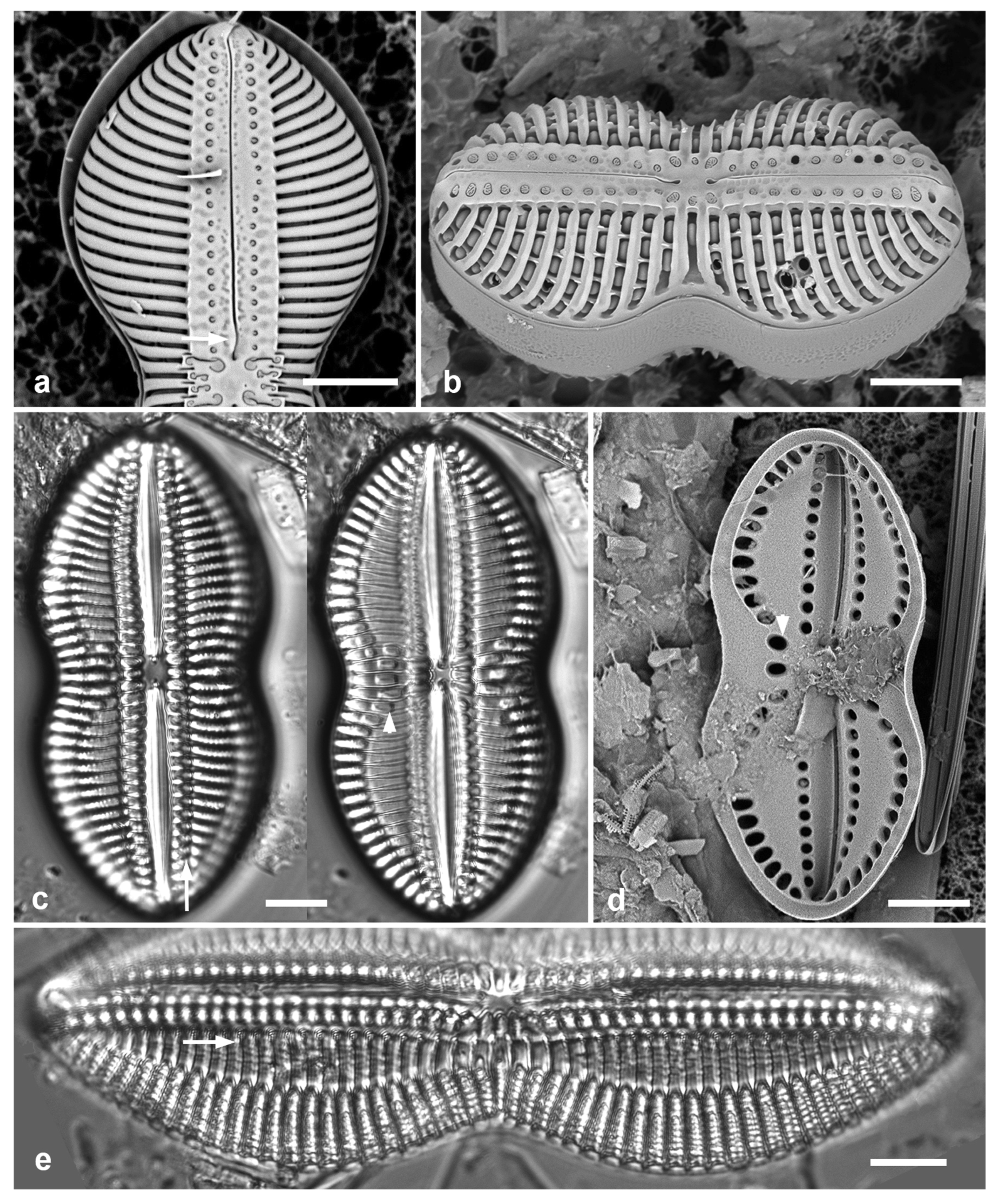
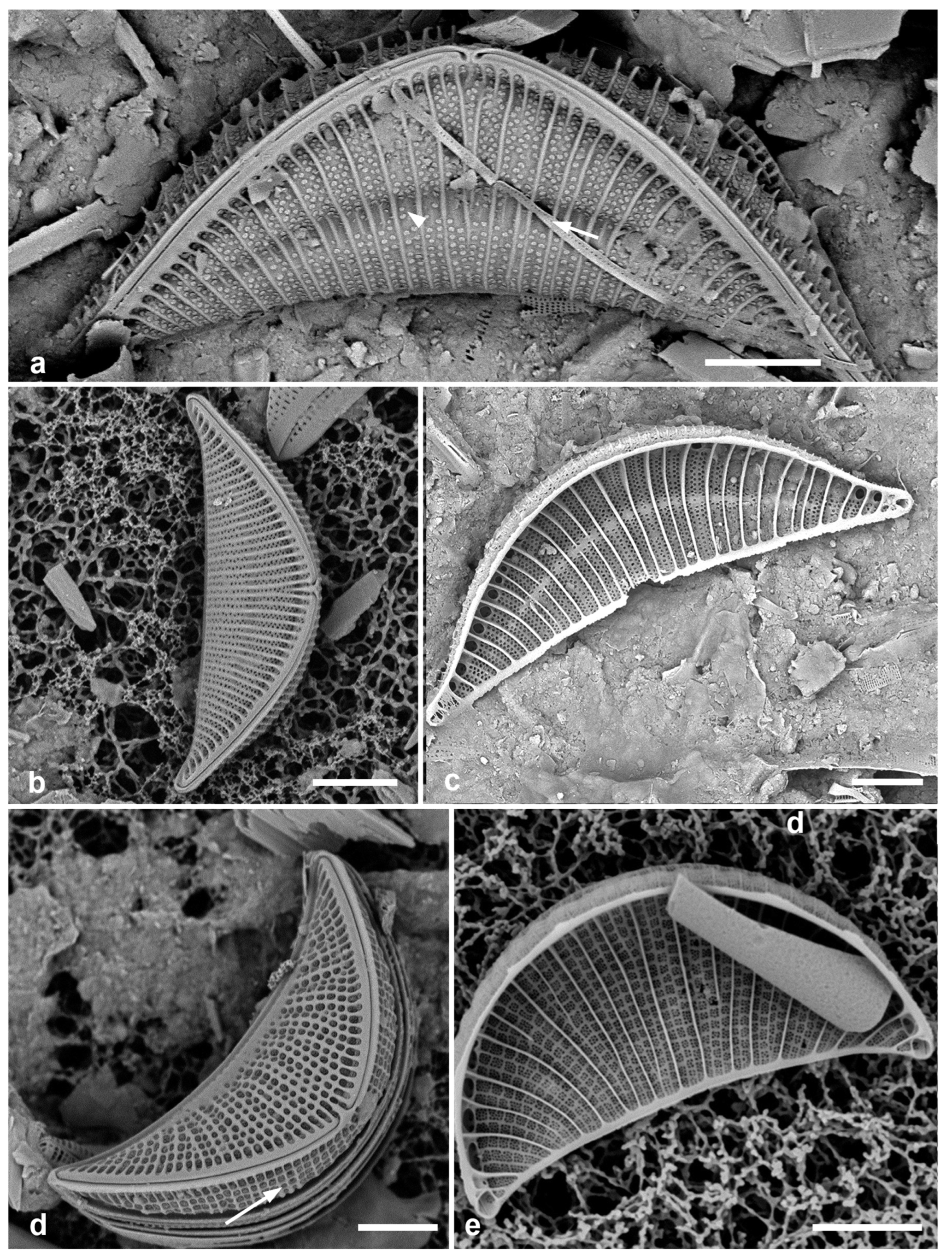
3.8. Asterolampra marylandica Ehrenberg 1844 Figure 5a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 249, pl. 5,
Figure 6); 585493
Yap samples: Y42-1
Dimensions: Diam. 49–131 µm.
Comments: Planktonic species.
PROBOSCIALES Medlin
Probosciaceae R.W.Jordan & Ligowski
3.9. Proboscia alata (Brightwell) Sunderström Figure 5b, c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 253, pl. 9, figs 1, 2); 216777
Yap samples: Y45-5, Y45-2
Dimensions: Conical valves 8–10 µm diam. at margin.
Comments: Planktonic species.
Rhizosoleniaceae De Toni
3.10. Rhizosolenia imbricata Brightwell 1858 Figure 5d–h
References: Hustedt 1927–1930, p. 584, Figure 332; Suneson & Sar 2007, pp. 634–636, figs 48–61
Yap samples: Y25B
Dimensions: Diam. 14 µm
Diagnostics: Valve (calyptra) conical and asymmetrical, with a narrow spine (outer extension of rimoportula) that fits into a groove. Large areolae in both valve and girdle bands. Girdle bands in lateral rows.
Comments: Only fragments seen; cells are 100–500 µm long (Hustedt 1927–1930). Distinguished from R. styliformis Brightwell by the lateral arrangement of girdle bands (vice dorsiventral) and coarser areolae. In Hustedt (1927–1930), the smaller size distinguished these specimens as var. shrubsolei (Cleve) Schröder, but this distinction is no longer recognized (Suneson & Sar 2007). New record for Micronesia. Planktonic species.
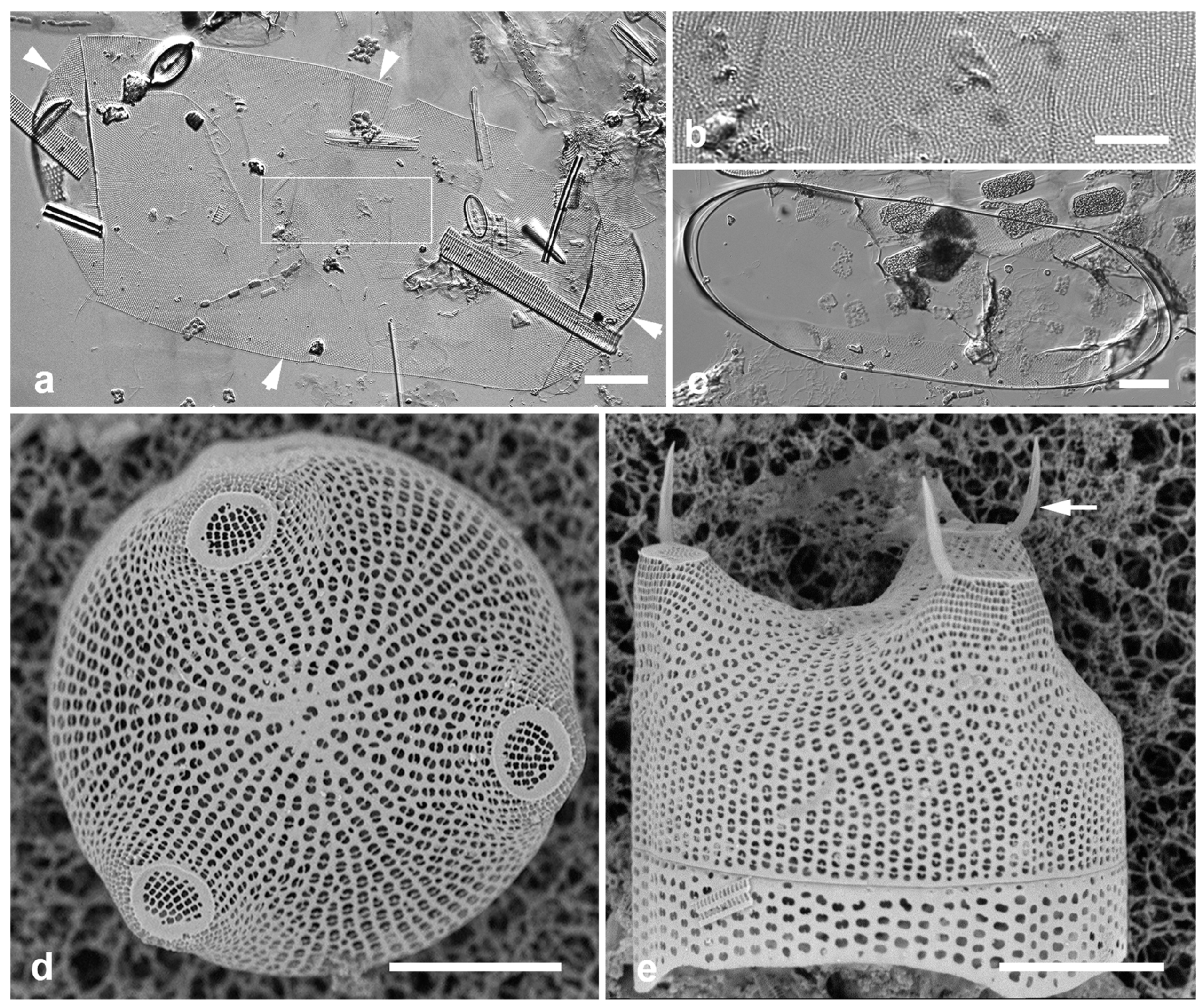
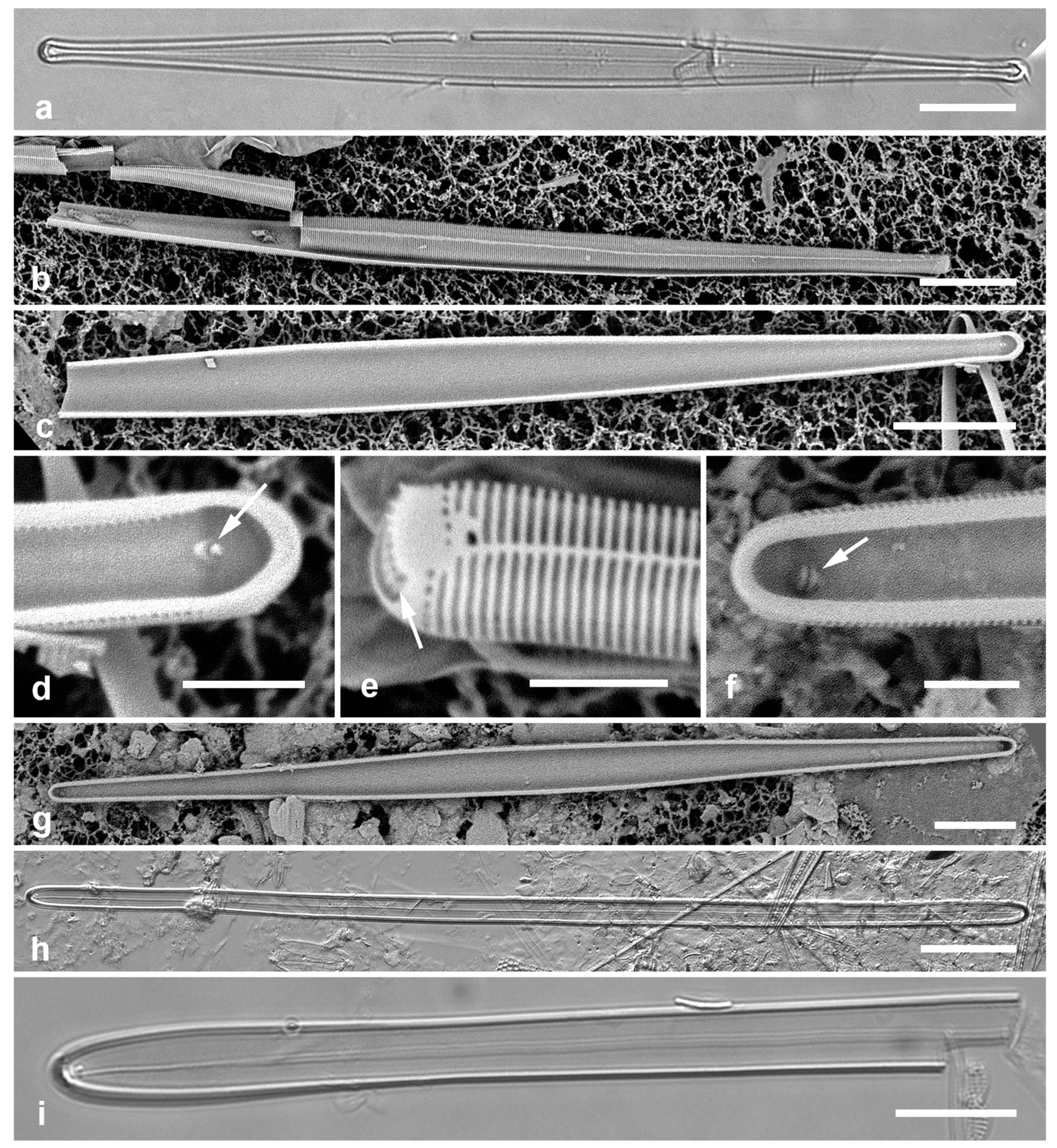
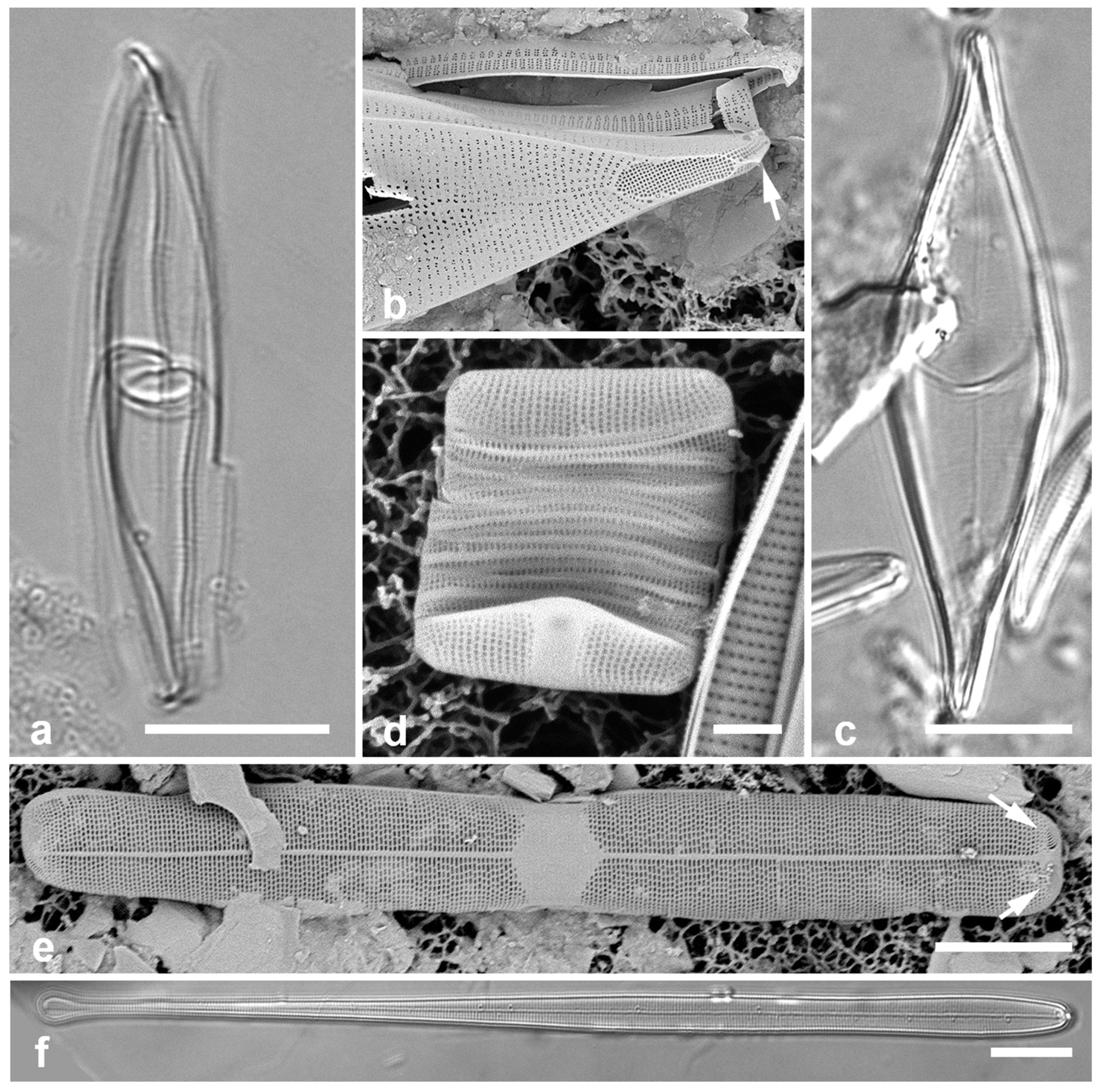
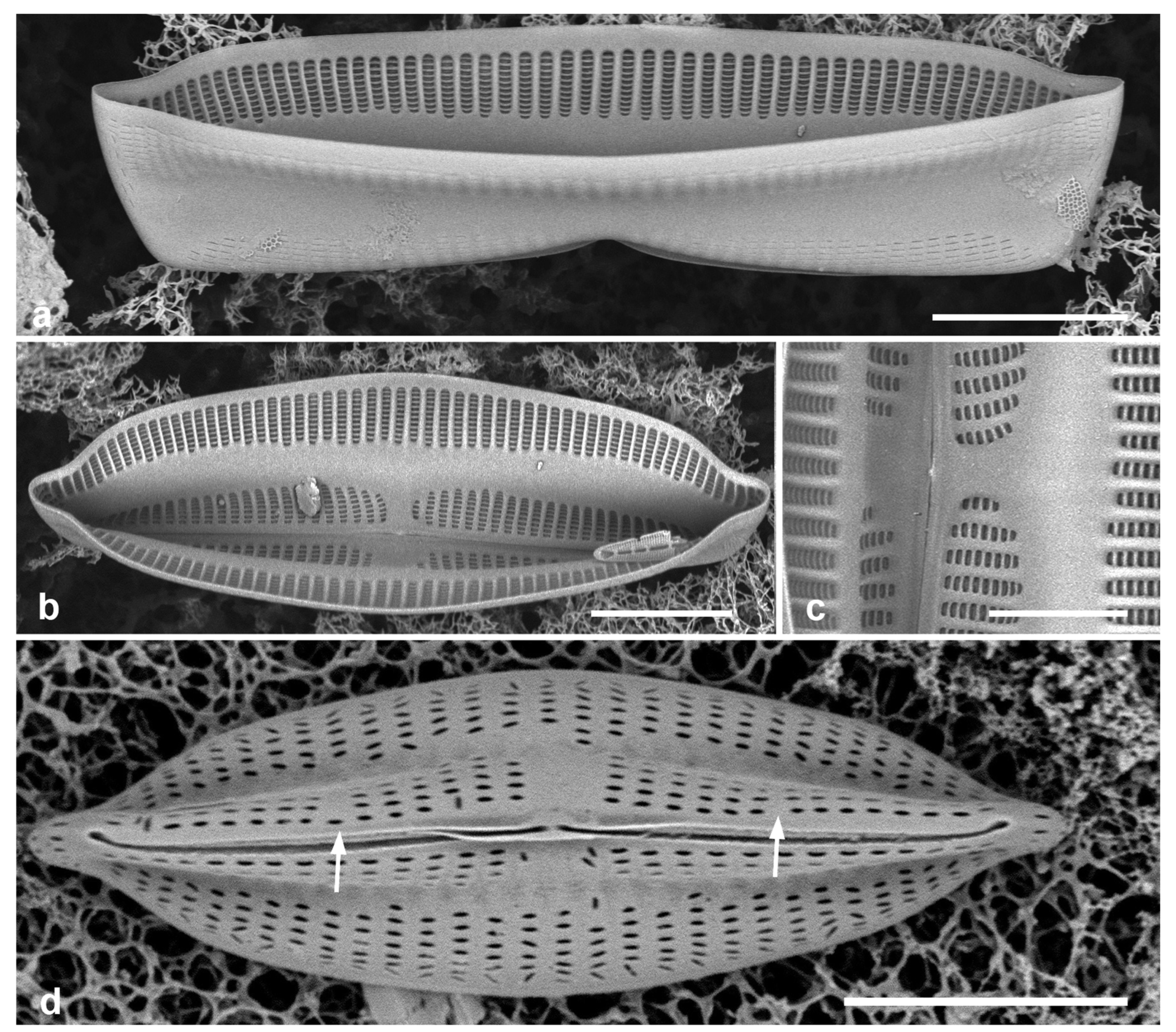
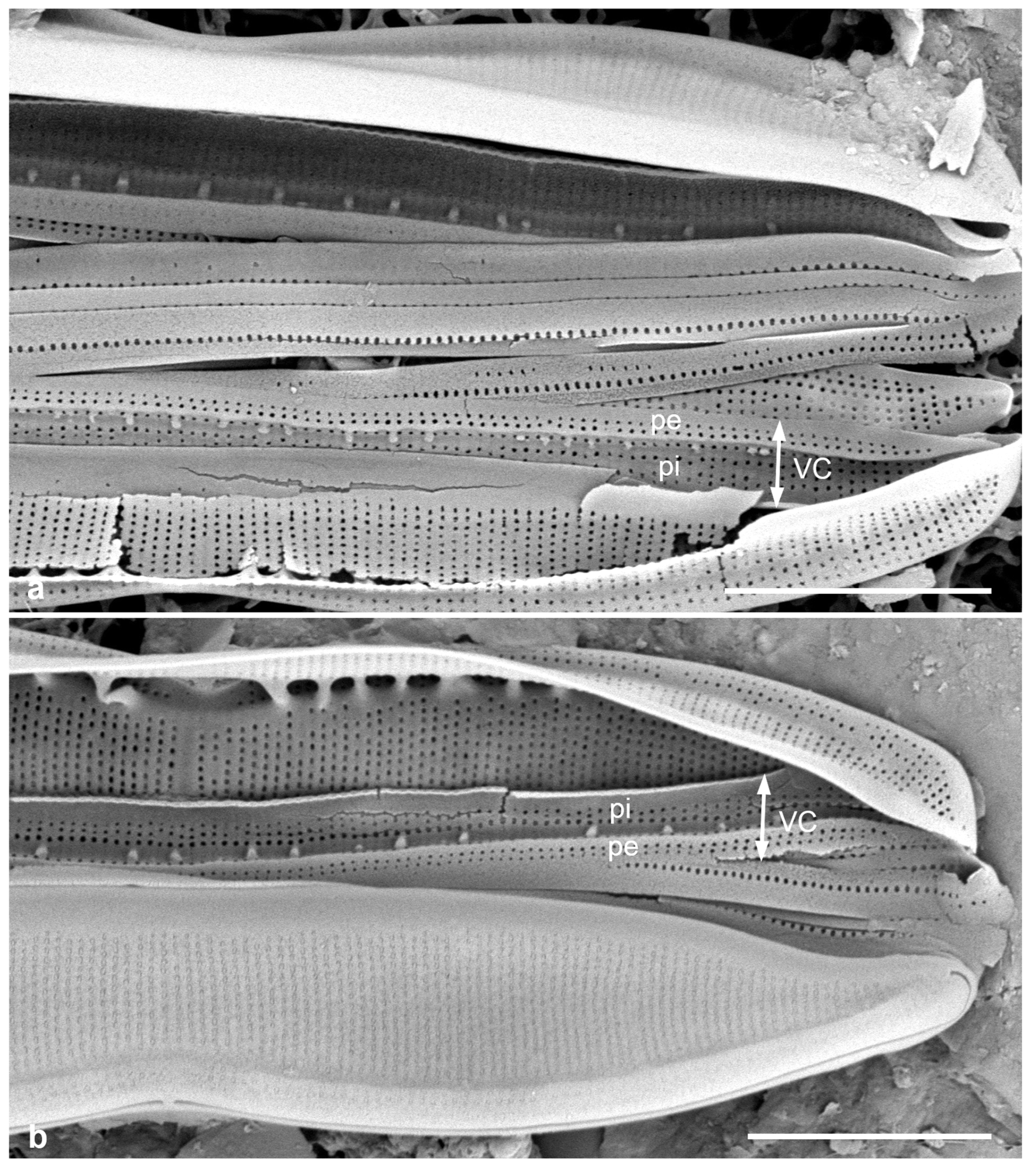
Figure 5.
(
a)
Asterolampra marylandica, valve in LM. (
b, c)
Proboscia alata valve and detail of tip, SEM. (
d–h)
Rhizosolenia imbricata. (
d)Valve and several girdle bands in dorsiventral view, LM. (
e) Girdle bands in lateral view, SEM. (
f) Long fragment of frustule showing arrangement of girdle bands in lateral rows, SEM. (
g, h) Details of valve (V) and attached girdle bands in lateral and slightly oblique view, SEM, showing the spine on one side (arrow in
Figure 5g; out of view to left in
Figure 5h) and the matching groove (arrow,
Figure 5h), extending onto the girdle. Scale bars (f) = 25 µm, (a, b, d, f, g) = 10 µm, (c, e) = 5 µm.
Figure 5.
(
a)
Asterolampra marylandica, valve in LM. (
b, c)
Proboscia alata valve and detail of tip, SEM. (
d–h)
Rhizosolenia imbricata. (
d)Valve and several girdle bands in dorsiventral view, LM. (
e) Girdle bands in lateral view, SEM. (
f) Long fragment of frustule showing arrangement of girdle bands in lateral rows, SEM. (
g, h) Details of valve (V) and attached girdle bands in lateral and slightly oblique view, SEM, showing the spine on one side (arrow in
Figure 5g; out of view to left in
Figure 5h) and the matching groove (arrow,
Figure 5h), extending onto the girdle. Scale bars (f) = 25 µm, (a, b, d, f, g) = 10 µm, (c, e) = 5 µm.
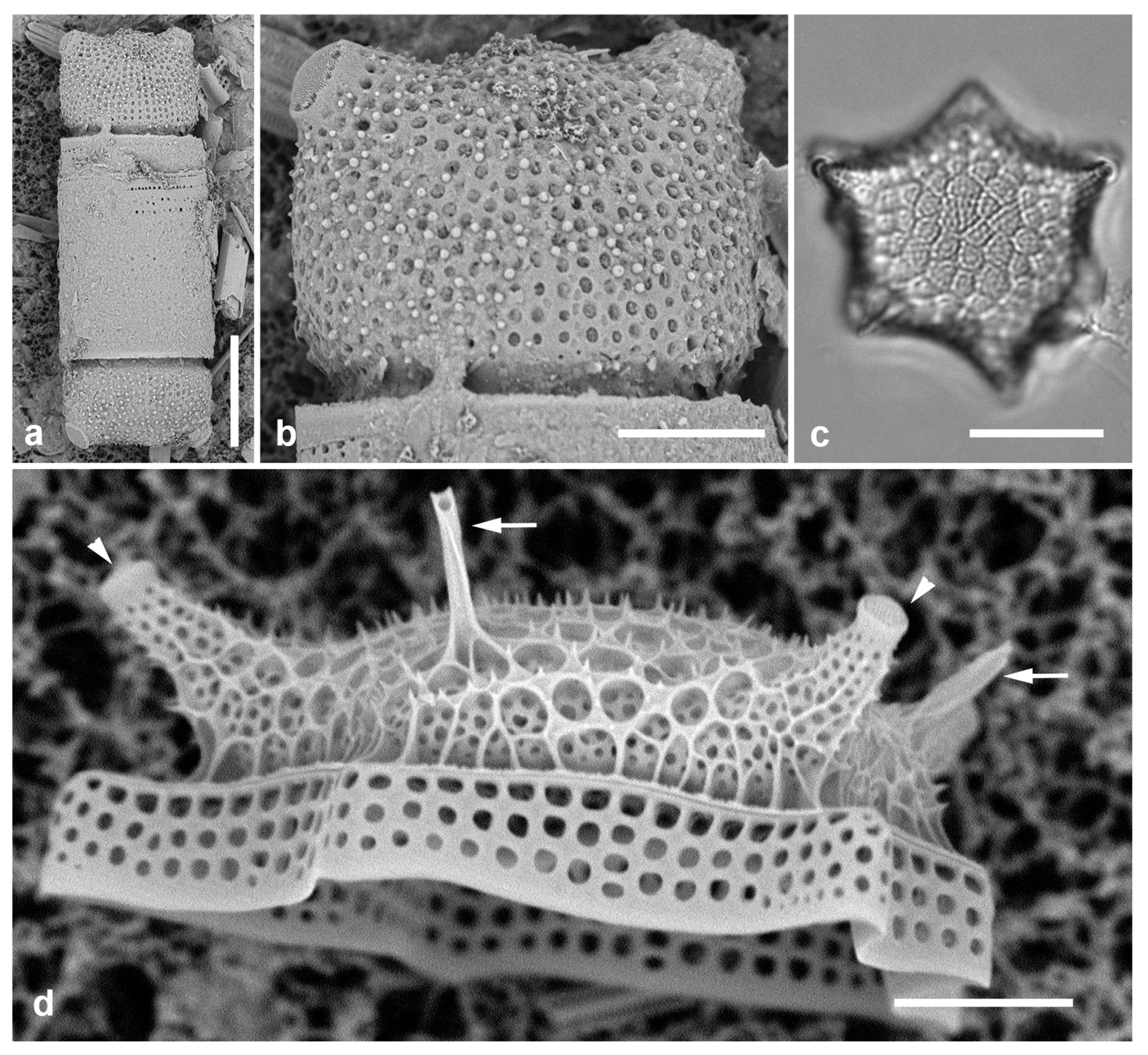
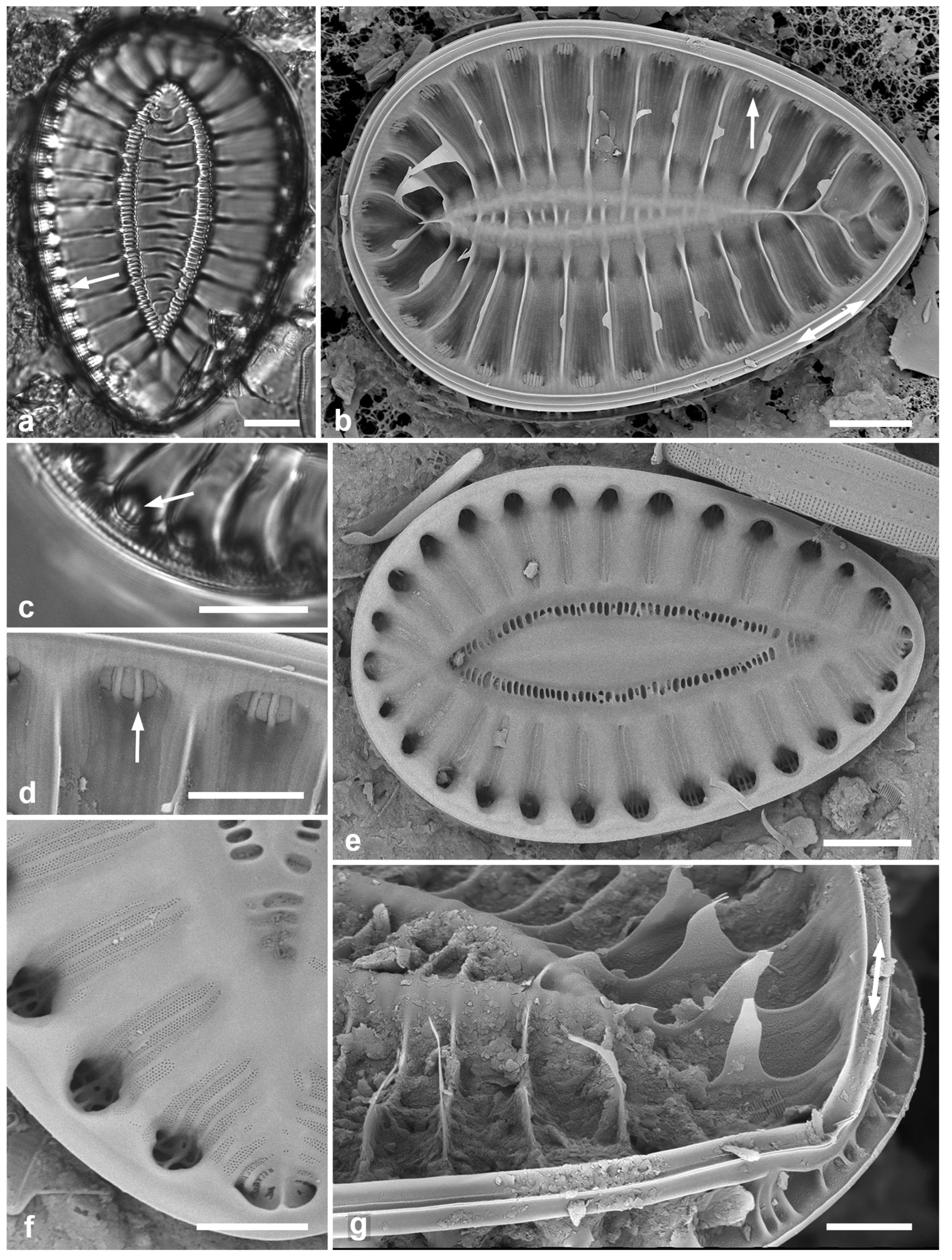
Figure 6.
(a–c). Hemiaulus sinensis valves in girdle view in LM and SEM. (d, e) Anaulus minutus, SEM. (f) Bacteriastrum furcatum, LM. Scale bars: (a, b, d, f) = 10 µm, Fig. (c, e) = 5 µm.
Figure 6.
(a–c). Hemiaulus sinensis valves in girdle view in LM and SEM. (d, e) Anaulus minutus, SEM. (f) Bacteriastrum furcatum, LM. Scale bars: (a, b, d, f) = 10 µm, Fig. (c, e) = 5 µm.
HEMIAULALES Round & R.M.Crawford
Hemiaulaceae Heiberg
3.11. Hemiaulus sinensis Greville 1865 Figure 6a–c
References: Hustedt 1927–1930, p. 875, Figure 519
Yap samples: Y25B, Y26C
Dimensions: Length (apical axis) 10–25 µm.
Diagnostics: Valve elliptical, a coarse network of large areolae; two moderately long apical processes with a spine at the end (these used to connect the cells in chains).
Comments: Planktonic. Many valves in Y25B. This species is distinguished from congeners by the combination of very large pores and lack of a central elevation. Hemiaulus hauckii Grunow was reported for Guam (Lobban et al. 2012).
ANAULALES Round & R.M.Crawford
Anaulaceae (Schütt) Lemmermann
3.12. Anaulus minutus Grunow in Van Heurck 1882 Figure 6d, e
References; Hustedt 1927–1930, p. 894, Figure 537; Witkowski et al. 2000, p. 23, pl.10, figs 33–35; Hein et al. 2008, p. 17, pl. 1,
Figure 4
Yap samples: Y26C, Y33A
Dimensions: Length 14 µm, width 8 µm
Diagnostics: Tiny elliptical valves divided by two pseudosepta, no evident areolae but a small elevation near each pole with opening.
Comments: New record for Micronesia.
CHAETOCEROTALES Round & R.M.Crawford
Chaetocerotaceae Ralfs in Prichard
3.13. Bacteriastrum furcatum Shadbolt 1853 Figure 6f
Previous Micronesia records: Chuuk (Park et al. 2022, p. 35, Figure 81)
Yap samples: Y26B
Dimensions: Diam. of disc 8–11 µm
Comments: Planktonic. The setae typically fork once, though this is not apparent in the LM image and in SEM images from Yap setae were all broken off near the base. Images from other islands show this.
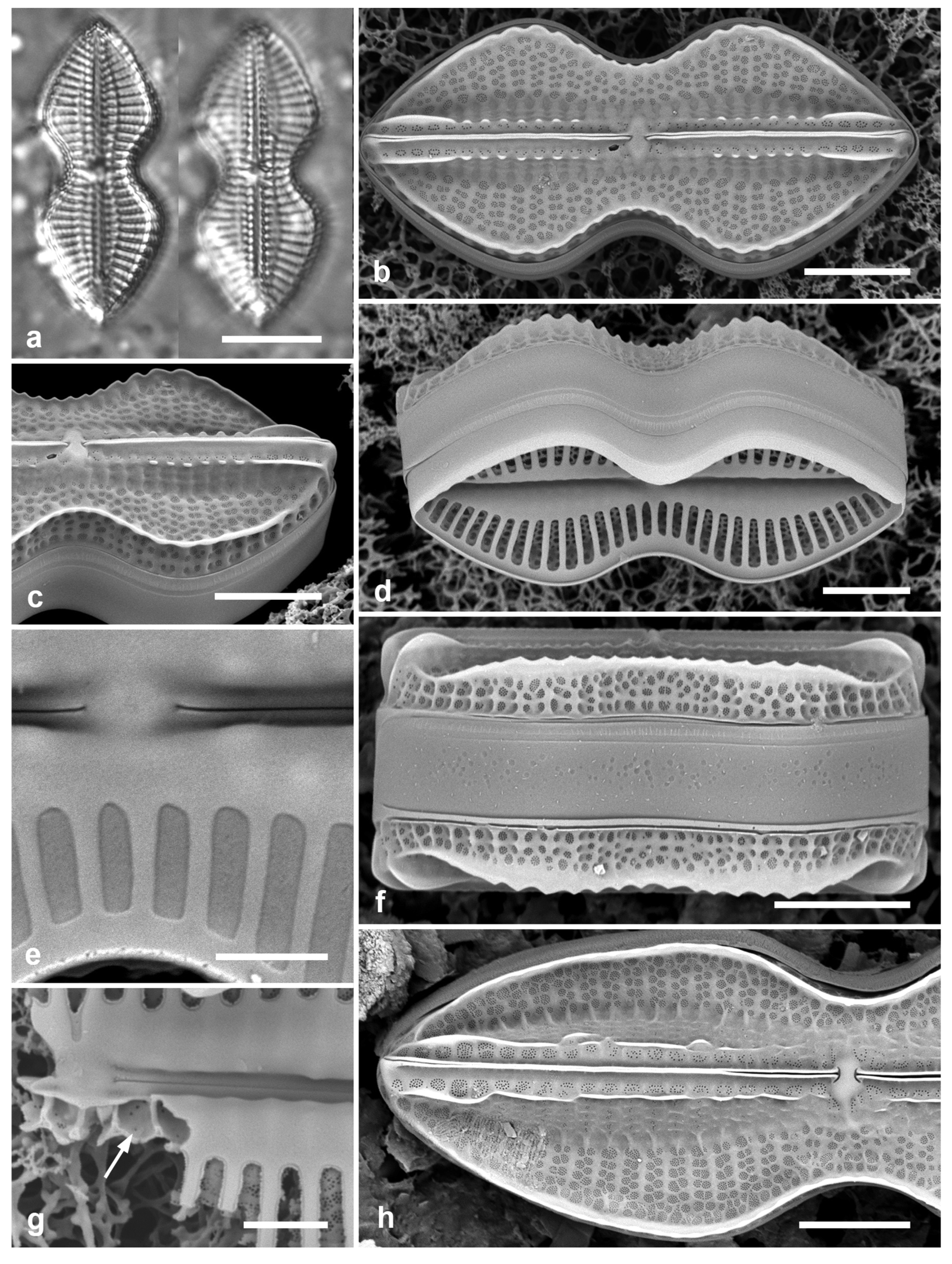
3.14. Chaetoceros peruvianus Brightwell 1856 Figure 7a–d
Previous Micronesia records: Guam (Lobban et al. 2012, p. 254, pl. 9, figs 4, 5); 426635
Yap samples: Y26B, Y26C
Dimensions: Diam. 18–24 µm
Comments: Planktonic, this species usually solitary rather than in chains (Hustedt 1927–1930); one of the few planktonic species commonly encountered in Guam.
THALASSIOSIRALES Glezer & Makarova
Lauderiaceae (Schütt) Lemmermann emend. Lobban
3.15. Disymmetria excentrica (Lobban) Lobban 2023 [b] Figure 7e
Synonym: Lauderia excentrica Lobban
Previous Micronesia records: Guam (Lobban 2015a, p. 6, figs 43–48, as Lauderia excentrica); 585487
Yap samples: Y41-8
Dimensions: 26 µm diameter, striae (measured by the costae below the reniform area) 15 in 10 µm.
Diagnostics: Discoidal, bipolar valves with numerous fultoportulae around the periphery and across the valve face; a reniform area of scattered pores set in one half of the valve face with striae and costae radiating from it. Only the periphery is pseudoloculate.
3.16. Disymmetria reticulata Lobban 2023 [b]
Previous Micronesia records: Palau, Guam, Yap (Lobban 2023b, figs 1–6, 8–16; Y42-1: figs 5, 6)
Dimensions: 26 µm diam., striae 10 in 10 µm.
Diagnostics: Discoidal, bipolar valves with pseudoloculate structure and fultoportulae in both periphery and reniform area; periphery wider at one pole.
Comment: Lobban (2023b) described this species as a new genus, transferred Lauderia excentrica into Disymmetria, and emended the family definition. This species differs from D. excentrica in having asymmetry in the peripheral zone as well as the valve face, in having a larger, pseudoloculate reniform area, and in the coarser stria density.
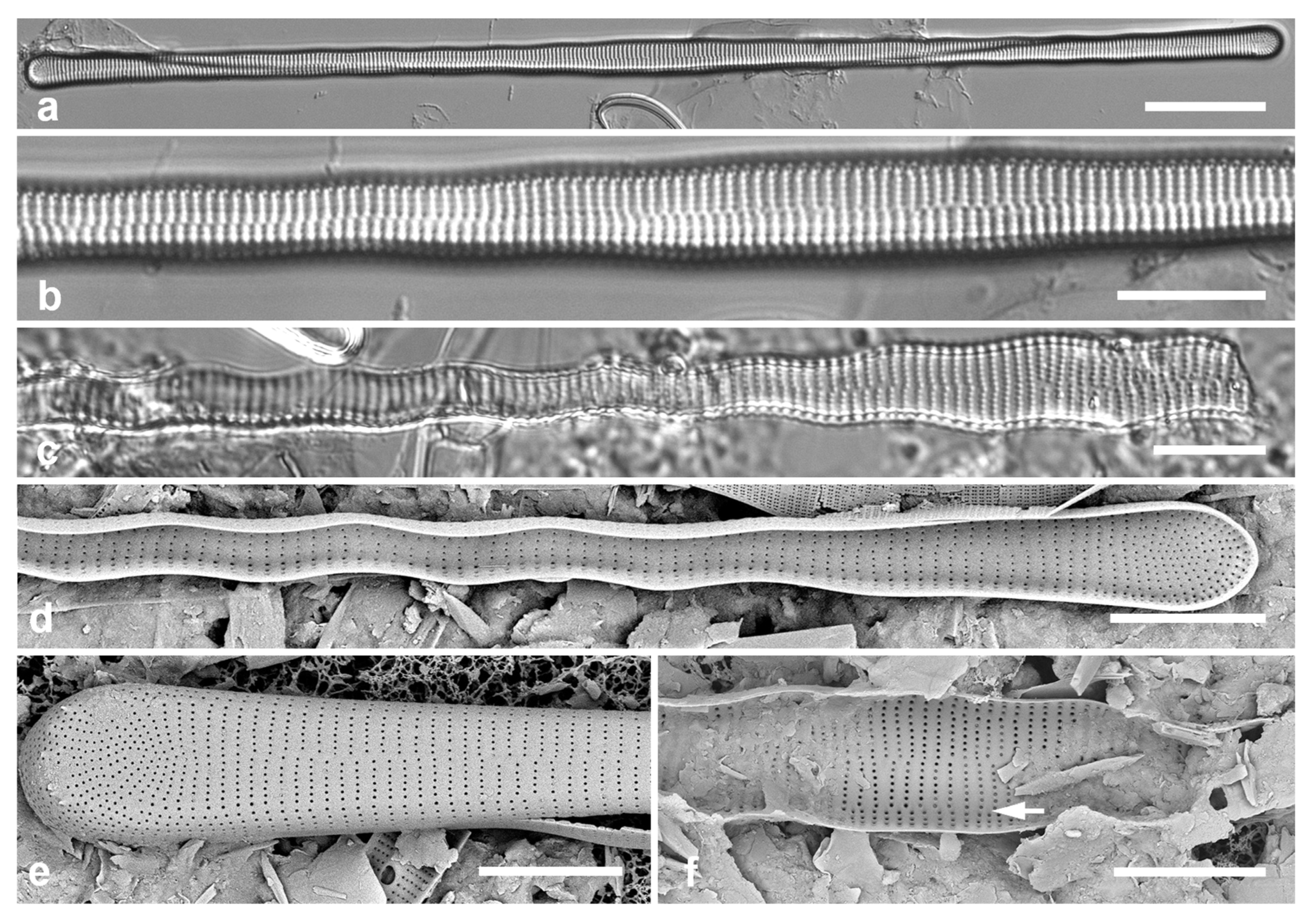
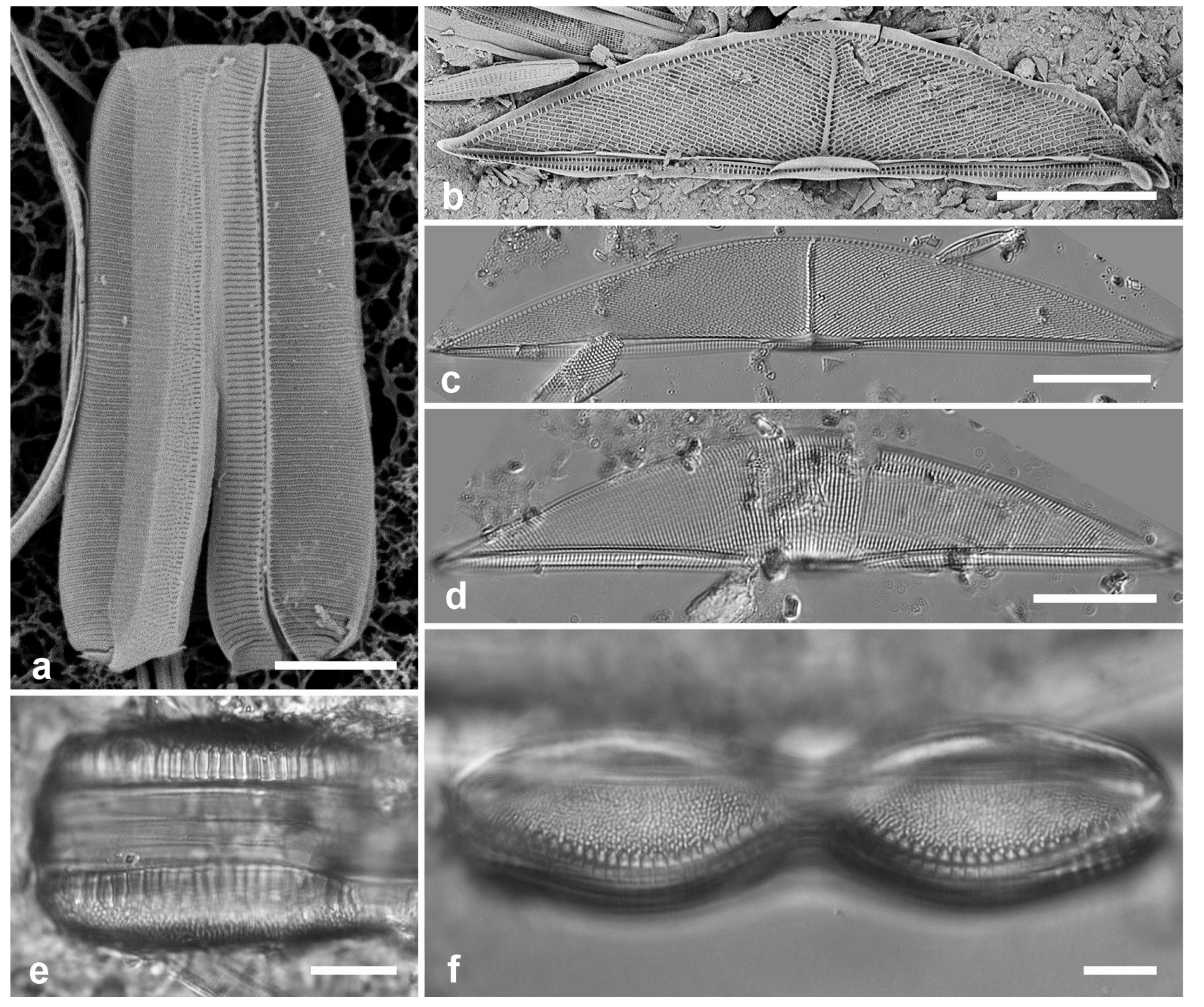
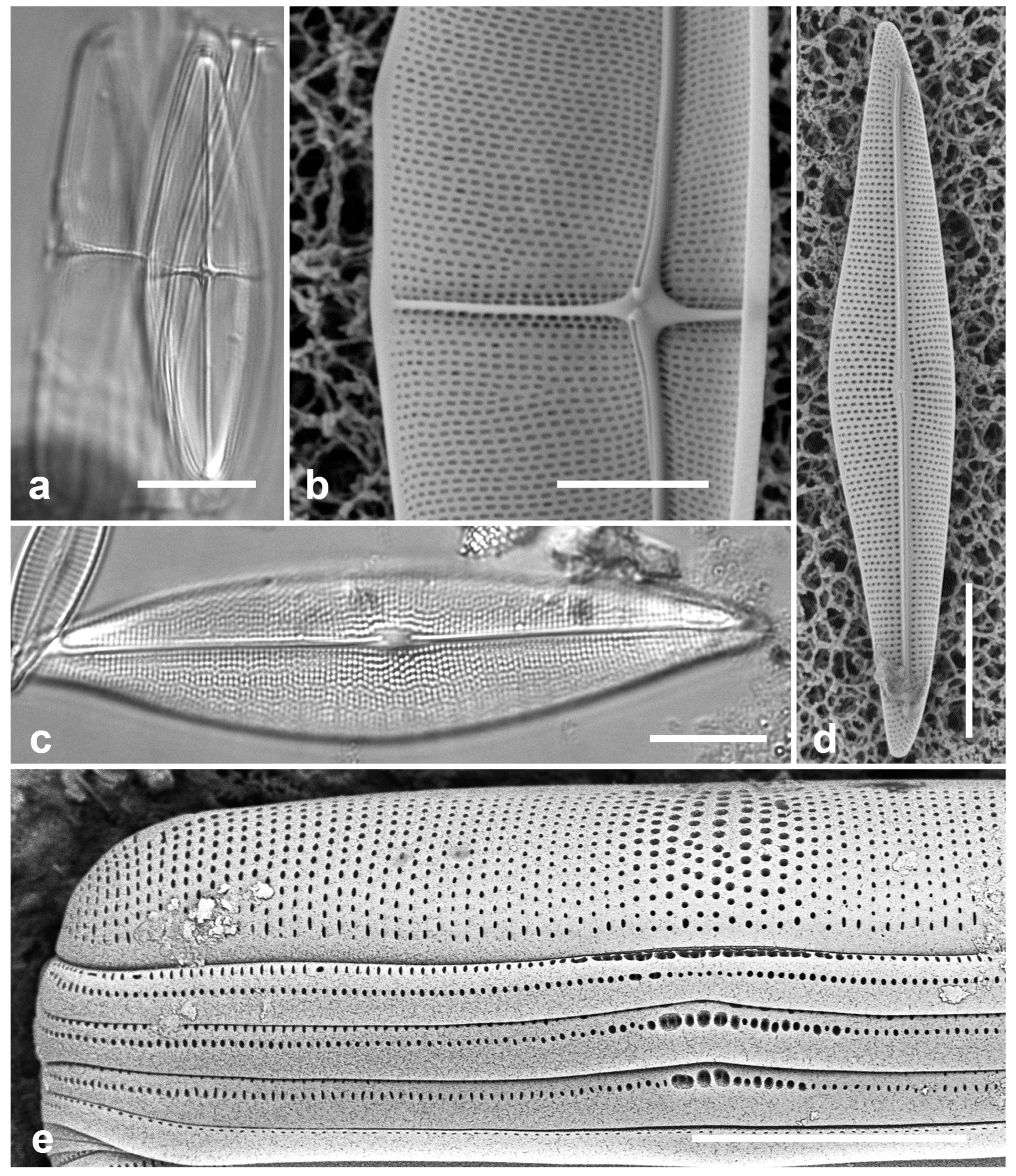
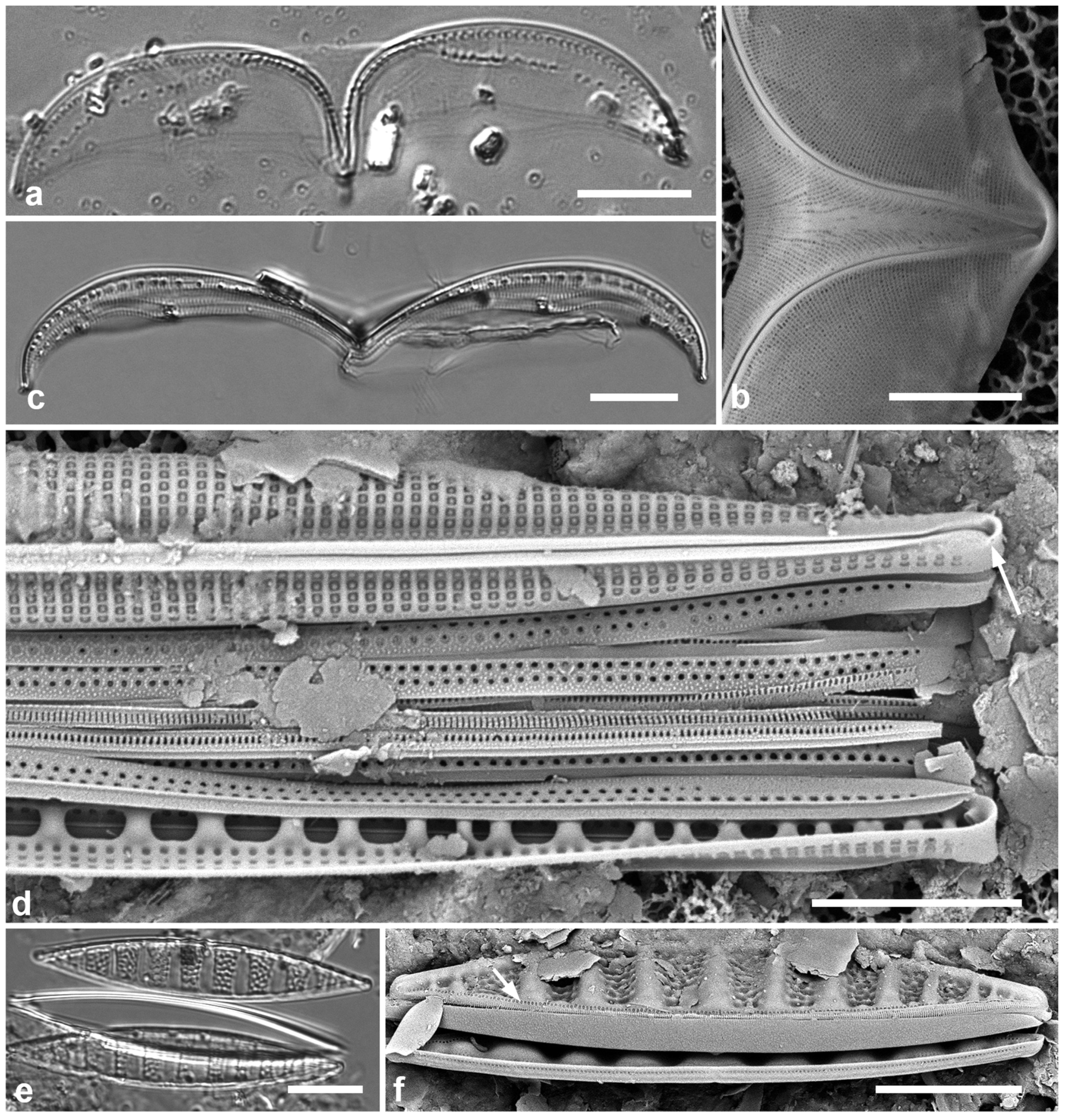
Figure 7.
(a–d) Chaetoceros peruvianus. (a) Upper valve in LM, showing base of setae. (b) Upper valve in SEM, in girdle view, showing large external rimoportula tube (arrow) between recurved setae. (c, d) Lower valve with straight setae and detail of pores and spines on seta. (e) Disymmetria excentrica, SEM. Scale bars: (a–c) = 10 µm, (e) = 5 µm, (d) = 2 µm.
Figure 7.
(a–d) Chaetoceros peruvianus. (a) Upper valve in LM, showing base of setae. (b) Upper valve in SEM, in girdle view, showing large external rimoportula tube (arrow) between recurved setae. (c, d) Lower valve with straight setae and detail of pores and spines on seta. (e) Disymmetria excentrica, SEM. Scale bars: (a–c) = 10 µm, (e) = 5 µm, (d) = 2 µm.
Skeletonemataceae Lebour
3.17. Skeletonema grevillei Sarno & Zingone 2005 Figure 8a–c
References: Zingone et al. 2005, p. 144, figs 5, 6
Yap samples: Y25B
Dimensions: Diam. 8–9 µm
Diagnostics: Cells connected in chains by a ring of long half-tubular structures called intercalary fultoportula processes (IFPPs), which join 1:1, occasionally 1:2, with knuckle-like junctions (
Figure 8b).
Comments:
Skeletonema grevillei was separated
S. costatum (Greville) Cleve emend Zingone & Sarno based on study of Greville’s type material from Hong Kong. Several series of silica ridges joining the IFPP bases (Zingone et al. 2005,
Figure 6F) distinguish this from several other recently-described
S. costatum-like species (Zingone et al. 2005, Sarno et al. 2005). As this species was only recently recognized, its known geographic distribution is still limited to locations in East Asia and a few places westward (Kooistra et al. 2008). Planktonic. New record for Micronesia.
CYMATOSIRALES Round & R.M.Crawford
Cymatosiraceae Hasle, Stosch & Syvertsen
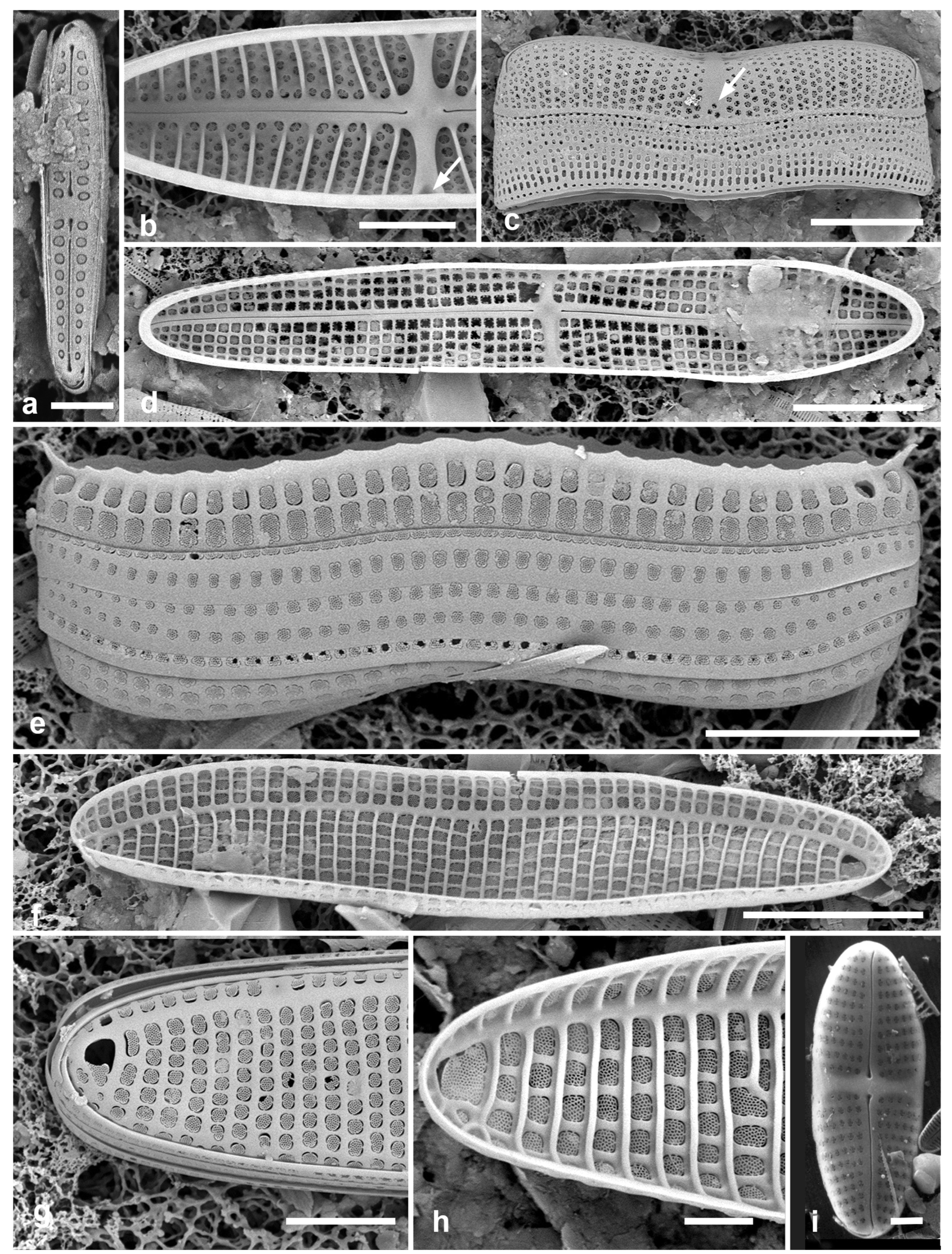
Figure 8.
(a–c) Skeletonema grevillei, SEM. (a) Portion of chain. (b) Two cells in chain connected by linked extensions of the fultoportulae, joined in the middle. (c) Oblique view of internal surface (center of valve missing), showing openings of the single rimoportula (arrow) and the ring of fultoportulae (arrowheads). (d–f) Unidentified Cymatosiraceae in LM and two views of a valve in SEM, (f) rotated and tilted 60°. Scale bars: (a) = 25 µm, (d) = 10 µm, (e, f) = 5 µm, (b, c) = 2 µm.
Figure 8.
(a–c) Skeletonema grevillei, SEM. (a) Portion of chain. (b) Two cells in chain connected by linked extensions of the fultoportulae, joined in the middle. (c) Oblique view of internal surface (center of valve missing), showing openings of the single rimoportula (arrow) and the ring of fultoportulae (arrowheads). (d–f) Unidentified Cymatosiraceae in LM and two views of a valve in SEM, (f) rotated and tilted 60°. Scale bars: (a) = 25 µm, (d) = 10 µm, (e, f) = 5 µm, (b, c) = 2 µm.
3.18. Unidentified genus 1. Figure 8d–f
Yap samples: Y26C
Dimensions: Length 28 µm, width 10–11 µm
Comments: This small species has been rarely seen in Yap samples but has been found in greater numbers in Jellyfish Lake, Palau (Lobban & Jordan 2010,
Figure 18.5o). We have been unable to place it in a genus but present these images in the hope that someone in the future can. Two specimens were found in surface sediment about 15 m subtidal in an area bordered by coral reefs but not far from mangrove forest.
[EUPODISCALES E.J.Cox (invalid)]
Odontellacae P.A.Sims, D.M.Williams & Ashworth
Comment: Mann in Adl et al. (2019) erected Subclass Odontellophycideae D.G.Mann to include Odeontella and Triceratium but no Order was specified by either Mann or Sims et al. (2018) for this Family. Cox (2015) had erected Eupodiscales nom. prov. for Eupodiscaceae Simonsen but a nom. prov. taxon is invalid; this Family had also included Lampriscus in our flora, removed by Mann to Biddulphiaceae. In AlgaeBase (Guiry & Guiry 2024), Odontellaceae remains in Eupodiscales.
3.19. Odontella obtusa Kützing 1844 Figure 9 a, b
Ref. illus: Hustedt 1927–1930, p. 848–849, Figure 502, as Biddulphia aurita var. obtusa (Kützing) Hustedt; Lavigne et al. 2015, p. 312, figs 34–43
Yap samples: Y16B
Dimensions: Length 33 µm (between ocelli).
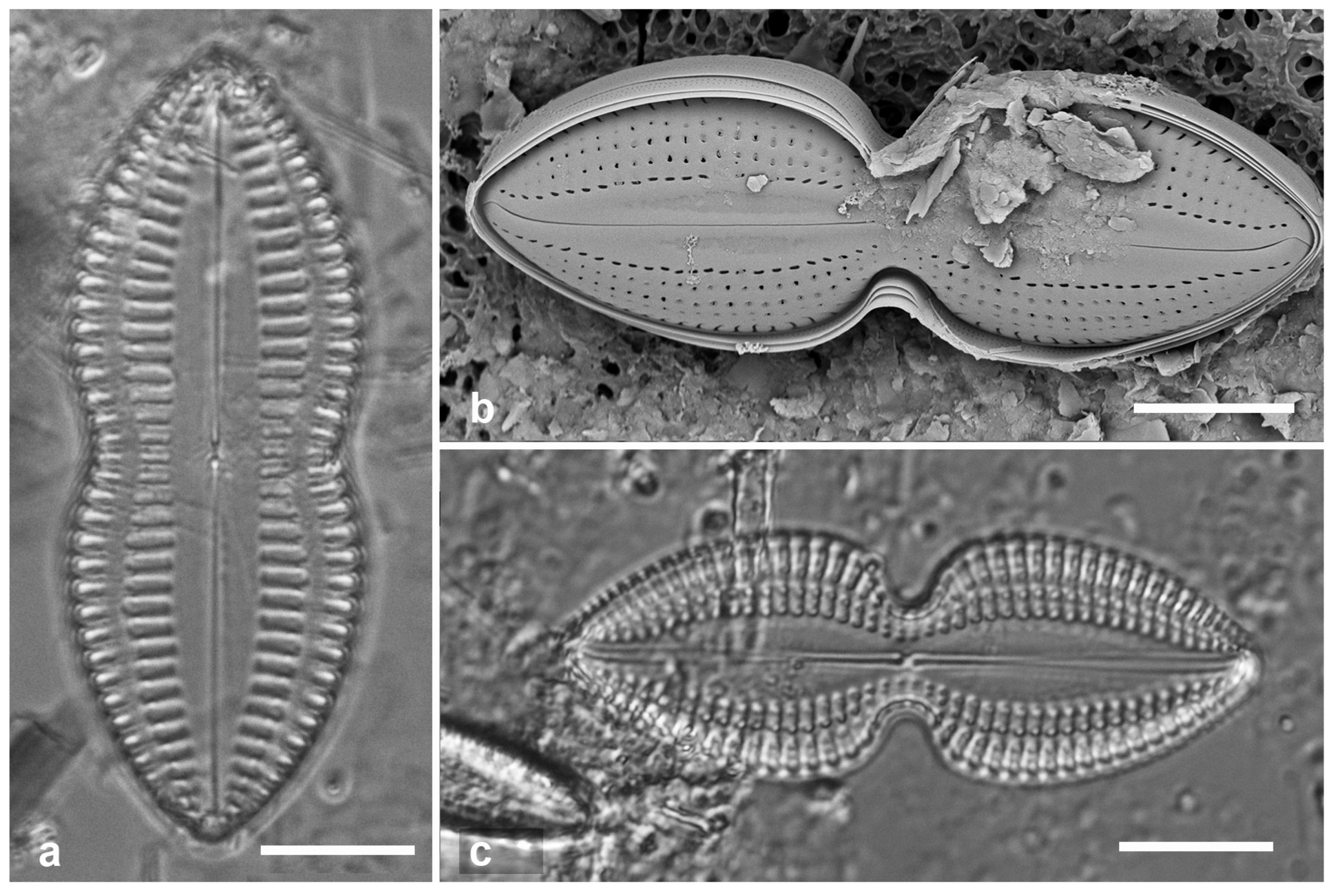
Figure 9.
(a, b). Odontella obtusa, SEM. (c, d) Pseudictyota dubia. (c) Valve in valve view, LM. (d) Valve in girdle view, SEM, showing ocelli (arrowhead), rimoportulae (arrow) and pseudoloculate structure. Scale bars: (a) = 25 µm, (b, c) = 10 µm, (d) = 5 µm.
Figure 9.
(a, b). Odontella obtusa, SEM. (c, d) Pseudictyota dubia. (c) Valve in valve view, LM. (d) Valve in girdle view, SEM, showing ocelli (arrowhead), rimoportulae (arrow) and pseudoloculate structure. Scale bars: (a) = 25 µm, (b, c) = 10 µm, (d) = 5 µm.
Diagnostics: Small cells with large areolae (not pseudoloculate – contrast Pseudictyota below), ocelli on low apical elevations (no higher than the central elevation).
Comments: Very small elliptical cells, but seemingly conforming to this taxon as shown in SEM by Lavigne et al. (2015). Odontella aurita (Lyngbye) Brébisson & Godey has been reported from Guam (Lobban et al. 2012) and Chuuk (Park et al. 2022). New record for Micronesia.
3.20. Pseudictyota dubia (Brightwell) P.A.Sims & D.M.Williams in Sims et al. 2018 Figure 9c, d
Synonym: Triceratium dubium Brightwell
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 130, figs 21–24); Chuuk (Park et al. 2018, p. 103,
Figure 3); 585454
Yap samples: Y25H-1, Y25H-2, Y37-8, Y41-7
Dimensions: Diam. 24–27 µm.
Comments: Three of the angles bear ocelli on long elevations, the other three bear rimoportulae with long tubes.
ARDISSONEALES Round emend Lobban & Ashworth
Ardissoneaceae Round emend Lobban & Ashworth
3.21. Ardissonea densistriata Lobban 2022 in Lobban et al. 2022 [b]
Previous Micronesia records: Marshall Is. and Yap (Lobban et al. 2022b,
Figure 2 [2B from Yap, Y42-1])
Additional Yap samples: Y41-1, Y42-3
Dimensions: Length 46–103 µm, width 7–10 µm, stria density 16–17 in 10 µm
Comments: Stria density nearly double that of. A. formosa. Ardissoneaceae are either known or expected to be epiphytic, often attached to seaweeds by stout mucilage pads.
3.22. Ardissonea formosa (Hantzsch) Grunow in Cleve & Grunow 1880
Previous Micronesia records: Guam and Yap (Navarro & Lobban 2009, p. 136; Lobban et al. 2012, p. 259, pl. 15, figs 4, 5, pl. 16, figs 1, 2; Lobban et al. 2022b,
Figure 1 [1E, F from Yap [Y39A], Y42-1]); 585467
Additional Yap samples: Y41-7
Dimensions: Length 201–300 µm, width 22–24 µm, stria density 8–10 in 10 µm
3.23. Ardissoneopsis appressata Lobban & Ashworth in Lobban et al. 2022 [b]
Previous Micronesia records: Guam (Lobban et al. 2012, p. 260, pl. 1, figs 1, 2, pl. 16, figs 6–8 (as Ardissonea fulgens var. gigantea (Lobarzewsky) Rabenhorst); Yap (Lobban et al. 2022b, figs 15, 16: Yap Y42-1: figs 15E–G); 585370
Additional Yap samples: Y36-1
Dimensions: Length 857 µm (560–900 µm), width 9–12 µm, striae 18–19 in 10 µm
Comments:
Ardisosoneopsis appressata forms flabellate colonies on sturdy mucilage pads (Lobban et al. 2012, pl. 1, Lobban et al. 2022b,
Figure 15A).
Ardisosoneopsis contains species that differ from
Ardissonea sensu stricto in having valves with a single wall and from
Synedrosphenia in having simple apical architecture. The double wall character can be discerned with diffulty in LM, the apical structure can be seen only with SEM, and very similar species are found in each genus (Lobban et al. 2022b). In particular, we have not claimed
Ardissoneopsis fulgicans Lobban & Ashworth for Yap because we have only LM, indistinguishable from
Synedrosphenia fulgens (Greville) Lobban & Ashworth, which has not been reported from Micronesia.
3.24. Ardissoneopsis gracilis Lobban in Lobban et al. 2022 [b] Figure 10 a, b
Previous Micronesia records: Guam, Yap, Chuuk, Majuro, Jaluit (Lobban et al. 2022b,
Figure 18; Yap Y36-2, no voucher)
Additional Yap samples: Y26C
Dimensions: Length 260 µm, width 7 µm at poles, 8 µm at center, stria density 11 in 10 µm
Comments: Fragments from Y26C (
Figure 10c–f) differ from the description of
A. gracilis in being slightly larger (center 9 µm wide, poles 7 µm wide, in between 5 µm, much longer, apparently 570 µm (half valve, not shown), 9–10 striae in 10 µm (11.5 at center), and more strongly undulate. These differences probably indicate another species, but the stria density is much lower than that of the undulate Honduras species
A. undosa (Grunow) Lobban & Ashworth, not yet seen in the Western Pacific. Without more material, especially copulae, the identity of these fragments cannot be confirmed.
Figure 10.
(a–f) Ardissoneopsis. (a, b) A. gracilis valve from Y36-2 in LM with central portion and one pole; detail of central portion, typical of the species. (c–f) Valve fragments from Y26C, possibly a different species but not A. undosa. (c) Mid portion with inflation, LM. (d) Internal SEM of pole, showing increased stria density at apex. (e) External view of apex, SEM, showing spines; broken edge shows lack of internal costae. (f) Internal aspect of central portion, SEM, showing location of annulus (arrow). Scale bars: (a)= 25 µm, (b–f) = 10 µm.
Figure 10.
(a–f) Ardissoneopsis. (a, b) A. gracilis valve from Y36-2 in LM with central portion and one pole; detail of central portion, typical of the species. (c–f) Valve fragments from Y26C, possibly a different species but not A. undosa. (c) Mid portion with inflation, LM. (d) Internal SEM of pole, showing increased stria density at apex. (e) External view of apex, SEM, showing spines; broken edge shows lack of internal costae. (f) Internal aspect of central portion, SEM, showing location of annulus (arrow). Scale bars: (a)= 25 µm, (b–f) = 10 µm.
3.25. Climacosphenia elegantissima Lobban in Lobban et al. 2022 [b] Figure 11a
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 136, figs 64, 65, as C. elongata; Lobban et al. 2022, p. 156, figs 26–28)
Yap samples: Y26B, Y26C, Y41-8
Dimensions: Length > 1000 µm, width across apex 25 µm, striae 27–28 in 10 µm
Comments: Differing from C. elongata J.W.Bailey (also present in Micronesia) in the very long, narrow stem and margins and sides of the annulus parallel toward the apex.
3.26. Climacosphenia scimiter A.Mann 1925 Figure 11b
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 150, as C. moniligera), Guam and Chuuk (Lobban et al. 2022b, p. 160, figs 29, 30)
Yap samples: Y41-7, Y41-8
Dimensions: Length 273 µm, width across apex 27 µm, striae 29 in 10 µm
Comments: This species, formerly dismissed as a curved form of C. moniligera Ehrenberg, is clearly distinguished from it by the delicate and seamless craticular bars on the valvocopula (Lobban et al. 2022b); C. monilgera was removed from the regional flora (Lobban & Witkowski 2024b) but may yet be found.
3.27. Grunowago pacifica Lobban & Ashworth in Lobban et al. 2022 [b] Figure 11c
Previous Micronesia records: Guam (Lobban et al. 2012 as Synedra bacillaris (Grunow) Hustedt; Lobban et al. 2022b, figs 20, 21); 585506
Yap samples: Y42-1
Dimensions: Length 272 µm, width 13 µm, striae 8 in 10 µm
Comments: This species of “big sticks” differs from other Ardissoneaceae in having a longitudinal costa along the apical axis. The species differs from Grunowago bacillaris (Grunow) Lobban & Ashworth in width and lanceolate valve outline, not reported from Micronesia.
Figure 11.
(a) Climacosphenia elegantissima, apical portion of valve and valvocopula showing parallel sides and annular lines until the larger space between craticular bars (arrow), LM. (b) Climacosphenia scimiter, valvocopula, LM. (c) Grunowago pacifica, SEM of valve interior and valvocopula, showing central costa. (d, e) Synedrosphenia gomphonema, LM. (f) Synedrosphenia licmophoropsis, apical and middle portions in LM, arrows indicate annulus. Scale bars: (a, b, d) = 25 µm, (c) = 20 µm, (e, f) = 10 µm.
Figure 11.
(a) Climacosphenia elegantissima, apical portion of valve and valvocopula showing parallel sides and annular lines until the larger space between craticular bars (arrow), LM. (b) Climacosphenia scimiter, valvocopula, LM. (c) Grunowago pacifica, SEM of valve interior and valvocopula, showing central costa. (d, e) Synedrosphenia gomphonema, LM. (f) Synedrosphenia licmophoropsis, apical and middle portions in LM, arrows indicate annulus. Scale bars: (a, b, d) = 25 µm, (c) = 20 µm, (e, f) = 10 µm.
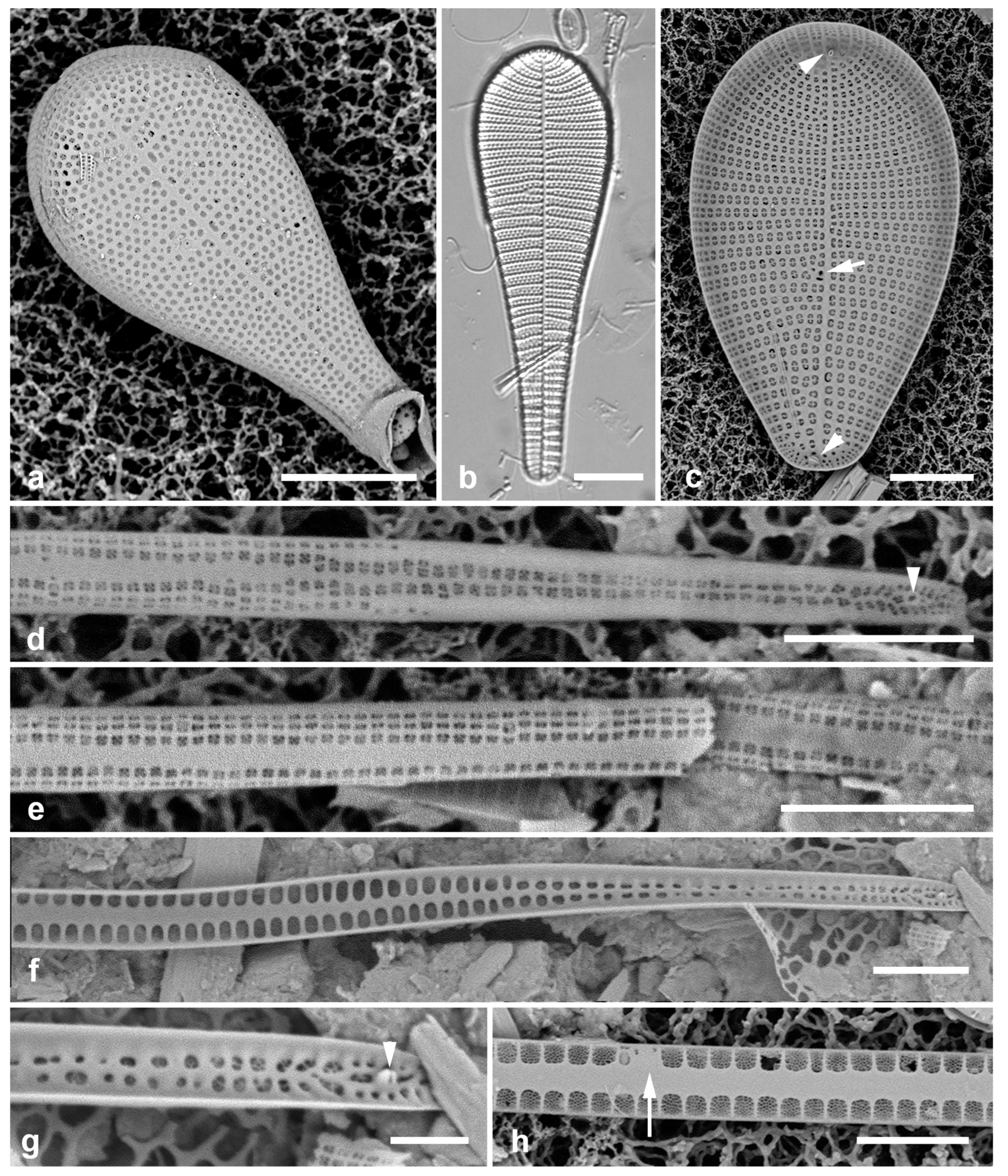
3.28. Synedrosphenia gomphonema (Janisch & Rabenhorst) Hustedt 1932 Figure 11d, e
Previous Micronesia records: Marshall Is., Chuuk, and Yap (Lobban et al. 2022b, figs 15, 16); Chuuk (Park et al. 2022, p. 35,
Figure 13)
Yap samples: Y42-1
Dimensions: Length 504 µm, width 29 µm.
Comments: This is the well-known species of Synedrosphenia, with a cuneate apex, still clearly distinct from other species added to the genus by Lobban et al. (2022b), which are not all tapered.
3.29. Synedrosphenia licmophoropsis Lobban in Lobban et al. 2022 [b] Figure 11f
Previous Micronesia records: Guam, Yap, Marshall Is. (Lobban et al. 2022b, figs 9, 10)
Yap samples: Y37-8
Dimensions: Length 600–735 µm, widening apically from 14 to 27 µm, striae 19 in 10 µm near apex
Comments: A very large species, like
S. gomphonema but with a broadly rounded apex, named for its resemblance to
Licmophora attenuata Lobban, Tharngan & Ashworth, (reported from Guam) from which it can be easily distinguished by the presence of the parallel lines of the annulus (
Figure 11f, arrows).
Figure 12.
(a) Toxarium hennedyanum, central portion showing smooth valve outline and field of scattered areolae inside the annulus; LM. (b) Toxarium cf. hennedyanum central and apical portions of a valve with no areolae inside the annulus (annulus along the valve margin with a line of areolae on each side); LM. (c) Toxarium undulatum, center and apical portions of valves showing undulating outline and scattered areolae inside the annulus at center and pole; SEM. Scale bars = 10 µm.
Figure 12.
(a) Toxarium hennedyanum, central portion showing smooth valve outline and field of scattered areolae inside the annulus; LM. (b) Toxarium cf. hennedyanum central and apical portions of a valve with no areolae inside the annulus (annulus along the valve margin with a line of areolae on each side); LM. (c) Toxarium undulatum, center and apical portions of valves showing undulating outline and scattered areolae inside the annulus at center and pole; SEM. Scale bars = 10 µm.
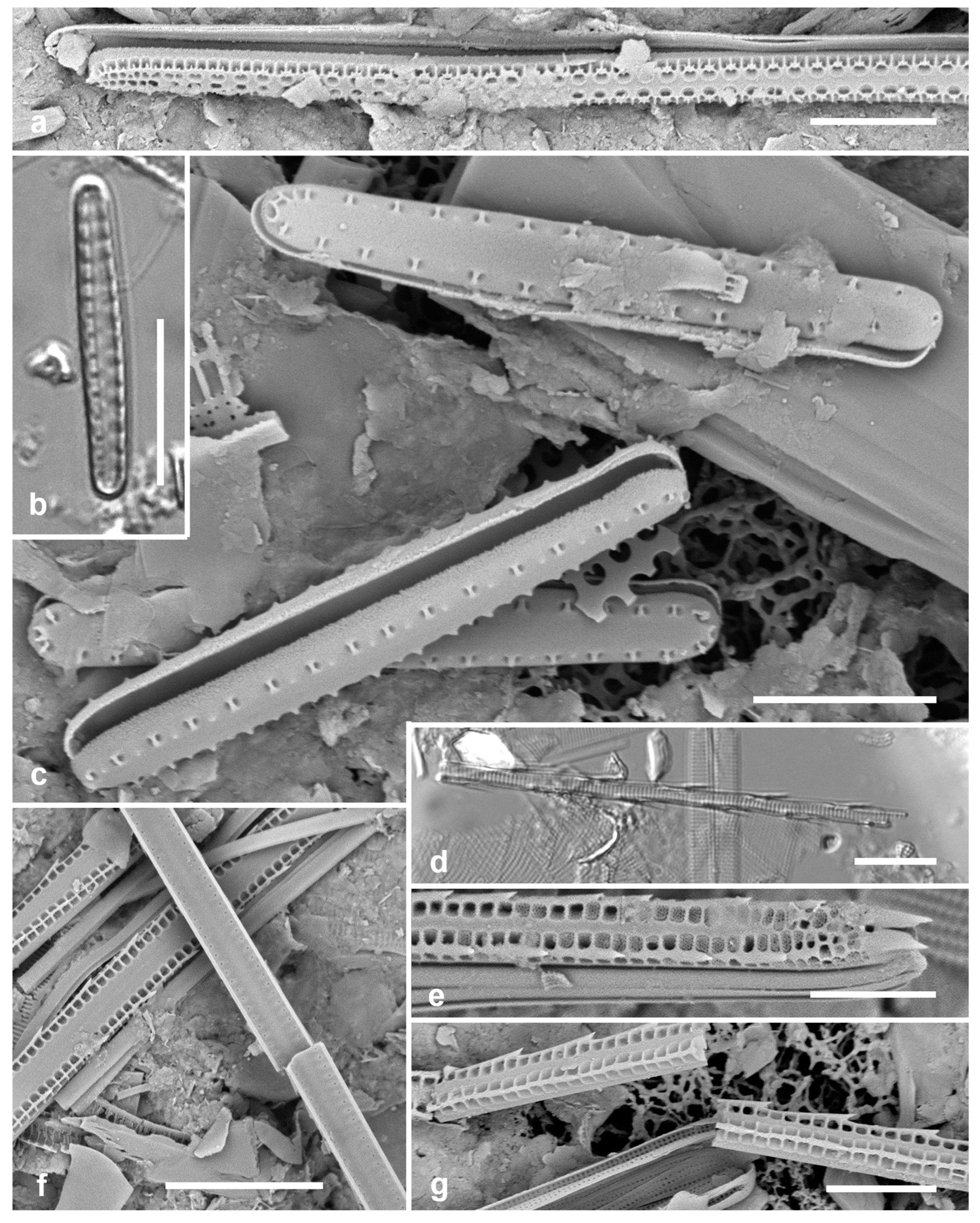
3.30. Toxarium hennedyanum (Gregory) Pelletan 1889 Figure 12a, b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 260, pl. 17, figs 1–5); Chuuk (Park et al. 2018, p. 105, figs 7a, b); Marshall Islands (Majuro, Jaluit, Bikar) (Lobban et al. 2022b, p. 45
Figure 22); 585471
Yap samples: Y26B, Y26C
Dimensions: Length 156–334 µm in Micronesia; width 7 µm across inflated center; striae 9–11 in 10 µm
Comment: The form lacking scattered areolae inside the annulus (
Figure 12b) was reported by Lobban et al. (2012) for Guam, but still has uncertain taxonomic status. Only observed as fragments in sediment samples but probably common as seaweed epiphyte like
T. undulatum (next entry).
3.31. Toxarium undulatum Bailey ex Bailey 1854 Figure 12c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 260, pl. 17, figs 6–8); Chuuk (Park et al. 2018, p. 105, figs 8a, b); Yap, Majuro, Jaluit (Lobban et al. 2022b, p. 49,
Figure 23, no voucher); 210620
Yap samples: Y26C, Y36-2, Y42-3
Dimensions: Length 450 µm, width 6 µm, striae 14 in 10 µm
Comments: Yap specimens shown have the characteristics of Guam materials, which, as noted by Lobban et al. (2022), may or may not correspond to authentic T. undulatum.
BIDDULPHIALES Krieger
Biddulphiaceae Kützing
Comment: Mann in Adl et al. (2019), following current phylogenetic trees, but with a need to create a taxonomy in which each genus is under a continuous set of higher taxa, pulled Biddulphiopsis and Lampriscus, without a formal Family or Order, into a new Subclass Chrysanthemodiscophycidae, along with Stictodiscales (including Chrysanthemodiscus and Stictocyclus, both common in Guam but not yet recorded in Yap) and Ardissoneaceae. For now, we follow AlgaeBase (Guiry & Guiry 2024) in classifying them in Biddulphiales: Biddulphiaceae (Subclass Biddulphiophycidae).
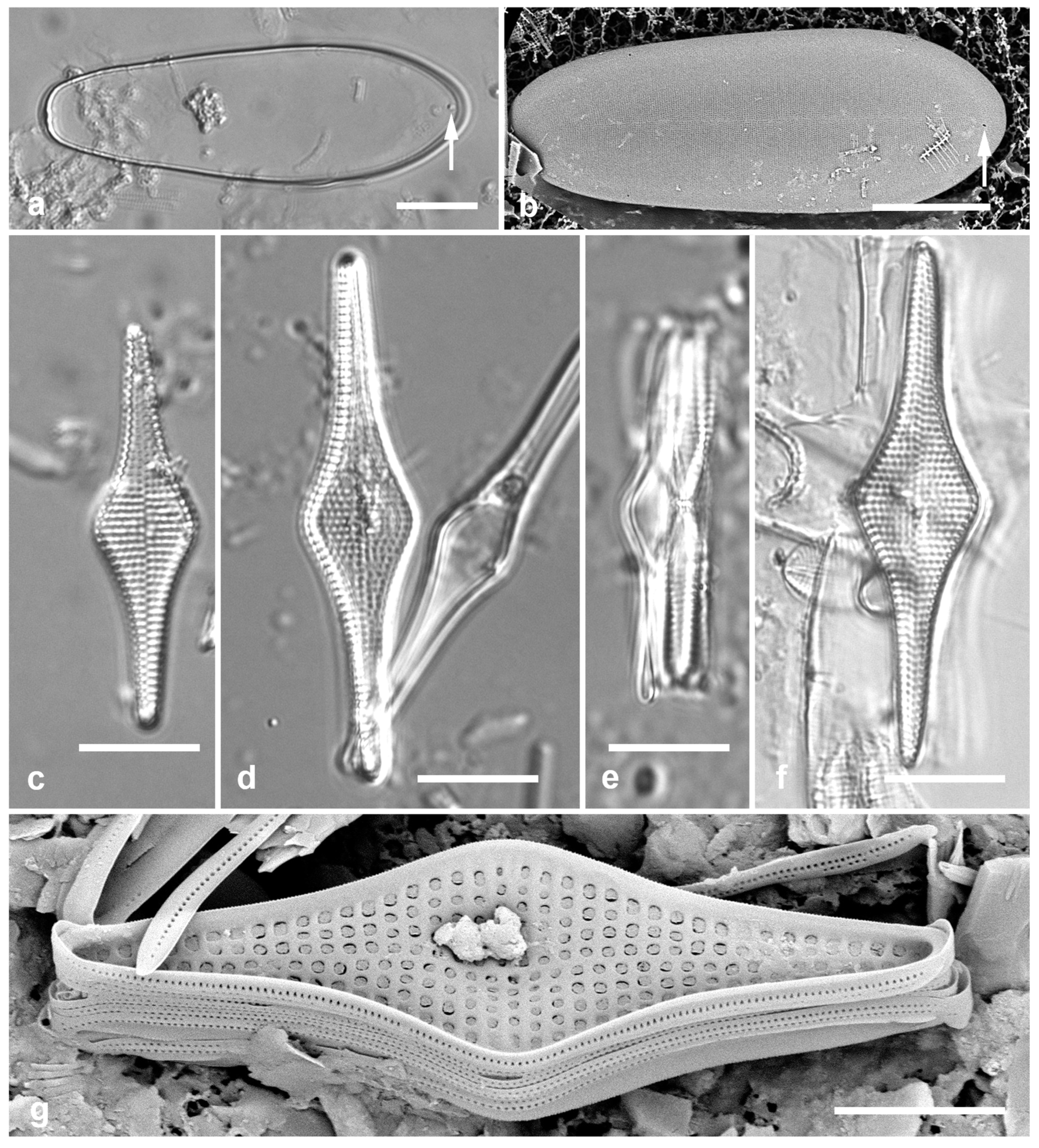
3.32. Biddulphia biddulphiana (J.E.Smith) Boyer 1901 Figure 13a
Previous Micronesia records: Guam (Navarro & Lobban, 2009, p. 133, figs 28–31); 585629
Yap samples: Y25H-2
Dimensions: Length 79 µm
3.33. Biddulphiella cuniculopsis Lobban, sp. nov. Figure 13e–h
Diagnostics: Distinguished from congeners by long apical elevations arising from a single elevation, in valve view elliptical-rhomboidal.
Holotype: Specimen at 9.2 mm E and 9.5 mm S of the mark on slide 1129, deposited at Diatom Collection, Academy of Natural Sciences of Drexel University, Philadelphia, accession number [pending].
Figure 13e.
Type locality: Epiphytic on seaweed in farmer fish territory, GU44AR-3. Coll. 12 Aug. 2012, C.S. Lobban & M. Schefter.
Morphology: Valve hemispherical to nearly spherical with very long apical elevations (
Figure 13e, h), flange extended slightly toward poles, having the shape of an eye when seen from below (
Figure 13f). Length 23–38 µm, width 27 µm. Very shallow diagonal sulci in the valve face/mantle (Figs 13f, g arrowheads). At least one rimoportula with long forked external tube (
Figure 13e); internal openings difficult to distinguish from interstitial pores (
Figure 13g) [in
B. tridens the fine slit is clearly visible (
Figure 13d inset; compare with Sims et al. 2022,
Figure 13F)]. Areolae ca. 5 in 10 µm, closed by volae and interspersed with conical papillae and interstitial pores (
Figure 13g). Very long apical elevations hyaline except for pore field at tips (pseudocelli) (Figs 13e, h). Copulae not seen.
Additional records: Y 26C, Y45-2
Etymology: From L. cuniculus, a rabbit, with reference to the resemblance to a rabbit’s head and ears.
PhycoBank registration: … [pending]
Comments: This species was first found in two collections from Yap taken at nearby locations (
Figure 1a) but 26 years apart but is typed on a subsequent Guam collection that yielded a good specimen in LM. This striking species is
Biddulphiella rather than
Biddulphia because of having sulci rather than costae (terminology of Sims et al. 2022). In comparison with
B. tridens and other living and fossil species, which have several to many domes separated by deep sulci,
B. cuniculopsis has only one dome, from which the apical elevations arise, barely separated by slight sulci.
3.34. Biddulphiella tridens (Ehrenberg) P.A.Sims & M.P.Ashworth in Sims et al. 2022 Figure 13b–d
Synonym: Biddulphia tuomeyi (J.W. Bailey) Roper
Previous Micronesian records: Palau (Lobban & Jordan 2010 as B. tuomeyi); Guam (Lobban & Witkowski 2024b)
Yap samples: Y26C, Y34E
Dimensions: Length 55–70 µm, width 23–28 µm.
Figure 13.
(
a)
Biddulpha biddulphiana, oblique valve in LM. (
b–d)
Biddulphiella tridens, SEM. (
b) Exterior view showing deep sulci, spines, and two rimoportula tubules (arrows). (
c) Valve in profile, tilt=80°. (
d) Valve as in (c), tilt=0°, inset showing internal rimoportula opening. (
e–h)
Biddulphiella cuniculopsis, n. sp. (
e) LM holotype valve from Guam at two focal planes in near-apical girdle view, showing rimoportula (arrow). (
f) LM specimen from Yap, showing sulcus (arrowhead). (
g, h) SEM of valve from Yap. (
g) Interior, tilt=40°, showing sulci (arrowheads) and possible rimoportula opening (arrows; compare
Figure 13e). (
h) Valve in lateral girdle view, showing typical shape; arrowhead = sulcus. Scale bars: (a–g) = 10 µm, (h) = 5 µm.
Figure 13.
(
a)
Biddulpha biddulphiana, oblique valve in LM. (
b–d)
Biddulphiella tridens, SEM. (
b) Exterior view showing deep sulci, spines, and two rimoportula tubules (arrows). (
c) Valve in profile, tilt=80°. (
d) Valve as in (c), tilt=0°, inset showing internal rimoportula opening. (
e–h)
Biddulphiella cuniculopsis, n. sp. (
e) LM holotype valve from Guam at two focal planes in near-apical girdle view, showing rimoportula (arrow). (
f) LM specimen from Yap, showing sulcus (arrowhead). (
g, h) SEM of valve from Yap. (
g) Interior, tilt=40°, showing sulci (arrowheads) and possible rimoportula opening (arrows; compare
Figure 13e). (
h) Valve in lateral girdle view, showing typical shape; arrowhead = sulcus. Scale bars: (a–g) = 10 µm, (h) = 5 µm.
Figure 14.
(a–c) Biddulphiopsis membranacea, LM. (a, b). Complete valve (edges indicated by arrowheads) and detail of center showing scattered pattern contrasting with radiating striae. (c) Copula with apical septa. (d, e) Lampriscus shadboltianus. Valves in valve and girdle view, respectively, SEM, showing the smooth outline of the nominate variety and the characteristic spines on the edges of the ocelli in this species (arrow). Scale bars: (a, c) = 25 µm, (b, d, e) = 10 µm.
Figure 14.
(a–c) Biddulphiopsis membranacea, LM. (a, b). Complete valve (edges indicated by arrowheads) and detail of center showing scattered pattern contrasting with radiating striae. (c) Copula with apical septa. (d, e) Lampriscus shadboltianus. Valves in valve and girdle view, respectively, SEM, showing the smooth outline of the nominate variety and the characteristic spines on the edges of the ocelli in this species (arrow). Scale bars: (a, c) = 25 µm, (b, d, e) = 10 µm.
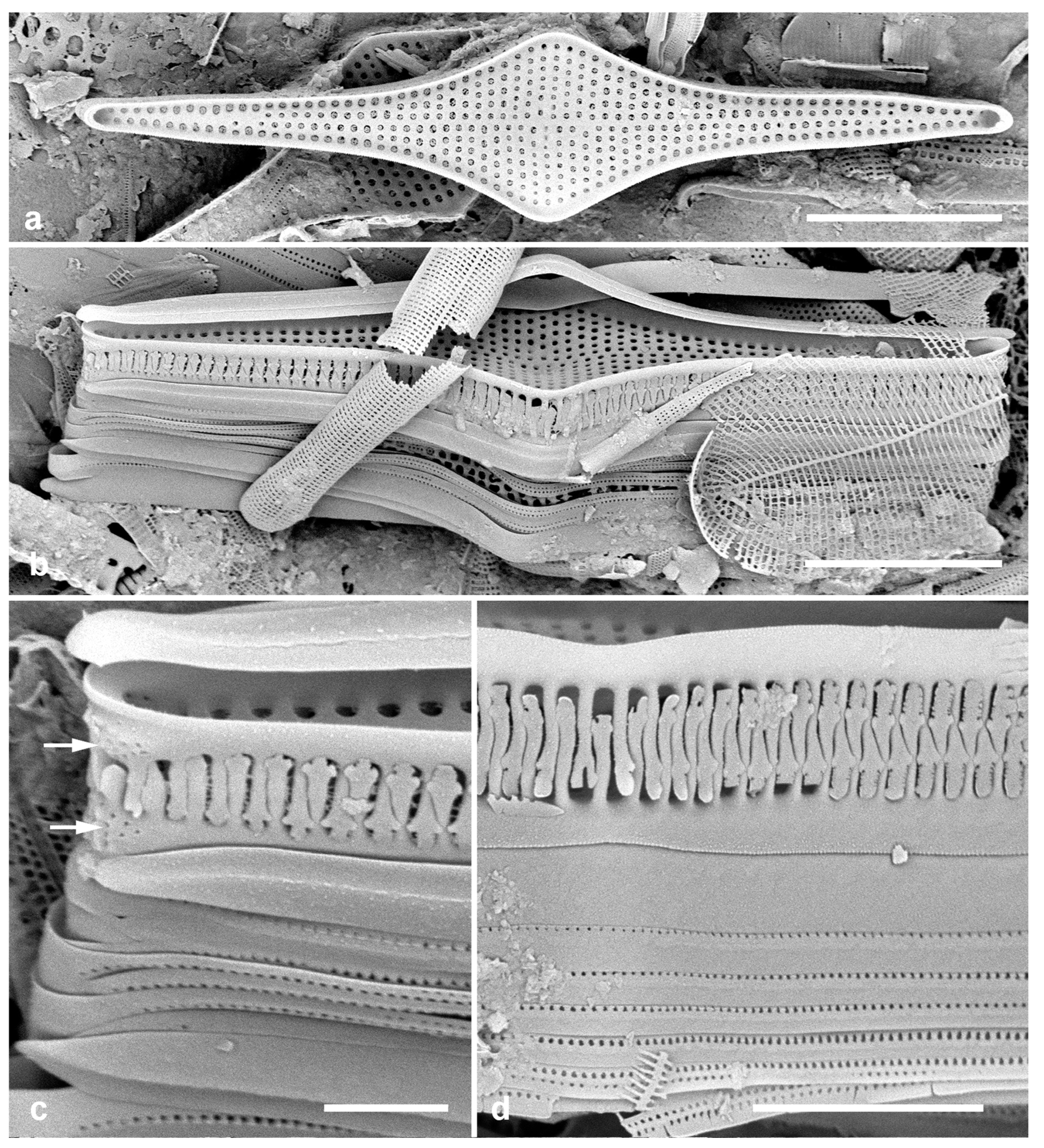
3.35. Biddulphiopsis membranacea (Cleve) von Stosch & Simonsen 1984 Figure 14a–c
Previous Micronesia records: Guam (Navarro & Lobban, 2009, p. 133, figs 6, 7); Chuuk (Park et al. 2022, p. 33, figs 74, 75); 585631
Yap samples: Y42-1
Dimensions: Length 257–288 µm, width including mantle 121 µm, copula 100 µm.
Diagnostics: Huge, delicate valves are elliptical, central zone of random areolae surrounded by radiating striae (
Figure 14b). Numerous rimportulae around the valve margin, especially at the apices.
Comments: In Guam, common in subtidal farmer fish territories along with other huge cells including Chrysanthemodiscus floriatus A.Mann and Stictocyclus stictodiscus (Grunow) Ross (Lobban & Navarro 2009) (species not found in the Yap samples analyzed to date). Easily missed during observations with oil immersion lenses. There is a second, smaller species present in Micronesia, B. titiana (Grunow) von Stosch & Simonsen.
3.36. Lampriscus shadboltianus (Greville) Peragallo & Peragallo 1902 Figure 14d, e
Previous Micronesia records: Chuuk (Park et al. 2022, p. 36, figs 14, 15)
Yap samples: Y42-1
Dimensions: Diam. 29–31 µm
Comments: Smoothly circular copulae show that this is the nominate variety rather than var. crenulatus Navarro, which was reported from Guam (Navarro & Lobban 2009, p. 133, figs 25, 26).
STRIATELLALES Round
Striatellaceae Kützing
3.37. Striatella unipunctata (Lyngbye) Agardh 1832 Figure 15a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 263, pl. 20, figs 4, 5); 210618
Yap samples: Y36-2, Y41-7, Y26C
Dimensions: Length 33–106 µm, width 12–37 µm, transverse striae 46 in 10 µm (counted from
Figure 15a)
Comment: The areolae are transapically elongated, so the oblique striae are about 20–24 in 10 µm, as given by Hustedt (1931–1959, p. 32) and others, thus visible in LM. Transverse striae countable only in SEM.
PLAGIOGRAMMALES E.J.Cox (“nom. prov.”)
Plagiogrammaceae De Toni
3.38. Neofragilaria anomala (Giffen) Witkowski & Dąbek in Li et al. 2015 Figure 15b, c
References: Li et al. 2015, p. 10, figs 6a–k
Previous Micronesia records: Guam (Lobban et al. 2012, p. 254, pl. 10, figs 1, 2, as Neofragilaria nicobarica Desikachary, Prasad & Prema); 505994 .
Yap samples: Y16B, Y37-8, Y36-1, Y41-7, -8
Dimensions: Length 12–32 µm, width 4–10 µm, striae 5.5–12.5
Comments: The specimen from Y16B (
Figure 15c) is uncharacteristically small, elliptical and more finely striate, so may not represent the same species.
3.39. Plagiogramma porcipellis Ashworth & Chunlian Li in Li et al. 2020 Figure 15 d–h
References: Foged (1978, pl. 7, figs 10–12) as P. staurophorum; Li et al. 2020, p. 16, figs 9j, k, 12
Previous Micronesia records: Guam (Lobban et al. 2012, p. 254, pl. 10, figs 3, 4 [non
Figure 5] as
P. staurophorum); 585470
Yap samples: Y26C
Dimensions: Length 33–45 µm, width 13 µm; 8 striae in 10 µm.
Comments: The specimens cited in Lobban et al. (2012) and Foged (1978) are much more finely striated (>15 in 10 µm) than P. staurophorum and the new name was applied by Li et al. (2020).
3.40. Plagiogramma subatomus Lobban, S.Konno, Y.Arai & R.W.Jordan in Lobban 2021
Previous Micronesia records: Guam, Yap, Chuuk, Jaluit (Lobban 2021, p. 236, figs 1–6; Y18E figs 5, 6); 585498
Additional Yap samples: Y36-4
Dimensions: Length 9 µm, width 5 µm, areolae ca. 17 in 10 µm
Figure 15.
Figure 81, Figure 82, Figure 83, Figure 84, Figure 85, Figure 86, Figure 87 and Figure 88. (a) Striatella unipunctata, SEM. (b, c) Neofragilaria anomala, internal views of two valves. (d–h). Plagiogramma porcipellis. (d) Two frustules in girdle view, LM. (e) Valve in LM. (f) Frustule in oblique view, SEM, showing spines, apical pores fields, central elevation, and broad valvocopula. (g) Detail of apical pore field and areolae, SEM. (h) Internal view of valve showing pseudoseptum and transverse costae. Scale bars: (a, d, e, f, h) = 10 µm, (b, g) = 5 µm. (c) = 2 µm.
Figure 15.
Figure 81, Figure 82, Figure 83, Figure 84, Figure 85, Figure 86, Figure 87 and Figure 88. (a) Striatella unipunctata, SEM. (b, c) Neofragilaria anomala, internal views of two valves. (d–h). Plagiogramma porcipellis. (d) Two frustules in girdle view, LM. (e) Valve in LM. (f) Frustule in oblique view, SEM, showing spines, apical pores fields, central elevation, and broad valvocopula. (g) Detail of apical pore field and areolae, SEM. (h) Internal view of valve showing pseudoseptum and transverse costae. Scale bars: (a, d, e, f, h) = 10 µm, (b, g) = 5 µm. (c) = 2 µm.
RHAPHONEIDALES Round
Psammodiscaceae Round & D.G.Mann
3.41. Psammodiscus nitidus (W.Gregory) Round & D.G.Mann 1980 Figure 16a, b
References: Watanabe et al. 2013, figs 1–18.
Previous Micronesia records: Guam (Lobban et al. 2012, p. 258, pl. 14, figs 1, 2); 585514
Yap samples: Y26C, Y34A
Dimensions: Diam. 17–21 µm
Diagnostics: Circular with large areolae. In SEM areolae covered with rotae supported by 2–3 spokes, presence of central pore, and usually one or more marginal or central rimoportulae.
Comment: A species recently reported from Kume I., Okinawa, P. calceatus Tsuy.Watanabe, Nagumo & Ji.Tanaka (Watanabe et al. 2013), differs ultrastructurally from P. nitidus but is probably not distinguishable in LM. In SEM there are 5–9 spokes, giving the areolae a different look than in P. nitidus. We have checked our sparse SEM images from Micronesia and none corresponds to P. calceatus, though most differ from P. nitidus in lacking both the central pore and any rimoportulae. Psammophilic.
Figure 16.
(a, b) Psammodiscus nitidus in LM and SEM external view. (c) Rhaphoneis castracanei, LM. (d) Perissonoë crucifera, SEM external view. (e) Bleakeleya notata, LM. (f) Perideraion montgomeryi, SEM, external. (g, h) Falcula paracelsiana, SEM external, detail of apex with slits. Scale bars: (h) = 25 µm, (a, c–e) = 10 µm, (b, f, g) = 5 µm.
Figure 16.
(a, b) Psammodiscus nitidus in LM and SEM external view. (c) Rhaphoneis castracanei, LM. (d) Perissonoë crucifera, SEM external view. (e) Bleakeleya notata, LM. (f) Perideraion montgomeryi, SEM, external. (g, h) Falcula paracelsiana, SEM external, detail of apex with slits. Scale bars: (h) = 25 µm, (a, c–e) = 10 µm, (b, f, g) = 5 µm.
Raphidoneidaceae Forti
3.42. Perissonoë crucifera (Kitton in Prichard) Desikachary, Gowthaman, Hema, A.K.S.K.Prasad & Prema 1987 Figure 16d
Synonym: Perissonoë cruciata (Janisch & Rabenhorst) G.W.Andrews & Stoelzel
Previous Micronesia records: Guam (Lobban et al. 2012, p. 258, pl. 14, figs 1, 2, as P. cruciata); 585511
Yap samples: Y26C
Dimensions: Width 20 µm
Comments: Andrews & Stoelzel (1984) established Perissonoë based on Amphitetras cruciata Janisch & Rabenhorst (1863); Desikachary et al. (1987) showed that Perissonoë crucifera Kitton in Prichard (1861) has priority and Williams (1988) was wrong about Amphitetras parvula Greville (1863) having priority. Psammophylic: this species was common in Y26C, along with other sand-dwelling and biofilm taxa such as Progonoia, Arcuatasigma, and several long, linear Homoeocladia spp. (The biofilm community has been observed in Guam, but Y26C had also collected a wide range of specimens from different habitats – see Discussion.)
3.43. Rhaphoneis castracanei Grunow in Van Heurck 1880 Figure 16c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 258, 181, pl. 14,
Figure 5); 585513
Yap samples: Y26C
Dimensions: Length 31 µm, width 23 µm
KOERNERELLALES Lobban & Ashworth
Koernerellaceae Lobban & Ashworth
3.44. Bleakeleya notata (Grunow in Van Heurck) F.E. Round in Round et al. 1990 Figure 16e
Previous Micronesia records: Guam (Lobban et al. 2011a, pp. 178, 181, figs 2, 6, 7, 14–20); 585275
Yap samples: Y36-1, Y41-7
Dimensions: Length 91 µm, width at base 9 µm
Comments: Loosely epiphytic, the necklace-like chains of this and Perideraion spp. loosely festooning seaweeds in sheltered waters.
3.45. Perideraion montgomeryi Lobban, Jordan & Ashworth in Lobban et al. 2011 [a] Figure 16f
Previous Micronesia records: Guam (Lobban et al. 2011a, p. 183, figs 4, 10, 11, 26–33); 585611
Yap samples: Y41-8
Dimensions: Length 36 µm, width 3 µm, striae 32 in 10 µm
FRAGILARIALES P.C.Silva
Fragilariaceae Greville
3.46. Divergita biformis Lobban 2021
Previous Micronesia records: Yap (type locality) (Lobban 2021, figs 26–28)
Yap samples: Y45-5
Dimensions: Length 76 µm, width 4 µm, striae 26–28 in 10 µm.
3.47. Falcula paracelsiana Voigt 1961 Figure 16g, h
Previous Micronesia records: Guam (Lobban et al. 2012, p. 255, pl. 11, figs 1–6); 585698
Yap samples: Y45-2
Dimensions: Length 118 µm, striae 32 in 10 µm.
Comments: This is the only Asia-Pacific species in the original descriptions by Voigt (1960a, 1961), but two species were reported from Japan (Sugawara et al. 2024), showing the ultratructural characters needed for taxonomy, and the identification of F. paracelsiana in Micronesia may warrant reconsideration with further specimens in SEM.
3.48. Hendeyella lineata Ashworth & Lobban in Li et al. 2016 Figure 17a, b
Previous Micronesia records: Guam (Li et al. 2016, p. 1025,
Figure 5a–l); 585751
Yap samples: Y37-8
Dimensions: Length 37 µm, width 5 µm, striae 10 in 10 µm
Diagnostics: Chains of linear valves tightly linked by stout spines.
Figure 17.
Hendeyella. (a, b) Hendeyella lineata in SEM. (a) Yap voucher (Y37-8). (b) Guam specimen (GU44BF-1A) showing broad spines (arrowhead), broad valvocopula (VC), ligulate copula (arrow) and apical pore fields. (c–f) Hendeyella rhombica. (c) Chain in girdle view with valve view, LM. (d) Chain in girdle view showing broad valvocopula (VC) and narrowly branched spines, SEM. (e, f) Valve interiors, SEM, the latter showing weak heteropolarity, SEM. Scale bars: (a, c) = 10 µm, (b, d–f) = 5 µm.
Figure 17.
Hendeyella. (a, b) Hendeyella lineata in SEM. (a) Yap voucher (Y37-8). (b) Guam specimen (GU44BF-1A) showing broad spines (arrowhead), broad valvocopula (VC), ligulate copula (arrow) and apical pore fields. (c–f) Hendeyella rhombica. (c) Chain in girdle view with valve view, LM. (d) Chain in girdle view showing broad valvocopula (VC) and narrowly branched spines, SEM. (e, f) Valve interiors, SEM, the latter showing weak heteropolarity, SEM. Scale bars: (a, c) = 10 µm, (b, d–f) = 5 µm.
Comments: Ribbons loosely associated with seaweeds. As with wild material from Guam, this linear valve (
Figure 17a) has four longitudinal rows of areolae, in contrast to cultured material which was more variable. However, linear specimens from Palau had different spines (Lobban, unpubl.), indicating that there may be more than one linear species in the region. No girdle views could be attributed to this species from Yap but
H. rhombica Ashworth was common in several samples (see next species).
Hendeyella is one of several genera of Fragilariales and Plagiogrammales that are hard to identify because the valves link tightly together and are uncommonly seen in valve view (Li et al. 2015, 2016, 2020).
Hendeyella valvocopulae are broad and hyaline with other copulae bearing large ligules on narrow bands (
Figure 17b, d).
3.49. Hendeyella rhombica Ashworth in Li et al. 2016 Figure 17c–f
References: Li et al. 2016, p. 1024,
Figure 3
Yap samples: Y18E, Y26C, Y37-8
Dimensions: Length 17–26 µm, width 7–8 µm, striae 10 in 10 µm
Diagnostics: Broadly lanceolate, slightly heteropolar valves tightly linked in chains with slender branched spines.
Comments: Valves are similar to H. dimeregrammopsis Ashworth, which is smaller (7.5–12 µm long, 4.4–5.8 µm wide) and less elliptical; on the basis of size we have assigned the specimens in valve view and the LM image to H. rhombica. The girdle view in SEM shows the distinctively slender spines of this species. New record for Micronesia.
3.50. Hyalosynedra laevigata (Grunow) D.M. Williams & F.E. Round 1986 Figure 18a–f
Previous Micronesia records: Guam (Sabir et al. 2018, pp. 9, 12, figs 7–19; non Navarro & Lobban 2009, p. 133, figs 28–31; Lobban & Witkowski 2024b, figs 63–65)
Yap samples: Y41-7, Y45-5
Dimensions: Length 53–195 µm, width 4–5 µm, striae 52 in 10 µm
Diagnostics: Narrowly lanceolate, hyaline cells, distinguished from Stricosus cardinalii Lobban & E.C.Theriot in SEM by only three rows of pores in ocellulimbus and asymmetrical halves of the narrow rimoportula.
Comments: Epiphytic.
Hyalosynedra has rather few taxonomic characters and the morphological and molecular study by Sabir et al. (2018) is hard to apply beyond the Red Sea region. Concurrently Belando et al. (2018) distinguished a new species,
H. lanceolata Belando, Jiménez & Aboal, which is narrower and has a lanceolate sternum; Joh (2021) reported this species from Jeju I., South Korea. We have seen long and short specimens with a lanceolate sternum but do not have enough information to determine whether either or both match
H. lanceolata in all respects. The specimen shown in Navarro & Lobban (2009) as
H. laevigata was reassigned to
H. cf.
al-turkii J.S.M.Sabir & E.C.Theriot (Lobban & Witkowski 2024b) but further study is needed to address the species in Micronesia, which are common and probably diverse. Moreover,
H. laevigata (
Figure 18a) is scarcely distinguishable in LM from
Stricosus cardinalii E.C.Theriot & Lobban (
Figure 18g), which was present in other Yap samples (the two genera can be distinguished in SEM internal views by the shape of the rimoportulae, Figs 18d vs. 18f). Nevertheless, specimens in Y41-7 showed the shallow ocellulimbus of
Hyalosynedra and the linear sternum of
H. laevigata; no specimens of
Stricosus were found in this sample in SEM. Internal views of a specimen from Y45-5 shows the asymmetrical (“parrot beak”) rimoportula (
Figure 18d). We have taken
Stricosus out of alphabetical order next, to make the contrast.
3.51. Stricosus cardinalii Lobban & E.C.Theriot in Sabir et al. 2018 Figure 18f, g
Previous Micronesia records: Guam (Sabir et al. 2018 p. 37, figs 99–103)
Yap samples: Y16B
Dimensions: Length 107–116 µm, width 6 µm; striae (from literature) 38–39 in 10 µm
Diagnostics: Narrowly lanceolate, hyaline cells, distinguished from Hyalosynedra laevigata in SEM by 6–7 rows of pores in ocellulimbus and symmetrical halves of the wide rimoportula.
Comments: See Hyalosynedra laevigata, above.
3.52. Stricosus harrisonii Lobban & E.C.Theriot in Sabir et al. 2018 Figure 18 h, i
Previous Micronesia records: Guam (Sabir et al. 2018 p. 38, figs 95–98; Lobban & Witkowski 2024b, figs 115–120)
Yap samples: Y18E, Y33A
Dimensions: Length 269 µm, width 7 µm; striae (from literature) 38–41 in 10 µm
Diagnostics: Very long, linear, hyaline valve; sternum and rimoportula visible in LM.
Figure 18.
Hyalosynedra vs.
Stricosus. (
a–f)
Hyalosynedra laevigata. (
a) Valve in LM (Y41-7). (
b) Fractured frustule in SEM (Y41-7), left-hand apex out of frame, shown in
Figure 18d. (
c, d) Internal view of valve fragment (Y45-5) and detail of apex showing asymmetrical rimoportula (arrow). (
e) Detail of apex external showing shallow ocellulimbus (arrow) and apical spines. (
f, g)
Stricosus cardinalii apex internal detail showing symmetrical rimoportula (arrow), and whole of same valve showing similarity of size and shape to
H. laevigata. (
h, i)
Stricosus harrisonii, LM. Scale bars: (h) = 25 µm, (a–c, g, i) = 10 µm, (d–f) = 2 µm.
Figure 18.
Hyalosynedra vs.
Stricosus. (
a–f)
Hyalosynedra laevigata. (
a) Valve in LM (Y41-7). (
b) Fractured frustule in SEM (Y41-7), left-hand apex out of frame, shown in
Figure 18d. (
c, d) Internal view of valve fragment (Y45-5) and detail of apex showing asymmetrical rimoportula (arrow). (
e) Detail of apex external showing shallow ocellulimbus (arrow) and apical spines. (
f, g)
Stricosus cardinalii apex internal detail showing symmetrical rimoportula (arrow), and whole of same valve showing similarity of size and shape to
H. laevigata. (
h, i)
Stricosus harrisonii, LM. Scale bars: (h) = 25 µm, (a–c, g, i) = 10 µm, (d–f) = 2 µm.
Comments: Identifiable in LM since there are presently no other hyaline cells of this size and shape known in the region. The full specimen shown here substantially extends the size ranges given in Sabir et al. (2018) and Lobban & Witkowski (2024b).
3.53. Neosynedra provincialis (Grunow) D.M. Williams & F.E. Round 1986 Figure 19 a, b
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 135, figs 46, 47); 585613
Yap samples: Y45-5
Dimensions: Length 59 µm, width 3 µm.
Figure 19.
(a, b) Neosynedra provincialis valve and detail of apex, SEM. (c, d). Neosynedra tortosa apex and valve, SEM. (e–h). Opephora pacifica. (e) Frustule in LM. (f, g) Valve interiors in SEM, showing size range and heteropolarity. (h) Apex external view showing pore field, SEM. (i, j) Synedra lata, SEM. Scale bars: (a, d–f, i, j) = 10 µm, (b, c) = 5 µm, (h) = 2 µm.
Figure 19.
(a, b) Neosynedra provincialis valve and detail of apex, SEM. (c, d). Neosynedra tortosa apex and valve, SEM. (e–h). Opephora pacifica. (e) Frustule in LM. (f, g) Valve interiors in SEM, showing size range and heteropolarity. (h) Apex external view showing pore field, SEM. (i, j) Synedra lata, SEM. Scale bars: (a, d–f, i, j) = 10 µm, (b, c) = 5 µm, (h) = 2 µm.
3.54. Neosynedra tortuosa (Grunow) D.M. Williams & F.E. Round 1986 Figure 19 c, d
Previous Micronesia records: Yap (Y16K) (Navarro & Lobban 2009, p. 135, figs 44, 45); Guam (Lobban et al. 2012, p. 255, pl. 12,
Figure 1); 585504
Additional Yap samples: Y25H-1, Y41-7
Dimensions: Length 85 µm, width 5 µm.
Comments: The apical pore fields differ between these two Neosynedra spp. Note that in N. tortosa the sternum is straight, even though the valve margin is undulate. The spelling of the specific epithet has been orthographically corrected in AlgaeBase from tortosa, which we used in earlier publications.
3.55. Synedra lata (Giffen) Witkowski in Witkowski et al. 2000 Figure 19 i, j
Previous Micronesia records: Guam (Lobban et al. 2012, p. 257, pl. 13, figs 3, 4); 585855
Yap samples: Y37-5, Y37-8, Y26C
Dimensions: Length 30–40 µm, width 6–10 µm, striae 14–17 in 10 µm
Diagnostics: Broadly lanceolate, rostrate valves with short, coarse striae only along the margin, rest of valve surface hyaline. Apical pore fields apparently not sunken.
Comments: Witkowski in Witkowski et al. (2000) made a “comb. nov.” (actually a stat. nov.) to raise the rank of lata within Synedra based on LM. In SEM, however, areolae and apical pores fields are characteristic of Tabularia rather than Synedra as presently understood (Williams & Karthick 2021) and our specimens may represent more than one species. Tabularia is a “paraphyletic assemblage” (D. Williams, pers. comm. 5 Apr. 2023) and S. lata has not yet been studied, so the correct placement must await further study of Tabularia and S. lata iteslf.
3.56. Tabularia parva (Kützing) D.M. Williams & F.E. Round 1986 Figure 20a–d
Previous Micronesia records: Guam (Lobban et al. 2012, p. 257, pl. 13, figs 6, 7); 585764
Yap samples: Y16B, Y42-1
Dimensions: Length 13–52 µm, width 4–5 µm, striae 20–22 in 10 µm
Diagnostics: Lanceolate cells with small apical ocellulimbi; broad, lanceolate sternum; biseriate striae on valve face and mantle with no break, areolae opposite; single rimoportula.
Comments: Identity of the Yap specimens as T. parva is uncertain, recognizing that there are other species with wide sterna yet to be resolved (including the Guam specimens cited above, which have a marginal break in the striae) and that we lack internal SEM images.
Staurosiraceae Medlin
3.57. Opephora pacifica (Grunow) Petit 1899 Figure 19 e–h
References: Sabbe & Vyverman 1995, p. 244–245, figs 45–53; Witkowski et al. 2000, p. 72, pl. 25, figs 18–26
Yap samples: Y16B
Dimensions: Length 20–44 µm, width 7–8 µm, striae 6–7 in 10 µm
Diagnostics: This species is distinguished from congeners by the very low stria density, lack of crossbars in the striae, and lack of spines (Sabbe & Wyverman 1995), and from Neofragilaria anomala by heteropolarity, wide sternum, and pores in the apical fields vs. slits.
Comments: New record for Micronesia.
RHABDONEMATALES Round & R.M.Crawford
Grammatophoraceae Lobban & Ashworth
3.58. Grammatophora angulosa Ehrenberg 1840 [a] Figure 20e
Previous Micronesia records: Guam (Lobban et al. 2012, p. 262, pl. 19, figs 1, 2); 585791
Yap samples: Y26C
Dimensions: Length 15 µm
Comments: Scarce and so far always very small in Micronesia.
3.59. Grammatophora oceanica Ehrenberg 1840 [b] Figure 20f, g
Previous Micronesia records: Guam (Lobban et al. 2012, p. 262, pl. 19, figs 3–5); Chuuk (Park et al. 2022, p. 36, correcting Park et al. 2018,
Figure 22); 210454
Yap samples: Y36-4
Dimensions: Length 33 µm, width 7 µm, striae 22 in 10 µm
Diagnostics: Valves linear-elliptical, not inflated in the middle; stria density 22–23 in 10 µm. Based on stria densities and the lack of inflation, these are G. oceanica rather than G. marina (Lyngbye) Kützing, but the two species, each with several named varieties, are very difficult to distinguish.
Comments: Forming epiphytic zig-zag chains, along with other species of Grammatophora.
Figure 20.
(a–d) Tabularia parva, SEM. (a, b) Large specimen and detail of striae. (c, d) Small specimens, (c) oblique, showing lack of break in striae at valve–mantle junction. (e) Grammatophora angulosa, girdle view showing characteristically hooked septa, LM. (f, g) Grammatophora oceanica, LM, valve view and girdle view. (h) Hyalosira tropicalis, SEM. (i) Microtabella interrupta, SEM of frustule showing valve interior and copulae with septa. Scale bars: (a, e–g, i) = 10 µm, (c, h) = 5 µm, (b, d) = 2 µm.
Figure 20.
(a–d) Tabularia parva, SEM. (a, b) Large specimen and detail of striae. (c, d) Small specimens, (c) oblique, showing lack of break in striae at valve–mantle junction. (e) Grammatophora angulosa, girdle view showing characteristically hooked septa, LM. (f, g) Grammatophora oceanica, LM, valve view and girdle view. (h) Hyalosira tropicalis, SEM. (i) Microtabella interrupta, SEM of frustule showing valve interior and copulae with septa. Scale bars: (a, e–g, i) = 10 µm, (c, h) = 5 µm, (b, d) = 2 µm.
3.60. Hyalosira pacifica Lobban in Lobban et al. 2022 [a]
Previous Micronesian records: Yap (Lobban et al. 2022a, p. 3, figs 3A–P; M-P from Y26A).
Yap samples: Y26A
Dimensions: Length 9–23 µm, width 3–5 µm, striae 22–24 in 10 µm
Diagnostics: Hyalosira pacifica is one of only three septate species known so far in the genus, the others being H. septata Bosak, Van de Vijver and Bizsel and H. hesperica Alvarez-Blanco & Blanco, both from the Mediterranean Sea (Lobban et al. 2021, 2022a). It differs from the former in striae densities of valve and copulae, and from the latter also in having two rimoportulae per valve.
Comment: Hyalosira pacifica type locality is Melbourne, Australia. Attached to seaweeds by mucilage pads.
3.61. Hyalosira tropicalis Navarro 1992 Figure 20h
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 136, figs 52, 53; Lobban et al. 2021, figs 7–9); 585278
Yap samples: Y41-8
Dimensions: Length 17 µm, width 5 µm, striae 24 in 10 µm
Diagnostics: Small, epiphytic, chain-forming cells, distinguished from congeners by the inflated valve outline and the uniseriate striae with large pores.
3.62. Microtabella interrupta (Ehrenberg) F.E. Round in Round et al. 1990 Figs 20i, 21a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 261, pl. 1,
Figure 3, pl. 18, figs 6, 7, as
Hyalosira interrupta (Ehrenberg) Navarro; Lobban & Ashworth 2014, figs 13 a–f); Chuuk (Park et al. 2022, p. 36, Figure 89); 585519
Yap samples: Y25H-1, Y25H-2, Y41-7
Dimensions: Length 19–38 µm, width 7 µm, striae 26 in 10 µm
Comments: Microtabella spp. are notable for the distended septa of the last-formed copulae on each half of the cingulum, which enclose the plastids, giving an interrupted appearance. However, Ehrenberg’s (1838) etymology referred to the space between the septa. The identity of the specimens we have been calling M. interrupta in this region is still under investigation, as the original illustrations (Tessella interrupta Kützing, see Jahn & Kusber 2004) do not show any inflated septa and because there are no SEM images of European M. interrupta.
3.63. Microtabella rhombica Lobban 2015 [c] Figure 21b, c
Previous Micronesia records: Guam (Lobban 2015c, figs 1–4); 586030
Yap samples: Y18E, Y26C
Dimensions: Length 37–52 µm, width 11–12 µm, striae 27–29 in 10 µm
Diagnostics: Rhombic valve much broader than M. interrupta but hard to distinguish in girdle view.
Comments: The discovery of this species showed that the patch of plastids held between inflated septa, is a generic character. Several unpublished species found in Saipan (CNMI) and Florida / U.S. Virgin Islands (Lobban, Ashworth & Frankovich in prep.) suggest that there may be still more species in Micronesia.
CYCLOPHORALES Round & R.M.Crawford
Cyclophoraceae Round & R.M.Crawford
3.64. Cyclophora minor Ashworth & Lobban in Ashworth et al. 2012 Figure 21d
Previous Micronesia records: Guam and Palau (Ashworth et al. 2012, p. 688, figs 4, 31–36); 586931
Yap samples: Y41-8
Dimensions: Length 8 µm, striae 44 in 10 µm
Diagnostics: Very small lanceolate cells with circular pseudoseptum on one or both valves.
Figure 21.
(a) Microtabella interrupta valve and girdle bands in LM. (b, c) Microtabella rhombica. (b) SEM, arrow points to apical spine. (c) Valve and girdle band in LM. (d) Cyclophora minor frustule in LM, one valve with pseudoseptum. (e). Cyclophora tenuis valve exterior in SEM, arrows indicate two parts of the apical slit field. (f) Licmophora flabellata valve in LM, showing multiple rimoportulae along sternum. Scale bars: (a–c, e, f) = 10 µm, (d) = 2 µm.
Figure 21.
(a) Microtabella interrupta valve and girdle bands in LM. (b, c) Microtabella rhombica. (b) SEM, arrow points to apical spine. (c) Valve and girdle band in LM. (d) Cyclophora minor frustule in LM, one valve with pseudoseptum. (e). Cyclophora tenuis valve exterior in SEM, arrows indicate two parts of the apical slit field. (f) Licmophora flabellata valve in LM, showing multiple rimoportulae along sternum. Scale bars: (a–c, e, f) = 10 µm, (d) = 2 µm.
3.65. Cyclophora tenuis Castracane 1878 Figure 21e
Previous Micronesian records: Guam and Palau (Ashworth et al. 2012, p. 686, figs 1, 5, 6, 14–18); 216820
Samples: Y34B
Dimensions: Length 76 µm, width 9 µm, striae 31 in 10 µm
Diagnostics: Broad valves, circular pseudoseptum on only one valve, apical slits in a U-shape field, often appearing as two patches in valve view.
Comments: Single specimen observed in a mangrove sample, but commonly found in the coral reef epiphytic community in Guam. Two narrower new species from Guam have been found elsewhere in Micronesia but not yet in Yap (C. castracanei Ashworth & Lobban in Majuro and C. tabellariformis Ashworth & Lobban in Palau).
LICMOPHORALES Round
Licmophoraceae Kützing
3.66. Licmophora flabellata (Greville) C.Agardh 1831 Figure 21f
Previous Micronesia records: Guam (Lobban et al. 2011, p. 20, figs 29–33); Chuuk (Park et al. 2022, p. 38, figs 24a, b); 297516
Yap samples: Y36-4, Y37-7
Dimensions: Length 60–150 µm, width 7–9 µm, striae 31–33
Comment: Well-known for attaching by strong mucilage stalks to seaweeds and other submerged surfaces, biofouling. All Licmophora spp. are epiphytic.
3.67. Licmophora cf. hastata Mereschkowsky 1901 Figure 22a–d
References: Mereschkowsky 1901–1902, p. 150,
Figure 10; Hustedt 1931–1959, p. 72, Figure 599, Honeywill 1998, p. 248, figs 14a–e
Yap samples: Y36-4, Y41-7
Dimensions: Length 38–50 µm, max. width 6–8 µm, striae 28–29 in 10 µm
Diagnostics: Distinguished by the abrupt narrowing at the apex and the moderate septum.
Description: Valves clavate, widest about one third the way from the apex, which is protracted or rostrate rather than acute and has a spine. Lower part tapered to a slightliy inflated basal pole; sternum wider at base, multiscissura with five slits. Areolae uniform, transapically elongate, 24 in 10 µm. Rimoportula on the mantle of at least one apex, other apex not observed. Copulae have not been documented before except for the septum on the valvocopula, described by Honeywill as shallow. The valvocopula in
Figure 22a, b —and thus the transition of the midrib from ab- to advalar side—is largely hidden but as much as can be seen of the open end of the band (
Figure 22c, VC) shows that the midrib ended already. The 1st pleura, closed at the base, has a slightly advalvar midrib that becomes wider as the band narrows toward the base (
Figure 22a, b); a single row of pores on the advalvar side continues around the closed end. The 2nd pleura has a broad midrib and thickened ligule (
Figure 22a arrowhead) and the open ends do not reach the basal pole.
Comments: New record for Micronesia. The identification of this species requires some justification for three reasons: (1) the great majority of Licmophora species so far identified in this region have been new species, suggesting a generic flora distinct from the European flora (Lobban & Santos 2022); (2) the key character usually used to pick this out is its supposedly unique shape, especially the acute apex; and (3) it was described by Mereschkowsky (1901–1902), whose descriptions and especially outline drawings leave much to be desired. Mereschkowsky even stated that this species and a more clavate variety differed only in shape from L. debilis. The shape of our specimens is between the nominate and clavate varieties. He described two other species with acute apices (L. rostrata Mer. and L. inflata Mer.), which Hustedt (1931–1959) dismissed. Hustedt observed both forms of L. hastata and L. debilis with LM and accepted them as different taxa. Honeywill (1998) found both species in Britain and provided the first SEM of them, adding ultrastructural characters such as the number of slits in the multiscissura at the basal pole. Mereschkowsky noted that L. hastata was frequent but scarce everywhere. Two specimens from Yap match the more detailed descriptions in Hustedt and Honeywill except for the shape, which may not be a reliable character, and the density of areolae in 10 µm (i.e., ours are slightly longer than theirs). Whereas specimens in their works are shown as having the widest part of the valve near the apex, as is usual in the genus, our specimens are widest further down. Ultrastructure of the girdle bands in the European populations and/or molecular data will be needed to establish whether the Yap populations are indeed L. hastata and for now we have qualified the identification with “cf.” Licmophora debilis is smaller, has many more areolae in 10 µm than L. hastata, and lacks the apical spine (Honeywill 1998). In Australian L. debilis, Lobban & Santos (2022, Figure 151) observed that the valvocopular midrib ended halfway along the band.
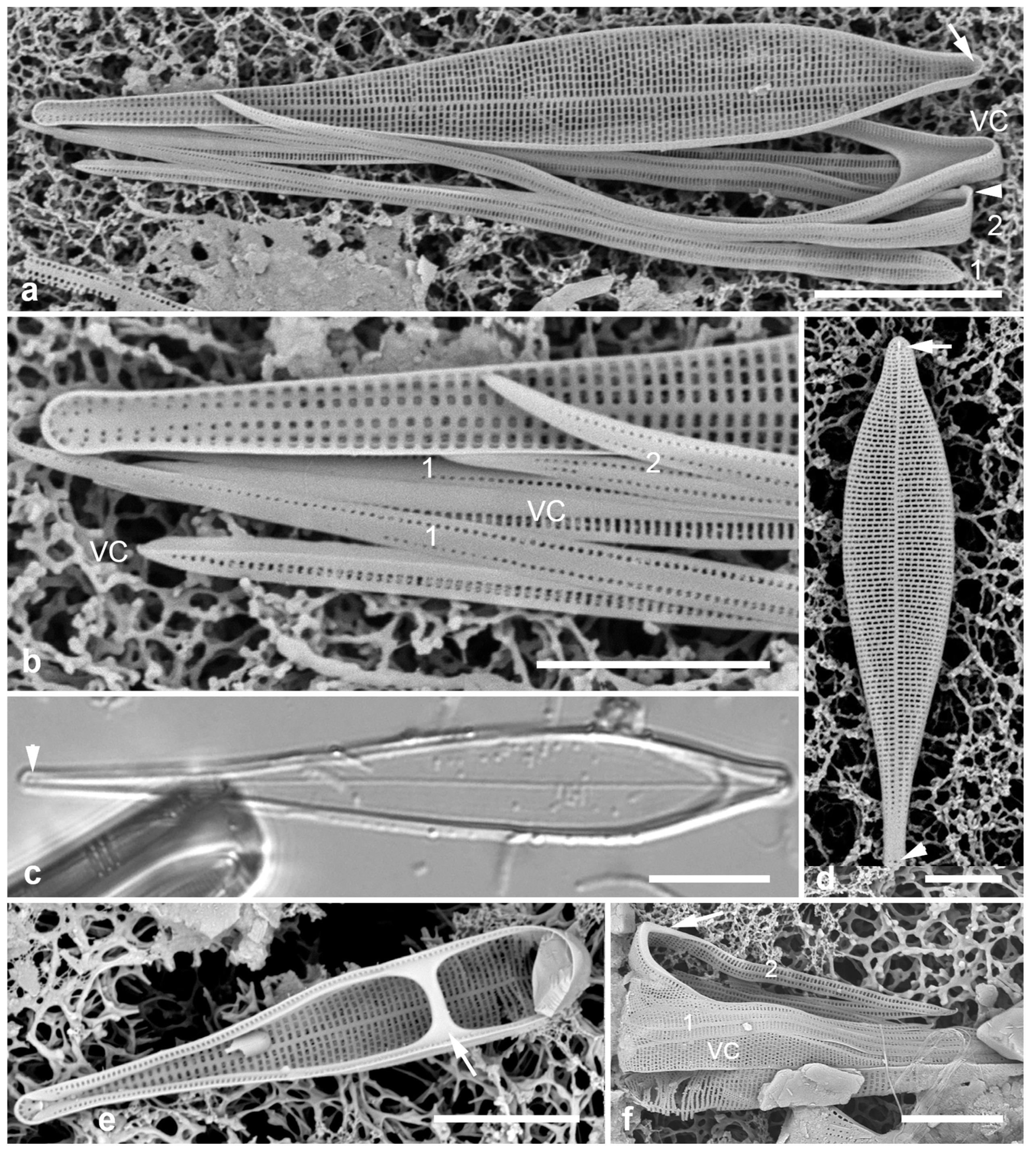
Figure 22.
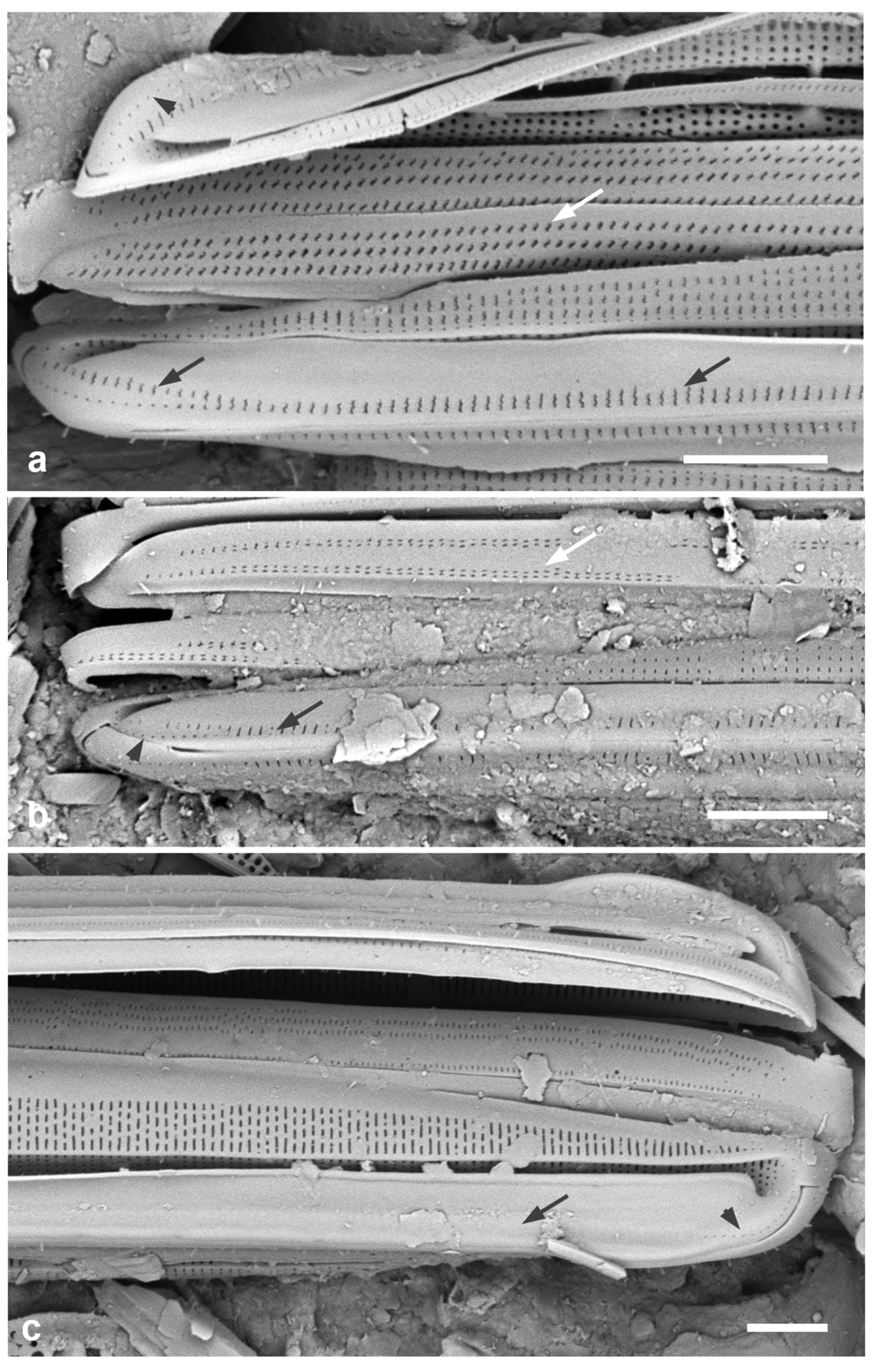
Licmophora. (a–d) L. hastata. (a, b) Valve interior with some of the girdle bands, SEM: valvocopula (VC), 1st pleura (1) and 2nd pleura (2); arrowhead on (a) points to thickened ligule on 2nd pleura, arrow to the rimoportula. (c) Valve with valvocopula (showing window in septum – arrowhead) in LM. (d) External SEM of valve with basal rimoportula opening (arrowhead), the five slits of the multiscissura below it; also showing apical pine (arrow). (e, f) L. johnwestii. (e) Interior view of valve with valvocopula showing the bridge-like septum (arrow) and the change in stria density between base and apex. (f) Partial frustule in girdle view, the valve across the bottom of image, showing valvocopula (VC), 1st pleura (1), and 2nd pleura (2) with shallow septum on abvalvar edge at apex. Scale bars: (a, c) = 10 µm, (b, d–f) = 5 µm.
Figure 22.
Licmophora. (a–d) L. hastata. (a, b) Valve interior with some of the girdle bands, SEM: valvocopula (VC), 1st pleura (1) and 2nd pleura (2); arrowhead on (a) points to thickened ligule on 2nd pleura, arrow to the rimoportula. (c) Valve with valvocopula (showing window in septum – arrowhead) in LM. (d) External SEM of valve with basal rimoportula opening (arrowhead), the five slits of the multiscissura below it; also showing apical pine (arrow). (e, f) L. johnwestii. (e) Interior view of valve with valvocopula showing the bridge-like septum (arrow) and the change in stria density between base and apex. (f) Partial frustule in girdle view, the valve across the bottom of image, showing valvocopula (VC), 1st pleura (1), and 2nd pleura (2) with shallow septum on abvalvar edge at apex. Scale bars: (a, c) = 10 µm, (b, d–f) = 5 µm.
3.68. Licmophora johnwestii Lobban and Emm.S.Santos 2022 Figure 22e, f
Previous Micronesia records: Yap (Lobban & Santos 2022, figs 11, 99–105, no Yap voucher).
Yap samples: Y26A, C
Dimensions: Length 17–21 μm, width 3.5–4 μm at septal bridge. Stria density different in basal and apical halves of the valve, 32–33 to 43–44 in 10 μm
Diagnostics: Small clavate cells distinguished from
L. debilis by the window in the septum, distinguished from other licmosphenioids known so far by its small size, transapically elongate areolae, and rapid change in stria density mid-valve. The shallow septum visible on the abvalvar edge of the 2nd pleura (
Figure 22f, arrow) confirms this character, unusual in the genus (see Lobban & Santos 2022, Figure 100).
Comment: The type locality for this distinctive species is Melbourne, Australia, where it was epiphytic on seaweed in an intertidal pool. The Yap samples, from subtidal sediment collections, were likely washed off the reef.
Figure 23.
Licmophora, cont. (a) L. peragallioides, LM, showing apical window in septum (arrow). (b) L. remulus, LM, showing regular areolae in the lamina. (c–e) L. romuli, SEM, valve with details of basal pole and lamina; (d) showing single line of areolae on each side of the sternum on the “stem” with short striae on the basal pole, and 8 slits in the multiscissura; (e) showing the centripetal loss of vimines on the lamina, resulting in shredding of the valve in acid cleaning. (f) L. undulata, LM, showing undulation (arrows), parallel sides of lower stem, and inflated base. Scale bars: (c) = 25 µm, (a, b, e, f) = 10 µm, (d) = 2 µm.
Figure 23.
Licmophora, cont. (a) L. peragallioides, LM, showing apical window in septum (arrow). (b) L. remulus, LM, showing regular areolae in the lamina. (c–e) L. romuli, SEM, valve with details of basal pole and lamina; (d) showing single line of areolae on each side of the sternum on the “stem” with short striae on the basal pole, and 8 slits in the multiscissura; (e) showing the centripetal loss of vimines on the lamina, resulting in shredding of the valve in acid cleaning. (f) L. undulata, LM, showing undulation (arrows), parallel sides of lower stem, and inflated base. Scale bars: (c) = 25 µm, (a, b, e, f) = 10 µm, (d) = 2 µm.
3.69. Licmophora peragallioides Lobban 2013 Figure 23a
Previous Micronesia records: Guam (Lobban 2013, p. 189, figs 3–21); 585709
Yap samples: Y41-8
Dimensions (from Guam): Length 68–109 µm, width 14–16 µm, striae 12–13 near base, 14–16 near apex.
Comments: Although recorded only as the valvocopula, it conforms to L. peragallioides.
3.70. Licmophora remulus Grunow 1867 Figure 23b
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 136, figs 54–57; Lobban et al. 2011b, p. 19–20, figs 26–28); Chuuk (Park et al. 2022, p. 38, figs 91–93); 585601
Yap samples: Y36-4
Dimensions: Length 84 (100–160) µm, width at apex 8 µm, striae 29–30 in 10 µm
Comments: Distinguished from L. romuli (see below) by the regular areolae in the lamina. Although Grunow (1867: 2) claimed that the “oar-like” shape of this species distinguished it from all congeners, there was in fact that second species in his material with the same shape but different areolae, which Lobban (2021) discovered initially in Guam.
3.71. Licmophora romuli Lobban 2021 Figure 23c–e
Previous Micronesia records: Guam (Lobban 2021, p. 238, figs 7–17)
Yap samples: Y41-7
Dimensions: Length 135–182 µm, width 11–12 µm, striae 34 in 10 µm
Diagnostics: With the classic spathulate outline of L. remulus but differing in the dearth of vimines in the apical part, giving it a shredded appearance in SEM; difficult to distinguish from L. remulus in LM.
Comments: Lobban (2021) noted that much of the variation in length is due to the stem length.
3.72. Licmophora undulata Macatugal, Tharngan & Lobban 2019 Figure 23f
Previous Micronesia records: Guam (Macatugal et al. 2019, p. 5, figs 3–5); 586913
Yap samples: Y26C
Dimensions: Length 171 µm, width near apex 13 µm, striae 30 in 10 µm
Diagnostics: The long “stem” has a distinct wave in it.
Comments: Single valve observed. Differs from
L. repanda Macatugal, Tharngan & Lobban (not yet recorded from Yap) in the more distinct wave (
Figure 23f arrows), nearly parallel sides in the “stem” and an inflated basal pole. The sternum remains straight through the margin undulations in both species.
3.73. Podocystis adriatica (Kützing) Ralfs in Prichard 1861 Figure 24a, b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 256, pl. 12, figs 2, 3); 585505
Yap samples: Y37-7, Y37-8, Y41-8
Dimensions: Length 38–64 µm, width 18–23 µm
Comment: Podocystis spp. attach to seaweeds by mucilage pads.
3.74. Podocystis spathulata (Shadbolt) Van Heurck 1896 Figure 24c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 256, pl. 12, figs 4–6); Chuuk (Park et al. 2018, p. 106,
Figure 19); 585486
Yap samples: Y25H-2, Y36-2, Y37-8, Y45-2
Dimensions: Length 54–78 µm, width 33–47 µm
THALASSIONEMATALES Round
Thalassionemataceae Round
Figure 24.
(a, b) Podocystis adriatica. (a) SEM, external, showing bi- to multiseriate striae. (b) LM showing costae between the striae. (c) Podocystis spathulata SEM, internal, showing absence of costae; this valve has apical and basal rimoportulae (arrowheads), the other valve would have only apical. There is also a characteristic pore near the sternum (arrow). (d, e) Lioloma delicatulum, SEM external valve faces, showing basal pole with rimoportula (arrowhead) and part of the middle of a very long valve. (f–h) Lioloma elongatum, SEM external valve faces, showing basal pole with rimoportula (arrowhead) and portion of the middle with a “bubble-shaped structure” (arrow). Scale bars: (a–c) = 10 µm, (d–f, h) = 5 µm, (g) = 2 µm.
Figure 24.
(a, b) Podocystis adriatica. (a) SEM, external, showing bi- to multiseriate striae. (b) LM showing costae between the striae. (c) Podocystis spathulata SEM, internal, showing absence of costae; this valve has apical and basal rimoportulae (arrowheads), the other valve would have only apical. There is also a characteristic pore near the sternum (arrow). (d, e) Lioloma delicatulum, SEM external valve faces, showing basal pole with rimoportula (arrowhead) and part of the middle of a very long valve. (f–h) Lioloma elongatum, SEM external valve faces, showing basal pole with rimoportula (arrowhead) and portion of the middle with a “bubble-shaped structure” (arrow). Scale bars: (a–c) = 10 µm, (d–f, h) = 5 µm, (g) = 2 µm.
3.75. Lioloma delicatulum (Cupp) Hasle 1996 Figure 24d, e
References: Hasle 2001, pp. 69–73, figs 252, 276–292 (esp. figs 277, 286, 288)
Yap samples: Y26B
Dimensions: Valves, 3 µm wide away from the apex, are said to be 900 µm long, but we did not find any complete valves.
Diagnostics: Very long slender valves without marginal spines, with several areolae in short striae on the mantle and valve face.
Comments: Planktonic. We observed only the basal pole. Lioloma differs from Thalassiothrix in lacking marginal spines and the genus name reflects this (“smooth margin”).
3.76. Lioloma elongatum (Grunow) Hasle 1996 Figure 24f–h
References: Hasle 2001, pp. 58–63, figs 208–235 (esp. 221–231)
Yap samples: Y26B
Dimensions: Valves, 3 µm wide away from the apex, are said to be 900–2000 µm long, but no complete valves found.
Diagnostics: Very long slender valves without marginal spines, with single large areolae on the valve face. The external view shows the location of one of the “bubble-shaped structures” (compare
Figure 24h with Hasle’s Figure 231).
Comments: We observed only the basal pole. This species differs from L. delicatulum in the number of areolae in a stria. Planktonic.
3.77. Thalassionema baculum Lobban 2021 Figure 25b, c
Ref. illus: Lobban 2021, figs 40–44
Previous Micronesia records: Guam (Lobban 2021, p. 244, figs 40–44)
Yap samples: Y26B, Y26C
Dimensions: Length 19 µm, width 2.5 µm, striae 10 in 10 µm
Diagnostics: Small, baton-like frustule, with single line of areolae along the valve margin, simple bar across each areola.
Comments: The only species in this genus commonly observed in the region, and probably the only benthic species.
3.78. Thalassionema synedriforme (Greville) Hasle 1999 Figure 25a
Refs.: Hasle 1999; Hasle 2001, p. 25, figs 68–84.
Yap samples: Y26C
Diagnostics: Hasle (2001) noted that the external openings are covered by structures that are especially elaborate close to the pointed pole compared to the rest of the valve and compared to other species. The apical pole has a spine (seen here), the basal pole has none.
Comments: Planktonic. Type locality: Hong Kong harbor.
3.79. Thalassiothrix gibberula Hasle 1996 Figure 25d–g
References: Hasle 2001, pp. 43–47, figs 133–156
Yap samples: Y26B.
Dimensions: Length > 830 µm, width 1.7–2.7 µm, marginal spines ca. 30 in 100 µm.
Diagnostics: Very long slender valves with dense marginal spines pointed toward the head pole, wide sternum with a longitudinal rib plus spines separating the areolae along the valve margin.
Comments: The shape of the foot pole (
Figure 25e) and the density of the spines distinguish this from
T. spathulata Hasle (Hasle 2001: 49). Planktonic.
Figure 25.
Figure 157–163. (a) Thalassionema synedriforme, SEM portion with apical spine. (b, c) Thalassionema baculum. (b). Frustule in LM. (c) Frustules in girdle view showing simple bars across areolae. (d–g) Thalassiothrix gibberula. (d) Portion of valve in LM showing prominent spines. (e) Basal pole with characteristic spines, SEM. (f) Portions of valves showing interior and exterior. (g) Fragments of valve in external view. Scale bars: (b, d, f) = 10 µm, (a, c, e, g) = 5 µm.
Figure 25.
Figure 157–163. (a) Thalassionema synedriforme, SEM portion with apical spine. (b, c) Thalassionema baculum. (b). Frustule in LM. (c) Frustules in girdle view showing simple bars across areolae. (d–g) Thalassiothrix gibberula. (d) Portion of valve in LM showing prominent spines. (e) Basal pole with characteristic spines, SEM. (f) Portions of valves showing interior and exterior. (g) Fragments of valve in external view. Scale bars: (b, d, f) = 10 µm, (a, c, e, g) = 5 µm.
Incertae Sedis
3.80. Gato hyalinus Lobban & Navarro 2013 Figure 26a, b
Previous Micronesia records: Guam (Lobban & Navarro 2013, p. 23, figs 1–31); Chuuk (Park et al. 2022, p. 38, Figure 98); 585595
Yap samples: Y37-7, Y37-8, Y36-1
Dimensions: Length 43–52 µm, width 16–18 µm, striae 60–70 in 10 µm (not resolved in LM)
Comment: Attached to seaweeds inside its own mucilage tubes. Acid-cleaned valves easily overlooked in LM because the large very finely striated valves resemble isolated girdle bands, but the apical rimoportula can be seen (
Figure 26a, arrow).
Figure 26.
Figure 164, Figure 165, Figure 166, Figure 167, Figure 168, Figure 169 and Figure 170. (a, b) Gato hyalinus, LM showing hyaline valve face with apical rimoportula (arrow), and SEM external valve face showing very fine striae and opening of apical rimoportula (arrow). (c–g). Glyphodesmis acus. (c–e) Yap specimens in LM (same scale). (f) Guam specimen (GU52P-7) in LM. (g) Yap specimen in SEM, internal face and narrow copulae. Scale bars: (a–f) = 10 µm, (g) = 5 µm..
Figure 26.
Figure 164, Figure 165, Figure 166, Figure 167, Figure 168, Figure 169 and Figure 170. (a, b) Gato hyalinus, LM showing hyaline valve face with apical rimoportula (arrow), and SEM external valve face showing very fine striae and opening of apical rimoportula (arrow). (c–g). Glyphodesmis acus. (c–e) Yap specimens in LM (same scale). (f) Guam specimen (GU52P-7) in LM. (g) Yap specimen in SEM, internal face and narrow copulae. Scale bars: (a–f) = 10 µm, (g) = 5 µm..
Figure 27.
Glyphodesmis acus, cont., Guam specimens (GU52P-7), SEM. (a) Valve, internal view. (b) Frustule, oblique internal view, showing interlocking spines between two valves, multiple copulae (some hyaline, others perforate), and elevated center. (c) Detail of same specimen to show apical pore fields (arrows). (d) Portion of different frustule in girdle view near center, showing copulae and overlapping spines. Scale bars: (a, b) = 10 µm, (d) = 5 µm, (c) = 2 µm.
Figure 27.
Glyphodesmis acus, cont., Guam specimens (GU52P-7), SEM. (a) Valve, internal view. (b) Frustule, oblique internal view, showing interlocking spines between two valves, multiple copulae (some hyaline, others perforate), and elevated center. (c) Detail of same specimen to show apical pore fields (arrows). (d) Portion of different frustule in girdle view near center, showing copulae and overlapping spines. Scale bars: (a, b) = 10 µm, (d) = 5 µm, (c) = 2 µm.
3.81. Glyphodesmis acus A.Mann 1925 Figs 26c–g, 27
References: Mann 1925, p. 78, pl. 16, figs 2, 3; Takano 1975, figs 1–16; Fryxell & Miller 1978, figs 11–19
Yap samples: Y26C
Dimensions: Length 32–43 µm, width 10–11 µm; striae 15 in 10 µm.
Diagnostics: Valves rhomboidal to linear–lanceolate with strongly inflated center, center of the valve elevated but without distinctive structure. Sternum absent. SEM shows small pore fields on valve apex and mantle but no ocellus. Interlocking spines at end of each stria, no apreaolae on mantle; spines resemble those of Hendeyella lineata. Copulae of various widths but generally narrow, 0–2 rows of pores on each. Apparently no rimoportulae. External valve face not seen.
Comments: Mann (1925) gave larger dimensions, 42–68 x 10–12 µm. Specimens like this have been observed a few times in Micronesian samples but too little material is available to correctly place it; however, it does not have the characters of
Glyphodesmis. It differs from
Hendeyella in the numerous narrow girdle bands (Figs 26g, 27b, d), even though the spines and small apical pore fields resemble those of
H. lineata (
Figure 17b). For comparison,
Figure 27 shows specimens from Guam from different angles, showing the spines.
Foged (1978, p. 67, pl. 7,
Figure 20) and Podzorski & Håkansson (1987, p. 32, pl. 6,
Figure 11) show light micrographs of specimens identified as
Glyphodesmis rhombica (Cleve) Simonsen. Meister (1932: 28,
Figure 64) recombined “
Fragilaria (?)
rhombica” Cleve into
F. inflata var.
rhombica (Cleve) Meister and later Simonsen (1974), recombined Cleve’s species as
Glyphodesmis rhombica (Cleve) Simonsen, based on a single specimen from a different region than all the other specimens he cites, on the grounds that it had a central nodule. Cleve (1901) noted that there were some “siliceous concretions” at the center, where the “obscurely punctate” striae were fainter. Our images (Figs 26d, f, g) suggest that Cleve was looking at debris. Simonsen (1974) synoymized
G. acus A.Mann (1925), reported as often common in the Philippines; Takano (1975) studied a bloom of
G. acus in Japan using SEM and observed a ring of spines around the central area; Fryxell & Miller (1978) also studied
G. acus with SEM, considering Simonsen’s synonymy “in doubt.” Mann was clear that there was no hyaline center but based his genus decision on the valve view, which, like our
Figure 26e shows a raised center and perhaps spines. Round et al. (1990: 240) show defining characters of
Glyphodesmis including apical ocelli on the valve face with additional pore field on the mantle below, a complex hyaline raised central nodule and wide, nearly hyaline copulae. In contrast, Takano (1975) and Fryxell & Miller (1978), as noted by Round et al. (1990), showed much different characters, as shown here. Fryxell & Miller (1978: 121) also noted taxonomic issues with the generic name, and these may have been compounded by recent progress in Plagiogrammales. In samples from the Marsall Islands another rhomboid species has been found with different ultrastructure that may help resolve generic issues. Pending further study, we follow Fryxell & Miller and assign the Yap specimens to Mann’s taxon. While only a few colony fragments have been found in Micronesia so far,
G. acus can be abundant, and ribbons should be sought among other ribbon-forming taxa in the region, such as
Hendeyella spp.
EUNOTIALES P.C.Silva
Eunotiaceae Kützing
3.82. Colliculoamphora gabgabensis Lobban 2015 [a] Figure 28a, b
Previous Micronesia records: Guam (Lobban 2015a, pp. 4–5, figs 33–40); 586033
Yap samples: Y36-5, Y37-8, Y41-8
Dimensions: Length 10 µm, striae 25 in 10 µm
LYRELLALES D.G.Mann
Lyrellaceae D.G.Mann
3.83. Lyrella clavata (Gregory) D.G.Mann in Round et al. 1990 Figure 28c
References: Hustedt 1961–1966, p. 444, Figure 1509a–c (as
Navicula clavata); Hendey 1964, plate 35,
Figure 13
Previous Micronesia records: Guam (Lobban et al. 2012, p. 263, pl. 20, figs 6–8, as Lyrella hennedyi (W.Smith) Stickle & D.G.Mann (including var. granulosa Grunow))
Yap samples: Y26C
Dimensions: Length 62–77 µm, width 38–45 µm, striae 14–15 in 10 µm
Comments: Hustedt (1961–1966: 444–453) illustrated many named forms of this species, but this appears closest to the nominate form, as also illustrated in Hendey (1964).
This species was identified in Lobban et al. (2012) as L. hennedyi, but according to Hustedt’s (1961–1966) key, the rostrate apices make this L. clavata. Like the Guam specimens, Yap specimen stria densities compare to 10–14 in 10 µm for L. clavata vs. 9–11 striae in 10 µm for L. hennedyi.
Figure 28.
(a, b) Colliculoamphora gabgabensis, frustule in SEM and valve in LM. (c) Lyrella clavata, LM. (d, e) Lyrella cf. rudiformis, SEM, valve exterior and detail of areolae. (f) Lyrella lyra, L.M. (g). Lyrella clavata valve exterior, SEM. Scale bars: (a, c, f, g) = 10 µm, (d) = 5 µm, (b) = 2 µm, (e) = 1 µm.
Figure 28.
(a, b) Colliculoamphora gabgabensis, frustule in SEM and valve in LM. (c) Lyrella clavata, LM. (d, e) Lyrella cf. rudiformis, SEM, valve exterior and detail of areolae. (f) Lyrella lyra, L.M. (g). Lyrella clavata valve exterior, SEM. Scale bars: (a, c, f, g) = 10 µm, (d) = 5 µm, (b) = 2 µm, (e) = 1 µm.
3.84. Lyrella lyra (Ehrenberg) Karayeva 1978 Figure 28f
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 150, Figure 134); 585617
Yap samples: Y25H-1, Y25H-2, Y36-1
Dimensions: Length 98 µm, width 33 µm, striae 13 in 10 µm
Diagnostics: Distinguished from other Lyrella spp. by the narrow, straight lateral sterna.
3.85. Lyrella cf. rudiformis (Hustedt) Gusliakov & Karaeva in Gusliakov et al. 1992 Figure 28d, e
References: Hustedt 1961–1966, p. 384, Figure 1471 (as
Navicula rudiformis); Gusliakov et al. 1992, p. 36, pl. 47, figs 7, 8; Siquieros Beltrones et al. 2021,
Figure 17d
Samples: Y26C
Dimensions: Length 22 µm, width 13 µm, striae 16 in 10 µm
Diagnostics: Small species with large areolae, narrow forcipate lateral sterna with three areolae on each side of the raphe-sternum, in contrast to the similar-size Fallacia hummii (Hustedt) D.G.Mann and the much larger L. rudis (Cleve) Gusliakov & Karaeva —Hustedt (1961–1966, pp. 378, 380, figs 1466, 1468). Differing from most Lyrella, the areolae are closed by cribra, suggesting that the genus assignment may be wrong if this is the same species.
Comments: We imaged several small cells in LM but none matched the published illustrations. These taxa illustrate the difficulty in distinguishing small Lyrella, Fallacia and Diploneis. Gusliakov et al. (1992) gave no rationale for the transfer of this, nor several other Navicula spp. they transferred to Lyrella. This species, originally described from Norway, has been reported by LM from diverse places (M.D. Guiry in Guiry & Guiry 2024) including the two references above. We present here SEM images of a species that may fit the description.
3.86. Moreneis cf. hexagona J.Park, Koh & Witkowski in Park et al. 2012 Figure 29a–c
Previous Micronesia records: Chuuk (J.S.Park et al. 2018, p. 108,
Figure 26); Guam (Lobban & Witkowski 2024b)
Yap samples: Y26C, Y42-1
Dimensions: Length 35 µm, width 13 µm, striae 16 in 10 µm
Comments: Yap specimens, like those from Chuuk (J.S.Park et al. 2018) are larger than Korean specimens, and J.Park et al. (2012) noted larger areolae along the raphe-sternum, not evident here. The single pore at the Voigt fault, described by J.Park et al. (2012) and shown in SEM from Guam (Lobban & Witkowski 2024b), is not evident in our Yap images (
Figure 29b). There are other undescribed species in Micronesia, including one with an undulate margin in Palau, and more work is needed complete the taxonomy of
Moreneis spp. in the region.
3.87. Petroneis granulata (Bailey) D.G. Mann in Round et al. 1990 Figure 29d
Previous Micronesia records: Guam (Lobban et al. 2012, p. 264, pl. 21,
Figure 1); Chuuk (Park et al. 2018, p. 110,
Figure 27); 585522
Yap samples: Y26B, Y37-7
Dimensions: Length 54 µm, width 25 µm, striae 11 in 10 µm
Comments: All these Lyrellaceae seem to be associated with sediments. According to AlgaeBase (Guiry & Guiry 2024), Mann’s combination is illegitimate.
3.88. Petroneis humerosa (Brébisson ex W. Smith) Stickle & D.G. Mann in Round et al. 1990 Figure 29e–g
Previous Micronesia records: Guam (Lobban 2015a, p. 11, figs 95–97); 586009
Yap samples: Y16B, Y25H-2
Dimensions: Length 46–58 µm, width 28–30 µm, striae 10 in 10 µm
Figure 29.
(a–c) Moreneis cf. hexagona. (a) Valve in LM. (b) Valve in SEM, external view showing characteristic central raphe endings (arrow). (c) Broken frustule in SEM showing interior covered foramina. Also note the single bar extending into each areola (arrow). (d) Petroneis granulata, LM. (e–g) Petroneis humerosa. (e) Valve in LM. (f) Frustule in SEM, external also showing copulae. (g) Valve internal surface in SEM. Scale bars: (a, d–g) = 10 µm, (b, c) = 5 µm.
Figure 29.
(a–c) Moreneis cf. hexagona. (a) Valve in LM. (b) Valve in SEM, external view showing characteristic central raphe endings (arrow). (c) Broken frustule in SEM showing interior covered foramina. Also note the single bar extending into each areola (arrow). (d) Petroneis granulata, LM. (e–g) Petroneis humerosa. (e) Valve in LM. (f) Frustule in SEM, external also showing copulae. (g) Valve internal surface in SEM. Scale bars: (a, d–g) = 10 µm, (b, c) = 5 µm.
Comments: SEM of P. humerosa shown by Jones et al. (2005) is entirely consistent with that of Micronesian specimens. They noted, however, that P. humerosa is “heterogeneous, varying significantly in outline, size and stria pattern, and may consist of several sibling species.”
MASTOGLOIALES D.G.Mann
Mastogloiaceae Mereschkowsky
Forty-three
Mastogloia species recorded from Yap in a separate paper (Lobban in press) are listed above in
Table 2.
3.89. Mastogloiopsis biseriata Lobban & Navarro 2012 Figure 30a
Previous Micronesia records: Guam (Lobban & Navarro 2012, p. 254, figs 5–29); 585701
Yap samples: Y26C
Dimensions: Length 25 µm, striae 33 in 10 µm.
Diagnostics: Resembling Mastogloia sect. Marginuluatae, especially the small, isopolar species M. guamensis Lobban (in press), but with apparently hollow flange on valvocopula in lieu of partecta. In LM, central area symmetrical and central striae more distant.
Comment: Single specimen, probably redeposited into this sediment sample. Validation of Simonsen’s sect. Marginuluatae was recently proposed by Lobban (in press).
Figure 30.
Figures 189–194. (a) Mastogloiopsis biseriata, frustule in girdle view, SEM. (b, c) Tetramphora decussata internal view in SEM, valve in LM. (d) Tetramphora intermedia, LM. (e, f) Dictyoneis cf. marginata, frustules in girdle and oblique views, LM. Scale bars: (b–d) = 25 µm, (e, f) = 10 µm, (a) = 5 µm.
Figure 30.
Figures 189–194. (a) Mastogloiopsis biseriata, frustule in girdle view, SEM. (b, c) Tetramphora decussata internal view in SEM, valve in LM. (d) Tetramphora intermedia, LM. (e, f) Dictyoneis cf. marginata, frustules in girdle and oblique views, LM. Scale bars: (b–d) = 25 µm, (e, f) = 10 µm, (a) = 5 µm.
3.90. Tetramphora decussata (Grunow) Stepanek & Kociolek 2016 Figure 30b, c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 298, pl. 1, figs 7–9, pl. 54,
Figure 5, pl. 55, figs 1–3, as
Amphora decussata Grunow); Chuuk (Park et al. 2022, figs 106, 107); 585570
Yap samples: Y25H-2, Y26B, Y26C
Dimensions: Length 115–144 µm, width 22–25 µm, dorsal striae 19 in 10 µm
Comment: Stepanek & Kociolek (2016) made the case for separating this genus from Amphora and for its placement in Mastogloiaceae.
3.91. Tetramphora intermedia (Cleve) Stepanek & Kociolek 2016 Figure 30d
Previous Micronesia records: Guam (Lobban 2015a, p. 2, figs 1–6, as
Amphora rhombica var.
intermedia); Chuuk (Park et al. 2022,
Figure 31); 585756
Yap samples: Y25H-2, Y42-1
Dimensions: Length 115 µm, width 22 µm, dorsal striae 14–18 in 10 µm.
DICTYONEIDALES D.G.Mann
Dictyoneidaceae D.G.Mann
3.92. Dictyoneis cf. marginata (F.W.Lewis) Cleve 1890 Figure 30e, f
References: Hustedt 1931–1959, p. 576–577, Figure 1009; Round et al. 1990, pp. 468–469, figs a–e
Yap samples: Y26C
Dimensions: Length 82–103 µm
Diagnostics: Panduriform frustules with coarse apparent poration caused by pseudoloculate structure; large openings along the margin suggestive of Mastogloia partecta.
Comments: Hein et al. (2008) show two forms that differ somewhat from the illustrations referenced above. Hustedt (1931–1959) commented that the genus has a small number of species difficult to distinguish. The specimens shown here were the only ones found and cannot be positively identified as D. marginata.
CYMBELLALES D.G.Mann
Rhoicospheniaceae Chen & Zhu
3.93. Gomphonemopsis littoralis (Hendey) Medlin 1986 Figure 31a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 264, pl. 22, figs 1–3); Chuuk (Park et al. 2018, p. 110,
Figure 33); 585636
Yap samples: Y41-7
Dimensions: Length 12 µm, width 2 µm, striae 20–21 in 10 µm.
Comments: Recent work in the South China Sea (Krzywda et al. 2019, Li et al. 2024) and Antarctic (Al-Handel et al. 2018), building on Medlin & Round (1986), suggest that this small specimen with circular areolae would still be classified as G. littoralis. Other species are also present in the region, and our flora needs re-examining in light of these recent papers.
ACHNANTHALES P.C.Silva
Achnanthaceae Kützing
3.94. Achnanthes armillaris (O.F.Müller) Guiry 2019 Figure 31b, c
Synonym Achnanthes longipes C.Agardh, nom. illeg.
Previous Micronesia records: Guam (Lobban et al. 2012, p. 285, pl. 39, figs 1–3); 585550
Yap samples: Y26C
Dimensions: Length 10–40 µm, width 8–10 µm, striae 12–13 in 10 µm (costae 6–8 in 10 µm)
Diagnostics: Striae appear biseriate because of internal costae; raphe valve stauros thick and forked on the mantle with areolae in the fork (
Figure 31 b, c), in contrast to
A. brevipes which has flat stauros with no fork and no internal costae (
Figure 31d). Raphe valves concave. Sternum valves also biseriate.
Comment: Achnanthes cf. brevipes and A. kuwaitensis are shown next for comparison. Lee et al. (2013) show SEM of A. armillaris (as A. longipes) from Korea.
3.95. Achnanthes cf. brevipes C.A. Agardh 1824 Figure 31 d, e, 32
Previous Micronesia records: Guam (Lobban et al. 2012, p. 284, pl. 38, figs 1–4); Chuuk (Park et al. 2022, p. 39,
Figure 26); 585355
Figure 31.
(a) Gomphonemopsis littoralis, SEM. (b, c) Achnanthes armillaris. (b) Internal view of raphe valve showing costae and stauros forked under mantle (arrow). (c) Girdle view of raphe valve and cingulum showing mantle areolae in stauros fork (arrow). (d, e) Achnanthes cf. brevipes. (d) Internal view of raphe valve showing flat stauros and lack of costae. (e) Frustule in girdle view showing mantle rim with apical spines of sternum valve. (f–h) Achnanthes kuwaitensis. (f) Internal view of sternum valve. (g) External detail of sternum valve showing apical obiculus (cribrum mostly missing). (h) Internal detail of sternum valve apex with intact cribrum in obiculus. (i) Achnanthes parvula, Yap voucher, SEM (courtesy of Nelson Navarro). Scale bars: (c–f) = 10 µm, (b, g) = 5 µm, (a, h, i) = 2 µm.
Figure 31.
(a) Gomphonemopsis littoralis, SEM. (b, c) Achnanthes armillaris. (b) Internal view of raphe valve showing costae and stauros forked under mantle (arrow). (c) Girdle view of raphe valve and cingulum showing mantle areolae in stauros fork (arrow). (d, e) Achnanthes cf. brevipes. (d) Internal view of raphe valve showing flat stauros and lack of costae. (e) Frustule in girdle view showing mantle rim with apical spines of sternum valve. (f–h) Achnanthes kuwaitensis. (f) Internal view of sternum valve. (g) External detail of sternum valve showing apical obiculus (cribrum mostly missing). (h) Internal detail of sternum valve apex with intact cribrum in obiculus. (i) Achnanthes parvula, Yap voucher, SEM (courtesy of Nelson Navarro). Scale bars: (c–f) = 10 µm, (b, g) = 5 µm, (a, h, i) = 2 µm.
Figure 32.
Achnanthes cf. brevipes, Pohnpei population (PN1-1). (a) General view of population on filamentous seaweed, frustules attached by raphe valves. (b) Frustule in girdle view showing rim with spines on sternum valve. Scale bars: (a) = 50 µm, (b) = 10 µm.
Figure 32.
Achnanthes cf. brevipes, Pohnpei population (PN1-1). (a) General view of population on filamentous seaweed, frustules attached by raphe valves. (b) Frustule in girdle view showing rim with spines on sternum valve. Scale bars: (a) = 50 µm, (b) = 10 µm.
Figure 33.
(a) Achnanthes (undescribed sp.?) frustule from Yap in girdle view, showing spines and large areolae on mantle (cribrum lost on left, obscured on right). (b) A. inflata (freshwater) showing difference in copulae as well as the inflated center; SV = sternum valve, RV = raphe valve (Yap voucher, courtesy of N. Navarro). Scale bars: (b) = 10 µm, (a) = 5 µm.
Figure 33.
(a) Achnanthes (undescribed sp.?) frustule from Yap in girdle view, showing spines and large areolae on mantle (cribrum lost on left, obscured on right). (b) A. inflata (freshwater) showing difference in copulae as well as the inflated center; SV = sternum valve, RV = raphe valve (Yap voucher, courtesy of N. Navarro). Scale bars: (b) = 10 µm, (a) = 5 µm.
Yap samples: Y16B, Y16N, Y26C
Dimensions: Length 36–77 µm, width 9–14 µm, striae 11 in 10 µm
Diagnostics: Valves uniseriate, no costae internally. Sternum valves weakly convex with prominent rim ending in a spine at each apex (Figs 31e, 32). Sternum not differentiated from the rim.
Comments: These specimens with rimmed sternum valves are unlike the type material of
A. brevipes var.
intermedia (Kützing) Cleve shown in Toyoda & Williams (2004, figs 14–22), where there is no rim, there is a clear, sternum, and the interior is costate. Park et al. (2022) reassigned the specimen in Park et al. (2018, p. 110,
Figure 34) as “closer to”
A. cuneata and reported
A. brevipes var.
brevipes based on discussion in Toyoda & Williams (2004) (see also Hustedt 1931–1959, p. 425, figs 877d, e). Our micrographs cannot be assigned to any described variety of
A. brevipes, based on recent floristic and taxonomic studies in temperate waters (Toyoda & Williams 2004, Lee et al. 2013), and perhaps cannot be placed within this species.
There were no large areolae at apices, in contrast to
A. kuwaitensis, but in one whole frustule found in Yap samples (
Figure 33a) there was a large areola at each apex,
on the mantle, not seen in Pohnpei or Guam populations and probably indicating a new species. Al-though the striae on the valve faces are hard to judge as bi- or uniseriate in this image, the deep mantle of the sternum valve (top) suggests there are internal costae between each
three striae. For further comparison, we show (
Figure 33b) a specimen of
A. inflata (Kützing) Grunow from Yap freshwater stream (reported but not vouchered in Navarro & Lobban 2009). In this, the copulae have a single row of small areolae, in contrast to the other taxa shown here.
3.96. Achnanthes kuwaitensis Hendey 1958 Figure 31 f–h
References: Hendey 1958, p. 55, pl. 6, figs 8–10
Dimensions: Length 43–49 µm, width 9–10 µm, striae 9–10 in 10 µm.
Diagnostics: the linear valve outline with the large obiculi at each end of the sternum valve, the sternum on the valeve face–mantle boundary, and the internal costae distinguish this species from A. brevipes and from other species with obiculi.
Comments: Very difficult to distinguish A. kuwaitensis and A. brevipes raphe valves, even in SEM. Achnanthes subconstricta and A. yaquinensis also have obiculi (Lee et al. 2013).
3.97. Achnanthes grunowii Toyoda & D.M.Williams in Toyoda et al. 2005 Figure 34
Synonym: Achnanthes javanica var. rhombica Grunow
References: Toyoda et al. 2005, figs 1–17.
Yap samples: Y26C
Dimensions: Length 49 µm, width, 33 µm, striae biseriate, 7 in 10 µm.
Comment: New records for Micronesia; also observed in Palau (
Figure 34b, c). Taxonomy and ultrastructure: Toyoda et al. (2005).
3.98. Achnanthes orientalis F. Meister 1937 Figure 35
non Achnanthes orientalis Petit 1904, nec Achnanthes orientalis Hustedt 1933
References: Meister 1935, p. 97, figs 45, 46
Yap samples: Y26C, Y41-8
Dimensions: Length 36–66 µm, width 8–12.5 µm; striae 13–15 in 10 µm
Diagnostics: Lanceolate–rhombic valves, slightly flexed toward poles, RV convex, SV concave; apices turned slightly in opposite directions, sometimes rostrate; raphe path sinuous; striae biseriate as seen in SEM. Stria densities similar on sternum and raphe valves, but striae shorter on SV, leaving a broad lanceolate hyaline area in the middle of the valve.
Comments: Also found in Palau (PW46,
Figure 35a), Chuuk (TK1A,
Figure 35e), and Guam (GU52O-4 Lobban, unpubl.). Meister’s species is illegitimate because of Petit’s precedent. Andrzej Witkowski had intended to develop a broader paper on South-East Asian
Planothidium spp. around this transfer, with possibly a new genus to include this species, but in view of his untimely death, it must be reported under the present name. New records for Micronesia.
3.99. Achnanthes parvula Kützing 1844 Figure 31i
References: Hustedt 1931–1959, p. 426, figs 877f–i; Archibald 1983, p. 24, figs 71–74; Lee et al. 2013, p. 399,
Figure 1Z, 4A
Previous Micronesia records: Yap (Y7I) (Navarro & Lobban 2009, p. 140 – no illustration, as Achnanthes brevipes var. parvula (Kützing) Cleve)
Dimensions: Length 20 µm, width 6.4 µm, striae 12 in 10 µm (Navarro & Lobban 2009)
Figure 34.
Achnanthes grunowii. (a) Raphe valve in LM. (b, c) Valves in SEM (Palau specimens, PW2009-22). (b) Sternum valve interior with valvocopula. (c) Raphe valve exterior (with Diploneis crispa). Scale bars: 10 µm.
Figure 34.
Achnanthes grunowii. (a) Raphe valve in LM. (b, c) Valves in SEM (Palau specimens, PW2009-22). (b) Sternum valve interior with valvocopula. (c) Raphe valve exterior (with Diploneis crispa). Scale bars: 10 µm.
Comments: No new specimens found but
Figure 31i provides the voucher for the previous record. This taxon is distinguished by its small size and oval shape (Hustedt 1931–1959, p. 424, Figure 877f–i) and is presently considered a separate species, as Kützing (1844) described it (Guiry & Guiry 2024). Lee et al. (2013,
Figure 1W–Y) show very broad sternum valves with obiculi.
Figure 35.
Achnanthes orientalis. (a) Exterior views of rostrate raphe and sternum valves (Palau, PW46). (b–d) Yap specimens with more typical apices in LM and SEM. (e) Part of frustule in oblique view showing apical curvature of sternum valve (Chuuk, TK1A). Scale bars: (a–d) = 10 µm, (e) = 5 µm.
Figure 35.
Achnanthes orientalis. (a) Exterior views of rostrate raphe and sternum valves (Palau, PW46). (b–d) Yap specimens with more typical apices in LM and SEM. (e) Part of frustule in oblique view showing apical curvature of sternum valve (Chuuk, TK1A). Scale bars: (a–d) = 10 µm, (e) = 5 µm.
Achnanthidiaceae D.G.Mann
3.100. Planothidium delicatulum (Kützing) Round & Bukhtiyarova 1996 Figure 36a–c
References: Witkowski et al. 2000, p. 118, pl. 46, figs 28, 29, pl. 48, figs 1, 2; John 2016, p. 95, Figure 121 a, b
Yap samples: Y16B
Dimensions: Length 10 µm, width 5 µm, striae ca. 12 in 10 µm.
Diagnostics: Small rostrate valves with radiate multiseriate striae, no central break in striae.
Comment: This “species” is a difficult complex of forms that have not yet been resolved (Round & Bukhtiyarova 1996, Potapova 2010). We have also observed an elliptical species.
Figure 36.
(a–c) Planothidium delicatulum, SEM: raphe valve (RV) interior and exterior, sternum valve interior. (d–f) Anorthoneis sp. (d) Sternum valve (SV) in LM. (e, f) SV in SEM, with and without papillae. (g) Cocconeis convexa, RV and SV in LM. Scale bars: (d, g) = 10 µm, (e, f) = 5 µm, (a–c) = 2 µm.
Figure 36.
(a–c) Planothidium delicatulum, SEM: raphe valve (RV) interior and exterior, sternum valve interior. (d–f) Anorthoneis sp. (d) Sternum valve (SV) in LM. (e, f) SV in SEM, with and without papillae. (g) Cocconeis convexa, RV and SV in LM. Scale bars: (d, g) = 10 µm, (e, f) = 5 µm, (a–c) = 2 µm.
Cocconeidaceae Kützing
3.101. Anorthoneis sp. Figure 36d–f
References: Pennesi et al. 2018
Previous Micronesia records: Chuuk (Park et al. 2022, p. 40, Figure 108, as Anorthoneis eurystoma Cleve)
Yap samples: Y16B, Y26C, Y34E
Dimensions: Length 17–25 µm, width 12–16 µm; radiate striae 18–19 in 10 µm (SV).
Diagnostics: Oval valves; sternum valve with oval hyaline zone in the middle.
Comments: Only SV observed. Several specimens in the samples all showed a symmetrical hyaline area and circular areolae. The specimens from Y34E had more scattered areolae toward the hyaline area, whereas in others, striae were consistent; papillae were numerous on most specimens. Although we previously identified this species as A. eurystoma, several features are inconsistent with that hypothesis: A. eurystoma is larger (30–35 x 23–28 µm), the SV markedly concave along the sternum, the areolae are in fact shown to be slit-like, the striae density is lower (12–14 along the margin). Papillae were not mentioned for any species by Pennesi et al. (2018). Park et al. (2022) also considered A. hyalina Hustedt but that has a strongly excentric hyaline area on the SV (best seen in LM, by a faint line along the sternum) and slit-like areolae, though it does have a flat-convex SV face, and is closer in size (24–36 x 20–28 µm); stria densities are 15–17 along the sternum. The only other species with a large hyaline area is A. pulex Sterrenburg, but it, like that of A. hyalina is strongly excentric and the valves are smaller (12–15.5 x 8–9.5 µm); the valve face is flat-convex; the areolae are circular. Specimens in Yap appear to be the same as the specimen from Chuuk, but in the absence of raphe valves, there is insufficient evidence to place them in any of the known species—although it seems close to specimens identified by Witkowski et al. (2000, pl. 54, figs 4–8) as A. eurystoma—nor to describe it as new. Anorthoneis vortex Sterrenburg was found in Guam (Lobban et al. 2012).
Figure 37.
(a, b) Cocconeis convexa, cont. (a) Sternum valve, exterior, SEM. (b) Raphe valve exterior (Majuro), showing convexity (whole frustule is curved to fit on algal filaments, so this lower surface is concave). (c) Cocconeis coronatoides, sternum valve, SEM. (d, e) Cocconeis dirupta, LM of RV and SV respectively. (f) Cocconeis heteroidea frustule at two focal planes in LM, showing RV and SV. (g, h) Berkeleya rutilans. Scale bars: (f) = 10 µm, (a–e, g, h) = 5 µm.
Figure 37.
(a, b) Cocconeis convexa, cont. (a) Sternum valve, exterior, SEM. (b) Raphe valve exterior (Majuro), showing convexity (whole frustule is curved to fit on algal filaments, so this lower surface is concave). (c) Cocconeis coronatoides, sternum valve, SEM. (d, e) Cocconeis dirupta, LM of RV and SV respectively. (f) Cocconeis heteroidea frustule at two focal planes in LM, showing RV and SV. (g, h) Berkeleya rutilans. Scale bars: (f) = 10 µm, (a–e, g, h) = 5 µm.
3.102. Cocconeis convexa Giffen 1967 Figs 36g, 37a, b
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 140, figs 73–76); Chuuk (Park et al. 2018, p. 116,
Figure 32); 585614
Yap samples: Y25H-1, Y25H-2, Y26C, Y37-8, Y37-7, Y36-1
Dimensions: Length 17–19 µm, width 13 µm; striae on SV 40 in 10 µm, on RV 21 in 10 µm
Diagnostics: SV with striae crossed by parallel longitudinal lines, evident in LM (
Figure 36g); RV with fine radiate striae, larger areolae near margin visible in LM.
3.103. Cocconeis coronatoides Riaux-Gobin & Romero 2011 Figure 37c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 286, pl. 39,
Figure 6); Chuuk (Park et al. 2022, p. 40, Figure 109); 585749
Yap samples: Y25H-1, Y25H-2, Y26C, Y34B, Y37-8, Y37-7, Y36-1, Y D-2
Dimensions: Length 29 µm, width 19 µm, striae 15 in 10 µm
Diagnostics: Distinguished by the papillae and the submarginal costa on outer SV surface.
3.104. Cocconeis dirupta Gregory 1857 [a] Figure 37d, e
Previous Micronesia records: Guam (Lobban et al. 2012, p. 286, pl. 40, figs 1, 2); 585552
Yap samples: Y25H-1, Y37-8, Y41-7
Dimensions: Length 11 µm, width 8 µm, striae 24 in 10 µm (SV), not resolved in LM on RV
Comments: We have at least
C. dirupta var.
dirupta (shown) and
C. sp. aff.
dirupta sensu Riaux-Gobin et al. (2011, p. 26, pl. 3, figs 9, 10, pl.41, figs 1–6) (and see Lobban et al. 2012, pl. 40,
Figure 3).
3.105. Cocconeis heteroidea Hantzsch 1863 Figure 37f
Previous Micronesia records: Guam (Lobban et al. 2012, p. 287, pl. 40, figs 6, 7, pl. 41, figs 1–3); Chuuk (Park et al. 2018, p. 116,
Figure 30, 31); 585750
Yap samples: Y25H-1, Y25H-2, Y37-8, Y41-7, Y41-8
Dimensions: Length 31–59 µm, width 20–48 µm, striae 18–21 in 10 µm (RV)
NAVICULALES Bessey
Berkeleyaceae D.G.Mann
3.106. Berkeleya rutilans (Trentepohl ex Roth) Grunow 1880 Figure 37g, h
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 18, Figure 77)
Yap samples: Y26A
Dimensions: Valves 18 µm long, 3.5 µm wide, striae 32 in 10 µm near along the raphe branches, coarser in middle.
Diagnostics: Cells elliptical to linear with rounded apices, central raphe endings distant, tuning-fork-shaped thickenings aroud raphe branches. Edlund et al. (in press) give a table comparing morphometrics of many Berkeleya spp.
Comments: Tube-dwelling diatom colonies are generally microscopic in Micronesia, with the exception of Homoeocladia martiana C.Agardh (Lobban & Tsuda 2003, Lobban et al. 2019) and small colonies of a species resembling B. hyalina (Round & Brooks) Cox (Lobban & Witkowski 2024), with only isolated valves of B. rutilans observed so far across Micronesia.
3.107. Climaconeis lorenzii Grunow 1862
Previous Micronesia records: Yap Y26C (Lobban et al. 2010, figs 33, 34 and Lobban 2021, p. 249, figs 62–69); Palau (Lobban et al. 2010,
Figure 11); 585599
Dimensions: Length 140–146 µm, width 7 µm, striae 15 in 10 µm
Diagnostics: Straight cells, central raphe endings deflected, no stauros; craticular bars present on valvocopula.
Comments: C. coxii is similar but its central raphe endings are straight. These are the only two known Climaconeis spp. with craticular bars. Lobban (2021) has table comparing morphometrics of 11 species and a key to all 21 published Climaconeis species, 13 of which have been found in Micronesia, including two described from Yap in that paper and summarized below.
3.108. Climaconeis minaegensis Lobban 2021
Previous Micronesia records: Yap and Marshall Islands (Lobban 2021, p. 249, figs 50–60)
Yap samples: Y18E
Dimensions: Length 228–247 µm, width 5.0 µm except 8.3 µm at center and 7.6 µm at apex. Striae 19 in 10 µm
Diagnostics: Valves long, linear but slightly bent along the apical plane, without craticular bars or stauros, silica ribs bordering the raphe, quadrate to transapically elongate areolae.
3.109. Climaconeis tarangensis Lobban 2021
Previous Micronesia records: Yap (Lobban 2021, p. 246, figs 45–49)
Dimensions: Length 121–124 µm, width 4.5 µm; stria density 2021 in 10 µm
Diagnostics: Curved species without craticular bars or stauros, differing from Climaconeis riddleae A.K.S.K. Prasad, in its lower stria density (20 vs 24–27 in 10 µm), regular apically rectangular areolae, and more strongly arcuate raphe.
Yap samples: Y26C
3.110. Parlibellus biblos (Cleve) E.J.Cox 1988 Figure 38a, b
Ref. illus: Hustedt 1931–1959, Figure 1178 (as Stauroneis biblos [Cleve] Hustedt); Cox 1988, p. 23
Previous Micronesia records: Guam (Lobban et al. 2012, p. 296, pl. 52, figs 4, 5, as Stauroneis retrostauron (Mann) Meister); Lobban & Witkowski 2024b; Chuuk (Park et al. 2022, p. 40, figs 119–121); 585620
Yap samples: Y36-2, Y37-7, Y42-1
Dimensions: Length 28–38 µm, width 4 µm, striae 37 in 10 µm
Comments: Lobban et al. (2012) identified this species as Stauroneis retrostauron, noting its similarity to Stauroneis biblos. The justification for the correction is given in Park et al. (2022). This species is unusual in Parlibellus for having a stauros.
3.111. Parlibellus hamulifer (Grunow) E.J.Cox 1988 Figure 38c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 289, pl. 42,
Figure 6); 585555
Yap samples: Y25H-1, Y26C, Y37-8, Y37-7
Dimensions: Length 47–80 µm, width 8–19 µm, striae 21–22 in 10 µm
Comments: Tube-dwelling species but very mobile and often encountered as solitary cells.
3.112. Parlibellus waabensis Lobban 2021 Figs 38d, 39
Previous Micronesia records: Yap (Y39A) (Lobban 2021, p. 252, figs 79–90,
Table 2)
Dimensions: Length 58–64 µm, width 18 µm, striae 22 in 10 µm, coarser in middle
Diagnostics: Relatively large compared to most congeners, but of similar size to P. hamulifer, differing from it in striae asymmetrically more widely spaced and sometimes biseriate in the middle, and copulae with larger pores at the center of the cell. It is clear in Figs 38e and 39e that the apparently large pores are 1–3 pores in pits, visible but overlooked in Lobban (2021, Figure 89).
Figure 38.
(a, b) Parlibellus biblos, LM and internal SEM. (c) Parlibellus hamulifer, LM and internal SEM. (d) Parlibellus waabensis, SEM of Palau specimen, girdle view showing spacing of central striae and larger pores on copulae near mid-cell. Scale bars: (a, c–e) = 10 µm, (b) = 5 µm.
Figure 38.
(a, b) Parlibellus biblos, LM and internal SEM. (c) Parlibellus hamulifer, LM and internal SEM. (d) Parlibellus waabensis, SEM of Palau specimen, girdle view showing spacing of central striae and larger pores on copulae near mid-cell. Scale bars: (a, c–e) = 10 µm, (b) = 5 µm.
Comments: Shown to be tube-dwelling. Described from a mud sample on a mangrove root but in a channel (Tagireeng) rather than a mangal. Broader habitat was not established. Since publishing this species in 2021, we have observed it in samples from Palau [PW(2021)4-7, Ngiwal, Babeldaob,
Figure 38e and 39b] and Pohnpei [PN1-1 (
Figure 39a, c–e) and PN1-9] that are new records for those entities and establish a mangrove habitat for this species and confirm the mucilage tubes.
Figure 39.
Parlibellus waabensis from Pohnpei (PN1-1) (a, c–e) and Palau [PW(2021)4-7] (b). (
a) Preserved cells in mucilage tube, LM. (
b, c) Frustules in girdle view showing the distinctive large pores in the girdle bands (arrows), LM. (
d) SEM of frustule in mucilage tube, the distinctive copula pores and wider striae spacing on center of cell visible through the dried mucilage (arrows). (
e) Girdle view of Pohnpei valve in SEM, showing slight differences from the Palau specimen in
Figure 38e, especially in the valve areolae and central striae. Scale bars: (a) = 20 µm, (b–e) = 10 µm.
Figure 39.
Parlibellus waabensis from Pohnpei (PN1-1) (a, c–e) and Palau [PW(2021)4-7] (b). (
a) Preserved cells in mucilage tube, LM. (
b, c) Frustules in girdle view showing the distinctive large pores in the girdle bands (arrows), LM. (
d) SEM of frustule in mucilage tube, the distinctive copula pores and wider striae spacing on center of cell visible through the dried mucilage (arrows). (
e) Girdle view of Pohnpei valve in SEM, showing slight differences from the Palau specimen in
Figure 38e, especially in the valve areolae and central striae. Scale bars: (a) = 20 µm, (b–e) = 10 µm.
Figure 40.
Progonoia spp. (a) Progonoia diatreta, LM. (b, c) Progonoia intercedens. Scale bars = 10 µm.
Figure 40.
Progonoia spp. (a) Progonoia diatreta, LM. (b, c) Progonoia intercedens. Scale bars = 10 µm.
Scoliotropidaceae Mereschkowsky
3.113. Progonoia diatreta Lobban 2015 [b] Figure 40a
Previous Micronesia records: Guam (Lobban 2015b, p. 249, figs 6, 18–29); 586096
Yap samples: Y26B, Y26C
Dimensions: Length 52 µm, width 20 µm, striae 7.5 in 10 µm
Comment: The two Progonoia spp are associated with biofilm on sediments.
3.114. Progonoia intercedens (A. Schmidt) Lobban 2015 [b] Figure 40b, c
Previous Micronesia records: Guam (Lobban 2015b, p. 248, figs 5, 7–17); 586097
Yap samples: Y26C, Y41-8
Dimensions: Length 45 µm, width 18 µm, striae 9 in 10 µm
Pinnulariaceae D.G.Mann
3.115. Caloneis egena (A. Schmidt) Cleve 1894 Figure 41a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 294, pl. 49, figs 3, 4); Chuuk (Park et al. 2022, p. 41, figs 38, 123); 585563
Yap samples: Y37-8 (LM)
Dimensions: Length 27 µm, width 5 µm, striae ca 35 in 10 µm
3.116. Caloneis ophiocephala (Cleve & Grove) Cleve 1894 Figure 41b
Ref. illus: Schmidt 1859–1966, pl. 212,
Figure 6
Yap samples: Y26C
Dimensions: Length 68 µm, width 14 µm, striae 18 in 10 µm
Diagnostics: With the unusual shape of C. egena but 2–3 times larger in all dimensions, the striae very clear. Reported from Java, China and Taiwan (Guiry & Guiry 2024).
Comment: New record for Micronesia.
Figure 41.
(a) Caloneis egena, LM. (b) Caloneis ophiocephala, LM. (c, d) Caloneis cf. petitiana, LM at two focal planes, SEM at 15 kV showing finely porous membranes over alveoli. Scale bars: (a–c) = 10 µm, (d) = 5 µm.
Figure 41.
(a) Caloneis egena, LM. (b) Caloneis ophiocephala, LM. (c, d) Caloneis cf. petitiana, LM at two focal planes, SEM at 15 kV showing finely porous membranes over alveoli. Scale bars: (a–c) = 10 µm, (d) = 5 µm.
3.117. Caloneis cf. petitiana (Grunow in Cleve) Cleve 1901 Figure 41 c, d
References: Cleve 1881, plate 3,
Figure 34 (as
Navicula petitiana)
Yap samples: Y26C, Y25H-2
Dimensions: Length 88 µm, width 17 µm, striae 12 in 10 µm
Comments: Cleve (1881) illustrated three similar species including this one based on drawings supplied by Grunow, but the illustrations of
N. petitiana by Schmidt 1894 in Schmidt et al. (1874–1959, pl. 212, figs 26–29) match more closely the positions of the longitudinal lines of striae as seen in the Yap specimen. Cleve (1881, pl. 3,
Figure 34) showed structure in the hemilanceolate axial areas, whereas Yap specimens have none. Cleve (1901) transferred this species to
Caloneis.
Diploneidaceae D.G.Mann
3.118. Diploneis carolinensis Lobban & Witkowski 2024 [a]
Previous Micronesia records: Palau and Yap (Lobban & Witkowski 2024a; Yap voucher
Figure 3C)
Yap sample: Y34A
Dimensions: Length 19–23 μm long, greatest width 9–11 μm, striae 12 in 10 μm
Comments: Park et al. (2022, p. 41,
Figure 37) reported
D. gravelleana from Chuuk, but Lobban & Witkowski (2024a) showed that in LM this species could not be distinguished from
D. carolinensis and the Chuuk record is more likely to be the latter based on biogeography.
3.119. Diploneis cerebrum Pennesi, Caputo & Lobban in Pennesi et al. 2017 Figure 42
Previous Micronesia records: Palau and Guam (Pennesi et al. 2017, p. 213, figs 66–75); Chuuk (Park et al. 2022, p. 41, figs 128, 129); 585262
Yap samples: Y41-7, Y41-8
Dimensions: Length 55 µm, width 10 µm, striae 8 in 10 µm
Diagnostics: Striae alveolate, hidden behind thickened virgae, one longitudinal rib dividing them (
Figure 42c, d arrows); raphe with small flap near central endings, canal areolae with “brain”-shaped volae, 4 of 6 central canal areolae larger and oblique but otherwise like the others. Internal foramina long and closed by hymenes (
Figure 42c).
3.120. Diploneis chersonensis (Grunow) Cleve 1892 in Schmidt et al. 1874–1959 Figure 43a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 289, pl. 44, figs 1–4); 585558
Yap samples: Y36-3
Dimensions: Length 39 µm, width 16 µm, striae 12 in 10 µm
Diagnostics: Striae divided by multiple parallel longitudinal ribs, not always visible in SEM, areolae over longitudinal canals small, circular, with volae attached at single point, leaving a c-shaped slit; central canal much different from D. cerebrum: eight areolae transversely elongated and opening by convoluted slits.
Comment: This species and D. claustra (see next), in contrast to D. cerebrum, have a distinct strip over the parallel longitinal canals, resembling a shirt placket.
3.121. Diploneis claustra Lobban & Pennesi in Pennesi et al. 2017 Figure 43b
Previous Micronesia records: Guam (Pennesi et al. 2017, p. 220, figs 115–117); 586263
Yap samples: Y26C
Dimensions: Length 30 µm, width 17 µm, striae 9 in 10 µm
Diagnosis: Areolae, closed by very finely porous cribra, clearly seen between virgae and vimenes, the virgae raised and angled, with support struts on the vimines (longitudinal ribs), reminiscent of a window shade, differing from D. weissflogii (A.Schmidt) Cleve and D. weissflogiopsis Lobban & Pennesi (see below) in the angled virgae and the placket-like appearance of the wall over the canals. Internal unknown but probably like D. weissflogii and D. weissflogiopsis in having one large, apparently open foramen per areola.
Figure 42.
Diploneis cerebrum. (a) LM. (b) External valve surface, SEM. (c) Interior valve surface, again showing the longitudinal rib. (d) Detail of central part of valve with detail of “brain-like” cribra over longitudinal canal areolae; double-headed arrow shows position of longitudinal rib, arrowhead the small wave in the raphe near the central endings and single arrow the central areolae over the canal (Chuuk specimen, TK28). Scale bars: (a–c) 10 µm, (d) = 5 µm.
Figure 42.
Diploneis cerebrum. (a) LM. (b) External valve surface, SEM. (c) Interior valve surface, again showing the longitudinal rib. (d) Detail of central part of valve with detail of “brain-like” cribra over longitudinal canal areolae; double-headed arrow shows position of longitudinal rib, arrowhead the small wave in the raphe near the central endings and single arrow the central areolae over the canal (Chuuk specimen, TK28). Scale bars: (a–c) 10 µm, (d) = 5 µm.
3.122. Diploneis crabro Ehrenberg 1854 (var. crabro) Figure 43c, d
Previous Micronesia records: Guam (Lobban et al. 2012, p. 290, pl. 45, figs 1, 2); 585557
Yap samples: Y18C
Dimensions: Length 84 µm, width 41 µm, striae 4.5 in 10 µm
Comments: Striae are alveolate opening externally by biseriate pores and internally by a single foramen (
Figure 43d). The short excavations in the lunula area between the canals and the striae (see Pennesi et al. 2017,
Figure 1) indicate that this is the nominate variety, which can range from 35 to over 200 µm long, according to Hustedt (1931–1959). This and its var.
excavata are the largest and most coarsely striated
Diploneis in the Micronesian flora.
This spectacular species is very variable with numerous named varieties and forms (Hustedt 1931–1959, figs 1028–1037) but generally rare and poorly studied, especially with SEM.
Figure 43.
Diploneis spp. (a) D. chersonensis, half of valve in SEM, showing flaps on raphe near central area (arrow). (b) D. claustra. (c, d) D. crabro, nominate variety. (c) LM at two focal planes showing small lunula area (arrow) and large internal foramina (arrowhead). (d) Internal SEM showing large foramina. (e) D. crabro var. excavata, LM, showing wide, excavated lunula area (arrow). Scale bars: (c–e) = 10 µm, (a, b) = 5 µm.
Figure 43.
Diploneis spp. (a) D. chersonensis, half of valve in SEM, showing flaps on raphe near central area (arrow). (b) D. claustra. (c, d) D. crabro, nominate variety. (c) LM at two focal planes showing small lunula area (arrow) and large internal foramina (arrowhead). (d) Internal SEM showing large foramina. (e) D. crabro var. excavata, LM, showing wide, excavated lunula area (arrow). Scale bars: (c–e) = 10 µm, (a, b) = 5 µm.
3.123. Diploneis crabro var. excavata Hustedt 1937 in Hustedt 1931–1959 Figure 43e
Previous Micronesia records: Guam (Pennesi et al. 2017, pp. 197–198, figs 1, 3–9); 585557
Yap samples: Y26C
Dimensions: Length 75–123 µm, width 25–42 µm, striae 4–6 in 10 µm
Diagnostics: Distinguished by the long excavations in the lunula area.
3.124. Diploneis craticula Pennesi, Caputo & Lobban in Pennesi et al. 2017 Figure 45a
Previous Micronesia records: Palau and Guam (Pennesi et al. 2017, p. 213, figs 76–84); 586264
Yap samples: Y41-7
Dimensions: Length 38 µm, width 11 µm, striae 15 in 10 µm
Diagnostics: Elliptical to slightly panduriform, transverse and longitudinal multiseriate striae; valve has only the basal silica layer, so internal aspect the same as external.
3.125. Diploneis denticulata Lobban & Prelosky, sp. nov. Figure 44
Diagnosis: Valves lanceolate-panduriform, striae biseriate, lunula with pitted but nonperforated surface, longitudinal canals and raphe raised on flat keel with costate edge; denticulate rim between valve face and vertical mantle.
Holotype: Specimen at 13.0 mmm E, 5.0 mm S of the mark on slide 3038, deposited at Diatom Collection, Academy of Natural Sciences of Drexel University, Philadelphia, accession number [pending].
Figure 44a. [This slide was designated as an isotype of
Gomphotheca marciae Lobban & Prelosky (2022) but is now transferred from GUAM to ANSP.]
Type locality: Y39A
Description: Valves lanceolate-panduriform (see Hendey 1964, text-
Figure 2:11) (
Figure 44a, b), 24–39 µm long, 15–18 µm wide, 11 striae in 10 µm, a more rounded form (39–43 µm long, 13–15 µm wide) in the same community (
Figure 44h). Valve face sloping into depressions (lunulae) on the side of the keel (
Figure 44c); valve face separated from mantle by strong, denticulate rim (
Figure 44b, c, f, h); keel raising longitudinal canals and raphe and bordered by irregular costae, especially near the apices (
Figure 44b, c, h). Keel completely flat (
Figure 44f). Striae of single alveolae, the inner side covered with a finely porous hymen (
Figure 44d, e). Externally, raphe bordered by costae, central and terminal endings straight. Longitudinal canals with large external pore fields, no internal foramina (
Figure 44b, d, h); canals possibly divided into sections (
Figure 44g). Copulae hyaline, or at most somewhat pitted (
Figure 44d, f).
Etymology: With reference to the toothed rim between valve face and mantle.
Phycobank registration: pending
Additional records: YAP: Y26C; PALAU: PW(2009)46; GUAM: GU55B-4.
Comments. This an unusual Diploneis because of the sharp, rimmed boundary between valve face and mantle, but it clearly has longitudinal canals and internally resembles species such as D. smithii. An image of this species, without origin notes, was published by Alcober Bosch (2015) as “Dictyoneis sp.” However, the external structure of Dictyoneis comprises a pseudoloculate network over most of the surface, and the internal aspect closely spaced transverse costae, e.g., Dictyoneis apapae Lobban & Witkowski (2024). In LM the new species somewhat resembles Navicula vesparella Mann = Diploneis vespa Cleve because of the rhomboidal half-cells. That taxon has a convoluted nomenclatural history (see Mann 1925, p. 124 and Hustedt 1931–1959, p. 643, Figure 1048B) but Hustedt considered it to be only a “species limiting variation” of D. dalmatica and noted its distribution as East Asia and Sunda Islands. Cleve’s (1894) material was 50 x 12 µm, 11 striae in 10 µm, Mann’s nearly twice that size. There is too little in those descriptions to assert that the present material belongs to D. vespa, and we therefore propose this as a new species.
Figure 44.
Diploneis denticulata sp. nov., SEM except (a). Yap specimens from Y34A and (f) Y26C. (a) Holotype specimen at two focal planes in LM. (b) Exterior valve. (c) Half of same valve with natural tilt to show surface relief. (d) Oblique view of frustule, apparently in division, showing toothed rim of epitheca and inner face of daughter hypotheca. (e) Detail of inner valve surface with central raphe endings and intact hymenes covering striae. (f) Girdle view of intact frustule showing flat raphe keel, vertical mantle and hyaline cingulum. (g) Fragment of broken valve including central nodule, showing chambering in the longitudinal canal (arrow). (h) Half of valve of rounded form. Scale bars: (a) = 10 µm, (b–d, f, h) = 5 µm, (e, g) = 2 µm.
Figure 44.
Diploneis denticulata sp. nov., SEM except (a). Yap specimens from Y34A and (f) Y26C. (a) Holotype specimen at two focal planes in LM. (b) Exterior valve. (c) Half of same valve with natural tilt to show surface relief. (d) Oblique view of frustule, apparently in division, showing toothed rim of epitheca and inner face of daughter hypotheca. (e) Detail of inner valve surface with central raphe endings and intact hymenes covering striae. (f) Girdle view of intact frustule showing flat raphe keel, vertical mantle and hyaline cingulum. (g) Fragment of broken valve including central nodule, showing chambering in the longitudinal canal (arrow). (h) Half of valve of rounded form. Scale bars: (a) = 10 µm, (b–d, f, h) = 5 µm, (e, g) = 2 µm.
Figure 45.
Diploneis spp. (a) D. craticula, SEM exterior. (b–d) D. papula, external views of frustules, oblique view showing domed cribra of areolae between virgae (arrow). (e) D. nitescens, SEM of valve exterior. (f) D. smithii, SEM of valve exterior. (g) D. smithii var. rhombica, LM. (h) D. suborbicularis, LM. Scale bars: (e–h) = 10 µm, (a–d) = 5µm.
Figure 45.
Diploneis spp. (a) D. craticula, SEM exterior. (b–d) D. papula, external views of frustules, oblique view showing domed cribra of areolae between virgae (arrow). (e) D. nitescens, SEM of valve exterior. (f) D. smithii, SEM of valve exterior. (g) D. smithii var. rhombica, LM. (h) D. suborbicularis, LM. Scale bars: (e–h) = 10 µm, (a–d) = 5µm.
3.126. Diploneis nitescens (W.Gregory) Cleve 1894 Figure 45e
References: Hustedt 1931–1959, pp. 537–538, Figure 1047; Witkowski et al. 2000, p. 189, pl. 90, figs 1–3, pl. 94,
Figure 1
Previous Micronesia records: Chuuk (Park et al. 2018, p. 116, Figure 78)
Yap samples: Y34H, Y34F, Y37-8, Y39A, Y26A, Y26B, Y26C
Dimensions: Length 43–66 µm, width 19–28 µm, striae 8–9 in 10 µm
Diagnostics: Distinguished from D. smithii (Brébisson) Cleve (see below) by the greater proportion of valve face over the longitudinal canals, i.e., 1/3 vs. 1/4–1/3.
3.127. Diploneis papula (A.Schmidt) Cleve 1894 Figure 45b–d
Previous Micronesia records: Guam (Pennesi et al. 2017, p. 205, figs 24–31); 586268
Yap samples: Y37-7, Y36-1, Y41-7, Y41-8
Dimensions: Length 21–24 µm, width 11–12 µm, striae 12–13 in 10 µm
Diagnostics: Oval to weakly constricted valves with areolae partly hidden below wide virgae. Two to three longitudinal ribs evident in LM; areolae covered with domed cribrum (
Figure 45d), each areola opening internally through one large foramen (Pennesi et al. 2017, figs 27, 30).
3.128. Diploneis smithii (Brébisson) Cleve 1894 Figure 45f
Previous Micronesia records: Guam (Lobban et al. 2012, p. 290, pl. 45, figs 3–6, pl. 46,
Figure 1); 585833
Yap samples: Y25H-1, Y41-7, -8
Dimensions: Length 35–99 µm, width 15–41 µm, striae 8–10 in 10 mm
Comments: Droop et al. (2000) refer to the D. smithii/D. fusca complex, to indicate that there are several very closely similar species, even within the U.K., where his work was done. How the tropical specimens relate to these must await molecular study. Idei et al. (2018) noted that their morphotype (deme) of D. smithii from Japan did not fit any of Droop’s demes. It is likely that the tropical species will get new names when this complex can eventually be sorted out.
3.129. Diploneis smithii var. rhombica Mereschkowsky 1902 Figure 45g
Previous Micronesia records: Palau (Pennesi et al. 2017, pp. 208–209, figs 39–44)
Yap samples: Y33A
Dimensions: Length 45 µm, width 24 µm, striae 11 in 10 mm
3.130. Diploneis suborbicularis (W.Gregory) Cleve 1894 Figure 45h
Previous Micronesia records: Guam (Lobban et al. 2012, p. 291, pl. 46, figs 2–4); 585834
Yap samples: Y25H-1, Y25H-2
Dimensions: Length 45 µm, width 26 µm, striae 8 in 10 mm
3.131. Diploneis weissflogii (A.Schmidt) Cleve 1894 Figure 46a, b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 291, pl. 46, figs 5–7); 585560
Yap samples: Y25H-1, Y25H-2, Y26B, Y37-8
Dimensions: Length 26–30 µm, width 11 µm, striae 9–10 in 10 µm
Comment: Two simulacra are now known that cannot be distinguished from
D. weissflogii in LM, i.e.,
D. weissflogiopsis (next taxon) and
D. claustra (see above). Externally, the areolae open by sunken cribra (Pennesi et al. 2017,
Figure 65). Internally, each areola of the valve wall opens by a large foramen, and there are no openings on the canals except for three small ones at each apex (Pennesi et al. 2017, figs 62, 63).
3.132. Diploneis weissflogiopsis Lobban & Pennesi in Pennesi et al. 2017 Figure 46c, d
Previous Micronesia records: Guam (Pennesi et al. 2017, p. 218, figs 108–114) 586266
Yap samples: Y18C
Dimensions: Length 27–43 µm, width 9–14 µm, striae 10–12 in 10 µm
Diagnostics: The striae are denser in this species but the differences between
D. weissflogii and
D. weissflogiopsis are mostly ultrastructural, and easier to see on interior views. There are three modified striae between the central raphe endings in the latter, each with one foramen, one in the former (
Figure 46b vs 46c, d), and external central nodule is smooth in the former, dimpled in the latter (
Figure 46d). Internal openings at apices of the longitudinal canals (
Figure 46a) are absent in
D. weissflogiopsis. (See Pennesi et al. 2017, Table 4.)
Figure 46.
(a–d) Diploneis weissflogii vs. D. weissflogiopsis, SEM. (a, b) D. weissflogii. (a) Internal view showing single foramen on each side of the central area (in rectangle), foramina at apex of canals (arrows), and hyaline copulae. (b) External valve face showing single modified stria between central raphe endings (in rectangle). (c, d) D. weissflogiopsis. (c) Valve showing three modified striae between raphe endings (in rectangle); the higher striae density is also evident in the comparison. (d) Detail of central area in slightly oblique view with the central dimple more evident (arrow). (e) Navicula consors. SEM. Scale bars: (e) =10 µm, (a–d) = 5 µm.
Figure 46.
(a–d) Diploneis weissflogii vs. D. weissflogiopsis, SEM. (a, b) D. weissflogii. (a) Internal view showing single foramen on each side of the central area (in rectangle), foramina at apex of canals (arrows), and hyaline copulae. (b) External valve face showing single modified stria between central raphe endings (in rectangle). (c, d) D. weissflogiopsis. (c) Valve showing three modified striae between raphe endings (in rectangle); the higher striae density is also evident in the comparison. (d) Detail of central area in slightly oblique view with the central dimple more evident (arrow). (e) Navicula consors. SEM. Scale bars: (e) =10 µm, (a–d) = 5 µm.
Naviculaceae Kützing
3.133. Cymatoneis sulcata (Greville) Cleve 1894 Figure 47a, b
Previous Micronesia records: Yap Y16E–Y16K (Navarro & Lobban 2009, p. 143, figs 82–89); Guam (Lobban et al. 2012, p. 292, pl. 47,
Figure 1); 585694
Additional Yap samples: Y25B
Dimensions: Length 32–50 µm, width 16– µm, striae 10–11 in 10 µm
Comments: Although the longitudinal costae on the valve face are more robust that those of the specimens shown by Round et al. (1990), this seems to be the species originally described by Greville (1865b: p. 235, pl. 3,
Figure 10) as
Navicula sulcata from New Caledonia material; Williams (1998, p. 41, pl. 47, figs 6, 7) shows the type, length 40 µm, though Greville described it as “minute.” The genus, established by Cleve (1894), is characterized by the valve being divided by one or several longitudinal ridges, and the raphe often slightly sigmoid and on a keel. Cleve (1894) also described a new species,
C. circumvallarta, from widespread localities including Labuan (Borneo). Mann (1925, pp. 72–73 pl. 15, figs 1–3) found the two previous species and three new species in the Philippines; there are now nine species, the most recently described being
C. margaritae Witkowski (Witkowski et al. 2000, p. 179, pl. 109, figs 9–17, SEM included). Two new species apparently fitting the genus definition have been recorded in Micronesia, one each in Palau and Yap, which differ from others in having longitudinal hyaline zones interrupting the striae. We describe these next and include new images of
C. sulcata (from Palau) for comparison.
Figure 47.
Cymatoneis spp., SEM. (a, b) C. sulcata, Palau specimens (PW46). Valve and girdle views showing strongly developed longitudinal ribs and hyaline cingulum. (c) C. belauensis, sp. nov. from Palau (PW46) valve view, showing longitudinal ridges with hyaline outer sides and ribs enclosing much of raphe. (d). Same valve naturally tilted, showing elevations of the valve. Scale bars: (a–c) = 10 µm, (d) = 5 µm.
Figure 47.
Cymatoneis spp., SEM. (a, b) C. sulcata, Palau specimens (PW46). Valve and girdle views showing strongly developed longitudinal ribs and hyaline cingulum. (c) C. belauensis, sp. nov. from Palau (PW46) valve view, showing longitudinal ridges with hyaline outer sides and ribs enclosing much of raphe. (d). Same valve naturally tilted, showing elevations of the valve. Scale bars: (a–c) = 10 µm, (d) = 5 µm.
3.134. Cymatoneis belauensis Lobban, sp. nov. Figs 47c, d, 48 a–c
Diagnosis: Valve face with lateral sterna joined by central area, vimenes not thickened into transverse costae, striae densely areolate, raphe branches partially bordered by ribs.
Holotype:
Figure 47c, from specimen on stub 226, according to Article 40.5 of the International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code: Turland et al. 2018).
Type locality: PALAU: Babeldaob Island, Ngaremlengui State, dock at Bkulangriil, 07°31.488′ N, 134°29.966′ E, sand sample rich in Carinasigma, sample PW2009-46. Coll. 11 April 2009, C.S. Lobban and M. Schefter.
Description: Lanceolate, naviculoid valve 52 µm long, 16 µm wide, deeply vaulted (
Figure 48a: height 8.8 µm) striae 10 in 10 µm, areolae 36 in 10 µm. Valve profile comprises a low peripheral zone striated by areolae comprising densely spaced apical slits in the outer surface, virgae and vimines unequally thickened on the inner side; no external costae either longitudinally or transversely (contrast
C. sulcata). Broad hyaline zones, appearing in valve view (
Figure 47c) as lateral sterna, rise up to the keel (Figs 47d, 48a), with striae resuming again along the raphe except at the central area, which is asymmetrically widened and joins the two lateral sterna. Striae around the apices change abruptly to vertically oriented, shorter areolae (
Figure 47d, arrow). Raphe raised on a keel, the highest point near the apices, the lowest point at the central endings, but the raphe bordered by ribs of increasing height toward the central endings (
Figure 47c, d). Central and terminal raphe endings apparently straight but raphe path overall slightly sinuous. Girdle band wide and hyaline, as in
C. sulcata (Figs 47c, d vs. 47 b).
Etymology: Named for the country where it was found.
Phycobank registration: pending.
Comments: The description is based on two specimens, both imaged as seen and tilted, the interior view specimen on the Phenom XL eucentric stage, the latter had tilted naturally between images taken two years apart. The internal central raphe ending is consistent with the images in Round et al. (1990).
3.135. Cymatoneis yapensis Lobban, sp. nov. Figure 48d
Diagnosis: Differing from C. belauensis in smaller size and sparser areolae in the striae.
Holotype:
Figure 48d, from specimen on stub 1465, according to Article 40.5 of the International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code: Turland et al. 2018).
Type locality: Yap State, Yap Island, Weloy Municipality, Maa’ Mangrove, approx: 9°32’25.62” N, 138° 5’14.45” E, adjacent to Nimpal Marine Protected Area, sample Y34A, subtidal mud from Sonneratia alba (white mangrove) pneumorrhiza, ca 20 cm from bottom, and likely in the salt wedge. Coll. C.S. Lobban, M. Schefter & T. Gorong, 28 May 2014
Description: Lanceolate valve, rostrate apices, 37 µm long, 12 µm wide, striae 9–10 in 10 µm, areolae widely spaced apically linear slits, ca. 30 in 10 µm. No transverse costae on the virgae, in contrast to
C. sulcata. Striae weakly radiating, interrupted by lateral sterna paralleling the valve border and connected to an asymmetrical central area; a few areolae on the rostrate tips. Raphe on keel, probably highest toward apex and lowest at central nodule but valve not seen in profile; raphe branches bordered by short ribs only near central nodule, each branch slightly curved, central and terminal endings deflected to the secondary side (note Voigt faults,
Figure 48d arrows). Valve interior and cingulum not seen.
Etymology: Named for the state where it was found.
Phycobank registration: pending.
Figure 48.
Cymatoneis spp., cont. (a–c) C. belauensis, internal aspects of a valve. (a) Valve as found, showing valve depth from outside and outer zone of the striae. (b, c) Same valve tilted 45°; (b) showing whole valve, (c) detail of central area with small central nodule. (d) C. yapensis, sp. nov. from Yap (Y34A). Scale bars: (a, b, d) = 10 µm, (c) = 5 µm.
Figure 48.
Cymatoneis spp., cont. (a–c) C. belauensis, internal aspects of a valve. (a) Valve as found, showing valve depth from outside and outer zone of the striae. (b, c) Same valve tilted 45°; (b) showing whole valve, (c) detail of central area with small central nodule. (d) C. yapensis, sp. nov. from Yap (Y34A). Scale bars: (a, b, d) = 10 µm, (c) = 5 µm.
3.136. Navicula consors A.Schmidt in Schmidt et al. 1874–1959 Figure 45e
Previous Micronesia records: Guam (Lobban et al. 2012, p. 293, pl. 47,
Figure 8); 585561
Yap samples: Y37-8, Y37-7, Y37-8, Y41-7
Dimensions: Length 64–73 µm, width 16–18 µm, striae 7 in 10 µm
3.137. Navicula plicatula Grunow 1894 Figure 49a, b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 293, pl. 48, figs 1–3); Chuuk (Park et al. 2022, p. 41,
Figure 45); 585839
Yap samples: Y26C
Dimensions: Length 95 µm, width 32 µm, striae 15 in 10 µm
3.138. Navicula tsukamotoi (Sterrenburg & Hinz) Yuhang Li & Kuidong Xu in Li et al. 2017 Figure 49c
References: Sterrenburg et al. 2015, pp. 151–152, figs 14, 15, 33–38; Li et al. 2017, pp. 454–457, figs 5–21; Lobban et al. 2020, p. 172, figs 51–62
Previous Micronesia records: Guam (Lobban et al. 2012, p. 291, pl. 47, figs 6, 7, as Haslea howeana (Hagelstein) Giffen) Chuuk (Park et al. 2022, p. 41, figs 132, 133);
Yap samples: Y25H-1, Y25H-2, Y26C, Y37-7, Y37-8, Y36-1, Y41-7
Figure 49.
(a, b) Navicula plicatula SEM external and internal aspects of valve. (c) Navicula tsukamotoi valve external, SEM. Scale bars = 10 µm.
Figure 49.
(a, b) Navicula plicatula SEM external and internal aspects of valve. (c) Navicula tsukamotoi valve external, SEM. Scale bars = 10 µm.
Dimensions: Length 59–65 µm, width 11 µm, striae 16–17 in 10 µm
Comments: The reasons for adopting this name for the Micronesian records, and the relationship between the two species was discussed by Lobban et al. (2020).
3.139. Trachyneis aspera (Ehrenberg) Cleve 1894 Figure 50a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 293, pl. 48, figs 4–9); Chuuk (Park et al. 2018, p. 121, Figure 103, 104); 585562
Yap samples: Y25H-2, Y26C, Y37-7, Y37-8, Y36-1
Dimensions: Length 45–70 µm, width 13–16 µm, striae 13–16 in 10 µm
3.140. Trachyneis velata (A. Schmidt) Cleve 1894 Figure 50b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 293, pl. 49, figs 1, 2); Chuuk (Park et al. 2018, p. 121, Figure 105); 585841
Yap samples: Y25H-1, Y37-7
Dimensions: Length 45–62 µm, width 13 µm, striae 16 in 10 µm
Plagiotropidaceae D.G.Mann
3.141. Plagiotropis lepidoptera (W.Gregory) Kuntze 1898 Figure 50c–g, 51
Previous Micronesia records: Guam (Lobban et al. 2012, p. 295, pl. 51, figs 5–8); Chuuk (Park et al. 2018, p. 124, Figure 119); 585846
Yap samples: Y25H-1, Y41-7, -8
Dimensions: Length 59 µm, width 14 µm, striae 22–23 in 10 µm
Comments: Central striae more widely spaced and there is a small central area. In our material paired areolae formed a transverse slit (
Figure 50e, arrow) rather than the chevron (<) seen in other species. There is a suprising diversity of
Plagiotropis in Micronesia, some with scuta (either a bilayered wall with external outgrowth,
Figure 51b, or monolayered valve wall pleated into a flap,
Figure 51c), some with conopea over the central raphe endings (
Figure 51d); not yet analyzed.
Figure 50.
(
a)
Trachyneis aspera. (
b)
Trachyneis velata. (
c–g)
Plagiotropis lepidoptera. (
c) LM at two focal planes. (
d, e) SEM of external valve faces showing major and minor sides and paired areolae (arrow,
Figure 50e). (
f, g) Central area with enlargement of central nodule. Scale bars: (a–d) = 10 µm, (f) = 5 µm, (e) = 2 µm, (g) = 1 µm.
Figure 50.
(
a)
Trachyneis aspera. (
b)
Trachyneis velata. (
c–g)
Plagiotropis lepidoptera. (
c) LM at two focal planes. (
d, e) SEM of external valve faces showing major and minor sides and paired areolae (arrow,
Figure 50e). (
f, g) Central area with enlargement of central nodule. Scale bars: (a–d) = 10 µm, (f) = 5 µm, (e) = 2 µm, (g) = 1 µm.
Figure 51.
Morphological characters in Plagiotropis across Micronesia. (a) Unidentified species with scuta (arrows) (Y34H). (b) Portion of a valve showing double wall with external silica flap (arrow) forming the scutum (PW1990-47). (c) Portion of a valve showing single wall pleated to produce the scutum (arrow) (GU68D-1B). (d) Specimen with conopeum over central raphe endings (arrow) (Y45-2). Scale bars: (a, d) = 10 µm, (b, c) = 2 µm.
Figure 51.
Morphological characters in Plagiotropis across Micronesia. (a) Unidentified species with scuta (arrows) (Y34H). (b) Portion of a valve showing double wall with external silica flap (arrow) forming the scutum (PW1990-47). (c) Portion of a valve showing single wall pleated to produce the scutum (arrow) (GU68D-1B). (d) Specimen with conopeum over central raphe endings (arrow) (Y45-2). Scale bars: (a, d) = 10 µm, (b, c) = 2 µm.
Pleurosigmataceae Mereschkowsky
3.142. Pleurosigma simulacrum Lobban & F.A.S. Sterrenberg in Lobban 2021 Figure 52a, b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 295, pl. 51, figs 32–34, as Pleurosigma intermedium W.Smith); Guam, Chuuk and Yap (Lobban 2021, p. 253, figs 91–101; Yap record Y37-8); 197753
Additional Yap samples: Y26B, Y26C, Y42-1
Dimensions: Length 110–133 μm, width 13–14 μm, transverse striae 26 in 10 μm
Diagnostics: One of four perfectly straight, narrow species in a genus otherwise sigmoid (Lobban 2021, Lobban & Witkowski 2024b).
Comments:
Figure 52a, b shows the Yap record reported in Lobban (2021).
3.143. Rhoicosigma parvum Hein & Lobban 2015 Figure 52c, d
Previous Micronesia records: Guam (Hein & Lobban 2015, figs 1–37); 585625
Yap samples: Y37-8, Y36-1, Y41-7
Dimensions: Length 48 μm, width 8 μm, transverse striae 27 in 10 μm
Diagostics: In this genus, valves bent across the pervalvar axis, the concave valve with sigmoid raphe, convex with nearly straight raphe. This species smaller than R. compactum (Greville) Grunow reported for Micronesia.
Figure 52.
(a, b) Pleurosigma simulacrum, Yap voucher specimen in SEM at two magnifications. (c, d) Rhoicosigma parvum. Convex valve with straight raphe in LM, concave valve with sigmoid raphe in SEM. (e–g) Schizostauron cf. trachyderma. (e) Raphe valve in LM. (f) Sternum valve in LM. (g) Sternum valve internal aspect in SEM. Scale bars: (a) = 20 µm, (c–g) = 10 µm, (b) = 5 µm.
Figure 52.
(a, b) Pleurosigma simulacrum, Yap voucher specimen in SEM at two magnifications. (c, d) Rhoicosigma parvum. Convex valve with straight raphe in LM, concave valve with sigmoid raphe in SEM. (e–g) Schizostauron cf. trachyderma. (e) Raphe valve in LM. (f) Sternum valve in LM. (g) Sternum valve internal aspect in SEM. Scale bars: (a) = 20 µm, (c–g) = 10 µm, (b) = 5 µm.
Stauroneidaceae D.G.Mann
3.144. Schizostauron cf. trachyderma (F.Meister) Górecka & Riaux-Gobin in Górecka et al. 2021 Figure 52e–g
Previous Micronesia records: Guam (Lobban et al. 2012, p. 285, pl. 38, figs 5, 6, as
Achnanthes citronella; Lobban & Witkowski 2024b); Chuuk (Park et al. 2022, p. 43,
Figure 35); 585825
Yap samples: Y25H-2
Dimensions: Length 32 µm, width 16 µm, striae 10 in 10 µm
Comments: As with the Guam record (Lobban & Witkowski 2024b), our species previously identified as Achnanthes citronella (Mann) Hustedt is a species of Schizostauron but we can so far only propose it as Schizostauron cf. trachyderma (F.Meister) Górecka & Riaux-Gobin. The difficulties in deciding whether it is that species, S. kajotkei Dabek, Górecka & Witkowski, or neither was discussed with reference to Chuuk specimens by Park et al. (2022), and the SEM images we have to date for this species in our region do not resolve the question. The raphe valve is similar to Moreneis hexagona J.Park, Koh & Witkowski but the central raphe endings are not deflected.
Figure 53.
Amphora spp. (a) A. arenaria, LM. (b, c) A. bigibba SEM of frustules, arrows showing rows of apically elongate areolae near raphe ledge. (c, d) A. bigibba var. interrupta. SEM of frustule in ventral view and isolated valve, external aspect. Scale bars: (a) = 10 µm, (b–d) = 5 µm.
Figure 53.
Amphora spp. (a) A. arenaria, LM. (b, c) A. bigibba SEM of frustules, arrows showing rows of apically elongate areolae near raphe ledge. (c, d) A. bigibba var. interrupta. SEM of frustule in ventral view and isolated valve, external aspect. Scale bars: (a) = 10 µm, (b–d) = 5 µm.
THALASSIOPHYSALES D.G.Mann
Catenulaceae Mereschkowsky
3.145. Amphora arenaria Donkin 1858 Figure 53a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 297, pl. 53, figs 5, 6); Chuuk (Park et al. 2018, p. 126, Figure 142); 585568
Yap samples: Yap samples: Y25H-1
Dimensions: Length 72 µm, width 11, striae ca. 25 in 10 µm
3.146. Amphora bigibba Grunow 1875 in Schmidt et al. 1874–1959 Figure 53b, c
References: Güttinger 1990, series 4, 2.05.03-4; Navarro et al. 2000, p. 114, pl. 23,
Figure 2
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 150, figs 135, 136); Chuuk (Park et al. 2022, Figure 128); 585618
Yap samples: Y25H-1, Y26B, Y37-8, Y41-7
Dimensions: Length 12–20 µm, width 4–5, dorsal striae 19–20 in 10 µm, ventral 31–36 in 10 µm
Diagnostics: Small cells with rostrate apices and a strong constriction in the middle of the dorsal side, hence the name bigibba.
Comments: There are two rows of areolae along the dorsal side of the sternum oriented along the apical axis (
Figure 53b, c, arrows), contrasting with the areolae on the rest of the dorsal surface. These images are consistent with the SEM images in Navarro & Lobban (2009). There are other some varieties of
A. bigibba and some other species of
Amphora with a central constriction, e.g., A.
kolbei, and the small size suggests there may hidden diversity within this species. Hustedt (1955: 40) gives a long and inconclusive discussion of
A. bigibba from multiple localities and includes a drawing (pl. 14,
Figure 24) of a specimen with long protracted apices and a central break in the striae, which resembles the specimens shown below as var.
interrupta.
3.147. Amphora bigibba var. interrupta (Grunow) Cleve 1895 Figure 53d, e
References: Grunow 1875 in Schmidt et al. 1874–1959, pl. 25,
Figure 65; Witkowski et al. 2000, p. 131, pl. 163, figs 27–30; Hein et al. 2008, p.41, pl.18, figs 5–7
Yap samples: Y26C
Dimensions: Length 23 µm, width 4 µm, dorsal striae 27 in 10 µm
Diagnostics: Bigibbous valve with long-protracted apices, strongly bent central raphe endings, and break in dorsal striae at center of valve. Dorsal areolae hidden by overlying virgae development leaving slit; ventral areolae absent. There is a longitudinal break in the striae near the raphe ledge.
Comments: The difference in ultrastructure clearly marks these as different species from the nominate variety (see previous entry), so LM alone will not suffice to sort this complex. Montgomery (1978, pl. 21) shows a valve identified as v. interrupta in (A) and one in (B) identified as the nominate form, lacking an interruption, but otherwise having exactly the structure as v. interrupta.
3.148. Amphora hyalina Kützing 1844
Previous Micronesia records: Yap (Y16K) (Navarro & Lobban 2009, p. 145, figs 97, 98; 586297
Dimensions: Length 40–45 µm, width 8 µm, striae 28–30 in 10 µm.
3.149. Amphora immarginata Nagumo 2003 Figure 54a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 298, pl. 56, figs 2–5); 585573
Yap samples: Y37-8, Y41-7
Dimensions: Length 26–43 µm, width 8–10 µm, dorsal and ventral striae 17–18 in 10 µm
3.150. Amphora obtusa W.Gregory 1857 [b] Figure 54b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 298, pl. 54, figs 3, 4); 585848
Yap samples: Y26C
Dimensions: Length 49–106 µm, width 14–16 µm, dorsal and ventral striae 19–23 in 10 µm
Figure 54.
(a) Amphora obtusa, LM. (b) Amphora immarginata, SEM valve external. (c) Amphora ostrearia var. vitrea, SEM valve external. (d, e) Amphora proteus, LM, vale and frustule. (f). Amphora spectabilis, LM. (g) Amphora subhyalina, SEM of frustule. Scale bars = 10 µm.
Figure 54.
(a) Amphora obtusa, LM. (b) Amphora immarginata, SEM valve external. (c) Amphora ostrearia var. vitrea, SEM valve external. (d, e) Amphora proteus, LM, vale and frustule. (f). Amphora spectabilis, LM. (g) Amphora subhyalina, SEM of frustule. Scale bars = 10 µm.
3.151. Amphora ostrearia var. vitrea (Cleve) Cleve 1895 Figure 54c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 299, pl. 56, figs 5, 6); Chuuk (Park et al. 2022, Figure 140); 585576
Yap samples: Y41-7
Dimensions: Length 64 µm, width 14 µm, dorsal and ventral striae 15 in 10 µm
3.152. Amphora cf. proteus W.Gregory 1857 [a] Figure 54d, e
References: Witkowski et al. 2000, pl. 162, figs 5, 6; Wachnika & Geiser 2007, p. 432, figs 174–176; Levkov 2009, p. 104, pl. 112, figs 1–9, pl. 248, figs 4–6
Previous Micronesia records: Chuuk (Park et al. 2018, p. 128, figs 132)
Yap samples: Y25H-1, Y18B
Dimensions: Length 31–36 µm, width 8 µm, dorsal striae 15 in 10 µm, ventral striae 12 in 10 µm
Comments: Small compared to specimens in Levkov (2009) and Wachnicka & Geiser (2007). Resembling A. immarginata (see above) and “Amphora sp. 3” (Park et al. 2018, Figure 131), and several other species. Levkov (2009) provided some SEM but identifications in Wachnicka & Geiser (2007), Park et al. (2018), and here are based on LM. Rare in our material, and A. proteus, originally described from Scotland, remains a poorly supported hypothesis for these specimens.
3.153. Amphora spectabilis W.Gregory 1857 [a] Figure 54f
References: Peragallo & Peragallo 1897–1908, p. 216, pl. 48,
Figure 8; Levkov 2009, p. 265, pl. 114, figs 1–5, pl. 252, figs 1–5
Previous Micronesia records: Guam and Palau (Lobban & Witkowski 2024b, p. 3, figs 3, 4)
Yap samples: Y26C
Dimensions: Length 50–78 µm, width 14–17 µm, dorsal striae 7 in 10 µm, ventral striae 14 in 10 µm
Comments: Lobban & Witkowski (2024b) show SEM of a Palau specimen that matches SEMs in Levkov (2009).
3.154. Amphora subhyalina Podzorski & Håkansson 1987 Figure 54g
Synonym: A. insulana Stepanek & Kociolek 2018
Previous Micronesia records: Guam (Lobban & Witkowski 2024b, p. 3, figs 5–7)
Yap samples: Y26C, Y41-8
Dimensions: Length 27–42 µm, width 6–7 µm, dorsal striae 34–36 in 10 µm, ventral striae 37 in 10 µm
Comments: Lobban & Witkowski (2024b) discussed the synonymy.
3.155. Halamphora exigua (W.Gregory) Levkov 2009
References: Peragallo & Peragallo 1897–1908, p. 230, pl. 50, figs 30, 31. (as Amphora exigua Greg.); Archibald 1983 pp. 47, 48; Levkov 2009, pp. 188, 189
Previous Micronesia records: Yap Y14G (Navarro & Lobban 2009, p. 145, no fig., as Amphora exigua Greg.)
Dimensions: Length 24 µm, striae 12–13 in 10 µm (fide Navarro & Lobban 2009)
Comments: This and H. turgida are both small, faint, rostrate species with similar stria densities. Both Archibald (1983) and Levkov (2009) commented on how poorly this species is known and therefore how difficult it is to identify correctly. We consider this record dubious.
3.156. Halamphora turgida (W.Gregory) Levkov 2009
References: Peragallo & Peragallo 1897–1908, p. 231, pl. 50, figs 33; Wachnicka & Geiser 2007, p. 421, Figure 122 (both as Amphora turgida Greg.); Levkov 2009, p. 240, pl. 102: 1–8, pl. 236, figs 3, 5, 6
Previous Micronesia records: Yap Y24B (Navarro & Lobban 2009, p. 145, no fig., as Amphora turgida Greg.)
Dimensions: Length 25 µm, striae 12–13 in 10 µm (fide Navarro & Lobban 2009)
Comments: This species does not have the identification issues of the preceeding species and Levkov (2009) gives SEM images. Peragallo & Peragallo (1897–1908) state that this species differs from H. exigua in the stronger silicification, the dorsal margin more curved, the apices more capitate (vs. rostrate), and striae coarser, 7 vs. 12–18 in 10 µm. However, the stria densities reported in Navarro & Lobban (2009) were 12–13 in 10 µm for both species. Similar in general appearance to “Amphora sp. 1” shown for Chuuk in Park et al. (2018, p. 128, Figure 129), but the stria density there was 28 in 10 µm.
Figure 55.
(a, b) Thalassiophysa hyalina. (a) Valve in LM. (b) Center of valve exterior in SEM. (c) Undatella lineata valve in LM. (d) “Bacillaria paradoxa” Group B, SEM showing characteristic T-shaped terminal raphe ending (arrow). (e, f) Cymatonitzschia marina, frustules in LM and SEM. Scale bars: (a, c, e, f) = 10 µm, (b, d) = 5 µm.
Figure 55.
(a, b) Thalassiophysa hyalina. (a) Valve in LM. (b) Center of valve exterior in SEM. (c) Undatella lineata valve in LM. (d) “Bacillaria paradoxa” Group B, SEM showing characteristic T-shaped terminal raphe ending (arrow). (e, f) Cymatonitzschia marina, frustules in LM and SEM. Scale bars: (a, c, e, f) = 10 µm, (b, d) = 5 µm.
Thalassiophysidaceae D.G.Mann
3.157. Thalassiophysa hyalina (Greville) Paddock & Sims 1981 Figure 55a, b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 300, pl. 2, figs 1–8, pl. 58,
Figure 1); 585575
Yap samples: Y25H-2
Dimensions: Length 112 µm, width 30 µm, striae ca. 80 in 10 µm
Incertae Sedis
3.158. Undatella lineata (Greville) Paddock & Sims 1980 Figure 55c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 299, pl. 57, figs 1, 2); 585755
Yap samples: Y26C, Y41-7
Dimensions: Length 79 µm, width ca. 9 µm, striae 27 in 10 µm, fibulae 6 in 10 µm
BACILLARIALES Hendey
Bacillariaceae Ehrenberg
3.159.“. Bacillaria paradoxa” Group B sensu Schmid 2007 Figure 55d
Previous Micronesia records: Guam (Lobban et al. 2012, p. 300, pl. 58, figs 2–6, pl. 59, figs 1, 2); Chuuk (Park et al. 2022, p. 44, figs 144, 145; 585742
Yap samples: Y25H-1, Y37-8
Dimensions: Length 76–113 µm, width 4–5 µm, striae 24 in 10 µm, fibulae 7–8 in 10 µm
Comments: There are also much longer cells (205–215 µm), possibly a different species. The marine “carpenter’s-rule” diatom taxonomy is still in limbo. We use the T-shaped terminal raphe endings (
Figure 55d, arrow) as a clue to this group vs. other
Bacillaria spp. also present but unidentified, e.g.,
B. socialis (W.Gregory) Ralfs.
3.160. Cymatonitzschia marina (Lewis) Simonsen 1974 Figure 55e, f
References: Cleve 1878, p. 14; pl. 4,
Figure 26, as “
Denticula (?)
antillarum Cl. & Grun.”; Meister 1937, pl. 10,
Figure 3, as
Nitzschia antillarum (Cleve & Grunow) F.Meister; Simonsen 1974, p. 56, pl. 41, figs 5–9; Al-Yamani & Saburova 2011, p. 142, pl. 146g–i
Yap samples: Y25H-2, Y26B, Y26C
Dimensions: Length 43–67 µm, width 8–9 µm
Diagnostics: Alternating transverse hyaline ridges and pitted depressions are characteristic (κύματος = wave); there are no striae, only a line of pores along the opposite edge from the raphe/keel, visible only in SEM (
Figure 55f, arrow).
3.161. Gomphotheca marciae Lobban & Prelosky 2022
Previous Micronesia records: Yap, Palau (Lobban & Prelosky 2022, figs 1–26)
Dimensions: Length 348–624 μm, width tapering uniformly from 9.5–12.3 μm near apex to 4.2–4.7 μm near base; striae 35–45 in 10 μm, fibulae 5.5–6.5 in 10 μm
Comments: This large and robust species is from mangrove habitat.
Homoeocladia spp.
Multiple species of
Homoeocladia were found in Y26C (Lobban et al. 2023), two described from that sample, others described from other islands. Of those, three with published Yap vouchers are listed first, then three without Yap vouchers are illustrated (
Figure 56a–c), followed by other species found in Yap. Thirty species have been described from Micronesia and differention is almost entirely based on fine structural details. Many lanceolate ones, including potential species complexes in
H. dagmannii and
H. electrae, have yet to be analyzed. The diagnostics given below do not include all the character states that combine to define each species. Short species are up to 25 µm long, moderately long species 25–50 µm, long species 51–120 µm, and extremely long species >150 µm.
Figure 56.
Homoeocladia spp. Yap vouchers, SEM. (a) H. schefteropsis, showing areolae in peri-raphe zone (black arrow) and squiggly areolae on copulae (white arrow). (b) H. coacervata, showing areolae in peri-raphe zone (black arrow) and stacked slits on copulae (white arrow). (c) H. micronesica, showing no areolae in peri-raphe zone (black arrow). A row of small pores is present on the apex in all three species (arrowhead). Scale bars = 2 µm.
Figure 56.
Homoeocladia spp. Yap vouchers, SEM. (a) H. schefteropsis, showing areolae in peri-raphe zone (black arrow) and squiggly areolae on copulae (white arrow). (b) H. coacervata, showing areolae in peri-raphe zone (black arrow) and stacked slits on copulae (white arrow). (c) H. micronesica, showing no areolae in peri-raphe zone (black arrow). A row of small pores is present on the apex in all three species (arrowhead). Scale bars = 2 µm.
3.162. Homoeocladia celaenopsis Lobban, Sison and Ashworth 2023
Previous Micronesia records: Palau, Yap (Lobban et al. 2023, p. 32, figs 20A–G (E– G Yap vouchers, Y26C)
Dimensions: Length 46–58 µm, width 6 µm, striae 50 in 10 µm, fibulae 3–4 in 10 µm
Diagnostics: Moderate length, lanceolate, areolae S-shaped, weakly spathulate, two rows of S-shaped pores along each side of the peri-raphe zone.
3.163. Homoeocladia radiata Lobban, Sison, and Ashworth 2023
Previous Micronesia records: Yap (Lobban et al. 2023, p. 9, figs 4A–E; type locality Y26C)
Dimensions: Length 39–51 µm, width 6 µm, striae 50 in 10 µm
Diagnostics: Moderate length, lanceolate, striae strongly radiate towards apices.
3.164. Homoeocladia vittaelatae Lobban, Sison and Ashworth 2023
Previous Micronesia records: Yap (Lobban et al. 2023, p. 25, figs 15A–I; type locality, Y26C)
Dimensions: Length 89–98 µm, width 11 µm, striae 47 in 10 µm, fibulae 3.5 in 10 µm
Diagnostics: Long, linear, nonspathulate, copulae deep and densely perforated (visible in LM).
3.165. Homoeocladia schefteropsis Lobban, Sison and Ashworth 2023 Figure 56a
Previous Micronesia records: Palau, Yap, Guam (Lobban et al. 2023, p. 34, figs 21A–H, 22; Yap record Y26C)
Dimensions: Length 27–40 µm, width 4.5 µm, striae 55–56 in 10 µm, fibulae 5 in 10 µm
Diagnostics: Moderate length, lanceolate, indistinguishable from H. coacervata (next taxon) except by sigmoid girdle band areolae.
3.166. Homoeocladia coacervata Lobban, Sison and Ashworth 2023 Figure 56b
Previous Micronesia records: Guam, Yap, Palau, Marshall Islands (Lobban et al. 2023, p. 36, figs 23A–F; Yap record Y26C)
Dimensions: Length 30–39 µm, width 3–4 µm, striae 55 in 10 µm, fibulae 6–7 in 10 µm
Diagnostics: Moderate length, lanceolate, areolae on the copulae in short stacks of straight slits.
3.167. Homoeocladia micronesica Lobban, Sison and Ashworth 2023 Figure 56c
Previous Micronesia records: Palau, Yap (Lobban et al. 2023, p. 24, figs 14A–E; Yap record Y26C)
Dimensions: Length 86 µm, width 7 µm, striae 44–46 in 10 µm, fibulae 4 in 10 µm
Diagnostics: Long, linear, spathulate, differing from others of similar size and shape in areolae character and position
3.168. Homoeocladia martiana C.Agardh 1827 Figure 57a, b
Previous Micronesia records: Guam (Lobban & Mann 1987, figs. 4–13, Lobban & Tsuda 2003, Lobban et al. 2019, p. 214, figs 56–62)
Yap samples: Y26C
Dimensions: Length about 230 µm, entire specimens not observed in this sediment sample; width 3.5 µm, striae 30 in 10 µm, fibulae 6–7 in 10 µm
Diagnostics: Extremely long and slender, easily distinguished from other
Homoeocladia species but there is a scutate simulacrum,
Nitzschia venerata Lobban (2023a), in which flaps grow toward the keel rather than from it (as in
Plagiotropis,
Figure 51a–c).
Comments: This a tube-dwelling species that can form stout colonies up to 10 cm length.
3.169. Homoeocladia tarangensis (Lobban) Lobban & Ashworth 2022
Previous Micronesia records: Yap (type locality Y26C) (Lobban 2021, p. 257, figs 103–110)
Dimensions: Length 109–194 µm, width 10–12 µm, striae 46 in 10 µm, fibulae irregular, 2 in 10 µm.
Diagnostics: Valves long, spathulate in girdle view because of apical keel extensions; valve linear with rib along the edge of the valve depression, small pores in peri-raphe zone, raphe bordered by ribs.
Comments: Several similar species were seen in Yap samples but insufficient material so far to describe them.
Figure 57.
Homoeocladia spp., SEM. (a, b) H. martiana valve fragment at two magnifications. (c, d) H. volvendirostrata, frustule at two magnifications. Scale bars: (a) = 25 µm, (b, c) = 5 µm, (c) = 2 µm.
Figure 57.
Homoeocladia spp., SEM. (a, b) H. martiana valve fragment at two magnifications. (c, d) H. volvendirostrata, frustule at two magnifications. Scale bars: (a) = 25 µm, (b, c) = 5 µm, (c) = 2 µm.
3.170. Homoeocladia volvendirostrata (Ashworth, Dᶏbek & Witkowski) Lobban & Ashworth 2022 Figure 57c, d
Previous Micronesia records: Guam (Lobban et al. 2019, p. 210, figs 14, 24, 35–45); 586930
Yap samples: Y37-8
Dimensions: Length 13–17 µm, width 1.7–3 µm, striae 52 in 10 µm, fibulae 7–11 in 10 µm
Diagnostics: Small, lanceolate-elliptical valves with long rostrate endings (named for resemblance to a rolling pin). Indistinguishable in LM from several rare forms of similar size and shape bearing thickened external costae in various combinations described in Lobban et al. (2023). These decorated forms have not yet been seen in Yap samples but are very likely to co-occur. Homoeocladia volvendirostrata is widespread but considered to be a complex of several still-unresolved taxa.
3.171. Nitzschia frustulum (Kützing) Grunow in Cleve & Grunow 1880
References: Peragallo & Peragallo 1898–1907, p. 286, pl. 73,
Figure 25; Navarro 1982, p. 53, pl. 34, figs 11, 12; Witkowski et al. 2000, p. 382, pl. 209, figs 13–17
Previous Micronesia records: Yap Y14G (Navarro & Lobban 2009, p. 145, no fig.)
Dimensions: Length 17 µm, width 2.4 µm, striae 20–22 in 10 µm, fibulae 10 in 10 µm
Comments: One of the very small linear/lanceolate species with faint striae; Witkowski et al. (2000: 382) comment that “There exist several apparently independent species in a complex of very difficult to distinguish forms,” and they list a wide range of size, fibula and stria densities. I include this species on Navarro’s authority.
Figure 58.
Nitzschia spp. (a–c) Nitzschia longissima, SEM. (a, b) Whole valve, external view, with detail of central portion. (c) Internal aspect near central area showing uninterrupted striae and character of fibulae. (d, e) N. maiae, SEM and LM of frustule. (f) N. marginulata var. didyma frustule SEM. Scale bars: (a) = 25 µm, (b–f) = 10 µm.
Figure 58.
Nitzschia spp. (a–c) Nitzschia longissima, SEM. (a, b) Whole valve, external view, with detail of central portion. (c) Internal aspect near central area showing uninterrupted striae and character of fibulae. (d, e) N. maiae, SEM and LM of frustule. (f) N. marginulata var. didyma frustule SEM. Scale bars: (a) = 25 µm, (b–f) = 10 µm.
3.172. Nitzschia longissima (Brébisson ex Kützing) Ralfs in Pritchard 1861 Figure 58a–c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 302, pl. 60,
Figure 9, pl. 61,
Figure 1); 585583
Yap samples: Y25H-2, Y26B, Y37-8, Y41-7
Dimensions: Length 204 µm, striae 34 in 10 µm.
Comments: Nitzschia rectilonga Takano (Takano 1983) is very similar; the differences were clarified by Shorenko et al. (2016) and the species reported from Chuuk by Park et al. (2022, p. 44, figs 149–151). One difference is interruption of striae in internal aspect, so that the SEM image in Lobban et al. (2012) shows N. rectilonga. There appear to additional similar species in our region, but they are scarce and not yet adequately studied.
3.173. Nitzschia maiae Lobban, Ashworth, Calaor & E.C.Theriot 2019 Figure 58d, e
Previous Micronesia records: Guam (Lobban et al. 2019, pp. 208–210, figs 13, 26–34); Chuuk (Park et al. 2022, p. 44, Figure 148); 586925
Yap samples: Y36-1, Y36-5. Y41-7, Y41-8.
Dimensions: Length 33 µm, width 4 µm, striae 37 in 10 µm, fibulae 17 in 10 µm
Diagnostics: Very small conopea only at the apices, lacking a valve depression, and easily distinguished from Homoeocladia spp. by the high fibula density.
Comments: Common in Y36-1. Although a conopeate Nitzschia (Lobban et al. 2019), N. maiae was not transferred to Homoeocladia because it lacks the defining structure, i.e., valve depressions and conopea the length of the valve (Lobban & Ashworth 2022).
3.174. Nitzschia marginulata var. didyma Grunow in Cleve & Grunow 1879 Figure 58f
Previous Micronesia records: Guam (Lobban et al. 2012, p. 303, pl. 61, figs 2, 3); Chuuk (Park et al. 2018, p. 129, Figure 161); 585584
Yap samples: Y34C, Y34H, Y34F, Y36-2, Y36-5
Dimensions: Length 27–36 µm, width 9–10 µm, striae 28 in 10 µm, fibulae 16 in 10 µm
Comments: Although we have been using this name for this taxon, common in Micronesia, it was named in a slide set and never formally described or illustrated, and the name is thus invalid. The taxon has been transferred to Trybionella by Haworth & Kelly 2002, but in an unpublished ms., which is also invalid (see AlgaeBase entries; Guiry & Guiry 2024). The nominate variety was transferred legitimately to Tryblionella by D.G.Mann in Round et al. (1990: 678). Transfer of var. didyma must await taxonomic study of the species, and our specimens may not be the same taxon.
3.175. Nitzschia obtusa f. parva Hustedt 1921 in A.Schmidt et al. (1874–1959)
References: Hustedt in Schmidt et al. 1874–1959, pl. 336, figs 25, 26; Navarro 1982, p. 55, pl. 35, figs 8–10
Previous Micronesia records: Yap Y3E (Navarro & Lobban 2009, p. 147, figs 99–101)
Dimensions: Length 35–40 µm, width 5–7 µm, striae 30–35 in 10 µm
Comments: Park et al. (2022, p. 45, figs 50, 146, 147) identified similar specimens from Chuuk as Nitzschia cf. clausii Hantzsch, somewhat longer, narrower and more finely striated that the specimens reported from Yap. The SEM images are very similar in the two papers; I consider the two populations to be conspecific but the two identification hypotheses requiring further study. Two larger taxa claimed for the Micronesian flora, in the same group according to Peragallo & Peragallo (1897–1908), are N. scalpelliformis Grunow (Park et al. 2018, p. 129, figs 148, 149 [LM]) and N. vidovichii Grunow (Navarro & Lobban 2009, p. 147, figs 102–104). According to AlgaeBase this is a nom. inval.
Figure 59.
Nitzschia pseudohybridopsis, sp. nov. (a–c) Range of size in LM, (c) = holotype. (d). Frustule in girdle view, SEM, showing ‘papillae’ on valvocopula. (e). Valve in valve view. Scale bars: (a–c) = 10 µm, (d, e) = 5 µm.
Figure 59.
Nitzschia pseudohybridopsis, sp. nov. (a–c) Range of size in LM, (c) = holotype. (d). Frustule in girdle view, SEM, showing ‘papillae’ on valvocopula. (e). Valve in valve view. Scale bars: (a–c) = 10 µm, (d, e) = 5 µm.
3.176. Nitzschia pseudohybridopsis Lobban, sp. nov. Figs 59, 60
Diagnosis: Nitzschiae dubiae differing from N. pseudohybrida in the sparser fibulae.
Holotype: Specimen at 8.4 mm E and 8.3 mm S of the mark on slide 2815 deposited at ANSP, accession number pending.
Figure 59c.
Type locality: YAP STATE: Maap Island, Wanead, 9° 36’ 15” N, 138° 11’ 0” E; estuarine, intertidal rocks from mouth of stream, sample Y16B. Coll. C.S. Lobban & M. Schefter, 21 Sep. 1988. Plentiful in this sample.
Figure 60.
Nitzschia pseudohybridopsis, sp. nov., cont. (a, b) SEM of frustules showing details of the valvocopula (VC) with small pars exterior (pe) and larger papillate pars interior (pi), (b) also showing part of the internal keel with central nodule. Scale bars: 5 µm.
Figure 60.
Nitzschia pseudohybridopsis, sp. nov., cont. (a, b) SEM of frustules showing details of the valvocopula (VC) with small pars exterior (pe) and larger papillate pars interior (pi), (b) also showing part of the internal keel with central nodule. Scale bars: 5 µm.
Description: Valves lanceolate with rostrate apices, moderately eccentric, arched keel, the central raphe endings in the constriction (Figs 59a–e, 60b). Length 26–47 µm, width 4.5 µm, striae 42–45 in 10 µm, irregular fibulae 9–11 in 10 µm, prominent in LM (
Figure 59a–c) and further apart across central nodule (
Figure 60b). Wide hyaline border along the valve face–mantle. Several simple girdle bands but valvocopula unusual in having extensive pars interior, with irregular row of ‘papillae’ (occasionally visible in LM:
Figure 59b, arrow) on outer side, i.e., against hyaline valve border, and curled upper edge (
Figure 60a, b).
Etymology: Named for its resemblance to N. pseudohybrida.
Phycobank registration: pending
Additional materials: Y26C
Comments:
Nitzschiae dubiae was redefined by Hustedt (1955, p. 44) to include
Nitzschiae bilobatae for species with a moderately eccentric keel, constricted in the middle, and he added four new species from his Beaufort, NC mud sample. The Yap specimens are close to
N. pseudohybrida Hustedt (1955, p. 45, pl. 15, figs 3, 4), which is 25–48 µm long, 3–6 µm wide, striae about 40 in 10 µm, and fibulae 10–16 in 10 µm. The fibulae seem more prominent in our material (
Figure 59a–c) than in Hustedt’s, described as “very small, not elongated.” It is impossible to judge the comparison between our micrographs and Hustedt’s drawings, or Hustedt’s descriptive terms, given his much more extensive experience with the genus. Because of the novel ultrastructure observed and the different ocean basins involved, we judge the more conservative hypothesis is that the species are different, until
N. pseudohybrida can be studied in SEM.
3.177. Nitzschia ventricosa J.L.Palmer in Kitton 1873 Figure 61a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 304, pl. 62, figs 1, 2); Chuuk (Park et al. 2018, p. 129, Figure 151); 585587
Yap samples: Y26B
Dimensions (from Guam records): Length 150 µm, width 10 µm, striae 34 in 10 µm, fibulae and costae in central inflation 7–10 in 10 µm
Comments: Only one fragment observed in Yap samples.
3.178. Psammodictyon constrictum (W.Gregory) D.G.Mann in Round et al. 1990 Figure 61b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 304, pl. 62, figs 3, 4, as Nitzschia constricta (Gregory) Grunow); Chuuk (Park et al. 2018, p. 131, Figure 165); 585860
Yap samples: Y25H-1, Y25H-2, Y37-7, Y37-8, Y36-1
Dimensions: Length 22 µm, width 9 µm, striae 16 in 10 µm, fibulae 16 in 10 µm
Comments: This is part of a complex that needs much work. As we understand this species, there is no break in the striae The group is sometimes called Tryblionella coarctata (Grunow) D.G. Mann complex but the loculate structure of the common species in Micronesia seems to best fit into Psammodictyon (Lobban 2015a).
3.179. Psammodictyon panduriforme (W.Gregory) D.G.Mann in Round et al. 1990 Figure 61d, e
Previous Micronesia records: Yap Y16E (Navarro & Lobban 2009, p. 147, figs 105, 106); Guam (Lobban et al. 2012, p. 304, pl. 62, figs 3, 4); Chuuk (Park et al. 2018, p. 132, Figure 167); 585588
Yap samples: Y25H-1, Y26C, Y37-7, Y36-1
Dimensions: Length 90–100 µm, width 25 µm, striae 16–18 in 10 µm, fibulae 8 in 10 µm
3.180. Psammodictyon pustulatum (Voigt ex Meister) Lobban 2015 [a] Figure 61c
Previous Micronesia records: Guam (Lobban 2015a, pp. 12–13, figs 118–128); 585861
Yap samples: Y18E, Y36-4, Y37-8
Dimensions: Length 27–32 µm, width 15–17 µm, striae 22 in 10 µm
Diagnostics: This species is similar in size and areola pattern to P. constrictum but differs in the relief of the valve surface and in the presence of a longitudinal break across the striae.
Figure 61.
(a) Nitzschia ventricosa, valve fragment, LM. (b) Psammodictyon constrictum frustule in SEM. (c) Psammodictyon pustulatum frustule in SEM. (d, e) Psammodictyon panduriforme, SEM. (d) Frustule showing exterior valve face. (e) Internal valve face. Scale bars: (a, d, e) = 10 µm, (b, c) = 5 µm.
Figure 61.
(a) Nitzschia ventricosa, valve fragment, LM. (b) Psammodictyon constrictum frustule in SEM. (c) Psammodictyon pustulatum frustule in SEM. (d, e) Psammodictyon panduriforme, SEM. (d) Frustule showing exterior valve face. (e) Internal valve face. Scale bars: (a, d, e) = 10 µm, (b, c) = 5 µm.
3.181. Tryblionella granulata (Grunow) D.G.Mann in Round et al. 1990 Figure 62a
Previous Micronesia records: Yap (Navarro & Lobban 2009, p. 145, no fig.); Guam (Lobban et al. 2012 p. 302, pl. 60,
Figure 4) (both as
Nitzschia granulata Grunow); Chuuk (Park et al. 2022, p. 46, figs 56, 57); 585582
Yap samples: Y7I, Y34A
Dimensions: Length 16–24 µm, width 6–9 µm, striae 7.5 in 10 µm
Comments:
Figure 62a shows Navarro’s image of the Y7I voucher.
Entomoneidaceae
3.182. Entomoneis yudinii Prelosky & Lobban, sp. nov. Figure 62b–g
Diagnosis: Moderately silicified compared to congeners with prominent areolae in uniseriate striae.
Holotype: Specimen at 17.3 mm E and 1.3 mm S of the mark on slide 3038, deposited at Diatom Collection, Academy of Natural Sciences of Drexel University, Philadelphia, accession number [pending].
Figure 61c.
Figure 62.
(a) Tryblionella granulata, frustule, SEM, Yap voucher, courtesy of Nelson Navarro. (b–g) Entomoneis yudinii, sp. nov. (b, c) Valves in valve view, LM; (c) = holotype. (d) Valve in valve view showing overall shape and features of exterior. (e) Detail of valve showing depressed area with infilled areolae and the faint asymmetrical stauros (arrows). (f) Detail of valve in (d) showing outer coverings of areolae and a grooved rib alongside the raphe slit (arrow). (g) Interior showing smaller inner foramina, also occluded, and part of the keel canal with fibulae (arrow). Scale bars: (b–d) = 10 µm, (a, e, g) = 5 µm, (f) = 2 µm.
Figure 62.
(a) Tryblionella granulata, frustule, SEM, Yap voucher, courtesy of Nelson Navarro. (b–g) Entomoneis yudinii, sp. nov. (b, c) Valves in valve view, LM; (c) = holotype. (d) Valve in valve view showing overall shape and features of exterior. (e) Detail of valve showing depressed area with infilled areolae and the faint asymmetrical stauros (arrows). (f) Detail of valve in (d) showing outer coverings of areolae and a grooved rib alongside the raphe slit (arrow). (g) Interior showing smaller inner foramina, also occluded, and part of the keel canal with fibulae (arrow). Scale bars: (b–d) = 10 µm, (a, e, g) = 5 µm, (f) = 2 µm.
Type locality: Yap State, Yap Island, Weloy Municipality, Maa’ Mangrove, approx: 9°32’25.62” N, 138° 5’14.45” E, adjacent to Nimpal Marine Protected Area, sample Y34A, subtidal mud from Sonneratia alba (white mangrove) pneumorrhiza, ca 20 cm from bottom, and likely in the salt wedge. Coll. C.S. Lobban, M. Schefter & T. Gorong, 28 May 2014.
Description: Valve lanceolate–panduriform, 35–48 µm long, 13–15 µm wide (
Figure 62b–d), with strongly bilobate keel and sigmoid raphe, striae 17–20 in 10 µm, areolae prominent in LM, infilled in circular area toward each apex where valve is pinched in. External areola coverings ca. 350 nm diameter (
Figure 62f), internal opening also covered, ca. 180 nm diameter (
Figure 62g). Faint stauros or fascia visible in SEM (
Figure 62d), Raphe with rib on each side, one side with a parallel groove (
Figure 62d, f). Girdle bands not observed.
Etymology: Named in honor of Lee Yudin, former Dean of College of Natural and Applied Sciences, University of Guam for his support of the Microscopy Teaching and Research Laboratory over many years and for mentoring Prelosky.
Phycobank registration: pending
Additional records: YAP: Y34B
Comments: Recent research on Entomoneis has turned up diverse planktonic communities of very delicate species that can only be resolved with combined morphological and genetic data (Mejdandžić et al. 2018); there are also more freshwater forms than indicated in Round et al. (1990), including the robust E. corrugata (Giffen) Witkowski, Lange-Bertalot & Metzeltin, reported from Yap by Navarro & Lobban 2009.
Rhopalodiaceae (Karsten) Topachevs’kyj & Oksiyuk
3.183. Epithemia guettingeri (K.Krammer) Lobban & JoonS.Park in Park et al. 2018 Figure 63a–c
Previous Micronesia records: Yap Y3E, Y16E (Navarro & Lobban 2009, p. 147, Figure 107, as
Rhopalodia gibberula (Ehrenberg) O.Müller]; Guam (Lobban & Jordan 2010,
Figure 18.5f, as
Rhopalodia guettingeri); Chuuk (Park et al. 2018, p. 132, figs 171–173); 585589
Additional Yap samples: Y26C, Y29C, Y34A, Y34B, Y36-5, Y39A
Dimensions: Length 19–54 µm, width 7–11 µm, striae ca. 30–40 in 10 µm
Diagnostics: This species and
Epithemia pacifica (K.Krammer) Lobban & JoonS.Park are very similar marine species, differentiated in several ways including the character of the apical “costa,” which in
E. pacifica is a thickened costa interrupting the areolae but not causing a depression in the outer surface, whereas in
E. guettingeri it is a wave, with the wall the same thickness and perforated by some areolae (Krammer in Lange-Bertalot & Krammer 1987:83, Krammer 1988:169) (
Figure 63a). There is also a marked difference in stria densities (15–18 in 10 µm in
E. pacifica) and areola densities (18–27 in 10 µm in
E. pacifica vs. 42–48 in 10 µm), so that the striae and puncta of
E. pacifica are clearly visible in LM and those of
E. guettingeri not.
Comments: Small specimens (19–25 µm) had the higher stria densities (
Figure 63b). The broad range of size and stria density here suggest we may be confounding two species. There are several potentially new species in the region that may be differentiated on areola characters at the ultrastructural level, which still require study.
3.184. Epithemia muscula Kützing 1844 Figure 63d, e
Previous Micronesia records: Guam (Navarro & Lobban 2009, p. 147, no illus., as Rhopalodia musculus (Kützing) O.Müller); 585641
Yap samples: Y37-8
Dimensions: Length 12–30 µm, width 21 µm, areolae 21 in 10 µm
Comment: In line with the conclusions of Ruck et al. (2016a, b), although not mentioned there, we revert the name to Kützing’s original.
Figure 63.
Epithemia spp. in SEM. (a–c) E. guettingeri. (a) Valve view of ventral surface, showing axial “costa” formed by narrow wave in surface (arrow), note areolae in the groove (arrowhead). Stria density about 30 along keel. (b) Small specimen, ca. 40 striae, axial costa not so clear. (c). Interior aspect of valve showing transverse costae and part of keel canal (Palau specimen PW(2022)1A-5). (d, e) Epithemia muscula, SEM. (d) Frustule in oblique dorsal view, showing narrow dorsal surface (arrow). (e) Interior aspect of valve showing numerous transverse costae and large cribrate areolae. Scale bars = 5 µm.
Figure 63.
Epithemia spp. in SEM. (a–c) E. guettingeri. (a) Valve view of ventral surface, showing axial “costa” formed by narrow wave in surface (arrow), note areolae in the groove (arrowhead). Stria density about 30 along keel. (b) Small specimen, ca. 40 striae, axial costa not so clear. (c). Interior aspect of valve showing transverse costae and part of keel canal (Palau specimen PW(2022)1A-5). (d, e) Epithemia muscula, SEM. (d) Frustule in oblique dorsal view, showing narrow dorsal surface (arrow). (e) Interior aspect of valve showing numerous transverse costae and large cribrate areolae. Scale bars = 5 µm.
3.185. Protokeelia cholnokyi (Giffen) F.E.Round & P.W.Basson 1995 Figure 64a, b
Previous Micronesia records: Guam (Lobban 2015a, p. 12, figs 114–117); Chuuk (Park et al. 2022, p. 46, Figure 159); 585622
Yap samples: Y26B, Y26C
Dimensions: Length 5–17 µm, width 4–7 µm, biseriate striae ca. in 10 µm
Comments: Lobban (2015a) commented on the taxonomy of the known species; the present material seems to agree with the Guam specimens.
Figure 64.
(a, b) Protokeelia cholnokyi valve in LM and frustule in SEM. (c) Auricula intermedia. (d) Campylodiscus giffenii valve, SEM. (e) Campylodiscus humilis valve, LM. Scale bars: (a, c, e) = 10 µm, (b, d) = 5 µm.
Figure 64.
(a, b) Protokeelia cholnokyi valve in LM and frustule in SEM. (c) Auricula intermedia. (d) Campylodiscus giffenii valve, SEM. (e) Campylodiscus humilis valve, LM. Scale bars: (a, c, e) = 10 µm, (b, d) = 5 µm.
Auriculaceae Hendey
3.186. Auricula complexa (W.Gregory) Cleve 1894
Previous Micronesia records: Yap (Y26B) (Navarro & Lobban 2009, p. 147, figs 112–114); Chuuk (Park et al. 2022, p. 48, figs 65, 160)
Dimensions: Length 45 µm, striae 18–20 in 10 µm
Comments: Lobban & Witkowski (2024b) confirmed this identity vs. Auricula densistriata Osada.
3.187. Auricula flabelliformis M.Voigt 1960 [b]
Previous Micronesia records: Yap (Y26B) and Guam (Lobban 2015a, p. 3, figs 10–12; figs 10, 11 Yap vouchers); Chuuk (Park et al. 2022, p. 49,
Figure 66); 585876
Additional Yap records: Y26C
Dimensions: Length 92–95 μm, width 69 μm, striae ca. 19 in 10 μm; fibulae ca. 8 in 10 μm.
Comments: There is another large Auricula in the samples not yet identified.
3.188. Auricula intermedia (Lewis) Cleve 1894 Figure 64c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 304, pl. 63, figs 3, 4); Chuuk (Park et al. 2022, p. 49,
Figure 67); 585590
Yap samples: Y26B, Y26C, Y41-7
Dimensions: Length 52 μm, width 19 μm, striae 18 in 10 μm
Surirellaceae Kützing
3.189. Campylodiscus brightwellii Grunow 1862
Previous Micronesia records: Yap 16B (Navarro & Lobban 2009, p. 150, figs 128, 129); 585866
Yap samples: Y25H-1, Y25H-2, Y42-1
Dimensions: Diam. 25–30 µm
3.190. Campylodiscus giffenii Lobban & JoonS.Park in Park et al. 2022 Figure 64d
Synonyms: Surirella scalaris Giffen
Campylodiscus scalaris (Giffen) Lobban & JoonS. Park
Previous Micronesia records: Guam (Lobban et al. 2012, p. 308, pl. 68, figs 4–6, pl. 69, figs 1, 2; Lobban 2015a, p. 3, figs 15, 17, both as Surirella scalaris); Chuuk (Park et al. 2018, p. 134, Figure 176, as Campylodiscus scalaris); 585356
Yap samples: Y34B, Y41-8
Dimensions: Length 10–13 µm, width 10 µm
Comments: Lobban (2015a) showed that while
C. giffenii and
C. fastuosus are very similar in valve morphology (compare LM in Lobban 2015a, figs 14 vs. 15),
C. giffenii, as a former
Surirella, had the axes of the two valves parallel (Lobban et al. 2012, pl. 68,
Figure 6), whereas the axes in
C. fastuosus were at right angles, as originally defined in
Campylodiscus (Lobban 2015a,
Figure 16). Park et al. (2022: 55) corrected their invalid combination,
Campylodiscus scalaris.
3.191. Campylodiscus humilis Greville 1865 [b] Figure 64e
Previous Micronesia records: Guam (Lobban et al. 2012, p. 306, pl. 66, figs 1–4); 585674
Yap samples: Y37-8
Dimensions: Diam. 23–29 μm
Comments: This and the following species probably belong in Coronia but have not been transferred.
3.192. Campylodiscus imperialis Greville 1860
Previous Micronesia records: Yap 16E (Navarro & Lobban 2009, p. 150, Figure 127); 586287
Dimensions: Diam. 40–50 μm
3.193. Campylodiscus neofastuosus Ruck & Nakov in Ruck et al. 2016 [b] Figure 66a
Previous Micronesia records: Guam (Lobban et al. 2012, p. 307, pl. 67, figs 2, 3, pl. 68, figs 1–3, as Surirella fastuosa Ehrenberg); Chuuk (Park et al. 2018, p. 132, Figure 175); 216754
Yap samples: Y25H-2, Y37-8, Y36-1, Y41-7, -8
Dimensions: Length 39–90 μm, width 28–71 µm
3.194. Campylodiscus ralfsii W.Smith 1853
Previous Micronesia records: Yap 16B (Navarro & Lobban 2009, p. 150, figs 128, 129); 216757
Dimensions: Diam. 60–70 µm
3.195. Campylodiscus tatreauae Prelosky & Lobban, sp. nov. Figure 65
Diagnosis: Differing from C. neofastuosus in the aqueduct-like rim (raphe-keel), more prominent, barred fenestrae, and silica fins on the infundibular costae.
Holotype: Specimen at 21.7 mm E and 10.3 mm S of the mark on slide 1471, deposited at Diatom Collection, Academy of Natural Sciences of Drexel University, Philadelphia, accession number [pending].
Figure 65a.
Type locality: Yap State, Yap Island, Weloy Municipality, Maa’ Mangrove, approx: 9°32’25.62” N, 138° 5’14.45” E, adjacent to Nimpal Marine Protected Area, sample Y34A, subtidal mud from Sonneratia alba (white mangrove) pneumorrhiza, ca. 20 cm from bottom, and likely in the salt wedge. Coll. C.S. Lobban, M. Schefter & T. Gorong, 28 May 2014
Description: Valves heteropolar, ovate, with a wider end and narrower end (central raphe endings at the wider pole). Length 72–89 µm long, 49–60 µm wide, infundibula 17 in 100 µm. As in its simulacrum,
C. neofastuosus (
Figure 65a), there is a variable zone in the middle, often crossed by irregular ribs but showing no external perforations; internally, there is a ring of pits (
Figure 65d, f), visible in LM, bounding this zone. Infundibula have long funnels, reaching almost to the central zone (
Figure 65e, f). Fenestrae are wide and crossed by 2–3 ribs, these are visible in LM as well as SEM (Figs 65a–d, arrows). The raphe rim has smooth channel on the upper side, reminiscent of an aqueduct (Figs 65b, g, double-headed arrows), in contrast to a simple ridge with densely spaced short ridges on the inner slope that resembles the edge of a pie crust pressed down with a fork in
C. neofastuosus (cf.
Figure 66a). This difference is invisible in LM, as is the third major difference, the presence, usually, of multiple silica fins arising from the infundibular ridges (Figs 65b, g). These seem prone to erode and may sometimes not develop but are prominent when present.
Etymology: Named in honor of Prelosky’s high-school science teacher and mentor, Linda Tatreau, whose Marine Mania program introduced many students to marine biology.
Phycobank registration:
Additional material: Y34B, Y34C; PALAU: PW(2021)1-1, PW(2021)4-7, PW(2022)1-1, PW(2022)1A-5; GUAM: GU58G-1.
Comments: Camypolydiscus neofastuosus is one of the most commonly encountered species, with well-known variation (e.g., López Fuerte et al. 2010, pl. 41, 42) and this new species would have been easily overlooked had it not been for seeing the silica fins in SEM. The ribbed fenestrae can be seen in LM with precise focusing but are unlikely to be visible in images taken without awareness of their possible existence. Thus, it is possible that this species is widespread, though we have so far found it only in three islands in Micronesia, all in samples from mud on mangrove pneumorrhizae.
Figure 65.
Campylodiscus tatreauae sp. nov. Yap (a–c) and Palau (d–g). (a) Holotype in LM showing barred fenestrae (arrow). (b) Valve exterior showing aqueduct-like rim (double-headed arrow), silica flaps, and barred fenestrae (arrow). (c, d) Details of margin with barred fenestrae (arrows) (c) in LM from another specimen on same slide, (d) in SEM (PW(2022)1A-5). (e) Valve interior. (f) Detail of valve interior (same specimen). (g) Oblique external view of valve showing rim (double-headed arrow) and silica flaps. Scale bars: (a–c, e) = 10 µm, (d, f, g) = 5 µm.
Figure 65.
Campylodiscus tatreauae sp. nov. Yap (a–c) and Palau (d–g). (a) Holotype in LM showing barred fenestrae (arrow). (b) Valve exterior showing aqueduct-like rim (double-headed arrow), silica flaps, and barred fenestrae (arrow). (c, d) Details of margin with barred fenestrae (arrows) (c) in LM from another specimen on same slide, (d) in SEM (PW(2022)1A-5). (e) Valve interior. (f) Detail of valve interior (same specimen). (g) Oblique external view of valve showing rim (double-headed arrow) and silica flaps. Scale bars: (a–c, e) = 10 µm, (d, f, g) = 5 µm.
Figure 66.
(
a)
Campylodiscus neofastuosus, valve in SEM, showing small fenestrae and “pie-crust” rim (arrows). (
b)
Coronia ambigua, LM. (
c)
Coronia decora var.
decora, LM, frustule showing two axes at right angles. (
d)
Coronia decora var.
pinnata valve, LM. (
e, f)
Hydrosilicon mitra, SEM. (
e) Yap voucher. (
f) Detail of middle of valve interior, Guam specimen, showing striae converging on transverse and longitudinal ribs (see also
Figure 66a, b). Scale bars: (a–e) = 10 µm, (f) = 5 µm.
Figure 66.
(
a)
Campylodiscus neofastuosus, valve in SEM, showing small fenestrae and “pie-crust” rim (arrows). (
b)
Coronia ambigua, LM. (
c)
Coronia decora var.
decora, LM, frustule showing two axes at right angles. (
d)
Coronia decora var.
pinnata valve, LM. (
e, f)
Hydrosilicon mitra, SEM. (
e) Yap voucher. (
f) Detail of middle of valve interior, Guam specimen, showing striae converging on transverse and longitudinal ribs (see also
Figure 66a, b). Scale bars: (a–e) = 10 µm, (f) = 5 µm.
3.196. Campylodiscus wallichianus Greville 1863
Previous Micronesia records: Yap 26B (Navarro & Lobban 2009, p. 150, figs 130–133); 586288
Dimensions: Diam. 75–90 μm
3.197. Coronia ambigua (Greville) Ruck & Guiry 2016 Figure 66b
Previous Micronesia records: Guam (Lobban et al. 2012, p. 305, pl. 63, figs 5, 6, pl. 64,
Figure 1, as
Campylodiscus ambiguus Greville); 585658
Yap samples: Y26B
Dimensions: Diam. 104 µm
3.198. Coronia decora (Brébisson) Ruck & Guiry 2016 Figure 66c
Previous Micronesia records: Guam (Lobban et al. 2012, p. 305, pl. 64, figs 5, 6, as Campylodiscus decorus Brébisson); Chuuk (Park et al. 2018, p.132, Figure 170); 585867
Yap samples: Y25H-2, Y26B, Y26C
Dimensions: Diam. 39–41 µm
3.199. Coronia decora var. pinnata (Peragallo) Lobban & J.S. Park in Park et al. 2018 Figure 66d
Previous Micronesia records: Guam (Lobban et al. 2012, p. 302, pl. 65, figs 1, 2); 585870
Yap samples: Y26B
Dimensions: Diam. 72–80 µm
3.200. Hydrosilicon mitra Brun 1891 Figs 66e, f, 67
Previous Micronesia records: Guam (Lobban et al. 2012, p. 306, pl. 66,
Figure 5); 585594
Yap samples: Y37-8
Dimensions: Length ca. 100 μm, width 50 μm, striae 17 in 10 μm
Comments: For Yap, single valve fragment observed in SEM (
Figure 66e). This species is extremely rarely seen, and we show here additional images of a complete valve from Guam (GU75A-4). As noted by Round et al. (1990, p. 636), there is a raphe around each half of the cell, meeting in the central constriction.
3.201. Petrodictyon gemma (Ehrenberg) D.G. Mann in Round et al. 1990 Figure 68a–c
References: Sterrenburg (2001)
Yap samples: Y34A
Dimensions: Length 75–76 µm, width 43 µm; striae 20 in 10 µm, areolae ca. 24 in 10 µm
Comments: The specimen shown in
Figure 68a has visible areolae, one of the main characters Sterrenburg (2001) used to distinguish this from the simulacrum species,
P. patrimonii (see next entry). Densities cited by Witkowski et al. (2000) for
P. gemma are 18–25 striae and 22–30 puncta in 10 µm. Sterrenburg (2001) also stated that
P. patrimonii differs in two further ways: (1) it has 3–6 small round portulae between the 1st order fibulae (i.e., those with the long transapical costae) (
Figure 67e inset), versus 2–3 large, round portulae in
P. gemma (
Figure 68c inset); (2) its 2nd order fibulae do not form fibular costae. The number and shape of portulae in Navarro & Lobban’s (2009, Figure 121) figure shows sections with 3–6 portulae, suggesting that this would be
patrimonii and we have counted it as such below.
3.202. Petrodictyon patrimonii (Sterrenburg) Sterrenburg 2001 Figure 68d–f
Previous Micronesia records: Yap Y16B (Navarro & Lobban 2009, p. 148, figs 115–122, as P. gemma); Guam (Lobban et al. 2012, p. 306, pl. 66, figs 2, 3); Chuuk (Park et al. 2022, Figure 161); 585602
Figure 67.
(a, b) Hydrosilicon mitra, Guam specimen, showing entire specimen, valve internal view, and higher magnification of constriction where the two raphe endings meet in the nodule and from whence the striae radiate towards the transverse and longitudinal costae. Scale bars: (a) = 10 µm, (b) = 5 µm.
Figure 67.
(a, b) Hydrosilicon mitra, Guam specimen, showing entire specimen, valve internal view, and higher magnification of constriction where the two raphe endings meet in the nodule and from whence the striae radiate towards the transverse and longitudinal costae. Scale bars: (a) = 10 µm, (b) = 5 µm.
Additional Yap samples: Y26B
Dimensions: Length 77–84 μm, width 15–29 μm, striae 30 in 10 μm
Comments: See P. gemma, above.
Figure 68.
Petrodictyon. (a–c) Petrodictyon gemma. (a) LM showing visible striae and areolae. (b) Half of external valve view, SEM. (c) Half of internal view, SEM, inset showing 2–3 portulae between fibulae. (d–f) Petrodictyon patrimonii. Fig. (d) LM showing striae not resolved. (e) Internal view, SEM, inset showing 3–6 portulae between fibulae. (f) Frustule (broken open), showing depth of mantle. Scale bars = 10 µm.
Figure 68.
Petrodictyon. (a–c) Petrodictyon gemma. (a) LM showing visible striae and areolae. (b) Half of external valve view, SEM. (c) Half of internal view, SEM, inset showing 2–3 portulae between fibulae. (d–f) Petrodictyon patrimonii. Fig. (d) LM showing striae not resolved. (e) Internal view, SEM, inset showing 3–6 portulae between fibulae. (f) Frustule (broken open), showing depth of mantle. Scale bars = 10 µm.